Abstract
Burn injuries are prevalent and life-threatening forms that contribute significantly to mortality rates due to associated wound infections. The management of burn wounds presents substantial challenges. Hydrogel exhibits tremendous potential as an ideal alternative to traditional wound dressings such as gauze. This is primarily attributed to its three-dimensional (3D) crosslinked polymer network, which possesses a high water content, fostering a moist environment that supports effective burn wound healing. Additionally, hydrogel facilitates the penetration of loaded therapeutic agents throughout the wound surface, combating burn wound pathogens through the hydration effect and thereby enhancing the healing process. However, the presence of eschar formation on burn wounds obstructs the passive diffusion of therapeutics, impairing the efficacy of hydrogel as a wound dressing, particularly in cases of severe burns involving deeper tissue damage. This review focuses on exploring the potential of hydrogel as a carrier for transdermal drug delivery in burn wound treatment. Furthermore, strategies aimed at enhancing the transdermal delivery of therapeutic agents from hydrogel to optimize burn wound healing are also discussed.
Keywords: Hydrogel, transdermal drug delivery, therapeutic carrier, burn wound, tissue regeneration
Graphical Abstract
1. Introduction
A burn is defined as an injury to the skin that occurs due to contact with heat, electricity, radiation, lasers, or chemicals (Tiwari, 2012). The severity of a burn can be determined by assessing the depth of the burn wound. Burn wounds are classified into four degrees: first-degree, superficial or deep-partial thickness second-degree, third-degree, and fourth-degree (Jeschke et al., 2020) (Table 1). First-degree burns only affect the superficial epidermis layer of the skin, resulting in quick recovery without medical attention and minimal scarring in about a week (Yao et al., 2021). Second-degree burns, which can be superficial-partial thickness or deep-partial thickness, involve injury to the epidermis and the superficial or deep layers of the dermis. Recovery time for second-degree burns is longer than that for first-degree burns, typically taking 2–3 weeks if there is no wound infection. Deep-partial thickness second-degree burns take approximately 1–2 weeks longer to heal compared to superficial-partial thickness burns. Surgery may be necessary for deep-partial thickness burns, and there is a possibility of scarring (Markiewicz-Gospodarek et al., 2022). Third-degree burns affect the epidermis, dermis, and even the hypodermis (subcutaneous tissue), while fourth-degree burns extend beyond the full thickness of the skin to involve muscles and bones. Both third and fourth-degree burns require surgical intervention. In summary, the severity of burns increases with the degree of the burn (Bai et al., 2022). Consequently, the duration of wound healing and the care required for burn wounds are heavily dependent on the severity of the burns (Tiwari, 2012; Jeschke et al., 2020; Bai et al., 2022) (Table 1).
Table 1.
Classification of burn by depth.
| Burn degree/thickness | Depth | Treatment | Healing time | |
|---|---|---|---|---|
| First (superficial) | Superficial epidermis | - Cool water irrigation - Application of burn creams or ointment |
With minimal scarring in about a week | |
| Second | superficial-partial thickness | Epidermis and superficial of dermis | - Use of topical antimicrobial creams or dressings to prevent infection | With minimal scarring, < 3 weeks if there is no wound infection |
| deep-partial thickness | Epidermis and deep dermis | - Use of topical antimicrobial creams or dressings to prevent infection - Surgical intervention may be necessary |
Possibility of scarring, 3–5 weeks | |
| Third (full thickness) | Epidermis, dermis, and hypodermis (subcutaneous tissue) | - Surgical intervention, including excision and skin grafting - Temporary coverage with dressing /skin substitute |
Scarring, >8 weeks | |
| Fourth (full thickness) | Beyond the full thickness of the skin to involve muscles and bones | - Surgical intervention, often including excision, debridement, reconstruction, and grafting | Scarring, >8 weeks | |
Burn injuries are complex and can progress from acute to chronic conditions (Barrett et al., 2019). The disruption of the skin’s integrity and innate immune system in burn wounds makes them susceptible to microbial invasion and growth leading to infection (Church et al., 2006). Infection is the most common systemic complication of burn injuries that contributes to delayed wound healing and chronic inflammation (Markiewicz-Gospodarek et al., 2022). Severe burns can even result in sepsis, a life-threatening condition that causes multi-organ failure (Caraballo & Jaimes, 2019). Additionally, severe burns can induce hypovolemic shock due to increased capillary permeability (Arbuthnot & Garcia, 2019). Other common complications of burn wounds, especially in severe cases, include eschar formation, scarring, and contractures (Goel & Shrivastava, 2010; Tiwari, 2012). These complications not only have long-term negative effects on patients’ physical and physiological health but also impact their mental well-being (Barrett et al., 2019). It is worth noting that most burn injury deaths are caused by complications rather than burns (Lee et al., 2014). Therefore, the primary goal of burn wound healing is to prevent or treat infection and other complications while promoting accelerated wound healing with minimal scarring (Stone et al., 2018).
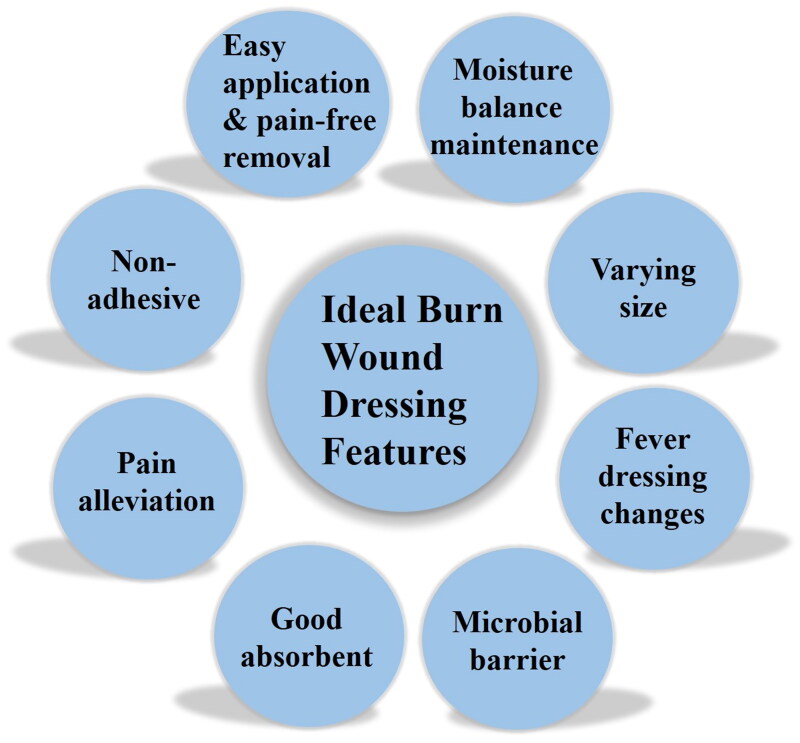
Wound dressing is the most commonly used approach to temporarily cover burn wounds, protecting against further injury and external infections, while facilitating wound healing (Madaghiele et al., 2014; Firlar et al., 2022). An online survey conducted among burn experts worldwide identified several desirable properties for an ideal burn wound dressing. Wound. It should be non-adhesive to the wound bed, easy to apply and remove, and allow for pain-free dressing changes. Moreover, it should possess anti-microbial, pain-relieving, and high absorbency properties. Additionally, wound dressings that require fewer changes and are available in various sizes are advantageous for burn wound management (Nischwitz et al., 2021). The concept of moist wound healing is also crucial in designing an optimal burn wound dressing (Benbow, 2008). The dressings should maintain an optimally moist environment to promote wound healing (Gupta et al., 2019) (Figure 1). Gauze is the widely used and cost-effective conventional wound dressing that covers the wound bed and absorbs wound exudates (Brumberg et al., 2021; Laurano et al., 2022). However, it has significant drawbacks. Gauze tends to adhere to the wound bed due to absorbed exudates, causing secondary trauma and pain during dressing removal (Dhivya et al., 2015; Farahani & Shafiee, 2021; Mirhaj et al., 2022). Additionally, gauze is a passive dressing that allows gases and moisture to pass through, making it challenging to maintain an appropriate moist environment for optimal wound healing (Brumberg et al., 2021). Thus, gauze is not an ideal choice for burn wound healing.
Figure 1.
Characteristics of an ideal burn wound dressing.
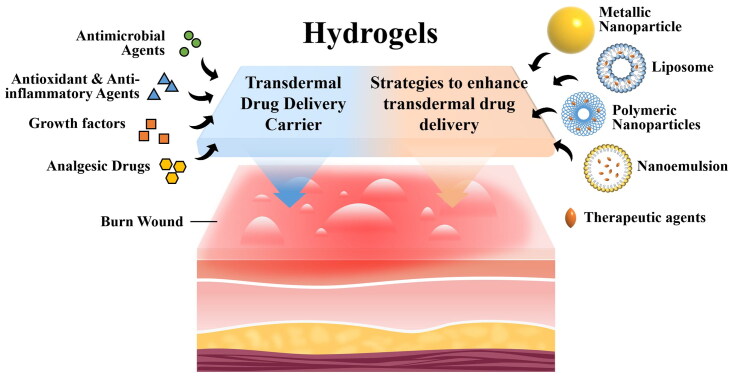
Over the past decade, various commercially available advanced wound dressings have been developed based on the concept of moist wound healing. These dressings are offered in various forms such as films, foams, hydrogels, and hydrocolloids (Gupta et al., 2019; Laurano et al., 2022; Mirhaj et al., 2022). Hydrogel has gained significant attention in the field of wound dressing due to its intrinsic hydrophilic 3D network. This network allows them to absorb wound exudates and create a moist environment that promotes wound healing. Furthermore, they are biocompatible and usually non-adhesive, facilitating easy application and removal from the wound (Yao et al., 2021). The 3D porous structure of hydrogels allows for the incorporation of antimicrobial and bioactive agents, which can effectively promote wound healing. Consequently, hydrogel dressings have the potential to revolutionize burn wound management and play a vital role in promoting burn wound healing. This review aims to emphasize the potential of hydrogels as carriers for transdermal delivery of drugs and therapeutic agents, specifically in the context of burn wound healing. Additionally, the review discusses the strategies employed to enhance the transdermal delivery of drugs and therapeutic agents, focusing on the utilization of hydrogel as a carrier.
2. Hydrogels as dressing for burn wounds
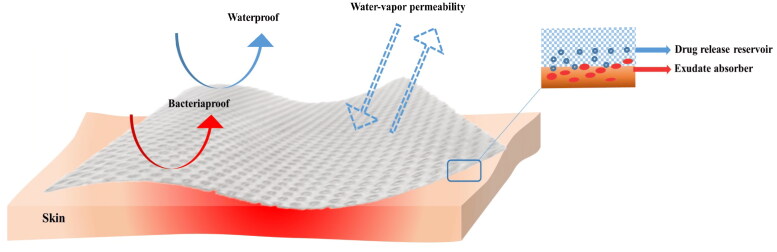
Hydrogels exhibit 3D, hydrophilic, insoluble structures composed of crosslinked polymer chains that undergo swelling in aqueous solutions without disintegration (Yao et al., 2021). Hydrogels can be synthesized from natural or synthetic polymers, or a combination of both (Jiann Chong et al., 2022). Polymeric hydrogels have garnered significant attention in wound dressing research due to their hydrophilic, ability to mimic the extracellular matrix (ECM), biocompatibility, and biodegradability (Madaghiele et al., 2014; Pan et al., 2021). Functioning as a physical barrier, hydrogel effectively protects the wound bed against external contamination while creating an ideal moist environment that facilitates wound repair. These hydrogels are considered inert (Stoica et al., 2020) (Figure 2). Moreover, the inherent high water content of hydrogels provides cooling and soothing effects, making them particularly suitable for burn wound dressings (1) (Stoica et al., 2020). Extensive literature has discussed the sources of polymers, crosslinking methods for hydrogel fabrication, and various types of hydrogels utilized for wound healing purposes (Fan et al., 2021; Pan et al., 2021; Firlar et al., 2022),
Figure 2.
Properties of hydrogel as a potential wound dressing for burn wound healing. Reprinted from an open-access source (Negut et al., 2018).
3. Hydrogels as transdermal drug delivery carriers for burn wound healing
Burn wound healing is often a complicated infection. Therefore, the conventional hydrogel dressings based on the concept of moist wound healing are insufficient in promoting burn wound healing when infection is present. The delivery of drugs and therapeutic agents, such as antimicrobial agents, growth factors, bioactive compounds, and stem cells, has demonstrated encouraging results in promoting burn wound healing (Rowan et al., 2015; Stoica et al., 2020; Shu et al., 2021; Yao et al., 2021). Given that burns can affect various body surfaces (Markiewicz-Gospodarek et al., 2022), transdermal drug delivery through the skin, the largest and easily accessible organ of the human body, is preferred for the localized or systematic administration and absorption of drugs and therapeutic agents for wound healing. Compared to the parenteral route, which involves needle-based injections, transdermal drug delivery is noninvasive, painless, and supports self-administration without professional assistance, thereby improving patient compliance (Alkilani et al., 2015). Moreover, by enabling the controlled release and permeation of drugs and therapeutic agents through the skin, transdermal delivery ensures localized and long-acting drug distribution at the wound site, while preventing systemic toxic complications and optimizing bioavailability by bypassing pre-systemic metabolism (Wang et al., 2021).
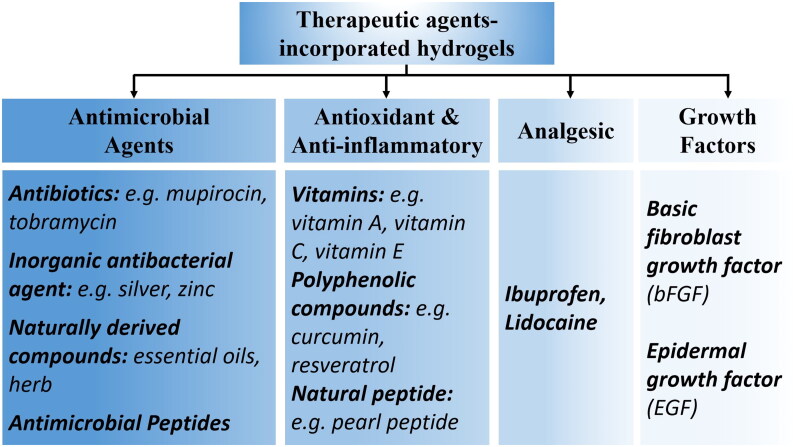
Hydrogels possess desirable characteristics, including biocompatibility, biodegradability, non-toxicity, lack of allergenicity, ease of application and removal, and high water content making them suitable as transdermal drug delivery carriers (Jacob et al., 2021). The hydration effect exerted by hydrogels on the skin enhances the penetration of therapeutics across the skin, facilitating the transdermal delivery of drugs (Kim et al., 2020). Additionally, drugs and therapeutic agents can be incorporated into the porous and crosslinked polymer network of hydrogels, allowing for controlled release through modulation of the hydrogel’s porosity, crosslinking degree, and swelling behavior (Johnson & Wang, 2015) (Figure 2). Moreover, external environmental stimuli such as temperature (Chen et al., 2022) and pH (Huang et al., 2022), can trigger drug release by modifying the chemical properties of the hydrogels (Firlar et al., 2022). This versatility further expands the application of hydrogels as wound dressing (Saghazadeh et al., 2018; Zhong et al., 2020). The drugs and therapeutic agents incorporated into the hydrogels can be categorized as follows: (i) antimicrobial agents, (ii) antioxidant and anti-inflammatory agents, (iii) analgesic drugs, and (iv) growth factors (Figure 3).
Figure 3.
Therapeutic agents-incorporated hydrogels that improve their versatility as a burn wound dressing.
3.1. Antimicrobial agents-incorporated hydrogels
Burn wound infection is the most prevalent complication of burn injuries, often caused by microorganisms such as Pseudomonas aeruginosa, S. aureus, and methicillin-resistant S. aureus (Church et al., 2006). This infection impedes wound healing and can be fatal for burn patients (Jeschke et al., 2020). Although inert hydrogel could act as a physical barrier against external microorganisms, it is insufficient to counteract microbial invasion and colonization (Yao et al., 2021). To address this issue, researchers have developed a strategy to incorporate antimicrobial agents into hydrogels.
The most common approach for combating wound infection is to include antibiotics in hydrogels, which can either kill or impede the growth of pathogens (Gupta et al., 2020). Various antibiotics, including tobramycin (Huang et al., 2022), ciprofloxacin (Dong et al., 2022; Zhu & Chen, 2022), gentamycin (Nuutila et al., 2020), mupirocin (Chen et al., 2022; Han et al., 2022), and vancomycin (Nuutila et al., 2020), have been incorporated into hydrogels to combat microbial infection in burn wounds. For instance, a tobramycin-loaded self-healing hydrogel was developed for the treatment of P. aeruginosa-infected burn wound healing. The hydrogel was composed of quaternized chitosan, oxidized dextran, and polydopamine-coated polypyrrole nanowires, linked together by Schiff base bonds. The release of tobramycin was sustained and controlled, triggered by the pH changes during the bacteria growth. When bacteria respire and ferment, they produce acidic products like carbonic acid and lactic acid. This results in a slightly acidic environment that promotes hydrogel degradation and the release of tobramycin. In vitro experiments showed that the sustained and controlled release of tobramycin effectively killed P. aeruginosa, Staphylococcus aureus, and Escherichia coli. It also demonstrated superior healing of bacterial-infected burn wounds compared to commercial products (Huang et al., 2022). However, the extensive use of antibiotics has led to the development of drug resistance in bacteria (Zhong et al., 2020; Bai et al., 2022). Therefore, in the past decade, antibiotic substitutes such as metal ions and naturally derived agents have emerged as leading candidates in the fight against wound infections (Zhong et al., 2020).
Inorganic antibacterial agents, including silver (Gupta et al., 2016; Low et al., 2016; Kumar & Ghosh, 2017; Banerjee et al., 2019), zinc (Li et al., 2017; Zhang et al., 2020; Tao et al., 2022), and copper (Klinkajon & Supaphol, 2014; Qian et al., 2022; Xia et al., 2022) ions are commonly used in the treatment of infected wounds. Among them, silver ions have long been recognized for their broad-spectrum antibacterial activity and lack of drug resistance, making them a viable alternative to antibiotics (Wahid et al., 2017; Laurano et al., 2022; Liu et al., 2022). In one study, an ionically crosslinked chitosan hydrogel was developed to function as a controlled release delivery system for silver (Ag+) ions (as silver nitrate, AgNO3) and tea tree oil (TTO). The controlled release was achieved through hydrogel degradation via erosion, thereby facilitating wound management. This hydrogel successfully demonstrated broad-spectrum antimicrobial activity against representative wound-infecting organisms, namely P. aeruginosa, S. aureus, and Candida albicans (Low et al., 2016). In another study, zinc (Zn) was incorporated into a gelatin-based hydrogel using an ionic crosslinking method. The release of Zn was achieved by the swelling of hydrogel, which demonstrated potent antibacterial activity against E. coli and S. aureus. This antibacterial effect was attributed to the increased levels of intracellular reactive oxygen species and the alteration of bacterial membrane permeability. Furthermore, it also improved the healing process of S. aureus-infected full-thickness wounds by reducing inflammation and enhancing re-epithelization and angiogenesis (Tao et al., 2022). In addition to the previously mentioned commonly used metal ion, Qin and colleagues discovered gallium-incorporated alginate hydrogel as a potential antimicrobial dressing. This is due to the release of the gallium ion (Ga3+) through the replacement of univalent metal cations in the surrounding environment, leading to gel degradation. This hydrogel exhibited significant antibacterial activity against E. coli and S. aureus in vitro and accelerated the healing of S. aureus infected wounds (Qin et al., 2022). Therefore, including metal ions in hydrogels holds the potential for burn wound dressing.
Currently, naturally derived antimicrobial agents extracted from plants or animals offer a viable approach for fabricating antibiotics-free hydrogel to treat wound infection (Zhong et al., 2020; Zhang et al., 2021; Ryall et al., 2022). Essential oils, which are bioactive secondary metabolites of plants possessing antibacterial, antifungal, antioxidant, and anti-inflammatory properties (Seow et al., 2014; De Luca et al., 2021), are excellent candidates for incorporation into hydrogels (Low et al., 2016; Jiji et al., 2019; Wang et al., 2021). Thymol, a phenol derivative of essential oils extracted from various plants, possesses potent antimicrobial properties. By integrating thymol into bacterial cellulose (BCT) hydrogel, an excellent antibacterial wound dressing against burn-specific infections was created. Compared to pure bacterial cellulose (BC) hydrogel, BCT hydrogel promotes faster burn wound closure in rat models (Jiji et al., 2019). Additionally, rhein, a bioactive anthraquinone extracted from the traditional Chinese herb rhubarb and possessing favorable antimicrobial and anti-inflammatory properties, was incorporated into designated hydrogels using the chemical crosslinking method (Yin et al., 2022) and the self-assembly technique (Li et al., 2022), respectively. Both rhein-incorporated silk fibroin (Yin et al., 2022) and aramid nanofibers-based (Li et al., 2022) hydrogels demonstrated satisfactory antibacterial efficiency. Furthermore, they promoted healing in bacteria-associated burn wound healing by reducing inflammation, and stimulating angiogenesis, thereby leading to the development of advanced antibacterial wound dressings for burn wounds.
In addition to the previously mentioned antimicrobial agents, antimicrobial peptides (AMPs) offer an alternative to antibiotics in fighting drug-resistant bacteria while being less prone to drug resistance (Bai et al., 2022). These peptides have been successfully incorporated into hydrogels for burn wound treatment (Ouyang et al., 2018; Zhou et al., 2023), enabling sustained and controlled release to maintain their bioactivity. For instance, in a study, a natural AMP called Jelleine-1 was physically self-assembled into the hydrogel. The formed hydrogel displayed excellent biocompatibility in both in vitro and in vivo settings. Moreover, it exhibited remarkable antimicrobial activity against S. aureus, MRSA, E. coli, and C. albicans in vitro. Due to its potent antimicrobial activity, Jelleine-1 promoted wound healing in a burn wound infected with MRSA (Zhou et al., 2023).
3.2. Antioxidant and anti-inflammatory agents-incorporated hydrogels
The healing of burn wounds is often complicated by excessive inflammation (Almasaudi, 2021; Markiewicz-Gospodarek et al., 2022). Therefore, the development of anti-inflammatory hydrogels is also crucial. Hydrogels loaded with antioxidant and anti-inflammatory agents, including vitamins (Soriano-Ruiz et al., 2020; Soriano et al., 2021), curcumin (Dang et al., 2019; Babaluei et al., 2023), polyphenolic compounds (Wang et al., 2019; Shao et al., 2022), and natural peptides (Liu et al., 2022), have shown promise in enhancing burn wound healing. For instance, tannic acid, a polyphenolic compound derived from plants known for its antioxidant and anti-inflammatory properties, was incorporated into a novel adhesive, hemostatic and biocompatible hydrogel composed of thioctic acid and phytic acid (TAPA). The presence of tannic acid in this hydrogel, referred to as TATAPA, exhibited better DPPH radical scavenging activity compared to the TAPA hydrogel alone. Moreover, TATAPA demonstrated satisfactory antibacterial and anti-inflammatory activities in a methicillin-resistant S.aureus (MRSA) infected burn wound, surpassing the performance of the TAPA hydrogel (Shao et al., 2022). Another study conducted by Liu et al. utilized the pearl peptides extracted from pearl powder to construct a hydrogel with strong antioxidant and antibacterial properties for burn wound healing. The inclusion of selenium-containing block-functionalized polyethylene glycol (PEG)/Polypropylene glycol (PPG) hydrogels with pearl peptide was found to enhance the viability of skin fibroblast by alleviating oxidative stress. Furthermore, the sustained release of pearl peptide from hydrogel, achieved through the degradation of the hydrogel via the breakage of the selenium-containing bond, demonstrated significant enhancement in the healing of deep partial thickness burn in the rat model. This enhancement was characterized by faster re-epithelialization, formation of granulation tissue, and angiogenesis compared to the group treated with pure hydrogel (Liu et al., 2022).
3.3. Analgesic drugs-incorporated hydrogels
Burn patients may endure more pain than other types due to frequent dressing changes (Laurano et al., 2022) and damage to the dermis that could touch the nerve endings (Yao et al., 2021). To address this issue, various strategies have been explored using hydrogels loaded with analgesic and anti-inflammatory drugs (Teoh et al., 2021). For example, ibuprofen, a non-steroidal anti-inflammatory and analgesic drug, is loaded onto alginate hydrogel fabricated via pressurized gas expanded liquid (PGX) technology. The controlled release of ibuprofen from the PGX-alginate/calcium hydrogel by re-dissolution greatly enhanced in vivo burn wound healing by suppressing inflammation (Johnson et al., 2020). Lidocaine is an analgesic drug that possesses an antioxidant effect (Lenfant et al., 2004; Jae et al., 2010; Laurano et al., 2022). In a recent study, lidocaine was added along with ciprofloxacin, an antimicrobial compound in a carbomer (CbCipLid) hydrogel for the treatment of second-degree burns. The release of lidocaine by diffusion provides an immediate anesthetic effect on the wound. Moreover, the combination of lidocaine release and ciprofloxacin release shortened the wound healing period by increasing the fibroblast proliferation and re-epithelialization rate (Sanchez et al., 2018).
3.4. Growth factors-incorporated hydrogels
The wound healing process is a complex and dynamic physiological process involving multiple cell types, growth factors, chemokines, and cytokines (Gupta et al., 2019). Growth factors (GFs) are endogenous signaling molecules that play a crucial role in wound healing by regulating cell growth, proliferation, migration, and differentiation (Barrientos et al., 2008; Park et al., 2017). However, in chronic and burn wounds, excessive degradation of endogenous GFs impaired the healing process (Zhang et al., 2016; Miricescu et al., 2021). To address this issue, the delivery of exogenous GFs to the wound site has been explored. Unfortunately, their effectiveness is limited due to their short half-life, low in vivo stability, and rapid elimination by wound exudates (Goh et al., 2016; Zhang et al., 2016; Divyashri et al., 2022). Polymeric hydrogels that provide sustained release of GFs and protect them from degradation have shown promising results in enhancing wound healing (Goh et al., 2016; Fan et al., 2021; Kushibiki et al., 2021; Wang et al., 2022). This approach is not only effective but also cost-effective, as high doses and frequent administration of GFs are avoided.
The major GFs involved in acute and chronic wound healing include epidermal growth factor (EFG), fibroblast growth factor (FGF), transforming growth factor-β (TGF- β), vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF) (Barrientos et al., 2008; Park et al., 2017; Gupta et al., 2019). Incorporating EGF (Rezaei et al., 2020; Wang et al., 2021) and bFGF (Mai et al., 2020; Chakrabarti et al., 2021) into hydrogels has been explored to promote burn wound healing. bFGF is well-known for its ability to stimulate angiogenesis, granulation tissue formation, re-epithelialization, and remodeling by promoting fibroblast and endothelial cell migration and proliferation (Gupta et al., 2019; Chakrabarti et al., 2021). In one study, collagen-silver sulfadiazine (AgSD) incorporated hydrogel with bFGF promoted burn wound healing in a rat model by stimulating fibroblast proliferation and alleviating burn wound inflammation. This was achieved through activation of the ERK and TRK pathway, leading to increased re-epithelization and granulation tissue formation (Chakrabarti et al., 2021). In another study, researchers demonstrated that the release of EGF from poly (N-isopropyl acrylamide) (PNIPAM) hydrogel loaded with vancomycin (VANCO) nanoparticles via hydrogel swelling supported fibroblast cell proliferation and growth. The EGF-containing hydrogel also stimulated better re-epithelialization, wound contraction rate, neovascular formation, and transforming growth factor-beta expression in a rat burn wound infected with S. aureus compared to untreated and pure hydrogel groups (Rezaei et al., 2020).
4. Strategies to enhance transdermal drug delivery for burn wound healing
Hydrogels have demonstrated potential as transdermal drug delivery carriers for burn wound healing due to their inherent high water content, which serves as a natural penetration enhancer (Karande & Mitragotri, 2009). However, the unique characteristics of burn wounds pose challenges to the efficacy of transdermal drug delivery. Eschar, a layer of stiff necrotic tissue that forms on the wound bed, is a local complication of the burn wound (Saghazadeh et al., 2018). The presence of eschar impedes the effective penetration of drugs and therapeutic agents incorporated in hydrogels into the deeper injured tissue (Tiwari, 2012; Cartotto, 2017; Souto et al., 2020). Additionally, the high exudate levels in burn wounds can further impact the bioactivity and bioavailability of penetrated drugs and therapeutic agents (Johnson & Wang, 2015; Whittam et al., 2016; Yao et al., 2021). Consequently, various approaches have been investigated to overcome these barriers and enhance the transdermal delivery of drugs and therapeutic agents through the eschar into the deeper wound area, as well as improve their bioavailability by utilizing hydrogels as carriers.
4.1. Nanoparticles (NPs)
NPs have been extensively investigated for their potential to enhance the transdermal delivery of drugs and therapeutic agents in wound healing applications (Souto et al., 2020; Huang et al., 2021; Shalaby et al., 2022). Due to their small size (1–100 nm), NPs significantly enhance the penetration of drugs and therapeutic agents into the wound (Souto et al., 2020; Blanco-Fernandez et al., 2021). It is crucial to maintain the concentration and bioactivity of drugs or therapeutics agents at the burn wound site for effective wound healing. While hydrogels provide control over the release rate of drugs and therapeutic agents, they suffer from the drawback of burst release (Johnson & Wang, 2015). NPs offer a solution for achieving controlled and sustained release of drugs and therapeutic agents, while also increasing the agents’ half-life by protecting them from intense proteolysis in the burn wound microenvironment (Blanco-Fernandez et al., 2021; Huang et al., 2021). All of these factors contribute to improving the bioactivity and bioavailability of these agents in the local wound tissues (Hussain et al., 2022). Therefore, NP-loaded hydrogel is an established strategy aimed at preventing and treating wound infections, as well as promoting re-epithelization. The NPs can be either non-polymeric or polymeric (Table 2).
Table 2.
Strategies for transdermal therapeutic agents delivery enhancement in burn wound healing by using hydrogel systems as the delivery carrier.
| Hydrogel composition | Nanoparticles/Nanoparticle-based carriers | Particle Size (nm) | Therapeutic agents | Burn wound model | Findings |
|---|---|---|---|---|---|
| Non-polymeric | |||||
| Oxidized dextran (ODex), adipic dihydrazide grafted hyaluronic acid (HA-ADH), and quaternized chitosan (HACC) | Silver (Ag) Reducing agent: Chitosan |
∼50–190 | – | P. aeruginosa infected burn wound in rat model | - Enhanced antibacterial effect against E. coli, S. aureus, and P. aeruginosa compared to the hydrogel only group in both in vitro and in vivo experiments. - Promoted burn wound healing, as evidenced by minimal scar formation, nearly complete re-epithelialization, well-organized collagen deposition, and significant suppression of inflammatory factors compared to other groups, including commercial Ag dressings containing 1.2% ionic silver (Chen et al., 2021). |
| Carbopol-940 | Silver (Ag) Reducing agent: Mimosa tenuifora (plant extract) |
∼21 | – | Second-degree burn wound in rat model | - Enhanced antibacterial effect against E. coli in vitro, surpassing that of the control gel, gel loaded with Mimosa tenuifora (MtE), and commercially available Ag NPs loaded gel. - Accelerated wound healing in comparison to the control gel, as well as gels loaded with MtE and commercially available Ag NPs (Martínez-Higuera et al., 2021). |
| Carbomer 934 P | Silver (Ag) | <20 | – | Acute burn injury in mouse model | - Enhanced antibacterial activity against MRSA and P. aeruginosa compared to both blank gel and commercially available silver nanoparticles gel. - Prevented bacterial colonization and effectively reduced inflammation, surpassing the effectiveness of both the blank gel and commercially available silver nanoparticle gel. - Accelerated wound healing, characterized by a faster wound closure rate, a higher level of re-epithelialization, increased collagen deposition, and reduced scar formation, in comparison to both the blank gel and a commercially available silver nanoparticles gel (Chen et al., 2020). |
| Ulvan (green algae Ulva Lactuca Linn extract) | Silver (Ag) Reducing agent: Ulvan |
∼13–22 | – | Second-degree thermal burn wound in rat model | - Enhanced antibacterial effect against S. epidermidis, E. coli, S. aureus, and P. aeruginosa compared to the hydrogel only group in in vitro experiment. - Accelerated wound closure during the initial 14 days following the burn injury compared to the hydrogel only group and negative control. - Improved re-epithelization compared to the hydrogel only group and negative control (Sulastri et al., 2023). |
| Sodium alginate and kappa-carrageenan with a concentration ratio of 60:40 | Silver (Ag) Reducing agent: Sodium alginate and kappa-carrageenan |
∼2–6 | – | Second-degree burn wound in mouse model | - Improved antibacterial effect against E. coli and S. aureus compared to the other hydrogels with varying ratios of sodium alginate and kappa-carrageenan concentrations. - Accelerated wound closure during the initial 14 days following the burn injury compared to the other hydrogels with varying ratios of sodium alginate and kappa-carrageenan concentrations. - Improved angiogenesis and collagen deposition at the wound site compared to the other hydrogels with varying ratios of sodium alginate and kappa-carrageenan concentrations (Zia et al., 2020). |
| Gelatin | Titanium dioxide (TiO2) | 15–25 | – | Third-degree burn wound in mouse model | - Increased antibacterial activity against S. aureus and P. aeruginosa compared to the gelatin gel only group and SSD 1% ointment in the in vitro experiment. - Promoted wound healing by facilitating wound contraction, stimulating angiogenesis, and promoting fibroblast proliferation and granulation tissue formation compared to only gelatin gel and silver SSD 1% ointment groups (Javanmardi et al., 2018). |
| Methylcellulose | Silver oxide (AgO) | – | – | Second-degree burn wound in rat model | - Enhanced antibacterial effect against E.coli, S. aureus, and K. pneumoniae compared to the hydrogel only group in in vitro experiment. - Promoted wound healing by suppressing inflammation, enhancing re-epithelialization and collagen deposition compared to control and the hydrogel only groups (Kim et al., 2018). |
| Carbopol | Nanoemulsion | ∼160–180 | Curcumin and resveratrol | Second-degree burn wound in rat model | - Improved curcumin and resveratrol skin penetration and retention. - Accelerated wound healing in burnt rats by enhancing antioxidant and anti-inflammatory potential, while also increasing collagen and amino acid levels in the skin compared to the control group, as well as only curcumin-loaded nanoemulgel and resveratrol-loaded nanoemulgel (Alyoussef et al., 2021). |
| Starch-g-poly (acrylic acid-co-acrylamide) with 0.3 w/v% of starch | Nanoemulsion | 175 | Myrtus oil | Second-degree burn wound in rat model | - Improved drug sustained release compared to the hydrogel sample containing 1 w/v% of starch. - Enhanced antibacterial effect against E.coli, Candida albican, and MRSA compared to the hydrogel sample with 1 w/v% of starch in in vitro experiment. - Accelerated wound healing compared to the control group, as well as the group treated with myrtus oil nanoemulsion-loaded hydrogel containing 1 w/v% starch and silver sulfadiazine 1% (Moradi et al., 2023). |
| Poloxamer (P407, P188) and polyvinyl alcohol (PVA) | Plant lipid droplets(CLD) | ∼133.5 | hFGF2 | Deep second-degree burn wound in mouse model | - Achieved controlled and sustained release of CLD-hFGF2 from hydrogel to promote cell proliferation and migration. - Accelerated wound healing rate compared to the control group, CLD-containing hydrogel group, and CLD-hFGF2 group. - Accelerated the re-epithelialization, promoted collagen deposition and angiogenesis compared to other treatment groups (Zhang et al., 2022). |
| Polymeric | |||||
| Poloxamer (P407) and polyvinyl alcohol (PVA) | Gelatin | ∼11 | Mupirocin | Third-degree burn wound in rat model | - Promoted antibacterial activity against S. epidermis, S.aureus, and P. aeruginosa compared to mupirocin ointment (Kamlungmak et al., 2020). - Enhanced wound healing by lowering inflammation as well as improving wound contraction, angiogenesis, and granulation tissue formation compared to the hydrogel only group and mupirocin ointment (Kamlungmak et al., 2021). |
| Poly(N-isopropylacrylamide) (PNIPAM) | Silk fibroin-sodium alginate | ∼200 | Vancomycin | MRSA-infected third-degree burn wound in rat model | - Showed good antibacterial activity against MRSA. - Promoted wound healing by lowering wound inflammation, accelerating angiogenesis, re-epithelialization, and collagen deposition with the synergistic effect of EGF loaded directly into the hydrogel (Rezaei et al., 2020). |
| Carbopol®934 | Polycaprolactone (PCL) | ∼225 | Fusidic acid (FA) | MRSA-infected burn wound in rat model | - Enhanced antibacterial activity against S.aureus, and MRSA compared to that of FA solution and commercially available FA cream. - Enhanced FA retention on skin surface compared to both FA-based hydrogel and the commercially available FA cream. - Accelerated wound contraction and re-epithelization, decreased the bacterial colonization in wounds compared to other treatment groups (Ullah et al., 2023). |
| Carboxymethyl chitosan (CMCS) and sodium alginate (SA) | Poly(lactic-co-glycolic acid) (PLGA) | ∼420 | bFGF | MRSA-infected burn wound in mouse model | - Promoted wound healing by accelerating wound closure, enhancing collagen deposition and re-epithelialization - Inhibited bacterial growth and controlled inflammation in both in vitro and in vivo experiments with the synergistic effect of porphyrin photosensitizer sinoporphyrin sodium loaded directly into the hydrogel (Mai et al., 2020). |
| Combination of non-polymeric and polymeric | |||||
| Recombinant methacrylated collagen and glycidyl methacrylated quaternary ammonium chitosan | Liposome and cyclodextrin (CD) metal organic skeleton loaded with ultrafine silver nanoparticles | ∼122 and ∼93 | Asiaticoside (AS) | E. coli and S. aureu-infected burn wound in rat model | - Enhanced antibacterial activity against E. coli and S. aureu in the presence of ultrafine silver nanoparticles within the hydrogel. - Promoted the in vitro cell migration and tube formation in the presence of AS-loaded liposomes within the hydrogel. - Accelerated the wound healing rate and lowered the bacteria remaining in the wound area compared to the control group, hydrogel only group, ultrafine silver nanapoarticles-loaded hydrogel group and commercially available Aquacel Ag group. - Enhanced wound healing by lowering inflammation, improving cell proliferation, collagen deposition and angiogenesis compared to the control group, hydrogel only group, ultrafine silver nanapoarticles-loaded hydrogel group and commercially available Aquacel Ag group (Feng et al., 2023). |
4.1.1. Non-polymeric NPs
4.1.1.1. Metal and metal oxide NPs
Metal and metal oxide NPs have been widely researched for their potential in promoting burn wound healing, thanks to their intrinsic antimicrobial activity. The incorporation of silver NPs (AgNPs) into hydrogel overcomes the safety and efficacy issues associated with silver ions-incorporating hydrogel. By incorporating AgNPs, the hydrogel can achieve prolonged and controlled release of AgNPs into the wound tissue (Bai et al., 2022). Additionally, due to their small size, AgNPs have better penetration capability in deeper wounds compared to silver ions. Moreover, they can increase the localized release of silver ions following intra-cellular uptake owing to their large surface area-to-volume ratio (Wilkinson et al., 2011; Alkilani et al., 2022; Shabatina et al., 2022). AgNPs incorporated hydrogels have shown promising outcomes in burn wound healing. In a study, AgNPs were incorporated into a hydrogel composed of oxidized dextran (ODex), adipic dihydrazide grafted hyaluronic acid (HA-ADH), and quaternized chitosan (HACC) (Ag@ODex/HA-ADH/HACC). The release of AgNPs due to the hydrogel degradation demonstrated significantly enhanced healing of P. aeruginosa-infected burn wounds compared to commercial Aquacel Ag dressings. The improved wound healing speed and well-organized collagen deposition were mainly attributed to the excellent antibacterial properties of AgNPs and the synergistic effects of chitosan. However, it should be noted that Ag@ODex/HA-ADH/HACC exhibited a significant cytotoxic effect by decreasing cell viability, possibly due to the toxicity of the AgNPs (Chen et al., 2021). To address the biocompatibility issue of AgNPs, Higuera and colleagues utilized Mimosa tenuiflora (MtE) plant extracts as a bio-reducing agent for AgNPs synthesis. AgNPs synthesized using MtE extracts (AgMt NPs) showed no cytotoxic effects on cells while maintaining comparable antibacterial activity to conventionally synthesized AgNPs. In a rat model of second-degree burn injuries AgMt NPs loaded gel demonstrated enhanced wound healing compared to MtE loaded gel, AgNPs loaded gel, gel only, and control groups (Martínez-Higuera et al., 2021).
Apart from metal NPs, metal oxide NPs with antimicrobial activity can also be delivered to the wound tissue by incorporating them into hydrogels. For example, titanium dioxide NPs (TiO2 NPs) were incorporated into a gelatin gel (TNG) for third-degree burn wound treatment. TNG exhibited significant antibacterial activity against S. aureus and P. aeruginosa, surpassing that of plain gelatin gel and 1% silver sulfadiazine (SSD) ointment. Animal healing results from a third-degree burn wound model confirmed the efficacy of TNG in accelerating burn wound healing by stimulating angiogenesis, fibroblast proliferation, and granulation tissue formation, and preventing wound infection (Javanmardi et al., 2018).
4.1.1.2. Lipid-based NPs
Lipid-based NPs, including liposomes, nanoemulsion, and lipid droplets, are commonly nonpolymeric NPs incorporated into hydrogels for the treatment of burn wounds. Among these, liposomes consist of unilamellar or multilamellar lipid bilayers that separate an aqueous phase (Yu et al., 2021; Alkilani et al., 2022). This unique feature allows liposomes to encapsulate both hydrophilic and hydrophobic drugs and therapeutic agents (Jeong et al., 2021). Due to their high absorption and retention at the lesion site, as well as their controlled drug-release capability, liposomes are widely used in transdermal drug delivery to enhance drug stability and bioactivity (Jeong et al., 2021; Yu et al., 2021). Studies have shown that liposomes, particularly nanoliposomes with size of around 150 nm, can penetrate deeper into the skin layers, which is beneficial for the treatment of severe burn wounds that extend beyond the epidermis into the dermis and hypodermis (El Maghraby et al., 2008). Furthermore, liposomes create a moist microenvironment at the wound site, promoting improved wound healing (Hussain et al., 2022; Xiong et al., 2023). In a study, asiaticoside (AS), a triterpenoid saponin derived from Centella asiatica, known for its anti-inflammatory and antioxidant properties was encapsulated into liposomes (LIP) to create asiaticoside-loaded liposomes (LIP@AS) with an average size of 121.10 ± 2.40 nm. This strategy aimed to improve the cell membrane permeability of the AS, as it has limited ability to penetrate the skin epidermis, thus restricting the therapeutic effect of local medications. The integrity and stability of LIP@AS were enhanced upon incorporation into a hydrogel formulated with recombinant methacrylated collagen and glycidyl methacrylated quaternary ammonium chitosan. This improvement in integrity and stability is supported by a decrease in the release rate of AS due to the hydrolysis of the ester bond in the hydrogel compared to liposomes alone. Furthermore, the inclusion of LIP@AS in the hydrogel significantly promoted the L929 cell migration and tube formation of HUVEC during in vitro experiments. To further enhance the antibacterial ability of the hydrogel, silver nanoparticles incorporated in a metal-organic framework were co-loaded into the hydrogel. This approach augmented the hydrogel’s antibacterial properties. In a rat burn-wound infection model, the healing efficacy of the hydrogel, with its synergistic combination of LIP@AS and silver nanoparticle incorporation, surpassed that of the commercially available Aquacel Ag dressing (Feng et al., 2023).
Nanoemulsions (NEs) are thermodynamically stable and heterogeneous colloidal systems consisting of an aqueous phase and an oil phase, which are further stabilized with surfactants and co-surfactants (Jeong et al., 2021). Due to their small droplet size, excellent skin wettability, and heterogeneity, NEs serve as ideal nanocarriers for enhancing transdermal delivery of both hydrophilic and hydrophobic bioactive compounds like nonsteroidal anti-inflammatory drugs (NSAIDs), anticancer drugs, and antioxidants (Rai et al., 2018; Miastkowska et al., 2020; Jeong et al., 2021). However, the low viscosity of NEs poses challenges to their topical application to wounds (Miastkowska et al., 2020). Therefore, incorporating bioactive-loaded NEs into a hydrogel system provides an optimal approach to maximize their effectiveness. Therefore, incorporating bioactive compounds-loaded NEs into a hydrogel system offers an optimal approach to maximize their effectiveness. The combination of NEs and hydrogel systems can create a moist wound healing environment, facilitate easy application and removal, and enable controlled and sustained release of NEs to promote wound healing (Miastkowska et al., 2020; Moradi et al., 2023). In a study by Alyoussef et al., the efficacy of a curcumin and resveratrol co-loaded nanoemulsion integrated with carbopol gel (nanoemulgel) was successfully demonstrated in the treatment of burn-induced wounds. Curcumin and resveratrol are polyphenolic compounds known for their antimicrobial and anti-inflammatory properties. However, challenges such as poor skin penetration, limited water solubility, and low stability have hindered their application in wound healing. To overcome these issues, curcumin and resveratrol were formulated into a nanoemulgel to enhance skin penetration. Results from skin deposition experiments revealed significant retention of curcumin and resveratrol in the skin after 48 hours, indicating that the integration of the nanoemulsion into the carbopol gel contributed to prolonged residence of the compounds in the skin and increased deposition over time. The improved skin penetration of the nanoemulgel enhanced its therapeutic activity in promoting burn healing (Alyoussef et al., 2021).
4.1.2. Polymeric NPs
Polymeric NPs are commonly utilized for drug encapsulation and as carriers for therapeutic agents. Polymeric NPs can be synthesized using biocompatible and biodegradable natural or synthetic polymers. Using polymeric NPs as a transdermal drug delivery offers several advantages, including the capacity to encapsulate both hydrophilic and hydrophobic drugs into NPs enabling controlled release. In a study conducted by Kamlungmak et al., gelatin, a biocompatible, biodegradable, and non-immunogenic natural polymer was employed to fabricate mupirocin-loaded NPs. The gelatin-based NPs loaded with mupirocin, which had a particle size of approximately 11 nm, were incorporated into thermoresponsive hydrogels composed of poloxamer (P407)-polyvinyl alcohol (PVA) hydrogels (referred to as MLH) to facilitate burn wound healing. The mupirocin-gelatin NPs loaded in hydrogel exhibited a more effective controlled release of mupirocin through diffusion compared to mupirocin ointment, thereby demonstrating superior antibacterial activity against S. aureus, S. epidermidis, P. aeruginosa, and E. coli (Kamlungmak et al., 2020). In addition, the biocompatible MLH demonstrated superior efficacy in promoting burn wound healing in a rat model compared to mupirocin ointment, as evidenced by enhanced wound contraction, angiogenesis, and granulation tissue formation (Kamlungmak et al., 2021).
In addition to natural polymer, a study has been conducted on the use of polycaprolactone (PCL), a biocompatible and biodegradable synthetic polymer that can be degraded by lipase produced by bacteria at the infection site. PCL was used to fabricate lipase-sensitive NPs loaded with fusidic acid (FA) (referred to as FA-NPs) to target the delivery of FA in MRSA-infected burn wounds. FA is a tetracyclic triterpenoid antibacterial drug derived from Fusidium coccineum, a naturally occurring fungus. The fabricated FA-NPs had a particle size of approximately 225 nm and exhibited sustained and on-demand release in the presence of lipase, which is secreted by pathogens such as MRSA. Upon incorporating FA-NPs into carbopol hydrogel for drug delivery, it demonstrated superior antibacterial activity against S. aureus, and MRSA surpassing that of FA solution and commercially available FA cream. Although it demonstrated lower permeation of FA through the skin layers compared to FA-based hydrogel, its ability to retain FA on the skin surface was superior to both FA-based hydrogel and the commercially available FA cream. In an MRSA-infected burn wound model, the biocompatible FA-NPs hydrogel exhibited superior wound treatment, as evidenced by a higher rate of wound contraction, a significant decrease in bacterial count, and accelerated re-epithelialization (Ullah et al., 2023).
5. Conclusion and perspectives
Herein, we explore the potential of hydrogel as a wound dressing and transdermal delivery carrier for burn wound healing. The 3D hydrophilic crosslinked polymer network of hydrogels makes them an attractive choice as wound-covering dressing, creating a moist environment for wound healing and offering a physical barrier against external harm. Additionally, the porous nature of hydrogels enables the incorporation of drugs and therapeutic agents to combat burn wound infections, which are common in severe burns and poorly managed wounds. These properties make hydrogels versatile in facilitating burn wound healing through transdermal delivery.
To enhance the transdermal drug delivery efficacy of hydrogels, one approach is to incorporate drug-laden nanocarriers into the hydrogel (passive transdermal delivery technique). However, effectively delivering hydrophilic and macromolecular therapeutic agents such as proteins and peptides through passive diffusion remains a challenge. To overcome this obstacle, a viable solution is to directly deliver therapeutic agents into the wound tissue below the eschar. For instance, combining passive and active transdermal delivery techniques, such as incorporating therapeutic agents loaded nanocarriers into hydrogel microneedles patches, could improve the overall efficacy of hydrogels as transdermal delivery carriers for burn wound healing. Previous studies have demonstrated that hydrogel microneedle patches loaded with bioactive agents effectively deliver and control the release of therapeutics, leading to improved wound healing in deeper tissues (Chi et al., 2020; Yuan et al., 2022). Although the application of microneedles for burn wound treatment is less explored, the strategy of hydrogel microneedle patches holds great potential for further research.
Furthermore, burn wound healing is a complex and dynamic process, influenced by factors such as the severity of burns (e.g. depth and percentage of total body surface area) and the patient’s condition (e.g. age, sex, and comorbidities). Considering the complexity of the burn wound healing process, researchers must design customizable hydrogel wound dressings tailored to each stage of healing and the specific needs of individual patients. Recently, 3D printing technology has been utilized in the development of 3D-printed dressings for burn wound treatment, taking advantage of their capability to create personalized wound dressings (Teoh et al., 2021). Leu et al. conducted a systematic study on different gelation ratios of alginate to fabricate and customize hydrogel wound dressings using 3D printing technology. They discovered that the optimized 3D-printed alginate-gelatin hydrogels demonstrated superior wound healing compared to non-printed hydrogels (Fayyazbakhsh et al., 2022, 2023). These findings prompted further research into incorporating bioactive borate glass for continuous hydration and treatment of second-degree burns. By incorporating borate glass, which regulates water release to maintain optimal wound moisture, the healing of second-degree burn wounds in a rat model was significantly improved in comparison to non-printed hydrogels with the same composition. Therefore, 3D printing technology holds immense potential as a versatile approach for fabricating hydrogels using bioink, which incorporates nanoparticles, stem cells, and bioactive agents for burn wound healing. In conclusion, there is still significant room for improvement in hydrogels as ideal burn wound dressings and their translation into clinical applications.
Funding Statement
This work was supported by National Key R&D Program of China (2019YFE0110400), National Natural Science Foundation of China (82272028, 81971621, 82102087), Key R&D Program of Hunan Province (2021SK2035), The Project of Science and Technology Innovation of Hunan Province (2021SK51807), Natural Science Foundation of Hunan Province (2022JJ30039, 2022JJ40392).
Author contributions
MeeiChyn Goh: literature search and data analysis, writing-original draft preparation, writing-review and editing. Meng Du: literature search and data analysis. Wang Rui Peng: literature search and data analysis. Phei Er Saw: conceptualization, writing-review, and editing. Zhiyi Chen: funding acquisition, supervision. All authors contributed and approved the submitted version.
Disclosure statement
No potential conflict of interest was reported by the authors.
Consent for publication
All authors agreed with the content of this manuscript and approved the submitted version.
References
- Alkilani AZ, McCrudden MTC, Donnelly RF. (2015). Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 7:1–15. doi: 10.3390/pharmaceutics7040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkilani AZ, Nasereddin J, Hamed R, et al. (2022). Beneath the skin: A review of current trends and future prospects of transdermal drug delivery systems. Pharmaceutics 14:1152. doi: 10.3390/pharmaceutics14061152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasaudi S. (2021). The antibacterial activities of honey. Saudi J Biol Sci 28:2188–96. Available at doi: 10.1016/j.sjbs.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyoussef A, El-Gogary RI, Ahmed RF, et al. (2021). The beneficial activity of curcumin and resveratrol loaded in nanoemulgel for healing of burn-induced wounds. J Drug Deliv Sci Technol 62:102360. Available at doi: 10.1016/j.jddst.2021.102360. [DOI] [Google Scholar]
- Arbuthnot MK, Garcia AV. (2019). Early resuscitation and management of severe pediatric burns. Semin Pediatr Surg 28:73–8. doi: 10.1053/j.sempedsurg.2019.01.013. [DOI] [PubMed] [Google Scholar]
- Babaluei M, Mottaghitalab F, Seifalian A, Farokhi M. (2023). Injectable multifunctional hydrogel based on carboxymethylcellulose/polyacrylamide/polydopamine containing vitamin C and curcumin promoted full-thickness burn regeneration. Int J Biol Macromol 236:124005. Available at doi: 10.1016/j.ijbiomac.2023.124005. [DOI] [PubMed] [Google Scholar]
- Bai Q, Zheng C, Chen W, et al. (2022). Current challenges and future applications of antibacterial nanomaterials and chitosan hydrogel in burn wound healing. Mater Adv 3:6707–27. doi: 10.1039/D2MA00695B. [DOI] [Google Scholar]
- Banerjee J, Seetharaman S, Wrice NL, et al. (2019). Delivery of silver sulfadiazine and adipose derived stem cells using fibrin hydrogel improves infected burn wound regeneration. PLoS One 14:e0217965. doi: 10.1371/journal.pone.0217965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LW, Fear VS, Waithman JC, et al. (2019). Understanding acute burn injury as a chronic disease. Burn Trauma 7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos S, Stojadinovic O, Golinko MS, et al. (2008). Growth factors and cytokines in wound healing. Wound Repair Regen 16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Benbow M. (2008). Exploring the concept of moist wound healing and its application in practice. Br J Nurs 17:S4, S6, S8 passim–S16. doi: 10.12968/bjon.2008.17.Sup6.30705. [DOI] [PubMed] [Google Scholar]
- Blanco-Fernandez B, Castaño O, Mateos-Timoneda MÁ, et al. (2021). Nanotechnology approaches in chronic wound healing. Adv Wound Care (New Rochelle) 10:234–56. doi: 10.1089/wound.2019.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumberg V, Astrelina T, Malivanova T, Samoilov A. (2021). Modern wound dressings: Hydrogel dressings. Biomedicines 9:1235. doi: 10.3390/biomedicines9091235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraballo C, Jaimes F. (2019). Organ dysfunction in sepsis: An ominous trajectory from infection to death. Yale J Biol Med 92:629–40. [PMC free article] [PubMed] [Google Scholar]
- Cartotto R. (2017). Topical antimicrobial agents for pediatric burns. Burn Trauma 5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Mazumder B, Rajkonwar J, et al. (2021). bFGF and collagen matrix hydrogel attenuates burn wound inflammation through activation of ERK and TRK pathway. Sci Rep 11:3357. Available at doi: 10.1038/s41598-021-82888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Yin H, Chen X, et al. (2020). Ångstrom-scale silver particle-embedded carbomer gel promotes wound healing by inhibiting bacterial colonization and inflammation. Sci Adv 6:eaba0942. doi: 10.1126/sciadv.aba0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Zhou Y, Dai J, et al. (2022). Calcium alginate/PNIPAAm hydrogel with body temperature response and great biocompatibility: Application as burn wound dressing. Int J Biol Macromol 216:686–97. Available at doi: 10.1016/j.ijbiomac.2022.07.019. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhang H, Yang X, et al. (2021). Preparation and application of quaternized chitosan-and agnps-base synergistic antibacterial hydrogel for burn wound healing. Molecules 26:4037. doi: 10.3390/molecules26134037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi J, Zhang X, Chen C, et al. (2020). Bioactive materials antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact Mater 5:253–9. Available at doi: 10.1016/j.bioactmat.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church D, Elsayed S, Reid O, et al. (2006). Burn wound infections. Clin Microbiol Rev 19:403–34. doi: 10.1128/CMR.19.2.403-434.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang LH, Huynh NT, Pham NO, et al. (2019). Injectable nanocurcumin-dispersed gelatin-pluronic nanocomposite hydrogel platform for burn wound treatment. Bull Mater Sci 42:71. doi: 10.1007/s12034-019-1745-0. [DOI] [Google Scholar]
- De Luca I, Pedram P, Moeini A, et al. (2021). Nanotechnology development for formulating essential oils in wound dressing materials to promote the wound-healing process: A review. Appl Sci 11:1713. doi: 10.3390/app11041713. [DOI] [Google Scholar]
- Dhivya S, Padma VV, Santhini E. (2015). Wound dressings – a review. Biomedicines 5:24–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divyashri G, Badhe RV, Sadanandan B, et al. (2022). Applications of hydrogel-based delivery systems in wound care and treatment: An up-to-date review. Polymers for Advanced Techs 33:2025–43. doi: 10.1002/pat.5661. [DOI] [Google Scholar]
- Dong M, Mao Y, Zhao Z, et al. (2022). Novel fabrication of antibiotic containing multifunctional silk fibroin injectable hydrogel dressing to enhance bactericidal action and wound healing efficiency on burn wound: In vitro and in vivo evaluations. Int Wound J 19:679–91. doi: 10.1111/iwj.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Maghraby GM, Barry BW, Williams AC. (2008). Liposomes and skin: From drug delivery to model membranes. Eur J Pharm Sci 34:203–22. doi: 10.1016/j.ejps.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Fan F, Saha S, Hanjaya-Putra D. (2021). Biomimetic hydrogels to promote wound healing. Front Bioeng Biotechnol 9:718377. doi: 10.3389/fbioe.2021.718377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahani M, Shafiee A. (2021). Wound healing: from passive to smart dressings. Adv Healthc Mater 10:e2100477. doi: 10.1002/adhm.202100477. [DOI] [PubMed] [Google Scholar]
- Fayyazbakhsh F, Khayat MJ, Leu MC. (2022). 3D-printed gelatin-alginate hydrogel dressings for burn wound healing: A comprehensive study. Int J Bioprint 8:618. doi: 10.18063/ijb.v8i4.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayyazbakhsh F, Khayat MJ, Sadler C, Day D. (2023). 3D-printed hydrogels dressings with bioactive borate glass for continuous hydration and treatment of second-degree burns. Int J Bioprinting 9(6):0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Liu Y, Chen Y, et al. (2023). Injectable antibacterial hydrogel with asiaticoside-loaded liposomes and ultrafine silver nanosilver particles promotes healing of burn-infected wounds. Adv Healthc Mater 12:e2203201. [DOI] [PubMed] [Google Scholar]
- Firlar I, Altunbek M, McCarthy C, et al. (2022). Functional hydrogels for treatment of chronic wounds. Gels 8:127. doi: 10.3390/gels8020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Shrivastava P. (2010). Post-burn scars and scar contractures. Indian J Plast Surg 43:S63–S71. doi: 10.4103/0970-0358.70724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh M, Hwang Y, Tae G. (2016). Epidermal growth factor loaded heparin-based hydrogel sheet for skin wound healing. Carbohydr Polym 147:251–60. Available at doi: 10.1016/j.carbpol.2016.03.072. [DOI] [PubMed] [Google Scholar]
- Gupta A, Briffa SM, Swingler S, et al. (2020). Synthesis of silver nanoparticles using curcumin-cyclodextrins loaded into bacterial cellulose-based hydrogels for wound dressing applications. Biomacromolecules 21:1802–11. doi: 10.1021/acs.biomac.9b01724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Kowalczuk M, Heaselgrave W, et al. (2019). The production and application of hydrogels for wound management: A review. Eur Polym J 111:134–51. Available at doi: 10.1016/j.eurpolymj.2018.12.019. [DOI] [Google Scholar]
- Gupta A, Low WL, Radecka I, et al. (2016). Characterisation and in vitro antimicrobial activity of biosynthetic silver-loaded bacterial cellulose hydrogels. J Microencapsul 33:725–34. doi: 10.1080/02652048.2016.1253796. [DOI] [PubMed] [Google Scholar]
- Han K, Bai Q, Zeng Q, et al. (2022). A multifunctional mussel-inspired hydrogel with antioxidant, electrical conductivity and photothermal activity loaded with mupirocin for burn healing. Mater Des 217:110598. doi: 10.1016/j.matdes.2022.110598. [DOI] [Google Scholar]
- Huang R, Hu J, Qian W, et al. (2021). Recent advances in nanotherapeutics for the treatment of burn wounds. Burn. Trauma 9:tkab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mu L, Zhao X, et al. (2022). Bacterial growth-induced tobramycin smart release self-healing hydrogel for Pseudomonas aeruginosa-infected burn wound healing. ACS Nano 16:13022–36. doi: 10.1021/acsnano.2c05557. [DOI] [PubMed] [Google Scholar]
- Hussain Z, Thu HE, Rawas-Qalaji M, et al. (2022). Recent developments and advanced strategies for promoting burn wound healing. J Drug Deliv Sci Technol 68:103092. Available at doi: 10.1016/j.jddst.2022.103092. [DOI] [Google Scholar]
- Jacob S, Nair AB, Shah J, et al. (2021). Emerging role of hydrogels in drug delivery systems, tissue engineering and wound management. Pharmaceutics 13:357. doi: 10.3390/pharmaceutics13030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jae ML, Jung KS, Ji SJ, et al. (2010). Antioxidant effect of lidocaine and procaine on reactive oxygen species-induced endothelial dysfunction in the rabbit abdominal aorta. Korean J Anesthesiol 59:104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanmardi S, Ghojoghi A, Divband B, Ashrafi J. (2018). Titanium dioxide nanoparticle/gelatin: a potential burn wound healing biomaterial. Wounds Compend Clin Res Pract 30:372–9. [PubMed] [Google Scholar]
- Jeong WY, Kwon M, Choi HE, Kim KS. (2021). Recent advances in transdermal drug delivery systems: a review. Biomater Res 25:24. doi: 10.1186/s40824-021-00226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke MG, van Baar ME, Choudhry MA, et al. (2020). Burn injury. Nat Rev Dis Primers 6:11. Available at: doi: 10.1038/s41572-020-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiann Chong ET, Ng JW, Lee P-C. (2022). Classification and medical applications of biomaterials–A mini review. Bio Integr 4(2):54–61. [Google Scholar]
- Jiji S, Udhayakumar S, Rose C, et al. (2019). Thymol enriched bacterial cellulose hydrogel as effective material for third degree burn wound repair. Int J Biol Macromol 122:452–60. doi: 10.1016/j.ijbiomac.2018.10.192. [DOI] [PubMed] [Google Scholar]
- Johnson K, Muzzin N, Toufanian S, et al. (2020). Drug-impregnated, pressurized gas expanded liquid-processed alginate hydrogel scaffolds for accelerated burn wound healing. Acta Biomater 112:101–11. Available at doi: 10.1016/j.actbio.2020.06.006. [DOI] [PubMed] [Google Scholar]
- Johnson N, Wang Y. (2015). Drug delivery systems for wound healing. Curr Pharm Biotechnol 16:621–9. doi: 10.2174/1389201016666150206113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamlungmak S, Nakpheng T, Kaewpaiboon S, et al. (2021). Safety and biocompatibility of mupirocin nanoparticle-loaded hydrogel on burn wound in rat model. Biol Pharm Bull 44:1707–16. doi: 10.1248/bpb.b21-00397. [DOI] [PubMed] [Google Scholar]
- Kamlungmak S, Rugmai S, Tinpun K, et al. (2020). Phase behavior, in vitro drug release, and antibacterial activity of thermoresponsive poloxamer–polyvinyl alcohol hydrogel-loaded mupirocin nanoparticles. J Appl Polym Sci 137:1–14. [Google Scholar]
- Karande P, Mitragotri S. (2009). Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim Biophys Acta 1788:2362–73. doi: 10.1016/j.bbamem.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Kim B, Cho H-E, Moon SH, et al. (2020). Transdermal delivery systems in cosmetics. Biomed Dermatol 4:1–12. doi: 10.1186/s41702-019-0053-z. [DOI] [Google Scholar]
- Kim MH, Park H, Nam HC, et al. (2018). Injectable methylcellulose hydrogel containing silver oxide nanoparticles for burn wound healing. Carbohydr Polym 181:579–86. Available at doi: 10.1016/j.carbpol.2017.11.109. [DOI] [PubMed] [Google Scholar]
- Klinkajon W, Supaphol P. (2014). Novel copper (II) alginate hydrogels and their potential for use as anti-bacterial wound dressings. Biomed Mater 9:045008. doi: 10.1088/1748-6041/9/4/045008. [DOI] [PubMed] [Google Scholar]
- Kumar PM, Ghosh A. (2017). Development and evaluation of silver sulfadiazine loaded microsponge based gel for partial thickness (second degree) burn wounds. Eur J Pharm Sci 96:243–54. Available at doi: 10.1016/j.ejps.2016.09.038. [DOI] [PubMed] [Google Scholar]
- Kushibiki T, Mayumi Y, Nakayama E, et al. (2021). Photocrosslinked gelatin hydrogel improves wound healing and skin flap survival by the sustained release of basic fibroblast growth factor. Sci Rep 11:23094. Available at doi: 10.1038/s41598-021-02589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurano R, Boffito M, Ciardelli G, Chiono V. (2022). Wound dressing products: A translational investigation from the bench to the market. Eng Regen 3:182–200. Available at doi: 10.1016/j.engreg.2022.04.002. [DOI] [Google Scholar]
- Lee KC, Joory K, Moiemen NS. (2014). History of burns: The past, present and the future. Burns Trauma 2:169–80. doi: 10.4103/2321-3868.143620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenfant F, Lahet JJ, Courderot-Masuyer C, et al. (2004). Lidocaine has better antioxidant potential than ropivacaine and bupivacaine: In vitro comparison in a model of human erythrocytes submitted to an oxidative stress. Biomed Pharmacother 58:248–54. doi: 10.1016/j.biopha.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Li J, Wang C, Han X, et al. (2022). Aramid nanofibers-reinforced rhein fibrous hydrogels as antibacterial and anti-inflammatory burn wound dressings. ACS Appl Mater Interfaces 14:45167–77. doi: 10.1021/acsami.2c12869. [DOI] [PubMed] [Google Scholar]
- Li Y, Han Y, Wang X, et al. (2017). Multifunctional hydrogels prepared by dual ion cross-linking for chronic wound healing. ACS Appl Mater Interfaces 9:16054–62. doi: 10.1021/acsami.7b04801. [DOI] [PubMed] [Google Scholar]
- Liu J, Jiang W, Xu Q, Zheng Y. (2022). Progress in antibacterial hydrogel dressing. Gels 8:503. doi: 10.3390/gels8080503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Chen Y, Zhu Q, et al. (2022). Antioxidant thermogelling formulation for burn wound healing. Chem Asian J 17:e202200396. [DOI] [PubMed] [Google Scholar]
- Low W, Kenward MA, Amin M, Martin C. (2016). Ionically crosslinked chitosan hydrogels for the controlled release of antimicrobial essential oils and metal ions for wound management applications. Medicines 3:8. doi: 10.3390/medicines3010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madaghiele M, Demitri C, Sannino A, Ambrosio L. (2014). Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burns Trauma 2:153–61. doi: 10.4103/2321-3868.143616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai B, Jia M, Liu S, et al. (2020). Smart hydrogel-based DVDMS/bFGF nanohybrids for antibacterial phototherapy with multiple damaging sites and accelerated wound healing. ACS Appl Mater Interfaces 12:10156–69. doi: 10.1021/acsami.0c00298. [DOI] [PubMed] [Google Scholar]
- Markiewicz-Gospodarek A, Kozioł M, Tobiasz M, et al. (2022). Burn wound healing: clinical complications, medical care, treatment, and dressing types: the current state of knowledge for clinical practice. Int J Environ Res Public Health 19:1338. doi: 10.3390/ijerph19031338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Higuera A, Rodríguez-Beas C, Villalobos-Noriega JMA, et al. (2021). Hydrogel with silver nanoparticles synthesized by Mimosa tenuiflora for second-degree burns treatment. Sci Rep 11:11312. Available at doi: 10.1038/s41598-021-90763-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miastkowska M, Kulawik-Pióro A, Szczurek M. (2020). Nanoemulsion gel formulation optimization for burn wounds: Analysis of rheological and sensory properties. Processes 8:1416. doi: 10.3390/pr8111416. [DOI] [Google Scholar]
- Mirhaj M, Labbaf S, Tavakoli M, Seifalian AM. (2022). Emerging treatment strategies in wound care. Int Wound J 19:1934–54. doi: 10.1111/iwj.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miricescu D, Badoiu SC, Stanescu-Spinu II, et al. (2021). Growth factors, reactive oxygen species, and metformin—Promoters of the wound healing process in burns? Int J Mol Sci 22:9512. doi: 10.3390/ijms22179512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi M, Barati A, Moradi S, Zarinabadi E. (2023). Synthesis and characterization of starch-based hydrogels containing myrtus oil nanoemulsion for wound dressings. Polym Bull Available at doi: 10.1007/s00289-023-04855-w. [DOI] [Google Scholar]
- Negut I, Grumezescu V, Grumezescu AM. (2018). Treatment strategies for infected wounds. Molecules 23:2392. doi: 10.3390/molecules23092392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nischwitz SP, Luze H, Popp D, et al. (2021). Global burn care and the ideal burn dressing reloaded—A survey of global experts. Burns 47:1665–74. Available at doi: 10.1016/j.burns.2021.02.008. [DOI] [PubMed] [Google Scholar]
- Nuutila K, Grolman J, Yang L, et al. (2020). Immediate treatment of burn wounds with high concentrations of topical antibiotics in an alginate hydrogel using a platform wound device. Adv Wound Care (New Rochelle) 9:48–60. doi: 10.1089/wound.2019.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang QQ, Hu Z, Lin ZP, et al. (2018). Chitosan hydrogel in combination with marine peptides from tilapia for burns healing. Int J Biol Macromol 112:1191–8. doi: 10.1016/j.ijbiomac.2018.01.217. [DOI] [PubMed] [Google Scholar]
- Pan Z, Ye H, Wu D. (2021). Recent advances on polymeric hydrogels as wound dressings. APL Bioeng 5:011504. doi: 10.1063/5.0038364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Hwang SR, Yoon IS. (2017). Advanced growth factor delivery systems in wound management and skin regeneration. Molecules 22:1259. doi: 10.3390/molecules22081259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Ji L, Xu W, et al. (2022). Copper-hydrazide coordinated multifunctional hyaluronan hydrogels for infected wound healing. ACS Appl Mater Interfaces 14:16018–31. doi: 10.1021/acsami.2c01254. [DOI] [PubMed] [Google Scholar]
- Qin J, Li M, Yuan M, et al. (2022). Gallium(III)-mediated dual-cross-linked alginate hydrogels with antibacterial properties for promoting infected wound healing. ACS Appl Mater Interfaces 14:22426–42. doi: 10.1021/acsami.2c02497. [DOI] [PubMed] [Google Scholar]
- Rai VK, Mishra N, Yadav KS, Yadav NP. (2018). Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J Control Release 270:203–25. doi: 10.1016/j.jconrel.2017.11.049. [DOI] [PubMed] [Google Scholar]
- Rezaei F, Damoogh S, Reis RL, et al. (2020). Dual drug delivery system based on pH-sensitive silk fibroin/alginate nanoparticles entrapped in PNIPAM hydrogel for treating severe infected burn wound. Biofabrication 13:015005. doi: 10.1088/1758-5090/abbb82. [DOI] [PubMed] [Google Scholar]
- Rowan MP, Cancio LC, Elster EA, et al. (2015). Burn wound healing and treatment: Review and advancements. Crit Care 19:243. Available at doi: 10.1186/s13054-015-0961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryall C, Duarah S, Chen S, et al. (2022). Advancements in skin delivery of natural bioactive products for wound management: A brief review of two decades. Pharmaceutics 14:1072. doi: 10.3390/pharmaceutics14051072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghazadeh S, Rinoldi C, Schot M, et al. (2018). Drug delivery systems and materials for wound healing applications. Adv Drug Deliv Rev 127:138–66. doi: 10.1016/j.addr.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MF, Breda SA, Soria EA, et al. (2018). Ciprofloxacin-lidocaine-based hydrogel: development, characterization, and in vivo evaluation in a second-degree burn model. Drug Deliv Transl Res 8:1000–13. doi: 10.1007/s13346-018-0523-7. [DOI] [PubMed] [Google Scholar]
- Seow YX, Yeo CR, Chung HL, Yuk HG. (2014). Plant essential oils as active antimicrobial agents. Crit Rev Food Sci Nutr 54:625–44. doi: 10.1080/10408398.2011.599504. [DOI] [PubMed] [Google Scholar]
- Shabatina T, Vernaya O, Shumilkin A, et al. (2022). Nanoparticles of bioactive metals/metal oxides and their nanocomposites with antibacterial drugs for biomedical applications. Materials (Basel) 15:3602. doi: 10.3390/ma15103602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaker DS, Ishak RAH, Ghoneim A, Elhuoni MA. (2019). Nanoemulsion: A review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Sci Pharm 87:17. doi: 10.3390/scipharm87030017. [DOI] [Google Scholar]
- Shalaby MA, Anwar MM, Saeed H. (2022). Nanomaterials for application in wound Healing: current state-of-the-art and future perspectives. J Polym Res 29:91. doi: 10.1007/s10965-021-02870-x. [DOI] [Google Scholar]
- Shao XH, Yang X, Zhou Y, et al. (2022). Antibacterial, wearable, transparent tannic acid-thioctic acid-phytic acid hydrogel for adhesive bandages. Soft Matter 18:2814–28. doi: 10.1039/d2sm00058j. [DOI] [PubMed] [Google Scholar]
- Shpichka A, Butnaru D, Bezrukov EA, et al. (2019). Skin tissue regeneration for burn injury. Stem Cell Res Ther 10:94. doi: 10.1186/s13287-019-1203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W, Wang Y, Zhang X, et al. (2021). Functional hydrogel dressings for treatment of burn wounds. Front Bioeng Biotechnol 9:788461. doi: 10.3389/fbioe.2021.788461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões D, Miguel SP, Ribeiro MP, et al. (2018). Recent advances on antimicrobial wound dressing: A review. Eur J Pharm Biopharm 127:130–41. doi: 10.1016/j.ejpb.2018.02.022. [DOI] [PubMed] [Google Scholar]
- Soriano JL, Calpena AC, Rodríguez-Lagunas MJ, et al. (2021). Endogenous antioxidant cocktail loaded hydrogel for topical wound healing of burns. Pharmaceutics 13:8. doi: 10.3390/pharmaceutics13010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano-Ruiz JL, Calpena-Campmany AC, Silva-Abreu M, et al. (2020). Design and evaluation of a multifunctional thermosensitive poloxamer-chitosan-hyaluronic acid gel for the treatment of skin burns. Int J Biol Macromol 142:412–22. doi: 10.1016/j.ijbiomac.2019.09.113. [DOI] [PubMed] [Google Scholar]
- Souto EB, Ribeiro AF, Ferreira MI, et al. (2020). New nanotechnologies for the treatment and repair of skin burns infections. Int J Mol Sci 21:393. doi: 10.3390/ijms21020393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica AE, Chircov C, Grumezescu AM. (2020). Hydrogel dressings for the treatment of burn wounds: An up-to-date overview. Materials (Basel) 13:2853. doi: 10.3390/ma13122853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone R, Natesan S, Kowalczewski CJ, et al. (2018). Advancements in regenerative strategies through the continuum of burn care. Front Pharmacol 9:672. doi: 10.3389/fphar.2018.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulastri E, Lesmana R, Zubair MS, et al. (2023). Ulvan/Silver nanoparticle hydrogel films for burn wound dressing. Heliyon 9:e18044. Available at doi: 10.1016/j.heliyon.2023.e18044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surowiecka A, Strużyna J, Winiarska A, Korzeniowski T. (2022). Hydrogels in burn wound management—A review. Gels 8:122. doi: 10.3390/gels8020122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao B, Lin C, Qin X, et al. (2022). Fabrication of gelatin-based and Zn2+-incorporated composite hydrogel for accelerated infected wound healing. Mater Today Bio 13:100216. Available at doi: 10.1016/j.mtbio.2022.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh JH, Mozhi A, Sunil V, et al. (2021). 3D printing personalized, photocrosslinkable hydrogel wound dressings for the treatment of thermal burns. Adv Funct Mater 31:2105932. [Google Scholar]
- Tiwari VK. (2012). Burn wound: How it differs from other wounds. Indian J Plast Surg 45:364–73. doi: 10.4103/0970-0358.101319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah N, Khan D, Ahmed N, et al. (2023). Lipase-sensitive fusidic acid polymeric nanoparticles based hydrogel for on-demand delivery against MRSA-infected burn wounds. J Drug Deliv Sci Technol 80:104110. doi: 10.1016/j.jddst.2022.104110. [DOI] [Google Scholar]
- Wahid F, Zhong C, Wang HS, et al. (2017). Recent advances in antimicrobial hydrogels containing metal ions and metals/metal oxide nanoparticles. Polymers (Basel) 9:636. doi: 10.3390/polym9120636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gao Y, Li H, et al. (2022). Effect of natural-based biological hydrogels combined with growth factors on skin wound healing. Nanotechnol Rev 11:2493–512. doi: 10.1515/ntrev-2022-0122. [DOI] [Google Scholar]
- Wang FY, Chen Y, Huang YY, Cheng CM. (2021). Transdermal drug delivery systems for fighting common viral infectious diseases. Drug Deliv Transl Res 11:1498–508. Available at doi: 10.1007/s13346-021-01004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liu Y, Cai K, et al. (2021). Antibacterial polysaccharide-based hydrogel dressing containing plant essential oil for burn wound healing. Burn Trauma 9:tkab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Huang X, Zheng H, et al. (2021). Nanomaterials applied in wound healing: Mechanisms, limitations and perspectives. J Control Release 337:236–47. doi: 10.1016/j.jconrel.2021.07.017. [DOI] [PubMed] [Google Scholar]
- Wang P, Huang S, Hu Z, et al. (2019). In situ formed anti-inflammatory hydrogel loading plasmid DNA encoding VEGF for burn wound healing. Acta Biomater 100:191–201. doi: 10.1016/j.actbio.2019.10.004. [DOI] [PubMed] [Google Scholar]
- Wang S, Li J, Ma Z, et al. (2021). A sequential therapeutic hydrogel with injectability and antibacterial activity for deep burn wounds’ cleaning and healing. Front Bioeng Biotechnol 9:794769. doi: 10.3389/fbioe.2021.794769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam AJ, Maan ZN, Duscher D, et al. (2016). Challenges and opportunities in drug delivery for wound healing. Adv Wound Care (New Rochelle) 5:79–88. doi: 10.1089/wound.2014.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson LJ, White RJ, Chipman JK. (2011). Silver and nanoparticles efficacy and safety. J Wound Care 20:543–9. doi: 10.12968/jowc.2011.20.11.543. [DOI] [PubMed] [Google Scholar]
- Xia H, Zhang Y, Xin H, et al. (2022). Metal–phenolic network-based polydopamine@Cu within a polyvinyl alcohol hydrogel film for improved infected wound healing through antibacterial and pro-angiogenesis activity. Mater Des 221:110904. Available at doi: 10.1016/j.matdes.2022.110904. [DOI] [Google Scholar]
- Xiao Y, Zhao H, Ma X, et al. (2022). Hydrogel dressing containing basic fibroblast growth factor accelerating chronic wound healing in aged mouse model. Molecules 27:6361. doi: 10.3390/molecules27196361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Zhang R, Zhou H, et al. (2023). Smart materials in medicine application of nanomedicine and mesenchymal stem cells in burn injuries for the elderly patients. Smart Mater Med 4:78–90. Available at doi: 10.1016/j.smaim.2022.08.001. [DOI] [Google Scholar]
- Yao Y, Zhang A, Yuan C, et al. (2021). Recent trends on burn wound care: Hydrogel dressings and scaffolds. Biomater Sci 9:4523–40. doi: 10.1039/d1bm00411e. [DOI] [PubMed] [Google Scholar]
- Yin C, Han X, Lu Q, et al. (2022). Rhein incorporated silk fibroin hydrogels with antibacterial and anti-inflammatory efficacy to promote healing of bacteria-infected burn wounds. Int J Biol Macromol 201:14–9. doi: 10.1016/j.ijbiomac.2021.12.156. [DOI] [PubMed] [Google Scholar]
- Yu YQ, Yang X, Wu XF, Fan YB. (2021). Enhancing permeation of drug molecules across the skin via delivery in nanocarriers: Novel strategies for effective transdermal applications. Front Bioeng Biotechnol 9:646554. doi: 10.3389/fbioe.2021.646554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Liu K, Jiang T, et al. (2022). GelMA/PEGDA microneedles patch loaded with HUVECs ‑ derived exosomes and Tazarotene promote diabetic wound healing. J Nanobiotechnology 20:147. Available at doi: 10.1186/s12951-022-01354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Chen S, Zhong L, et al. (2020). Zn2+-loaded TOBC nanofiber-reinforced biomimetic calcium alginate hydrogel for antibacterial wound dressing. Int J Biol Macromol 143:235–42. doi: 10.1016/j.ijbiomac.2019.12.046. [DOI] [PubMed] [Google Scholar]
- Zhang X, Qin M, Xu M, et al. (2021). The fabrication of antibacterial hydrogels for wound healing. Eur. Polym. J 146:110268. doi: 10.1016/j.eurpolymj.2021.110268. [DOI] [Google Scholar]
- Zhang Y, He W, Zhang S, et al. (2022). Poloxam thermosensitive hydrogels loaded with hFGF2-linked camelina lipid droplets accelerate skin regeneration in deep second-degree burns. Int J Mol Sci 23:12716. doi: 10.3390/ijms232112716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang T, He J, Dong J. (2016). Growth factor therapy in patients with partial-thickness burns: A systematic review and meta-analysis. Int Wound J 13:354–66. doi: 10.1111/iwj.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Xiao H, Seidi F, Jin Y. (2020). Natural polymer-based antimicrobial hydrogels without synthetic antibiotics as wound dressings. Biomacromolecules 21:2983–3006. doi: 10.1021/acs.biomac.0c00760. [DOI] [PubMed] [Google Scholar]
- Zhou J, Cha R, Wu Z, et al. (2023). An injectable, natural peptide hydrogel with potent antimicrobial activity and excellent wound healing-promoting effects. Nano Today 49:101801. doi: 10.1016/j.nantod.2023.101801. [DOI] [Google Scholar]
- Zhu L, Chen L. (2022). Facile design and development of nano-clustery graphene-based macromolecular protein hydrogel loaded with ciprofloxacin to antibacterial improvement for the treatment of burn wound injury. Polym Bull (Berl) 79:7953–68. doi: 10.1007/s00289-021-03875-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zia T, Usman M, Sabir A, et al. (2020). Development of inter-polymeric complex of anionic polysaccharides, alginate/k-carrageenan bio-platform for burn dressing. Int J Biol Macromol 157:83–95. doi: 10.1016/j.ijbiomac.2020.04.157. [DOI] [PubMed] [Google Scholar]