Abstract
Rationale
The prevalence and diagnostic utility of bronchodilator responsiveness (BDR) in a real-life setting is unclear.
Objective
To explore this uncertainty in patients aged ⩾12 years with physician-assigned diagnoses of asthma, asthma and chronic obstructive pulmonary disease (COPD), or COPD in NOVELTY, a prospective cohort study in primary and secondary care in 18 countries.
Methods
The proportion of patients with a positive BDR test in each diagnostic category was calculated using 2005 (ΔFEV1 or ΔFVC ⩾12% and ⩾200 ml) and 2021 (ΔFEV1 or ΔFVC >10% predicted) European Respiratory Society/American Thoracic Society criteria.
Measurements and Main Results
We studied 3,519 patients with a physician-assigned diagnosis of asthma, 833 with a diagnosis of asthma + COPD, and 2,436 with a diagnosis of COPD. The prevalence of BDR was 19.7% (asthma), 29.6% (asthma + COPD), and 24.7% (COPD) using 2005 criteria and 18.1%, 23.3%, and 18.0%, respectively, using 2021 criteria. Using 2021 criteria in patients diagnosed with asthma, BDR was associated with higher fractional exhaled nitric oxide; lower lung function; higher symptom burden; more frequent hospital admissions; and greater use of triple therapy, oral corticosteroids, or biologics. In patients diagnosed with COPD, BDR (2021) was associated with lower lung function and higher symptom burden.
Conclusions
BDR prevalence in patients with chronic airway diseases receiving treatment ranges from 18% to 30%, being modestly lower with the 2021 than with the 2005 European Respiratory Society/American Thoracic Society criteria, and it is associated with lower lung function and greater symptom burden. These observations question the validity of BDR as a key diagnostic tool for asthma managed in clinical practice or as a standard inclusion criterion for clinical trials of asthma and instead suggest that BDR be considered a treatable trait for chronic airway disease.
Keywords: asthma, BDR, chronic obstructive pulmonary disease, diagnosis
At a Glance Commentary
Scientific Knowledge on the Subject
The prevalence and diagnostic utility of bronchodilator responsiveness (BDR) in a real-life setting is unclear. In 2021, the European Respiratory Society/American Thoracic Society task force recommended that BDR be defined as a change (Δ) of FEV1 or FVC >10% predicted, replacing the previous 2005 definition (ΔFEV1 or ΔFVC ⩾12% and ⩾200 ml). How the use of these two criteria impacts the prevalence and diagnostic utility of BDR in clinical practice is unclear.
What This Study Adds to the Field
This cross-sectional analysis of 6,788 participants from 18 countries with physician-assigned labels of asthma, asthma and chronic obstructive pulmonary disease, or chronic obstructive pulmonary disease in primary and secondary care showed that the prevalence of a positive BDR in patients with chronic airway diseases ranged from 18.0% to 29.6%, being modestly lower with the 2021 compared with the 2005 European Respiratory Society/American Thoracic Society criteria, and was associated with worse lung function and greater symptom burden. These observations question the validity of BDR as a key diagnostic criterion for asthma managed in clinical practice or as a standard inclusion criterion for clinical trials of asthma. We suggest that it should instead be considered a treatable trait in clinical practice.
Asthma and chronic obstructive pulmonary disease (COPD) are the two most prevalent airway diseases. Their clinical presentations often overlap, so accurate diagnosis is often difficult. This is relevant because they need different treatment approaches. In patients with asthma, the use of inhaled corticosteroids (ICS) is mandatory, based on the risk of hospitalization and death with use of bronchodilators (BDs) alone, whereas in patients with COPD, long-acting muscarinic antagonists or long-acting β-agonists (with or without ICS) are the cornerstone of pharmacologic treatment (1, 2).
Traditionally, enhanced bronchodilator responsiveness (BDR) has been considered diagnostic of asthma, although it is recognized that positive BDR may also occur in some patients with COPD (3). According to the 2005 European Respiratory Society (ERS)/American Thoracic Society (ATS) guideline criteria, BDR was defined by an increase in FEV1 or FVC of ⩾12% and ⩾200 ml from baseline (4). More recently, in 2021, the ERS/ATS Technical Standard Task Force recommended that BDR be defined as a change (Δ) in FEV1 or FVC of >10% relative to the predicted value (5). This new criterion was intended to minimize the sex and height differences in assessing BDR and to reduce the influence of baseline lung function. It was based on epidemiological data in healthy adults showing that the upper limit (95th percentile) of the range of responses was between 10.1% and 11.6% of predicted for ΔFEV1 and between 9.6% and 10.2% of predicted for ΔFVC (6, 7). The inclusion by both task forces of FVC, as an alternative to FEV1, was based on the evidence that FVC better reflects the physiological process of air trapping (8–12). Whether the 2021 ERS/ATS criteria result in better differentiation of asthma from COPD in clinical practice or for eligibility for clinical trials is unknown. The aim of this study was to compare the prevalence, diagnostic utility, and associated clinical characteristics of BDR in adult patients with physician-assigned asthma, asthma and COPD (referred to as asthma + COPD in this article), or COPD in a large, global, real-life clinical practice setting.
Methods
Participants
We analyzed data from participants in NOVELTY (Observational Study of Obstructive Lung Disease), a noninterventional, prospective cohort study of patients with physician-diagnosed or suspected asthma and/or COPD aged over 12 years from 18 countries (13). Patients were recruited between July 2016 and March 2018 from both primary and secondary care sites. Recruitment to the study was stratified by physician-assigned severity; no specific diagnostic or severity criteria were provided to physicians. Details of the study have been reported previously (13, 14).
The analysis population includes all patients at sites who undertook pre- and post-BDR testing at the baseline visit with salbutamol (albuterol) or terbutaline as the BD. The patient flow diagram is shown in the online supplement (see Figure E1 in the online supplement).
Participants in the study were grouped according to a physician-assigned diagnosis of asthma, asthma + COPD, and COPD. A sensitivity analysis was undertaken in the subsets of patients considered as having high-likelihood asthma or COPD based on the basis of the following criteria:
-
•
High-likelihood asthma: physician diagnosis of asthma only, asthma symptom onset before age 40, less than 5 pack-years of smoking exposure
-
•
High-likelihood COPD: physician diagnosis of COPD only, COPD symptom onset at age greater than 40, at least 10 pack-years of smoking exposure, post-BD FEV1/FVC <0.7
BD Testing and Criteria
Measurement of FEV1 and FVC was undertaken by spirometry, performed by sites trained to the relevant ERS/ATS standards (15). Spirometers were provided to 253 sites (Masterscope, Clario) or performed on accredited site spirometers when available. Spirometry was undertaken at baseline and again 15 to 30 minutes after administration of a BD, with the doses used according to local practice. The present analyses were limited to patients for whom the BD was a short-acting β-agonist (nebulized or inhaled salbutamol/albuterol or terbutaline). Patients were instructed to withhold their short-acting bronchodilation medication(s) for at least 6 hours before visits when performing reversibility testing and long-acting BDs (with or without ICS) for 12–24 hours, depending on whether the patient was using twice- or once-daily therapy.
Positive BDR was defined using the following ERS/ATS criteria:
-
•
ERS/ATS 2005: change in FEV1 or FVC ⩾12% and ⩾200 ml relative to pre-BD (ΔFEV1 or ΔFVC ⩾12% and ⩾200 ml) (4)
-
•
ERS/ATS 2021: change in FEV1 or ΔFVC >10% relative to the corresponding percent predicted (ΔFEV1 or ΔFVC >10% predicted) (5)
In addition, we evaluated the following:
-
•
Change in FEV1 ⩾12% and ⩾200 ml relative to pre-BD (ΔFEV1 ⩾12% and ⩾200 ml)
-
•
Change in FVC ⩾12% and ⩾200 ml relative to pre-BD (ΔFVC ⩾12% and ⩾200 ml)
-
•
Change in FEV1 >10% relative to the corresponding percent predicted FEV1 (ΔFEV1 >10% predicted)
-
•
Change in FVC >10% relative to the corresponding percent predicted FVC (ΔFVC >10% predicted)
-
•
Change in FEV1 ⩾15% and ⩾400 ml relative to pre-BD (ΔFEV1 ⩾15% and ⩾400 ml)
The predicted values for FEV1 and FVC were derived from the ERS Global Lung Function Initiative reference equations (16).
Statistical Analysis
Statistical analysis was performed using the R version 4.1.0 statistical package. P values are displayed for descriptive purposes and are based on the chi-square test for the comparison of categorical variables and one-way ANOVA or Kruskal-Wallis H-test for normal or nonnormal continuous variables, respectively. P values were considered significant at the 0.05 α-level. Missing values were excluded from denominators for proportions. Logistic regression models (unadjusted and adjusted) were developed to assess the associations of BDR with other clinical characteristics at baseline according to each BDR definition, separately within asthma and COPD. Results are reported as odds ratios (ORs) with 95% confidence intervals (CIs). Adjusted ORs (for age and sex) are provided in the online supplement. Receiver operating characteristic curve analysis was used to assess the utility of each BDR criterion for distinguishing physician-assigned asthma from COPD. Results are reported as sensitivity, specificity, and area under the curve. BDR prevalence and discrimination analyses were repeated in the subgroups of “high-likelihood” asthma or COPD, as defined earlier. The absolute change in FEV1 with the BD (mean and interquartile range) is presented by deciles of pre-BD FEV1 percent predicted for different disease groupings. In post hoc analyses, the association of baseline BDR (following the 2005 and 2021 definitions) with the probability of experiencing one or more moderate/severe exacerbations during 1-year follow-up was presented by calculation of ORs, based on unadjusted and adjusted logistic regression models fitted within each diagnosis group separately, and the overall group that included all diagnoses.
Results
We included 6,788 patients in this analysis, 3,519 with a physician-assigned diagnosis of asthma, 833 with a diagnosis of asthma + COPD, and 2,436 with a diagnosis of COPD (Table 1 and Figure E1). For asthma, a minority of participants had a positive BDR, regardless of the BDR definition used (Table 1). With the 2021 criteria (ΔFEV1 or ΔFVC >10% predicted), the proportions with positive BDR for asthma, asthma + COPD, and COPD were 18.1%, 23.3%, and 18.0%, respectively. These proportions were lower than when the 2005 criteria (ΔFEV1 or ΔFVC ⩾12% and ⩾200 ml) were used, particularly for patients with asthma + COPD and COPD (19.7% for asthma, 29.6% for asthma + COPD, and 24.7% for COPD). For the criterion of ΔFEV1 >10% predicted, the proportion with a positive BDR was lower for COPD, whereas for ΔFVC >10% predicted, the proportion with a positive BDR was lower for asthma. When omitting FVC from the 2005 criteria (i.e., defining BDR positive based on ΔFEV1 ⩾12% and ⩾200 ml), the proportions with positive BDR (17%, 23.6%, and 14.1% for asthma, asthma + COPD, and COPD, respectively) were similar to those for ΔFEV1 or ΔFVC >10% predicted for asthma and for asthma + COPD but lower for COPD.
Table 1.
Distribution of BDR and Discrimination Statistics, by Physician-assigned Diagnosis for Different BDR Definitions
| Asthma and COPD (n = 833) | Asthma vs. COPD |
||||
|---|---|---|---|---|---|
| BDR Definition | Asthma (n = 3,519) | COPD (n = 2,436) | AUC (95% CI) | Sensitivity, Specificity | |
| ΔFEV1 or ΔFVC ⩾12% and ⩾200 ml | 631/3,196 (19.7%) | 224/757 (29.6%) | 552/2,239 (24.7%) | 0.597 (0.572, 0.622) | 0.55, 0.30 |
| ΔFEV1 ⩾12% and ⩾200 ml | 548/3,229 (17.0%) | 179/760 (23.6%) | 317/2,242 (14.1%) | 0.659 (0.629, 0.690) | 0.58, 0.21 |
| ΔFVC ⩾12% and ⩾200 ml | 298/3,228 (9.2%) | 143/758 (18.9%) | 409/2,256 (18.1%) | 0.592 (0.562, 0.622) | 0.42, 0.43 |
| ΔFEV1 ⩾15% and ⩾400 ml | 228/3,229 (7.1%) | 42/760 (5.5%) | 52/2,242 (2.3%) | 0.727 (0.652, 0.803) | 0.80, 0.07 |
| ΔFEV1 >10% pred | 482/3,176 (15.2%) | 114/750 (15.2%) | 177/2,207 (8.0%) | 0.571 (0.555, 0.586) | 0.15, 0.92 |
| ΔFVC >10% pred | 279/3,175 (8.8%) | 130/748 (17.4%) | 326/2,221 (14.7%) | 0.578 (0.562, 0.593) | 0.09, 0.86 |
| ΔFEV1 or ΔFVC >10% pred | 569/3,143 (18.1%) | 174/747 (23.3%) | 396/2,204 (18.0%) | 0.505 (0.490, 0.521) | 0.18, 0.82 |
| High-likelihood asthma or COPD (1) | Asthma HL (n = 1,820) | COPD HL (n = 1,260) | AUC (95% CI) | Sensitivity, Specificity | |
| ΔFEV1 or ΔFVC ⩾12% and ⩾200 ml | 351/1,658 (21.2%) | 338/1,222 (27.7%) | 0.619 (0.586, 0.652) | 0.54, 0.26 | |
| ΔFEV1 ⩾12% and ⩾200 ml | 313/1,681 (18.6%) | 176/1,223 (14.4%) | 0.703 (0.664, 0.742) | 0.56, 0.17 | |
| ΔFVC ⩾12% and ⩾200 ml | 153/1,676 (9.1%) | 260/1,227 (21.2%) | 0.602 (0.562, 0.642) | 0.42, 0.40 | |
| ΔFEV1 ⩾15% and ⩾400 ml | 148/1,681 (8.8%) | 24/1,223 (2.0%) | 0.765 (0.673, 0.857) | 0.80, 0.04 | |
| ΔFEV1 >10% pred | 275/1,652 (16.6%) | 89/1,203 (7.4%) | 0.592 (0.572, 0.613) | 0.16, 0.93 | |
| ΔFVC >10% pred | 134/1,647 (8.1%) | 210/1,207 (17.4%) | 0.614 (0.593, 0.635) | 0.08, 0.83 | |
| ΔFEV1 or ΔFVC >10% pred | 315/1,629 (19.3%) | 242/1,202 (20.1%) | 0.508 (0.487, 0.530) | 0.19, 0.80 | |
Definition of abbreviations: AUC = area under the curve; BDR = bronchodilator responsiveness; CI = confidence interval; COPD = chronic obstructive pulmonary disease; HL = high likelihood; ROC = receiver operating characteristic; pred = predicted.
Δ denotes the difference between the pre- and post-bronchodilator values. This table shows the distribution of BDR for different BDR definitions across patients diagnosed with asthma and/or COPD (top) and among patients with high-likelihood asthma or COPD (bottom). The last column displays results from a ROC curve analysis evaluating the ability to discriminate between asthma-only and COPD-only patients (1). High-likelihood asthma was defined as physician diagnosis of asthma only, asthma symptom onset before age 40, and less than 5 pack-years of smoking exposure. High-likelihood COPD was defined as physician diagnosis of COPD only, COPD symptom onset at age greater than 40, at least 10 pack-years smoking exposure, and post-bronchodilator FEV1/FVC <0.7.
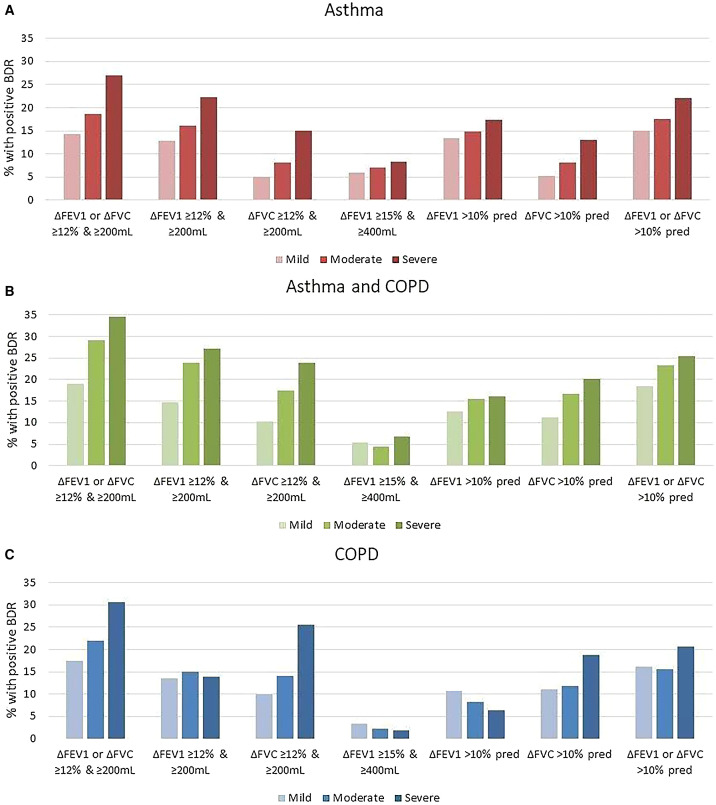
For each BDR criterion, the proportions with a positive result in the asthma and/or COPD diagnostic groups were similar when a broad or high-likelihood definition was used (Table 1). For asthma and for asthma + COPD, the proportion with positive BDR increased with increasing physician-assessed severity across the different BDR criteria (Figure 1 and Table E1). In contrast, for COPD, this trend was less marked (Figure 1 and Table E1).
Figure 1.
(A–C) Prevalence of bronchodilator responsiveness (BDR) according to different BDR definitions among patients with physician-assigned asthma, COPD, or both. COPD = chronic obstructive pulmonary disease; pred = predicted.
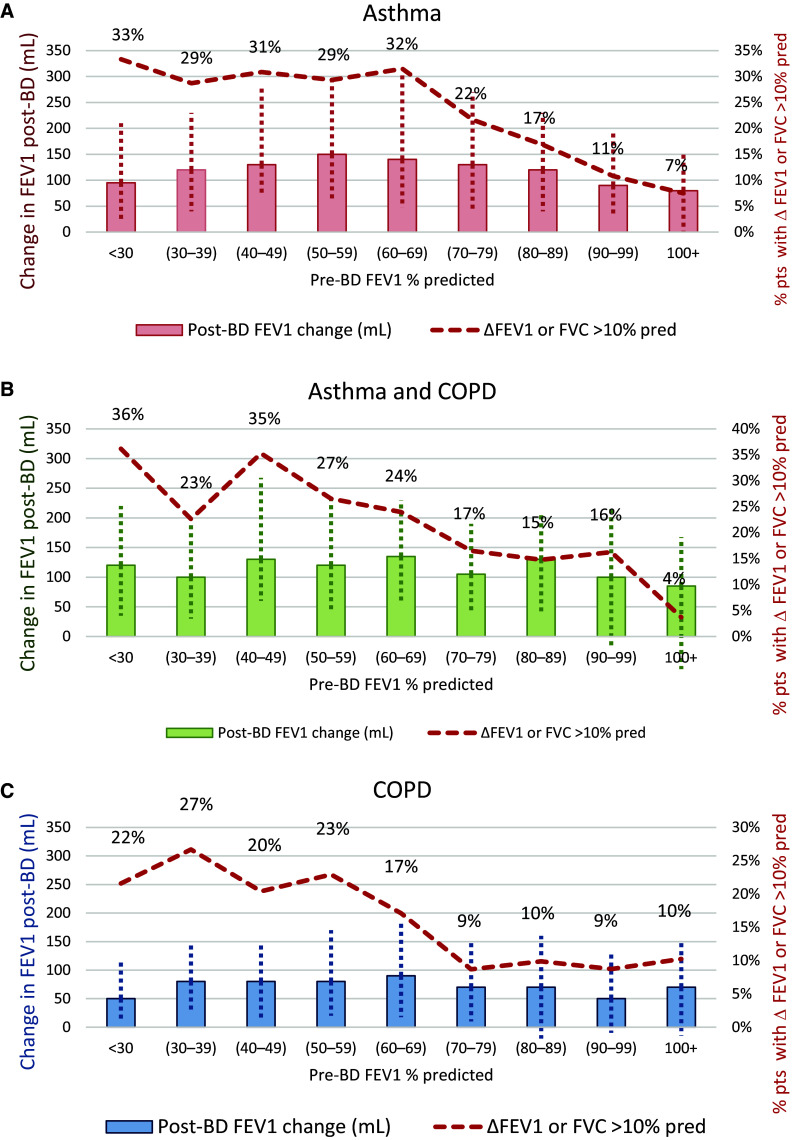
For asthma, asthma + COPD, and COPD, the proportion with positive BDR increased with progressively lower pre-BD FEV1 percent predicted for the ΔFEV1 or ΔFVC >10% predicted (Figure 2) and ΔFEV1 or ΔFVC ⩾12% and ⩾200 ml criteria (Figure E2). The relationship between the likelihood of having a positive BDR and baseline FEV1 for the individual components of change in FEV1 and change in FVC for each criterion is shown in Figures E3–E6, respectively. The magnitude of the change in FEV1 post-BD was similar in patients with asthma and asthma + COPD but greater than in those with COPD alone for both ΔFEV1 or ΔFVC >10% predicted (Figure 2) and ΔFEV1 or ΔFVC ⩾12% and ⩾200 ml criteria (Figure E2).
Figure 2.
(A–C) Relative change in FEV1 post-bronchodilator (post-BD) and frequency in bronchodilator responsiveness (BDR) positive patients by pre-BD FEV1 percent predicted, defining BDR positive as post-BD ΔFEV1 or ΔFVC >10% predicted (European Respiratory Society/American Thoracic Society 2021 criteria). The columns show the median change in FEV1 (ml) after BD (BDR). The vertical dotted lines correspond to the interquartile range. The red line and percentages correspond to the proportions of patients meeting BDR positive criteria defined as ΔFEV1 or ΔFVC >10% predicted. The numbers in parentheses correspond to (min-max) for each decile of pre-BD FEV1 % predicted. COPD = chronic obstructive pulmonary disease; pred = predicted.
The ability to discriminate between asthma and COPD diagnoses varied according to the BDR criteria used (Table 1). The ΔFEV1 or ΔFVC >10% predicted criterion had low sensitivity and high specificity, whereas the criterion of ΔFEV1 or ΔFVC ⩾12% and ⩾200 ml had modest sensitivity and low specificity. This discriminatory ability remained poor when the high-likelihood definitions were used (Table 1).
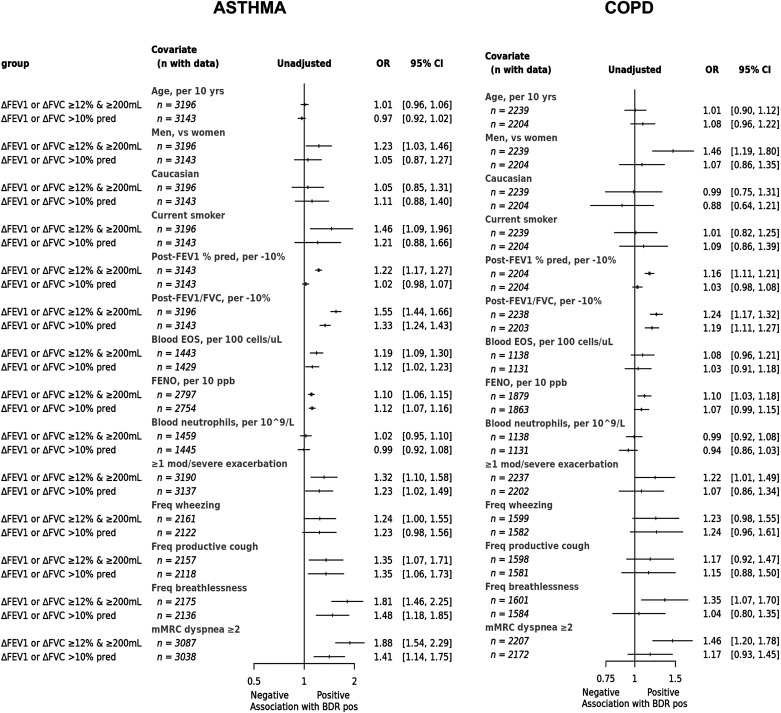
In participants with a diagnosis of asthma, positive BDR (using the 2021 criterion of ΔFEV1 or ΔFVC >10% predicted) was associated with lower post-BD lung function; higher fractional exhaled nitric oxide (FeNO); worse respiratory symptoms; greater likelihood of hospital admission for an exacerbation in the previous 12 months; and higher proportions treated with triple therapy, maintenance oral corticosteroids, or biologics than those with negative BDR (Table 2). The proportions with a diagnosis of chronic bronchitis, emphysema, bronchiectasis, or other pulmonary or extrapulmonary comorbidities were low and similar between groups. These differences in the characteristics of patients with asthma who were BDR positive versus negative were broadly similar when assessing individual components of the BDR criteria (ΔFEV1 >10% predicted or ΔFVC >10% predicted [Tables E2 and E3]) or the ΔFEV1 or ΔFVC ⩾12% and ⩾200 ml criterion, although the positive groups had a stronger T2 signal with higher blood eosinophil counts, in addition to the higher FeNO (Table E4). The characteristics for the individual components ΔFEV1 ⩾12% and ⩾200 ml or ΔFVC ⩾12% and ⩾200 ml are shown in Tables E5 and E6. The ORs for the association of BDR (2005 and 2021 criteria) with baseline clinical characteristics in patients with asthma are shown in Figure 3. These associations were very similar when adjusted for age and sex (Figure E7).
Table 2.
Characteristics of Patients with a Physician Diagnosis of Asthma or COPD, by BDR Status Based on ΔFEV1 or ΔFVC >10% Predicted
| Variable | Asthma (ΔFEV1 and ΔFVC ⩽10% pred) (n = 2,574) | Asthma (ΔFEV1 or ΔFVC >10% pred) (n = 569) | P Value | COPD (ΔFEV1 and ΔFVC ⩽10% pred) (n = 1,808) | COPD (ΔFEV1 or ΔFVC >10% pred) (n = 396) | P Value |
|---|---|---|---|---|---|---|
| Age, yr | 50.65 (17.20) | 49.61 (18.70) | 66.82 (9.10) | 67.47 (8.92) | ||
| Men | 964/2,574 (37.5%) | 220/569 (38.7%) | 1,111/1,808 (61.4%) | 250/396 (63.1%) | ||
| White race | 2,046/2,574 (79.5%) | 462/569 (81.2%) | 1,579/1,808 (87.3%) | 340/396 (85.9%) | ||
| BMI, kg/m2 | 28.19 (6.75) | 28.12 (6.16) | 27.64 (6.18) | 26.96 (5.62) | * | |
| Smoking status | ||||||
| Current smoker | 202/2,574 (7.8%) | 53/569 (9.3%) | 502/1,808 (27.8%) | 117/396 (29.5%) | ||
| Former smoker | 748/2,574 (29.1%) | 149/569 (26.2%) | 1,198/1,808 (66.3%) | 254/396 (64.1%) | ||
| Never smoker | 1,624/2,574 (63.1%) | 367/569 (64.5%) | 108/1,808 (6.0%) | 25/396 (6.3%) | ||
| Years since diagnosis | 18.99 (17.44) | 20.32 (17.37) | 7.91 (8.76) | 7.23 (6.37) | ||
| Age at symptom onset | 28.39 (20.71) | 26.14 (20.48) | * | 53.25 (16.66) | 53.35 (16.87) | |
| Lung function | ||||||
| Post FEV1, % pred | 86.98 (20.42) | 86.14 (20.72) | 60.42 (23.16) | 59.06 (21.96) | ||
| Post FEV1/FVC, % | 75.84 (11.75) | 71.43 (12.15) | *** | 56.63 (16.33) | 52.13 (15.96) | *** |
| Post FEV1/FVC below LLN | 490/2,574 (19.0%) | 206/569 (36.2%) | *** | 1,153/1,807 (63.8%) | 311/396 (78.5%) | *** |
| Post FEF25–75, % pred | 83.28 (38.33) | 74.41 (39.27) | *** | 48.73 (35.59) | 43.34 (36.65) | * |
| Biomarkers | ||||||
| Blood EOS, cells/μl | 220.65 (171.72) | 239.89 (168.60) | 175.10 (112.42) | 194.12 (162.64) | * | |
| Blood EOS, cells/μl | * | |||||
| <150 | 483/1,183 (40.8%) | 90 (32.6%) | 455/922 (49.3%) | 104/216 (48.1%) | ||
| 150–299 | 430/1,183 (36.3%) | 112 (40.6%) | 356/922 (38.6%) | 78/216 (36.1%) | ||
| 300+ | 270/1,183 (22.8%) | 74 (26.8%) | 111/922 (12.0%) | 34/216 (15.7%) | ||
| ⩾300 cells/μl | 270/1,183 (22.8%) | 74 (26.8%) | 111/922 (12.0%) | 34/216 (15.7%) | ||
| FeNO, ppb | 30.11 (27.54) | 40.98 (36.51) | *** | 19.48 (16.52) | 21.61 (21.33) | * |
| FeNO, ⩾25 ppb | 997/2,326 (42.9%) | 298/515 (57.9%) | *** | 374/1,540 (24.3%) | 84/330 (25.5%) | |
| Blood neutrophils, 109/L | 4.53 (1.84) | 4.50 (1.95) | 5.07 (1.83) | 4.88 (1.86) | ||
| Exacerbations and symptoms | ||||||
| ⩾1 mod/severe exacerbation last 12 mo | 834/2,569 (32.5%) | 211/568 (37.1%) | 683/1,806 (37.8%) | 156/396 (39.4%) | ||
| ⩾1 admission for exacerbation last 12 mo | 97/2,569 (3.8%) | 42/568 (7.4%) | ** | 229/1,806 (12.7%) | 50/396 (12.6%) | |
| SGRQ total score | 29.98 (20.58) | 35.82 (22.12) | *** | 41.89 (21.77) | 45.17 (20.81) | ** |
| Freq wheezing last 3 mo | 567/1,744 (32.5%) | 141/378 (37.3%) | ** | 616/1,311 (47.0%) | 142/271 (52.4%) | |
| Freq productive cough last 3 mo | 423/1,741 (24.3%) | 114/377 (30.2%) | *** | 489/1,310 (37.3%) | 110/271 (40.6%) | |
| Freq breathlessness last 3 mo | 585/1,755 (33.3%) | 162/381 (42.5%) | *** | 681/1,312 (51.9%) | 144/272 (52.9%) | |
| mMRC dyspnea grade ⩾2 | 503/2,479 (20.3%) | 148/559 (26.5%) | *** | 964/1,783 (54.1%) | 225/389 (57.8%) | |
| Respiratory medications | ||||||
| Reliever only | 246/2,348 (10.5%) | 63/526 (12.0%) | 116/1,616 (7.2%) | 35/350 (10.0%) | ||
| ICS (any) | 1,990/2,348 (84.8%) | 441/526 (83.8%) | 938/1,616 (58.0%) | 194/350 (55.4%) | ||
| ICS + LABA, no LAMA | 1,379/2,348 (58.7%) | 272/526 (51.7%) | ** | 230/1,616 (14.2%) | 46/350 (13.1%) | |
| Triple ICS + LABA + LAMA (open/fixed) | 302/2,348 (12.9%) | 89/526 (16.9%) | *** | 616/1,616 (38.1%) | 118/350 (33.7%) | |
| LAMA + LABA, no ICS | 31/2,348 (1.3%) | 8/526 (1.5%) | 547/1,616 (33.8%) | 118/350 (33.7%) | ||
| Add-on treatments | 644/2,348 (27.4%) | 124/526 (23.6%) | 122/1,616 (7.5%) | 28/350 (8.0%) | ||
| OCS maintenance | 63/2,348 (2.7%) | 26/526 (4.9%) | *** | 18/1,616 (1.1%) | 6/350 (1.7%) | |
| Biologics | 221/2,348 (9.4%) | 71/526 (13.5%) | ** | — | 1/350 (0.3%) | |
| Medical history | ||||||
| Chronic bronchitis | 33/2,574 (1.3%) | 7/569 (1.2%) | 66/1,808 (3.7%) | 13/396 (3.3%) | ||
| Emphysema | 45/2,574 (1.7%) | 13/569 (2.3%) | 728/1,808 (40.3%) | 164/396 (41.4%) | ||
| Bronchiectasis | 133/2,574 (5.2%) | 24/569 (4.2%) | 117/1,808 (6.5%) | 31/396 (7.8%) | ||
| Obstructive sleep apnea | 196/2,574 (7.6%) | 34/569 (6.0%) | 166/1,808 (9.2%) | 42/396 (10.6%) | ||
| Allergy (excluding food/drug) | 1807/2,574 (70.2%) | 395/569 (69.4%) | 302/1,808 (16.7%) | 62/396 (15.7%) | ||
| Chronic rhino-/sinusitis | 1581/2,574 (61.4%) | 334/569 (58.7%) | 254/1,808 (14.0%) | 41/396 (10.4%) | * | |
| Nasal/sinus polyps | 147/2,574 (5.7%) | 36/569 (6.3%) | 13/1,808 (0.7%) | 2/396 (0.5%) | ||
| CHD (MI or HF) | 29/2,574 (1.1%) | 11/569 (1.9%) | 139/1,808 (7.7%) | 29/396 (7.3%) | ||
| Type 2 diabetes | 206/2,574 (8.0%) | 47/569 (8.3%) | 293/1,808 (16.2%) | 54/396 (13.6%) | ||
| Hypertension | 588/2,574 (22.8%) | 127/569 (22.3%) | 813/1,808 (45.0%) | 177/396 (44.7%) | ||
| GERD | 372/2,574 (14.5%) | 60/569 (10.5%) | * | 264/1,808 (14.6%) | 42/396 (10.6%) | * |
| Osteoarthritis/unspecified arthritis | 209/2,574 (8.1%) | 39/569 (6.9%) | 195/1,808 (10.8%) | 50/396 (12.6%) | ||
| Osteoporosis | 108/2,574 (4.2%) | 27/569 (4.7%) | 114/1,808 (6.3%) | 22/396 (5.6%) | ||
| Thyroid disease | 193/2,574 (7.5%) | 44/569 (7.7%) | 168/1,808 (9.3%) | 27/396 (6.8%) | ||
| Depression or anxiety | 337/2,574 (13.1%) | 75/569 (13.2%) | 271/1,808 (15.0%) | 51/396 (12.9%) |
Definition of abbreviations: BMI = body mass index; CHD = coronary heart disease; COPD = chronic obstructive pulmonary disease; EOS = eosinophils; FEF25–75 = forced expiratory flow, midexpiratory phase; FeNO = fractional exhaled nitric oxide; Freq = frequency; GERD = gastroesophageal reflux disease; HF = heart failure; ICS = inhaled corticosteroids; LABA = long-acting β-agonists; LAMA = long-acting muscarinic antagonists; LLN = lower limit of normal; MI = myocardial infarction; mMRC = modified Medical Research Council; OCS = oral corticosteroids; pred = predicted; SGRQ = St. George’s Respiratory Questionnaire.
P values are based on the chi-square test for categorical variables and one-way ANOVA or Kruskal-Wallis H-test for normal or nonnormal continuous variables, respectively. Numbers without percent correspond to the standard deviation. Asterisks indicate level of statistical significance as follows: ***P < 0.001; **P < 0.01; *P < 0.05; otherwise, the field is left empty.
Figure 3.
Odds ratios for the association of clinical characteristics with BDR in patients with physician diagnosed asthma or COPD. ORs are derived from a logistic regression model with the BDR as the outcome and the clinical characteristic as the predictor (no covariate adjustments). BDR = bronchodilator responsiveness; CI = confidence interval; EOS = eosinophils; FeNO = fractional exhaled nitric oxide; mMRC = modified Medical Research Council; ORs = odds ratios.
In participants with a diagnosis of COPD, positive BDR (using the criterion of ΔFEV1 or ΔFVC >10%) was associated with a modestly worse degree of airflow obstruction and worse health status (St. George’s Respiratory Questionnaire total score) but not clinical characteristics other than those with a negative BDR (Table 2). The proportion with a diagnosis of chronic bronchitis, emphysema, bronchiectasis, or other pulmonary or extrapulmonary comorbidities was similar. The characteristics of participants with COPD who were BDR positive versus negative were broadly similar when the ΔFEV1 or ΔFVC ⩾12% and ⩾200 ml criterion was used, although the positive group had a higher FeNO (Table E4). The ORs for the association of BDR (2005 and 2021 criteria) with baseline clinical characteristics in patients with COPD are shown in Figure 3. As with asthma, these associations were largely the same when adjusted for age and sex (Figure E8).
BDR defined by the ΔFEV1 or ΔFVC ⩾12% and ⩾200 ml criterion was associated with an increased probability of experiencing one or more moderate or severe exacerbations in the subsequent year, with adjusted ORs of 1.19 (95% CI, 1.02–1.37) overall and 1.31 (95% CI, 1.06–1.64) in the asthma subgroup (Table E7). BDR defined by ΔFEV1 or ΔFVC >10% was not associated with exacerbation risk overall (OR, 1.04; 95% CI, 0.88–1.22) or in the diagnostic subgroups (Table E7).
Discussion
This cross-sectional analysis of the NOVELTY study shows that 1) the prevalence of BDR in patients treated for chronic airway disease in a large, global, real-life setting was approximately 18–30%; 2) the prevalence of BDR was modestly lower with the 2021 compared with the 2005 ERS/ATS criteria; 3) neither set of criteria discriminated asthma from COPD on the basis of physician-assigned diagnoses, with a positive BDR test occurring in similar proportions across the populations with labels of asthma, asthma + COPD, and COPD; 4) regardless of the criterion used to define BDR, the sensitivity and/or specificity for the diagnosis of asthma versus COPD was poor, and most patients with a diagnosis of asthma receiving treatment did not exhibit BDR on a single test; and, finally, 5) a positive BDR test was associated with worse lung function and a greater symptom burden. Collectively, these observations question the validity of a single measurement of BDR as a diagnostic tool. We propose that in adult patients with established chronic airway disease receiving treatment, if the diagnosis is reviewed, a positive BDR test should not be considered a fundamental requirement for the diagnosis of asthma or a standard inclusion criterion for randomized controlled trials (RCTs) of asthma. Rather, we suggest that BDR should be considered a treatable trait in the clinical management of patients with chronic airway diseases (17–20).
Interpretation of Novel Findings
It had been shown that BDR may be present in only a minority of adults with a clinical diagnosis of asthma using the 2005 ERS/ATS and other criteria (21–26). This study shows that this finding also applies to the new 2021 ERS/ATS criteria. Similarly, the observation that those with an asthma or a COPD diagnosis have broadly similar proportions with BDR (3, 23, 24) was also confirmed with the 2021 ERS/ATS criteria. However, contrasting findings have been reported from the COPDGene (Genetic Epidemiology of COPD) study in the United States, in which the proportions of patients with COPD who had BDR were 33% and 45% for the 2005 and 2021 ERS/ATS criteria, respectively (27), and a database study of children and adults from China, in which the proportions of patients with asthma who had BDR with the 2005 and 2021 ERS/ATS criteria were 63% and 53%, respectively, and 31% and 23%, respectively, for COPD (28). The reasons for the differences are uncertain but may relate to differing patient populations, different long-term treatments such as proportion receiving ICS, or differing use of BDR as a diagnostic tool for asthma in clinical practice.
The prevalence of BDR was higher in patients with a diagnosis of asthma + COPD, regardless of the criteria used. Although there are a number of possible explanations for this, one may be that when faced with a patient with COPD, the presence of BDR may enhance the probability of making a concurrent diagnosis of asthma. The criterion of ΔFEV1 ⩾15% and ⩾400 ml had the best sensitivity for differentiating asthma from COPD, although at the cost of a very low proportion in the asthma population and very low specificity differentiating asthma from COPD. These properties support the guideline recommendation that the greater the degree of BDR, the greater the likelihood that a patient has asthma (1, 29, 30).
Using the criterion of an increase in FEV1 of >10% predicted, 15% of participants with asthma and 8% with COPD had a positive test result. For the criterion of an increase in FVC of >10% predicted, a different pattern was observed in which 9% and 15% of participants with asthma and with COPD, respectively, had a positive BDR test result. Thus, the different components that make up this 2021 BDR criterion have different properties. Although a BDR criterion of change in FEV1 based on percent predicted reduces the influence of baseline lung function (6, 31, 32), we observed that the likelihood of having a positive BDR rose as baseline FEV1 fell, regardless of the criterion applied; intriguingly, the drivers for this differed between asthma and COPD, with the change in FVC percent predicted rather than FEV1 percent predicted predominating in more severe COPD. The low number of positive responses at pre-BD values close to normal suggests a ceiling effect; the larger proportion of positive responses at lower pre-BD values is likely to be a function of both reduced airway caliber producing proportionately larger changes in airway resistance for the same degree of airway smooth muscle relaxation and the smaller starting values for predicted lung function in more severe disease.
The differences in characteristics between patients with asthma based on BDR positivity suggest that BDR might be considered a treatable trait in asthma. The patients with asthma with BDR had worse post-BD lung function, higher FeNO, and a greater burden from respiratory symptoms and more hospital admissions, despite higher levels of treatment with triple therapy, continuous oral corticosteroids, and biologics. For COPD, BDR positivity was associated with modestly worse airflow obstruction and a greater burden from respiratory symptoms, consistent with the increased rate of lung function decline in patients with COPD with BDR (33, 34). The commonality of worse lung function and symptom burden with BDR positivity across both diagnostic groups is likely to related, to some extent, to the progressively greater magnitude of FEV1 change with lower pre-BD FEV1 percent predicted. The association between positive BDR and higher FeNO in asthma (2005 and 2021 criteria) and COPD (2005 criteria) is consistent with previous findings in asthma (21) and COPD (35). Because a raised FeNO is a marker of eosinophilic inflammation and in symptomatic patients short-term responsiveness to corticosteroids in asthma and COPD (36), this suggests that BDR positivity may represent a treatable trait associated with more severe airway disease and ICS responsiveness, regardless of the diagnostic label.
Further support for BDR representing a treatable trait came from the post hoc longitudinal analyses, which showed that BDR defined by the ΔFEV1 or ΔFVC ⩾12% and ⩾200 ml criteria was associated with about a 20% increased probability of experiencing one or more moderate or severe exacerbations in the subsequent 12 months overall, with the association most marked in the asthma group. In contrast, this longitudinal association was not observed with the ΔFEV1 or ΔFVC >10% criterion, suggesting an important clinical difference between these criteria. Importantly, these findings suggest that using the 2005 BDR criterion for eligibility in RCTs has enriched the populations for exacerbators.
Clinical Implications
There are two main clinical implications from these findings. First, there is no specific cut point for any of the BDR criteria to clearly differentiate asthma from COPD in treated patients with long-standing disease. It is reasonable to conclude that the higher the degree of BDR, the greater the probability of asthma, with the caveat that most patients with asthma receiving ICS-containing treatment will not show BDR by any criteria, particularly on a single day of testing. For clinical practice, objective confirmation of the diagnosis of asthma in patients receiving treatment may require stepping down or cessation of ICS (1, 37).
Second, the standard practice of requiring positive BDR as an inclusion criterion in major RCTs in asthma means that the evidence base derived from the RCTs is not generalizable to the management of the broad asthma population. Indeed, it has been reported that only a median of 6% of participants with current asthma receiving treatment met the eligibility criteria (including but not limited to BDR) for major asthma RCTs cited in the 2005 Global Initiative for Asthma strategy report (38). This indicates that most patients with current asthma receiving treatment in the community would not have been eligible for these RCTs. Similarly, only a median of 10% of patients with severe asthma were found to be eligible for enrollment in the phase III trials of biologic therapies because of the requirement for BDR and other criteria, indicating that the vast majority were excluded from trial participation by criteria designed to reconfirm questionable diagnostic labels rather than by biomarker criteria that predict responsiveness to treatment (39). Notably, in these studies, average BDR is typically 25% to 35% of baseline FEV1, a level found in only a very small proportion of NOVELTY patients with a label of severe asthma (14). Comparable findings have been observed in COPD, in which over 90% of patients with COPD in the community who were taking medication did so on the basis of RCTs for which they would have not been eligible (40).
Strengths and Potential Limitations
The fact that our analysis includes over 6,000 adult patients with a range of chronic airway diseases recruited in primary and specialized clinics around the globe is a clear strength to explore the prevalence, diagnostic value, and associated clinical characteristics of BDR because it ensures both adequate power and generalizability across populations. Our analysis, however, has some potential limitations. First, BDR was assessed on a single occasion, as might occur in clinical practice, rather than repeatedly, so it was not possible to determine the stability of this trait. Previous studies in COPD have reported that BDR exhibited significant day-to-day variability (41–43). Second, children under the age of 12 were not included in the NOVELTY study, so our observations relate only to adolescents and adults.
The diagnostic groups were based on the physicians’ diagnosis or suspected diagnosis of asthma, asthma and COPD, and COPD to ensure generalizability to clinical practice. Sensitivity analyses based on more stringent criteria were undertaken to define subgroups with a high-likelihood diagnosis of asthma and COPD and showed results similar to those derived from the broader diagnostic criteria. The inclusion of participants with established obstructive lung disease, with a mean 20 years and 7 years since diagnosis in the asthma and COPD groups, respectively, means that the findings are not generalizable to BDR at the time of the initial diagnosis, which is when BDR testing is primarily intended to be used. It is known that the magnitude of BDR may reduce over time as part of the natural history of asthma (6, 44, 45) and that ICS treatment reduces BDR in asthma (46). These factors may account for the previous observation in two cohorts with adult-onset asthma that the proportion with a positive BDR test result (using the criterion of ΔFEV1 ⩾12% and ⩾200 ml) at the time of diagnosis was 24% (47), modestly higher than the proportion observed in the present analysis for the equivalent definition. In a subsequent analysis within one of these cohorts, the proportion of steroid-naive patients with newly diagnosed asthma with a positive BDR test result (using the criterion of ΔFEV1 ⩾12% and ⩾200 ml) increased from 36% overall to 56% if baseline obstruction (pre-BD FEV1/FVC <0.7) was present, indicating that the utility of BDR testing is enhanced if baseline airflow obstruction is present (48).
We have undertaken multiple statistical comparisons, so those not directly related to the primary outcomes can be considered exploratory. Furthermore, we have been careful not to make detailed comparisons between the characteristics of the BDR positive and negative groups defined by the different BDR criteria and their individual FEV1 or FVC components because of the large number of characteristics presented. However, our data add to evidence that different subgroups defined by components of BDR may have different clinical characteristics (49–51).
Conclusions
In a real-life setting, positive BDR occurs in less than one-fourth of treated patients with chronic airway disease, is similar regardless of the diagnostic criteria used (ERS/ATS 2005 or 2021), does not have clinical utility in discriminating a physician-assigned diagnosis of asthma from COPD, and is associated with worse lung function and more symptom burden. We propose, therefore, that positive BDR should not be considered an essential requirement for the diagnosis of asthma and that positive BDR should no longer be considered a mandatory inclusion criterion for clinical trials of asthma. Rather, BDR should be considered a treatable trait in patients with chronic airway diseases.
Footnotes
Supported by AstraZeneca.
Author Contributions: Conception and design: all authors, with R.H. writing the first draft of the protocol. Analysis and/or interpretation: all authors, with E.R. taking primary responsibility for the statistical analyses. Drafting the manuscript for important intellectual content: all authors, with R.B. writing the first draft.
Data-Sharing Statement: Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data-sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli can be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. The AstraZeneca Vivli member page is also available, outlining further details: https://vivli.org/ourmember/astrazeneca/. The NOVELTY protocol is available at https://astrazenecagrouptrials.pharmacm.com.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202308-1436OC on November 29, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Global Initiative for Asthma. 2023. www.ginasthma.org
- 2. Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, et al. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD executive summary. Am J Respir Crit Care Med . 2023;207:819–837. doi: 10.1164/rccm.202301-0106PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calverley PM, Albert P, Walker PP. Bronchodilator reversibility in chronic obstructive pulmonary disease: use and limitations. Lancet Respir Med . 2013;1:564–573. doi: 10.1016/S2213-2600(13)70086-9. [DOI] [PubMed] [Google Scholar]
- 4. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J . 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 5. Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J . 2022;60:2101499. doi: 10.1183/13993003.01499-2021. [DOI] [PubMed] [Google Scholar]
- 6. Quanjer PH, Ruppel GL, Langhammer A, Krishna A, Mertens F, Johannessen A, et al. Bronchodilator response in FVC is larger and more relevant than in FEV1 in severe airflow obstruction. Chest . 2017;151:1088–1098. doi: 10.1016/j.chest.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 7. Tan WC, Vollmer WM, Lamprecht B, Mannino DM, Jithoo A, Nizankowska-Mogilnicka E, et al. BOLD Collaborative Research Group Worldwide patterns of bronchodilator responsiveness: results from the Burden of Obstructive Lung Disease study. Thorax . 2012;67:718–726. doi: 10.1136/thoraxjnl-2011-201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen C, Jian W, Gao Y, Xie Y, Song Y, Zheng J. Early COPD patients with lung hyperinflation associated with poorer lung function but better bronchodilator responsiveness. Int J Chron Obstruct Pulmon Dis . 2016;11:2519–2526. doi: 10.2147/COPD.S110021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han MK, Wise R, Mumford J, Sciurba F, Criner GJ, Curtis JL, et al. NETT Research Group Prevalence and clinical correlates of bronchoreversibility in severe emphysema. Eur Respir J . 2010;35:1048–1056. doi: 10.1183/09031936.00052509. [DOI] [PubMed] [Google Scholar]
- 10. Lee JS, Huh JW, Chae EJ, Seo JB, Ra SW, Lee JH, et al. Response patterns to bronchodilator and quantitative computed tomography in chronic obstructive pulmonary disease. Clin Physiol Funct Imaging . 2012;32:12–18. doi: 10.1111/j.1475-097X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 11. Rodríguez-Carballeira M, Heredia JL, Rué M, Quintana S, Almagro P. The bronchodilator test in chronic obstructive pulmonary disease: interpretation methods. Respir Med . 2007;101:34–42. doi: 10.1016/j.rmed.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 12. Walker PP, Calverley PM. The volumetric response to bronchodilators in stable chronic obstructive pulmonary disease. COPD . 2008;5:147–152. doi: 10.1080/15412550802092928. [DOI] [PubMed] [Google Scholar]
- 13. Reddel HK, Gerhardsson de Verdier M, Agustí A, Anderson G, Beasley R, Bel EH, et al. Prospective observational study in patients with obstructive lung disease: NOVELTY design. ERJ Open Res . 2019;5:00036-02018. doi: 10.1183/23120541.00036-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reddel HK, Vestbo J, Agustí A, Anderson GP, Bansal AT, Beasley R, et al. NOVELTY study investigators Heterogeneity within and between physician-diagnosed asthma and/or COPD: NOVELTY cohort. Eur Respir J . 2021;58:2003927. doi: 10.1183/13993003.03927-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J . 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3–95-yr age range: the Global Lung Function 2012 equations. Eur Respir J . 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J . 2016;47:410–419. doi: 10.1183/13993003.01359-2015. [DOI] [PubMed] [Google Scholar]
- 18. Agustí A, Bafadhel M, Beasley R, Bel EH, Faner R, Gibson PG, et al. on behalf of all participants in the seminar Precision medicine in airway diseases: moving to clinical practice. Eur Respir J . 2017;50:1701655. doi: 10.1183/13993003.01655-2017. [DOI] [PubMed] [Google Scholar]
- 19. Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al. After asthma: redefining airways diseases. Lancet . 2018;391:350–400. doi: 10.1016/S0140-6736(17)30879-6. [DOI] [PubMed] [Google Scholar]
- 20. McDonald VM, Fingleton J, Agusti A, Hiles SA, Clark VL, Holland AE, et al. participants of the Treatable Traits Down Under International Workshop; Treatable Traits Down Under International Workshop participants Treatable traits: a new paradigm for 21st century management of chronic airway diseases: Treatable Traits Down Under International Workshop report. Eur Respir J . 2019;53:1802058. doi: 10.1183/13993003.02058-2018. [DOI] [PubMed] [Google Scholar]
- 21. Janson C, Malinovschi A, Amaral AFS, Accordini S, Bousquet J, Buist AS, et al. Bronchodilator reversibility in asthma and COPD: findings from three large population studies. Eur Respir J . 2019;54:1900561. doi: 10.1183/13993003.00561-2019. [DOI] [PubMed] [Google Scholar]
- 22. Tuomisto LE, Ilmarinen P, Lehtimäki L, Tommola M, Kankaanranta H. Immediate bronchodilator response in FEV1 as a diagnostic criterion for adult asthma. Eur Respir J . 2019;53:1800904. doi: 10.1183/13993003.00904-2018. [DOI] [PubMed] [Google Scholar]
- 23. Appleton SL, Adams RJ, Wilson DH, Taylor AW, Ruffin RE, North West Adelaide Cohort Health Study Team Spirometric criteria for asthma: adding further evidence to the debate. J Allergy Clin Immunol . 2005;116:976–982. doi: 10.1016/j.jaci.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 24. Kesten S, Rebuck AS. Is the short-term response to inhaled beta-adrenergic agonist sensitive or specific for distinguishing between asthma and COPD? Chest . 1994;105:1042–1045. doi: 10.1378/chest.105.4.1042. [DOI] [PubMed] [Google Scholar]
- 25. Selvanathan J, Aaron SD, Sykes JR, Vandemheen KL, FitzGerald JM, Ainslie M, et al. Canadian Respiratory Research Network Performance characteristics of spirometry with negative bronchodilator response and methacholine challenge testing and implications for asthma diagnosis. Chest . 2020;158:479–490. doi: 10.1016/j.chest.2020.03.052. [DOI] [PubMed] [Google Scholar]
- 26. Fingleton J, Weatherall M, Beasley R. Bronchodilator responsiveness: interpret with caution. Thorax . 2012;67:667–668. doi: 10.1136/thoraxjnl-2012-201966. [DOI] [PubMed] [Google Scholar]
- 27. Bhatt SP, Fortis S, Bodduluri S. New guidelines for bronchodilator responsiveness in COPD: a test in search of a use. Am J Respir Crit Care Med . 2022;206:1042–1044. doi: 10.1164/rccm.202203-0458LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y, Lin J, Wang Z, Wang Z, Tan L, Liu S, et al. Bronchodilator responsiveness defined by the 2005 and 2021 ERS/ATS criteria in patients with asthma as well as chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis . 2022;17:2623–2633. doi: 10.2147/COPD.S385733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scottish Intercollegiate Guidelines Network/British thoracic Society British Guidelines on the management of asthma. https://www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/
- 30. Beasley R, Beckert L, Fingleton J, Hancox RJ, Harwood M, Hurst M, et al. Asthma and Respiratory Foundation NZ Adolescent and Adult Asthma Guidelines 2020: a quick reference guide. N Z Med J . 2020;133:73–99. [PubMed] [Google Scholar]
- 31. Ward H, Cooper BG, Miller MR. Improved criterion for assessing lung function reversibility. Chest . 2015;148:877–886. doi: 10.1378/chest.14-2413. [DOI] [PubMed] [Google Scholar]
- 32. Brand PL, Quanjer PH, Postma DS, Kerstjens HA, Koëter GH, Dekhuijzen PN, et al. The Dutch Chronic Non-Specific Lung Disease (CNSLD) Study Group Interpretation of bronchodilator response in patients with obstructive airways disease. Thorax . 1992;47:429–436. doi: 10.1136/thx.47.6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. ECLIPSE Investigators Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med . 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 34. Fortis S, Quibrera PM, Comellas AP, Bhatt SP, Tashkin DP, Hoffman EA, et al. Subpopulations and Intermediate Outcome Measures in COPD Study Investigators Bronchodilator responsiveness in tobacco-exposed people with or without COPD. Chest . 2023;163:502–514. doi: 10.1016/j.chest.2022.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Papi A, Romagnoli M, Baraldo S, Braccioni F, Guzzinati I, Saetta M, et al. Partial reversibility of airflow limitation and increased exhaled NO and sputum eosinophilia in chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2000;162:1773–1777. doi: 10.1164/ajrccm.162.5.9910112. [DOI] [PubMed] [Google Scholar]
- 36. Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med . 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aaron SD, Vandemheen KL, FitzGerald JM, Ainslie M, Gupta S, Lemière C, et al. Canadian Respiratory Research Network Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA . 2017;317:269–279. doi: 10.1001/jama.2016.19627. [DOI] [PubMed] [Google Scholar]
- 38. Travers J, Marsh S, Williams M, Weatherall M, Caldwell B, Shirtcliffe P, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax . 2007;62:219–223. doi: 10.1136/thx.2006.066837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brown T, Jones T, Gove K, Barber C, Elliott S, Chauhan A, et al. Wessex Severe Asthma Cohort (WSAC) team; Members of the Wessex Severe Asthma Cohort (WSAC) team Randomised controlled trials in severe asthma: selection by phenotype or stereotype. Eur Respir J . 2018;52:1801444. doi: 10.1183/13993003.01444-2018. [DOI] [PubMed] [Google Scholar]
- 40. Travers J, Marsh S, Caldwell B, Williams M, Aldington S, Weatherall M, et al. External validity of randomized controlled trials in COPD. Respir Med . 2007;101:1313–1320. doi: 10.1016/j.rmed.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 41. Calverley PM, Burge PS, Spencer S, Anderson JA, Jones PW, for the ISOLDE Study Investigators Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax . 2003;58:659–664. doi: 10.1136/thorax.58.8.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hanania NA, Celli BR, Donohue JF, Martin UJ. Bronchodilator reversibility in COPD. Chest . 2011;140:1055–1063. doi: 10.1378/chest.10-2974. [DOI] [PubMed] [Google Scholar]
- 43. Albert P, Agusti A, Edwards L, Tal-Singer R, Yates J, Bakke P, et al. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax . 2012;67:701–708. doi: 10.1136/thoraxjnl-2011-201458. [DOI] [PubMed] [Google Scholar]
- 44. Kainu A, Lindqvist A, Sarna S, Lundbäck B, Sovijärvi A. FEV1 response to bronchodilation in an adult urban population. Chest . 2008;134:387–393. doi: 10.1378/chest.07-2207. [DOI] [PubMed] [Google Scholar]
- 45. Johannessen A, Lehmann S, Omenaas ER, Eide GE, Bakke PS, Gulsvik A. Post-bronchodilator spirometry reference values in adults and implications for disease management. Am J Respir Crit Care Med . 2006;173:1316–1325. doi: 10.1164/rccm.200601-023OC. [DOI] [PubMed] [Google Scholar]
- 46. Kerstjens HA, Brand PL, Quanjer PH, van der Bruggen-Bogaarts BA, Koëter GH, Postma DS, Dutch CNSLD Study Group Variability of bronchodilator response and effects of inhaled corticosteroid treatment in obstructive airways disease. Thorax . 1993;48:722–729. doi: 10.1136/thx.48.7.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tommola M, Won HK, Ilmarinen P, Jung H, Tuomisto LE, Lehtimäki L, et al. Relationship between age and bronchodilator response at diagnosis in adult-onset asthma. Respir Res . 2020;21:179. doi: 10.1186/s12931-020-01441-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tuomisto LE, Ilmarinen P, Lehtimäki L, Niemelä O, Tommola M, Kankaanranta H. Clinical value of bronchodilator response for diagnosing asthma in steroid-naïve adults. ERJ Open Res . 2021;7:00293-02021. doi: 10.1183/23120541.00293-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fortis S, Comellas A, Make BJ, Hersh CP, Bodduluri S, Georgopoulos D, et al. COPDGene Investigators–Core Units: Administrative Center, COPDGene Investigators–Clinical Centers: Ann Arbor VA Combined forced expiratory volume in 1 second and forced vital capacity bronchodilator response, exacerbations, and mortality in chronic obstructive pulmonary disease. Ann Am Thorac Soc . 2019;16:826–835. doi: 10.1513/AnnalsATS.201809-601OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hansen JE, Dilektasli AG, Porszasz J, Stringer WW, Pak Y, Rossiter HB, et al. A new bronchodilator response grading strategy identifies distinct patient populations. Ann Am Thorac Soc . 2019;16:1504–1517. doi: 10.1513/AnnalsATS.201901-030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barjaktarevic IZ, Buhr RG, Wang X, Hu S, Couper D, Anderson W, et al. NHLBI SubPopulations and InteRmediate Outcome Measures In COPD Study (SPIROMICS) Clinical significance of bronchodilator responsiveness evaluated by forced vital capacity in COPD: SPIROMICS Cohort Analysis. Int J Chron Obstruct Pulmon Dis . 2019;14:2927–2938. doi: 10.2147/COPD.S220164. [DOI] [PMC free article] [PubMed] [Google Scholar]