From the Authors:
We thank Eleuteri and colleagues for recognizing the importance of our study on the role of high-dose inhaled nitric oxide (iNO) in improving systemic oxygenation in patients with COVID-19 (1). We appreciate the opportunity to engage in a meaningful dialogue about our work and have provided responses to each of the three comments raised by Eleuteri and colleagues.
First, maintaining oxygenation remains the cornerstone of the management of acute respiratory distress syndrome (ARDS). Several large, robust clinical trials support that maneuvers which improve oxygenation are associated with a decreased risk of mortality in patients with ARDS (2–4). Although multiorgan failure contributes significantly to mortality in patients with ARDS, including those with COVID-19, addressing hypoxemia remains a crucial aspect of patient care. It is conceivable that certain subgroups of patients with ARDS, particularly Black patients with nitric oxide cyclic guanosine 3′,5′-monophosphate system suppression or those with specific clinical characteristics or phases of ARDS, might derive more substantial advantages from iNO therapy. Exploring these nuances in patient selection and refining treatment protocols could be pivotal in unraveling the full therapeutic potential of iNO.
Second, we agree that NO production is increased in inflammatory conditions through the inducible nitric oxide synthase pathway (5). This induction of NO synthesis and production of reactive oxygen species in inflammation serves as a mediator of the immune response (5). However, iNO is unlikely to play a role in the inflammatory response because of its rapid degradation (6). On the contrary, iNO has been shown to have potent antiinflammatory action, which improves allograft function after liver transplant (7, 8) and reduces kidney injury in cardiac surgery (8). Specifically, during hemolysis, NO has been shown to react rapidly with plasma-free oxyhemoglobin to form methemoglobin, thereby reducing the oxidative burden (6). This antioxidant action of iNO led to the hypothesis that iNO reduces the risk of postoperative risk of acute kidney injury in cardiac surgery patients in whom cardiopulmonary bypass was used (9). Cardiopulmonary bypass is associated with hemolysis, and the increase in oxyhemoglobin leads to precipitation of acute kidney injury secondary to oxidative damage (9). Administration of iNO was hypothesized to convert oxyhemoglobin into methemoglobin and reduce the risk of postoperative acute kidney injury (9). This randomized controlled trial of 240 patients showed that iNO reduced the risk of postoperative acute kidney injury in cardiac surgery patients by 22% through its hypothesized antioxidant action (9). Furthermore, longitudinal measures of inflammatory markers such as C-reactive protein (CRP) and ferritin were only available in a subset of the current study population. Among the 63 patients with CRP values and 58 patients with ferritin values, we found that the change in CRP (mean difference by treatment group, 29.6; 95% confidence interval, −15.1, 74.3) and ferritin (mean difference by treatment group, −169.7; 95% confidence interval, −737.9, 399.4) concentrations at 48 hours were similar in both study arms (Table 1). Further studies are needed to assess the role of inflammatory markers in guiding the selection of patients with ARDS who would benefit the most from treatment with iNO therapy.
Table 1.
Change in Inflammatory Markers at 48 Hours, Stratified by Study Arm
| Unadjusted Analysis |
Adjusted Analysis* |
||||||
|---|---|---|---|---|---|---|---|
| Marker | Group (n) | Mean (95% CI)† | Group Difference (95% CI) | P Value | Mean (95% CI)† | Group Difference (95% CI) | P Value |
| CRP | NO (32) | 31.8 (−3.5, 67.2) | 27.8 (−22.5, 78.2) | 0.27 | 33.8 (1.0, 66.7) | 29.6 (−15.1, 74.3) | 0.18 |
| Control (31) | 4.0 (−31.9, 39.9) | 4.2 (−31.5, 39.8) | |||||
| Ferritin | NO (30) | −50.2 (−454.1, 353.7) | −24.1 (−605.3, 557.2) | 0.93 | −176.9 (−587.9, 234.1) | −169.7 (−737.9, 399.4) | 0.55 |
| Control (28) | −26.1 (−444.1, 391.9) | −7.7 (−445.1, 429.7) | |||||
Definition of abbreviations: CI = confidence interval; CRP = C-reactive protein; NO = nitric oxide.
Adjusted for age, age2, sex, race, and body mass index.
Values depict least squares mean and 95% confidence interval.
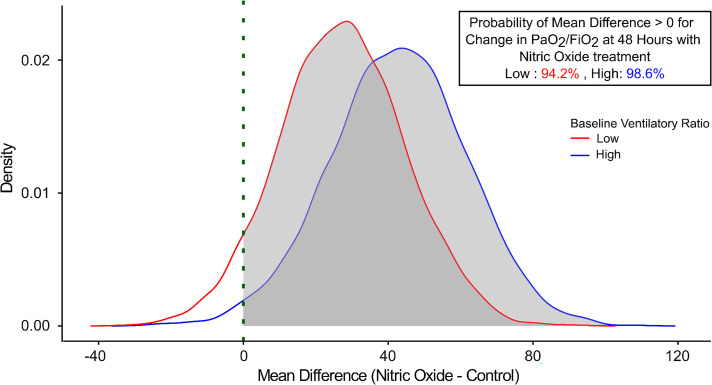
Third, we agree that COVID-19 ARDS is associated with microvascular thrombosis and microangiopathy, leading to profound perfusion abnormalities, as highlighted in our article (10). We were interested in investigating the impact of iNO on the ventilatory ratio in our trial participants (1). We found that iNO did not improve the ventilatory ratio at 48 hours (1). In addition, we also present a post hoc analysis of the effect of iNO on improving oxygenation at 48 hours stratified by the ventilatory ratio. The study population was stratified using the median ventilatory ratio of 1.54 into low (n = 42 in the treatment arm and 48 in the control arm) and high (n = 45 in the treatment arm and 47 in the control arm) ventilatory ratio. We found that the probability that iNO would increase the PaO2/FiO2 ratio at 48 hours was similar in both study groups, at 94.2% in the low ventilatory ratio group and 98.6% in the high ventilatory ratio group (Figure 1). Therefore, patients with severe ARDS (PaO2/FiO2 ratio, <100 mm Hg) may reap the greatest benefit of iNO therapy, regardless of their ventilatory ratio. Precision phenotyping of ARDS based on various factors, such as clinical, physiological, and molecular markers, to better understand and tailor treatment approaches for specific subtypes or manifestations of the condition is warranted.
Figure 1.
Posterior probability curves for change in PaO2/FiO2 ratio at 48 hours with inhaled nitric oxide therapy stratified by ventilatory ratio.
Footnotes
Supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health awards (R01HL160982, R01HL163852, R01HL163081, and K23HL146887) (P.A.) and by the Reginald Jenney Endowment Chair at Harvard Medical School, Sundry Funds at Massachusetts General Hospital, and laboratory funds of the Anesthesia Center for Critical Care Research of the Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital (L.B.).
Originally Published in Press as DOI: 10.1164/rccm.202311-2112LE on December 21, 2023
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Di Fenza R, Shetty NS, Gianni S, Parcha V, Giammatteo V, Safaee Fakhr B, et al. Nitric Oxide Investigators High-dose inhaled nitric oxide in acute hypoxemic respiratory failure due to COVID-19: a multicenter phase II trial. Am J Respir Crit Care Med . 2023;208:1293–1304. doi: 10.1164/rccm.202304-0637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. PROSEVA Study Group Prone positioning in severe acute respiratory distress syndrome. N Engl J Med . 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 3. DesPrez K, McNeil JB, Wang C, Bastarache JA, Shaver CM, Ware LB. Oxygenation saturation index predicts clinical outcomes in ARDS. Chest . 2017;152:1151–1158. doi: 10.1016/j.chest.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Goligher EC, Kavanagh BP, Rubenfeld GD, Adhikari NK, Pinto R, Fan E, et al. Oxygenation response to positive end-expiratory pressure predicts mortality in acute respiratory distress syndrome. A secondary analysis of the LOVS and ExPress trials. Am J Respir Crit Care Med . 2014;190:70–76. doi: 10.1164/rccm.201404-0688OC. [DOI] [PubMed] [Google Scholar]
- 5. Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J . 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ichinose F, Roberts JD, Jr, Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation . 2004;109:3106–3111. doi: 10.1161/01.CIR.0000134595.80170.62. [DOI] [PubMed] [Google Scholar]
- 7. Lang JD, Jr, Smith AB, Brandon A, Bradley KM, Liu Y, Li W, et al. A randomized clinical trial testing the anti-inflammatory effects of preemptive inhaled nitric oxide in human liver transplantation. PLoS One . 2014;9:e86053. doi: 10.1371/journal.pone.0086053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lang JD, Jr, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest . 2007;117:2583–2591. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lei C, Berra L, Rezoagli E, Yu B, Dong H, Yu S, et al. Nitric oxide decreases acute kidney injury and stage 3 chronic kidney disease after cardiac surgery. Am J Respir Crit Care Med . 2018;198:1279–1287. doi: 10.1164/rccm.201710-2150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Villalba JA, Hilburn CF, Garlin MA, Elliott GA, Li Y, Kunitoki K, et al. Vasculopathy and increased vascular congestion in fatal COVID-19 and acute respiratory distress syndrome. Am J Respir Crit Care Med . 2022;206:857–873. doi: 10.1164/rccm.202109-2150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]