Summary:
Background:
The human endometrium undergoes recurring cycles of growth, differentiation, and breakdown in response to sex hormones. Dysregulation of epithelial-stromal communication during hormone-mediated signaling may be linked to myriad gynecological disorders for which treatments remain inadequate. Here, we describe a completely defined, synthetic extracellular matrix that enables co-culture of human endometrial epithelial and stromal cells in a manner that captures healthy and disease states across a simulated menstrual cycle.
Methods:
We parsed cycle-dependent endometrial integrin expression and matrix composition to define candidate cell-matrix interaction cues for inclusion in a polyethylene glycol (PEG)-based hydrogel crosslinked with matrix metalloproteinase-labile peptides. We semi-empirically screened a parameter space of biophysical and molecular features representative of the endometrium to define compositions suitable for hormone-driven expansion and differentiation of epithelial organoids, stromal cells, and co-cultures of the two cell types.
Findings:
Each cell type exhibited characteristic morphological and molecular responses to hormone changes when co-encapsulated in hydrogels tuned to a stiffness regime similar to the native tissue and functionalized with a collagen-derived adhesion peptide (GFOGER) and a fibronectin-derived peptide (PHSRN-K-RGD). Analysis of cell-cell crosstalk during IL1B-induced inflammation revealed dysregulation of epithelial proliferation mediated by stromal cells.
Conclusions:
Altogether, we demonstrate the development of a fully synthetic matrix to sustain the dynamic changes of the endometrial microenvironment and support its applications to understand menstrual health and endometriotic diseases.
Graphical Abstract

eTOC Blurb:
Gnecco and Brown et al develop a synthetic extracellular matrix that establishes a novel co-culture model of endometrial stromal and epithelial organoids in a controlled and tunable biomaterial. This allows the study of hormone-mediated processes of the human menstrual cycle in vitro and analysis of cell-cell and cell-extracellular matrix interactions.
Introduction:
The endometrium is the mucosal lining of the uterus in which the establishment and maintenance of pregnancy occurs1. It is a highly dynamic tissue that undergoes spatial and temporal changes in response to endocrine signaling by the ovarian sex hormones estradiol (E2) and progesterone (P4). Cyclical changes in these hormones dictate the timing and functional capabilities of the endometrium to support nidation by driving cell-specific morphological and biochemical processes2. Histologically, the endometrium is primarily composed of the hormone-responsive epithelium and specialized reticular fibroblasts (stromal cells) embedded in a dynamic extracellular matrix (ECM).1,3,4 Endocrine-induced reproductive function is mediated through both direct and indirect mechanisms governed by the crosstalk between these cell populations.5–8 The idealized 28-day human menstrual cycle is characterized by three distinct phases in which the endometrium undergoes E2-mediated tissue regeneration (the ‘proliferative phase’) followed by a 14-day P4-dominant phase (the ‘secretory phase’) that governs the differentiation processes necessary for the successful establishment of pregnancy (Fig 1A). In the absence of embryo implantation, a sharp withdrawal of hormones triggers a cascade of inflammatory processes that result in the shedding of the endometrial tissue (the ‘menstrual phase’) after which the cycle initiates again.1,4 Despite the fundamental roles E2 and P4 play in maintaining reproductive functions, a thorough understanding of the cellular mechanisms driving these processes remains elusive due in part to a lack of physiological models that recapitulate the complex multi-cellular human condition.1,9
Figure 1. A tissue-inspired multi-omic approach elucidates the design parameters to engineer a fully synthetic PEG hydrogel for the endometrium.
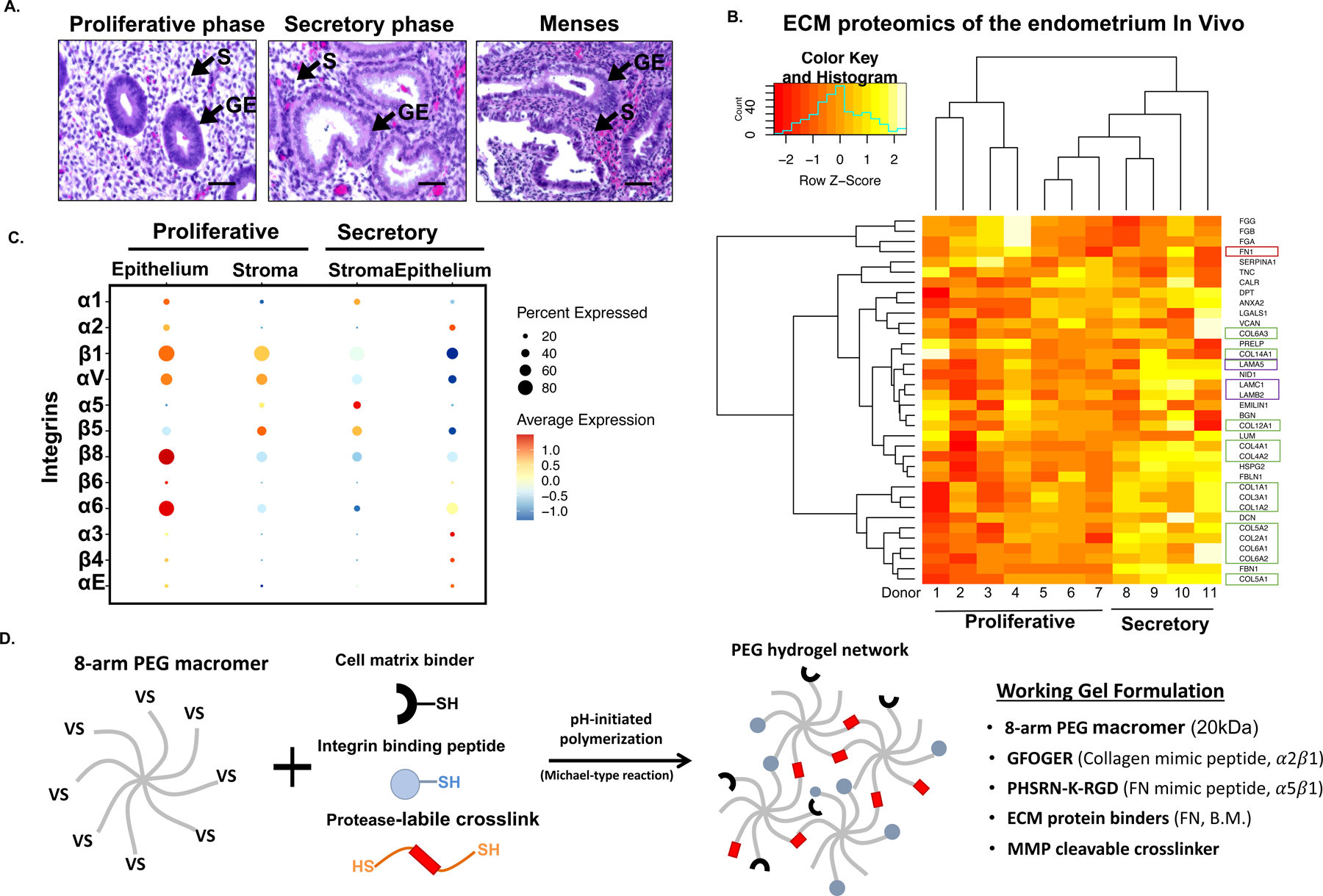
(A) Representative histological hematoxylin and eosin (H&E) stained samples of the menstrual cycle phases. Arrows (▲) indicate stromal stromal fibroblasts (S) and the glandular epithelium (GE). (B) Heatmap of proteomic analysis of the human endometrium showing ECM proteins (matrisome) across the menstrual cycle (n=11 donors). Box colors indicated ECM protein associations: green corresponds to collagens; red to fibronectin; and purple to laminins. (C) Dot plot analysis of specific integrins expression from single cell RNA-seq data (n = 6 donors, 6,659 cells). Examination of stromal and epithelial component integrin expression across the menstrual cycle (proliferative and secretory phases). Circle size and color indicate P-value and variation about the means of the average expression value for each integrin chain in all cells (D) Schematic of the overall strategy to design and fabricate a synthetic ECM for the endometrium. 8-arm 20kDa PEG macromers are functionalized with cell matrix binders (FN, B.M.), integrin adhesion peptides, and polymerized with a protease-labile crosslinker. Descriptive outline of the key peptides utilized in the synthetic ECM formulation.
Experimental models capable of recapitulating epithelial-stromal crosstalk are necessary to understand human reproductive physiology and pathology. Endometrial stromal cells (ESCs) are routinely used for experimentation in reproductive biology due to their ability to be cultured and expanded in vitro, however culturing epithelium has been more challenging. Recently, protocols to expand and culture primary endometrial epithelial cells as endometrial epithelial organoids (EEOs) have transformed the experimental landscape for in vitro analysis of endometrial behavior.10,11 Organoids are self-organizing 3D cell structures that retain many physiologically relevant and functional features of their native tissues of origin.12 Thus, EEOs provide opportunities to investigate human reproductive function, understand disease pathogenesis13, and test therapeutic compounds for clinical applications.10,11 EEOs are typically generated from isolated primary endometrial glands embedded in Matrigel, a complex, murine tumor-derived ECM primarily comprising heterogenous basement membrane (BM) proteins and growth factors (GFs), together with a defined culture medium designed to enrich the expansion of the stem/progenitor compartment.14,15 Cultured in Matrigel, clonally-generated EEOs retain columnar epithelial architecture of the native tissue and form polarized cyst-like structures that are genomically stable, hormone-responsive, and cell-heterogenous.10,11,16 However, the reliance on Matrigel to culture organoids presents a critical obstacle for numerous experimental, analytical, and therapeutic applications for dynamic and hormone-sensitive tissues such as the endometrium.17,18 Matrigel’s inherent lot-to-lot variability, poorly-defined molecular composition, limited biophysical properties, and poor suitability for co-culture of epithelia with stromal cells have driven efforts to develop synthetic ECMs that are fully defined, modular, and tunable.13,19–27
Although endometrial epithelia and stroma are often co-cultured in naturally-derived ECM hydrogels28–30, these models are limited by rapid ECM breakdown, which ultimately leads to highly abbreviated experimental conditions and limited control over the biophysical and molecular ECM compositions that can reliably support the morphogenic and functional behavior of multiple cell types. Recently, the development of fully or semi-synthetic ECMs to culture enteric organoids were reported for both murine24,31 and human18,22,27,31 tissues. We have previously demonstrated that poly(ethylene glycol) (PEG)-based hydrogel systems can be applied to model some aspects of the endometrial mucosal barrier,26 and developed a completely synthetic hydrogel formulation for the culture, differentiation and passaging of human intestinal enteroids that also supported culture of endometrial epithelial cells27. This synthetic ECM also supports the culture of pancreatic tumor organoids with a complex stroma.32 Despite these advances, a fully-defined synthetic ECM that can support the long-term culture and function of multiple endometrial cell populations, especially under the dynamic conditions of hormone variation in a simulated menstrual cycle, is still an unmet need.
Inspired by cell-matrix interactions in native tissue, we designed and synthesized a synthetic ECM that targets cell-specific integrins on epithelia and stroma, supports cell-secreted ECM deposition, and mimics the biophysical properties of the endometrium in health and disease. We show that this bio-labile PEG-based synthetic ECM supports the 3D co-culture of primary human EEOs and ESCs and recapitulates characteristic phenotypic properties of the endometrial responses to sex hormone changes across a simulated 15-day menstrual cycle in vitro. We used this 3D co-culture model to parse stromal-epithelial crosstalk and disease-related phenotypes in response to inflammatory cues.
Results
Design of a fully synthetic ECM guided by multi-omic evaluation of the human endometrium.
The ECM composition of the endometrium, along with cell-matrix receptor expression, are regulated by sex steroids throughout the menstrual cycle,33,34 giving rise to dramatic shifts in tissue structure and morphology (Fig 1A). The overarching design goal for our synthetic ECM is to define a minimal set of biophysical and biomolecular cues required to support the dynamic phenotypic functions of heterogenous endometrial cell populations, over weeks of culture.
As a first step, we performed a targeted proteomic analysis (LC-MS/MS, N=11) of the endometrial ECM-associated proteins (matrisome) across the menstrual cycle to benchmark the ECM composition in the native tissue (Fig 1B). Consistent with immunohistochemical reports34–36, FN abundance was greater in the proliferative phase, while fibrillin, collagen and laminin proteins increased during the secretory phase, corresponding to the differentiation of stromal fibroblasts, epithelial gland maturation, and vascular remodeling in response to progesterone (Fig 1B).
Our initial studies demonstrating the establishment of endometrial organoids in a synthetic ECM27 were guided in part by existing histologic analysis of endometrial integrin expression patterns from a subset of the 24 known integrins.36–43 We sought additional insights into cell population-specific integrin expression by probing a single cell RNA-sequencing dataset44 (scRNAseq, N=6; Fig 1C; Fig S1 in Data File 1) to profile the integrin expression across the endometrial proliferative or secretory phase epithelia and stroma in addition to the immune and vascular cellular compartments. Across all cells in the data set, transcripts for all eighteen α chains and all eight β chains, except integrin β3, were detected (data not shown). Integrin β3, a contested marker of fertility that appears in the secretory phase45–47, was also absent in another endometrial scRNAseq data set.48 Consistent with the immunohistochemistry literature38, αV was robustly expressed by epithelia and stroma mostly during the proliferative phase. Its primary binding partner β5 was primarily expressed by the stroma across the cycle (Fig S1A in Data File 1), while β6 was sparse except in proliferative phase epithelia (Fig S1A in Data File 1). Unexpectedly, integrin β8, an additional αV partner that has not previously been characterized via immunohistochemistry in the endometrium, was robustly expressed in several epithelial and some stromal subpopulations (Fig 1C; Fig S1A in Data File 1). Also consistent with the immunohistochemistry data36,39,41–43 integrin β1 was robustly expressed by both epithelia and stroma across the cycle. Expression of the collagen-binding α1 dimer (at lower levels, especially in proliferative epithelia) along with strong expression of α2 in the epithelia was also observed, while the stroma primarily expressed α5 (Fig 1C, Fig S1B in Data File 1). Finally, laminin-binding α6 and its heterodimer β1 was robustly expressed in all epithelia, while expression in the stroma was higher in the proliferative phase (Fig S1 in Data File 1). We investigated the expression of a subset of these integrins in cultured endometrial cells via qPCR confirming comparable expression of β1, α1, α2 and αv in both stromal and epithelial cells, but significantly greater expression of α5 and α8 in stromal cells (Fig S1C in Data File 1).
The minimal synthetic ECM formulation must provide integrin-engaging adhesions cues that support cell viability immediately post-encapsulation upon dissociation from tissue or a prior culture state49,50, while enabling remodeling and stabilization of cell-produced ECM that provides a fuller spectrum of adhesion cues as the culture progresses. We thus include the collagen-derived peptide GFOGER, recognized by the α1β1 (expressed on stroma and epithelia) and α2β1 integrin heterodimers (expressed on epithelia),51–53,54 together with the fibronectin-derived PHSRN-K-RGD peptide4,26 as an RGD ligand for αVβ1 and α5β1 (Fig 1D). This ligand incorporates the PHSRN synergy site recognized by α5β1 heterodimer primarily expressed by the stroma and, to a lesser extent, by the secretory epithelium (Fig 1C).
As cells remodel the local microenvironment, proteolytically degrading the synthetic ECM, they deposit cell-specific ECM, creating an additional dominant source of adhesion cues for a broad spectrum of receptors as the culture progresses. To sequester the cell-produced ECM and enhance its interactions with the synthetic ECM, we incorporated peptides that bind to basement membrane components produced by epithelia (and some decidual fibroblasts) and also to fibronectin produced by stromal cells, as previously described.26,27,32 This strategy obviates the need to include laminin-derived peptides.26,27,32
To synthesize the synthetic ECM, we first functionalized an 8-arm PEG macromers with a combination of GFOGER and PHSRN-K-RGD (“MIX”) in defined ratios (see below), then crosslinked this macromer solution, in the presence of cells, with a protease-degradable crosslinking peptide (“CL-LW”) to enable cell-dependent matrix remodeling, migration and proliferation during hormone-driven morphogenesis.26,55,56 Using this basic framework, individual synthetic ECM parameters (e.g., matrix stiffness) were systematically and independently varied to mimic aspects of healthy and disease states (Fig 1D). Altogether, a tissue-inspired approach was implemented to design a synthetic matrix that would initiate and maintain the culture of EEOs and ESC in a controlled environment that mimics the molecular and biophysical properties of the endometrial ECM.
Synthetic ECM supports 3D co-culture of primary human endometrial stromal and epithelial cells.
A semi-empirical screen of synthetic ECM properties yields a cue-response phenotypic landscape for endometrial epithelial organoids.
We previously demonstrated that a particular formulation of synthetic ECM designed for enteric organoids also supported endometrial epithelial organoids27; however, the impact of the biophysical properties and peptide composition on organoid phenotype and function was largely unexplored. These first-generation hydrogels27 incorporated only one adhesion ligand (i.e., GFOGER) and were substantially stiffer (~2000 Pa, corresponding to 5wt% PEG gels) than Matrigel (~150–443 Pa)21,57 or the native endometrial tissue (~250 Pa)58. Thus, to better understand EEO generation in synthetic matrices, we probed the biophysical parameters that more closely mimicked native endometrial stiffness and additionally interrogated the consequences of integrin ligand composition. We first generated a tissue bank of EEOs from 8 endometrial donors with and without endometriotic disorders (Table 1 and Fig S2A in Data File 1) and defined a panel of gels with varying elastic moduli ranging from “soft” gels (3wt% PEG, ~300 Pa), comparable to native stiffness of the endometrium, to the “stiffest” gels (7wt% PEG, ~6,000 Pa; Fig 2A). To test these formulations, we dissociated organoids to single cells, passaged them into synthetic matrix or Matrigel, and followed the emergence into single cell-derived endometrial epithelial organoids (“scEEO”, Fig S2B in Data File 1). In agreement with our previous work27, employing synthetic hydrogels functionalized with GFOGER was sufficient to support the growth of scEEOs, even in a relatively stiff (5wt% PEG) environment. However, scEEOs generated in stiffer gels developed a crenelated morphology (Fig 2B; Fig S4 in Data File 1) compared to those cultured in softer conditions (3wt% PEG), and this was consistent across all donors regardless of the donor’s disease state (data not shown).
Figure 2. Dual integrin ligand functionalization in softer synthetic matrix regimes enhance EEOs generation.
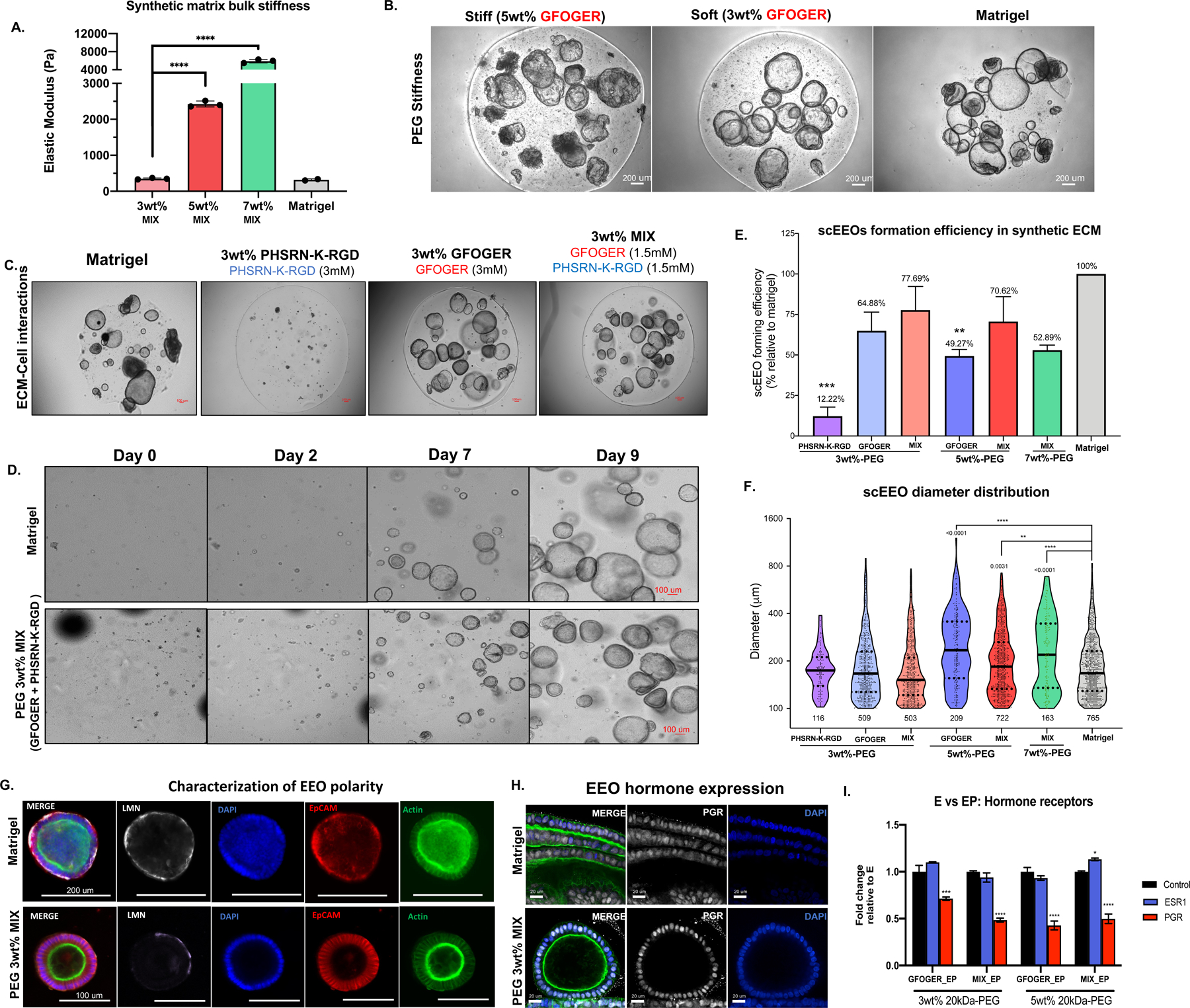
(A) Biomechanical properties (elastic moduli, Pa, N=3 gels) of synthetic hydrogel formulations, where “soft” = 3wt% (~300 Pa), “stiff” = 5wt% (~2kPa) and “stiffest” = 7wt% (~6kPa). (B) Representative images of scEEO and morphology in stiff (5wt%) in soft (3wt%) PEG hydrogels functionalized with only GFOGER (3mM) compared to Matrigel. (C) Day-15 images of hydrogel formulations comparing adhesion peptides reveals that GFOGER is necessary to generate scEEOs. Dual-adhesion-peptide functionalization with GFOGER (1.5 mM) and PHSRN-K-RGD (1.5 mM) is sufficient for robust scEEO generation. (D) Time-lapse images of 10-day cultures of EEO derived from single cells in synthetic hydrogels (PEG 3wt%-MIX). (E) Quantification of the scEEO formation efficiency in synthetic hydrogels relative to Matrigel (N=8). (F) Quantification of the total lumenized EEO (>100 um diameter) count and EEO diameter distribution after 10 days in culture. (G) Characterization of EEO morphology and polarity of EEOs generated in synthetic ECM (3wt%-MIX) compared to Matrigel via immunofluorescent (IF) analysis of ECM deposition for laminin (LMN) and F-actin localization after 14 days of culture in soft (3%) PEG MIX hydrogel and in Matrigel. (H-I) Characterization of protein and transcriptomic expression of hormone receptors in EEOs generated in synthetic hydrogels. (H) Representative images of PGR staining countered stained with F-actin (green) and DAPI (nuclei) and (I) qPCR analysis of mRNA expression of PGR and ESR1 in EEOs treated with either E2 (E) or E2 + MPA (EP) for 14 days. Analysis compares ESR1, PGR values relative to matching E treatment groups (control). Significance is indicated as *p<0.05, **p<0.01, ***p<0.001.
Anticipating the integration of α5β1-expressing stromal cells, we then evaluated the influence of an additional integrin ligand on scEEO growth behavior and morphologies. We compared ECM formulations incorporating nominal concentrations of GFOGER (3mM), PHSRN-K-RGD (3mM), or a MIX of both peptide (1.5 mM each) in the soft (~300 Pa, 3wt% PEG) gel regime. Biomechanical properties were not significantly affected by differences in integrin ligand composition (data not shown). As expected, GFOGER was necessary to generate scEEOs in synthetic matrices (Fig 2C; Fig S3 in Data File 1), presumably through engagement of α1β1 and α2β1, and although PHSRN-K-RGD conferred minimal scEEO generation alone the incorporation of both adhesion ligands in the MIX formulation robustly promoted the growth of the scEEO despite the lower (1.5 mM vs 3 mM) concentration of GFOGER in the MIX (1.5 mM GFOGER + 1.5 mM PHSRN-K-RGD) formulation. When visualized by brightfield imaging on sequential days after seeding single cells, organoids appeared to emerge more slowly in PEG matrices (soft, 3wt%) than in Matrigel, yet they retained similar morphologies and diameters by day 9 of culture (Fig 2D) suggesting that the 3wt% gels function with comparable efficacy to Matrigel.
Having confirmed the requirement for GFOGER, and comparable scEEO emergence in both GFOGER-only and MIX gels, we next compared how matrix stiffnesses impacted scEEO emergence by varying the PEG polymer content from 3wt% (~300 Pa) to 7wt% (~6 kPa) to mimic physiologic58 and pathologic59 uterine regimes, respectively (Fig 2E–F). The efficiency of scEEO formation, as assessed by the number of organoids at day 14 relative to Matrigel, was slightly inversely correlated with matrix stiffness (Fig 2E). Moreover, organoid diameter distributions in stiffer matrices, where organoids were sparser, skewed slightly higher in the 5wt% and 7wt% gels compared to softer 3wt% matrices (Fig 2F). Consistently, the organoids cultured in stiffer matrices (5wt% and 7wt%) manifested the meandering, crenelated morphology described above, but not in softer PEG matrices or Matrigel (Fig S4 in Data File 1). These results suggest that EEO clonal establishment is robust across a range of biophysical properties (Fig 2E–F) and provide a preliminary indication that tissue stiffness influences epithelial morphology and behavior.
Based on these results, we focused further investigations using the more physiologically relevant 3wt%-MIX gels to characterize scEEO architecture, hormone response and cellular heterogeneity in the synthetic ECM. PEG-derived scEEOs exhibited proper polarity based on basolateral laminin (LMN) deposition and apical F-actin accumulation, comparable to those generated in Matrigel (Fig 2G). Moreover, scEEOs generated in synthetic matrices retained their epithelial origin and architecture as assessed by EpCAM and F-actin staining (Fig S5A–B in Data File 1), were mitotically active (Fig S5C in Data File 1), and exhibited appropriate response to the sex steroids E2 (1nM, 14 days) or the synthetic progestin (MPA, 500 nM, 14 days). As demonstrated by immunohistochemical staining of progesterone receptors (PGR), EEOs cultured in both hydrogels express PGR when maintained with E2 (Fig 2H). Treatment with progestin reduced nuclear staining of PGR while maintaining estrogen receptor (ESR1) (Fig S5E in Data File 1). Downregulation of PGR RNA transcript, but not ESR1, was also in response to progestin treatment by qPCR (Fig 2I). To confirm scEEO maturation, live-imaging and immunostaining revealed distinct populations of motile ciliated and secretory epithelial cells (Fig S5D in Data File 1; Video S1–S2 in Data File 2). These results were consistent across several tissue donors (N=8). Altogether, these results justified the use of soft (3wt%) PEG hydrogels, functionalized with a dual presentation of GFOGER and PHSRN-K-RGD (MIX), as a functional synthetic ECM to generate and maintain long-term cultures of endometrial organoids (Fig 1D).
Establishment and characterization of ESC 3D cultures in synthetic ECM
Our results examining organoids in synthetic matrices demonstrated that both the biophysical and the molecular properties of the hydrogels are important to promote adequate scEEO growth and function; therefore, we set out to evaluate, in parallel, how adhesive cues and matrix stiffness influenced ESC behavior in 3D (Fig 3A). Embedded ESCs cultured in the gels were viable in all conditions tested (GFOGER, PHSRN-K-RGD, or MIX for the 3–7% PEG) and maintained comparable cell numbers across all conditions (data not shown). However, ESCs adopted a more characteristic stellate morphology in softer (3wt%) hydrogel microenvironments compared to the stiffer (5wt%) gels which constrained fibroblast spreading and outgrowth (Fig 3B; 7% not shown). Furthermore, in support of previous studies26, hydrogels containing PHSRN-K-RGD further provided a modest increase in stromal dispersion and spreading, as assessed by the fluorescence projected area covered on day 7 of culture, compared to matrices containing GFOGER alone where cells retained a more clumped distribution (Fig 3C).
Figure 3. Development and characterization of a synthetic endometrial stromal cell culture.

(A) Schematic of the workflow for culturing ESCs in 3D using a synthetic hydrogel. (B) Maximum intensity projection images of F-actin (green) reveal ESC remodeling (cell elongation and dispersion) at day 7 of culture in stromal (serum containing) media impacted primarily by hydrogel stiffness (20X magnification) as quantified in (C). (D) Time-lapse images of embedded ESCs in synthetic matrix (PEG) compared to the natural-derivedhydrogels Collagen I (~4 mg/mL) and Matrigel (8 mg/mL) allow for long-term stable cultures. (E) Brightfield images of stromal decidualization morphology in synthetic ECMs as a function of matrix stiffness (3wt%, 5wt% and 7wt% MIX) after 14 days of EP treatment and in standard ESC media or EEO medium (see Methods). (F) Biochemical measurement of prolactin (PRL) in the spent media (EEO media) from 15-day cultures in (E) compared to standard 2D polystyrene cultures (N=4). (G) Represenatitive fluorescent staining of DNA synthesis (EdU incorporation for 24 hrs hrs prior to fixation at day 15 as a function of simulated cycle phase and inflammation cue, IL1B (H) Quantification of mean EdU+ staining from cultures shown in (G) shows IL1B suppresses DNA synthesis of ESCs (n=3) (I) Quantification of apoptosis (cleaved caspase-3+) in ESC cells stimulated with homrone and IL1B treatment groups at day 15 of culture relative to total nuclei count (N=3) (J) Inflammatory challenge with IL1B significantly suppressed PRL secretion in ESC cultures (E+ = E + IL1B, PE+= PE+IL1B, W = hormone withdrawal) (N=6). (K) Vimentin stain (red) of ESC morphology cultured in synthetic and natural hydrogels. IL1B (1ng/mL) treated ESC in synthetic hydrogel provided as a control for inflamed morphology after 6 days. Significance is indicated as *p<0.05, **p<0.01, ***p<0.001. Scale bars are 50 mm, unless otherwise noted.
Although the ESCs can enzymatically remodel the synthetic matrices due to the incorporation of the MMP cleavable crosslinker, PEG gels remained intact at the 15-day time point, whereas cultures in Matrigel or collagen I gels have shrunken and at least partly disintegrated by this time point (Fig 3D). This ability to maintain long-term cultures enabled us to assess stromal function in the gels by maintaining the 3D cultures under sex hormone stimulation throughout the 15-day experiment to mimic the proliferative (E2 1nM, 15-days, “E”), the secretory (E + 500 nM synthetic progestin MPA, “PE”), or menstrual (12 days of PE treatment followed by hormone withdrawal and treatment with 10mM RU-486,72 hrs, “W”) phases of the menstrual cycle. In response, subsets of ESCs underwent decidualization in response to progestin treatment, a differentiation process necessary for the establishment of pregnancy, characterized by the secretion of pro-gestational protein prolactin (PRL) and morphological changes toward an epithelial-like decidual cell.60–62 Although increased matrix stiffness curtailed ESC elongation in the E-containing medium before decidualization, ESC developed characteristic rounded epithelioid morphologies in response to progestin in the MIX gels, in both standard ESC culture media as well as EEO media (Fig 3E). Detection of PRL secretion in the spent media confirmed that ESC embedded in 3D gel underwent robust decidualization across all matrix stiffnesses response to progestin treatment (Fig 3F), suggesting that stiffness is not a strong influence on this metric of stromal hormone sensitivity.
We next investigated the effects of exogenous treatment with interleukin-1β (IL1B), a prototypical inflammatory cytokine implicated in the pathogenesis of endometriosis,63 on ESC phenotype in the synthetic gels using a dose of 1 ng/mL (57 pM) as this concentration is well above the reported 0.2 ng/mL IC50 for suppressing the decidualization response of primary human stromal cells in vitro.64,65 Compared to control groups, which exhibited robust proliferation at day-15 in the 3wt% MIX hydrogel in both E and PE media with 50–60% of cells incorporating EdU over a 24-hour incubation period (Fig 3G–H), IL1B suppressed ESC mitotic activity resulting in ~5% of cells incorporating EdU (Fig 3G) and induced an increase in apoptosis (Fig 3I). Furthermore, treatment with IL1B also dramatically suppressed PRL production by day 15 even in the presence of the progestin MPA (Fig 3J). Brightfield and immunofluorescent examination of stromal morphology in response to the inflammatory cue revealed a drastic change toward an elongated morphology, characterized by a thin architecture (Fig S6A–B in Data File 1) and decreased cell surface area (Fig S6C in Data File 1). This change was attributed to an apparent increase in ECM remodeling capacity characterized by increased cellular motility (Videos S4–S13 in Data File 3). These results suggest that despite a reduction of mitotic activity, IL1B induces a more activated ESC phenotype in 3D. In line with this result, morphological assessment revealed that ESC cultured in Matrigel also developed the phenotype akin to those exogenously treated with IL1B (Fig 3K), highlighting the limitations conferred by Matrigel to study the stroma28. Altogether, these results demonstrate that the relatively soft, dual integrin ligand (3wt% MIX) synthetic matrix formulation is suitable as a “one size fits all” scaffold for culturing both stromal cells and epithelial organoids.
Engineered synthetic matrices support the stable co-culture of EEO and ESC, capturing the temporal physiologic processes of the human menstrual cycle
In the endometrium, both stromal and epithelial cells express hormone receptors for E2 and P4, yet proper tissue-level function is mediated by the crosstalk between these cell types.66–69 Parsing this crosstalk in vitro using Matrigel - which contains numerous exogenous growth factors, rapidly degrades, and largely lacks the physiologic ECM molecules for stromal cells – is arguably a fraught endeavor. Thus, we used the synthetic matrices described to develop a co-culture model using defined cell ratios that were shown to approximate those observed in vivo (Fig S7 in Data File 1) to interrogate these dynamic phenomena. Donor matched EEOs and ESCs populations (N=12) were co-cultured in the synthetic ECM (3wt%-MIX) using a fully defined common media (see Methods) and maintained for up to 15 days of culture (Fig 4A). Immunostaining demonstrated the persistence of morphologically well-defined stromal and epithelial populations (Fig 4B; Video S3 in Data File 2). All cultures were viable, with minimal cell death, throughout the length of the experiments as demonstrated by live/dead staining (Fig S12 in Data File 1) at day 15. Co-cultures were exposed to hormone stimulation designed to mimic the proliferative (E2, ‘E’), secretory (E2 + MPA, ‘PE’), or menstrual (72 hr PE withdrawal + RU-486 (10 µM, ‘W’) phases of the idealized human menstrual cycle (Fig 4C–D).
Figure 4. Establishment of a co-culture model recapitulates the molecular signature of the human menstrual cycle in vitro.

(A) Schematic and representative images of the co-cultures. EEOs (10 intact organoids/µL) and ESC (10k cells/µL) are expanded separately and embedded in the synthetic ECM, cultured as 3 µl droplets and maintained for 15 days of culture. A dual-adhesion peptide synthetic ECM (3wt% 20kDa-PEG) functionalized with GFOGER (1.5 mM) and PHSRN-K-RGD (1.5mM) supports the establishment of the EEOs and ESC co-culture model. (B) Representative 3D images of day-15 endometrial EEOs (EpCAM = green) and ESC (Vimentin = red) in the co-cultures (scale = 100 µm). Images acquired from Video S3 in Data File 3. (C) Schematic and timeframe of the idealized 28-day menstrual cycle in vivo. (D) Experimental design for the validation of the co-culture endometrial model. Treatment groups are designed to mimic the hormonal changes in the phases of the menstrual cycle, including a pharmacologic induction of the menstruation using a PGR antagonist (RU-486, 10 µM). ‘Inflamed’ groups were co-treated with IL1B (1ng/mL) throughout the length of the experiment. (E-G) Transcriptomic (bulk RNAseq) analysis of the co-culture models (n= 7). (E) Gene ontology (GO) analysis of the top significantly enriched GO terms that are downregulated or upregulated in response to progestin treatment. Cocultures treated with E2 + MPA (“PE”) have gene ontology (GO) expression profiles that align with the secretory phase of the menstrual cycle. GSEA analysis of hallmark pathway gene sets show pathway changes consistent with corresponding phases of the menstrual cycle. All images and transcriptomic analysis were performed on day-15 co-cultures in the synthetic 3wt% 20kDa-PEG-Mix hydrogels (N=8). Data is shown with the −log of their P values.
To determine whether the PEG-based co-culture model could phenocopy canonical molecular pathways involved in the human menstrual cycle, we characterized the co-cultures (N = 5 donors) by both transcriptomic and functional analysis after hormonal treatment. First, we implemented bulk RNA sequencing (n=7) and demonstrated that after 15 days, co-cultures were transcriptionally distinct from either ESC or EEO monocultures (Fig S8B, Fig S19 in Data File 1). Further analysis using a generalized linear model compared gene expression between ESC monocultures and co-cultures using Benjamini-Hochberg (BH) multiple hypothesis test correcting70, resulted in 7,821 genes to be differentially expressed at an adjusted p-value threshold of 1×10−5 (Fig S8C in Data File 1). These findings are suggestive of a crosstalk between these stromal and epithelial populations when cultured together.
Next, we analyzed the response of co-cultures with respect to hormonal stimulation. Further transcriptomic analysis of the co-cultures by hierarchical clustering demonstrated that the expression of endometrium-associated genes segregated samples by hormonal treatment rather than donor variability (N=2 donors), and adequately reproduced gene expression profiles associated with their respective menstrual cycle phases (Fig S9A in Data File 1). Across the menstrual cycle, P4 is known to regulate numerous cellular processes that lead to the preparation of implantation including immune-modulatory, proteolytic MMP activity and cellular differentiation of the epithelial and stromal compartments toward a secretory phenotype.1,71,72 To identify the molecular functions, biological processes, and pathways differentially regulated by the experimental hormone treatments, we performed Gene Ontology (GO) analysis and detected key reproductive biological processes associated with progesterone signaling to be significantly (p>0.05) enriched in the progestin-treated secretory and menses groups, including ECM composition and receptor signaling activity (Fig 4E). Gene network visualizations of these pathways confirmed that, in response to hormone treatment, the co-cultures reproduced P4-induced expression of specific secretory phase associated genes including PRL, PAEP, and ECM-associated genes and downregulation of CPM, MMP9, and cytokine-mediated inflammatory genes (Fig S9B in Data File 1). Finally, Gene Set Enrichment Analysis (GSEA) further confirmed recapitulation of canonical menstruation-associated hallmarks (Fig S10D in Data File 1), including significant upregulation of senescence, inflammatory66, and hypoxia73 in the withdrawal treatment group compared to E or PE (Fig 4F). To expand on these transcriptomic findings, we also observed expression of matrix metalloproteinases (MMPs), including those primarily produced by either the stromal (e.g., MMP-2) or epithelial populations (e.g., MMP-7, MMP-26).11,74 To corroborate these transcriptomic findings, we also measured temporal changes in matrix metalloproteinases (MMPs) from the spent media (Fig S14C in Data File 1). Together, we co-cultures recapitulated similar temporal expression trends that are observed in vivo75,76, specifically a reduced MMP-1, -3 and -10 expression in the secretory phase compared to proliferative group and reduced MMP-2 compared to the menses group (Fig S10C in Data File 1).
Finally, we experimentally validated the co-culture model using functional assays to assess the morphologic and biochemical changes observed across the human menstrual cycle. First, we examined whether the model captured morphologic features of secretory endometrial glands in response to hormonal stimulation observed in vivo (Fig 5A). Progestin treatment caused changes in EEO morphology characterized by thickening of the pseudo-stratified columnar epithelial layer and increased epithelial invaginations (Fig 5B; Fig S10A in Data File 1). Moreover, epithelial secretory function was demonstrated by positive staining of progestogen-associated endometrial protein (PAEP), a marker of the secretory epithelium, in the lumen of the organoids treated with progestin (Fig 5C; Fig S10B in Data File 1). Similarly, a robust induction of stromal decidualization in response to progestin also occurred in the co-cultures by day 15 of the experiment resulting in increased secretion of PRL in the secretory groups and reduced in the menses group (Fig 5D). Moreover, the characteristic negative regulation of PGR during the secretory phase compared to the proliferative phase observed in vivo77,78 was demonstrated via immunostaining in both epithelial and stromal populations in vitro (Fig 5E). In accordance with literature observations6, the temporal analysis across the 15 days of treatment showed that co-cultures maintained in baseline E2 conditions showed no significant increase in PRL (Fig 5F), but co-cultures treated with the progestin MPA showed a significant increase in PRL production starting 9 days after MPA treatment initiation (Fig 5F). Finally, we also observed a significant (p=0.031, N=3) doubling of apoptosis (Fig 5G–H; Fig S11 in Data File 1) in epithelial glands of the co-cultures in the menses groups (3.06 Casp+/cm2) compared to the secretory groups (1.37 Casp+/cm2) mimicking the increased cell death observed during the induction of menstrual phase in vivo.79,80
Figure 5. Functional validation of the co-culture model recapitulates morphologic and biochemical cycle-dependent reproductive processes.

(A) Representative histological (IF) characterization of the proliferative and secretory phases of the menstrual cycle in vivo reveals morphologic and biochemical changes. Vimentin (red), F-Actin (Green), DAPI (blue). (B) Characterization of the epithelial morphology in co-cultures at day 15 of culture by IF reveals glandular maturation and invagination of EEOs in response to progestin treatment groups. (C) IF staining of PAEP (glycodelin) in co-cultures recapitulates the epithelial secretory phenotype. (D) Stromal decidualization was assessed by measuring prolactin secretion in the medium across 15 days of culture. PRL secretion is induced by PE treatment but suppressed in menses treatment group. (E) PGR staining in the co-cultures at day 15 of treatment. (F) Temporal profiles of decidualization normalized to fold change relative to day 0 were performed by measuring spent media (N=12) and can be suppressed by IL1B treatment (1 ng/mL, E+, PE+). (G-H) Cleaved caspase-3 (Casp-3) IF in the co-cultures as a marker of apoptosis (G) and quantified in as positive staining per organoid area (H). All images are of day-15 co-cultures in the synthetic ECM 3wt% 20kDa-PEG-Mix hydrogels. Significance is indicated as *p<0.05, **p<0.01, ***p<0.001.
Altogether, these results demonstrate the establishment of a co-culture model of the endometrium can recapitulate in vitro several of the hormone-induced phenotypic, morphologic, and biochemical changes associated with the idealized human menstrual cycle and could be used to investigate on reproductive function in mechanistic fashion.
Progesterone action is disrupted by IL1B treatment in the co-culture model
The suppression of stromal cell response to progestin in the presence of IL1B (Figs 3H, J) motivated us to analyze the molecular and phenotypic responses of epithelia and co-cultured stromal and epithelial cells to IL1B, hypothesizing that outcomes in the latter case would likely be influenced by stromal-epithelial cross talk. Separate proliferative, secretory, and menses treatment groups were co-stimulated with IL1B (1 ng/mL) throughout the 15-day experiment (Fig 4D). First, in accord to the results described above (Fig S6 in Data File 1), the stromal population in co-cultures also developed an elongated (inflamed) morphology (Fig S15 in Data File 1). Furthermore, co-cultures exposed to the inflammatory stimulant IL1B showed suppression of a stromal decidualization response. Control co-cultures showed a canonical increase in production of PRL following progestin exposure through the 15 days of treatment (Fig 5F); however, PRL production in co-cultures treated with IL1B showed no difference in the presence or absence of progestin by day 15 despite an unexpected spike in PRL production in the immediate 3 days after stimulation with the inflammatory cue which fell to negligible levels for the remainder of the 15 days (Fig. 5F). Analysis of the inflamed (IL1B treated) co-cultures by bulk transcriptomic (Fig S13 in Data File 1) and multiplex immunoassay (Fig S14 in Data File 1) analysis confirmed the decreased PRL expression observed at the protein level (Fig S13B in Data File 1). Gene expression analysis in a generalized linear model using 8,423 genes expressed across all samples comparing controls to IL1B stimulated cocultures (Fig S13A in Data File 1) revealed an expected global up-regulation of several additional pro-inflammatory cytokines and chemokines (e.g., IL6, TNF-α, CCL2, CCL8, CCL5) and down-regulation of anti-inflammatory cytokines (e.g., IL10) in the IL1B-treated groups (Fig S13B in Data File 1). Hallmark pathway enrichment analysis of the unstimulated and IL1B-stimulated co-cultures detected disruptions of progesterone-regulated pathways including the downregulation of ECM structural constituent, and upregulation of inflammatory receptor-ligand signaling processes (Fig S13C in Data File 1). Immunostaining revealed that co-cultures with IL1B, compared to the untreated controls, further increased apoptosis in the menses phase (Fig 5H; Fig S11 in Data File 1), an observation that was corroborated by a separate assay of cell death (Fig S12 in Data File 1). These findings show that IL1B can disrupt progesterone actions. We confirmed this via immunostaining for PGR expression in the co-cultures which showed reduced PGR signal, even in the E-containing media, (Fig 6A) in both the epithelial and stromal populations.
Figure 6. Functional analysis of inflammatory cue (IL1B) repsonses in co-cultures in synthetic hydrogel reveals the intitiation of the endometriotic phenotype mediated by cell-cell communication.

(A) 3D maximum intensity projections of PGR expresison in E2-only treated co-cultures in response to IL1B stimulation (B-C) IL1B stimulation (1 ng/mL) induces morphological changes in EEOs. Representative IF images (B) and quantification (C) of epithelial height (distance from base to apical edge of the epithelium, N=3). (D) Schematic of donor matched EEO monoculture and co-culture in IL1B treatment groups. (E) Represenative images of time-lapse co-cultures stimulated with IL1B reveals enlarged EEO diameter by day 7 of culture. Quantication of the mean fold-change of EEO diameter across 15-days of cultures from daily time-lapse images in co-cultures (black) and monocultures (red) across treatment conditions (E, PE, W) and IL1B-treated (E+, PE+) groups. Fold-change denotes the mean increase in diameter relative to day 1 of culture. Analysis compares the mean EEO growth rate between monoculture vs coculture (n=4; E + IL-1 p=0.0076; PE + IL1B p=0.0026). (G) Immunostaining analysis of DNA synthesis in co-cultures and EEO monocultures by EdU incorporation in response to hormones and IL1B treatment (N=3). (H-I) Quantification of proliferative profiles calculated as the number of EdU+ cells per organoid area (mm2) in (H) organoid monocultures and (I) co-cultures. (J) Working model of the cellular mechanisms driving epithelial and stromal communication in the endometrium under physiologic (hormones) and pathogenic (IL1B-treated) conditions. All images are of day-15 co-cultures in the synthetic ECM 3wt% 20kDa-PEG-MIX hydrogels. Significance is indicated as *p<0.05, **p<0.01, ***p<0.001.
Lastly, stimulating the co-cultures with IL1B caused a significant thinning of epithelial morphology causing a shift from the pseudo-columnar architecture in the controls toward a squamous cell structure with thin apical F-actin staining (Fig 6B). Whereas progestin treatment significantly increased cell height in the control co-cultures, as characterized by the distance from apical to basolateral side (E vs PE; p=0.0098), in the IL1B-treated groups, epithelial cells were thinner than in controls (PE vs PE+IL1B; p <0.0001) and were relatively unaffected by progestin (Fig 6C). This flattening phenomenon closely mimics the de-differentiation events observed in the wound repair cell response of the intestine.81 The change in morphology of the epithelial cells was accompanied by a slight, but non-significant (p=0.127), increase in spacing between nuclei compared to the round nuclei in the controls at the single cell level (Fig 6B; Fig S16A in Data File 1), suggesting the flattened cell in the IL1B-treated groups have a slightly increase in projected cell area compared to the controls to compensate for the reduction in cell height while keeping similar volume. An alternate metric of projected cell area, the density of nuclei counted per area of organoid monolayer surface, was comparable in control and IL1B-treated co-cultures (Fig S16B in Data File 1) though analysis of the morphometric changes cell volume is a topic for a future investigation. Altogether, these results highlight the extent by which IL1B can induce the overexpression of additional pro-inflammatory cytokines associated with reproductive diseases82–84 and can potentiate progesterone resistance, a phenomenon that is a characteristic feature of endometriotic disease in vivo.85,86
IL1B treatment disrupts epithelial proliferation via a stromal-dominant communication.
Chronic inflammation of ectopic lesions is a characteristic feature of endometriosis; wherein endometrial epithelial and stromal cells are found growing outside the uterus.87 We thus next assessed whether inflammation altered epithelial proliferation in ways that may illuminate mechanisms of disease. Tissue-recombinant murine models have previously demonstrated that estrogen and progesterone mediate epithelial mitogenic processes indirectly through signals from the hormone-responsive stromal fibroblast,69,88,89 therefore we investigated whether IL1B exerts its effect via similar mechanisms. To test this, we measured the effects of IL1B treatment on epithelial growth, in epithelial monocultures and in co-cultures (Fig 6D; N=4 donors, matched cultures), using both morphological analysis of organoid diameters and measuring DNA synthesis.
First, we measured changes in organoid size (outer diameter) across the 15 days of culture as a proxy for growth rates (Fig S17A in Data File 1). As was observed with endometrial stromal cells in monoculture (Fig 3G–H) IL1B treatment significantly (PE vs PE+IL1B; p=0.0121) suppressed the growth of the epithelial organoid diameter in monoculture, compared to controls (Fig S17A in Data File 1), thus we hypothesized that IL1B would negatively impact epithelial mitotic processes in the co-cultures. Unexpectedly, when co-cultured with stroma, IL1B treatment caused an observable increase in epithelial organoid diameter compared to untreated controls (Fig 6E). Quantification of the organoid diameter across the 15 days of culture demonstrated that IL1B stimulation positively impacts scEEO diameter growth in the co-cultures, but not in the monocultures (Coculture vs monoculture; p=0.0026) resulting in a mean fold change in organoid diameter of 7.3 and 3.9-fold in the co-cultures and monocultures, respectively (Fig 6F).
As scEEO diameter is only a proxy for changes in cell number, the mitotic activity of these cultures, at day 15, was examined by measuring EdU incorporation by the organoids after 24 hours of incubation (Fig 6G). In EEO monocultures, a non-significant (p > 0.05) trend toward reduction of DNA synthesis in scEEOs upon treatment with IL1B (E=1822; PE=2333; E+IL1B=1185; PE+IL1B=1407 EdU+/mm2; N=3) and a surprising trend toward an increase in DNA synthesis in the presence of progestin compared to E-only conditions (Fig 6H) was observed. In stark contrast, stimulation with IL1B resulted in a greater than 3-fold increase (PE = 228; PE+IL1B = 978 EdU+/mm2; P= 0.0014; N=4) of scEEO DNA synthesis in co-cultures compared to controls (Fig 6I), consistent with the observed increase in organoid diameters in IL1B-treated co-cultures (Fig 6E). Further, unlike monocultures, the proliferative index of scEEOs in co-culture decreased in progestin-treated controls compared to E-only controls, as would be expected in the transition from proliferative to secretory phase in vivo90. These phenotypic changes in epithelial mitotic activity mirror the increased epithelial proliferative profiles observed in the eutopic and ectopic endometriotic tissues in vivo.91,92 Although the scEEO monocultures appeared to have a greater proliferative index than the co-cultures in some groups, this difference could be attributed to the contribution of the ESC in mediating hormone signaling, as shown in other models.93
Perhaps because of this increased growth rate, IL1B stimulation in co-cultures resulted in greater organoid collapse by day 15 and could also explain the ~1.5-fold (p=0.012) increase in apoptosis (Casp-3+ staining) observed in the epithelial (Fig 5G) populations that resulted in an overall increase in cell death (ethidium homodimer staining, Fig S12 in Data File 1). Altogether, these results support a working model by which inflammatory signals and sex hormone signaling in the endometrium are partially mediated indirectly via the stroma (Fig 6J).
Discussion:
The endometrium is a marvelous example of regenerative biology wherein sex hormones mediate rapid growth and maturation of the tissue, accompanied by equally dynamic changes in ECM-cell interactions that are both mechanically and molecularly linked to reproductive function.94 These remarkable regenerative properties also contribute to common debilitating diseases like endometriosis, for which new therapies are desperately needed. Here, we developed a model for dissecting molecular and phenotypic consequences of endometrial epithelial-stromal crosstalk in long term (>15 day) cultures of patient-derived endometrial cells by defining a completely synthetic extracellular matrix hydrogel that is tailored to both replace Matrigel for organoid culture, and simultaneously to support stromal culture. We then used this model to show how the hormone-dependent behaviors of the endometrial epithelium in co-culture with stroma diverge from those in monoculture, observing for example that the pro-inflammatory cue IL1B appears to drive the endometrial co-cultures, but not monocultures, to a state that phenocopies features of diseases like endometriosis.83,87
The synthetic ECM described here overcomes certain limitations of organoid technologies and other co-culture approaches for parsing cell-cell communication in general, especially in the endometrium. Natural matrices like Matrigel and collagen – which have been used either alone for co-culture28,29,61,95 or combined in creative ways96,97 – include many extraneous signaling molecules that may drown out the signals produced by the cells they support. This limitation is underscored by the inability to design experiments that require longer term stable cultures, as is the case for hormone signaling, or require specific ECM stiffness. The main constituent of this synthetic ECM, PEG, is a blank slate known for its relative lack of interaction with proteins. Thus, growth factors, cytokines, and other molecules produced by each cell type can freely dominate the cell-cell communication networks. By design, the synthetic ECM comprises only a minimal set of biological cues: two integrin ligands, two ECM-binding proteins, and a peptide crosslinker, all produced by standard, well-defined chemical peptide synthesis methods. These minimal cues, which were modified from a previous formulation used for organoids27 and monolayer cell line cultures26, include ligands for a spectrum of integrins expressed differentially by epithelial and stroma (see Results) populations and enable cell-mediated remodeling of the microenvironment as well as the accumulation of cell-produced ECM. By demonstrating that this synthetic matrix is sufficient to generate scEEOs from the original primary glands without the need to first expand in Matrigel (Fig S18 in Data File 1), we provide a hydrogel alternative to alleviate the dependency on Matrigel. Because the synthetic matrix is relatively simple and is chemically defined, it is straightforward to implement reproducibly for the culture of organoids and co-cultures. Reproducibility within and between labs is highly desirable to analyze phenotypes of patient-derived tissue models due to the inherent biological variability in the samples themselves. Finally, although only an initial characterization is provided here, the synthetic ECM arguably offers greater potential to mimic and study certain features of endometrial diseases compared to ECM-free co-culture models98,99, as its biophysical properties can be tuned systematically to mimic the “soft” regimes of healthy endometrium or the “stiff” regimes of the myometrium100 or fibrotic tissue (Fig 2A).
Steroid hormone-driven stromal-epithelial crosstalk in the endometrium has been illustrated in mouse models involving cell type-specific receptor knockouts in the eutopic endometria71,77 and recombinant implantation of tissue fragments in the renal capsule,69,93 but crosstalk in normal and diseased human co-cultures has been more challenging to parse due to the disparate matrix environments preferred by each cell type101,102, and the relative short-lived nature of cultures in Matrigel or collagen. For example, work by Rawlings and co-workers showed stromal-epithelial crosstalk enabled a simplified medium to support an elegant collagen gel-embedded “assembloid” co-culture model that captured both normal and drug-skewed co-evolution of the stromal population to subpopulations of decidual and acutely senescent fibroblasts61. They thus defined a tantalizing model for analysis of embryo implantation if the limitations of the culture longevity, which disintegrate before the entire sequence of implantation steps is realized under some conditions, and the limited access organoid apical interface, can be overcome61. Thus, the long-term capacity of co-cultures in our synthetic hydrogel may provide one approach to extending the assembloid model if similar evolution of fibroblasts populations can be demonstrated. In concordance with a different short term (7-day) co-culture model, employing EEOs cultured in Matrigel in a Transwell membrane above a coverslip coated with stroma103, we observed the expected suppression of proliferation by E2 and P4 compared to E2 in control co-cultures (Fig 6F;Fig S17 in Data File 1). However, we are also able to observe more pronounced effects at the two-week time point, especially in cultures treated with IL1B (Fig 6F; Fig S17 in Data File 1) and provide evidence that the inflammatory cytokine IL1B can also modulate epithelial proliferation via the stroma (Fig 6J).
Indeed, the response of co-cultures to an inflammatory cue at extended time points is one of the most striking findings in this study, as it may shed light on inflammatory disorders such as endometriosis. We chose the cytokine IL1B as a prototypical modulator of both physiological inflammation in the eutopic endometrium104 and in pathophysiological inflammation in endometriosis lesions63, recognizing that our co-culture system configuration is similar to a lesion model in its cyst-like structure of epithelia. IL1B is produced by myeloid cells, endothelia, epithelia, and fibroblasts throughout the body105,106. Upon binding to the IL1 receptor type 1 (IL1R1) and recruitment of IL1R3, IL1B signals through the NF-kB pathway and through JNK, ERK1/2 and p38 to the AP1 complex to trigger production of additional cytokines, including IL-6, IL-8, and IL-1.105,106 The canonical pathways activated by IL1B are shared by other interleukins, cell stressors, and pathogens, making IL1B a reasonable proxy for other triggers of innate inflammatory signaling105. IL1B signaling is regulated at multiple levels, including: competition for binding to IL1R1 by the decoy ligand IL1ra; neutralization by shed receptor sIL1R1; neutralization by binding to the non-signaling receptor IL1R2; and downregulation of IL1R1 by receptor-mediated endocytosis64,105,106.
In the human endometrium, expression of IL1B is present across the menstrual cycle and is implicated in the physiology of implantation and maintenance of pregnancy, with conflicting reports on how alterations in expression levels in the endometrium, uterine fluid and systemic circulation are correlated with fertility.104,107–114 Furthermore, the presence of IL1B is consistently implicated in the pathophysiology of endometriosis, though its contributions to lesion phenotypes and symptoms remain challenging to dissect in a landscape of large clinical variation in both patient and lesion characteristics, and the complex dynamic interplay between the entire network of regulatory molecules involved in IL1B action63–65; nevertheless, inhibition of IL1B is the focus of an ongoing clinical trial in endometriosis patients (NCT03991520).
The reported ranges (0.01 – 2 pM) of IL1B concentration in plasma113,115,116 and uterine fluid108,117 are far below the reported 100–1000 pM KD values for receptor binding106, though ~25 pM values in the saliva of menopausal women are close.118 In vitro culture models that investigate endometrial cell responses to IL1B typically employ ligand concentrations in the range of 0.1–10 ng/mL (5.7–570 pM), with a reported IC50 for inhibiting decidual responses of human endometrial stromal cells of 0.2 ng/mL (11 pM).64,65 We had previously observed that the concentration of IL1B in the local microenvironment of hydrogel-encapsulated endometrial cells reaches a steady state about 20–25% lower than the external concentration in the culture medium when dosed at 10 ng/mL55, suggesting that cells may be internalizing and degrading the ligand, thus we chose a ligand concentration of 1 ng/mL (57 pM) for stimulating gel-encapsulated cells to ensure a chronic tonic stimulus mimicking what might be present in an endometriosis lesion, given the gradients produced in 3D tissues by ligand diffusion and consumption. When the co-culture became exposed to this concentration of IL1B, organoid diameter (Fig 6F) and epithelia mitotic activity (Fig 6I) are both increased, even in the presence of MPA compared to the E2 condition, whereas the canonical response to progestin resulted in a decrease in epithelial proliferation in the control co-cultures and in monocultures. We speculate that this observation of an IL1B-MPA synergy may have clinical relevance for the observed failure of some endometriosis patients to respond to progestin therapies. Moreover, greater proliferation indices are observed in the eutopic endometrium of endometriotic patients, compared to controls90,92. Similar to our observations about IL1B inducing profound epithelial morphological thinning along with increased proliferation, in deep infiltrating endometriosis lesions, an “invasive front” characterized by highly flattened epithelia with greater proliferative index than epithelia in a more quiescent core has been described.119 While more translational validation is warranted to confirm mechanistically similar behaviors, the results are suggestive that the model may capture features that are important clinically.
Importantly, the timely and acute induction of inflammatory mediators are also well known to be critical for maintaining normal reproductive physiology during implantation.104 This may explain why a transient (day 3) spike in PRL secretion was observed in the co-cultures in response to IL1B as acute-phase inflammatory insults that activate the nuclear factor–kappaB (NF-KB) system, a master regulator of cell survival and proliferation in endometriotic disease120, have been shown to also regulate PGR expression in vivo63,77. IL1b can enhance stromal decidualization121 and blastocyst implantation35,122 during short exposure times, suggesting IL1B may have a biphasic response. Because most assays were performed at end point measurements (day 15) in this study, future mechanistic studies that dissect the timing (acute vs chronic), abundance (low vs high concentration), and synergism with sex hormones (estrogenic vs progestin conditions) will be critical to fully understand the extent by which IL1B expression can positively promote physiologic (e.g., implantation) or induce pathologic processes (e.g., endometriosis, infertility)86,120, and ultimately will help identify therapeutic approaches for targeting the disease while maintaining reproductive function (Fig 6J).
Finally, we are just beginning to explore how the properties of the synthetic ECM may enable more complex tissue-level components beyond the organoid-stroma culture described herein. This relatively simple model does not reflect the tissue-like architecture and heterogenous cell densities that makes up the in vivo tissue. Although we focused on primarily on paracrine crosstalk, and not on cell-cell contact as a method for cell communication in this study, we did observe that stromal cells can migrate through the gel to become juxtaposed by the epithelia (Fig 6B). Nonetheless, alternative approaches, including spheroid aggregate cultures embedded in the synthetic ECM, may be better suited to directly investigate communication via cell-cell contact. Furthermore, while we did not aim to mimic tissue architecture, we observed that organoids close to the gel-liquid interface sometimes merge with each other and erupt to form mature epithelial organoid-monolayers structures that are more representative the glandular-luminal epithelial tissue-like structures of a true endometrial mucosal barrier (Fig S21 in Data File 1). Thus, we are now defining protocols that foster efficient and reproducible organoid fusion model. Finally, while this model employed two key cellular components of the endometrium, a parallel effort to represent the heterogenous cell populations of the endometrial microenvironment by combining the immune component to the stroma and epithelia, as we have described for pancreatic tumors32, is on-going with the goal of incorporating these complex endometrial models to perfusable blood vessels in microfluidic devices, thereby extending our initial findings that we can form microvascular networks in synthetic PEG gels.123,124 We speculate that the co-culture model described here can be productively extended by the research community as a platform to mechanistically parse stromal-epithelial crosstalk in the human endometrium.
Limitations of study:
There are a few limitations to this study. First, the sample size for scRNAseq and proteomic analysis is not sufficient to make any definitive conclusion about the different phases of the menstrual cycle endometrium. Only subset from datasets were used to help design the model and were not intended to atlas of the endometrium. Secondly, synthetic hydrogel design only includes two peptides designed to engage the integrins expressed by the stromal and epithelial populations. Although this bottom-up approach was sufficient to support the metrics we set to achieve in this study, the role of additional components of the ECM biomaterials remains unexplored. Similarly, as discussed, the endometrial microenvironment is composed of many different cell populations, in this study only the epithelial and stromal populations were examined. Third, most of the analysis of this study were performed at end-point measurements at day-15 to accommodate for study design; thus, future studies can be designed with greater temporal granularity as well analysis of the cell populations with single cell resolution. Fourth, this study is not intended to recreate the intact regenerative capacity and architecture of the native endometrium, but rather a model than recapitulates hormone-mediated features of the menstrual cycle in vitro thereby enabling the study of endometrial cell-communication and ECM-cell interactions. Finally, enrollment did not consider general population demographics including the social-economic status, ethnicity and disease status and included donors undergoing treatment for a type of uterine disorders. While the use of heterogenous populations demonstrated the potential of hydrogel to function on cells obtained from distinct clinical phenotypes and cycle phases, the impact of disease status on cell behavior and patient diagnosis was not examined in this study. Prospective studies with appropriate power analysis will use this hydrogel platform to investigate this in more detail.
STAR methods
RESOURCE AVAILABILITY
Lead contact:
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Linda Griffith (griff@mit.edu).
Materials availability:
Hydrogel reagents and peptides described in the manuscript are commercially available. The reagents to make the synthetic hydrogel are in the process of being commercially licensed with the purpose of providing accessibility of these gels to the community. For immediate availability, a Material Transfer Agreement may be required to provide reagents due to transfer restrictions.
Data and code availability:
Single cell RNA and Bulk RNA sequencing data have been deposited into the database of Genotypes and Phenotypes (dbGAP) and are available under dbGaP accession phs003326.v1.p1. Proteomics data was deposited into PRIDE, and processed data into the Gene Expression Omnibus (GEO). All data and links are being compiled into a central study site, which can be accessed here: “Integrating endometrial proteomic and single cell transcriptomic pipelines reveals distinct menstrual cycle and endometriosis-associated molecular profiles”: https://fairdomhub.org/studies/1139. Accession numbers are listed in the key resources table.
Imaging data have been deposited at Omero, an Open microscopy image repository client-specific repository, and bioassay data are publicly available as of the date of publication and can be found in https://fairdomhub.org/studies/1139.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Vimentin | abcam | ab8978 |
| DAPI | ThermoFischer | R37606 |
| AlexaFluor™ 488 phalloidin | ThermoFischer | R37110 |
| EpCAM | Abcam | ab7504 |
| Laminin (LMN) | Abcam | ab11575 |
| Cleaved Caspase-3 | Abcam | ab2302 |
| Progesterone receptor (PGR) | Abcam | ab16661 |
| Progestagen associated endometrial protein (PAEP) | Abcam | ab270454 |
| LiveDead Viability Kit | Invitrogen | R37601 |
| acetylated 𝛼-Tubulin- AF594 | Abcam | ab195889 |
| Ki67 | Abcam | ab15580 |
| Vimentin-AF594 | Abcam | ab154207 |
| Vimentin-AF647 | Abcam | ab195878 |
| Goat pAb to Rb IgG-AF594 | Abcam | ab150080 |
| Goat pAb to Ms IgG-AF647 | Abcam | ab150115 |
| smooth muscle actin (SMA) | Invitrogen | MA-37027 |
| E-cad | R&D | AF748 |
| ESRalpha (ESR1) | Abcam | ab16660 |
| EpCAM | Abcam | ab218448 |
| Bacterial and virus strains | ||
| N/A | ||
| Biological samples | ||
| Human uterine tissues | This paper | Protocol number IRB-P001994 |
| Chemicals, peptides, and recombinant proteins | ||
| Mifepristone (RU-486) | Sigma | M8046 |
| 17-β estradiol | Sigma | 50-28-2 |
| Medroxyprogesterone acetate (MPA) | Sigma | 71-58-9 |
| PEG-20 (8-arm 20kDa PEG-VS) | Jenkem | 8ARM(TP)-VS |
| XL-IA | (Ac)-GCRD-LPRTG-GPQGIAGQ-DRCG-(Am) | Custom peptide Boston Open Labs (Cambridge, MA), GenScript (Piscataway, NJ), or CPC Scientific (Sunnyvale, CA). |
| PHSRN-K-RGD | (Ac)-PHSRNGGGK-(GGG-ERCG-(Am))-GGRGDSPY-(Am) | Custom peptide Boston Open Labs (Cambridge, MA), GenScript (Piscataway, NJ), or CPC Scientific (Sunnyvale, CA). |
| GFOGER | ‘‘(Ac)-GGYGGGPG(GPP)5GFOGER(GPP)5GPC-(Am)[45,46] | Custom peptide Boston Open Labs (Cambridge, MA), GenScript (Piscataway, NJ), or CPC Scientific (Sunnyvale, CA). |
| FN-binder | (Am)-GCRE-TLQPVYEYMVGV-(Ac) | Custom Peptide Boston Open Labs (Cambridge, MA), GenScript (Piscataway, NJ), or CPC Scientific (Sunnyvale, CA). |
| Critical commercial assays | ||
| Click-iT™ EdU Cell Proliferation Kit | Thermo Fisher | C10424-647 |
| Directzol RNA Mini-Prep kit | Zymo Research | R2051 |
| High-Capacity RNA-to-cDNA Kit | ThermoFisher Scientific | 4387406 |
| PureLink DNase Set | Thermofisher Scientific | 12185010 |
| Prolactin ELISA duoset | R&D Systems/Fisher | DY682 |
| Matrigel Phenol free | Fisher/Corning | 356231 |
| TMT10plex kit | Pierce | 90110 |
| barcoded mRNA capture beads | ChemGenes | N/A |
| Human XL Cytokine Luminex Performance Panel Premixed Kit | R&D Systems | LKTM014 |
| Luminex Performance Human MMP Magnetic Panel | R&D Systems | LMPM000 |
| Maxima H Minus Reverse Transcriptase | ThermoFisher | EP0751 |
| KAPA Hifi PCR Mastermix | Kappa Biopsystems | KR0368 |
| Deposited data | ||
| Bulk RNAseq data and single cell RNA sequencing data | This paper, and Baugh and Goods et al.44 | https://fairdomhub.org/studies/1139; dbGAP Accession number is phs003326.v1.p1. |
| Raw imaging data | This paper. | https://fairdomhub.org/studies/1139 |
| Proteomics | Baugh and Goods et al.44 | https://fairdomhub.org/studies/1139 |
| Experimental models: Cell lines | ||
| Human: Primary endometrial epithelial cells (EEOs) | This paper | Protocol number IRB-P001994 |
| Human: Primary endometrial stromal cells (ESCs) | This paper | Protocol number IRB-P001994 |
| Experimental models: Organisms/strains | ||
| N/A | ||
| Oligonucleotides | ||
| GAPDH | Hs02786624_g1 | 157 |
| PGR | Hs01556702_m1 | 77 |
| ESR1 | Hs01046816_m1 | 65 |
| PAEP | Hs01046123_g1 | 66 |
| ITGA2 | Hs00158127_m1 | 67 |
| ITGA5 | Hs01547673_m1 | 54 |
| ITGB4 | Hs00236216_m1 | 65 |
| ITGA6 | Hs01041011_m1 | 64 |
| ITGA3 | Hs01076879_m1 | 85 |
| ITGA1 | Hs00235006_m1 | 87 |
| ITGB2 | Hs00164957_m1 | 76 |
| ITGAV | Hs00233808_m1 | 64 |
| ITGA8 | Hs00233321_m1 | 89 |
| ITGB1 | Hs01127536_m1 | 74 |
| Recombinant DNA | ||
| N/A | ||
| Software and algorithms | ||
| ImageJ | Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997-2018. | https://imagej.nih.gov/ij/ |
| Imaris (RRID:SCR_007370) | Imaris 9.7 (Bitplane) | http://www.bitplane.com/imaris/imaris |
| GraphPad Prism (RRID:SCR_002798) | Graph pad Prism 8 | https://www.graphpad.com/ |
| Other | ||
| N/A | ||
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Tissue Acquisition and Isolation of Human Endometrial Cells.
All participants provided informed consent in accordance with a protocol approved by the Partners Human Research Committee and the Massachusetts Institute of Technology Committee on the Use of Humans as Experimental Subjects (Protocol number IRB-P001994). Endometrial tissue was obtained from pipelle biopsies from reproductive age women (N=18, ages 18–45; Table 1) undergoing laparoscopic surgery for non-malignant gynecologic indications. Study enrollment was limited to pre-menopausal women and excluded patients with an irregular or ambiguous cycle, or a history of hormone use in the 3 months prior to surgery. Cycle phase dating was performed as described elsewhere4 by histological analysis (Fig S20 in Data File 1). Endometrial epithelial glands and stromal cells were isolated by enzymatic digestion and filter-separated as previously described125, resulting in ≥ 95% purity as assessed by positive staining for vimentin and morphological assessment. Briefly, single stromal cells were separated from intact epithelial glands fragments and cultured separately. Stromal cells were expanded in traditional 2D cultures (as described below) and epithelial glands were cultured as organoids using protocols adapted from Turco et al.11 All cells were passaged a maximum of 5 times for all experimental in vitro assessments. For this study, we only included all female (individuals with a uterus). Limited or no metadata information regarding clinical and ethnic or socioeconomic background was available for some patients.
Cell Isolation and Endometrial Organoid Generation.
Primary cells were utilized from the donors described and used for in vitro culture and analysis. 15 different endometrial donors were used for isolation and cell culture of those 12 were used to establish co-culture models. Stromal cells were cultured and maintained in phenol red-free DMEM/F12 with 5% charcoal-stripped calf serum, 1 nM 17-β estradiol (E2, Sigma Aldrich) and 1× Pen-Strep solution (Sigma Aldrich). Some cell cultures were treated with 500 nM synthetic progesterone medroxyprogesterone acetate (MPA, Sigma Aldrich), or the progesterone antagonist mifepristone (RU-486 10 µM). Primary endometrial epithelial organoids (EEOs) were generated from the primary epithelial fragments (p0) according to established protocols and maintained in endometrial epithelial organoid expansion medium (EEO medium).10,11 EEO media is modified from the protocols established by Turco et al.11 Briefly, EEO media was defined as the minimal set of components needed to establish organoid cultures expansion and was composed of advanced basal media cocktail11 (ABM, 1X); rhEGF (50 ng/mL, Corning); rhNoggin (100 ng/mL, Peprotech); rhRspondin-1 (200 ng/mL, R&D systems); rhFGF-10 (50 ng/mL, Peprotech); E2 (1nM); Nicotinamide (1mM); Insulin-Transferrin-Selenium (ITS, 1%, Invitrogen); N-Acetyl-L-Cysteine (1.25 mM); TGFB pathway inhibitor (A83-01, 500 nM, Peprotech); and Rock inhibitor Y-27632, (ROCKi, 10 µM, Tocris). For seeding, epithelial cells, as fragments or single cell suspensions, were embedded in 70% Matrigel and cultured for 6 days, with media was changed every two days (Fig S2 in Data File 1). For passaging, 6-day old organoids were incubated in Cell Recovery Solution (CRS, ThermoFisher) for 30 mins at 4°C to dissolve Matrigel, then pelleted and digested with Tryp-LE supplemented with 1:100 DNAse (40k units, Sigma) for 15 minutes in a 37°C water bath followed by mechanical dissolution to generate single cells. For expansion purposes, single epithelial cells were seeded in Matrigel at a density of 1,000 cells/µL in 60µL droplets in non-tissue culture treated plates. We refer to organoids generated from single cells, in regular expansion or in experiments, as scEEOs. For co-culture experiments of ESC and scEEOs, ROCKi was omitted from EEO media since intact organoids were utilized to establish the co-cultures. ESCs retained hormone sensitivity and morphological characteristic when cultured in this EEO-based common media (See Results). Due to the presence of progesterone in the N2 and B27 supplements in the advanced basal media a homemade formulation of EEO media termed neutral EEO (nEEO) media that omitted P4 in the supplements was also explored in some experiments. All media changes for the co-cultures occurred every 3 days for up to 15 days unless noted otherwise. Spent conditioned media from these cultures was collected and stored at −80°C for downstream analysis. Sex of cells were all female because this population has a uterus and endometrial tissue.
METHOD DETAILS
PEG hydrogel materials and peptides.
8-arm poly(ethylene glycol) (PEG) macromers (20 kDa) functionalized with vinyl sulfone (20 kDa PEG-VS) were purchased from JenKem Technology (Beijing, China). All peptides were custom synthesized and purified (>95%) by Boston Open Labs (Cambridge, MA), GenScript (Piscataway, NJ), or CPC Scientific (Sunnyvale, CA). Peptides used in these studies include: a dithiol crosslinking peptide containing a matrix metalloproteinase (MMP)-sensitive substrate (Ac)GCRD-LPRTG-GPQGIWGQ-DRCG(Am) (CL-LW); fibronectin (FN)-derived peptide containing both the canonical RGD motif from the 10th FN type III domain as well as the PHSRN synergy site from the 9th FN Type III repeat in a branched configuration akin to the biophysical presentation in FN, (Ac)PHSRNGGGK-GGGERCG(Ac) -GGRGDSPY(Am) (PHSRN-K-RGD);56,126,127 a Collagen-derived peptide, (Ac)GGYGGGPG(GPP)5GFOGER(GPP)5GPC(Am) (GFOGER);22,40 a peptide with affinity for sequestering cell-produced FN, (Ac)KKGCRE-TLQPVYEYMVGV(Am) (FN-binder);128 and a peptide with affinity for sequestering the basement membrane proteins type IV collagen and laminin, (Ac)GCRE-ISAFLGIPFAEPPMGPRRFLPPEPKKP(Am) (BM-binder).129 All peptides were reconstituted in acidic (pH 5.5) Milli-Q water (Millipore). The concentration of free thiols in all peptides was determined using Ellman’s reagent (Sigma Aldrich).
Fabrication of synthetic PEG extracellular matrix.
Synthetic matrices were assembled using materials at the concentrations indicated throughout the text. Briefly, 8-arm 20 kDa PEG-VS macromers were prepared in a 10 w/w% solution in ultra pure water. Macromers were used at concentrations ranging from 14.4–33.6 mM VS (nominally, 3–7 wt%). Macromers were functionalized (fPEG-VS) via a Michael-type addition reaction with the integrin (PHSRN-K-RGS, GFOGER) and matrix-binding (FN-binder, BM-binder) by adding 10% of the total solution volume in 10x PBS + 1M HEPES, pH 8.2, vortex-mixing, and reacting at room temperature for 30 minutes. Note that lyophilized peptides containing HCl salt can be very acidic in stock solutions, so the pH of the final reaction mix should be maintained at 7.8 by adjusting the pH of the buffer solution. During functionalization, cells were counted and pelleted at the appropriate number for the target final cell densities (10,000 ESC and 10 EEO per µL of gel solution). Following functionalization, care was taken to ensure that all cell media was removed without disturbing the cell pellet, and pellets were gently resuspended in fPEG-VS. Upon resuspension, cross linker (CL-LW) was added to the mixture at a stoichiometric ratio of 0.45 thiol:VS to initiate gelation. The solution was gently mixed and plated accordingly, typically in 3 µL droplets in non-tissue culture treated 96-well plates unless otherwise specified in the text. Cell-fPEG-VS mixture was incubated for 25 minutes in a humidified incubator at 37°C until gelation was complete.
Rheological characterization of hydrogels.
To measure bulk bio-mechanical properties of crosslinked gels, 25 µL of each gel matrix mixture was loaded into a 1 mL syringe that had the tip cut off at the 0.1 mL mark. The matrix was allowed to gel at 37 °C for 25 min and then extruded from the open syringe as a cylindrical disc and moved to a 24 well plate that contained 400 µL of 1X PBS. The plate was incubated for 24 hours in a humidified incubator at 37 °C, 95% air, and 5% CO2, to allow equilibrium swelling to occur prior to rheological characterization. This procedure generates hydrogel discs of 1.4 mm in thickness. The discs were sandwiched between an 8 mm sandblasted parallel plate geometry and sandblasted base. The shear modulus was determined by performing small-strain oscillatory shear measurements on an Anton Parr MCR 302 rheometer. The mechanical response was recorded by performing frequency sweep measurements (0.1–10 Hz) at a constant strain (0.05), at 37 °C. The elastic modulus (E) is reported as a measure of bulk matrix mechanical properties derived from the recorded storage modulus (G’) in the linear elastic regime where: 𝐸 = 2𝐺′(1 + 𝜈) with the assumption that 𝜈 = 0.5 for ideal elastic hydrogel materials.
EEO generation in synthetic hydrogels.
Endometrial organoids were established and expanded in Matrigel according to established protocols11 with some minor modifications. Lumenized intact organoids with a diameter greater than 100 µm were harvested with cold CRS. Matrigel dollops were manually detached with a wide bore pipette tip, making sure to fully scrape the entire well to lift off the entire gel. The dollops were resuspended in cold CRS (5mL) for at least 30 mins on ice to dissolve remaining Matrigel, followed by centrifugation at 500–1000g, which resulted in a clear pellet of organoids. Organoids were broken into single cell suspensions using 1mL of prewarmed TrypLE Express (ThermoFisher) supplemented with DNAase (10µl, 1:100) and continuous mechanical agitation until a single cell suspension of epithelial cells was observed. Single cells were counted using a hemocytometer then resuspended in the functionalized PEG precursor (fPEG-VS) solutions prior to the addition of the crosslinker. In parallel, single cells were resuspended in Matrigel that served as an experimental control during matrix evaluation. In both cases, cells were encapsulated at a density of 500 cells/µL of matrix. Cell-matrix suspensions were seeded as 3 µL droplets on a non-tissue treated 96 well plate and allowed to polymerize for 25 min in a humidified incubator at 37 °C, 95% air, and 5% CO2. Complete gelation was examined visually on a test gel droplet prior to the addition of media. Gel droplets were maintained in 100 µL of expansion EEO media supplemented with ROCKi (10 µM). Media was changed every three days.
Generation of co-culture model.
Six-day old intact organoids (~100 µm diameter) were grown and expanded in Matrigel and harvested as described above. To maintain scEEO integrity, organoids were incubated in CRS until Matrigel was fully dissolved. Careful resuspension of intact scEEOs in DMEM/F12 was performed to prevent disrupting scEEO integrity. In parallel, donor matched ESCs were expanded using traditional 2D polystyrene cell culture plates and grown to 80% confluency. ESCs were lifted from 2D culture plates using trypsin (1X) and processed to generate a single cell suspension. Intact scEEOs and ESCs were prepared in parallel to encapsulate both cell types simultaneously and establish a co-culture in the PEG matrix solution. Cells were encapsulated at a density of 10,000 ESC and 10 intact scEEOs per µL of matrix. This epithelial-stromal ratio (approximately 1:1) was determined experimentally based on the cell ratios used to generate established endometrial xenograft models (sub-renal capsule) described elsewhere130,131 and validated using whole-mount 3D image analysis of endometrial tissue we performed (see Results). Embedded cells in fPEG-vs were seeded in 3 µL droplets and allowed to polymerize as described above in non-tissue culture treated 96-well plates. Co-culture was maintained in EEO media without Y-27632, a common media recipe utilized in other co-culture studies61, unless otherwise noted. Media was changed every three days and maintained for up to 15 days. It was noted that some basal components of the organoid medium contained P4 (N2 and B27) which may impact hormone responsive behavior, so a P4-reduced ‘neutral’ formulation of this organoid medium (nEEO medium) that completely lacked P4 was tested in some co-cultures. Both media formulations were sufficient to maintain support cell culture and induced hormone-mediated responses (Fig S19 in Data File 1); however, as expected, co-cultures maintained in nEEO media conferred a more robust response to progestin. Matching monocultures of ESCs-only or scEEOs-only were established in parallel for some experiments. Cultures were imaged daily.
EEO efficiency and diameter quantification.
To quantify organoid diameter and total organoid formation efficiency, single epithelial cells were encapsulated in fPEG-VS hydrogels or Matrigel and cultured over 14 days. scEEO formation efficiency was calculated as the percentage of organoids with a clear lumen with a diameter (>100 um) relative to Matrigel. 4X brightfield (BF) z-stack images of 10-day old endometrial organoids were captured using an EVOS M500 microscope (Invitrogen). The images were processed in Fiji using the time lapse Gaussian-based stacker focuser plugin to generate maximum intensity projections. Images were manually analyzed in Fiji to obtain a diameter and organoid count distribution for each condition. Daily images of co-cultures were manually curated to obtain relative growth rates of the organoids in single culture (N=3) or co-culture (N=8) by measuring scEEO diameter and quantifying the daily fold change relative to day 1 of culture (Videos S4–S16 in Data File 4).
Cell viability and proliferation analysis.
Co-culture cell viability and cell death was measured using the LIVE/DEAD™ Cell Imaging Kit (488/570) (Thermofisher Scientific) according to the manufacturers protocol. Briefly, at day-15 prior to fixation, cells were incubated the reagents provided by the kit and incubated at 37 °C, 95% air, and 5% CO2 for 20 minutes. Images were captured using Keyence BZ-X800 Fluorescence Microscope. Cell proliferation was assessed by EdU incorporation using the Click‐iT Plus EdU imaging kit with Alexa Fluor 594. All co-culture and monoculture experiments were treated with EdU (5-ethynyl-2′-deoxyuridine, 20 μM) for a 24-hour incubation period at 37°C, 95% air, 5% CO2 prior to fixation at day 15 of culture. Click-iT reaction cocktail was prepared as described by the manufacturer (ThermoFisher Scientific). Cells were incubated for 1 hr at RT protected from light and counter stained with DAPI (1 mg/mL, 1:2000). Images were captured using a ZEISS confocal Laser Scanning Microscope (LSM 880) and analyzed by 3D image processing was performed using the Surface and Spots function in Imaris (Bitmap) software (9.6.0). At least three individual organoids were analyzed per donor, and the proliferative index was measured as the number of EdU+ positive cells per organoid surface area (mm2). ESC proliferative index was calculated as the EdU+ cells relative to the total number of DAPI stained nuclei.
RNA isolation for qPCR and RNA-seq.
ESCs and intact scEEOs cultured in Matrigel or synthetic hydrogels were used for molecular analysis. After 14 days of culture, intact scEEOs were released from Matrigel and the synthetic ECM, using Cell Recovery Solution, or recombinant Sortase, respectively. Intact scEEO-only cultures, ESC-only cultures, or co-cultures were pelleted, resuspended in TRIzol reagent (ThermoFisher Scientific), and then stored at −80°C until processing. RNA was extracted using the Directzol RNA Mini-Prep kit (Zymo Research) per the manufacturer’s protocols with the inclusion of an on-column DNase step using the PureLink DNase Set (ThermoFisher Scientific). At least eight replicates (3 µl droplets) were pooled together to obtain sufficient RNA. cDNA was synthesized from ~1 µg of total RNA using the High-Capacity RNA-to-cDNA Kit (ThermoFisher Scientific, 4387406) per manufacturer’s protocols. TaqMan Fast Advanced Master Mix (ThermoFisher Scientific) was used in congruence with the cell-specific probes for qPCR. Gene expression was determined using the StepOnePlus real-time PCR system (Applied Biosystems) and calculated using the ΔΔCt method using GraphPad Prism. Gene expression was first normalized using the housekeeping GAPDH gene in each sample, then the relative fold change relative to experimental control. High Throughput 3’ Digital Gene Expression (3’DGE) RNAseq was performed on the isolated RNA using NovaSeq flowcells at the BioMicroCenter (MIT) and processed using the BMC/BCC 1.8 pipeline (cocultures n=7; ESC monocultures, n=6).
Protein measurement by ELISA and Luminex.
Spent media was stored at −80°C until analyzed by ELISA for prolactin (R&D Systems). Protocols provided by the manufacturer were adapted to allow ELISAs to be performed in a 384-well plate (ThermoFisher) to minimize medium (sample) volume needed.26 Multiplexed Luminex assays were performed to measure cytokines in 48- or 72-hour undiluted conditioned medium throughout the co-culture period using the magnetic Human Luminex XL Cytokine 45-PLEX pre-mixed kit (R&D systems). Protocols provided by the manufacturer were adapted to allow the assay to be performed in a 384-well plate to avoid introducing batch variability. Ten-point standard curves with culture medium and assay buffer blanks were included for quantification. Conversion from mean fluorescence intensity (MFI) values to absolute concentration was performed by interpolating 5-parameter logistical fits of standard curves for each analyte. Following the same downstream analysis protocol, secreted matrix metalloproteinases (MMP) concentrations were measured using the magnetic Human MMP Magnetic Luminex Performance Assay (R&D Systems). This assay required dilutions of cell culture supernatants in fresh culture medium at either 2x or 200x to detect the reported analytes within a quantifiable range.
Immunostaining and image analysis.
Hydrogel samples were fixed in 4% paraformaldehyde (Electron Microscopy Sciences) for 30 mins, permeabilized in 0.2% TritonX-100 in PBS (Sigma) for at least 30 mins at room temperature, and then blocked overnight with blocking buffer (1% BSA (Sigma), 5% normal donkey serum (Electron Microscopy Sciences) in PBS) at 4°C. Hydrogels were incubated with primary antibodies overnight while rocking at 4°C, rinsed with blocking buffer 3 times (5 min each while rocking at RT), and treated with secondary-conjugated antibodies overnight while rocking at 4°C. Specific antibodies and relevant controls can be found in the Key Resources Table and supplementary table of reagents (Table S2 in Data File 1). Images were captured using a ZEISS confocal Laser Scanning Microscope (LSM 880) or a Keyence BZ-X800 Fluorescence Microscope. Image analysis was performed using Fiji open software and 3D image processing was performed using Imaris (Bitmap) software (9.6.0).
Endometrial ECM proteomics.
Endometrial tissue processing for proteomics, proteomics data collection and analysis is described elsewhere (in preparation)44. In this study, the proteomic processing and pipelines were applied to 3 additional donors (N=11). Briefly, fresh endometrial biopsies were collected and processed using 50 µl of RapiGest (Waters Corporation) reconstituted in 50 mM NH4HCO3 at a concentration of 2mg/ml to digest the endometrial biopsy sample for 24 hours at 37°C. Next, urea (ThermoFisher) and dithiothreitol (ThermoFisher) were added to the sample to bring the mixture of a final concentration of 4 mM and 5 mM, respectively. Finally, iodoacetamide (MilliporeSigma) was added to the sample, for a final concentration of 15 mM and kept in the dark, at 37°C, for 30 minutes. Samples were frozen at −80°C until all patient samples had been collected and processed. Samples were lyophilized overnight and then reconstituted in NH4HCO3. Samples were digested with Trypsin Gold, mass spectrometry grade (Promega), using a ratio of 1 µg trypsin to 50 µg sample protein as quantified using a Pierce BCA Protein Assay Kit (ThermoFisher). Samples were digested overnight at room temperature using a rotor for mixing and then placed in a speed vacuum to reduce sample volume. Dried down digested peptide samples were labeled with TMT10plex kit (Pierce). After labeling, the samples were mixed and purified with peptide desalting spin columns (Pierce). 1/5 of the sample was used for one LC/MS/MS analysis (Thermo Exploris480 mass spectrometer). The dried peptide mix was reconstituted in a solution of 2% formic acid (FA) for MS analysis. Peptides were loaded with the autosampler directly onto a 50cm EASY-Spray C18 column (ThermoFisher). All MS/MS samples were analyzed using Sequest (ThermoFisher; version IseNode in Proteome Discoverer 2.3.0.523). Sequest was set up to search uniprot_human_reviewed_032120.fasta (version March 21, 2020) assuming the digestion enzyme trypsin. Scaffold Q+ (version Scaffold_5.0.1, Proteome Software Inc.) was used to quantitate Label Based Quantitation peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 10.0% probability to achieve an FDR less than 1.0% by the Percolator posterior error probability calculation.132 Normalization was performed iteratively (across samples and spectra) on intensities, as described in Statistical Analysis of Relative Labeled Mass Spectrometry Data from Complex Samples Using ANOVA.133 Medians were used for averaging. The protein list was then compared to the curated human matrisome (http://matrisome.org/) list to look for ECM proteins. Samples were normalized using a mixed aliquot of all patient samples that was analyzed in each LC/MS-MS run. Overlapping ECM proteins found in all 3 LC/MS-MS runs were identified and the Limma package134 in R was used to remove batch to batch effects.
Single cell RNA sequencing (scRNAseq).
scRNAseq data collection, processing and analysis as described elsewhere (in preparation)44 and is available (see Data and Code Accessibility). Data was downloaded and Seurat (V3) was used to create dot plots and violin plots of specific genes of interest on scaled data.
Bulk RNAseq analysis of in vitro co-cultures.
Aligned RNAseq transcript expected counts were analyzed in R (version 4.0.0) where data was modeled using the edgeR package (version 3.34.0).135,136 Counts were normalized by library size and filtered to exclude genes with less than 20 transcripts across all samples. Principal component analysis was performed using data batch-corrected by donor via the ComBat-seq method of the sva package (version 3.13) in R, and each gene was scaled to have unit variance before modeling.137 Differential gene expression was calculated by modeling transcript data in a quasi-likelihood negative binomial generalized log-linear model using the edgeR function “glmQLFit”. The use of a generalized log-linear model allowed for differences between hormone treatment groups to be compared while accounting for inter-donor variability. Genes were ranked by fold change in expression between hormone treatment groups to produce a ranked list of genes for gene set enrichment analysis (GSEA) (http://www.broadinstitute.org/gsea/index.jsp). Gene ontology (GO) term analysis was performed on ranked gene lists of genes with pvalues <= 0.05. No adjustment was performed at this step due to the low sample number, high variability, and intent to use the RNA sequencing as a hypothesis generation step. The R package topGO (version 2.44.0) was used for GO term identification and the package clusterProfiler (version 4.0.2) was used for visualizations. A curated list of menstrual related genes was assembled from existing literature.62,138 A heatmap of log2 transformed data was created using the R package pheatmap (https://CRAN.R-project.org/package=pheatmap; version 1.0.12) with the “complete” clustering method. Analysis was performed on 7 co-culture experiments comprised of 5 donors, 2 independent technical replicates and results were compared between experiments that were maintained in nEEO media (N=2) or traditional EEO media (N=5).
QUANTIFICATION AND STATISTICAL ANALYSIS.
Data are expressed as average ± standard error of the mean (SEM). Statistical analysis was performed using GraphPad Prism 8. Unpaired t-tests assuming unequal variances and two-way ANOVAs with appropriate post-tests were performed. Holm-Sidak's multiple comparison of the mean or two-tailed Mann-Whitney tests when comparing two groups. qPCR data was analyzed using multiple t-tests. Specific sample sizes for each assay is specified in the figure legends or methods. Statistical significance was defined as * p < 0.05, ** p < 0.01, *** p < 0.0001, and ****P < 0.0001.
Supplementary Material
Highlights:
Developed a tissue-inspired synthetic extracellular matrix for human endometrial cells.
PEG hydrogel enables stable co-culture of endometrial stroma and epithelial organoids.
Coculture model recapitulates key hormone-mediated phases of the human menstrual cycle.
The model supports the study of cellular crosstalk in reproductive health and disease.
Context and Significance:
Signals by sex hormones in the human menstrual cycle are primarily mediated by two cell populations, stroma and epithelium; however, a lack of models that enable their study has hindered advances in the field. Researchers from MIT establish a new synthetic extracellular matrix that enables to study simultaneously endometrial epithelial organoids and stromal cells. This model mimics key processes across the human menstrual cycle in vitro and introduces a synthetic platform that enables the study of cell-cell and cell-matrix communication in a controlled, long-term and tunable environment in 3D. This study will allow to advance our understanding of the mechanisms regulating human menstrual health and diseases.
Acknowledgements:
We thank the Newton Wellesley Hospital staff for facilitating a tissue collection program with MIT and the patients that donated their tissues for our research. We thank Histology Facility at the Koch Institute at MIT for their assistance with histological processing of tissues. We thank Profs. Kevin Osteen and Kaylon Bruner-Tran at Vanderbilt University for their support. We thank Ellen Kan (MIT) and Elizabeth Marr (Tufts University) for their technical help. J.S.G. was supported by an Environmental Toxicology Training Grant (NIH T32 ES007020) and from the Bill and Melinda Gates Foundation. Additional funding was provided by The John and Karine Begg Foundation, the Manton Foundation, and NIH U01 (EB029132).
Funding:
This work was supported by The John and Karine Begg Foundation, the Manton Foundation, and NIH U01 (EB029132).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests statement: L.G.G. and V.H.G. have a patent application pending related to the hydrogel system. The rest of the authors have no competing interests.
Supplementary Data File 1, related to Figures 1–6 and STAR Methods.
Supplementary Data File 2, related to Figure 2.
Supplementary Data File 3, related to Figure 5.
Supplementary Data File 4, related to Figure 6.
References:
- 1.Aplin JD (1989). Cellular Biochemistry of the Endometrium. In Biology of the Uterus (Karger Publishers), pp. 89–129. 10.1007/978-1-4684-5589-2_6. [DOI]
- 2.Aplin JD, and Ruane PT (2017). Embryo-epithelium interactions during implantation at a glance. J. Cell Sci 130, 15–22. 10.1242/jcs.175943. [DOI] [PubMed] [Google Scholar]
- 3.Loy SLY and S T (2005). Normal cycling Endometrium:Molecular, Cellular, and Histologic Perspectives. In Endometriosis in Clinical Practice, pp. 1–29.
- 4.Noyes RW, Hertig AT, and Rock J (1975). Dating the endometrial biopsy. Am. J. Obstet. Gynecol 122, 262–263. 10.1016/S0002-9378(16)33500-1. [DOI] [PubMed] [Google Scholar]
- 5.Roy A, and Matzuk MM (2011). Reproductive tract function and dysfunction in women. Nat. Rev. Endocrinol 7, 517–525. 10.1038/nrendo.2011.79. [DOI] [PubMed] [Google Scholar]
- 6.Brosens JJ, Hayashi N, and White JO (1999). Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology 140, 4809–4820. 10.1210/endo.140.10.7070. [DOI] [PubMed] [Google Scholar]
- 7.Kelleher AM, Demayo FJ, and Spencer TE (2019). Uterine Glands: Developmental Biology and Functional Roles in Pregnancy. Endocr. Rev 40, 1424–1445. 10.1210/er.2018-00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunha GR, Cooke PS, and Kurita T (2004). Role of stromal-epithelial interactions in hormonal responses. Arch. Histol. Cytol 67, 417–434. 10.1679/aohc.67.417. [DOI] [PubMed] [Google Scholar]
- 9.Cameron IT, Fraser IS, and Smith SK (Stephen K) (1998). Clinical disorders of the endometrium and menstrual cycle (Oxford University Press; ). [Google Scholar]
- 10.Boretto M, Cox B, Noben M, Hendriks N, Fassbender A, Roose H, Frédé F, Amant F, Timmerman D, Tomassetti C, et al. (2017). Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. dev.biologists.org. 10.1242/dev.148478. [DOI] [PubMed]
- 11.Turco MY, Gardner L, Hughes J, Cindrova-Davies T, Gomez MJ, Farrell L, Hollinshead M, Marsh SGE, Brosens JJ, Critchley HO, et al. (2017). Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol 19, 568–577. 10.1038/ncb3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clevers H (2016). Modeling Development and Disease with Organoids. Cell 165, 1586–1597. 10.1016/j.cell.2016.05.082. [DOI] [PubMed] [Google Scholar]
- 13.Boretto M, Maenhoudt N, Luo X, Hennes A, Boeckx B, Bui B, Heremans R, Perneel L, Kobayashi H, Van Zundert I, et al. (2019). Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat. Cell Biol 21, 1041–1051. 10.1038/s41556-019-0360-z. [DOI] [PubMed] [Google Scholar]
- 14.Sato T, Stange DE, Ferrante M, Vries RGJ, Van Es JH, Van Den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, et al. (2011). Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772. 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 15.Fujii M, Matano M, Toshimitsu K, Takano A, Mikami Y, Nishikori S, Sugimoto S, and Sato T (2018). Human Intestinal Organoids Maintain Self-Renewal Capacity and Cellular Diversity in Niche-Inspired Culture Condition. Cell Stem Cell 23, 787–793.e6. 10.1016/j.stem.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald HC, Dhakal P, Behura SK, Schust DJ, and Spencer TE (2019). Self-renewing endometrial epithelial organoids of the human uterus. Proc. Natl. Acad. Sci. U. S. A 116, 23132–23142. 10.1073/pnas.1915389116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura T, and Sato T (2018). Advancing Intestinal Organoid Technology Toward Regenerative Medicine. CMGH 5, 51–60. 10.1016/j.jcmgh.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruz-Acuña R, Quirós M, Farkas AE, Dedhia PH, Huang S, Siuda D, García-Hernández V, Miller AJ, Spence JR, Nusrat A, et al. (2017). Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol 19, 1326–1335. 10.1038/ncb3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 20.Talbot NC, and Caperna TJ (2015). Proteome array identification of bioactive soluble proteins/peptides in Matrigel: relevance to stem cell responses. Cytotechnology 67, 873–883. 10.1007/s10616-014-9727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes CS, Postovit LM, and Lajoie GA (2010). Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10, 1886–1890. 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 22.Cruz-Acuña R, and García AJ (2017). Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions. Matrix Biol 57–58, 324–333. 10.1016/j.matbio.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capeling MM, Czerwinski M, Huang S, Tsai YH, Wu A, Nagy MS, Juliar B, Sundaram N, Song Y, Han WM, et al. (2019). Nonadhesive Alginate Hydrogels Support Growth of Pluripotent Stem Cell-Derived Intestinal Organoids. Stem Cell Rep 12, 381–394. 10.1016/j.stemcr.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gjorevski N, Sachs N, Manfrin A, Giger S, Bragina ME, Ordóñez-Morán P, Clevers H, and Lutolf MP (2016). Designer matrices for intestinal stem cell and organoid culture. Nat. Publ. Group 539, 560–564. 10.1038/nature20168. [DOI] [PubMed] [Google Scholar]
- 25.Kratochvil MJ, Seymour AJ, Li TL, Paşca SP, Kuo CJ, and Heilshorn SC (2019). Engineered materials for organoid systems. Nat. Rev. Mater 4, 606–622. 10.1038/s41578-019-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook CD, Hill AS, Guo M, Stockdale L, Papps JP, Isaacson KB, Lauffenburger DA, and Griffith LG (2017). Local remodeling of synthetic extracellular matrix microenvironments by co-cultured endometrial epithelial and stromal cells enables long-term dynamic physiological function. Integr. Biol. U. K 9, 271–289. 10.1039/c6ib00245e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernandez-Gordillo V, Kassis T, Lampejo A, Choi GH, Gamboa ME, Gnecco JS, Brown A, Breault DT, Carrier R, and Griffith LG (2020). Fully synthetic matrices for in vitro culture of primary human intestinal enteroids and endometrial organoids. Biomaterials 254, 120125. 10.1016/j.biomaterials.2020.120125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnold JT, Kaufman DG, Seppälä M, and Lessey BA (2001). Endometrial stromal cells regulate epithelial cell growth in vitro: A new co-culture model. Hum. Reprod 16, 836–845. 10.1093/humrep/16.5.836. [DOI] [PubMed] [Google Scholar]
- 29.Abbas Y, Brunel LG, Hollinshead MS, Fernando RC, Gardner L, Duncan I, Moffett A, Best S, Turco MY, Burton GJ, et al. (2020). Generation of a three-dimensional collagen scaffold-based model of the human endometrium. Interface Focus 10, 20190079. 10.1098/rsfs.2019.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osteen KG, Rodgers WH, Gaire M, Hargrove JT, Gorstein F, and Matrisian LM (1994). Stromal-epithelial interaction mediates steroidal regulation of metalloproteinase expression in human endometrium. Proc. Natl. Acad. Sci. U. S. A 91, 10129–10133. 10.1073/pnas.91.21.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rezakhani S, Gjorevski N, and Lutolf MP (2020). Low-Defect Thiol-Michael Addition Hydrogels as Matrigel Substitutes for Epithelial Organoid Derivation. Adv. Funct. Mater 30, 2000761. 10.1002/adfm.202000761. [DOI] [Google Scholar]
- 32.Below CR, Kelly J, Brown A, Humphries JD, Hutton C, Xu J, Lee BY, Cintas C, Zhang X, Hernandez-Gordillo V, et al. (2021). A microenvironment-inspired synthetic three-dimensional model for pancreatic ductal adenocarcinoma organoids. Nat. Mater 10.1038/s41563-021-01085-1. [DOI] [PMC free article] [PubMed]
- 33.O’Connor BB, Pope BD, Peters MM, Ris-Stalpers C, and Parker KK (2020). The role of extracellular matrix in normal and pathological pregnancy: Future applications of microphysiological systems in reproductive medicine. Exp. Biol. Med 245, 1163–1174. 10.1177/1535370220938741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aplin JD, and Jones CJP (1989). Extracellular Matrix in Endometrium and Decidua. In Placenta as a Model and a Source, Genbačev O, Klopper A, and Beaconsfield R, eds. (Springer US; ), pp. 115–128. 10.1007/978-1-4613-0823-2_12. [DOI] [Google Scholar]
- 35.Gellersen B, and Brosens JJ (2014). Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr. Rev 35, 851–905. 10.1210/er.2014-1045. [DOI] [PubMed] [Google Scholar]
- 36.Béliard A, Donnez J, Nisolle M, and Foidart JM (1997). Localization of laminin, fibronectin, E-cadherin, and integrins in endometrium and endometriosis. Fertil. Steril 67, 266–272. 10.1016/S0015-0282(97)81909-7. [DOI] [PubMed] [Google Scholar]
- 37.Bischof P, Redard M, Gindre P, Vassilakos P, and Campana A (1993). Localization of alpha 2, alpha 5 and alpha 6 integrin subunits in human endometrium, decidua and trophoblast. Eur. J. Obstet. Gynecol. Reprod. Biol 51, 217–226. 10.1016/0028-2243(93)90038-E. [DOI] [PubMed] [Google Scholar]
- 38.Puy LA, Pang C, and Librach CL (2002). Immunohistochemical analysis of alphavbeta5 and alphavbeta6 integrins in the endometrium and endometriosis. Int. J. Gynecol. Pathol. Off. J. Int. Soc. Gynecol. Pathol 21, 167–177. 10.1097/00004347-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Tabibzadeh S (1992). Patterns of expression of integrin molecules in human endometrium throughout the menstrual cycle. Hum. Reprod 7, 876–882. 10.1093/oxfordjournals.humrep.a137753. [DOI] [PubMed] [Google Scholar]
- 40.Wojtowicz AM, Shekaran A, Oest ME, Dupont KM, Templeman KL, Hutmacher DW, Guldberg RE, and García AJ (2010). Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials 31, 2574–2582. 10.1016/j.biomaterials.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Creus M, Balasch J, Ordi J, Fábregues F, Casamitjana R, Quinto L, Coutifaris C, and Vanrell JA (1998). Integrin expression in normal and out-of-phase endometria. Hum. Reprod 13, 3460–3468. 10.1093/humrep/13.12.3460. [DOI] [PubMed] [Google Scholar]
- 42.Aplin JD, Spanswick C, Behzad F, Kimber SJ, and Vićovac L (1996). Molecular aspects of implantation: Integrins β5, β3 and αv are apically distributed in endometrial epithelium. Mol. Hum. Reprod 2, 527–534. 10.1093/molehr/2.7.527. [DOI] [PubMed] [Google Scholar]
- 43.Lessey BA, Damjanovich L, Coutifaris C, Castelbaum A, Albeida SM, and Buck CA (1992). Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J. Clin. Invest 90, 188–195. 10.1172/JCI115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baugh LM, Goods BA, Gnecco JS, Bae Y, Retchin M, Tzouanas CN, Loring M, Isaacson K, Shalek AK, Lauffenburger DA, et al. (2022). Integrating endometrial proteomic and single cell transcriptomic pipelines reveals distinct menstrual cycle and endometriosis-associated molecular profiles. medRxiv, 2022.01.29.22269829. 10.1101/2022.01.29.22269829. [DOI]
- 45.Lessey BA, and Castelbaum AJ (2002). Integrins and implantation in the human. Rev. Endocr. Metab. Disord 3, 107–117. 10.1023/a:1015450727580. [DOI] [PubMed] [Google Scholar]
- 46.Casals G, Ordi J, Creus M, Fábregues F, Carmona F, Casamitjana R, and Balasch J (2012). Expression pattern of osteopontin and αvβ3 integrin during the implantation window in infertile patients with early stages of endometriosis. Hum. Reprod 27, 805–813. 10.1093/humrep/der432. [DOI] [PubMed] [Google Scholar]
- 47.Coughlan C, Sinagra M, Ledger W, Li TC, and Laird S (2013). Endometrial integrin expression in women with recurrent implantation failure after in vitro fertilization and its relationship to pregnancy outcome. Fertil. Steril 100, 825–830. 10.1016/j.fertnstert.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Alonso L, Handfield L-F, Roberts K, Nikolakopoulou K, Fernando RC, Gardner L, Woodhams B, Arutyunyan A, Polanski K, Hoo R, et al. (2021). Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat. Genet 53, 1698–1711. 10.1038/s41588-021-00972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hynes RO (2002). Integrins: Bidirectional, Allosteric Signaling Machines. Cell 110, 673–687. 10.1016/S0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 50.Hehlgans S, Haase M, and Cordes N (2007). Signalling via integrins: implications for cell survival and anticancer strategies. Biochim. Biophys. Acta 1775, 163–180. 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 51.Fields GB (2010). Synthesis and biological applications of collagen-model triple-helical peptides. Org. Biomol. Chem 8, 1237–1258. 10.1039/B920670A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carafoli F, Hamaia SW, Bihan D, Hohenester E, and Farndale RW (2013). An Activating Mutation Reveals a Second Binding Mode of the Integrin α2 I Domain to the GFOGER Motif in Collagens. PLOS ONE 8, e69833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khoshnoodi J, Pedchenko V, and Hudson BG (2008). Mammalian collagen IV. Microsc. Res. Tech 71, 357–370. 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gardner H (2014). Integrin α1β1. Adv. Exp. Med. Biol 819, 21–39. 10.1007/978-94-017-9153-3_2. [DOI] [PubMed] [Google Scholar]
- 55.Valdez J, Cook CD, Ahrens CC, Wang AJ, Brown A, Kumar M, Stockdale L, Rothenberg D, Renggli K, Gordon E, et al. (2017). On-demand dissolution of modular, synthetic extracellular matrix reveals local epithelial-stromal communication networks. Biomaterials 130, 90–103. 10.1016/j.biomaterials.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cambria E, Renggli K, Ahrens CC, Cook CD, Kroll C, Krueger AT, Imperiali B, and Griffith LG (2015). Covalent Modification of Synthetic Hydrogels with Bioactive Proteins via Sortase-Mediated Ligation. Biomacromolecules 16, 2316–2326. 10.1021/acs.biomac.5b00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soofi SS, Last JA, Lliensiek SJ, Nealey PF, and Murphy CJ (2009). Elastic modulus of Matrigel as determined by AFM. J. Struct. Biol 167, 216–219. 10.1016/j.jsb.2009.05.005.The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abbas Y, Carnicer-Lombarte A, Gardner L, Thomas J, Brosens JJ, Moffett A, Sharkey AM, Franze K, Burton GJ, and Oyen ML (2019). Tissue stiffness at the human maternal-fetal interface. Hum. Reprod 34, 1999–2008. 10.1093/humrep/dez139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Myers KM, and Elad D (2017). Biomechanics of the human uterus. Wiley Interdiscip. Rev. Syst. Biol. Med 9, 1–20. 10.1002/wsbm.1388. [DOI] [PubMed] [Google Scholar]
- 60.Wetendorf M, and DeMayo FJ (2012). The progesterone receptor regulates implantation, decidualization, and glandular development via a complex paracrine signaling network. Mol. Cell. Endocrinol 357, 108–118. 10.1016/j.mce.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rawlings TM, Makwana K, Taylor DM, Molè MA, Fishwick KJ, Tryfonos M, Odendaal J, Hawkes A, Zernicka-Goetz M, Hartshorne GM, et al. (2021). Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. eLife 10. 10.7554/eLife.69603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lucas ES, Vrljicak P, Muter J, Diniz-da-Costa MM, Brighton PJ, Kong C-S, Lipecki J, Fishwick KJ, Odendaal J, Ewington LJ, et al. (2020). Recurrent pregnancy loss is associated with a pro-senescent decidual response during the peri-implantation window. Commun. Biol 3, 37. 10.1038/s42003-020-0763-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sikora J, Ferrero S, Mielczarek-Palacz A, and Kondera-Anasz Z (2018). The Delicate Balance between the Good and the Bad IL-1 Proinflammatory Effects in Endometriosis. Curr. Med. Chem 25, 2105–2121. 10.2174/0929867325666180111093547. [DOI] [PubMed] [Google Scholar]
- 64.Yu J, Berga SL, Zou W, Yook DG, Pan JC, Andrade AA, Zhao L, Sidell N, Bagchi IC, Bagchi MK, et al. (2017). IL-1β Inhibits Connexin 43 and Disrupts Decidualization of Human Endometrial Stromal Cells Through ERK1/2 and p38 MAP Kinase. Endocrinology 158, 4270–4285. 10.1210/en.2017-00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu J, Berga SL, Zou W, and Taylor RN (2019). Interleukin-1β inhibits estrogen receptor-α, progesterone receptors A and B and biomarkers of human endometrial stromal cell differentiation: implications for endometriosis. Mol. Hum. Reprod 25, 625–637. 10.1093/molehr/gaz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Critchley HO, Maybin JA, Armstrong GM, and Williams ARW (2020). Physiology of the endometrium and regulation of menstruation. Physiol. Rev 10.1152/physrev.00031.2019. [DOI] [PubMed]
- 67.Mehasseb MK, Panchal R, Taylor AH, Brown L, Bell SC, and Habiba M (2011). Estrogen and progesterone receptor isoform distribution through the menstrual cycle in uteri with and without adenomyosis. Fertil. Steril 95, 2228–2235, 2235.e1. 10.1016/j.fertnstert.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 68.Cunha GR, Bigsby RM, Cooke PS, and Sugimura Y (1985). Stromal-epithelial interactions in adult organs. Cell Differ 17, 137–148. 10.1016/0045-6039(85)90481-6. [DOI] [PubMed] [Google Scholar]
- 69.Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, and Cunha GR (1997). Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc. Natl. Acad. Sci. U. S. A 94, 6535–6540. 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benjamini Y, and Hochberg Y (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol 57, 289–300. 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 71.Bigsby RM (2002). Control of growth and differentiation of the endometrium: The role of tissue interactions. In Annals of the New York Academy of Sciences, pp. 110–117. 10.1111/j.1749-6632.2002.tb02771.x. [DOI] [PubMed]
- 72.Osteen KG, Igarashi TM, and Bruner-Tran KL (2003). Progesterone action in the human endometrium: induction of a unique tissue environment which limits matrix metalloproteinase (MMP) expression. Front. Biosci. J. Virtual Libr 8, d78–86. 10.2741/938. [DOI] [PubMed] [Google Scholar]
- 73.Reavey JJ, Walker C, Nicol M, Murray AA, Critchley HOD, Kershaw LE, and Maybin JA (2021). Markers of human endometrial hypoxia can be detected in vivo and ex vivo during physiological menstruation. Hum. Reprod. Oxf. Engl 36, 941–950. 10.1093/humrep/deaa379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bruner KL, Rodgers WH, Gold LI, Korc M, Hargrove JT, Matrisian LM, and Osteen KG (1995). Transforming growth factor β mediates the progesterone suppression of an epithelial metalloproteinase by adjacent stroma in the human endometrium. Proc. Natl. Acad. Sci. U. S. A 92, 7362–7366. 10.1073/pnas.92.16.7362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goffin F, Munaut C, Frankenne F, Perrier D’Hauterive S, Béliard A, Fridman V, Nervo P, Colige A, and Foidart J-M (2003). Expression pattern of metalloproteinases and tissue inhibitors of matrix-metalloproteinases in cycling human endometrium. Biol. Reprod 69, 976–984. 10.1095/biolreprod.103.015933. [DOI] [PubMed] [Google Scholar]
- 76.Curry TEJ, and Osteen KG (2003). The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr. Rev 24, 428–465. 10.1210/er.2002-0005. [DOI] [PubMed] [Google Scholar]
- 77.Large MJ, and DeMayo FJ (2012). The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol. Cell. Endocrinol 358, 155–165. 10.1016/j.mce.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H, Critchley HO, Kelly RW, Shen D, and Baird DT (1998). Progesterone receptor subtype B is differentially regulated in human endometrial stroma. Mol. Hum. Reprod 4, 407–412. 10.1093/molehr/4.4.407. [DOI] [PubMed] [Google Scholar]
- 79.Kokawa K, Shikone T, and Nakano R (1996). Apoptosis in the human uterine endometrium during the menstrual cycle. J. Clin. Endocrinol. Metab 81, 4144–4147. 10.1210/jcem.81.11.8923873. [DOI] [PubMed] [Google Scholar]
- 80.Armstrong GM, Maybin JA, Murray AA, Nicol M, Walker C, Saunders PTK, Rossi AG, and Critchley HOD (2017). Endometrial apoptosis and neutrophil infiltration during menstruation exhibits spatial and temporal dynamics that are recapitulated in a mouse model. Sci. Rep 7, 17416. 10.1038/s41598-017-17565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miyoshi H, VanDussen KL, Malvin NP, Ryu SH, Wang Y, Sonnek NM, Lai C, and Stappenbeck TS (2017). Prostaglandin E2 promotes intestinal repair through an adaptive cellular response of the epithelium. EMBO J 36, 5–24. 10.15252/embj.201694660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y, Nicholes K, and Shih IM (2020). The Origin and Pathogenesis of Endometriosis. Annu. Rev. Pathol. Mech. Dis 15, 71–95. 10.1146/annurev-pathmechdis-012419-032654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vercellini P, Viganò P, Somigliana E, and Fedele L (2014). Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol 10, 261–275. 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 84.Horne AW, and Saunders PTK (2019). SnapShot: Endometriosis. Cell 179, 1677-1677.e1. 10.1016/j.cell.2019.11.033. [DOI] [PubMed] [Google Scholar]
- 85.Bulun SE, Cheng Y-H, Yin P, Imir G, Utsunomiya H, Attar E, Innes J, and Julie Kim J (2006). Progesterone resistance in endometriosis: Link to failure to metabolize estradiol. Mol. Cell. Endocrinol 248, 94–103. 10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 86.Al-Sabbagh M, Lam EW-F, and Brosens JJ (2012). Mechanisms of endometrial progesterone resistance. Mol. Cell. Endocrinol 358, 208–215. 10.1016/j.mce.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 87.Zondervan KT, Becker CM, and Missmer SA (2020). Endometriosis. N. Engl. J. Med 382, 1244–1256. 10.1056/NEJMra1810764. [DOI] [PubMed] [Google Scholar]
- 88.Chung D, Gao F, Jegga AG, and Das SK (2015). Estrogen mediated epithelial proliferation in the uterus is directed by stromal Fgf10 and Bmp8a. Mol. Cell. Endocrinol 400, 48–60. 10.1016/j.mce.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Winuthayanon W, Lierz SL, Delarosa KC, Sampels SR, Donoghue LJ, Hewitt SC, and Korach KS (2017). Juxtacrine Activity of Estrogen Receptor α in Uterine Stromal Cells is Necessary for Estrogen-Induced Epithelial Cell Proliferation. Sci. Rep 7, 8377. 10.1038/s41598-017-07728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wingfield M, Macpherson A, Healy DL, and Rogers PA (1995). Cell proliferation is increased in the endometrium of women with endometriosis. Fertil. Steril 64, 340–346. 10.1016/s0015-0282(16)57733-4. [DOI] [PubMed] [Google Scholar]
- 91.Lenz J, Chvatal R, Fiala L, Konecna P, and Lenz D (2021). Comparative immunohistochemical study of deep infiltrating endometriosis, lymph node endometriosis and atypical ovarian endometriosis including description of a perineural invasion. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czechoslov 165, 69–79. 10.5507/bp.2020.006. [DOI] [PubMed] [Google Scholar]
- 92.Colón-Caraballo M, García M, Mendoza A, and Flores I (2019). Human Endometriosis Tissue Microarray Reveals Site-specific Expression of Estrogen Receptors, Progesterone Receptor, and Ki67. Appl. Immunohistochem. Mol. Morphol. AIMM 27, 491–500. 10.1097/PAI.0000000000000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kurita T, Young P, Brody JR, Lydon JP, O’Malley BW, and Cunha GR (1998). Stromal progesterone receptors mediate the inhibitory effects of progesterone on estrogen-induced uterine epithelial cell deoxyribonucleic acid synthesis. Endocrinology 139, 4708–4713. 10.1210/endo.139.11.6317. [DOI] [PubMed] [Google Scholar]
- 94.Griffith LG, and Swartz MA (2006). Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol 7, 211–224. 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 95.Stejskalová A, Fincke V, Nowak M, Schmidt Y, Borrmann K, von Wahlde M-K, Schäfer SD, Kiesel L, Greve B, and Götte M (2021). Collagen I triggers directional migration, invasion and matrix remodeling of stroma cells in a 3D spheroid model of endometriosis. Sci. Rep 11, 4115. 10.1038/s41598-021-83645-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuperman T, Gavriel M, Gotlib R, Zhang Y, Jaffa A, Elad D, and Grisaru D (2020). Tissue-engineered multi-cellular models of the uterine wall. Biomech. Model. Mechanobiol 10.1007/s10237-020-01296-6. [DOI] [PubMed]
- 97.Park DW, Choi DS, Ryu H-S, Kwon HC, Joo H, and Min CK (2003). A well-defined in vitro three-dimensional culture of human endometrium and its applicability to endometrial cancer invasion. Cancer Lett 195, 185–192. 10.1016/s0304-3835(03)00131-9. [DOI] [PubMed] [Google Scholar]
- 98.Wiwatpanit T, Murphy AR, Lu Z, Urbanek M, Burdette JE, Woodruff TK, and Kim JJ (2020). Scaffold-Free Endometrial Organoids Respond to Excess Androgens Associated With Polycystic Ovarian Syndrome. J. Clin. Endocrinol. Metab 105, 769–780. 10.1210/clinem/dgz100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wendel JRH, Wang X, Smith LJ, and Hawkins SM (2020). Three-Dimensional Biofabrication Models of Endometriosis and the Endometriotic Microenvironment. Biomedicines 8. 10.3390/biomedicines8110525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fang S, McLean J, Shi L, Vink J-SY, Hendon CP, and Myers KM (2021). Anisotropic Mechanical Properties of the Human Uterus Measured by Spherical Indentation. Ann. Biomed. Eng 49, 1923–1942. 10.1007/s10439-021-02769-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bentin-Ley U, Pedersen B, Lindenberg S, Falck Larsen J, Hamberger L, and Horn T (1994). Isolation and culture of human endometrial cells in a three-dimensional culture system. J. Reprod. Fertil 101, 327–332. 10.1530/jrf.0.1010327. [DOI] [PubMed] [Google Scholar]
- 102.Bentin-Ley U, Lindenberg S, Horn T, and Larsen JF (1995). Ultrastructure of endometrial epithelial cells in a three-dimensional cell culture system for human implantation studies. J. Assist. Reprod. Genet 12, 632–638. 10.1007/BF02212588. [DOI] [PubMed] [Google Scholar]
- 103.Bläuer M, Heinonen PK, Martikainen PM, Tomás E, and Ylikomi T (2005). A novel organotypic culture model for normal human endometrium: Regulation of epithelial cell proliferation by estradiol and medroxyprogesterone acetate. Hum. Reprod 20, 864–871. 10.1093/humrep/deh722. [DOI] [PubMed] [Google Scholar]
- 104.Simón C, Mercader A, Gimeno MJ, and Pellicer A (1997). The interleukin-1 system and human implantation. Am. J. Reprod. Immunol. N. Y. N 1989 37, 64–72. 10.1111/j.1600-0897.1997.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 105.Weber A, Wasiliew P, and Kracht M (2010). Interleukin-1 (IL-1) pathway. Sci. Signal 3, cm1. 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 106.Boraschi D, and Tagliabue A (2013). The interleukin-1 receptor family. Semin. Immunol 25, 394–407. 10.1016/j.smim.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 107.von Wolff M, Thaler CJ, Strowitzki T, Broome J, Stolz W, and Tabibzadeh S (2000). Regulated expression of cytokines in human endometrium throughout the menstrual cycle: dysregulation in habitual abortion. Mol. Hum. Reprod 6, 627–634. 10.1093/molehr/6.7.627. [DOI] [PubMed] [Google Scholar]
- 108.Inagaki N, Stern C, McBain J, Lopata A, Kornman L, and Wilkinson D (2003). Analysis of intra-uterine cytokine concentration and matrix-metalloproteinase activity in women with recurrent failed embryo transfer. Hum. Reprod. Oxf. Engl 18, 608–615. 10.1093/humrep/deg139. [DOI] [PubMed] [Google Scholar]
- 109.Boomsma CM, Kavelaars A, Eijkemans MJC, Lentjes EG, Fauser BCJM, Heijnen CJ, and Macklon NS (2009). Endometrial secretion analysis identifies a cytokine profile predictive of pregnancy in IVF. Hum. Reprod. Oxf. Engl 24, 1427–1435. 10.1093/humrep/dep011. [DOI] [PubMed] [Google Scholar]
- 110.Lei K, Georgiou EX, Chen L, Yulia A, Sooranna SR, Brosens JJ, Bennett PR, and Johnson MR (2015). Progesterone and the Repression of Myometrial Inflammation: The Roles of MKP-1 and the AP-1 System. Mol. Endocrinol 29, 1454–1467. 10.1210/me.2015-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang H, Shi G, Li M, Fan H, Ma H, and Sheng L (2018). Correlation of IL-1 and HB-EGF with endometrial receptivity. Exp. Ther. Med 16, 5130–5136. 10.3892/etm.2018.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salama KM, Alloush MK, and Al Hussini RM (2020). Are the cytokines TNF alpha and IL 1Beta early predictors of embryo implantation? Cross sectional study. J. Reprod. Immunol 137, 102618. 10.1016/j.jri.2019.102618. [DOI] [PubMed] [Google Scholar]
- 113.Zhao Y, Zhang T, Guo X, Wong CK, Chen X, Chan YL, Wang CC, Laird S, and Li TC (2021). Successful implantation is associated with a transient increase in serum pro-inflammatory cytokine profile followed by a switch to anti-inflammatory cytokine profile prior to confirmation of pregnancy. Fertil. Steril 115, 1044–1053. 10.1016/j.fertnstert.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 114.Jørgensen H, Fedorcsak P, Isaacson K, Tevonian E, Xiao A, Beste M, Qvigstad E, Lauffenburger D, and Griffith L (2022). Endometrial cytokines in patients with and without endometriosis evaluated for infertility. Fertil. Steril 117, 629–640. 10.1016/j.fertnstert.2021.11.024. [DOI] [PubMed] [Google Scholar]
- 115.Di Iorio A, Ferrucci L, Sparvieri E, Cherubini A, Volpato S, Corsi A, Bonafè M, Franceschi C, Abate G, and Paganelli R (2003). Serum IL-1beta levels in health and disease: a population-based study. “The InCHIANTI study”. Cytokine 22, 198–205. 10.1016/s1043-4666(03)00152-2. [DOI] [PubMed] [Google Scholar]
- 116.DiVasta AD, Stamoulis C, Gallagher JS, Laufer MR, Anchan R, and Hornstein MD (2021). Nonhormonal therapy for endometriosis: a randomized, placebo-controlled, pilot study of cabergoline versus norethindrone acetate. FS Rep 2, 454–461. 10.1016/j.xfre.2021.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Llarena NC, Richards EG, Priyadarshini A, Fletcher D, Bonfield T, and Flyckt RL (2020). Characterizing the endometrial fluid cytokine profile in women with endometriosis. J. Assist. Reprod. Genet 37, 2999–3006. 10.1007/s10815-020-01989-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rahnama M, Jastrzębska I, Jamrogiewicz R, and Kocki J (2013). IL-1α and IL-1β levels in blood serum and saliva of menopausal women. Endocr. Res 38, 69–76. 10.3109/07435800.2012.713425. [DOI] [PubMed] [Google Scholar]
- 119.García-Solares J, Dolmans MM, Squifflet JL, Donnez J, and Donnez O (2018). Invasion of human deep nodular endometriotic lesions is associated with collective cell migration and nerve development. Fertil. Steril 110, 1318–1327. 10.1016/j.fertnstert.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 120.González-Ramos R, Defrère S, and Devoto L (2012). Nuclear factor-kappaB: a main regulator of inflammation and cell survival in endometriosis pathophysiology. Fertil. Steril 98, 520–528. 10.1016/j.fertnstert.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 121.Strakova Z, Srisuparp S, and Fazleabas AT (2000). Interleukin-1beta induces the expression of insulin-like growth factor binding protein-1 during decidualization in the primate. Endocrinology 141, 4664–4670. 10.1210/endo.141.12.7810. [DOI] [PubMed] [Google Scholar]
- 122.Yoshinaga K, Davies C, White K, Caron K, Golos T, Fazleabas A, Paria B, Mor G, Paul S, Ye X, et al. (2014). Interdisciplinary collaborative team for blastocyst implantation research: inception and perspectives. Am. J. Reprod. Immunol. N. Y. N 1989 71, 1–11. 10.1111/aji.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Brown A, He H, Trumper E, Valdez J, Hammond P, and Griffith LG (2020). Engineering PEG-based hydrogels to foster efficient endothelial network formation in free-swelling and confined microenvironments. Biomaterials 243, 119921. 10.1016/j.biomaterials.2020.119921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sofman M, Brown A, Griffith LG, and Hammond PT (2021). A modular polymer microbead angiogenesis scaffold to characterize the effects of adhesion ligand density on angiogenic sprouting. Biomaterials 264, 120231. 10.1016/j.biomaterials.2020.120231. [DOI] [PubMed] [Google Scholar]
- 125.Osteen KG, Hill GA, Hargrove JT, and Gorstein F (1989). Development of a method to isolate and culture highly purified populations of stromal and epithelial cells from human endometrial biopsy specimens. Fertil. Steril 52, 965–972. 10.1016/S0015-0282(16)53160-4. [DOI] [PubMed] [Google Scholar]
- 126.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, and Hubbell JA (2003). Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. U. S. A 100, 5413–5418. 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kuhlman W, Taniguchi I, Griffith LG, and Mayes AM (2007). Interplay between PEO tether length and ligand spacing governs cell spreading on RGD-modified PMMA-g-PEO comb copolymers. Biomacromolecules 8, 3206–3213. 10.1021/bm070237o. [DOI] [PubMed] [Google Scholar]
- 128.Gao X, and Groves MJ (1998). Fibronectin-binding peptides. I. Isolation and characterization of two unique fibronectin-binding peptides from gelatin. Eur. J. Pharm. Biopharm 45, 275–284. 10.1016/S0939-6411(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 129.Johnson G, and Moore SW (2004). Identification of a structural site on acetylcholinesterase that promotes neurite outgrowth and binds laminin-1 and collagen IV. Biochem. Biophys. Res. Commun 319, 448–455. 10.1016/j.bbrc.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 130.Kurita T, Medina R, Schabel AB, Young P, Gama P, Parekh TV, Brody J, Cunha GR, Osteen KG, Bruner-Tran KL, et al. (2005). The activation function-1 domain of estrogen receptor α in uterine stromal cells is required for mouse but not human uterine epithelial response to estrogen. Differentiation 73, 313–322. 10.1111/j.1432-0436.2005.00033.x. [DOI] [PubMed] [Google Scholar]
- 131.Cunha GR, and Baskin L (2016). Use of sub-renal capsule transplantation in developmental biology. Differentiation 91, 4–9. 10.1016/j.diff.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Käll L, Storey JD, and Noble WS (2008). Non-parametric estimation of posterior error probabilities associated with peptides identified by tandem mass spectrometry. Bioinformatics 24, 42–48. 10.1093/bioinformatics/btn294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oberg AL, Mahoney DW, Eckel-Passow JE, Malone CJ, Wolfinger RD, Hill EG, Cooper LT, Onuma OK, Spiro C, Therneau TM, et al. (2008). Statistical analysis of relative labeled mass spectrometry data from complex samples using ANOVA. J. Proteome Res 7, 225–233. 10.1021/pr700734f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, and Smyth GK (2015). limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43, e47–e47. 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Robinson MD, McCarthy DJ, and Smyth GK (2009). edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McCarthy DJ, Chen Y, and Smyth GK (2012). Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res 40, 4288–4297. 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Y, Parmigiani G, and Johnson WE (2020). ComBat-seq: batch effect adjustment for RNA-seq count data. NAR Genomics Bioinforma 2, 1–10. 10.1093/nargab/lqaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang W, Vilella F, Alama P, Moreno I, Mignardi M, Isakova A, Pan W, Simon C, and Quake SR (2020). Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat. Med 26, 1644–1653. 10.1038/s41591-020-1040-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Single cell RNA and Bulk RNA sequencing data have been deposited into the database of Genotypes and Phenotypes (dbGAP) and are available under dbGaP accession phs003326.v1.p1. Proteomics data was deposited into PRIDE, and processed data into the Gene Expression Omnibus (GEO). All data and links are being compiled into a central study site, which can be accessed here: “Integrating endometrial proteomic and single cell transcriptomic pipelines reveals distinct menstrual cycle and endometriosis-associated molecular profiles”: https://fairdomhub.org/studies/1139. Accession numbers are listed in the key resources table.
Imaging data have been deposited at Omero, an Open microscopy image repository client-specific repository, and bioassay data are publicly available as of the date of publication and can be found in https://fairdomhub.org/studies/1139.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Vimentin | abcam | ab8978 |
| DAPI | ThermoFischer | R37606 |
| AlexaFluor™ 488 phalloidin | ThermoFischer | R37110 |
| EpCAM | Abcam | ab7504 |
| Laminin (LMN) | Abcam | ab11575 |
| Cleaved Caspase-3 | Abcam | ab2302 |
| Progesterone receptor (PGR) | Abcam | ab16661 |
| Progestagen associated endometrial protein (PAEP) | Abcam | ab270454 |
| LiveDead Viability Kit | Invitrogen | R37601 |
| acetylated 𝛼-Tubulin- AF594 | Abcam | ab195889 |
| Ki67 | Abcam | ab15580 |
| Vimentin-AF594 | Abcam | ab154207 |
| Vimentin-AF647 | Abcam | ab195878 |
| Goat pAb to Rb IgG-AF594 | Abcam | ab150080 |
| Goat pAb to Ms IgG-AF647 | Abcam | ab150115 |
| smooth muscle actin (SMA) | Invitrogen | MA-37027 |
| E-cad | R&D | AF748 |
| ESRalpha (ESR1) | Abcam | ab16660 |
| EpCAM | Abcam | ab218448 |
| Bacterial and virus strains | ||
| N/A | ||
| Biological samples | ||
| Human uterine tissues | This paper | Protocol number IRB-P001994 |
| Chemicals, peptides, and recombinant proteins | ||
| Mifepristone (RU-486) | Sigma | M8046 |
| 17-β estradiol | Sigma | 50-28-2 |
| Medroxyprogesterone acetate (MPA) | Sigma | 71-58-9 |
| PEG-20 (8-arm 20kDa PEG-VS) | Jenkem | 8ARM(TP)-VS |
| XL-IA | (Ac)-GCRD-LPRTG-GPQGIAGQ-DRCG-(Am) | Custom peptide Boston Open Labs (Cambridge, MA), GenScript (Piscataway, NJ), or CPC Scientific (Sunnyvale, CA). |
| PHSRN-K-RGD | (Ac)-PHSRNGGGK-(GGG-ERCG-(Am))-GGRGDSPY-(Am) | Custom peptide Boston Open Labs (Cambridge, MA), GenScript (Piscataway, NJ), or CPC Scientific (Sunnyvale, CA). |
| GFOGER | ‘‘(Ac)-GGYGGGPG(GPP)5GFOGER(GPP)5GPC-(Am)[45,46] | Custom peptide Boston Open Labs (Cambridge, MA), GenScript (Piscataway, NJ), or CPC Scientific (Sunnyvale, CA). |
| FN-binder | (Am)-GCRE-TLQPVYEYMVGV-(Ac) | Custom Peptide Boston Open Labs (Cambridge, MA), GenScript (Piscataway, NJ), or CPC Scientific (Sunnyvale, CA). |
| Critical commercial assays | ||
| Click-iT™ EdU Cell Proliferation Kit | Thermo Fisher | C10424-647 |
| Directzol RNA Mini-Prep kit | Zymo Research | R2051 |
| High-Capacity RNA-to-cDNA Kit | ThermoFisher Scientific | 4387406 |
| PureLink DNase Set | Thermofisher Scientific | 12185010 |
| Prolactin ELISA duoset | R&D Systems/Fisher | DY682 |
| Matrigel Phenol free | Fisher/Corning | 356231 |
| TMT10plex kit | Pierce | 90110 |
| barcoded mRNA capture beads | ChemGenes | N/A |
| Human XL Cytokine Luminex Performance Panel Premixed Kit | R&D Systems | LKTM014 |
| Luminex Performance Human MMP Magnetic Panel | R&D Systems | LMPM000 |
| Maxima H Minus Reverse Transcriptase | ThermoFisher | EP0751 |
| KAPA Hifi PCR Mastermix | Kappa Biopsystems | KR0368 |
| Deposited data | ||
| Bulk RNAseq data and single cell RNA sequencing data | This paper, and Baugh and Goods et al.44 | https://fairdomhub.org/studies/1139; dbGAP Accession number is phs003326.v1.p1. |
| Raw imaging data | This paper. | https://fairdomhub.org/studies/1139 |
| Proteomics | Baugh and Goods et al.44 | https://fairdomhub.org/studies/1139 |
| Experimental models: Cell lines | ||
| Human: Primary endometrial epithelial cells (EEOs) | This paper | Protocol number IRB-P001994 |
| Human: Primary endometrial stromal cells (ESCs) | This paper | Protocol number IRB-P001994 |
| Experimental models: Organisms/strains | ||
| N/A | ||
| Oligonucleotides | ||
| GAPDH | Hs02786624_g1 | 157 |
| PGR | Hs01556702_m1 | 77 |
| ESR1 | Hs01046816_m1 | 65 |
| PAEP | Hs01046123_g1 | 66 |
| ITGA2 | Hs00158127_m1 | 67 |
| ITGA5 | Hs01547673_m1 | 54 |
| ITGB4 | Hs00236216_m1 | 65 |
| ITGA6 | Hs01041011_m1 | 64 |
| ITGA3 | Hs01076879_m1 | 85 |
| ITGA1 | Hs00235006_m1 | 87 |
| ITGB2 | Hs00164957_m1 | 76 |
| ITGAV | Hs00233808_m1 | 64 |
| ITGA8 | Hs00233321_m1 | 89 |
| ITGB1 | Hs01127536_m1 | 74 |
| Recombinant DNA | ||
| N/A | ||
| Software and algorithms | ||
| ImageJ | Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/, 1997-2018. | https://imagej.nih.gov/ij/ |
| Imaris (RRID:SCR_007370) | Imaris 9.7 (Bitplane) | http://www.bitplane.com/imaris/imaris |
| GraphPad Prism (RRID:SCR_002798) | Graph pad Prism 8 | https://www.graphpad.com/ |
| Other | ||
| N/A | ||


