Abstract
Purpose:
The purpose of this study was to investigate the effect of integrated evaluation of resting static computed tomography perfusion (CTP) and coronary computed tomography angiography (CCTA)–derived fractional flow reserve (FFRCT) on therapeutic decision-making and predicting major adverse cardiovascular events (MACEs) in patients with suspected coronary artery disease.
Materials and Methods:
In this post hoc analysis of a prospective trial of CCTA in patients assigned to either CCTA or CCTA plus FFRCT arms, 500 patients in the CCTA plus FFRCT arm were analyzed. Both resting static CTP and FFRCT were evaluated by using the conventional CCTA. Perfusion defects in the myocardial segments with ≥50% degree of stenosis in the supplying vessels were defined as resting static CTP positive, and any vessel with an FFRCT value of ≤0.80 was considered positive. Patients were divided into 3 groups: (1) negative CTP-FFRCT match group (resting static CTP-negative and FFRCT-negative group); (2) mismatch CTP-FFRCT group (resting static CTP-positive and FFRCT-negative or resting static CTP-negative and FFRCT-positive group); and (3) positive CTP-FFRCT match group (resting static CTP-positive and FFRCT-positive group). We compared the revascularization-to-invasive coronary angiography ratio and the MACE rate among 3 subgroups at 1- and 3-year follow-ups. The adjusted Cox hazard proportional model was used to assess the prognostic value of FFRCT and resting static CTP to determine patients at risk of MACE.
Results:
Patients in the positive CTP-FFRCT match group were more likely to undergo revascularization at the time of invasive coronary angiography compared with those in the mismatch CTP-FFRCT group (81.4% vs 57.7%, P=0.033) and the negative CTP-FFRCT match group (81.4% vs 33.3%, P=0.001). At 1- and 3-year follow-ups, patients in the positive CTP-FFRCT match group were more likely to have MACE than those in the mismatch CTP-FFRCT group (10.5% vs 4.2%, P=0.046; 35.6% vs 9.4%, P<0.001) and the negative CTP-FFRCT match group (10.5% vs 0.9%, P<0.001; 35.6% vs 5.4%, P<0.001). A positive CTP-FFRCT match was strongly related to MACE at 1-year (hazard ratio=8.06, P=0.003) and 3-year (hazard ratio=6.23, P<0.001) follow-ups.
Conclusion:
In patients with suspected coronary artery disease, the combination of FFRCT with resting static CTP could guide therapeutic decisions and have a better prognosis with fewer MACE in a real-world scenario.
Key Words: myocardial ischemia, fractional flow reserve, perfusion, coronary artery disease, computed tomography angiography
Coronary artery disease (CAD) is a primary cause of death around the world.1 Invasively physiology-guided coronary revascularization (REV) remains a mainstay of treatment for CAD. Evaluation of the functional significance of a coronary lesion rather than the anatomic severity of coronary stenosis is an important step in guiding decisions of REV and determining prognosis. Invasive fractional flow reserve (FFR) is the accepted gold standard for the assessment of the hemodynamic severity of CAD and has been shown to improve clinical outcomes during REV.2 However, due to its invasiveness and high cost, its clinical application is limited to patients with suspected CAD. Importantly, primary results of the International Study of Comparative Health Effectiveness With Medical and Invasive Approaches (ISCHEMIA trial) found no statistical evidence of a benefit of the invasive strategy on ischemic cardiovascular events or death among patients with stable CAD.3 Thus, guidelines recommend that noninvasive functional imaging tests will be more valuable in evaluating the hemodynamic significance of coronary stenoses to reduce the number of patients without obstructive CAD, who undergo invasive coronary angiography (ICA).2,4,5
With advances in computed tomography (CT) systems and postprocessing techniques, CT-based noninvasive functional imaging methods, such as coronary computed tomography angiography (CCTA)–derived FFR (FFRCT) and CT myocardial perfusion imaging, have emerged as recently developed methods for the functional assessment of CAD.6 As shown in our previous studies, many patients with FFRCT≤0.80 had no poor clinical outcomes during 1- and 2-year follow-ups.7,8 The combination of FFRCT and stress dynamic computed tomography perfusion (CTP) has shown promise for further improving the accuracy of CCTA in the evaluation of stable chest pain and is associated with clinical outcomes.9,10 However, stress dynamic CTP requires stress-inducing pharmacology with some potential contraindications, increased scanner hardware requirements, and higher radiation exposure. Compared with stress dynamic CTP, resting static CTP has the potential to become the most widely used imaging modality for the evaluation of myocardial perfusion in the routine clinical setting because every CCTA can be used for mining resting static CTP data without the aforementioned shortcomings of dynamic stress CTP.11,12 However, to date, the clinical impact of the combination of resting static CTP and FFRCT remains unknown.
Therefore, we hypothesize that combined FFRCT and resting static CTP can provide incremental value in patients with CAD by allowing a 1-stop-shop anatomical and functional evaluation.11 The purpose of this study was to evaluate the effect of integrated FFRCT and resting static CTP on guiding therapeutic management and predicting adverse outcomes in patients with CAD in a real-world scenario.
MATERIALS AND METHODS
Subjects
This trial was a single-center prospective 2-arm controlled study of CCTA in patients with suspected CAD referred for CCTA as the first-line diagnostic test. The primary results have been reported previously.7 In brief, 1184 patients with 25% to 80% coronary stenosis on CCTA were assigned to anatomical CCTA alone (CCTA alone arm, n=593) or anatomical CCTA plus FFRCT group (FFRCT arm, n=591). This secondary post hoc study analyzed the data in the FFRCT arm. Of the 1184 enrolled patients, 591 patients were assigned to the FFRCT group, and 566 were successfully sent for FFRCT analysis.
CCTA Examination and Image Analysis
CCTA was performed using a dual-source CT scanner (Somatom Definition Flash; Siemens Healthineers) in all 566 patients according to the societal guideline.13 CCTA was performed using prospective electrocardiographic triggering at 30% to 80% of the R-R interval. The scan parameters are given as follows: tube voltage: 100 to 120 kVp; effective tube current: 370 mAs; detector collimation: 64×2×0.6 mm; and temporal resolution: 75 ms; 60 mL of iopromide (Ultravist 370 mg I/mL; Bayer Schering Pharma) was injected into an antecubital vein with a flow rate of 4 to 5 mL/s. Then, 40 mL saline was injected with the same flow rate.
All CCTA images were transferred to a dedicated workstation (Syngo.Via; Siemens) for image postprocessing. CCTA studies were interpreted independently by 2 observers (Long Jiang Zhang and Hong Yan Qiao, with 22 and 10 y of experience in CCTA, respectively) with a half-day interval, blinded to all participants’ clinical data and all other modalities. The readers were able to access various image postprocessing and display tools. Maximal degree of stenosis (DS) was recorded as follows: mild (25% to 49%), moderate (50% to 69%), and severe (≥70%). A ≥50% DS was considered as obstructive CAD recommended by the Society of Cardiovascular Computed Tomography on a per-participant basis.14
FFRCT Measurement
FFRCT calculations were blindly and independently conducted on CCTA datasets using a software prototype (cFFR, version 3.0.1; Siemens Healthcare). The software is based on the machine learning platform for the noninvasive computation of FFR values using existing CCTA data, and the detailed algorithm has been reported in previous studies.7,15,16 An observer (Hong Yan Qiao with 5 y of experience in FFRCT analysis), who was blinded to all participants’ baseline clinical data, measured lesion-specific FFRCT values at 2 to 4 cm distal to coronary stenosis in real time. Any vessel with an FFRCT value of ≤0.80 was considered positive.17 Additional FFRCT-positive cutoff values of ≤0.75 and ≤0.70 were also used for this subgroup analysis, respectively. A gray zone was defined for the range of FFRCT>0.70≤0.80.18 Interobserver agreement was evaluated by selecting 100 consecutive subjects analyzed by the same observers (Long Jiang Zhang and Hong Yan Qiao).7 Excellent interobserver agreement of the FFRCT analysis was reported (intraclass correlation coefficients [ICCs]=0.82, 95% CI: 0.78-0.86, and P<0.001) in our previous study.7
Resting Static CTP Studies
All resting static CTP were reconstructed using routine CCTA datasets and analyzed using a resting static CTP software prototype (CT Cardiac Function, Syngo.Via Frontier; Siemens). The scientific basis of this resting static CTP evaluation has been described previously.19 This software allows fully automatic segmentation of the left ventricle for noninvasive evaluation of myocardial perfusion using 2 series of CCTA data (both diastole and systole phases). Polar maps were automatically generated. Resting static CTP images were evaluated according to a standard 17-segment model by visual analysis according to the American Heart Association (AHA) myocardial segment model.20 One reader (Su Yu Li with 2 y of experience in CCTA) independently evaluated the resting static CTP for evaluation of perfusion defects, blinded to the clinical and FFRCT findings. Perfusion defects were defined as resting static CTP positive when a red overlay in the myocardium polar map had ≥50% DS in the corresponding supplying vessels.12,19 We evaluated interobserver agreement by selecting 60 consecutive subjects analyzed by 2 observers (Su Yu Li and Rui Zuo, with 2 and 1 y of experience in CCTA, respectively). The analysis was repeated after 6 months to calculate intraobserver variability by the same observer (Su Yu Li) blinded to the identities of the patients and the timing of the studies.
Evaluation of CCTA Combined With FFRCT and Resting Static CTP
All patients were classified into 3 subgroups according to resting static CTP and FFRCT results: (1) negative CTP-FFRCT match group, that is, resting static CTP-negative and FFRCT-negative group; (2) mismatch CTP-FFRCT group, that is, resting static CTP-positive and FFRCT-negative or resting static CTP-negative and FFRCT-positive group; and (3) positive CTP-FFRCT match group, that is, resting static CTP-positive and FFRCT-positive group. For the FFRCT analysis, we adjusted FFRCT thresholds of ≤0.80, ≤0.75, and ≤0.70 to observe the interaction of myocardial perfusion and hemodynamic significance of coronary stenosis, respectively.
Clinical Endpoints
Clinical follow-ups were conducted at 90 days, 1, and 3 years after enrollment. The primary endpoint was the REV-to-ICA ratio at 90 days. The secondary endpoint was the occurrence of major adverse cardiovascular events (MACEs), including all-cause mortality, nonfatal myocardial infarction, and acute coronary syndrome (ACS) leading to unplanned REV and stroke at 1- and 3-year follow-ups.5,7
Statistical Analysis
IBM SPSS, version 21.0 (SPSS) and MedCalc 3.0 (MedCalc Software) were used for the statistical analyses. Continuous data were presented as mean±SD or median (interquartile range), as appropriate. Categorical data were presented as numbers or proportions. We calculated the power of this study using Power Analysis and Sample Size (PASS) 2008 Statistical Software (version 15.0). Based on this, a sample size of 488 patients can provide 100% power at a 1-year follow-up, and 402 patients can provide 100% power at a 3-year follow-up (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/JTI/A259). Interobserver and intraobserver agreements of resting static CTP were analyzed with ICC analysis. The nonobstructive CAD rate and the REV-to-ICA ratio were compared among groups with the χ2 or Fisher exact test. The incidence of MACE was compared using the Chi-square or Fisher exact test. The ICA rates and incidence of MACE were analyzed when the participants were divided into 3 subgroups according to FFRCT values (≤0.70, >0.70≤0.80, and >0.80). Cumulative probabilities of MACE were compared among the negative CTP-FFRCT match group, the mismatch CTP-FFRCT group, and the positive CTP-FFRCT match groups by using the Kaplan-Meier analysis and the log-rank test. The effect size of 3 groups on MACE was analyzed with a Cox proportional hazard survival model, adjusting for age, sex, hypertension, diabetes mellitus, hyperlipidemia, present smoking, and presenting chest pain symptoms at baseline with a P-value <0.10 in univariate comparisons. Relative risks were expressed as a hazard ratio (HR) with 95% CIs. A 2-sided level of P-value<0.05 was considered statistically significant.
RESULTS
Baseline Patient Characteristics
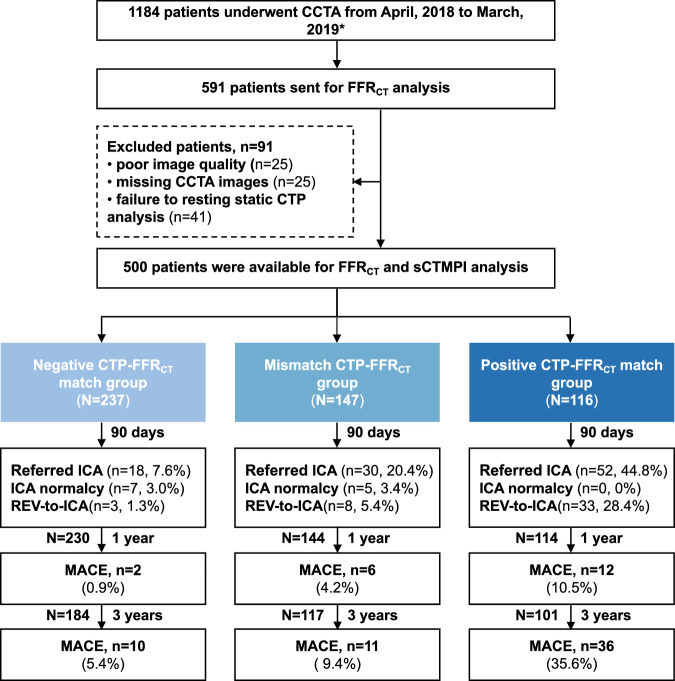
A total of 66 (11.7%, 66/566) patients were excluded due to failure to conduct resting static CTP calculations (n=41) and incomplete CCTA datasets (n=25), and 500 patients were included in this substudy (Fig. 1). The patients’ demographics are provided in Table 1. Patients had a mean age of 62.4±10.6 years and 67.8% were male. Of the 500 patients, with FFRCT ≤0.80 as the cutoff value, 47.4% (n=237) were in the negative CTP-FFRCT match group, 29.4% (n=147) in the mismatch CTP-FFRCT group, and 23.2% (n=116) in the positive CTP-FFRCT match group. With FFRCT≤0.80 as the cutoff value, patients in the positive CTP-FFRCT match group were more likely to present with ≥50% DS and ≥70% DS compared with those in the mismatch CTP-FFRCT group (98.3% vs 70.7%, P<0.001; 70.7% vs 7.0%, P<0.001, respectively) and those in the negative CTP-FFRCT match group (47.4% vs 9.5%, P<0.001; 9.5% vs 0%, P<0.001, respectively) compared with those in the positive CTP-FFRCT match group. One-year follow-up was successfully obtained in 488 patients (97.6%) and 3-year follow-up in 402 (80.4%) patients (Fig. 1). Excellent interobserver and intraobserver reproducibilities for resting static CTP were shown with ICCs (95% CI) of 0.967 (0.865-0.992) and 0.980 (0.901-0.995), respectively.
FIGURE 1.
Study flowchart. *The prospective cohort screening 2677 patients undergoing CCTA with 1493 patients excluded due to coronary stenosis <25% or >80% (n=1242), declined to participate (n=53), prior coronary stents (n=101), and poor image quality (n=97), and 1184 patients were recruited finally.
TABLE 1.
Baseline Characteristics of Participants
| Variables | All | Negative CTP-FFRCT match group (n=237) | Mismatch CTP-FFRCT group (n=147) | Positive CTP-FFRCT match group (n=116) | P |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Age (mean±SD) (y) | 62.4±10.6 | 61.5±10.5 | 63.0±11.4 | 63.3±10.6 | 0.236 |
| Sex (men) | 339 (67.8) | 150 (63.3) | 100 (68.0) | 89 (76.7) | 0.040 |
| Diabetes | 117 (23.4) | 52 (21.9) | 36 (24.5) | 29 (25.0) | 0.762 |
| Hypertension | 317 (63.4) | 149 (62.9) | 95 (64.6) | 73 (62.9) | 0.935 |
| Smokers | 124 (24.8) | 62 (26.2) | 31 (26.7) | 31 (26.7) | 0.460 |
| Hypercholesterolemia | 217 (43.4) | 109 (46.0) | 61 (41.5) | 47 (40.5) | 0.533 |
| Chest pain | 449 (89.8) | 205 (86.5) | 139 (94.6) | 105 (90.5) | 0.106 |
| Typical angina | 91 (18.2) | 41 (17.3) | 26 (17.7) | 24 (20.7) | 0.727 |
| Atypical angina | 215 (43.0) | 92 (38.8) | 67 (45.6) | 56 (48.3) | 0.182 |
| Nonanginal chest pain | 50 (10.0) | 22 (9.3) | 18 (12.2) | 10 (8.6) | 0.548 |
| Dyspnea/palpitation | 91 (18.2) | 50 (21.1) | 26 (17.7) | 15 (12.9) | 0.036 |
| ≥50% DS | 234 (46.8) | 16 (7.0) | 104 (70.7) | 114 (98.3) | <0.001 |
| ≥70% DS | 69 (13.8) | 0 | 14 (9.5) | 55 (47.4) | <0.001 |
Values are mean±SD and n (%).
(1) Negative CTP-FFRCT match group (resting static CTP-negative and FFRCT-negative group); (2) mismatch CTP-FFRCT group (resting static CTP-positive and FFRCT-negative or resting static CTP-negative and FFRCT-positive group); and (3) positive CTP-FFRCT match group (resting static CTP-positive and FFRCT-positive group).
Impact of FFRCT and Resting Static CTP on Downstream Clinical Management
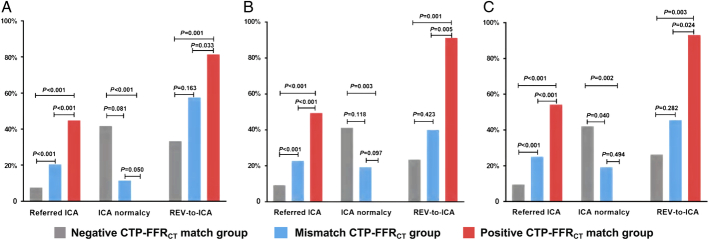
During the 90-day follow-up period, 20.4% (102/500) of the patients were referred for ICA. Of those referred patients, 79.4% (81/102) actually underwent ICA with 64.2% (52/81) undergoing subsequent coronary REV (percutaneous coronary intervention, n=46; coronary artery bypass grafting, n=6). With FFRCT≤0.80 as the cutoff value, patients in the positive CTP-FFRCT match group were more likely to be referred to ICA (44.8% vs 20.4%, P<0.001), actually receive ICA (37.1% vs 17.7%, P<0.001), and undergo REV at the time of ICA (81.4% vs 57.7%, P=0.033) compared with those in the mismatch CTP-FFRCT group (Table 2, Fig. 2A). Patients in the negative CTP-FFRCT match group were less likely to be referred to ICA (7.6% vs 44.8%, P<0.001), actually receive ICA (5.1% vs 37.1%, P<0.001), and undergo REV at the time of ICA (33.3% vs 81.4%, P=0.001) compared with those in the positive CTP-FFRCT match group. The nonobstructive CAD rate in ICA was the lowest in the positive CTP-FFRCT match group compared with those in the mismatch CTP-FFRCT group (0 vs 11.5%, P=0.050) and the negative CTP-FFRCT match group (0% vs 41.7%, P<0.001). No significant differences in the REV-to-ICA ratio (81.4% vs 57.7%, P=0.163) and the nonobstructive CAD rate in ICA (11.5% vs 41.7%, P=0.081) were found between the mismatch CTP-FFRCT group and the negative CTP-FFRCT match group (Table 2, Fig. 2A). Similar results were found when FFRCT-positive cutoff values were adjusted to ≤0.75 and ≤0.70 (Table 2, Figs. 2B, C).
TABLE 2.
Impact of FFRCT and Resting Static CTP on Therapeutic Decision-making
| n (%) | ||||
|---|---|---|---|---|
| Variables | Negative CTP-FFRCT match group | Mismatch CTP-FFRCT group | Positive CTP-FFRCT match group | P |
| Cutoff of FFRCT=0.80 | ||||
| N | 237 | 147 | 116 | |
| Referred ICA rate | 18 (7.6) | 30 (20.4) | 52 (44.8) | <0.001 |
| Actual ICA rate | 12 (5.1) | 26 (17.7) | 43 (37.1) | <0.001 |
| ICA normalcy rate* | 5 (41.7) | 3 (11.5) | 0 | <0.001 |
| REV-to-ICA ratio* | 4 (33.3) | 15 (57.7) | 35 (81.4) | <0.001 |
| Cutoff of FFRCT=0.75 | ||||
| N | 259 | 162 | 79 | |
| Referred ICA rate | 24 (9.3) | 37 (22.8) | 39 (49.4) | <0.001 |
| Actual ICA rate | 17 (6.6) | 30 (18.5) | 34 (43.0) | <0.001 |
| ICA Normalcy rate* | 5 (29.4) | 3 (10.0) | 0 | <0.001 |
| REV-to-ICA ratio* | 7 (41.2) | 16 (53.3) | 29 (85.3) | <0.001 |
| Cutoff of FFRCT=0.70 | ||||
| N | 274 | 167 | 59 | |
| Referred ICA rate | 26 (9.5) | 42 (25.1) | 32 (54.2) | <0.001 |
| Actual ICA rate | 19 (6.9) | 33 (19.8) | 29 (49.2) | <0.001 |
| ICA normalcy rate* | 6 (31.6) | 2 (6.1) | 0 | <0.001 |
| REV-to-ICA ratio* | 8 (42.1) | 19 (57.6) | 25 (86.2) | <0.001 |
(1) Negative CTP-FFRCT match group (resting static CTP-negative and FFRCT-negative group); (2) mismatch CTP-FFRCT group (resting static CTP-positive and FFRCT-negative or resting static CTP-negative and FFRCT-positive group); (3) positive CTP-FFRCT match group (resting static CTP-positive and FFRCT-positive group).
The denominator is the number of patients in whom ICA was actually performed.
FIGURE 2.
Ninety-day outcomes of different CTP-FFRCT groups. A, FFRCT-positive cutoff value was ≤0.80. B, FFRCT-positive cutoff value was adjusted to ≤0.75. C, FFRCT-positive cutoff value was adjusted to ≤0.70.
When the patients were divided into 3 subgroups according to FFRCT values (≤0.70, the gray zone, and >0.80), the referred and actual rates for ICA and the REV-to-ICA ratios were higher in patients with positive resting static CTP compared with those with negative resting static CTP (Supplemental Table 2, Supplemental Digital Content 2, http://links.lww.com/JTI/A260). The nonobstructive CAD rate in ICA was slightly lower among those in the positive resting static CTP group than in those in the negative resting static CTP group among all 3 subgroups (Supplemental Table 2, Supplemental Digital Content 2, http://links.lww.com/JTI/A260); however, these differences were not significant (all P>0.05) (Supplemental Table 2, Supplemental Digital Content 2, http://links.lww.com/JTI/A260).
MACEs
MACE occurred in 20 patients (4.1%) at a 1-year follow-up and 57 patients (14.2%) at a 3-year follow-up. We analyzed all clinical factors and imaging parameters with potential association with MACEs. In univariable analysis, we found that DS ≥ 50% (HR=3.40; 95% CI: 1.88-6.14; and P<0.001), FFRCT (HR=5.03; 95% CI: 2.74-9.23; and P<0.001), and resting static CTP (HR=3.30; 95% CI: 1.86-5.84; P<0.001) were all significant predictors for MACE at 3-year follow-up, which were subsequently included in multivariable logistic regression analysis. According to multivariable logistic regression analysis, only FFRCT (HR=3.77; 95% CI: 1.93-7.35; and P<0.001) was identified as an independent risk factor for 3-year MACE. Similar results were found at 1-year follow-up. The details of logistic regression analysis are presented in Supplemental Table 3 (Supplemental Digital Content 3, http://links.lww.com/JTI/A261). Resting static CTP, DS ≥ 50%, and FFRCT achieved a c-index of 0.65, 0.70, and 0.74 to predict MACE, respectively. Compared with FFRCT, adding resting static CTP to FFRCT resulted in a numerically higher c-index for predicting all causes of MACE, but there was no statistical difference (c-index: 0.75 and P=0.331) (Supplemental Table 4, Supplemental Digital Content 4, http://links.lww.com/JTI/A262).
MACE rates were higher in patients in the positive CTP-FFRCT match group compared with those in the mismatch CTP-FFRCT group at 1-year (10.5% vs 4.2%, P=0.046) and 3-year follow-ups (35.6% vs 9.4%, P<0.001) (Table 3, Fig. 3). Patients in the positive CTP-FFRCT match group were more likely to have MACE than those in the negative CTP-FFRCT match group at 1-year (10.5% vs 0.9%, P<0.001) and 3-year follow-ups (35.6% vs 5.4%, P<0.001) (Table 3, Fig. 3). Similar results were found when FFRCT thresholds were adjusted to 0.75 and 0.70 (all P<0.05) (Table 3, Fig. 3).
TABLE 3.
Impact of FFRCT and Resting Static CTP on MACE at 1- and 3-Year Follow-ups
| 1-y follow-up, n (%) | 3-y follow-up, n (%) | |||||
|---|---|---|---|---|---|---|
| Variables | Negative CTP-FFRCT match group | Mismatch CTP-FFRCT group | Positive CTP-FFRCT match group | Negative CTP-FFRCT match group | Mismatch CTP-FFRCT group | Positive CTP-FFRCT match group |
| Cutoff of FFRCT=0.80 | ||||||
| N | 230 | 144 | 114 | 184 | 117 | 101 |
| MACE* | 2 (0.9) | 6 (4.2) | 12 (10.5) | 10 (5.4) | 11 (9.4) | 36 (35.6) |
| All-Cause Death | 1 (0.4) | 0 | 0 | 4 (2.2) | 4 (3.4) | 6 (5.9) |
| Nonfatal MI | 0 | 1 (0.7) | 3 (2.6) | 4 (2.2) | 1 (0.9) | 6 (5.9) |
| Unplanned REV | 1 (0.4) | 5 (3.5) | 9 (7.9) | 2 (1.1) | 5 (4.3) | 17 (16.8) |
| Stroke | 0 | 0 | 0 | 0 | 1 (0.9) | 7 (6.9) |
| Cutoff of FFRCT=0.75 | ||||||
| N | 252 | 148 | 78 | 205 | 129 | 68 |
| MACE* | 3 (1.2) | 7 (4.7) | 10 (12.8) | 12 (5.9) | 18 (14.0) | 27 (39.7) |
| All-Cause Death | 1 (0.4) | 0 (0.0) | 0 (0.0) | 4 (2.0) | 4 (3.1) | 6 (8.8) |
| Nonfatal MI | 0 | 1 (0.7) | 3 (3.8) | 4 (2.0) | 2 (1.5) | 5 (7.4) |
| Unplanned REV | 2 (0.8) | 6 (4.1) | 7 (9.0) | 3 (1.5) | 8 (6.2) | 13 (19.1) |
| Stroke | 0 | 0 | 0 | 1 (0.5) | 4 (3.1) | 3 (4.4) |
| Cutoff of FFRCT=0.70 | ||||||
| N* | 267 | 162 | 59 | 218 | 132 | 52 |
| MACE | 4 (1.5) | 7 (4.3) | 9 (15.3) | 14 (6.4) | 19 (14.4) | 24 (46.2) |
| All-cause death | 1 (0.4) | 0 | 0 | 5 (2.3) | 4 (3.0) | 5 (9.6) |
| Nonfatal MI | 1 (0.4) | 0 | 3 (5.1) | 5 (2.3) | 2 (1.5) | 4 (7.7) |
| Unplanned REV | 2 (0.7) | 7 (4.3) | 6 (10.2) | 3 (1.4) | 9 (6.8) | 12 (23.1) |
| Stroke | 0 | 0 | 0 | 1 (0.5) | 4 (3.0) | 3 (5.8) |
Twelve patients were excluded due to follow-up loss during a 1-year follow-up, and 98 patients were excluded due to follow-up loss during a 3-year follow-up. Thus, 488 and 402 patients were finally included in the final 1- and 3-year follow-up cohorts, respectively.
MI indicates myocardial infarction.
FIGURE 3.
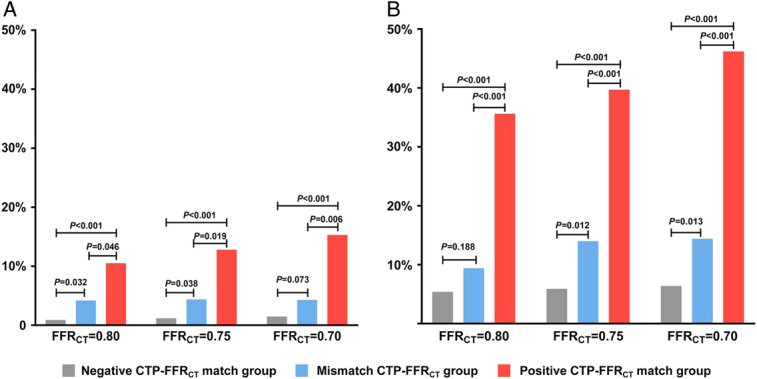
MACE rate at 1- and 3-year follow-ups in FFRCT subgroups. A, Comparison of MACE rate in 3 subgroups at 1-year follow-up. B, Comparison of MACE rate in 3 subgroups at 3-year follow-up.
When FFRCT thresholds were adjusted to 0.75 and 0.70, the differences were more significant between the mismatch CTP-FFRCT group and the negative CTP-FFRCT match group at 3-year follow-up (all P<0.05). The rates of ACS leading to unplanned REV in the positive CTP-FFRCT match group were the highest among all causes of MACE when FFRCT-positive cutoff values were adjusted to 0.80, 0.75, and 0.70 (Table 3).
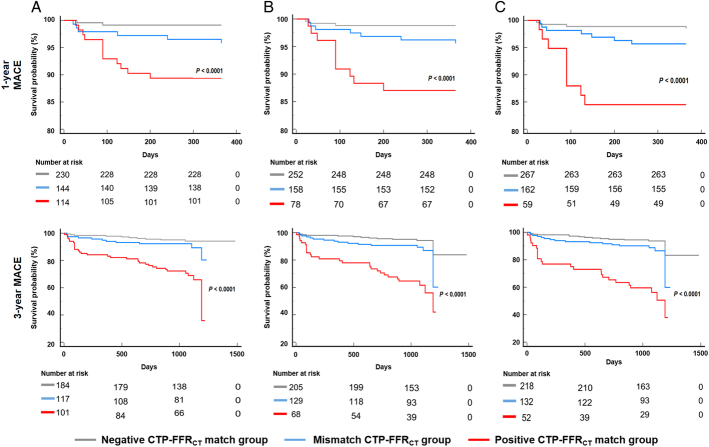
In multivariate Cox regression models adjusting for age, sex, hypertension, diabetes mellitus, hyperlipidemia, present smoking, and presenting chest pain symptoms, patients in the positive CTP-FFRCT match group were strongly predisposed to MACE compared with patients in the negative CTP-FFRCT match group during 1-year (HR=8.06; 95% CI: 2.22-29.27; and P=0.003) and 3-year (HR=6.23; 95% CI: 3.04-12.79; and P<0.001) follow-ups (Table 4, Fig. 4). Similar results were found when the FFRCT-positive cutoff values were adjusted to FFRCT thresholds of 0.75 and 0.70 (Table 4, Fig. 4). Representative cases are illustrated in Figure 5.
TABLE 4.
Multivariate Cox Regression Analyses for the Association Between Hemodynamic Metrics of Coronary Stenoses and 1- and 3-Year Outcomes
| 1-y MACE (N=488)* | 3-y MACE (N=402)* | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P | HR (95% CI) | P |
| Cutoff of FFRCT=0.80 | ||||
| Negative CTP-FFRCT match group | Reference | Reference | ||
| Mismatch CTP-FFRCT group | 2.35 (0.56-9.92) | 0.081 | 1.52 (0.64-3.63) | 0.342 |
| Positive CTP-FFRCT match group | 8.06 (2.22-29.27) | 0.003 | 6.23 (3.04-12.79) | <0.001 |
| Cutoff of FFRCT=0.75 | ||||
| Negative CTP-FFRCT match group | Reference | Reference | ||
| Mismatch CTP-FFRCT group | 2.98 (0.75-11.75) | 0.119 | 1.74 (0.82-3.69) | 0.148 |
| Positive CTP-FFRCT match group | 8.76 (2.31-33.19) | 0.001 | 8.37 (4.15-16.89) | <0.001 |
| Cutoff of FFRCT=0.70 | ||||
| Negative CTP-FFRCT match group | Reference | Reference | ||
| Mismatch CTP-FFRCT group | 2.29 (0.66-7.93) | 0.193 | 1.70 (0.84-3.44) | 0.143 |
| Positive CTP-FFRCT match group | 8.45 (2.40-29.74) | 0.001 | 9.35 (4.67-18.72) | <0.001 |
Adjusted for age, sex, diabetes, hypertension, smoking, hypercholesterolemia, typical angina, atypical angina, nonanginal chest pain, and dyspnea.
FIGURE 4.
Kaplan-Meier survival curves showing MACEs at 1 and 3 years. A, FFRCT thresholds of 0.80. B, FFRCT thresholds of 0.75. C, FFRCT thresholds of 0.70. MACE: all-cause mortality, myocardial infarction, ACS leading to unplanned REV, and stroke.
FIGURE 5.
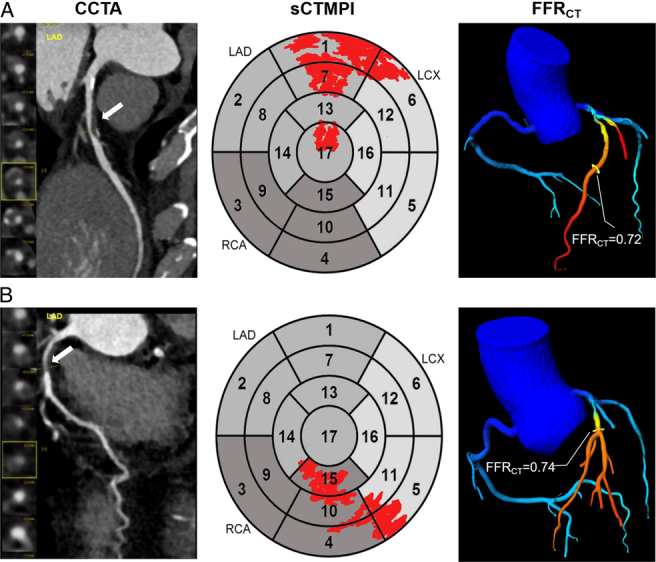
Representative cases with CCTA, FFRCT, and resting static CTP resting static CTP. A, A 62-year-old man experienced a nonfatal myocardial infarction at a 1-year follow-up. CCTA with multiplanar reformation shows severe stenosis in the proximal segment of the LAD (arrow) with perfusion defects in segments 1 and 7 in resting static CTP resting static CTP and positive FFRCT (0.72). B, A 58-year-old woman had no MACE during a 3-year follow-up. CCTA with multiplanar reformation shows stenosis degree >50% in the proximal LAD (arrow) without perfusion defect in the corresponding myocardial segments in the polar map and positive FFRCT (0.74). LAD indicates left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery; sCTMPI, static computed tomography myocardial perfusion imaging.
According to different FFRCT cutoff values (≤0.70, the gray zone, and >0.80), patients with positive resting static CTP had a higher MACE rate than those with negative resting static CTP among all subgroups at one- and 3-year follow-ups (Supplemental Table 5, Supplemental Digital Content 5, http://links.lww.com/JTI/A263), respectively. At 3-year follow-up, the rate of MACE in patients with FFRCT ≤ 0.70 and positive resting static CTP was significantly higher than in those with FFRCT ≤ 0.70 and negative resting static CTP (46.2% vs 17.6%, P=0.047) (Supplemental Table 5, Supplemental Digital Content 5, http://links.lww.com/JTI/A263). No difference was found between positive resting static CTP and negative resting static CTP subgroups at one- and 3-year follow-ups in patients with the FFRCT values in the range of the gray zone (both P>0.05) (Supplemental Table 5, Supplemental Digital Content 5, http://links.lww.com/JTI/A263).
DISCUSSION
In this post hoc analysis of a prospective study of CCTA among patients with suspected CAD, we demonstrated the incremental value of the integration of FFRCT and resting static CTP, which allows functional perfusion evaluation for determining the downstream hemodynamic significance of lesions for guiding treatment decisions and evaluating the validity of stenosis-specific interventions in a real-world scenario. Our data suggest that combining resting static CTP with FFRCT may improve the efficiency of referral to ICA by increasing the rate of subsequent REVs. Importantly, we demonstrated that the integration of positive FFRCT and resting static CTP had a better predictive value for MACE than a mismatched FFRCT and resting static CTP or a negative FFRCT and resting static CTP.
Current guidelines recommend that CCTA is an excellent “gatekeeper” for ICA referral, especially by enhancing its value through methods such as FFRCT and CTP, which further improves the accuracy of CCTA by identifying candidates who might receive unnecessary ICA.4,21–24 Some studies highlighted the potential for FFRCT to increase the efficiency of subsequent coronary REV.8,25 In the CRESCENT II trial, adding CTP when CCTA revealed a >50% stenosis resulted in fewer ICA without a class I indication for REV. However, the integration of FFRCT and CTP was not included in previous studies.8,25–28 Although we did not find that resting static CTP alone had an independent prognosis value compared with FFRCT, this study did demonstrate that patients with FFRCT ≤0.80 and positive resting static CTP were substantially more likely to undergo REV compared with those in the resting static CTP-positive and FFRCT-negative or resting static CTP-negative and FFRCT-positive groups. Our findings further demonstrated that the hemodynamic significance of CAD evaluation was more important for mechanical REV by combining myocardial perfusion with the functional significance of coronary stenosis by FFRCT. Importantly, we directly analyzed the data from the conventional diagnostic CCTA datasets without concomitant increased contrast material use or radiation dose. However, in our study, 4 patients in the negative CTP-FFRCT match group underwent REV due to coronary stenosis of ≥80% in ICA and a chest pain symptom, which is rational for clinical management according to comprehensive phenotype. In a post hoc analysis of the PROMISE trial, adding information on FFRCT to CCTA improved the efficiency of referral to ICA by lowering the number of ICA and decreasing the nonobstructive CAD rate in ICA29 In our study, the integration of resting static CTP and FFRCT caused a modest net increase in referral for and actual use of ICA. This is notable because the resting static CTP results were not available to physicians during the trial, while the FFRCT results guided clinical management. Meanwhile, a higher REV efficiency was reported in the positive CTP-FFRCT match group. Thus, robust evidence-based recommendations await well-conducted prospective studies in this arena.
The prognostic value of the integration of both FFRCT and resting static CTP was demonstrated in our prospective cohort. Many previous studies have reported a definitive link between FFRCT and patient prognosis.24,30,31 Building on the 90-day experience,32 the 1-year clinical outcomes of the ADVANCE registry highlighted the favorable prognosis associated with a negative FFRCT (>0.80) with significantly lower cardiovascular death and myocardial infarction rate among those participants compared with those with a positive FFRCT (0.80% vs 0.20%; P=0.01).24 However, FFRCT only evaluates the hemodynamic significance of coronary stenosis rather than myocardial functional status. Thus, the combination of the hemodynamic significance of coronary stenosis and myocardial perfusion evaluation can provide incremental value in this clinical setting. We observed that the patients with matched positive resting static CTP and FFRCT results were more likely to suffer from MACE compared with those in the negative CTP-FFRCT match group at 1- and 3-year follow-ups (HR=8.06 and 6.23, and both P<0.05). Our observed MACE rates were higher than the ones in the ADVANCE registry (1.2%)24 and the CONSERVE study (4.6%).30 It can be explained that the patients who were both resting static CTP-positive and FFRCT-positive in our study represented cases with relatively severe myocardial ischemia. Meanwhile, invasive FFR was not utilized in our study, and no additional noninvasive functional tests were utilized subsequent to CCTA to help guide treatment decisions. Therefore, the benefits seen in the positive resting static CTP and FFRCT match group might be attributable to the ability to discriminate lesion-specific ischemia, allowing for optimal management, thus reducing the incidence of ACS leading to unplanned REV.
The additional value of resting static CTP over FFRCT might increase especially in patients in the “gray zone” of FFRCT, in which empirical watchful waiting for ICA is often chosen, due to the low prevalence of invasive FFR ≤0.80.18 Previous studies reported that the conventional cutoff value of FFRCT ≤0.80 and the range of gray zone were associated with low specificity and a high false-positive rate with invasive FFR ≤0.80 as the reference standard for hemodynamically significant ischemia.9,18,33,34 Our study found that a positive resting static CTP can slightly reduce the rate of ICA yielding nonobstructive CAD and increase the REV-to-ICA ratio in patients within the gray zone of FFRCT and FFRCT ≤0.70 but without statistical significance. The main reason may be that these patients with suspected CAD did not have relevant myocardial ischemia.
Our study has some limitations. First, this was a retrospective post hoc analysis of a single-center prospective 2-arm study, and the results of resting static CTP were not available to caregivers and did not affect clinical decision-making. Second, this prospective study enrolled patients with 25% to 80% coronary stenosis on CCTA; thus, our study results cannot be generalized to all CAD patients. Third, the rate of 3-year follow-up loss was relatively high; however, our study sample size was adequately powered. Finally, this study did not use stress CTP to evaluate myocardial ischemia because of the potentially severe complications of stress CTP. The use of resting static CTP is convenient and safe, especially in a real-world scenario.
In conclusion, this study shows that, in patients with CAD, the addition of resting static CTP to CCTA plus FFRCT resulted in an increased rate of subsequent coronary REV and a better prognosis with fewer MACE than FFRCT or resting static CTP alone. Multicenter prospective, randomized, controlled trials with long-term follow-up are needed to further validate our study results.
Supplementary Material
Footnotes
S.Y.L. and J.Z contributed equally to this work.
Supported by the National Natural Science Foundation of China (8217933 for Chun Xiang Tang).
The authors declare no conflict of interest.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.thoracicimaging.com.
Contributor Information
Su Yu Li, Email: 635752769@qq.com.
Jian Zhong, Email: dbzqzyyzj@163.com.
Hong Yan Qiao, Email: xiaoqiao986308@163.com.
U. Joseph Schoepf, Email: schoepf@musc.edu.
Tilman Emrich, Email: emrich@musc.edu.
W. Nicholas Butler, Email: butlewil@musc.edu.
Rui Zuo, Email: zuorui44456@163.com.
Yi Xue, Email: shellyxy0213@qq.com.
Ya Liu, Email: 1522002373@qq.com.
Li Yan Dai, Email: 1207989807@qq.com.
Chang Sheng Zhou, Email: njzhouyisheng@qq.com.
Guang Ming Lu, Email: cjr.luguangming@vip.163.com.
Chun Xiang Tang, Email: 522956587@qq.com.
Long Jiang Zhang, Email: kevinzhlj@163.com.
REFERENCES
- 1.Nowbar AN, Gitto M, Howard JP, et al. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12:e005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016;134:e123–e155. [DOI] [PubMed] [Google Scholar]
- 3.Maron DJ, Hochman JS, Reynolds HR, et al. ISCHEMIA research group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narula J, Chandrashekhar Y, Ahmadi A, et al. SCCT 2021 expert consensus document on coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2021;15:192–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hicks KA, Tcheng JE, Bozkurt B, et al. 2014 ACC/AHA Key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task force on clinical data standards (writing committee to develop cardiovascular endpoints data standards). Circulation. 2015;22:1041–1144. [DOI] [PubMed] [Google Scholar]
- 6.Patel P, Emrich T, Schoepf UJ, et al. Comprehensive computed tomography imaging of vessel-specific and lesion-specific myocardial ischemia. J Thorac Imaging. 2021. doi: 10.1097/RTI.0000000000000592. [DOI] [PubMed] [Google Scholar]
- 7.Qiao HY, Tang CX, Schoepf UJ, et al. One-year outcomes of CCTA alone versus machine learning-based FFRCT for coronary artery disease: a single-center, prospective study. Eur Radiol. 2022;32:5179–5188. [DOI] [PubMed] [Google Scholar]
- 8.Qiao HY, Tang CX, Schoepf UJ, et al. Impact of machine learning-based coronary computed tomography angiography fractional flow reserve on treatment decisions and clinical outcomes in patients with suspected coronary artery disease. Eur Radiol. 2020;30:5841–5851. [DOI] [PubMed] [Google Scholar]
- 9.Coenen A, Rossi A, Lubbers MM, et al. Integrating CT myocardial perfusion and CT-FFR in the work-up of coronary artery disease. JACC Cardiovasc Imaging. 2017;10:760–770. [DOI] [PubMed] [Google Scholar]
- 10.van Assen M, Duguay TM, Litwin SE, et al. The feasibility, tolerability, safety, and accuracy of low-radiation dynamic computed tomography myocardial perfusion imaging with regadenoson compared with single-photon emission computed tomography. J Thorac Imaging. 2021;36:345–352. [DOI] [PubMed] [Google Scholar]
- 11.Punzo B, Cavaliere C, Maffei E, et al. Narrative review of cardiac computed tomography perfusion: insights into static rest perfusion. Cardiovasc Diagn Ther. 2020;10:1946–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danad I, Szymonifka J, Schulman-Marcus J, et al. Static and dynamic assessment of myocardial perfusion by computed tomography. Eur Heart J Cardiovasc Imaging. 2016;17:836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr. 2016;10:435–449. [DOI] [PubMed] [Google Scholar]
- 14.Cury RC, Abbara S, Achenbach S, et al. CAD-RADS(TM) coronary artery disease—Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr. 2016;10:269–281. [DOI] [PubMed] [Google Scholar]
- 15.Zhang LJ, Tang C, Xu P, et al. Chinese Society of Radiology . Coronary computed tomography angiography-derived fractional flow reserve: an expert consensus document of Chinese society of radiology. J Thorac Imaging. 2022;37:385–400. [DOI] [PubMed] [Google Scholar]
- 16.Chen YC, Zhou F, Wang YN, et al. Optimal measurement sites of coronary-computed tomography angiography-derived fractional flow Reserve: The insight from China CT-FFR study. J Thorac Imaging. 2023;38:194–202. [DOI] [PubMed] [Google Scholar]
- 17.Nørgaard BL, Fairbairn TA, Safian RD, et al. Coronary CT angiography-derived fractional Flow reserve testing in patients with stable coronary artery disease: recommendations on interpretation and reporting. Radiol Cardiothorac Imaging. 2019;1:e190050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumura-Nakano Y, Kawaji T, Shiomi H, et al. Optimal cutoff value of fractional flow reserve derived from coronary computed tomography angiography for predicting hemodynamically significant coronary artery disease. Circ Cardiovasc Imaging. 2019;12:e008905. [DOI] [PubMed] [Google Scholar]
- 19.Patel AR, Bamberg F, Branch K, et al. Society of cardiovascular computed tomography expert consensus document on myocardial computed tomography perfusion imaging. J Cardiovasc Comput Tomogr. 2020;14:87–100. [DOI] [PubMed] [Google Scholar]
- 20.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. [DOI] [PubMed] [Google Scholar]
- 21.Brandt V, Schoepf UJ, Aquino GJ, et al. Impact of machine-learning-based coronary computed tomography angiography-derived fractional flow reserve on decision-making in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Radiol. 2022;32:6008–6016. [DOI] [PubMed] [Google Scholar]
- 22.Williams MC, Moss A, Nicol E, et al. Cardiac CT improves outcomes in stable coronary heart disease: results of recent clinical trials. Curr Cardiovasc Imaging Rep. 2017;10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams MC, Hunter A, Shah ASV, et al. Use of coronary computed tomographic angiography to guide management of patients with coronary disease. J Am Coll Cardiol. 2016;67:1759–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel MR, Nørgaard BL, Fairbairn TA, et al. 1-year impact on medical practice and clinical outcomes of FFRCT: The ADVANCE Registry. JACC Cardiovasc Imaging. 2020;13(pt 1):97–105. [DOI] [PubMed] [Google Scholar]
- 25.Lubbers M, Coenen A, Kofflard M, et al. Comprehensive cardiac CT with myocardial perfusion imaging versus functional testing in suspected coronary artery disease: the multicenter, randomized CRESCENT-II trial. JACC Cardiovasc Imaging. 2018;11:1625–1636. [DOI] [PubMed] [Google Scholar]
- 26.Schoepf UJ, van Assen M. FFR-CT and CT myocardial perfusion imaging: friends or foes? JACC Cardiovasc Imaging. 2019;12:2472–2474. [DOI] [PubMed] [Google Scholar]
- 27.Nørgaard BL, Terkelsen CJ, Mathiassen ON, et al. Coronary CT angiographic and flow reserve-guided management of patients with stable ischemic heart disease. J Am Coll Cardiol. 2018;72:2123–2134. [DOI] [PubMed] [Google Scholar]
- 28.Pontone G, Baggiano A, Andreini D, et al. Dynamic stress computed tomography perfusion with a whole-heart coverage scanner in addition to coronary computed tomography angiography and fractional flow reserve computed tomography derived. JACC Cardiovasc Imaging. 2019;12:2460–2471. [DOI] [PubMed] [Google Scholar]
- 29.Lu MT, Ferencik M, Roberts RS, Lee KL, et al. Noninvasive FFR derived from coronary CT angiography: management and outcomes in the PROMISE trial. JACC Cardiovasc Imaging. 2017;10:1350–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang HJ, Lin FY, Gebow D, et al. Selective referral using CCTA versus direct referral for individuals referred to invasive coronary angiography for suspected CAD: a randomized, controlled, open-label trial. JACC Cardiovasc Imaging. 2019;12(pt 2):1303–1312. [DOI] [PubMed] [Google Scholar]
- 31.Douglas PS, De Bruyne B, Pontone G, et al. 1-year outcomes of FFRCT-guided care in patients with suspected coronary disease: the PLATFORM study. J Am Coll Cardiol. 2016;68:435–445. [DOI] [PubMed] [Google Scholar]
- 32.Fairbairn TA, Nieman K, Akasaka T, et al. Real-world clinical utility and impact on clinical decision-making of coronary computed tomography angiography-derived fractional flow reserve: lessons from the ADVANCE registry. Eur Heart J. 2018;39:3701–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nørgaard BL, Hjort J, Gaur S, et al. Clinical use of coronary CTA-derived FFR for decision-making in stable CAD. JACC Cardiovasc Imaging. 2017;10:541–550. [DOI] [PubMed] [Google Scholar]
- 34.Kruk M, Wardziak Ł, Demkow M, et al. Workstation-based calculation of CTA-based FFR for intermediate stenosis. JACC Cardiovasc Imaging. 2016;9:690–699. [DOI] [PubMed] [Google Scholar]