Abstract
Background
Reduced choroidal perfusion is hypothesized to play a role in the pathogenesis of normal tension glaucoma. Thus the impact of antiglaucomatous eye drops on ocular perfusion has been the focus of recent research and the subject of intensive investigations. The present study investigates whether topically applied latanoprost or bimatoprost influence ocular perfusion in patients with normal tension glaucoma and compares these effects with that changes detected after the treatment with dorzolamide.
Methods
Ocular hemodynamics were assessed by color Doppler imaging (CDI) shortly before and after a one-month treatment with either latanoprost, bimatoprost or dorzolamide. Primary end-points of the study were peak systolic and end-diastolic blood flow velocities in the short posterior ciliary artery (SPCA) under the new therapy. Intraocular pressure (IOP) and additional perfusion parameters in the SPCA and other retrobulbar vessels were tracked as observational parameters. n = 42 patients with normal tension glaucoma were enrolled in the study.
Results
Systolic and diastolic blood flow velocities in the SPCA showed no significant alteration after the treatment with latanoprost or bimatoprost. Dorzolamide lead to increase of peak systolic velocity. IOP was reduced by all three agents in a range reported in the literature.
Conclusion
Topically applied latanoprost and bimatoprost act in a hemodynamically neutral manner and have the capability to lower IOP even in patients with normal tension glaucoma and low initial IOP level. Dorzolamide accelerates blood flow in systole. None of the tested compounds has a negative impact on hemodynamics in the short posterior ciliary arteries.
Background
Traditionally atrophy of the optic nerve head in glaucoma patients has been related to a chronically increased intraocular pressure (IOP).[1] In the majority of glaucoma patients increased IOP levels can be measured. At the same time there is a significant number of patients who develop the major features of glaucoma, meaning progressive visual field loss and atrophy of the optic nerve head, although the IOP is in the normal range below 21 mmHg in these individuals. [2-6] This variant of primary open angle glaucoma has been termed as normal tension glaucoma and the glaucomatous changes have been – at least in part – attributed to reduced perfusion of the optic nerve head by different authors. [6-8] Thus patients with normal tension glaucoma constitute a particularly interesting group for investigating pharmacological effects on ocular perfusion.
Up to now therapy of normal tension glaucoma does not differ substantially from the therapy of classical primary open angle glaucoma with increased IOP level: in both cases the focus is set on lowering IOP.[9,10] There is a tendency for defining lower target IOPs individually for each patient and optimizing ocular hemodynamics is still rated as complementary. Recently, several systemic approaches have been evaluated, e.g. oral administration of magnesium or the calcium antagonist nimodipine.[11,12] In addition, the hemodynamic effects of locally applied antiglaucomatous drugs are subject to intensive investigations within the last years: [13-16] Dorzolamide, an inhibitor of the carbonic anhydrase has shown the most promising effects on ocular hemodynamics. [17-19] In contrast, brinzolamide did not to alter ocular perfusion.[20] ß-receptor antagonists influence parameters of ocular hemodynamics differently depending on the compound used: betaxolol and carteolol lower vascular resistance while timolol seems to lead to an increased vascular tone.[15] The α2-receptor agonist brimonidine did not affect ocular perfusion in primary open angle glaucoma patients.[21] Derivatives of prostaglandins have become more and more popular for the treatment of glaucoma patients since they are very effective in lowering IOP and have to be applied only once a day.[22] Up to now there are predominantly four substances in clinical use: latanoprost, bimatoprost, travoprost and unoprostone. Only few studies have addressed the influence of prostaglandin analogues on ocular perfusion so far.
Prostaglandins constitute a large group of compounds produced endogenously with various and partially contrary effects on vascular tone.[23,24] Certain prostaglandins, such as PGI2 and PGE2, are potent vasodilators; others like PGF2α constricts arteries. [25-27] Investigations of the vasoactive effects of prostaglandins are complicated by the species dependency of their effects on vascular tone.[28] Latanoprost derives from PGF2α and has been reported to improve the perfusion of the optic nerve head[13], although in vitro studies indicate that latanoprost in contrast to its chemical origin PGF2α exerts no or minimal effects on vascular tone in the primate eye.[28] Bimatoprost has been demonstrated to have a vasoconstricting effect on isolated porcine ciliary arteries in vitro.[29] It is unknown whether this effect is of relevance in vivo and in humans.
The aim of the present study is to compare the hemodynamic effects of bimatoprost and latanoprost in patients with normal tension glaucoma. The effects were compared with the hemodynamic properties of dorzolamide, which has repeatedly been shown to improve ocular blood flow and can therefore be used as a reference compound for the evaluation of hemodynamic effects of antiglaucomatous eye drops. [17-19] It is hypothesized that these compounds lead to changes in systolic and diastolic blood flow velocities within the short posterior cilary artery. This vessel constitutes the main blood supply to the optic nerve head and alterations in its perfusion might play an important role in the pathogenesis and progression of normal tension glaucoma.
Methods
The study was performed in accordance to institutional, national, and international guidelines and was approved by the local ethics committee. The study was designed as an interventional, randomized, prospective, institutional, single-blinded, controlled, clinical trial.
Color Doppler imaging
Color Doppler imaging (CDI) was performed with a Sonoline Elegra Advanced System (Siemens, Erlangen, Germany) using a phased array transducer type 7.5L40 (Siemens, Erlangen, Germany) as described previously.[30] Ultrasound frequency was 6.5 MHz in the pulsed Doppler mode. The transducer was carefully set on the closed eye lid without exerting pressure on the bulb. Acoustic coupling between transducer and skin was optimized by a carbomeric gel (Vidisic®, Dr. Mann Pharma, Germany). After identification of the optic nerve as a landmark the following vessels were investigated in the color Doppler mode: central retinal artery (CRA), short and long posterior ciliary arteries (SPCA and LPCA) and ophthalmic artery (OA). Blood flow in the CRA was measured along its course through the optic nerve. Velocities in the SPCA and LPCA were recorded before entering the sclera. Flow velocity in the OA was measured close to its crossing of the optic nerve. The angle between transducer and orientation of the vessel was corrected. Gain and threshold were adjusted individually for each examination until noise disappeared and then kept constant during the entire examination. Pulse repetition frequency (PRF) was minimized to avoid aliasing (typically 5208 Hz for the OA, and 2500 Hz for the SPCA and LPCA in the Doppler mode). Sample size for all vessels was set constantly to 1.5 mm.
The changes of blood flow velocities over the course of a cardiac cycle were recorded continuously. Peak systolic velocity (PSV) and end diastolic velocity (EDV) can be determined directly and pulsatility index (PI) and resistive index (RI) are calculated automatically by the CDI software.
Patients
Consecutive patients with progressive normal tension glaucoma were randomized either to the latanoprost or to the bimatoprost group. Progression was defined as progressive excavation of the optic disc (funduscopic and controlled by the Heidelberg retina tomography – HRT) and/or progressive visual field loss in the Humphrey perimeter over the last 6–12 months. Glaucoma progression was assessed by the same glaucoma specialist (M.K.) in all patients. The patients underwent CDI measurements of ocular perfusion of the right eye by CDI shortly before and 3 to 5 weeks after initiation a local therapy with either latanoprost or bimatoprost. Both eye drops were applied once a day between 6 p.m. and 8 p.m. Normal tension glaucoma patients with unchanged excavation of the optic disc in funduscopy and HRT as well as stable visual field over the last 6–12 months served as negative controls. The glaucoma parameters in the control group were assessed by the same glaucoma specialist (M.K.) like in the therapy group. In the control group two subsequent CDI examinations were performed with an interval of 3 to 5 weeks. An additional group of patients with progressive normal tension glaucoma received dorzolamide eye drops three times a day, which has been reported to enhance blood flow in retrobulbar vessels. [17-19] This group was recruited to demonstrate that the CDI-methodology used is suitable to detect changes in retrobulbar blood flow. Regarding the assessment of progression the same criteria were applied as for the other study groups. CDI-examinations in the dorzolamide group were carried out shortly before and 3 to 5 weeks after initiation of the dorzolamide therapy.
All measurements in both therapy and control groups were carried out at 4 p.m. ± 2 h as a standardized time point to ensure best possible comparability. This specific time point was chosen due to organizational reasons. Prior to CDI intraocular pressure was measured. All examinations were performed with the patient sitting in an upright position and all the patients rested for several minutes before the examination started. CDI measurements for this study were performed by two experienced investigators (O.Z. and E.T.M.). To exclude investigator dependent effects, both CDI measurements in one patient were performed by the same physician. The study group membership and the individual therapy of the patients were masked for the CDI-investigator.
Patients with an intraocular pressure higher than 21 mmHg prior to therapy as well as patients with cardiovascular diseases were not included in the study. Systemic medication of the patients did not change between their first and second presentation. Patients who had ophthalmic surgery to the right eye were excluded from the study.
Bimatoprost
Lumigan® eye drops from Pharm Allergan (Ettlingen, Germany) were used in the bimatoprost treatment group. Lumigan® contains 0.3 mg/ml bimatoprost. The solvent contained benz alkonium chloride, sodium chloride, di sodium hydrogene phosphate 7H2O, citric acid, hydrochloric acid or sodium hydroxide (for pH adjustment), and water.
Latanoprost
Latanoprost (50 μg/ml) was obtained from Pharmacia Pfizer (Karlsruhe, Germany) as Xalatan®. Further ingredients are benz alkonium chloride, sodium chloride, sodium dihydrogene phosphate 1H2O, sodium monohydrogene phosphate, and water.
Dorzolamide
Dorzolamide was obtained in form of Trusopt® from MSD-Chibret, Munich, Germany. Trusopt® containes 22.26 mg dorzolamide hydrochloride per 1 ml. Further ingredients of Trusopt® are hydroxyethylcellulose, D-mannitole, sodium citrate, sodium hydroxide, benzalkonium chloride and water as solvent.
Statistics
Statistical analysis of the data was done with SPSS 10.0. All data are given as mean ± standard error of means (SEM). Student's t-test for paired data was used. It was hypothesized that the tested compounds would influence peak systolic and end-diastolic blood flow velocities (PSV and EDV) in the short posterior ciliary artery (SPCA). Thus two statistical tests were performed in each of the four study groups resulting in eight parallel statistical tests in total. Performing multiple statistical tests in a study necessitates the correction of the P-value to reach a significance level of 0.05. Therefore the Bonferroni-adjustment was applied and P < 0.006 is considered to be significant (double sided). All other parameters except EDV and PSV in SPCA were evaluated in a descriptive manner. Thus no P-values will be presented for these observational parameters.
In an a-priori-power-analysis the sample size was calculated. H1 was defined by an increase of flow velocity by 50% and a standard deviation of 35%. This definition was applied because preliminary data indicated a change in that range caused by dorzolamide. To reach a statistic power of 0.80 or more at least n = 9 individuals for PSV and n = 8 individuals for EDV per treatment group are required. For power analysis, the tool G*POWER V. 2.0 of F. Faul and E. Erdfelder [31] was applied.
Results
11 patients were treated with bimatoprost, 10 patients with latanoprost. Initially 9 patients were enrolled in the negative control group, but only 8 could be evaluated. One patient had to be excluded, because he did not return to the second examination due to personal reasons. 12 additional patients received dorzolamide. All data shown was obtained from measurements of the right eye of the patients.
Intraocular pressure (IOP)
Intraocular pressure was reduced from 14.6 ± 1.7 to 11.4 ± 0.8 mmHg under therapy with bimatoprost; latanoprost led to a decrease from 14.8 ± 2.2 to 12.3 ± 1.5 mmHg. Dorzolamide decreased the IOP in the dorzolamide group from 16.8 ± 1.6 to 14.8 ± 0.9 mmHg. In the negative control group intraocular pressure was 13.2 ± 1.7 mmHg at first presentation and 12.5 ± 1.9 mmHg at second presentation 3 to 5 weeks later (fig. 1).
Figure 1.
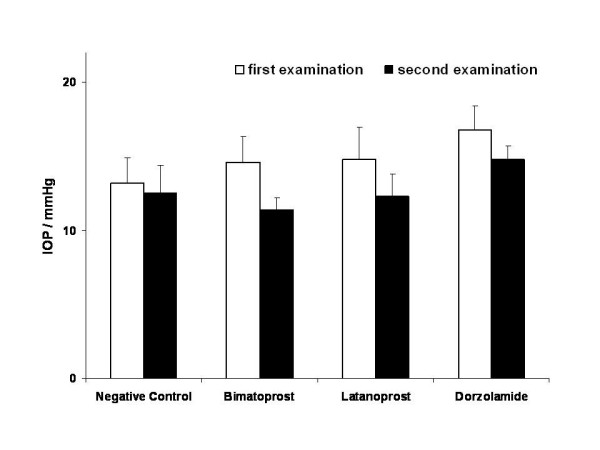
Intraocular pressure changes. Intraocular pressure (IOP) at first and second examination. IOP was an observational parameter and was not subjected to statistical testing.
Color Doppler imaging (CDI)
Neither bimatoprost nor latanoprost showed a statistically significant influence on peak systolic or enddiastolic velocities in the short posterior ciliary artery (fig. 2 and 3). All other parameters assessed by CDI measurements in the central retinal artery, short and long posterior ciliary arteries and ophthalmic artery appeared to be stable. Table 1 gives a detailed overview of the quantitative results obtained by CDI before and after treatment with latanoprost and table 2 does this for bimatoprost. In the negative control group all parameters were stable over time (table 3). Dorzolamide lead to a significant acceleration of systolic blood flow in the short posterior ciliary artery (table 4).
Figure 2.
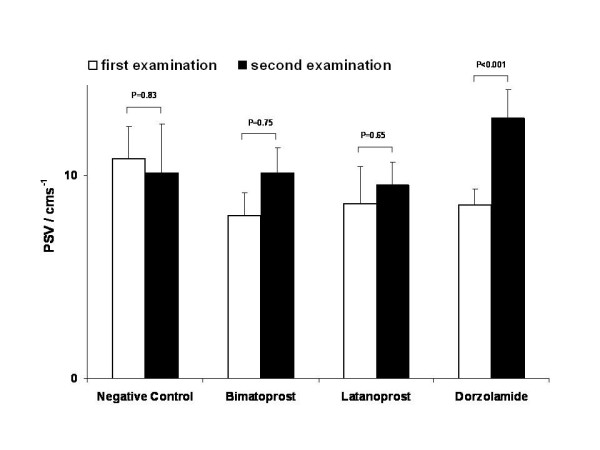
Peak systolic velocity. Peak systolic velocity (PSV) at first and second examination. In tendency, both compounds led to a slight increase of PSV, which was statistically not significant. Dorzolamide led to a statistical increase of PSV.
Figure 3.
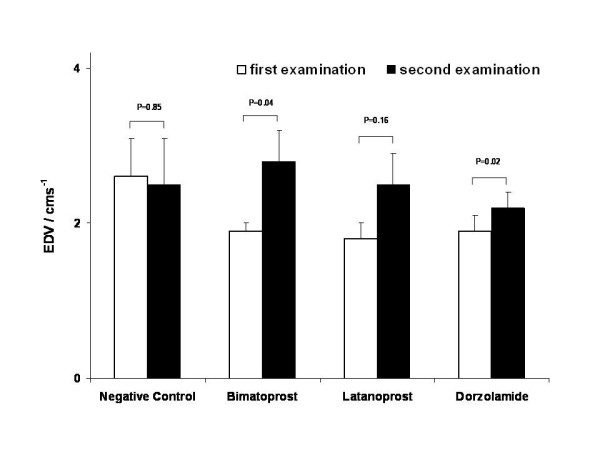
End diastolic velocity. End-diastolic velocity (EDV) at first and second examination. In tendency, both compounds led to a slight increase of EDV, which was statistically not significant.
Table 1.
Influence of bimatoprost on retrobulbar hemodynamics. Influence of bimatoprost on ocular perfusion. Overview of the results from CDI measurements before and one month after regular application of bimatoprost: Increase of blood flow velocities were statistically not significant (n = 11). Bold printed measures were subjected to statistical testing; all other parameters were observational only.
| PSV (cms-1) | EDV (cms-1) | RI | PI | ||
| OA | before bimatoprost | 31.6 ± 3.5 | 3.5 ± 0.9 | 0.89 ± 0.02 | 2.76 ± 0.20 |
| after bimatoprost | 38.5 ± 4.8 | 4.2 ± 0.7 | 0.87 ± 0.03 | 2.53 ± 0.21 | |
| CRA | before bimatoprost | 7.9 ± 0.9 | 1.4 ± 0.2 | 0.79 ± 0.04 | 2.22 ± 0.31 |
| after bimatoprost | 8.1 ± 0.5 | 1.7 ± 0.1 | 0.79 ± 0.02 | 1.90 ± 0.10 | |
| SPCA | before bimatoprost | 8.0 ± 1.1 | 1.9 ± 0.1 | 0.71 ± 0.03 | 1.61 ± 0.15 |
| after bimatoprost | 10.1 ± 1.2 | 2.8 ± 0.4 | 0.75 ± 0.01 | 1.66 ± 0.05 | |
| LPCA | before bimatoprost | 14.8 ± 3.0 | 3.0 ± 0.7 | 0.78 ± 0.03 | 1.90 ± 0.17 |
| after bimatoprost | 16.8 ± 2.2 | 3.0 ± 0.3 | 0.80 ± 0.02 | 1.90 ± 0.12 |
Abbreviations: OA – Ophthalmic artery, CRA – central retinal artery, SPCA – short posterior ciliary artery, LPCA – long posterior ciliary artery, PSV – peak systolic velocity, EDV – enddiastolic velocity, RI – resistive index, PI – pulsatility index.
Table 2.
Influence of latanoprost on retrobulbar hemodynamics. Influence of latanoprost on ocular perfusion. Overview of the results from CDI measurements before and one month after regular application of latanoprost: Increase of blood flow velocities were statistically not significant (n = 10). Bold printed measures were subjected to statistical testing; all other parameters were observational only.
| PSV (cms-1) | EDV (cms-1) | RI | PI | ||
| OA | before latanoprost | 31.7 ± 3.1 | 4.6 ± 0.9 | 0.87 ± 0.02 | 2.59 ± 0.15 |
| after latanoprost | 39.0 ± 10.1 | 6.2 ± 1.2 | 0.83 ± 0.01 | 2.14 ± 0.09 | |
| CRA | before latanoprost | 7.6 ± 0.6 | 1.5 ± 0.1 | 0.78 ± 0.01 | 1.96 ± 0.10 |
| after latanoprost | 8.4 ± 0.9 | 1.7 ± 0.1 | 0.79 ± 0.02 | 1.95 ± 0.13 | |
| SPCA | before latanoprost | 8.6 ± 1.8 | 1.8 ± 0.2 | 0.74 ± 0.03 | 1.75 ± 0.13 |
| after latanoprost | 9.5 ± 1.1 | 2.5 ± 0.4 | 0.73 ± 0.03 | 1.60 ± 0.11 | |
| LPCA | before latanoprost | 13.1 ± 1.5 | 2.5 ± 0.3 | 0.81 ± 0.02 | 1.96 ± 0.14 |
| after latanoprost | 13.0 ± 1.9 | 2.5 ± 0.4 | 0.78 ± 0.04 | 1.90 ± 0.21 |
Abbreviations: OA – Ophthalmic artery, CRA – central retinal artery, SPCA – short posterior ciliary artery, LPCA – long posterior ciliary artery, PSV – peak systolic velocity, EDV – enddiastolic velocity, RI – resistive index, PI – pulsatility index.
Table 3.
Influence of dorzolamide on retrobulbar hemodynamics. Ocular perfusion in the dorzolamide group. CDI measurements obtained from the patient group treated with dorzolamide (n = 12). The time period between between first and second measurement was 3–5 weeks. Data are given as mean ± standard deviation. Bold printed measures were subjected to statistical testing; all other parameters were observational only.
| PSV (cms-1) | EDV (cms-1) | RI | PI | ||
| OA | before dorzolamide | 29.6 ± 1.2 | 3.3 ± 0.4 | 0.89 ± 0.02 | 2.82 ± 0.25 |
| after dorzolamide | 37.2 ± 3.5 | 4.4 ± 0.7 | 0.88 ± 0.02 | 2.64 ± 0.20 | |
| CRA | before dorzolamide | 7.3 ± 0.8 | 1.4 ± 0.1 | 0.80 ± 0.02 | 1.98 ± 0.18 |
| after dorzolamide | 8.5 ± 0.8 | 1.8 ± 0.2 | 0.78 ± 0.02 | 1.97 ± 0.24 | |
| SPCA | before dorzolamide | 8.5 ± 0.8 | 1.9 ± 0.2 | 0.77 ± 0.02 | 1.79 ± 0.11 |
| after dorzolamide | 12.8 ± 1.4 * | 2.2 ± 0.2 | 0.80 ± 0.03 | 1.91 ± 0.13 | |
| LPCA | before dorzolamide | 10.9 ± 0.8 | 2.0 ± 0.3 | 0.73 ± 0.07 | 1.80 ± 0.22 |
| after dorzolamide | 16.9 ± 1.7 | 2.9 ± 0.3 | 0.83 ± 0.01 | 1.95 ± 0.12 |
Asterisk (*) denotes statistic significance (P < 0.006). Abbreviations: OA – Ophthalmic artery, CRA – central retinal artery, SPCA – short posterior ciliary artery, LPCA – long posterior ciliary artery, PSV – peak systolic velocity, EDV – enddiastolic velocity, RI – resistive index, PI – pulsatility index.
Table 4.
CDI measurements in the control group. Ocular perfusion in the control group. CDI measurements obtained from the control group (n = 8). The time period between between first and second measurement was 3–5 weeks. Local and systemic medication was not changed between the two examinations of the patient. Data are given as mean ± standard deviation. Bold printed measures were subjected to statistical testing; all other parameters were observational only.
| PSV (cms-1) | EDV (cms-1) | RI | PI | ||
| OA | first examination | 29.3 ± 2.6 | 3.7 ± 0.6 | 0.87 ± 0.02 | 2.57 ± 0.29 |
| second examination | 30.6 ± 3.2 | 5.4 ± 1.0 | 0.83 ± 0.02 | 2.08 ± 0.20 | |
| CRA | first examination | 8.2 ± 1.5 | 1.8 ± 0.4 | 0.76 ± 0.03 | 1.93 ± 0.19 |
| second examination | 7.5 ± 0.9 | 1.4 ± 0.1 | 0.80 ± 0.03 | 2.14 ± 0.21 | |
| SPCA | first examination | 10.8 ± 1.6 | 2.6 ± 0.5 | 0.75 ± 0.02 | 1.76 ± 0.17 |
| second examination | 10.1 ± 2.4 | 2.5 ± 0.6 | 0.75 ± 0.02 | 1.55 ± 0.09 | |
| LPCA | first examination | 18.0 ± 3.8 | 3.1 ± 0.8 | 0.82 ± 0.04 | 2.01 ± 0.21 |
| second examination | 18.0 ± 4.0 | 3.6 ± 0.9 | 0.77 ± 0.01 | 1.69 ± 0.12 |
Abbreviations: OA – Ophthalmic artery, CRA – central retinal artery, SPCA – short posterior ciliary artery, LPCA – long posterior ciliary artery, PSV – peak systolic velocity, EDV – enddiastolic velocity, RI – resistive index, PI – pulsatility index.
Discussion
Based on the presented results bimatoprost or latanoprost and have no significant influence on hemodynamics in the short posterior ciliary artery has to be rejected, although a tendency toward improved peak systolic and end diastolic blood flow velocities was detected in both treatment groups compared with the negative control group.
To our knowledge the present study is the first to directly compare hemodynamic effects of bimatoprost and latanoprost in patients with normal tension glaucoma. An altered blood flow velocity due to a vasoconstricting activity of bimatoprost reported from Allemann and colleagues[29] is not detectable in humans by CDI measurements. These differences might be explained by the in vitro system used by Allemann, who worked with isolated ciliary arteries from pigs. Apart from potential differences between species, the balance between neuronal, endothelial and myogenic influences on vascular tone is disturbed in such an in vitro setting. In vitro studies for latanoprost revealed minimal vasoactivity.[28] The chemical origin of latanoprost is the prostaglandin PGF2α, which increases arterial tone. [25-27] Bimatoprost is a derivative of the prostamide F2α, which is enzymatically formed from PGF2α and should act similar like PGF2α, but has a lower receptor affinity.[32] How vasoconstriction influences ocular perfusion depends on its site of action: Constriction of pre-capillary sphincters will cause a deterioration of blood flow. On the other hand, moderate constriction of the entire arterial system of an organ will increase perfusion pressure. From this theoretic view prostaglandins could influence ocular blood flow in positive and negative direction or both effects neutralize each other.
For latanoprost a handful of studies dealing with its effects on ocular perfusion have been published. Some of these studies state positive effects.[13,33,34] All these studies have in common that acute and short-term effects from less than an hour up to one week after application of the drug were observed. In accordance with the present results, studies with an extended follow up did not find any mid- or long-term effect of latanoprost on ocular hemodynamics.[13,35,36] A possible explanation for the difference between long- and short-term effects might be the acute reduction of intraocular pressure, which will result in an increased ocular perfusion pressure and leads to an improvement of ocular hemodynamics. For comparison with the presented results one has to point out that the previous reports were carried out with patients suffering from primary open angle glaucoma with initially increased IOP. Thus it is difficult to differentiate whether the observed improvement of ocular perfusion can be attributed to a primary effect of the active agent on the ocular vasculature or is secondary to IOP-reduction. In the setting of the present study the absolute IOP reduction in relation to ocular perfusion pressure is low and does affect ocular perfusion pressure only by approximately 5%. Retrobulbar blood flow velocities are directly proportional to ocular perfusion pressure. Subsequently, the effect of such a change in intraocular pressure on retrobulbar blood flow velocities is low, particularly compared to the changes seen after the administration of dorzolamide. Nevertheless, the tendency of an increase of retrobulbar blood flow velocity is visible in all treated groups.
Patients with normal tension glaucoma are therefore a particularly interesting group for the clinical investigation of hemodynamic effects of antiglaucomatous eye drops since results are less skewed by changes in IOP. In addition, a reduction in ocular perfusion might play a major role in the pathogenesis of normal tension glaucoma.[37] Reversal of this condition could be of great clinical benefit for this patient group and deterioration of ocular perfusion is expected to accelerate progression of glaucomatous optic neuropathy. Therefore normal tension glaucoma requires a therapy which ideally improves hemodynamics of the eye, but at least does not alter it in a negative way.
Although not primary end-point of the present study, the results indicate that latanoprost and bimatoprost have the capability to reduce IOP in patients with normal tension glaucoma, despite the fact that initial IOP levels before the treatment are low. Relative IOP-reduction in the present study is smaller with previous reports on treatment of patients with primary open angle glaucoma.[22,38,39] This is most likely due to the low initial intraocular pressure in the patient population with normal tension glaucoma. Studies on the IOP-lowering effect of prostaglandins, predominantly latanoprost, in normal tension glaucoma find an IOP-reduction in a similar magnitude. [40-42] This can be taken as an indicator for good compliance of the patients and it can be concluded that both tested compounds have the capability to reliably decrease IOP even in the lower range.
Some ophthalmologists doubt the reliability of CDI, but the reliability of the CDI method has repeatedly been shown in vitro[4] and in vivo[43]. Experiments performed by our group are in accordance with these findings.[44] The positive control with dorzolamide in the present study underlines the applicability of CDI for assessing pharmacological effects on ocular perfusion. To resolve the potential problem of investigator dependency both CDI-examinations for each patient were performed by the same investigator. In addition, the CDI procedure was performed in a highly standardized manner in our laboratory minimizing investigator dependency of the results.
The precise and clinically relevant evaluation of ocular perfusion still poses a great challenge. Quantitative determination of total ocular blood flow with a single method is not possible, but combination of different methods is proposed to give a better approximation.[45] This fact limits the explanatory power of this and other studies addressing ocular perfusion in ophthalmological diseases. Aim of the present study was to focus on glaucoma patients and it is hypothesized that glaucoma is associated with a localized disturbance of ocular hemodynamics at the optic disc. The optic disc is supplied with blood by the posterior ciliary arteries. The most reliable method for evaluation of hemodynamics in the short posterior ciliary artery is color Doppler imaging. Blood flow velocity in the short posterior ciliary arteries is influenced on the one hand by the vascular tone of the vessel itself but also by the resistance of the dependent downstream vasculature. Thus blood flow velocities in the short posterior ciliary artery reflect also the hemodynamics at the level of the optic disc. The influence of the tested compounds on perfusion of the entire eye cannot be answered by the present study.
Recently it has been shown that the correlation between measurements of ocular hemodynamics with different methodologies is limited.[30] Thus differences in outcome between studies investigating the effects of the same compound on ocular perfusion might at least partially be explained by the variability between the methodologies. Color Doppler imaging (CDI) measures blood flow velocities. Measurement of ocular blood flow by the method of Langham (LOBF) is based on assessing pulse synchronal oscillations of the IOP and calculate ocular perfusion from its amplitude by mathematical algorithms.[46] From the parameter determined by CDI only the pulsatility index correlates with the perfusion index determined by LOBF.[30] Therefore the comparison of the results of the present study e.g. with the work by Georgopoulus et al.[33] is limited.
Conclusion
In summary no significant influence of latanoprost and bimatoprost on ocular hemodynamics was detected in the present study. In accordance with previous reports dorzolamide led to an increase of blood flow velocity in systole. A suitable compound for the treatment of normal tension glaucoma should at least act hemodynamically neutral. This minimum requirement is fulfilled by all three tested compounds. Only dorzolamide was capable to improve perfusion in the posterior ciliary arteries. When comparing both prostaglandin-like eye drops, they showed similar effects on ocular hemodynamics and IOP. Subsequently latanoprost and bimatoprost can be considered as equivalent in the treatment of normal tension glaucoma. However, in order to comprehensively treat the pathogenic disturbances leading or at least contributing to normal tension glaucoma there is still a great demand for a pharmacological compound which significantly enhances perfusion of the optic nerve head.
Competing interests
M.K. received a grant from Pharm-Allergan (manufacturer of bimatoprost) in 2003. This grant is not related to the present study. Neither the present study nor the present manuscript is financed by this grant. There are no further commercial relationships for any author.
Authors' contributions
OZ designed and co-ordinated the study. ETM and OZ performed the CDI measurements. JR and AW documented the data and did under supervision of OZ the statistical analysis. LW and PG were additionally involved in editing the manuscript and interpreting the data. PG contributed knowledge about basic science aspects of prostaglandin action on vasculature. GR and MK supported the other authors in interpreting the results. MK as glaucoma specialist was additionally responsible for patient recruitment and randomization.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The authors would like to gratefully acknowledge Birgit Bischoff and Fatima Icagic for their excellent technical support in the study.
Contributor Information
Oliver Zeitz, Email: zeitz@uke.uni-hamburg.de.
Eike T Matthiessen, Email: e.matthiessen@uke.uni-hamburg.de.
Juliane Reuss, Email: julianereuss@compuserve.de.
Anne Wiermann, Email: hanni@hanni.org.
Lars Wagenfeld, Email: wagenfeld@arcor.de.
Peter Galambos, Email: peter.galambos@web.de.
Gisbert Richard, Email: augenklinik@uke.uni-hamburg.de.
Maren Klemm, Email: klemm@uke.uni-hamburg.de.
References
- Goldmann H. Open-angle glaucoma. Br J Ophthalmol. 1972;56:242–248. doi: 10.1136/bjo.56.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD, Javitt J, Singh K. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090–1095. doi: 10.1001/archopht.1991.01080080050026. [DOI] [PubMed] [Google Scholar]
- Pillunat LE, Stodtmeister R, Marquardt R, Mattern A. Ocular perfusion pressures in different types of glaucoma. Int Ophthalmol. 1989;13:37–42. doi: 10.1007/BF02028635. [DOI] [PubMed] [Google Scholar]
- Klingmuller V, Schmidt KG, von Ruckmann A, Koch B, Stein A. [Doppler sonography of the short posterior ciliary artery in patients with primary open angle glaucoma] Ultraschall Med. 2000;21:32–37. doi: 10.1055/s-2000-8928. [DOI] [PubMed] [Google Scholar]
- Maumenee AE. Causes of optic nerve damage in glaucoma. Robert N. Shaffer lecture. Ophthalmology. 1983;90:741–752. doi: 10.1016/s0161-6420(83)34493-6. [DOI] [PubMed] [Google Scholar]
- Schmidt KG, Ruckmann AV, Mittag TW, Hessemer V, Pillunat LE. Reduced ocular pulse amplitude in low tension glaucoma is independent of vasospasm. Eye. 1997;11:485–488. doi: 10.1038/eye.1997.131. [DOI] [PubMed] [Google Scholar]
- Schumann J, Orgul S, Gugleta K, Dubler B, Flammer J. Interocular difference in progression of glaucoma correlates with interocular differences in retrobulbar circulation. Am J Ophthalmol. 2000;129:728–733. doi: 10.1016/S0002-9394(99)00481-X. [DOI] [PubMed] [Google Scholar]
- Boehm AG, Pillunat LE, Koeller U, Katz B, Schicketanz C, Klemm M, Richard G. Regional distribution of optic nerve head blood flow. Graefes Arch Clin Exp Ophthalmol. 1999;237:484–488. doi: 10.1007/s004170050266. [DOI] [PubMed] [Google Scholar]
- Collaborative normal tension glaucoma study group The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:498–505. doi: 10.1016/S0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- Collaborative normal tension glaucoma study group Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126:487–497. doi: 10.1016/S0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- Piltz JR, Bose S, Lanchoney D. The effect of nimodipine, a centrally active calcium antagonist, on visual function and mascular blood flow in patients with normal-tension glaucoma and control subjects. J Glaucoma. 1998;7:336–342. [PubMed] [Google Scholar]
- Gaspar AZ, Gasser P, Flammer J. The influence of magnesium on visual field and peripheral vasospasm in glaucoma. Ophthalmologica. 1995;209:11–13. doi: 10.1159/000310566. [DOI] [PubMed] [Google Scholar]
- Tamaki Y, Nagahara M, Araie M, Tomita K, Sandoh S, Tomidokoro A. Topical latanoprost and optic nerve head and retinal circulation in humans. J Ocul Pharmacol Ther. 2001;17:403–411. doi: 10.1089/108076801753266785. [DOI] [PubMed] [Google Scholar]
- Tamaki Y, Araie M, Tomita K, Nagahara M, Sandoh S, Tomidokoro A. Effect of topical unoprostone on circulation of human optic nerve head and retina. J Ocul Pharmacol Ther. 2001;17:517–527. doi: 10.1089/10807680152729202. [DOI] [PubMed] [Google Scholar]
- Altan-Yaycioglu R, Turker G, Akdol S, Acunas G, Izgi B. The effects of beta-blockers on ocular blood flow in patients with primary open angle glaucoma: a color Doppler imaging study. Eur J Ophthalmol. 2001;11:37–46. doi: 10.1177/112067210101100108. [DOI] [PubMed] [Google Scholar]
- Schmidt KG, von Ruckmann A, Becker R, Pillunat LE. [Ocular pulse amplitude, intraocular pressure and beta blocker/carbonic anhydrase inhibition in combined therapy of primary open-angle glaucoma] Klin Monatsbl Augenheilkd. 1999;215:361–366. doi: 10.1055/s-2008-1034733. [DOI] [PubMed] [Google Scholar]
- Schmidt KG, von Ruckmann A, Pillunat LE. Topical carbonic anhydrase inhibition increases ocular pulse amplitude in high tension primary open angle glaucoma. Br J Ophthalmol. 1998;82:758–762. doi: 10.1136/bjo.82.7.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KG, Dick B, von Ruckmann A, Pillunat LE. [Ocular pulse amplitude and local carbonic anhydrase inhibition] Ophthalmologe. 1997;94:659–664. doi: 10.1007/s003470050179. [DOI] [PubMed] [Google Scholar]
- Pillunat LE, Bohm AG, Koller AU, Schmidt KG, Klemm M, Richard G. Effect of topical dorzolamide on optic nerve head blood flow. Graefes Arch Clin Exp Ophthalmol. 1999;237:495–500. doi: 10.1007/s004170050268. [DOI] [PubMed] [Google Scholar]
- Klemm M, Zeitz O, Reuss J, Matthiessen ET, Richard G. [Therapy of normal tension glaucoma: effect of brinzolamide on ocular haemodynamics] Klin Monatsbl Augenheilkd. 2003;220:330–333. doi: 10.1055/s-2003-39432. [DOI] [PubMed] [Google Scholar]
- Schmidt KG, Klingmuller V, Gouveia SM, Osborne NN, Pillunat LE. Short posterior ciliary artery, central retinal artery, and choroidal hemodynamics in brimonidine-treated primary open-angle glaucoma patients. Am J Ophthalmol. 2003;136:1038–1048. doi: 10.1016/S0002-9394(03)00631-7. [DOI] [PubMed] [Google Scholar]
- Parrish RK, Palmberg P, Sheu WP. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003;135:688–703. doi: 10.1016/S0002-9394(03)00098-9. [DOI] [PubMed] [Google Scholar]
- Terragno NA. Prostaglandins, antidiuretic hormone and renin angiotensin system. Hypertension. 1981;3:II–65-70. doi: 10.1161/01.hyp.3.6_pt_2.ii-65. [DOI] [PubMed] [Google Scholar]
- Bassenge E. Endothelial function in different organs. Prog Cardiovasc Dis. 1996;39:209–228. doi: 10.1016/s0033-0620(96)80002-8. [DOI] [PubMed] [Google Scholar]
- Kaley G, Hintze TH, Panzenbeck M, Messina EJ. Role of prostaglandins in microcirculatory function. Adv Prostaglandin Thromboxane Leukot Res. 1985;13:27–35. [PubMed] [Google Scholar]
- Nakayama K, Osol G, Halpern W. Differential contractile responses of pressurized porcine coronary resistance-sized and conductance coronary arteries to acetylcholine, histamine and prostaglandin F2 alpha. Blood Vessels. 1989;26:235–245. doi: 10.1159/000158771. [DOI] [PubMed] [Google Scholar]
- Maigaard S, Forman A, Andersson KE. Different responses to prostaglandin F2 alpha and E2 in human extra- and intramyometrial arteries. Prostaglandins. 1985;30:599–607. doi: 10.1016/0090-6980(85)90024-3. [DOI] [PubMed] [Google Scholar]
- Stjernschantz J, Selen G, Astin M, Resul B. Microvascular effects of selective prostaglandin analogues in the eye with special reference to latanoprost and glaucoma treatment. Prog Retin Eye Res. 2000;19:459–496. doi: 10.1016/S1350-9462(00)00003-3. [DOI] [PubMed] [Google Scholar]
- Allemann R, Flammer J, Haefliger IO. Vasoactive properties of bimatoprost in isolated porcine ciliary arteries. Klin Monatsbl Augenheilkd. 2003;220:161–164. doi: 10.1055/s-2003-38167. [DOI] [PubMed] [Google Scholar]
- Zeitz O, Matthiessen E, Richard G, Klemm M. Estimation of choroid perfusion by colour Doppler imaging vs. other methods. Ultrasound Med Biol. 2002;28:1023. doi: 10.1016/S0301-5629(02)00555-0. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E. G*Power http://www.psycho.uni-duesseldorf.de/aap/projects/gpower/
- Matias I, Chen J, Petrocellis LD, Bisogno T, Ligresti A, Fezza F, Krauss AH, Shi L, Protzman CE, Li C, Liang Y, Nieves AL, Kedzie KM, Burk RM, Di Marzo V, Woodward DF. Prostaglandin-ethanolamides (prostamides): in vitro pharmacology and metabolism. J Pharmacol Exp Ther. 2004;309:745–757. doi: 10.1124/jpet.103.061705. [DOI] [PubMed] [Google Scholar]
- Georgopoulos GT, Diestelhorst M, Fisher R, Ruokonen P, Krieglstein GK. The short-term effect of latanoprost on intraocular pressure and pulsatile ocular blood flow. Acta Ophthalmol Scand. 2002;80:54–58. doi: 10.1034/j.1600-0420.2002.800111.x. [DOI] [PubMed] [Google Scholar]
- Geyer O, Man O, Weintraub M, Silver DM. Acute effect of latanoprost on pulsatile ocular blood flow in normal eyes. Am J Ophthalmol. 2001;131:198–202. doi: 10.1016/S0002-9394(00)00797-2. [DOI] [PubMed] [Google Scholar]
- Arend O, Harris A, Wolter P, Remky A. Evaluation of retinal haemodynamics and retinal function after application of dorzolamide, timolol and latanoprost in newly diagnosed open-angle glaucoma patients. Acta Ophthalmol Scand. 2003;81:474–479. doi: 10.1034/j.1600-0420.2003.00122.x. [DOI] [PubMed] [Google Scholar]
- Harris A, Migliardi R, Rechtman E, Cole CN, Yee AB, Garzozi HJ. Comparative analysis of the effects of dorzolamide and latanoprost on ocular hemodynamics in normal tension glaucoma patients. Eur J Ophthalmol. 2003;13:24–31. doi: 10.1177/112067210301300104. [DOI] [PubMed] [Google Scholar]
- Flammer J, Orgul S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard JP, Stefansson E. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/S1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- Cantor LB. Bimatoprost: a member of a new class of agents, the prostamides, for glaucoma management. Expert Opin Investig Drugs. 2001;10:721–731. doi: 10.1517/13543784.10.4.721. [DOI] [PubMed] [Google Scholar]
- Gandolfi SA, Cimino L. Effect of bimatoprost on patients with primary open-angle glaucoma or ocular hypertension who are nonresponders to latanoprost. Ophthalmology. 2003;110:609–614. doi: 10.1016/S0161-6420(02)01891-2. [DOI] [PubMed] [Google Scholar]
- Hoyng PF, Kitazawa Y. Medical treatment of normal tension glaucoma. Surv Ophthalmol. 2002;47 Suppl 1:S116–24. doi: 10.1016/S0039-6257(02)00322-3. [DOI] [PubMed] [Google Scholar]
- Liu CJ, Ko YC, Cheng CY, Chiu AW, Chou JC, Hsu WM, Liu JH. Changes in intraocular pressure and ocular perfusion pressure after latanoprost 0.005% or brimonidine tartrate 0.2% in normal-tension glaucoma patients. Ophthalmology. 2002;109:2241–2247. doi: 10.1016/S0161-6420(02)01247-2. [DOI] [PubMed] [Google Scholar]
- McKibbin M, Menage MJ. The effect of once-daily latanoprost on intraocular pressure and pulsatile ocular blood flow in normal tension glaucoma. Eye. 1999;13 ( Pt 1):31–34. doi: 10.1038/eye.1999.6. [DOI] [PubMed] [Google Scholar]
- Baxter GM, Williamson TH. Color Doppler imaging of the eye: normal ranges, reproducibility, and observer variation. J Ultrasound Med. 1995;14:91–96. doi: 10.7863/jum.1995.14.2.91. [DOI] [PubMed] [Google Scholar]
- Matthiessen ET, Zeitz O, Richard G, Klemm M. Reproducibility of blood flow velocity measurements using colour decoded Doppler imaging. Eye. 2004;18:400–405. doi: 10.1038/sj.eye.6700651. [DOI] [PubMed] [Google Scholar]
- Rechtman E, Harris A, Kumar R, Cantor LB, Ventrapragada S, Desai M, Friedman S, Kagemann L, Garzozi HJ. An update on retinal circulation assessment technologies. Curr Eye Res. 2003;27:329–343. doi: 10.1076/ceyr.27.6.329.18193. [DOI] [PubMed] [Google Scholar]
- Langham ME, To'Mey KF. A clinical procedure for the measurements of the ocular pulse-pressure relationship and the ophthalmic arterial pressure. Exp Eye Res. 1978;27:17–25. doi: 10.1016/0014-4835(78)90049-0. [DOI] [PubMed] [Google Scholar]


