Abstract
Objectives:
Checkpoint inhibitors have been proposed for sepsis following reports of increased checkpoint molecule expression in septic patients. To determine whether clinical studies investigating checkpoint molecule expression provide strong evidence supporting trials of checkpoint inhibitors for sepsis.
Data Sources:
PubMed, EMBASE, Scopus, Web of Science, inception through October 2019.
Study Selection:
Studies comparing checkpoint molecule expression in septic patients versus healthy controls or critically ill nonseptic patients or in sepsis nonsurvivors versus survivors.
Data Extraction:
Two investigators extracted data and evaluated study quality.
Data Synthesis:
Thirty-six studies were retrieved. Across 26 studies, compared with healthy controls, septic patients had significantly (p ≤ 0.05) increased CD4+ lymphocyte programmed death-1 and monocyte programmed death-ligand-1 expression in most studies. Other checkpoint molecule expressions were variable and studied less frequently. Across 11 studies, compared with critically ill nonseptic, septic patients had significantly increased checkpoint molecule expression in three or fewer studies. Septic patients had higher severity of illness scores, comorbidities, and mortality in three studies providing analysis. Across 12 studies, compared with septic survivors, nonsurvivors had significantly increased expression of any checkpoint molecule on any cell type in five or fewer studies. Of all 36 studies, none adjusted for nonseptic covariates reported to increase checkpoint molecule expression.
Conclusions:
Although sepsis may increase some checkpoint molecule expression compared with healthy controls, the data are limited and inconsistent. Further, data from the more informative patient comparisons are potentially confounded by severity of illness. These clinical checkpoint molecule expression studies do not yet provide a strong rationale for trials of checkpoint inhibitor therapy for sepsis.
Keywords: checkpoint molecule expression, flow cytometry methods, sepsis
Sepsis is a leading cause of critical illness and mortality worldwide (1), prompting continued investigation into its pathogenesis and treatment. It has been proposed that the septic immune response includes early inflammation accompanied by rapid and prolonged immunosuppression that can impair microbial clearance (2). One putative mechanism underlying this suppression is increased immune checkpoint molecule expression (CME) (2-4). CME can counter lymphocyte activation (5) by suppressing T cell proliferation and inflammatory cytokine production and reducing T cell longevity (6, 7). Conversely, disruption of these pathways with checkpoint inhibitors (CPIs) produces sustained inflammatory responses potentially beneficial for select clinical conditions (7). Blockade of the checkpoint molecules programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and cytotoxic T lymphocyte antigen-4 (CTLA-4) has proven efficacious for cancer (8, 9) and shown promise for chronic viral and protozoal infections (5, 10).
The pathogenic role of CME in sepsis and its utility as a diagnostic or therapeutic target is under investigation. However, septic patients differ in age, comorbidities, and severity of underlying infection, and many patients initially thought to have sepsis are later found to have critical illness of noninfectious causes. These variables may influence CME, and the effectiveness of CPIs in a heterogeneous septic patient population rests on the likelihood that CME is uniform and has similar harmful effects across most patients. Studies have now compared CME in septic patients versus healthy controls (HCs) or critically ill nonseptic (CINS) patients and in septic nonsurvivors versus survivors. Findings from these studies reportedly provided a basis for two phase I trials of checkpoint inhibition in sepsis and interest in this therapeutic approach continues (11, 12). To date, however, there has been no systematic review of the literature to assess how well existing published data support clinical trials of CPI therapy for sepsis. We therefore performed a systematic review of studies comparing CME in septic patients to either HC or CINS subjects, or in sepsis nonsurvivors compared with survivors. We evaluated the studies’ methodologic strengths in assessing the utility of CME in discriminating septic patients from other comparison groups and septic survivors from nonsurvivors in order to determine whether alterations in CME associated with sepsis provide a strong rationale to perform clinical trials of CPIs in sepsis.
MATERIALS AND METHODS
This systematic review was prepared using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guidance for literature review and data extraction (Appendix 1, Supplemental Digital Content 1, http://links.lww.com/CCM/F620) (13).
Literature Search and Study Selection
Using published guidelines (13) and search strategies in Appendix 2 (Supplemental Digital Content 2, http://links.lww.com/CCM/F621), two authors (P.Q.E., P.T.-P.) identified relevant studies in PubMed, EMBASE, Scopus, and Web of Science from inception through October 2019 without language restrictions. Studies comparing cell-surface expression or serum levels of at least one of the immune checkpoint molecules PD-1, PD-L1, CTLA-4, or B- and T-lymphocyte attenuator (BTLA) in septic patients versus HCs or CINS patients or in sepsis nonsurvivors versus survivors were analyzed. Author consensus resolved uncertainty about study inclusion.
Data Extraction and Quality Assessment
Two investigators (L.M.B., P.T.-P.) extracted study data using an a priori designed standardized extraction form. Included data points are defined in Appendix 3 (Supplemental Digital Content 3, http://links.lww.com/CCM/F622). Methodologic elements related to CME measurement were also extracted.
Scoring of Flow Cytometric Methodology and Reporting
All studies measuring CME on circulating immune cells employed flow cytometry but methods varied. Based partly on consensus recommendations regarding standards for the validity and reproducibility of flow cytometric techniques, a scoring system was developed to grade laboratory methodology impacting interpretation of flow cytometric results (14). See Appendix 3 (Supplemental Digital Content 3, http://links.lww.com/CCM/F622) for full description of scoring system. Scores greater than 50% were judged “strong” and less than or equal to 50% as “weak.”
Baseline Patient Characteristics Reporting
Studies were examined to determine whether data for nonsepsis factors potentially altering CME were provided and statistically compared between groups. These factors included age, presence of comorbidities, illness severity score (e.g., Acute Physiology and Chronic Health Evaluation II), and mortality (when applicable).
Differences in CME and Soluble Concentrations Across Studies
For each comparison, less than or equal to four studies provided mean/median data with a measure of dispersion for the same checkpoint molecule and cell population or soluble concentration. Measures of dispersion included sd, sem, and interquartile range and are subsequently referred to as variability. These limited numbers precluded meta-analysis although these data are provided in Supplementary Table 1 (Supplemental Digital Content 4, http://links.lww.com/CCM/F623) and Supplementary Table 2 (Supplemental Digital Content 5, http://links.lww.com/CCM/F624) and a box plot of comparable data are provided in Supplementary Figure 1 (Supplemental Digital Content 6, http://links.lww.com/CCM/F625). However, all studies reported whether CME (presented as percent positive cells of the parent population, mean fluorescence intensity [MFI], or both) or the soluble checkpoint molecule concentration was increased, decreased, or not different between at least two study groups. Therefore, for each comparison, data are presented as the number of studies which reported that there were significant (p ≤ 0.05) increases, decreases, or no significant difference in expression for the checkpoint molecule and cell type investigated or the soluble concentration measured. For studies presenting CME as both cell frequency and MFI, or on more than 1 day, if either measure was significantly different between groups on any day of measurement, the study was recorded as demonstrating a significant change.
RESULTS
Search Results
Of 6,833 retrieved reports, 36 studies were included (Appendix 4, Supplemental Digital Content 7, http://links.lww.com/CCM/F626) (15-50). Twenty-nine studies compared septic versus HC subjects, 15 septic versus CINS patients, and 16 septic nonsurvivors versus survivors. Thirty-one studies measured cell-surface CME and seven soluble checkpoint molecule concentrations (two included both). Study characteristics including the checkpoint molecules and immune cell types investigated, timepoint of measurements, study design, numbers of subjects, and inclusion and exclusion criteria are summarized in Table 1 and Supplementary Table 3 (Supplemental Digital Content 8, http://links.lww.com/CCM/F627).
TABLE 1.
Summary of Study Characteristics
| Study Groups Compared |
Checkpoint Molecule, Cell Type, and Source |
||||||
|---|---|---|---|---|---|---|---|
| References | Septic vs Healthy Control |
Septic vs Critically Ill Nonseptic |
Septic Survivors vs Nonsurvivors |
Checkpoint Molecule |
Cell Type or Plasma |
Day of Samplinga |
Total Subjects |
| Cell-surface checkpoint molecule expression studies | |||||||
| Manjuck et al (15) | Yes | Yes | No | CTLA-4 | Lymphocyte, monocyte | 1 | 39 |
| Monneret et al (16) | Yes | No | Yes | CTLA-4 | Lymphocyte | 7–10 | 20 |
| Huang et al (17) | Yes | No | No | PD-1 | Monocyte | 1–2; 3–4; 5–6 | 10 |
| Roger et al (18) | Yes | Yes | No | CTLA-4 | Lymphocyte | 1 | 68 |
| Boomer et al (19) | No | Yes | No | PD-1, PD-L1 CTLA-4, BTLA |
Tissue lymphocyte, monocyte | 4b | 89 |
| Guignant et al (20) | Yes | Yes | Yes | PD-1, PD-L1, PD-L2 | Lymphocyte, monocyte | 1–2 | 126 |
| Zhang et al (21) | Yes | No | No | PD-1, PD-L1 | Lymphocyte, monocyte | 1 | 41 |
| Boomer et al (22) | Yes | No | No | PD-1, CTLA-4, BTLA | Lymphocyte | 1 | 36 |
| Roger et al (23) | Yes | No | No | CTLA-4 | Lymphocyte | NR | 63 |
| Shubin et al (24) | No | Yes | No | BTLA | Monocyte, PMN | NR | 27 |
| Shubin et al (25) | Yes | Yes | No | BTLA | Lymphocyte | 21c | 45 |
| Chang et al (26) | No | Yes | No | PD-1, PD-L1 | Lymphocyte, monocyte | 1–3; 4–7; 8–12; 13–21 | 58 |
| Gao et al (27) | Yes | No | No | PD-1, CTLA-4 | Lymphocyte | NR | 367 |
| Huang et al (28) | Yes | Yes | No | PD-1 | Lymphocyte | 0 | 70 |
| Pan et al (29) | Yes | No | Yes | PD-1, PD-L1 | Lymphocyte, monocyte | 1 | 86 |
| Shao et al (30) | Yes | Yes | Yes | BTLA | Lymphocyte, monocyte | 1 | 336 |
| Wang et al (31) | Yes | No | No | PD-L1 | PMN, Lymphocyte | NR | 51 |
| Jia et al (32) | Yes | No | Yes | PD-1 | Lymphocyte | NR | 295 |
| Patera et al (33) | Yes | Yes | No | PD-1, PD-L1 | Monocyte, PMN | 1–3 | 51 |
| Shao et al (34) | Yes | No | Yes | PD-1, PD-L1 | Lymphocyte, monocyte | 4 | 164 |
| Liao et al (35) | Yes | No | No | PD-1 | Lymphocyte | 1 | 152 |
| Liu et al (36) | Yes | No | Yes | PD-1 | Lymphocyte | 1 | 139 |
| Tomino et al (37) | Yes | No | No | PD-1 | Lymphocyte | 1; 3; 7 | 28 |
| Liu et al (38) | Yes | No | Yes | BTLA | Lymphocyte | 0 | 146 |
| Ferreira da Mota et al (39) | Yes | No | Yes | PD-1, PD-L1 | Monocyte | 0 | 92 |
| Tai et al (40) | No | No | Yes | PD-1, PD-L1 | Lymphocyte, monocyte | 0 | 177 |
| Wilson et al (41) | Yes | No | Yes | PD-1, PD-L1, PD-L2 | Lymphocyte, monocyte | 0 | NR |
| Xia et al (42) | Yes | No | No | PD-1, PD-L1, PD-L2 | Lymphocyte, monocyte | 1 | 43 |
| Mouillaux et al (43) | Yes | No | No | PD-1 | Lymphocyte | 1; 3; 7 | 65 |
| Niu et al (44) | No | Yes | No | PD-1 | Lymphocyte | 1; 5 | 44 |
| Zhao et al (45) | Yes | No | Yes | PD-1, PD-L1 | Lymphocyte, monocyte | 1 | 157 |
| Soluble checkpoint molecule concentration studies | |||||||
| Lange et al (46) | Yes | Yes | Yes | sPD-1, soluble CTLA-4, sBTLA | Plasma | 1 | 160 |
| Liu et al (47) | Yes | No | Yes | sPD-1, sPD-L1 | Plasma | 1 | 120 |
| Banerjee et al (48) | No | Yes | Yes | sPD-1, sPD-L1 | Plasma | 0; 3 | 60 |
| Monaghan et al (49) | No | Yes | No | sBTLA | Plasma | 0–1 | 60 |
| Wilson et al (41) | Yes | No | No | sPD-1, sPD-L1 | Plasma | 0 | NR |
| Zhao et al (50) | Yes | Yes | Yes | sPD-1 | Plasma | 0 | 595 |
| Zhao et al (45) | Yes | No | Yes | sPD-1, sPD-L1 | Plasma | 1; 7 | 157 |
BTLA = B- and T-lymphocyte attenuator, CTLA-4 = cytotoxic T lymphocyte antigen-4, NR = not reported, PD-1 = programmed death-1, PD-L1 = programmed death-ligand 1, PD-L2 = programmed death-ligand 2, PMN = polymorphonuclear leukocyte, sBTLA = soluble B- and T-lymphocyte attenuator, sPD-1 = soluble programmed death-1, sPD-L1 = soluble programmed death-ligand 1.
Day of (0) or following (≥ 1) sepsis diagnosis unless otherwise specified.
Samples obtained at time of death from sepsis following mean duration of sepsis of 4 d (range, 1–40 d); control spleen samples obtained following brain death declaration or at emergency splenectomy following trauma; and control lung samples obtained from transplant donor lungs or nontumor involved tissue from malignant lobectomies.
Samples from median sampling times post-ICU admission of 4 d (range, 0–43 d) systemic inflammatory response syndrome patients and 21 d (range, 3–83 d) septic patients.
All studies were prospective observational designs.
Sepsis Versus Healthy Control Subjects
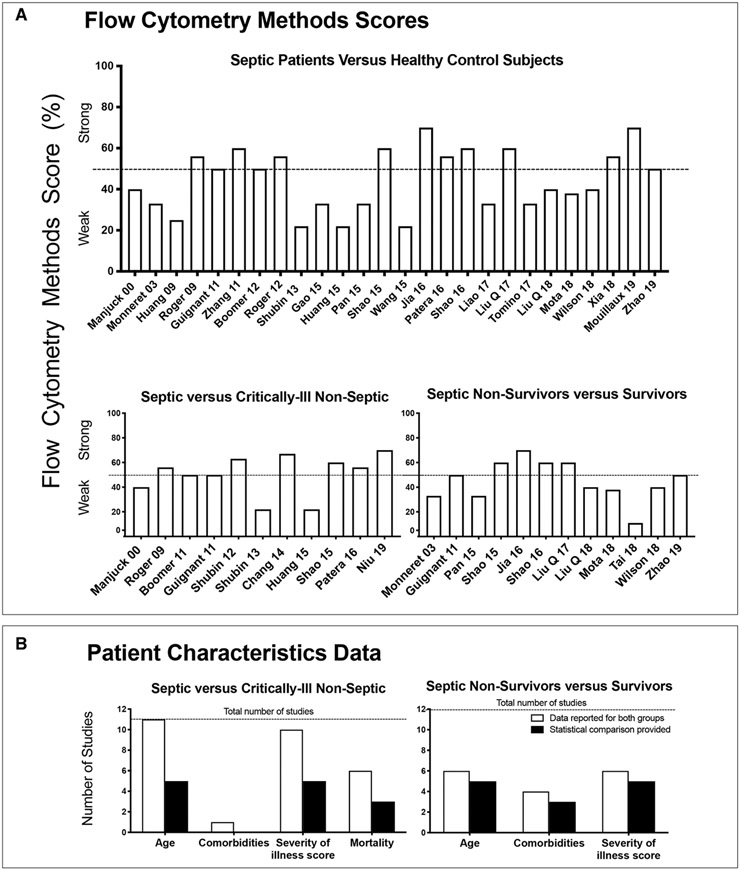
Twenty-six studies compared cell-surface CME in sepsis versus HCs (Table 1; and Supplementary Table 3, Supplemental Digital Content 8, http://links.lww.com/CCM/F627). Twenty-four studies provided the timing of sample acquisition, with most studies (21) collecting samples within 24 hours of study enrollment. Three studies provided serial measurements. For individual flow score elements, see Figure 1. Together, 10 of 24 studies (42%) reported greater than 50% of these elements (Fig. 1 and Fig. 2A).
Figure 1.
Summary of whether studies did (yes in green) or did not (no in red) report on the 10 individual components of the flow cytometry methods score and the overall score. Red cells indicate that an item was not reported/provided for the given study. Green cells indicate an item was reported/provided for the given study. Yellow cells indicate that an item is not applicable to the given study. §Only total WBC provided; #mean provided but no error; ¶mean provided for sepsis versus healthy control (HC), but not for nonsurvivors versus survivors; and @mean provided for sepsis versus HC. BTLA = B- and T-lymphocyte attenuator, CTLA-4 = cytotoxic T lymphocyte antigen-4, lymph = lymphocyte, MFI = mean fluorescence intensity, mono = monocyte, NA = not applicable, PD-1 = programmed death-1, PD-L1 = programmed death-ligand 1, PMN = polymorphonuclear leukocyte.
Figure 2.
Study methods and patient characteristics. Flow cytometry methods scores (A) and baseline patient characteristics data (B) for cell-surface checkpoint molecule expression studies comparing septic patients to healthy control subjects or critically ill nonseptic patients or septic nonsurvivors versus survivors. A, Each bar shows for each study noted on the x-axis, the flow cytometry methods score on the y-axis. The score represents the percentage of the number of criteria of the methods score (maximum 8 to 10) a study reported on or employed (see Materials and Methods and Appendix 3 [Supplemental Digital Content 3, http://links.lww.com/CCM/F622] for methods criteria). A score greater than 50% was considered strong. B, Each open bar represents the number of studies that provided the data noted on the x-axis for both study groups (either septic and nonseptic critically ill patients or septic nonsurvivor and survivor), and the closed bar represents the number of studies that provided a statistical analysis comparing these data for these study groups. The horizontal dotted line shows the total number of studies that were reviewed for each patient comparison.
Ten studies provided ages of septic and HC subjects and eight studies reported the two groups were age-matched. Only five studies reported the frequency of baseline comorbidities potentially affecting CME in sepsis patients (22, 34, 36, 37, 41).
Sepsis was associated with significant increases in PD-1 expression on CD3+/CD4+ lymphocytes in 13 studies and no difference in one (Fig. 3, A and D; Supplementary Fig. 2, A, D, G, and J, Supplemental Digital Content 9, http://links.lww.com/CCM/F628; Supplementary Table 4, Supplemental Digital Content 10, http://links.lww.com/CCM/F629). CD8+ lymphocyte or monocyte PD-1 expression increased with sepsis in seven and five studies, respectively, and was not different in two studies each. Sepsis-associated changes in PD-L1, CTLA-4, and BTLA expression on lymphocytes were investigated less frequently and were variable with greater than or equal to 20% of studies of each checkpoint molecule and cell type showing significant decreases or no change. Monocyte PD-L1 expression was increased in seven studies. Only two studies reported neutrophil CME. PD-L1 expression was increased and not different in one each.
Figure 3.
Changes in checkpoint molecule expression associated with sepsis. A–F, The number of studies reporting either a statistically significant increase (black bars), significant decrease (white bars), or no significant difference (gray bar) in the checkpoint molecule programmed death-ligand 1 (PD-1), programmed death-ligand 1 (PD-L1), cytotoxic T lymphocyte antigen-4 (CTLA-4), or B- and T-lymphocyte attenuator (BTLA) on CD3+ or CD4+ lymphocytes or monocytes in studies that compared this expression in septic patients versus healthy control (HC) subject (A and D), septic versus critically ill nonseptic (CINS) patients (B and E), or septic nonsurvivors (NS) versus survivors (S) (C and F). Numbers in parentheses denote the number of studies which provided mean with variability data. For studies presenting checkpoint molecule expression (CME) as both cell frequency and mean fluorescence intensity (MFI), or that presented CME on more than 1 d after presentation, if either measure was significantly different in septic versus control patients or in septic NS versus S on any day measurements were made, the study was recorded as demonstrating a significant change (see Materials and Methods). †One study (32) reported no difference in %BTLA+ in septic versus HC, but decreased %BTLA+ in severe sepsis or septic shock versus HC, and increased BTLA MFI in septic versus HC, but no difference in BTLA MFI in severe sepsis or septic shock versus HC. This study received notation for both an increase and decrease. *One study displayed the indicated change by cell frequency but there was no difference by MFI. For detailed results, please see Supplementary Table 4 (Supplemental Digital Content 10, http://links.lww.com/CCM/F629), Supplementary Table 5 (Supplemental Digital Content 12, http://links.lww.com/CCM/F631), Supplementary Table 6 (Supplemental Digital Content 13, http://links.lww.com/CCM/F632), Supplementary Table 7 (Supplemental Digital Content 14, http://links.lww.com/CCM/F633), and Supplementary Table 8 (Supplemental Digital Content 14, http://links.lww.com/CCM/F634).
Five studies investigated soluble checkpoint molecule levels, see Appendix 5 (Supplemental Digital Content 11, http://links.lww.com/CCM/F630) and Supplementary Table 5 (Supplemental Digital Content 12, http://links.lww.com/CCM/F631) for results.
Septic Versus Critically Ill Nonseptic Controls
Eleven studies compared cell-surface CME in septic versus CINS patients (Table 1; and Supplementary Table 3, Supplemental Digital Content 8, http://links.lww.com/CCM/F627). Individual CINS definitions are listed in Supplementary Table 3 (Supplemental Digital Content 8, http://links.lww.com/CCM/F627), but generally included ICU patients without evidence of infection or sepsis. Eight studies assayed CME within the first day after enrollment, and two provided data at multiple timepoints.
For details on the individual flow score elements, see Figure 1. Together, six of the 11 studies reported greater than 50% of these elements (Fig. 1 and Fig. 2A).
Ages were reported for both study groups in all 11 cell-surface CME studies, severity of illness measure in 10, mortality rates in six, and comorbidities in one. Only five studies provided statistical analysis comparing these parameters (Fig. 2B; and Supplementary Table 6, Supplemental Digital Content 13, http://links.lww.com/CCM/F632). Severity of illness scores and mortality were greater with sepsis in three of the five studies providing statistics for the former and in the two of three studies for the latter. In the only study providing comorbidity data for both groups, the frequencies of diabetes, morbid obesity, renal, respiratory, or liver disease were all higher in septic patients but analysis was not provided.
In contrast to the relatively large number of studies reporting increased PD-1 expression in septic versus HCs, when compared with CINS patients, sepsis was associated with increased PD-1 expression in only three studies and no significant difference in one (Fig. 3, B and E; and Supplementary Fig. 2, B, E, H, and K, Supplemental Digital Content 9, http://links.lww.com/CCM/F628; Supplementary Table 7 (Supplemental Digital Content 14, http://links.lww.com/CCM/F633). Sepsis was associated with increases in PD-1 expression on CD8+ lymphocytes and monocytes in two and one study, respectively, and no difference in one study for monocytes. Neutrophil, lymphocyte, and monocyte expression of PD-L1, CTLA-4, or BTLA were studied in less than or equal to two studies each and the results were variable (details in Supplementary Table 7, Supplemental Digital Content 14, http://links.lww.com/CCM/F633).
For the four soluble checkpoint molecule studies, comorbidity data are provided in Supplementary Table 7 (Supplemental Digital Content 14, http://links.lww.com/CCM/F633) and results in Supplementary Figure 2 (Supplemental Digital Content 9, http://links.lww.com/CCM/F628), Appendix 5 (Supplemental Digital Content 11, http://links.lww.com/CCM/F630), and Supplementary Table 5 (Supplemental Digital Content 15, http://links.lww.com/CCM/F631).
One study compared CME on tissue immune cells from patients who died of sepsis versus cells from CINS patients harvested at the time of surgery or death (19). Splenic CD4+ PD-1 and dendritic cell PD-L1 expression were increased in septic patients (Supplementary Table 7, Supplemental Digital Content 16, http://links.lww.com/CCM/F633). Expression by other cells or of other molecules were not significantly different.
Sepsis Nonsurvivors Versus Sepsis Survivors
Twelve studies compared CME in septic nonsurvivors versus survivors (Table 1; and Supplementary Table 3, Supplemental Digital Content 7, http://links.lww.com/CCM/F626). Ten studies assayed CME within the first day after study enrollment. One study provided two timepoints of analysis. For details on individual flow score elements, see Figure 1. Four of the 12 studies (33%) reported greater than 50% of these elements (Fig. 1 and Fig. 2A).
Baseline characteristics data were provided for both groups for age and illness severity measures in six studies and comorbidities in four of these six (Fig. 2B; and Supplementary Table 6, Supplemental Digital Content 13, http://links.lww.com/CCM/F632). Five studies provided statistical analyses and nonsurvivors were significantly older than survivors in one study and had higher severity of illness scores in all five. Four studies provided comorbidity data, and of the three with statistical analysis, one documented more comorbidities in sepsis nonsurvivors.
Sepsis nonsurvivors had increased CD4+ PD-1 expression in five studies and no significant difference in two (Fig. 3, C and F; Supplementary Fig. 2, C, F, I, and L, Supplemental Digital Content 9, http://links.lww.com/CCM/F628; and Supplementary Table 8, Supplemental Digital Content 14, http://links.lww.com/CCM/F634). Nonsurvivors PD-1 expression was increased on CD8+ lymphocytes in four studies and on monocytes in one study but decreased in another. CD4+ lymphocyte PD-L1 expression was increased in one study and not different in three. CTLA-4 or BTLA were studied in less than or equal to two studies each and in most studies were not different or decreased in nonsurvivors. Monocyte PD-L1 expression was increased in nonsurvivors in five studies but decreased in one.
For the five soluble checkpoint molecule studies, comorbidity data are provided in Supplementary Table 6 (Supplemental Digital Content 13, http://links.lww.com/CCM/F632) and results in Supplementary Figure 2 (Supplemental Digital Content 9, http://links.lww.com/CCM/F628), Appendix 5 (Supplemental Digital Content 11, http://links.lww.com/CCM/F630), and Supplementary Table 5 (Supplemental Digital Content 12, http://links.lww.com/CCM/F631).
Covariate Analysis and CME Relationship With Sepsis Outcomes, Severity, and Microbial Clearance
No study provided an adjusted effect of sepsis on CME or soluble marker levels based on important baseline covariates. Five studies provided area under the receiver operating characteristics curve (AUC) estimates for the relationship between CME on circulating cells and sepsis mortality (Supplementary Table 9, Supplemental Digital Content 16, http://links.lww.com/CCM/F635). These estimates were between 0.57 and 0.74 (fair to poor), except for two studies examining PD-L1 on monocytes in which the AUCs were 0.85 and 0.849, but these estimates lacked variability and did not report whether results were based on univariate or multivariate logistic regression. The AUC estimates for soluble checkpoint molecules in three studies were 0.71 for soluble PD-L1 and 0.73–0.87 for soluble PD-1 (sPD-1). The AUC for sPD-1 increased to 0.843 when combined with Mortality in Emergency Department Sepsis score and procalcitonin measurement. In the only study testing it (30) compared with HC subjects, the frequency of BTLA+CD4+ cells was not different in patients with sepsis but was actually decreased in those with severe sepsis or septic shock, while BTLA MFI was increased in patients with sepsis but not in those with severe sepsis or septic shock. No study examined whether increased CME levels in septic patients were associated with worsened microbial clearance.
DISCUSSION
This systematic review suggests that clinical CME studies do not presently provide a strong rationale for trials of CPI therapy in sepsis due to limited data for relevant patient groups and important outcomes, failure to control for confounding clinical factors, and weak flow cytometry methodology. CME changes were most consistent when comparing septic patients to HCs, but were variable when comparing septic to CINS patients and septic nonsurvivors to survivors.
The association between sepsis and cell-surface CME was investigated in the greatest number of studies when compared with HCs. These findings suggest that sepsis may be associated with increases in lymphocyte PD-1 and monocyte PD-L1, but provide no evidence that this is unique to the pathogenesis of sepsis or contributes to sepsis lethality.
Studies comparing septic versus CINS patients are more informative regarding the uniqueness of increased CME for sepsis. Notably, 10 studies provided no baseline comorbidity data to demonstrate that nonseptic conditions potentially altering CME (51-58) were similar between groups. Comorbidities were more frequent in septic patients when studied. Severity of illness and mortality were also greater in the sepsis group. Since conditions present in CINS patients can themselves stimulate CME (e.g., trauma, surgery), increases in CME may have been related to differences in the severity of underlying acute illness and not the presence of sepsis (3, 59, 60). In a propensity score matched analysis from the CHEST trial, critically ill septic and nonseptic patients had comparable outcomes when carefully matched for baseline characteristics and severity of illness (61). This highlights the importance of selecting an appropriate control population and matching of relevant clinical markers in future sepsis trials and observational studies, particularly those assessing the value of immunologic markers as therapeutic targets.
In 12 studies comparing septic nonsurvivors and survivors, CME was variable or not investigated extensively. Lymphocyte PD-1 expression and monocyte PD-L1 expression again showed the most consistent association between increased expression and sepsis mortality. Only three studies reported baseline comorbidities and provided statistical analysis. Comorbidities were increased in septic nonsurvivors in one.
Notably, no study adequately adjusted for baseline covariates (age, comorbidities, or severity of illness). Additionally, the timing of sample collection differed among studies, which is important to the kinetics of this axis (62). Most studies collected samples within 1 day of enrollment, representing the early septic response, although duration of antecedent illness prior to presentation was not reported. Relatively few studies provided serial CME data, which may be more informative given the dynamic nature of sepsis.
In cancer, the best predictor of response to therapy is PD-L1 expression in tumor tissue (63). However, analysis of CME in septic patients must rely on circulating or extravascular fluid cells, which may not be equivalent (64). Comparable data from oncology patients are limited (51, 65-67). Overall, no circulating immune cell CME phenotype has been identified that clearly predicts therapeutic responses. Notably, in the two recent phase I trials investigating CPIs in sepsis, no measures of CME were reported by the investigators. Low absolute lymphocyte count was an inclusion criterion, and no dose-related changes were seen with treatment. Monocyte human leukocyte antigen-DR expression increased over the study period in both, but there was no commensurate difference in serum cytokine levels (11, 12). Based on these data and the prior animal sepsis literature, it is unclear if elevated CME predicts responsiveness to CPI therapy in sepsis (68). If immune checkpoint inhibition remains under investigation, more detailed analyses of these signaling pathways must be performed across multiple subsets of patients with diverse backgrounds and infectious etiologies. Without this type of epidemiologic data and improved pre-clinical models, it is difficult to predict a priori which therapeutic strategies will have a desirable effect or could predispose to dangerous immune-related adverse events (IRAEs). For example, combination therapy with anti-CTLA-4 and anti-PD-1 in multiple cancer trials resulted in improved anti-tumor responses but also in significantly higher rate and broader range of IRAEs compared with monotherapy (69).
As availability of flow cytometric technology improves, a commitment to more rigorous experimental design and reporting of results is needed to facilitate high quality, consistently reproducible translational investigations prior to initiating clinical trials of CPI therapy for sepsis. For studies utilizing immunologic endpoints such as CME to support trials of CPI for sepsis, we suggest that several laboratory and methodological issues be considered. First, studies must employ consistent and rigorous flow cytometry methods so that sepsis effects can be reliably compared across studies. Several groups such as the Minimum Information for Biological and Biological Information foundry and International Society for Advancement of Cytometry endorsed the Minimum Information about a Flow Cytometry Experiment guideline. This guideline sought to standardize the details of experimental methodology that should be reported in scientific publications to improve reproducibility. However, this guideline is broad and does not include measures allowing similar interpretation of data across studies (14). Similarly, the Minimum Quality Threshold in Pre-Clinical Sepsis Studies consensus (70) was limited to experimental procedures and did not include any aspect of sample collection, laboratory processes, or data analysis. In synthesizing flow cytometry data across a large number of observational trials, we discovered a wide array of laboratory methods including investigations of differing cell populations, inconsistent gating strategies, and incomplete data reporting. These limitations hindered meta-analysis and possible clinical application of the data.
To address these limitations and improve interpretation of results and comparison across studies, we have provided a 10-item flow cytometry score highlighting what we believe are important and attainable characteristics of flow cytometry experiments in sepsis studies (Appendix 3, Supplemental Digital Content 3, http://links.lww.com/CCM/F622; and Fig. 1). Second, studies comparing septic to CINS patients or septic nonsurvivors to survivors must control for differences in nonseptic characteristics influencing CME such as comorbidities, age, and severity of illness. Studies of septic patients compared with healthy subjects provide minimal insight into the unique immunopathology of sepsis, and results obtained from these studies should be validated by comparison with cohorts of nonseptic critically ill patients. Third, studies should investigate whether CME levels actually correlate with impaired microbial clearance, a central hypothesis for CPI efficacy in sepsis. Although difficult to study in humans, preclinical models testing efficacy of CPIs should incorporate bacterial cultures, ideally of both local and distant sites, to document effects on microbial clearance.
CONCLUSIONS
Although the present studies suggest that sepsis is associated with increases in CME on circulating immune cells compared with HCs, they do not clearly demonstrate that CME differs in septic versus CINS patients or in septic nonsurvivors versus survivors. Studies employing standardized CME measurement and assessing the sensitivity and specificity of these measures after adjusting for influential covariates are necessary prior to trials targeting CME diagnostically or therapeutically in septic patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank Judith Welsh, BSN, MLS for her contribution in designing and performing the literature search.
Intramural funding from the National Institutes of Health supported this work.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
REFERENCES
- 1.Singer M, Deutschman CS, Seymour CW, et al. : The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315:801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Monneret G, Payen D: Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013; 13:862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavaillon JM, Eisen D, Annane D: Is boosting the immune system in sepsis appropriate? Crit Care 2014; 18:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Opal S: New therapeutics for sepsis. Int J Infect Dis 2016; 45:64 [Google Scholar]
- 5.Attanasio J, Wherry EJ: Costimulatory and coinhibitory receptor pathways in infectious disease. Immunity 2016; 44:1052–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buchbinder EI, Desai A: CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016; 39:98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honda T, Egen JG, Lämmermann T, et al. : Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity 2014; 40:235–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callahan MK, Postow MA, Wolchok JD: Targeting T cell co-receptors for cancer therapy. Immunity 2016; 44:1069–1078 [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Han X: Anti-PD-1/PD-L1 therapy of human cancer: Past, present, and future. J Clin Invest 2015; 125:3384–3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao M, Valentini D, Dodoo E, et al. : Anti-PD-1/PD-L1 therapy for infectious diseases: Learning from the cancer paradigm. Int J Infect Dis 2017; 56:221–228 [DOI] [PubMed] [Google Scholar]
- 11.Hotchkiss RS, Colston E, Yende S, et al. : Immune checkpoint inhibition in sepsis: A phase 1b randomized, placebo-controlled, single ascending dose study of antiprogrammed cell death-ligand 1 antibody (BMS-936559). Crit Care Med 2019; 47:632–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotchkiss RS, Colston E, Yende S, et al. : Immune checkpoint inhibition in sepsis: A phase 1b randomized study to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of nivolumab. Intensive Care Med 2019; 45:1360–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group: Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med 2009; 151:264–269, W64 [DOI] [PubMed] [Google Scholar]
- 14.Lee JA, Spidlen J, Boyce K, et al. : MIFlowCyt: The minimum information about a flow cytometry experiment. Cytometry A 2008; 73:926–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manjuck J, Saha DC, Astiz M, et al. : Decreased response to recall antigens is associated with depressed costimulatory receptor expression in septic critically ill patients. J Lab Clin Med 2000; 135:153–160 [DOI] [PubMed] [Google Scholar]
- 16.Monneret G, Debard AL, Venet F, et al. : Marked elevation of human circulating CD4+CD25+ regulatory T cells in sepsis-induced immunoparalysis. Crit Care Med 2003; 31:2068–2071 [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Venet F, Wang YL, et al. : PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A 2009; 106:6303–6308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roger PM, Hyvernat H, Breittmayer JP, et al. : Enhanced T-cell apoptosis in human septic shock is associated with alteration of the costimulatory pathway. Eur J Clin Microbiol Infect Dis 2009; 28:575–584 [DOI] [PubMed] [Google Scholar]
- 19.Boomer JS, To K, Chang KC, et al. : Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011; 306:2594–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guignant C, Lepape A, Huang X, et al. : Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care 2011; 15:R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Li JB, Lou JS, et al. : Upregulation of programmed death-1 on T cells and programmed death ligand-1 on monocytes in septic shock patients. Crit care 2011; 15:R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boomer JS, Shuherk-Shaffer J, Hotchkiss RS, et al. : A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit Care 2012; 16:R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roger PM, Hyvernat H, Ticchioni M, et al. : The early phase of human sepsis is characterized by a combination of apoptosis and proliferation of T cells. J Crit Care 2012; 27:384–393 [DOI] [PubMed] [Google Scholar]
- 24.Shubin NJ, Chung CS, Heffernan DS, et al. : BTLA expression contributes to septic morbidity and mortality by inducing innate inflammatory cell dysfunction. J Leukoc Biol 2012; 92:593–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shubin NJ, Monaghan SF, Heffernan DS, et al. : B and T lymphocyte attenuator expression on CD4+ T-cells associates with sepsis and subsequent infections in ICU patients. Crit Care 2013; 17:R276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang K, Svabek C, Vazquez-Guillamet C, et al. : Targeting the programmed cell death 1: Programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit Care 2014; 18:R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao DN, Yang ZX, Qi QH: Roles of PD-1, Tim-3 and CTLA-4 in immunoregulation in regulatory T cells among patients with sepsis. Int J Clin Exp Med 2015; 8:18998–19005 [PMC free article] [PubMed] [Google Scholar]
- 28.Huang H, Xu R, Lin F, et al. : High circulating CD39(+) regulatory T cells predict poor survival for sepsis patients. Int J Infect Dis 2015; 30:57–63 [DOI] [PubMed] [Google Scholar]
- 29.Pan T, Liu Z, Yin J, et al. : Notch signaling pathway was involved in regulating programmed cell death 1 expression during sepsis-induced immunosuppression. Mediators Inflamm 2015; 2015:539841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao R, Li CS, Fang Y, et al. : Low B and T lymphocyte attenuator expression on CD4+ T cells in the early stage of sepsis is associated with the severity and mortality of septic patients: A prospective cohort study. Crit Care 2015; 19:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang JF, Li JB, Zhao YJ, et al. : Up-regulation of programmed cell death 1 ligand 1 on neutrophils may be involved in sepsis-induced immunosuppression: An animal study and a prospective case-control study. Anesthesiology 2015; 122:852–863 [DOI] [PubMed] [Google Scholar]
- 32.Jia Y, Zhao Y, Li C, et al. : The expression of programmed death-1 on CD4+ and CD8+ T lymphocytes in patients with type 2 diabetes and severe sepsis. PLoS One 2016; 11:e0159383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patera AC, Drewry AM, Chang K, et al. : Frontline science: Defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J Leukoc Biol 2016; 100:1239–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao R, Fang Y, Yu H, et al. : Monocyte programmed death ligand-1 expression after 3-4 days of sepsis is associated with risk stratification and mortality in septic patients: A prospective cohort study. Crit Care 2016; 20:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao XL, Feng T, Zhang JQ, et al. : Phenotypic changes and impaired function of peripheral γδ T cells in patients with sepsis. Shock 2017; 48:321–328 [DOI] [PubMed] [Google Scholar]
- 36.Liu Q, An L, Qi Z, et al. : Increased expression of programmed cell death-1 in regulatory T-cells of patients with severe sepsis and septic shock: An observational clinical study. Scand J Immunol 2017; 86:408–417 [DOI] [PubMed] [Google Scholar]
- 37.Tomino A, Tsuda M, Aoki R, et al. : Increased PD-1 expression and altered T cell repertoire diversity predict mortality in patients with septic shock: A preliminary study. PLoS One 2017; 12:e0169653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Q, Lu Y, An L, et al. : B- and T-lymphocyte attenuator expression on regulatory T-cells in patients with severe sepsis. Chin Med J (Engl) 2018; 131:2637–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira da Mota NV, Brunialti MKC, Santos SS, et al. : Immunophenotyping of monocytes during human sepsis shows impairment in antigen presentation: A shift toward nonclassical differentiation and upregulation of FCgammaRi-receptor. Shock (Augusta, Ga) 2018; 50:293–300 [DOI] [PubMed] [Google Scholar]
- 40.Tai H, Xing H, Xiang D, et al. : Monocyte programmed death ligand-1, a predicator for 28-day mortality in septic patients. Am J Med Sci 2018; 355:362–367 [DOI] [PubMed] [Google Scholar]
- 41.Wilson JK, Zhao Y, Singer M, et al. : Lymphocyte subset expression and serum concentrations of PD-1/PD-L1 in sepsis - pilot study. Crit Care 2018; 22:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia Q, Wei L, Zhang Y, et al. : Immune checkpoint receptors Tim-3 and PD-1 regulate monocyte and T lymphocyte function in septic patients. Mediators Inflamm 2018; 2018:1632902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mouillaux J, Allam C, Gossez M, et al. : TCR activation mimics CD127low PD-1high phenotype and functional alterations of T lymphocytes from septic shock patients. Crit Care 2019; 23:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niu B, Zhou F, Su Y, et al. : Different expression characteristics of LAG3 and PD-1 in sepsis and their synergistic effect on T cell exhaustion: A new strategy for immune checkpoint blockade. Front Immunol 2019; 10:1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y, Jia Y, Li C, et al. : Predictive value of soluble programmed death-1 for severe sepsis and septic shock during the first week in an intensive care unit. Shock 2019; 51:289–297 [DOI] [PubMed] [Google Scholar]
- 46.Lange A, Sundén-Cullberg J, Magnuson A, et al. : Soluble B and T lymphocyte attenuator correlates to disease severity in sepsis and high levels are associated with an increased risk of mortality. PLoS One 2017; 12:e0169176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu M, Zhang X, Chen H, et al. : Serum sPD-L1, upregulated in sepsis, may reflect disease severity and clinical outcomes in septic patients. Scand J Immunol 2017; 85:66–72 [DOI] [PubMed] [Google Scholar]
- 48.Banerjee D, Monaghan S, Zhao R, et al. : Soluble programmed cell death protein-1 and programmed cell death ligand-1 in sepsis. Crit Care 2018; 22:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monaghan SF, Banerjee D, Chung CS, et al. : Changes in the process of alternative RNA splicing results in soluble B and T lymphocyte attenuator with biological and clinical implications in critical illness. Mol Med 2018; 24:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y, Jia Y, Li C, et al. : The risk stratification and prognostic evaluation of soluble programmed death-1 on patients with sepsis in emergency department. Am J Emerg Med 2018; 36:43–48 [DOI] [PubMed] [Google Scholar]
- 51.Wang Z, Aguilar EG, Luna JI, et al. : Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med 2019; 25:141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canter RJ, Le CT, Beerthuijzen JMT, et al. : Obesity as an immune-modifying factor in cancer immunotherapy. J Leukoc Biol 2018; 104:487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun P, Jin Q, Nie S, et al. : Unlike PD-L1, PD-1 is downregulated on partial immune cells in type 2 diabetes. J Diabetes Res 2019; 2019:5035261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilkinson TMA: Immune checkpoints in chronic obstructive pulmonary disease. Eur Respir Rev 2017; 26:170045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elias R, Hartshorn K, Rahma O, et al. : Aging, immune senescence, andimmunotherapy: A comprehensive review. Sem Onc 2018; 45:187–200 [DOI] [PubMed] [Google Scholar]
- 56.Elias R, Karantanos T, Sira E, et al. : Immunotherapy comes of age: Immune aging & checkpoint inhibitors. J Geriatr Oncol 2017; 8:229–235 [DOI] [PubMed] [Google Scholar]
- 57.Vukmanovic-Stejic M, Sandhu D, Seidel JA, et al. : The characterization of varicella zoster virus-specific T cells in skin and blood during aging. J Invest Dermatol 2015; 135:1752–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weyand CM, Berry GJ, Goronzy JJ: The immunoinhibitory PD-1/PD-L1 pathway in inflammatory blood vessel disease. J Leukoc Biol 2018; 103:565–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cavaillon JM: Concomitant inflammation and immunosuppression. Shock (Augusta, Ga) 2016; 46:11–12 [Google Scholar]
- 60.Xu P, Zhang P, Sun Z, et al. : Surgical trauma induces postoperative T-cell dysfunction in lung cancer patients through the programmed death-1 pathway. Cancer Immunol Immunother 2015; 64:1383–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson K, Taylor C, Jan S, et al. : Health-related outcomes of critically ill patients with and without sepsis. Intensive Care Med 2018; 44:1249–1257 [DOI] [PubMed] [Google Scholar]
- 62.Drewry AM, Samra N, Skrupky LP, et al. : Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 2014; 42:383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daud AI, Wolchok JD, Robert C, et al. : Programmed death-ligand 1 expression and response to the anti-programmed death 1 anti-body pembrolizumab in melanoma. J Clin Oncol 2016; 34:4102–4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davies R, O’Dea K, Gordon A: Immune therapy in sepsis: Are we ready to try again? J Intensive Care Soc 2018; 19:326–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamphorst AO, Pillai RN, Yang S, et al. : Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A 2017; 114:4993–4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou J, Mahoney KM, Giobbie-Hurder A, et al. : Soluble PD-L1 as a biomarker in malignant melanoma treated with checkpoint blockade. Cancer Immunol Res 2017; 5:480–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagato T, Ohkuri T, Ohara K, et al. : Programmed death-ligand 1 and its soluble form are highly expressed in nasal natural killer/T-cell lymphoma: A potential rationale for immunotherapy. Cancer Immunol Immunother 2017; 66:877–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Busch LM, Sun J, Cui X, et al. : Checkpoint inhibitor therapy in preclinical sepsis models: A systematic review and meta-analysis. Intensive Care Med Exp 2020; 8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boutros C, Tarhini A, Routier E, et al. : Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol 2016; 13:473–486 [DOI] [PubMed] [Google Scholar]
- 70.Osuchowski MF, Ayala A, Bahrami S, et al. : Minimum Quality Threshold in Pre-Clinical Sepsis Studies (MQTiPSS): An international expert consensus initiative for improvement of animal modeling in sepsis. Infection 2018; 46:687–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.