Abstract
Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces various functions, including the proliferation and differentiation of a broad range of hematopoietic cells. We previously reported that at least two distinct pathways are involved in human GM-CSF receptor signaling; both require the box 1 region of the common β subunit (βc). This region is essential for the activation of JAK2, which is necessary for all the biological functions of GM-CSF. The activation of JAK2 by GM-CSF leads to rapid tyrosine phosphorylation of cellular proteins, including the βc. However, the significance of βc phosphorylation with regard to the regulation of signaling molecules and the expression of GM-CSF functions is less well understood. Here we investigated the role of the cytoplasmic tyrosine residues of the βc by using a series of βc mutants expressed in murine BA/F3 cells. A mutant βc with all eight cytoplasmic tyrosines converted to phenylalanine (Fall) activated JAK2 but not SHP-2, MAPK cascades, STAT5, or the c-fos promoter in BA/F3 cells, and it did not effectively induce proliferation. Adding back each tyrosine to Fall revealed that Tyr577, Tyr612, and Tyr695 are involved in the activation of SHP-2, MAPK cascades, and c-fos transcription, while every tyrosine, particularly Tyr612, Tyr695, Tyr750, and Tyr806, facilitated STAT5 activation. Impaired growth was also restored, at least partly, by any of the tyrosines. These results provide evidence that βc tyrosines possess distinct yet overlapping functions in activating multiple signaling pathways induced by GM-CSF.
Cytokines have specific biological functions, including proliferation, differentiation, and functional modulation, in target cells expressing their cognate receptors (2). Thus, most cytokine receptors are coupled with multiple signaling pathways, which act in concert to govern the functional specificity of a particular cytokine. How each cytokine regulates multiple signals downstream of its receptor is less well understood.
Although most cytokine receptors do not possess intrinsic tyrosine kinase domains, they do interact with one or more nonreceptor tyrosine kinases. Stimulation with their cognate ligands results in rapid and reversible tyrosine phosphorylation of multiple proteins, including the receptors themselves (43). The importance of tyrosine phosphorylation in cytokine signaling has been suggested by findings from various experiments in which tyrosine kinase inhibitors were used. Many signaling molecules with SH2 (Src homology 2) and/or PTB (phosphotyrosine binding) domains, such as Shc, SHPs (SH2-containing protein tyrosine phosphatases), and signal transducers and activators of transcription (STATs), have been reported to be recruited onto various cytokine receptors following ligand stimulation (43). As is the case for growth factor receptors with an intrinsic tyrosine kinase domain, tyrosine residues of cytokine receptors are likely to play critical roles in regulating downstream signaling pathways by being phosphorylated and hence by providing specific recognition motifs for SH2 domain and/or PTB domain-containing proteins.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a pleiotropic cytokine which supports proliferation, survival, and differentiation of hematopoietic progenitor cells; it also enhances the multiple functions of mature neutrophils, macrophages, and eosinophils (4, 14). A functional, high-affinity receptor for GM-CSF is composed of α and β subunits, both belonging to the type I cytokine receptor superfamily (or hematopoietin receptor family) (5, 15, 19, 23). The β subunit, which is also shared by the interleukin 3 (IL-3) and IL-5 receptors (and is thereby termed the common β subunit [βc]), has a relatively large cytoplasmic domain and plays a pivotal role in signal transduction (30).
GM-CSF binding induces the formation of a complex between α and β subunits, which then triggers the activation of several tyrosine kinases, including JAK2 (17, 37, 45). A series of experiments with a dominant-negative type of JAK2 revealed that the activity of JAK2 is necessary for all the biological functions expressed by GM-CSF (46). For JAK2 activation, the membrane-proximal region of the βc containing the box 1 motif is necessary and sufficient. GM-CSF induces in target cells the expression of early-response genes, such as c-fos, c-jun, and c-myc (30). For induction of the c-fos gene, not only JAK2 activation but also a membrane-distal region of the βc containing several tyrosine residues is required (22). Since GM-CSF stimulation results in tyrosine phosphorylation of the receptor βc subunit (11, 39) as well as proteins with SH2 and/or PTB domain(s), such as Shc, SHP-2, Vav, c-Cbl, and STAT5 (29, 34, 35, 41, 50), a possible role of the βc tyrosines in signaling was considered. We reported that GM-CSF-induced activation of the c-fos promoter by β589, a truncated mutant βc, was significantly diminished by substitution of a single tyrosine at position 577 (Tyr577), thereby indicating an important role of this tyrosine in signaling (22). However, the full-length βc with the same mutation at Tyr577 transduced signals sufficient to activate the c-fos promoter, suggesting that other functional domains, probably tyrosine residues, also transmit signals. Tyrosine phosphorylation of SHP-2 (previously termed PTP1D, SH-PTP2, or Syp) (1), a phosphotyrosine phosphatase proposed to be involved in Ras activation (7, 26), correlated well with this phenomenon; that is, SHP-2 phosphorylation was mediated either by Tyr577 or by other functional domains independent of this tyrosine. Although the significance of tyrosine phosphorylation of SHP-2 in Ras activation remains to be determined, it results in the association of SHP-2 with an adapter molecule, GRB2 (7, 26). These results suggest the existence of multiple pathways from the βc to c-fos transcription, probably through Ras, but the functional domain, other than Tyr577, that is responsible for these events remains to be clarified.
To better understand the pathways and the regulatory mechanism of GM-CSF signaling, we examined the roles of cytoplasmic tyrosines of the human βc using various mutants with one or more of these residues replaced by phenylalanine. The signaling potential of these mutants was analyzed by reconstituting high-affinity receptors in combination with the wild-type α subunit in murine BA/F3 cells. Our evidence shows that all eight βc tyrosines play critical roles in transmitting GM-CSF-induced signals for cell proliferation, with each one possessing distinct yet overlapping functions.
MATERIALS AND METHODS
Cells and culture.
A murine interleukin-3 (mIL-3)-dependent pro-B-cell line, BA/F3, was maintained in RPMI 1640 medium supplemented with 5% fetal calf serum, 50 U of penicillin per ml, 50 μg of streptomycin per ml, and 0.25 ng of mIL-3 per ml. Factor depletion was done with the same medium but without mIL-3 for cultures maintained at 37°C for 5 to 6 h. Endogenous murine βc (AIC2B) in BA/F3 cells can functionally interact with the human GM-CSF (hGM-CSF) receptor (hGM-CSFR) α subunit at high concentrations (100 ng/ml or more) of hGM-CSF and support proliferation (23). In this study, we used 10 ng of hGM-CSF per ml for stimulation unless otherwise indicated. Purified recombinant hGM-CSF produced in Escherichia coli was provided by Schering-Plough Corp. mIL-3 expressed in the silkworm (Bombyx mori) was purified as described previously (31).
Antibodies.
Rat monoclonal antibody against the human βc, 5A5, was prepared as described previously (49) and used for fluorescence-activated cell scanning (FACS) analysis. Antiphosphotyrosine monoclonal antibody (clone 4G10; 05-321) and antiserum against JAK2 (06-255) were obtained from Upstate Biotechnology, Inc. (Lake Placid, N.Y.), and antisera against the hGM-CSFR α subunit (sc-458), SHP-2 (sc-280), Raf-1 (sc-227), ERK2 (sc-154), and JNK1 (sc-474) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Rabbit polyclonal antibody against STAT5 (N1) was kindly provided by Hiroshi Wakao (Helix Research Institute, Kisarazu, Japan).
Plasmids.
The c-fos promoter–luciferase gene fusion plasmid contains the human c-fos promoter fragment (−404 to +41) fused to the luciferase fragment and was constructed as described previously (48). A serum response element (SRE)-chloramphenicol acetyltransferase (CAT) construct containing a 25-bp oligonucleotide of the c-fos SRE site inserted upstream of the mouse IL-3 promoter linked to the CAT gene (13) and a β-casein promoter–luciferase construct consisting of a luciferase gene under the control of a minimal β-casein promoter (34) were kindly provided by Kozo Kaibuchi (Nara Advanced Institute of Science and Technology, Ikoma, Japan) and Hiroshi Wakao (Helix Research Institute), respectively. A plasmid encoding the glutathione S-transferase (GST)–c-Jun fusion protein [pGEX2T-cJun(1-223)] (20) was a kind gift from Michael Karin (University of California, San Diego).
The hGM-CSFR βc (KH97) cDNA was originally cloned into the pME18S vector as described previously (19). The βc mutants used in this study were constructed by introducing point mutations by use of sequential PCR steps (3) with appropriately designed oligonucleotide primers. Tyrosine-coding codons TAC (at 450, 452, 577, 612, and 866) and TAT (at 695, 750, and 806) were changed to phenylalanine-coding codons TTC and TTT, respectively. These PCR-amplified fragments, alone or in combination, were used to replace the tyrosine-containing sequences of the wild-type βc in the pME18S vector by use of intrinsic restriction enzyme sites within the cDNA. The accuracy of all the nucleotide sequences of the fragments derived from the PCR was confirmed by dideoxy sequencing with an automated sequencer (Applied Biosystems Inc.).
Transient transfection and reporter gene assays.
Plasmid DNAs were transiently transfected into BA/F3 cells by electroporation. For each transfection, 3 μg of various βc mutant cDNAs or control vector (pME18S), in combination with either c-fos–luciferase (3 μg), SRE-CAT (10 μg), or β-casein–luciferase (15 μg), was used. BA/F3 cells which stably expressed the wild-type hGM-CSFR α subunit (3 × 106 cells) were washed twice with OPTI-MEM (Life Technologies, Inc.), resuspended in 200 μl of OPTI-MEM, and then mixed with plasmid DNAs. An electronic pulse was given with a Gene Pulser (Bio-Rad) set at 960 μF and 200 V. Cells were recovered, maintained in complete medium for about 12 h, and then separated into three aliquots. After factor depletion for 6 h in a mIL-3-free medium containing 5% fetal calf serum, the cells were stimulated with hGM-CSF (10 ng/ml), mIL-3 (1 ng/ml), or no cytokine and then harvested. Proteins were extracted from the cells by three cycles of freezing and thawing. The protein concentration was determined with bicinchoninic acid protein assay reagent (Pierce, Rockford, Ill.) according to the manufacturer’s instructions. Luciferase activity was measured with a luminometer (Lumat model LB9501; Berthold Japan K. K., Tokyo, Japan) and luciferase assay substrate (Promega, Madison, Wis.). CAT activity was measured by a diffusion assay as described previously (40). Data were subjected to a statistical analysis, and the P values were determined by Scheffe’s multiple-comparison test. P < 0.05 was considered statistically significant.
Establishment of stable transfectants.
The expression plasmid for the βc (13.5 μg) was transfected together with the pME18S vector containing a neomycin resistance gene (1.5 μg) by electroporation into BA/F3 cells which stably expressed the wild-type hGM-CSFR α subunit as described previously (20). After selection with G418 (1 mg/ml) for about 10 days, surface expression of the transfected βc gene products of the G418-resistant clones was confirmed by FACScan (Becton Dickinson) flow cytometry with anti-βc antibody 5A5. At least two independent clones were established for each of the βc mutants, and these clones were used in this study.
Ligand binding assay.
BA/F3 transfectants were incubated with 125I-Bolton-Hunter-labeled recombinant hGM-CSF (NEN Life Science) at 4°C for 2 h in the presence or absence of a 100-fold excess of unlabeled hGM-CSF in Hanks’ balanced salt solution (Life Technologies) containing 0.01% bovine serum albumin and 0.02% NaN3. Cell-bound 125I-hGM-CSF was separated from the free ligand by centrifugation through an oil layer (di-n-butyl phthalate–dioctyl phthalate [1:1.5]; Nacalai Tesque, Inc., Kyoto, Japan). The radioactivities associated with the cell-bound and free fractions were determined with a gamma counter (model 5550; Packard).
Preparation of protein samples.
To analyze tyrosine phosphorylation of JAK2, SHP-2, or STAT5, immunoprecipitation was done with the corresponding antibody as described previously (22).
For a mobility shift assay of Raf-1 or ERK2, total cell lysates of BA/F3 transfectants were prepared as follows. Factor-deprived cells were resuspended in factor-free medium at 106 cells/ml and then transferred into 1.5-ml tubes. The cells were incubated at 37°C for 30 min and then either left unstimulated or stimulated with 10 ng of hGM-CSF per ml. After stimulation, the cells were precipitated by a brief centrifugation and then lysed in lysis buffer A (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% Triton X-100, 30 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 50 mM NaF, 1 mM phenylmethylsulfonyl fluoride [PMSF], 2 μg of leupeptin per ml, 1 μg of pepstatin A per ml) for 1 h at 4°C. Cell lysates were centrifuged to remove the insoluble material, and the supernatants were mixed with an equal volume of 2× sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) sample buffer (2× SDS-PAGE sample buffer is 125 mM Tris-HCl [pH 6.8], 4% SDS, 20% glycerol, 2% 2-mercaptoethanol, and 0.01 mg of bromophenol blue per ml), boiled for 5 min, and then subjected to SDS-PAGE.
Western blot analysis.
Protein samples were electrophoresed on an SDS-polyacrylamide gel, electrophoretically transferred onto an Immobilon polyvinylidene difluoride membrane (Millipore), and then subjected to Western blot analysis with appropriate antibodies and enhanced chemiluminescence detection reagent (Amersham) as described previously (20).
JNK assay.
c-Jun N-terminal kinase/stress-activated protein kinase (JNK) activity was determined by an in vitro kinase assay with purified recombinant GST–c-Jun as a substrate as described previously (28). Briefly, factor-deprived BA/F3 transfectants (3 × 106 cells) were either left unstimulated or stimulated with 10 ng of hGM-CSF per ml at 37°C. Cells were lysed in lysis buffer B (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40, 1 mM sodium orthovanadate, 1 mM PMSF) at 4°C for 1 h. Cell lysates were centrifuged to remove the insoluble material, and then endogenous JNK was immunoprecipitated by incubation with a polyclonal antibody prebound to protein A-Sepharose (Pharmacia Biotech, Inc.) at 4°C for 2 h. The immunocomplexes were washed twice with lysis buffer B and twice with kinase assay washing buffer (20 mM HEPES [pH 7.5], 50 mM NaCl, 2.5 mM MgCl2, 0.1 mM EDTA, 0.05% Triton X-100). The kinase reaction was initiated by the addition of kinase buffer (20 mM HEPES [pH 7.5], 20 mM MgCl2, 20 mM β-glycerol phosphate, 1 mM sodium orthovanadate, 20 μM dithiothreitol, 20 μM ATP) containing 1 μg of purified GST–c-Jun(1-223) and 25 μM [γ-32P]ATP (10 Ci/mmol) to a final volume of 20 μl. After 20 min of incubation at 30°C, the reaction was terminated by the addition of SDS-PAGE sample buffer and boiling for 5 min. Samples were separated by SDS-PAGE, and the phosphorylation of GST–c-Jun was examined with a FUJI image analyzer (model BAS-2000).
Cell proliferation assays.
Short-term cell proliferation was measured by a colorimetric assay with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), originally developed by Mosmann (32), as described previously (22).
To analyze long-term proliferation, BA/F3 transfectants were deprived of mIL-3 for 6 h and then cultured in the absence or presence of either hGM-CSF (10 ng/ml) or mIL-3 (1 ng/ml). Viable cell numbers were counted by a trypan blue dye exclusion assay.
RESULTS
Multiple tyrosine residues transmit signals leading to activation of the c-fos promoter.
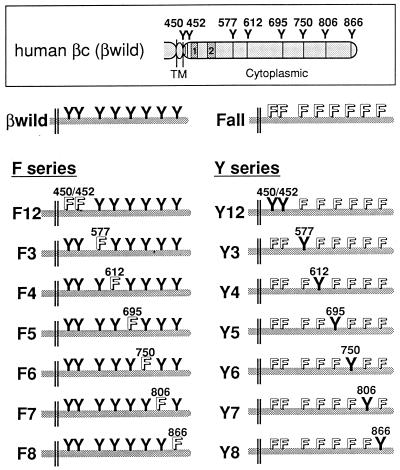
We have found that GM-CSF-induced activation of SHP-2 and the c-fos promoter is mediated by Tyr577 as well as by other functional residues of the βc located distal to amino acid 589 (22). This distal region contains five tyrosine residues (Fig. 1). The βc also has two more tyrosines within its cytoplasmic domain (thus, eight in total) just adjacent to the membrane-spanning region (Fig. 1). Their functional significance has not been investigated. To search for the possible involvement of these βc tyrosines in mediating GM-CSF signaling, we carried out a mutational analysis of all eight cytoplasmic tyrosines of the βc by phenylalanine substitution. The potential of the βc mutants to transduce signals was analyzed by reconstituting high-affinity GM-CSF receptors with the wild-type human α subunit in mIL-3-dependent BA/F3 cells.
FIG. 1.
Schematic structures of the βc mutants used in this study. The structures of the wild-type βc (boxed) and the βc mutants are shown, with extracellular portions abbreviated. Y and F, tyrosines and introduced phenylalanines, respectively (their amino acid positions are indicated); TM, transmembrane region; 1 and 2, box 1 and box 2 motifs, respectively. Note that the F3 mutant was previously termed βwild;Y577F (22, 46).
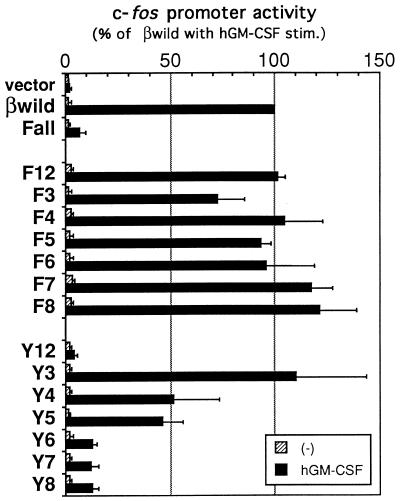
We first needed to know the requirement of βc tyrosines for activation of the c-fos promoter, and for this we used a transient transfection assay. A reporter plasmid carrying the c-fos promoter fused to the luciferase gene was cotransfected with receptor cDNAs. After stimulation with hGM-CSF, cell lysates were prepared, and their luciferase activities were determined (Fig. 2). Substitution of all eight tyrosines together (the Fall mutant; Fig. 1) remarkably diminished the potential of the βc to transduce c-fos promoter activation signals. In contrast, all of the mutants containing only a single mutated tyrosine, with the remaining residues intact (the F series mutants; Fig. 1), activated the c-fos promoter, which means that all of the individual tyrosine residues are dispensable for c-fos promoter activation. Thus, hGM-CSF-induced activation of the c-fos promoter is mediated through multiple cytoplasmic tyrosines of βc.
FIG. 2.
Multiple tyrosine residues transmit signals leading to activation of the c-fos promoter. Activation of the c-fos promoter by each βc mutant was measured by a transient transfection assay. The c-fos promoter–luciferase fusion construct was cotransfected with mutant βc cDNA into BA/F3 cells expressing the wild-type hGM-CSFR α subunit as described in Materials and Methods. After 6 h of factor depletion, cells were left unstimulated (−) or were stimulated with 10 ng of hGM-CSF per ml or 1 ng of mIL-3 per ml for 6 h at 37°C. Cell lysates were prepared, and their luciferase activities were measured with a luminometer. The c-fos promoter activity was calculated by dividing the luminescence intensity (relative light units per minute per microgram of total protein) of cells with no stimulation or hGM-CSF stimulation (stim.) by that of cells with mIL-3 stimulation and is presented as a percentage of that for the wild-type βc. All values are the averages of at least three experiments, and standard deviations are shown as error bars. Statistical significances of the differences in the c-fos promoter activities between hGM-CSF-stimulated samples were as follows: wild-type βc and Fall or any of the Y series mutants except for Y3, significant at P < 0.01; Y3 and Y4 or Y5, significant at P < 0.01; and Y4 and Y5, not significant.
We then constructed a series of mutants, namely, the Y series mutants (Fig. 1); each of these mutants contains only a single intact tyrosine residue, with the remaining tyrosines mutated. Using these Y series mutants, we examined whether each of the βc tyrosine residues alone could transmit signals leading to activation of the c-fos promoter. As shown in Fig. 2, the Y3 mutant, which possesses Tyr577 as the sole cytoplasmic tyrosine, activated the c-fos promoter to a level comparable to that seen with the wild-type βc, consistent with results obtained previously for truncation mutant β589 (22). In addition to the Y3 mutant, Y4 and Y5, possessing Tyr612 and Tyr695, respectively, also activated the promoter partially, albeit above the basal level. Therefore, three distinct tyrosine residues within the βc, namely, Tyr577, Tyr612, and Tyr695, can independently transduce signals activating the c-fos promoter.
Activation of JAK2 does not require βc tyrosine residues.
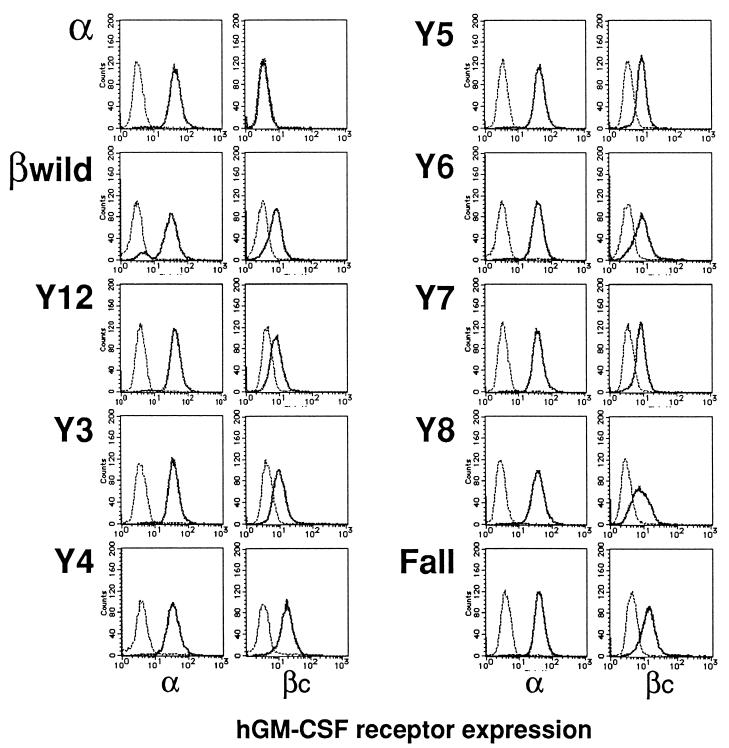
To assess the relationship of various signaling molecules involved in GM-CSF signaling with the βc tyrosines, we established stable transfectants of BA/F3 cells expressing the hGM-CSFR composed of the wild-type α subunit and a mutant βc subunit. Surface expression of the exogenously introduced receptors was examined by FACS analysis, and we confirmed that the levels of expression of both α and βc subunits were not significantly different among the transfectants (Fig. 3). To confirm that the βc mutants used in this study are capable of reconstituting high-affinity receptors, we performed ligand binding assays using 125I-labeled hGM-CSF. The Fall mutant clones exhibited high-affinity binding sites for hGM-CSF, with Kd values of 76 to 90 pM, comparable to those obtained with the wild-type human βc (Kd, 92 pM). These results indicate that the βc tyrosine residues are not required for the formation of high-affinity receptors for hGM-CSF.
FIG. 3.
Surface expression of the exogenously introduced hGM-CSFR subunits on BA/F3 transfectants. BA/F3 stable transfectants were incubated with either antibodies against the hGM-CSFR α and βc subunits (thick lines in left and right panels, respectively) or isotype control antibodies (thin lines). Cells were then stained with appropriate fluorescein isothiocyanate-conjugated secondary antibodies and subjected to FACS analysis. Surface expression of the F series mutants of the βc was also examined and confirmed to occur at similar levels (data not shown). Note that two or more stable clones were established for each of the mutants and that the results for one representative clone are shown here.
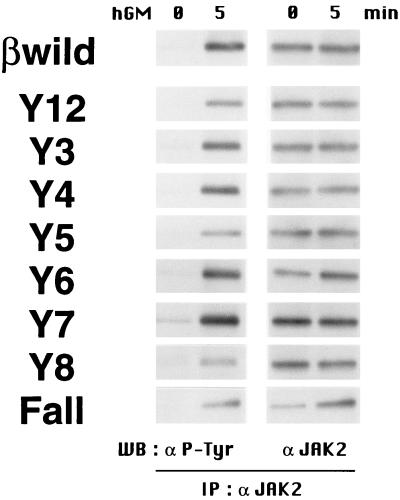
First, we checked the activation of tyrosine kinase JAK2, which is considered to be an initial step in receptor activation. BA/F3 stable transfectants were stimulated with hGM-CSF, and immunoprecipitation was performed with an anti-JAK2 antibody. Tyrosine phosphorylation of JAK2 was analyzed by Western blot analysis with an antiphosphotyrosine antibody. As shown in Fig. 4, JAK2 was activated by all of the βc mutants, including Fall, which contains no cytoplasmic tyrosines. Therefore, βc tyrosine residues are not required for JAK2 activation.
FIG. 4.
Activation of JAK2 does not require βc tyrosine residues. Factor-deprived BA/F3 stable transfectants (107 cells/sample) were left unstimulated or were stimulated with 10 ng of hGM-CSF per ml for 5 min at 37°C. Cells were lysed, and immunocomplexes with anti-JAK2 antibodies were precipitated. Protein samples were separated by SDS-PAGE, transferred onto polyvinylidene difluoride membranes, and subjected to Western blot (WB) analysis with antibodies against phosphotyrosine (4G10, left) or JAK2 (right). IP, immunoprecipitation.
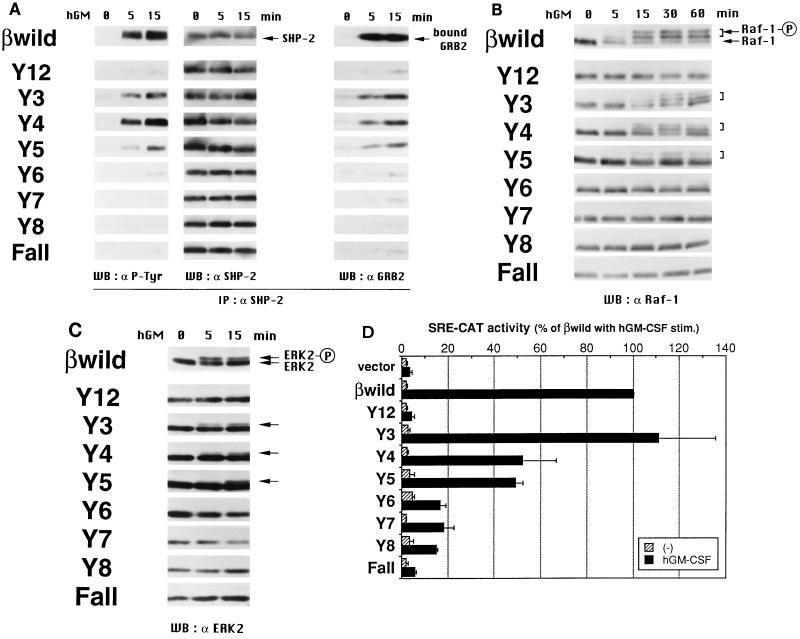
Tyr577, Tyr612, and Tyr695 induce SHP-2 phosphorylation and activate the Raf-1 and ERK2 pathway leading to the c-fos SRE site.
In earlier work, we analyzed two signaling molecules, Shc and SHP-2, which function as positive regulators in Ras activation. We found that Tyr577 is essential for tyrosine phosphorylation of Shc, whereas that of SHP-2 is mediated by Tyr577 as well as by other functional sites (22). To determine the possible involvement of SHP-2 with Tyr612 and/or Tyr695, both of which positively regulate c-fos promoter activation signals, tyrosine phosphorylation of SHP-2 in BA/F3 transfectants expressing various βc mutants was analyzed by immunoprecipitation and Western blot analyses (Fig. 5A). Among the Y series mutants, Y4 induced tyrosine phosphorylation of SHP-2 at a level similar to that induced by the wild-type βc. SHP-2 phosphorylation was also induced by Y3 and Y5, although at a slightly lower level. This phosphorylation correlated with coimmunoprecipitation of the adapter protein GRB2 with SHP-2, as revealed by reprobing of the same membrane with an anti-GRB2 antibody, thereby implying a relationship with Ras activation. These observations show that Tyr612 and Tyr695, in addition to Tyr577, can induce tyrosine phosphorylation of SHP-2 and its subsequent association with GRB2.
FIG. 5.
Tyr577, Tyr612, and Tyr695 activate SHP-2 and the Raf-1 and ERK2 pathway leading to the c-fos SRE site. (A) Tyrosine phosphorylation of SHP-2 and its association with GRB2. Factor-deprived BA/F3 stable transfectants (107 cells/sample) were stimulated with 10 ng of hGM-CSF per ml at 37°C for the indicated times. Cells were lysed, and immunocomplexes with anti-SHP2 antibodies were precipitated. Protein samples were separated by SDS-PAGE, transferred onto polyvinylidene difluoride (PVDF) membranes, and subjected to Western blot (WB) analysis with antibodies against phosphotyrosine (4G10, left), SHP-2 (center), or GRB2 (right). IP, immunoprecipitation. (B and C) Phosphorylation of Raf-1 and ERK2 examined by mobility shift in SDS-PAGE. Factor-deprived BA/F3 stable transfectants (105 cells/sample) were stimulated with 10 ng of hGM-CSF per ml at 37°C for the indicated times. Total cell lysates were prepared, separated by SDS-PAGE, transferred onto PVDF membranes, and subjected to Western blot analysis with antibodies against Raf-1 (B) or ERK2 (C). The band marked with a circled P in each panel corresponds to the phosphorylated molecule. (D) Activation of transcription through the SRE site determined by a transient transfection assay. A plasmid containing the c-fos SRE site fused to the CAT coding region was cotransfected with mutant βc cDNA into BA/F3 cells expressing the wild-type hGM-CSFR α subunit. After 6 h of factor depletion, cells were left unstimulated or were stimulated with 10 ng of hGM-CSF per ml or 1 ng of mIL-3 per ml for 10 h at 37°C. Cell lysates were prepared, and their CAT activities were measured by a diffusion assay. Data were normalized by dividing the CAT activity (counts per minute per microgram of protein) of cells with no stimulation or hGM-CSF stimulation (stim.) by that of cells with mIL-3 stimulation and are presented as a percentage of that for the wild-type βc. All values are the averages of three experiments, and standard deviations are shown as error bars. Statistical significances of the differences in the SRE-CAT activities between hGM-CSF-stimulated samples were as follows: wild-type βc and any of the mutants except for Y3, significant at P < 0.05; Fall and Y3, Y4, or Y5, significant at P < 0.05; Y3 and Y4 or Y5, significant at P < 0.05; and Y4 and Y5, not significant.
To confirm that SHP-2 phosphorylation results in activation of the Ras pathway, we examined the activation of molecules known to function downstream of Ras. We analyzed the phosphorylation of serine/threonine kinase Raf-1 by looking for a mobility shift in SDS-PAGE. Total cell lysates were prepared from hGM-CSF-treated cells and separated by SDS-PAGE, followed by Western blot analysis with an anti-Raf-1 antibody. As shown in Fig. 5B, the Y3, Y4, and Y5 mutants, which induced SHP-2 phosphorylation, also induced Raf-1 phosphorylation. We also examined the phosphorylation of ERK2, a member of the mitogen-activated protein kinase (MAPK) family, again by looking for a mobility shift in SDS-PAGE. The result obtained was similar to that for the phosphorylation of Raf-1 (Fig. 5C). The Y3, Y4, and Y5 mutants, but no other mutants, were capable of inducing the phosphorylation of ERK2. Our findings support the model that SHP-2 functions as a positive regulator for activation of the Ras, Raf-1, and ERK2 pathway in GM-CSF signaling.
Among the target molecules to be phosphorylated by activated extracellular signal-regulated kinases (ERKs) in the nucleus are the ternary complex factors (TCFs) (42). TCFs bind to the SRE in the c-fos promoter region and activate transcription of the c-fos gene. To determine if activation signals leading to the c-fos promoter from βc tyrosines involve the Ras pathway and the SRE site, we performed transient transfection assays using the SRE-CAT construct as a reporter gene. The result (Fig. 5D) was an expression pattern for the reporter gene similar to that obtained with the c-fos–luciferase reporter gene (Fig. 2). The Y3 mutant activated transcription through the SRE site at a level comparable to that seen with the wild-type βc. In addition, partial but clearly evident activation was also observed with the Y4 and Y5 mutants. These results imply that the activation of c-fos transcription by GM-CSF is mediated mainly through the SRE site.
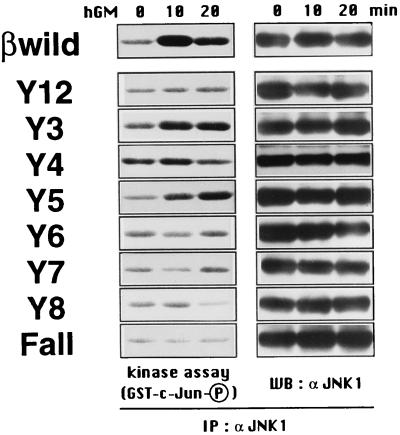
Tyr577, Tyr612, and Tyr695 activate JNK.
We recently obtained evidence that hGM-CSF activates JNK, a member of the MAPK family, in BA/F3 transfectants, and this activation requires a membrane-proximal region including box 1 and a more distal region of the βc (28). We also showed in that report that any one of the F series mutants, but not the Fall mutant, activates JNK, which means that multiple tyrosines play a role in the activation of JNK. To further delineate the mechanism of JNK activation by GM-CSF, we examined the requirement for receptor tyrosine residues using the Y series mutants (Fig. 6). JNK was immunoprecipitated from cell lysates after GM-CSF treatment, and an in vitro kinase assay was performed with GST–c-Jun as a substrate. Among the Y series mutants, Y3, Y4, and Y5 were capable of activating JNK. Thus, GM-CSF regulates the JNK pathway through the same set of βc tyrosines as that used for the ERK pathway.
FIG. 6.
Tyr577, Tyr612, and Tyr695 activate JNK. Factor-deprived BA/F3 stable transfectants (8 × 106 cells/sample) were stimulated with 10 ng of hGM-CSF per ml at 37°C for the indicated times. Cells were lysed, and immunocomplexes with anti-JNK1 antibodies were precipitated. Protein samples were divided into two portions, and one part (equivalent to 3 × 106 cells) was subjected to the in vitro kinase assay with GST–c-Jun as a substrate (left) as described in Materials and Methods. The other part of the samples (equivalent to 5 × 106 cells) was used for Western blot (WB) analysis to determine JNK1 protein levels (right). The circled P means that the bands correspond to the phosphorylated GST–c-Jun. IP, immunoprecipitation.
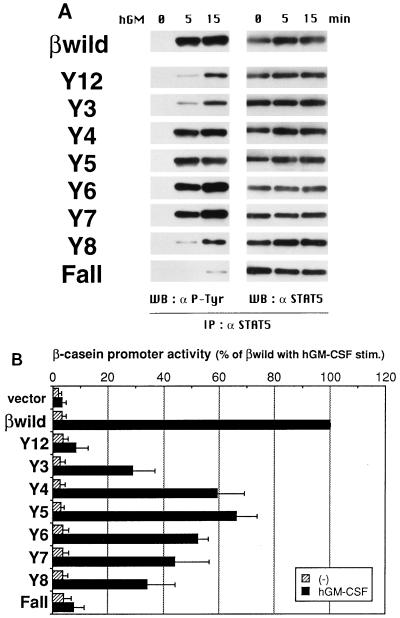
Tyrosine residues of the βc are required for STAT5 activation.
Among the STAT family members, STAT5A and STAT5B are activated by GM-CSF in BA/F3 cells, and in a previous study with C-terminally truncated mutants, it was noted that this activation seemed to be independent of βc tyrosine residues (34). However, the possible involvement of the most proximal tyrosines (Tyr450 and Tyr452) would need to be ruled out. With other cytokines, such as IL-2, erythropoietin, or growth hormone, the activation of STAT5 has been shown to depend on specific tyrosine residues in their receptor subunits (10, 16, 18, 24, 27, 38). Thus, we examined the possibility that the βc tyrosines also play a role in the activation of STAT5 by GM-CSF. Tyrosine phosphorylation of STAT5, which is required for dimer formation, was assessed by immunoprecipitation and subsequent Western blot analysis. The antibody used here recognizes both STAT5A and STAT5B. As shown in Fig. 7A, the induction of STAT5 phosphorylation was significantly diminished, although not completely abrogated, when all eight tyrosines of the βc were substituted (Fall), thereby demonstrating the requirement of tyrosine residues for βc-mediated phosphorylation of STAT5. Similar results were obtained in gel shift assays with the mammary gland factor (MGF) binding site in the β-casein promoter as a probe (data not shown). To further delineate the tyrosines involved, we analyzed the Y series mutants and found that Y4, Y5, Y6, and Y7 were capable of inducing STAT5 phosphorylation to the same extent as wild-type βc. Moreover, the level of phosphorylation induced by the Y12, Y3, and Y8 mutants was slight but was significantly higher than that induced by Fall. These data suggest that any of the eight tyrosines can independently contribute to the activation of STAT5, albeit to different extents.
FIG. 7.
Tyrosine residues of the βc are required for STAT5 activation. (A) Tyrosine phosphorylation of STAT5. Factor-deprived BA/F3 stable transfectants (5 × 106 cells/sample) were stimulated with 10 ng of hGM-CSF per ml at 37°C for the indicated times. Cells were lysed, and immunocomplexes were precipitated with anti-STAT5 antibodies. Protein samples were separated by SDS-PAGE, transferred onto polyvinylidene difluoride membranes, and subjected to Western blot (WB) analysis with antibodies against phosphotyrosine (4G10, left) or STAT5 (right). Two independent stable clones for each of the Y series mutants and three for the Fall mutant were analyzed and showed essentially the same results (not shown). IP, immunoprecipitation. (B) Activation of the β-casein promoter by each βc mutant was measured by a transient transfection assay. The β-casein promoter–luciferase fusion construct was cotransfected with mutant βc cDNA into BA/F3 cells expressing the wild-type hGM-CSFR α subunit as described in Materials and Methods. After 6 h of factor depletion, cells were left unstimulated or were stimulated with 10 ng of hGM-CSF per ml or 1 ng of mIL-3 per ml for 6 h at 37°C. Cell lysates were prepared, and their luciferase activities were measured with a luminometer. The β-casein promoter activity was calculated by dividing the luminescence intensity (relative light units per minute per microgram of total protein) of cells with no stimulation or hGM-CSF stimulation (stim.) by that of cells with mIL-3 stimulation and are presented as a percentage of that for the wild-type βc. All values are the averages of three experiments, and standard deviations are shown as error bars. Statistical significances of the differences in the β-casein promoter activities between hGM-CSF-stimulated samples were as follows: wild-type βc and any of the mutants, significant at P < 0.05; Fall and Y4, Y5, Y6, or Y7, significant at P < 0.05; Y12 and Y8, not significant; and Y7 and Y6 or Y8, not significant.
Since tyrosine phosphorylation may not be sufficient for STAT5 to induce transcriptional activation, we used a transient transfection assay and the β-casein promoter–luciferase reporter gene for related determinations. The transactivation capacity of STAT5 was seen to depend on βc tyrosines in a manner similar although not identical to that seen for STAT5 phosphorylation. The Fall mutant showed little expression of the reporter gene, while all of the Y series mutants, except for Y12, were capable of inducing significantly higher expression (Fig. 7B). However, we detected no marked difference between the Fall and Y12 mutants. The Y4, Y5, Y6, and Y7 mutants induced STAT5 phosphorylation to similar extents, which were apparently higher than that induced by the Y8 mutant (Fig. 7A), yet the transactivation capacity of Y7 appeared to be weaker than those of Y4, Y5, and Y6 and was not significantly different from that of Y8. Nonetheless, our results clearly indicate that GM-CSF-induced activation of STAT5 depends, to a considerable extent, on multiple tyrosine residues of the βc. As expected, each of the F series mutants was capable of inducing transactivation of the β-casein promoter to a level comparable to that seen with wild-type βc (data not shown), indicating that none of the βc tyrosine residues is indispensable for this signaling event.
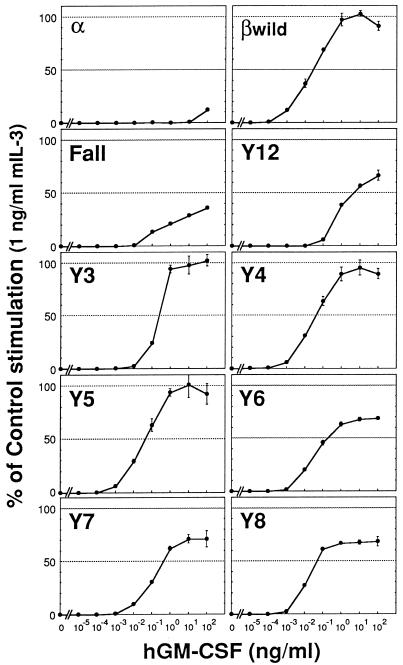
Tyrosine residues of the βc are required for a maximal proliferative response to hGM-CSF.
Studies carried out with a dominant-negative type of STAT5 suggested that this molecule has a role in IL-3-induced proliferation (33). As we found that βc tyrosines are important for βc-mediated STAT5 activation, we next asked whether or not they are also involved in transmitting growth-promoting signals. As shown in Fig. 8, the potential of the βc to induce short-term proliferation of BA/F3 cells, as examined by an MTT assay, was severely impaired in the absence of all of the cytoplasmic tyrosines (Fall). This result indicates that the βc tyrosines are necessary for GM-CSF-stimulated proliferation, at least to induce a maximal response. Further analyses with BA/F3 cells expressing Y series mutants showed that this deficiency of Fall was restored, at least partly, by the presence of any one (or two, in the case of Y12) of the βc tyrosines. In particular, the Y3, Y4, and Y5 mutants induced a relatively higher level of growth response than did the other mutants. Therefore, each of the βc tyrosines can independently and in a different manner mediate growth-promoting signals.
FIG. 8.
Tyrosine residues of the βc are necessary for a maximal proliferative response to hGM-CSF. Shown is short-term proliferation of BA/F3 transfectants expressing the hGM-CSFR α subunit together with no βc (α), the wild-type βc (βwild), Fall, or the Y series mutants. The BA/F3 transfectants were factor deprived for 6 h and then cultured for 24 h in the presence of 0 to 100 ng of hGM-CSF per ml or 0 to 10 ng of mIL-3 per ml. Cell growth was examined by the MTT colorimetric assay. The vertical axis indicates the relative MTT reduction value normalized to the value for cells incubated with 1 ng of mIL-3 per ml. All values are the averages of triplicate samples, and standard deviations are shown as error bars. All of the transfectants exhibited a similar dose-dependent response to mIL-3 stimulation (not shown). Similar results were obtained in three separate experiments.
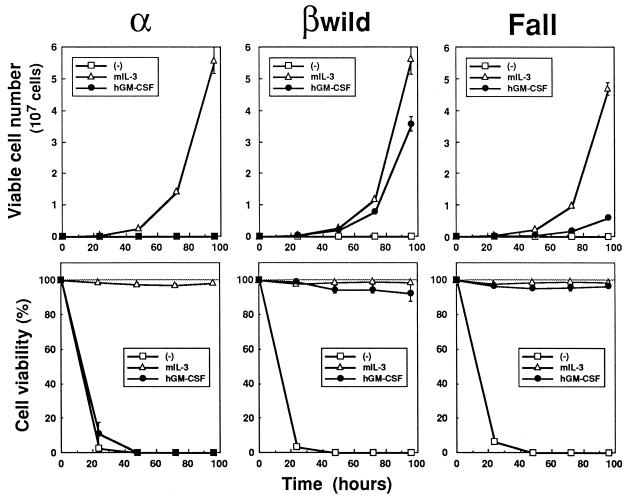
To determine whether or not the impairment of the Fall mutant in growth promotion was due to a defect in maintaining cell viability, we further examined the long-term proliferation and survival of BA/F3 transfectants by a trypan blue dye exclusion assay. In the presence of 10 ng of hGM-CSF per ml, BA/F3 cells expressing the Fall mutant kept on proliferating, albeit at a lower growth rate, and showed no significant loss of viability (Fig. 9). The viability of the cells was maintained at the same level for more than 1 week (data not shown). Consistent with this result, all of the Y series mutants also possessed the capability of supporting cell viability (data not shown).
FIG. 9.
The Fall mutant can support the survival of BA/F3 transfectants. Shown are long-term proliferation (upper panels) and the viability (lower panels) of BA/F3 transfectants expressing the hGM-CSFR α subunit together with no βc (α), the wild-type βc (βwild), or the Fall mutant (Fall). Factor-deprived BA/F3 transfectants (105 cells) were seeded in the absence or presence of either hGM-CSF (10 ng/ml) or mIL-3 (1 ng/ml). Viable cell numbers were measured by a trypan blue dye exclusion assay. All values are the averages of triplicate samples, and standard deviations are shown as error bars. Three independent stable clones were examined for the Fall mutant and gave similar results.
DISCUSSION
In the present work, we focused attention on cytoplasmic tyrosine residues within the βc and analyzed their function in GM-CSF signaling. Substituting all eight cytoplasmic tyrosines together severely impaired the potential of the βc to transduce various signals, thereby indicating the critical requirement of βc tyrosines for signaling. Because the Fall mutant still activated JAK2, loss of this function likely did not result from unfavorable changes in structural integrity. None of the F series mutants, lacking only one tyrosine, showed any significant defect in activating the c-fos promoter or in growth promotion (Fig. 2 and data not shown), consistent with our previous results indicating that c-fos activation can be mediated independently by either Tyr577 or other functional residues located C terminal to this tyrosine (22). Therefore, we constructed the Y series mutants by adding back each and every tyrosine in the background of the Fall mutant.
Among the eight tyrosines of the βc, three tyrosines, Tyr577, Tyr612, and Tyr695, are involved in and can individually induce SHP-2 phosphorylation, its association with GRB2, and activation of the putative downstream Raf and ERK pathway, resulting in transactivation of the c-fos promoter. We and others previously showed that Tyr577 is necessary for tyrosine phosphorylation of Shc (12, 22), another molecule implicated in the activation of Ras through the GRB2-Sos complex. Thus, Tyr577 utilizes both Shc and SHP-2 to stimulate this pathway, while Tyr612 or Tyr695 induces the phosphorylation of only SHP-2. This result may partly account for the finding that the Y3 mutant can activate the c-fos promoter sufficiently and more strongly than Y4 and Y5. The c-fos promoter contains several cis regulatory elements, and our results strongly suggest that GM-CSF-dependent transcription of c-fos is mediated mainly through the SRE site. Consistent with the present results, our recent study done to dissect the c-fos promoter region also revealed a critical role for the SRE site in hGM-CSF-induced transcriptional activation (47). The activation of JNK was also mediated by the same set of βc tyrosines as that needed for the phosphorylation of SHP-2, Raf-1, and ERK2 and the induction of c-fos transcription, suggesting that these events are related. It was reported that Ras activation is necessary but not sufficient for IL-3 stimulation of JNK1 (44). JNKs phosphorylate and hence activate the transcription factors c-Jun, ATF2, and Elk-1, which is a member of the TCFs (9, 51). It is thus possible that the JNK pathway is also involved in GM-CSF-induced activation of the c-fos promoter through the SRE site.
The activation of STAT5 by GM-CSF was also seen to depend on βc tyrosines, but the requirements differed. The level of STAT5 phosphorylation was dramatically decreased by a lack of all of the βc tyrosines (Fall) but was increased when any one (or two, in the case of Y12) tyrosine was added back, with each showing a unique extent of recovery. Interestingly, the Y12 mutant induced a markedly stronger phosphorylation of STAT5 than did Fall, while these two mutants showed little transactivation of the β-casein promoter. Since the activity of STAT5 is subject to regulation concerning Ser phosphorylation (6), the Y12 mutant may not be able to transmit that signal. Notably, we consistently observed that the Fall mutant could induce low but detectable levels of STAT5 activation and SHP-2 phosphorylation in response to GM-CSF stimulation (Fig. 5A and 7A). Since Fall is capable of activating JAK2 (Fig. 4), it is possible that JAK2 and/or other tyrosine kinases, which can be activated independently of the βc tyrosine residues, play a substantial role in phosphorylating STAT5 and SHP-2. Nonetheless, efficient phosphorylation can only be accomplished in the presence of the receptor tyrosines by means of recruitment of these molecules onto the receptor and hence their close proximity to the kinases.
Our evidence shows that the βc tyrosines play critical roles in transmitting growth-promoting signals, as the Fall mutant was severely impaired in stimulating cell proliferation. We obtained a similar result for [3H]thymidine incorporation (45a). This defect was restored, at least partly, by the presence of any one of the βc tyrosines. Dominant-negative STAT5 inhibits but does not completely suppress IL-3-driven proliferation of BA/F3 cells, thereby suggesting an important role for STAT5 in mitogenic signals (33). Taken together, these findings may indicate that increased activation of STAT5 by the presence of any one tyrosine results in improved growth-promoting activity of the Y series mutants. Although the activation of STAT5 by the Y12 mutant is ambiguous, as mentioned above, it is possible that even a low level of STAT5 activation is sufficient to accelerate growth promotion, while the transactivation of the β-casein promoter requires a much higher level of STAT5 activation and/or additional signaling events. Alternatively, Tyr450 or Tyr452 could stimulate proliferation by recruiting a molecule other than STAT5. It should be noted that, among the Y series mutants, Y3, Y4, and Y5 induced relatively higher levels of growth response than did the other mutants. This finding correlates with the induction of SHP-2 phosphorylation, MAPK pathway activation, and c-fos transcription. Thus, these signaling events, although not necessarily required for growth promotion, may play a role in inducing optimal cell proliferation under physiological conditions.
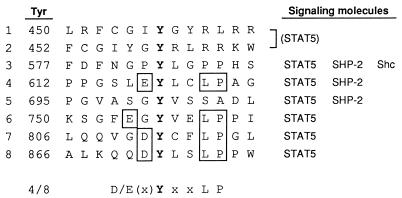
In general, specificity in the recognition of a particular phosphotyrosine residue by an SH2 or PTB domain is predominantly defined by a primary sequence surrounding that residue. Four of the eight cytoplasmic tyrosines of the βc, namely, Tyr612, Tyr750, Tyr806, and Tyr866, possess a leucine and a proline at positions +3 and +4, respectively, and an acidic residue at position −1 or −2 (Fig. 10). Since these four tyrosines were all involved in the activation of STAT5, it is conceivable that these Y-X-X-L-P motifs are recognized by the SH2 domain of STAT5 or of another adapter molecule which in turn binds STAT5. Consistent with this suggestion, many of the tyrosines of the erythropoietin (EPO) receptor and the IL-2 receptor β subunit involved in STAT5 activation also possess a leucine at position +3 (10, 16, 24, 27, 38). However, STAT5 was also activated by the other βc tyrosines, surrounded by relatively different motifs. It is possible that these tyrosines recruit STAT5 in different ways, presumably through distinct adapter proteins. Alternatively, the STAT5 SH2 domain could bind relatively divergent motifs, including those with and without a leucine at position +3. This notion is supported by the sequence surrounding the Tyr694 of STAT5 (Y-V-K-P-Q), which should be recognized by the STAT5 SH2 domain to form a dimer.
FIG. 10.
Tyrosine-containing motifs in the human βc subunit. Amino acid sequences surrounding the βc tyrosines are aligned, with signaling molecules activated by each tyrosine on the right. The conserved residues among four of the eight βc tyrosines (the Y-X-X-L-P motif, bottom) are boxed.
In the case of SHP-2, our results showed that Tyr577, Tyr612, and Tyr695 are involved in its phosphorylation. It has been predicted that the N-terminal SH2 domain of SHP-2 binds to phosphotyrosines followed by a β-branched residue at position +1 and a hydrophobic residue at +3, comprising the consensus sequence Y-V/I/T-X-V/L/I (8). In addition, the involvement of residues N terminal and more C terminal to these residues, especially a hydrophobic residue (leucine, phenylalanine, or proline) at position +5 and valine, leucine, or glycine at position −2, in high-affinity binding has also been suggested (21, 25). The sequence surrounding Tyr612 (L-G-Y612-L-C-L-P-A; Fig. 10) matches these predictions well; thus, there may be a direct interaction of this tyrosine with SHP-2. Notably, among the four tyrosines with the Y-X-X-L-P motif mentioned above, only Tyr612 could induce SHP-2 phosphorylation, while all four were involved in STAT5 activation, confirming the role of residues outside the former consensus sequence (Y-V/I/T-X-V/L/I [8]) in defining the specificity in SHP-2 interactions.
Taken together, STAT5 activation and SHP-2 phosphorylation are mediated by multiple βc tyrosines. The sets of tyrosines required for these molecules are distinct, yet they overlap. Their surrounding motifs are not necessarily uniform either. These molecules seem to be regulated through “redundant” tyrosines of the βc. In terms of signaling molecules that the individual tyrosines activate, it is intriguing that several βc tyrosines have pleiotropic functions rather than a specific one. This finding is most remarkable with Tyr577, which is involved in the activation of STAT5 and the phosphorylation of SHP-2 and Shc. With Tyr612 and Tyr695, STAT5 activation and SHP-2 phosphorylation were observed. Further analyses are required to determine whether each of these molecules can independently interact with the same tyrosine or whether these molecules associate with another common molecule which interacts directly with the βc tyrosine, thereby functioning as a docking protein. Alternatively, these molecules might be activated sequentially along the same pathway. We have no clear evidence of a direct interaction of either STAT5 or SHP-2 with the βc, while a possible interaction between Tyr577 and the Shc PTB domain has been reported (36). It seems likely that adapter proteins play a role in recruiting these molecules onto the βc. Therefore, it is of great importance to define the molecules directly interacting with βc tyrosines. These studies, together with our present data showing the critical roles of the βc tyrosines, will pave the way for a better understanding of the signaling mechanisms of GM-CSF, IL-3, and IL-5.
ACKNOWLEDGMENTS
We thank D. Chida for helpful suggestions, K. Hagino for technical assistance, and M. Ohara and M. Dahl for critical comments on the manuscript.
This work was supported in part by CREST (Core Research for Evolutional Science and Technology) of the Japan Science and Technology Corporation and by a grant-in-aid for scientific research on priority areas from the Ministry of Education, Science, Sports and Culture of Japan. T.I. is a recipient of a research fellowship from the Japan Society for the Promotion of Science for Young Scientists.
REFERENCES
- 1.Adachi M, Fischer E H, Ihle J, Imai K, Jirik F, Neel B, Pawson T, Shen S, Thomas M, Ullrich A, Zhao Z. Mammalian SH2-containing protein tyrosine phosphatases. Cell. 1996;85:15. doi: 10.1016/s0092-8674(00)81077-6. [DOI] [PubMed] [Google Scholar]
- 2.Arai K, Lee F, Miyajima A, Miyatake S, Arai N, Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1989. [Google Scholar]
- 4.Baldwin G C. The biology of granulocyte-macrophage colony-stimulating factor: effects on hematopoietic and nonhematopoietic cells. Dev Biol. 1992;151:352–367. doi: 10.1016/0012-1606(92)90175-g. [DOI] [PubMed] [Google Scholar]
- 5.Bazan J F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci USA. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beadling C, Ng J, Babbage J W, Cantrell D A. Interleukin-2 activation of STAT5 requires the convergent action of tyrosine kinases and a serine/threonine kinase pathway distinct from the Raf1/ERK2 MAP kinase pathway. EMBO J. 1996;15:1902–1913. [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett A M, Tang T L, Sugimoto S, Walsh C T, Neel B G. Protein-tyrosine-phosphatase SHPTP2 couples platelet-derived growth factor receptor beta to Ras. Proc Natl Acad Sci USA. 1994;91:7335–7339. doi: 10.1073/pnas.91.15.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Case R D, Piccione E, Wolf G, Benett A M, Lechleider R J, Neel B G, Shoelson S E. SH-PTP2/Syp SH2 domain binding specificity is defined by direct interactions with platelet-derived growth factor beta-receptor, epidermal growth factor receptor, and insulin receptor substrate-1-derived phosphopeptides. J Biol Chem. 1994;269:10467–10474. [PubMed] [Google Scholar]
- 9.Cavigelli M, Dolfi F, Claret F-X, Karin M. Induction of c-fos expression through JNK-mediated TCF/Elk-1 phosphorylation. EMBO J. 1995;14:5957–5964. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damen J E, Wakao H, Miyajima A, Krosl J, Humphries R K, Cutler R L, Krystal G. Tyrosine 343 in the erythropoietin receptor positively regulates erythropoietin-induced cell proliferation and Stat5 activation. EMBO J. 1995;14:5557–5568. doi: 10.1002/j.1460-2075.1995.tb00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duronio V, Clark-Lewis I, Federsppiel B, Wieler J S, Schrader J W. Tyrosine phosphorylation of receptor β subunits and common substrates in response to interleukin-3 and granulocyte-macrophage colony-stimulating factor. J Biol Chem. 1992;267:21856–21863. [PubMed] [Google Scholar]
- 12.Durstin M, Inhorn R C, Griffin J D. Tyrosine phosphorylation of Shc is not required for proliferation or viability signaling by granulocyte-macrophage colony stimulating factor in hematopoietic cell lines. J Immunol. 1996;157:534–540. [PubMed] [Google Scholar]
- 13.Fukumoto Y, Kaibuchi K, Oku N, Hori Y, Takai Y. Activation of the c-fos serum-response element by the activated c-Ha-ras protein in a manner independent of protein kinase C and cAMP-dependent protein kinase. J Biol Chem. 1990;265:774–780. [PubMed] [Google Scholar]
- 14.Gasson J C. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991;77:1131–1145. [PubMed] [Google Scholar]
- 15.Gearing D P, King J A, Gough N M, Nicola N A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989;8:3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gobert S, Chretien S, Gouilleux F, Muller O, Pallard C, Dusanter-Fourt I, Groner B, Lacombe C, Gisselbrecht S, Mayeux P. Identification of tyrosine residues within the intracellular domain of the erythropoietin receptor crucial for STAT5 activation. EMBO J. 1996;15:2434–2441. [PMC free article] [PubMed] [Google Scholar]
- 17.Hanazono Y, Chiba S, Sasaki K, Mano H, Miyajima A, Arai K, Yazaki Y, Hirai H. c-fps/fes protein-tyrosine kinase is implicated in a signaling pathway triggered by granulocyte-macrophage colony-stimulating factor and interleukin-3. EMBO J. 1993;12:1641–1646. doi: 10.1002/j.1460-2075.1993.tb05809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen L H, Wang X, Kopchick J J, Bouchelouche P, Nielsen J H, Galsgaard E D, Billestrup N. Identification of tyrosine residues in the intracellular domain of the growth hormone receptor required for transcriptional signaling and Stat5 activation. J Biol Chem. 1996;271:12669–12673. doi: 10.1074/jbc.271.21.12669. [DOI] [PubMed] [Google Scholar]
- 19.Hayashida K, Kitamura T, Gorman D M, Arai K, Yokota T, Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci USA. 1990;87:9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 21.Huyer G, Li Z M, Adam M, Huckle W R, Ramachandran C. Direct determination of the sequence recognition requirements of the SH2 domains of SH-PTP2. Biochemistry. 1995;34:1040–1049. doi: 10.1021/bi00003a039. [DOI] [PubMed] [Google Scholar]
- 22.Itoh T, Muto A, Watanabe S, Miyajima A, Yokota T, Arai K. Granulocyte-macrophage colony-stimulating factor provokes RAS activation and transcription of c-fos through different modes of signaling. J Biol Chem. 1996;271:7587–7592. doi: 10.1074/jbc.271.13.7587. [DOI] [PubMed] [Google Scholar]
- 23.Kitamura T, Hayashida K, Sakamaki K, Yokota T, Arai K, Miyajima A. Reconstitution of functional receptors for human granulocyte/macrophage colony-stimulating factor (GM-CSF): evidence that the protein encoded by the AIC2B cDNA is a subunit of the murine GM-CSF receptor. Proc Natl Acad Sci USA. 1991;88:5082–5086. doi: 10.1073/pnas.88.12.5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klingmuller U, Bergelson S, Hsiao J G, Lodish H F. Multiple tyrosine residues in the cytosolic domain of the erythropoietin receptor promote activation of STAT5. Proc Natl Acad Sci USA. 1996;93:8324–8328. doi: 10.1073/pnas.93.16.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C H, Kominos D, Jacques S, Margolis B, Schlessinger J, Shoelson S E, Kuriyan J. Crystal structures of peptide complexes of the amino-terminal SH2 domain of the Syp tyrosine phosphatase. Structure. 1994;2:423–438. doi: 10.1016/s0969-2126(00)00044-7. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Nishimura R, Kashishian A, Batzer A G, Kim W J, Cooper J A, Schlessinger J. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol Cell Biol. 1994;14:509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin J X, Migone T S, Tsang M, Friedmann M, Weatherbee J A, Zhou L, Yamauchi A, Bloom E T, Mietz J, John S, Leonard W J. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 28.Liu R, Itoh T, Arai K, Watanabe S. Activation of c-Jun N-terminal kinase by human granulocyte macrophage-colony stimulating factor in BA/F3 cells. Biochem Biophys Res Commun. 1997;234:611–615. doi: 10.1006/bbrc.1997.6643. [DOI] [PubMed] [Google Scholar]
- 29.Matsuguchi T, Inhorn R C, Carlesso N, Xu G, Druker B, Griffin J D. Tyrosine phosphorylation of p95Vav in myeloid cells is regulated by GM-CSF, IL-3 and steel factor and is constitutively increased by p210BCR/ABL. EMBO J. 1995;14:257–265. doi: 10.1002/j.1460-2075.1995.tb06999.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 30.Miyajima A, Mui A L-F, Ogorochi T, Sakamaki K. Receptors for granulocyte-macrophage colony-stimulating factor, interleukin-3, and interleukin-5. Blood. 1993;82:1960–1974. [PubMed] [Google Scholar]
- 31.Miyajima A, Schreurs J, Otsu K, Kondo A, Arai K, Maeda S. Use of the silkworm, Bombyx mori, and an insect baculovirus vector for high-level expression and secretion of biologically active mouse interleukin-3. Gene. 1987;58:273–281. doi: 10.1016/0378-1119(87)90382-9. [DOI] [PubMed] [Google Scholar]
- 32.Mosmann T. Rapid colorimetric assays for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 33.Mui A L, Wakao H, Kinoshita T, Kitamura T, Miyajima A. Suppression of interleukin-3-induced gene expression by a C-terminal truncated Stat5: role of Stat5 in proliferation. EMBO J. 1996;15:2425–2433. [PMC free article] [PubMed] [Google Scholar]
- 34.Mui A L, Wakao H, O’Farrell A M, Harada N, Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J. 1995;14:1166–1175. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Odai H, Sasaki K, Iwamatsu A, Hanazono Y, Tanaka T, Mitani K, Yazaki Y, Hirai H. The proto-oncogene product c-Cbl becomes tyrosine phosphorylated by stimulation with GM-CSF or Epo and constitutively binds to the SH3 domain of Grb2/Ash in human hematopoietic cells. J Biol Chem. 1995;270:10800–10805. doi: 10.1074/jbc.270.18.10800. [DOI] [PubMed] [Google Scholar]
- 36.Pratt J C, Weiss M, Sieff C A, Shoelson S E, Burakoff S J, Ravichandran K S. Evidence for a physical association between the Shc-PTB domain and the beta c chain of the granulocyte-macrophage colony-stimulating factor receptor. J Biol Chem. 1996;271:12137–12140. doi: 10.1074/jbc.271.21.12137. [DOI] [PubMed] [Google Scholar]
- 37.Quelle F W, Sato N, Witthuhn B A, Inhorn R C, Eder M, Miyajima A, Griffin J D, Ihle J N. JAK2 associates with the βc chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol Cell Biol. 1994;14:4335–4341. doi: 10.1128/mcb.14.7.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quelle F W, Wang D, Nosaka T, Thierfelder W E, Stravopodis D, Weinstein Y, Ihle J N. Erythropoietin induces activation of Stat5 through association with specific tyrosines on the receptor that are not required for a mitogenic response. Mol Cell Biol. 1996;16:1622–1631. doi: 10.1128/mcb.16.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakamaki K, Miyajima I, Kitamura T, Miyajima A. Critical cytoplasmic domains of the common β subunit of the human GM-CSF, IL-3 and IL-5 receptors for growth signal transduction and tyrosine phosphorylation. EMBO J. 1992;11:3541–3549. doi: 10.1002/j.1460-2075.1992.tb05437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 41.Sato N, Sakamaki K, Terada N, Arai K, Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common β subunit responsible for different signaling. EMBO J. 1993;12:4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- 43.Taniguchi T. Cytokine signaling through nonreceptor protein tyrosine kinases. Science. 1995;268:251–255. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- 44.Terada K, Kaziro Y, Satoh T. Ras-dependent activation of c-Jun N-terminal kinase/stress-activated protein kinase in response to interleukin-3 stimulation in hematopoietic BaF3 cells. J Biol Chem. 1997;272:4544–4548. doi: 10.1074/jbc.272.7.4544. [DOI] [PubMed] [Google Scholar]
- 45.Torigoe T, O’Connor R, Santoli D, Reed J C. Interleukin-3 regulates the activity of the LYN protein-tyrosine kinase in myeloid-committed leukemic cell lines. Blood. 1992;80:617–624. [PubMed] [Google Scholar]
- 45a.Watanabe, S. Unpublished result.
- 46.Watanabe S, Itoh T, Arai K. JAK2 is essential for activation of c-fos and c-myc promoters and cell proliferation through the human granulocyte-macrophage colony-stimulating factor receptor in BA/F3 cells. J Biol Chem. 1996;271:12681–12686. doi: 10.1074/jbc.271.21.12681. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe S, Kubota H, Sakamoto K M, Arai K. Characterization of cis-acting sequences and trans-acting signals regulating early growth response 1 and c-fos promoters through the granulocyte-macrophage colony-stimulating factor receptor in BA/F3 cells. Blood. 1997;89:1197–1206. [PubMed] [Google Scholar]
- 48.Watanabe S, Mui A L-F, Muto A, Chen J X, Hayashida K, Yokota T, Miyajima A, Arai K. Reconstituted human granulocyte-macrophage colony-stimulating factor receptor transduces growth-promoting signals in mouse NIH 3T3 cells: comparison with signalling in BA/F3 pro-B cells. Mol Cell Biol. 1993;13:1440–1448. doi: 10.1128/mcb.13.3.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe Y, Kitamura T, Hayashida K, Miyajima A. Monoclonal antibody against the common β subunit (βc) of the human interleukin-3 (IL-3), IL-5, and granulocyte-macrophage colony-stimulating factor receptors shows upregulation of βc by IL-1 and tumor necrosis factor-α. Blood. 1992;80:2215–2220. [PubMed] [Google Scholar]
- 50.Welham M J, Dechert U, Leslie K B, Jirik F, Schrader J W. Interleukin (IL)-3 and granulocyte/macrophage colony-stimulating factor, but not IL-4, induces tyrosine phosphorylation, activation, and association of SHPTP2 with Grb2 and phosphatidylinositol 3′-kinase. J Biol Chem. 1994;269:23764–23768. [PubMed] [Google Scholar]
- 51.Whitmarsh A J, Shore P, Sharrocks A D, Davis R J. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]