Abstract
Microcrystal Electron Diffraction (MicroED) enables structure determination of very small crystals that are much too small to be of use for other conventional diffraction techniques. MicroED has been used to determine the structures of many proteins and small organic molecules, and the technique can be performed on most standard cryo-TEM instruments equipped with high-speed detectors capable of collecting electron diffraction data. Here we present protocols for MicroED sample preparation and data collection for protein microcrystals.
Keywords: Microcrystal electron diffraction, MicroED, protein crystallography, structural biology, sample preparation
1. Introduction
Microcrystal electron diffraction (MicroED) is a method for collecting electron diffraction data using a cryo-transmission electron microscope (cryo-TEM) (1,2). MicroED makes it possible to collect high-resolution electron diffraction data sets from extremely small three-dimensional crystals that would otherwise be unusable for conventional X-ray diffraction methods (3,4). Since its initial development, MicroED has been successfully used on a variety of protein (5–10), peptide (11–16), and small molecule targets (17–21). Sample preparation methods can vary depending on the type of crystal being studied, and here we will present methods for preparing protein crystals samples for MicroED analysis.
When working with protein samples it is critical that the crystals remain hydrated; therefore, protein microcrystals are vitrified in liquid ethane in order to ensure they are preserved in a frozen hydrated state within the cryo-TEM. Sample preparation equipment and procedures for MicroED are similar to those used in single particle cryo-electron microscopy (cryo-EM) (22,23). Briefly, microcrystals are applied onto a carbon-coated electron microscopy (EM) grid (e.g. Quantifoil) and excess solution is blotted away using filter paper. Following blotting, the crystal loaded EM grid is immediately vitrified in liquid ethane and either loaded into the cryo-TEM or stored under liquid nitrogen for future use. While the protocols presented here will focus on more standard sample preparation methods, there are also other procedures that can be used including crystal fragmentation (6) and cryo-focus ion beam (cryo-FIB) milling (24,25). Readers are encouraged to refer to other sources for details on how to use these other methods for sample preparation.
Once the sample is inserted into the microscope, the quality of the sample is screened, and data is collected from the best diffracting crystals on the grid. While there are recent methods for automated data collection (26–28) and new user interfaces for MicroED data collection, this chapter will focus on the manual method for MicroED data collection that does not require additional software besides the standard cryo-TEM control interface. Data collection begins by screening the grid for the presence of suitable microcrystals on the grid. Suitable crystals are those which have a defined shape and are not too close to the EM grid bars or other crystals, which would limit the range of data that could be collected (Fig 1). Following the identification of a suitable crystal, an initial diffraction pattern is collected, and its quality is assessed. If the diffraction is of high-quality (Fig 2), a continuous rotation data set (7) should be collected from this crystal. This involves tilting the stage of the cryo-TEM to high-tilt and then starting a steady and continuous rotation of the stage. Diffraction data are recorded using a high-speed detector as the crystal continuously tilts in the beam. These diffraction data sets are then processed using standard data processing workflows (29,30)
Figure 1.
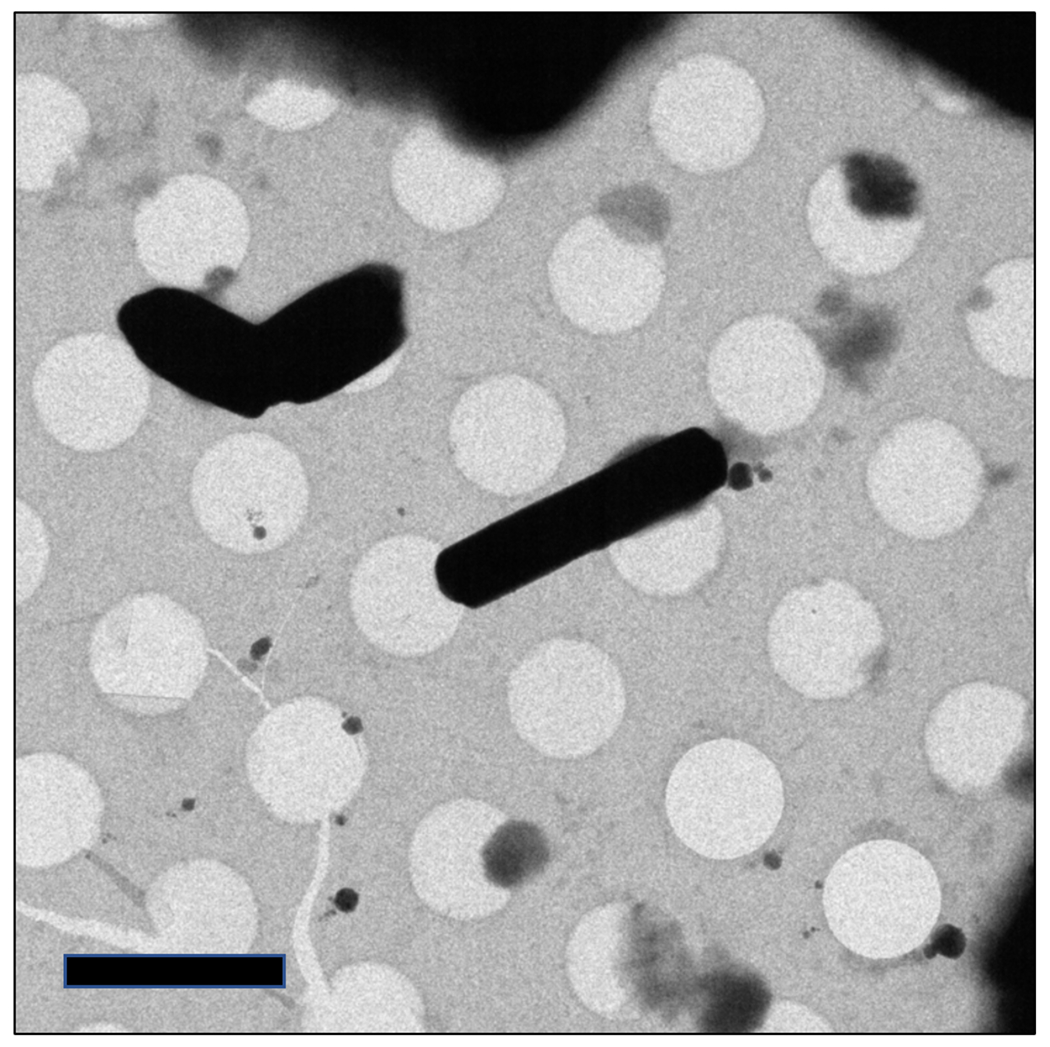
Images of protein microcrystals as seen under a typical medium magnification search. Crystals show well-defined edges and are well-dispersed on the grid. Scale bar represents 4μm.
Figure 2.
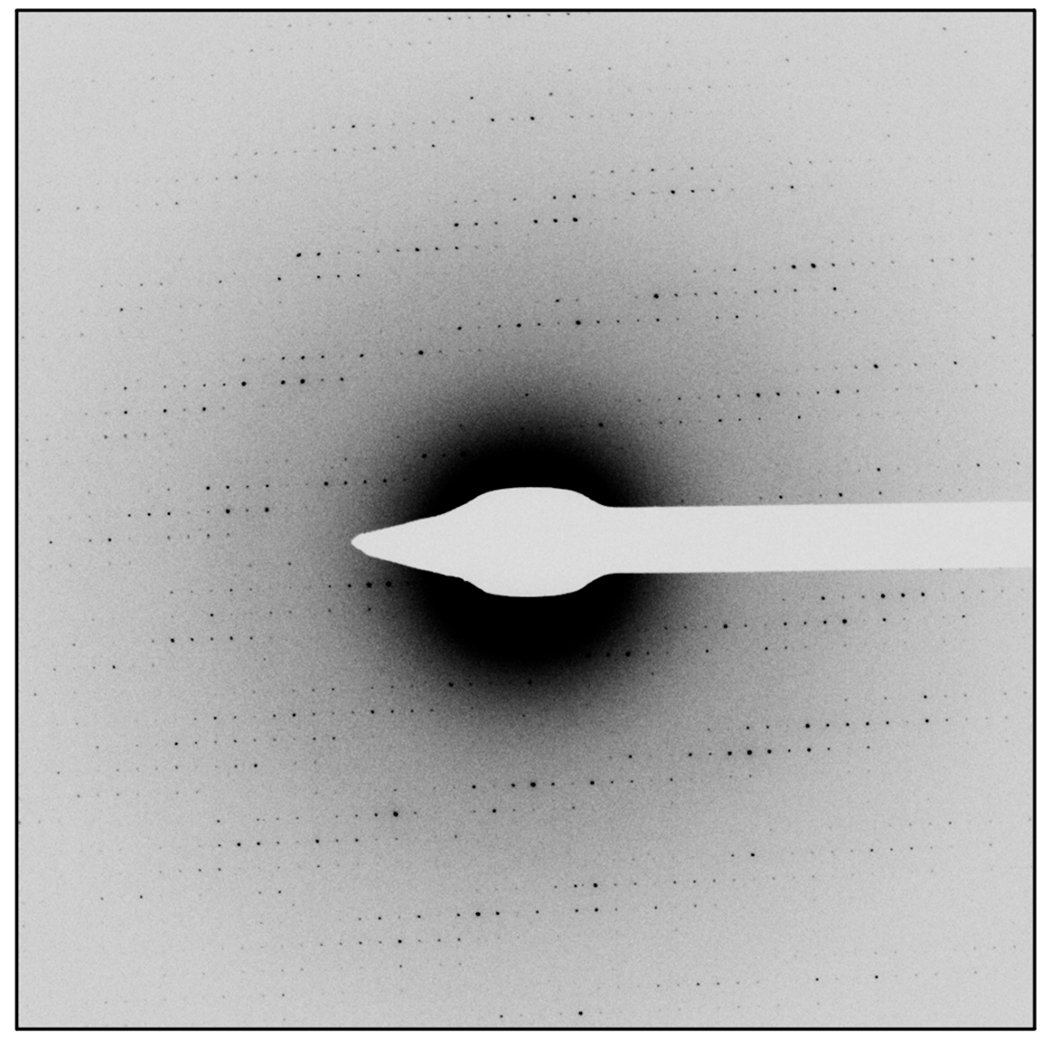
Example of high-quality diffraction data from a protein microcrystal. Spots are sharp and well-separated and extend to high-resolution.
2. Materials
Holey carbon or continuous carbon coated EM grids (copper; 200 – 400 mesh)
Protein microcrystals
Glow discharge system
Clean glass microscope slides
Plunge freezing apparatus (Note 1)
Locking Fine-tip tweezers for plunge freezing apparatus
Blotting paper for plunge freezing apparatus
Liquid Nitrogen
Liquid Ethane
Pipettes capable of dispensing in the range of 1 to 20 μL
Cryo grid box
Cryo-TEM (Note 2)
High-speed detector for the collection of electron diffraction data (Note 3)
3. Methods
Here we will present the generic workflow for MicroED sample preparation and data collection when working with protein crystals. The procedures start after the identification of protein microcrystals (31,22,32,33) and ends with the collection of continuous rotation MicroED data sets that can be processed and used for structure determination (22). It is recommended that first time users of MicroED attempt these protocols with known standards in order to become familiar with the process. Suggested protein standards are lysozyme and proteinase K and there are several publications where these samples have been analyzed by MicroED (6,34,7,9).
3.1. Sample preparation
Use clean tweezers to place carbon coated EM grids onto a clean glass microscope slide.
Use a glow discharge unit and follow the manufacturers protocol to clean the surface of the carbon.
Use tweezers to flip the grids over on the glass slide and repeat step 2 to treat the opposite surface of the grid.
Assemble the components of the plunge freezing coolant container according to the specific manufacturer’s instructions. Fill container with liquid nitrogen and allow the temperature to stabilize. Add additional liquid nitrogen as needed.
Fill the reservoir where the grid will be plunged with liquid ethane according to manufacturer’s instructions.
Use the locking fine-tip tweezers for plunge freezing apparatus to pick up an EM grid prepared following step 3 and place tweezers on the lab bench.
Use a pipette to aspirate 2-3 μL of solution containing microcrystals (Note 4). Dispense solution onto the EM grid.
Allow crystal solution to rest on the surface of the grid for 30 seconds to 1 minute.
In some cases, microcrystal solution is applied to both sides. If crystals will only be deposited on one side of the grid, skip this step. To deposit crystals on the other side of the grid as well, flip over the tweezers holding the EM grid that has just had microcrystal solution applied to it, and repeat step 7-8 to deposit on to the other side of the grid.
Attach fine-tip tweezers holding the EM grid and protein microcrystal sample to the plunge freezing device.
Blot and vitrify the sample using the plunge freezing device (Note 5). Place the vitrified sample into the cryo grid box that is under liquid nitrogen. Ensure that once the grid has been vitrified it always remains at liquid nitrogen temperatures.
Repeat steps 6 – 11 to make multiple samples. Additional samples should be made to screen around blotting time, volume of crystals dispensed on grid, and microcrystal treatment strategies (e.g. cryo-protectants, dilutions).
Once all samples have been made, store the cryo grid boxes containing the prepared EM grids under liquid nitrogen until loading into a cryo-TEM for analysis.
3.2. Screening microcrvstal diffraction quality
Follow protocols for the cryo-TEM to align the microscope in both imaging and diffraction mode (Note 6).
Load grids into a cryo-TEM equipped with a high-speed detector for diffraction data collection (see Notes 2 and 3).
When the samples have been loaded in the cryo-TEM, examine the grid under low magnification (~100 to 200x). This step is to examine the overall thickness of the grid and verify presence of protein microcrystals. It is common to observe an uneven distribution of crystals and overall thickness. If there are no visible regions of the grid because grid is too thick everywhere, or if there are no crystals visible on the grid, either load a new grid or repeat the sample preparation steps to prepare new samples with different blotting or sample handling conditions.
Note areas of the grid which are promising (i.e. thin enough for the beam to penetrate and holding crystals. Move the stage to one of these grid squares and increase the magnification to a medium level (approximately 500 – 1000x). The field of view should be approximately one grid square (see Fig 1).
Locate promising crystals on the grid square. If no crystals are visible when zooming in after step 4, return to step 4 and move to a new grid square.
When a promising crystal is identified (well-defined edges and well-separated from other crystals or the grid bars) on the grid square, move the crystal to the center of the detector. The eucentric height should be accurately set so that the crystal does not move as the stage is rotated.
Insert the selected area aperture.
Insert the beam stop, unless the detector equipped on the cryo-TEM does not require the use of a beam stop.
Blank the beam and switch the microscope into diffraction mode that has been set up to collect low-dose diffraction patterns.
Unblank the beam and collect a still exposure diffraction pattern from the single crystal.
Assess the quality of the diffraction pattern. If the reflections are sharp, well-separated, and extend to high-resolution (Fig 2), proceed to the next section to collect a continuous rotation data set from this crystal. If the crystal shows poor diffraction, or no diffraction, switch the microscope back to imaging mode, remove the selected area aperture and beam stop, and return to step 5.
3.3. MicroED data collection
Once a crystal has shown high-quality diffraction from a single exposure. Return the microscope to imaging to visualize the crystal on the grid square again (500 – 1000x magnification). Remove the selected area aperture and beam stop.
Tilt the stage in both the positive and negative directions to ensure the crystal remains centered while rotating and to determine the maximum possible tilt range that can be collected without the crystal being obscured by other crystals or the bars of the grid. If the crystals move while rotating, correct the Z-height to return the crystal to the eucentric position.
Once the tilt range to be collected has been confirmed in step 2, tilt the grid to the maximum tilt angle of this range. Put in the selected area aperture and beam stop. Blank the beam.
Based on the manufacturer and model, set up the stage rotation such that when data collection begins, the stage can be rotated slowly from the starting tilt angle to the end tilt angle (Note 7).
According to manufactures direction, set the high-speed detector up for data to be collected in a movie mode (Note 8).
Return the microscope to diffraction mode used for collecting low-dose diffraction patterns
Unblank the beam and begin the rotation of the stage. Once the stage begins to rotate, begin recording with the high-speed detector.
Once the data set has been collected, return the stage to 0° tilt, remove the beam stop and the selected area aperture.
Repeat the procedures presented in 3.2 and 3.3 to collect data sets from multiple crystals on the grid. Generally, multiple crystals are needed for structure determination. Therefore, it is recommended to collect as many high-quality data sets as possible from grid that shows high-quality crystals. Following the successful use of these methods on suitable samples, the user will have collected several high-quality MicroED data sets that can be processed by standard programs and procedures (35,29,30).
Acknowledgments
The Nannenga lab is supported by the National Institutes of Health grant R01GM124152 and R21GM135784, the Air Force Office of Scientific Research grant FA9550-18-1-0012, and the National Science Foundation award 1942084. We acknowledge the use of facilities within the LeRoy Eyring Center for Solid State Science at Arizona State University, Tempe, AZ, specifically the use of the Titan Krios and the funding of this instrument by NSF MRI 1531991.
4. Notes
There is not one specific plunge freezing device required for MicroED. Many types of automated or manual plunge freeze devices can be used for vitrification of samples for MicroED. Users are encouraged to use whichever device they prefer.
In principle, any cryo-TEM capable of low-dose data collection can be used for MicroED. Much of the previous work on MicroED has used Thermo Fisher cryo-TEMs (e.g. TF-20, Titan Krios), however other manufactures such as JEOL can be used as well. One of the required features of the cryo-TEM is the ability to load vitrified samples into the grid either with an autoloader system or a compatible cryo-holder. The second required feature is the ability to collect data under very low-dose conditions and to be able to switch between the three aligned low-dose modes: low-magnification search, medium-magnification search, and diffraction data collection mode. Low magnification search should be approximately 100 – 200x and will be used to visualize large areas of the grid at once. Medium magnification mode will be around 500 – 1000x (enough to see one grid square) and is used to identify crystals on the grid. Alternatively, medium-magnification search can be performed in over-focused diffraction mode. Both search modes should be set up to have the absolute minimum amount of dose required to visualize crystals on the grid. The third mode, diffraction data collection mode, is performed while the microscope is in diffraction mode and should be set such that the exposure is on the order of 0.01 e−/Å2/s. It is critical that the diffraction data collection mode is very well aligned with the medium magnification search mode, so that when a crystal is centered in the search mode, it is also centered in the beam for diffraction data collection mode.
A requirement for continuous rotation MicroED data collection is that the detector used must be have a high dynamic range, be sensitive, and be able to collect high-speed data. Several camera systems have been used for MicroED including high-speed scintillator based CMOS detectors (17,16,7,8), direct electron detectors (35), and hybrid-pixel detectors (10,36,37).
In some cases crystals must be diluted using the precipitant solution. In the cases where the crystals grow in highly viscous solutions (e.g. high-molecular weight PEGs), dilution in the presence of less viscous (e.g. low-molecular weight PEGs) is required. This is generally a trial-and-error process where the user optimizes the ability to blot the solution with the stability of the microcrystals in these solutions. For sensitive crystals, pipetting should be kept as gentle as possible. If crystals are stable yet too large, there are protocols for fragmenting by pipetting, and other means, available (6).
Blotting time is another variable that must be screened to obtain optimal samples. Ideally, the crystals will have a thin layer of vitrified solution surrounding them, and the rest of the grid will be easily penetrated by the electron beam. If blotting time is too long, too much solution may be removed, and crystal quality can be affected. If blotting time is too short, the grid may be too thick and the electron beam will not penetrate. It is recommended to try a wide range of blotting time (e.g 2 – 20s) and multiple blotting procedures where the sample is blotted more than once before plunge freezing.
It is critical that the microscope be well-aligned for the collection of high-quality MicroED data. Also, the medium magnification search mode must be aligned with the diffraction data collection mode so that when a crystal is located in medium magnification search, it can be exposed by the diffraction mode and data can be collected.
The rotation rate of the microscope is a parameter that can be adjusted depending on the crystal samples being studied. By adjusting the rotation rate along with the integration time on the high-speed detector, the user can control how many degrees of rotation occurs for each diffraction frame in the data set. For example, if the rotation rate on the stage is 0.1°/s and the frame rate of the detector is set to 4s, each frame in the data set will consist of a 0.4° sampling of reciprocal space.
5. References
- 1.Nannenga BL (2020) MicroED methodology and development. Struct Dyn 7 (1):014304. doi: 10.1063/1.5128226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nannenga BL, Gonen T (2019) The cryo-EM method microcrystal electron diffraction (MicroED). Nature Methods 16 (5):369–379. doi: 10.1038/s41592-019-0395-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff AM, Young ID, Sierra RG, Brewster AS, Martynowycz MW, Nango E, Sugahara M, Nakane T, Ito K, Aquila A, Bhowmick A, Biel JT, Carbajo S, Cohen AE, Cortez S, Gonzalez A, Hino T, Im D, Koralek JD, Kubo M, Lazarou TS, Nomura T, Owada S, Samelson AJ, Tanaka T, Tanaka R, Thompson EM, van den Bedem H, Woldeyes RA, Yumoto F, Zhao W, Tono K, Boutet S, Iwata S, Gonen T, Sauter NK, Fraser JS, Thompson MC (2020) Comparing serial X-ray crystallography and microcrystal electron diffraction (MicroED) as methods for routine structure determination from small macromolecular crystals. IUCrJ 7 (Pt 2):306–323. doi: 10.1107/S205225252000072X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zatsepin NA, Li C, Colasurd P, Nannenga BL (2019) The complementarity of serial femtosecond crystallography and MicroED for structure determination from microcrystals. Current Opinion in Structural Biology. doi: 10.1016/j.sbi.2019.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purdy MD, Shi D, Chrustowicz J, Hattne J, Gonen T, Yeager M (2018) MicroED structures of HIV-1 Gag CTD-SP1 reveal binding interactions with the maturation inhibitor bevirimat. Proceedings of the National Academy of Sciences:201806806. doi: 10.1073/pnas.1806806115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Cruz MJ, Hattne J, Shi D, Seidler P, Rodriguez J, Reyes FE, Sawaya MR, Cascio D, Weiss SC, Kim SK, Hinck CS, Hinck AP, Calero G, Eisenberg D, Gonen T (2017) Atomic-resolution structures from fragmented protein crystals with the cryoEM method MicroED. Nat Methods 14 (4):399–402. doi: 10.1038/nmeth.4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nannenga BL, Shi D, Leslie AG, Gonen T (2014) High-resolution structure determination by continuous-rotation data collection in MicroED. Nat Methods 11 (9):927–930. doi: 10.1038/nmeth.3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nannenga BL, Shi D, Hattne J, Reyes FE, Gonen T (2014) Structure of catalase determined by MicroED. Elife 3:e03600. doi: 10.7554/eLife.03600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi D, Nannenga BL, ladanza MG, Gonen T (2013) Three-dimensional electron crystallography of protein microcrystals. Elife 2:e01345. doi: 10.7554/eLife.01345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu H, Lebrette H, Clabbers MTB, Zhao J, Griese JJ, Zou X, Högbom M (2019) Solving a new R2lox protein structure by microcrystal electron diffraction. Science Advances 5 (8):eaax4621. doi: 10.1126/sciadv.aax4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warmack RA, Boyer DR, Zee C-T, Richards LS, Sawaya MR, Cascio D, Gonen T, Eisenberg DS, Clarke SG (2019) Structure of amyloid-β (20-34) with Alzheimer’s-associated isomerization at Asp23 reveals a distinct protofilament interface. Nature Communications 10 (1):3357. doi: 10.1038/s41467-019-11183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krotee P, Griner SL, Sawaya MR, Cascio D, Rodriguez JA, Shi D, Philipp S, Murray K, Saelices L, Lee J, Seidler P, Glabe CG, Jiang L, Gonen T, Eisenberg DS (2018) Common fibrillar spines of amyloid-beta and human islet amyloid polypeptide revealed by microelectron diffraction and structure-based inhibitors. J Biol Chem 293 (8):2888–2902. doi: 10.1074/jbc.M117.806109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallagher-Jones M, Glynn C, Boyer DR, Martynowycz MW, Hernandez E, Miao J, Zee CT, Novikova IV, Goldschmidt L, McFarlane HT, Helguera GF, Evans JE, Sawaya MR, Cascio D, Eisenberg DS, Gonen T, Rodriguez JA (2018) Sub-angstrom cryo-EM structure of a prion protofibril reveals a polar clasp. Nat Struct Mol Biol 25 (2):131–134. doi: 10.1038/s41594-017-0018-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krotee P, Rodriguez JA, Sawaya MR, Cascio D, Reyes FE, Shi D, Hattne J, Nannenga BL, Oskarsson ME, Philipp S, Griner S, Jiang L, Glabe CG, Westermark GT, Gonen T, Eisenberg DS (2017) Atomic structures of fibrillar segments of hIAPP suggest tightly mated beta-sheets are important for cytotoxicity. Elife 6. doi: 10.7554/eLife.19273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawaya MR, Rodriguez J, Cascio D, Collazo MJ, Shi D, Reyes FE, Hattne J, Gonen T, Eisenberg DS (2016) Ab initio structure determination from prion nanocrystals at atomic resolution by MicroED. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1606287113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez JA, Ivanova MI, Sawaya MR, Cascio D, Reyes FE, Shi D, Sangwan S, Guenther EL, Johnson LM, Zhang M, Jiang L, Arbing MA, Nannenga BL, Hattne J, Whitelegge J, Brewster AS, Messerschmidt M, Boutet S, Sauter NK, Gonen T, Eisenberg DS (2015) Structure of the toxic core of alpha-synuclein from invisible crystals. Nature. doi: 10.1038/nature15368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine AM, Bu G, Biswas S, Tsai EHR, Braunschweig AB, Nannenga BL (2020) Crystal structure and orientation of organic semiconductor thin films by microcrystal electron diffraction and grazing-incidence wide-angle X-ray scattering. Chem Commun (Camb). doi: 10.1039/d0cc00119h [DOI] [PubMed] [Google Scholar]
- 18.van Genderen E, Clabbers MT, Das PP, Stewart A, Nederlof I, Barentsen KC, Portillo Q, Pannu NS, Nicolopoulos S, Gruene T, Abrahams JP (2016) Ab initio structure determination of nanocrystals of organic pharmaceutical compounds by electron diffraction at room temperature using a Timepix quantum area direct electron detector. Acta Crystallogr A Found Adv 72 (Pt 2):236–242. doi: 10.1107/S2053273315022500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruene T, Wennmacher JTC, Zaubitzer C, Holstein JJ, Heidler J, Fecteau-Lefebvre A, De Carlo S, Muller E, Goldie KN, Regeni I, Li T, Santiso-Quinones G, Steinfeld G, Handschin S, van Genderen E, van Bokhoven JA, Clever GH, Pantelic R (2018) Rapid Structure Determination of Microcrystalline Molecular Compounds Using Electron Diffraction. Angew Chem Int Ed Engl 57(50):16313–16317. doi: 10.1002/anie.201811318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ting CP, Funk MA, Halaby SL, Zhang Z, Gonen T, van der Donk WA (2019) Use of a scaffold peptide in the biosynthesis of amino acid–derived natural products. Science 365 (6450):280. doi: 10.1126/science.aau6232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones CG, Martynowycz MW, Hattne J, Fulton TJ, Stoltz BM, Rodriguez JA, Nelson HM, Gonen T (2018) The CryoEM Method MicroED as a Powerful Tool for Small Molecule Structure Determination. ACS Central Science 4 (11):1587–1592. doi: 10.1021/acscentsci.8b00760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi D, Nannenga BL, de la Cruz MJ, Liu J, Sawtelle S, Calero G, Reyes FE, Hattne J, Gonen T (2016) The collection of MicroED data for macromolecular crystallography. Nat Protoc 11 (5):895–904. doi: 10.1038/nprot.2016.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carragher B, Cheng Y, Frost A, Glaeser RM, Lander GC, Nogales E, Wang HW (2019) Current outcomes when optimizing ‘standard’ sample preparation for single-particle cryo-EM. J Microsc 276 (1):39–45. doi: 10.1m/jrni.12834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martynowycz MW, Zhao W, Hattne J, Jensen GJ, Gonen T (2019) Collection of Continuous Rotation MicroED Data from Ion Beam-Milled Crystals of Any Size. Structure 27 (3):545–548.e542. doi: 10.1016/j.str.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duyvesteyn HME, Kotecha A, Ginn HM, Hecksel CW, Beale EV, de Haas F, Evans G, Zhang P, Chiu W, Stuart DI (2018) Machining protein microcrystals for structure determination by electron diffraction. Proc Natl Acad Sci U S A 115 (38):9569–9573. doi: 10.1073/pnas.1809978115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de la Cruz MJ, Martynowycz MW, Hattne J, Gonen T (2019) MicroED data collection with SerialEM. Ultramicroscopy 201:77–80. doi: 10.1016/j.ultramic.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang B, Zou X, Smeets S (2019) Automated serial rotation electron diffraction combined with cluster analysis: an efficient multi-crystal workflow for structure determination. IUCrJ 6 (Pt 5):854–867. doi: 10.1107/S2052252519007681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cichocka MO, Angstrom J, Wang B, Zou X, Smeets S (2018) High-throughput continuous rotation electron diffraction data acquisition via software automation. J Appl Crystallogr 51 (Pt 6):1652–1661. doi: 10.1107/S1600576718015145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hattne J, Reyes FE, Nannenga BL, Shi D, de la Cruz MJ, Leslie AG, Gonen T (2015) MicroED data collection and processing. Acta Crystallogr A Found Adv 71 (Pt 4):353–360. doi: 10.1107/S2053273315010669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clabbers MTB, Gruene T, Parkhurst JM, Abrahams JP, Waterman DG (2018) Electron diffraction data processing with DIALS. Acta Crystallogr D Struct Biol 74 (Pt 6):506–518. doi: 10.1107/S2059798318007726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson HP, Lin G, Barnes CO, Sutkeviciute I, Krzysiak T, Weiss SC, Reynolds S, Wu Y, Nagarajan V, Makhov AM, Lawrence R, Lamm E, Clark L, Gardella TJ, Hogue BG, Ogata CM, Ahn J, Gronenborn AM, Conway JF, Vilardaga JP, Cohen AE, Calero G (2016) Transmission electron microscopy for the evaluation and optimization of crystal growth. Acta Crystallogr D Struct Biol 72 (Pt 5):603–615. doi: 10.1107/S2059798316001546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barnes CO, Kovaleva EG, Fu X, Stevenson HP, Brewster AS, DePonte DP, Baxter EL, Cohen AE, Calero G (2016) Assessment of microcrystal quality by transmission electron microscopy for efficient serial femtosecond crystallography. Arch Biochem Biophys 602:61–68. doi: 10.1016/j.abb.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson HP, Makhov AM, Calero M, Edwards AL, Zeldin OB, Mathews II, Lin G, Barnes CO, Santamaria H, Ross TM, Soltis SM, Khosla C, Nagarajan V, Conway JF, Cohen AE, Calero G (2014) Use of transmission electron microscopy to identify nanocrystals of challenging protein targets. Proc Natl Acad Sci U S A 111 (23):8470–8475. doi: 10.1073/pnas.1400240111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hattne J, Shi D, de la Cruz MJ, Reyes FE, Gonen T (2016) Modeling truncated pixel values of faint reflections in MicroED images. J Appl Crystallogr 49 (Pt 3):1029–1034. doi: 10.1107/S1600576716007196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hattne J, Martynowycz MW, Penczek PA, Gonen T (2019) MicroED with the Falcon III direct electron detector. IUCrJ 6 (Pt 5):921–926. doi: 10.1107/S2052252519010583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tinti G, Frojdh E, van Genderen E, Gruene T, Schmitt B, de Winter DAM, Weckhuysen BM, Abrahams JP (2018) Electron crystallography with the EIGER detector. IUCrJ 5 (Pt 2):190–199. doi: 10.1107/S2052252518000945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clabbers MTB, van Genderen E, Wan W, Wiegers EL, Gruene T, Abrahams JP (2017) Protein structure determination by electron diffraction using a single three-dimensional nanocrystal. Acta Crystallogr D Struct Biol 73 (Pt 9):738–748. doi: 10.1107/S2059798317010348 [DOI] [PMC free article] [PubMed] [Google Scholar]


