Keywords: development, function, kidney, organoid, physiology
Abstract
Kidney organoids are three-dimensional structures generated from pluripotent stem cells (PSCs) that are capable of recapitulating the major structures of mammalian kidneys. As this technology is expected to be a promising tool for studying renal biology, drug discovery, and regenerative medicine, the functional capacity of kidney organoids has emerged as a critical question in the field. Kidney organoids produced using several protocols harbor key structures of native kidneys. Here, we review the current state, recent advances, and future challenges in the functional characterization of kidney organoids, strategies to accelerate and enhance kidney organoid functions, and access to PSC resources to advance organoid research. The strategies to construct physiologically relevant kidney organoids include the use of organ-on-a-chip technologies that integrate fluid circulation and improve organoid maturation. These approaches result in increased expression of the major tubular transporters and elements of mechanosensory signaling pathways suggestive of improved functionality. Nevertheless, continuous efforts remain crucial to create kidney tissue that more faithfully replicates physiological conditions for future applications in kidney regeneration medicine and their ethical use in patient care.
NEW & NOTEWORTHY Kidney organoids are three-dimensional structures derived from stem cells, mimicking the major components of mammalian kidneys. Although they show great promise, their functional capacity has become a critical question. This review explores the advancements and challenges in evaluating and enhancing kidney organoid function, including the use of organ-on-chip technologies, multiomics data, and in vivo transplantation. Integrating these approaches to further enhance their physiological relevance will continue to advance disease modeling and regenerative medicine applications.
INTRODUCTION
The kidneys play a critical role in maintaining the internal environment of organisms by maintaining fluid and electrolyte balance, and eliminating toxins (1). The nephron of the kidney, composed of the glomerulus and the renal tubules, is surrounded by a stroma containing fibroblasts and immune cells (2, 3) and is intimately associated with a highly organized vasculature (4). These cells and structures are uniquely organized in different regions along the cortico-medullary axis of the kidney (5, 6). Within the nephrons, the glomeruli produce ∼180 L of ultrafiltrate per day (5), which then undergoes reabsorption and secretion processes in sequential tubular segments to produce the final urine that is expelled into the renal pelvis (6). This mature organization of nephrons in each adult kidney with highly specialized anatomical organization requires, early in organogenesis, reciprocal inductive interactions between progenitor cells of the ureteric bud and metanephric mesenchyme (7). Developing model systems that recapitulate the functions of the human kidney holds great promise for understanding the mechanisms of functional decline in the kidney, as well as strategies to restore healthy function in disease states and replace damaged kidneys (8). This area of research is of significant interest to the kidney community, including patients and nephrologists.
As our understanding of kidney organoids continues to expand, one of the key questions that arise is regarding their functional capacity when generated in vitro. Researchers in the field of organoid development aim to cultivate three-dimensional (3-D) structures from pluripotent stem cells that can differentiate and mimic the structure and function of a mammalian kidney. Current kidney organoids partially replicate the complexity of renal tissue by containing important components such as endothelial cells, stromal cells, podocytes, and tubules. The proportions of each cell type vary depending on the specific differentiation protocol used (9). However, their ability to function and form a connected system for urine drainage remains largely unknown. Claude Bernard, a renowned physiologist, famously stated, “The function of an organ or apparatus should be determined by its structure” (10). Yet, this first needs to be confirmed with proper functional evaluation in a new technology such as kidney organoids. In alignment with the RBK Consortium’s goal “to coordinate and support studies that will result in the ability to generate or repair nephrons that can function within the kidney” (11), a Functional Assay Working Group was established to catalog functional assays optimized to demonstrate successful function in organoids and other renal model systems, as mentioned in previous reviews (12, 13). In this perspective, we provide an overview of the recent advances in the characterization and optimization of the functional capacity of organoids, including strategies that replicate physiological processes by stimulating their maturation. Based on the results of these studies, the path forward must be prioritized to identify mechanisms to accelerate the functional maturation of organoids in the future.
STRUCTURE AND FUNCTION OF THE KIDNEYS AND THE KIDNEY ORGANOIDS: HOW DO THEY COMPARE?
Several experimental procedures have been established to generate kidney organoids, resulting in differences in structure and cellular composition (9, 14–21). However, they share common phenotypic features including cellular types and structures found in mammalian kidneys (Fig. 1A). To confirm the physiological relevance of kidney organoids, characterization of its structural organization and the function of each specific segment is necessary. An inherent limitation to the functional analysis pertains to the 3-D configuration of these miniature organs lacking a proper inflow and outflow. These are theoretically necessary for the study of filtration, reabsorption, and secretion processes. Research groups have aimed to overcome these challenges by using various assays, and the key functional findings related to each segment are summarized in Fig. 2 and in this section.
Figure 1.
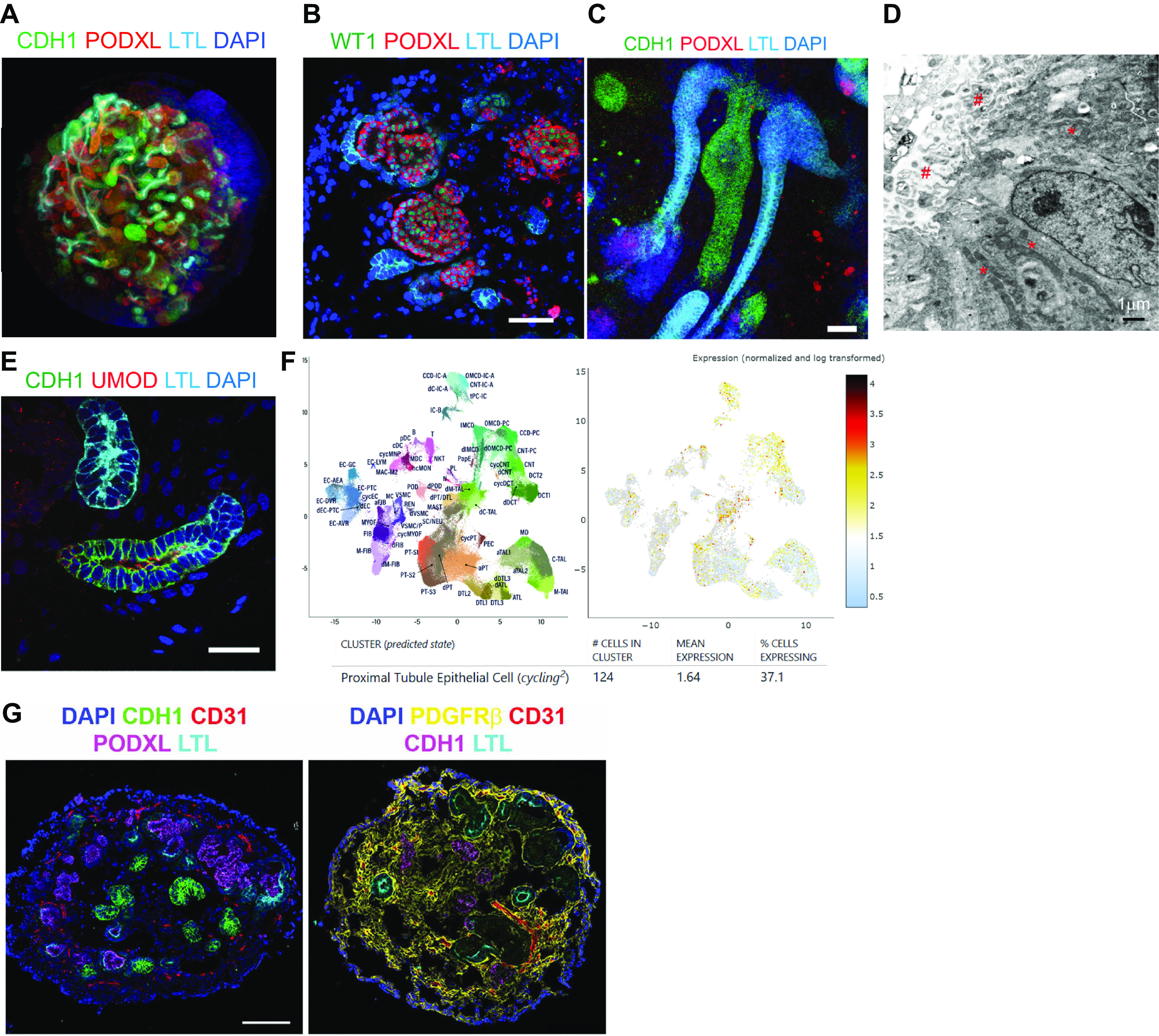
Kidney organoid structures and cellular components. A: three-dimensional (3-D) images of kidney organoids on day 49 of differentiation. B and C: immunostaining displaying organoid glomeruli (B) and tubules (C). D: an electron microscope image showing brush border-like structures (#) and mitochondria (*) in organoid tubules. E: immunofluorescence microscopy displaying segments of proximal, loops of Henle, and distal tubules. F: single-nuclear RNA sequencing of healthy human kidneys from the Human Biomolecular Atlas Program (HuBMAP) and Kidney Precision Medicine Project (KPMP) revealing the expression of cadherin-1 (CDH1) in proliferating proximal tubules, loops of Henle, and distal nephrons. G: immunostaining showing organoid vasculature and stromal cells. The images of A were kindly provided by Ken Hiratsuka, B–E were from Morizane et al. (14), and G were from Gupta et al. (22).
Figure 2.
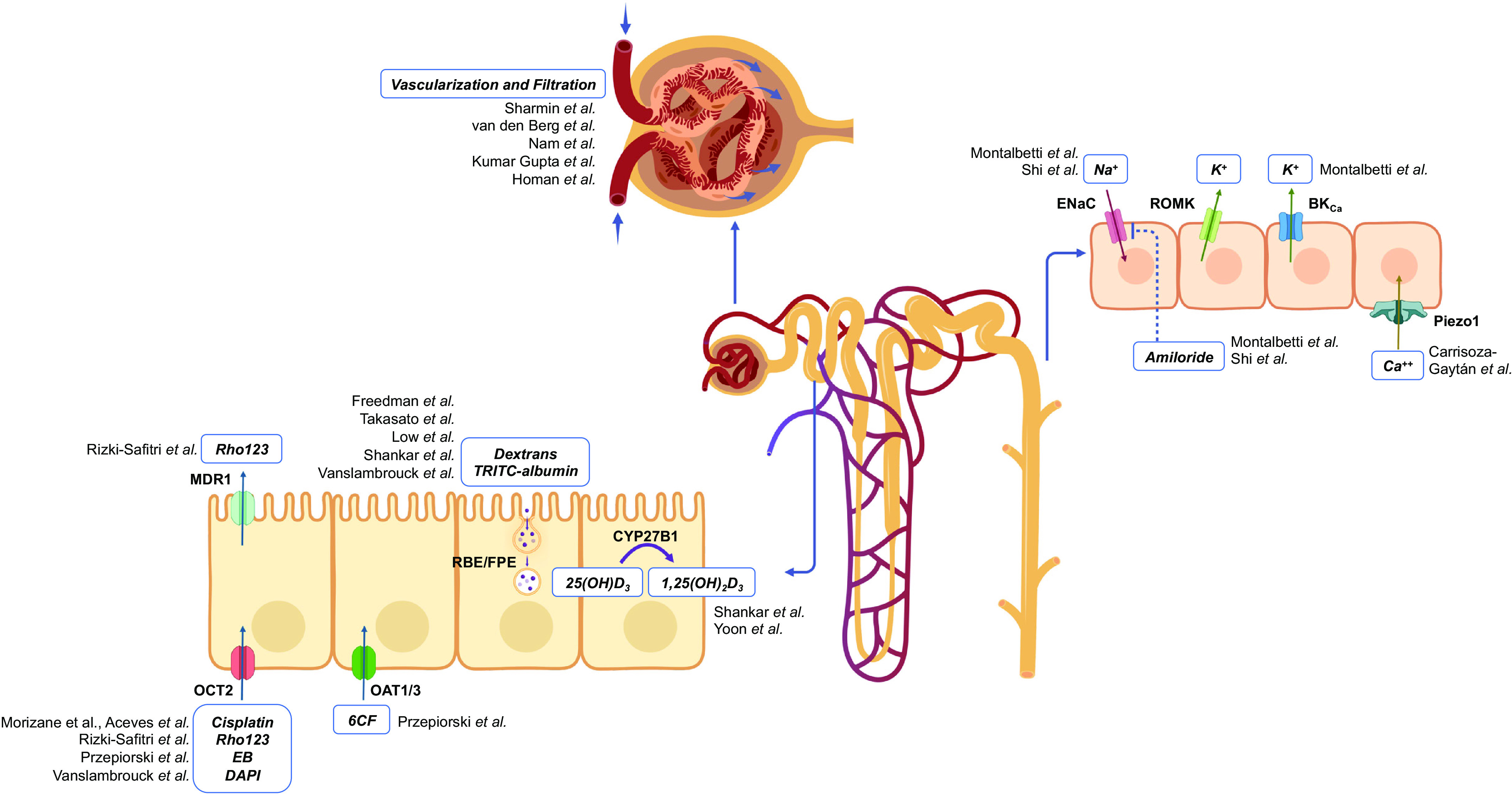
Functions identified in the kidney organoids in each nephron segment. An illustration summarizing studies of kidney organoid function in each nephron segment is shown. BKCa, large-conductance Ca2+-activated K+ channel; EB, ethidium bromide; ENaC, epithelial Na+ channel; FPE, fluid-phase endocytosis; OCT2, organic cation transporter 2; RBE, receptor-based endocytosis; Rho123, rhodamine 123; ROMK, renal outer medullary K+ channel; 6CF, 6-carboxyfluorescein.
Glomerulus
Structure in kidney organoids.
Kidney organoids display round-shaped structures with clustered cells expressing podocalyxin and nephrin indicative of podocytes, surrounded by a lumen separating the cluster from an epithelium resembling the parietal layer of the Bowman’s capsule. Foot processes have also been observed by electron microscopy (14). These glomerulus-like structures, however, usually lack polarity and vascularization that mimics the typical glomerular capillary tuft when cultured under static conditions. Along the same line, stromal cells suggestive of mesangial cells are usually not observed in these structures (Fig. 1B).
Glomerular filtration.
The glomerular filtration barrier within the fully differentiated mammalian kidney is composed of three layers arranged in multiple loops that confer a large area able to support the filtration of massive volumes of plasma. A specific fenestrated endothelium within the capillary loops is surrounded by the glomerular basement membrane, itself covered by the podocyte foot processes interconnected by slit diaphragms (8). Both cell types are arranged in close communication with the supporting mesangial cells and matrix. Due to Starling forces and the particular architecture of this barrier, plasma is filtered to produce ultrafiltrate containing water and solutes of low molecular weight. In kidney organoids maintained under static conditions, as this architecture is usually not seen, an effective filtration process is quite unlikely. To date, there is no reported evidence of glomerular filtration occurring within kidney organoids generated in vitro, except in cases where they have been transplanted into animals (summarized below in Response to In Vivo Transplantation) (21, 23–28).
Proximal Tubule
Structure in kidney organoids.
A tubular structure with columnar epithelium is usually observed contiguous with these podocyte clusters. These tubular structures bind Lotus tetragonolobus lectin (LTL) along the brush border of their apical membranes, similar to observations made in the proximal tubule (PT) of adult human kidneys (18). Accordingly, ultrastructural analysis of this epithelium confirmed the presence of an apical brush border as well as cells densely packed with mitochondria (14), suggestive of the high energetic demand of PT cells (Fig. 1, C and D). Detailed ultrastructural analyses have not been performed to date to identify features indicative of discrete segments of the PT, namely S1 and S2 within the convoluted PT (pars convoluta) and S3, within the straight PT (pars recta) (29). It is thus not known if the current kidney organoid protocols achieve PT subsegment differentiation.
Proximal tubule absorption and secretion.
Due to its large surface area of exchange, the fully differentiated PT of the mammalian kidney is the site of bulk reabsorption of low-molecular-weight molecules, amino acids, glucose, water, phosphate, potassium, bicarbonate, and sodium (30). The segment notably accounts for the reabsorption of ∼70% of the filtered load of sodium and water both through the paracellular route, mediated by claudin-2, and through transcellular pathways, via multiple cotransporters and exchangers localized to the apical or basolateral membranes. On the other hand, low-molecular-weight molecules are reabsorbed through receptor-based endocytosis that is mediated by megalin and/or cubilin, or fluid-phase endocytosis. These two endocytic pathways of the PT are usually assessed by labeled cargo uptake, and in particular fluorescent low-molecular-weight dextran for fluid-phase endocytosis (31). The other central role of the PT is its secretory clearance of small molecules that are directly delivered by the peritubular capillaries to the basolateral side of the epithelium. Waste products and toxins, in particular protein-bound drugs that are not directly filtrated by the glomerulus, are mainly secreted via organic anion or cation transporters (OAT and OCT) (32). The transporters implicated in the reabsorption or the secretion of solutes are differentially expressed in the three segments of the PT. OCT2, for instance, exhibits higher expression levels in the S3 segment compared with the S1/S2 segments. It serves as the primary transporter of cisplatin at the basolateral membrane, which explains the drug’s accumulation in the PT. This accumulation is specifically responsible for the susceptibility of proximal tubules to cisplatin-induced nephrotoxicity (33).
Unlike the complex architecture of glomeruli, which has not been perfectly replicated under static conditions in kidney organoids, the LTL-positive tubular segments in organoids exhibit striking structural and ultrastructural similarities with PT. These similarities were confirmed by single-cell RNA sequencing revealing the expression of specific PT transporters and receptors within LTL-positive epithelium, such as cubilin and OAT1 (34). Several pieces of evidence strengthen the hypothesis of PT functionality in kidney organoids. Cargo uptake has been demonstrated specifically in this segment in several reports (15, 16, 21). Regarding PT drug uptake and secretion, Przepiorski et al. (35) reported in vitro OAT-dependent transport of six carboxyfluorescein (6-CF). Consistent with these findings, Rizki-Safitri et al. (36) demonstrated the transepithelial dynamic transit of rhodamine 123 by live imaging, from its basolateral OCT2-dependent uptake to its luminal multidrug resistance mutation 1 (MDR1)-dependent excretion. Through a protocol aiming at simultaneously increasing the nephron progenitor population and delaying nephron initiation, Vanslambrouck et al. (37) generated kidney organoids with an enhanced PT population able to increase the uptake of albumin. In addition, single-cell RNA sequencing data suggested the presence of different PT populations bearing some resemblance to S1, S2, and S3 segments. PT enhancement occurred, however, at the cost of a reduced distal tubule population compared with their standard differentiation protocol. Future efforts should be directed at more precisely evaluating these different processes along the length of LTL-positive segments in kidney organoids to detect potential axial functional differences suggestive of the existence of S1, S2, and S3-like PT segments (30, 31).
Endocrine functions.
The PT also plays an important endocrine role through its response to parathyroid hormone (PTH) and the production of active vitamin D in the PT cells in mammalian kidneys. Kidney organoids have been reported to express the PTH receptor PTH1R and the 1 α-hydroxylase CYP27B1, which catalyzes the synthesis of active vitamin D (38). The expression of both increases with organoid maturation, and 1,25-vitamin D is produced and accumulates in culture media in response to PTH, consistent with functional calcium metabolism in kidney organoids (39).
Loop of Henle-Distal Nephron
Structure in kidney organoids.
A second tubular epithelium is found in kidney organoids, with cells expressing cadherin-1 (CDH1). In human kidneys, CDH1 is expressed in the loop of Henle (LOH), the distal convoluted tubule, the connecting tubule, and the collecting duct (18). Double positive (LTL and CDH1) segments are occasionally observed (13), which appear to correspond to cycling proximal tubular cells (represented by enrichment of cell cycle genes) in healthy human kidneys according to the single-cell human kidney atlas generated by Human Biomolecular Atlas Program (HuBMAP) and Kidney Precision Medicine Project (KPMP) (Fig. 1, E and F) (40, 41). Although discernable morphological differences allow renal physiologists to identify and microdissect discrete segments of the mammalian kidney under a binocular loupe (6) or by light microscopy, specific features have not been assigned thus far to tubules in kidney organoids. However, single-cell gene expression profiling revealed cell identities that correspond to each segment, similar to human data (41–43). For instance, the LOH cluster within kidney organoids expressed SLC12A1, which encodes NKCC2 and marks distal straight tubules. Interestingly, developmental genes such as dickkopf WNT signaling pathway inhibitor 1 (DKK1) were also coexpressed in this segment, suggesting an immature state (43).
Water and electrolyte handling.
The lack of functional homogeneity of the CDH1-positive segment makes it challenging to assess as a whole. In the fully differentiated mammalian kidney, the mature LOH is subdivided into a thin descending [descending thin limb (DTL)]], ascending thin (ATL), and thick ascending limb (TAL), arranged in a specific hairpin shape. Together with this structural arrangement, the differential permeability to water via aquaporin (AQP)1 expression in the DTL and to sodium via NKCC2 expression in the TAL, allows the establishment of the axial osmotic gradient necessary for urine concentration by the countercurrent multiplication process (44). The TAL also accounts for the reabsorption of ∼20% of the filtered load of sodium and is the major site of calcium and magnesium paracellular reabsorption as well (6).
Still in the mature mammalian kidney, the LOH is followed by a segment called the distal convoluted tubule (DCT) which, although short, plays a major role in sodium balance hence extracellular volume homeostasis, through fine-tuning of the activity of the apical sodium-chloride cotransporter (NCC) (45). Finally, two consecutive segments, namely the connecting tubule (CNT), and immediately downstream, the collecting duct (CD) display very similar cellular composition but interestingly originate from two different embryological progenitors, respectively, the metanephric mesenchyme and the ureteric bud. However, there may be contributions of resident CD cell types from both the lineages in some parts of the CNT (46). The CNT and CD are crucial for the final fine-tuning of ion and water balance. They consist of two major cell populations (47). Principal cells (PC) mediate sodium reabsorption via the apical epithelial Na+ channel (ENaC), potassium secretion via the renal outer medullary K+ (ROMK) channel, and water reabsorption via AQP2. Adjacent intercalated cells (IC) are further subdivided into type A (IC-A) and type B (IC-B) cells, predominantly involved in acid and bicarbonate secretion, respectively, but also contributing to net urinary potassium excretion via the large-conductance (BK) channel (48, 49). IC-A has an apical H+-ATPase and a basolateral chloride/bicarbonate exchanger (AE1), and IC-B has a basolateral H+-ATPase and an apical chloride/bicarbonate exchanger (pendrin) (6).
The majority of these transport proteins have been identified in kidney organoids at the mRNA/protein level in several reports (50–52), including those focused on the maturation of ureteric bud-derived organoids (51, 53–55). It is only recently that the function of some of them has been demonstrated. Montalbetti et al. (56) confirmed the expression of ENaC, ROMK, and BK channels in kidney organoids and characterized their electrophysiological signatures by patch-clamp analyses. Shi et al. (57) reseeded their ureteric bud-derived CD kidney organoids onto a two-dimensional (2-D) epithelial model allowing them to observe an amiloride-sensitive transepithelial voltage and current suggestive of an effective ENaC-mediated sodium transport. Despite the identification of cell identities corresponding to some of the distal nephron segments, the anatomic organization of LOH in a discrete cortico-medullary axis has not yet been achieved in kidney organoids. Moreover, as the distal segments are a small fraction of the tubular epithelial cells found in the kidney organoids, their study and functionality are inevitably constrained by their limited quantity. New differentiation strategies are thus warranted to broaden the fraction of these cell types while maintaining the more proximal segments of the nephron.
Interstitium
Structure in kidney organoids.
Kidney organoids contain mesenchyme-derived stroma-like cells as demonstrated by their nuclear MEIS1 expression (58, 59) and PDGFR membrane and cytoplasmic expression (Fig. 1G) (22). These cells are found embedded within the spaces between the aforementioned parts of the nephrons and in the periphery of the organoids. Their number tends to increase in later-stage organoids (60). The distinct zonation observed in stromal cells in rodent kidneys has not yet been reported in kidney organoids (61).
Endocrine functions.
Interstitial cells include fibroblast-like cells, pericytes, and mesangial cells (62). Their two main endocrine functions are the production of renin in the juxta-glomerular apparatus and erythropoietin (EPO) throughout the cortex and the outer medulla in mammalian kidneys. In this respect, Shankar et al. (23) observed the production of renin by kidney organoids, which was further stimulated in vitro by cAMP. The expression of renin was more pronounced in a pericyte subset cluster by single-cell sequencing. Regarding the response to the renin-angiotensin system, the expression of the angiotensin II type 1 receptor (AT1R) and the angiotensin II type 2 receptor (AT2R) appear to be sequential, the former being strongly expressed in the early stages of differentiation, whereas the latter peaking at day 12 after initiation of induced pluripotent stem cell (iPSC) differentiation (63). In addition, treatment with angiotensin II during the middle phase of differentiation seems to increase the expression of podocyte markers such as WT1 and podocalyxin, suggestive of improved maturation (63). Finally, although no studies have shown kidney organoids produce functional EPO to date, EPO-producing cells have been engineered from human-induced pluripotent stem cells that respond to hypoxia (64).
Vasculature
Structure in kidney organoids.
When cultured under static conditions, kidney organoids contain cells expressing endothelial markers such as platelet and endothelial cell adhesion molecule (PECAM)-1 (CD31) located in the interstitium (Fig. 1G). Although they generally do not penetrate the glomeruli, these cells are organized in capillary-like 3-D networks without a distinct lumen (16). Thus current organoid protocols need substantial improvement in achieving vascular cell type and functional diversity.
ENHANCING KIDNEY ORGANOID MATURATION: THE KEY TO IMPROVING FUNCTION?
Response to Shear Stress
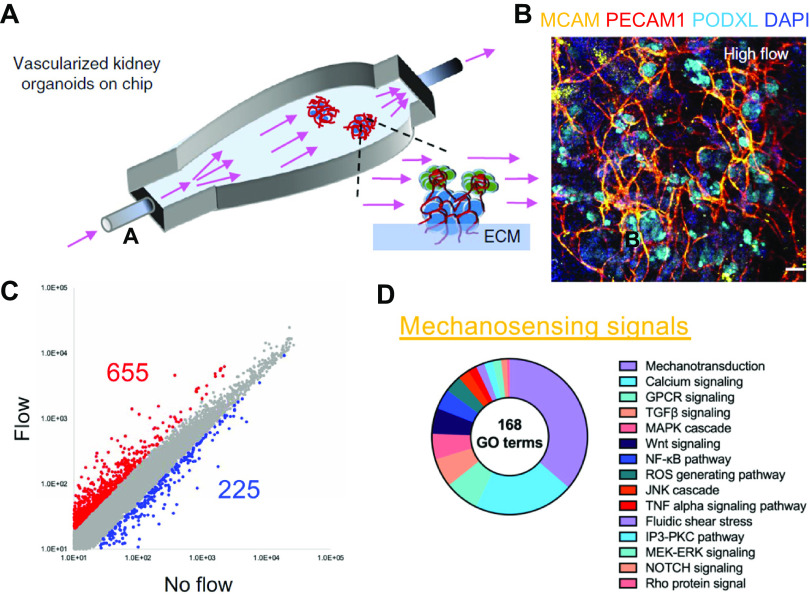
As kidneys receive 25% of the cardiac output, renal cells are submitted to continuous flow of decreasing rate along with vascular branching. Vascular flow is critical to deliver vital nutrients and oxygen to cells but also confers a direct effect on cellular homeostasis (65). Luminal flow rate subjects epithelial cells in the discrete nephron segments to hydrodynamic forces (66). Fluid shear stress has been shown to play an essential role in organ and vascular maturation during embryogenesis and angiogenesis (67–69), partly mediated by calcium signaling (70–72). This observation along with findings that vasculature facilitates the formation of the liver bud from stem cell-derived multiple lineages (73), led to the investigation of the role of superfusate flow on kidney organoid differentiation and maturation (Fig. 3A). Kidney organoid superfusion led first to an increase in vascular density within the organoids (Fig. 3B), second to trigger migration of endothelial cells into the developing renal corpuscle, and third to enhanced tubular maturation as demonstrated by the increased expression of specific PT transporters (74). Recently, Aceves et al. (76) confirmed the observations of an upregulation of drug transporters induced by fluid flow and cisplatin-induced nephrotoxicity in kidney organoids (14). Their study demonstrated the basolateral OCT2-dependent uptake of cisplatin in an organ-on-a-chip model using PT-like epithelium isolated from kidney organioids, as previously described (76).
Figure 3.
Flow-induced vascularization and mechanosensing biological processes. A: an illustration of the kidney organoid-on-chip model. B: immunostaining displaying vascularized kidney organoids under flow. C and D: differentially expressed genes in kidney organoids cultured under flow and their GO terms implicated in mechanosensing signals. The images of A and B were taken from Homan et al. (74) and C and D were from Hiratsuka et al. (75). GPCR, G protein-coupled receptor; ROS, reactive oxygen species; TGF, transforming growth factor.
The mechanisms by which shear stress accelerates kidney organoid function are suggested by transcriptomic analyses and are currently under investigation (Fig. 3, C and D) (75). Although increases in superfusate flow may stimulate differentiation by continuously removing morphogen inhibitors and/or ensuring a steady supply of essential nutrients, other flow-dependent physical cues may be at play. Sensing of luminal fluid shear stress in the fully differentiated proximal tubule has been attributed to the mechanical perturbation of brush border microvilli that are made of actin filament bundles (66). In the distal nephron, primary cilia have been proposed as key mechanosensors (77). An interesting finding is that the mutations in primary ciliary proteins can result in the loss of epithelial polarity, disrupted proliferation, and the formation of cysts, including in kidney organoids (78). This pathophysiological process was validated by inducing cyst formation upon the initiation of fluid flow in a kidney organoid model of autosomal recessive polycystic kidney disease. Furthermore, this process was linked to mechanosensory signals related to RAC1 and FOS (75).
Other factors mediate mechanosensation as well. Luminal flow-induced transient increases in intracellular calcium concentration have been identified in both principal and intercalated cells in acutely deciliated cortical CDs (CCDs) of the mature kidney, albeit lacking the typical immediate high-amplitude spike proposed to be secondary to inositol (1,4,5)-trisphosphate (IP3)-mediated release (66, 79, 80). In fact, recent studies have revealed the presence of basolateral PIEZO1 channels in the mammalian kidney (81) as well as in isolated organoid tubules (82). Specifically, single-organoid tubules subject to an increase in luminal or basolateral (superfusate) flow exhibited a prompt increase in intracellular calcium concentration with the response to luminal flow increasing with advancing days of organoid culture. This maturation of Ca2+ mobilization was associated with a gradual increase in PIEZO1 channel expression in the cell membranes over time during long-term organoid culture (82). Note that Piezo1 is expressed in endothelial cells of developing blood vessels in mice and has been shown to be required for proper vascular development during gestation (83).
Response to In Vivo Transplantation
Our ability to reproduce a physiological environment in vitro, needed for optimal viability and health of engineered organs, is still limited. An integrated approach with specific in vivo assays is needed to demonstrate functional maturity of kidney organoids and to optimize their maturation. It may also show sustainability by transplanting them into immunodeficient animals to prevent rejection (84). Vascular engraftment and fusion with the host along with accelerated maturation and global vascularization have been shown in several reports, as well as effective dextran uptake by the transplanted organoids (21, 23–28). Li et al. (85) have also shown significant creatinine accumulation in kidney organoid cysts in vivo, a finding that might be suggestive of effective glomerular filtration and/or tubular fluid concentration. Endocrine functions might also be improved following transplantation, as highlighted by the upregulation of renin production in transplanted kidney organoids (23, 86).
Recent Advances in the Ureteric Bud, Vascular and Stroma Differentiation
One strategy that might enhance the functional maturation of kidney organoids is to integrate nephron progenitor-derived and ureteric bud-derived organoids. This approach has been reported to create an architecture closer to mammalian kidneys (51, 55, 87, 88). Stromal cells play an important role in kidney development as well (61, 89, 90). Recently, Tanigawa et al. (86) showed, utilizing the latter approach, that adding stromal progenitors further improves organoid architecture and specialized stromal cell differentiation. These integrated approaches of incorporating and optimizing different lineages hold promise to make better organized and mature organoids with a remaining challenge of creating a contiguous tubular system of urine flow.
RESOURCES AND DATA FOR iPSC AND KIDNEY ORGANOID RESEARCH
Genetically engineered induced pluripotent stem cell lines (iPSCs) are being extensively utilized in the advancement of kidney organoid research. These iPSC lines, both control (parent) and reporter cell lines specifically designed to label major nephron cell types of proximal, distal, and collecting tubules, are being generated and used for various purposes. These include optimizing differentiation protocols and conducting biological experiments, as mentioned in the preceding sections. To ensure the quality and standardization of iPSC lines for kidney research, the ReBuilding a Kidney (RBK) consortium has taken the initiative. They have established a framework for authenticating iPSC lines, which involves validating the targeted integration of reporters, assessing genetic and chromosomal integrity, and confirming the ability to form kidney organoids. As part of this initiative, the RBK has created a central iPSC repository (https://www.atlas-d2k.org/resources/cell-lines/), from which investigators can request both parent and reporter cell lines (Table 1). Currently, several iPSC lines are already available (91). In collaboration with the ATLAS-D2K Center (data center for RBK and GUDMAP) and the Washington University Pediatric Center of Excellence in Nephrology (https://pcen.wustl.edu/), a new development enables the distribution of each vial of these cell lines to researchers free of charge (Table 1). This collaborative effort establishes an infrastructure that will continue to expand and incorporate new iPSC lines donated by investigators. It aims to accelerate research efforts in kidney organoids and regenerative medicine.
Table 1.
Resources for human kidney organoids
| Resource Name | Resource Type | Website |
|---|---|---|
| Washington University Human iPS Core | iPS cells | https://pcen.wustl.edu |
| Atlas D2K center | Cell line resources, Protocols, Functional assays handbook, Omics data | https://www.atlas-d2k.org/ |
| KPMP Kidney Tissue Atlas | Single-cell transcriptome data (human kidney) | https://atlas.kpmp.org/ |
| HuBMAP | Spatial multiomic maps (healthy human organs) | https://portal.hubmapconsortium.org/ |
| CellxGene by CZI | Atlas of healthy and injured cell states and niches (human kidney) | https://cellxgene.cziscience.com/ |
| Kidney Interactive Transcriptomics | Single-cell transcriptome data (human kidney Mouse kidney organoids) | https://humphreyslab.com/SingleCell/ |
| ENCODE study | Functional genome data (human kidney organoids) | https://www.encodeproject.org/ |
| Gene Expression Omnibus | Single-cell and bulk transcriptome data (human kidney organoids, human fetal and adult kidneys) | https://www.ncbi.nlm.nih.gov/geo/ (e.g., GSE164647) |
HuBMAP, Human Biomolecular Atlas Program; KPMP, Kidney Precision Medicine Project.
Importantly, the provision of standardized resources and protocols will enhance quality control and reduce technical variability when working with highly sophisticated procedures such as the creation of kidney organoids. In this perspective, there is rapid progress in generating single-cell and spatial multiomic datasets from the human kidney that have begun to be publicly available including the Kidney Tissue Atlas KPMP (41), the Kidney Interactive Transcriptomics (KIT) datasets (92), the ENCODE study (93), HuBMAP (94), or the NCBI Gene Expression Omnibus data set of functional genomics (95), originating from human kidney tissue as well as from kidney organoids. These resources, along with the Handbook of Functional Assays, offer promising approaches to help inform improved organoid maturation and functionality (Table 1).
CHALLENGES AND FUTURE DIRECTION
Kidney organoids offer unparalleled prospects for exploring renal physiology and diseases in human cells ex vivo, representing a groundbreaking avenue for scientific inquiry. Nonetheless, there exist notable obstacles that demand immediate attention and resolution. Ethical considerations necessary to guide responsible organoid use have been summarized by previous reviews (96, 97). Ethical issues are mainly divided according to their use in research or clinics, the latter being still a distant prospect in the field of kidney organoids. Regarding their use in research, the first issue is the use of human embryonic stem cells (hESCs). To obtain hESCs, the first step involves the destruction of a 5-day-old preimplantation embryo (blastula stage), which raises the debate on the moral status of the embryo. The alternative use of human-derived iPSCs is not devoid of ethical concerns either. Researchers thus also need ethical guidance to ensure the responsible use of these resources, including donor’s informed consent requirement, and the use of approved repositories (98).
Foremost among the technical and scientific challenges is the issue of batch variations in kidney organoid differentiation, as elucidated by Phipson et al. (99). The maintenance of human pluripotent stem cells (hPSCs) necessitates vigilant monitoring and careful passaging, precisely timed to initiate cellular differentiation effectively. To achieve successful organoid differentiation, multiple intricate steps must be undertaken, incorporating an assortment of multiple growth factors. Consequently, this process entails labor-intensive daily feeding and the meticulous administration of various growth factors and small molecules. These technical complexities significantly contribute to the intricacy of organoid research, causing unpredictable batch variations. This inherent nature of organoid research poses a considerable challenge when striving to obtain reproducible outcomes across disparate laboratories. Moreover, the development of multiple differentiation protocols aimed at generating kidney organoids from hPSCs further adds to the complexity of the field. As a result, it becomes crucial to establish clear and standardized metrics for successful organoid differentiation.
The advancements achieved through the RBK activities have propelled the development and functional exploration of kidney organoids to a significant extent. Consequently, we are now at a stage where it is both appropriate and necessary to establish key metrics that define successful differentiation. These metrics serve as crucial benchmarks, ensuring that kidney organoids possess the essential cellular components required for accurate representation and study of nephrons and interstitial cells (Fig. 4). To meet these metrics, kidney organoids must comprise the fundamental cellular constituents of nephrons, such as podocytes, proximal tubules, LOHs, and distal nephrons (including distal convoluted tubules and connecting tubules). Importantly, the segments need to be arranged in a contiguous manner. Another important aspect of the cellular arrangement is the establishment of cellular polarity, with distinct luminal and basal sides, which is essential for functional studies and proper modeling of kidney physiology. In addition, interstitial cells, encompassing stromal cells and endothelial cells, must be present within the kidney organoids. Their inclusion is paramount to emulate the microenvironment of the kidney accurately and enable comprehensive investigations of cellular interactions and signaling pathways within the organoid system. Moreover, for the organoids to be effective tools in toxicity studies and pharmacological research, the proximal tubules must express key drug transporters, such as OCT2, OAT1/3, and MDR1. These transporters play a crucial role in drug metabolism and elimination, making their presence essential for the accurate evaluation of drug responses and toxicity assessments. Although progress has been made in defining these metrics, there remains a need for further studies to establish the specific benchmarks for LOHs and distal nephrons. By conducting these studies, we will be able to refine our understanding of these critical components, ensuring that kidney organoids encompass a comprehensive representation of the nephron structure and function.
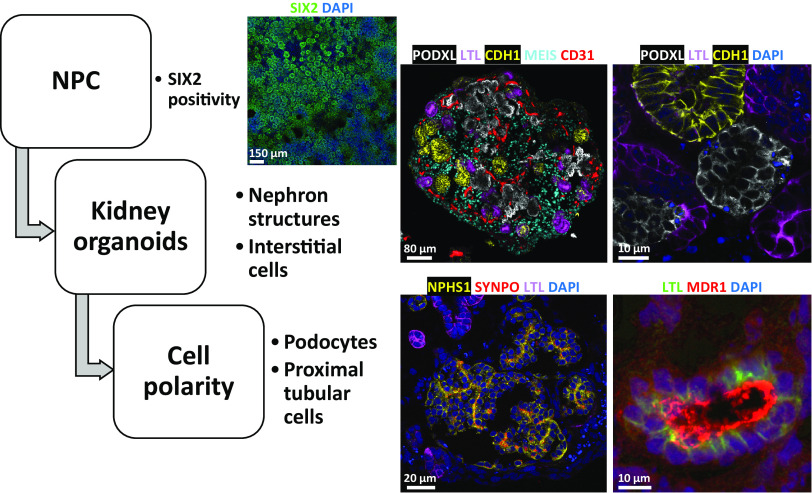
Figure 4.
Key metrics for successful differentiation of kidney organoids. At days 7–9 of differentiation, the nephron progenitor cells (NPC) express SIX2, as shown by the immunostaining in two-dimensional (2-D) cultures. Matured three-dimensional (3-D) organoids must comprise podocyte clusters, proximal tubular epithelial cells, and distal tubular epithelial cells, as evidenced by podocalyxin (PODXL), Lotus tetragonolobus lectin (LTL), and cadherin 1 (CDH1) staining, respectively. They also bear CD31-positive endothelial cells and MEIS-positive interstitial stromal cells. Cellular polarity must be verified, with apical staining of PODXL within the podocyte clusters, conversely to nephrin (NPHS1) and synaptopodin (SYNPO) basal staining, and luminal staining of MDR1 in the proximal tubular epithelial cells.
CONCLUSIONS: TOWARD THE PRODUCTION AND EXPULSION OF URINE
The exploration of kidney organoid functionality paves the way for this highly dynamic research field to advance to the next level. Confirmation of physiological function utilizing optimized and easily performed functional assays is necessary and should be considered as quality control of kidney organoids. In this perspective, the framework when studying kidney organoid function is summarized in Table 2. Progress is still needed to optimize/enhance/refine organoid architecture, structure, and functional maturation of these tissues, and many challenges remain. The physiological relevance of kidney organoids is critical in justifying their use as a relevant experimental model of disease. Translation of knowledge gained from the rigorous morphological and functional analyses of maturing organoids promises to provide additional insight into the structure and function of the metanephric kidney in development, health, and disease. A vital step toward achieving physical and functional maturity in kidney organoids involves understanding cell type diversity, spatial relationships, and gene expression profiles across different stages of life, ranging from pediatric to adult. Various atlas efforts are underway to delineate these aspects. This knowledge will serve as a crucial roadmap for assessing the degree of maturity achieved in kidney organoids and for engineering functional subsegments of the nephron. Ultimately, it is anticipated that these efforts will enable the production of engineered functional kidneys for transplantation.
Table 2.
Practice points to assess kidney organoid function and future directions
| Practice Points | Research Agenda |
|---|---|
| Assess the structure • Quality control of the organoids • Staining of the nephron segments |
Improve the segment identification • Identification of the different parts of the distal nephron, Improve the architecture • Developmental insight |
| Assess the function • Live imaging of epithelial transport • Transepithelial voltage and current • Microperfusion of microdissected tubules • Patch-clamp analysis of specific transporters |
Improve the tools for glomerular filtration and tubular secretion and reabsorption assessment • in vitro with live organoid imaging • in vivo on experimental models |
| Assess the maturity • Gene expression profiles: developmental vs. differentiated genes. |
Enhance the maturation • Bioengineered tools • Flow-enhanced maturation • In vivo maturation • Improved differentiation methods |
GRANTS
This work was supported by National Institutes of Health (NIH) Award DP2EB029388/DK133821 (to R.M.), NIH Grants R01DK038470 (to L.M.S), R01DK129285 (to L.M.S), UC2DK126023 (to L.M.S. and R.M.), U01DK114933 (to S.J.), U54DK134301 (to S.J.), P50DK133943 (to S.J.), U24DK135157 (to S.J.), U01EB028899/DK127587 (to R.M.), and R21DK129909 (to R.M.), the French National Research Agency ANR-22-CE14-0077-01, the Monahan Foundation in collaboration with the Fulbright program, and the Servier Institute (to N.T.).
DISCLOSURES
R.M. is an inventor on a patent related to this work filed by the President and Fellows of Harvard College and Mass General Brigham (PCT/US2018/036677). R.M. holds a stock option in Trestle Biotherapeutics. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
N.T., L.M.S., S.J., and R.M. conceived and designed conceived and designed the review; N.T. N.T. wrote the first draft of the manuscript; N.T. and R.M. analyzed data; N.T. and R.M. prepared figures; N.T., L.M.S., S.J., and R.M. drafted manuscript; N.T., L.M.S., S.J., and R.M. edited and revised manuscript; N.T., L.M.S., S.J., and R.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank RBK members for the insightful discussion about kidney organoids and their functional assessment and Dr. Haruka Oishi and Ken Hiratsuka for kidney organoid images. BioRender was used for the design of the figures.
REFERENCES
- 1. Vize PD, Smith HW. A Homeric view of kidney evolution: a reprint of H.W. Smith’s classic essay with a new introduction. Anat Rec A Discov Mol Cell Evol Biol 277: 344–354, 2004. doi: 10.1002/ar.a.20017. [DOI] [PubMed] [Google Scholar]
- 2. Lemley KV, Kriz W. Anatomy of the renal interstitium. Kidney Int 39: 370–381, 1991. doi: 10.1038/ki.1991.49. [DOI] [PubMed] [Google Scholar]
- 3. Park JG, Na M, Kim MG, Park SH, Lee HJ, Kim DK, Kwak C, Kim YS, Chang S, Moon KC, Lee DS, Han SS. Immune cell composition in normal human kidneys. Sci Rep 10: 15678, 2020. [Erratum in Sci Rep 11: 4313, 2021]. doi: 10.1038/s41598-020-72821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marsh DJ, Postnov DD, Sosnovtseva OV, Holstein-Rathlou NH. The nephron-arterial network and its interactions. Am J Physiol Renal Physiol 316: F769–F784, 2019. doi: 10.1152/ajprenal.00484.2018. [DOI] [PubMed] [Google Scholar]
- 5. Deen WM, Lazzara MJ, Myers BD. Structural determinants of glomerular permeability. Am J Physiol Renal Physiol 281: F579–F596, 2001. doi: 10.1152/ajprenal.2001.281.4.F579. [DOI] [PubMed] [Google Scholar]
- 6. Tabibzadeh N, Crambert G. Mechanistic insights into the primary and secondary alterations of renal ion and water transport in the distal nephron. J Intern Med 293: 4–22, 2023. doi: 10.1111/joim.13552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shah MM, Tee JB, Meyer T, Meyer-Schwesinger C, Choi Y, Sweeney DE, Gallegos TF, Johkura K, Rosines E, Kouznetsova V, Rose DW, Bush KT, Sakurai H, Nigam SK. The instructive role of metanephric mesenchyme in ureteric bud patterning, sculpting, and maturation and its potential ability to buffer ureteric bud branching defects. Am J Physiol Renal Physiol 297: F1330–F1341, 2009. [Erratum in Am J Physiol Renal Physiol 298: F1285, 2010. doi: 10.1152/ajprenal.00125.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tekguc M, Gaal RCV, Uzel SGM, Gupta N, Riella LV, Lewis JA, Morizane R. Kidney organoids: a pioneering model for kidney diseases. Transl Res 250: 1–17, 2022. doi: 10.1016/j.trsl.2022.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morizane R, Bonventre JV. Kidney organoids: a translational journey. Trends Mol Med 23: 246–263, 2017. doi: 10.1016/j.molmed.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernard C. Introduction à l’étude de la médecine expérimentale (Online). (Champs Classiques). Paris, France: Flammarion, 2013, p. 384. [Google Scholar]
- 11. Oxburgh L, Carroll TJ, Cleaver O, Gossett DR, Hoshizaki DK, Hubbell JA, Humphreys BD, Jain S, Jensen J, Kaplan DL, Kesselman C, Ketchum CJ, Little MH, McMahon AP, Shankland SJ, Spence JR, Valerius MT, Wertheim JA, Wessely O, Zheng Y, Drummond IA. (Re)Building a kidney. J Am Soc Nephrol 28: 1370–1378, 2017. doi: 10.1681/ASN.2016101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rizki-Safitri A, Traitteur T, Morizane R. Bioengineered kidney models: methods and functional assessments. Function (Oxf) 2: zqab026, 2021. doi: 10.1093/function/zqab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freedman BS. Physiology assays in human kidney organoids. Am J Physiol Renal Physiol 322: F625–F638, 2022. doi: 10.1152/ajprenal.00400.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33: 1193–1200, 2015. doi: 10.1038/nbt.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, Peters DT, Lu J, Baccei A, Siedlecki AM, Valerius MT, Musunuru K, McNagny KM, Steinman TI, Zhou J, Lerou PH, Bonventre JV. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 6: 8715, 2015. doi: 10.1038/ncomms9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526: 564–568, 2015. [Erratum Nature 536: 238, 2016]. doi: 10.1038/nature15695. [DOI] [PubMed] [Google Scholar]
- 17. Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14: 53–67, 2014. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 18. Przepiorski A, Sander V, Tran T, Hollywood JA, Sorrenson B, Shih JH, Wolvetang EJ, McMahon AP, Holm TM, Davidson AJ. A simple bioreactor-based method to generate kidney organoids from pluripotent stem cells. Stem Cell Rep 11: 470–484, 2018. doi: 10.1016/j.stemcr.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Przepiorski A, Crunk AE, Holm TM, Sander V, Davidson AJ, Hukriede NA. A simplified method for generating kidney organoids from human pluripotent stem cells. J Vis Exp 2021: e62452. 2021. doi: 10.3791/62452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garreta E, Prado P, Tarantino C, Oria R, Fanlo L, Martí E, Zalvidea D, Trepat X, Roca-Cusachs P, Gavaldà-Navarro A, Cozzuto L, Campistol JM, Izpisúa Belmonte JC, Hurtado Del Pozo C, Montserrat N. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat Mater 18: 397–405, 2019. doi: 10.1038/s41563-019-0287-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Low JH, Li P, Chew EGY, Zhou B, Suzuki K, Zhang T, Lian MM, Liu M, Aizawa E, Rodriguez Esteban C, Yong KSM, Chen Q, Campistol JM, Fang M, Khor CC, Foo JN, Izpisua Belmonte JC, Xia Y. Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell 25: 373–387.e9, 2019. doi: 10.1016/j.stem.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta N, Matsumoto T, Hiratsuka K, Garcia Saiz E, Galichon P, Miyoshi T, Susa K, Tatsumoto N, Yamashita M, Morizane R. Modeling injury and repair in kidney organoids reveals that homologous recombination governs tubular intrinsic repair. Sci Transl Med 14: eabj4772, 2022. [Erratum in Sci Transl Med 14: eadd0524, 2022]. doi: 10.1126/scitranslmed.abj4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shankar AS, Du Z, Mora HT, van den Bosch TPP, Korevaar SS, Van den Berg-Garrelds IM, Bindels E, Lopez-Iglesias C, Clahsen-van Groningen MC, Gribnau J, Baan CC, Danser AHJ, Hoorn EJ, Hoogduijn MJ. Human kidney organoids produce functional renin. Kidney Int 99: 134–147, 2021. [Erratum in Kidney Int 100: 1346–1347, 2021]. doi: 10.1016/j.kint.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 24. Sharmin S, Taguchi A, Kaku Y, Yoshimura Y, Ohmori T, Sakuma T, Mukoyama M, Yamamoto T, Kurihara H, Nishinakamura R. Human induced pluripotent stem cell-derived podocytes mature into vascularized glomeruli upon experimental transplantation. J Am Soc Nephrol 27: 1778–1791, 2016. doi: 10.1681/ASN.2015010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, Lievers E, Koning M, Vanslambrouck JM, Koster AJ, Howden SE, Takasato M, Little MH, Rabelink TJ. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Rep 10: 751–765, 2018. doi: 10.1016/j.stemcr.2018.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nam SA, Seo E, Kim JW, Kim HW, Kim HL, Kim K, Kim TM, Ju JH, Gomez IG, Uchimura K, Humphreys BD, Yang CW, Lee JY, Kim J, Cho DW, Freedman BS, Kim YK. Graft immaturity and safety concerns in transplanted human kidney organoids. Exp Mol Med 51: 1–13, 2019. [Erratum in Exp Mol Med 52: 180, 2020]. doi: 10.1038/s12276-019-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumar Gupta A, Sarkar P, Wertheim JA, Pan X, Carroll TJ, Oxburgh L. Asynchronous mixing of kidney progenitor cells potentiates nephrogenesis in organoids. Commun Biol 3: 231, 2020. doi: 10.1038/s42003-020-0948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van den Berg CW, Koudijs A, Ritsma L, Rabelink TJ. In vivo assessment of size-selective glomerular sieving in transplanted human induced pluripotent stem cell-derived kidney organoids. J Am Soc Nephrol 31: 921–929, 2020. doi: 10.1681/ASN.2019060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhuo JL, Li XC. Proximal nephron. Compr Physiol 3: 1079–1123, 2013. doi: 10.1002/cphy.c110061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boron WF. Acid-base transport by the renal proximal tubule. J Am Soc Nephrol 17: 2368–2382, 2006. doi: 10.1681/ASN.2006060620. [DOI] [PubMed] [Google Scholar]
- 31. Schuh CD, Polesel M, Platonova E, Haenni D, Gassama A, Tokonami N, Ghazi S, Bugarski M, Devuyst O, Ziegler U, Hall AM. Combined structural and functional imaging of the kidney reveals major axial differences in proximal tubule endocytosis. J Am Soc Nephrol 29: 2696–2712, 2018. doi: 10.1681/ASN.2018050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang K, Kestenbaum B. Proximal tubular secretory clearance. Clin J Am Soc Nephrol 13: 1291–1296, 2018. doi: 10.2215/CJN.12001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Filipski KK, Mathijssen RH, Mikkelsen TS, Schinkel AH, Sparreboom A. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther 86: 396–402, 2009. doi: 10.1038/clpt.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Czerniecki SM, Cruz NM, Harder JL, Menon R, Annis J, Otto EA, Gulieva RE, Islas LV, Kim YK, Tran LM, Martins TJ, Pippin JW, Fu H, Kretzler M, Shankland SJ, Himmelfarb J, Moon RT, Paragas N, Freedman BS. High-throughput screening enhances kidney organoid differentiation from human pluripotent stem cells and enables automated multidimensional phenotyping. Cell Stem Cell 22: 929–940.e4, 2018. doi: 10.1016/j.stem.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Przepiorski A, Vanichapol T, Espiritu EB, Crunk AE, Parasky E, McDaniels MD, Emlet DR, Salisbury R, Happ CL, Vernetti LA, MacDonald ML, Kellum JA, Kleyman TR, Baty CJ, Davidson AJ, Hukriede NA. Modeling oxidative injury response in human kidney organoids. Stem Cell Res Ther 13: 76, 2022. doi: 10.1186/s13287-022-02752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rizki-Safitri A, Gupta N, Hiratsuka K, Kobayashi K, Zhang C, Ida K, Satlin LM, Morizane R. Live functional assays reveal longitudinal maturation of transepithelial transport in kidney organoids. Front Cell Dev Biol 10: 978888, 2022. doi: 10.3389/fcell.2022.978888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vanslambrouck JM, Wilson SB, Tan KS, Groenewegen E, Rudraraju R, Neil J, Lawlor KT, Mah S, Scurr M, Howden SE, Subbarao K, Little MH. Enhanced metanephric specification to functional proximal tubule enables toxicity screening and infectious disease modelling in kidney organoids. Nat Commun 13: 5943, 2022. doi: 10.1038/s41467-022-33623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shankar AS, van den Berg SAA, Tejeda Mora H, Du Z, Lin H, Korevaar SS, van der Wal R, van den Bosch TPP, Clahsen-van Groningen MC, Gribnau J, Hoorn EJ, Baan CC, Hoogduijn MJ. Vitamin D metabolism in human kidney organoids. Nephrol Dial Transplant 37: 190–193, 2021. doi: 10.1093/ndt/gfab264. [DOI] [PubMed] [Google Scholar]
- 39. Yoon SH, Meyer MB, Arevalo C, Tekguc M, Zhang C, Wang JS, Castro Andrade CD, Strauss K, Sato T, Benkusky NA, Lee SM, Berdeaux R, Foretz M, Sundberg TB, Xavier RJ, Adelmann CH, Brooks DJ, Anselmo A, Sadreyev RI, Rosales IA, Fisher DE, Gupta N, Morizane R, Greka A, Pike JW, Mannstadt M, Wein MN. A parathyroid hormone/salt-inducible kinase signaling axis controls renal vitamin D activation and organismal calcium homeostasis. J Clin Invest 133: e163627, 2023. doi: 10.1172/JCI163627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hansen J, Sealfon R, Menon R, Eadon MT, Lake BB, Steck B, , et al. A reference tissue atlas for the human kidney. Sci Adv 8: eabn4965, 2022. doi: 10.1126/sciadv.abn4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lake BB, Menon R, Winfree S, Hu Q, Melo Ferreira R, Kalhor K, , et al. An atlas of healthy and injured cell states and niches in the human kidney. Nature 619: 585–594, 2023. doi: 10.1038/s41586-023-05769-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu H, Humphreys BD. Single cell sequencing and kidney organoids generated from pluripotent stem cells. Clin J Am Soc Nephrol 15: 550–556, 2020. doi: 10.2215/CJN.07470619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 23: 869–881.e8, 2018. doi: 10.1016/j.stem.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bankir L, Figueres L, Prot-Bertoye C, Bouby N, Crambert G, Pratt JH, Houillier P. Medullary and cortical thick ascending limb: similarities and differences. Am J Physiol Renal Physiol 318: F422–F442, 2020. doi: 10.1152/ajprenal.00261.2019. [DOI] [PubMed] [Google Scholar]
- 45. McCormick JA, Ellison DH. Distal convoluted tubule. Compr Physiol 5: 45–98, 2015. doi: 10.1002/cphy.c140002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gupta N, Morizane R. Kidney development to kidney organoids and back again. Semin Cell Dev Biol 127: 68–76, 2022. doi: 10.1016/j.semcdb.2021.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lindström NO, Sealfon R, Chen X, Parvez RK, Ransick A, De Sena Brandine G, Guo J, Hill B, Tran T, Kim AD, Zhou J, Tadych A, Watters A, Wong A, Lovero E, Grubbs BH, Thornton ME, McMahon JA, Smith AD, Ruffins SW, Armit C, Troyanskaya OG, McMahon AP. Spatial transcriptional mapping of the human nephrogenic program. Dev Cell 56: 2381–2398.e6, 2021. doi: 10.1016/j.devcel.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ray EC, Carrisoza-Gaytan R, Al-Bataineh M, Marciszyn AL, Nkashama LJ, Chen J, Winfrey A, Griffiths S, Lam TR, Flores D, Wu P, Wang WHui, Huang CL, Subramanya AR, Kleyman TR, Satlin LM. L-WNK1 is required for BK channel activation in intercalated cells. Am J Physiol Renal Physiol 321: F245–F254, 2021. doi: 10.1152/ajprenal.00472.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carrisoza-Gaytan R, Ray EC, Flores D, Marciszyn AL, Wu P, Liu L, Subramanya AR, Wang W, Sheng S, Nkashama LJ, Chen J, Jackson EK, Mutchler SM, Heja S, Kohan DE, Satlin LM, Kleyman TR. Intercalated cell BKα subunit is required for flow-induced K+ secretion. JCI Insight 5: e130553, 2020. doi: 10.1172/jci.insight.130553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hiratsuka K, Monkawa T, Akiyama T, Nakatake Y, Oda M, Goparaju SK, Kimura H, Chikazawa-Nohtomi N, Sato S, Ishiguro K, Yamaguchi S, Suzuki S, Morizane R, Ko SBH, Itoh H, Ko MSH. Induction of human pluripotent stem cells into kidney tissues by synthetic mRNAs encoding transcription factors. Sci Rep 9: 913, 2019. doi: 10.1038/s41598-018-37485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Uchimura K, Wu H, Yoshimura Y, Humphreys BD. Human pluripotent stem cell-derived kidney organoids with improved collecting duct maturation and injury modeling. Cell Rep 33: 108514, 2020. doi: 10.1016/j.celrep.2020.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wilson SB, Howden SE, Vanslambrouck JM, Dorison A, Alquicira-Hernandez J, Powell JE, Little MH. DevKidCC allows for robust classification and direct comparisons of kidney organoid datasets. Genome Med 14: 19, 2022. doi: 10.1186/s13073-022-01023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zeng Z, Huang B, Parvez RK, Li Y, Chen J, Vonk AC, Thornton ME, Patel T, Rutledge EA, Kim AD, Yu J, Grubbs BH, McMahon JA, Pastor-Soler NM, Hallows KR, McMahon AP, Li Z. Generation of patterned kidney organoids that recapitulate the adult kidney collecting duct system from expandable ureteric bud progenitors. Nat Commun 12: 3641, 2021. doi: 10.1038/s41467-021-23911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mae SI, Ryosaka M, Sakamoto S, Matsuse K, Nozaki A, Igami M, Kabai R, Watanabe A, Osafune K. Expansion of human iPSC-derived ureteric bud organoids with repeated branching potential. Cell Rep 32: 107963, 2020. doi: 10.1016/j.celrep.2020.107963. [DOI] [PubMed] [Google Scholar]
- 55. Howden SE, Wilson SB, Groenewegen E, Starks L, Forbes TA, Tan KS, Vanslambrouck JM, Holloway EM, Chen YH, Jain S, Spence JR, Little MH. Plasticity of distal nephron epithelia from human kidney organoids enables the induction of ureteric tip and stalk. Cell Stem Cell 28: 671–684.e6, 2021. doi: 10.1016/j.stem.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Montalbetti N, Przepiorski AJ, Shi S, Sheng S, Baty CJ, Maggiore JC, Carattino MD, Vanichapol T, Davidson AJ, Hukriede NA, Kleyman TR. Functional characterization of ion channels expressed in kidney organoids derived from human induced pluripotent stem cells. Am J Physiol Renal Physiol 323: F479–F491, 2022. doi: 10.1152/ajprenal.00365.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shi M, McCracken KW, Patel AB, Zhang W, Ester L, Valerius MT, Bonventre JV. Human ureteric bud organoids recapitulate branching morphogenesis and differentiate into functional collecting duct cell types. Nat Biotechnol 41: 252–261, 2023. doi: 10.1038/s41587-022-01429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chang-Panesso M, Kadyrov FF, Machado FG, Kumar A, Humphreys BD. Meis1 is specifically upregulated in kidney myofibroblasts during aging and injury but is not required for kidney homeostasis or fibrotic response. Am J Physiol Renal Physiol 315: F275–F290, 2018. doi: 10.1152/ajprenal.00030.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lindström NO, Guo J, Kim AD, Tran T, Guo Q, De Sena Brandine G, Ransick A, Parvez RK, Thornton ME, Baskin L, Grubbs B, McMahon JA, Smith AD, McMahon AP. Conserved and divergent features of mesenchymal progenitor cell types within the cortical nephrogenic niche of the human and mouse kidney. J Am Soc Nephrol 29: 806–824, 2018. doi: 10.1681/ASN.2017080890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Combes AN, Zappia L, Er PX, Oshlack A, Little MH. Single-cell analysis reveals congruence between kidney organoids and human fetal kidney. Genome Med 11: 3, 2019. doi: 10.1186/s13073-019-0615-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. England AR, Chaney CP, Das A, Patel M, Malewska A, Armendariz D, Hon GC, Strand DW, Drake KA, Carroll TJ. Identification and characterization of cellular heterogeneity within the developing renal interstitium. Development 147: dev190108, 2020. doi: 10.1242/dev.190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kurtz A. Endocrine functions of the renal interstitium. Pflugers Arch 469: 869–876, 2017. doi: 10.1007/s00424-017-2008-9. [DOI] [PubMed] [Google Scholar]
- 63. Yanofsky SM, Dugas CM, Katsurada A, Liu J, Saifudeen Z, El-Dahr SS, Satou R. Angiotensin II biphasically regulates cell differentiation in human iPSC-derived kidney organoids. Am J Physiol Renal Physiol 321: F559–F571, 2021. doi: 10.1152/ajprenal.00134.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hitomi H, Kasahara T, Katagiri N, Hoshina A, Mae SI, Kotaka M, Toyohara T, Rahman A, Nakano D, Niwa A, Saito MK, Nakahata T, Nishiyama A, Osafune K. Human pluripotent stem cell-derived erythropoietin-producing cells ameliorate renal anemia in mice. Sci Transl Med 9: eaaj2300, 2017. doi: 10.1126/scitranslmed.aaj2300. [DOI] [PubMed] [Google Scholar]
- 65. Raghavan V, Weisz OA. Flow stimulated endocytosis in the proximal tubule. Curr Opin Nephrol Hypertens 24: 359–365, 2015. doi: 10.1097/MNH.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weinbaum S, Duan Y, Satlin LM, Wang T, Weinstein AM. Mechanotransduction in the renal tubule. Am J Physiol Renal Physiol 299: F1220–F1236, 2010. doi: 10.1152/ajprenal.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol 4: a008300, 2012. doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huang Y, Qian JY, Cheng H, Li XM. Effects of shear stress on differentiation of stem cells into endothelial cells. World J Stem Cells 13: 894–913, 2021. doi: 10.4252/wjsc.v13.i7.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Galie PA, Nguyen DH, Choi CK, Cohen DM, Janmey PA, Chen CS. Fluid shear stress threshold regulates angiogenic sprouting. Proc Natl Acad Sci USA 111: 7968–7973, 2014. doi: 10.1073/pnas.1310842111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jaffe LF. Organization of early development by calcium patterns. Bioessays 21: 657–667, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 71. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2000. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 72. Webb SE, Miller AL. Calcium signalling during embryonic development. Nat Rev Mol Cell Biol 4: 539–551, 2003. doi: 10.1038/nrm1149. [DOI] [PubMed] [Google Scholar]
- 73. Camp JG, Sekine K, Gerber T, Loeffler-Wirth H, Binder H, Gac M, Kanton S, Kageyama J, Damm G, Seehofer D, Belicova L, Bickle M, Barsacchi R, Okuda R, Yoshizawa E, Kimura M, Ayabe H, Taniguchi H, Takebe T, Treutlein B. Multilineage communication regulates human liver bud development from pluripotency. Nature 546: 533–538, 2017. doi: 10.1038/nature22796. [DOI] [PubMed] [Google Scholar]
- 74. Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, Lewis JA, Morizane R. Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16: 255–262, 2019. doi: 10.1038/s41592-019-0325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hiratsuka K, Miyoshi T, Kroll KT, Gupta NR, Valerius MT, Ferrante T, Yamashita M, Lewis JA, Morizane R. Organoid-on-a-chip model of human ARPKD reveals mechanosensing pathomechanisms for drug discovery. Sci Adv 8: eabq0866, 2022. doi: 10.1126/sciadv.abq0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Aceves JO, Heja S, Kobayashi K, Robinson SS, Miyoshi T, Matsumoto T, Schäffers OJM, Morizane R, Lewis JA. 3D proximal tubule-on-chip model derived from kidney organoids with improved drug uptake. Sci Rep 12: 14997, 2022. doi: 10.1038/s41598-022-19293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nigro EA, Boletta A. Role of the polycystins as mechanosensors of extracellular stiffness. Am J Physiol Renal Physiol 320: F693–F705, 2021. doi: 10.1152/ajprenal.00545.2020. [DOI] [PubMed] [Google Scholar]
- 78. Cruz NM, Reddy R, McFaline-Figueroa JL, Tran C, Fu H, Freedman BS. Modelling ciliopathy phenotypes in human tissues derived from pluripotent stem cells with genetically ablated cilia. Nat Biomed Eng 6: 463–475, 2022. [Erratum in Nat Biomed Eng 6: 1086, 2022]. doi: 10.1038/s41551-022-00880-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Carrisoza-Gaytán R, Wang L, Schreck C, Kleyman TR, Wang WH, Satlin LM. The mechanosensitive BKα/β1 channel localizes to cilia of principal cells in rabbit cortical collecting duct (CCD). Am J Physiol Renal Physiol 312: F143–F156, 2017. doi: 10.1152/ajprenal.00256.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li X, Hu J, Zhao X, Li J, Chen Y. Piezo channels in the urinary system. Exp Mol Med 54: 697–710, 2022. doi: 10.1038/s12276-022-00777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dalghi MG, Clayton DR, Ruiz WG, Al-Bataineh MM, Satlin LM, Kleyman TR, Ricke WA, Carattino MD, Apodaca G. Expression and distribution of PIEZO1 in the mouse urinary tract. Am J Physiol Renal Physiol 317: F303–F321, 2019. doi: 10.1152/ajprenal.00214.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Carrisoza-Gaytan R, Kroll KT, Hiratsuka K, Gupta NR, Morizane R, Lewis JA, Satlin LM. Functional maturation of kidney organoid tubules: Piezo1-mediated Ca2+ signaling. Am J Physiol Cell Physiol 324: C757–C768, 2023. doi: 10.1152/ajpcell.00288.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, Li YS, Chien S, Patapoutian A. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc Natl Acad Sci USA 111: 10347–10352, 2014. doi: 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Konoe R, Morizane R. Strategies for improving vascularization in kidney organoids: a review of current trends. Biology 12: 503, 2023. doi: 10.3390/biology12040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Li Z, Araoka T, Wu J, Liao HK, Li M, Lazo M, Zhou B, Sui Y, Wu MZ, Tamura I, Xia Y, Beyret E, Matsusaka T, Pastan I, Rodriguez Esteban C, Guillen I, Guillen P, Campistol JM, Izpisua Belmonte JC. Culture supports long-term expansion of mouse and human nephrogenic progenitors. Cell Stem Cell 19: 516–529, 2016. doi: 10.1016/j.stem.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tanigawa S, Tanaka E, Miike K, Ohmori T, Inoue D, Cai CL, , et al. Generation of the organotypic kidney structure by integrating pluripotent stem cell-derived renal stroma. Nat Commun 13: 611, 2022. [Erratum in Nat Commun 14: 1874, 2023]. doi: 10.1038/s41467-022-28226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Taguchi A, Nishinakamura R. Higher-order kidney organogenesis from pluripotent stem cells. Cell Stem Cell 21: 730–746.e6, 2017. doi: 10.1016/j.stem.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 88. Tsujimoto H, Kasahara T, Sueta SI, Araoka T, Sakamoto S, Okada C, Mae SI, Nakajima T, Okamoto N, Taura D, Nasu M, Shimizu T, Ryosaka M, Li Z, Sone M, Ikeya M, Watanabe A, Osafune K. A modular differentiation system maps multiple human kidney lineages from pluripotent stem cells. Cell Rep 31: 107476, 2020. doi: 10.1016/j.celrep.2020.03.040. [DOI] [PubMed] [Google Scholar]
- 89. Drake KA, Chaney CP, Das A, Roy P, Kwartler CS, Rakheja D, Carroll TJ. Stromal β-catenin activation impacts nephron progenitor differentiation in the developing kidney and may contribute to Wilms tumor. Development 147: dev189597, 2020. doi: 10.1242/dev.189597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Das A, Tanigawa S, Karner CM, Xin M, Lum L, Chen C, Olson EN, Perantoni AO, Carroll TJ. Stromal-epithelial crosstalk regulates kidney progenitor cell differentiation. Nat Cell Biol 15: 1035–1044, 2013. [Erratum in Nat Cell Biol 15: 1260, 2013]. doi: 10.1038/ncb2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vanslambrouck JM, Wilson SB, Tan KS, Soo JY, Scurr M, Spijker HS, Starks LT, Neilson A, Cui X, Jain S, Little MH, Howden SE. A toolbox to characterize human induced pluripotent stem cell-derived kidney cell types and organoids. J Am Soc Nephrol 30: 1811–1823, 2019. doi: 10.1681/ASN.2019030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wu H, Malone AF, Donnelly EL, Kirita Y, Uchimura K, Ramakrishnan SM, Gaut JP, Humphreys BD. Single-cell transcriptomics of a human kidney allograft biopsy specimen defines a diverse inflammatory response. J Am Soc Nephrol 29: 2069–2080, 2018. doi: 10.1681/ASN.2018020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Luo Y, Hitz BC, Gabdank I, Hilton JA, Kagda MS, Lam B, Myers Z, Sud P, Jou J, Lin K, Baymuradov UK, Graham K, Litton C, Miyasato SR, Strattan JS, Jolanki O, Lee JW, Tanaka FY, Adenekan P, O'Neill E, Cherry JM. New developments on the Encyclopedia of DNA Elements (ENCODE) data portal. Nucleic Acids Res 48: D882–D889, 2020. doi: 10.1093/nar/gkz1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jain S, Pei L, Spraggins JM, Angelo M, Carson JP, Gehlenborg N, Pei L, Spraggins JM, Angelo M, Carson JP, Gehlenborg N, Ginty F, Gonçalves JP, Hagood JS, Hickey JW, Kelleher NL, Laurent LC, Lin S, Lin Y, Liu H, Naba A, Nakayasu ES, Qian WJ, Radtke A, Robson P, Stockwell BR, Van de Plas R, Vlachos IS, Zhou M; HuBMAP Consortium; Börner K, Snyder MP. Advances and prospects for the Human BioMolecular Atlas Program (HuBMAP). Nat Cell Biol 25: 1089–1100, 2023. doi: 10.1038/s41556-023-01194-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, Robertson CL, Serova N, Davis S, Soboleva A. NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Res 41: 41: D991–D995, 2013. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Haase K, Freedman BS. Once upon a dish: engineering multicellular systems. Development 147: dev188573, 2020. doi: 10.1242/dev.188573. [DOI] [PubMed] [Google Scholar]
- 97. de Jongh D, Massey EK, Bunnik EM. Organoids: a systematic review of ethical issues. Stem Cell Res Ther 13: 337, 2022. doi: 10.1186/s13287-021-02613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. King NM, Perrin J. Ethical issues in stem cell research and therapy. Stem Cell Res Ther 5: 85, 2014. doi: 10.1186/scrt474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Phipson B, Er PX, Combes AN, Forbes TA, Howden SE, Zappia L, Yen HJ, Lawlor KT, Hale LJ, Sun J, Wolvetang E, Takasato M, Oshlack A, Little MH. Evaluation of variability in human kidney organoids. Nat Methods 16: 79–87, 2019. doi: 10.1038/s41592-018-0253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]