Abstract
Scientific interest in ischaemic heart disease (IHD) in women has grown considerably over the past 2 decades. A substantial amount of the literature on this subject is centred on sex differences in clinical aspects of IHD. Many reports have documented sex-related differences in presentation, risk profiles, and outcomes among patients with IHD, particularly acute myocardial infarction. Such differences have often been attributed to inequalities between men and women in the referral and treatment of IHD, but data are insufficient to support this assessment. The determinants of sex differences in presentation are unclear, and few clues are available as to why young, premenopausal women paradoxically have a greater incidence of adverse outcomes after acute myocardial infarction than men, despite having less-severe coronary artery disease. Although differential treatment on the basis of patient sex continues to be described, the extent to which such inequalities persist and whether they reflect true disparity is unclear. Additionally, much uncertainty surrounds possible sex-related differences in response to cardiovascular therapies, partly because of a persistent lack of female-specific data from cardiovascular clinical trials. In this Review, we assess the evidence for sex-related differences in the clinical presentation, treatment, and outcome of IHD, and identify gaps in the literature that need to be addressed in future research efforts.
Introduction
Mortality related to cardiovascular disease (CVD) has declined in most Western countries since the 1960s; however, CVD remains the leading cause of death for both women and men and a major cause of morbidity.1 Ischaemic heart disease (IHD), the most common form of CVD, is also the single most frequent cause of death in Western countries in both women and men.1 Nevertheless, IHD in women was neglected by physicians until the early 1990s, when reports emerged that treatment of women with this disease was less aggressive than in men.2 Major efforts have since been made worldwide by the World Heart Federation, the National Heart, Lung, and Blood Institute, and the Centers for Disease Control and Prevention to increase awareness of heart health and promote healthy lifestyles among women.3,4 However, despite growing attention to the issue of IHD in women, many questions underlying sex differences in clinical manifestations and management of the disease remain unanswered.4 For example, the clinical relevance of angina among women is unclear, and whether women have more adverse IHD-related outcomes than men do, or even a different expression of the disease, is still to be established. Such information is missing, because most studies have been focused on men with the under-representation or exclusion of women. Studies of women, such as the Women’s Ischemia Syndrome Evaluation (WISE), the Nurses’ Health Study, and the Women’s Health Initiative, have not included a male comparison group. Moreover, even when data were available, few publications have included analysis of data by sex. In this Review, we discuss the current evidence for sex-related differences in the clinical presentation, treatment, and outcome of IHD in Western countries, and identify gaps in the literature that need to be addressed in future research.
Clinical presentation
Symptoms as predictors of IHD
The clinical presentation of IHD differs between women and men. Compared with men, women are more likely to develop angina as their first manifestation of IHD (47% versus 32%) and less likely to present with an acute myocardial infarction (AMI; 6% versus 10%).5 This presentation pattern, which was initially reported in the USA in the 1980s by the Framingham Heart Study investigators,6 was confirmed by the results of a 2008 meta-analysis of data from 31 countries showing that women have, on average, a 20% higher rate of angina than men, irrespective of their rate of coronary death.7 In the setting of acute coronary syndrome (ACS), women and men have different clinical profiles and presentation, with fewer women than men presenting with ST-segment elevation myocardial infarction (STEMI), but more presenting with unstable angina.8,9
Presence of chest pain, particularly if ‘typical’ in character, is a predictor of obstructive coronary artery disease (CAD) in both women and men, although fewer women than men have obstructive CAD in each category of chest pain (typical, atypical, or nonanginal chest pain).10 ‘Typical’ symptoms of angina (Box 1) are also strong predictors of ACS in women.11 Women with IHD are often thought to be less likely than men to present with chest pain, but this notion is not substantiated by the latest research.12–16 Among patients with a presumed or established diagnosis of IHD, the majority of both women and men present with chest pain, with similar proportions by sex. Women, however, report a larger number of additional, nonspecific symptoms (Box 1), which can complicate the presentation. In addition, minor sex-related differences might exist in other chest pain descriptors.12–16 Physicians should investigate symptoms indicative of cardiac ischaemia in women, and not dismiss them as being noncardiac in origin.17–19
Box 1 |. Symptoms of ischaemic heart disease*.
‘Typical’ symptoms
Crushing substernal chest pain
Diaphoresis
‘Nonspecific’ symptoms
Epigastric pain
Intermammary pain
Intrascapular pain
Right arm pain
Fatigue
Nausea
Shortness of breath
Sleep disturbance
*Women are more likely to experience nonspecific than typical symptoms.
Symptom recognition and awareness
Awareness that IHD is the leading cause of death is low among women. A survey of women in the USA showed that, although awareness has improved since 1997, almost half of women remain unaware that IHD is the leading cause of death among their sex.20 Until the turn of the 21st century, fewer CAD-related public health messages were targeted towards women than men and, consequently, women might have been less likely to modify their lifestyles in a cardioprotective manner.21 This approach is likely to have affected cardiac prevention among women, and has contributed to the popular view that young and middle-aged women are ‘immune’ to heart disease. Only about half of women in the USA would call the emergency services if they thought they were experiencing symptoms of a heart attack.21 Fukuoka et al. reported that <40% of women and men hospitalized for AMI interpreted their symptoms as cardiac in origin.22 Thus, the lack of appropriate identification of IHD-related symptoms in women continues to be an important public-health concern. This problem, in conjunction with the less clear-cut symptom presentation in women than in men, represents a substantial challenge for the prevention, timely identification, and management of IHD in women.
Sex, age, and clinical characteristics
Sex differences in the presentation of IHD are more pronounced among younger patients than older patients. In the Euro Heart Survey23 of ACS, which involved >10,000 patients, women aged <65 years were more likely than men of a similar age to present with unstable angina, and less likely to have STEMI, whereas presentation was similar in women and men aged ≥65 years. In the US National Registries of Myocardial Infarction,12 women aged <65 years were more likely than men of similar age to present without chest pain (Figure 1) and to have a history of diabetes mellitus, heart failure, or stroke, and a higher Killip class (an index of acute heart failure). These sex differences in presentation were less pronounced or absent among older patients.12 Although sex differences in chest pain presentation were more marked in patients aged <65 years, older men and women were much more likely than younger patients to present without chest pain. About 50% of patients aged ≥75 years presented without chest pain.12 The reasons for these differences are not understood.
Figure 1 |.
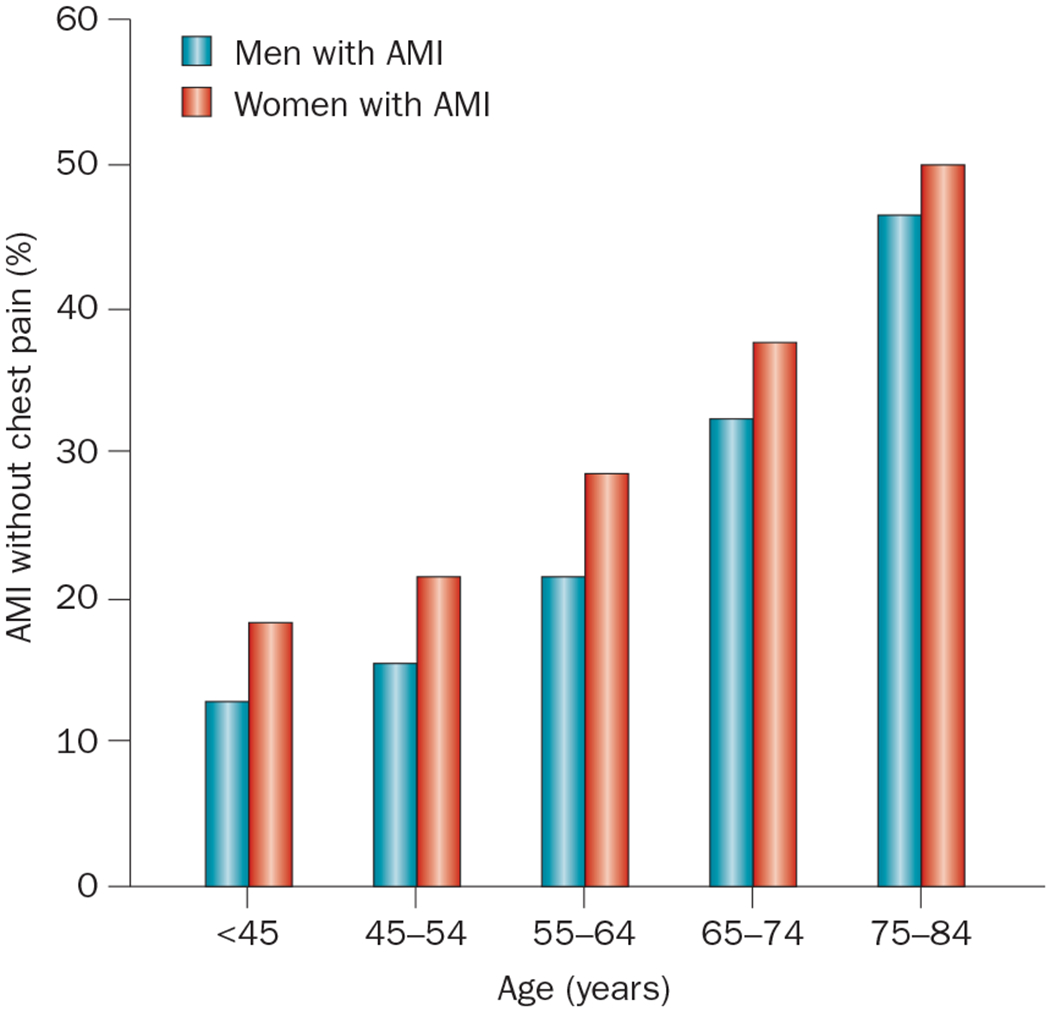
Differences in presentation of AMI among 1,143,513 patients (481,581 of whom were women) in the National Registry of Myocardial Infarction (1994–2006). Women with AMI were more likely than men with AMI to present without chest pain or discomfort, across all age groups.12 Abbreviation: AMI, acute myocardial infarction.
Diagnostic strategy
Physicians tend to underestimate cardiovascular risk in women, which can lead to missed or delayed diagnoses of IHD.24,25 Part of the problem is that the diagnosis of IHD in women is hampered by difficulties arising from sex-related differences in the prevalence and clinical manifestations of this disease and its risk factors.26 For a substantial proportion of women presenting for evaluation of chest pain, traditional diagnostic strategies that focus on detection of severe coronary stenosis are likely to be inadequate. Unique challenges in the evaluation of IHD in women include greater symptom burden, higher rate of functional disability, and lower prevalence of obstructive CAD than in men.
Exercise electrocardiography
Exercise electrocardiography (ECG) is the diagnostic test most-frequently performed to assess suspected IHD. Using the threshold of 1.0 mm ST-segment depression to define abnormality, the diagnostic accuracy is lower in women (sensitivity and specificity ranging from 60% to 70%) than in men (up to ~80%).27 The reduced accuracy is, in part, related to the fact that women are more likely than men to have functional impairment, precluding them from achieving adequate exercise-induced stress. In fact, women are often incapable of performing >5 metabolic equivalents of treadmill exercise, leading to inadequate heart-rate responses.28,29 Additional causes of diminished accuracy of ECG stress testing in women include ST-segment abnormalities related to the menstrual cycle or other hormonal changes, such as those that occur during the perimenopause,30 and a lower QRS voltage in comparison with men. The diagnostic accuracy for assessing IHD can be improved by using an imaging modality.
Stress echocardiography
As for exercise ECG, exercise stress testing using echocardiographic techniques can be suboptimal in women owing to decreased exercise tolerance compared with men. Pharmacological stress testing (using dobutamine or dipyridamole) is preferred in women with reduced exercise capacity.24 In addition, the accuracy of stress echocardiography depends on the experience of the operator. For optimal interpretation, rapid assessment of multiple echocardiographic views at peak heart rate is essential. Despite these limitations, stress echocardiography is a highly accurate technique for the detection of IHD in women, with a sensitivity of 85% and a specificity of 75%.28,29,31
Coronary angiography
The traditional diagnostic strategy of coronary angiography focusing on the detection of coronary obstruction with a lumen stenosis of ≥50% can be inadequate in women, who are more likely than men to have chest pain with a normal or near-normal coronary angiogram (Figures 2 and 3).32–34 Even among patients with AMI, a large proportion (32%) were found to have a normal coronary angiogram, and an additional 15% had <50% stenosis in any of the major vessels.35 These proportions are much higher in women than in men.17 Functional testing performed during angiography can provide an improved understanding of the mechanisms that account for chest pain in patients with a normal or near-normal angiogram.17–19,36 Coronary artery function is most commonly and safely assessed by intracoronary infusion of acetylcholine.37 Reduced vasodilatory response of the coronary microcirculation or paradoxical vasoconstriction of the epicardial coronary vessels are signs of coronary artery dysfunction (Figure 4).38
Figure 2 |.
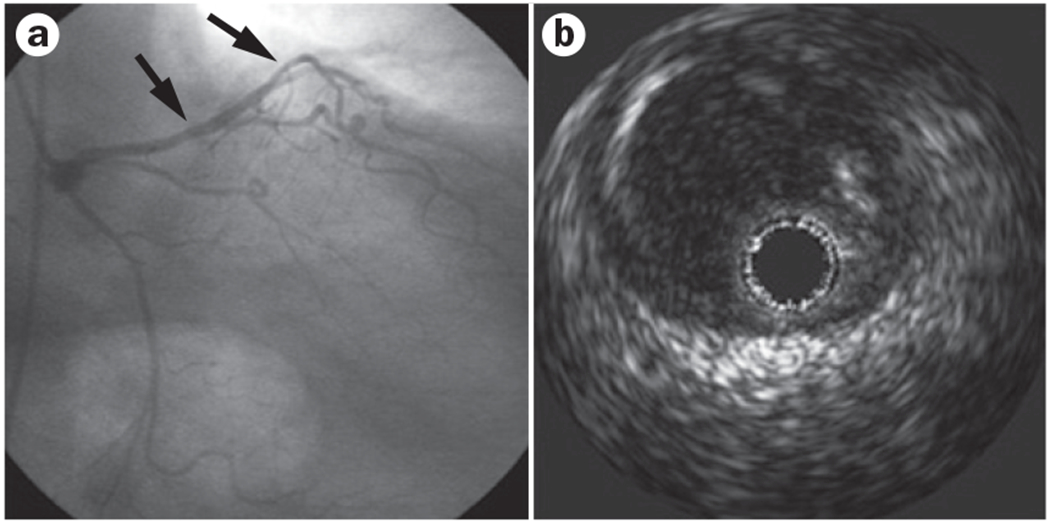
Underestimation of stenosis by angiography in a woman aged 65 years. a | Coronary angiography shows diffuse minor luminal irregularities (arrows) in the mid left anterior descending artery. b | Intravascular ultrasonography image at the site of luminal irregularity showing diffuse occult eccentric atherosclerosis (grey area adjacent to the dark lumen; positive remodelling).
Figure 3 |.
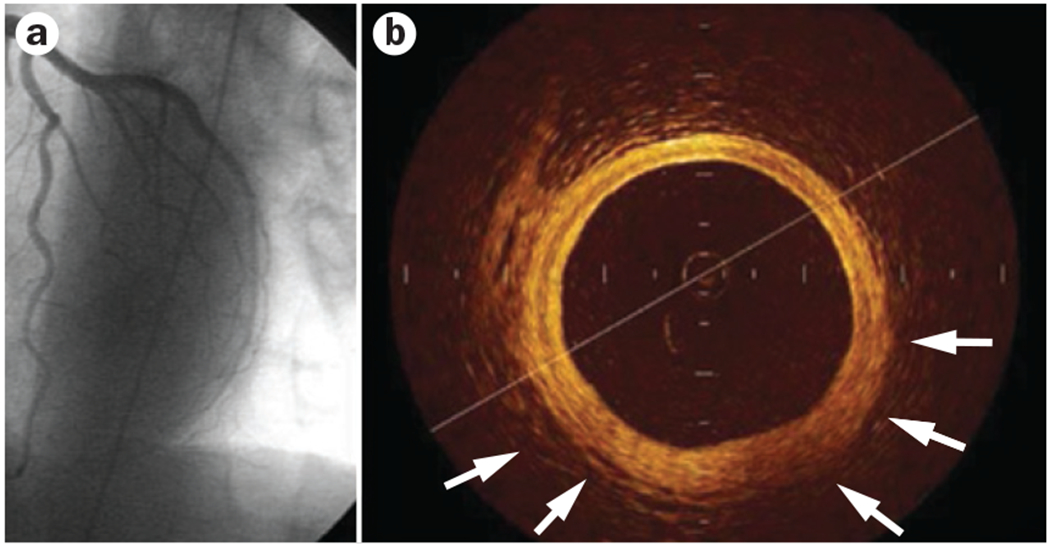
Intracoronary imaging with optical coherence tomography in a woman aged 55 years. a | Left anterior descending artery. Normal angiographic result after acute coronary syndrome. b | Optical coherence tomography shows the three-layer appearance of normal vessel wall. Intimal thickening and fibrous appearance of plaque is shown (arrows).
Figure 4 |.
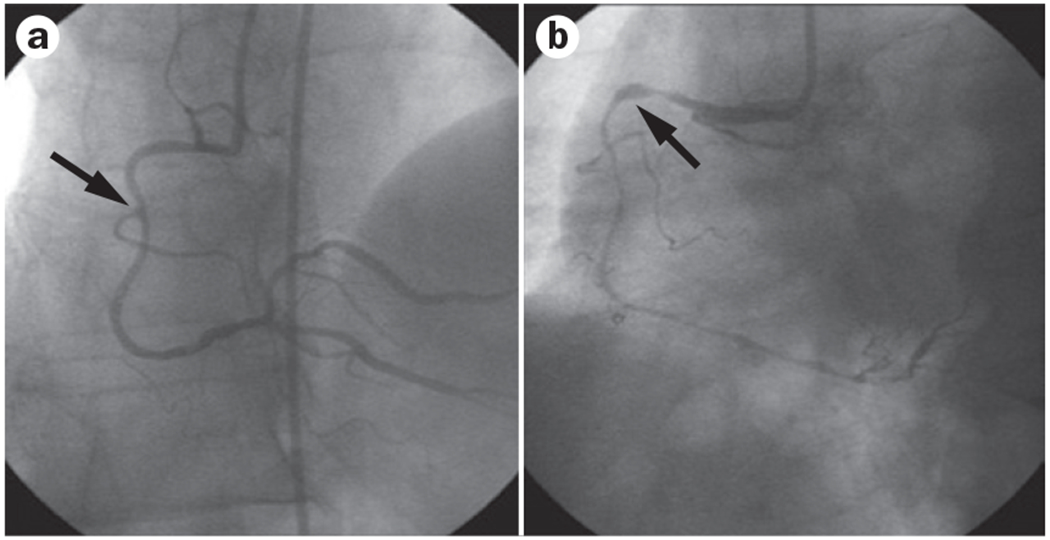
Acetylcholine testing in a woman aged 55 years. a | Coronary angiography shows diffuse minor luminal irregularities (arrow) at the proximal site of the right coronary artery. b | After administration of acetylcholine, coronary angiography shows diffuse subocclusive vasoconstriction (arrow) indicating severe endothelial dysfunction.
Coronary CT angiography
Coronary CT angiography (CCTA) has comparable diagnostic accuracy in women and men for the detection of obstructive coronary stenosis at thresholds of both 50% and 70%.39 In addition, CCTA can be used to detect non-obstructive atherosclerotic lesions that would not have been detected with functional cardiac imaging, which is of critical importance in women given their lower prevalence of obstructive disease17 and the suboptimal performance of traditional noninvasive tests.28,29 Studies of patients with CAD assessed using CCTA showed that women had a greater risk of death from three-vessel or left-main disease than men, with similar mortality from nonobstructive and obstructive one-vessel or two-vessel CAD.40–42 Importantly, the assessment of nonobstructive CAD using CCTA can improve risk prediction beyond patient demographic data, traditional risk factors for CAD, and the Framingham risk score.41
Cardiac MRI
Cardiac MRI has emerged as a novel operator-independent diagnostic tool allowing the diagnosis of structural heart disease and ischaemia. Wall motion can be assessed after administration of dobutamine, analogous to stress echocardiography protocols, to evaluate myocardial viability and ischaemia.43,44 Data specific to women are not available; however, in a meta-analysis, stress-induced wall-motion abnormality imaging with MRI demonstrated a sensitivity of 83% and a specificity of 86% compared with coronary angiography.25 MRI perfusion imaging had a sensitivity of 0.91 and a specificity of 0.81.25 MRI imaging with late gadolinium enhancement provides additional information by enabling detection and quantification of myocardial scar tissue.45,46 MRI has been used to demonstrate subendocardial hypoperfusion during the intravenous administration of adenosine in women with chest pain and nonobstructive CAD.47–49 Cardiac MRI, therefore, can help to define the aetiology of chest pain in patients with a negative ECG, negative cardiac enzymes, and intermediate risk of IHD.
Myocardial perfusion SPECT
The assessment of stress-induced myocardial perfusion abnormalities using single-photon emission compute tomography (SPECT) is the most-commonly used nuclear-based technique for the investigation of both women and men presenting with chest pain.50 Overall, the diagnostic accuracy of SPECT is higher than for exercise ECG testing and reaches a sensitivity of 85% and a specificity of 70%.28,29,31 The accuracy of this method is, however, reduced in women with limited exercise capability. For this reason, pharmacological stress using adenosine or dipyridamole is often recommended. In addition, to reduce soft-tissue attenuation artefacts (owing to fat deposits or breast tissue) the higher-energy technetium (99mTc) radioisotope is preferred to thallium (201Tl) in women.51 Another challenge with the use of SPECT imaging in women relates to the smaller size of the heart than in men and, therefore, potentially smaller areas of myocardium with reduced perfusion that might be missed by currently available SPECT cameras with limited spatial resolution. Computer algorithms for attenuation correction of SPECT imaging have resulted in dramatic improvement in diagnostic accuracy for women with chest pain.52
Specific manifestations of IHD
Stable angina
The early results of the Framingham Heart Study6 suggested that women with angina had much more favourable outcomes than their male counterparts, with fewer events such as AMI. However, these early results must be viewed in light of the diagnostic limitations of the time, including limited use of routine coronary angiography.6 Contemporary data from a large community-based study indicate that women and men of all ages presenting with stable angina have increased coronary mortality relative to the general population.53 Women with angina who were aged <75 years, however, had higher standardized mortality ratios for CAD than men; among those aged 55–64 years, for example, the mortality ratio was 4.7 in women and 2.4 in men.53 In the Euro Heart Survey of Stable Angina,54 women were twice as likely as men die or experience nonfatal AMI during the 1-year follow-up period. These investigations negate the notion that stable angina is a benign condition in women.
Recommendations for the management of stable angina are similar in women and men, despite uncertainties about sex differences in response to treatment, and include adopting a healthy lifestyle; medication to decrease ischaemia and chest pain; secondary prevention with aspirin, β-blockers, statins, and angiotensin-converting-enzyme (ACE) inhibitors; coronary revascularization (when indicated); and cardiac rehabilitation.55 The majority of patients with stable angina recruited to clinical trials are male, which makes deriving conclusions about benefits specific to women difficult. Despite similarities in treatment recommendations between the sexes, the use of diagnostic procedures and secondary prevention therapies for angina are reported to be lower in women than men in community-based studies.54,56 These data suggest that stable angina remains underinvestigated and undertreated in women. Perceived difficulties in the diagnosis of IHD among women might explain some of these differences as described above. Currently, after a definite diagnosis of ‘clinically significant’ CAD is made, guideline-driven therapies for secondary prevention should be applied equally in men and women.57
Acute coronary syndromes
From the mid-1980s onwards, studies consistently documented higher unadjusted complication rates and mortality after ACS in women than in men.58 However, after differences in age and comorbidity are accounted for, the evidence in the literature for sex differences in prognosis seems to be inconsistent.9 The reason for these discrepancies is likely to be that higher mortality after ACS in women than in men does not occur across the whole patient population, but only in specific subgroups.58
Women with STEMI have worse outcomes than their male counterparts.59 By contrast, after adjusting for age, no sex differences in outcome are evident among patients with non-ST-segment elevation myocardial infarction (NSTEMI).9,60,61 Among patients with unstable angina, women actually have a significantly better prognosis than men after adjustment for age and risk factors.9,62 Reasons for differences in outcomes might be related to the underlying pathophysiology. The less extensive the arterial obstruction the better the prognosis, and women are more likely to have nonobtructive lesions than men in the clinical setting of unstable angina.8 On the other hand, STEMI is often secondary to acute coronary thrombus formation on a ruptured or eroded atherosclerotic plaque. This process can lead more often, or more suddenly, to total occlusion in women than men owing to smaller vessels in women and their tendency to develop plaque erosion, contributing to the poorer prognosis of women with STEMI.63
Thrombolytic therapy in patients with STEMI has been shown to reduce mortality to a similar degree in men and women.64 In addition, primary percutaneous coronary intervention (PCI) for patients with STEMI is as effective in women as in men.65 However, the efficacy and safety of an early invasive strategy in women with unstable angina or NSTEMI is controversial. The problem is partly caused by the low number of women included in randomized controlled trials. Since 2009, the proportion of women included in government-sponsored and industry-sponsored cardiovascular clinical trials has not increased.66 A meta-analysis of contemporary trials did not show a survival benefit for an early invasive strategy in women with unstable angina or NSTEMI.67 The benefits of this intervention seem to be enhanced in women presenting with increased levels of cardiac biomarkers, such as the troponins, whereas the use of this strategy has no benefit and might cause harm in low-risk women.68
Women with ACS derive benefit from many pharmacological treatments, including aspirin, β-blockers, clopi-dogrel, and oral anticoagulants.69 In the setting of early coronary revascularization, platelet glycoprotein IIb/IIIa inhibitors provide similar benefits in women as in men, although these agents are associated with an increased bleeding risk in women.70 However, among patients with ACS not routinely scheduled for early revascularization, glycoprotein IIb/IIIa inhibitors benefit men but are associated with an overall lack of efficacy in women.71 Nevertheless, the subgroup of women with an elevated level of troponin T or I (≥0.1 μg/l) derive a similar benefit from glycoprotein IIb/IIIa inhibitors to men.71 These data support the concept that women with unstable angina or NSTEMI benefit from an early invasive strategy and adjunctive glycoprotein IIb/IIIa use only if they have features indicative of high-risk, such as elevated levels of cardiac biomarkers. Additionally, these data suggest that women with unstable angina or AMI are a heterogeneous group with diverse pathophysiology underlying their chest pain, and thus have varied responses to treatment.
For many decades, women with ACS have been reported to receive less-aggressive treatment than men.23,72 In the past 5–8 years, however, reported differences in treatment by sex were small, particularly among patients with unstable angina or NSTEMI.61 As the size of cardiovascular databases continues to grow, even tiny differences can become statistically significant, thus ‘clinical significance’ becomes increasingly relevant.73 In the Get With the Guidelines–Coronary Artery Disease database, the difference between the sexes in use of aspirin and β-blockers was only 2 percentage points.61 In other contemporary databases, such as CRUSADE,74 CURE,75 GRACE,76 Medicare,35 and the National Registry of Myocardial Infarction,77 such differences were similarly small. Larger differences by sex have been reported in reperfusion therapy among patients with STEMI, although results are varied.35,61,77 In part, these gaps in treatment by sex might derive from concerns about the increased risk of bleeding among women. Larger treatment differences between the sexes are usually reported for less evidence-based procedures, such as coronary catheterization and revascularization.35,74,75,77 Whether these differences reflect true disparities in care is unclear, because the less-frequent use of revascularization in women might be explained by their lower prevalence of obstructive coronary disease at catheterization or their higher bleeding risks than in men.74,75
Perhaps the most pertinent issue is not whether treatments for ACS are the same in women and in men, but whether they should be the same. Medical treatment can have different benefits in men and women. For example, the benefits of statins in women with AMI,78 and for primary prevention of cardiovascular events,79 might be less than in men. The same might be true for ACE inhibitors in patients with left ventricular systolic dysfunction.80 By contrast, β-blockers substantially improve survival in women with AMI, with possibly a greater benefit than in men.81 According to the Committee on Understanding the Biology of Sex and Sex Differences, which was convened by the Institute of Medicine in 2001, these differences mean that a given therapy can no longer be assumed to have the same efficacy in men and women.82 Additional research will be needed before guidelines specific to women can be developed.
Coronary artery spasm and variant angina
The concept that the epicardial coronary arteries can go into spasm, provoking angina, AMI, and even sudden death is well established.83 Coronary vasospasm is the likely mechanism of variant angina, which is characterized by anginal symptoms at rest, absent or mild atherosclerosis of the major coronary arteries, and ST-segment elevation on the ECG during the anginal episode.92 Variant angina is thought to be more common in women than in men,85 although some cohort studies suggest a male preponderance.86,87 Transient sympathovagal imbalance and reduced bioavailability of nitric oxide or other vasoactive substances have been suggested as underlying mechanisms of coronary vasospasm.88–90
The long-term prognosis in patients with variant angina seems to be better than in those with obstructive CAD.91 Survival is 95% at 1 year and 89% at 5 years.84,92 However, nonfatal AMI occurs in 25% of the population, most commonly within 3 months of diagnosis.93 Concurrence of CAD and incidence of serious arrhythmias are strong predictors of mortality.94 No significant differences in clinical characteristics and outcomes exist between men and women.
Limited data exist on the effectiveness of treatments for variant angina, and no data are available on sex-related treatment indications for this condition. In a study performed >2 decades ago, patients with variant angina treated with diltiazem, nifedipine, or verapamil had better outcomes than those who received other medical therapies.91
‘Cardiac syndrome X’/nonobstructive CAD
Chest pain with a ‘normal’ or ‘near-normal’ coronary angiogram is present in 10–25% of women presenting with ACS, compared with 6–10% of men.8 Presentation of this syndrome ranges from uncomplicated chest pain to severe ischaemia and AMI, and yet the various terms32–34 (Box 2) are often used to describe different clinical presentations.95 The term ‘microvascular angina’ is the most-appropriate description of this syndrome.96 However, the notion of ‘normal’ coronary arteries should be reconsidered in light of the intravascular ultrasonography substudy from WISE,97 which showed that 80% of a sample of 100 women with seemingly normal coronaries had definite coronary atherosclerosis concealed by positive remodelling.
Box 2 |. Terms used to describe nonobstructive CAD*.
Angina with ‘normal’ or ‘near-normal’ coronary arteries17
Vasotonic angina34
Cardiac syndrome X147
Sensitive heart148
Microvascular angina149
Nonobstructive CAD150
*Effort-induced angina, positive stress test, positive single-photon emission computed tomography for myocardial ischaemia, ‘normal’ or ‘near-normal’ coronary arteries on angiography. Abbreviation: CAD, coronary artery disease.
Generally, the variability in patient populations and investigative methods makes summarizing the literature and drawing meaningful conclusions difficult.17 The definition of ‘cardiac syndrome X’ is particularly deceiving. Reliably identifying by angiography those patients who have coronary arteries free from any atherosclerotic plaque is not possible; indeed, the majority are likely to have some plaque (Figures 2 and 3). In women presenting with chest pain, consideration should be given to the ‘microvascular ischaemia’ hypothesis,98,99 in which myocardial ischaemia occurs in the absence of obstructive epicardial CAD. Research conducted over the past decade has shown that both angiographically smooth coronary arteries and diffuse nonobstructive CAD are associated with a significantly increased risk of major adverse cardiac events and all-cause mortality compared with healthy individuals without IHD, even after adjusting for traditional cardiac risk factors.17,18,36,100 The concept of microvascular disease is currently an area of intense basic and clinical investigation.
Both abnormal coronary vasomotion and microvascular disease have been implicated in the adverse outcomes associated with cardiac syndrome X or nonobstructive coronary disease.101–104 For example, in WISE,105 abnormal coronary vasomotion in response to acetylcholine was independently linked to adverse cardiovascular outcomes in 163 women with a clinical indication for coronary angiography, most of whom had no (or only mild) epicardial CAD. In a study of 42 women with de novo angina, reversible myocardial ischaemia on SPECT, and a normal coronary angiogram, Bugiardini et al. reported that coronary vasoconstriction provoked by intracoronary acetylcholine infusion was predictive of the development of coronary atherosclerosis at 10-year follow-up.106 In a further analysis of WISE,107 endothelium-independent vasodilatory reserve in response to adenosine, an index of microvascular function, was significantly related to increased risk of major adverse events among women with chest pain without obstructive CAD. Other investigators have reported similar findings.108,109 Notably, however, most studies of this condition have included either only women, or only a small population of men. Therefore, whether sex differences exist in outcomes of patients with nonobstructive CAD is unclear.76
No randomized trials of therapies for the reduction of adverse cardiac events in patients with angina and ‘normal’ coronary arteries have been conducted, and available data on adverse patient outcomes are limited to cohort studies. β-Blockers have been shown to be highly effective for reduction of chest-pain episodes during daily life.110 These drugs might counteract the proischaemic effects of increased adrenergic tone, or might simply reduce myocardial oxygen demand. Statins and ACE inhibitors improve endothelial dysfunction, can counteract oxidative stress, and might be of benefit in patients with a ‘normal’ angiogram.17,111 In small, placebo-controlled trials, cilazapril and enalapril improved exercise treadmill performance in such patients.112,113 In one study, improved coronary flow reserve was reported in patients taking enalapril.114 In the clinical setting of ACS, patients with nonobstructive coronary disease should be treated in the same way as those with obstructive disease. However, women and men were not examined separately in these studies, partly because the majority of patients were women. Thus, sex differences in the treatment of patients with nonobstructive CAD have not been adequately investigated.
Stress-related cardiomyopathy
Stress-related cardiomyopathy, also known as transient apical ballooning syndrome or Takotsubo cardiomyopathy, almost exclusively affects postmenopausal women and is a condition of sudden, severe, and reversible left ventricular dysfunction triggered by acute emotional stress.115 The incidence of stress-related cardiomyopathy in men is low (0–18% of patients presenting with chest pain).115 A clear nexus exists between this syndrome and severe emotional or physical stress.116 The syndrome is characterized by exaggerated sympathetic stimulation with an elevated plasma catecholamine level, which is thought to induce myocardial stunning as a result of transient vasospasm, microvascular dysfunction, and oxidative stress.117
The prognosis of stress-related cardiomyopathy is favourable among patients who survive the initial acute phase of heart failure, and symptoms usually resolve without long-term sequelae. This condition can, however, lead to serious complications, such as cardiogenic shock (6.5%), ventricular tachycardia (1.6%), and death (3.2%).118 Other rarer, but no less serious, complications include ventricular septal defect, ventricular fibrillation, pneumothorax, left ventricle rupture, and stroke. Whether sex differences exist in outcomes among patients with stress-related cardiomyopathy is unknown.
Limited data exist on the effectiveness of treatments for stress-related cardiomyopathy, and no data are available on sex-related treatment indications for this condition. The use of β-blockers in the acute phase of stress-related cardiomyopathy is a matter of debate119 and, because apical ballooning increases the risk of cardiac rupture, treatment with aspirin or heparin is also controversial. No specific treatment is recommended for the left ventricular failure that characterizes this syndrome, because cardiac function usually normalizes within a few weeks.
Revascularization procedures
CABG surgery
Women undergoing CABG surgery have higher operative mortality than men.120,121 This difference has been attributed, in part, to the smaller body size and smaller coronary vessels in women than men, as well as to a higher surgical risk owing to increased age and the presence of comorbidities, such as diabetes and hypertension.122 In addition, surgery in women is more-often performed on an emergency basis than in men, which adds to the risk.121 In a large cohort of patients undergoing CABG surgery, women had less-severe CAD and higher left ventricular ejection fraction than men at all ages.123 Nevertheless, in-hospital mortality was twofold higher for women than men in the youngest age group (<50 years; 3.4% versus 1.1%) and 1.5-fold higher among patients aged 50–69 years (2.6% versus 1.1%). In older patients (aged ≥75 years) the difference in mortality between the sexes was less pronounced.123 These findings parallel those for AMI, in that the excess mortality risk for women is more-pronounced in younger age groups.123
Percutaneous coronary intervention
PCI is reported to be less successful in women than in men. In the National Heart, Lung, and Blood Institute Coronary Angioplasty Registry, 546 women (out of 2,136 patients) who underwent PCI experienced more-frequent complications and had considerably higher procedural mortality than men (2.6% versus 0.3%).124,125 In women who survived the initial procedure, 4-year survival was similar to men.124,125 Differences in outcomes have declined over time. In BARI,126 an analysis of 489 women and 1,340 men revealed similar early and late mortality after PCI. Comparable outcomes for both sexes have also been reported in other studies published in the past decade, in which the focus has been on new treatment strategies, such as the use of drug-eluting stents.127,128
Coronary artery size is thought to have a role in worsening outcome among women undergoing PCI,129 because women have smaller coronary arteries than men even when accounting for differences in body size.130 However, women exhibit a lower risk of restenosis after coronary stenting.131 Therefore, other, as yet unknown, factors are likely to have a role in the differential response to PCI between the sexes.132
Sex, age, and outcomes in IHD
Women who experience an AMI at a young age or in middle-age (that is, those aged <65 years, and particularly those aged <55 years) have a worse prognosis than men in the same age group (Figure 5),133 with mortality in women declining with increasing age.134 Among older patients (aged ≥65 years), women tend to have better outcomes than men. Originally reported by Vaccarino and colleagues,134 the poorer prognosis in women of a young age has been confirmed in many population-based and registry studies,133,135–137 and is observed both in patients with STEMI and in those with NSTEMI.60 A similar pattern of adverse outcomes for young women is observed for recovery138,139 and mortality61 after CABG surgery, and also for complications and death after PCI.129,140 The adverse outcome for younger women is, however, less apparent in datasets from randomized clinical trials,9 probably because of enrolment criteria.
Figure 5 |.
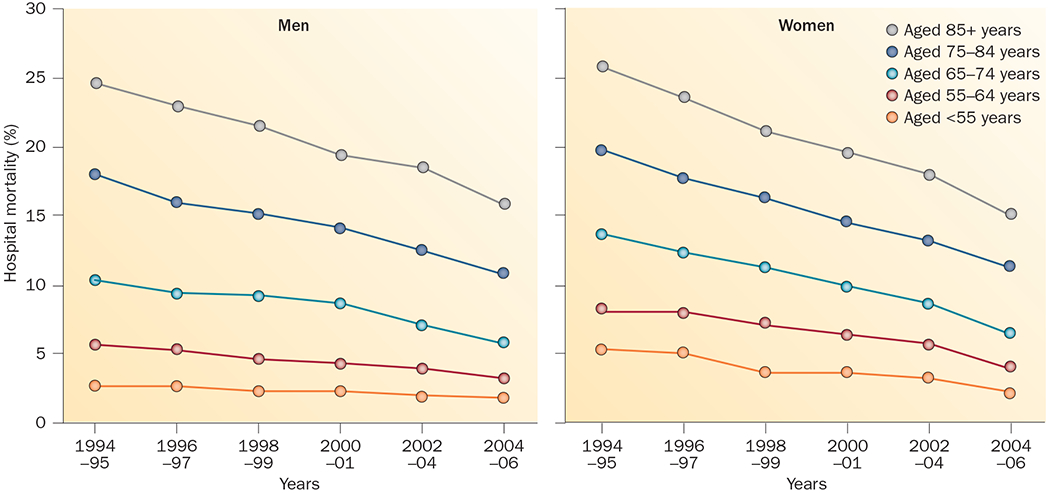
Temporal trends in hospital mortality by sex and age. In the National Registry of Myocardial Infarction (1994–2006), improvement in hospital mortality after myocardial infarction was greater in women than in men. The reduction in mortality relative to men was most pronounced among young women (aged <55 years). As a result, the higher mortality of young women with myocardial infarction compared with men considerably narrowed, but was still present.133
The reason that sex-related differences in outcomes of patients with AMI and those who undergo revascularization procedures are observed only in young and middle-aged individuals is unclear. Women aged <50 years, the majority of whom are likely to be premenopausal, might be expected to be protected, rather than at increased risk, compared with age-matched men in terms of survival and cardiovascular risk.134,141 Indeed, in these studies younger women have less-severe CAD, a greater incidence of preserved systolic function and, in the AMI studies, smaller infarcts compared with men of similar age.134,141 A higher proportion of out-of-hospital CAD-related deaths among men might be a contributory factor.142,143 For example, MacIntyre et al. found that female sex increased the probability of surviving to reach hospital, and this outweighed the excess risk of death occurring in younger women after hospitalization.144 The lower prehospital mortality in women than men, however, is not a consistent finding.141,145 Unaccounted comorbidities and risk factors might provide additional reasons for the differences in outcome. Cigarette smoking has been associated with higher risk of AMI and increased CAD-related mortality among women than men.146 Differences in presentation might also have an effect on outcomes. Among those presenting without chest pain, young women have higher hospital mortality than men in the same age group, and these sex differences decrease or even reverse with advancing age.12 Alternatively, unknown nontraditional pathologies, such as microvascular disease, or risk factors more common in women than in men, such as psychological stress and social disadvantage, might be involved. Although we do not have a full explanation for the excess mortality risk in young women, the sex difference has narrowed over time.141 This change suggests that environmental or behavioural causes of sex differences in outcomes are more important than biological factors.
Conclusions
Important differences exist between women and men in the clinical presentation, response to treatment, and outcomes for IHD, for which few clear explanations have been proposed. Issues concerning presentation and recognition of ischaemic symptoms in women continue to raise questions regarding the specificity and sensitivity of these symptoms and the accuracy of available tests in this population. Many questions remain to be addressed by future research. What is the true extent of sex differences in symptom recognition and awareness of IHD risk? Are sex differences in presentation a reflection of differences in the pathophysiology, or rather differences in recognition or expression of cardiac symptoms between women and men? Why do young women paradoxically have more adverse outcomes after AMI and coronary procedures, despite having less-severe CAD, than men of the same age? A better understanding of these issues might lead to the development of new strategies for prevention, detection, and treatment of IHD specifically tailored to women and, therefore, improve the clinical management of women with IHD.
Key points.
Important differences exist between women and men in clinical presentation, recognition of symptoms by patients and physicians, outcome, and response to treatment for ischaemic heart disease (IHD)
Among patients with IHD, environmental or behavioural causes of sex-related differences in outcomes might be more important than biological factors
Onset of IHD in women, manifesting as an acute myocardial infarction before the age of 65 years, is associated with adverse outcomes compared with men of a similar age
A traditional diagnostic strategy, focusing on detection of severe coronary stenoses, is likely to be inadequate in women
Additional invasive testing aimed at determining endothelial coronary dysfunction might be useful to risk-stratify women with chest pain and minimal or no obstructive coronary artery disease
Review criteria.
The MEDLINE and EMBASE databases were searched for articles published in the English language, using the following key words: “sex differences”, “women”, “pathophysiology”, “diagnosis”, “therapy”, and “prognosis” in combination with “angina”, “myocardial infarction”, “angina with normal coronary arteries”, “cardiac syndrome X”, “stress-related cardiomyopathy”, “nonobstructive coronary disease”, “variant angina”, “percutaneous coronary intervention”, and “CABG surgery”. Reference lists of published articles and abstracts of meeting presentations were also reviewed.
Acknowledgements
The authors of this Review are members of the European Society of Cardiology Working Group on Coronary Pathophysiology and Microcirculation, and acknowledge the European Society of Cardiology for financial support. Dr Vaccarino is supported by the National Institutes of Health, grant K24HL077506.
Footnotes
Competing interests
The authors declare no competing interests.
Contributor Information
Viola Vaccarino, Emory University Rollins School of Public Health and School of Medicine, USA.
Lina Badimon, Cardiovascular Research Centre, CSIC-ICCC, Spain.
Roberto Corti, University Hospital, Zurich, Switzerland.
Cor de Wit, Institut für Physiologie, Universität zu Lübeck, Germany.
Maria Dorobantu, Floreasca Emergency Hospital, Romania.
Olivia Manfrini, Department of Experimental, Diagnostics and Specialized Medicine, Section of Cardiology, Policlinico Sant’Orsola-Malpighi, 40138 Bologna, Italy.
Akos Koller, University of Pecs, Pécs, Hungary.
Axel Pries, Charité-Universitätsmedizin Berlin, Germany.
Edina Cenko, Department of Experimental, Diagnostics and Specialized Medicine, Section of Cardiology, Policlinico Sant’Orsola-Malpighi, 40138 Bologna, Italy.
Raffaele Bugiardini, Department of Experimental, Diagnostics and Specialized Medicine, Section of Cardiology, Policlinico Sant’Orsola-Malpighi, 40138 Bologna, Italy.
References
- 1.Go AS et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 127, e6–e245 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poon S. et al. Bridging the gender gap: insights from a contemporary analysis of sex-related differences in the treatment and outcomes of patients with acute coronary syndromes. Am. Heart J 163, 66–73 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Rajadurai J. et al. Women’s cardiovascular health: perspectives from South-East Asia. Nat. Rev. Cardiol 9, 464–77 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Vaccarino V. Ischemic heart disease in women: many questions, few facts. Circ. Cardiovasc. Qual. Outcomes 3, 111–115 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murabito JM, Evans JC, Larson MG & Levy D Prognosis after the onset of coronary heart disease. An investigation of differences in outcome between the sexes according to initial coronary disease presentation. Circulation 88, 2548–2555 (1993). [DOI] [PubMed] [Google Scholar]
- 6.Lerner DJ & Kannel WB Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am. Heart J 111, 383–390 (1986). [DOI] [PubMed] [Google Scholar]
- 7.Hemingway H. et al. Prevalence of angina in women versus men: a systematic review and meta-analysis of international variations across 31 countries. Circulation 117, 1526–1536 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Hochman JS et al. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. N. Engl. J. Med 341, 226–232 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Berger JS et al. Sex differences in mortality following acute coronary syndromes. JAMA 302, 874–882 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genders TS et al. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. Eur. Heart J 32, 1316–1330 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Milner KA, Funk M, Arnold A & Vaccarino V Typical symptoms are predictive of acute coronary syndromes in women. Am. Heart J 143, 283–288 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Canto JG et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA 307, 813–822 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Milner KA et al. Gender differences in symptom presentation associated with coronary heart disease. Am. J. Cardiol 84, 396–399 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Bugiardini R. Women, ‘nonspecific’ chest pain, and normal or near-normal coronary angiograms are not synonymous with favourable outcome. Eur. Heart J 27, 1387–1389 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Kreatsoulas C, Shannon HS, Giacomini M, Velianou JL & Anand SS Reconstructing angina: cardiac symptoms are the same in women and men. JAMA Intern. Med 173, 829–833 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Mackay MH, Ratner PA, Johnson JL, Humphries KH, Buller CE Gender differences in symptoms of myocardial ischaemia. Eur. Heart J 32, 3107–3114 (2011). [DOI] [PubMed] [Google Scholar]
- 17.Bugiardini R & Bairey Merz CN Angina with “normal” coronary arteries: a changing philosophy. JAMA 293, 477–84 (2005). [DOI] [PubMed] [Google Scholar]
- 18.Bugiardini R. et al. Angina, “normal” coronary angiography, and vascular dysfunction: risk assessment strategies. PLoS Med .4, e12 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw LJ, Bugiardini R & Merz CN Women and ischemic heart disease: evolving knowledge. J. Am. Coll. Cardiol 54, 1561–1575 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosca L, Mochari-Greenberger H, Dolor RJ, Newby LK & Robb KJ Twelve-year follow-up of American women’s awareness of cardiovascular disease risk and barriers to heart health. Circ. Cardiovasc. Qual. Outcomes 3, 120–127 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosca L et al. Fifteen-year trends in awareness of heart disease in women: results of a 2012 American Heart Association national survey. Circulation 127, 1254–1263 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuoka Y. et al. Is severity of chest pain a cue for women and men to recognize acute myocardial infarction symptoms as cardiac in origin? Prog. Cardiovasc. Nurs, 22, 132–137 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Rosengren A et al. Sex, age, and clinical presentation of acute coronary syndromes. Eur. Heart J 25, 663–670 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Mulvagh SL et al. Contrast echocardiography: current and future applications. J. Am. Soc. Echocardiogr 13, 331–342 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Nandalur KR, Dwamena BA, Choudhri AF, Nandalur MR & Carlos RC Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J. Am. Coll. Cardiol 50, 1343–1353 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC). Prevalence of heart disease—United States, 2006–2010. MMWR Morb. Mortal. Wkly Rep 60, 1377–1381 (2011). [PubMed] [Google Scholar]
- 27.Gibbons RJ et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). Circulation 106, 1883–1892 (2002). [DOI] [PubMed] [Google Scholar]
- 28.Shaw LJ et al. The value of estimated functional capacity in estimating outcome: results from the NHBLI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. J. Am. Coll. Cardiol 47, S36–S43 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Mieres JH et al. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation 111, 682–696 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Grzybowski A. et al. How to improve noninvasive coronary artery disease diagnostics in premenopausal women? The influence of menstrual cycle on ST depression, left ventricle contractility, and chest pain observed during exercise echocardiography in women with angina and normal coronary angiogram. Am. Heart J 156, 964.e1–964.e5 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Shaw LJ et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) study: part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J. Am. Coll. Cardiol 47 (Suppl.), S4–S20 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Lanza GA & Crea F Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation 121, 2317–2325 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Maseri A, Crea F, Kaski JC & Crake T Mechanisms of angina pectoris in syndrome X.J. Am. Coll. Cardiol 17, 499–506 (1991). [DOI] [PubMed] [Google Scholar]
- 34.Bugiardini R, Pozzati A, Ottani F, Morgagni GL & Puddu P Vasotonic angina: a spectrum of ischemic syndromes involving functional abnormalities of the epicardial and microvascular coronary circulation. J. Am. Coll. Cardiol 22, 417–425 (1993). [DOI] [PubMed] [Google Scholar]
- 35.Gan SC et al. Treatment of acute myocardial infarction and 30-day mortality among women and men. N. Engl. J. Med 343, 8–15 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Bugiardini R, Manfrini O & De Ferrari GM Unanswered questions for management of acute coronary syndrome: risk stratification of patients with minimal disease or normal findings on coronary angiography. Arch. Intern. Med 166, 1391–1395 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Scanlon PJ et al. ACC/AHA guidelines for coronary angiography: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. Circulation 99, 2345–2357 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Ong P et al. High prevalence of a pathological response to acetylcholine testing in patients with stable angina pectoris and unobstructed coronary arteries. The ACOVA Study (Abnormal COronary VAsomotion in patients with stable angina and unobstructed coronary arteries). J. Am. Coll. Cardiol 59, 655–662 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Budoff MJ et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicentre ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J. Am. Coll. Cardiol 52, 1724–1732 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Beigel R et al. Prognostic implications of nonobstructive coronary artery disease in patients undergoing coronary computed tomographic angiography for acute chest pain. Am. J. Cardiol 111, 941–945 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Lin FY et al. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2-centre study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J. Am. Coll. Cardiol 58, 510–519 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Min JK et al. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicentre CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicentre Registry) of 23,854 patients without known coronary artery disease. J. Am. Coll. Cardiol 58, 849–860 (2011). [DOI] [PubMed] [Google Scholar]
- 43.Martinez MW et al. Myocardial infarction with normal coronary arteries: a role for MRI? Clin. Chem 53, 995–996 (2007). [DOI] [PubMed] [Google Scholar]
- 44.Steg PG et al. Impact of collateral flow to the occluded infarct-related artery on clinical outcomes in patients with recent myocardial infarction: a report from the randomized occluded artery trial. Circulation 121, 2724–2730 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolff SD et al. Myocardial first-pass perfusion magnetic resonance imaging: a multicentre dose-ranging study. Circulation 110, 732–737 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Schwitter J et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur. Heart J 29, 480–489 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Panting JR et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N. Engl. J. Med 346, 1948–1953 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Schwitter J Extending the frontiers of cardiac magnetic resonance. Circulation 118, 109–112 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Reynolds HR et al. Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation 124, 1414–1425 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mieres JH et al. American Society of Nuclear Cardiology consensus statement: Task Force on Women and Coronary Artery Disease—the role of myocardial perfusion imaging in the clinical evaluation of coronary artery disease in women [correction]. J. Nucl. Cardiol 10, 95–101 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Klocke FJ et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging–executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to revise the 1995 guidelines for the clinical use of cardiac radionuclide imaging). Circulation 108, 1404–1418 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Matsunari I. et al. Attenuation-corrected rest thallium-201/stress technetium 99m sestamibi myocardial SPECT in normals. J. Nucl. Cardiol 5, 48–55 (1998). [DOI] [PubMed] [Google Scholar]
- 53.Hemingway H. et al. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA 295, 1404–1411 (2006). [DOI] [PubMed] [Google Scholar]
- 54.Daly C. et al. Gender differences in the management and clinical outcome of stable angina. Circulation 113, 490–498 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Fox K. et al. Guidelines on the management of stable angina pectoris: executive summary: the Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur. Heart J 27, 1341–1381 (2006). [DOI] [PubMed] [Google Scholar]
- 56.Crilly M, Bundred P, Hu X, Leckey L & Johnstone F Gender differences in the clinical management of patients with angina pectoris: a cross-sectional survey in primary care. BMC Health Serv. Res 7, 142 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnston N, Schenck-Gustafsson K & Lagerqvist B Are we using cardiovascular medications and coronary angiography appropriately in men and women with chest pain? Eur. Heart J 32, 1331–1336 (2011). [DOI] [PubMed] [Google Scholar]
- 58.Vaccarino V, Krumholz HM, Berkman LF & Horwitz RI Sex differences in mortality after myocardial infarction. Is there evidence for an increased risk for women? Circulation 91, 1861–1871 (1995). [DOI] [PubMed] [Google Scholar]
- 59.Wenger NK, Shaw LJ & Vaccarino V Coronary heart disease in women: update 2008. Clin. Pharmacol. Ther 83, 37–51 (2008). [DOI] [PubMed] [Google Scholar]
- 60.Champney KP et al. The joint contribution of sex, age and type of myocardial infarction on hospital mortality following acute myocardial infarction. Heart 95, 895–899 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jneid H. et al. Sex differences in medical care and early death after acute myocardial infarction. Circulation 118, 2803–2810 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Capewell S. et al. Short-term and long-term outcomes in 133,429 emergency patients admitted with angina or myocardial infarction in Scotland, 1990–2000: population-based cohort study. Heart 92, 1563–1570 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fuster V. Elucidation of the role of plaque instability and rupture in acute coronary events. Am. J. Cardiol 76, 24C–33C (1995). [DOI] [PubMed] [Google Scholar]
- 64.Fibrinolytic Therapy Trialists’ (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1,000 patients. Lancet 343, 311–322 (1994). [PubMed] [Google Scholar]
- 65.Tamis-Holland JE et al. Benefits of direct angioplasty for women and men with acute myocardial infarction: results of the Global Use of Strategies to Open Occluded Arteries in Acute Coronary Syndromes Angioplasty (GUSTO II-B) angioplasty substudy. Am. Heart J 147, 133–139 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Kim ES & Menon V Status of women in cardiovascular clinical trials. Arterioscler. Thromb. Vac. Biol 29, 279–283 (2009). [DOI] [PubMed] [Google Scholar]
- 67.Bavry AA et al. Invasive therapy along with glycoprotein IIb/IIIa inhibitors and intracoronary stents improves survival in non-ST-segment elevation acute coronary syndromes: a meta-analysis and review of the literature. Am. J. Cardiol 93, 830–835 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Glaser R. et al. Benefit of an early invasive management strategy in women with acute coronary syndromes. JAMA 288, 3124–3129 (2002). [DOI] [PubMed] [Google Scholar]
- 69.Lansky AJ et al. Percutaneous coronary intervention and adjunctive pharmacotherapy in women: a statement for healthcare professionals from the American Heart Association. Circulation 111, 940–953 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Cho L et al. Clinical benefit of glycoprotein IIb/IIIa blockade with abciximab is independent of gender: pooled analysis from EPIC, EPILOG and EPISTENT trials. Evaluation of 7E3 for the prevention of ischemic complications. Evaluation in percutaneous transluminal coronary angioplasty to improve long-term outcome with abciximab GP IIb/IIIa blockade. Evaluation of platelet IIb/IIIa inhibitor for stent. J. Am. Coll. Cardiol 36, 381–386 (2000). [DOI] [PubMed] [Google Scholar]
- 71.Boersma E. et al. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: a meta-analysis of all major randomised clinical trials. Lancet 359, 189–198 (2002). [DOI] [PubMed] [Google Scholar]
- 72.Healy B. The Yentl syndrome. N. Engl. J. Med 325, 274–276 (1991). [DOI] [PubMed] [Google Scholar]
- 73.Bugiardini R, Oestrada JL, Nikus K, Hall AS & Manfrini O Gender bias in acute coronary syndromes. Curr. Vasc. Pharmacol 8, 276–284 (2010). [DOI] [PubMed] [Google Scholar]
- 74.Blomkalns AL et al. Gender disparities in the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: large-scale observations from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines) national quality improvement initiative. J. Am. Coll. Cardiol 45, 832–837 (2005). [DOI] [PubMed] [Google Scholar]
- 75.Anand SS et al. Differences in the management and prognosis of women and men who suffer from acute coronary syndromes. J. Am. Coll. Cardiol 46, 1845–1851 (2005). [DOI] [PubMed] [Google Scholar]
- 76.Dey S. et al. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart 95, 20–26 (2009). [DOI] [PubMed] [Google Scholar]
- 77.Vaccarino V et al. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N. Engl. J. Med 353, 671–682 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karp I, Chen SF & Pilote L Sex differences in the effectiveness of statins after myocardial infarction. CMAJ 176, 333–338 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Walsh JM & Pignone M Drug treatment of hyperlipidemia in women. JAMA 291, 2243–2252 (2004). [DOI] [PubMed] [Google Scholar]
- 80.Shekelle PG et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J. Am. Coll. Cardiol 41, 1529–1538 (2003). [DOI] [PubMed] [Google Scholar]
- 81.First International Study of Infarct Survival Collaborative Group. Randomised trial of intravenous atenolol among 16,027 cases of suspected acute myocardial infarction: ISIS-1. Lancet 2, 57–66 (1986). [PubMed] [Google Scholar]
- 82.Institute of Medicine (eds Wizemann T & Pardue M) Exploring the Biological Contributions to Human Health: Does Sex Matter? (The National Academy Press, 2001). [PubMed] [Google Scholar]
- 83.Aldea GS et al. Effect of gender on postoperative outcomes and hospital stays after coronary artery bypass grafting. Ann. Thorac. Surg 67, 1097–1103 (1999). [DOI] [PubMed] [Google Scholar]
- 84.Stern S & Bayes de Luna A Coronary artery spasm: a 2009 update. Circulation 119, 2531–2534 (2009). [DOI] [PubMed] [Google Scholar]
- 85.Selzer A, Langston M, Ruggeroli C & Cohn K Clinical syndrome of variant angina with normal coronary arteriogram. N. Engl. J. Med 295, 1343–1347 (1976). [DOI] [PubMed] [Google Scholar]
- 86.Seung-Woon R et al. The impact of gender difference on angiographic characteristics during intracoronary acetylcholine provocation test in Korean patients [abstract TCT-437]. J. Am. Coll. Cardiol 60 (Suppl. B), B124 (2012). [Google Scholar]
- 87.Bory M et al. Coronary artery spasm in patients with normal or near normal coronary arteries. Long-term follow-up of 277 patients. Eur. Heart J 17, 1015–1021 (1996). [DOI] [PubMed] [Google Scholar]
- 88.Pozzati A, Pancaldi LG, Di Pasquale G, Pinelli G & Bugiardini R Transient sympathovagal imbalance triggers “ischemic” sudden death in patients undergoing electrocardiographic Holter monitoring. J. Am. Coll. Cardiol 27, 847–852 (1996). [DOI] [PubMed] [Google Scholar]
- 89.Yoo SY & Kim JY Recent insights into the mechanisms of vasospastic angina. Korean Circ. J 39, 505–511 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Egashira K. et al. Basal release of endothelium-derived nitric oxide at site of spasm in patients with variant angina. J. Am. Coll. Cardiol 27, 1444–1449 (1996). [DOI] [PubMed] [Google Scholar]
- 91.Walling A et al. Long-term prognosis of patients with variant angina. Circulation 76, 990–997 (1987). [DOI] [PubMed] [Google Scholar]
- 92.Mishra PK Variations in presentation and various options in management of variant angina. Eur. J. Cardiothorac. Surg 29, 748–759 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Waters DD et al. Factors influencing the long-term prognosis of treated patients with variant angina. Circulation 68, 258–265 (1983). [DOI] [PubMed] [Google Scholar]
- 94.Ong P, Athanasiadis A, Borgulya G, Voehringer M & Sechtem U 3-year follow-up of patients with coronary artery spasm as cause of acute coronary syndrome: the CASPAR (coronary artery spasm in patients with acute coronary syndrome) study follow-up. J. Am. Coll. Cardiol 57, 147–152 (2011). [DOI] [PubMed] [Google Scholar]
- 95.Asbury EA, Creed F & Collins P Distinct psychosocial differences between women with coronary heart disease and cardiac syndrome X. Eur. Heart J 25, 1695–1701 (2004). [DOI] [PubMed] [Google Scholar]
- 96.Camici PG & Crea F Coronary microvascular dysfunction. N. Engl. J. Med 356, 830–840 (2007). [DOI] [PubMed] [Google Scholar]
- 97.Khuddus MA et al. An intravascular ultrasound analysis in women experiencing chest pain in the absence of obstructive coronary artery disease: a substudy from the National Heart, Lung and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE). J. Interv. Cardiol 23, 511–519 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wenger NK Women and coronary heart disease: a century after Herrick: understudied, underdiagnosed, and undertreated. Circulation 126, 604–611 (2012). [DOI] [PubMed] [Google Scholar]
- 99.Bairey Merz CN et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J. Am. Coll. Cardiol 47 (3 Suppl.), S21–S29 (2006). [DOI] [PubMed] [Google Scholar]
- 100.Jespersen L. et al. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J 33, 734–744 (2012). [DOI] [PubMed] [Google Scholar]
- 101.Kaski JC et al. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J. Am. Coll. Cardiol 25, 807–814 (1995). [DOI] [PubMed] [Google Scholar]
- 102.Gulati M. et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch. Intern. Med 169, 843–850 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johnson BD et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 109, 2993–2999 (2004). [DOI] [PubMed] [Google Scholar]
- 104.Oerlemans JG, Lagro-Janssen AL & Bakx C Angina pectoris and normal coronary arteries: prevalence and prognosis in men and women [Dutch]. Ned. Tijdschr. Geneeskd 144, 522–527 (2000). [PubMed] [Google Scholar]
- 105.von Mering GO et al. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE). Circulation 109, 722–725 (2004). [DOI] [PubMed] [Google Scholar]
- 106.Bugiardini R, Manfrini O, Pizzi C, Fontana F & Morgagni G Endothelial function predicts future development of coronary artery disease: a study of women with chest pain and normal coronary angiograms. Circulation 109, 2518–2523 (2004). [DOI] [PubMed] [Google Scholar]
- 107.Pepine CJ et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia: results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) Study. J. Am. Coll. Cardiol 55, 2825–2832 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Britten MB, Zeiher AM & Schächinger V Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron. Artery Dis 15, 259–264 (2004). [DOI] [PubMed] [Google Scholar]
- 109.Fragasso G. et al. Coronary slow-flow causing transient myocardial hypoperfusion in patients with cardiac syndrome X: long-term clinical and functional prognosis. Int. J. Cardiol 137, 137–144 (2009). [DOI] [PubMed] [Google Scholar]
- 110.Bugiardini R, Borghi A, Biagetti L & Puddu P Comparison of verapamil versus propranolol therapy in syndrome X. Am. J. Cardiol 63, 286–290 (1989). [DOI] [PubMed] [Google Scholar]
- 111.Xhyheri B & Bugiardini R Diagnosis and treatment of heart disease: are women different from men? Prog. Cardiovasc. Dis 53, 227–236 (2010). [DOI] [PubMed] [Google Scholar]
- 112.Kaski JC, Rosano G, Gavrielides S & Chen L Effects of angiotensin-converting enzyme inhibition on exercise-induced angina and ST segment depression in patients with microvascular angina. J. Am. Coll. Cardiol 23, 652–657 (1994). [DOI] [PubMed] [Google Scholar]
- 113.Nalbantgil I et al. Therapeutic benefits of cilazapril in patients with syndrome X. Cardiology 89, 130–133 (1998). [DOI] [PubMed] [Google Scholar]
- 114.Pauly DF et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am. Heart J 162, 678–684 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Akashi YJ, Nef HM, Mollmann H & Ueyama T Stress cardiomyopathy. Annu. Rev. Med 61, 271–286 (2010). [DOI] [PubMed] [Google Scholar]
- 116.Yoshioka T et al. Clinical implications of midventricular obstruction and intravenous propranolol use in transient left ventricular apical ballooning (Tako-tsubo cardiomyopathy). Am. Heart J 155, 526.e1–526.e7 (2008). [DOI] [PubMed] [Google Scholar]
- 117.Wittstein IS et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med 352, 539–548 (2005). [DOI] [PubMed] [Google Scholar]
- 118.Bielecka-Dabrowa A. et al. Takotsubo cardiomyopathy—the current state of knowledge. Int. J. Cardiol 142, 120–125 (2010). [DOI] [PubMed] [Google Scholar]
- 119.Akashi YJ, Goldstein DS, Barbaro G & Ueyama T Takotsubo cardiomyopathy: a new form of acute, reversible heart failure. Circulation 118, 2754–2762 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Edwards FH, Carey JS, Grover FL, Bero JW & Hartz RS Impact of gender on coronary bypass operative mortality. Ann. Thorac. Surg 66, 125–131 (1998). [DOI] [PubMed] [Google Scholar]
- 121.Weintraub WS, Wenger NK, Jones EL, Craver JM & Guyton RA Changing clinical characteristics of coronary surgery patients. Differences between men and women. Circulation 88, II79–II86 (1993). [PubMed] [Google Scholar]
- 122.Woods SE, Noble G, Smith JM & Hasselfeld K The influence of gender in patients undergoing coronary artery bypass graft surgery: an eight-year prospective hospitalized cohort study. J. Am. Coll. Surg 196, 428–434 (2003). [DOI] [PubMed] [Google Scholar]
- 123.Vaccarino V, Abramson JL, Veledar E & Weintraub WS Sex differences in hospital mortality after coronary artery bypass surgery: evidence for a higher mortality in younger women. Circulation 105, 1176–1181 (2002). [DOI] [PubMed] [Google Scholar]
- 124.Kelsey SF et al. Results of percutaneous transluminal coronary angioplasty in women. 1985–1986 National Heart, Lung, and Blood Institute’s Coronary Angioplasty Registry. Circulation 87, 720–727 (1993). [DOI] [PubMed] [Google Scholar]
- 125.Holubkov R. et al. Angina 1 year after percutaneous coronary intervention: a report from the NHLBI Dynamic Registry. Am. Heart J 144, 826–833 (2002). [DOI] [PubMed] [Google Scholar]
- 126.Jacobs AK et al. Better outcome for women compared with men undergoing coronary revascularization: a report from the Bypass Angioplasty Revascularization Investigation (BARI). Circulation 98, 1279–1285 (1998). [DOI] [PubMed] [Google Scholar]
- 127.Thompson CA et al. Gender-based differences of percutaneous coronary intervention in the drug-eluting stent era. Catheter. Cardiovasc. Interv 67, 25–31 (2006). [DOI] [PubMed] [Google Scholar]
- 128.Abbott JD et al. Gender-based outcomes in percutaneous coronary intervention with drug-eluting stents (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am. J. Cardiol 99, 626–631 (2007). [DOI] [PubMed] [Google Scholar]
- 129.Argulian E. et al. Gender differences in short-term cardiovascular outcomes after percutaneous coronary interventions. Am. J. Cardiol 98, 48–53 (2006). [DOI] [PubMed] [Google Scholar]
- 130.Yang F, Minutello RM, Bhagan S, Sharma A & Wong SC The impact of gender on vessel size in patients with angiographically normal coronary arteries. J. Interv. Cardiol 19, 340–344 (2006). [DOI] [PubMed] [Google Scholar]
- 131.Mehilli J. et al. Gender and restenosis after coronary artery stenting. Eur. Heart J 24, 1523–1530 (2003). [DOI] [PubMed] [Google Scholar]
- 132.Dickerson JA, Nagaraja HN & Raman SV Gender-related differences in coronary artery dimensions: a volumetric analysis. Clin. Cardiol 33, E44–E49 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vaccarino V. et al. Sex differences in mortality after acute myocardial infarction: changes from 1994 to 2006. Arch. Intern. Med 169, 1767–1774 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vaccarino V, Parsons L, Every NR, Barron HV & Krumholz HM Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N. Engl. J. Med 341, 217–225 (1999). [DOI] [PubMed] [Google Scholar]
- 135.Andrikopoulos GK et al. Younger age potentiates post myocardial infarction survival disadvantage of women. Int. J. Cardiol 108, 320–325 (2006). [DOI] [PubMed] [Google Scholar]
- 136.Koek HL et al. Short- and long-term prognosis after acute myocardial infarction in men versus women. Am. J. Cardiol 98, 993–999 (2006). [DOI] [PubMed] [Google Scholar]
- 137.Radovanovic D. et al. Gender differences in management and outcomes in patients with Acute Coronary Syndromes: results on 20,290 patients from the AMIS Plus Registry. Heart 93, 1369–1375 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vaccarino V. et al. Sex differences in health status after coronary artery bypass surgery. Circulation 108, 2642–2647 (2003). [DOI] [PubMed] [Google Scholar]
- 139.Vaccarino V. et al. Gender differences in recovery after coronary artery bypass surgery. J. Am. Coll. Cardiol 41, 307–314 (2003). [DOI] [PubMed] [Google Scholar]
- 140.Abramson JL, Veledar E, Weintraub WS & Vaccarino V Association between gender and in-hospital mortality after percutaneous coronary intervention according to age. Am. J. Cardiol 91, 968–971 (2003). [DOI] [PubMed] [Google Scholar]
- 141.Rosengren A. et al. Sex differences in survival after myocardial infarction in Sweden; data from the Swedish National Acute Myocardial Infarction Register. Eur. Heart J 22, 314–322 (2001). [DOI] [PubMed] [Google Scholar]
- 142.Russo AM et al. Influence of gender on arrhythmia characteristics and outcome in the Multicentre UnSustained Tachycardia Trial. J. Cardiovasc. Electrophysiol 15, 993–998 (2004). [DOI] [PubMed] [Google Scholar]
- 143.Salomaa V. et al. Decline in out-of-hospital coronary heart disease deaths has contributed the main part to the overall decline in coronary heart disease mortality rates among persons 35 to 64 years of age in Finland: the FINAMI study. Circulation 108, 691–696 (2003). [DOI] [PubMed] [Google Scholar]
- 144.MacIntyre K. et al. Gender and survival: a population-based study of 201,114 men and women following a first acute myocardial infarction. J. Am. Coll. Cardiol 38, 729–735 (2001). [DOI] [PubMed] [Google Scholar]
- 145.Centers for Disease Control and Prevention (CDC). State-specific mortality from sudden cardiac death—United States, 1999. MMWR Morb. Mortal. Wkly Rep 51, 123–126 (2002). [PubMed] [Google Scholar]
- 146.Njolstad I, Arnesen E & Lund-Larsen PG Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction. A 12-year follow-up of the Finnmark Study. Circulation 93, 450–456 (1996). [DOI] [PubMed] [Google Scholar]
- 147.Kemp HG Jr, Vokonas PS, Cohn PF & Gorlin R. The anginal syndrome associated with normal coronary arteriograms. Report of a six year experience. Am. J. Med 54, 735–742 (1973). [DOI] [PubMed] [Google Scholar]
- 148.Cannon RO 3rd et al. Abnormal cardiac sensitivity in patients with chest pain and normal coronary arteries. J. Am. Coll. Cardiol 16, 1359–1366 (1990). [DOI] [PubMed] [Google Scholar]
- 149.Cannon RO 3rd & Epstein SE “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. Am. J. Cardiol 61, 1338–1343 (1988). [DOI] [PubMed] [Google Scholar]
- 150.Reis SE et al. Coronary flow velocity response to adenosine characterizes coronary microvascular function in women with chest pain and no obstructive coronary disease. Results from the pilot phase of the Women’s Ischemia Syndrome Evaluation (WISE) study. J. Am. Coll. Cardiol 33, 1469–1475 (1999). [DOI] [PubMed] [Google Scholar]


