Abstract
Inkjet printing is a more sustainable and scalable fabrication method than spin coating for producing perovskite solar cells (PSCs). Although spin-coated SnO2 has been intensively studied as an effective electron transport layer (ETL) for PSCs, inkjet-printed SnO2 ETLs have not been widely reported. Here, we fabricated inkjet-printed, solution-processed SnOx ETLs for planar PSCs. A champion efficiency of 17.55% was achieved for the cell using a low-temperature processed SnOx ETL. The low-temperature SnOx exhibited an amorphous structure and outperformed high-temperature crystalline SnO2. The improved performance was attributed to enhanced charge extraction and transport and suppressed charge recombination at ETL/perovskite interfaces, which originated from enhanced electrical and optical properties of SnOx, improved perovskite film quality, and well-matched energy level alignment between the SnOx ETL and the perovskite layer. Furthermore, SnOx was doped with Cu. Cu doping increased surface oxygen defects and upshifted energy levels of SnOx, leading to reduced device performance. A tunable hysteresis was observed for PSCs with Cu-doped SnOx ETLs, decreasing at first and turning into inverted hysteresis afterwards with increasing Cu doping level. This tunable hysteresis was related to the interplay between charge/ion accumulation and recombination at ETL/perovskite interfaces in the case of electron extraction barriers.
Keywords: inkjet printing, SnOx, Cu doping, perovskite solar cells, hysteresis, low-temperature solution process
1. Introduction
Perovskite solar cells (PSCs) have achieved a power conversion efficiency (PCE) reaching 26% after a fast development during the past decade [1]. However, there is still a lack of a reliable large-scale manufacturing method that can reduce the production cost of PSCs and simultaneously offer PSC device performance comparable to that of conventional spin coating. Inkjet printing is considered as an alternative deposition method to spin coating because of its potential for large-scale manufacturing [2]. Moreover, its drop-on-demand technology produces almost no material waste. In recent years, partially inkjet-printed PSCs have been demonstrated [3–5] while inkjet-printed SnO2 electron transport layers (ETLs) have not been widely reported. Rohnacher et al. fabricated SnOx ETLs using inkjet printing and achieved a PCE of 18.8% with severe hysteresis [6]. Ghahremani et al. reported an optimal PCE of 13.08% for devices using inkjet-printed SnO2 ETLs [7]. In our previous work, we achieved a PCE of 17.37% for inkjet-printed SnO2 ETLs prepared from a commercial colloidal SnO2 dispersion [8]. More studies with detailed analysis are required to move closer to large-scale device applications.
As a key component of PSCs, ETLs play an important role in transporting electrons and blocking holes [9]. For a long time, TiO2 has served as the most widely used electron transport material because of its abundance and favourable energy band alignment with perovskites [10]. However, TiO2 has its own limitations. For example, its photoactivity deteriorates device stability under ultraviolet (UV) illumination; a high sintering temperature (greater than 450°C) is required for proper crystallinity of TiO2, which is not compatible with flexible PSCs [11,12]. Thereafter, SnO2 has attracted considerable attention because of its potential to overcome the disadvantages of TiO2. SnO2 has a slightly wider band gap (above 3.6 eV) than TiO2, allowing for a longer device stability upon exposure to UV light [13]. It also has an electron mobility of up to 240 cm2 V−1 s−1, which is 100 times higher than that of TiO2 [12]. Regarding the fabrication of SnO2 ETLs, a high-temperature sintering process is not a must. Low-temperature processed SnO2 ETLs have been reported to deliver high device performance [14–16], even superior to that of their high-temperature processed counterparts [17,18]. Up to now, the main strategy for boosting the performance and stability of SnO2 based devices has been to suppress energy loss within SnO2 [19,20] or at the ETL/perovskite interface [21–23]. Doping is a direct and effective method of modifying the properties of SnO2, such as the conductivity and work function, which can facilitate electron extraction and transport [24]. It is also easily compatible with a solution process. For the selection of doping elements, a broad range of dopants has been tested, such as Li [25], Ga [26], Nb [27], Zn [28] and Sb [29]. To the best of our knowledge, there are only two reports in which Cu-doped SnO2 was used as an ETL for PSCs. Zhou et al. applied Cu-doped SnO2 ETLs in planar PSCs and achieved an enhanced PCE of 21% (19.63% for undoped SnO2-based PSCs) and improved long-term stability [30]. In the other study [31], based on a low-temperature solution-processed Cu-doped SnO2 ETL, the PSC device exhibited a PCE of 11.29% and a fill factor (FF) of 73.38% compared to the undoped device with a PCE of 8.32% and an FF of 59.9%. Therefore, there is a need for more comprehensive studies on the effects of Cu doping on device performance to unveil the underlying mechanisms.
In this study, we conducted a systematic investigation of the application of inkjet-printed solution-processed SnOx ETLs in planar PSCs. The effect of the annealing temperature of SnOx ETLs was studied, and low-temperature amorphous SnOx outperformed high-temperature crystalline SnO2. The amorphous SnOx enabled the formation of high-quality perovskite films. SnOx also exhibited improved electrical and optical properties and better energy level alignment with the perovskite layer, facilitating charge transport and reducing charge recombination at ETL/perovskite interfaces. An optimum PCE of 17.55% was achieved for planar PSCs using inkjet-printed amorphous SnOx ETLs. Afterwards, Cu was incorporated into SnOx. Upon Cu doping, SnOx exhibited increased surface defects and upshifted energy levels, and the perovskite film exhibited increased defects, resulting in reduced device performance. A tunable hysteresis was observed for PSCs based on Cu-doped SnOx ETLs with variations in the Cu doping level, originating from the interaction of ion/charge accumulation and recombination at ETL/perovskite interfaces in the case of extraction barriers.
2. Material and methods
2.1. Materials
All chemicals were purchased off the shelf and were used without further modifications. Tin(IV) acetate (Sn(CH3CO2)4) and copper(II) acetate (Cu(CO2CH3)2, 99.99%) were purchased from Sigma–Aldrich (Darmstadt, Germany). Lead iodide (PbI2, 99.99%) and lead bromide (PbBr2, >98.0%) were purchased from TCI (Tokyo, Japan). Formamidinium iodide (FAI, CH(NH2)2I, >98%) and methylammonium bromide (MABr, CH3NH3Br, >98%) were purchased from Dyenamo (Stockholm, Sweden) and Sigma–Aldrich (Darmstadt, Germany), respectively. Spiro-OMeTAD (99.8%) was purchased from Borun New Material Technology (Ningbo, China). Bis(trifluoromethane)sulfonimide lithium salt (LiTFSI, 99.95%), FK 209 Co(III) TFSI salt (FK 209, 98%), and 4-tert-butylpyridine (TBP, 98%) were purchased from Sigma–Aldrich (Darmstadt, Germany).
2.2. Inkjet printing of pristine and Cu-doped SnOx thin layers
A customized drop-on-demand inkjet printing system was used in our laboratory, which was designed for the piezoelectric-driven printheads from XaarJet [32]. The inkjet printing of SnOx and Cu-doped SnOx was performed under ambient conditions using XJ126/80 printheads with 126 active nozzles and a drop volume of 80 pL. More technical information on the printheads is presented in electronic supplementary material, table S1. A customized waveform was used with a peak voltage of 20 V and a jetting pulse of 20 µs. The printing frequency was set as 283.46 Hz and the printing resolution was 360 dpi.
SnOx thin films were inkjet-printed using inks with tin(IV) acetate dissolved in a solution consisting of 2-propanol and propylene glycol (9/1, v/v). A few drops of ethanolamine were added to improve solubility of tin acetate. For printing, a substrate was placed on a pre-heated printing stage at 60°C; 5 min after printing, the substrate was transferred to a furnace and annealed at 220°C for 1 h. Cu-doped SnOx was fabricated using a process similar to that of pristine SnOx. A certain amount of Cu acetate solution, containing 0.25 M Cu acetate dissolved in a mixed solvent of 2-propanol and propylene glycol (9/1, v/v), was added to the Sn acetate solution. The amount of the added Cu acetate solution was determined based on the desired doping level. The mixed solution was then printed, and the resulting film was annealed at 220°C for 1 h.
2.3. Device fabrication
Fluorine-doped tin oxide (FTO) glass substrates (14 Ω sq−1, Pilkington TEC) were cut into pieces with dimensions of 25 mm × 15 mm. Each piece was etched at the edge with Zn powder and 2 M HCl aqueous solution. After that, the substrates were sonicated in the sequence of 5% deconex water solution, deionized water, acetone, and 2-propanol for 15 min. Before inkjet printing of the ETL, the FTO substrate was pre-heated at 500°C for 30 min (pre-heating treatment) and afterwards cooled down to room temperature. A compact SnOx or Cu-doped SnOx ETL was fabricated as described above. For fabrication of the perovskite layer, we followed the procedure reported in [8,33]. To prepare the perovskite precursor, 1.1 M PbI2, 1 M FAI, 0.2 M PbBr2 and 0.2 M MABr were dissolved in a mixed solvent (N,N-dimethylformamide/dimethyl sulfoxide, 4/1 v/v). From that volume, 75 µl of the perovskite precursor was spin-coated at 4500 rpm for 30 s, and meanwhile 125 µl of chlorobenzene was dripped onto the perovskite film 15 s before the end of spin coating. The resulting perovskite film was immediately transferred to a hotplate and dried at 100°C for 30 min. A hole transport layer was prepared by spin-coating at 4000 rpm for 30 s using a precursor containing 70 mM Spiro-OMeTAD, 20 mM LiTFSI, 200 mM TBP, and 2 mM FK 209 in chlorobenzene. Finally, an Au electrode with a thickness of 80 nm was deposited via thermal evaporation (Edwards Auto 306).
2.4. Characterization
The surface morphology and cross-sectional microstructure were analysed via a combined focused ion beam/scanning electron microscope (FIB/SEM, FEI Nova 600 Nanolab, ThermoFisher, Eindhoven, Netherlands). The X-ray diffraction (XRD) analysis was performed using an X-ray diffractometer (Siemens D5000, Siemens, Munich, Germany) with the use of Cu Kα1 radiation (λ = 1.5406 Å). The characterized areas of the solar cells were defined by masks of 0.126 cm2 and illuminated under an AM 1.5G solar simulator (Newport 91160–1000) with an incident light density of 100 mW cm−2. Photocurrent density–voltage (J–V) data were collected by a Keithley 2400 unit with a scan rate of 125 mV s−1. X-ray photoelectron spectroscopy (XPS) and ultraviolet photoelectron spectroscopy (UPS) measurements were conducted using a K-Alpha XPS/UPS system manufactured by Thermo Scientific. For XPS analysis, the spectra were obtained using a monochromatized Al Kα line with a photon energy (hν) of 1486.6 eV. For UPS analysis, a He1 ultraviolet light source with an energy of 21.22 eV was employed. The valence band photoelectron signal originated from the top 2–3 nm of the sample surface, and the electronic work function of the material surface was measured. Sn M5,4-edge and O K-edge X-ray absorption spectroscopy (XAS) measurements were conducted on BL7.3.1 and 8.0.1.4 at the Advanced Light Source (ALS). The ultraviolet–visible (UV/Vis) absorption and transmission spectra were obtained using a Lambda 750 spectrophotometer. The conductivity of the ETLs was measured using a two-probe method [33], and the current–voltage (I–V) characteristics were collected by a Keithley 2400 unit. The steady-state photoluminescence (PL) of the perovskite films was investigated using a CARY Eclipse fluorescence spectrophotometer with an excitation wavelength of 450 nm.
3. Results and discussion
3.1. Inkjet-printed solution-processed SnOx electron transport layer
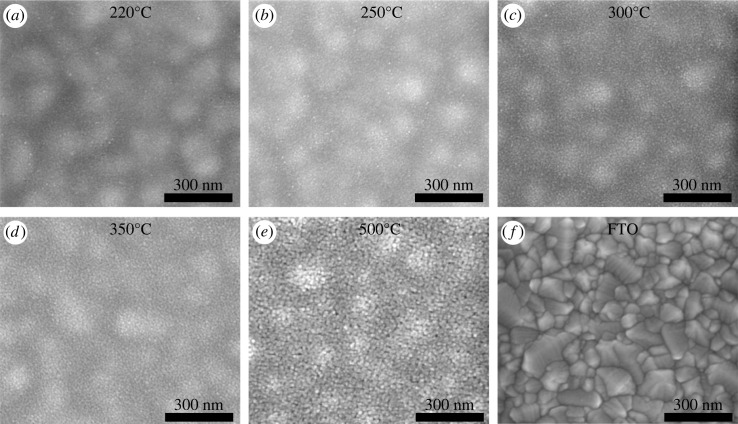
In this work, we prepared SnOx thin films using precursor inks consisting of tin(IV) acetate. The drying mechanism of these inkjet-printed thin films is different from that of their spin-coated counterparts prepared from SnCl2 or tin(IV) isopropoxide precursors [17,18]. Therefore, the effect of the annealing temperature was studied to find the optimal annealing temperature for SnOx ETLs. A schematic diagram of the inkjet-printed solution-processed SnOx is shown in electronic supplementary material, figure S1. Figure 1 shows top-view SEM images of the printed films annealed at various temperatures. All the printed films were uniform and continuous, with no pinholes observed. Obvious granular features appeared at 300°C. The particles became larger and the films exhibited more porosity when the temperature was further increased to 500°C. This was confirmed by the XRD patterns displayed in figure 2a. The printed SnOx thin films annealed below 300°C exhibited an amorphous phase with no diffraction peaks detected. The characteristic peaks attributed to tetragonal rutile crystalline phases of SnO2 (JCPDS 41-1445) were observed at and above 300°C [17,34]. The intensities of these peaks increased with increasing annealing temperature.
Figure 1.
Top-view SEM images of inkjet-printed SnOx thin films annealed at various temperatures: (a) 220°C, (b) 250°C, (c) 300°C, (d) 350°C, and (e) 500°C. A bare FTO substrate is shown in (f). Note that larger spherical features in (a–e) are from the FTO beneath the SnOx films, which has a substantially higher roughness (f).
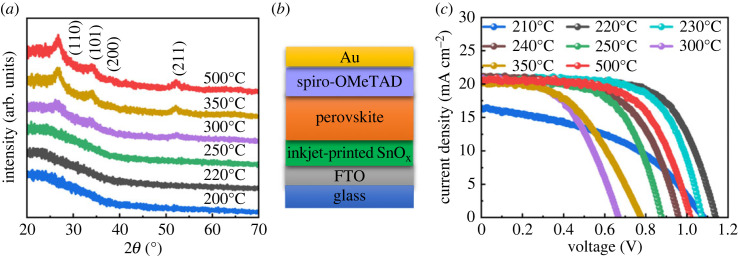
Figure 2.
(a) Temperature-dependent XRD patterns of SnOx thin films inkjet-printed on glass substrates; (b) device configuration and (c) J–V curves of PSCs based on inkjet-printed SnOx ETLs annealed at various temperatures.
Planar PSCs (figure 2b) were then fabricated using inkjet-printed SnOx ETLs annealed at various temperatures ranging from 210°C to 500°C. The J–V curves of the devices are shown in figure 2c. The photovoltaic parameters and their statistical distributions are presented in detail in electronic supplementary material, table S2 and figure S2, respectively. The cells based on 220°C-annealed SnOx ETLs achieved the highest PCE of 15.70% (table 1). When the annealing temperature decreased from 220°C to 200°C, the short-circuit current density (JSC) and FF both decreased dramatically, and JSC almost dropped to 0 at 200°C. The reason could be that tin acetate did not decompose completely at 200°C, and thus the residuals of the acetate could hinder the charge transfer and act as recombination sites. When the annealing temperature increased from 220°C to 500°C, JSC changed slightly, whereas the open-circuit voltage (VOC) and FF varied significantly and showed the same trend, first decreasing and then increasing. Thus, PSCs based on 300°C-annealed SnOx showed the lowest PCE of 7.03%, which could be due to the phase transfer of the resulting SnOx from amorphous to crystalline at 300°C [6]. The PCE then increased to 13.17% when the annealing temperature increased from 300°C to 500°C. Table 1 also demonstrates an important parameter, the hysteresis index (HI). This is used to describe the current–voltage hysteresis and is defined as HI = (PCE|reverse – PCE|forward)/PCE|reverse, where PCE|reverse and PCE|forward represent the PCE obtained from the reverse-scan and forward-scan J–V characteristics, respectively. PSCs with low-temperature (e.g. 220°C) annealed SnOx ETLs exhibited suppressed hysteresis compared to those with high-temperature (e.g. 500°C) annealed SnO2 ETLs.
Table 1.
Photovoltaic parameters of PSCs with inkjet-printed SnOx ETLs annealed at 220°C and 500°C.
| scan direction | PCE (%) | VOC (V) | JSC (mA cm−2) | FF (%) | HI (%) | Rs (Ω cm2) | Rsh (Ω cm2) | A | J0 (mA cm−2) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 220°C | reverse | 15.70 | 1.14 | 20.95 | 65.7 | 15.3 | 3.54 | 1100 | 4.03 | 3.73 × 10−4 |
| (SnOx) | forward | 13.30 | 1.12 | 20.82 | 57.3 | |||||
| 500°C | reverse | 13.17 | 1.02 | 20.64 | 62.9 | 22.5 | 3.90 | 650 | 4.15 | 1.56 × 10−3 |
| (HT-SnO2) | forward | 10.21 | 0.91 | 20.81 | 53.9 |
The results presented above suggest that the low-temperature annealed SnOx ETL outperformed its high-temperature counterpart, which are consistent with reported results [17,18]. We then conducted additional experiments and characterizations to reveal the origins of the improved performance of the low-temperature annealed SnOx ETLs. For simplicity, further on we only discuss the results for the low-temperature (220°C) annealed SnOx and the high-temperature (500°C) annealed SnO2 (HT-SnO2).
XPS measurements were conducted to investigate the chemical states of SnOx and HT-SnO2. As seen in figure 3a, SnOx showed two characteristic peaks at 495.7 eV and 487.3 eV (ΔE = 8.4 eV), which were assigned to Sn 3d3/2 and Sn 3d5/2, respectively. SnOx also exhibited a peak at 931.2 eV in the O 1s XPS spectrum (figure 3b). These XPS results suggest that Sn was in the +4 valence state in SnOx. There was no peak shift observed between SnOx and HT-SnO2 in Sn 3d and O 1s XPS spectra, which indicates that Sn was also in the +4 state in HT-SnO2. Moreover, XAS measurements were performed to confirm the amorphous nature of SnOx. In the Sn M5,4-edge XAS spectra of HT-SnO2 (figure 3c), two sets of triplet peaks (a, b, c) and (d, e, f) arose from 3d5/2 and 3d3/2 to 5p transitions, respectively. In figure 3d, peaks x and y corresponded to the hybridization of O 2p orbitals with Sn 5s and Sn 5p orbitals, respectively [35]. The Sn M5,4-edge and O K-edge spectral features of HT-SnO2 are consistent with the typical characteristics of crystalline SnO2 in earlier reports [34,36,37]. For the SnOx sample, significant differences were observed in the Sn M5,4-edge spectral features. Peaks a and b broadened and merged into one peak h, and peaks d and e broadened and merged into i as well. This spectral broadening is related to the lack of long-range order in the structure of SnOx and the presence of uncoordinated surface tin atoms [38,39]. An additional pre-edge peak g appeared, which was assigned to the surface states originating from uncoordinated surface atoms, oxygen vacancies, or surface reconstruction [38–40]. As observed in the O K-edge spectrum of SnOx, the spectral broadening was consistent with that in the Sn M5,4-edge spectrum and was attributed to the presence of the surface states. These Sn M5,4-edge and O K-edge spectral features of SnOx are consistent with those of the amorphous SnOx reported previously [37,41,42], confirming an amorphous structure without long-range order for the low-temperature annealed SnOx.
Figure 3.
(a) Sn 3d XPS, (b) O 1s XPS, (c) Sn M5,4-edge XAS, (d) O K-edge XAS, and (e) optical transmission spectra of SnOx and HT-SnO2; (f) energy level alignment of the PSC device with a SnOx or HT-SnO2 ETL; (g,h) SEM images and (i) XRD patterns of the perovskite films deposited on SnOx and HT-SnO2.
The amorphous structure of SnOx can be beneficial for the charge transport within ETLs. The grain boundary scattering within the nanocrystalline HT-SnO2 could lead to electron transport losses, which would be less severe in the amorphous SnOx [43,44]. This was verified by the improved conductivity of SnOx (electronic supplementary material, figure S3). The conductivity of SnOx was estimated to be 2.96 × 10−5 S cm−1, which was slightly higher than that of HT-SnO2 (2.46 × 10−5 S cm−1). This enhanced conductivity can contribute to the improved JSC. UV/Vis measurements were performed to examine the optical properties of SnOx and HT-SnO2 (figure 3e). SnOx and HT-SnO2 both showed an optical transmittance above 80% in the visible region (380–750 nm), which confirmed the transparency of the two samples. They also improved the optical transmission properties of FTO substrates because of the good antireflection ability of SnO2 thin films [44,45]. Compared to HT-SnO2, SnOx exhibited a slightly enhanced transmittance, which could allow more light to reach the perovskite absorber to generate carriers, contributing to the enhanced JSC of the devices based on SnOx ETLs [45]. Furthermore, the optical band gap (Eg) can be determined by Tauc's relationship, which is described by , where α is the optical absorption coefficient, hν is the photon energy, and C is a material constant [46]. The values of the band gap extrapolated from Tauc's plots (electronic supplementary material, figure S4) were 3.76 eV and 3.72 eV for SnOx and HT-SnO2, respectively. The enlarged band gap of SnOx was consistent with the enhanced transmittance.
UPS measurements were performed to investigate the energy band levels of SnOx and HT-SnO2 (electronic supplementary material, figure S5a). The work function can be determined by the formula [22], where EF is the Fermi level, Eonset is the onset, and Ecutoff is the cutoff of the UPS spectrum. The work function of SnOx and HT-SnO2 was calculated to be 6.76 eV and 7.02 eV, respectively. The valence band maximum (EVBM) of SnOx and HT-SnO2 was measured to be 1.22 eV and 2.22 eV below the Fermi level (electronic supplementary material, figure S5b), and then the EVBM values were calculated to be −7.98 eV and −9.24 eV, respectively. Their conduction band minimums (ECBM) were further determined to be −4.22 eV and −5.52 eV using the equation . The energy band positions of the perovskite layer were obtained from the literature [47]. The energy band alignment diagram for PSCs based on SnOx and HT-SnO2 ETLs is shown in figure 3f. Compared to HT-SnO2, SnOx showed upshifted energy band levels and thus possessed an ECBM much closer to that of the perovskite layer, enabling more efficient electron extraction and less VOC losses at the SnOx/perovskite interfaces. Moreover, the ECBM of HT-SnO2 was very close to the EVBM of the perovskite layer, which could result in severe recombination of the electron–hole pairs at the HT-SnO2/perovskite interfaces [48]. Therefore, SnOx exhibited a better energy level alignment with the perovskite layer, thereby facilitating electron extraction and reducing recombination losses at the SnOx/perovskite interfaces [49,50]. This could be the main reason why SnOx ETLs offered improved VOC and suppressed hysteresis compared with HT-SnO2 ETLs.
The film morphology of the perovskite layers deposited on SnOx and HT-SnO2 was also investigated. As shown in the SEM images (figure 3g,h), the perovskite film deposited on SnOx exhibited a larger average grain size of 293 nm than that of 242 nm for the perovskite film deposited on HT-SnO2 (electronic supplementary material, figure S6). A few gaps appeared for the perovskite film deposited on HT-SnO2. In the XRD patterns of the two perovskite films illustrated in figure 3i, three major peaks located at 14.3°, 28.6°, and 32.0° were assigned to (001), (002), and (012) crystal planes, respectively [51,52]. Compared to the HT-SnO2/perovskite film, the SnOx/perovskite film displayed increased relative intensity at both (001) and (002) peaks (electronic supplementary material, table S3), indicating enhanced crystallinity of the perovskite film with preferential growth along (001) and (002) planes [52,53]. Overall, the data showed that the SnOx/perovskite film exhibited enlarged grains, improved morphology, and enhanced crystallinity. This might be attributed to the improved morphology of the amorphous SnOx, thereby boosting the formation and crystallization of the perovskite film. The improved film quality of the perovskite layer can facilitate charge transport and reduce carrier recombination at grain boundaries, leading to enhanced device performance [54]. PL measurements were performed to investigate the charge transport kinetics at ETL/perovskite interfaces (electronic supplementary material, figure S7). The faster quenching indicated more efficient charge extraction and transport at the SnOx/perovskite interface. Furthermore, the recombination characteristics of PSCs with SnOx and HT-SnO2 ETLs were obtained by fitting their J–V curves using an ideal diode mode: (electronic supplementary material, figure S8), where Jph is the photo-induced current density, J0 is the recombination current density, e is the elementary charge, Rs is the series resistance, A is the ideality factor, K is the Boltzmann constant, and T is the thermodynamic temperature [55]. The shunt resistance (Rsh) is calculated as at . The values of these parameters are summarized in table 1. J0 and the ideality factor both decreased, indicating suppressed charge recombination in the cells with SnOx ETLs [45,55]. The reduced Rs and increased Rsh was also indicative of effective electron extraction and suppressed charge recombination at SnOx/perovskite interfaces [43]. To summarize, the enhanced charge extraction and transport and suppressed charge recombination were ascribed to the enhanced electrical and optical properties of the amorphous SnOx, improved film quality of the perovskite layer, and good energy level matching between the SnOx ETL and the perovskite layer. Consequently, PSCs with SnOx ETLs achieved improvements in all photovoltaic parameters (VOC, JSC, FF and PCE) and suppression of hysteresis compared to those with HT-SnO2 ETLs.
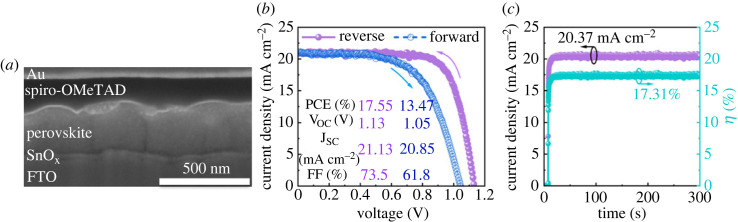
The surface wettability of the substrates is crucial to film formation in the inkjet-printing deposition process, especially in a low-temperature solution process. Specifically, we fabricated three groups of PSCs using SnOx ETLs printed on FTO substrates without surface treatment, with ultraviolet/ozone (UVO) treatment, and with pre-heating treatment. As shown in electronic supplementary material, figure S9 and table S4, the device performance was improved by applying either UVO or pre-heating treatment. Both UVO and pre-heating treatment can remove the organic residuals on the FTO substrates and improve the wettability of the FTO substrates. This enabled the uniform deposition of SnOx on the FTO substrates (electronic supplementary material, figure S9) and improved the interfacial contact between SnOx and FTO, contributing to reduced recombination and thus improved performance with suppressed hysteresis [56,57]. Therefore, pre-heating treatment is a simple alternative to UVO cleaning in terms of substrate surface processing. We further optimized the performance of SnOx-based devices by controlling the thickness of the SnOx ETL, which was realized by adjusting the concentration of the precursor inks. As shown in figure 4a and electronic supplementary material, figure S10, the average thickness of the SnOx ETL increased from 0 to 35 nm and 75 nm when the precursor concentration increased from 0 to 0.05 M and 0.1 M. Accordingly, the device performance varied with the thickness variation of the SnOx ETL (electronic supplementary material, figure S11, figure S12, and table S5). The highest PCE of 17.55%, along with a forward-scan PCE of 13.47% (figure 4b), was obtained for the cell based on a SnOx ETL with a thickness of approximately 35 nm. We also obtained a stabilized photocurrent density of 20.37 mA cm−2 and stabilized power output of 17.31% over 5 min (figure 4c). Moreover, PSCs with SnOx ETLs exhibited improved performance with suppressed hysteresis compared with those without an ETL, regardless of the thickness of the SnOx ETL. This proved that the inkjet-printed SnOx as an ETL had the capability of separating charges, i.e. transporting electrons and blocking holes [17].
Figure 4.
(a) Cross-sectional FIB/SEM image, (b) reverse-scan and forward-scan J–V curves, and (c) steady-state photocurrent density and stabilized efficiency (at a bias of 0.85 V) for the champion cell based on the inkjet-printed SnOx ETL prepared from a 0.05 M precursor ink.
In comparable reports focused on inkjet-printed SnO2 ETLs, Ghahremani et al. achieved a champion PCE of 13.08% [7], and Rohnacher et al. obtained a champion efficiency of 18.8% (12.0% in the forward scan) with a stabilized PCE of 15.2% over 5 min [6]. Our devices exhibited an improved stabilized efficiency and suppressed hysteresis compared to those in the latter report, although they did not deliver a PCE higher than 18.8%. Overall, we successfully fabricated solution-processed SnOx ETLs as effective ETLs for planar PSCs by inkjet printing, which is expected to promote large-scale fabrication of PSCs.
3.2. Inkjet-printed solution-processed Cu : SnOx electron transport layer ETL
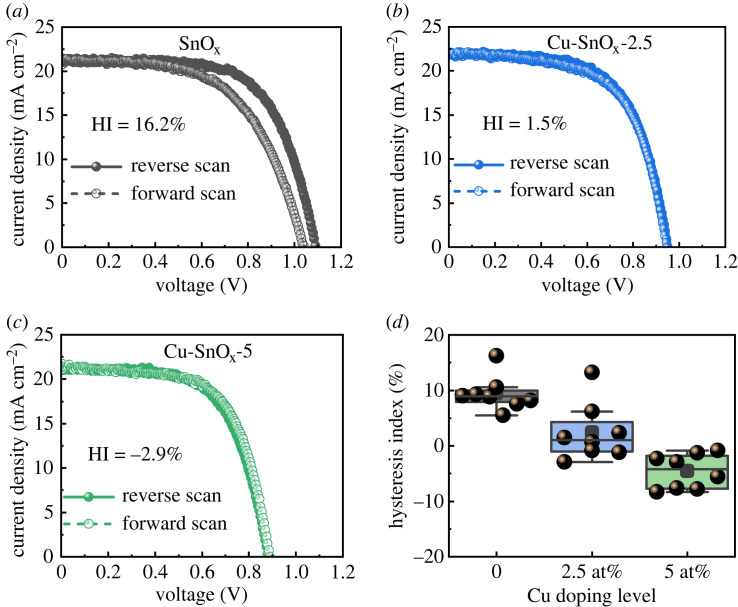
Inkjet-printed solution-processed SnOx has been established as an effective ETL for planar PSCs while there is still a motivation to further optimize this SnOx ETL. The solution process used in the present work allows for doping SnOx by adding a dopant precursor directly into the Sn precursor inks. Herein, we introduced Cu dopant into SnOx ETLs and investigated the effects of Cu doping on the device performance. Various Cu : SnOx ETLs were prepared from inks with different Cu concentrations of 2.5 at%, 5 at%, 7.5 at% and 10 at%. We then fabricated PSCs employing these Cu : SnOx ETLs and obtained J–V characteristics of the devices (figure 5 and electronic supplementary material, figure S13). VOC and FF decreased after Cu doping, while JSC first increased and then decreased with increasing Cu doping level (electronic supplementary material, figure S14 and table S6). As a result, the devices with Cu : SnOx ETLs produced lower PCEs than those with pristine SnOx ETLs. Interestingly, the hysteresis index decreased dramatically from 16.2% to 1.5% when the Cu concentration increased from 0 to 2.5 at%. Afterwards, an inverted hysteresis phenomenon appeared, i.e. the PCE for the forward scan surpassed that for the reverse scan, when the Cu doping level further increased to 5 at% and higher levels. The inverted hysteresis mainly originated from the improved FF for the forward scan compared to that for the reverse scan (electronic supplementary material, figure S14). In addition, we fabricated PSCs using 500°C-annealed Cu : SnOx ETLs. The devices exhibited reduced performance compared to those with 220°C-annealed counterparts (electronic supplementary material, figure S15). This result is consistent with that for the undoped SnOx.
Figure 5.
Reverse-scan and forward-scan J–V characteristics of PSCs based on (a) pristine SnOx, (b) 2.5 at% Cu : SnOx, and (c) 5 at% Cu : SnOx; (d) distribution of the hysteresis index for PSCs based on pristine SnOx and Cu : SnOx ETLs.
To reveal the origin of the effects of Cu doping on the device performance, various characterizations and measurements were conducted. The XRD patterns in electronic supplementary material, figure S16, show that no crystalline peaks were observed for Cu : SnOx. All the Cu : SnOx thin films exhibited dense and compact surfaces with no pinholes (electronic supplementary material, figure S17). The SEM images also imply that Cu doping did not have a significant influence on the film morphology. From figure 6a and electronic supplementary material, figure S18, the peaks of the O 1s and Sn 3d XPS spectra shifted to lower binding energies upon Cu doping, indicating a strong chemical interaction between Cu and SnOx [26]. As seen in figure 6b, the Cu 2p peaks were detected for 5 at% Cu : SnOx while the signal was very weak for 2.5 at% Cu : SnOx. The energy peak at 932.2 eV indicated the presence of Cu+ oxidation state, and the observable satellite features at 942.3 eV represented the +2 state of Cu [30]. Therefore, there were two oxidation states of Cu+ and Cu2+ in Cu : SnOx. Moreover, both Sn M5,4-edge and O K-edge XAS spectra only exhibited intensity variations in the characteristic peaks upon Cu doping (electronic supplementary material, figure S19). The XPS and XAS results suggested that SnOx was successfully doped with Cu. As shown in figure 6a, the O 1s XPS peak of SnOx can be deconvoluted into two peaks. The peak at 531.2 eV originated from lattice oxygen (OL) and the other peak at 532.6 eV was assigned to oxygen vacancies or adsorbed hydroxyl groups (OV) [58,59]. The peak ratio of OV to total oxygen increased from 24.11% to 30.92% when the Cu doping concentration increased from 0 to 2.5 at%, and then significantly increased to 52.53% when the Cu doping level increased to 5 at% (electronic supplementary material, table S7). These surface oxygen defects (oxygen vacancies or hydroxyl groups) could cause carrier recombination and poor charge transfer for PSCs [30,60,61].
Figure 6.
(a) O 1s and (b) Cu 2p XPS spectra for pristine, 2.5 at%, and 5 at% Cu-doped SnOx; (c) energy level alignment of the pristine, 2.5 at%, and 5 at% Cu-doped SnOx ETL with the perovskite layer.
The surface chemistry of ETLs can influence the formation and crystallinity of the perovskite layers [30]. For the perovskite film deposited on Cu-doped SnOx compared to that deposited on SnOx (electronic supplementary material, figure S20, and figure 3g), the average crystal grain size decreased, and a few white PbI2 spots appeared. This was confirmed by the XRD patterns (electronic supplementary material, figure S21), in which the intensity ratio of the peak at 14.3° for perovskite to the peak at 12.9° for PbI2 decreased upon Cu doping (electronic supplementary material, table S8). Thus, Cu-doped SnOx exerted a negative effect on the film quality of the perovskite layers, resulting in recombination losses and poor charge transport. This effect was further investigated by PL measurements of the perovskite layers (electronic supplementary material, figure S22). The higher PL intensity represented more severe charge recombination and worse charge transport ability at the Cu-doped SnOx/perovskite interfaces [26,31]. The Cu-SnOx-5/perovskite film exhibited a much higher PL intensity than the Cu-SnOx-2.5/perovskite film, which might be mainly attributed to the presence of a substantial number of oxygen defects on the surface of the 5 at% Cu-doped SnOx. Consequently, PSCs based on Cu-doped SnOx ETLs suffered from degradation in device performance (VOC, FF, and PCE). With a few exceptions, the devices with 2.5 at% Cu-doped SnOx offered an enhanced JSC compared to those with pristine SnOx, which was attributed to the improved conductivity (electronic supplementary material, figure S23) [25].
The optical transmittance of the Cu-doped samples decreased slightly compared to that of the pristine SnOx (electronic supplementary material, figure S24a). Then, the values of the optical band gap extracted from Tauc's plots (electronic supplementary material, figure S24b) were 3.72 eV and 3.70 eV for 2.5 at% and 5 at% Cu-doped SnOx, respectively. The reduced band gap could originate from the increased surface defect states upon Cu doping [62]. Furthermore, the energy band positions of Cu-doped SnOx were obtained from the UPS and valence band XPS measurements (electronic supplementary material, figure S25 and table S9). As shown in figure 6c, the conduction band minimum of the two Cu-doped SnOx ETLs was significantly upshifted to become shallower than that of the perovskite layer, which could form unfavourable electron extraction barriers and thus cause charge/ion accumulation at the ETL/perovskite interface [63]. The conduction band offset between Cu-doped SnOx and perovskite increased when the Cu doping level increased from 2.5 at% to 5 at%, leading to increased charge/ion accumulation at the ETL/perovskite interface [64,65]. This could account for the increase in HI (absolute value). The decrease in HI from 0 to 2.5 at% was probably due to the enhanced conductivity which might alleviate the hysteresis by balancing the electron flux and hole flux with PSCs [25,66].
The origin of normal hysteresis has been attributed to ion migration and accumulation at interfaces, which screens the electric field and leads to a decrease in VOC and FF in the forward scan [67–69]. According to a previous study [70], in the case of extraction barriers, these accumulated ionic charges also formed dipole layers with piled-up electronic charges at the ETL/perovskite interface, which enhanced the electric field and improved the FF for the forward scan, resulting in an inverted hysteresis. Therefore, the observed inverted hysteresis in our devices was related to the charge/ion accumulation induced by the energetic extraction barriers at Cu-doped SnOx/perovskite interfaces. Besides, the relatively low scan rate (125 mV s−1) used in this work also allowed the occurrence of a slow ion migration process, thus intensifying the impact of extraction barriers on charge extraction, leading to an inverted hysteresis [65,70]. Furthermore, the hysteretic behaviour transferred from normal hysteresis to inverted hysteresis when the Cu doping level increased from 2.5 at% to 5 at%, although there were energy barriers at both Cu-doped SnOx/perovskite interfaces. A similar phenomenon was reported by Rong et al., who achieved tunable hysteresis for carbon-based PSCs by adjusting the thickness of a compact TiO2 ETL [71]. They ascribed the origin of this phenomenon to the interplay between the slow dynamics of charge accumulation and changes in recombination rates. In our work, in addition to the changes in charge/ion accumulation induced by the enlarged conduction band offset, the recombination losses at the ETL/perovskite interfaces also varied, originating from variations in the quantity of oxygen defects on the Cu-doped SnOx surfaces and defects on the perovskite films with increasing Cu content. Therefore, the transformation of hysteresis from normal to inverted can be attributed to the interplay between charge/ion accumulation and recombination at the Cu-doped SnOx/perovskite interfaces. The exact origin of this phenomenon is still under investigation.
Our results regarding the effect of Cu doping were quite different from those reported in the literature [30,31], in which Cu-doped SnO2 based PSCs exhibited an improved PCE with normal hysteresis. The disparity in the device performance could originate from the differences in the morphological, structural, electronic, and optical properties between our Cu-doped SnOx and their Cu-doped SnO2. Our results imply that elemental doping does not always lead to a favourable performance improvement. The selection of the doping element and the concentration of the selected dopant are of great importance in determining the PSC device performance. This work demonstrates a method for tuning the hysteresis of SnOx-based PSCs through Cu doping. An acceptable PCE of 13.15% with a low hysteresis of 1.5% was obtained for the device with a 2.5 at% Cu-doped SnOx ETL. However, further investigations including stability measurements are required to verify the advantages of this low hysteresis.
4. Conclusion
In summary, we have successfully fabricated solution-processed inkjet-printed SnOx as effective ETLs for planar PSCs. The effect of the annealing temperature of the SnOx ETL on the device performance was studied. The low-temperature annealed amorphous SnOx ETLs outperformed their high-temperature annealed crystalline counterparts. The improved device performance originated from enhanced charge extraction and transport and suppressed charge recombination at ETL/perovskite interfaces, which was attributed to the enhanced electrical and optical properties of the amorphous SnOx, improved film quality of the perovskite layer, and better energy level alignment between the SnOx ETL and perovskite layer. Through further optimization of the substrate surface treatment and the film thickness of the SnOx ETL, a champion efficiency of 17.55% was achieved. Thereafter, Cu was incorporated into SnOx. Upon Cu doping, SnOx showed increased surface defects and upshifted energy levels, and the perovskite film also exhibited increased defects, resulting in the reduction in the device performance. Moreover, the devices with Cu-doped SnOx ETLs exhibited tunable hysteresis, which was suppressed and then transformed into an inverted hysteresis with increasing Cu doping concentration. The reduced hysteresis for the 2.5 at% Cu-doped SnOx ETLs was probably due to the enhanced conductivity. The inverted hysteresis was related to the energetic extraction barriers at Cu-doped SnOx/perovskite interfaces. Therefore, the transformation of hysteresis from normal to inverted can be attributed to the interplay between charge/ion accumulation and recombination at Cu-doped SnOx/perovskite interfaces in the case of extraction barriers. This work provides an approach to fabricate ETLs in a sustainable and scalable manner and a platform for the fabrication of fully inkjet-printed PSCs in future work.
Acknowledgments
Prof. James M. Gardner and Dr Mahboubeh Jamshidi are sincerely acknowledged for their help with some of the characterizations.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material [72].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
D.L.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, visualization, writing—original draft, writing—review and editing; F.Y.: investigation; C.D.: investigation, writing—review and editing; J.G.: resources, supervision, writing—review and editing; J.J.U.: resources; L.B.: conceptualization, funding acquisition, investigation, methodology, project administration, resources, supervision, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This research was funded by China Scholarship Council and Jernkontoret (Stiftelsen Jernkontorsfonden för Bergsvetenskaplig Forskning). This research used resources of the Advanced Light Source, which is a DOE Office of Science User Facility under Contract no. DE-AC02-05CH11231. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract no. DE-AC02-05CH11231.
References
- 1.National Renewable Energy Laboratory. 2023. Best research-cell efficiencies. See https://www.nrel.gov/pv/interactive-cell-efficiency.html (accessed 29 June 2023).
- 2.Hopkinson N, Smith PJ. 2017. Industrial 3D inkjet printing/additive manufacturing. In Handbook of industrial inkjet printing (ed. Zapka W), pp. 649-660. Weinheim, Germany: Wiley-VCH. [Google Scholar]
- 3.Schackmar F, Eggers H, Frericks M, Richards BS, Lemmer U, Hernandez-Sosa G, Paetzold UW. 2021. Perovskite solar cells with all-inkjet-printed absorber and charge transport layers. Adv. Mater. Technol. 6, 2000271. ( 10.1002/admt.202000271) [DOI] [Google Scholar]
- 4.Eggers H, Schackmar F, Abzieher T, Sun Q, Lemmer U, Vaynzof Y, Richards BS, Hernandez-Sosa G, Paetzold UW. 2020. Inkjet-printed micrometer-thick perovskite solar cells with large columnar grains. Adv. Energy Mater. 10, 1903184. ( 10.1002/aenm.201903184) [DOI] [Google Scholar]
- 5.Gheno A, Huang Y, Bouclé J, Ratier B, Rolland A, Even J, Vedraine S. 2018. Toward highly efficient inkjet-printed perovskite solar cells fully processed under ambient conditions and at low temperature. Sol. RRL 2, 1800191. ( 10.1002/solr.201800191) [DOI] [Google Scholar]
- 6.Rohnacher V, et al. 2021. Analytical study of solution-processed tin oxide as electron transport layer in printed perovskite solar cells. Adv. Mater. Technol. 6, 2000282. ( 10.1002/admt.202000282) [DOI] [Google Scholar]
- 7.Ghahremani AH, Ratnayake D, Sherehiy A, Popa DO, Druffel T. 2021. Automated fabrication of perovskite photovoltaics using inkjet printing and intense pulse light annealing. Energy Technol. 9, 2100452. ( 10.1002/ente.202100452) [DOI] [Google Scholar]
- 8.Lu D, Zhang W, Kloo L, Belova L. 2021. Inkjet-printed electron transport layers for perovskite solar cells. Materials 14, 7525. ( 10.3390/ma14247525) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang T, He Q, Yu J, Chen A, Zhang Z, Pan J. 2022. Recent progress in improving strategies of inorganic electron transport layers for perovskite solar cells. Nano Energy 104, 107918. ( 10.1016/j.nanoen.2022.107918) [DOI] [Google Scholar]
- 10.Lu H, Tian W, Gu B, Zhu Y, Li L. 2017. TiO2 electron transport bilayer for highly efficient planar perovskite solar cell. Small 13, 1701535. ( 10.1002/smll.201701535) [DOI] [PubMed] [Google Scholar]
- 11.Zhou J, et al. 2022. SnO2 quantum dot-modified mesoporous TiO2 electron transport layer for efficient and stable perovskite solar cells. ACS Appl. Energy Mater. 5, 3052-3063. ( 10.1021/acsaem.1c03681) [DOI] [Google Scholar]
- 12.Jiang Q, et al. 2016. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells. Nat. Energy 2, 16177. ( 10.1038/nenergy.2016.177) [DOI] [Google Scholar]
- 13.Deng K, Chen Q, Li L. 2020. Modification engineering in SnO2 electron transport layer toward perovskite solar cells: efficiency and stability. Adv. Funct. Mater. 30, 2004209. ( 10.1002/adfm.202004209) [DOI] [Google Scholar]
- 14.Ke W, et al. 2015. Low-temperature solution-processed tin oxide as an alternative electron transporting layer for efficient perovskite solar cells. J. Am. Chem. Soc. 137, 6730-6733. ( 10.1021/jacs.5b01994) [DOI] [PubMed] [Google Scholar]
- 15.Blackburn D, Routledge T, O'kane M, Cassella EJ, Game OS, Catley TE, Wood CJ, Mcardle T, Lidzey DG. 2022. Low-temperature, scalable, reactive deposition of tin oxide for perovskite solar cells. Sol. RRL 6, 2200263. ( 10.1002/solr.202200263) [DOI] [Google Scholar]
- 16.Cheng N, Yu Z, Li W, Lei B, Zi W, Xiao Z, Zhao Z, Zong P-A. 2022. Low temperature processed SnO2 electron transporting layer from tin oxalate for perovskite solar cells. ACS Appl. Energy Mater. 5, 15 385-15 391. ( 10.1021/acsaem.2c03002) [DOI] [Google Scholar]
- 17.Jung K-H, Seo J-Y, Lee S, Shin H, Park N-G. 2017. Solution-processed SnO2 thin film for a hysteresis-free planar perovskite solar cell with a power conversion efficiency of 19.2%. J. Mater. Chem. A 5, 24 790-24 803. ( 10.1039/C7TA08040A) [DOI] [Google Scholar]
- 18.Ke W, Zhao D, Cimaroli AJ, Grice CR, Qin P, Liu Q, Xiong L, Yan Y, Fang G. 2015. Effects of annealing temperature of tin oxide electron selective layers on the performance of perovskite solar cells. J. Mater. Chem. A 3, 24 163-24 168. ( 10.1039/C5TA06574G) [DOI] [Google Scholar]
- 19.Zhong JX, et al. 2022. Room temperature fabrication of SnO2 electrodes enabling barrier-free electron extraction for efficient flexible perovskite photovoltaics. Adv. Funct. Mater. 32, 2200817. ( 10.1002/adfm.202200817) [DOI] [Google Scholar]
- 20.Xing Y, et al. 2022. LiF-modified SnO2 electron transport layer improves the performance of carbon-based all-inorganic CsPbIBr2 perovskite solar cells. Energy Fuels 36, 13 179-13 186. ( 10.1021/acs.energyfuels.2c02797) [DOI] [Google Scholar]
- 21.Wang L, et al. 2022. Robust interfacial modifier for efficient perovskite solar cells: reconstruction of energy alignment at buried interface by self-diffusion of dopants. Adv. Funct. Mater. 32, 2204725. ( 10.1002/adfm.202204725) [DOI] [Google Scholar]
- 22.Wang J, Wang Z, Chen S, Jiang N, Yuan L, Zhang J, Duan Y. 2022. Reduced surface hydroxyl and released interfacial strain by inserting CsF anchor interlayer for high-performance perovskite solar cells. Sol. RRL 7, 2200960. ( 10.1002/solr.202200960) [DOI] [Google Scholar]
- 23.Liu C, Guo M, Su H, Zhai P, Xie K, Liu Z, Zhang J, Liu L, Fu H. 2022. Highly improved efficiency and stability of planar perovskite solar cells via bifunctional phytic acid dipotassium anchored SnO2 electron transport layer. Appl. Surf. Sci. 588, 152943. ( 10.1016/j.apsusc.2022.152943) [DOI] [Google Scholar]
- 24.Song J, Zhang W, Wang D, Deng K, Wu J, Lan Z. 2019. Colloidal synthesis of Y-doped SnO2 nanocrystals for efficient and slight hysteresis planar perovskite solar cells. Sol. Energy 185, 508-515. ( 10.1016/j.solener.2019.04.084) [DOI] [Google Scholar]
- 25.Park M, Kim J-Y, Son HJ, Lee C-H, Jang SS, Ko MJ. 2016. Low-temperature solution-processed Li-doped SnO2 as an effective electron transporting layer for high-performance flexible and wearable perovskite solar cells. Nano Energy 26, 208-215. ( 10.1016/j.nanoen.2016.04.060) [DOI] [Google Scholar]
- 26.Wang R, Wu J, Wei S, Zhu J, Guo M, Zheng Q, Wei M, Cheng S. 2022. Gadolinium-doped SnO2 electron transfer layer for highly efficient planar perovskite solar cells. J. Power Sources 544, 231870. ( 10.1016/j.jpowsour.2022.231870) [DOI] [Google Scholar]
- 27.Halvani Anaraki E, et al. 2018. Low-temperature Nb-doped SnO2 electron-selective contact yields over 20% efficiency in planar perovskite solar cells. ACS Energy Lett. 3, 773-778. ( 10.1021/acsenergylett.8b00055) [DOI] [Google Scholar]
- 28.Ye H, Liu Z, Liu X, Sun B, Tan X, Tu Y, Shi T, Tang Z, Liao G. 2019. 17.78% efficient low-temperature carbon-based planar perovskite solar cells using Zn-doped SnO2 electron transport layer. Appl. Surf. Sci. 478, 417-425. ( 10.1016/j.apsusc.2019.01.237) [DOI] [Google Scholar]
- 29.Kim J, Murdoch BJ, Partridge JG, Xing K, Qi D-C, Lipton-Duffin J, Mcconville CF, Van Embden J, Gaspera ED. 2020. Ultrasonic spray pyrolysis of antimony-doped tin oxide transparent conductive coatings. Adv. Mater. Interfaces 7, 2000655. ( 10.1002/admi.202000655) [DOI] [Google Scholar]
- 30.Zhou X, et al. 2022. Solution-processed Cu-doped SnO2 as an effective electron transporting layer for high-performance planar perovskite solar cells. Appl. Surf. Sci. 584, 152651. ( 10.1016/j.apsusc.2022.152651) [DOI] [Google Scholar]
- 31.Bahadur J, Ghahremani AH, Martin B, Pishgar S, Sunkara MK, Druffel T, Pal K. 2022. Solution-processed Cu:SnO2 as an efficient electron transport layer for fabrication of low-temperature planar perovskite solar cell under ambient conditions. IEEE J. Photovoltaics 12, 1162-1169. ( 10.1109/JPHOTOV.2022.3162340) [DOI] [Google Scholar]
- 32.Fang M. 2012. Properties of multifunctional oxide thin films deposited by ink-jet printing. Doctoral thesis, KTH Royal Institute of Technology, Stockholm, Sweden. [Google Scholar]
- 33.Chen C, et al. 2017. Cu(II) complexes as p-type dopants in efficient perovskite solar cells. ACS Energy Lett. 2, 497-503. ( 10.1021/acsenergylett.6b00691) [DOI] [Google Scholar]
- 34.Bae J-Y, Park J, Kim HY, Kim H-S, Park J-S. 2015. Facile route to the controlled synthesis of tetragonal and orthorhombic SnO2 films by mist chemical vapor deposition. ACS Appl. Mater. Interfaces 7, 12 074-12 079. ( 10.1021/acsami.5b02251) [DOI] [PubMed] [Google Scholar]
- 35.Minohara M, Kikuchi N, Yoshida Y, Kumigashira H, Aiura Y. 2019. Improvement of the hole mobility of SnO epitaxial films grown by pulsed laser deposition. J. Mater. Chem. C 7, 6332-6336. ( 10.1039/C9TC01297D) [DOI] [Google Scholar]
- 36.Wang D, Yang J, Li X, Wang J, Li R, Cai M, Sham TK, Sun X. 2012. Observation of surface/defect states of SnO2 nanowires on different substrates from X-ray excited optical luminescence. Cryst. Growth Des. 12, 397-402. ( 10.1021/cg2011919) [DOI] [Google Scholar]
- 37.Haeberle J, Machulik S, Janowitz C, Manzke R, Gaspar D, Barquinha P, Schmeißer D. 2016. Gap states in the electronic structure of SnO2 single crystals and amorphous SnOx thin films. J. Appl. Phys. 120, 105101. ( 10.1063/1.4962313) [DOI] [Google Scholar]
- 38.Jaiswal MK, Kumar R, Kanjilal D, Dong CL, Chen CL, Asokan K, Ojha S. 2015. Studies of dense electronic excitation-induced modification in crystalline Fe-doped SnO2 thin films. Appl. Surf. Sci. 332, 726-735. ( 10.1016/j.apsusc.2014.12.182) [DOI] [Google Scholar]
- 39.Kucheyev SO, Baumann TF, Sterne PA, Wang YM, Van Buuren T, Hamza AV, Terminello LJ, Willey TM. 2005. Surface electronic states in three-dimensional SnO2 nanostructures. Phys. Rev. B 72, 035404. ( 10.1103/PhysRevB.72.035404) [DOI] [Google Scholar]
- 40.Baumann TF, Kucheyev SO, Gash AE, Satcher JH Jr. 2005. Facile synthesis of a crystalline, high-surface-area SnO2 aerogel. Adv. Mater. 17, 1546-1548. ( 10.1002/adma.200500074) [DOI] [Google Scholar]
- 41.Sharma V, Vyas R, Bazylewski P, Chang GS, Asokan K, Sachdev K. 2016. Probing the highly transparent and conducting SnOx/Au/SnOx structure for futuristic TCO applications. RSC Adv. 6, 29 135-29 141. ( 10.1039/C5RA24422F) [DOI] [Google Scholar]
- 42.Nesov SN, Bolotov VV, Korusenko PM, Povoroznyuk SN, Vilkov OY. 2016. Interfacial interaction in a composite based on multi-walled carbon nanotubes and amorphous tin oxide. Phys. Solid State 58, 997-1003. ( 10.1134/S1063783416050164) [DOI] [Google Scholar]
- 43.Zhang X, et al. 2022. Amorphous TiO2 film with fiber like structure: an ideal candidate for ETL of perovskite solar cells. Mater. Lett. 324, 132684. ( 10.1016/j.matlet.2022.132684) [DOI] [Google Scholar]
- 44.Zhang L, et al. 2023. Amorphous F-doped TiOx caulked SnO2 electron transport layer for flexible perovskite solar cells with efficiency exceeding 22.5%. Adv. Funct. Mater. 33, 2213961. ( 10.1002/adfm.202213961) [DOI] [Google Scholar]
- 45.Yang G, et al. 2017. Reducing hysteresis and enhancing performance of perovskite solar cells using low-temperature processed Y-doped SnO2 nanosheets as electron selective layers. Small 13, 1601769. ( 10.1002/smll.201601769) [DOI] [PubMed] [Google Scholar]
- 46.Tauc J, Menth A. 1972. States in the gap. J. Non-Cryst. Solids 8, 569-585. ( 10.1016/0022-3093(72)90194-9) [DOI] [Google Scholar]
- 47.Song S, Kang G, Pyeon L, Lim C, Lee G-Y, Park T, Choi J. 2017. Systematically optimized bilayered electron transport layer for highly efficient planar perovskite solar cells (η = 21.1%). ACS Energy Lett. 2, 2667-2673. ( 10.1021/acsenergylett.7b00888) [DOI] [Google Scholar]
- 48.Huang X, Hu Z, Xu J, Wang P, Wang L, Zhang J, Zhu Y. 2017. Low-temperature processed SnO2 compact layer by incorporating TiO2 layer toward efficient planar heterojunction perovskite solar cells. Sol. Energy Mater. Sol. Cells 164, 87-92. ( 10.1016/j.solmat.2017.02.010) [DOI] [Google Scholar]
- 49.Noh YW, Lee JH, Jin IS, Park SH, Jung JW. 2019. Tailored electronic properties of Zr-doped SnO2 nanoparticles for efficient planar perovskite solar cells with marginal hysteresis. Nano Energy 65, 104014. ( 10.1016/j.nanoen.2019.104014) [DOI] [Google Scholar]
- 50.Wang P, et al. 2021. Cobalt chloride hexahydrate assisted in reducing energy loss in perovskite solar cells with record open-circuit voltage of 1.20 V. ACS Energy Lett. 6, 2121-2128. ( 10.1021/acsenergylett.1c00443) [DOI] [Google Scholar]
- 51.Jeon NJ, Noh JH, Yang WS, Kim YC, Ryu S, Seo J, Seok SI. 2015. Compositional engineering of perovskite materials for high-performance solar cells. Nature 517, 476-480. ( 10.1038/nature14133) [DOI] [PubMed] [Google Scholar]
- 52.Xu Z, et al. 2019. A thermodynamically favored crystal orientation in mixed formamidinium/methylammonium perovskite for efficient solar cells. Adv. Mater. 31, 1900390. ( 10.1002/adma.201900390) [DOI] [PubMed] [Google Scholar]
- 53.Alexander A, Kamalon VP, Dev VV, Raees AM, Reghunathan S, Nair PR, Namboothiry MAG. 2023. Enhancing the efficiency and stability of perovskite solar cells through defect passivation and controlled crystal growth using allantoin. ACS Appl. Mater. Interfaces 15, 58 406-58 415. ( 10.1021/acsami.3c13591) [DOI] [PubMed] [Google Scholar]
- 54.Song Z, Xu W, Wu Y, Liu S, Bi W, Chen X, Song H. 2020. Incorporating of lanthanide ions into perovskite film for efficient and stable perovskite solar cells. Small 16, 2001770. ( 10.1002/smll.202001770) [DOI] [PubMed] [Google Scholar]
- 55.Dong J, Shi J, Li D, Luo Y, Meng Q. 2015. Controlling the conduction band offset for highly efficient ZnO nanorods based perovskite solar cell. Appl. Phys. Lett. 107, 073507. ( 10.1063/1.4929435) [DOI] [Google Scholar]
- 56.Ke W, et al. 2015. Efficient hole-blocking layer-free planar halide perovskite thin-film solar cells. Nat. Commun. 6, 6700. ( 10.1038/ncomms7700) [DOI] [PubMed] [Google Scholar]
- 57.Méndez PF, Muhammed SKM, Barea EM, Masi S, Mora-Seró I. 2019. Analysis of the UV–ozone-treated SnO2 electron transporting layer in planar perovskite solar cells for hHigh performance and reduced hysteresis. Sol. RRL 3, 1900191. ( 10.1002/solr.201900191) [DOI] [Google Scholar]
- 58.Yi Z, Xiao B, Li X, Luo Y, Jiang Q, Yang J. 2023. Novel dual-modification strategy using Ce-containing compounds toward high-performance flexible perovskite solar cells. Nano Energy 109, 108241. ( 10.1016/j.nanoen.2023.108241) [DOI] [Google Scholar]
- 59.Gao F, et al. 2023. Porous organic cage induced spontaneous restructuring of buried interface toward high-performance perovskite photovoltaic. Adv. Funct. Mater. 33, 2211900. ( 10.1002/adfm.202211900) [DOI] [Google Scholar]
- 60.Mohamad Noh MF, Arzaee NA, Safaei J, Mohamed NA, Kim HP, Mohd Yusoff AR, Jang J, Mat Teridi MA. 2019. Eliminating oxygen vacancies in SnO2 films via aerosol-assisted chemical vapour deposition for perovskite solar cells and photoelectrochemical cells. J. Alloys Compd. 773, 997-1008. ( 10.1016/j.jallcom.2018.09.273) [DOI] [Google Scholar]
- 61.Bai G, et al. 2019. High performance perovskite sub-module with sputtered SnO2 electron transport layer. Sol. Energy 183, 306-314. ( 10.1016/j.solener.2019.03.026) [DOI] [Google Scholar]
- 62.Babu B, Kadam AN, Ravikumar RVSSN, Byon C. 2017. Enhanced visible light photocatalytic activity of Cu-doped SnO2 quantum dots by solution combustion synthesis. J. Alloys Compd. 703, 330-336. ( 10.1016/j.jallcom.2017.01.311) [DOI] [Google Scholar]
- 63.Correa Baena JP, et al. 2015. Highly efficient planar perovskite solar cells through band alignment engineering. Energy Environ. Sci. 8, 2928-2934. ( 10.1039/C5EE02608C) [DOI] [Google Scholar]
- 64.García-Rosell M, Bou A, Jiménez-Tejada JA, Bisquert J, Lopez-Varo P. 2018. Analysis of the influence of selective contact heterojunctions on the performance of perovskite solar cells. J. Phys. Chem. C 122, 13 920-13 925. ( 10.1021/acs.jpcc.8b01070) [DOI] [Google Scholar]
- 65.Pham ND, et al. 2019. Tailoring crystal structure of FA0.83Cs0.17PbI3 perovskite through guanidinium doping for enhanced performance and tunable hysteresis of planar perovskite solar cells. Adv. Funct. Mater. 29, 1806479. ( 10.1002/adfm.201806479) [DOI] [Google Scholar]
- 66.Heo JH, Han HJ, Kim D, Ahn TK, Im SH. 2015. Hysteresis-less inverted CH3NH3PbI3 planar perovskite hybrid solar cells with 18.1% power conversion efficiency. Energy Environ. Sci. 8, 1602-1608. ( 10.1039/C5EE00120J) [DOI] [Google Scholar]
- 67.Tress W, Marinova N, Moehl T, Zakeeruddin SM, Nazeeruddin MK, Grätzel M. 2015. Understanding the rate-dependent J–V hysteresis, slow time component, and aging in CH3NH3PbI3 perovskite solar cells: the role of a compensated electric field. Energy Environ. Sci. 8, 995-1004. ( 10.1039/C4EE03664F) [DOI] [Google Scholar]
- 68.Chen B, et al. 2015. Impact of capacitive effect and ion migration on the hysteretic behavior of perovskite solar cells. J. Phys. Chem. Lett. 6, 4693-4700. ( 10.1021/acs.jpclett.5b02229) [DOI] [PubMed] [Google Scholar]
- 69.Li C, Tscheuschner S, Paulus F, Hopkinson PE, Kießling J, Köhler A, Vaynzof Y, Huettner S. 2016. Iodine migration and its effect on hysteresis in perovskite solar cells. Adv. Mater. 28, 2446-2454. ( 10.1002/adma.201503832) [DOI] [PubMed] [Google Scholar]
- 70.Tress W, Correa Baena JP, Saliba M, Abate A, Graetzel M. 2016. Inverted current–voltage hysteresis in mixed perovskite solar cells: polarization, energy barriers, and defect recombination. Adv. Energy Mater. 6, 1600396. ( 10.1002/aenm.201600396) [DOI] [Google Scholar]
- 71.Rong Y, et al. 2017. Tunable hysteresis effect for perovskite solar cells. Energy Environ. Sci. 10, 2383-2391. ( 10.1039/C7EE02048A) [DOI] [Google Scholar]
- 72.Lu D, Yang F, Dun C, Guo J, Urban JJ, Belova L. 2024. Inkjet-printed SnOx as an effective electron transport layer for planar perovskite solar cells and the effect of Cu doping. Figshare. ( 10.6084/m9.figshare.c.7074851) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lu D, Yang F, Dun C, Guo J, Urban JJ, Belova L. 2024. Inkjet-printed SnOx as an effective electron transport layer for planar perovskite solar cells and the effect of Cu doping. Figshare. ( 10.6084/m9.figshare.c.7074851) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material [72].