Highlights
-
•
Fragrant rapeseed oils were prepared by enzymatic methods to produce flavor substances.
-
•
The nutty/toasted flavor correlated mainly with 2,6-dimethylpyrazine, 2,5-dimethyl-pyrazine, 2-methyl-pyrazine, and trimethylpyrazine,
-
•
The correlation between flavor and quality indicators was analyzed.
Keywords: Fragrant rapeseed oil, Maillard reaction, Amino acids, Pyrazines
Abstract
Enzymatically prepared aromatic oils commonly have high purity and aroma quality. However, amino acid type and content vary greatly according to the type of oil, which impacts overall aroma and quality. In this study, the effects of lysine (Lys), arginine (Arg), proline (Pro), and glutamic (Glu) acid on physicochemical indices, nutrients, hazardous substances, fatty acid composition, and flavor during fragrant rapeseed oil (FRO) enzymatic preparation were investigated using the Maillard reaction (MR). In the lysine-treated group, the unsaturated fatty acids (93.16 %), α-tocopherol (183.06 mg/kg), γ-tocopherol (404.37 mg/kg), and δ-tocopherol (12.69 mg/kg) contents were the highest, whereas the acid value (1.27 mg/g) and moisture (0.10 %) and benzo[a]pyrene (1.45 μg/kg) contents were the lowest. Sensory evaluation showed that lysine effectively enhanced FRO flavor by enhancing the nutty/toasted flavor (4.80 scores). Principle component analysis (PCA) showed that the nutty/toasted flavor correlated mainly with 2,6-dimethylpyrazine, 2,5-dimethyl-pyrazine, 2-methyl-pyrazine, and trimethylpyrazine, nutty/toasted flavor strength increased with pyrazine content, which were the highest in the lysine group (24.02 μg/g). This study provides a guide for FRO preparation by adding external MR prerequisites.
1. Introduction
Rapeseed, palm, and soybean oils are the top three most popular edible oils among global consumers, owing to their high nutritional value and distinct flavor(Y. F. Zhang et al., 2020). Rapeseed oil contains a well-balanced ratio of unsaturated fatty acids (USFA), including oleic, linoleic, and linolenic acids, resulting in high oxidative stability and beneficial nutritional properties(Wu et al., 2019). Furthermore, the contents of phenolic compounds, tocopherol, and phytosterol in rapeseed oil offer additional health benefits(Chew, 2020). With socioeconomic development, the type of oil chosen by consumers is based on health and nutrition, in addition to flavor. Consequently, fragrant rapeseed oils (FROs) with a high nutritional value and strong flavor have a high market demand.
Generally, flavor rapeseed oils are extracted by roasting and pressing the plant seeds at high temperatures. Several complex chemical reactions occur during high-temperature roasting, involving lipids, proteins, sugars, and other components, including volatile flavor components as secondary specific components in oils and fats. Among these, the Maillard reaction (MR) of amino acids and sugars is the key factor, resulting in the unique aroma(Y. F. Zhang et al., 2021) of rapeseed oil. However, high-temperature roasting can easily produce harmful substances, such as benzo[a]pyrene (BaP)(Gao et al., 2022)etc. FROs have been prepared using enzymatic methods, as the MR between amino acids and reducing sugars during thermal processing after enzymatic hydrolysis produces substances(Yuan, Tu, Gao, Hu, & He, 2020)involved in dictating oil flavor. Moreover, oil flavor intensity is higher and BaP content is lower, providing additional human health benefits(Shibao & Bastos, 2011). However, enzymatic preparation of concentrated FROs has many problems, such as enzyme species selection, controlled reaction conditions, and limitations in the level of production technology, which restrict the development of concentrated FRO development.
Most foods contain sugars, amino acids, and other precursors; thus, MR can easily occur during food processing and storage. MR pathways and results are influenced by a variety of factors, including reactant characteristics (type and concentration of sugar/amino acid residues), pH, heating temperature, water activity, and time (Kathuria, Hamid, Gautam, & Thakur, 2023). The type of reducing sugar and amino acid is the main factor influencing the formation of various flavors. Previous studies have focused on the formation mechanism and flavor presentation of different classes of reducing sugars and amino acids in the MR. For example, Ma concluded that the MR between histidine and glucose generates numerous pyrazine flavoring substances that contribute significantly to the flavor of sesame oil(Ma et al., 2022). Zou(Zou, Gao, He, & Yang, 2018)investigated the effect of bioproducts from the MR between reducing sugars and primary amino acids in the volatile composition of wheat germ oil during roasting. The MR rates of common reducing sugars are glucose > fructose > sucrose, among which glucose is commonly used as a raw material for MR due to its high reactivity, easy availability, and low cost. The types of amino acids differ according to oil type: oleic peanut kernels contain mainly glutamic acid, aspartic acid, arginine, leucine, and phenylalanine(Latif, Pfannstiel, Makkar, & Becker, 2013), whereas sesame seeds contain serine, cystine, arginine, and lysine(Liu, Han, Wang, Zheng, & Wang, 2020). However, comprehensive studies on the types of amino acids for volatiles in rapeseed oil are limited.
Recent research on FROs has primarily focused on process optimization(Ye & Liu, 2023) and volatile matter analysis(Jing et al., 2020, Yu et al., 2021, Yu et al., 2021); however, the effect of amino acids in MR on flavor remains unclear. In this study, the changes in the amino acid content of rapeseed meal before and after roasting were determined, and amino acids and glucose with large variations in content were selected for FRO preparation. A preliminary evaluation to discuss the possible mechanisms by which different amino acid types affect the quality and flavor of FROs was carried out through physicochemical indices, fatty acid composition, and volatile flavor compounds.
2. Materials and methods
2.1. Materials
The rapeseed meal and refined rapeseed oil were provided by COFCO Co., Ltd. Corporation Nutrition and Health Research Institute. The papain (20000 U/g) and flavor protease (15000 U/g) were purchased from Shanghai Yuanye Biotechnology Co., Ltd. Lysine (Lys), Arginine (Arg), Proline (Pro), and Glutamic (Glu) acid were purchased from Soleibao Biotechnology Co., Ltd. Commercially available group of fragrant rapeseed oil purchased from supermarkets. The standards of 1,2-pichlorobenzene, amino acid mixed standard, sterol mixed standard, 37 fatty acid methyl esters were purchased from Sigma-Aldrich Co. Ltd. (USA). The standards of α-tocopherol, γ-tocopherol, δ-tocopherol were purchased from Anpel Experimental Technology Co., Ltd. (Shanghai, China). All other reagents and solvents were purchased from China National Pharmaceutical Group Co., Ltd. (Hubei, China).
2.2. Preparation of fragrant rapeseed oil
Oil samples were prepared according to the method described by Yuan(Yuan et al., 2020) with suitable modifications. Rapeseed meal (30 g) was crushed, sieved, roasted at 135 ℃, cooled to room temperature, and mixed with distilled water (90 mL). The resulting mixture was divided into four equal parts; 0.5 g of Lys, Arg, Pro, or Glu was added to form four amino acid groups and stirred. Subsequently, the pH was adjusted (Lys and Arg pH to 9; Glu pH to 4; and Pro pH to 7), and 2.5 % of flavor protease and papain (3:1 m/m) were added to the solution. The mixture was shaken in a water bath at 200–900 r/min and 50 ℃, and subsequently centrifuged. The supernatant was obtained by enzymatic hydrolysis, processed (15 g), and 75 g of refined rapeseed oil was added. The resulting mixture was heated, stirred, centrifuged, and filtered to obtain FRO.
2.3. Determination of amino acids in rapeseed meal
Amino acids were determined according to the official methods of analysis of the Association of Official Agricultural Chemists (AOAC). Rapeseed meal (0.1 g) was accurately weighed and placed in the ampoule. First, 10.0 mL and 6.0 mol/L HCl were added to a bottle containing the ampoule and rapeseed meal solution. After filling with nitrogen, the bottle was sealed with an alcohol blowtorch and placed in a drying oven at 110.0 ± 2 ℃ for 24 h. Subsequently, the solution was cooled, filtered, and transferred to a 25 mL volumetric flask. The volume was set to scale, shaken well, and strained; 1.0 mL was extracted into a special tube of concentrator, placed in a sample vacuum concentrator, and vacuumed at 45.0 ℃. After drying, the sample was added to 2.0 mL of 0.02 mol/L HCl, mixed, and filtered. Determination was performed on an automatic amino acid analyzer with a column (50 mm × 4.6 mm × 7 μm). During the analysis process, the eluent and reaction flow rates were 0.45 mL/min and 0.25 mL/min, and the column and reaction temperatures were 57.0 ∼ 74.0 ℃ and 130.0 ℃, respectively. The detection wavelengths were 570 nm and 440 nm. The results were calculated by peak area and quantified by an external standard method.
2.4. Basic physicochemical indicators
The acid value (AV), tocopherol, iodine value (IV), peroxide value (POV), moisture content, sterol and fatty acid was determined according to the Cd 3d–63, Ce 8–89, Ch 2a–94, Ba 2a-38,Cd 1–25, Ce 12–16, Ce 1–62 official recommended by AOCS. Method for determination of BaP concerning BS EN 16619:2015.
2.5. Phenols content
Polyphenol content was analyzed by the Folin-Ciocalteu reagent method described by Romano et al(Romano et al., 2022). A polar extract of flavored rapeseed oil (5 mL) was obtained and mixed with 0.5 mL Folin-Ciocalteu reagent; the solution was kept in the dark for 3 min. Subsequently, 1 mL of 10 % sodium carbonate was added and the mixture was allowed to react in the dark for 2 h; absorbance at 765 nm was measured. The quantitative results were calculated using the analytical curve of gallic acid and expressed as mg/kg gallic acid of oil sample.
2.6. Determination of flavor compounds
The assay of flavor compounds was performed as previously described(Pang, Cao, Kang, Jiang, & Cao, 2021) and optimized slightly. A 5.0 g FRO sample was added into a 20 mL headspace bottle sealed with A silicone padded lid. Then, 7.0 μL, 0.45 mg/mL of 2-octanolwas added to the sample as internal standard. The aging extraction head was inserted and preheated at a constant temperature of 50℃ for 30 min. Then the divinylbenzene/carboxymethyl/ polydimethylsiloxane (DVB/CAR/PDMS, 50.0 μm × 30.0 μm × 1.0 cm) extraction tip was inserted into the extraction flask for 30 min at 50℃ and kept 1.0 cm above the sample to collect volatiles. After adsorption for 30 min, the extraction head was inserted into the GC inlet, where thermal desorption at 250℃ was allowed to proceed for 3 min before data collection.
Gas chromatography–mass spectrometry (GC–MS) (7890A-5975C, Agilent Technologies) equipped with a HP-5MS capillary column (30.0 m × 0.25 mm × 0.25 μm) was employed to analyze the samples. Analyses were carried out in splitless mode with helium as the carrier gas at a flow rate of 1 mL/min. The oven temperature was initially held at 40 ℃ for 3 min, increased to 120 ℃ at a rate of 4 ℃/min, and then increased to 240 ℃ at a rate of 6 ℃/min, held at 240 ℃ for 9 min. Ionization was performed in electron impact mode at 70 eV. Chromatograms were collected in full scan mode with a mass scan range of m/z 30–550.
Qualitative identification of compounds was assigned by retention indices, and their recorded mass spectra were matched to the MS NIST14 library (NIST18, version 2.2, National Institute of Standards and Technology, Gaithersburg, MD, USA). The volatile compounds were tentatively identified using the GC–MS spectra. Compounds with ≤ 85 % similarity to the NIST library were not considered. The concentration of major volatile compounds was calculated based on the internal standard method (2-octanol).
2.7. Sensory analysis
A sensory evaluation panel was composed of trained personnel, who have been engaged in the evaluation of oil flavor for a long time. The panel consists of 4 women and 6 men from China, which aged between 20 and 30 years old. The ethical approval is not required by national laws, and all panelist provided written informed consent. The oil samples were placed in 20 mL cups, labelled at random, and heated to 40 ℃ to volatilize the odors. All panelists gave an assessment score for the intensity of five flavor attributes (fatty flavor, nutty/toasted flavor, spicy flavor, burnt-like flavor and grassy flavor). Data each sample simultaneously. The scores were based on a 5-point hedonic scale: where 0 represented the lowest score and 5 the highest score. The average values were calculated based on all scores.
2.8. Odor activity value(OAV)
OAV, calculated by dividing the concentrations of the compounds with a given aroma by its sensory thresholds obtained from literature, was used to quantify the contribution of the compound to the overall oil aroma, and was positively correlated with this contribution. Only compounds with OAV>1 were considered to individually contribute to the oil aroma. The greater the OAV value within a certain range, the greater the contribution of this component to the overall odor.
2.9. Statistical analysis
The data were reported as mean values ± standard deviation (SD) of three measurements. Experimental data were analyzed by using SPSS software to perform one-way analysis of variance, the significance of differences was analyzed using Duncan's multiple comparisons The influence of different levels of each factor on the detection indicators was analyzed by one-way analysis of variance, the differences between individual means were considered significant at p < 0.05. Origin 8.1 (Origin Lab Corporation, Northampton, United Stated) was used to draw and analyze the linear fitting of the standard curve. Principal component analysis of characteristic flavor substances was performed using SPSS (IBM Corp., Armonk, United States).
3. Results and discussion
3.1. Effect of rapeseed meal roasting on amino acid content
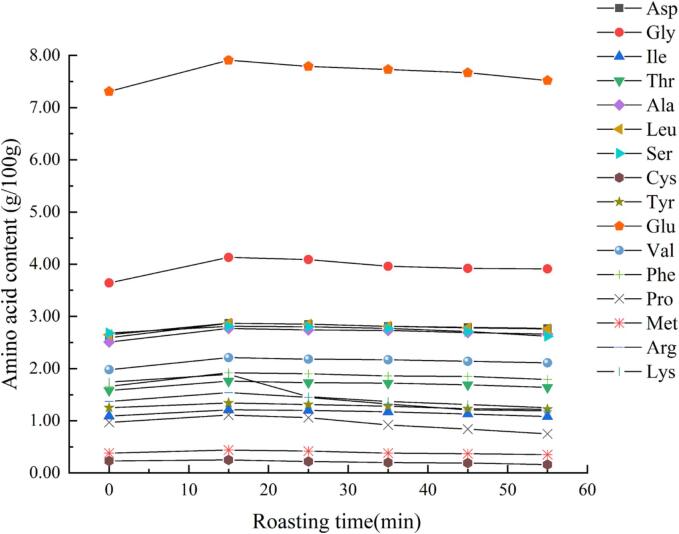
Fig. 1 shows the amino acid content of rapeseed meal during roasting. Sixteen amino acids were detected in rapeseed meal, the contents of which increased and then subsequently decreased during the roasting process; the highest levels were reached at 5 min. The increase in amino acid content was likely due to protein hydrolysis caused by increased temperatures during roasting. Notably, the amino acid content decreased after roasting for > 5 min. Amino acids play an important role in the process of the MR. during which sugars and peptides are broken down into carbon and amino groups and react. This process results in the removal of the amino group and exposure of the free carboxyl residue, which eventually leads to a decrease in free amino group content. The largest decrease was observed in lysine content (-0.64 %) followed by Glu, Pro and Arg (0.39 %, 0.36 %, and 0.35 %, respectively). It was deduced that the main amino acids in MR were Lys, Glu, Pro and Arg.
Fig. 1.
The changes in amino acid content in rapeseed meal during roasting. Asp, Aspartic acid; Gly, Glycine acid; Ile, l-isoleucine acid; Thr, Threonine acid; Ala, Alanine acid; Leu, Leucine acid; Ser, Serine acid; Cys, Cysteine acid; Tyr, Tyrosine acid; Glu, Glutamate acid; Val, Valine acid; Phe, Phenylalanine acid; Pro, Proline acid; Met, Methionine acid; Arg, Arginine acid; Lys, Lysine acid.
3.2. Effects of amino acid on oil physicochemical indicators
As shown in Table 1, the differences in AV between the four added amino acid groups and control group are not significant (p < 0.05). According to EU legislation, AV and PV must not exceed 2.5 and 10 mg/g(Maszewska, Florowska, Matysiak, Marciniak-Lukasiak, & Dluzewska, 2018), respectively, and all five groups of oils met these standards. The iodine value (IV) of the four prepared FROs was higher than that of the control group; however, the difference was not significant (p < 0.05). Moisture content is an important indicator in FROs, as water is a common MR medium and is a key factor influencing the MR route and final MR products(Wei et al., 2019). Thus, high water content may affect flavor. As shown in Table 1, the moisture contents of the five FROs ranges from 0.10 % to 0.17 %. The moisture content of the lysine group was 0.10 %, which was lower than those of the other three FRO types.
Table 1.
Nutrients, physicochemical properties, BaP, fatty acid composition.
| Control | Glu | Lys | Pro | Arg | |
|---|---|---|---|---|---|
| α-Tocopherol (mg/kg) | 166.89 ± 0.61b | 170.55 ± 1.98b | 183.06 ± 6.73a | 166.85 ± 1.87b | 176.44 ± 0.32a |
| γ-Tocopherol (mg/kg) | 403.01 ± 0.28a | 396.56 ± 3.38b | 404.37 ± 3.35a | 402.33 ± 1.61a | 400.28 ± 2.76a |
| δ-Tocopherol (mg/kg) | 12.62 ± 0.31a | 12.61 ± 0.57a | 12.69 ± 0.98a | 12.64 ± 0.21a | 12.74 ± 0.00a |
| Total Tocopherols (mg/kg) | 582.52 ± 0.64b | 579.72 ± 5.94b | 600.11 ± 8.51a | 581.82 ± 0.48b | 589.46 ± 2.43a |
| Polyphenols (mg/kg) | 19.52 ± 2.18c | 18.76 ± 0.38c | 19.41 ± 0.40c | 21.78 ± 1.68b | 22.43 ± 0.13b |
| Sterols (mg/kg) | 753.46 ± 9.57a | 797.50 ± 0.70a | 751.03 ± 1.03c | 745.00 ± 1.41c | 782.29 ± 0.23b |
| BaP (μg/kg) | 1.47 ± 0.02b | 1.54 ± 0.01a | 1.45 ± 0.03b | 1.49 ± 0.01b | 1.57 ± 0.01a |
| AV (mg/g) | 1.31 ± 0.02b | 1.31 ± 0.02b | 1.27 ± 0.04b | 1.29 ± 0.02b | 1.37 ± 0.02a |
| IV (g/100 g) | 98.03 ± 0.76c | 100.77 ± 1.45b | 101.43 ± 1.33b | 99.43 ± 0.91c | 100.17 ± 1.24d |
| PV (g/100 g) | 0.12 ± 0.00a | 0.12 ± 0.00a | 0.12 ± 0.00a | 0.13 ± 0.01a | 0.12 ± 0.00a |
| Moisture content (%) | 0.17 ± 0.00a | 0.13 ± 0.00b | 0.10 ± 0.00c | 0.12 ± 0.00b | 0.16 ± 0.00a |
| Fatty acid content (%) | |||||
| C16:0 | 6.90 ± 0.09b | 4.53 ± 0.18b | 4.03 ± 0.10a | 4.88 ± 0.55a | 5.06 ± 0.66b |
| C18:0 | 2.88 ± 0.06b | 2.74 ± 0.11b | 2.81 ± 0.12b | 2.54 ± 0.14b | 1.83 ± 0.08b |
| C18:1 | 2.88 ± 0.06b | 2.74 ± 0.11b | 2.81 ± 0.12b | 2.54 ± 0.14b | 1.83 ± 0.08b |
| C18:2 | 19.82 ± 0.19c | 19.90 ± 0.54b | 19.65 ± 0.05c | 21.52 ± 0.12b | 19.72 ± 0.09c |
| C18:3 | 7.62 ± 0.18b | 7.14 ± 0.51a | 7.40 ± 0.22c | 7.62 ± 0.29b | 7.26 ± 0.26a |
| C20:1 | 1.22 ± 0.21a | 2.15 ± 0.15a | 2.43 ± 0.28a | 2.59 ± 0.22b | 1.25 ± 0.26b |
| C22:1 | 4.30 ± 0.26c | 4.59 ± 0.40b | 4.14 ± 0.38a | 4.76 ± 0.23a | 4.72 ± 0.74b |
| SFA | 9.77 ± 0.26c | 7.27 ± 0.08b | 6.84 ± 0.10a | 7.42 ± 0.65a | 6.89 ± 0.63b |
| MUFA | 64.53 ± 2.93c | 65.69 ± 0.85b | 66.11 ± 0.19a | 63.44 ± 0.46b | 66.13 ± 0.86a |
| PUFA | 27.43 ± 0.08c | 27.04 ± 0.76b | 27.05 ± 0.18d | 29.14 ± 0.40c | 26.98 ± 0.35c |
Values in the table are mean ± standard deviation, and superscript letters indicate significant differences in the same row of data (P < 0.05).
3.3. Fatty acid composition
Changes in fatty acids in the FROs of the various experimental groups are shown in Table 1. Seven fatty acids were identified: palmitic(C16:0), stearic(C18:0), oleic(C18:1), linoleic(C18:2), linolenic(C18:3), eicosenoic (C20:1), and erucic (C22:1) acids. Linoleic acid content was the highest (19.65 %–22.31 %). The difference between the fatty acid contents of four amino acid groups and the control group was not significant (p < 0.05); however, the Pro group had the highest linoleic acid content (21.52 %). The FROs contained 91.96 %–93.16 % USFA and 6.84 %–11.54 % saturated fatty acids (SFA). Daily USFA consumption has been shown to aid in various physiological functions, such as lowering blood lipids, antithrombosis, and immune regulation, which are beneficial to human health(Chew, 2020). Thus, high USFA levels are beneficial for the body. As shown in Table 1, the Lys group has the highest USFA content (93.16 %) among the five samples.
3.4. Determination of BaP and nutrients in FROs
Three tocopherols (including α-, γ- and δ-tocopherols) were observed in all FRO samples (Table 1), with γ-Tocopherol (396.56–404.37 mg/kg) accounting for > 60 % of the total tocopherol content (579.72–601.19 mg/kg). Total tocopherol content was higher in the Lys group (404.37 mg/kg) than in the Arg, Pro, and Glu groups; the α-, γ-, and δ-tocopherol contents were marginally higher than those in all other groups. Tocopherols are highly heat-sensitive; during the MR, high-temperature heating degrades the tocopherol structure in oils and fats, which affects their overall stability. In addition, external addition of amino acids leads to a higher MR intensity and accompanying temperature change, resulting in a greater heat-loss of tocopherols(Nurkhuzaiah, Babji, Rosli, & Foo, 2015).
The Arg group had the highest polyphenol content (22.43 mg/kg) (Table 1). This may be due to the difference in arginine residues(Z. Y. Zhang et al., 2018)that leads to different MR pathways and results, which produces more compounds such as reductones and melanin analog antioxidants relative to the other amino acids. Furthermore, to some extent, polyphenols have some synergistic effect with these MR Products under the same conditions; therefore, the polyphenol content in the Arg group was relatively high.
As FRO preparation requires high-temperature treatment, a higher heating temperature significantly increases BaP formation(Kim, Kim, & Shin, 2021). However, BaP is highly carcinogenic and therefore should be present in oil at the lowest possible level. According to the Commission Regulation EC No. 835/2011, BaP content in FROs should be < 2 µg/kg for safe human consumption. In the present study, mean individual BaP levels ranged from 1.17 to 1.57 µg/kg in the control sample, with the lowest BaP content observed in lysine-treated rapeseed oil samples (1.45 μg/kg). Thus, the BaP content observed in all samples complied with this regulation.
3.5. Sensory evaluation of FROs
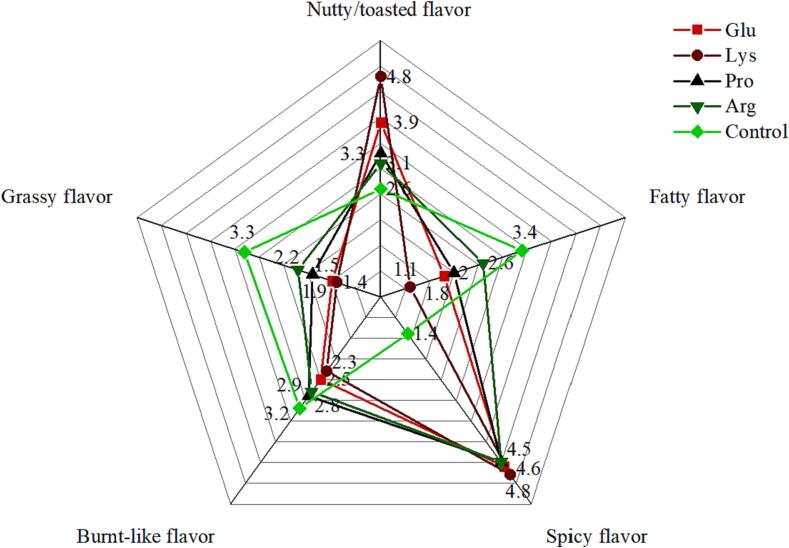
Fig. 2 shows the sensory properties of the different FROs according to flavor: nutty/toasted, burnt-like, spiciness, fatty, and grassy. The small differences observed in burnt taste scores among the five groups may be due to the different classes of amino acids added with the MR during sample preparation, as different amino acids produce different flavor substances during MR. By comparison, the Lys group had the highest nutty/roasted flavor score (4.80 scores). The addition of Lys made the MR process more adequate and increased flavor intensity, resulting in the highest rating.
Fig. 2.
Sensory evaluation of FROs.
3.6. Flavor substance composition of FROs
The composition of the FRO-flavoring substances is shown in Table 2. Gas chromatography–mass spectrometry (GC–MS) detected and identified 23 volatile compounds as main aroma contributors, including pyrazines, furans, aldehydes, ketones, acids, ethers, and nitriles. Pyrazines consist of eight volatile compounds: 2-Ethyl-pyrazine, 2-ethyl-6-methyl-pyrazine, 2-methyl-Pyrazine, 2,5-Dimethyl-pyrazine, 3-ethyl-2,5-dimethyl-pyrazine, 2,6-dimethyl-Pyrazine, 2-ethyl-5-methyl-pyrazine and trimethyl-pyrazine, and are the main volatile flavor components in roasted oil seeds and their products, which usually present nutty and roasted flavors(Y. F. Zhang et al., 2023). In the present study, the Lys group had the highest pyrazine content (24.02 μg/g), corresponding to the high nutty/toasted flavor scores previously reported (Fig. 2). The OAV was used as the target value for flavor evaluation; that is, the greater the OAV, the greater the contribution to the overall flavor of the sample. In the Lys group, the OAV of 2-ethyl-6-methylpyrazine was 70.5. During the MR, sulfur-containing amino acids are degraded via Strecker synthesis to produce hydrogen sulfide and ammonia, which provide precursors for the formation of several heterocyclic flavors; the flavors are subsequently oxidized to produce pyrazine. In food products, pyrazines are generated from free amino acids and peptides, and carbonyl compounds as precursors. Glucose degradation produces dicarbonyl compounds, which subsequently combine with free amino acids or peptides under alkaline conditions and react to form α-amino ketones, i.e., Strecker amino acid synthesis in the MR process. These α-amino ketones are condensed to form different types of pyrazines(Scalone, Lamichhane, Cucu, De Kimpe, & De Meulenaer, 2019). Lysine is an alkaline amino acid; therefore, its addition may lead to a more adequate MR. In contrast to reducing sugars, amino groups are important in determining the backbone of pyrazine structures because nitrogen atoms in pyrazines are derived from amino compounds. In general, hydroxy and basic amino acids are relatively reactive compared to non-polar and acidic amino acids. Of these, lysine and lysine-containing peptides are highly inhibitory and produce pyrazines at fairly high levels. During heat treatment, ammonia is released from both amino acids and the corresponding amide bond in the peptide—a phenomenon known as deamidation. Deamidation mainly undergoes direct hydrolysis of the amide bond, followed by β-shift involving the generation of succinate intermediate. Ammonia generated via deamidation has been shown to participate in pyrazine formation and accelerate MR by reacting directly with dicarbonyl compounds(H. Yu et al., 2021, Yu et al., 2021).
Table 2.
Volatile chemical composition of FROs.
| Content (μg/g) | OAV | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | RI | Threshold | Control | Market | Pro | Lys | Glu | Arg | Control | Market | Pro | Lys | Glu | Arg | |
| 1 | 2-Ethyl pyrazine | 930 | 0.25 | 2.05 | 1.88 | / | 1.27 | / | 1.69 | 8.20 | 7.52 | <1 | 5.08 | <1 | 6.76 |
| 2 | Pentanal | 935 | 0.05 | 2.43 | / | / | / | 4.78 | 2.12 | 48.60 | <1 | <1 | <1 | 95.60 | 42.40 |
| 3 | Hexanal | 1083 | 0.07 | 2.17 | 1.28 | 1.82 | 1.45 | 1.90 | 1.98 | 31.00 | 18.29 | 26.00 | 20.71 | 27.14 | 28.29 |
| 4 | Heptanal | 1174 | 0.05 | 2.79 | / | 3.12 | / | / | 4.53 | 55.80 | <1 | 62.40 | <1 | <1 | 90.60 |
| 5 | Furan, 2-pentyl- | 1231 | 0.10 | 1.38 | 0.46 | 1.55 | / | 1.76 | / | 13.80 | 4.60 | 15.50 | <1 | 17.60 | <1 |
| 6 | (E)-2-Heptenal | 1243 | 0.04 | / | 2.07 | / | 2.60 | 2.70 | / | <1 | 51.75 | <1 | 65.00 | 67.50 | <1 |
| 7 | Pyrazine, 2-methyl | 1266 | 6.00 | 2.30 | 2.87 | 2.47 | 7.57 | 4.92 | 2.89 | <1 | <1 | <1 | 1.26 | <1 | <1 |
| 8 | 2,5-Dimethyl--pyrazine | 1320 | 2.00 | 3.82 | 4.01 | 4.28 | 4.65 | 3.90 | 3.90 | 1.91 | 2.01 | 2.14 | 2.33 | 1.95 | 1.95 |
| 9 | 2,6-Dimethylpyrazine | 1328 | 0.40 | 2.74 | 3.34 | 2.14 | 2.55 | / | 2.25 | 6.85 | 8.35 | 5.35 | 6.38 | <1 | 5.63 |
| 10 | 2-ethyl-5-methylpyrazine | 1341 | 0.02 | 1.03 | 3.41 | 5.40 | / | 4.98 | 1.98 | 51.50 | 170.50 | 270.00 | <1 | 249.00 | 99.00 |
| 11 | (E)-2-Octenal | 1345 | 0.006 | 3.10 | 2.21 | 2.05 | 1.81 | / | 0.71 | 775.00 | 552.50 | 512.50 | 452.50 | <1 | 177.50 |
| 12 | 1-Hexanol | 1360 | 0.45 | 1.45 | 2.72 | 3.08 | 3.11 | 2.85 | 2.67 | 3.22 | 6.04 | 6.84 | 6.91 | 6.33 | 5.93 |
| 13 | 2-ethyl-6-methylpyrazine | 1386 | 0.04 | 2.25 | 3.58 | 5.46 | 2.82 | 4.81 | 3.33 | 56.25 | 89.50 | 136.50 | 70.50 | 120.25 | 83.25 |
| 14 | Nonanal | 1391 | 0.26 | 3.17 | 2.27 | 2.00 | 1.19 | 2.42 | 2.82 | 12.19 | 8.73 | 7.69 | 4.58 | 9.31 | 10.85 |
| 15 | Trimethylpyrazine | 1406 | 0.29 | 0.95 | 3.43 | / | 3.53 | 2.12 | 1.14 | 3.28 | 11.83 | <1 | 12.17 | 7.31 | 3.93 |
| 16 | Acetic acid | 1452 | 0.50 | 1.50 | 1.19 | 1.49 | 0.99 | 1.48 | 1.30 | 3.00 | 2.38 | 2.98 | 1.98 | 2.96 | 2.60 |
| 17 | Furfural | 1461 | 3.00 | 3.19 | 4.69 | 4.11 | 4.58 | 3.74 | 4.01 | 1.06 | 1.56 | 1.37 | 1.53 | 1.25 | 1.34 |
| 18 | 3-Ethyl-2,5-dimethylpyrazine | 1464 | 0.08 | 0.97 | 0.74 | 2.80 | 1.63 | 2.12 | 0.57 | 12.13 | 9.25 | 35.00 | 20.38 | 26.50 | 7.13 |
| 19 | 2-Acetylfuran | 1501 | 1.00 | 0.18 | 0.92 | 1.23 | 1.97 | 1.20 | 1.14 | <1 | <1 | 1.23 | 1.97 | 1.20 | 1.14 |
| 20 | Benzaldehyde | 1508 | 0.06 | 1.57 | 2.43 | 2.29 | 5.62 | 2.12 | 2.01 | 26.17 | 40.50 | 38.17 | 93.67 | 35.33 | 33.50 |
| 21 | Furaneol | 1669 | 5.00 | 2.25 | / | 1.31 | 0.60 | 1.42 | 2.02 | <1 | <1 | <1 | <1 | <1 | <1 |
| 22 | 2(5H)-Furanone | 1767 | 0.04 | 0.91 | 1.85 | 3.42 | 4.27 | 2.32 | 1.44 | 22.75 | 46.25 | 85.50 | 106.75 | 58.00 | 36.00 |
| 23 | Benzenepropanenitrile | 2041 | 0.02 | 2.40 | 3.22 | 0.97 | 2.21 | 1.63 | 0.60 | 120.00 | 161.00 | 48.50 | 110.50 | 81.50 | 30.00 |
Note: “/” is not detect.
Furans (such as furfural and 2-acetylfuran) contain a furan ring in their molecular structure and are important flavor intermediates in foods(Wan et al., 2015). Most furans are formed from dicarbonyl intermediates during the intermediate or final stages of the MR, in the presence or absence of amino acids. Furfural is a product obtained from the decomposition of 3- or 1-deoxyribonucleosides. Depending on the reaction conditions (e.g., sugar and amino acid types), the formation pathways of furanic compounds may become more complex. In terms of the four amino acid groups, the highest OAV values (1.53) were observed for furfural in the Lys group. In the present study, the FRO samples of the Lys group contained more furfural (4.58 μg/g) than those of the Pro (4.11 μg/g), Glu (3.74 μg/g), Arg (4.01 μg/g), and control groups (3.19 μg/g), consistent with the Lys group sensory evaluation results. Thus, the changes observed during the MR may be due to the loss of some functional groups —namely, NH2 from lysine—, which may result in the generation of new functional groups of MRPs, such as C O for Amadori compounds, C N for pyrazines, and C N for Schiff bases. In the subsequent MR stages, different flavor substances are formed(Wan et al., 2015).
3.7. Principal component analysis
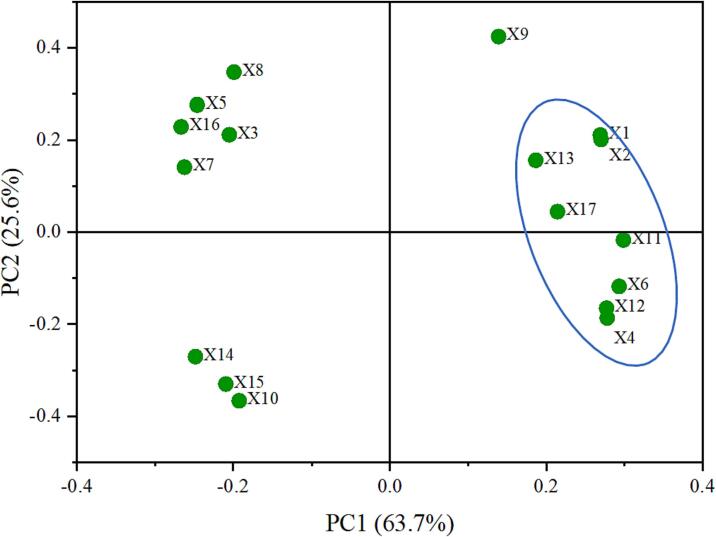
Principal component analysis (PCA) was performed to assess associations among the 17 quality indices of the four reducing amino acid groups (Fig. 3). Indicator selection was based on the screening and comparison of different flavor substances present at the core of nutrients, harmful substances, and various flavor substances. These two principal components (PCs) accounted for 85 % of the total variability, and were therefore selected as indices. The first PC explained 63.7 % of the variance and indicators, reflecting nutrients, flavor substances, and flavor scores. The second PC contributed 25.6 % of the total change, reflecting the metrics of BaP and fatty/burnt-like flavor. The PCA results showed that X4 (Nutty/toasted flavor) was more strongly correlated with X11(Pyrazine, 2-methyl), X12(2,5-Dimethyl--pyrazine), X13(2,6-Dimethylpyrazine), and X17(Trimethylpyrazine).Table 2 shows that the Lys group had the highest pyrazine content, including 2-methyl-pyrazine (7.57 %), 2,5-dimethyl-pyrazine (4.65 %), 2,6-dimethylpyrazine (2.55 %), and trimethylpyrazine (3.53 %). The PCA plot shows that X1(α-Tocopherol), X2(Total Tocopherols), and X4(Nutty/toasted flavor) have a high positive correlation, indicating that the nutrients in the oil had a marked impact on flavor. These findings provide data to support future research examining oil flavor determinants.
Fig. 3.
PCA loading plot of aromatic substances. X1 α-Tocopherol; X2 Total Tocopherols; X3 BaP; X4 Nutty/toasted flavor; X5 Fatty flavor; X6 Spicy flavor; X7 Burnt-like flavor; X8 Grassy flavor; X9 2-Ethylpyrazine; X10 2-pentylFuran; X11 Pyrazine, 2-methyl; X12 2,5-Dimethyl-pyrazine; X13 2,6-Dimethylpyrazine; X14 2-ethyl-5-methylpyrazine; X15 2-ethyl-6-methylpyrazine; X16 Nonanal; X17 Trimethylpyrazine.
For overall FRO quality evaluation, the four groups of samples were analyzed by a PCA system in combination with flavor and nutritional safety considerations. The results of the integrated scores in Table 3 show that the integrated scores of the Lys group are higher, thus confirming that the Lys group is the highest in terms of flavor and nutritional quality.
Table 3.
Main ingredient score and ranking of comprehensive score of different fragrant rapeseed oil.
| Groups | PC1 | PC2 | Scores | Sequence |
|---|---|---|---|---|
| Glu | −1.08289 | −1.84952 | −2.93241 | 3 |
| Lys | 4.85387 | −0.10964 | 4.74423 | 1 |
| Pro | −2.45099 | −0.98627 | −3.43726 | 4 |
| Arg | −1.31999 | 2.94543 | 1.62544 | 2 |
4. Conclusions
In this study, we investigated MR substrates and explored the effect of their participation in MR on the flavor of rapeseed meal before and after roasting by examining its amino acid contents; selecting Pro, Lys, Glu, and Arg; and then adding each of these amino acids to the enzyme solution to prepare FROs by MR. The differences in physicochemical parameters, micronutrients, fatty acids, BaP, and flavor substances between FRO and commercially available aromatic rapeseed oil were analyzed. The AV (1.27–1.31 mg/g), PV (4.70–4.97 mmol/kg), IV (98.03–101.43 g/100 g), moisture content (0.10–0.17 %), and BaP content (1.45–1.57 μg/kg) of the prepared oils were standardized. The contents of tocopherols (579.72 ∼ 600.11 mg/kg) and polyphenols (18.76 ∼ 22.43 mg/kg) in the oil were high, and the content of USFA was high, with the highest content being 93.16 % in the Lys group. The group with extra Lys had the highest content of pyrazine-flavoring substances and the highest rating for nutty/toasted flavors (4.80 scores). The dominance of lysine in the FROs prepared for MR was also determined. This study provides a guideline for the preparation of intensely flavored rapeseed oil using MR. This study also has some shortcomings in that the exact mechanism of synthesis of flavor products from the different amino acids in the MR is unclear, and only the flavor substances studied so far have been examined.
Ethical statement
The ethical approval is not required by national laws. All panelist provided written informed consent, and their anonymous information will be published in this article.
CRediT authorship contribution statement
Pan Gao: Writing – review & editing, Writing – original draft, Funding acquisition. Bobo Sun: Writing – original draft. Zhe Chen: Methodology. Qiaona Yuan: Data curation. Wu Zhong: Formal analysis. Jiaojiao Yin: Formal analysis. Chuanrong Hu: Project administration. Dongping He: Resources. Xingguo Wang: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors gratefully acknowledge financial support from China Scholarship Council and Key Laboratory of Edible Oil Quality and Safety for State Market Regulation. This work was supported by the project of Key Laboratory for Deep Processing of Major Grain and Oil, Ministry of Education (DZLY2023003).
Data availability
The authors do not have permission to share data.
References
- Chew S.C. Cold-pressed rapeseed oil: Chemistry and functionality. Food Research International. 2020;131:13. doi: 10.1016/j.foodres.2020.108997. [DOI] [PubMed] [Google Scholar]
- Gao P., Zheng Y.L., Liu H., Yang W., Hu C.R., He D.P. Effects of roasting and deodorisation on 3-monochloropropane-1, 2-diol esters, 3, 4-benzopyrene and trans fatty acids in peanut oil. Food Additives and Contaminants Part a-Chemistry Analysis Control Exposure & Risk Assessment. 2022;39(3):451–461. doi: 10.1080/19440049.2021.2022772. [DOI] [PubMed] [Google Scholar]
- Jing B.Y., Guo R., Wang M.Z., Zhang L.Y., Yu X.Z. Influence of seed roasting on the quality of glucosinolate content and flavor in virgin rapeseed oil. Lwt-Food Science and Technology. 2020;126:8. doi: 10.1016/j.lwt.2020.109301. [DOI] [Google Scholar]
- Kathuria, D., Hamid, Gautam, S., & Thakur, A. (2023). Maillard reaction in different food products: Effect on product quality, human health and mitigation strategies. Food Control, 153, 13. doi: 10.1016/j.foodcont.2023.109911.
- Kim D.Y., Kim B., Shin H.S. Reduction of Polycyclic Aromatic Hydrocarbons (PAHs) in Sesame Oil Using Cellulosic Aerogel. Foods. 2021;10(3):12. doi: 10.3390/foods10030644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latif S., Pfannstiel J., Makkar H.P.S., Becker K. Amino acid composition, antinutrients and allergens in the peanut protein fraction obtained by an aqueous enzymatic process. Food Chemistry. 2013;136(1):213–217. doi: 10.1016/j.foodchem.2012.07.120. [DOI] [PubMed] [Google Scholar]
- Liu H.M., Han Y.F., Wang N.N., Zheng Y.Z., Wang X.D. Formation and Antioxidant Activity of Maillard Reaction Products Derived from Different Sugar-amino Acid Aqueous Model Systems of Sesame Roasting. Journal of Oleo Science. 2020;69(4):391–401. doi: 10.5650/jos.ess19336. [DOI] [PubMed] [Google Scholar]
- Ma G., He S.D., Liu S.Y., Zhang Z.Y., Zhang T., Wang L., Sun H.J. Application of Maillard Reaction Products Derived Only from Enzymatically Hydrolyzed Sesame Meal to Enhance the Flavor and Oxidative Stability of Sesame Oil. Molecules. 2022;27(24):20. doi: 10.3390/molecules27248857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maszewska M., Florowska A., Matysiak K., Marciniak-Lukasiak K., Dluzewska E. The study of palm and rapeseed oil stability during frying. Journal of Applied Botany and Food Quality. 2018;91:103–108. doi: 10.5073/jabfq.2018.091.014. [DOI] [Google Scholar]
- Nurkhuzaiah K., Babji A.S., Rosli W.I.W., Foo S.P. TOCOPHEROL AND TOCOTRIENOL CONTENTS OF CHICKEN NUGGETS BLENDED WITH RED PALM OILS BEFORE AND AFTER FRYING. Journal of Oil Palm Research. 2015;27(1):82–89. [Google Scholar]
- Pang M., Cao L.L., Kang S.M., Jiang S.T., Cao L.L. Controlled Release of Flavor Substances from Sesame-Oil-Based Oleogels Prepared Using Biological Waxes or Monoglycerides. Foods. 2021;10(8):17. doi: 10.3390/foods10081828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano R., Aiello A., De Luca L., Pizzolongo F., Durazzo A., Lucarini M., Santini A. Deep-frying purple potato <i>Purple Majesty</i> using sunflower oil: Effect on the polyphenols, anthocyanins and antioxidant activity. Heliyon. 2022;8(5):8. doi: 10.1016/j.heliyon.2022.e09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalone G.L.L., Lamichhane P., Cucu T., De Kimpe N., De Meulenaer B. Impact of different enzymatic hydrolysates of whey protein on the formation of pyrazines in Maillard model systems. Food Chemistry. 2019;278:533–544. doi: 10.1016/j.foodchem.2018.11.088. [DOI] [PubMed] [Google Scholar]
- Shibao J., Bastos D.H.M. Maillard reaction products in foods: Implications for human health. Revista De Nutricao-Brazilian Journal of Nutrition. 2011;24(6):895–904. doi: 10.1590/s1415-52732011000600010. [DOI] [Google Scholar]
- Wan Y., Li H.X., Fu G.M., Chen X.Y., Chen F., Xie M.Y. The relationship of antioxidant components and antioxidant activity of sesame seed oil. Journal of the Science of Food and Agriculture. 2015;95(13):2571–2578. doi: 10.1002/jsfa.7035. [DOI] [PubMed] [Google Scholar]
- Wei C.K., Ni Z.J., Thakur K., Liao A.M., Huang J.H., Wei Z.J. Color and flavor of flaxseed protein hydrolysates Maillard reaction products: Effect of cysteine, initial pH, and thermal treatment. International Journal of Food Properties. 2019;22(1):84–99. doi: 10.1080/10942912.2019.1573830. [DOI] [Google Scholar]
- Wu Y., Zhou R.S., Wang Z.G., Wang B., Yang Y.J., Ju X.R., He R. The effect of refining process on the physicochemical properties and micronutrients of rapeseed oils. Plos One. 2019;14(3):16. doi: 10.1371/journal.pone.0212879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z., Liu Y.F. Polyphenolic compounds from rapeseeds (Brassica napus L.): The major types, biofunctional roles, bioavailability, and the influences of rapeseed oil processing technologies on the content. Food Research International. 2023;163:32. doi: 10.1016/j.foodres.2022.112282. [DOI] [PubMed] [Google Scholar]
- Yu H., Zhang R.Y., Yang F.W., Xie Y.F., Guo Y.H., Yao W.R., Zhou W.B.A. Control strategies of pyrazines generation from Maillard reaction. Trends in Food Science & Technology. 2021;112:795–807. doi: 10.1016/j.tifs.2021.04.028. [DOI] [Google Scholar]
- Yu J., Wang M.Z., Zhang M., Liu Y.F., Li J.W. Effect of infrared ray roasting on oxidation stability and flavor of virgin rapeseed oils. Journal of Food Science. 2021;86(7):2990–3000. doi: 10.1111/1750-3841.15792. [DOI] [PubMed] [Google Scholar]
- Yuan Q.N., Tu M.J., Gao P., Hu C.R., He D.P. Comparative Analysis of Rapeseed Oils Prepared by Three Different Methods. Journal of Oleo Science. 2020;69(12):1641–1648. doi: 10.5650/jos.ess20188. [DOI] [PubMed] [Google Scholar]
- Zhang Y.F., Wu Y.Q., Chen S.R., Yang B.B., Zhang H., Wang X.G., Jin Q.Z. Flavor of rapeseed oil: An overview of odorants, analytical techniques, and impact of treatment. Comprehensive Reviews in Food Science and Food Safety. 2021;20(4):3983–4018. doi: 10.1111/1541-4337.12780. [DOI] [PubMed] [Google Scholar]
- Zhang Y.F., Zhen C., Zhao B.X., Zhou S.M., Jiang Y.R., Wang X.G., Zhang Y.Y. Comparative characterization of key odorants and aroma profiles of fragrant rapeseed oil under different roasting conditions. Food Research International. 2023;163:9. doi: 10.1016/j.foodres.2022.112195. [DOI] [PubMed] [Google Scholar]
- Zhang Y.F., Zhu Y., Shi L.K., Guo Y., Wei L., Zhang H., Jin Q.Z. Physicochemical properties and health risk assessment of polycyclic aromatic hydrocarbons of fragrant rapeseed oils in China. Journal of the Science of Food and Agriculture. 2020;100(8):3351–3359. doi: 10.1002/jsfa.10368. [DOI] [PubMed] [Google Scholar]
- Zhang Z.Y., Elfalleh W., He S.D., Tang M.M., Zhao J.L., Wu Z.Y., Sun H.J. Heating and cysteine effect on physicochemical and flavor properties of soybean peptide Maillard reaction products. International Journal of Biological Macromolecules. 2018;120:2137–2146. doi: 10.1016/j.ijbiomac.2018.09.082. [DOI] [PubMed] [Google Scholar]
- Zou Y.P., Gao Y.Y., He H., Yang T.K. Effect of roasting on physico-chemical properties, antioxidant capacity, and oxidative stability of wheat germ oil. Lwt-Food Science and Technology. 2018;90:246–253. doi: 10.1016/j.lwt.2017.12.038. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.