Highlights
-
•
The volatile metabolites of fermented milk were analyzed by SPME-GC-MS.
-
•
Strains isolated from goat yogurt promote the level of ketones and aldehydes.
-
•
The strain of different source was different contribution for yoghurt flavor metabolite.
Chemical compounds studied in this article: 2,3-Butanedione (PubChem CID: 650); Acetoin (PubChem CID: 179); 2-Undecanone (PubChem CID: 8163); Heptanal (PubChem CID: 8130); Hexanal (PubChem CID: 6184); Nonanal (PubChem CID: 31289); 2-Nonanone (PubChem CID: 13187); Acetic acid (PubChem CID: 176); 2-Heptanone (PubChem CID: 8051); 3-Methyl-1-butanol (PubChem CID: 31260)
Keywords: Lactococcus lactis subsp. lactis, Fermented milk, Different naturally fermented milk isolates, Volatile metabolites
Abstract
Lactococcus lactis subsp. lactis (L. lactis subsp. lactis) is a commonly used starter cultures in fermented dairy products, contributing distinct flavor and texture characteristics with high application value. However, the strains from different isolates have different contributions to milk fermentation. Therefore, this study aimed to investigate the influence of L. lactis subsp. lactis isolated from various sources on the volatile metabolites present in fermented milk. In this study, L. lactis subsp. lactis from different isolation sources (yogurt, koumiss and goat yogurt) was utilized as a starter culture for fermentation. The volatile metabolites of fermented milk were subsequently analyzed by headspace solid phase microextraction gas chromatography-mass spectrography (HS-SPME-GC-MS). The results indicated significant differences in the structure and abundance of volatile metabolites in fermented milk produced with different isolates (R2Y = 0.96, Q2 = 0.88). Notably, the strains isolated from goat yogurt appeared to enhance the accumulation of ketones (goat yogurt vs yogurt milk: 50 %; goat yogur vs koumiss: 27.3 %)and aldehydes (goat yogurt vs yogurt milk: 21.4 %; goat yogurt vs koumiss: 54.5 %) in fermented milk than strains isolated from koumiss and yogurt milk. It significantly promoted the production of 8 flavor substances (1 substance with OAV ≥ 1 and 6 substances with OAV > 0.1) and enhanced the biosynthesis of valine, leucine, and isoleucine. This study provides valuable insights for the application of Lactococcus lactis subsp. lactis isolated from different sources in fermented dairy production and screening of potential starter cultures.
1. Introduction
Fermented dairy products, particularly yogurt, represent a unique category of nutritious, and beneficial acidic curd-like products derived from milk and dairy products through the fermentation process facilitated by lactic acid bacteria (Min et al., 2020). Among the microbial communities responsible for this fermentation, lactobacillus strains stand out due to their various probiotic properties that can confer probiotic functions to fermented milk production. Traditionally, Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus are frequently employed as starter cultures in fermented milk. The metabolites produced by these two species during milk fermentation can promote each other’s growth and contribute to the formation of essential flavor substances in fermented milk products (Wang et al., 2020).
The flavour of fermented dairy products is mainly derived from a series of complex microbiological and biochemical changes, the biochemical changes are classified into primary (glycolysis, proteolysis and lipolysis) and secondary (metabolism of amino acids and fatty acids) events (Chen et al., 2022). The decomposition and metabolism of sugars, fats and proteins in milk by fermenting strains and their enzymes, e.g. various enzymes convert lactose into lactic acid, while generating galactose, and also produce flavouring substances such as oligosaccharides, polysaccharides, acetaldehyde, diacetyl, butanone and acetone (Tian et al., 2023). Lipases catalyze the hydrolysis of triglycerides, liberating short-chain free fatty acids (FFAs) with low perception thresholds. Proteolysis can directly affect the flavour of fermented dairy products by contributing to the release of lipid compounds from the milk matrix and providing free amino acids (FAAs) as precursors for the biosynthesis of various aromatic compounds. Free fatty acids and the degradation of free fatty acids are important secondary biochemical events in the cheese ripening process leading to the production of various volatiles including esters, lactones, ketones and volatile sulphur-containing compounds. Previous studies have identified C4 compounds as a typical source of yoghurt flavour, including 2,3-butanedione, ethynyl ketone and 2,3-butanediol (Chen et al., 2017, Chen et al., 2022, Liu et al., 2022, Tian et al., 2022).
The distinct flavor and texture of fermented milk are shaped by the strains of bacteria employed during fermentation. Variations in the bacterial isolates can significantly impact the organoleptic properties of the end product. For instance, Lactobacillus casei Zhang (LCZ) isolated from Xilingole dairy koumiss, can produce greater quantity of flavor compounds such as acetaldehyde and 2, 3-butanedione during the fermentation process, thereby enhancing the flavor of fermented milk (Sun et al., 2022). Similarly, when Bifidobacterium animalis Probio-M8 isolated from human colostrum was used to ferment goat milk, it significantly increased the content of organic acids, reduced the “goat” flavor-related substances, and improved the overall flavor of fermented milk (Guo et al., 2022). Furthermore, Bifidobacterium lactis V9 isolated from the gastrointestinal tract of a healthy Mongolian child, as a fermenting agent, positively affected the production of flavor substances such as diacetyl, 3-ethyl-2-methylpentane and 6-ethyl-2-methyloctane at the end of fermentation process in fermented milk (Wang, Zhao et al., 2021). Notably, Lactobacillus plantarum P9 isolated from naturally fermented sour porridge in Bayannur, Inner Mongolia, significantly altered fatty acid levels and some bioactive peptides, benefiting the sensory quality and functional properties of fermented milk (Gauglitz et al., 2020).
The Lactococcus lactis subsp. lactis (L. lactis subsp. lactis), the most representative strain of its genus, has long been recognized for its significant contributions to the production of various fermented foods, including cheese, fermented milk, and sauerkraut, among others. Owing to its exceptional acid and fragrance production characteristics, it plays a pivotal role in shaping the flavor, texture and acidity of the final product (Dan et al., 2019). Therefore, it is imperative to understand the effects of L. lactis subsp. lactis from different isolates on metabolite production during milk fermentation, as this will facilitate the screening of strains with specific fermentation characteristics.
In this study, L. lactis subsp. lactis isolated from different dairy isolate sources was employed as fermenters to ferment milk. The volatile metabolites of fermented milk were analyzed by headspace solid phase microextraction gas chromatography-mass spectrometry (HS-SPME-GC-MS). This approach aimed to elucidate the effects of L. lactis subsp. lactis from different isolation sources on the volatile metabolites of fermented milk. By identifying L. lactis subsp. lactis strains that demonstrate superior fermentation traits, our findings are positioned to expedite advancements in fermented dairy product research. Ultimately, such contributions aspire to rejuvenate and sustain the growth trajectory of China's starter culture and fermented dairy sectors.
2. Materials and methods
2.1. Strains, materials, and equipment
Three isolates of L. lactis subsp. lactis were acquired from the Lactic Acid Bacteria Collection Center (LABCC) of the Inner Mongolia Agricultural University, Hohhot, China. These isolates were derived from traditional fermented food: yogurt, koumiss, and goat yogurt.
Whole milk powder was purchased from Fonterra (New Zealand), while sucrose was obtained from Tianjin Fengchuan Chemical Reagent Technology Co., Ltd. (China). M17 broth medium was obtained from Qingdao High-tech Industrial Park Haibo Biotechnology Co., Ltd. (China). 1,2-Dichlorobenzene, acetone, methanol, and C3-C9 n-alkane mixture were acquired from Sigma-Aldrich (USA).
The equipment used in this study was provided by the Key Laboratory of Dairy Biotechnology and Engineering at Inner Mongolia Agricultural University, including a gas chromatography-mass spectrometry system (7890B GC-5977A MSD, Agilent, USA), a capillary column (HP-5MS, 30 m length, 0.25 mm diameter, 0.25 µm film thickness, Agilent, USA), a SPME fiber (50/30 μm CVB/CAR/PDMS, Supelco, USA), a high-pressure homogenizer (SRH60-90, Shanghai Zhonglu Homogenizer Co., Ltd. China), and a biochemical incubator (Shanghai Yiheng Technology Co., Ltd. China).
2.2. Strains activation and enumeration
Three isolates of L. lactis subsp. lactis were inoculated initially into M17 broth medium for activation. The cultures were incubated at 30 °C for 24 h under anaerobic conditions. The activated strains were then isolated and purified on M17 agar plates, followed by anaerobic culture at 30°C for 48 h. Subsequently, the strains were subcultured in an M17 broth medium for three generations with a 2 % inoculum to ensure maximum strain viability. After the third generation, the culture medium was centrifuged at 3500 × g for 15 min to collect the bacterial pellet. The collected pellet was washed twice with phosphate buffered-saline (PBS), resuspended in normal saline, diluted for enumeration, and stored at 4 °C for later use.
2.3. Preparation of fermented milk
Fermented milk was prepared by dissolving 11.5 % whole milk powder (containing, per 100 g, 39.1 g of lactose, 26.8 g of fat, and 25.0 g of protein) and 6.5 % sucrose in sterile distilled water at 50 °C with thorough mixing. The mixture was homogenized at 65 °C, and 90Mpa using a high-pressure homogenizer, heated at 95 °C for 5 min to pasteurize, then cooled to 30 °C. The homogenized milk was portioned into sterile beakers, each containing 200 mL. L. lactis subsp. lactis was added to each beaker with a viable cell count of 5 × 106 CFU/ mL. Fermentation was conducted at 30°C with triplicate samples for each isolate. The pH value of fermented milk was monitored throughout the process. When culture suspension pH reached 4.6, fermentation was stopped. The yoghurt was then transferred to 4 °C for further fermentation before being stored. The volatile flavor substances of the stored fermented milk were later analyzed by GC–MS (Wang,Sun et al., 2021).
2.4. Determination of volatile flavor substances
The volatile flavor compounds in the fermented milk produced by different strains were quantitatively analyzed by HS-SPME-GC-MS. The adsorbed volatile compounds were desorbed from the SPME fibers and separated on an HP-5MS column (30 m length, 0.25 mm diameter, 0.25 µm film thickness, Agilent, USA). For the analysis, 20 mL of fermented milk was transferred into a 100 mL gas cleaning bottle, to which 1 µL of 1, 2-dichlorobenzene solution was added as an internal standard solution. The final concentration of internal standard solution in each sample was 10 µg/L. The SPME extraction head was positioned at GC inlet, conditioned at 250 °C for 5 min and then placed over the sample bottle to capture volatile compounds. The extraction temperature was 50 °C with spin bar agitation at 300 rpm for 60 min. Subsequently, the samples were desorbed at 250 °C for 3 min, and each sample was analyzed three times. The GC conditions were as follows: 35 °C for 5 min, then increased to 140 °C at 5 °C/min for 2 min, and finally increased to 250 °C at a rate of 10 °C/min. High-purity helium was used as the carrier gas with a flow rate of 1 mL/min. The MS detector was operated in electron impact mode at 150 °C with an electron energy of 70 Ev. The ion source temperature and transfer line temperatures were 230 °C and 280 °C, respectively. The mass scan range was m/z 35–500 with an emission current of 100 μA, and a detection voltage of 1.4 kV. There was no solvent delay. Volatile compounds were identified by matching of their mass spectra with those of a commercial database (National Institute of Standards Technology Mass Spectral Database) and compared with the retention indices of the authentic control compounds or the retention indices recorded in the literature. All identified compounds were semi-quantified as peak areas in the total ion chromatogram, and relative abundances were standardized by dividing peak areas of volatiles by that of the internal standard from the same sample, and were given as means based on independent duplicates. The concentration of each component flavor substance was calculated using 1,2-dichlorobenzene solution as an internal standard, calculated according to Eq. (1) (Min et al., 2020).The RI value is calculated to determine the volatile compounds and RI was calculated according to Eq. (2). The key volatile flavour compounds in fermented milks were identified using an aroma activity value (OAV), and the OAV of each component was calculated from its volatile flavour content versus a threshold value for that substance in water. The odor activity value (OAV) of the selected odor was calculated according to Eq. (3). Higher OAV values contribute more to the overall flavour of fermented milk samples (Gu et al., 2022).
| (1) |
Where Ci is concentration of the substance to be measured in the sample, Cs is concentration of internal standard, Ai is chromatographic peak area of the substance to be measured in the sample, As is chromatographic peak area of the internal standard.
| (2) |
Where RT(Z), RT(Z + 1), and RT(X), are the retention times of n-alkanes with carbon atoms Z, Z + 1 and X to be measured.
| (3) |
Where C is the quantitated concentration and OT is over their respective odor threshold.
2.5. Data analysis
The obtained raw experimental data were organized in Microsoft excel and analyzed using SPSS 21.0 (IBM Corporation, Armonk, NY, USA) and OriginPro7.5 (Origin Lab Corporation, Northampton, MA, USA) software. The data were standardized prior to multivariate statistical analysis. Principal component analysis (PCA) and partial least squares discriminant analysis (PLSDA) were employed to visualize differences and differentiation of metabolic profiles between samples. Heat map visualization and hierarchical cluster analysis were utilized to analyze the quantitative relationship of metabolic profiles under different conditions. Metabolic pathway analysis of different metabolites was performed using the KEGG pathway database (https://www.genome.jp/kegg/tool/map_pathway2.html). Pathway topology analysis was performed with the pathway analysis module in MetaboAnalyst (https://www.metaboanalyst.ca/MetaboAnalyst/upload/PathUploadView.xhtml) (Sun et al., 2021).
3. Results
3.1. Comparative analysis of volatile metabolite profiles of fermented milk derived from different natural isolates
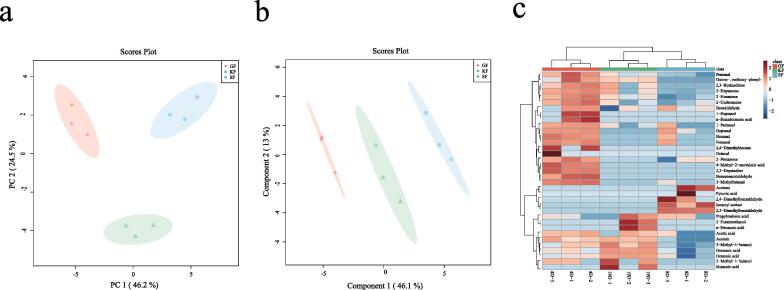
L. lactis subsp. lactis isolated from yogurt milk, koumiss and goat yogurt were employed for fermentation experiments. Subsequent metabolic profiles of these fermented milk were compared and analyzed using GC–MS technology. This analysis identified a total of 35 volatile metabolites present in the fermented milk samples (refer to Table 1 for content details). These metabolites included 5 alcohols, 8 ketones, 10 aldehydes, 9 carboxylic acids, 1 aromatic hydrocarbon, and 2 esters. Principal component analysis (PCA) showed a clear separation of the samples into 3 clusters based on the isolated source (Fig. 1a). Partial least squares discriminant analysis (PLS-DA) further distinguished the metabolic differences between the 3 groups (Fig. 1b), with high R2Y (0.96) and Q2 (0.88) values indicating significant dissimilarities. Cluster analysis also segregated the samples into 3 clusters corresponding to the isolate sources (Fig. 1c). Overall, the results demonstrate that L. lactis subsp. lactis isolates from different natural sources (yogurt milk, koumiss, and goat yogurt) contribute distinct volatile flavor profiles to fermented dairy products. The strain-specific production of flavor compounds should be considered when selecting starters for tailored sensory properties of fermented milks.
Table 1.
Volatile compounds quantified by HS-SPME-GC-MS of fermented milk from different isolates.
| NO. | Volatiles | RT (min) | RI | RI reference | Threshold (μg/L) | SF (μg/L) | OAV | GF (μg/L) | OAV | KF (μg/L) | OAV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2-Methyl-1-butanol | 3.9 | 713 | 722 | 320 | 0 | 0 | 2 ± 2 | 0.01 | 5.01 ± 5.01 | 0.02 |
| 2 | 3-Methyl-1-butanol | 3.83 | 704 | 710 | 4 | 0.1 ± 0.2 | 0.03 | 6 ± 4 | 1.5 | 35.94 ± 28.62 | 9 |
| 3 | Isoamyl acetate | 9.03 | 868 | 870 | 3 | 0.4 ± 0.2 | 0.1 | 0 | 0 | 0 | 0 |
| 4 | 1-Pentanol | 4.68 | — | — | 4000 | 0.4 ± 0.7 | 0.0001 | 1.6 ± 0.3 | 0.0004 | 0.2 ± 0.2 | 0.0001 |
| 5 | 2-Furanmethanol | 8.52 | — | — | 300 | 0 | 0 | 0 | 0 | 1.6 ± 1.6 | 0.005 |
| 6 | 1-Heptanol | 12.74 | 965 | 964 | 330 | 0 | 0 | 0.2 ± 0.2 | 0.001 | 0 | 0 |
| 7 | 2,3-Butanedione | 1.76 | — | — | 2.3 | 0 | 0 | 17 ± 7 | 7.4 | 7 ± 7 | 3.0 |
| 8 | 2-Heptanone | 9.62 | 871 | 863 | 140 | 3.8 ± 0.3 | 0.03 | 13.7 ± 1.4 | 0.1 | 8 ± 4 | 0.06 |
| 9 | 2-Nonanone | 16.9 | 1088 | 1088 | 5 | 1.5 ± 0.4 | 0.3 | 4.8 ± 0.8 | 1 | 2.8 ± 0.5 | 0.6 |
| 10 | 2-Pentanone | 2.82 | 651 | 653 | 2150 | 0.2 ± 0.1 | 0.0001 | 0.7 ± 0.3 | 0.0003 | 0.1 ± 0 | 0.0001 |
| 11 | 2-Undecanone | 22.9 | 1289 | 1291 | 255 | 0.3 ± 0.1 | 0.001 | 0.9 ± 0.2 | 0.004 | 0.4 ± 0.2 | 0.002 |
| 12 | 4-Methyl-2-oxovaleric acid | 11.6 | 99.58 | NS | — | 0 | — | 5.7 ± 1.2 | — | 0 | — |
| 13 | Acetic acid | 3.69 | 602 | 602 | 2000 | 3 ± 5 | 0.002 | 20 ± 6 | 0.01 | 13 ± 13 | 0.007 |
| 14 | Acetone | 1.39 | — | — | 7 | 0.5 ± 0.5 | 0.07 | 0 | 0 | 0 | 0 |
| 15 | Acetoin | 4.9 | 709 | 706 | 55 | 0.5 ± 0.8 | 0.01 | 15 ± 9 | 0.3 | 16 ± 2 | 0.3 |
| 16 | 2,3-Heptandione | 7.5 | — | — | — | 0 | — | 0.8 ± 0.5 | — | 0 | — |
| 17 | Benzaldehyde | 12.19 | 932 | 933 | 3 | 0.6 ± 0.3 | 0.2 | 0.8 ± 0.5 | 0.3 | 0.3 ± 0.3 | 0.1 |
| 18 | 2,4-Dimethylbenzaldehyde | 20.64 | — | — | 350 | 0.3 ± 0.4 | 0.001 | 0 | 0 | 0 | 0 |
| 19 | 2,5-Dimethylbenzaldehyde | 20.64 | — | — | — | 0.2 ± 0.01 | — | 0 | — | 0 | — |
| 20 | Benzeneacetaldehyde | 15.21 | 1048 | NS | 4 | 0 | 0 | 0.6 ± 0.2 | 0.15 | 0 | 0 |
| 21 | 3-Methylbutanal | 2.74 | 632 | 632 | 5.4 | 0.1 ± 0.2 | 0.02 | 5.6 ± 1.1 | 1.04 | 0 | 0 |
| 22 | Butanoic acid | 6.18 | 973 | 973 | 240 | 0 | 0 | 0 | 0 | 0.3 ± 0.3 | 0.001 |
| 23 | 2,4-Dimethyldecane | 15.74 | 1038 | NS | — | 0 | — | 0.3 ± 0.2 | — | 0 | — |
| 24 | Heptanal | 10.05 | 899 | 898 | 3 | 0.2 ± 0.3 | 0.07 | 0.7 ± 0.1 | 0.2 | 0 | 0 |
| 25 | Hexanal | 5.98 | — | — | 4.5 | 0.2 ± 0.4 | 0.4 | 1 ± 0.2 | 0.2 | 0 | 0 |
| 26 | Hexanoic acid | 13.5 | 978 | 977 | 3000 | 0.3 ± 0.3 | 0.0001 | 1.2 ± 0.5 | 0.0004 | 4.5 ± 0.2 | 0.002 |
| 27 | n-Decanoic acid | 24.9 | 1349 | 1344 | 3000 | 0 | 0 | 0 | 0 | 0.3 ± 0.3 | 0.0001 |
| 28 | n-Hexadecanoic acid | 36 | — | — | — | 0 | — | 0.4 ± 0.3 | — | 0 | — |
| 29 | Nonanal | 17.3 | 1085 | 1086 | 1 | 0.1 ± 0.2 | 0.1 | 0.4 ± 0.1 | 0.4 | 0 | 0 |
| 30 | Octanal | 13.9 | 999 | 999 | 0.7 | 0 | 0 | 0.1 ± 0.2 | 0.14 | 0 | 0 |
| 31 | Octanoic acid | 19.6 | 1174 | 1174 | 500 | 0.3 ± 0.3 | 0.0006 | 0.6 ± 0.2 | 0.001 | 1.9 ± 0.1 | 0.004 |
| 32 | Oxime-, methoxy-phenyl- | 10.9 | 916 | NS | — | 0 | — | 2.5 ± 2.1 | — | 1.1 ± 0.1 | — |
| 33 | Pentanal | 2.9 | 668 | 668 | 20 | 0.3 ± 0.1 | 0.02 | 1.6 ± 0.5 | 0.08 | 0.7 ± 0.2 | 0.04 |
| 34 | Propylmalonic acid | 13.0 | 785 | NS | — | 0.1 ± 0.1 | — | 0.1 ± 0.1 | — | 0.4 ± 0.4 | — |
| 35 | Pyruvic acid | 2.8 | 659 | NS | — | 0.1 ± 0.1 | — | 0 | — | 0 | — |
a Excluding the effects of different samples, values were standardized by dividing peak areas of volatiles by that of internal standard from the same sample and were given as means based on independent triplicates. The retention indices (RI) of unknown compounds in the HP-5MS column (Agilent Technologies Inc., Palo Alto, CA, United States) calculated against the GC–MS retention time of n-alkanes (C3–C25). RI from the NIST Chemistry WebBook database (https://webbook.nist.gov/chemistry). Threshold in water, was cited from the book: Odour thresholds: Compilations of flavour threshold values in water and other media. –, not detected; NS, not found in any database.
Fig. 1.
Volatile metabolomics characteristics of different isolates at the end of fermentation. (a) Analysis of volatile metabolic data at the end of fermentation of different isolate source strains using principal component analysis (PCA). (b) Partial least squares discriminant analysis (PLS-DA) was used to analyze the metabolic data of the different isolated source strains at the end of fermentation. (c) Heat map and cluster analysis of volatile metabolites of the samples with color ranges representing the relative abundance of volatile metabolites. (GF = Fermented milk of goat yogurt isolate; KF = Fermented milk of koumiss isolate; SF = Fermented milk of yogurt milk isolate).
3.2. Effects of yogurt milk and koumiss isolates on the metabolic profile of fermented milk
The volatile metabolites between the fermented milk of koumiss isolate (KF) and fermented milk of yogurt milk isolate (SF) groups were compared according to the criteria established in the multivariate statistical analysis described above. The OPLS-DA model was applied to identify compounds responsible for distinct metabolomic patterns. The R2Y and Q2 values were 0.97 and 0.86, respectively, indicating a strong model with a close fit to the data. A clear trend of separation was observed in the PCA and OPLS-DA between the SF and KF group samples (Fig. 2a, 2b), suggesting significant differences in the effects of yogurt milk isolates and koumiss isolates on fermented milk metabolites. A volcano plot was utilized to identify and visualize differential metabolites between the two groups. A total of 8 significant differential metabolites were identified (P < 0.05&VIP > 1&FC > 2 or < 0.5; Fig. 2c), with 6 volatiles exhibiting higher levels in the KF group than in the SF group (Fig. 2d). These included 2 alcohol compounds (3-methyl-1-butanol, 2-Furanmethanol), 2 carboxylic acids (hexanoic acid, octanoic acid), 1 ketone compound (acetoin), 1 ldehydae compound (pentanal,), and 1 aromatic hydrocarbon. These findings suggest that L. lactis subsp. lactis isolated from koumiss is more conducive to the production of alcohol metabolites compared to those isolated from yogurt milk.
Fig. 2.
Comparison of volatile metabolites in fermented milk between SF group and KF group at the end of fermentation. (a) Principal component analysis (PCA) plots of volatile metabolites of fermented milk in SF and KF groups at the end of fermentation. (b) Orthogonal partial least squares discriminant analysis (OPLS-DA) plots of volatile metabolites in SF and KF fermented milks at the end of fermentation. (c) Partial least squares discriminant analysis (PLS-DA) plots of volatile metabolites in SF and KF fermented milks at the end of fermentation. (d) Heat map of volatile metabolites of fermented milk in SF and KF groups at the end of fermentation. (SF = Fermented milk of yogurt milk isolate; KF = Fermented milk of koumiss isolate).
3.3. Effects of goat yogurt and yogurt milk isolates on the metabolic profile of fermented milk
Similarly, a comparison of the volatile metabolites between fermented milk of goat yogurt isolate (GF) and SF groups was conducted using OPLS-DA model. The R2Y and Q2 values were 0.97 and 0.91, respectively, indicating a good model fit. The GF and SF samples showed clear separation in the PCA and OPLS-DA score plots (Fig. 3a, 3b), suggesting significantly different effects of goat yogurt and yogurt milk on fermented milk metabolites. A volcano plot identified and visualized differential metabolites between two groups. A total of 16 significant differential metabolites were identified (P < 0.05&VIP > 1&FC > 2 or < 0.5; Fig. 3c), with 14 volatiles present at higher levels in GF compared to SF (Fig. 3d). Notably, these compounds comprised 7 ketone compounds (2,3-heptandione, 2-heptanone, 2,3-butanedione, 2-nonanone, 2-pentanone, acetoin, 2-undecanone), 3 aldehyde compounds (benzeneacetaldehyde, pentanal, 3-methylbutanal), 1 alcohol compounds (3-methyl-1-butanol), 2 carboxylic acids (4-methyl-2-oxovaleric acid, acetic acid), and 1 ester compound (oxime-, methoxy-phenyl-). These results indicate that L. lactis subsp. lactis isolated from goat yogurt promotes better production of ketone and aldehyde metabolites than isolates from yogurt milk, ketone and aldehyde metabolites accounted for 50 % and 21.4 % respectively.
Fig. 3.
Comparison of volatile metabolites in fermented milk between GF group and SF group at the end of fermentation. (a) Principal component analysis (PCA) plots of volatile metabolites in fermented milk from GF and SF groups at the end of fermentation. (b) Orthogonal partial least squares discriminant analysis (OPLS-DA) of volatile metabolites in fermented milk from GF and SF groups at the end of fermentation. (c) Partial least squares discriminant analysis (PLS-DA) plots of volatile metabolites in fermented milk from GF and SF groups at the end of fermentation. (d) Heat map of volatile metabolites in fermented milk of GF and SF groups at the end of fermentation. (GF = Fermented milk of goat yogurt isolate; SF = Fermented milk of yogurt milk isolate).
3.4. Effects of goat yogurt and koumiss isolates on the metabolic profile of fermented milk
The metabolic profiles of fermented milk samples in the GF and KF groups were analyzed using the Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) model. Both R2Y and Q2 values indicated a strong model fit, with values of 0.97 and 0.90, respectively. PCA and OPLS-DA plots illustrated a distinct separation between the GF and KF groups, affirming the significant influence of the source of L. lactis subsp. lactis on the volatile metabolites in fermented milk (Fig. 4a, 4b). A volcano plot was utilized to identify and visualize differential metabolites between two groups. A total of 13 significant differential metabolites were identified (P < 0.05&VIP > 1&FC > 2 or < 0.5; Fig. 4c), with the levels of 11 volatiles being higher in GF than in KF (Fig. 4d). These included 6 aldehyde compounds (heptanal, nonanal, hexanal, 3-methylbutanal, benzeneacetaldehyde, pentanal), 3 ketone compounds (2,3-heptandion, 2-pentanone, 2-undecanone), 1 alcohol compound (1-pentanol), 1 carboxylic acid (4-methyl-2-oxovaleric acid). These findings suggest that L. lactis subsp. lactis isolated from goat yogurt is more favorable for the production of aldehyde and ketone metabolites than those isolated from koumiss, accounted for 54.5 % and 27.3 % respectively.
Fig. 4.
Comparison of volatile metabolites in fermented milk between GF group and KF group at the end of fermentation. (a) Principal component analysis (PCA) plots of volatile metabolites in fermented milk from GF and KF groups at the end of fermentation. (b) Orthogonal partial least squares discriminant analysis (OPLS-DA) plots of volatile metabolites in fermented milk from GF and KF groups at the end of fermentation. (c) Partial least squares discriminant analysis (PLS-DA) of volatile metabolites of fermented milk from GF and KF groups at the end of fermentation. (d) Heat map of volatile metabolites in fermented milk of GF and KF groups at the end of fermentation. (GF = Fermented milk of goat yogurt isolate; KF = Fermented milk of koumiss isolate).
3.5. Volatile metabolite variation among different isolation sources
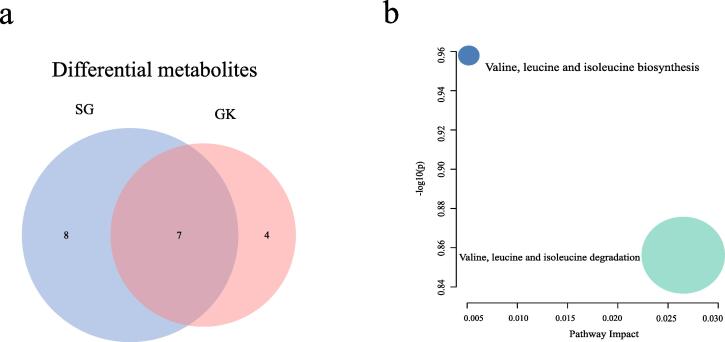
The metabolomic pathways of L. lactis subsp. lactis isolated from goat yogurt displayed a pronounced propensity for the production of ketone and aldehyde metabolites than those isolated from yoghurt milk or koumiss. Therefore, significantly increased differential metabolites in the GF group were analyzed using Venny (Fig. 5a). SG and GK shared 7 metabolites, which were enriched through pathway analysis in MetaboAnalyst 5.0. Two pathways were higher in GF group compared SF and KF groups: Valine, leucine, and isoleucine biosynthesis, and valine, leucine and isoleucine degradation (Fig. 5b). This suggests a potential metabolic preference in L. lactis subsp. lactis isolated from goat yogurt.
Fig. 5.
Goat yogurt isolates significantly increased volatile metabolites. (a) Comparison of the differential metabolites of SG and GK to using Venny. (b) Comparison of differential metabolites between SG and GK using partial least squares discriminant analysis.
3.6. Key volatile metabolites and metabolic pathway
We constructed metabolic pathways for 10 important volatile metabolites based on 2,3-butanedione, acetoin, 2-nonanone, 2-undecanone, heptanal, hexanal, nonanal, 3-methylbutanal, acetic acid and 4-methyl-2-oxovaleric acid (Fig. 6). The Fig. 6 shows that these substances are more abundant in the GF group. The OAV values of these substances were also calculated and compared (refer to Table 1 for details). 2,3-butanedione, 2-nonanone and 3-methylbutanal were the key flavour substances (OAV ≥ 1) in the GF group, and 3-methyl-1-butanol and 2,3-butanedione were the key flavour substances in the KF group. In addition, the substances with OAV > 0.1 were found to be 70 % in the GF group, which was higher than the other two groups (30 % in both the SF group and KF group).
Fig. 6.
Metabolic pathways of important volatile metabolites at the end of fermentation. The known metabolic pathways of the 10 volatile metabolites in the present study.
4. Discussion
L. lactis subsp. lactis as a fundamental industrial starter, plays a crucial role in milk fermentation. Its paramount role in milk fermentation is underscored by its ability to efficiently convert lactose into lactic acid, thus not only reducing the fermentation duration but also suppressing pathogenic growth. Moreover, its proteolytic activity and amino acid conversion also contribute to the final texture and flavor of dairy products (Dan, Jin et al., 2018). The aroma of yogurt, which includes volatiles initially present in milk and compounds produced during fermentation and storage, is a significant factor affecting yogurt quality (Moineau-Jean et al., 2020). To date, more than 90 flavor compounds have been detected in fermented milk, encompassing various volatile compounds such as acids, aldehydes, ketones, alcohols, esters, and hydrocarbons (Dan,Wang et al., 2017). L. lactis subsp. lactis from different isolation sources contribute differently to fermented milk. For instance, strains isolated from koumiss and goat yogurt have been attributed to imparting unique flavors and enhancing the aromatic compound content in fermented milk. Those isolated from yogurt are renowned for conferring fruit and malt aromas (Gallegos et al., 2017, Li et al., 2020, Tang et al., 2020, Tian et al., 2023). In this paper, we aimed to investigate the effects of volatile metabolites on fermented milk produced by L. lactis subsp. lactis isolated from different naturally fermented milk sources. HS- SPME-GC-MS technique was employed to elucidate the influence of these varied isolates on the volatile flavor compounds of fermented milk after fermentation. Our analysis identified in fermented milk, including 5 alcohols, 8 ketones, 10 aldehydes, 9 carboxylic acids, 1 aromatics and 2 esters. These findings align with previous studies on volatile flavor compounds including acids, aldehydes, ketones, alcohols, esters, hydrocarbons and other compounds identified in fermented milk of L. lactis subsp. lactis (Li et al., 2020).
The analysis of volatile metabolites from different natural fermented milk isolates revealed that strains from various isolation sources (yogurt milk, koumiss, and goat yogurt) contribute differently to the flavor of fermented milk. This study found that the strain isolated from koumiss was more likely to produce alcohol. Koumiss, fermented from mare's milk, contains more sugar than cow’s milk. During fermentation, sugar is converted into ethanol through the starter culture, resulting in higher ethanol content in koumiss. At the same time, sour mare's milk is also known as koumiss, has a high ethanol content, and strains isolated from this koumiss may have evolved to be more suitable for ethanol production. Consequently, the fermented milk of the koumiss isolate strain may produce more alcohol (Afzaal et al., 2021, Meng et al., 2021, Rakhmanova et al., 2021). These alcohol compounds may be related to lactose metabolism, methyl ketone reduction, and amino acid metabolism. 3-methyl butanol can impart a pleasant aroma to fresh cheese. Hexanol, heptanol and nonanol are the primary flavor compounds in fermented milk, Simultaneously, alcohols eventually react with acids and esters, making alcohols essential in fermented milk (Dan et al., 2019). The strains isolated from sour goat milk are more likely to produce ketones and aldehydes. Goat milk, which serves as the base material for sour goat milk fermentation, is rich in bioactive peptides and lipids (short and medium chain fatty acids and phospholipids) derived from its proteins and other bioactive ingredients, such as oligosaccharides, hormones, cytokines, and other base substances (Mituniewicz-Małek et al., 2019, Verruck et al., 2019, dos Santos et al., 2023). Ketones, primarily formed by the oxidation of free fatty acids, the Maillard reaction, and the degradation of amino acids, are known for their significant impact on the aroma of most dairy products (Beltrán-Barrientos et al., 2019). In this study, keto compounds such as 2, 3-butanedione, acetoin, 2-heptanone, 2-nononone and 2-undecone were identified in the fermented milk samples. Aldehydes greatly influence the flavor of fermented milk. A crucial pathway for acetaldehyde formation in yogurt involves threonine aldolase, which aids in decomposing threonine into acetaldehyde and glycine. Acetaldehyde is the primary compound that imparts yogurt its typical aroma, giving it a green apple or nutty flavor (Peng et al., 2022). Other volatile metabolites like carboxylic acids, which predominantly emanate lipolysis, proteolysis, or lactose fermentation, are notable. These acidic compounds, which encompass acetic acid, butyric acid, caproic acid, heptanoic acid, caprylic acid and n-capric acid, not only enhance the aroma but also contribute significantly to the sour taste of fermented milk (Dan,Wang et al., 2017). Notably, caproic acid is a significant source of smell and flavor in yogurt. It enhances the taste and smell of yogurt and other types of fermented milk by imparting a “flowery, pungent” taste (Papaioannou et al., 2021). Esters, produced mainly by esterification of fatty acids and alcohols, contribute to the flavor of fermented milk, while alcohols in yogurt combine with free amino acids to form esters. Ethyl esters, in particular, have been identified as key agents in imparting fruity characteristics to dairy products. Most esters provide fruity and floral flavors in fermented milk and attenuate the pungent and astringent flavors of fatty acids and amines (Dan et al., 2019).
Compared to the koumiss and yogurt groups, Lactococcus lactis isolated from goat yogurt produced ketone and aldehyde metabolites more effectively. These metabolites play a pivotal role in the biosynthesis and degradation pathway of valine, leucine, and isoleucine. Interestingly, this metabolic pathway of amino acids has been identified as crucial for strain growth (Wang,Zhao et al., 2021). Metabolites of leucine, isoleucine, and valine, such as 2-methylpropionic acid, 3-methylbutyric acid, 2-methylbutyric acid, and 3-methylbutyric acid, are converted from alpha-keto-isocaproic acid (Guo et al., 2021). We also analyzed 10 other important volatile metabolites, including 2,3-butanedione, acetoin, 2-nonanone, 2-undecanone, heptanal, hexanal, nonanal, 3-methylbutanal, acetic acid, 4-methyl-2-oxovaleric acid and constructed their metabolic pathway. 2,3-butanedione and acetoin are common metabolites of citric acid metabolism and contribute to the perception of butter and cream flavor in dairy products. Methyl ketones such as 2-heptanone and 2-nononone contribute to the moldy surface flavor of blue-veined cheese. 2-undecone imparts fermented milk a floral, herbal flavor (Papaioannou et al., 2021). In relation to the role of aldehydes in fermented milk, hexanal produces a fresh grassy aroma, heptanal offers a rich fat flavor, and nonanal introduces citrus and fatty overtones. Notably, hexanal is a transient compound, primarily due to its reactive chemical nature, often reducing to either acidic compounds or alcohols. Another derivative of amino acid degradation is 3-methylbutanal, a branched aldehyde compound derived enzymatically from isoleucine and leucine and recognized for its potent aroma in fermented milk, Significantly, acetic acid emerges as a primary by-product of LAB fermentation (Dan et al., 2018, Dan et al., 2018). In addition, the key flavour substances were analysed in this study using the aroma activity value (OAV). It is generally accepted that substances with an OAV ≥ 1 make a significant contribution to the flavour of fermented milk and that substances with 0.1 ≤ OAV < 1 are important modifiers of the overall flavour of fermented milk. This study found that substances with OAV ≥ 1 (e.g. 2,3-butanedione, 2-nonanone and 3-methylbutanal) were higher in the GF group than in the other two groups. The percentage of substances with OAV > 0.1 (e.g. 2,3-butanedione, 2-nonanone, 3-methylbutanal, acetoin, heptanal, hexanal and nonanal) was also much higher in the GF group than in the KF and SF groups. This shows that these substances contribute to the aroma and are high in the GF group of species (Yang et al., 2023). Moreover, as seen from the Fig. 6, the contents of these substances are generally high in the fermented milk group of goat yogurt, with only the acetoin content being low. In this study, it can be seen that 2.3-butanedione is more enriched. It was found that the combination of 2,3-butanedione and acetoin can provide mild and pleasant butter taste of yogurt, while acetoin tends to reduce the irritation of 2, 3-butanedione. Acetoin can be converted from 2,3-butanedione through diacetyl reductase. Diacetyl reductase activity may have decreased after fermentation, limiting 2,3-butanedione conversion to acetoin and causing 2,3-butanedione accumulation (Peng et al., 2022).
5. Conclusion
In conclusion, this study investigated the effects of different isolation sources of L. lactis subsp. lactis on the volatile metabolite profiles of fermented milk. The HS-SPME-GC-MS analysis revealed significant variations in the volatile compositions of milk fermented with strains isolated from koumiss, goat yogurt, and regular yogurt. Compared to the other strains, the goat yogurt isolates notably enhanced the formation of crucial flavor compounds like aldehydes, ketones, and organic acids in fermented milk. It mainly stimulated the production of aroma-contributing such as 3-methylbutanal, 2,3-butanedione, acetoin, 2-undecanone, heptanal, hexanal, nonanal, 2-nonanone, acetic acid and 4-methyl-2-oxovaleric acid. Moreover, metabolic profiling indicated that strains isolated from goat yogurt significantly promoted the biosynthesis of the branched-chain amino acids valine, leucine, and isoleucine. Overall, this study demonstrates that the isolation source of L. lactis subsp. lactis can markedly influence the volatile metabolite production during milk fermentation, which has important implications for starter culture selection in dairy fermentations. The metabolomic data provides valuable insights into the effects of different isolates on flavor formation and guides the targeted application of strains for enhanced aroma development in fermented dairy foods.
CRediT authorship contribution statement
Xia Yu: Writing – original draft, Investigation, Validation, Visualization. Yaru Sun: Writing – original draft, Investigation, Validation, Visualization. Xin Shen: Writing – review & editing. Weicheng Li: Investigation. Hongyu Cai: Investigation. Shuai Guo: Investigation. Zhihong Sun: Conceptualization, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was funded by the Research Fund for the National Key R&D Program of China (2022YFD2100700), the earmarked fund for CARS36, the Basic Research Expenses for universities directly under the Inner Mongolia Autonomous Region in 2022 (BR22-14-01), the Yunnan Workstation of Academicians and Experts (202105AF150055), and National Dairy Science and Technology Innovation Center (2022- Open Project-6).
Data availability
Data will be made available on request.
References
- Afzaal M., Saeed F., Anjum F., Waris N., Husaain M., Ikram A., Ateeq H., Muhammad Anjum F., Suleria H. Nutritional and ethnomedicinal scenario of koumiss: A concurrent review. Food Science & Nutrition. 2021;9(11):6421–6428. doi: 10.1002/fsn3.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán-Barrientos L.M., Garcia H.S., Reyes-Díaz R., Estrada-Montoya M.C., Torres-Llanez M.J., Hernández-Mendoza A., González-Córdova A.F., Vallejo-Cordoba B. Cooperation between Lactococcus lactis NRRL B-50571 and NRRL B-50572 for aroma formation in fermented milk. Foods. 2019;8(12) doi: 10.3390/foods8120645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zhao S., Hao G., Yu H., Tian H., Zhao G. Role of lactic acid bacteria on the yogurt flavour: A review. International Journal of Food Properties. 2017;20(sup1):S316–S330. doi: 10.1080/10942912.2017.1295988. [DOI] [Google Scholar]
- Chen Xiao G.u., Zixuan P.Y., Young Q.S. What happens to commercial camembert cheese under packaging? Unveiling biochemical changes by untargeted and targeted metabolomic approaches. Food Chemistry. 2022;383 doi: 10.1016/j.foodchem.2022.132437. [DOI] [PubMed] [Google Scholar]
- Dan T., Chen H., Li T., Tian J., Ren W., Zhang H., Sun T. Influence of Lactobacillus plantarum P-8 on fermented milk flavor and storage stability. Frontiers in Microbiology. 2018;9:3133. doi: 10.3389/fmicb.2018.03133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan T., Jin R., Ren W., Li T., Chen H., Sun T. Characteristics of milk fermented by Streptococcus thermophilus MGA45-4 and the profiles of associated volatile compounds during fermentation and storage. Molecules. 2018;23(4):878. doi: 10.3390/molecules23040878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan T., Ren W., Liu Y., Tian J., Chen H., Li T., Liu W. Volatile flavor compounds profile and fermentation characteristics of milk fermented by Lactobacillus delbrueckii subsp. bulgaricus. Frontiers in Microbiology. 2019;10:2183. doi: 10.3389/fmicb.2019.02183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan T., Wang D., Jin R.L., Zhang H.P., Zhou T.T., Sun T.S. Characterization of volatile compounds in fermented milk using solid-phase microextraction methods coupled with gas chromatography-mass spectrometry. Journal of Dairy Science. 2017;100 doi: 10.3168/jds.2016-11528. [DOI] [PubMed] [Google Scholar]
- Dan T., Wang D., Wu S., Jin R., Ren W., Sun T. Profiles of volatile flavor compounds in milk fermented with different proportional combinations of Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus. Molecules. 2017;22(10) doi: 10.3390/molecules22101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dos Santos W.M., Guimarães Gomes A.C., de Caldas S., Nobre M., Souza Pereira Á.M., dos Santos V., Pereira E., Olbrich dos Santos K.M., Florentino E.R., Alonso Buriti F.C. Goat milk as a natural source of bioactive compounds and strategies to enhance the amount of these beneficial components. International Dairy Journal. 2023;137 doi: 10.1016/j.idairyj.2022.105515. [DOI] [Google Scholar]
- Gallegos J., Arce C., Jordano R., Arce L., Medina L.M. Target identification of volatile metabolites to allow the differentiation of lactic acid bacteria by gas chromatography-ion mobility spectrometry. Food Chemistry. 2017;220:362–370. doi: 10.1016/j.foodchem.2016.10.022. [DOI] [PubMed] [Google Scholar]
- Gauglitz J.M., Aceves C.M., Aksenov A.A., Aleti G., Almaliti J., Bouslimani A., Brown E.A., Campeau A., Caraballo-Rodríguez A.M., Chaar R., da Silva R.R., Demko A.M., Di Ottavio F., Elijah E., Ernst M., Ferguson L.P., Holmes X., Jarmusch A.K., Jiang L., Kang K.B., Koester I., Kwan B., Li J., Li Y., Melnik A.V., Molina-Santiago C., Ni B., Oom A.L., Panitchpakdi M.W., Petras D., Quinn R., Sikora N., Spengler K., Teke B., Tripathi A., Ul-Hasan S., van der Hooft J.J.J., Vargas F., Vrbanac A., Vu A.Q., Wang S.C., Weldon K., Wilson K., Wozniak J.M., Yoon M., Bandeira N., Dorrestein P.C. Untargeted mass spectrometry-based metabolomics approach unveils molecular changes in raw and processed foods and beverages. Food Chemistry. 2020;302 doi: 10.1016/j.foodchem.2019.125290. [DOI] [PubMed] [Google Scholar]
- Gu Z., Jin Z., Schwarz P., Rao J., Chen B. Uncovering aroma boundary compositions of barley malts by untargeted and targeted flavoromics with HS-SPME-GC-MS/olfactometry. Food Chemistry. 2022;394 doi: 10.1016/j.foodchem.2022.133541. [DOI] [PubMed] [Google Scholar]
- Guo S., Chen M., Wu T., Liu K., Zhang H., Wang J. Probiotic Bifidobacterium animalis ssp. lactis Probio-M8 improves the properties and organic acid metabolism of fermented goat milk. Journal of Dairy Science. 2022;105(12):9426–9438. doi: 10.3168/jds.2022-22003. [DOI] [PubMed] [Google Scholar]
- Guo S., Wu T., Peng C., Wang J., Sun T., Zhang H. Metabolic footprint analysis of volatile metabolites by gas chromatography-ion mobility spectrometry to discriminate between different fermentation temperatures during Streptococcus thermophilus milk fermentation. Journal of Dairy Science. 2021;104(8):8541–8553. doi: 10.3168/jds.2020-19555. [DOI] [PubMed] [Google Scholar]
- Li W., Ren M., Duo L., Li J., Wang S., Sun Y., Li M., Ren W., Hou Q., Yu J., Sun Z., Sun T. Fermentation Characteristics of Lactococcus lactis subsp. lactis Isolated From Naturally Fermented Dairy Products and Screening of Potential Starter Isolates. Frontiers in Microbiology. 2020;11:1794. doi: 10.3389/fmicb.2020.01794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Yang P., Wang H., Song H. Identification of odor compounds and odor-active compounds of yogurt using DHS, SPME, SAFE, and SBSE/GC-O-MS. LWT. 2022;154 doi: 10.1016/j.lwt.2021.112689. [DOI] [Google Scholar]
- Yuecheng M., Xuliang C., Zhehang S., Yanhua L.i., Dongdong C., Sheng F., Jie C. Exploring core microbiota responsible for the production of volatile flavor compounds during the traditional fermentation of Koumiss. LWT. 2021;135 doi: 10.1016/j.lwt.2020.110049. [DOI] [Google Scholar]
- Min Z., Yunyun J., Miao C., Zhennai Y. Characterization and ACE Inhibitory Activity of Fermented Milk with Probiotic Lactobacillus plantarum K25 as Analyzed by GC-MS-Based Metabolomics Approach. Journal of Microbiology and Biotechnology. 2020;30(6):903–911. doi: 10.4014/jmb.1911.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mituniewicz-Małek A., Zielińska D., Ziarno M. Probiotic monocultures in fermented goat milk beverages – sensory quality of final product. International Journal of Dairy Technology. 2019;72 doi: 10.1111/1471-0307.1257. [DOI] [Google Scholar]
- Moineau-Jean A., Raymond Y., Sabik H., Graveline N., Champagne C.P., Roy D., LaPointe G. Effect of manufacturing processes and storage on aroma compounds and sensory properties of yoghurt. International Dairy Journal. 2020;105 doi: 10.1016/j.idairyj.2020.104662. [DOI] [Google Scholar]
- Papaioannou, G., Kosma, I., Badeka, A. V., & Kontominas, M. G.. (2021). Profile of volatile compounds in dessert yogurts prepared from cow and goat milk, using different starter cultures and probiotics. 10(12): 3153. https://doi.org/10.3390/foods10123153. [DOI] [PMC free article] [PubMed]
- Peng C., Yao G., Sun Y., Guo S., Wang J., Mu X., Sun Z., Zhang H. Comparative effects of the single and binary probiotics of Lacticaseibacillus casei Zhang and Bifidobacterium lactis V9 on the growth and metabolomic profiles in yogurts. Food Research International. 2022;152 doi: 10.1016/j.foodres.2021.110603. [DOI] [PubMed] [Google Scholar]
- Rakhmanova A., Wang T., Xing G., Ma L., Hong Y., Lu Y., Xin L., Xin W., Zhu Q., Lü X. Isolation and identification of microorganisms in Kazakhstan koumiss and their application in preparing cow-milk koumiss. Journal of Dairy Science. 2021;104(1):151–166. doi: 10.3168/jds.2020-18527. [DOI] [PubMed] [Google Scholar]
- Sun Y., Peng C., Wang J., Guo S., Sun Z., Zhang H. Mesopic fermentation contributes more to the formation of important flavor compounds and increased growth of Lactobacillus casei Zhang than does high temperature during milk fermentation and storage. Journal of Dairy Science. 2022;105(6):4857–4867. doi: 10.3168/jds.2021-20949. [DOI] [PubMed] [Google Scholar]
- Sun Y., Peng C., Wang J., Sun H., Guo S., Zhang H. Metabolic footprint analysis of volatile metabolites to discriminate between different key time points in the fermentation and storage of starter cultures and probiotic Lactobacillus casei Zhang milk. Journal of Dairy Science. 2021;104(3):2553–2563. doi: 10.3168/jds.2021-20949. [DOI] [PubMed] [Google Scholar]
- Tang H., Ma H., Hou Q., Li W., Xu H., Liu W., Sun Z., Haobisi H., Menghe B. Profiling of koumiss microbiota and organic acids and their effects on koumiss taste. BMC Microbiology. 2020;20(1):85. doi: 10.1186/s12866-020-01773-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Jing Y., Yu H., Huang J., Yuan H., Lou X., Wang B., Xu Z., Chen C. Effect of alsD deletion and overexpression of nox and alsS on diacetyl and acetoin production by Lacticaseibacillus casei during milk fermentation. Journal of Dairy Science. 2022;105(4):2868–2879. doi: 10.3168/jds.2021-21163. [DOI] [PubMed] [Google Scholar]
- Huaixiang T., Xiong Juanjuan Y.u., Haiyan C.C., Xinman L. Flavor optimization in dairy fermentation: From strain screening and metabolic diversity to aroma regulation. Trends in Food Science & Technology. 2023;141 doi: 10.1016/j.tifs.2023.104194. [DOI] [Google Scholar]
- Tian H., Yang R., Sun X., Yu H., Huang J., Yuan H., Lou X., Yuan Z., Chen C. Screening of goaty flavor-inhibiting lactic acid bacteria and their effects on the flavor profiles of goat milk cakes. Food Bioscience. 2023;53 doi: 10.1016/j.fbio.2023.102504. [DOI] [Google Scholar]
- Verruck S., Dantas A., Prudêncio E.S. Functionality of the components from goat’s milk, recent advances for functional dairy products development and its implications on human health. Journal of Functional Foods. 2019;52:243–257. doi: 10.1016/j.jff.2018.11.017. [DOI] [Google Scholar]
- Wang J., Sun H., Guo S., Sun Y., Kwok L.Y., Zhang H., Peng C. Comparison of the effects of single probiotic strains Lactobacillus casei Zhang and Bifidobacterium animalis ssp. lactis Probio-M8 and their combination on volatile and nonvolatile metabolomic profiles of yogurt. Journal of Dairy Science. 2021;104(7):7509–7521. doi: 10.3168/jds.2020-20099. [DOI] [PubMed] [Google Scholar]
- Wang J., Zhao W., Guo S., Sun Y., Yao K., Liu Z., Sun Z., Kwok L.Y., Peng C. Different growth behaviors and metabolomic profiles in yogurts induced by multistrain probiotics of Lactobacillus casei Zhang and Bifidobacterium lactis V9 under different fermentation temperatures. Journal of Dairy Science. 2021;104(10):10528–10539. doi: 10.3168/jds.2021-20352. [DOI] [PubMed] [Google Scholar]
- Wang, X., Kristo, E., & LaPointe, G. (2020). Adding apple pomace as a functional ingredient in stirred-type yogurt and yogurt drinks. Food Hydrocolloids. 100: Not Available. https://doi.org/10.1016/j.foodhyd.2019.105453.
- Yang, W., Liu, J., Liu, H., Zhang, Q., Lv, Z., & Jiao, Z. (2023). Characterization of strawberry purees fermented by Lactobacillus spp. based on nutrition and flavor profiles using LC-TOF/MS, HS-SPME-GC/MS and E-nose. LWT 189: 115457. https://doi.org/10.1016/j.lwt.2023.115457.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.