Highlights
-
•
DP and CMDP were used to improve the emulsifying properties of WPI.
-
•
The stability of oil-in-water emulsions was increased by DP-WPI and CMDP-WPI.
-
•
DP and CMDP effectively enhanced the antioxidant capacity of emulsions during storage.
-
•
CMDP-WPI exhibited better effects on the stability of emulsions than DP-WPI.
Keywords: Dandelion polysaccharide, Carboxymethylation, Whey protein isolate, Emulsifying property, Antioxidant capacity
Abstract
In this study, the effects of dandelion polysaccharide (DP) and its carboxymethylated derivative (CMDP) on the emulsifying characteristics and antioxidant capacities of emulsions stabilized by whey protein isolate (WPI) were determined. The addition of both DP and CMDP reduced the particle size and zeta potential of the emulsions. Using 1.0 % WPI and 1.0 % CMDP as emulsifier, the emulsifying activity index (EAI) and emulsifying stability index (ESI) were 32.61 ± 0.11 m2/g and 42.58 ± 0.13 min, respectively, which were higher than the corresponding values of 27.19 ± 0.18 m2/g and 36.17 ± 0.15 min with 1.0 % WPI and 1.0 % DP. Fourier-transform infrared spectroscopy (FT-IR), far-ultraviolet circular dichroism (Far-UV CD), and fluorescence (FS) spectra analyses confirmed that the α-helix and β-sheet structures in WPI-polysaccharide complexes were reduced compared with those in pure WPI, whereas the random-coil content was enhanced by the addition of polysaccharides. Moreover, DP and CMDP effectively improved the antioxidant capacity and inhibited oxidation of the emulsions during storage. Therefore, DP and its carboxymethylated derivative exhibit great potential to be applied in the emulsion-based delivery system.
Introduction
Oil-in-water (O/W) emulsions are unstable systems where oil droplets are dispersed in an aqueous medium. One effective method to improve the O/W emulsion stability is to use proteins as emulsifiers. Whey protein isolate (WPI), as one of the most important whey protein products, is extensively applied in food processing owing to its high nutritional quality, comprising more than 90 % protein, and good functional properties, including gel, foaming, and emulsifying properties (Shi, et al., 2021). However, natural WPI is sensitive to temperature and has poor oxidative stability, and the emulsifying ability of WPI is greatly influenced by environmental factors (particularly pH and ions) (Bai et al., 2019, Zhang et al., 2021, Zhang et al., 2021). To resolve this issue, there have been extensive research efforts toward identifying effective measures to improve the functional properties of WPI.
Previous studies showed that the ability of WPI to stabilize O/W emulsions can be effectively enhanced by non-covalent interactions with polysaccharides (Sarraf et al., 2021, Li et al., 2022, Li and Meeren, 2022, Hong et al., 2022). Wang et al. (2020) reported that soy hull polysaccharides could delay the flocculation of droplets and improve the stability of emulsions against creaming. Nooshkam and Varidi (2021) also found that emulsions stabilized using protein-polysaccharide complexes with a homogenous distribution had smaller droplet sizes than those obtained using protein alone, and a higher interfacial protein concentration and better viscosity were also obtained with the protein–polysaccharides complex (Nooshkam & Varidi, 2021). Over the past five years, we have been studying various aspects of dandelion polysaccharide (DP), a main component of dandelion roots. DP has a molecular weight of 10.82 kDa, comprising six monosaccharides: mannose, rhamnose, galacturonic acid, glucose, galactose, and arabinose (Li et al., 2023). We previously confirmed that DP has antioxidant activity and immune-enhancing activity (Wang et al., 2021). We also found that DP had inhibitory effects on the pasting behaviors of corn starch and decreased the digestibility of corn starch (Gao et al., 2021). Importantly, DP could promote the formation of WPI gels and improve their water holding capacity (WHC) (Li et al., 2023). These physicochemical properties indicated that the DP might have advantages for the formation and stability of emulsions.
Carboxymethylation modification has been shown to increase the water solubility and bioactivity of native polysaccharides (Xie et al., 2021, Zhao et al., 2022, Duan et al., 2020). Wei et al. found that the carboxymethylation of corn fiber gums enhanced the physical stability of pea protein dispersions (Wei, Lou, Cai, Li, & Zhang, 2022). Wang et al. further showed that carboxymethylation could increase the water solubility and antioxidant activity of the polysaccharide from Tremella fuciformis (Wang, Zhang, & Zhao, 2015). Similarly, we found that the DP derivative (CMDP) obtained after carboxymethylation showed better thermal performance and superior gelling properties. CMDP also showed a stronger influence on the WHC and formation of WPI gels than that of unmodified DP (Li et al., 2023). However, the effects of DP and CMDP on the emulsifying characteristics of O/W emulsions stabilized by WPI have not been reported to date. Accordingly, the aim of this study was to further investigate the emulsifying properties of two polysaccharides and determine the influence of carboxymethylation modification on the emulsifying capacity of DP. In addition, we compared the effects of DP and CMDP on the antioxidant ability of O/W emulsions.
Materials and methods
Materials
WPI (purity, 99 %) was purchased from XABC Biotech Co. (Xian, Shanxi, China). Dandelion roots (Taraxacum officinale) were purchased from a local drugstore (Harbin, Heilongjiang, China). Soybean oil (relative density of 0.920, iodine value of 130 g/100 g, saponification value of 190 mg/g) was purchased from a local natural food market (Harbin, Heilongjiang, China). Chloroacetic acid and isopropyl alcohol were obtained from J & K Scientific Ltd. (Beijing, China). All chemicals were of analytical grade.
Preparation of DP and CMDP
DP was obtained by ultrasonic-assisted extraction and purification on a D4006 macroporous resin column. CMDP was prepared after carboxymethylation modification by a solvent method. The detailed procedure and technological conditions were described in our previous report (Li et al., 2023).
Preparation and structure characterization of WPI-polysaccharide complexes
Preparation of WPI-polysaccharide complexes
Deionized water was applied to prepare WPI solutions (1.0 %, w/v), followed by magnetic stirring for 2 h, and then the polysaccharides (DP or CMDP) were added to the WPI solution to obtain mixed solutions with different concentrations (0 %, 0.25 %, 0.50 %, 0.75 %, and 1.0 %, w/v). The mixtures were stirred at 400 rpm for 2 min to dissolve the polysaccharides completely.
FT-IR
The WPI-polysaccharide complexes with different polysaccharide concentrations were prepared according to the method described in Section 2.3.1. After freeze-drying, the samples were mixed with potassium bromide, ground into a powder, and recorded on an FT-IR spectrometer (FTS135, PerkinElmer Corporation, Hopkinton, MA, USA) over a range of 4000–400 cm−1 with a resolution of 2 cm−1. The transmissivity of original spectra was analyzed using OMNIC software (Version 8.0, Thermo Nicolet, New York, NY, USA).
Far-UV CD
The WPI sample (10 mg) was dissolved in 100 mL phosphate-buffered saline (PBS; pH 7.0, 0.01 mol/L), and DP or CMDP was added to obtain complexes with different polysaccharide concentrations (0 %, 0.25 %, 0.50 %, 0.75 %, and 1.0 %, w/v). The mixed solutions were stirred at 400 rpm for 2 min using a magnet stirrer and then analyzed using a J-815CD instrument (JASCO, Japan) at 25 °C. The test conditions were as follows: wavelength range of 190–250 nm, xenon lamp (150 W), scan rate of 100 nm/min, bandwidth of 1 nm, and cumulative time of 3. The secondary structure of proteins was analyzed using the CDpro program.
FS analysis
Sample solutions were prepared as described in Section 2.3.3 and then recorded on a fluorescence spectrophotometer (HITACHI F-4700, Osaka, Japan) with an excitation wavelength of 290 nm. The emission wavelength ranged from 300 to 400 nm, the light source was a xenon lamp, the excitation slit width was 5 nm, and the scan rate was 240 nm/min.
Determination of the contact angle and surface tension
The contact angles (θ, air–water) of the polysaccharide (1.0 %, w/v), WPI (1.0 %, w/v), and WPI-polysaccharide complexes (0 %, 0.25 %, 0.50 %, 0.75 %, and 1.0 %, w/v) were measured by the sitting-drop method using a contact-angle goniometer (Theta Lite, Biolin, Finland) at 25 °C (Liu et al., 2024). The surface tension of the polysaccharide, WPI, and WPI-polysaccharide complexes was measured by the hanging-drop method according to the instrument manual.
Preparation of emulsions stabilized by WPI-polysaccharide complexes
The emulsions were prepared according to the method of Iqbal et al. (Iqbal, Xu, Huang, & Chen, 2019). First, the polysaccharides (DP or CMDP) were added to the WPI solution (1.0 %, w/v) to obtain mixed solutions with different polysaccharide concentrations (0 %, 0.25 %, 0.50 %, 0.75 %, and 1.0 %, w/v). The mixed solutions were stirred at 400 rpm for 2 min using a magnet stirrer and then the soybean oil were added to the mixtures to generate 30 % oil in the final emulsion at room temperature. Lab-scale manufacturing of emulsions was carried out at 10000 rpm for 2 min using a high-speed homogenizer (FT200-SH, Shanghai, China).
Determination of the emulsion characteristics
Emulsifying activity and stability
The EAI and ESI were calculated according to turbidimetric method with minor modifications (Erçelebi & Ibanoğlu, 2009). The newly prepared emulsion (50 μL) was diluted with sodium dodecyl sulfate solution (5 mL, 0.1 % w/v). The absorbance (A0) of the mixture at 500 nm was measured immediately and then measured again after 10 min. EAI and ESI were calculated using the following formulas:
| (1) |
| (2) |
where N is the dilution factor (N = 100), C is the protein concentration (g/mL), φ is the proportion of the oil phase in the emulsion, and A0 and A10 are the absorbance of the emulsion at 0 min and 10 min, respectively.
Surface morphology
Freshly prepared emulsions were placed on slides and then covered with coverslips. An optical microscope (XSP-19C digital microscope, Batto Instruments Ltd., China) with a 40 × oil-immersion objective was used to capture images of the materials.
Particle size
Droplet sizes and the particle distribution of the fresh emulsions and the polysaccharides (1.0 %, w/v) were measured using a laser-diffraction particle size distribution analyzer (FJUL-1076, Batto Instruments Ltd., Shanghai, China). Before analysis, all the samples were diluted with 0.01 mol/L PBS and then stirred for 2 min to ensure complete dispersion. All measurements were performed at room temperature.
Zeta potential
The zeta potential of emulsions and the polysaccharides (1.0 % w/v) were determined by a Zetasizer Nano ZS90 instrument (Malvern Instrument Co., Ltd., Malvern, UK) at room temperature. The samples used for measurements were diluted with 0.01 mol/L phosphate buffer (1:100).
Rheological property measurements of emulsions
The prepared fresh emulsions samples were placed on the bottom plate of a rheometer (DHR-1, MAS Instruments Inc., USA); a 1-mm parallel plate was set up with a gap of 0.5 mm at 25 °C. After 1 min of equilibrium, the steady shear experiment was executed using the rheometer within the range of 0.1–––100 s−1. The following power-law equation (Equation (3) was used to calculate the viscoelastic properties of the samples:
| (3) |
where τ, k, γ, and n denote the shear stress (Pa), consistency index (Pa・sn), shear rate (s−1), and flow behavior index, respectively.
The linear viscoelastic region was measured with the strain ranging from 0.1 % to 100 % at a constant frequency of 1 Hz. The result showed 1 % strain can be used as the dynamic oscillatory measurement. Then frequency sweep experiments of emulsions at a fixed shear strain (1 %) were performed to determine changes in elastic (G′) and viscous (G″) moduli with an increasing frequency range (0.1–––10 rad/s).
Determination of the influence of polysaccharides on emulsion antioxidant capacities
Radical-scavenging activities
The 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS)-scavenging abilities of the sample solutions (WPI-polysaccharide complexes and emulsions stabilized by the WPI-polysaccharide complexes) at different polysaccharide concentrations (0 %, 0.25 %, 0.50 %, 0.75 %, and 1.0 %, w/v) were determined using 96-well plates. With gentle stirring, a 7 mmol ABTS solution was added to 2.45 mmol K2S2O8 solution (1:1, v/v) for 16 h in the dark and then the mixture was diluted in PBS solution until the absorbance reached 0.70 ± 0.02 at 734 nm. The absorbance of the reaction mixture comprising 100.0 μL samples and the 3 mL ABTS-PBS solution was measured at 734 nm using a microplate reader (Multiskan FC, Thermo Fisher, USA). Vitamin C (Vc) was set as the positive control. The ABTS radical-scavenging activity was calculated according to a previously reported method (Li et al., 2021).
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging capacities of the samples were also evaluated according to the method of a previous study with modification (Cai, Chen, Yi, & Zou, 2019). Briefly, 100 μL of the sample solutions (WPI-polysaccharide complexes and emulsions stabilized by the WPI-polysaccharide complexes) at different polysaccharide concentrations (0 %, 0.25 %, 0.50 %, 0.75 %, and 1.0 %, w/v) were prepared and mixed with DPPH-ethanol solution (0.3 mmol, 2.0 mL). The mixture was shaken vigorously and stored at 37 ℃ for 30 min in the dark. The absorbance of the sample was recorded at 517 nm and Vc was used as the reference material.
Ferric-reducing antioxidant power
The ferric-reducing antioxidant power was determined according to a previous report with appropriate modification (Li et al., 2021). In brief, 100 µL of sample solutions (WPI-polysaccharide complexes and emulsions stabilized by WPI- polysaccharide) were added to 0.5 mL of the potassium ferricyanide reagent and then incubated at 50 ℃ for 20 min. The reaction was terminated by adding 0.5 mL trichloroacetic acid (10.0 %, w/v). After centrifugation, 0.5 mL of the subnatant was added to 0.6 mL FeCl3 reagent (0.1 %, w/v). The absorbance value of the filtrate was measured at 700 nm. The reduction capacity of Fe3+ is proportional to the absorbance value.
Statistical analysis
Measurements were carried out in triplicates and data are expressed as mean ± standard deviation. Data analysis was performed using SAS version 9.4 software (SAS Institute Inc., Cary, NC, USA). One-way analysis of variance was used as the statistical analysis technique. Statistical significance was established at p < 0.05.
Results
Physicochemical properties of DP and CMDP
The native polysaccharide from dandelion roots was obtained and labeled DP. After modification, the CMDP was prepared. Both DP and CMDP contained the same monosaccharides (d-mannose, l-rhamnose, l-arabinose, d-glucose, d-galactose, and d-galacturonic acid) with different molar ratios. The molecular weight of CMDP was 69,800 Da, which was markedly lower than that of DP at 108,200 Da. In addition, CMDP had better solubility (93.66 ± 1.02 %) than DP (76.66 ± 1.22 %). These detailed data have been published in our previous study (Li et al., 2023).
Structure characterization of WPI-polysaccharide complexes
FT-IR analysis
The FT-IR spectra of WPI and WPI-polysaccharide complexes are presented in Fig. 1A and Fig. 1B, respectively. WPI presented three characteristic bands, including an amide I band at 1700–1600 cm−1, an amide II band at 1550–1500 cm−1, and an amide III band at 1300–1200 cm−1 (Du, Chen, Jiang Yang, & Fu, 2022). When the polysaccharide was added to WPI, the characteristic absorption peaks of WPI were not changed significantly, indicating that no new covalent bonds were produced between WPI and the polysaccharide. All samples exhibited strong and broad bands between 3700 and 3100 cm−1, which were attributed to O—H and N—H stretching vibrations. The absorption peaks of WPI-DP and WPI-CMDP complexes increased from 3353 cm−1 to 3360 cm−1 and 3368 cm−1, respectively, when the polysaccharide concentration was 1.0 %, indicating the existence of hydrogen bonding between WPI and the polysaccharide (Wang, Zhao, Jiang, Ban, & Wang, 2023). The interaction between CMDP and WPI was stronger due to the introduction of carboxymethyl groups; thus, the influence of CMDP on WPI was greater than that of DP under the same conditions. Moreover, the amide I band changed from 1654 cm−1 to 1645 cm−1 and 1644 cm−1 after the addition of DP and CMDP, respectively. This result confirmed that the binding of the polysaccharide to WPI caused the protein to unscrew and transform into a random-coil structure.
Fig. 1.
FT-IR spectra of pure WPI, WPI-DP complexes and WPI-CMDP complexes.
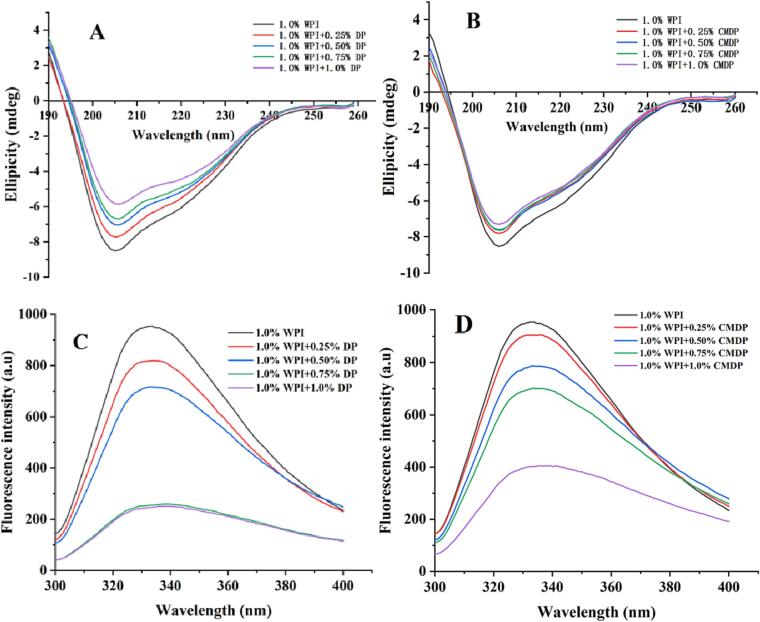
Far-UV CD analysis
Far-UV (190–260 nm) CD measurement is commonly used to analyze the changes of the secondary structure of proteins caused by combining with other polymers (Qu et al., 2018). As shown in Fig. 2A and Fig. 2B, the positive band within 190–200 nm and the negative band at 216 nm confirmed the existence of the β-sheet structure. The negative bands at 208 nm and 222 nm indicated the existence of α-helix structures. The contents of α-helices, β-sheets, and random coils in different samples were shown in Table 1, the α-helix and β-sheet structures in WPI-polysaccharide complexes were reduced compared with those in pure WPI, whereas the content of random coils was enhanced. This was consistent with the results of FT-IR spectroscopy. When the concentration of the polysaccharide was 1.0 %, the content of β-sheet structures decreased from 31.36 ± 0.10 % in WPI to 27.02 ± 0.15 % in the WPI-DP complex and to 27.36 ± 0.12 % in the WPI-CMDP complex. The content of α-helix structures decreased from 18.62 ± 0.02 % in WPI to 16.34 ± 0.02 % in the WPI-DP complex and 15.95 ± 0.11 % in the WPI-CMDP complex. These data demonstrated that DP produced a more significant effect on the β-sheet structure, whereas CMDP had a greater impact on the α-helix structure at the same polysaccharide concentrations. However, these differences were not particularly strong. It is possible that CMDP introduced a larger amount –OH and –COOH groups, thereby causing a greater reduction in α-helices to unordered structures.
Fig. 2.
Far-UV CD spectra of WPI-DP (A) and WPI-CMDP (B) complexes; FS spectra of WPI-DP (C) and WPI-CMDP (D) complexes.
Table 1.
Contents of the secondary structures in WPI and WPI-polysaccharide complexes.
| Sample | β-sheet (%) | α-helix (%) | Random coil (%) | R2 |
|---|---|---|---|---|
| 1.0 %WPI | 31.36 ± 0.10a | 18.62 ± 0.02a | 14.60 ± 0.05a | 0.996 |
| 1.0 %WPI + 0.25 %DP | 29.67 ± 0.08b | 16.77 ± 0.05b | 17.25 ± 0.02b | 0.997 |
| 1.0 %WPI + 0.50 %DP | 29.45 ± 0.06b | 15.68 ± 0.12c | 17.26 ± 0.06b | 0.999 |
| 1.0 %WPI + 0.75 %DP | 27.85 ± 0.04c | 17.11 ± 0.06d | 18.38 ± 0.03c | 0.996 |
| 1.0 %WPI + 1.0 %DP | 27.02 ± 0.15d | 16.34 ± 0.02e | 18.34 ± 0.05c | 0.995 |
| 1.0 %WPI + 0.25 %CMDP | 30.22 ± 0.13e | 17.32 ± 0.08f | 16.22 ± 0.12c | 0.998 |
| 1.0 %WPI + 0.50 %CMDP | 29.17 ± 0.09f | 16.02 ± 0.05 g | 17.90 ± 0.08d | 0.998 |
| 1.0 %WPI + 0.75 %CMDP | 26.92 ± 0.08 g | 16.15 ± 0.08 g | 18.84 ± 0.10e | 0.999 |
| 1.0 %WPI + 1.0 %CMDP | 27.36 ± 0.12 h | 15.95 ± 0.11 h | 18.78 ± 0.05f | 0.999 |
Notes: Values with different letters represented differences (p < 0.05) in the same column.
FS analysis
The fluorescence emission spectra of pure WPI and the polysaccharide-WPI complexes were examined for the evaluation of conformational changes. As shown in Fig. 2C and Fig. 2D, WPI exhibited a fluorescence emission maximum (λmax) at 335 nm. With the increasing amounts of DP and CMDP from 0 to 1.0 %, the λmax of WPI-DP complexes increased from 335 to 339 nm and that of WPI-CMDP complexes reached 340 nm. This red shift indicated that the polysaccharide changed the molecular structure of proteins through non-covalent interactions, which was more apparent in the WPI-CMDP complex. This was consistent with the FT-IR results.
In addition, the fluorescence intensity of the WPI-DP and WPI-CMDP complexes decreased as the polysaccharide concentration increased. This effect was attributed to covalent grafting between the polysaccharide and WPI molecule, resulting in burying of the fluorophore (Xue, Li, Zhu, Wang, & Pan, 2013). Since the molecular weight of DP is slightly greater than that of CMDP, the longer polysaccharide chain could produce a more obvious effect (Sheng et al., 2017).
Contact angle and surface tension of WPI-polysaccharide complexes
A lower contact angle is typically associated with a better hydrophilic characteristic and the formation of compact droplets on the liquid surface (Jha, et al., 2022). As shown in Fig. 3A, the contact angles of WPI-polysaccharide complexes gradually decreased with the addition of polysaccharides. At the same concentrations, the contact angles of WPI-CMDP complexes were smaller than those of WPI-DP complexes. The contact angle of 1.0 % WPI was 32.65°, however, with addition of 1.0 % polysaccharide, the contact angles of WPI-DP and WPI-CMDP were 9.79° and 5.93°, respectively. These data confirmed that CMDP had stronger ability to improve the hydrophilicity of WPI than DP.
Fig. 3.
(A) Contact angle and surface tension of WPI-polysaccharide complexes (a: 1.0 %WPI, b: 1.0 %WPI + 0.25 %DP, c: 1.0 %WPI + 0.50 %DP, d: 1.0 %WPI + 0.75 %DP, e: 1.0 %WPI + 1.0 %DP, f: 1.0 %WPI + 0.25 %CMDP, g: 1.0 %WPI + 0.50 %CMDP, h: 1.0 %WPI + 0.75 %CMDP, i: 1.0 %WPI + 1.0 %CMDP); (B) EAI and ESI of emulsions after adding DP; (C) EAI and ESI of emulsions after adding CMDP.
As shown in Fig. 3A, after mixing the WPI with the polysaccharide, the surface tension was reduced to a greater extent than that of the WPI alone, and the decreasing trend showed a positive correlation with the polysaccharide concentration. In addition, CMDP showed a stronger effect on decreasing the surface tension than DP under the same concentrations. This indicated that the interaction between WPI and the polysaccharide increased the exposure of hydrophobic groups, resulting in the decrease of surface tension (Kato & Nakai, 1980). CMDP has stronger interaction forces with amino acids for the introduction of hydrophilic carboxyl groups; thus, exposure of the hydrophobic groups of WPI after complexation with CMDP was enhanced. This effect is in line with the finding of our previous study (Li et al., 2023).
Effects of DP and CMDP on the emulsion characteristics
Emulsifying activity and stability
EAI and ESI are important indicators of the functional characteristics of an emulsion, which can reflect the ability of the complex to form an emulsified layer and its stability at the O/W interface (Tian et al., 2023). As shown in Fig. 3B and Fig. 3C, both the EAI and ESI values of the emulsions improved with an increasing concentration of the two polysaccharides, and the effect of CMDP was better than that of DP. With a 1.0 % polysaccharide concentration, the EAI and ESI of the WPI-CMDP complex emulsions were 32.61 ± 0.11 m2/g and 42.58 ± 0.13 min, respectively, whereas those of WPI-DP were 27.19 ± 0.18 m2/g and 36.17 ± 0.15 min, respectively. Therefore, CMDP was more suitable for improvement of WPI emulsification than DP. Hong et al. reported that WPI-dextran conjugates prepared via the Maillard reaction had better emulsifying activity than WPI, with an EAI of 17.5 m2/g and ESI of 22.5 min obtained when using a 1.0 % (w/w) WPI-dextran concentration (Hong, Xiao, Li, Li & Xie). This result demonstrated that the WPI-DP and WPI-CMDP complexes have a similar function to that of these previously reported WPI-dextran conjugates.
Particle sizes of emulsions
As shown in Fig. 4, with the addition of WPI-DP and WPI-CMDP, the particle sizes of the emulsions were markedly decreased compared to those of emulsions with addition of pure WPI. Smaller and more homogeneous droplets were also observed in the emulsions containing the polysaccharides. At the same concentration, the emulsion droplets stabilized by WPI-CMDP were smaller than those of emulsions stabilized by WPI-DP. A previous study demonstrated that a smaller particle size was associated with better stability of emulsions (Martínez-Padilla, Sosa-Herrera, & Osnaya-Becerril, 2021). The particle diameters and specific surface areas of the emulsions are summarized in Table 2, clearly demonstrating that the particle diameters were decreased and the specific surface areas were increased with the addition of polysaccharides. The particle sizes of emulsions using WPI-CMDP were smaller than those of emulsions prepared with the addition of WPI-DP at all tested concentrations, indicating that WPI-CMDP had better effects on the stabilizing the emulsions. The specific surface area was inversely correlated with the particle diameter, and particles with a smaller diameter and higher specific surface area resulted in smaller droplets (Fig. 4). Moreover, at concentrations of 1.0 % WPI and 1.0 % polysaccharide, the emulsions stabilized with WPI-CMDP had larger specific surface areas (474.49 ± 0.02 m2/m3) than those obtained using WPI-DP (404.87 ± 0.01 m2/m3), which were both higher than the mean surface area of 201.08 ± 0.11 m2/m3 obtained when using 1.0 % WPI alone. These data showed that WPI-CMDP had a greater contribution to the formation of the emulsions than WPI-DP.
Fig. 4.
Particle size distribution and observation diagram.
Table 2.
Particle size parameters and Zeta potentials of emulsions.
| Sample | Particle diameter (μm) | Specific surface area (m2/m3) | Zeta potential (mV) |
|---|---|---|---|
| 1.0 %DP | 1.48 ± 0.02a | 684.06 ± 0.21a | −8.36 ± 0.45a |
| 1.0 %CMDP | 0.95 ± 0.02b | 729.54 ± 0.21b | −18.68 ± 0.45b |
| 1.0 %WPI | 90.45 ± 0.01c | 201.08 ± 0.11c | −14.46 ± 0.81c |
| 1.0 %WPI + 0.25 %DP | 73.67 ± 0.02d | 229.99 ± 0.12d | −14.53 ± 0.45d |
| 1.0 %WPI + 0.50 %DP | 63.87 ± 0.01e | 270.64 ± 0.11e | −15.16 ± 1.05e |
| 1.0 %WPI + 0.75 %DP | 55.37 ± 0.02f | 323.87 ± 0.02f | −15.56 ± 1.60f |
| 1.0 %WPI + 1.0 % DP | 49.14 ± 0.01 g | 404.87 ± 0.01 g | −16.43 ± 0.74 g |
| 1.0 %WPI + 0.25 %CMDP | 56.27 ± 0.02f | 314.03 ± 0.21 h | −16.43 ± 1.53 g |
| 1.0 %WPI + 0.50 %CMDP | 48.05 ± 0.01 h | 405.33 ± 0.21i | −17.03 ± 0.95 h |
| 1.0 %WPI + 0.75 %CMDP | 45.46 ± 0.02i | 427.06 ± 0.07j | −17.83 ± 0.47i |
| 1.0 %WPI + 1.0 %CMDP | 43.60 ± 0.01 g | 474.49 ± 0.02 k | −17.93 ± 0.36j |
Notes: Values with different letters in the same column represented differences (p < 0.05).
Zeta potential of emulsions
Electrostatic repulsion can strongly affect the stability of emulsions; when the droplet carries a more negative charge, the electrostatic repulsion between the droplets increases and the droplet becomes more stable (Zhong, et al., 2020). A previous study demonstrated that the negative zeta-potential values of WPI were ascribed to carboxyl ionization (–COOH) occurring under neutral conditions (Wang, Pan, Chiou, Li, & Ding, 2022). As illustrated in Table 2, all of the particles exhibited negative charges and the absolute zeta potential was improved with an increase in the concentration of the added polysaccharide. With addition of 1.0 % polysaccharide, the zeta potentials decreased from –14.46 ± 0.81 mV in the emulsion stabilized by pure WPI to –16.43 ± 0.74 mV and –17.93 ± 0.36 mV in the emulsions stabilized by WPI-DP and WPI-CMDP, respectively. Notably, the zeta potentials of 1.0 % CMDP and 1.0 % DP were –18.68 ± 0.45 and –8.36 ± 0.45 mV, respectively. Therefore, CMDP was a more effective stabilizer than DP under the same conditions, which may be attributed to the effects of the carboxymethyl groups.
Rheological properties of the emulsions
Apparent viscosity
As shown in Fig. 5A, the apparent viscosities of all the emulsions steeply decreased when the shear rate increased. This shear-thinning behavior might indicate deformation and disruption of the emulsion droplets with the increase of shear rate (Zhang et al., 2021, Zhang et al., 2021). In this process, the originally entangled macromolecules would separate, leading to a reduction in the resistance to flow, resulting in decreased viscosity. When the concentration of the polysaccharides increased, the apparent viscosity also increased. On the one hand, the addition of polysaccharides reduced the particle sizes of the emulsion and improved the specific surface areas, thereby increasing the droplet number and interaction between droplets to result in an improvement of the apparent viscosity. On the other hand, the binding of polysaccharides to WPI caused the protein to unscrew and transform into a random-coil structure, thereby promoting the formation of larger aggregates, which might also be responsible for the increase in apparent viscosity.
Fig. 5.
Rheological properties of the fresh emulsions stabilized WPI and WPI-polysaccharide at different concentrations. (A) Shear viscosity versus shear rate profiles; (B) The G′ and G″ of emulsions stabilized WPI-DP with the increase of angular frequency; (C) The G′ and G″ of emulsions stabilized WPI-CMDP with the increase of angular frequency.
According to the power-law model, the n values of all samples were less than one, confirming that all the emulsions were non-Newtonian fluids. In addition, the n values were negatively correlated with the polysaccharide concentration and the k values demonstrated an increasing tendency with the polysaccharide concentration (data not shown). These results confirmed that both DP and CMDP could improve the pseudoplasticity of the emulsions. Overall, the apparent viscosities of emulsions stabilized by WPI-polysaccharide complexes were almost equal to those of the emulsions stabilized by WPI alone. There was no significant difference between the effects of the two polysaccharides.
Dynamic rheology
As shown Fig. 5B and Fig. 5C, the G′ and G′′ values of all emulsions increased with improvement of the angular frequency in the range of 0.1–10 rad/s. The G′ value was considerably higher than the G′′ value and gradually improved with the increase of polysaccharides concentration. This result confirmed that both DP and CMDP were conducive to promote the formation of elastic gel-like emulsions and can effectively enhance the stability of the emulsions (Wong, et al., 2021). CMDP exhibited slightly better effects than DP at the same concentration, although there was no significant difference between the two polysaccharides. A reasonable explanation might be that the introduction of carboxymethyl groups from CMDP increased the intermolecular interactions among WPI-polysaccharide complexes, which was beneficial for the elasticity of the emulsion. However, since the molecular weight of CMDP was lower than that of DP, it was easier for DP to form a network structure with WPI due to the longer chain length. These complexes would then be more likely to collide with each other, resulting in an increase of elasticity.
Influence of polysaccharides on the antioxidant capacities of emulsions
The antioxidant activities of the emulsions were first investigated by testing the scavenging abilities against free radicals. As shown in Fig. 6A and Fig. 6B, the scavenging activities of all the samples on ABTS and DPPH radicals were dose-dependent within the tested concentration range. Emulsions stabilized by WPI-CMDP presented better scavenging activities than those stabilized by WPI-DP under the same concentrations. With the addition of 1.0 % polysaccharide, the ABTS radical-scavenging activities of DP, the emulsion stabilized by WPI-DP, CMDP, and the emulsion stabilized by WPI-CMDP were 45.21 ± 1.91 %, 50.17 ± 1.07 %, 70.17 ± 1.44 %, and 80.18 ± 1.91 %, respectively. Similarly, the corresponding DPPH radical-scavenging activities were 36.11 ± 1.42 %, 60.17 ± 1.33 %, 66.19 ± 1.91 %, and 75.23 ± 1.47 %, respectively. This result confirmed that the emulsion stabilized by WPI-CMDP exhibited better scavenging abilities against free radicals than that stabilized by WPI-DP. The reason might be that the antioxidant activity of the polysaccharide was significantly improved after carboxymethylation modification.
Fig. 6.
The antioxidant capacities of the emulsions. (A) ABTS radical scavenging ability; (B) DPPH radical scavenging ability; (C) Ferric-reducing antioxidant power.
In addition, the ferric-reducing antioxidant power of the WPI-stabilized emulsions was demonstrated (Fig. 6C), with a dose-dependent relationship between polysaccharide concentration and reducing power observed. With addition of 1.0 % polysaccharide, the absorbance values of DP, the emulsion stabilized by WPI-DP, CMDP, and the emulsion stabilized by WPI-CMDP were 0.6 ± 0.027, 0.65 ± 0.022, 0.71 ± 0.026, and 0.82 ± 0.024, respectively. This result indicated that the reducing power of CMDP was stronger than that of DP; correspondingly, WPI-CMDP exhibited a better effect on stabilizing the emulsion than WPI-DP.
Discussion
Proteins stabilize emulsions through their adsorption at the interface to provide a combination of electrostatic and steric repulsion of the oil droplets (Ge, Iqbal, Kirk, & Chen, 2021). When proteins and polysaccharides are mixed in O/W emulsions, there is a possibility that the protein-polysaccharide complex could lead to better adsorption on the O/W interface and form a dense interfacial film to prevent the aggregation of oil droplets (Shao et al., 2020). Another possibility is that the proteins will be adsorbed on the O/W interface and the polysaccharide molecules will be adsorbed on the surface of protein-coated droplets; consequently, the polysaccharides will increase the viscosity of the aqueous phase, thus preserving the desired textural properties of the emulsion (Erçelebi & Ibanoğlu, 2010). In the present study, we found that the stability of O/W emulsions prepared using WPI-polysaccharide complexes was better than that of emulsions obtained using pure WPI, with the main advantages reflected in the smaller particle size and lower zeta potential of the former emulsions. The EAI and ESI of the emulsions increased significantly with increasing DP and CMDP concentrations. CD, FT-IR, and FS results confirmed that the secondary structure of the protein changed with the addition of polysaccharides. Moreover, the G' value of the emulsion was frequency-dependent and rose with the increase of polysaccharide concentration. Collectively, these results indicated that the ability of WPI- polysaccharide complexes to stabilize O/W emulsions was significantly better than that of the pure WPI. The reason for this improvement might be that the binding between the polysaccharide and protein via electrostatic molecular associations increased the exposure of hydrophobic moieties of WPI, thereby improving the adsorption behavior of proteins at the O/W interface. Under the same conditions, WPI-CMDP had a greater contribution to the stability of the emulsions than WPI-DP. This effect was attributed to the introduced carboxymethyl group, which increased electrostatic interactions and hydrogen bonding between the polysaccharide and WPI. Another possible reason is the lower molecular weight of CMDP, which caused the exposure of more functional groups such as hydroxyl groups. Therefore, the electrostatic repulsion between droplets was further improved to resist the attractive interaction (Van der Waals interaction) and prevented the formation of larger droplets (Delahaije, Hilgers, Wierenga, & Gruppen, 2017). Our previous study also showed that these two factors were helpful for formation of the better network structure in the WPI-CMDP gel than in the WPI-DP gel (Li et al., 2023).
There are some limitations of this study that should be acknowledged. The current results only represent preliminary evidence for the application of DP in an emulsion system. The O/W emulsions stabilized by the WPI-polysaccharide complex are dependent on many factors, including pH, ionic strength, and temperature (Antonov, Moldenaers, & Cardinaels, 2022). Therefore, the stability of emulsions at the pH approaching the isoelectric point of the protein with high ionic strength and under high temperature will be studied in future. In addition, the safety of DP has not yet been confirmed, which is another aspect to be addressed in our follow-up research.
Conclusion
In present study, the use of WPI-DP and WPI-CMDP in O/W emulsions narrowed the droplet size distribution and increased their stability against aggregation.
The secondary structure of the protein was changed with addition of polysaccharide and non-covalent interactions existed between WPI and polysaccharide. Under the same conditions, CMDP was more helpful for the enhancement of emulsifying properties than DP by the introduction of carboxymethyl group because of the enhanced electrostatic interactions and hydrogen bonding between polysaccharide and WPI. Overall, this work provides valuable information for the development of emulsions stabilized by WPI-polysaccharide complexes and expands utilization of carboxymethylated polysaccharides in the food industry.
CRediT authorship contribution statement
Yujun Han: Data curation, Investigation, Methodology. Lianyu Li: Investigation, Validation, Writing – original draft. Fangming Wei: Validation. Fengjie Zhang: Software, Writing – original draft. Zhaoyang Pan: Validation. Yanhui Wei: Investigation. Libo Wang: Supervision, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the Natural Science Foundation of Heilongjiang Province (LH2020C026) and Scholar backbone Project of Northeast Agricultural University (No. 19XG02).
Data availability
The data that has been used is confidential.
References
- Antonov Y.A., Moldenaers P., Cardinaels R. Binding of lambda carrageenan to bovine serum albumin and non-equilibrium effects of complexation. Food Hydrocolloids. 2022;126 [Google Scholar]
- Bai L.T., Song Y., Li Q.M., Pan L.H., Zha X.Q., Luo J.P. Emulsifying and physicochemical properties of lotus root amylopectin-whey protein isolate conjugates. LWT-Food Science and Technology. 2019;111:345–354. [Google Scholar]
- Cai L., Chen B., Yi F., Zou S. Optimization of extraction of polysaccharide from dandelion root by response surface methodology: Structural characterization and antioxidant activity. International Journal of Biological Macromolecules. 2019;140:907–919. doi: 10.1016/j.ijbiomac.2019.08.161. [DOI] [PubMed] [Google Scholar]
- Delahaije R.J.B.M., Hilgers R.J., Wierenga P.A., Gruppen H. Relative contributions of charge and surface coverage on pH-induced flocculation of protein stabilized emulsions. Colloids & Surfaces A: Physicochemical & Engineering Aspects. 2017;521:153–160. [Google Scholar]
- Duan S., Zhao M., Wu B., Wang S., Yang Y., Xu Y., et al. Preparation, characteristics, and antioxidant activities of carboxymethylated polysaccharides from blackcurrant fruits. International Journal of Biological Macromolecules. 2020;155:1114–1122. doi: 10.1016/j.ijbiomac.2019.11.078. [DOI] [PubMed] [Google Scholar]
- Erçelebi E.A., Ibanoğlu E. Characterization of phase separation behavior, emulsion stability, rheology, and microstructure of egg white-polysaccharide mixtures. Journal of Food Science. 2009;74(6):506–512. doi: 10.1111/j.1750-3841.2009.01252.x. [DOI] [PubMed] [Google Scholar]
- Erçelebi E.A., Ibanoğlu E. Effects of pectin and guar gum on creaming stability, microstructure and rheology of egg yolk plasma-stabilized emulsions. European Food Research and Technology. 2010;231:297–302. [Google Scholar]
- Gao J., Zhu L., Huang J., Li L., Yang Y., Xu Y., et al. Effect of dandelion root polysaccharide on the pasting, gelatinization, rheology, structural properties and in vitro digestibility of corn starch. Food Function. 2021;12:7029–7039. doi: 10.1039/d1fo00507c. [DOI] [PubMed] [Google Scholar]
- Ge A., Iqbal S., Kirk T.V., Chen X.D. Modulating the rheological properties of oil-in-water emulsions using controlled WPI-polysaccharide aggregation in aqueous phases. Journal of Food Engineering. 2021;297 [Google Scholar]
- Hong Z., Xiao N., Li L., Li Y., Xie X. Glycation of whey protein isolate and emulsions prepared by conjugates. Journal of Food Engineering. 2022;316 [Google Scholar]
- Iqbal S., Xu Z., Huang H., Chen X.D. Controlling the rheological properties of oil phases using controlled protein-polysaccharide aggregation and heteroaggregation in water-in-oil emulsions. Food Hydrocolloids. 2019;96:278–287. [Google Scholar]
- Jha S., Malviya R., Fuloria S., Sundram S., Subramaniyan V., Sekar M., et al. Characterization of microwave-controlled polyacrylamide graft copolymer of tamarind seed polysaccharide. Polymers (Basel) 2022;14:1037. doi: 10.3390/polym14051037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A., Nakai S. Hydrophobicity determined by a fluorescence probe method and its correlation with surface properties of proteins. Biochimica et Biophysica Acta (BBA)-General Subjects. 1980;624(1):13–20. doi: 10.1016/0005-2795(80)90220-2. [DOI] [PubMed] [Google Scholar]
- Li H., Meeren P.V.D. Sequential adsorption of whey proteins and low methoxy pectin at the oil-water interface: An interfacial rheology study. Food Hydrocolloids. 2022;128 [Google Scholar]
- Li J., Geng S., Zhen S., Lv X., Liu B. Fabrication and characterization of oil-in-water emulsions stabilized by whey protein isolate/phloridzin/sodium alginate ternary complex. Food Hydrocolloids. 2022;129 [Google Scholar]
- Li L., Zhang F., Zhu L., Yang Y., Xu Y., Wang L., et al. Carboxymethylation modification, characterization of dandelion root polysaccharide and its effects on gel properties and microstructure of whey protein isolate. International Journal of Biological Macromolecules. 2023;242 doi: 10.1016/j.ijbiomac.2023.124781. [DOI] [PubMed] [Google Scholar]
- Li Q., Shi J., Du X., McClements D.J., Chen X., Duan M., et al. Polysaccharide conjugates from Chin brick tea (Camellia sinensis) improve the physicochemical stability and bioaccessibility of β-carotene in oil-in-water nanoemulsions. Food Chemistry. 2021;357 doi: 10.1016/j.foodchem.2021.129714. [DOI] [PubMed] [Google Scholar]
- Liu C., Wang Y., Dai X., Zhang Y., Yang Y., Jiang B., et al. Post-self-assemble of whey protein isolation nanofibrils and its contribution to the stability of pickering emulsion. Food Hydrocolloids. 2024;151 [Google Scholar]
- Martínez-Padilla L.P., Sosa-Herrera M.G., Osnaya-Becerril M. Effect of the konjac glucomannan concentration on the rheological behaviour and stability of sodium caseinate oil-in-water emulsions. International Dairy Journal. 2021;117 [Google Scholar]
- Nooshkam M., Varidi M. Physicochemical stability and gastrointestinal fate of β-carotene-loaded oil-in-water emulsions stabilized by whey protein isolate-low acyl gellan gum conjugates. Food Chemistry. 2021;347 doi: 10.1016/j.foodchem.2021.129079. [DOI] [PubMed] [Google Scholar]
- Qu W., Zhang X., Han X., Wang Z., He R., Ma H. Structure and functional characteristics of rapeseed protein isolate-dextran conjugates. Food Hydrocolloids. 2018;82:329–337. [Google Scholar]
- Sarraf M., Naji-Tabasi S., Beig-babaei A. Influence of calcium chloride and pH on the soluble complex of basil seed and xanthan gum with whey protein concentrate. Semantic scholar. 2021 doi: 10.22541/AU.162227689.91706758/V1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao P., Feng J., Sun P., Xiang N., Lu B., Qiu D. Recent advances in improving stability of food emulsion by plant polysaccharides. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109376. [DOI] [PubMed] [Google Scholar]
- Sheng L., Su P., Han K., Chen J., Cao A., Zhang Z., et al. Synthesis and structural characterization of lysozyme-pullulan conjugates obtained by the Maillard reaction. Food Hydrocolloids. 2017;71:1–7. [Google Scholar]
- Shi R., Li T., Li M., Munkh-Amgalan G., Qayum A., Bilawal A., et al. Consequences of dynamic high-pressure homogenization pretreatment on the physicochemical and functional characteristics of citric acid-treated whey protein isolate. LWT-Food Science and Technology. 2021;136 [Google Scholar]
- Tian R., Han X., Tian B., Li G., Sun L., Tian S., et al. Effects of covalent binding of different polyphenols on structure, rheology and functional properties of whey protein isolate. LWT-Food Science and Technology. 2023;184 [Google Scholar]
- Wang Q., Pan M., Chiou Y., Li Z., Ding B. Surface characteristics and emulsifying properties of whey protein/nanoliposome complexes. Food Chemistry. 2022;384 doi: 10.1016/j.foodchem.2022.132510. [DOI] [PubMed] [Google Scholar]
- Wang S., Yang J., Shao G., Qu D., Zhao H., Yang L., et al. Soy protein isolated-soy hull polysaccharides stabilized O/W emulsion: Effect of polysaccharides concentration on the storage stability and interfacial rheological properties. Food Hydrocolloids. 2020;101 [Google Scholar]
- Wang L., Li L., Gao J., Huang J., Yang Y., Xu Y., et al. Characterization, antioxidant and immunomodulatory effects of selenized polysaccharides from dandelion roots. Carbohydrate Polymers. 2021;260 doi: 10.1016/j.carbpol.2021.117796. [DOI] [PubMed] [Google Scholar]
- Wang X., Zhang Z., Zhao M. Carboxymethylation of polysaccharides from Tremella fuciformis for antioxidant and moisture-preserving activities. International Journal of Biological Macromolecules. 2015;72:526–530. doi: 10.1016/j.ijbiomac.2014.08.045. [DOI] [PubMed] [Google Scholar]
- Wei Y., Lou N.H., Cai Z., Li R., Zhang H. Carboxymethylated corn fiber gums efficiently improve the stability of native and acidified aqueous pea protein dispersions. Food Hydrocolloids. 2022;133 [Google Scholar]
- Wong S.K., Low L.E., Supramaniam J., Manickam S., Wong T.W., Pang C.H., et al. Physical stability and rheological behavior of Pickering emulsions stabilized by protein-polysaccharide hybrid nanoconjugates. Nanotechnology Reviews. 2021;10(1):1293–1305. [Google Scholar]
- Xie L., Shen M., Wang Z., Xie J. Structure, function and food applications of carboxymethylated polysaccharides: A comprehensive review. Trends in Food Science & Technology. 2021;118:539–557. [Google Scholar]
- Xue F., Li C., Zhu X., Wang L., Pan S. Comparative studies on the physicochemical properties of soy protein isolate-maltodextrin and soy protein isolate-gum acacia conjugate prepared through Maillard reaction. Food Research International. 2013;51(2):490–495. [Google Scholar]
- Zhang X., Qi B., Xie F., Hu M., Sun Y., Han L., Li L., Zhang S., Li Y. Emulsion stability and dilatational rheological properties of soy/whey protein isolate complexes at the oil-water interface: Influence of pH. Food Hydrocolloids. 2021;113 [Google Scholar]
- Zhang B., Meng R., Li X.L., Liu W.J., Cheng J.S., Wang W. Preparation of Pickering emulsion gels based on kappa-carrageenan and covalent crosslinking with EDC: Gelation mechanism and bioaccessibility of curcumin. Food Chemistry. 2021;357 doi: 10.1016/j.foodchem.2021.129726. [DOI] [PubMed] [Google Scholar]
- Zhao T., Guo Y., Yan S., Li N., Ji H., Hu Q., et al. Preparation, structure characterization of carboxymethylated schisandra polysaccharides and their intervention in immunotoxicity to polychlorinated biphenyls. Process Biochemistry. 2022;115:30–41. [Google Scholar]
- Zhong Y., Xiang X., Wang X., Zhang Y., Hu M., Chen T., et al. Fabrication and characterization of oil-in-water emulsions stabilized by macadamia protein isolate/chitosan hydrochloride composite polymers. Food Hydrocolloids. 2020;103 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.