Abstract
Introduction
Fever is one of the postoperative complications of endoscopic submucosal dissection (ESD) and its derivative technology. However, there are few studies on risk factors for fever after ESD and its derivative technology. The aim of this study was to determine the incidence and related risk factors after ESD and its derivative technology for gastric lesions.
Materials and methods
A retrospective review of patients with gastric lesions who were treated by ESD and its derivative technology in our hospital from January 2014 to January 2019 was conducted.
Results
A total of 1955 patients were included in the present study. A total of 451 (23.1 %) patients presented with fever after ESD and its derived techniques. The highest fever temperature was 37.6 ± 3.12 °C, and the number of days with fever was 1.48 ± 0.85. Through single factor and multiple factor analysis, age (OR: 1.261, 95% CI: 1.009–1.576, p < 0.05), procedure time (OR: 1.457, 95% CI: 1.053–2.016, p < 0.05), postoperative gastric tube placement (OR: 2.098, 95% CI: 1:616–2.723, p < 0.05), intraoperative hemorrhage (OR: 1.537, 95% CI: 1.196–1.974, p < 0.05) and perforation (OR: 1.970, 95% CI: 1.531–2.535, p < 0.05) were independent risk factors for postoperative fever.
Conclusion
Age ≥56 years old, procedure time ≥60 min, gastric tube placement, intraoperative hemorrhage and perforation were independent risk factors for postoperative fever after gastric ESD and its derivative technology. Attention should be given to such patients to minimize the risk of postoperative fever.
Keywords: Endoscopic submucosal dissection, Gastric lesions, Gastric tube placement, Fever, Risk factors
1. Introduction
Endoscopic submucosal dissection (ESD) and its derivative technology, such as endoscopic submucosal excavation (ESE) and endoscopic full-thickness resection (EFTR), is now a highly acceptable endoscopic approach for the treatment of gastric mucosal and submucosal lesions, such as early gastric cancer, leiomyomas, and neuroendocrine tumors [[1], [2], [3]]. Compared with traditional surgical treatments, ESD and its derivative technology decrease the incidence of complications, such as bleeding and infections, and shorten the duration of hospitalization [4]. However, ESD and its derivative technology are also associated with some complications, such as bleeding, perforation, and fever [1]. Compared with bleeding and perforation, fever after ESD and its derivative technology of gastric lesions is a common and easily provoked complication that causes patient anxiety or results in overmedication but is of less concern, with an incidence of up to 24.8% [5].
Clinically, it is often believed that postoperative fever is related to infection. Elevated inflammation indicators, such as white blood cell count and C-reactive protein (CRP), can often be observed after ESD and its derivative technology [6]. For this reason, some doctors use antibiotics prophylactically after the operation of ESD and its derivative technology [7]. However, the use of antibiotics not only increases the risk of complications such as allergies, intestinal flora disorders, and liver and kidney function damage but also increases the cost of hospitalization, which adds to the financial burden of patients [8,9]. Additionally, postoperative fever can also cause anxiety about the success of the operation, which is not conducive to the recovery of the patient and the normalization of subsequent treatment [10]. Therefore, it is clinically important to explore the risk factors for postoperative fever after ESD and its derivative technology so that doctors can identify patients who are prone to fever after ESD and its derivative technology and inform them in advance.
Currently, the exact mechanism still needs to be investigated further, and there have been few reports about the risk factors for fever after ESD and its derivative technology for gastric lesions. A previous study showed that age and resection diameter were risk factors for fever in patients without pneumonia after ESD [5]. However, the sample size of the study was small, which would lead to a possible bias in the results of the study. Thus, the risk factors for pyrexia after gastric ESD and its derivative technology and the management strategies remain to be fully clarified and defined, respectively. The aim of this study was to clarify the incidence and risk factors for fever after gastric ESD and its derivative technology.
2. Materials and methods
2.1. Participants
This is a retrospective study performed at the Department of Gastroenterology, the First Affiliated Hospital of Nanchang University in China. Patients who underwent ESD, ESE or EFTR for gastric lesions at our department between January 2014 and January 2019 were enrolled. The exclusion criteria were as follows: (1) age younger than 18 years or older than 85 years; (2) the use of antibiotics within 3 weeks before ESD or ESE or EFTR; (3) immunodeficiency status; (4) serious cardiovascular, cerebrovascular, or hepatorenal diseases; (5) transfer to the surgical department for partial or total gastrectomy during the ESD or ESE or EFTR procedure; (6) fever (temperature>37.5 °C) or infection before the procedure; (7) patients with incomplete demographic data; and (8) pregnancy or lactation. This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University ((2022)CDYFYYLK(07–013)), and informed consent was obtained from every patient.
2.2. Relevant definitions
The ESD, ESE or EFTR procedure time was defined as the period from the intraoperative marking time to the withdrawal time. Intraoperative bleeding refers to bleeding >2 G/dl from the preoperative level of hemoglobin diluted to the first day after ESD, ESE or EFTR. Perforation was defined as other organs, extraluminal fat, or extraluminal space outside the muscle layer that could be seen through endoscopy during the ESD, ESE or EFTR procedure, regardless of air accumulation in the abdominal cavity, retroperitoneum or mediastinum. En bloc resection was defined as the endoscopic removal of a lesion in one piece and the acquisition of a single specimen. Fever was defined as a temperature >37.5 °C after the ESD, ESE or EFTR procedure (regardless of the duration of fever). Postoperative bleeding refers to hematemesis or melenas during hospitalization after ESD, ESE or EFTR, unstable vital signs or decreased hemoglobin levels by 2 g/dL compared with preoperative levels, and the requirement of endoscopic hemostasis treatment. Postoperative perforation was defined as free abdominal gas found in the abdominal plain film or CT scan of the patient after ESD, ESE or EFTR or a perforation visible under the endoscope after repeat endoscopic examination.
2.3. Gastric ESD and its derivative technology procedure
Before the ESD, ESE or EFTR procedure, patients underwent an endoscopic ultrasound (EUS) test with a radial-scanning echo endoscopy unit (UM240; Olympus Co., Ltd., Tokyo, Japan) or a 12-Fr catheter probe (UM-3R, 12 MHz; Olympus Co., Ltd., Tokyo, Japan) to identify the size, shape and layer of origin of the tumor. In addition, abdominal computerized tomography (CT) was performed to evaluate the tumor location, the growth pattern (intra/extraluminal) and the possibility of lateral growth or distant metastasis. All ESD, ESE or EFTR procedures were performed by experienced endoscopists with more than 10 years of experience. A single-channel endoscope (GIF-Q260J; Olympus Co., Ltd., Tokyo, Japan) was used in this procedure. After intravenous anesthesia with propofol, routine vital sign monitoring was performed. After identifying the gastric lesions through endoscopy, dots were marked around them with argon plasma coagulation (APC, 40 W soft coagulation). Then, 250 ml glycerol fructose, 2–3 ml indigo carmine and 1 ml 1:10,000 epinephrine were injected into the submucosal layer to elevate the lesion. The superficial mucosa was incised along the outer edge of the marker point by endoscopists using a hook knife (KD 620LR, Olympus). Subsequently, an IT knife-2 (KD 611 L, Olympus) was used to gradually separate the submucosal layer and lesion, and a snare (SD-230U-20; Olympus) was used to help with the removal of the lesion if necessary. If the gastric lesions originated from the submucosal layer or superficial muscularis propria (MP) layer, endoscopic submucosal excavation (ESE) was used. ESE is the derivative technology of ESD. On the basis of ESD technology, the submucosa and part of the muscularis propria at the base of the tumor were gradually peeled off. If the tumor was located in the deep MP layer with an extraluminal growth pattern or was closely adhered to the serosal layer, endoscopic full-thickness resection (EFTR) was chosen for safe and complete tumor removal. The difference between EFTR and ESE was the need for active perforation. To achieve complete resection, the tumor, adjacent MP and serosa were removed. hot biopsy forceps (FD-410LR; Olympus) or argon plasma coagulation (APC 300, ERBE) was used for intraoperative hemostasis. If there was active perforation caused by tumor excavation, titanium clips (HX-610-135; Aomori Olympus) or an over-the-scope clip system (OTSCs, Ovesco Endoscopy AG, Tübingen, Germany) was used to close the perforation. Fig. 1, Fig. 2 show two cases of ESD performed in our hospital. After removing the lesions, a gastric tube was placed based on the experience of the endoscopists to reduce gastric pressure for at least 24 h. All specimens were measured and immersed in formalin and were sent to the pathology department for immediate identification of the nature of the lesion.
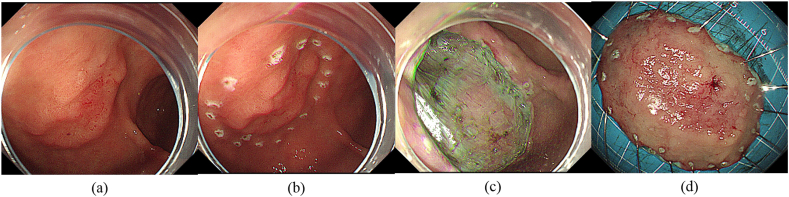
Fig. 1.
Case 1 underwent endoscopic submucosal dissection for early gastric cancer at the junction of the antrum and body. (a) A bump was seen in the anterior wall at the junction of the gastric antrum and body (endoscopic ultrasound showed that it originated from the mucosa layer and was 3.0 cm in diameter). (b) Dots were marked around them with APC. (c) The post-ESD wound has no defect left. (d) The mass.
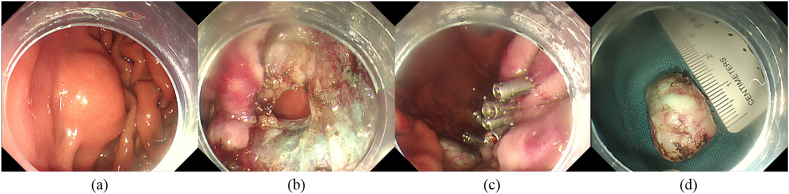
Fig. 2.
Case 1 underwent endoscopic full-thickness resection for a gastric mesenchymal tumor in the fundus. (a) A bump was seen in the fundus (endoscopic ultrasound showed that it originated from the muscular layer and grew extraluminally, 2.0 cm in diameter). (b) The post-ESD wound has no defect left. A perforation was observed. (c) The wound was large and closed with titanium clips. (d) The mass, 2.0 cm in diameter.
2.4. Postoperative management
Patients were sent to our ward after recovery from anesthesia and were asked to fast for 2–5 days. All patients received infusions (electrolytes, etc.), gastric mucosal protective agents and proton pump inhibitors (PPIs). The gastric tube was removed according to each patient's condition. If patients had a fever after ESD, ESE or EFTR, they were treated according to the experience of the doctors, which included clinical observation, physical cooling or empirical antibiotic treatment. Additionally, their temperature was recorded once a day until it returned to normal. If patients did not have any complications after ESD, ESE or EFTR, they were permitted to gradually return to a normal diet.
2.5. Statistical analysis
For the baseline characteristics, categorical variables were expressed as frequency and proportion, and statistical analysis was performed using Chi-square tests or Fisher's exact test. Continuous variables were represented as mean ± standard deviation (SD) or median interquartile (IQR) depending on whether they follow a normal distribution, and Student's t-test or nonparametric tests were used for statistical analysis. Univariate analysis was performed to assess the risk factors associated with fever, and those with a P value of <0.20 were incorporated into the multivariate analysis. The results are presented as odds ratios (ORs) with 95% confidence intervals (95% CIs). P < 0.05 was considered statistically significant. Statistical analyses were performed using R statistical software 3.6.1 (www.r-project.org).
3. Results
3.1. Patient characteristics
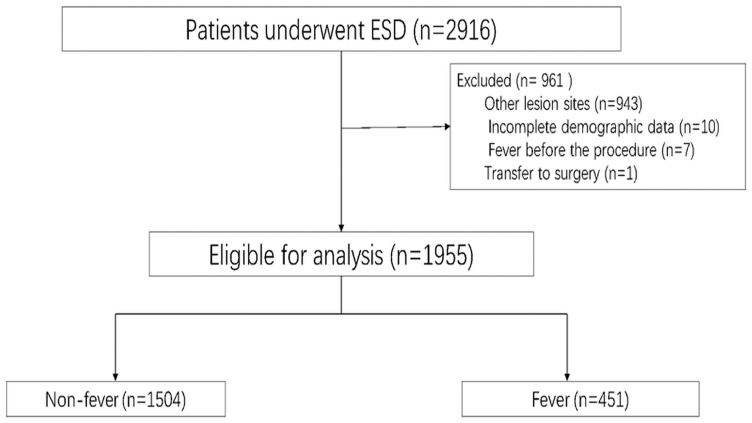
A total of 2916 patients underwent ESD, ESE or EFTR during the study period at our center. Among these patients, 1955 patients (Fig. 3) with gastric lesions were included in the present study. The median age of patients receiving gastric ESD and its derivative technology was 53 years old (IQR: 45–61), and 63.9% were male. The number of lesions in the upper 1/3 of the stomach, middle 1/3 of the stomach and lower 1/3 of the stomach was 685 (35.1%), 587 (30%) and 683 (34.9%), respectively. The median tumor size was 10 mm (IQR: 7–15), and the median procedure time was 33 min (IQR: 21–42). Intraoperative bleeding occurred in 484 (24.8%) patients, and 535 (27.4%) patients experienced perforation during the procedure. The rate of en bloc resection was 94.2% at our center. The most common pathology after gastric ESD and its derivative technology was stromal tumor (30.2%), followed by leiomyoma (22.1%) and heterotopic pancreas (10.2%). A total of 60.6% of patients had a gastric tube after ESD, ESE or EFTR, and the average number of days of gastric tube placement was 3.7 ± 1.06. Six (0.3%) patients had postoperative hemorrhage. In 3 of these patients, the bleeding was successfully stopped through emergency endoscopy, and 3 recovered after conservative treatment; none of the 6 patients received transfusions. None of the patients experienced perforation postoperatively. A total of 26 (1.3%) patients had postoperative abdominal pain and recovered after observation and analgesic treatment; 71 (3.6%) patients had nausea and vomiting after the procedure and improved after intramuscular injection of metoclopramide hydrochloride. The median length of hospital stay was 7 days (IQR: 6–9), as shown in Table 1.
Fig. 3.
The flowchart of patients included in the present study.
Table 1.
Baseline characteristics.
| Characteristic | N = 1955 | |
|---|---|---|
| Age [years, median (IQR)] | 53 (45–61) | |
| Gender [No. (%)] | ||
| Female | 705 (36.1) | |
| Male | 1250 (63.9) | |
| Coexisting diseases [No. (%)] | ||
| Diabetes | 51 (2.6) | |
| Hypertension | 234 (12) | |
| History of abdominal operation | 179 (9.2) | |
| ESD location [No. (%)] | ||
| Upper 1/3 stomach | 685 (35.1) | |
| Middle 1/3 stomach | 587 (30) | |
| Lower 1/3 stomach | 683 (34.9) | |
| Tumor size [mm, median (IQR)] | 10 (7–15) | |
| Pathology [No. (%)] | ||
| Adenoma | 163 (8.3) | |
| Adenocarcinoma | 96 (4.9) | |
| Hyperplastic polyp | 168 (8.6) | |
| Heterotopic pancreas | 200 (10.2) | |
| Lipoma | 44 (2.3) | |
| Leiomyoma | 432 (22.1) | |
| Neuroendocrine tumor | 22 (1.1) | |
| Schwannoma | 18 (0.9) | |
| Fibroma | 39 (2) | |
| Stromal tumor | 590 (30.2) | |
| inflammation | 117 (6) | |
| Other | 69 (3.5) | |
| Intraoperative bleeding [No. (%)] | 484 (24.8) | |
| Perforation [No. (%)] | 535 (27.4) | |
| Procedure time [min, median (IQR)] | 33 (21–42) | |
| En bloc resection [No. (%)] | 1842 (94.2) | |
| Postoperative fever days (mean ± SD) | 1.48 ± 0.85 | |
| Highest body temperature (°C, mean ± SD) | 37.6 ± 3.12 | |
| Type of antibiotic used | ||
| Second generation cephalosporin | 89 (42.68%) | |
| Third generation cephalosporin | 55 (26.07%) | |
| 4-quinolones | 5 (2.37%) | |
| Carbapenems | 61 (28.91%) | |
| Cephamycin | 1 (0.47%) | |
| Gastric tube placement [No. (%)] | 1184 (60.6) | |
| Duration of gastric tube (days, mean ± SD) | 3.7 ± 1.06 | |
| Duration of hospitalization [days, median (IQR)] | 7 (6–9) | |
| Postoperative adverse events [No. (%)] | ||
| Perforation | 0 | |
| Abdominal pain | 26 (1.3) | |
| Nausea and vomiting | 71 (3.6) | |
| Secondary endoscopy [No. (%)] | 3 (0.15) | |
| Mortality [No. (%)] | 0 |
3.2. Comparison between the fever and nonfever groups
There were no significant differences between the fever and nonfever groups in terms of sex, diabetes, hypertension, history of abdominal operation, pathology or en bloc resection. Patients in the fever group were older (54 vs 52, p = 0.005) and had a higher rate of intraoperative bleeding (34.8% vs 21.7%, p < 0.001), a higher rate of perforation (43.9% vs 22.4%, p < 0.001), a longer procedure time (37 vs 31, p < 0.001), a higher rate of gastric tube placement (78% vs 55.3%, p < 0.001) and a longer duration of hospitalization (8 vs 7, p < 0.001), as shown in Table 2.
Table 2.
Comparition of baseline between fever group and nonfever group.
| Variable | Nonfever group (n = 1504) | Fever group (n = 451) | P value |
|---|---|---|---|
| Median age [years, median (IQR)] | 52 (44–61) | 54 (46–62) | 0.005 |
| Gender [No. (%)] | 0.128 | ||
| Female | 941 (63) | 301 (67) | |
| Male | 556 (37) | 149 (33) | |
| Coexisting diseases [No. (%)] | |||
| Diabetes | 35 (2.3) | 16 (3.5) | 0.157 |
| Hypertension | 175 (11.6) | 59 (13.1) | 0.082 |
| History of abdominal operation | 139 (9.2) | 40 (8.9) | 0.832 |
| ESD location [No. (%)] | <0.001 | ||
| Upper 1/3 stomach | 502 (33.4) | 183 (40.6) | |
| Middle 1/3 stomach | 428 (28.5) | 159 (35.3) | |
| Lower 1/3 stomach | 574 (38.2) | 109 (24.2) | |
| Tumor size [mm, median (IQR)] | 10 (7–15) | 10 (7–18) | 0.001 |
| Tumor size [mm, mean ± SD] | 12 ± 9 | 14 ± 11 | <0.001 |
| Pathology [No. (%)] | 0.731 | ||
| Mucosa/Submucosa | 1169 (77.7) | 354 (78.5) | |
| Muscularis | 335 (22.3) | 97 (21.5) | |
| Intraoperative bleeding [No. (%)] | 327 (21.7) | 157 (34.8) | <0.001 |
| Perforation [No. (%)] | 337 (22.4) | 198 (43.9) | <0.001 |
| Procedure time[min, median (IQR)] | 31 (20–39) | 37 (28–51) | <0.001 |
| En bloc resection [No. (%)] | 1422 (94.5) | 420 (93.1) | 0.258 |
| Gastric tube placement [No. (%)] | 832 (55.3) | 352 (78) | <0.001 |
| Duration of hospitalization [days, median (IQR)] | 7 (6–8) | 8 (7–10) | <0.001 |
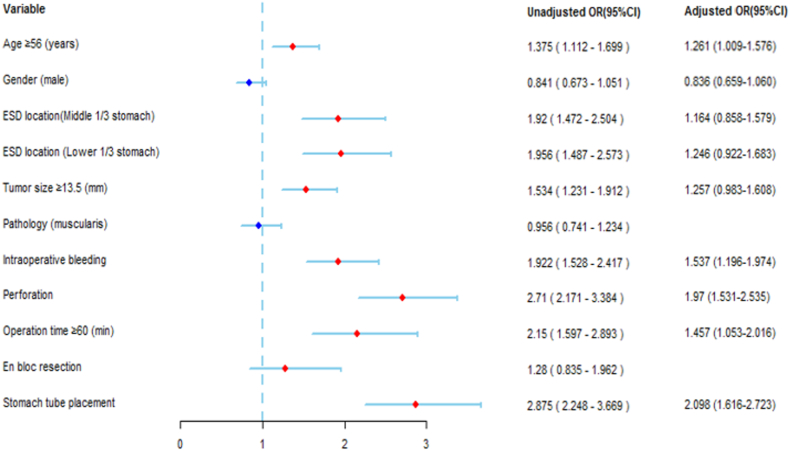
The risk factors for fever after ESD and its derivative technology procedure are reported in Table 3, Table 4 and Fig. 4. According to the univariable analysis, age ≥56 years old, ESD location, tumor size ≥13.5 mm, intraoperative bleeding, perforation, procedure time ≥60 min and gastric tube placement were risk factors for fever (Table 3). According to the multivariable analysis, age ≥56 years old (OR: 1.261; 95% CI: 1.009–1.576; P = 0.041), intraoperative bleeding (OR: 1.537; 95% CI: 1.196–1.974; P = 0.001), perforation (OR: 1.970; 95% CI: 1.531–2.535; P < 0.001), procedure time ≥60 min (OR: 1.457; 95% CI: 1.053–2.016; P = 0.023) and gastric tube placement (OR: 2.098; 95% CI: 1.616–2.723; P < 0.001) were independent risk factors for fever (Table 4).
Table 3.
Univariable analysis for fever.
| Variable | B | OR | 95% CI | P value |
|---|---|---|---|---|
| Age ≥56 (years) | 0.318 | 1.375 | 1.112–1.699 | 0.003 |
| Gender (male) | −0.173 | 0.841 | 0.673–1.051 | 0.128 |
| ESD location | ||||
| Upper 1/3 stomach | – | – | – | ref |
| Middle 1/3 stomach | 0.652 | 1.92 | 1.472–2.504 | <0.001 |
| Lower 1/3 stomach | 0.671 | 1.956 | 1.487–2.573 | <0.001 |
| Tumor size ≥13.5 (mm) | 0.428 | 1.534 | 1.231–1.912 | <0.001 |
| Pathology (muscularis) | −0.045 | 0.956 | 0.741–1.234 | 0.731 |
| Intraoperative bleeding | 0.653 | 1.922 | 1.528–2.417 | <0.001 |
| Perforation | 0.997 | 2.71 | 2.171–3.384 | <0.001 |
| Operation time ≥60 (min) | 0.765 | 2.15 | 1.597–2.893 | <0.001 |
| En bloc resection | 0.247 | 1.28 | 0.835–1.962 | 0.258 |
| Gastric tube palcement | 1.055 | 2.875 | 2.248–3.669 | <0.001 |
Table 4.
Multivariable analysis for fever.
| Variable | B | OR | 95% CI | P value |
|---|---|---|---|---|
| Age ≥56 (years) | 0.232 | 1.261 | 1.009–1.576 | 0.041 |
| Gender (male) | −0.179 | 0.836 | 0.659–1.060 | 0.139 |
| ESD location | ||||
| Upper 1/3 stomach | – | ref | – | – |
| Middle 1/3 stomach | 0.152 | 1.164 | 0.858–1.579 | 0.33 |
| Lower 1/3 stomach | 0.22 | 1.246 | 0.922–1.683 | 0.152 |
| Tumor size ≥13.5 (mm) | 0.229 | 1.257 | 0.983–1.608 | 0.069 |
| Intraoperative bleeding | 0.43 | 1.537 | 1.196–1.974 | 0.001 |
| Perforation | 0.678 | 1.97 | 1.531–2.535 | <0.001 |
| Operation time ≥60 (min) | 0.376 | 1.457 | 1.053–2.016 | 0.023 |
| Gastric tube placement | 0.741 | 2.098 | 1.616–2.723 | <0.001 |
Fig. 4.
Risk factors for fever after the ESD procedure presented as a forest plot. OR, odds ratio; CI, confidence interval.
4. Discussion
With the development of endoscopic technology, ESD and its derivative technology have become the standard methods for the treatment of early gastric cancer and [11]. Under strict indications, ESD and its derivative technology are safe and effective [12,13]. Compared with surgery, ESD and its derivative technology have less trauma, fewer complications, and lower cost [13]. Compared with endoscopic mucosal resection (EMR), which is also an endoscopic treatment method, ESD and its derivative technology can achieve en bloc resection, regardless of the location, shape, and coexistence of ulcers [14,15]. However, ESD and its derivative technology require more detailed operating techniques and are more difficult to perform [14]. Thus, compared with EMR, the procedure time of ESD and its derivative technology is longer, and operation-related complications are more likely to occur [12]. Fever is a common adverse reaction associated with ESD and its derivative technology, and there are few studies related to the problem of fever after ESD and its derivative technology [3]. Currently, the mechanism of fever after ESD and its derivative technology is unclear, and some studies suggest that fever after ESD and its derivative technology may be caused by bacteremia, especially for patients with perforation, and the incidence of bacteremia will be increased [6,16,17]. Therefore, several guidelines recommend prophylactic antibiotic treatment for patients after ESD and its derivative technology [[18], [19], [20]]. However, several recent studies have also shown that the probability of detecting bacteremia in blood cultures of patients with fever after ESD and its derivative technology is extremely low and that prophylactic antibiotic use after ESD, ESE or EFTR is unnecessary [6,17,21]. In this study, we observed that the mean number of days with fever after ESD, ESE or EFTR was 1.48 ± 0.85 days, and the mean fever temperature was 37.6 ± 3.12 °C, which suggests that the overall temperature and duration of fever after ESD, ESE or EFTR are not very high. According to the clinical experience of our center, patients with fever after ESD, ESE or EFTR can return to normal even without antibiotics. Therefore, we do not favor antibiotic treatment for such patients unless there is a clear indication of positive blood cultures. Although there is more evidence that postoperative fever after ESD and its derivative technology does not require special treatment, it is still clinically important to look for risk factors for postoperative fever after ESD and its derivative technology, identify patients who are prone to fever after ESD and its derivative technology, and inform or counsel them in advance for their postoperative recovery.
In this study, fever was defined as the occurrence of an axillary temperature >37.5 during hospitalization after ESD. Our study found that the fever rate of gastric lesions after ESD and its derivative technology was 23.1% (451/1955), which is similar to a previous study [5]. Univariate and multivariate analyses showed that age ≥56 years, procedure time ≥60 min, postoperative gastric tube placement, intraoperative bleeding and perforation were risk factors for fever after ESD and its derivative technology for gastric lesions. In our study, age ≥56 years was one of the risk factors. It may be that elderly patients often have basic lung disease or low immunity; hence, these conditions may delay the healing time of the wound surface and increase the probability of infection in the body, resulting in an increased incidence of postoperative fever. Intraoperative bleeding and perforation are common complications of ESD and its derivative technology. During the process of intraoperative bleeding and hemostasis, excessive electrocautery may be the reason for fever. In addition, bleeding will damage the endoscopic field of vision, resulting in an increased procedure time and other intraoperative complications. Gastric tube placement is another risk factor [22]. Long gastric tube insertion may increase the risk of iatrogenic infections, which may lead to postoperative fever.
This study has the following advantages. First, the sample size included in this study is relatively larger than other studies. In addition, this study included more comprehensive risk factors that may affect postoperative fever after gastric ESD. This study also had some limitations. First, this study was a retrospective study. Some patients did not have complete routine blood, CRP, procalcitonin or other inflammation indicator tests, so the relationship among fever, bacteremia and inflammatory responses could not be evaluated well after ESD and its derivative technology. Second, not all patients included in the study underwent a CT scan of the chest and abdomen after ESD and its derivative technology, so the relationship between fever and pneumonia could not be evaluated. Therefore, more large-scale, multicenter and prospective research is still needed. Third, the history of smoking and alcohol consumption of patients was not collected in this study, but according to the results of previous studies [17,21], smoking and alcohol consumption have a small effect on postoperative fever after ESD; therefore, the results of this study are still reliable.
In summary, this study found that age ≥56 years, operative time ≥60 min, postoperative gastric tube placement, intraoperative bleeding, and perforation were risk factors for postoperative fever in gastric ESD and its derivative techniques. For patients with these risk factors, medical staff should inform them in advance of the possibility of fever and give them more attention or psychological counseling in the postoperative period. Intraoperative bleeding and perforation should be treated promptly to minimize the occurrence of adverse reactions, including fever, after ESD and its derivative technology.
Ethics statement
This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University ((2022)CDYFYYLK(07–013)).
Picture statement
Participants consented to have these images published.
Funding sources
This work was supported by the Science and Technology Project of Jiangxi Provincial Health Commission (202210425).
CRediT authorship contribution statement
Yongkang Lai: Writing – original draft, Project administration, Methodology, Investigation, Formal analysis. Qian Zhang: Writing – original draft, Resources, Investigation, Formal analysis, Data curation. Foqiang Liao: Writing – original draft, Methodology, Formal analysis, Data curation. Xiaolin Pan: Writing – review & editing, Supervision, Project administration, Data curation, Conceptualization. Zhenhua Zhu: Writing – original draft, Resources, Project administration, Investigation, Conceptualization. Shunhua Long: Supervision, Software, Project administration, Investigation. Xiaojiang Zhou: Writing – review & editing, Validation, Supervision, Methodology, Formal analysis. Guohua Li: Supervision, Resources, Project administration, Data curation. Yin Zhu: Writing – review & editing, Supervision, Methodology, Investigation. Youxiang Chen: Software, Resources, Conceptualization. Xu Shu: Writing – review & editing, Supervision, Project administration, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We are grateful for the support of Jiangxi Clinical Research Center for Gastroenterology (20201ZDG02007).
Contributor Information
Yongkang Lai, Email: 409275844@qq.com.
Qian Zhang, Email: 1321179529@qq.com.
Foqiang Liao, Email: 459048754@qq.com.
Xiaolin Pan, Email: xlpan16@126.com.
Zhenhua Zhu, Email: zhuzhenhua19820122@163.com.
Shunhua Long, Email: 94212174@qq.com.
Xiaojiang Zhou, Email: yfyzxj1970@163.com.
Guohua Li, Email: liguohua98@sohu.com.
Yin Zhu, Email: zhuyin27@sina.com.
Youxiang Chen, Email: chenyx102@126.com.
Xu Shu, Email: jxmushx@126.com.
References
- 1.Japanese gastric cancer treatment guidelines 2018 . fifth ed. vol. 24. 2021. pp. 1–21. (Gastric Cancer). PMID:32060757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manta R., Galloro G., Pugliese F., Angeletti S., Caruso A., Zito F.P., et al. Endoscopic submucosal dissection of gastric Neoplastic lesions: an Italian, multicenter study. J. Clin. Med. 2020;9(3) doi: 10.3390/jcm9030737. PMID:32182894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai Y., Zhang Q., Pan X., Zhu Z., Long S., Zhou X., et al. Antibiotics for fever in patients without perforation after gastric endoscopic submucosal dissection and endoscopic submucosal excavation may be unnecessary: a propensity score-matching analysis. BMC Gastroenterol. 2021;21(1):64. doi: 10.1186/s12876-021-01602-1. PMID:33579207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H., Feng L.Q., Bian Y.Y., Yang L.L., Liu D.X., Huo Z.B., et al. Comparison of endoscopic submucosal dissection with surgical gastrectomy for early gastric cancer: an updated meta-analysis. World J. Gastrointest. Oncol. 2019;11(2):161–171. doi: 10.4251/wjgo.v11.i2.161. PMID:30788042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakanishi T., Araki H., Ozawa N., Takada J., Kubota M., Imai K., et al. Risk factors for pyrexia after endoscopic submucosal dissection of gastric lesions. Endosc. Int. Open. 2014;2(3):E141–E147. doi: 10.1055/s-0034-1377274. PMID:26134960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato M., Kaise M., Obata T., Yonezawa J., Toyoizumi H., Yoshimura N., et al. Bacteremia and endotoxemia after endoscopic submucosal dissection for gastric neoplasia: pilot study. Gastric Cancer. 2012;15(1):15–20. doi: 10.1007/s10120-011-0050-4. PMID:21559862. [DOI] [PubMed] [Google Scholar]
- 7.Draganov P.V., Wang A.Y., Othman M.O., Fukami N. AGA Institute clinical practice update: endoscopic submucosal dissection in the United States. Clin. Gastroenterol. Hepatol. 2019;17(1):16–25.e11. doi: 10.1016/j.cgh.2018.07.041. PMID:30077787. [DOI] [PubMed] [Google Scholar]
- 8.Nazli A., He D.L., Liao D., Khan M.Z.I., Huang C., He Y. Strategies and progresses for enhancing targeted antibiotic delivery. Adv. Drug Deliv. Rev. 2022;189 doi: 10.1016/j.addr.2022.114502. PMID:35998828. [DOI] [PubMed] [Google Scholar]
- 9.Westphal J.F., Vetter D., Brogard J.M. Hepatic side-effects of antibiotics. J. Antimicrob. Chemother. 1994;33(3):387–401. doi: 10.1093/jac/33.3.387. PMID:8040106. [DOI] [PubMed] [Google Scholar]
- 10.Dionigi R., Dionigi G., Rovera F., Boni L. Postoperative fever. Surg. Infect. 2006;7(Suppl 2):S17–S20. doi: 10.1089/sur.2006.7.s2-17. PMID:16895496. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt A., Abe S., Kumaravel A., Vargo J., Saito Y. Indications and techniques for endoscopic submucosal dissection. Am. J. Gastroenterol. 2015;110(6):784–791. doi: 10.1038/ajg.2014.425. PMID:25623656. [DOI] [PubMed] [Google Scholar]
- 12.Pimentel-Nunes P., Libânio D., Bastiaansen B.A.J., Bhandari P., Bisschops R., Bourke M.J., et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European society of gastrointestinal endoscopy (ESGE) guideline - update 2022. Endoscopy. 2022;54(6):591–622. doi: 10.1055/a-1811-7025. PMID:35523224. [DOI] [PubMed] [Google Scholar]
- 13.Chiu P.W., Teoh A.Y., To K.F., Wong S.K., Liu S.Y., Lam C.C., et al. Endoscopic submucosal dissection (ESD) compared with gastrectomy for treatment of early gastric neoplasia: a retrospective cohort study. Surg. Endosc. 2012;26(12):3584–3591. doi: 10.1007/s00464-012-2371-8. PMID:22678176. [DOI] [PubMed] [Google Scholar]
- 14.Fujishiro M. Endoscopic submucosal dissection for stomach neoplasms. World J. Gastroenterol. 2006;12(32):5108–5112. doi: 10.3748/wjg.v12.i32.5108. PMID:16937520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishizawa T., Yahagi N. Endoscopic mucosal resection and endoscopic submucosal dissection: technique and new directions. Curr. Opin. Gastroenterol. 2017;33(5):315–319. doi: 10.1097/mog.0000000000000388. PMID:28704212. [DOI] [PubMed] [Google Scholar]
- 16.Nelson D.B., Sanderson S.J., Azar M.M. Bacteremia with esophageal dilation. Gastrointest. Endosc. 1998;48(6):563–567. doi: 10.1016/s0016-5107(98)70036-7. PMID:9852444. [DOI] [PubMed] [Google Scholar]
- 17.Itaba S., Iboshi Y., Nakamura K., Ogino H., Sumida Y., Aso A., et al. Low-frequency of bacteremia after endoscopic submucosal dissection of the stomach. Dig. Endosc. 2011;23(1):69–72. doi: 10.1111/j.1443-1661.2010.01066.x. PMID:21198920. [DOI] [PubMed] [Google Scholar]
- 18.Ono H., Yao K., Fujishiro M., Oda I., Nimura S., Yahagi N., et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig. Endosc. 2016;28(1):3–15. doi: 10.1111/den.12518. PMID:26234303. [DOI] [PubMed] [Google Scholar]
- 19.Fernández-Esparrach G., Á Calderón, Dela-Peña J., Díaz-Tasende J.B., Esteban J.M., Gimeno-García A.Z., et al. Endoscopic submucosal dissection. Sociedad Española de Endoscopia Digestiva (SEED) clinical guideline. Rev. Esp. Enferm. Dig. 2014;106(2):120–132. doi: 10.4321/s1130-01082014000200007. PMID:24852737. [DOI] [PubMed] [Google Scholar]
- 20.Hoteya S., Yamashita S., Kikuchi D., Nakamura M., Fujimoto A., Matsui A., et al. Endoscopic submucosal dissection for submucosal invasive gastric cancer and curability criteria. Dig. Endosc. 2011;23(1):30–36. doi: 10.1111/j.1443-1661.2010.01040.x. PMID:21198914. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Chen Y., Shu X., Zhu Y., Li G., Hong J., et al. Prophylactic antibiotics may be unnecessary in gastric endoscopic submucosal dissection due to the low incidence of bacteremia. Surg. Endosc. 2020;34(9):3788–3794. doi: 10.1007/s00464-019-07143-9. PMID:31617087. [DOI] [PubMed] [Google Scholar]
- 22.Saito I., Tsuji Y., Sakaguchi Y., Niimi K., Ono S., Kodashima S., et al. Complications related to gastric endoscopic submucosal dissection and their managements. Clin Endosc. 2014;47(5):398–403. doi: 10.5946/ce.2014.47.5.398. PMID:25324997. [DOI] [PMC free article] [PubMed] [Google Scholar]