Abstract
Individuals with kidney failure undergoing hemodialysis are at elevated risk for thromboembolic events. Factor (F) XI, which is in the intrinsic pathway of coagulation, is emerging as an attractive target for new anticoagulants that may be safer than existing agents. Osocimab—an inhibitory FXIa antibody—is a potential treatment option for such patients. We conducted a phase 2b, double-blind, placebo-controlled trial, in which 704 participants (448 male, 256 female) with kidney failure undergoing hemodialysis were randomized to receive lower- or higher-dose osocimab or placebo. In total, 686 participants (436 male, 250 female) received treatment for ≤18 months (planned minimal treatment period of 6 months). The co-primary outcomes were clinically relevant bleeding (a composite of major and clinically relevant nonmajor bleeding) and a composite of the incidence of moderate, severe or serious adverse events. Clinically relevant bleeding occurred in 16/232 (6.9%) and 11/224 (4.9%) participants who received lower- and higher-dose osocimab, respectively, and in 18/230 participants (7.8%) who received a placebo. For the composite adverse event endpoint, incidences were 51%, 47% and 43% in the lower-dose osocimab, higher-dose osocimab and placebo groups, respectively. These results suggest that osocimab is associated with a low risk of bleeding and is generally well tolerated in this population; findings that require confirmation in larger trials. ClinicalTrials.gov identifier, NCT04523220.
Subject terms: Drug development, End-stage renal disease
In a phase 2 trial involving patients with kidney failure who were undergoing hemodialysis, treatment with osocimab—an antibody targeting coagulation factor XIa—did not lead to increased rates of clinically relevant bleeding or an increased risk of adverse events as compared to placebo, suggesting the possibility that factor XIa inhibitors may be safer in this patient population than currently available anticoagulants.
Main
Chronic kidney disease, which affects almost 10% of the global population, is a major cause of morbidity and mortality1. Diabetes and hypertension are common causes of kidney failure2. Most individuals with kidney failure who are managed with hemodialysis are at risk of major adverse vascular events such as myocardial infarction, stroke and other thromboembolic events2,3. Although long-term anticoagulation therapy has the potential to prevent these complications, its use remains understudied because people with kidney failure are also at increased risk of major bleeding4. Around one in seven such individuals will experience a major bleed within 3 years of dialysis initiation5, and the bleeding risk is further increased, up to tenfold, when people with kidney failure requiring hemodialysis are treated with warfarin6. Furthermore, a recent trial comparing warfarin with apixaban for stroke prevention in patients with atrial fibrillation on regular hemodialysis reported comparably high rates of major bleeding (9.7% versus 8.5%)7. Therefore, with limited evidence supporting the use of warfarin or direct oral anticoagulants in this patient population, there remains an unmet need for safer anticoagulants for the prevention of thromboembolic events.
Inhibitors of factor XI or its activated form, factor XIa, may be safer than the currently available anticoagulants because factor XI seems to be more important for thrombosis than for hemostasis8. Thus, individuals with reduced factor XI levels are at lower risk for thrombosis than those with normal levels9, but rarely experience serious bleeding8. Conversely, high levels of factor XI increase the risk for thrombosis8,10,11. Therefore, factor XI has emerged as a target for potentially safer anticoagulants and may be a particularly attractive target in individuals with kidney failure requiring regular dialysis3.
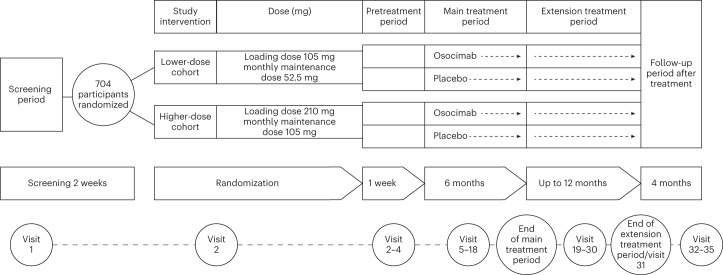
Osocimab is a long-acting, fully human inhibitory antibody directed against factor XIa12,13. In a phase 2 dose-finding study in individuals undergoing total knee arthroplasty, a single intravenous dose of osocimab administered before surgery was superior, or after surgery was noninferior, to enoxaparin for prevention of postoperative venous thromboembolism14. Furthermore, osocimab was associated with low rates of clinically relevant bleeding, the composite of major and clinically relevant nonmajor bleeding14. To address the unmet need for safer anticoagulants for patients with end-stage kidney disease, we conducted the CONVERT trial (ClinicalTrials.gov identifier, NCT04523220) to compare the rates of clinically relevant bleeding with osocimab and placebo in participants with kidney failure who were undergoing hemodialysis (Fig. 1).
Fig. 1. Study design.
Overview of the study design. Participants with kidney failure undergoing hemodialysis were randomized to receive lower- or higher-dose osocimab or placebo for ≤18 months. The follow-up period ended 5 months after the last study intervention and 4 months after the end of the main or extension treatment period.
Results
Patient disposition
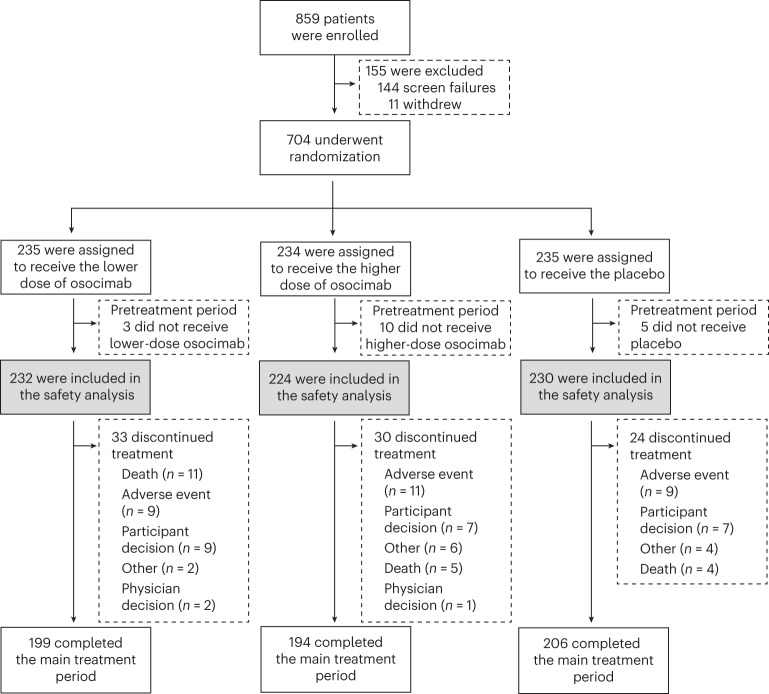
From August 2020 to April 2021, a total of 704 participants from 147 sites in 19 countries were randomized. After the exclusion of 18 participants who did not receive the study drug, 686 individuals started the main 6-month treatment period and were included in the analyses (Fig. 2). The main treatment period was completed by 199 participants in the lower-dose osocimab group (84.7%), 194 participants in the higher-dose osocimab group (82.9%) and 206 participants in the placebo group (87.7%). The extended treatment period was completed by 178 participants in the lower-dose osocimab group (75.5%), 174 participants in the higher-dose osocimab group (74.4%) and 176 participants in the placebo group (74.9%). The median previous duration on maintenance dialysis was 4.0 years (interquartile range, 2.0–7.2 years). Overall, 249 participants (36.3%) had known atherosclerotic disease and 290 (42.3%) were taking aspirin. The baseline characteristics and median duration of study treatment were similar across groups (Table 1).
Fig. 2. Patient disposition.
Summary of patient flow in the phase 2b CONVERT trial.
Table 1.
Baseline demographics and clinical characteristics of study participants
| Characteristic | Lower-dose osocimab (n = 232) | Higher-dose osocimab (n = 224) | Placebo (n = 230) |
|---|---|---|---|
| Age, median (range) (years) | 61 (28–91) | 61 (25–90) | 60 (24–90) |
| Age group, n (%) | |||
| <60 years | 104 (44.8) | 103 (46.0) | 108 (47.0) |
| 60–75 years | 98 (42.2) | 84 (37.5) | 94 (40.9) |
| >75 years | 30 (12.9) | 37 (16.5) | 28 (12.2) |
| Male sex, n (%) | 143 (61.6) | 143 (63.8) | 150 (65.2) |
| Body mass indexa, n (%) | |||
| 25–30 kg m−2 | 62 (26.7) | 87 (38.8) | 93 (40.4) |
| ≥30 kg m−2 | 74 (31.9) | 65 (29.0) | 62 (27.0) |
| White, n (%) | 191 (82.3) | 185 (82.6) | 185 (80.4) |
| Geographic region, n (%) | |||
| Western Europe | 36 (15.5) | 37 (16.5) | 35 (15.2) |
| Eastern Europe | 133 (57.3) | 121 (54.0) | 123 (53.4) |
| Asia Pacific | 21 (9.0) | 19 (8.4) | 19 (8.2) |
| North America | 39 (16.8) | 37 (16.5) | 37 (16.1) |
| Australia and Israel | 3 (1.2) | 10 (4.4) | 16 (6.9) |
| Dialysis duration, median (interquartile range) years | 4.05 (2.0–7.2) | 4.0 (2.0–7.5) | 3.85 (1.8–7.0) |
| Dialysis access, n (%) | |||
| Fistula | 191 (82.3) | 180 (80.4) | 189 (82.2) |
| Graft | 22 (9.5) | 20 (8.9) | 13 (5.7) |
| Catheter | 19 (8.2) | 24 (10.7) | 28 (12.2) |
| Heparin use during dialysis, n (%) | 221 (95.3) | 218 (97.3) | 218 (94.8) |
| Etiology of kidney disease, n (%) | |||
| Diabetes | 65 (28.0) | 53 (23.6) | 59 (25.6) |
| Hypertension | 51 (22.0) | 67 (30.0) | 50 (21.7) |
| Glomerulonephritis | 32 (13.8) | 30 (13.4) | 33 (14.3) |
| Pyelonephritis | 11 (4.7) | 10 (4.5) | 10 (4.3) |
| Polycystic kidney disease | 23 (9.9) | 23 (10.3) | 21 (9.1) |
| Other | 50 (21.5) | 41 (18.3) | 57 (24.8) |
| Diabetes, n (%) | 90 (38.8) | 82 (36.6) | 87 (37.8) |
| Hypertension, n (%) | 213 (91.8) | 212 (94.6) | 215 (93.5) |
| Coronary heart disease, n (%) | 56 (24.1) | 61 (27.2) | 47 (20.4) |
| Myocardial infarction, n (%) | 17 (7.3) | 15 (6.7) | 11 (4.8) |
| Peripheral artery disease, n (%) | 18 (7.8) | 23 (10.3) | 22 (9.6) |
| Stroke, n (%) | 12 (5.1) | 18 (7.7) | 18 (7.7) |
| Atrial fibrillation, n (%) | 15 (6.5) | 17 (7.6) | 14 (6.1) |
| Previous cardiovascular eventb, n (%) | 37 (15.9) | 43 (19.2) | 40 (17.4) |
| Atherosclerosisc, n (%) | 81 (34.9) | 86 (38.4) | 82 (35.7) |
| History of venous thromboembolism, n (%) | 8 (3.4) | 10 (4.5) | 10 (4.3) |
| Platelet count, mean (s.d.) × 109 l−1 | 209 (62) | 209 (56) | 212 (58) |
| Hemoglobin, median (interquartile range) (mg dl−1) | 10.9 (10.2–11.8) | 11.1 (10.4–11.8) | 11.0 (10.4–11.7) |
| Low-dose aspirin, n (%) | 97 (41.8) | 95 (42.4) | 98 (42.6) |
| Duration of study drug administration, median (interquartile range) (months) | 9.1 (7.0–11.7) | 9.2 (7.1–11.7) | 9.0 (7.1–11.4) |
| Length of study, n (%) | |||
| ≤6 months | 37 (15.9) | 33 (14.7) | 26 (11.3) |
| >6–9 months | 76 (32.8) | 73 (32.6) | 89(38.7) |
| >9 months | 119 (51.3) | 118 (52.7) | 115 (50.0) |
Percentages may not total 100 because of rounding.
aBody mass index is the weight in kilograms divided by the square of the height in meters.
bPrevious cardiovascular events include stroke, transient ischemic attack, myocardial infarction, deep vein thrombosis and pulmonary embolism.
cAtherosclerosis was defined as a history of ischemic stroke, transient ischemic attack, unstable angina, myocardial infarction, peripheral artery disease or aortic aneurysm.
Primary outcomes
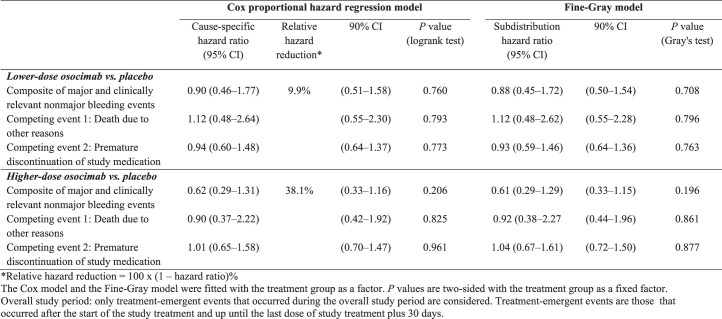
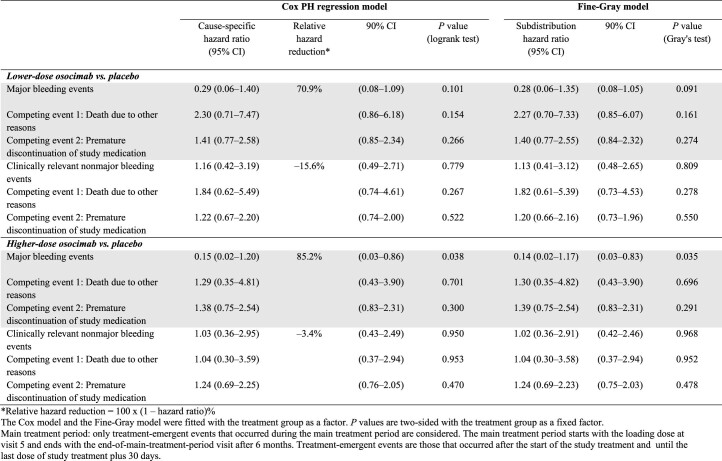
Clinically relevant bleeding (the composite of major and clinically relevant nonmajor bleeding) occurred in 16 of 232 participants (6.9%) who received lower-dose osocimab, 11 of 224 (4.9%) who received higher-dose osocimab and 18 of 230 (7.8%) who received placebo (Table 2). Major bleeding occurred in three (1.3%), two (0.9%) and seven (3.0%) participants in the lower- and higher-dose osocimab and placebo groups, respectively. In those over 75 years of age, clinically relevant bleeding per 100 person-years was 20 (90% confidence interval (CI), 7–39) events for lower-dose osocimab, 8 (90% CI, 1–18) events for higher-dose osocimab and 23 (90% CI, 8–44) events for placebo. Corresponding values for individuals on low-dose aspirin were 12 (90% CI, 6–20), 7 (90% CI, 3–13) and 16 (90% CI, 9–25) events per 100 person-years, respectively. Cause-specific hazard ratios and subdistribution hazard ratios for the primary outcomes are provided in Extended Data Tables 1–4.
Table 2.
Safety and efficacy outcomes
| Lower-dose osocimab (n = 232) | Higher-dose osocimab (n = 224) | Placebo (n = 230) | |
|---|---|---|---|
| Clinically relevant bleeding (primary outcome) | |||
| n (%) | 16 (6.9) | 11 (4.9) | 18 (7.8) |
| Events (90% CI) per 100 patient-years | 9.7 (6.1–14.1) | 6.7 (3.7–10.3) | 10.8 (7.0–15.3) |
| Composite of moderate, severe or serious adverse events (primary outcome) | |||
| n (%) | 118 (50.9) | 106 (47.3) | 99 (43.0) |
| Major bleeding | |||
| n (%) | 3 (1.3) | 2 (0.9) | 7 (3.0) |
| Events (90% CI) per 100 patient-years | 1.8 (0.5–3.8) | 1.2 (0.2–2.9) | 4.2 (2.0–7.1) |
| Clinically relevant nonmajor bleeding | |||
| n (%) | 13 (5.6) | 9 (4.0) | 11 (4.8) |
| Events (90% CI) per 100 patient-years | 7.9 (4.7–11.8) | 5.5 (2.9–8.8) | 6.6 (3.7–10.2) |
| Types of major bleeding, n (%) | |||
| Gastrointestinal | 1 (0.4) | 0 | 1 (0.4) |
| Urogenital (kidney or bladder) | 0 | 0 | 2 (0.9) |
| Intracranial | 0 | 0 | 1 (0.4) |
| Eye (intraocular or retinal) | 1 (0.4) | 0 | 2 (0.9) |
| Skin (any vascular access site) | 0 | 2 (0.9) | 0 |
| Respiratory (pulmonary) | 1 (0.4) | 0 | 0 |
| Procedural | 0 | 0 | 1 (0.4) |
| Types of clinically relevant nonmajor bleeding, n (%) | |||
| Gastrointestinal | 1 (0.4) | 1 (0.4) | 0 |
| Epistaxis | 2 (0.9) | 1 (0.4) | 2 (0.9) |
| Urogenital | 3 (1.3) | 2 (0.9) | 1 (0.4) |
| Skin | 4 (1.7) | 2 (0.9) | 2 (0.9) |
| Vascular access site | 2 (0.9) | 4 (1.8) | 6 (2.6) |
| Conjunctival | 1 (0.4) | 0 | 0 |
| Major adverse vascular eventsa | |||
| n (%) | 3 (1.3) | 6 (2.7) | 7 (3.0) |
| Events (90% CI) per 100 patient-years | 1.7 (0.5–3.7) | 3.6 (1.6–6.3) | 4.1 (1.9–6.9) |
| Type of event, n (%) | |||
| Myocardial infarction | 2 (0.9) | 4 (1.8) | 4 (1.7) |
| Ischemic stroke | 1 (0.4) | 1 (0.4) | 2 (0.9) |
| Major amputation | 0 | 0 | 1 (0.4) |
| Systemic embolism | 0 | 1 (0.4) | 0 |
| Atherosclerotic subgroupa | |||
| n/n (%) | 2/81 (2.5) | 2/86 (2.3) | 6/82 (7.3) |
| Events (90% CI) per 100 patient-years | 3.7 (0.7–8.7) | 3.3 (0.6–7.8) | 11.0 (4.8–19.2) |
| Dialysis circuit clotting, n (%) | |||
| Score of 2 or 3 | 68 (29.3) | 61 (27.2) | 95 (41.3) |
| Score of 3 | 4 (1.7) | 4 (1.8) | 10 (4.3) |
| Access thrombosis, n (%) | 6 (2.6) | 8 (3.6) | 11 (4.8) |
| Serious adverse events, n (%) | |||
| Serious adverse event | 70 (30.2) | 64 (28.6) | 63 (27.4) |
| Serious adverse event leading to discontinuation of study drug | 8 (3.4) | 10 (4.5) | 13 (5.7) |
| All-cause death | |||
| n (%) | 12 (5.2%) | 9 (4.0%) | 11 (4.8%) |
| Events (90% CI) per 100 patient-years | 7.0 (4.0–10.6) | 5.4 (2.8–8.6) | 6.4 (3.6–9.8) |
| Injection-site reactions, n (%) | 14 (6.0) | 17 (7.6) | 1 (0.4) |
Incidences are reported by the number of participants having the specific event up to 30 days after the end of study treatment for that individual.
aVascular death (due to myocardial infarction, stroke, pulmonary or systemic embolism), nonfatal myocardial infarction or stroke, major amputation for vascular etiology, acute limb ischemia and symptomatic venous thromboembolism or incidence of thrombosis of arteriovenous fistulas or grafts.
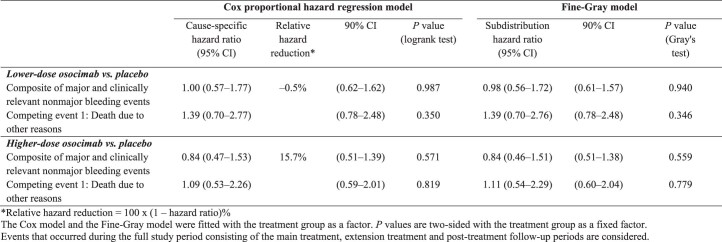
Extended Data Table 1.
Cause-specific hazard ratios and subdistribution hazard ratios for treatment-emergent primary outcome (composite of major and clinically relevant nonmajor bleeding) and competing events (safety analysis set)
Extended Data Table 4.
Cause-specific hazard ratios and subdistribution hazard ratios for treatment-emergent major bleeding events, clinically relevant nonmajor bleeding events, and competing events (safety analysis set)
The incidences of moderate, severe or serious adverse events were 51% (n = 118), 47% (n = 106) and 43% (n = 99) in the lower-dose osocimab, higher-dose osocimab and placebo groups, respectively (Table 2).
Secondary outcomes
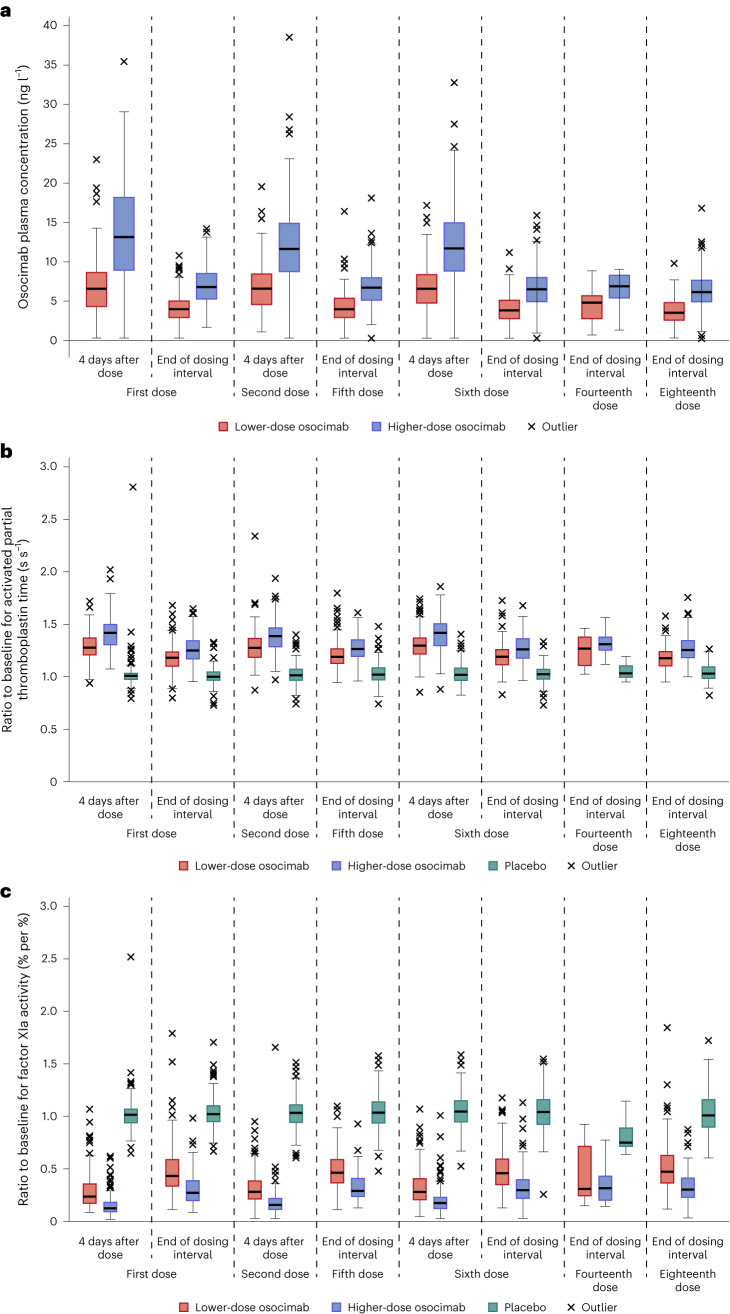
Prespecified secondary outcomes were osocimab plasma levels, prolongation of the activated partial thromboplastin time and inhibition of factor XIa. There were dose-dependent changes in these variables and their timecourses were comparable from the first to the last measured dose (Fig. 3).
Fig. 3. Osocimab plasma concentrations and effects of osocimab treatment on the activated partial thromboplastin time and inhibition of factor XIa activity.
a, Box plots showing osocimab concentrations. b, Activated partial thromboplastin times relative to baseline values. c, Inhibition of factor XIa activity relative to baseline values. Medians are indicated by the horizontal lines in the boxes; boxes indicate 25th and 75th percentiles; the vertical lines extend to a maximum distance of 1.5 interquartile ranges; values outside of this range are plotted separately. Analyses are based on the pharmacodynamic analysis set, n = 686 (lower-dose osocimab, n = 232; higher-dose osocimab, n = 224; placebo, n = 230).
Safety
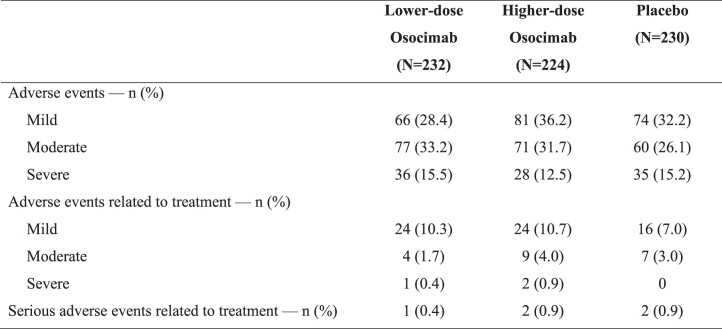
Injection-site reactions occurred in 14, 17 and 1 participant in the lower-dose osocimab, higher-dose osocimab and placebo groups, respectively, resulting in study drug discontinuation by two participants from each osocimab group and no participants in the placebo group. Further details regarding the incidence of treatment-related serious adverse events are provided in Extended Data Table 5.
Extended Data Table 5.
Summary of Adverse Events
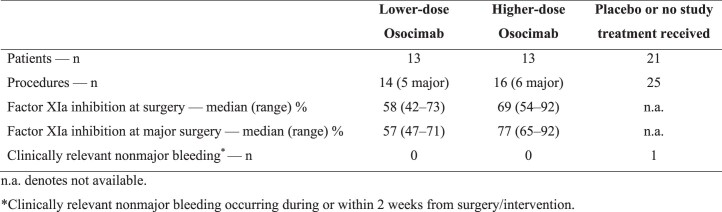
In total, 26 participants received lower- or higher-dose osocimab (n = 13 each) within 30 days preceding major surgery or intervention (Extended Data Table 6). The predicted median factor XIa inhibition levels at the time of surgery or intervention were 58% and 69% with the lower and higher osocimab doses, respectively, and no bleeding events were reported during or within 2 weeks of surgery or intervention. Of those randomized to placebo or randomized to osocimab but who did not receive any treatment, 21 participants underwent major surgery or intervention. There was one procedure-related, clinically relevant nonmajor bleed after dialysis catheter removal in the placebo group.
Extended Data Table 6.
Surgical Interventions Within One Month After Administration of Study Drug, Extent of Factor XI Inhibiton, and Associated Bleeding Outcomes*
Exploratory outcomes
For the prespecified exploratory outcomes, major adverse vascular events occurred in 3 of 232 (1.3%) participants who received lower-dose osocimab, 6 of 224 (2.7%) who received higher-dose osocimab and 7 of 230 (3.0%) who received placebo. In participants with known atherosclerotic disease, major adverse vascular events occurred in 4 of 167 (2.4%) individuals who received osocimab and in 6 of 82 (7.3%) who received placebo. No participant experienced symptomatic venous thromboembolism.
Clotting of the dialysis circuit was scored in each participant at every study visit (0, no clot; 1, trace of clot; 2, intermediate between 1 and 3; and 3, fully clotted system necessitating interruption of hemodialysis session). Dialysis circuit clotting scores of 2 or 3 at any visit were reported in 29.3% and 27.2% of participants in the lower- and higher-dose osocimab groups, respectively, and in 41.3% of those in the placebo group. The relative risk of moderate-to-complete clotting at one or more visits was significantly lower in both osocimab groups than in the placebo group (lower dose versus placebo, 0.71 (95% CI, 0.54–0.93; P = 0.0085); higher dose versus placebo, 0.66 (95% CI, 0.49–0.87; P = 0.0021)).
Sensitivity analyses
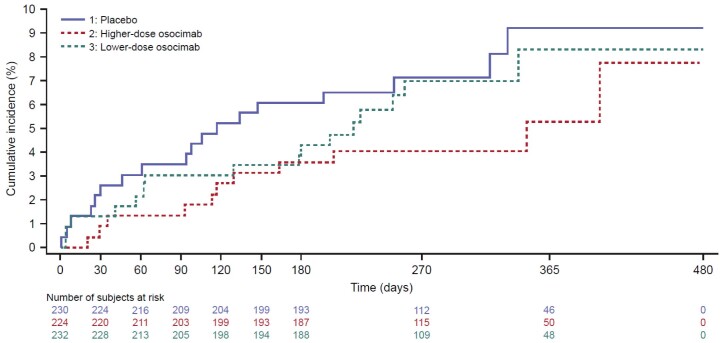
Aalen–Johansen estimates of the cumulative incidences (that is, the expected proportions of participants with an outcome over time, taking competing risks into account) for the primary outcomes and the exploratory efficacy outcome are provided in Extended Data Figs. 1–3.
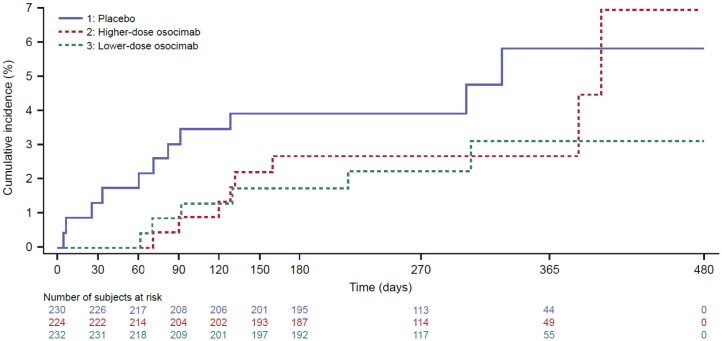
Extended Data Fig. 1. Cumulative incidence risk (Aalen–Johansen) of the time-to-first treatment-emergent composite of major and clinically relevant nonmajor bleeding (primary outcome; safety analysis set).
Cumulative incidence (%) = Aalen–Johansen estimates of the cumulative probability for an event, calculated as 100 x (1 minus the Aalen–Johansen estimate of the survival function). Overall study period: Only treatment-emergent events that occurred during the overall study period are considered. Treatment-emergent events are those that occurred after the start of the study treatment and until the last dose of the study treatment plus 30 days.
Extended Data Fig. 3. Cumulative incidence risk (Aalen–Johansen) of time-to-first arteriovenous fistula or graft thrombosis (exploratory outcome; safety analysis set).
Cumulative incidence (%) = Aalen–Johansen estimates of the cumulative probability for an event, calculated as 100 x (1 minus the Aalen–Johansen estimate of the survival function). Overall study period: Only treatment-emergent events that occurred during the overall study period are considered. Treatment-emergent events are those that occurred after the start of the study treatment and until the last dose of the study treatment plus 30 days.
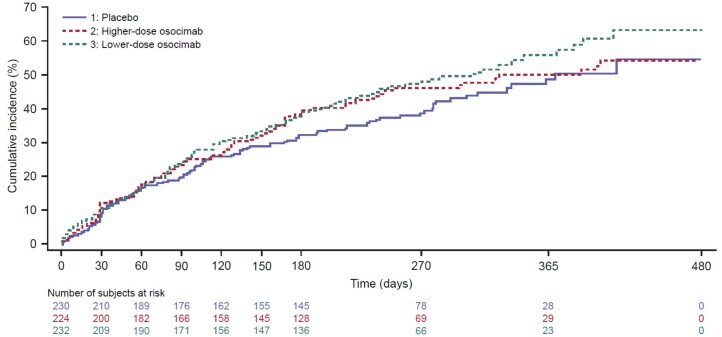
For the primary outcome of clinically relevant bleeding, the cumulative incidence risks over the main treatment period were 4.3% (90% CI, 2.48–6.90) for lower-dose osocimab, 3.57% (90% CI, 1.91–6.04) for higher-dose osocimab and 6.09% (90% CI, 3.84–9.04) for placebo. For the second primary outcome of the composite of moderate, severe or serious adverse events over the main treatment period, the cumulative incidence risks were 38.37% (90% CI, 33.10–43.60) for lower-dose osocimab, 38.84% (90% CI, 33.46–44.17) for higher-dose osocimab and 32.17% (90% CI, 27.16–37.28) for placebo.
For the exploratory efficacy outcome of the incidence of arteriovenous fistula or graft thrombosis over the main treatment period, the cumulative incidence risks were 1.7% (90% CI, 0.70–3.61) for lower-dose osocimab, 2.68% (90% CI, 1.29–4.91) for higher-dose osocimab and 3.91% (90% CI, 2.18–6.43) for placebo.
Discussion
Individuals with kidney failure requiring hemodialysis are at increased risk of bleeding3,4. The results of the primary outcomes of this phase 2b trial suggest that, compared with placebo, osocimab does not increase the risk of clinically relevant bleeding or the risk of moderate, severe or serious adverse events. No bleeding events were reported in the small number of osocimab-treated individuals who underwent major surgery or intervention, including the 13 participants who underwent kidney transplantation. Therefore, osocimab seems to be associated with a low rate of clinically relevant bleeding in individuals requiring hemodialysis, which is similar to what was previously reported with osocimab in subjects undergoing elective knee arthroplasty14. These findings were observed despite the fact that osocimab prolongs the activated partial thromboplastin time in a dose-dependent manner.
Low event rates precluded exploratory analysis of efficacy regarding the incidence of major adverse vascular events. However, the exploratory analysis of dialysis circuit clotting, which was assessed in all participants, revealed that both doses of osocimab were associated with a significant reduction in the risk of moderate-to-complete dialysis circuit clotting compared with placebo, providing proof of concept that osocimab has antithrombotic effects beyond those of heparin15. Extracorporeal circuits activate factor XII and trigger clotting via the intrinsic pathway16. By inhibiting factor XIa, which is activated by factor XIIa, osocimab seems to be able to attenuate this process to a greater extent than heparin.
At present, there are no safe and effective anticoagulants for the prevention of thromboembolic events in individuals with kidney failure requiring hemodialysis. Prospective and observational studies have reported little or no benefit and increased bleeding in those receiving oral anticoagulants17,18. Two randomized trials comparing vitamin K antagonists with apixaban in individuals with kidney failure undergoing hemodialysis who had atrial fibrillation were able to recruit only 154 and 97 participants, respectively, and were therefore underpowered to yield definitive results7,19. The current findings and those of a trial comparing fesomersen—an antisense oligonucleotide that reduces the hepatic synthesis of factor XI—with placebo in individuals with end-stage kidney disease requiring hemodialysis20, raise the possibility that factor XI inhibitors may be safer than currently available anticoagulants.
Like the above-mentioned apixaban trials7,19, the composite of major and clinically relevant nonmajor bleeding was a primary outcome of the current study. Osocimab is an experimental agent and, as such, adverse events were also included as a primary safety outcome. Stroke and systemic embolism were key secondary outcomes in the apixaban trials because the patients had atrial fibrillation, whereas most of the patients enrolled in the present study did not have atrial fibrillation. Given that the incidences of stroke and systemic embolism are lower in those without atrial fibrillation, a broader range of thromboembolic events was included as exploratory outcomes. Furthermore, because osocimab was compared with a placebo in this trial, the only patients with atrial fibrillation who were eligible for enrollment were those who had been deemed unsuitable for anticoagulation therapy.
Some methodological aspects of our trial require comment. The strengths of the study include the double-blind trial design, the extended duration of treatment and the consistent findings with lower and higher doses of osocimab, which render it unlikely that the reported safety findings reflect a play of chance. Nonetheless, because of the modest sample size, additional studies are needed to assess the safety of osocimab. Finally, although end-stage kidney disease is more prevalent in older than in younger individuals, the prevalence of end-stage kidney disease in those aged 45 to 64 years has increased by 56% over the past 20 years15. This may explain why the mean age of participants in the present study was 61 years. Additional studies are needed to assess the safety of osocimab more thoroughly in older subjects.
In summary, compared with placebo, osocimab did not increase the risk of clinically relevant bleeding in individuals with kidney failure requiring hemodialysis. Appropriately powered phase 3 trials are needed to determine whether osocimab reduces the risk of thromboembolic events in this vulnerable and understudied population.
Methods
Additional information about authors, study sites and investigators, and outcome definitions is provided in the Supplementary Appendix.
Study design and oversight
This phase 2b, randomized, double-blind, parallel-group trial compared two subcutaneous osocimab dosing regimens with a placebo in individuals with kidney failure requiring hemodialysis (Fig. 1). This multicenter study enrolled participants globally, including in North America, Europe, Asia and Australia.
An academic Steering Committee, in collaboration with the sponsor (Bayer), was responsible for the design and oversight of the study. The sponsor was responsible for data collection, maintenance and analysis. An Institutional Review Board at each participating center approved the protocol and all participants provided informed consent. This study was conducted in accordance with the consensus ethical principles derived from international guidelines including the Declaration of Helsinki and Council for International Organizations of Medical Sciences (CIOMS) International Ethical Guidelines, applicable ICH Good Clinical Practice Guidelines and all applicable laws and regulations.
The Steering Committee was blinded to treatment assignment as were the members of a Central Independent Adjudication Committee, who adjudicated all deaths, suspected bleeds, and cardiovascular or thromboembolic events. An independent Data and Safety Monitoring Committee periodically reviewed trial outcomes and adverse events with the support of an independent Statistical Analysis Center.
The protocol and accompanying documents are available with the full text of this article online.
Participants
Individuals with kidney failure were eligible for inclusion if they were 18 years of age or older and were undergoing hemodialysis (at least three times per week for a minimum of 9 h per week) and stable for at least 3 months. Patients with atrial fibrillation who were not considered to be candidates for therapeutic anticoagulation by their treating physicians were eligible for inclusion.
Participants were eligible for inclusion if all the following criteria were met:
≥18 years of age
End-stage kidney disease undergoing hemodialysis (including hemodiafiltration) for ≥3 months and stable, in the view of the investigator
Body weight ≥50 kg
Men and women were eligible; contraceptive use should be consistent with local regulations regarding the methods of contraception for those participating in clinical studies
Capable of providing signed informed consent
Participants were not eligible for inclusion if any of the following criteria applied:
Recent (<6 months before screening) clinically significant bleeding
Hemoglobin <9.0 g dl−1
Platelet count <100 × 109 l−1
Activated partial thromboplastin time or prothrombin time above the upper limit of normal
Hepatic disease associated with alanine aminotransferase over three times the upper limit of normal, or total bilirubin over two times the upper limit of normal with direct bilirubin over 20% of the total
Sustained uncontrolled hypertension (diastolic blood pressure ≥100 mmHg and/or systolic blood pressure ≥180 mmHg)
Known intracranial neoplasm, arteriovenous malformation or aneurysm
Known bleeding disorders
Recent (<3 months before screening) thromboembolic event
Recent (<3 months before screening) major surgery or scheduled major surgery during study participation
Scheduled living donor renal transplant during study participation
Persistent heart failure, as classified by the New York Heart Association classification of III or higher
Receiving antiplatelet therapy, except acetylsalicylic acid ≤150 mg per day
Receiving anticoagulation in therapeutic doses, other than standard anticoagulation during the hemodialysis procedure
Life expectancy <6 months
Active malignancy requiring treatment during study participation (except nonmelanoma skin cancer or cervical carcinoma in situ)
Known hypersensitivity to the investigational drug or to inactive constituents of the study drug
Participation in another clinical study with an investigational medicinal product within 30 days or within five half-lives of such, whichever is longer, before randomization and during the study
Any other conditions, which, in the opinion of the investigator or sponsor, would render the individual unsuitable for inclusion
Randomization and study treatment
Participants were randomized between lower-dose osocimab and lower-dose placebo in a ratio of 2:1, and higher-dose osocimab and higher-dose placebo in a ratio of 2:1. Different placebos were needed because a larger volume was administered in the higher-dose group than in the lower-dose group. Participants were centrally assigned using an interactive web-response system and covariate-adaptive randomization. Covariates included geographical region, age, previous major adverse cardiovascular event, dialysis access via catheter, low-dose aspirin use, diabetes and atrial fibrillation. After randomization, there was a 1-week pretreatment period where participants underwent three hemodialysis sessions to establish a baseline for adverse events without treatment. Participants then received osocimab in a lower- or higher-dose regimen or placebo for 6 months in the main treatment period followed by an extension period of up to 12 months, or until the last study participant had completed their 6-month main treatment period. The lower-dose osocimab regimen consisted of a 105 mg loading dose followed by a monthly maintenance dose of 52.5 mg, and the higher-dose regimen consisted of a 210 mg loading dose followed by a monthly maintenance dose of 105 mg. A matching placebo was provided in identical-appearing vials. Treatments were administered subcutaneously in the abdomen no more than 1 h before dialysis. Heparin was administered as per usual during the dialysis sessions. The maximum duration of treatment was 18 months, and the trial was stopped when the last randomized participant completed 6 months of treatment.
Study outcomes
Primary outcomes
The primary outcomes were (1) clinically relevant bleeding, namely the composite of major and clinically relevant nonmajor bleeding, and (2) the composite of moderate or severe adverse events and serious adverse events. Bleeding was classified as major if it was overt and associated with a decrease in hemoglobin of 2 g dl−1 or more; necessitated transfusion of two or more units of blood; occurred in a critical area or organ; or contributed to death. Overt bleeding not meeting these criteria, but that necessitated medical examination or intervention, or had clinical consequences, was classified as clinically relevant nonmajor bleeding. If neither set of criteria was met, bleeding was classified as minor.
Prespecified secondary outcomes were assessments of the change from baseline in osocimab concentration and key pharmacodynamic parameters, namely activated partial thromboplastin time and inhibition of factor XIa. Osocimab concentrations were measured by immunoassay, activated partial thromboplastin times were measured using C.K. Prest—a kaolin activator (Diagnostica Stago)—and factor XIa inhibition was quantified using a proprietary fluorogenic assay. Assays were conducted after in vitro neutralization of heparin to eliminate the potential effects of heparin in these assays.
Exploratory outcomes
Prespecified exploratory outcomes included the incidence of major adverse vascular events, the composite of vascular death due to myocardial infarction, stroke or pulmonary or systemic embolism; nonfatal myocardial infarction or stroke; major amputation of vascular etiology; acute limb ischemia and symptomatic venous thromboembolism.
Additionally, the incidence of arteriovenous fistula or graft thrombosis, and clotting of the dialysis circuit was assessed at every study visit as a prespecified exploratory outcome. Dialysis circuit clotting in the filter and air trap was assessed at the end of the hemodialysis procedure by study personnel blinded to treatment allocation. Clotting scores were assigned using a visual scoring system (0, no clot; 1, trace of clot; 2, intermediate between 1 and 3; and 3, fully clotted system necessitating interruption of hemodialysis session).
Statistical analysis
The major objectives of this study were to document the incidences of major and clinically relevant nonmajor bleeding for assessment of safety and arterial and venous thrombotic events for exploration of efficacy. There were no formal hypotheses. All analyses were descriptive and there was no formal a priori sample size calculation. Based on historic operational experience, of approximately 600 participants assigned randomly to study intervention, 555 participants would be expected to complete the main treatment period (assuming a true incidence rate of 15 participants lost to follow up per 100 participant years across all groups). Data were analyzed using SAS base v.9.4 (SAS/STAT v.14.3).
The efficacy and safety analyses were performed in the safety analysis set, defined as all randomized participants who received at least one dose of study medication. Safety and efficacy outcomes included all events that occurred during study treatment and up to 30 days after the last study drug administration. Outcomes were described by incidence proportions and cause-specific incidence rates per 100 person-years and their 90% CIs. Cause-specific hazard ratios were estimated with Cox proportional hazards model, subdistribution hazard ratios were estimated by Fine–Gray subdistribution hazards model. Because of the exploratory nature of these analyses, no multiplicity adjustment was performed. The number of participants who had a clotting score of 2 or 3 (moderate-to-complete dialysis circuit clotting) at one or more visits during the overall treatment period was compared between treatment groups by calculating relative risks and 95% CIs, where CIs were determined with the SAS procedure PROC FREQ by inverting two separate one-sided exact tests that were based on the score statistic (post hoc analysis; Farrington–Manning score statistic)21,22.
Sensitivity analyses
The cumulative incidence functions for the event-of-interest as well as the associated competing events together with the corresponding CIs were estimated for each treatment arm using Aalen–Johansen estimators. The competing events for the primary endpoints were death and premature discontinuation of exposure to assigned treatment. The cumulative incidences were estimated for time-to-event endpoints by Aalen–Johansen estimators with the competing event. The differences of the Aalen–Johansen estimators between high-dose osocimab and placebo and low-dose osocimab and placebo are presented with 90% CIs.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41591-023-02794-7.
Supplementary information
Supplementary appendix including author affiliations, Steering Committee, Data Monitoring Committee, IECs and IRBs, Clinical Events Adjudication Committee, study sites and investigators, and outcome definitions.
Acknowledgements
We thank all the participants in the trial and their families as well as the study site investigators. Medical writing support was provided by A. Jones and E. Aldera of Oxford PharmaGenesis, Oxford, UK, with financial support from Bayer. This study was funded by Bayer AG.
Extended data
Extended Data Fig. 2. Cumulative incidence risk (Aalen–Johansen) of the time-to-first treatment-emergent moderate, severe, or serious adverse event (primary outcome; safety analysis set).
Cumulative incidence (%) = Aalen–Johansen estimates of the cumulative probability for an event, calculated as 100 x (1 minus the Aalen–Johansen estimate of the survival function). Overall study period: Only treatment-emergent events that occurred during the overall study period are considered. Treatment-emergent events are those that occurred after the start of the study treatment and until the last dose of the study treatment plus 30 days.
Extended Data Table 2.
Cause-specific hazard ratios and subdistribution hazard ratios for primary outcome (composite of major bleeding and clinically relevant nonmajor bleeding) and competing events (safety analysis set)
Extended Data Table 3.
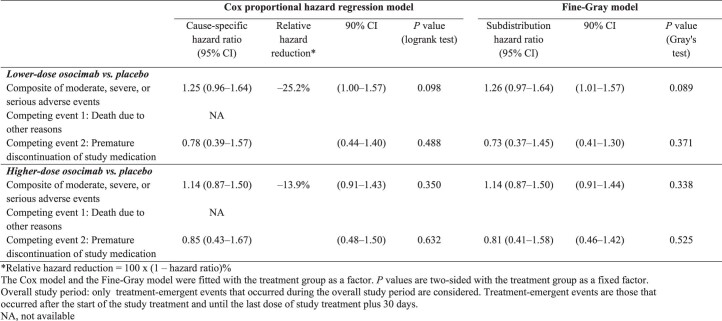
Cause-specific hazard ratios and subdistribution hazard ratios for the treatment-emergent composite of moderate, severe, or serious adverse events (primary outcome) and competing events (safety analysis set)
Author contributions
J.I.W. and L.B.T. drafted the initial manuscript. J.H. and L.B.T. made substantial contributions to the acquisition of data and Á.F.P. made substantial contributions to the analysis of data. All authors had full access to the study data and contributed to the critical review and revision of the manuscript and had final responsibility for the decision to submit it for publication.
Peer review
Peer review information
Nature Medicine thanks Kris Rogers, Alexander Zarbock and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Michael Basson, in collaboration with the Nature Medicine team.
Data availability
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA ‘Principles for responsible clinical trial data sharing.’ This pertains to the scope, timepoint and process of data access. As such, Bayer commits to sharing clinical trial data at the patient and study level upon request from qualified research personnel, as well as protocols from clinical trials for medicines and indications approved in the United States and European Union (necessary for conducting legitimate research). This applies to data on new medicines and indications that have been approved by the European Union and United States regulatory agencies on or after 1 January 2014. Interested researchers can use www.vivli.org to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information about the Bayer criteria for listing studies and other relevant information is provided in the member section of the portal. Data access to anonymized patient-level data, protocols and clinical study reports will be granted after approval by an independent scientific review panel. Data will be made available within 6 months after signing the Data Use Agreement to researchers who provide a methodologically sound proposal. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
Competing interests
L.B.T., J.H., Á.F.P. and D.K. are employees of Bayer; J.I.W. holds the Canada Research Chair (Tier I) in Thrombosis and the Heart and Stroke Foundation J.F. Mustard Chair in Cardiovascular Research at McMaster University, and has served as a consultant and received honoraria from Alnylam, Alexion, Anthos, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Janssen, Merck, PhaseBio, Regeneron, Servier and VarmX; J.F. reports consultancy or speaker honoraria from Alnylam, AstraZeneca, Bayer, Boehringer Ingelheim, Calliditas, Chinook, Novartis, Omeros, Travere, VeraTx and Vifor; he also serves on data safety monitoring boards of trials by Novo Nordisk and Visterra. J.F. is an associate editor of Kidney International; K.A.A.F. reports grant funding from Bayer and AstraZeneca and consulting for Bayer, Medscape, Baim Institute for Clinical Research and the Thrombosis Research Institute; D.L.B. reports the following relationships: Advisory Boards of Angiowave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia (now a subsidiary of Bristol Myers Squibb), NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences and Stasys; Board of Directors of Angiowave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care and TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Consultant: Broadview Ventures; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the National Institutes of Health (NIH)-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (co-chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and US national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Patent: Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women’s Hospital who assigned to Lexicon; neither I nor Brigham and Women’s Hospital receive any income from this patent); Research Funding: Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia (now a subsidiary of Bristol Myers Squibb), NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene and 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Takeda; R.T. has received royalties from Thermo Fisher Scientific, and consulting fees from the US Food and Drug Administration and Fresenius Medical Care; he has served in a data safety committee for Alnylam, and scientific advisory committees for Aggamin, Bayer, Comanche Biopharma and Hero; and he is on the board of directors for CAMP4, and is an equity holder with Aggamin, CAMP4, Comanche Biopharma and Hero; W.C.W. holds the Gordon A. Cain Chair in Nephrology at Baylor College of Medicine, and has served as a consultant and has received honoraria from Akebia/Otsuka, Ardelyx, AstraZeneca, Bayer, Boehringer Ingelheim/Lilly, GlaxoSmithKline, Merck Sharp & Dohme/Merck, Pharmacosmos, Reata, Unicycive and Zydus.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
A full list of members and their affiliations appears in the Supplementary Information.
Extended data
is available for this paper at 10.1038/s41591-023-02794-7.
Supplementary information
The online version contains supplementary material available at 10.1038/s41591-023-02794-7.
References
- 1.GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–1252. doi: 10.1016/S0140-6736(16)32064-5. [DOI] [PubMed] [Google Scholar]
- 3.Eikelboom J, Floege J, Thadhani R, Weitz JI, Winkelmayer WC. Anticoagulation in patients with kidney failure on dialysis: factor XI as a therapeutic target. Kidney Int. 2021;100:1199–1207. doi: 10.1016/j.kint.2021.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Reinecke H, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J. Am. Soc. Nephrol. 2009;20:705–711. doi: 10.1681/ASN.2007111207. [DOI] [PubMed] [Google Scholar]
- 5.Sood MM, et al. The three-year incidence of major hemorrhage among older adults initiating chronic dialysis. Can. J. Kidney Health Dis. 2014;1:21. doi: 10.1186/s40697-014-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jun M, et al. The association between kidney function and major bleeding in older adults with atrial fibrillation starting warfarin treatment: population based observational study. Br. Med. J. 2015;350:h246. doi: 10.1136/bmj.h246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pokorney SD, et al. Apixaban for patients with atrial fibrillation on hemodialysis: a multicenter randomized controlled trial. Circulation. 2022;146:1735–1745. doi: 10.1161/CIRCULATIONAHA.121.054990. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher WA, Luettgen JM, Quan ML, Seiffert DA. Inhibition of factor XIa as a new approach to anticoagulation. Arterioscler Thromb. Vasc. Biol. 2010;30:388–392. doi: 10.1161/ATVBAHA.109.197178. [DOI] [PubMed] [Google Scholar]
- 9.Preis M, et al. Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood. 2017;129:1210–1215. doi: 10.1182/blood-2016-09-742262. [DOI] [PubMed] [Google Scholar]
- 10.Meijers JC, Tekelenburg WL, Bouma BN, Bertina RM, Rosendaal FR. High levels of coagulation factor XI as a risk factor for venous thrombosis. N. Engl. J. Med. 2000;342:696–701. doi: 10.1056/NEJM200003093421004. [DOI] [PubMed] [Google Scholar]
- 11.Cushman M, O’Meara ES, Folsom AR, Heckbert SR. Coagulation factors IX through XIII and the risk of future venous thrombosis: the Longitudinal Investigation of Thromboembolism Etiology. Blood. 2009;114:2878–2883. doi: 10.1182/blood-2009-05-219915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beavers CJ, Wayne NB. Osocimab: a novel agent in preventing venous thromboembolism. J. Cardiovasc. Pharmacol. 2020;76:645–649. doi: 10.1097/FJC.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer M, Buchmueller A, Dittmer F, Straßburger J, Wilmen A. Allosteric inhibition as a new mode of action for BAY 1213790, a neutralizing antibody targeting the activated form of coagulation factor XI. J. Mol. Biol. 2019;431:4817–4833. doi: 10.1016/j.jmb.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Weitz JI, et al. Effect of osocimab in preventing venous thromboembolism among patients undergoing knee arthroplasty: the FOXTROT randomized clinical trial. JAMA. 2020;323:130–139. doi: 10.1001/jama.2019.20687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United States Renal Data System. 2022 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States (National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2022).
- 16.Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI. Medical device-induced thrombosis: what causes it and how can we prevent it? J. Thromb. Haemost. 2015;13:S72–S81. doi: 10.1111/jth.12961. [DOI] [PubMed] [Google Scholar]
- 17.Mavrakanas TA, Garlo K, Charytan DM. Apixaban versus no anticoagulation in patients undergoing long-term dialysis with incident atrial fibrillation. Clin. J. Am. Soc. Nephrol. 2020;15:1146–1154. doi: 10.2215/CJN.11650919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahal, K., Kunwar, S., Rijal, J., Schulman, P. & Lee, J. Stroke, major bleeding, and mortality outcomes in warfarin users with atrial fibrillation and chronic kidney disease: a meta-analysis of observational studies. Chest149, 951–959 (2016). [DOI] [PubMed]
- 19.Reinecke, H. et al. A randomized controlled trial comparing apixaban to the vitamin K antagonist phenprocoumon in patients on chronic hemodialysis: the AXADIA-AFNET 8 study. Circulation147, 296–309 (2023). [DOI] [PMC free article] [PubMed]
- 20.Winkelmayer, W. C., for the RE-THINC investigators. Antithrombotic treatment with fesomersen vs. placebo in patients with ESKD on hemodialysis (ESKD-HD). In Proc. ASN Kidney Week TH–PO976 (American Society of Nephrology, 2022).
- 21.Chan IS, Zhang Z. Test-based exact confidence intervals for the difference of two binomial proportions. Biometrics. 1999;55:1202–1209. doi: 10.1111/j.0006-341X.1999.01202.x. [DOI] [PubMed] [Google Scholar]
- 22.Farrington CP, Manning G. Test statistics and sample size formulae for comparative binomial trials with null hypothesis of non-zero risk difference or non-unity relative risk. Stat. Med. 1990;9:1447–1454. doi: 10.1002/sim.4780091208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary appendix including author affiliations, Steering Committee, Data Monitoring Committee, IECs and IRBs, Clinical Events Adjudication Committee, study sites and investigators, and outcome definitions.
Data Availability Statement
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the EFPIA/PhRMA ‘Principles for responsible clinical trial data sharing.’ This pertains to the scope, timepoint and process of data access. As such, Bayer commits to sharing clinical trial data at the patient and study level upon request from qualified research personnel, as well as protocols from clinical trials for medicines and indications approved in the United States and European Union (necessary for conducting legitimate research). This applies to data on new medicines and indications that have been approved by the European Union and United States regulatory agencies on or after 1 January 2014. Interested researchers can use www.vivli.org to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information about the Bayer criteria for listing studies and other relevant information is provided in the member section of the portal. Data access to anonymized patient-level data, protocols and clinical study reports will be granted after approval by an independent scientific review panel. Data will be made available within 6 months after signing the Data Use Agreement to researchers who provide a methodologically sound proposal. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.