Abstract
Phorbol ester treatment of quiescent Swiss 3T3 cells leads to cell proliferation, a response thought to be mediated by protein kinase C (PKC), the major cellular receptor for this class of agents. We demonstrate here that this proliferation is dependent on the activation of the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) cascade. It is shown that dominant-negative PKC-α inhibits stimulation of the ERK/MAPK pathway by phorbol esters in Cos-7 cells, demonstrating a role for PKC in this activation. To assess the potential specificity of PKC isotypes mediating this process, constitutively active mutants of six PKC isotypes (α, β1, δ, ɛ, η, and ζ) were employed. Transient transfection of these PKC mutants into Cos-7 cells showed that members of all three groups of PKC (conventional, novel, and atypical) are able to activate p42 MAPK as well as its immediate upstream activator, the MAPK/ERK kinase MEK-1. At the level of Raf, the kinase that phosphorylates MEK-1, the activation cascade diverges; while conventional and novel PKCs (isotypes α and η) are potent activators of c-Raf1, atypical PKC-ζ cannot increase c-Raf1 activity, stimulating MEK by an independent mechanism. Stimulation of c-Raf1 by PKC-α and PKC-η was abrogated for RafCAAX, which is a membrane-localized, partially active form of c-Raf1. We further established that activation of Raf is independent of phosphorylation at serine residues 259 and 499. In addition to activation, we describe a novel Raf desensitization induced by PKC-α, which acts to prevent further Raf stimulation by growth factors. The results thus demonstrate a necessary role for PKC and p42 MAPK activation in 12-O-tetradecanoylphorbol-13-acetate induced mitogenesis and provide evidence for multiple PKC controls acting on this MAPK cascade.
To date, 11 members of the protein kinase C (PKC) superfamily have been identified (for reviews, see references 13, 28, 45, and 52). On the basis of their biochemical properties and sequence homologies, they have been divided into three groups: the conventional PKCs (cPKC-α, -β1, -β2, and -γ), which are activated in a diacylglycerol (DAG)- and calcium-dependent manner; the calcium-independent but DAG-dependent novel PKCs (nPKC-δ, -ɛ, -η, -θ, and -μ, also termed PKD); and a third group consisting of atypical PKCs (aPKC-ζ and -ι/λ). The members of this last group of isotypes are unresponsive to DAG and calcium and, in contrast to c- and nPKCs, do not respond to phorbol esters. The existence of this large family of PKC isotypes suggests that individual PKC isotypes likely have specific roles in signal transduction. We have been interested in determining if such specificity exists in the case of the extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK) cascade, by which PKC may mediate some of its effects on cell growth and differentiation.
The MAPK cascade, which involves the kinases Raf, MAPK/ERK kinase (MEK), and ERK/MAPK, is ubiquitously expressed in mammalian cells and serves to couple various cell surface stimuli to the alteration of cell function. This cascade is implicated in both regulated cell proliferation (induced by growth factors) and deregulated proliferation (e.g., Ras transformation) as well as the control of differentiation (33, 54). Such actions are elicited at least in part through the translocation of activated MAPK to the nucleus, where it phosphorylates target molecules such as the transcription factors Elk-1 and SAP1, which consequently leads to alterations in gene expression (24).
The mechanisms involved in the activation events for this MAPK cascade have been studied extensively and are well established for MEK and p42 MAPK (ERK2). In both cases, two phosphorylations within the activation loop of the kinase are required for activation, and these are catalyzed by the immediate upstream kinase (4, 47, 59). For instance, for p42 MAPK, it was shown that MEK phosphorylates a threonine (T) and a tyrosine (Y) residue within a characteristic TEY motif, causing activation. In contrast to these activation mechanisms, the regulation of Raf has proven substantially more complex. This protein kinase is regulated in part through interaction with membrane-associated GTP-Ras and in part by phosphorylation (33, 37, 42). Furthermore, it is possible that other modifications and/or associations, such as dimerization of Raf molecules or association with 14-3-3 proteins, regulate Raf function (17, 19, 29, 35). Among the mechanisms involved, there is evidence for the operation of both PKC-dependent and PKC-independent pathways of Raf activation in response to agonists (49). Much evidence for the involvement of PKC in Raf activation comes from the action of the tumor promoters of the phorbol ester class. Acute treatment with phorbol esters leads to a rapid activation of p42 MAPK in most cell types (25, 50). Since PKC is the major receptor for these tumor promoters, it has been implicated in the activation of the ERK/MAPK pathway and the consequent triggering of cellular responses such as cell differentiation and proliferation (18, 23, 40, 41, 44). More-direct evidence for the involvement of PKC in regulating this pathway has come from coexpression studies in insect cells, which have reported that PKC-α, -β1/2, and -γ alone can induce Raf autophosphorylation, peptide phosphorylation, or MEK phosphorylation (38, 51).
While early studies employed diverse criteria for assessment of Raf activation, the identification of MEK-1 as a physiological substrate for Raf has provided a robust assay for agonist-induced Raf activation. Using this assay and employing dominant-negative and constitutively active PKC mutants, we here define the potential for these proteins in activation of the MAPK cascade, which is a prerequisite in the mediation of PKC effects, e.g., cell proliferation. We demonstrate here that PKC can be rate limiting for MAPK activation in mammalian cells. Furthermore, it is shown that all PKC isotypes tested (α, β1, δ, ɛ, η, and ζ) have the capacity to activate p42 MAPK and MEK. Additionally, we have been able to show that there are at least two mechanisms involved in activation of the ERK/MAPK pathway by PKCs: cPKC-α and nPKC-η use a Raf-dependent pathway to activate MEK and MAPK, while aPKC-ζ leads to MEK activation in a manner independent of Raf activation. Furthermore, PKC-α (and PKC-β1) is shown to induce a novel desensitization effect in c-Raf activation which prevents further activation by growth factors. These data indicate the operation of a distinct control by conventional PKCs of Raf function. In light of the effects of PKC isotypes on c-Raf mutants, the mechanism of PKC-dependent activation of this pathway is discussed.
MATERIALS AND METHODS
Plasmid constructs.
The cDNAs of the constitutively active PKC mutants carry deletions or point mutations in the N-terminal pseudosubstrate region. In PKC-α (48) and -β1, amino acids (aa) 22 to 28 are deleted; in PKC-δ, Ala-147 is exchanged for Glu; PKC-ɛ lacks aa 156 to 162; and PKC-η (12) and -ζ carry deletions from aa 155 to 171 and from aa 116 to 122, respectively. PKC-α, -β1, -δ, and -ζ mutants were expressed via the pCO2 vector, and PKC-ɛ and -η were expressed via pMT2 and pKS1, respectively. The dominant-negative mutant PKCα(T/A)3 has been described previously (6).
The myc-p42 MAPK was described by Howe et al. (27); the myc-MEK-1 construct is from S. Cowley and C.J.M. (10a). The myc epitope-tagged constructs for c-Raf1 (pEFHmRaf-1) and RafCAAX (pEFHmRafCAAX) were described previously (37). The myc-Raf S259A construct, in which the serine at position 259 has been replaced by alanine, was generated by PCR-directed mutagenesis of the pEFHmRaf plasmid. myc-Raf S499A was subcloned from the pKSRaf S499A plasmid (kindly provided by D. K. Morrison) into the pEFHmRaf construct.
Recombinant proteins.
Recombinant glutathione S-transferase–p42 MAPK was expressed and purified according to the protocol of Stokoe et al. (53). Purification of recombinant glutathione S-transferase–MEK–His protein was described by Alessi et al. (4).
Immunoblotting.
Immunoprecipitated proteins were resuspended in Laemmli sample buffer (without reducing agents), heated at 95°C for 10 min, and loaded onto sodium dodecyl sulfate (SDS)–10% polyacrylamide minigels. After electrophoresis, the proteins were transferred onto polyvinylidene difluoride membranes by the semidry method. The membranes were blocked in phosphate-buffered saline (PBS) containing 5% dry milk (fat reduced) and 0.1% Tween 20 for 1 h and then incubated with various primary antibodies for at least 8 h at 4°C. After three washes with PBS, a horseradish peroxidase-coupled anti-rabbit antibody (dilution, 1:5,000) was employed for 40 min, and nonspecifically bound material was washed off with several changes of PBS–0.1% Tween 20 before the protein was detected by using the Amersham ECL system.
A polyclonal antiserum against p42 MAPK was described earlier (1). c-Raf1 protein was detected with a polyclonal antiserum from Santa Cruz Biotechnology. A polyclonal antiserum against MEK-1 (human) was raised in rabbits against the C-terminal peptide G-L-N-Q-P-S-T-P-T-H-A-A-G-V.
Cell culture, transfection, and immunoprecipitation.
Cos-7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) in humified air with 10% CO2 at 37°C. For transfection, cells were treated as follows. On day 1, 1.125 × 106 Cos-7 cells were seeded on a 10-cm-diameter plate in DMEM supplemented with 10% fetal calf serum (FCS; Gibco). On day 2, the cells were washed twice in DMEM (without FCS) and then further grown in DMEM–0.1% FCS. On day 3, cells were transfected by the calcium phosphate method of Maniatis et al. (36) with 40 μg of PKC DNA and 5 μg of a reporter construct (myc-p42 MAPK, myc-MEK-1, myc-Raf, myc-RafCAAX, Raf S259A, or Raf S499A) without changing the medium. All DNAs used for transfections were purified over two CsCl gradients. After transfection (45 to 48 h), cells either were left untreated or were treated with 20% serum plus 400 nM 12-O-tetradecanoylphorbol-13-acetate (TPA; Sigma) for 10 min (in the case of Raf transfections) or 20 min (for p42 MAPK and MEK-1 transfections) and harvested into 600 μl of lysis buffer (buffer A; 1% Triton X-100, 50 mM HEPES [pH 7.5], 150 ml NaCl, 20 mM NaF, 50 μg of phenylmethylsulfonyl fluoride per ml, 100 μM Na3 VO4, 125 μg of aprotinin per ml, 250 μg of leupeptin per ml, 1 μM microcystin). After 20 min of preclearing with a protein A-Sepharose (Sigma) suspension (1% final concentration), 4 μg of 9E10 antibody (16) and 40 μl of a 50% suspension of protein G-Sepharose beads (Sigma) were added to the lysates, which then were incubated for 1 to 2 h at 4°C.
Thymidine incorporation into Swiss 3T3 cells.
Swiss 3T3 cells were cultured as described by Olivier and Parker (46). The incorporation of [3H]thymidine was performed as described by Withers et al. (57). The MEK-1 protein kinase inhibitor PD 098059 (Calbiochem) was used at a final concentration of 30 μM.
Analysis of myc-p42 MAPK activity.
Immunoprecipitates were washed three times in buffer A (see above) and once in kinase assay buffer (buffer B; 30 mM Tris [pH 7.5], 5 mM MgCl2, 2 mM MnCl2). In vitro kinase assays were carried out for 30 min at 25°C in 30 μl of buffer B supplemented with 10 μM ATP, 1 μCi of [γ-32P]ATP (Amersham), and 10 μg of myelin basic protein (MBP; Sigma) as a substrate. The kinase reaction was stopped with 15 μl of 4× SDS sample buffer, and samples were separated on an SDS–15% polyacrylamide minigel. The lower half of the gel was stained with Coomassie blue and dried down, and 32P incorporation into MBP was quantitated with a PhosphorImager (Molecular Dynamics). The contents of the upper part of the gel, containing immunoprecipitated myc-p42 MAPK protein, were transferred onto polyvinylidene difluoride membranes and detected with an anti-p42 MAPK polyclonal antiserum. The amount of protein was determined by scanning the Western blot and processing the data with the Adobe Photoshop program. MBP phosphorylation was normalized to the amount of myc-p42 MAPK in each sample and thus expressed as specific activity in arbitrary units.
Analysis of myc-MEK activity.
Immunoprecipitates were washed as described above for myc-p42 MAPK. For the two-step in vitro kinase assay, myc-MEK protein bound to protein G beads was incubated for 15 min at 30°C in 40 μl of buffer C (25 mM HEPES [pH 7.5], 10 mM MgCl2, 0.1 mM EDTA, 1.5 mM dithiothreitol, 0.1 mM ATP, and 0.3 μg of recombinant p42 MAPK) to activate p42 MAPK. Five microliters of [γ-32P]ATP (0.3 μCi) and 20 μg of MBP were added, and after a further 15-min incubation, 50% of the reaction mixture was spotted onto P81 paper, washed three times in 10% acetic acid, and quantitated by Cherenkov counting. The remaining 50% of the reaction mixture was run on a 15% polyacrylamide minigel and immunodetected with an anti-MEK antiserum. MBP phosphorylation was normalized as described above for p42 MAPK and is expressed as specific activity in arbitrary units.
Analysis of myc-Raf activity.
In vitro kinase assays of myc-Raf (wild-type and mutant proteins) were performed in a coupled kinase assay as described by Leevers et al. (34). Quantitation of the amount of Raf protein in each sample was performed in a manner analogous to the quantitation of p42 MAPK.
RESULTS
Phorbol ester-induced cell proliferation depends on activation of the ERK/MAPK pathway.
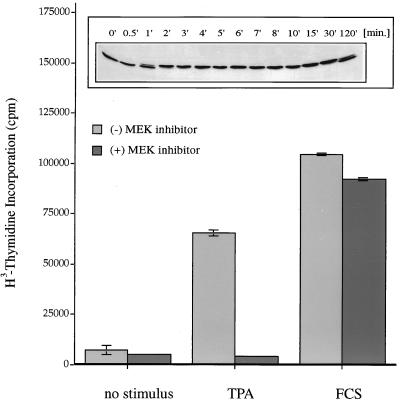
Phorbol ester treatment of fibroblasts leads to the immediate activation of p42 MAPK and long-term responses, e.g., stimulation of cellular proliferation (Fig. 1). To investigate whether activation of the ERK/MAPK pathway is a requirement for the induction of cell division by phorbol esters, we measured thymidine incorporation into Swiss 3T3 fibroblasts after challenging the cells with the phorbol ester TPA or FCS (as a positive control). Both stimuli induce proliferation efficiently; however, whereas serum-induced thymidine incorporation is essentially unaffected by the presence of the specific MAPK kinase inhibitor PD 098059 (3), TPA-induced cell proliferation is completely abolished in the presence of this agent. We conclude that phorbol ester-induced cell proliferation is dependent on the action of the ERK/MAPK pathway.
FIG. 1.
Effects of phorbol ester on Swiss 3T3 cells. Phorbol ester treatment induces DNA synthesis via the ERK/MAPK pathway. Quiescent Swiss 3T3 cells were treated for 40 h with 400 nM TPA or 20% fetal calf serum or left without stimulus in the presence (+) or absence (−) of the MEK-1 inhibitor PD 098059 (30 μM). DNA synthesis was assessed by measuring [3H]thymidine incorporation. Each value is the mean ± the standard error of the mean for three dishes representative of two independent experiments. (Inset) Phorbol ester treatment activates p42 MAPK. Quiescent Swiss 3T3 cells were treated with 400 nM TPA for different time intervals, and p42 MAPK activation was measured by mobility shift analysis of the p42 MAPK protein in a 10% polyacrylamide gel as a readout for kinase activity. Phorbol ester treatment of Swiss 3T3 cells causes a rapid activation of p42 MAPK (1 min) which is sustained for up to 2 h.
Dominant-negative PKC-α(T/A)3 inhibits activation of p42 MAPK by TPA.
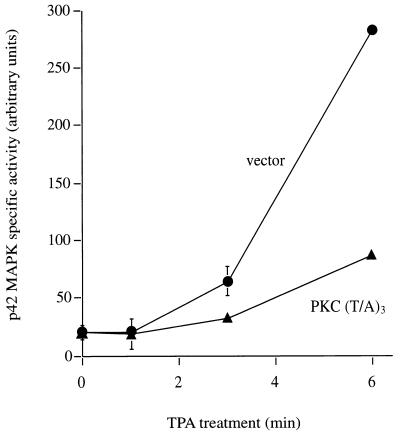
Biological responses observed after phorbol ester treatment are usually attributed to activation of the PKC family, the major cellular target for phorbol esters. However, other receptors have been identified, such as n-chimaerin and Vav, and therefore it was deemed important to determine whether phorbol ester-induced stimulation of the ERK/MAPK cascade is indeed mediated by PKC or another TPA-responsive intermediary (2, 22). We therefore assessed whether the activation of p42 MAPK by TPA is mediated by cellular PKC. For this purpose, a dominant-negative PKC-α mutant (alpha is the predominant PKC isotype in Cos-7 cells) was employed; this mutant competitively inhibits the functional maturation of endogenous PKC molecules in these cells (10). When cells that express the dominant-negative mutant PKC-α(T/A)3 are treated with TPA, the rate of p42 MAPK activation is markedly inhibited in comparison to that in vector-transfected control cells (Fig. 2). This dominant-negative PKC-α(T/A)3 construct has a broad specificity of action and cannot be used to distinguish PKC isotype specificity (19a). Nevertheless, its action implies a rate-limiting role for PKC in p42 MAPK activation in these cells. At later time points (≥18 min), the extent of p42 MAPK activation is the same in control- and mutant-transfected cells (data not shown), indicating that, consistent with the activity of this dominant-negative mutant (10), this is a partial inhibition which is not due to any nonspecific, cytotoxic effect.
FIG. 2.
Dominant-negative PKC-α inhibits p42 MAPK activation after TPA treatment. Serum-starved Cos-7 cells were cotransfected with myc-p42 MAPK and dominant-negative PKC-α(T/A)3 or with empty vector as a control. After transfection (48 h), cells were stimulated for 0, 1, 3, and 6 min with TPA (250 nM) and myc-p42 MAPK activity was determined in immune complex kinase assays with MBP as a substrate. Activities were normalized to myc-p42 MAPK protein levels in the immune complex. For each time point, triplicate samples were used, and the standard error is indicated (error bars) when it is >8% of the corresponding mean value. By the t test, the 6-min time points result in a P value of less than 0.001.
p42 MAPK and MEK-1 are activated by various PKC isotypes.
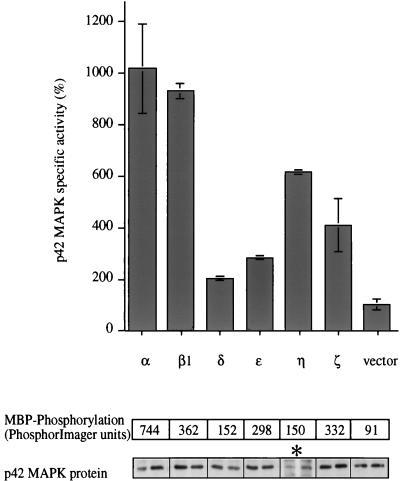
TPA treatment activates the majority of PKC isotypes (α, β1/2, γ, δ, ɛ, η, and θ), while the class of aPKCs (ζ and λ/ι) is unresponsive to phorbol esters. To examine which members of the PKC superfamily can regulate the ERK/MAPK cascade, constitutively active mutants of the α, β1, δ, ɛ, η, and ζ isotypes were used. All of these mutants carry a short deletion (α, β1, ɛ, η, and ζ) or point mutation (δ) in the pseudosubstrate sequences within the N-terminal regulatory domains of the proteins, such that the enzymes are locked in their active conformation (26). We and others have shown previously that these mutants have a substantially increased level of activity in the absence of cofactors compared to the wild-type proteins and that they induce a variety of biological effects when overexpressed in different cell contexts, e.g., induction of nitric oxide synthase or stimulation of ANF-, TRE/AP-1, and NF-AT-1-regulated promoter activities (11, 12, 14, 20, 48). Expression of a single active mutant in combination with a myc-tagged p42 MAPK construct in Cos-7 cells (which endogenously express PKC-α, -β1, -ɛ, and -ζ) permitted examination of PKC isotype specificity in vivo. As shown in Fig. 3, each of the PKC isotypes tested is able to activate p42 MAPK in vivo. It should be noted that this assay does not allow absolute potencies to be determined. (All PKC constructs express the appropriate proteins; however, we are unable to define an absolute level of expression for comparison of different isotypes, since antisera with known titers are not available. It should be noted, however, that absolute concentrations may have little bearing on localization, and it is the latter that ultimately influences downstream events [31].) The observations reflect the potential for each isotype to stimulate the MAPK cascade in vivo.
FIG. 3.
p42 MAPK is activated by various PKC isotypes in vivo. Six different constitutively active PKC isotypes representing the conventional (α, and β1), novel (δ, ɛ, and η), and atypical (ζ) subclasses of this family and empty vector were cotransfected with myc-p42 MAPK into Cos-7 cells. Duplicate dishes were harvested 48 h after transfection, myc-p42 MAPK was immunoprecipitated, and its activity was determined. myc-p42 MAPK activity is presented in arbitrary units as a function of protein expression. The panels below the graph show the amounts of substrate phosphorylation expressed as units of the PhosphorImager scanner (Molecular Dynamics) (upper panel) and the amounts of protein which was present in the reactions (duplicates; lower panel). Results from one of three similar experiments are shown. The stimulation of a control sample, transfected with empty vector and myc-p42 MAPK, by a mixture of TPA (400 nM) and FCS (20%) 20 min before harvesting of the cells resulted in myc-p42 MAPK activation of between 14- and 83-fold depending on the individual experiment (data not shown). The asterisk indicates a longer exposure of the Western blot showing myc-p42 MAPK protein in the PKC-η-transfected cells than for the other blots.
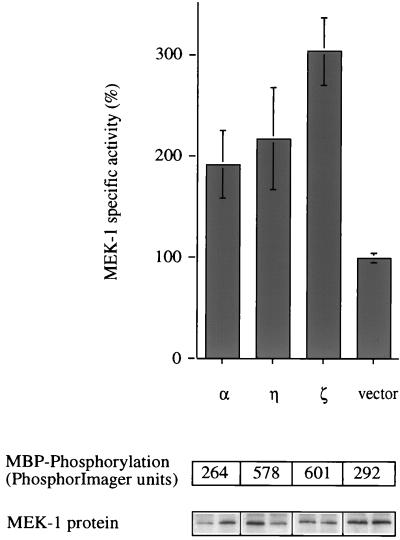
To dissect the pathway further, we examined whether constitutively active PKC-α, -η, and -ζ are able to activate the p42 MAPK upstream activator MEK-1 (these isoforms were chosen as, respectively, representatives of the cPKC, nPKC, and aPKC subclasses of PKC [see reference 13]). A tagged version of MEK-1, myc-MEK-1, was coexpressed with each of the active PKC mutants, and 45 h after transfection, cells were lysed and MEK-1 was immunoprecipitated. A coupled in vitro kinase assay with recombinant p42 MAPK and MBP was used to measure MEK-1 activity. Figure 4 shows that all three isotypes tested have the potential to activate MEK-1.
FIG. 4.
PKC-α, -η, and -ζ activate MEK-1 in vivo. Constitutively active forms of PKC-α, -η, and -ζ and empty vector were coexpressed with a myc-tagged MEK-1 construct in Cos-7 cells. Cells were cultured for 48 h before they were harvested and myc-MEK-1 was immunoprecipitated. Myc-MEK-1 activity was determined in a coupled in vitro kinase assay with recombinant p42 MAPK protein and MBP as a substrate. The panels below the graph represent 32P incorporation into MBP (upper panel) and the amount of myc-MEK-1 enzyme present (duplicates; lower panel) in each reaction. Each value is the average for duplicate samples. Error bars indicate standard error. Stimulation of a control sample, which was transfected with empty vector and myc-MEK-1, by a mixture of TPA (400 nM) and FCS (20%) for 20 min before harvesting resulted in 8- to 10-fold myc-MEK-1 activation (data not shown). The data shown are from one of two similar experiments.
PKC-α and -η, but not PKC-ζ, are able to activate c-Raf1.
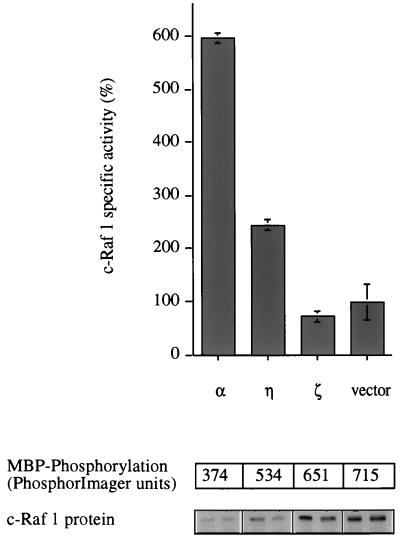
The ability of PKC-α, -η, and -ζ to induce MEK-1 activity prompted an investigation of Raf activation. It is well established that c-Raf1 is activated when cells are challenged with TPA, although the exact mechanism is still controversial (5, 7, 39, 43). myc-tagged c-Raf1 was immunoprecipitated after coexpression with constitutively active PKC mutants, and its activity was measured by reconstituting the phosphorylation cascade (myc-Raf → MEK → MAPK → MBP) with recombinant enzymes in vitro (34). It was observed that while cPKC-α and nPKC-η activate c-Raf1, aPKC-ζ is incapable of doing so (Fig. 5). Consistent with this, Cai et al. have recently reported that a constitutively active PKC-ɛ mutant also activates c-Raf1 (8). These observations provide direct evidence that certain PKC isotypes have an intrinsic potential to cause c-Raf1 activation in mammalian cells.
FIG. 5.
c-Raf 1 is activated by PKC-α and -η but not by PKC-ζ. Constitutively active constructs of PKC-α, -η, and -ζ, and empty vector were cotransfected with a myc-tagged c-Raf1 construct into Cos-7 cells. Forty-five hours after transfection, the myc-Raf protein was immunoprecipitated and its activity was measured in a coupled in vitro kinase assay with recombinant MEK and p42 MAPK proteins and MBP as a substrate. The amount of substrate phosphorylation (upper panel) and the quantity of myc-Raf enzyme in each reaction (duplicates; lower panel) are shown in the pairs of panels below the graph. Stimulation of a control sample, which was transfected with empty vector and myc-Raf, by a mixture of TPA (400 nM) and FCS (20%) for 10 min before harvesting resulted in 6- to 22-fold myc-Raf activation (data not shown). The data shown are from one of three similar experiments.
The inability of PKC-ζ to activate c-Raf1 indicates that a mechanism independent of Raf activation is involved in its activation of MEK (see above). We excluded the possibility that PKC-ζ activates MEK-1 directly in an in vitro kinase assay with recombinant MEK protein and recombinant PKC-ζ or activated PKC-ζ (data not shown). In addition, A-Raf, which is also expressed in Cos-7 cells, was shown not to mediate the effect of PKC-ζ on MEK, since cotransfection with PKC-ζ did not induce activation of A-Raf; a similar lack of activation was obtained for B-Raf. These results indicate a distinct pathway operating via PKC-ζ to activate MEK-1. However, it should be noted that this pathway is unlikely to account for TPA-induced MEK-1 activation since this agonist does not act on PKC-ζ (21, 56).
Mechanism of c-Raf1 activation by PKC-α and -η.
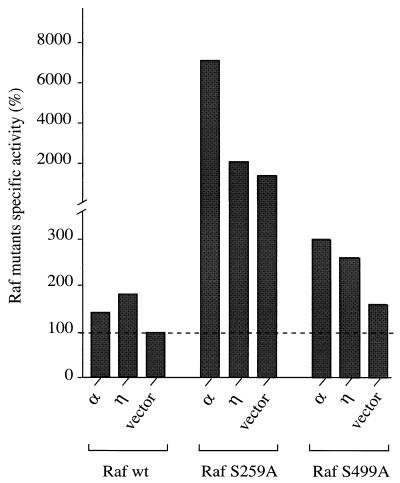
PKC has been shown to phosphorylate c-Raf1 on several serine residues in vivo and in vitro (9), but only the two sites Ser-259 and Ser-499 have been implicated in Raf activation by direct phosphorylation through PKC (32). To assess the role of direct phosphorylation in Raf activation, the two Raf mutants S259A and S499A were coexpressed with PKC-α and -η and activation was measured. If phosphorylation of these two sites by PKC was indeed responsible for activation of Raf, point mutants with a substitution of Ala for Ser-259 or Ser-499 would be expected to be unresponsive to the action of constitutively active PKC-α or -η. Interestingly, both mutants displayed activation levels similar to those observed for wild-type Raf in response to these two PKC isotypes (Fig. 6) (see below for a discussion of the enhanced response of Raf S259A). The results indicate that phosphorylation at these two sites is not required for activation by PKC.
FIG. 6.
PKC-α and -η are able to activate Raf S259A and Raf S499A. Cos-7 cells were cotransfected with constructs of constitutively active PKC-α and -η or empty vector in combination with a myc-tagged wild-type (wt) c-Raf1 or mutant Raf construct c-Raf S259A or c-Raf S499A. After transfection (40 h), the cells were harvested, the wild-type or mutant Raf proteins were immunoprecipitated, and their activities were measured in a coupled in vitro kinase assay. Substrate phosphorylation was normalized to the amount of Raf protein in each kinase reaction; Raf activation is presented as a percentage of the activity of the empty vector control. Stimulation of control samples, which were transfected with empty vector and either myc-Rafwt, myc-Raf S259A, or myc-Raf S499A, by a mixture of TPA (400 nM) and FCS (20%) for 10 min before harvesting resulted in 6-fold, 4- to 10-fold, and 7- to 9-fold activation, respectively (data not shown). This experiment was carried out in duplicate and is representative of three independent experiments.
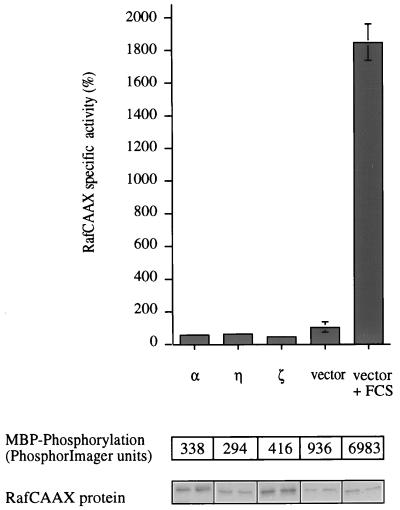
One of the essential steps in Raf activation, which is mediated by the small GTPase p21Ras, is its translocation to the plasma membrane, where it accumulates multiple phosphates on Ser, Thr, and Tyr sites (37, 42). To determine whether PKC might influence this or a later step, we investigated the PKC-induced activation of a membrane-targeted c-Raf (RafCAAX). Although this Raf enzyme has a higher intrinsic activity than wild-type Raf, it still responds to serum stimulation (34). While PKC-α and -η are both able to activate c-Raf1, no effect on RafCAAX could be observed (Fig. 7).
FIG. 7.
A membrane-localized mutant of Raf cannot be activated by constitutively active PKCs. Cos-7 cells were cotransfected with myc-RafCAAX and either constitutively active PKC-α, -η, or -ζ empty vector. Forty-five hours after transfection, control cells were either left untreated or stimulated for 10 min with 20% serum and 400 nM TPA as indicated and then harvested. A fraction of the total cell lysate was used to immunoprecipitate myc-RafCAAX and to determine its activity in a coupled assay that depends on the addition of recombinant MEK and p42 MAPK and MBP. Substrate phosphorylation (upper panel) was normalized to the amount of protein in each sample (duplicates; lower panel). Stimulation of a control sample, which was transfected with empty vector and myc-RafCAAX, by a mixture of TPA (400 nM) and FCS (20%) for 10 min before harvesting resulted in 3- to 8-fold myc-RafCAAX activation (data not shown). This experiment was carried out in duplicate and is representative of three independent assays.
PKC-α activates the ERK/MAPK pathway but inhibits further activation by serum.
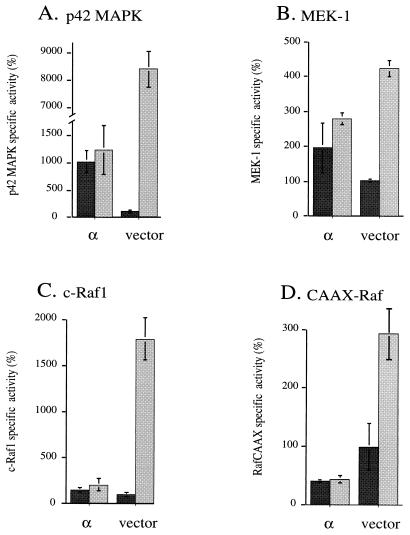
The data in Fig. 3, 4, and 5, respectively, show that constitutively active PKC-α can activate p42 MAPK, MEK-1, and c-Raf1. To compare this activation to the effect of a well-characterized stimulus for Raf, transfected cells were treated with a combination of potent Raf activators. While serum-TPA increases p42 MAPK activity still further in cells containing constitutively active PKC-δ, -ɛ, -η, or -ζ (data not shown) or vector controls, cells expressing active PKC-α (Fig. 8) and PKC-β1 (data not shown) are dramatically inhibited in their serum-TPA response. This desensitization effect of PKC-α could be detected as well at the level of MEK-1, c-Raf, and RafCAAX (Fig. 8). Thus, in addition to their activating effect on the MAPK pathway, PKC-α (and PKC-β1) have a second effect which limits further activation of c-Raf1 by growth factors and other TPA-responsive, endogenous PKC isotypes.
FIG. 8.
PKC-α exerts a desensitization effect at different levels of the MAPK cascade. Cos-7 cells were cotransfected with constitutively active PKC-α (α) or empty vector in combination with a myc epitope-tagged p42 MAPK (A), myc-MEK-1 (B), myc-c-Raf1 (C), or myc-RafCAAX (D). After 40 h of expression, half of the samples were stimulated with 20% serum and 400 nM TPA for 20 min in the case of p42 MAPK and MEK-1 or 10 min in the case of c-Raf 1 and RafCAAX; unstimulated cells are presented in dark gray, and serum-TPA-stimulated cells are presented in light gray. After immunoprecipitation of the myc-tagged proteins, the activities of p42 MAPK, MEK-1, c-Raf1, and RafCAAX were determined. All data shown have been normalized to the amount of reporter construct expressed in each sample. Each assay was done in duplicate.
DISCUSSION
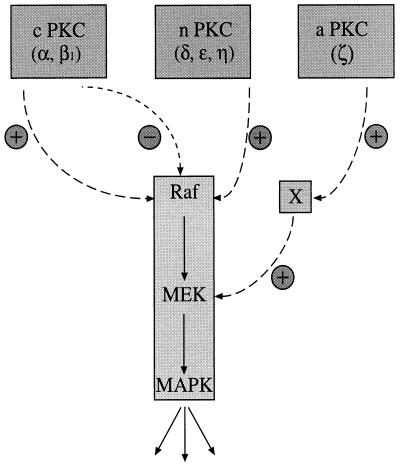
The results presented here demonstrate that PKC can control MAPK activation and, furthermore, that the mechanism of activation shows some isotype specificity. While cPKC-α and nPKC-η both show an ability to activate the MAPK cascade via c-Raf, aPKC-ζ activates this cascade by a mechanism independent of c-Raf1 activation. Thus, distinct subclasses of PKC may account for two independent signalling pathways to MEK and, hence, MAPK activation. For the activation of Raf itself, the inability of PKC-α or PKC-η to activate RafCAAX implies that PKC might be involved in controlling the membrane association of c-Raf. How this control operates is not evident; however, the activation of the c-Raf mutants S259A and S499A by these two PKC isotypes shows that PKC-dependent activation does not operate via direct phosphorylation at these sites. Finally, a novel desensitization process is shown to operate through PKC-α (and PKC-β1) that prevents c-Raf activation by the otherwise-potent serum-TPA cocktail of agonists. In contrast to activation, the inhibitory effect of PKC-α on growth factor (serum) activation of c-Raf still operates on RafCAAX. The emerging pattern of PKC control of c-Raf and the MAPK pathway is summarized in Fig. 9; this diagram illustrates both the positive and negative inputs of PKC into this cascade.
FIG. 9.
Model illustrating how different PKC isotypes activate the ERK/MAPK cascade. cPKCs (α and β1) have the potential to activate c-Raf1 in vivo while at the same time blocking further activation by growth factors and other PKC isotypes. PKC-δ, -ɛ, and -η, representing isotypes of the novel subclass of the PKC superfamily, activate the MAPK cascade but show no desensitization effect. aPKC-ζ differs from the other PKC isotypes with regard to MEK activation. While PKC-α and -η signal to MEK via Raf, PKC-ζ works through a Raf-independent pathway.
Many previous studies invoking PKC involvement in activation of the MAPK cascade have relied on the action of phorbol esters. However, it has become clear that targets other than PKC, some with pharmacological properties indistinguishable from those of PKC, exist (30). Such alternate targets confound the rigorous conclusions drawn from the use of phorbol esters. Here we have used a dominant-negative PKC-α mutant that competitively inhibits the priming of endogenous PKC to demonstrate that PKC can be causally involved in MAPK activation. The MAPK response to stimulation in the presence of PKC-α(T/A)3 is suppressed. Within the first 6 min of stimulation, p42 MAPK activation is inhibited by >75%. However, by 18 min there is no significant difference from the vector control. This demonstrates that PKC plays a rate-limiting role in the acute response to TPA. The fact that at later time points the effect of PKC-α(T/A)3 is no longer observed is consistent with the finding that the inhibition of endogenous PKC is incomplete (10, 19a).
The specificity of the PKC-α dominant-negative mutant employed here is broad with respect to the entire PKC family. Hence, no particular TPA-sensitive isotype can be implicated through this paradigm. The finding that in fact all of the PKC isotypes tested have the potential to activate the pathway in mammalian cells suggests an apparent redundancy of function. However, this does not seem to be the case at a molecular level. Each of the three PKC isotypes studied (PKC-α, -η, and -ζ) has specific effects on the MAPK pathway. Activation of the pathway by PKC-η is the most straightforward and can be rationalized by its activation of c-Raf1. In the case of PKC-ζ, while MEK-1 and p42 MAPK are activated, no effect on c-Raf1 is observed, indicating a mechanism of action independent of Raf activation. A-Raf, but not B-Raf, was detected in the cells employed in this study. To determine if an alternate Raf pathway was involved, A-Raf was coexpressed with PKC-ζ, but again no activation was observed (data not shown). The data clearly indicate that PKC-ζ acts independently of Raf. It has been reported previously that PKC-ζ can associate with and activate MEK-1 directly in vitro (15); however, we were unable to confirm this observation with baculovirus-expressed PKC-ζ or mammalian-expressed activated PKC-ζ (unpublished observations). Furthermore, no complex of PKC-ζ with MEK could be detected. It is proposed that PKC-ζ acts through an as-yet-unidentified factor that acts on MEK-1. We cannot exclude the possibility that other PKC isotypes also signal via this pathway.
For PKC-α, c-Raf1 appears to mediate activation of MEK and p42 MAPK; however, this action is complicated by a negative input that limits c-Raf1 activation. In marked contrast to the activation process, this negative effect also acts on the membrane-targeted form of c-Raf1, the RafCAAX mutant. This indicates that the negative input is unrelated to the constitutive PKC-α activation process and more likely reflects an active desensitization directed by PKC-α (and PKC-β1 [data not shown]). Our preliminary evidence indicates that the c-Raf1 S259A and S499A mutants are not sensitive to PKC-α-dependent desensitization; the phosphorylation of these sites by PKC is thus implicated in the downmodulation of activity rather than stimulation of activity. However, in the absence of a detailed understanding of c-Raf1 control, this conclusion remains speculative.
In comparing the efficacies of different PKC isotypes in the activation of p42 MAPK, MEK, and c-Raf1, it is evident that there are relative differences at each level of the hierarchy. While some intrinsic variation exists for c-Raf1 activation—e.g., PKC-ζ operates via a distinct pathway—it might be expected that the relative potencies for MEK and p42 MAPK activation would be the same. In the absence of other controls, this would be a reasonable view; however, controls affected by phosphatases and perhaps scaffolding proteins are also likely to be influenced by the PKC isotypes, and as observed for c-Raf1, this may well operate in a selective manner.
During revision of this manuscript, Cai et al. provided evidence for the redundant action of PKC-ɛ and PKC-α in the activation of c-Raf (8). Their conclusion is broadly similar to that here; i.e., multiple PKC isotypes can activate c-Raf (aPKC was not tested previously [8]). However, there are clear distinctions with respect to the proposed mechanism of activation. In particular, the in vitro studies of Cai et al. suggest a direct mechanism of activation, contrary to the results here. The basis for this discrepancy is not evident; however, it is notable that the in vitro studies of Cai et al. were performed with PKC preparations that appear to be less than 1% pure. One intriguing possibility is that these recombinant PKCs initially copurify with an intermediate component responsible for Raf activation.
With respect to the mechanism of c-Raf1 activation observed here, the inability of both PKC-α and PKC-η to activate the RafCAAX mutant is consistent with the notion that membrane association bypasses the PKC-dependent step. This lack of response for membrane-targeted c-Raf1 may be either because some direct PKC-Raf effect is complete for the RafCAAX mutant (e.g., the membrane location is sufficient to promote full phosphorylation by a PKC-dependent event) or because the actual recruitment of Raf to the membrane is controlled by PKC. Direct phosphorylation by PKC is unlikely to contribute, since the c-Raf1 S259A and S499A mutants were found to behave like wild-type c-Raf1 with respect to activation. As with serum stimulation of c-Raf1, there is no stable activation-dependent association with membranes, since no increase in particulate c-Raf1 is observed after activation by constitutively active PKC in vivo (data not shown); thus, any controlled recruitment would have to be a transient response.
For PKC-α, we noted an increased response for the c-Raf1 S259A mutant that is consistent with a role for phosphorylation of this site in desensitization; no such increased response was observed for PKC-η, which does not cause desensitization. Such a conclusion would be consistent with the increased activation of Raf S259A noted previously (42). It is not evident why the S499A mutant, which also appears to show a loss of PKC-α-induced desensitization, did not behave the same way as the S259A mutant. It would seem that phosphorylations of these two sites are not simply equivalent in their consequence to c-Raf1 function.
A recent study of the control of MAPK by activated PKC isotypes concluded that there was specificity of action on the pathway acting at the level of MEK (55). Specifically, Ueda et al. found that PKC-δ was the only activator of MEK and that PKC-α and -ɛ could not stimulate MEK activation. The reason for the differences between their data and the data presented here is not obvious. However, we have noted that the levels of expression of the activated PKC mutants are generally much lower than those of their wild-type counterparts. This phenomenon may well reflect a higher rate of turnover of these activated proteins (characteristic of the TPA-activated PKCs [58]), and this will vary in different cell types and under different culture conditions. It is therefore possible that the distinction seen by Ueda et al. (55) for PKC-δ reflects its more effective expression compared to other activated PKC isotypes employed by these workers. This point emphasizes the fact that the use of activated PKC isotypes in investigations of cell functions, is limited by expression, provides evidence of the potential for activation, and cannot imply a necessary physiological role. Nevertheless, the finding that the dominant-negative PKC-α inhibits activation of the p42 MAPK pathway indicates that one or more of the PKC isotypes expressed in Cos-7 cells do indeed control activation.
In conclusion, it has been shown here that at least three types of control of the MAPK pathway can be exerted by members of the PKC gene family. PKC-ζ acts independently of Raf activation to trigger MEK and p42 MAPK activation. This effect is indirect and implies a distinct PKC-ζ-controlled pathway to MEK. PKC-η also causes activation of MEK and p42 MAPK; however, this is coincident with activation of c-Raf1. The activation of c-Raf1 does not require two defined PKC phosphorylation sites (S259 and S499), and the RafCAAX data would be consistent with operation of PKC via a membrane targeting mechanism. Finally, PKC-α behaves like PKC-η with respect to activation of c-Raf1; however, PKC-α also induces a refractory state in the entire pathway that seems to operate through control of c-Raf1. This negative control may require phosphorylation at the defined PKC sites. A clearer understanding of the precise molecular mechanisms involved in these regulatory events will no doubt evolve from a detailed understanding of Raf (and MEK) control; it will then be possible to address the specific situations in which PKCs function in the activation of the MAPK cascade.
ACKNOWLEDGMENTS
We are grateful to D. K. Morrison for kindly providing Raf expression constructs. We thank L. V. Dekker, C. J. Fernandez, B. M. Marte, H. Mellor, and K. A. F. Reif for scientific advice and critical reading of the manuscript.
REFERENCES
- 1.Adams P D, Parker P J. TPA-induced activation of MAP kinase. FEBS Lett. 1991;290:77–82. doi: 10.1016/0014-5793(91)81230-6. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed S, Kozma R, Monfries C, Hall C, Lim H H, Smith P, Lim L. Human brain n-chimaerin cDNA encodes a novel phorbol ester receptor. Biochem J. 1990;272:767–773. doi: 10.1042/bj2720767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 4.Alessi D R, Saito Y, Campbell D G, Cohen P, Sithanandam G, Rapp U, Ashworth A, Marshall C J, Cowley S. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J. 1994;13:1610–1619. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avruch J, Zhang X F, Kyriakis J M. Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994;19:279–283. doi: 10.1016/0968-0004(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 6.Bornancin F, Parker P. Phosphorylation of threonine-638 critically controls the dephosphorylation and inactivation of protein kinase C-α. Curr Biol. 1996;6:1114–1123. doi: 10.1016/s0960-9822(02)70678-7. [DOI] [PubMed] [Google Scholar]
- 7.Burgering B M, Bos J L. Regulation of Ras-mediated signalling: more than one way to skin a cat. Trends Biochem Sci. 1995;20:18–22. doi: 10.1016/s0968-0004(00)88944-6. [DOI] [PubMed] [Google Scholar]
- 8.Cai H, Smola U, Wixler V, Eisenmann-Tappe I, Diaz-Meco M T, Moscat J, Rapp U, Cooper G M. Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol Cell Biol. 1997;17:732–741. doi: 10.1128/mcb.17.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll M P, May W S. Protein kinase C-mediated serine phosphorylation directly activates Raf-1 in murine hematopoietic cells. J Biol Chem. 1994;269:1249–1256. [PubMed] [Google Scholar]
- 10.Cazaubon S M, Parker P J. Identification of the phosphorylated region responsible for the permissive activation of protein kinase C. J Biol Chem. 1993;268:17559–17563. [PubMed] [Google Scholar]
- 10a.Cowley, S., and C. J. Marshall. Unpublished data.
- 11.Decock J B J, Gillespie-Brown J, Parker P J, Sugden P H, Fuller S J. Classical, novel and atypical isoforms of PKC stimulate ANF- and TRE/AP-1-regulated promoter activity in ventricular cardiomyocytes. FEBS Lett. 1994;356:275–278. doi: 10.1016/0014-5793(94)01283-0. [DOI] [PubMed] [Google Scholar]
- 12.Dekker L V, McIntyre P, Parker P J. Mutagenesis of the regulatory domain of rat protein kinase C-η: a molecular basis for restricted histone kinase activity. J Biol Chem. 1993;268:19498–19504. [PubMed] [Google Scholar]
- 13.Dekker L V, Parker P J. Protein kinase C—a question of specificity. Trends Biochem Sci. 1994;19:73–77. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 14.Diaz-Guerra M J, Bodelon O G, Velasco M, Whelan R, Parker P J, Bosca L. Up-regulation of protein kinase C-ɛ promotes the expression of cytokine-inducible nitric oxide synthase in RAW 264.7 cells. J Biol Chem. 1996;271:32028–32033. doi: 10.1074/jbc.271.50.32028. [DOI] [PubMed] [Google Scholar]
- 15.Diaz-Meco M, Dominguez I, Sanz L, Dent P, Lozano J, Municio M M, Berra E, Hay R T, Sturgill T W, Moscat J. ζ PKC induces phosphorylation and inactivation of IκB-α in vitro. EMBO J. 1994;13:2842–2848. doi: 10.1002/j.1460-2075.1994.tb06578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evan G I, Lewis G K, Ramsay G, Bishop J M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrar M, Alberolaila J, Perlmutter R. Activation of the Raf-1 kinase cascade by coumermycin-induced dimerization. Nature. 1996;383:178–181. doi: 10.1038/383178a0. [DOI] [PubMed] [Google Scholar]
- 18.Finkenzeller G, Marme D, Hug H. Inducible overexpression of human protein kinase C alpha in NIH 3T3 fibroblasts results in growth abnormalities. Cell Signal. 1992;4:163–177. doi: 10.1016/0898-6568(92)90080-r. [DOI] [PubMed] [Google Scholar]
- 19.Freed E, Symons M, MacDonald S G, McCormick F, Ruggieri R. Binding of 14-3-3 proteins to the protein kinase Raf and effects on its activation. Science. 1994;265:1713–1716. doi: 10.1126/science.8085158. [DOI] [PubMed] [Google Scholar]
- 19a.Garcia-Paramio, P., and P. J. Parker. Unpublished data.
- 20.Genot E M, Parker P J, Cantrell D A. Analysis of the role of protein kinase C-alpha, protein kinase C-epsilon, and protein kinase C-zeta in T-cell activation. J Biol Chem. 1995;270:9833–9839. doi: 10.1074/jbc.270.17.9833. [DOI] [PubMed] [Google Scholar]
- 21.Goodnight J, Kazanietz M G, Blumberg P M, Mushinski J F, Mischak H. The cDNA sequence, expression pattern and protein characteristics of mouse protein kinase C-ζ. Gene. 1992;122:305–311. doi: 10.1016/0378-1119(92)90219-f. [DOI] [PubMed] [Google Scholar]
- 22.Gulbins E, Coggeshall K M, Baier G, Telford D, Langlet C, Baier-Bitterlach G, Bonnefoy-Berard N, Burn P, Wittinghofer A, Altman A. Direct stimulation of Vav guanine nucleotide exchange activity for Ras by phorbol esters and diglycerides. Mol Cell Biol. 1994;14:4749–4758. doi: 10.1128/mcb.14.7.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hass R, Bartels H, Topley N, Hadam M, Kohler L, Goppelt S M, Resch K. TPA-induced differentiation and adhesion of U937 cells: changes in ultrastructure, cytoskeletal organization and expression of cell surface antigens. Eur J Cell Biol. 1989;48:282–293. [PubMed] [Google Scholar]
- 24.Hill C S, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 25.Hoshi M, Nishida E, Sakai H. Characterization of a mitogen-activated, Ca2+-sensitive microtubule-associated protein-2 kinase. Eur J Biochem. 1989;184:477–486. doi: 10.1111/j.1432-1033.1989.tb15040.x. [DOI] [PubMed] [Google Scholar]
- 26.House C, Kemp B E. Protein kinase C contains a pseudosubstrate prototope in its regulatory domain. Science. 1987;238:1726–1728. doi: 10.1126/science.3686012. [DOI] [PubMed] [Google Scholar]
- 27.Howe L R, Leevers S J, Gómez N, Nakielny S, Cohen P, Marshall C J. Activation of the MAP kinase pathway by the protein kinase Raf. Cell. 1992;71:335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- 28.Hug H, Sarre T F. Protein kinase C isoenzymes—divergence in signal transduction. Biochem J. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irie K, Gotoh Y, Yashar B M, Errede B, Nishida E, Matsumoto K. Stimulatory effects of yeast and mammalian 14-3-3 proteins on the Raf protein kinase. Science. 1994;265:1716–1719. doi: 10.1126/science.8085159. [DOI] [PubMed] [Google Scholar]
- 30.Kazanietz M G, Bustelo X R, Barbacid M, Kolch W, Mischak H, Wong G, Pettit G R, Bruns J D, Blumberg P M. Zinc finger domains and phorbol ester pharmacophore. J Biol Chem. 1994;269:11590–11594. [PubMed] [Google Scholar]
- 31.Kiley S C, Adams P D, Parker P J. Cloning and characterisation of phorbol ester differentiation-resistant U937 cell variants. Cell Growth Differ. 1997;8:221–230. [PubMed] [Google Scholar]
- 32.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marme D, Rapp U R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 33.Leevers S J, Marshall C J. Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J. 1992;11:569–574. doi: 10.1002/j.1460-2075.1992.tb05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leevers S J, Paterson H F, Marshall C J. Requirement for ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994;369:411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- 35.Luo Z, Tzivion G, Belshaw P, Vavvas D, Marshall M, Avruch J. Oligomerization activates c-Raf-1 through a Ras-dependent mechanism. Nature. 1996;383:181–185. doi: 10.1038/383181a0. [DOI] [PubMed] [Google Scholar]
- 36.Maniatis T, Sambrook J, Fritsch E F. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Marais R, Light Y, Paterson H F, Marshall C J. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 1995;14:3136–3145. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marquardt B, Frith D, Stabel S. Signaling from TPA to MAP kinase requires protein kinase C, Raf and MEK; reconstitution of the signaling pathway in-vitro. Oncogene. 1994;9:3213–3218. [PubMed] [Google Scholar]
- 39.Marshall C. Cell signaling—Raf gets it together. Nature. 1996;383:127–128. doi: 10.1038/383127a0. [DOI] [PubMed] [Google Scholar]
- 40.Mischak H, Goodnight J, Kolch W, Martin-Baron G, Schaechtle C, Kazanietz M G, Blumberg P M, Pierce J H, Mushinski J F. Overexpression of protein kinase C-δ and protein kinase C-ɛ in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993;268:6090–6096. [PubMed] [Google Scholar]
- 41.Mischak H, Pierce J H, Goodnight J, Kazanietz M G, Blumberg P M, Mushinski J F. Phorbol ester-induced myeloid differentiation is mediated by protein kinase C-α and -δ. J Biol Chem. 1993;268:20110–20115. [PubMed] [Google Scholar]
- 42.Morrison D K, Heidecker G, Rapp U R, Copeland T D. Identification of the major phosphorylation sites of the Raf-1 kinase. J Biol Chem. 1993;268:17309–17316. [PubMed] [Google Scholar]
- 43.Morrison D K, Kaplan D R, Rapp U, Roberts T M. Signal transduction from membrane to cytoplasm: growth factors and membrane-bound oncogene products increase Raf-1 phosphorylation and associated protein kinase activity. Proc Natl Acad Sci USA. 1988;85:8855–8859. doi: 10.1073/pnas.85.23.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray N R, Baumgardner G P, Burns D J, Fields A P. Protein kinase C isotypes in human erythroleukemia (K562) cell proliferation and differentiation. Evidence that beta II protein kinase C is required for proliferation. J Biol Chem. 1993;268:15847–15853. [PubMed] [Google Scholar]
- 45.Nishizuka Y. Intracellular signalling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 46.Olivier A R, Parker P J. Bombesin, platelet-derived growth factor, and diacylglycerol induce selective-membrane association and down-regulation of protein kinase C isotypes in Swiss 3T3 cells. J Biol Chem. 1994;269:2758–2763. [PubMed] [Google Scholar]
- 47.Payne D M, Rossomando A J, Martino P, Erickson A K, Her J-H, Shabanowitz J, Hunt D F, Weber M J, Sturgill T W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase) EMBO J. 1991;10:885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pears C J, Kour G, House C, Kemp B E, Parker P J. Mutagenesis of the pseudosubstrate site of protein kinase C leads to activation. Eur J Biochem. 1990;194:89–94. doi: 10.1111/j.1432-1033.1990.tb19431.x. [DOI] [PubMed] [Google Scholar]
- 49.Qiu Z H, Leslie C C. Protein kinase C-dependent and -independent pathways of mitogen-activated protein kinase activation in macrophages by stimuli that activate phospholipase-A2. J Biol Chem. 1994;269:19480–19487. [PubMed] [Google Scholar]
- 50.Rossomando A J, Payne D M, Weber M J, Sturgill T W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci USA. 1989;86:6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sözeri O, Vollmer K, Liyanage M, Frith D, Kour G, Mark III G E, Stabel S. Activation of the c-raf protein kinase by protein kinase C phosphorylation. Oncogene. 1992;7:2259–2262. [PubMed] [Google Scholar]
- 52.Stabel S, Parker P J. Protein kinase C. Pharmacol Ther. 1991;51:71–95. doi: 10.1016/0163-7258(91)90042-k. [DOI] [PubMed] [Google Scholar]
- 53.Stokoe D, Campbell D G, Nakielny S, Hidaka H, Leevers S J, Marshall C, Cohen P. MAPKAP kinase-2, a novel protein kinase activated by mitogen-activated protein kinase. EMBO J. 1992;11:3985–3994. doi: 10.1002/j.1460-2075.1992.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288:351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C delta activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 56.Ways D K, Cook P P, Webster C, Parker P J. Effect of phorbol esters on PKC-ζ. J Biol Chem. 1992;267:4799–4805. [PubMed] [Google Scholar]
- 57.Withers D J, Bloom S R, Rozengurt E. Dissociation of cAMP-stimulated mitogenesis from activation of the mitogen-activated protein kinase cascade in Swiss 3T3 cells. J Biol Chem. 1995;270:21411–21419. doi: 10.1074/jbc.270.36.21411. [DOI] [PubMed] [Google Scholar]
- 58.Young S, Parker P J, Ullrich A, Stabel S. Down-regulation of protein kinase C is due to an increased rate of degradation. Biochem J. 1987;244:775–779. doi: 10.1042/bj2440775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang F, Strand A, Robbins D, Cobb M H, Goldsmith E J. Atomic structure of the MAP kinase ERK2 at 2.3 Å resolution. Nature. 1994;367:704–711. doi: 10.1038/367704a0. [DOI] [PubMed] [Google Scholar]