Abstract
Lipoblastoma is a rare benign tumour which originates from embryonic fat tissue mainly affecting children below 3 years of age and is exceptionally rare in the thoracic cavity. Preoperative imaging is used to assess the extent of disease and aid surgical planning but definitive diagnosis is usually by histopathological examination. Complete surgical excision is the treatment of choice. Because of the tendency for these lesions to recur, regular follow-up is mandatory even with presumed complete excision. In this study, we report the case of a 3-year-old boy who presented with a huge intrathoracic tumour occupying the whole of the left hemithorax. A complete resection of the tumour was undertaken, with histopathology report confirming the diagnosis of lipoblastoma. The relevant literature review was done. At 6 months of follow-up, there was no recurrent tumour on imaging and the child was thriving well.
Keywords: Lipoblastoma, Intrathoracic tumour, Surgical excision, Histopathology
Introduction
Lipoblastoma is a rare benign tumour that arises from embryonic fat [1, 2]. Occurring most commonly in infancy and early childhood, it accounts for 1–2% of all benign paediatric tumours. It can arise almost anywhere within the soft tissue where there are mesenchymal cells, occurring more commonly in the extremities followed by the trunk and less commonly in the head and neck. It is exceptionally rare in the thoracic cavity [3] and since its first description in 1926, very few cases have been reported in the literature. Intrathoracic lipoblastomas are mostly asymptomatic, especially when the size is small. Large lipoblastoma within the chest cavity can show symptoms, but they may be incidentally discovered at a routine medical examination and in an imaging study done for some other reason. We report a case of massive intrathoracic mass in a 3-year-old male child that was removed successfully which turned out to be a lipoblastoma on pathologic examination. Important aspects of diagnosis and management specific to this rare entity along with the review of literature have been discussed in this report.
Case report
A 3-year-old male child presented in our hospital with complaints of chest pain, bouts of dry cough, and occasional shortness of breath since 1 month before admission. He had normal developmental milestones and maintained a body weight of about 12 kg. There was no significant past medical history or any relevant family history. On physical examination the vitals were stable, respiratory rate was 32 breaths per min, and saturating 94% on room air. The patient was found to have dullness on percussion along with absent breath sounds in left chest.
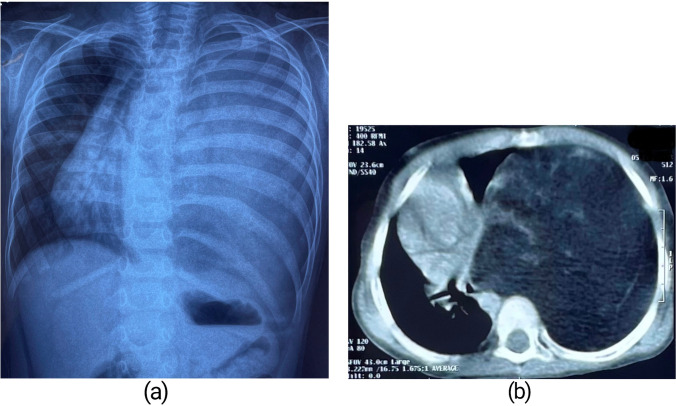
Chest X-ray posteroanterior view revealed a huge homogenously opacified lesion completely occupying the left hemithorax with gross mediastinal shift towards the right side (Fig. 1a). Contrast-enhanced computerized tomography (CECT) of the thorax confirmed a large heterogeneous fat density lesion measuring 125 mm × 106 mm in cross section and 175 mm in craniocaudal dimension, occupying the entire left hemithorax causing significant mass effect with near complete compressive collapse of the left lung sparing only part of the upper lobe and mediastinal shift to the right side (Fig. 1b). The lesion appeared well marginated, and had internal septations and strands with few areas of focal calcification. The left hemidiaphragm was pushed downwards and inverted by the lesion. There was no extra-thoracic extension. Computerized tomography (CT)–guided fine-needle aspiration cytology (FNAC) smears from the lesion showed lobules of mature adipocytes with few capillaries and scanty foci of haemorrhage. Laboratory workup was normal with regard to white cell count, haemoglobin, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR). An electrocardiogram showed normal sinus rhythm with an isoelectric pattern.
Fig. 1.
(a) Chest X-ray showing complete opacification of left hemithorax. (b) CT scan showing large heterogeneous fat density in left hemithorax
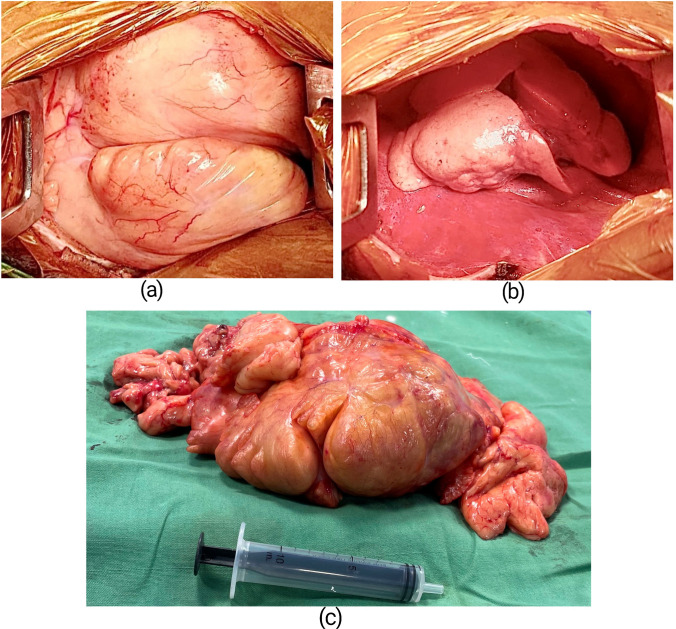
Based on these investigation findings, a left posterolateral thoracotomy was done through the left 5th intercostal space under general anaesthesia and single lumen endotracheal intubation with standby provision for cardiopulmonary bypass in the event of intraoperative haemodynamic deterioration. The thoracotomy revealed a massive lobulated, smooth surfaced, yellowish, soft tissue tumour mass occupying almost entire left hemithorax (Fig. 2a) compressing entirely the left lower lobe and partially the left upper lobe of the lung. The tumour was attached to the parietal pleura against the 7th intercostal space with a pedicle of around 3 cm in diameter. There was minimal adhesion to the surrounding structures but without any definite attachment. The entire mass was successfully extirpated (Fig. 2b) using blunt and electrocautery dissection after ligation and division of its pedicle. The resected specimen (Fig. 2c) weighed about 830 g and the dimensions nearly matched the CECT thorax measurements. The cut section was capsulated with variable lobulation, and soft yellowish and areas of haemorrhage. Histopathological examination of the tissue revealed lobules of adipocytes separated by intersecting fibrovascular septa (Fig. 3a). Adipocytes showed the range of maturation from primitive mesenchymal spindle cells to univacuolated and multivacuolated lipoblasts along with few stellate cells and areas of myxoid change (Fig. 3b). There was no significant atypia. The findings were consistent with lipoblastoma. The tissue sent for immunohistochemistry study came out to be immunoreactive for S100 protein and cluster of differentiation (CD) 34 with low reactivity to methylation-inhibited binding protein 1 (MIB-1).
Fig. 2.
(a) Intraopertive picture showing the large mass as seen through a thoracotomy. (b) Thoracic cavity after complete resection of the mass. (c) The resected specimen
Fig. 3.
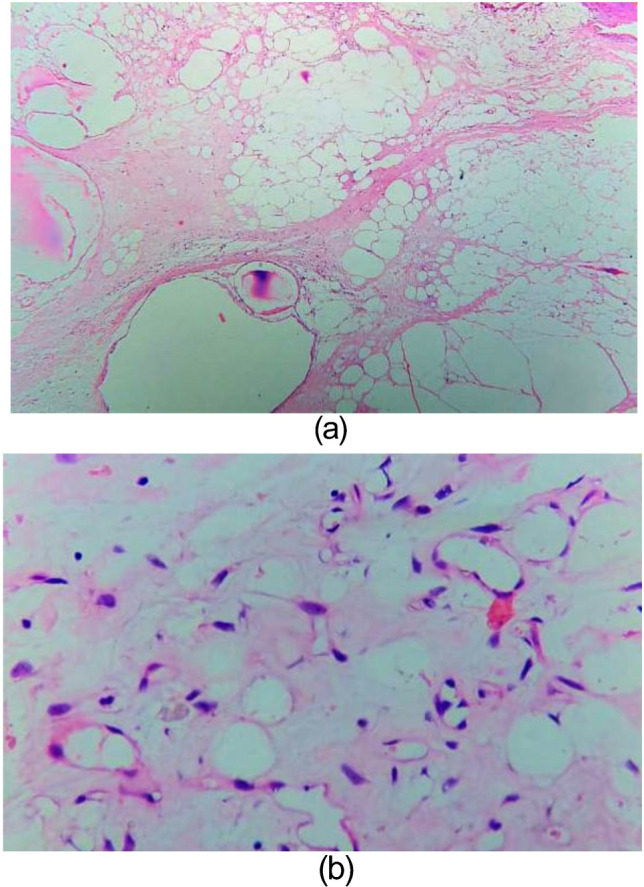
Microscopic sections of the mass showing (a) Lobulated sheets of adipocytes. (b) Adipocytes in a range of maturation
The postoperative recovery of the patient was uneventful. Chest X-ray done on the 3rd postoperative day showed complete re-expansion of the left lung with the mediastinal structures reverting nearly to their normal position. The chest drain was removed soon after and he was discharged on the 7th postoperative day with the final diagnosis of circumscribed lipoblastoma. At 6 months after surgery, he is symptom-free and with a normal chest X-ray. Further follow-up at regular intervals is planned with clinical examination and imaging studies.
Discussion
Lipoblastoma is a rare tumour of infancy and early childhood. It was first described by Jaffe in 1926 as tumours of embryonal white fat [1]. The pathogenesis of lipoblastoma involves embryonic white fatty cells that continue to proliferate and develop during the postnatal period [4].Chung and Enzinger in 1973 described the morphologic criteria for the diagnosis of this tumour for the first time and proposed the two forms of lipoblastoma: the more common localized encapsulated type and the diffuse multicentric type, lipoblastomatosis [5].
Infants and children less than 3 years of age account for more than 90% of the reported cases with a male to female ratio of about 3:1 [1, 2]. In more than 80% of the patients, lipoblastoma occurs in the extremities. The trunk is the second most common location while less common sites are the head and neck, mediastinum, retroperitonium, and mesentery [3, 6, 7]. Lipoblastoma occurring in the intrathoracic location is exceptionally rare [3]. In agreement with published literatures epidemiologically, our case was a 3-year-old male child with a huge intrathoracic mass who presented with chiefly with chest pain, bouts of dry cough, and dyspnoea. These symptoms can be attributed to the size of the tumour causing mass effect.
On CT scan, lipoblastoma presents as a nonspecific heterogeneous fat density lesion with enhancing septations and no or minimal calcification [8]. These features can mimic other benign or malignant lipomatous tumours and this means that differentiation between the various adipose tissue tumours cannot be made by CT scan [1, 9]. Magnetic resonance imaging (MRI) is the method of choice for preoperative diagnosis of lipoblastoma, which in addition to assessing tumour location, size, adjacent organs, and the surgical resection site, shows a characteristic intermediate to high signal intensity on T1-weighted images according to the amount of immature fat [1, 8]. Computed tomography scan done in our case showed a large, heterogeneous fat density intrathoracic lesion causing mass effect. However MRI was not done as it is much more time consuming with difficulty in keeping the child still, and would have needed him to be sedated which would have been risky, given his medical condition. FNAC also was not conclusive as it only showed lobules of fat cells. Hence, we did not have definite preoperative diagnosis other than assuming it being a large lipomatous lesion.
Treatment of choice for lipoblastoma is complete tumour excision with a clear plane of dissection from surrounding tissues [1–3, 7]. In our case also, the tumour was easily detached from the surrounding lung and mediastinal structures.
The white to yellow cut surface of a lipoblastoma shows variable lobulation and myxoid change. Histological presentation is that of a typical lobular pattern with white fat and variable amount of myxoid tissue demarcated by bands of fibrous tissue. The fat cells display a range of differentiation from multivacuolated lipoblasts to mature adipocytes. Histologic variations also include a subtle zonal architecture of fat maturation, abundant myxoid material, primitive mesenchymal cells, a focal plexiform vascular pattern, and multinucleated cells [10]. These findings are somehow similar to myxoid liposarcoma, but differ in the absence of pleomorphism, atypia, and hyperchromasia [1, 3]. A similar pathological picture was noted in our case thereby pointing to the diagnosis. Immunohistochemically, most traditional markers show relatively limited specificity and do not usually distinguish between benign and malignant proliferations and hence can be used only as a an ancillary tool in diagnosis [11]. Thus, a careful histological evaluation by an experienced pathologist, aware of all diagnostic entities and their histological spectra, is vital as was done in our case [11].
Although it is a benign tumour which carries an excellent prognosis after complete excision with no reported metastasis or malignant transformation [1, 2], it has the capability to grow rapidly with a recurrence rate of 14–25% [7] which necessitates long term follow-up with clinical examination and imaging. In our case, there was a complete excision with no recurrence at 6 months of follow-up.
Conclusion
Lipoblastoma is a rare soft tissue tumour occurring in young children and be should be considered in the differential diagnosis of soft fatty masses of children within the thorax and mediastinum. It is benign and quick growing which can have an insidious presentation when developing in internal, less visible anatomical spaces. Preoperative imaging is not diagnostic but is beneficial to assess the extent of disease and aid surgical planning. Definitive diagnosis is usually by histopathological examination. It carries an excellent prognosis after complete excision. Even with presumed complete excision, there is a tendency of lipoblastoma to recur which makes regular follow-up mandatory.
Funding
None.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials. Any additional data if needed, that may support the findings of this study, are available from the corresponding author upon request.
Declarations
Ethics approval
The institutional committee exempted this from need for ethical approval.
Informed consent statement
Obtained from the patient’s parents for surgery and scientific publication.
Conflict of interest
None declared by any of the authors.
Statement of human and animal rights
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Geramizadeh B, Javadi F, Foroutan HR. Intrathoracic lipoblastoma in a 15-month-old infant. Rare Tumors. 2011;3:e51. doi: 10.4081/rt.2011.e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spinelli C, Costanzo S, Severi E, Giannotti G, Massart F. A thoracic wall lipoblastoma in a 3-month-old infant: a case report and review of the literature. J PediatrHematol Oncol. 2006;28:594–600. doi: 10.1097/01.mph.0000212977.13749.c3. [DOI] [PubMed] [Google Scholar]
- 3.Gülhan SS, Adams PY, Sarica EA, Turut H, Agackiran Y. Chest wall lipoblastoma in a seven-month-old girl: a case report. J Pediatr Surg. 2004;39:1414–1417. doi: 10.1016/j.jpedsurg.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 4.Spătaru RI, Cîrstoveanu C, Iozsa DA, Enculescu A, Tomescu LF, Șerban D. Lipoblastoma: diagnosis and surgical considerations. Exp Ther Med. 2021;22:903. doi: 10.3892/etm.2021.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung EB, Enzinger FM. Benign lipoblastomatosis. An analysis of 35 cases. Cancer. 1973;32:482–92. doi: 10.1002/1097-0142(197308)32:2<482::AID-CNCR2820320229>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 6.Safavi M, Akhlaghi N, Ashjaei B. Large intrathoracic lipoblastoma. Asian Cardiovasc Thorac Ann. 2021;29:858–861. doi: 10.1177/02184923211038407. [DOI] [PubMed] [Google Scholar]
- 7.Moaath A, Raed E, Mohammad R, Mohammad S. Lipoblastoma: a rare mediastinal tumor. Ann Thorac Surg. 2009;88:1695–1697. doi: 10.1016/j.athoracsur.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 8.Ambusaidi FM, Al-Mammari L, Al-Brashdi Y, Al-Shamsi RM. Imaging features of lipoblastoma. Int J PediatrAdolesc Med. 2022;9:69–72. doi: 10.1016/j.ijpam.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CW, Chang WC, Lee HS, Ko KH, Chang CC, Huang GS. MRI features of lipoblastoma: differentiating from other palpable lipomatous tumor in pediatric patients. Clin Imaging. 2010;34:453–457. doi: 10.1016/j.clinimag.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Coffin CM, Lowichik A, Putnam A. Lipoblastoma (LPB): a clinicopathologic and immunohistochemical analysis of 59 cases. Am J Surg Pathol. 2009;33:1705–1712. doi: 10.1097/PAS.0b013e3181b76462. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen M. Immunohistochemistry of soft tissue tumours - review with emphasis on 10 markers. Histopathology. 2014;64:101–118. doi: 10.1111/his.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials. Any additional data if needed, that may support the findings of this study, are available from the corresponding author upon request.