Abstract
An important problem in designing any large network is the assembly of systems that are resilient to change. From a chemical point of view, an analogy can be used where one requires supramolecular assemblies to maintain their dimensionality combined with limited structural perturbation in response to variation in its intermolecular framework. The identification of hydrogen-bonded framework patterns within experimentally known supramolecular assemblies that are structurally robust to disruption and selective hydrogen substitution are envisioned to act as a supramolecular blueprint or template for metal-ion retroinsertion. Here, we report the formation of a large neutral discrete pseudo-spherical coordination capsule assembled from 6 pyrogallol[4]arene ligands and 24 Cu(II) metal ions. Amazingly, this coordination capsule is structurally analogous to its hydrogen-bonded counterpart. This result shows a robust ability of pyrogallol[4]arene molecules to self-assemble into large hexameric cage structures from either the hydrogen-bonding or metal-ligand coordination process. The identification of robust supramolecular assemblies that conserve their structure in response to interchangeability between hydrogen-bonded networks for metal coordination, or inversely, represents an important advancement in supramolecular design.
Keywords: coordination, self-assembly, hexamer
Inspired by the architectural beauty and robustness found within nature, the design of large multicomponent spheroid cage assemblies on the nanometer scale is becoming increasing feasible. These large supramolecular architectures often resemble and conform to the simple geometrical shapes described by Platonic or Archimedean solids (1). Indeed, such structural morphologies have attracted tremendous interest because of their practical applications for encapsulating various guest molecules (2). Synthetic chemists have principally relied on various self-assembly methods in their construction, such as hydrogen bonds (3) or metal-ligand coordination (4), although some notable covalent methods have recently been reported (5).
A popular strategy for designing large multicomponent cage assemblies is the metal-directed self-assembly approach, advocated chiefly by Fujita and coworkers (6-9) and Stang and coworkers (10-12). This method has mainly focused on the geometrical permutation of various triangular pyridine-like bridging ligands driven by coordination to cis-protected Pd metal centers. Nevertheless, for the construction of discrete assemblies with large molecular cavities, the molecular paneling approach by Fujita and coworkers (13) has received the most success. Indeed, an amazingly large discrete functionalized spherical coordination network assembled from 12 metal ions and 24 exo-multidentate ligands was recently reported (14). However, an inherent complication of using n-dimensional pyridine-like spacer multidentate ligands in coordination is the resultant high positive charge combined with porous surfaces within these supramolecular assemblies. The counterions usually occupy valuable space within the cavity. These coordination capsules have no hydrogen-bonded analogues.
A well designed alternative widely used for creating metal-bound cage assemblies is metal coordination to various bowl-shaped resorcin[4]arene ligands. Although there are numerous reports of metal-bound resorcin[4]arene-like cage assemblies, most are dimeric and highly charged in nature (15-22). Indeed, the construction of larger coordination cage structures involving the assembly of resorcin[4]arene-like ligands greater than two is still in its infancy (23, 24).
Methods
C-propan-3-ol pyrogallol[4]arene 1 was prepared by procedures described in ref. 25. C-propan-3-ol pyrogallol[4]arene (0.20 g) 1 was treated with four equivalents of Cu(NO3)2·3H2O (0.25 g) in a mixture of acetone (5 ml) and water (2 ml), with a 71% yield. A single red crystal suitable for x-ray analysis was obtained in a period of 24 h. Cr ystal data for Cu24(C40H39O16)6·11(CH3COCH3)·48(H2O), Mr = 7,527.13, monoclinic, a = 24.6232 (3), b = 36.2982 (5), c = 38.7685 (5) Å, β = 91.298 (2)°, V = 34641 (9) Å3, space group C2/c, Z = 4, ρcalc = 1.443 g cm-3, λ(MoKα) = 0.70930 Å, F (000) = 14872, T = 173 (2) K; 38,373 unique reflections (2φ ≤ 56°), of which 9,893 were observed [Io > 2σ(I)]. Final R factors: R1 = 0.0974, wR2 = 0.2952 for 1,979 parameters. Data were collected on a Bruker SMART 1000 charge-coupled device diffractometer (Billerica, MA) with MoKα radiation by using the ω-scan mode. Data were corrected for absorption by using the sadabs program (Bruker), and structure solution and refinement were performed by using the shelx-97 software package (http://shelx.uni-ac.gwdg.de/SHELX). All nonhydrogen atoms and nonguest molecules were refined anisotropically, whereas the hydrogen atoms were included at geometrically calculated positions and allowed to ride on their parent atoms. The hydrogen atoms from water molecules and those of the pyrogallol OH groups were not assigned. Details of the x-ray structure determination are available from the Cambridge Crystallographic Data Centre (deposition no. CCDC 248306). Positive and negative MALDI-TOF MS was carried out with a matrix constructed from 2,5-dihydroxybenzoic acid in 1:1 ratio of MeCN/H2O.
Results and Discussion
The resorcin[4]arene and pyrogallol[4]arene macrocycles have been shown to have a great deal of robustness in forming large spheroid hexameric cage assemblies in both solution and solid state (26-38). Indeed, this property has inspired and contributed to numerous theoretical and experimental studies of its host-guest relationships. In the solid state, six pyrogallol[4]arene macrocycles enclose a large spherical cavity of ≈1,200 Å3 with a hydrogen-bonding framework reminiscent of a small rhombicuboctahedron. Motivated by a copper β-cyclodextrin structure (39), it was envisaged that the pyrogallol[4]arene macrocycle (Fig. 1) could behave as a multidentate ligand. Interestingly, close inspection of the previously reported hydrogen-bonded solid-state hexameric structure 3 shows that there are 24 regioselective chelate sites on its framework (29). Deprotonation from the upper-rim phenol groups within these assemblies for metal-ion retroinsertion would result in the desired structural preservation of the hexameric capsule while introducing useful inorganic functionality.
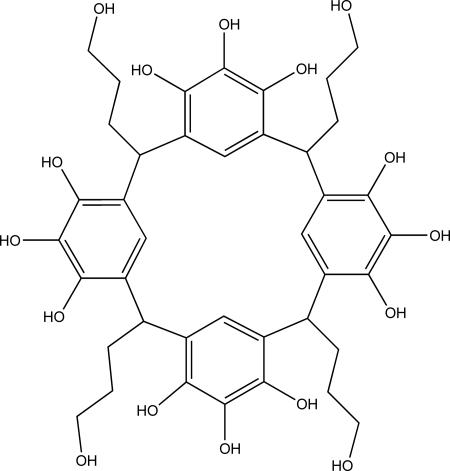
Fig. 1.
C-propan-3-ol pyrogallol[4]arene ligand 1.
C-propan-3-ol pyrogallol[4]arene 1 (Fig. 1), a multidentate pro-ligand, was treated with four equivalents of Cu(NO3)2·3H2O in a mixture of acetone and water. The metal-directed self-assembly resulted in the formation of a large neutral coordination capsule [Cu24(H2O)x(C40H40O16)6⊂(acetone)n] 2 where n = 1-6 (Fig. 2). The spheroid coordination capsule 2 was characterized by both single crystal x-ray analysis and MALDI-TOF MS.
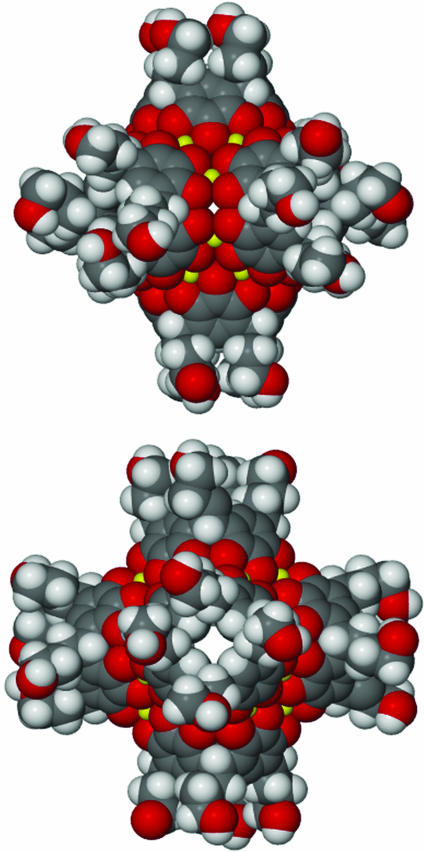
Fig. 2.
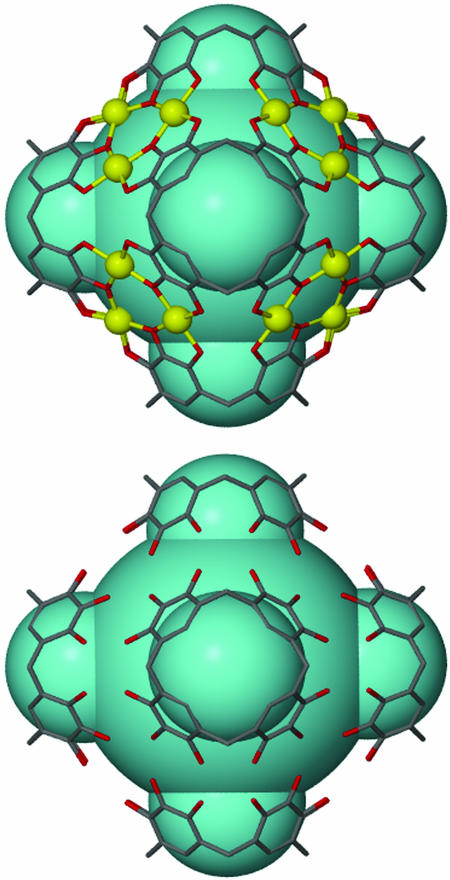
Different views of the space-filling representation of the hexameric coordination capsule 2 showing the metal-directed self-assembly of 6 pyrogallol[4]arenes and 24 Cu(II) ions. Any axial copper-coordinated water molecules were removed for clarity. Yellow, copper; red, oxygen.
Single crystal x-ray analysis shows 2 is assembled from 30 components: 6 pyrogallol[4]arene ligands and 24 Cu(II) ions. The previously reported hydrogen-bonded pyrogallol[4]arene hexameric structures are seamed together by 72 hydrogen bonds, 48 of which are intermolecular, or 12 hydrogen bonds per macrocycle. Retroinsertion of 24 Cu(II) metal ions into the hexameric framework results in substitution of 48 protons. All four of the equatorial positions on each copper center are occupied by oxygen atoms from the macrocycle upper rim. It should be noted that 24 phenol groups remain to participate in hydrogen bonding. Thus, a combination of 96 Cu-O coordination bonds and 24 intramolecular hydrogen bonds, 4 per macrocycle of the type O-H...O-, seam the framework together (O...O = 2.400-2.448 Å). Combination of hydrogen bonds and metal-ligand coordination that hold large multicomponent cage assemblies together are limited. An exquisite exception is the large spherical cavities produced by the self-assembly of 2,5-pyridinedicarboxylate with tin metal ions (40). However, tessellation of this space-filling truncated octahedra resulted in the formation of an infinite lattice network rather than discrete-like capsules.
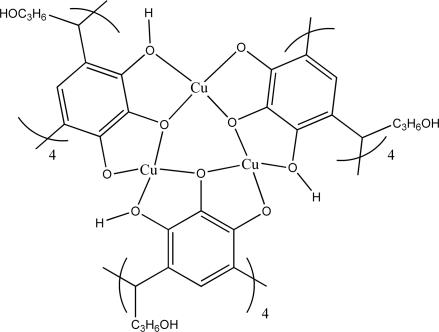
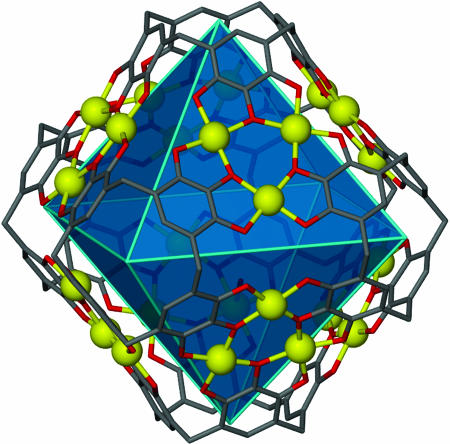
Alternatively, a useful description of our large coordination capsule 2 is held together by 8 six-membered [Cu3O3] planar arrays (Cu...O = 1.911-1.980 Å, O-Cu-O = 85.67-98.23°, Cu-O-Cu = 140.96-144.78°) (Fig. 3). However, structure 2 also may be viewed as an octahedron with each of its eight faces capped by a six-membered [Cu3O3] array (Fig. 4). Interestingly, each Cu(II) metal center resides on the vertices of a truncated cube that has eight triangle and six octagonal facets (Fig. 5). In recent years, the supramolecular approach has been beneficially used in the design and positioning of metal ions into novel framework topologies. Indeed, specific metal-ion arrays are of critical importance to new functions as exemplified by the intriguing magnetic properties of a two-dimensional Kagomé lattice (41) constructed by self-assembly.
Fig. 3.
One of the eight planar six-membered [Cu3O3] frameworks that hold 2 together.
Fig. 4.
Hypothetical octahedron within 2 showing each face capped by a six-membered [Cu3O3] array.
Fig. 5.
The structural framework similarity between the metal- and hydrogen-directed self-assembly process of six pyrogallol[4]arenes. (Upper) Stick bond representation of coordination capsule 2. (Lower) Stick bond representation of the previously determined hydrogen-bonded capsules 3. Hydrogen atoms and alkyl tails are removed for clarity.
The large coordination capsule 2 represents the inorganic counterpart of the hydrogen-bonded structure 3 (29). Remarkably, capsules 2 and 3 exhibit similar metrical parameters and dimensions, even in the face of the loss of 48 protons and the gain of 24 Cu(II) ions (Fig. 5). Both structures are arranged such that the centroids of the six pyrogallol[4]arene ligand components reside on the vertices of a hypothetical octahedron and enclose 1,200 Å3 of chemical space (42).† The unit edge length of the octahedron within 2 (ligandcentroid-ligandcentroid = average 12.626 Å) and 3 (average 12.482 Å) are remarkably similar for such large multicomponent systems (Fig. 4). These pyrogallol[4]arene hydrogen-bonded assemblies function as a supramolecular blueprint by allowing metal-ion additions while preserving the desired structural framework, consequently showing remarkable structural conservation. The ability of multicomponent assemblies to preserve structural function presents enhanced generality and robustness in supramolecular design. To our knowledge, there are limited reports known where such large discrete multicomponent closed assemblies illustrate limited structural change in response to such dramatic change in their intermolecular interactions. Indeed, one of the practical properties of structural preservation within supramolecular assemblies is the interchangeability and control of desired function. For example, hydrogen-bonded capsules are preferred when reversible encapsulation is needed, whereas metal coordination based on the same structural motif offers greater stability and rigidity.
There is growing excitement in the prospect of assembling the next generation of molecular devices by marrying the intrinsic structural specificity obtained from self-assembly with the properties of inorganic components (43). Indeed, various robust mutant versions of the cowpea mosaic virus, comprised of 60n identical protein subunits, were used as a structural template to bind gold nanoparticles at specific recognition sites on its surface (44).
The inherent advantage of the supramolecular blueprint approach is the potential to assemble large neutral supramolecular assemblies with no requirement for counterions as the positive charge obtained from the metal ions is counterbalanced with deprotonated phenol groups. To our knowledge, structure 2 represents the largest neutral discrete self-assembled coordination capsule constructed to date. Although the idea of metal coordination to various complementary bowl-shaped subunits has been extensively studied, our systems represent, to our knowledge, the first example of a discrete hexameric coordination capsule (15-22).
An often overlooked complication of using the hydrogen-bonding self-assembly of bowl-shaped molecules to enclose spheroid space is that the resulting hydrogen bonds used in seaming these assemblies together are usually arranged on the surface wall. Consequently steric constraints are the dominant force in organizing guest molecules, although beautiful exceptions do exist (45). The axial Cu(II) coordination sites within 2 are ideally suited orthogonal to the surface wall to affect the organizational arrangement of its guest molecules. However, Jahn-Teller distortion of the axial positions combined with the quality of crystal data and high degree of molecular disorder within 2, the solid-state structure could not confidently determine the number of axial copper coordinated water molecules. Thus, despite the introduction of 24 copper ions into the walls of the nanocapsule, the definitive location of all of the guest molecules entombed within the cavity is somewhat ambiguous. However, the contents of 2 were successfully characterized by MALDI-TOF analysis.
The (+)-MALDI mass spectra of 2 indicated that each individual capsule encapsulates different mixtures of water and acetone (see the supporting information, which is published on the PNAS web site). Of significant interest were two peaks that demonstrated the presence of 24 water molecules trapped within the cavity. Peaks at m/z = 6,677.68 and 6,735.59 corresponded closely to the calculated ionized molecular fragments of [Cu24(C40H40O16)6+(acetone)+24H2O+H]+, m/z = 6,677.24, and [Cu24(C40H40O16)6+2(acetone)+24H2O+H]+, m/z = 6,735.28, respectively. The effective volume taken up by these guest molecules within 2 is 34 and 39%, respectively, which yields a corresponding density of 0.678 and 0.758 g·cm-3 (42). Thus, the 24 axial coordination sites that orientate toward the center of the cavity allude to the presence of a metal-coordinated water cluster (H2O)24 in a truncated cube geometry despite the fact that a tetrakaidecahedral water cage structure based on fullerene-like topology, 12 pentagonal and 2 hexagonal facets, is favored (46). Indeed, confinement of water clusters within enclosed space is of great interest (47). For example, the potential to isolate and encapsulate a solvated electron within an enclosed discrete cavity is of sufficient interest. Interestingly, electron solvation of the [(H2O)24]- system has been theoretically studied (48).
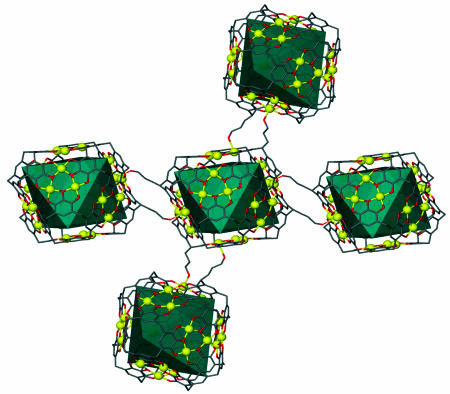
There are limited examples of large discrete multicomponent spheroid assemblies with large molecular cavities that contain functional groups on its periphery surface (14). Our elegant metal-directed self-assembly of 24 Cu(II) metal ions with 6 pyrogallol[4]arene macrocycles resulted in 24 hydroxyl groups attached at the nanocapsule periphery. It is envisioned that these functional groups can act as potential recognition sites. Interestingly, in the solid state, these periphery hydroxyl groups connect to adjacent hexameric capsules via copper-hydroxyl coordination (Cu...O = 2.400-2.504 Å). The capsules arrange themselves into a pseudo-hexagonal close-packed array. Within each individual hexameric capsule 2, four of its hypothetical octahedral faces connect to four adjacent octahedra capsules by face-to-face alignment (Fig. 6). Within each octahedral face-to-face alignment, one hydroxyl tail from each face coordinates to its neighbor. The remaining hydroxyl tails are not involved in coordination, although there is hydrogen bonding to water molecules.
Fig. 6.
Part of the solid-state molecular packing within 2. Hydroxyl alkyl tails not involved in metal-ligand coordination to adjacent capsules were removed for clarity.
The use of pyrogallol[4]arenes represents an important advancement in coordination cage design, demonstrating its robust ability to self-assemble into large spherical hexameric structures from both the hydrogen-bonding and metal-ligand coordination processes. This elegant approach to constructing large coordination cage assemblies with multicomponent ligands greater than two involves the use of a robust supramolecular template obtained by hydrogen self-assembly. A prerequisite for this supramolecular blueprint approach is the identification of hydrogen-bonded networks within multicomponent assemblies that are structurally robust to chelation. Thus, the supramolecular hydrogen-bonded pyrogallol[4]arene assemblies can function as a supramolecular blueprint by allowing selective metal-ion additions while preserving its desired structural framework of enclosed space.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation.
Author contributions: J.L.A. designed research; R.M.M. performed research; R.M.M. and G.W.V.C. analyzed data; and R.M.M. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The x-ray structure determinations were deposited in the Cambridge Structural Database, Cambridge Crystallographic Data Centre, Cambridge CB2 1EZ, United Kingdom (CSD reference no. 248306).
Footnotes
Maximum free volume within the cavities was calculated with guest molecules removed. The program mcavity (www.x-seed.net/cavity.html) assumed that the molecular volume of acetone and water is 61.3 and 14.7 Å3, respectively.
References
- 1.MacGillivray, L. R. & Atwood, J. L. (1999) Angew. Chem. Int. Ed. 38, 1018-1033. [DOI] [PubMed] [Google Scholar]
- 2.Hof, F., Craig, S. L., Nuckolls, C. & Rebek, J., Jr. (2002) Angew. Chem. Int. Ed. 41, 1488-1508. [DOI] [PubMed] [Google Scholar]
- 3.Conn, M. M. & Rebek, J., Jr. (1997) Chem. Rev. 97, 1647-1668. [DOI] [PubMed] [Google Scholar]
- 4.Leininger, S., Olenyuk, B. & Stang, P. J. (2000) Chem. Rev. 100, 853-908. [DOI] [PubMed] [Google Scholar]
- 5.Barrett, E. S., Irwin, J. L., Edwards, A. J. & Sherburn, M. S. (2004) J. Am. Chem. Soc. 126, 16747-16749. [DOI] [PubMed] [Google Scholar]
- 6.Fujita, M., Oguro, D., Miyazawa, M., Oka, H., Yamaguchi, K. & Ogura, K. (1995) Nature 378, 469-471. [Google Scholar]
- 7.Fujita, M. (1998) Chem. Soc. Rev. 27, 417-425. [Google Scholar]
- 8.Takeda, N., Umemoto, K., Yamaguchi, K. & Fujita, M. (1999) Nature 398, 794-796. [Google Scholar]
- 9.Fujita, M., Umemoto, K., Yoshizawa, M., Fujita, N., Kusukawa, T. & Biradha, K. (2001) Chem. Commun., 509-518.
- 10.Olenyuk, B., Fechtenkötter, A. & Stang, P. J. (1998) J. Chem. Soc. Dalton Trans., 1707-1728.
- 11.Olenyuk, B., Levin, M. D., Whiteford, J. A., Shield, J. E. & Stang, P. J. (1999) J. Am. Chem. Soc. 121, 10434-10435. [Google Scholar]
- 12.Olenyuk, B., Whiteford, J. A., Fechtenkötter, A. & Stang, P. J. (1999) Nature 398, 796-799. [DOI] [PubMed] [Google Scholar]
- 13.Chand, D. K., Biradha, K., Fujita, M., Sakamoto, S. & Yamaguchi, K. (2002) Chem. Commun., 2486-2487.
- 14.Tominaga, M., Suzuki, K., Kawano, M., Kusukawa, T., Ozeki, T., Sakamoto, S., Yamaguchi, K. & Fujita, M. (2004) Angew. Chem. Int. Ed. 43, 5621-5625. [DOI] [PubMed] [Google Scholar]
- 15.Jacopozzi, P. & Dalcanale, E. (1997) Angew. Chem. Int. Ed. 36, 613-615. [Google Scholar]
- 16.Fox, O. D., Dalley, N. K. & Harrison, R. G. (1998) J. Am. Chem. Soc. 120, 7111-7112. [Google Scholar]
- 17.Fox, O. D., Leung, J. F.-Y., Hunter, J. M., Dalley, N. K. & Harrison, R. G. (2000) Inorg. Chem. 39, 783-790. [DOI] [PubMed] [Google Scholar]
- 18.Zhong, Z., Ikeda, A., Ayabe, M., Shinkai, S., Sakamoto, S. & Yamaguchi, K. (2001) J. Org. Chem. 66, 1002-1008. [DOI] [PubMed] [Google Scholar]
- 19.Park, S. J. & Hong, J.-I. (2001) Chem. Commun., 1554-1555. [DOI] [PubMed]
- 20.Cotton, F. A., Lei, P., Lin, C., Murillo, C. A., Wang, X., Yu, S.-Y. & Zhang, Z.-X. (2004) J. Am. Chem. Soc. 126, 1518-1525. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi, K., Yamada, Y., Yamanaka, M., Sei, Y. & Yamaguchi, K. (2004) J. Am. Chem. Soc. 126, 13896-13897. [DOI] [PubMed] [Google Scholar]
- 22.Pinalli, R., Cristini, V., Sottili, V., Geremia, S., Campagnolo, M., Caneschi, A. & Dalcanale, E. (2004) J. Am. Chem. Soc. 126, 6516-6517. [DOI] [PubMed] [Google Scholar]
- 23.Fox, O. D., Drew, M. G. B. & Beer, P. D. (2000) Angew. Chem. Int. Ed. 39, 135-140. [DOI] [PubMed] [Google Scholar]
- 24.Fox, O. D., Drew, M. G. B., Wilkinson, E. J. S. & Beer, P. D. (2000) Chem. Commun., 391-392.
- 25.Gibb, B. C., Chapman, R. G. & Sherman, J. C. (1996) J. Org. Chem. 61, 1505-1509. [Google Scholar]
- 26.Gerkensmeier, T., Iwanek, W., Agena, C., Fröhlich, R., Kotila, S., Näther, C. & Mattay, J. (1999) Eur. J. Org. Chem., 2257-2262.
- 27.MacGillivray, L. R. & Atwood, J. L. (1997) Nature 389, 469-472. [Google Scholar]
- 28.Atwood, J. L., Barbour, L. J. & Jerga, A. (2001) Chem. Commun., 2376-2377. [DOI] [PubMed]
- 29.Cave, G. W. V., Antesberger, J., Barbour, L. J., McKinlay, R. M. & Atwood, J. L. (2004) Angew. Chem. Int. Ed. 43, 5263-5266. [DOI] [PubMed] [Google Scholar]
- 30.Shivanyuk, A. & Rebek, J., Jr. (2001) Proc. Natl. Acad. Sci. USA 98, 7662-7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shivanyuk, A. & Rebek, J., Jr. (2001) Chem. Commun., 2424-2425. [DOI] [PubMed]
- 32.Shivanyuk, A. & Rebek, J., Jr. (2003) J. Am. Chem. Soc. 125, 3432-3433. [DOI] [PubMed] [Google Scholar]
- 33.Yamanaka, M., Shivanyuk, A. & Rebek, J., Jr. (2004) J. Am. Chem. Soc. 126, 2939-2943. [DOI] [PubMed] [Google Scholar]
- 34.Avram, L. & Cohen, Y. (2002) Org. Lett. 4, 4365-4368. [DOI] [PubMed] [Google Scholar]
- 35.Avram, L. & Cohen, Y. (2002) J. Am. Chem. Soc. 124, 15148-15149. [DOI] [PubMed] [Google Scholar]
- 36.Avram, L. & Cohen, Y. (2003) Org. Lett. 5, 3329-3332. [DOI] [PubMed] [Google Scholar]
- 37.Avram, L. & Cohen, Y. (2003) J. Am. Chem. Soc. 125, 16180-16181. [DOI] [PubMed] [Google Scholar]
- 38.Avram, L. & Cohen, Y. (2004) J. Am. Chem. Soc. 126, 11556-11563. [DOI] [PubMed] [Google Scholar]
- 39.Fuchs, R., Habermann, N. & Klüfers, P. (1993) Angew. Chem. Int. Ed. 32, 852-854. [Google Scholar]
- 40.García-Zarracino, R. & Höpfl, H. (2004) Angew. Chem. Int. Ed. 43, 1507-1511. [DOI] [PubMed] [Google Scholar]
- 41.Moulton, B., Lu, J., Hajndl, R., Hariharan, S. & Zaworotko, M. J. (2002) Angew. Chem. Int. Ed. 41, 2821-2824. [DOI] [PubMed] [Google Scholar]
- 42.Barbour, J. L. (2003) mcavity, Program for Calculating the Molecular Volume of Closed Capsules (University of Missouri, Columbia, MO).
- 43.Lee, S.-W., Mao, C., Flynn, C. E. & Belcher, A. M. (2002) Science 296, 892-895. [DOI] [PubMed] [Google Scholar]
- 44.Blum, A. S., Soto, C. M., Wilson, C. D., Cole, J. D., Kim, M., Gnade, B., Chatterji, A., Ochoa, W. F., Lin, T., Johnson, J. E. & Ratna, B. R. (2004) Nano Lett. 4, 867-870. [Google Scholar]
- 45.Atwood, J. L., Barbour, L. J. & Jerga, A. (2002) Proc. Natl. Acad. Sci. USA 99, 4837-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ludwig, R. (2001) Angew. Chem. Int. Ed. 40, 1808-1827. [PubMed] [Google Scholar]
- 47.Barbour, L. J., Orr, G. W. & Atwood, J. L. (1998) Nature 393, 671-673. [Google Scholar]
- 48.Khan, A. (2004) J. Chem. Phys. 121, 280-284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.