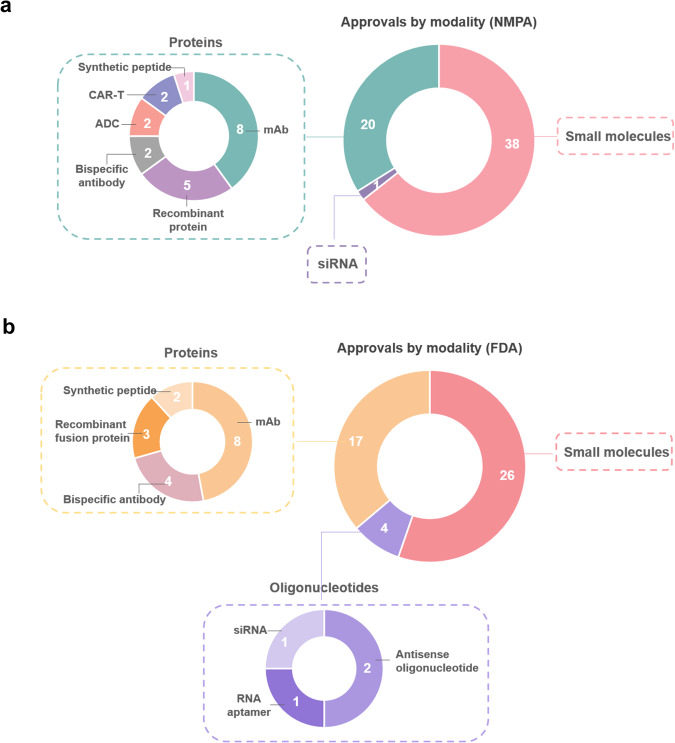
According to the official website of China’s National Medicines and Pharmaceutical Administration (NMPA), a total of 87 novel drugs were approved in China in 2023, with targeted drugs accounting for 67.8% of the total, amounting to 59 drugs (Fig. 1a; Table 1) [https://www.nmpa.gov.cn/yaopin/ypjgdt/index.html]. Notably, domestic innovation is flourishing, with five first-in-class drugs, including Glumetinib, a c-Met inhibitor from Haihe Biopharma; Leritrelvir, a 3CL protease inhibitor from Raynovent; Anaprazole, a proton pump inhibitor from Xuanzhu Biopharm; Pegol-Sihematide, an EPO drug from Hansoh Pharma; and Zuberitamab from BioRay Biopharmaceutical Co., Ltd. Additionally, the world’s first allosteric inhibitor targeting TYK2, Sotyktu (deucravacitinib), has been approved for the treatment of psoriasis and Selumetinib, a MEK inhibitor co-developed by AstraZeneca and Merck Sharp & Dohme (MSD), became the first approved drug in China for the treatment of neurofibromatosis type I (NF1). Beyond these drugs, the approval of novel drug types such as CAR-T cell products, siRNA, monoclonal antibodies, dual antibodies, and ADCs in China is a significant development in the country’s pharmaceutical industry (Fig. 1a). Notably, Equecabtagene autoleucel, jointly developed by IASO Biotherapeutics and Innovent Biologics, became the first approved BCMA-targeted CAR-T cell therapy product in China. Additionally, Inaticabtagene autoleucel developed by Juventas was the first approved CAR-T cell therapy product in the field of leukemia treatment in China. In parallel, the acceleration in the rate of new drug approvals in China is also noteworthy. Glofitamab, for example, was approved in China only five months after its approval in the United States. Furthermore, numerous clinical trials for novel drugs are currently underway in China, and it is anticipated that these new medications will soon bring significant benefits to Chinese patients.
Fig. 1.
NMPA and FDA-approved targeted drugs by modality, 2023. a Small molecules and protein-based drugs comprised a significant majority of NMPA approvals, while oligonucleotides only represented a single case. b The majority of FDA approvals for targeted drugs consist of small molecules and protein-based drugs, with small molecules being approved as new molecular entities (NMEs). Approved in four cases, oligonucleotides were also classified as new molecular entities (NMEs). Source: NMPA, FDA and Nature Reviews Drug Discovery (10.1038/d41573-024-00001-x)
Table 1.
Targeted drugs approved by the FDA and NMPA in 2023
| No. | Brand name | Active ingredient | Approval date | Target/Activity | Approved use on approval date | Drug class | Company |
|---|---|---|---|---|---|---|---|
| FDA-approval | |||||||
| 1 | Leqembi | Lecanemab-irmb | 1/6/2023 | Amyloid β (Aβ) | To treat Alzheimer’s disease | Monoclonal antibody (mAb) | Eisai, Biogen |
| 2 | Brenzavvy | Bexagliflozin | 1/20/2023 | Highly selective SGLT2 inhibitor | To enhance glycemic control in adults with type 2 diabetes, as a supplement to diet and exercise. | Small molecule | TheracosBio |
| 3. | Jaypirca | Pirtobrutinib | 1/27/2023 | BTK inhibitor | To treat relapsed or refractory Mantle cell lymphoma (MCL) or Chronic lymphocytic leukemia (CLL). | Small molecule | Eli Lilly and Company |
| 4 | Orserdu | Elacestrant | 1/27/2023 | Estrogen receptor antagonist | To treat ESR1-mutated ER+/HER2- advanced or metastatic breast cancer. | Small molecule | Stemline Therapeutics |
| 5 | Jesduvroq | Daprodustat | 2/1/2023 | Reversible inhibitor of HIF-PH1, PH2 and PH3 | To treat anemia that is caused by chronic kidney disease (CKD) in dialysis patients. | Small molecule | GlaxoSmithKline |
| 6 | Lamzede | Velmanase alfa-tycv | 2/16/2023 | Mannose-6-phosphate receptor | To treat non-central nervous system manifestations of alpha-mannosidosis | Recombinant fusion protein | Chiesi Farmaceutici S.p.A. |
| 7 | Filspari | Sparsentan | 2/17/2023 | ETAR and AT1R antagonist. | To lower protein in the urine (proteinuria) in adults with primary IgA nephropathy. | Small molecule | Travere Therapeutics, Inc. |
| 8 | Skyclarys | Omaveloxolone | 2/28/2023 | Nrf2 activator | To treat Friedrich’s ataxia | Small molecule | Reata Pharmaceuticals, Inc. |
| 9 | Zavzpret | Zavegepant | 3/9/2023 | Calcitonin gene-related peptide (CGRP) receptor antagonist | To treat migraine | Small molecule | Pfizer Inc. |
| 10 | Zynyz | Retifanlimab-dlwr | 3/22/2023 | PD-1 | To treat metastatic or recurrent locally advanced Merkel cell carcinoma (MCC). | Monoclonal antibody (mAb) | Incyte Corporation |
| 11 | Joenja | Leniolisib | 3/24/2023 | PI3Kδ inhibitor |
For the treatment of activated phosphoinositide 3-kinase delta (PI3Kδ) syndrome (APDS). |
Small molecule | Pharming Group N.V. |
| 12 | Qalsody | Tofersen | 4/25/2023 | SOD1 | To treat amyotrophic lateral sclerosis (ALS) with superoxide dismutase 1 (SOD1) mutation. | Gene therapy: antisense oligonucleotide (ASO) | Biogen MA Inc. |
| 13 | Veozah | Fezolinetant | 5/12/2023 | NK3 receptor antagonist | To reduce moderate to severe vasomotor symptoms (hot flashes and night sweats) caused by menopause | Small molecule | Astellas Pharma US, Inc. |
| 14 | Epkinly | Epcoritamab-bysp | 5/19/2023 | CD20, CD3 | To treat diffuse large B-cell lymphoma (DLBCL) that returned or didn’t respond after 2 or more prior treatments | Bispecific antibody | Genmab US, Inc. |
| 15 | Xacduro | Sulbactam, Durlobactam | 5/23/2023 | β-lactamase inhibitor | To treat hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia caused by susceptible isolates of Acinetobacter baumannii-calcoaceticus complex | Small molecule | Entasis Therapeutics Ltd. |
| 16 | Paxlovid | Nirmatrelvir, Ritonavir | 5/25/2023 | Nirmatrelvir is a main protease inhibitor, ritonavir is an HIV-1 protease inhibitor and CYP3A inhibitor | To treat mild-to-moderate COVID-19 | Small molecule | Pfizer Inc. |
| 17 | Inpefa | Sotagliflozin | 5/26/2023 | SGLT2 and SGLT1 inhibitor | To treat heart failure, type 2 diabetes mellitus, chronic kidney disease, and other cardiovascular risk factors | Small molecule | Lexicon Pharmaceuticals, Inc. |
| 18 | Columvi | Glofitamab-gxbm | 6/15/2023 | CD20, CD3 | To treat relapsed or refractory diffuse large B-cell lymphoma (DLBCL) | Bispecific antibody | Genentech, Inc. |
| 19 | Litfulo | Ritlecitinib | 6/23/2023 | JAK3/TEC inhibitor | To treat severely patchy hair loss | Small molecule | Pfizer Inc. |
| 20 | Rystiggo | Rozanolixizumab-noli | 6/26/2023 | FcRn | To treat adults with generalized myasthenia gravis (gMG) who are acetylcholine receptor (anti-AChR) antibody positive or muscle-specific tyrosine kinase (anti-MuSK) antibody positive. | Monoclonal antibody (mAb) | UCB, Inc. |
| 21 | Ngenla | Somatrogon-ghla | 6/27/2023 | GH receptor | To treat pediatric Growth Hormone deficiency. | Recombinant fusion protein | Pfizer Inc. |
| 22 | Beyfortus | Nirsevimab-alip | 7/17/2023 | RSV F protein | To prevent respiratory syncytial virus (RSV) lower respiratory tract disease | Monoclonal antibody (mAb) | Sanofi Pasteur, Inc. and AstraZeneca AB |
| 23 | Vanflyta | Quizartinib | 7/20/2023 | FLT3 inhibitor | For patients with newly diagnosed FLT3-ITD positive acute myeloid leukemia (AML). | Small molecule | Daiichi Sankyo, Inc. |
| 24 | Xdemvy | Lotilaner | 7/25/2023 | Gamma-aminobutyric acid (GABA)-gated chloride channel inhibitor | To treat Demodex blepharitis | Small molecule | Tarsus Pharmaceuticals, Inc. |
| 25 | Zurzuvae | Zuranolone | 8/4/2023 |
Gamma-aminobutyric acid (GABA) A receptor positive modulator |
To treat postpartum depression | Small molecule | Sage Therapeutics, Inc., Biogen Inc. |
| 26 | Izervay | Avacincaptad pegol | 8/4/2023 | Complement protein C5 inhibitor | To treat geographic atrophy (GA). | Gene therapy: RNA aptamer | IVERIC bio, Inc. |
| 27 | Talvey | Talquetamab-tgvs | 8/9/2023 | CD3 receptor and G protein-coupled receptor class C group 5 member D (GPRC5D) | To treat adults with relapsed or refractory multiple myeloma who have received at least four prior therapies | Bispecific antibody | Janssen Biotech, Inc. |
| 28 | Elrexfio | Elranatamab-bcmm | 8/14/2023 | B-cell maturation antigen (BCMA) and CD3 | To treat adults with relapsed or refractory multiple myeloma who have received at least four prior lines of therapy | Bispecific antibody | Pfizer, Inc. |
| 29 | Sohonos | Palovarotene | 8/16/2023 | RARγ agonist | To treat fibrodysplasia ossificans progressive. | Small molecule | Ipsen Biopharmaceuticals, Inc. |
| 30 | Veopoz | Pozelimab-bbfg | 8/18/2023 | Complement protein C5 | To treat CD55-deficient protein-losing enteropathy, also known as CHAPLE disease. | Monoclonal antibody (mAb) | Regeneron Pharmaceuticals, Inc. |
| 31 | Aphexda | Motixafortide | 9/8/2023 | CXCR4 inhibitor | To use with filgrastim (G-CSF) to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in patients with multiple myeloma | Synthetic peptide analog | BioLineRx |
| 32 | Ojjaara | Momelotinib | 9/15/2023 | JAK1/JAK2, mutant JAK2V617F and ACVR1 inhibitor | To treat anemia in adults with intermediate or high-risk myelofibrosis. | Small molecule | GlaxoSmithKline |
| 33 | Exxua | Gepirone | 9/22/2023 | 5HT1A receptors agonists | To treat major depressive disorder | Small molecule | Mission Pharmacal Company |
| 34 | Rivfloza | Nedosiran | 9/29/2023 | lactate dehydrogenase A (LDHA) | To lower urinary oxalate levels in patients 9 years and older with primary hyperoxaluria type 1 and relatively preserved kidney function | Gene therapy: siRNA | Pyramid Laboratories, Inc. |
| 35 | Velsipity | Etrasimod | 10/12/2023 | Sphingosine 1-phosphate (S1P) receptor modulator | To treat moderately to severely active ulcerative colitis in adults | Small molecule | Pfizer Inc. |
| 36 | Zilbrysq | Zilucoplan | 10/17/2023 | Complement protein C5 | To treat generalized myasthenia gravis (gMG) in patients who are AChR antibody positive . | Synthetic peptide analog | UCB, Inc. |
| 37 | Bimzelx | Bimekizumab | 10/17/2023 | Interleukin-17 A/F antagonist | To treat moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy | Monoclonal antibody (mAb) | UCB, Inc. |
| 38 | Agamree | Vamorolone | 10/26/2023 | Glucocorticoid receptor | To treat Duchenne muscular dystrophy | Small molecule | Santhera Pharmaceuticals (USA), Inc. |
| 39 | Omvoh | Mirikizumab-mrkz | 10/26/2023 | p19 subunit of IL-23 | To treat ulcerative colitis | Monoclonal antibody (mAb) | Eli Lilly and Company |
| 40 | Loqtorzi | Toripalimab-tpzi | 10/27/2023 | PD-1 | To treat advanced nasopharyngeal carcinoma (NPC). | Monoclonal antibody (mAb) | Coherus BioSciences, Inc. |
| 41 | Fruzaqla | Fruquintinib | 11/8/2023 | VEGFR1/2/3 inhibitor | To treat refractory, metastatic colorectal cancer | Small molecule | Takeda Pharmaceuticals America, Inc. |
| 42 | Augtyro | Repotrectinib | 11/15/2023 | ROS1, TRKA, TRKB, and TRKC inhibitor | To treat ROS1-positive non-small cell lung cancer | Small molecule | Bristol-Myers Squibb Company |
| 43 | Ryzneuta | Efbemalenograstim | 11/16/2023 | CSF-3R agonist | For the treatment of chemotherapy-induced neutropenia | Recombinant fusion protein | Evive Biotechnology Singapore PTE. Ltd. |
| 44 | Truqap | Capivasertib | 11/16/2023 | AKT1, AKT2 and AKT3 inhibitor | To treat breast cancer | Small molecule | AstraZeneca Pharmaceuticals LP |
| 45 | Ogsiveo | Nirogacestat | 11/27/2023 | Gamma secretase inhibitor | To treat adults with progressing desmoid tumors who require systemic treatment | Small molecule | SpringWorks Therapeutics, Inc. |
| 46 | Fabhalta | Iptacopan | 12/5/2023 | Complement Factor B inhibitor | To treat paroxysmal nocturnal hemoglobinuria | Small molecule | Novartis Pharmaceuticals Corporation |
| 47 | Wainua | Eplontersen | 12/21/2023 | TTR mRNA | To treat polyneuropathy of hereditary transthyretin-mediated amyloidosis | Gene therapy: antisense oligonucleotide (ASO) | AstraZeneca Pharmaceuticals LP |
| NMPA-approval | |||||||
| 1 | Polivy | Polatuzumab Vedotin-Piiq | 1/10/2023 | CD79B | For the treatment of diffuse large B-cell lymphoma | ADC | Roche China Holding Ltd. |
| 2 | Exkivity | Mobocertinib | 1/11/2023 | EGFR inhibitor | For the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) that has progressed during or after platinum-containing chemotherapy and carries an insertion mutation in exon 20 of the epidermal growth factor receptor (EGFR). | Small molecule | Takeda Pharmaceutical Co., Ltd. |
| 3 | Kispali | Ribociclib | 1/19/2023 | CDK4/6 inhibitor | For the treatment of breast cancer | Small molecule | Beijing Novartis Pharma Ltd. |
| 4 | Simnotrelvir/Ritonavir | 1/28/2023 |
HIV-1 protease, SARS-CoV 3CLpro inhibitor |
For the treatment of adult patients with mild to moderate novel coronavirus infection (COVID-19) | Small molecule | Simcere Pharmaceutical (Hainan) Co., Ltd. | |
| 5 | Deuremidevir | 1/29/2023 | RdRp inhibitor | For the treatment of adult patients with mild to moderate novel coronavirus infection (COVID-19) | Small molecule | Shanghai Wangshi Biomedical Technology Co., Ltd. | |
| 6 | ZEPOSIA | Ozanimod | 1/31/2023 | S1PR1/5 regulator | For the treatment of multiple sclerosis | Small molecule | Bristol-Myers Squibb (China) Investment Co. Ltd. |
| 7 | Keverprazan | 2/14/2023 | Potassium-competitive acid blockers | For the treatment of duodenal ulcer and reflux oesophagitis | Small molecule | Jiangsu Carephar Pharmaceutical Co., Ltd. | |
| 8 | Enhertu | Fam-trastuzumab deruxtecan-NXK | 2/21/2023 | HER2 antagonist | For the treatment of breast cancer | ADC | Daiichi Sankyo (China) Holdings Co., Ltd. |
| 9 | Adebrelimab | 2/28/2023 | PD-L1 | For the first-line treatment of extensive-stage small cell lung cancer (ES-SCLC) in combination with chemotherapy | Monoclonal antibody (mAb) | Shanghai Shengdi Pharmaceutical Co., Ltd. | |
| 10 | Glumetinib | 3/08/2023 | c-MET inhibitor | To treat locally advanced or metastatic non-small cell lung cancer with a mesenchymal-epithelial transforming factor (MET) exon 14 skipping mutation. | Small molecule | Haihe Biopharma Co., Ltd. | |
| 11 | Calquence | Acalabrutinib | 3/21/2023 | BTK inhibitor | For the treatment of adult patients with metachronous lymphoma (MCL) who have received at least one prior therapy | Small molecule | AstraZeneca Investment (China) Co., Ltd. |
| 12 | Leritrelvir | 3/23/2023 | SARS-CoV-2 3CLpro inhibitor | For the treatment of adult patients with mild-to-moderate novel coronavirus infection (COVID-19) | Small molecule | Guangdong Raynovent Biotech Co., Ltd. | |
| 13 | Koselugo | Selumetinib | 4/28/2023 | MEK1/2 inhibitor | For the treatment of neurofibromatosis type 1 | Small molecule | AstraZeneca Investment (China) Co., Ltd. |
| 14 | Olinvyk | Oliceridine | 4/28/2023 | μ opioid agonist | For the treatment of acute pain | Small molecule | Jiangsu Nhwa Pharmaceutical Co., Ltd. |
| 15 | Ryzneuta | Efbemalenograstim alfa | 5/06/2023 | CSF-3R agonist | For the treatment of chemotherapy-induced neutropenia | Recombinant fusion protein | Evive Biopharmaceutical (Beijing) Ltd. |
| 16 | Alfosbuvir | 5/12/2023 | HCV NS5B RNA-dependent RNA polymerase inhibitor | For the treatment of primary or interferon-treated genotypes 1, 2, 3, and 6 chronic hepatitis C virus (HCV) infection in adults with or without compensated cirrhosis | Small molecule | Nanjing Sanhome Pharmaceutical Co., Ltd. | |
| 17 | Zuberitamab | 5/12/2023 | CD20 | For the treatment of CD20-positive diffuse large B-cell lymphoma (DLBCL) | Monoclonal antibody (mAb) | BioRay Biopharmaceutical Co., Ltd. | |
| 18 | Aliqopa | Copanlisib | 5/19/2023 | PI3Kα/δ inhibitor | For adult patients with relapsed or refractory follicular lymphoma (FL) who have received at least two previous systemic treatments | Small molecule | Bayer Healthcare Co., Ltd. |
| 19 | ILUMYA | Tildrakizumab-ASMN | 5/26/2023 | IL-23p19 | For the treatment of plaque psoriasis | Monoclonal antibody (mAb) | Shenzhen Kangzhe Biotech Co., Ltd. |
| 20 | LIVMARLI | Maralixibat Chloride | 5/29/2023 | IBAT inhibitor | For the treatment of Alagille syndrome | Small molecule | Beihai Kangcheng (Suzhou) Biopharmaceutical Co., Ltd. |
| 21 | Befotertinib | 5/29/2023 | EGFR T790M inhibitor | For the treatment of patients with locally advanced or metastatic non-small cell lung cancer who are positive for the EGFR T790M mutation | Small molecule | Betta Pharmaceuticals Co., Ltd. | |
| 22 | Vorolanib | 6/07/2023 | Multi-target kinase (VEGFR2、KIT、PDGFR、FLT3 and RET) inhibitor | For the treatment of patients with advanced renal cell carcinoma who have failed prior tyrosine kinase inhibitor therapy | Small molecule | Betta Pharmaceuticals Co., Ltd. | |
| 23 | Aligrin | Bilastine | 6/21/2023 | H1 receptor antagonist | For the treatment of urticaria in adolescents and adults aged 12 years and over | Small molecule | A. Menarini China Holding Co., Ltd. |
| 24 | Anaprazole | 6/21/2023 | Proton pump inhibitor | For the treatment of duodenal ulcers | Small molecule | Xuanzhu (Beijing) Pharmaceutical Technology Co., Ltd. | |
| 25 | Iruplinalkib | 6/27/2023 | ALK inhibitor | For the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) that is mesenchymal lymphoma kinase (ALK)-positive | Small molecule | Qilu Pharmaceutical Co., Ltd. | |
| 26 | Retagliptin | 6/27/2023 | DPP-4 inhibitor | For improving glycaemic control in adults with type 2 diabetes | Small molecule | Jiangsu Hengrui Pharmaceuticals Co., Ltd. | |
| 27 | Carbaglu | Carglumic acid | 6/27/2023 | CPS1 activator | For the treatment of hyperammonaemia | Small molecule | Recordati (Beijing) Pharmaceutical Co., Ltd |
| 28 | Vivjoa | Oteseconazole | 6/27/2023 | Fungal CYP51 inhibitor | For the treatment of severe vulvovaginal candidiasis (VVC) | Small molecule | eVenus Pharmaceutical Laboratories, Inc. |
| 29 | Mulpleta | Lusutrombopag | 6/27/2023 | TPO receptor agonist | For the treatment of chronic liver disease with thrombocytopenia | Small molecule | Suzhou Ceclor Pharmaceutical Co., Ltd. |
| 30 | Telpegfilgrastim | 6/30/2023 | CSF-3R agonist | For the treatment of febrile neutropenia | Recombinant protein | Xiamen Amoytop Biotech Co., Ltd. | |
| 31 | Equecabtagene autoleucel | 6/30/2023 | B-cell maturation antigen (BCMA) | For the treatment of adult patients with relapsed or refractory multiple myeloma | CAR-T | Nanjing IASO Biopharmaceutical Co., Ltd. | |
| 32 | Pegol-Sihematide | 6/30/2023 | EPO receptor agonists | For the treatment of anemia caused by chronic kidney disease | Synthetic peptide analog | Jiangsu Hansoh Pharmaceutical Co., Ltd. | |
| 33 | Wakix | Pitolisant | 6/30/2023 | H3 receptor antagonist | For the treatment of narcolepsy | Small molecule | Citrine Pharmaceutical (Shanghai) Co., Ltd. |
| 34 | Remitch | Nalfurafine | 6/30/2023 | κ opioid receptor agonist | For the treatment of pruritus | Small molecule | Shenyang Sunshine Pharmaceutical Co., Ltd. |
| 35 | Vocabria | Cabotegravir | 7/11/2023 | HIV integrase inhibitor | For the treatment of HIV infection | Small molecule | GlaxoSmithKline (China) Investment Co., Ltd. |
| 36 | REZUROCK | Belumosudil | 8/01/2023 | ROCK1/2 inhibitor | For the treatment of chronic graft-versus-host disease | Small molecule | BioNova Pharmaceuticals (Shanghai) Ltd. |
| 37 | Tafolecimab | 8/15/2023 | PCSK9 inhibitor | For the treatment of primary hypercholesterolemia (including heterozygous familial and non-familial hypercholesterolemia) and mixed dyslipidemia | Monoclonal antibody (mAb) | Innovent Biologics (Suzhou) Co., Ltd. | |
| 38 | Leqvio | Inclisiran | 8/22/2023 | PCSK9 | For the treatment of hypercholesterolemia or mixed dyslipidemia | siRNA | Beijing Novartis Pharma Ltd. |
| 39 | Sunvozertinib | 8/22/2023 | EGFR tyrosine kinase inhibitor | For the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with EGFR exon 20 insertion mutations | Small molecule | Dizal Pharmaceutical Co., Ltd. | |
| 40 | MARGENZA | Margetuximab | 8/29/2023 | HER2 antagonist | For the treatment of HER2-positive breast cancer | Monoclonal antibody (mAb) | Zai Lab Ltd. |
| 41 | Narlumosbart | 9/05/2023 | RANKL inhibitor | For the treatment of adult patients with giant cell tumours of bone that are not surgically resectable or whose surgical resection may result in severe functional impairment | Monoclonal antibody (mAb) | Shanghai JMT Biological Technology Co., Ltd. | |
| 42 | Lysodren | Mitotane | 9/05/2023 | Steroid hormone receptor antagonist | For the treatment of adrenocortical carcinoma | Small molecule | Jiedi Pharmaceutical Technology (Shanghai) Co., Ltd. |
| 43 | Nexviazyme | Avalglucosidase alfa | 9/28/2023 | α-glucosidase | For the treatment of glycogen storage disease type II | Recombinant protein | Sanofi (China) Investment Co., Ltd. |
| 44 | Sotyktu | Deucravacitinib | 10/18/2023 | TYK2 inhibitor | For adult patients with moderate to severe plaque psoriasis who are suitable for systemic therapy or phototherapy | Small molecule | Bristol-Myers Squibb Company |
| 45 | Litfulo | Ritlecitinib | 10/19/2023 | JAK3/TEC inhibitor | To treat severely patchy hair loss | Small molecule | Pfizer Inc. |
| 46 | Kineret | Anakinra | 10/27/2023 | IL1R1 | For the treatment of periodic fever syndrome | Recombinant protein | Supi Pharmaceutical (Shanghai) Co., Ltd. |
| 47 | Aponermin | 11/01/2023 | DR4/DR5 agonist | For the treatment of relapsed or refractory multiple myeloma | Recombinant protein | Wuhan Hiteck Biological Pharma Co., Ltd. | |
| 48 | Columvi | Glofitamab | 11/07/2023 | CD20, CD3 | For the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma who have received at least two or more lines of systemic therapy | Bispecific antibody | Roche Pharma (Schweiz) AG |
| 49 | Inaticabtagene autoleucel | 11/07/2023 | CD19 | For the treatment of relapsed or refractory B-cell acute lymphoblastic leukaemia in adults. | CAR-T | Juventas Cell Therapy Ltd. | |
| 50 | Vebreltinib | 11/14/2023 | c-Met inhibitor | For the treatment of locally advanced or metastatic non-small cell lung cancer | Small molecule | Beijing Purunao Biotechnology Co., Ltd. | |
| 51 | XENLETA | Lefamulin | 11/14/2023 | 50S subunit | For the treatment of community acquired pneumonia | Small molecule | Sumitomo Pharmaceuticals (Suzhou) Co., Ltd. |
| 52 | Adasuve | Loxapine | 11/21/2023 | 5-HT2A receptor, D2 receptor antagonist | For the treatment of adult schizophrenia or bipolar I disorder | Small molecule | Zhaoke Pharmaceutical (Hefei) Co., Ltd. |
| 53 | Atilotrelvir/Ritonavir | 11/24/2023 | HIV-1 protease, SARS-CoV-2 3CLpro inhibitor | For the treatment of COVID-19 | Small molecule | Fujian Guangsheng Zhonglin Biotechnology Co., Ltd. | |
| 54 | Dimdazenil | 11/28/2023 | GABAA receptor positive allosteric modulator | Short-term treatment for patients with insomnia | Small molecule | Zhejiang Jingxin Pharmaceutical Co., Ltd. | |
| 55 | Tepmetko | Tepotinib | 12/05/2023 | c-Met inhibitor | For the treatment of non-small cell lung cancer | Small molecule | Merck Serono (Beijing) Pharmaceutical R&D Co., Ltd. |
| 56 | Vabysmo | Faricimab | 12/13/2023 | Ang2, VEGF-A | For the treatment of diabetic macular oedema | Bispecific antibody | Roche China Holding Ltd. |
| 57 | Livtencity | Maribavir | 12/19/2023 | UL97 inhibitor | For the treatment of cytomegalovirus infections | Small molecule | Takeda (China) International Trading Co., Ltd. |
| 58 | Socazolimab | 12/19/2023 | PD-L1 | For the treatment of uterine cervical cancer | Monoclonal antibody (mAb) | Zhaoke (Guangzhou) Oncology Pharmaceutical Ltd. | |
| 59 | Beyfortus | Nirsevimab | 12/26/2023 | RSV F protein | To prevent respiratory syncytial virus (RSV) lower respiratory tract disease | Monoclonal antibody (mAb) | AstraZeneca Investment (China) Co., Ltd. |
During the year, the FDA’s Centre for Drug Evaluation and Research (CDER) approved 55 novel drugs, surpassing 37 in 2022 and second only to 59 in 20181, [https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2023]. Of these novel drugs, 47, or 85.5%, were targeted therapies, reflecting a clear trend towards more personalized and effective treatment options. Within this category, 26 were small molecules, 8 were monoclonal antibodies, 4 were bispecific antibodies, 3 were recombinant fusion proteins, 4 were small nucleic acid drugs (including 2 antisense oligonucleotides, 1 RNA aptamer, and 1 siRNA), and 2 were synthetic peptide analogs (Fig. 1b; Table 1). The targets of these new drugs span a range of biological processes, including kinases, cytokines, enzymes, receptors, ion channels, and proteasomes. This diversity highlights the ongoing efforts to develop innovative therapies that target the root causes of various diseases. In terms of indications approved for marketing, cancer remains the leading focus of research and development, with 14 new cancer therapies, representing 29.7% of the total. Immune system disorders followed closely behind, accounting for 8 approved therapies (17%) while neurological disorders ranked third, with 7 approved therapies (14.9%). Diseases of the blood and lymphatic system, endocrine and metabolic diseases, and infectious diseases each accounted for four cases (8.5%) (Fig. 1b). This distribution highlights the diverse range of therapeutic areas targeted by novel drug development. Notably, there has been a gradual increase in targeted therapeutics for rare diseases, such as Pompe’s disease and paroxysmal sleep hemoglobinuria. These diseases often lack effective treatment options, making the development of targeted therapies particularly important for improving patient outcomes. Overall, the data presented in this article highlights the significant progress being made in the field of drug development, with a particular emphasis on targeted therapies. The increasing approval of novel drugs offers hope to patients with various diseases, and further research and development is expected to lead to even more effective and personalized treatment options in the future.
It is heartening to observe that the FDA has granted approved three innovative Chinese new drugs: Loqtorzi (toripalimab), Fruzaqla (fruquintinib) and Ryzneuta (efbemalenograstim). Toripalimab is a monoclonal antibody targeting PD-1, marks the first FDA-approved drug for the treatment of nasopharyngeal cancer. Fruquintinib, approved for the treatment of metastatic colorectal cancer, is unique as the first and only highly selective inhibitor approved in the U.S. for comprehensive inhibition of all three VEGF receptor kinases, regardless of a patient’s biomarker status. Efbemalenograstim, approved for the treatment of neutropenia in oncology patients receiving anti-cancer drugs, stands out as the only long-acting G-CSF (granulocyte colony-stimulating factor) product approved in both China and the U.S., showcasing its global relevance and potential impact on patient care2. As a result, Yifan Pharmaceutical Co., Ltd. has become the first innovative biopharmaceutical company in China to be approved by the FDA as a Marketed Access Holder (MAH), marking a significant milestone in the company’s journey towards global leadership in biopharmaceutical innovation.
Innovation in mechanism-based therapies reached new heights in the past year, with numerous drugs earning the distinction of “First-In-Class". These groundbreaking therapies either represent the first treatment option for a specific disease or introduce a novel mechanism of action. One remarkable example is Daprodustat, the first oral drug approved by the FDA for the treatment of anemia with chronic kidney disease (CKD) in dialysis patients3. Daprodustat reversibly inhibits HIF-PH1/2/3, thereby increasing HIF levels. This, in turn, stimulates the expression of genes essential for red blood cell production, such as EPO and VEGF. Another notable development is the approval of Fezolinetant, the world’s first non-hormone targeted drug, for the treatment of moderate to severe hot flashes caused by menopause. Unlike hormonal drugs commonly used to manage these symptoms, Fezolinetant works primarily by antagonizing the neurokinin-3 (NK3) receptor which offers patients a better quality of life with fewer side effects and more pronounced therapeutic effects.
Furthermore, 2023 marked a significant milestone in gene therapy with the approval of four groundbreaking nucleic acid drugs. Among them, Qalsody (tofersen) stands out as the first and currently only gene therapy designed to target the underlying pathogenesis of amyotrophic lateral sclerosis (ALS). Tofersen, an antisense oligonucleotide (ASO), specifically targets mRNA produced by the SOD1 mutant gene, halting the production of the toxic SOD1 protein and slowing the progression of ALS. Another noteworthy ASO therapy, Wainua (eplantersen), has been approved for marketing to inhibit the production of the TTR protein for the treatment of both hereditary and non-hereditary amyloidosis polyneuropathy. This innovative approach utilizes antisense oligonucleotide ligand coupling (LICA) technology, coupling ASO drugs to ligand molecules that bind to specific receptors on the cell surface. In addition, Izervay (avacincaptad pegol) represents a novel complement C5 protein inhibitor and the second FDA-approved RNA aptamer4. The approval of Avacincaptad pegol signifies the emergence of a potentially transformative innovator in the field of Geographic Atrophy (GA). Lastly, Rivfloza (nedosiran) was approved by the FDA in September 2023 as the second siRNA drug worldwide for the treatment of primary hyperoxaluria type 1 (PH1). This latest development showcases the increasing impact of gene therapy in treating previously intractable diseases and offers new hope for patients suffering from debilitating conditions.
In the realm of antibody therapeutics, we have witnessed the emergence of four groundbreaking bispecific antibodies capable of binding to two distinct epitopes or antigens simultaneously. This unique dual-action mechanism enhances target specificity, paving the way for innovative immunotherapy advancements. Notable examples include the Epcoritamab and Glofitamab, which target both CD20 and CD3, showcasing the potential of this approach in treating a range of diseases.
Targeted therapies continue to constitute the majority of approved therapies worldwide. However, immunotherapy has emerged as a significant avenue in cancer treatment in recent years, particularly when combined with targeted therapies, leading tosuperior therapeutic outcomes. This combination intervenes in the tumor’s immune escape mechanisms, thereby enhancing the immune cells’ attack capabilities and ultimately enhancing the efficacy of immunotherapy. Future advancements in targeted therapies will involve the identification and development of novel targets that specifically target immune escape mechanisms and interfere with immune cell-tumor interactions to improve immunotherapy effectiveness. Furthermore, the utilization of technologies such as RNA interference, gene editing, and other intracellular targeting methods will enable more precise therapeutic strategies.
In 2024, Signal Transduction and Targeted Therapy will continue to push the boundaries of targeted therapies, introducing innovative and disease-focused solutions. With a strong focus on clinical research, our aim is to publish more groundbreaking papers on novel therapeutic targets, signaling pathways, and effective new therapies especially drug discovery with clinical applications.
Competing interests
The authors declare no competing interests.
Contributor Information
Wenjing Wang, Email: wangwenjing@wchscu.cn.
Qiu Sun, Email: sunqiu@scu.edu.cn.
References
- 1.Mullard, A. 2023 FDA approvals. Nat. Rev. Drug Discov.10.1038/d41573-024-00001-x (2024). [DOI] [PubMed]
- 2.Blair HA. Efbemalenograstim alfa: first approval. Drugs. 2023;83:1125–1130. doi: 10.1007/s40265-023-01911-7. [DOI] [PubMed] [Google Scholar]
- 3.Allison, S. J. Daprodustat for anaemia in CKD. Nat. Rev. Nephrol. 18, 3 (2022). [DOI] [PubMed]
- 4.Kang C. Avacincaptad pegol: first approval. Drugs. 2023;83:1447–1453. doi: 10.1007/s40265-023-01948-8. [DOI] [PubMed] [Google Scholar]