Abstract
Reverse transcription of the Saccharomyces cerevisiae Ty1 retrotransposon is primed by tRNAiMet base paired to the primer binding site (PBS) near the 5′ end of Ty1 genomic RNA. The 10-nucleotide PBS is complementary to the last 10 nucleotides of the acceptor stem of tRNAiMet. A structural probing study of the interactions between the Ty1 RNA template and the tRNAiMet primer showed that besides interactions between the PBS and the 3′ end of tRNAiMet, three short regions of Ty1 RNA, named boxes 0, 1, and 2.1, interact with the T and D stems and loops of tRNAiMet. To determine if these sequences are important for the reverse transcription pathway of the Ty1 retrotransposon, mutant Ty1 elements and tRNAiMet were tested for the ability to support transposition. We show that the Ty1 boxes and the complementary sequences in the T and D stems and loops of tRNAiMet contain bases that are critical for Ty1 retrotransposition. Disruption of 1 or 2 bp between tRNAiMet and box 0, 1, or 2.1 dramatically decreases the level of transposition. Compensatory mutations which restore base pairing between the primer and the template restore transposition. Analysis of the reverse transcription intermediates generated inside Ty1 virus-like particles indicates that initiation of minus-strand strong-stop DNA synthesis is affected by mutations disrupting complementarity between Ty1 RNA and primer tRNAiMet.
The Saccharomyces cerevisiae Ty retrotransposons display structural and functional similarities to retroviruses (6). Like retroviruses, they alternate their genetic material between RNA and DNA (4, 23). Reverse transcription (RT) of genomic RNA into double-stranded DNA is carried out by the retroelement-encoded reverse transcriptase, which has an absolute requirement for primers to initiate DNA synthesis. The first step of RT is the synthesis of minus-strand strong-stop DNA, which proceeds from the 3′ hydroxyl group of a primer tRNA annealed at the primer binding site (PBS) of the element’s RNA.
The retrotransposon Ty1 has a PBS with 10 bases of complementarity to the last 10 nucleotides of the acceptor stem of tRNAiMet (3). Chapman et al. (8) have demonstrated genetically that tRNAiMet is used as a primer for minus-strand strong-stop DNA synthesis and is essential for Ty1 transposition. We recently completed a structural probing study of the conformation of the specific complex formed between the template, Ty1 RNA, and the primer, tRNAiMet (12). Our results showed that besides interactions between the PBS and the 3′ end of tRNAiMet, three short regions of Ty1 RNA, named boxes 0, 1, and 2.1, interact with the T and D stems and loops of tRNAiMet. In the extended complex, 30 bases of Ty1 RNA are base paired with primer tRNAiMet. Some preliminary data showed that mutations in the boxes disrupting some of the Watson-Crick base pairs between Ty1 RNA and the T and D regions of tRNAiMet had severe effects on transposition of the Ty1 element (27). In a recent study, Keeney et al. (16) identified determinants in the T loop and arm and D arm of tRNAiMet that are critical for Ty1 retrotransposition. To determine if extended base pairing between tRNAiMet and the genomic RNA is important in the RT pathway of the Ty1 retrotransposon, we have tested the effects of mutations in Ty1 RNA and tRNAiMet on transposition. Here we report that base pairing between the Ty1 RNA boxes and the complementary sequences in the T and D stems and loops of tRNAiMet is essential for Ty1 retrotransposition. A drastic effect on transposition was observed when as few as 2 bp between primer tRNAiMet and Ty1 RNA were disrupted. Transposition was rescued by compensatory mutations restoring base pairing between the primer and the template. To determine at which step of the transposition pathway the extended interactions between Ty1 RNA and tRNAiMet were required, the RT intermediates generated inside the Ty1 virus-like particles (VLPs) were analyzed. Our results indicate that the first step of the RT pathway (i.e., initiation of minus-strand strong-stop DNA synthesis) is affected by mutations disrupting complementarity between the Ty1 boxes and tRNAiMet.
MATERIALS AND METHODS
Plasmids.
Plasmid pJEF1105 (4), kindly provided by J. D. Boeke, is a high-copy-number (2μm) plasmid marked with URA3 and containing a Ty1-neo element fused to the GAL1 promoter. Plasmid pFS1 was constructed by ligating a 180-bp BamHI-HindIII fragment containing the wild-type IMT4 gene from plasmid pBY140 (24) to the 2μm URA3-marked vector pFL44s (7) digested with BamHI and HindIII. Plasmids pIMT, bearing wild-type or mutant tRNAiMet genes, were constructed by ligating a 180-bp BamHI-HindIII fragment containing the wild-type IMT4 or mutant imt4 gene into the BamHI-HindIII sites of YEp351 (a 2μm LEU2 plasmid).
Yeast strains and media.
Culture media and growth conditions were as described previously (22). Yeast strain AGY9 (MATa leu2Δ1 ura3-52 trp1Δ63 his4-539 lys2-801 spt3-202), kindly provided by J. D. Boeke, was used to prevent transcription of endogenous Ty1 element; transcription of GAL1-promoted elements is unaffected in the spt3-202 background (21). Yeast strain ASB-pFS1 (MATα ura3-52 trp1Δ1 leu2-3,112 imt1::TRP1 imt2::TRP1 imt3::TRP1 imt4::TRP1/pFS1), derived from ASB217-32C (24), was transformed with one of the LEU2-marked pIMT plasmids bearing a mutant tRNAiMet gene (pIMTG54, pIMTC60, or pIMTC54C60). To test if mutant tRNAiMet could function in initiation of translation, the ASB-pFS1 strain containing the URA3-marked pFS1 plasmid and one of the LEU2-marked pIMT plasmids bearing a mutant tRNAiMet gene was patched onto synthetic complete (SC)-Leu medium and replica plated on a medium containing 5-fluoro-orotic acid (5-FOA). Growth on the 5-FOA medium indicated that the URA3-marked plasmid carrying the wild-type IMT4 gene was lost and that the mutant tRNAiMet gene on the LEU2-marked pIMT plasmid was able to initiate translation (see Fig. 1).
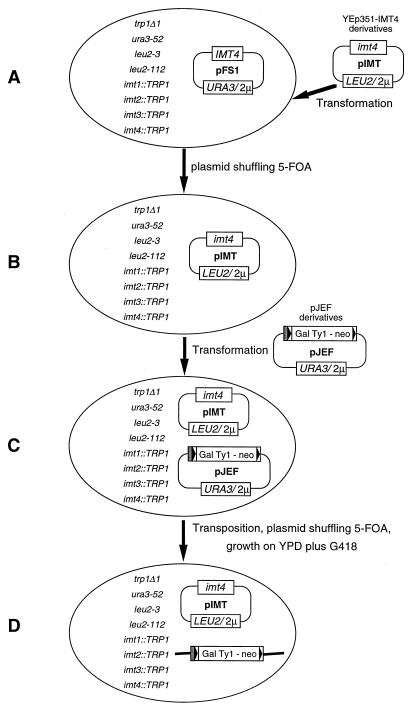
FIG. 1.
Genetic assay for the function of extended interactions between primer tRNAiMet and Ty1 RNA for Ty1 transposition in yeast strain ASB. (A) In yeast strain ASB, all four copies of the tRNAiMet genes (IMT1 to IMT4) are disrupted by TRP1 (24). A wild-type IMT4 gene cloned on an extrachromosomal URA3-marked plasmid is supplied to allow growth of the cell. (B) The wild-type IMT4 gene is replaced by a mutant imt4 gene cloned on a LEU2-marked plasmid by selection on 5-FOA. (C) The yeast strain containing the mutant imt4 gene is transformed by a neomycin-marked wild-type or mutant Ty1 element cloned on a URA3 plasmid. (D) Transposition is induced by growth on SC-Ura-Leu galactose medium. Cells are then grown on YPD to allow for plasmid loss. Cells that have lost the Ty1-neo plasmid are selected on a medium containing 150 μg of 5-FOA per ml. Cells are finally replica plated to YPD containing 150 μg of G418 per ml to identify colonies that have undergone transposition.
Mutagenesis.
Site-directed mutagenesis was performed as described by Kunkel (17). Mutations in the Ty1 element were made as previously described with the following oligodeoxyribonucleotides: Ty1 B0G (5′GCGCCTGTGCTTCGGGTACTTCTAAG3′), Ty1 B1C (5′GTCCACACAAATCAAGACCCGTTAGACG3′), Ty1 B0GB1G (5′GCGCCTGTGCTTCGGGTACTTCTAAG3′ and 5′GTCCACACAAATCAAGAGCCGTTAGACG3′), Ty1 B1CC (5′GTCCACACAAATCAAGACCCCTTAGACG3′), and Ty1 B2.1 (5′CCGTTAGACGTTTCAGCAAGTAAAACAGAAG3′).
Mutations in the IMT4 gene were done after subcloning a 180-bp BamHI-HindIII fragment containing the IMT4 gene from plasmid pBY140 (24) in phagemid pSL1180 (Pharmacia) digested with BamHI and HindIII. The tRNAiMet mutants were made with the following oligodeoxyribonucleotides: imt4-C60 (5′GCGCCGCTCGGGTTCGATCCGAGGAC3′), imt4-G54 (5′CTCGGTTTCGACCCGAGGACATCAGGG3′), and imt4-C54C60 (5′GCGCCGCTCGGGTTCGAGCCGAGGACATCAGGG3′). Mutant phagemids were identified by sequence analysis. Plasmids bearing the mutant imt4 genes were constructed by ligating the 180-bp BamHI-HindIII fragment from mutant phagemid DNA into the BamHI-HindIII sites of YEp351.
Transposition assays.
The transposition assay (see Fig. 1) was performed as described by Chapman et al. (8). Yeast strains ASB-pIMT harboring wild-type or mutant Ty1 elements were patched onto SC-Ura plates containing 2% glucose. Yeast strain AGY9 harboring the wild-type or mutant tRNAiMet gene plus wild-type or mutant Ty1 elements were patched onto SC-Ura-Leu or SC-Ura plates containing 2% glucose. After 2 days of growth at 30°C, the patches were replica plated to SC-Ura plates containing 4% galactose (yeast strain ASB) or to SC-Ura-Leu or SC-Ura plates containing 2% galactose (yeast strain AGY9). An excess of galactose and a longer incubation time were used to allow growth of strain ASB because the transport of galactose into these cells is reduced. Following 5 days of growth at 22°C for strain ASB and 3 days for strain AGY9, the cells were replica plated to nonselective yeast extract-peptone-dextrose (YPD) medium to allow for plasmid loss. Following 1 or 2 days of growth at 30°C, the patches were replica plated to SC-glucose medium containing 1 mg of 5-FOA per ml and incubated for 1 day at 30°C to select for cells that had lost the plasmid containing the URA3 gene (5). For each Ty1 construct or Ty1-tRNAiMet combination, two independent transformants were assayed qualitatively for their transposition phenotype. For the qualitative transposition test represented in Fig. 4, a serial dilution of a given amount (270 cells per μl for strain ASB and 1,140 cells per μl for strain AGY9) of Ura− cells was done. Five microliters of each dilution was spotted onto YPD plates containing 150 μg of G418 per ml and incubated at 30°C for 2 days. With this amount of Ura− cells, and given the transposition rate of the wild-type Ty1 element, 400 neomycin-resistant colonies were expected in the first spot of the wild-type Ty1 element. For quantitative assays, cell patches were scraped into 5 ml of NaCl, 0.15 M, and the concentration was determined by counting. One hundred microliters of the diluted suspension, at 104 cells/ml, was plated onto SC–5-FOA medium and incubated for 2 days at 30°C. Cells were finally replica plated to YPD containing 150 μg of G418 per ml to identify colonies that had undergone transposition of the Ty1-neo element. Transposition is expressed as the number of Neor Ura− yeast colonies divided by the total number of Ura− colonies. The frequency of transposition of the wild-type Ty1 element was 30% in strain ASB and 7% in strain AGY9. The results presented in Table 1 represent pooled quantitative data from two independent transformants.
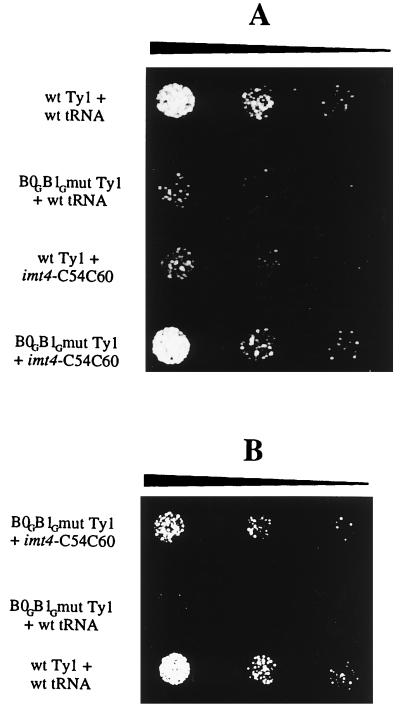
FIG. 4.
Compensatory mutations restore transposition defects. A qualitative transposition assay of yeast strain ASB (A) or AGY9 (B) containing various combinations of tRNAiMet genes and Ty1 elements on YPD plates containing 150 μg of G418 per ml is shown. A given amount of Ura− selected cells (270 cells per μl for ASB and 1,140 cells per μl for AGY9) was suspended in 1 ml of sterile water, fivefold dilutions were prepared, and 5 μl from each dilution was spotted on YPD-G418 plates to identify cells that had undergone transposition of the neomycin-marked Ty element.
TABLE 1.
Transposition of various Ty1 RNA-tRNAiMet combinationsa
| Ty1 | tRNAiMet | ASB
|
AGY9
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Neor Ura− colonies/ Ura− coloniesb
|
Transposition frequency (%) | Relative transposition | Neor Ura− colonies/Ura− colonies
|
Transposition frequency (%) | Relative transposition | ||||
| Expt 1 | Expt 2 | Expt 1 | Expt 2 | ||||||
| wt | wt | 254/752 | 273/1,050 | 30 | 1 | 77/1,120 | 68/960 | 7 | 1 |
| B0G mut | wt | 118/905 | 153/1,028 | 14 | 0.47 | 22/950 | 23/1,010 | 2.3 | 0.33 |
| B1C mut | wt | 120/761 | 147/980 | 15 | 0.50 | 23/1,100 | 18/860 | 2.1 | 0.30 |
| B0GB1G mut | wt | 48/1,040 | 57/1,012 | 5 | 0.17 | 1/1,096 | 0/780 | 0.09 | 0.01 |
| B1CC mut | wt | nd | nd | nd | nd | 2/800 | 1/640 | 0.20 | 0.03 |
| B2.1 mut | wt | nd | nd | nd | nd | 3/842 | 1/804 | 0.24 | 0.03 |
| B2.2 mut | wt | nd | nd | nd | nd | 36/604 | 59/955 | 6 | 0.86 |
| wt | imt4-C60 | 75/602 | 120/1,030 | 12 | 0.40 | na | na | na | na |
| wt | imt4-G54 | 91/859 | 106/898 | 11 | 0.37 | na | na | na | na |
| wt | imt4-C54C60 | 53/810 | 67/917 | 7 | 0.23 | na | na | na | na |
| B0G mut | imt4-C60 | 200/680 | 248/883 | 29 | 0.96 | 65/1,005 | 60/952 | 6.4 | 0.90 |
| B1C mut | imt4-G54 | 168/705 | 210/954 | 23 | 0.77 | 67/1,018 | 58/867 | 6.6 | 0.93 |
| B0GB1G mut | imt4-C54C60 | 230/874 | 166/760 | 24 | 0.80 | 60/970 | 59/944 | 6.2 | 0.88 |
The effects of mutations in tRNAiMet and Ty1 RNA were tested in two yeast strains, ASB and AGY9. wt, wild type; mut, mutant; nd, not determined; na, not applicable (in strain AGY9, wild-type tRNAiMet, which is always expressed, supports transposition of the wild-type Ty1 element).
Data are sums of colonies from three independent measurements.
Analysis of minus-strand strong-stop DNA by RT-PCR.
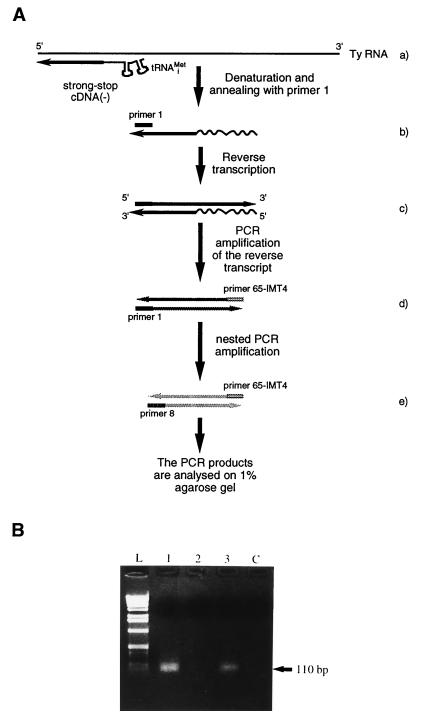
Ty1 VLPs were purified with a sucrose step gradient as described by Eichinger and Boeke (11) with minor modifications (22). Extraction of nucleic acids from VLPs was performed as described elsewhere (22). Nucleic acids from equal amounts of purified VLPs were denatured, reverse transcribed, and amplified by two rounds of PCR amplification. The reverse transcription step was done with avian myeloblastosis virus RT (AMV-RT) in a 20-μl reaction mixture containing 10 mM Tris-HCl (pH 7.8), 50 mM KCl, 5 mM MgCl2, 1 mM each deoxynucleoside triphosphate (dNTP), 0.01 μg of RNA, 4 μM Ty1-specific primer spanning positions 249 to 270 of Ty1-H3 (primer 1; 5′CTTCTAGTATATTCTGTATACC3′), 20 U of AMV-RT, and 5 U of RNasin. Reverse transcription was done at 42°C for 60 min. After denaturation of AMV-RT at 95°C for 5 min, the reverse transcription mixture was adjusted to a final volume of 100 μl containing 10 mM Tris-HCl (pH 7.8), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM each dNTP, a 2 μM concentration of the appropriate primers, and 2.5 U of Goldstar DNA polymerase (Eurogentec). A first PCR round was performed with a primer complementary to the tRNAiMet-derived part of the reverse transcription product (oligodeoxynucleotide 65-IMT4 with the same sequence as that of nucleotides 1 to 24 of tRNAiMet [5′AGCGCCGTGGCGCAGTGGAAGCGC3′]) and with primer 1. PCR was performed with the following thermal profile: 1 min of denaturation at 94°C, 1 min of annealing at 56°C, and 30 s of elongation at 72°C repeated 25 times. A second round of PCR was done with a nested Ty1-specific primer spanning positions 295 to 315 of Ty1-H3 (primer 8; 5′TGGAATCCCAACAATTATCTC3′) and primer 65-IMT4. A 2-μl volume of the first PCR mix was used for the second round of PCR amplification. The thermal profile of the second PCR amplification was the same as that described above, except that the annealing temperature was 53°C. The PCR products were analyzed by gel electrophoresis on a 1% agarose gel. For each reaction, a control was done without AMV-RT.
Strong-stop DNA intermediate labeling in vitro.
Purified VLPs were incubated with [α-32P]dTTP, [α-32P]dATP, and all four unlabeled deoxyribonucleotide triphosphates under conditions that allowed for RT (50 mM Tris-HCl [pH 7.8], 50 mM KCl, 10 mM MgCl2, and 6 mM β-mercaptoethanol). Reaction products were deproteinized with proteinase K in the presence of 0.5% sodium dodecyl sulfate and run on a DNA sequencing gel.
Analysis of Ty1 VLP DNA by Southern blotting.
Extraction of DNA from VLPs, electrophoresis on agarose gels, blotting, and hybridization with a 5′-end-labeled oligonucleotide probe specific for the R region of plus-strand DNA intermediates were performed as described previously (14).
RESULTS
Transposition assay.
Transposition of the Ty1 element was studied in two yeast strains, AGY9 and ASB. AGY9 is an spt3 strain deficient for the transcription of endogenous Ty1 elements (21). ASB is a yeast strain in which all genomic tRNAiMet genes (IMT1 to IMT4) are disrupted (24). In this strain a wild-type IMT4 gene cloned on an extrachromosomal plasmid must be supplied to allow growth of the cells. To study the effects of mutations in tRNAiMet on transposition of Ty1 elements and to avoid competition between wild-type and mutant tRNAiMet, the wild-type IMT4 gene, which was cloned on a URA3-marked plasmid, was replaced by a mutant imt4 gene cloned on a LEU2-marked plasmid by selection on a medium containing 5-FOA, which selects against cells expressing the URA3-marked plasmid (Fig. 1). The cells surviving are those which have lost the URA3-marked plasmid containing the wild-type IMT4 gene and are able to grow in the presence of the mutant imt4 gene cloned onto the LEU2-marked plasmid. Yeast strains containing the wild-type or mutant tRNAiMet gene were then transformed by wild-type or mutant neomycin-marked Ty1 elements cloned onto a 2μm high-copy-number plasmid. The resulting transformants were assayed for transposition as described in Materials and Methods.
Mutations disrupting complementarity between the boxes of Ty1 RNA and primer tRNAiMet affect transposition of Ty1 elements.
The structural model of the Ty1 RNA-tRNAiMet complex previously described by Friant et al. (12) is presented in Fig. 2. In this model, the PBS and boxes 0, 1, and 2.1 anneal to complementary sequences in the T and D arms and loops of tRNAiMet. Box 2.2, which comprises 9 nucleotides complementary to the D region of tRNAiMet, is not annealed to primer tRNAiMet. We have introduced mutations into the boxes of Ty1 RNA or the portions of tRNAiMet complementary to the boxes in order to disrupt some Watson-Crick base pairs of the Ty1 RNA-tRNAiMet initiation complex. The nucleotide changes in tRNAiMet were made so that they would not impair its function as an initiator tRNA. Mutations in Ty1 RNA were made so that they either did not change its coding sequence or, in the case of the Ty1 B0G mutant, made a V-to-G amino acid change at position 20 of the TyA protein. The Ty1 RNA-tRNAiMet combinations tested are shown in Fig. 3. The levels of wild-type Ty1 RNA–mutant tRNA, mutant Ty1 RNA–wild-type tRNA, and mutant Ty1 RNA-mutant tRNA transposition relative to wild-type Ty1 RNA–wild-type tRNA transposition are listed in Table 1. A dramatic effect on transposition was observed when 2 or more base pairs of the Ty1 RNA-tRNAiMet complex were disrupted. In strain ASB, the transposition frequency was reduced five- to six-fold for double mutants in which 1 bp between box 0 and tRNAiMet and 1 bp between box 1 and tRNAiMet were disrupted. The same transposition defect was observed whether the nucleotide changes were done in the Ty1 RNA boxes (the Ty1 B0GB1G mutant) or in tRNAiMet (imt4-C54C60). Mutations disrupting only 1 bp of the primer-template complex had less effect on transposition; the lowest activity obtained for a point mutant (imt4-G54) was threefold lower than the wild type.
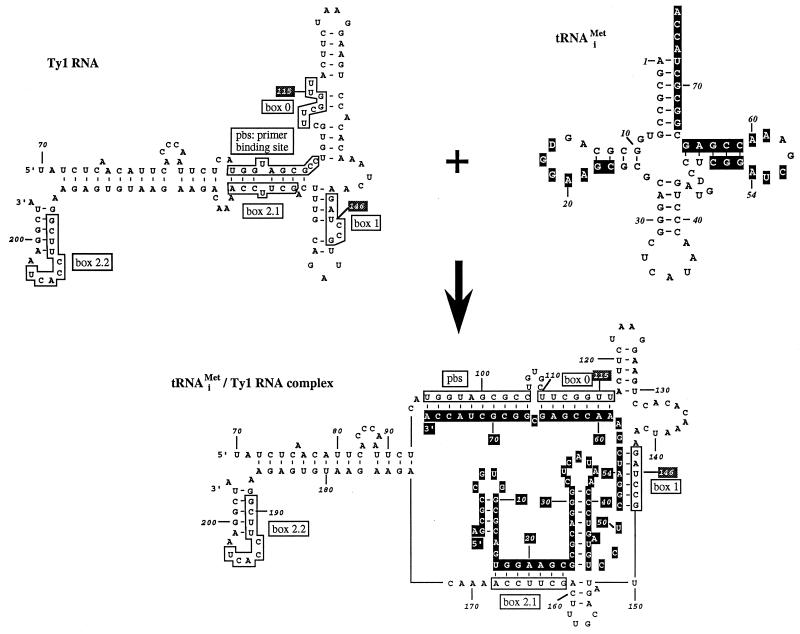
FIG. 2.
Structural model of Ty1 RNA, tRNAiMet, and the Ty1 RNA-tRNAiMet complex derived from the structural probing study described by Friant et al. (12). The PBS is complementary to 10 nucleotides at the 3′ end of tRNAiMet. Boxes 0, 1, 2.1, and 2.2 are complementary to parts of the T and D stems and loops of tRNAiMet. The regions of tRNAiMet complementary to the PBS and the boxes are shown in white on black in the secondary structure of tRNAiMet. In the Ty1 RNA-tRNAiMet complex, all of the nucleotides of tRNAiMet are white on black.
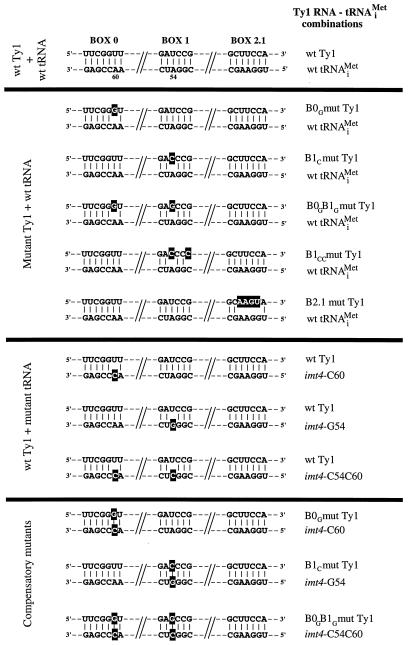
FIG. 3.
Complementarity between Ty1 boxes 0, 1, and 2.1 and tRNAiMet. Mutations (nucleotides in white on black) in Ty1 RNA or tRNAiMet that disrupt base pairing between the boxes and the complementary sequences of tRNAiMet impair transposition. The transposition defect is overcome when base pairing between mutant (mut) tRNAiMet and mutant Ty1 RNA is restored by compensatory mutations. Transposition frequencies of the Ty1 RNA-tRNAiMet combinations shown here are listed in Table 1. wt, wild-type.
The transposition frequencies of Ty1 RNA mutants in yeast strains ASB and AGY9 were compared. The results are qualitatively similar for the two strains. However, the transposition frequency of the mutants relative to the wild type was increased in strain AGY9. This is most striking for the Ty1 B0GB1G mutant, whose transposition frequency was reduced 100-fold in AGY9, whereas it was reduced only 6-fold in ASB. One difference between the two strains is that the transcription of endogenous chromosomal Ty elements was highly reduced in strain AGY9, whereas endogenous Ty elements were fully active in strain ASB. Thus, in yeast strain ASB, Ty1 RNA transcribed from the chromosomal DNA can be copackaged into VLPs with RNA produced from the Ty1-neo element cloned onto the 2μm vector. During RT of the RNA contained in these hybrid VLPs, minus-strand strong-stop DNA initiated on wild-type RNA transcribed from chromosomal RNA can be transferred intermolecularly on the mutant RNA and yield some preintegrative double-stranded DNA bearing the neomycin marker gene. This would explain the high background of G418-resistant colonies in strain ASB compared to strain AGY9.
Ty1 elements with mutations in boxes 2.1 and 2.2 were also tested for transposition in strain AGY9. Boxes 2.1 and 2.2 comprise, respectively, 7 (5′GCUUCCA3′) and 9 (5′GCUUCCACU3′) nucleotides complementary to the same region of tRNAiMet. The same mutations were done in the two boxes: 5′GCUUCCA3′ was changed to 5′GCAAGUA3′. Interestingly, mutations in box 2.1 had a severe effect on transposition, whereas mutations in box 2.2 only slightly affected transposition (relative transposition, 0.86). This result correlates with the fact that box 2.1 anneals to primer tRNAiMet in the model of the RT complex presented in Fig. 2, whereas box 2.2 does not interact with tRNAiMet despite its sequence similarity to box 2.1.
Compensatory mutations restore defects of Ty1 transposition.
To unambiguously demonstrate that the transposition defect observed with Ty1 RNA and tRNAiMet mutants is due to the disruption of base pairing between the Ty1 RNA boxes and the complementary sequences of tRNAiMet, mutant Ty1 elements were combined with mutant tRNAiMet in order to restore perfect complementarity between boxes 0 and 1 and the T loop region of tRNAiMet. In yeast strain ASB, imt4-C60 was combined with the Ty1 B0G mutant, imt4-G54 was combined with the Ty1 B1C mutant, and imt4-C54C60 was combined with the Ty1 B0GB1G mutant. In all cases, compensatory mutations restored transposition to near-wild-type levels (Table 1). This is illustrated in the qualitative transposition assay represented in Fig. 4; cells containing the wild-type element or the compensatory mutants exhibit a high level of Ty1 transposition, as indicated by growth on YPD-G418 plates, whereas cells containing the Ty1 double mutant alone or the tRNAiMet double mutant alone are markedly reduced in the number of G418-resistant colonies.
Compensatory mutations in strain AGY9 also were constructed and analyzed. Strain AGY9 was first transformed by a mutant Ty1 element (B0, B1, or B0B1), and transposition frequency was measured. The compensatory tRNAiMet mutant was then introduced into the same transformant to check for rescue of transposition. If transposition was restored, this would rule out that the Ty1 element had picked up a mutation elsewhere in Ty1. As indicated in Table 1, the relative transposition increased to 0.88 when the Ty1 B0GB1G mutant was combined with imt4-C54C60, compared to 0.01 in a strain containing the Ty1 B0GB1G mutant alone. When Ty1 B0G or Ty1 B1C mutants were combined with imt4-C60 or imt4-G54, the relative transposition increased to 0.90 and 0.93 compared to 0.30 and 0.33, respectively, for the Ty1 mutants alone. It is worth noting that the wild-type tRNAiMet present in strain AGY9 does not prevent the rescue of transposition by mutant tRNAiMet when combined with mutant Ty1 RNA. This suggests the absence of competition between the two tRNAs for binding to mutated Ty1 RNA and can be explained by our previous in vitro results (12) showing that annealing of wild-type tRNAiMet to Ty1 RNA bearing mutations in boxes 0 and 1 was abolished. These results demonstrate that base pairing between the boxes of Ty1 RNA and primer tRNAiMet is essential for Ty1 transposition and thus plays a role in one of the steps of the transposition pathway. The possibility that mutations in Ty1 RNA or tRNAiMet affect priming of DNA synthesis is analyzed below.
DNA synthesis is affected by mutations disrupting complementarity between the boxes of Ty1 RNA and tRNAiMet.
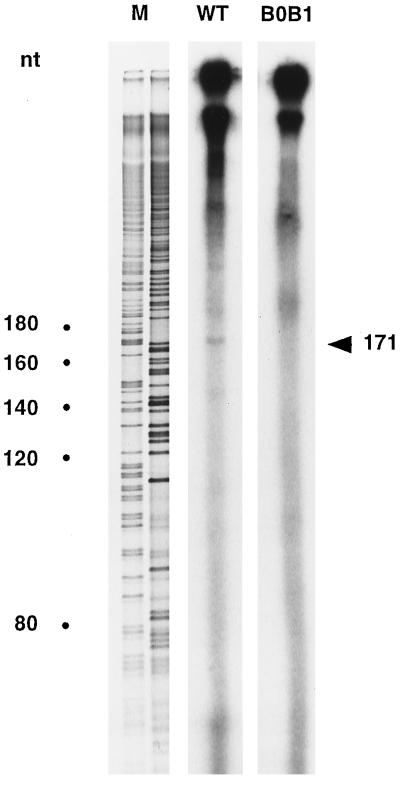
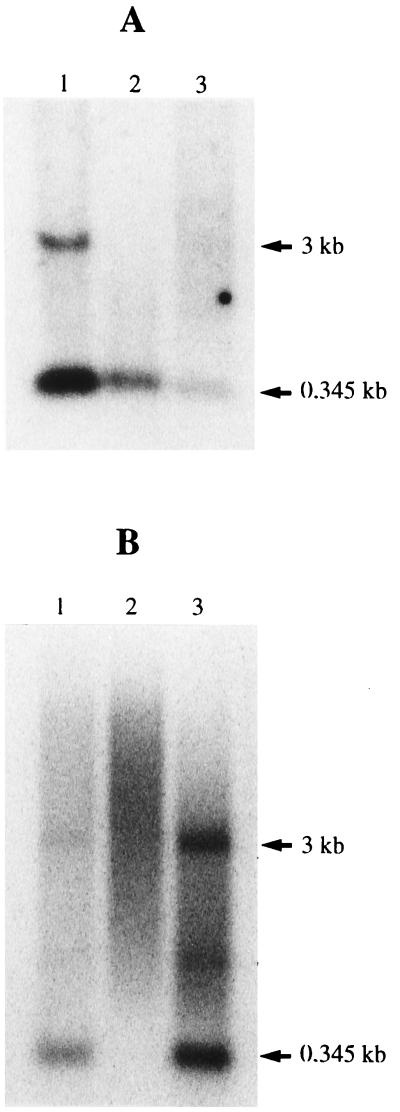
To determine if DNA synthesis is affected by mutations disrupting complementarity between the boxes of Ty1 RNA and tRNAiMet, an RT-PCR technique was used to detect minus-strand strong-stop DNA produced in wild-type or mutant VLPs. Nucleic acids extracted from purified wild-type or mutant VLPs produced in yeast strain AGY9 were amplified with a combination of primers (Fig. 5A), which allows specific amplification of minus-strand strong-stop DNA attached to its tRNAiMet moiety. As shown in Fig. 5B, lane 2, a very small amount of minus-strand strong-stop DNA was detected in a Ty1 double mutant (the Ty1 B0GB1G mutant) whose transposition frequency was reduced to background levels. Minus-strand strong-stop DNA synthesis was restored in a yeast strain harboring tRNAiMet with compensatory mutations (Fig. 5B, lane 3). To investigate whether the bands observed on the agarose gel were specific Ty1 minus-strand strong-stop DNA, the DNA was recovered from the agarose gel, cloned into plasmid pGEM-T (Promega), and sequenced. Our sequencing results (data not shown) unambiguously demonstrate that the 110-bp DNA fragments visualized on the agarose gel were generated by PCR amplification of the minus-strand DNA attached to the tRNAiMet primer. In particular, we found that the tRNAiMet double mutant is indeed attached to the minus-strand DNA in AGY9 yeast cells harboring the Ty1 B0GB1G mutant and the imt4-C54C60 mutant. The RT-PCR result was confirmed by examination of the Ty1 transposition intermediates labeled in vitro, as described by Chapman et al. (8). VLPs were purified from yeast cells transformed with plasmids bearing wild-type or B0GB1G mutant Ty1 elements and incubated with [α-32P]dTTP, [α-32P]dATP, and all four unlabeled deoxyribonucleotide triphosphates under conditions that allowed for RT. When this experiment was done with wild-type VLPs, a labeled species of the expected size (171 nucleotides, corresponding to 95 nucleotides of DNA covalently attached to the tRNAiMet primer of 76 nucleotides) was observed (Fig. 6). For the B0GB1G mutant VLP, the presence of minus-strand strong-stop DNA was not observed, in agreement with the result of the RT-PCR experiment. To confirm that DNA synthesis was affected by mutations in Ty1 RNA or tRNAiMet, we also determined the level of plus-strand DNA intermediates produced in VLPs by DNA blotting and hybridization with an oligodeoxyribonucleotide probe specific for plus-strand DNA. The levels of plus-strand DNA intermediates in yeast strains containing an imt4-C54C60 mutant or a Ty1 B0GB1G mutant and in yeast strains in which the Ty1 RNA and tRNAiMet bearing compensatory mutations were combined were determined (Fig. 7). Our results show that the levels of plus-strand strong-stop DNA produced in strain ASB (Fig. 7A) or AGY9 (Fig. 7B) containing the Ty1 or tRNAiMet mutant are very low (Fig. 7A, lanes 2 and 3, and Fig. 7B, lane 2). Compensatory mutations restore a wild-type level of plus-strand strong-stop DNA (Fig. 7A, lane 1, and Fig. 7B, lane 1). These results correlate with the transposition results and confirm that extended interactions between the Ty1 boxes and primer tRNAiMet are required for efficient DNA synthesis of the Ty1 element.
FIG. 5.
Minus-strand strong-stop DNA synthesized in Ty1 VLPs. (A) Nucleic acids extracted from purified VLPs are denatured. Only the genomic Ty1 RNA and the minus-strand strong-stop DNA are shown (a). The minus-strand strong-stop DNA attached to primer tRNAiMet is annealed to a specific primer (primer 1) complementary to positions 249 to 270 in the U5 region of Ty1-H3 (b). Minus-strand DNA and the attached tRNAiMet are reverse transcribed (c). A first PCR round is performed with primer 1 and a primer complementary to the tRNAiMet-derived part of the RT product (primer 65-IMT4) (d). A second round of PCR is done with a nested Ty1-specific primer (primer 8) complementary to positions 295 to 315 in the U5 region and primer 65-IMT4. (B) Ethidium bromide-stained agarose gel of the RT-PCR products of nucleic acids extracted from yeast strain AGY9 harboring a wild-type Ty1 element plus a wild-type IMT4 gene (lane 1), the Ty1 B0GB1G mutant plus a wild-type IMT4 gene (lane 2), and the Ty1 B0GB1G mutant plus the imt4-C54C60 mutant bearing compensatory mutations (lane 3). Lane C is a control without reverse transcriptase.
FIG. 6.
In vitro labeling of minus-strand strong-stop DNA. Wild-type (WT) and B0GB1G mutant VLPs were incubated with all four deoxyribonucleotide triphosphates, [α-32P]dATP, and [α-32P]dTTP under conditions that allowed for RT. Reaction products were deproteinized and run on a DNA sequencing gel. A sequencing reaction (lane M) was used to generate size markers (sizes are indicated in nucleotides [nt]). A 171-base species is observed with wild-type VLPs, whereas this molecule is not observed with B0GB1G mutant VLPs.
FIG. 7.
Plus-strand DNA synthesized in Ty1 VLPs. DNA extracted from purified VLPs was analyzed by Southern blotting with a radiolabeled probe specific for the R region. The 0.345-kb band is the plus-strand strong-stop DNA initiated at polypurine tract 1 (PPT1), and the 3-kb band is the plus-strand DNA initiated at PPT2. (A) Plus-strand DNA from yeast strain ASB harboring the Ty1 B0GB1G mutant plus the imt4-C54C60 mutant bearing compensatory mutations (lane 1), the Ty1 B0GB1G mutant (lane 2), and the imt4-C54C60 mutant (lane 3). (B) Plus-strand DNA from yeast strain AGY9 harboring the Ty1 B0GB1G mutant plus the imt4-C54C60 mutant bearing compensatory mutations (lane 1), the Ty1 B0GB1G mutant (lane 2), and the wild-type Ty1 element (lane 3).
DISCUSSION
In this study, we have demonstrated genetically that extended interactions between Ty1 RNA and primer tRNAiMet are required for Ty1 transposition. We have shown that nucleotide changes disrupting a few Watson-Crick base pairs between tRNAiMet and Ty1 RNA have a severe effect on Ty1 transposition. Compensatory mutations which restore complementarity between the two RNAs rescue Ty1 transposition. Rescue of transposition in strains combining mutant Ty1 RNA and mutant tRNAiMet that restores perfect base pairing between the primer and the template indicates that the mutant Ty1 RNA and tRNAiMet are both packaged into VLPs. Thus, the low transposition frequency of Ty1 mutants is not due to a packaging deficiency. In addition, according to Keeney et al. (16), tRNAiMet bearing transposition-inactivating mutations in the T region (at positions A60 or A64 and U50) does not show an encapsidation defect. We thus checked whether DNA synthesis was affected in the mutant VLPs. Analysis of DNA intermediates produced in mutant Ty1 VLPs indicates that priming of DNA synthesis is affected by these mutations, suggesting that formation of a specific complex between the tRNAiMet primer and the Ty1 genomic RNA is a prerequisite step in initiation of RT. In keeping with our results, Keeney et al. (16) have shown that mutations in the T arm and loop of tRNAiMet have dramatic effects on transposition. For two double mutants in which A60/A54 was changed to C60/T54 or T60/C54, the transposition frequency was reduced to background levels. These mutations disrupt base pairing between tRNAiMet and a U residue in box 0 and a U residue in box 1 of Ty1 RNA. This is consistent with our finding that Ty1 transposition is reduced in a B0GB1G mutant. Using interspecies hybrid initiator tRNA molecules, Keeney et al. (16) have implicated nucleotides in the D arm as additional recognition determinants. Here, we demonstrate that mutations in box 2.1 which disrupt interactions between Ty1 RNA and the D arm and loop of tRNAiMet affect transposition.
For several retroelements it has been proposed that interactions between the tRNA primer and RNA template are not limited to annealing of the 3′ end of the primer with the PBS but are extended to other regions of the tRNA primer and RNA template. It has been suggested that these extended primer-template interactions might be important for efficient initiation of RT. In vivo and in vitro studies of Rous sarcoma virus reveal that interactions between 7 bases of the T loop of primer tRNATrp and sequences in the U5 region of the retroviral RNA are required for efficient initiation of RT (9, 13) and that a specific secondary structure of the initiation complex is necessary for efficient RT (1, 2). Sequence and structure comparisons of the 5′ regions of several retroviral RNAs indicate that similar interactions may exist in other retroviruses (18). For human immunodeficiency virus type 1 (HIV-1), an enzymatic and chemical probing study of the conformation of the HIV-1 RNA-tRNA3Lys complex reveals a compact structure in which most of the anticodon loop, the 3′ strand of the anticodon stem, and the 5′ part of the variable loop of tRNA3Lys interact with viral RNA sequences 12 to 39 nucleotides upstream of the PBS (15). It has been suggested that these interactions produce a specific complex preferentially recognized by reverse transcriptase or may be involved in the annealing or encapsidation of the primer tRNA and therefore may play a role in initiation of RT.
Studies from several laboratories have established that HIV-1 can use different tRNA species to initiate RT if the PBS of the viral RNA is made complementary with the 3′-terminal 18 nucleotides of alternate tRNAs (10, 19, 26). However, the alternate tRNAs were not stable and the PBS reverted back to a wild-type sequence complementary to tRNA3Lys. Thus, it was assumed that complementarity between the primer tRNA and the PBS was not sufficient for preferential use of a tRNA in HIV-1 RT and that interaction between a region in the HIV-1 genome and the anticodon loop of tRNA3Lys was necessary to maintain the selective use of the primer tRNA. Wakefield et al. (25) have now been able to show that a proviral genome containing a PBS complementary to the 3′ end of tRNAHis and sequences upstream of the PBS complementary to the anticodon loop are able to maintain a PBS complementary to tRNAHis for over 4 months. This experiment clearly demonstrates that interactions between the primer tRNA anticodon loop and viral sequences in U5 contribute to the specificity of the tRNA used in RT in vivo.
Recently, a systematic mutagenesis analysis of the region including the PBS of Schizosaccharomyces pombe retrotransposon Tf1 also indicated that an extensive RNA structure is required for the cleavage reaction that generates the primer for Tf1 RT (20).
The finding that specific extended primer-template interactions are required for efficient initiation of RT of several retrotransposons and retroviruses suggests a common mechanism for replication of these retroelements. The extended interactions are certainly involved in the formation and stabilization of the primer-template complex. It is also very likely that the secondary or tertiary structure of the complex must be specifically recognized by reverse transcriptase to initiate RT. Further structural studies are now required to understand how the specific primer-template interacts with reverse transcriptase and to identify all of the molecular determinants involved in replication of these retroelements.
ACKNOWLEDGMENTS
We thank J. S. Lodmell for critical reading of the manuscript and T. M. Menees for suggesting that we sequence the PCR products.
This work was supported in part by grants from the Association pour la Recherche contre le Cancer (ARC) and from the Ligue contre le Cancer, Comité Départemental du Haut-Rhin. A. S. Byström was supported by grant BU-04856-309 from the Swedish Natural Science Research Council and grant 3516-B95-02XBB from the Swedish Cancer Society.
REFERENCES
- 1.Aiyar A, Cobrinik D, Zheng G E, Kung H-J, Leis J. Interaction between retroviral U5 RNA and the TYC loop of the tRNATrp primer is required for efficient initiation of reverse transcription. J Virol. 1992;66:2464–2472. doi: 10.1128/jvi.66.4.2464-2472.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiyar A, Ge Z, Leis J. A specific orientation of RNA secondary structure is required for initiation of reverse transcription. J Virol. 1994;68:611–618. doi: 10.1128/jvi.68.2.611-618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boeke J D, Eichinger D, Castrillon D, Fink G R. The Saccharomyces cerevisiae genome contains functional and nonfunctional copies of transposon Ty1. Mol Cell Biol. 1988;8:1432–1442. doi: 10.1128/mcb.8.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeke J D, Garfinkel D J, Styles C A, Fink G R. Ty elements transpose through an RNA intermediate. Cell. 1985;40:491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- 5.Boeke J D, Lacroute F, Fink G R. A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoroorotic acid resistance. Mol Gen Genet. 1984;197:345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- 6.Boeke J D, Sandmeyer S. Yeast transposable elements. In: Broach J R, Jones E W, Pringle J, editors. The molecular and cellular biology of the yeast Saccharomyces cerevisiae. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1991. pp. 193–261. [Google Scholar]
- 7.Bonneaud N, Ozier-Kalogeropoulos O, Li G, Labouesse M, Minvielle-Sebastia L, Lacroute F. A family of low and high copy replicative, integrative and single-stranded S. cerevisiae/E. coli shuttle vectors. Yeast. 1991;7:609–615. doi: 10.1002/yea.320070609. [DOI] [PubMed] [Google Scholar]
- 8.Chapman K B, Byström A S, Boeke J D. Initiator methionine tRNA is essential for Ty1 transposition in yeast. Proc Natl Acad Sci USA. 1992;89:3236–3240. doi: 10.1073/pnas.89.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordell B, Swanstrom R, Goodman H M, Bishop J M. tRNATrp as primer for RNA-directed DNA polymerase: structural determinants and function. J Biol Chem. 1979;254:1866–1874. [PubMed] [Google Scholar]
- 10.Das A T, Klaver B, Berkhout B. Reduced replication of human immunodeficiency virus type 1 mutants that use reverse transcription primers other than the natural tRNA3Lys. J Virol. 1995;69:3090–3097. doi: 10.1128/jvi.69.5.3090-3097.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eichinger D J, Boeke J D. The DNA intermediate in yeast Ty1 element transposition copurifies with virus-like particles: cell-free Ty1 transposition. Cell. 1988;54:955–966. doi: 10.1016/0092-8674(88)90110-9. [DOI] [PubMed] [Google Scholar]
- 12.Friant S, Heyman T, Wilhelm M L, Wilhelm F X. Extended interactions between the primer tRNAiMet and genomic RNA of the yeast Ty1 retrotransposon. Nucleic Acids Res. 1996;24:441–449. doi: 10.1093/nar/24.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haseltine W A, Kleid D G, Panet A, Rothenberg E, Baltimore D. Ordered transcription of RNA tumor virus genomes. J Mol Biol. 1976;106:109–131. doi: 10.1016/0022-2836(76)90303-x. [DOI] [PubMed] [Google Scholar]
- 14.Heyman T, Agoutin B, Friant S, Wilhelm F X, Wilhelm M L. Plus-strand DNA synthesis of the yeast retrotransposon Ty1 is initiated at two sites, PPT1 next to the 3′ LTR and PPT2 within the pol gene. PPT1 is sufficient for Ty1 transposition. J Mol Biol. 1995;253:291–303. doi: 10.1006/jmbi.1995.0553. [DOI] [PubMed] [Google Scholar]
- 15.Isel C, Ehresmann C, Keith G, Ehresmann B, Marquet R. Initiation of reverse transcription of HIV-1: secondary structure of the HIV-1 RNA/tRNA3Lys (template/primer) complex. J Mol Biol. 1995;247:236–250. doi: 10.1006/jmbi.1994.0136. [DOI] [PubMed] [Google Scholar]
- 16.Keeney J B, Chapman K B, Lauermann V, Voytas D F, Äström S U, von Pawel-Rammingen U, Byström A, Boeke J D. Multiple molecular determinants for retrotransposition in a primer tRNA. Mol Cell Biol. 1995;15:217–226. doi: 10.1128/mcb.15.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunkel T A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leis J, Aiyar A, Cobrinik D. Regulation of initiation of reverse transcription of retroviruses. In: Skalka A M, Goff S P, editors. Reverse transcriptase. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 33–47. [Google Scholar]
- 19.Li X, Mak E J, Arts Z, Gu L, Kleinman L, Wainberg M A, Parniak M A. Effects of alterations of primer binding site sequences on human immunodeficiency virus type 1 replication. J Virol. 1994;68:6198–6206. doi: 10.1128/jvi.68.10.6198-6206.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin J-H, Levin H L. A complex structure in the mRNA of Tf1 is recognized and cleaved to generate the primer of reverse transcription. Genes Dev. 1997;11:270–285. doi: 10.1101/gad.11.2.270. [DOI] [PubMed] [Google Scholar]
- 21.Mathias S L, Scott A F, Kazazian H H J, Boeke J D, Gabriel A. Reverse transcriptase encoded by a human transposable element. Science. 1991;254:1808–1810. doi: 10.1126/science.1722352. [DOI] [PubMed] [Google Scholar]
- 22.Pochart P, Agoutin B, Fix C, Keith G, Heyman T. A very poorly expressed tRNASer is highly concentrated together with replication primer initiator tRNAMet in the yeast Ty1 virus-like particles. Nucleic Acids Res. 1993;21:1517–1521. doi: 10.1093/nar/21.7.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varmus H. Retroviruses. Science. 1988;240:1427–1435. doi: 10.1126/science.3287617. [DOI] [PubMed] [Google Scholar]
- 24.von Pawel-Rammingen U, Aström S, Byström A S. Mutational analysis of conserved positions potentially important for initator tRNA function in Saccharomyces cerevisiae. Mol Cell Biol. 1992;12:1432–1442. doi: 10.1128/mcb.12.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakefield J K, Kang S M, Morrow C D. Construction of a type 1 human immunodeficiency virus that maintains a primer binding site complementary to tRNAHis. J Virol. 1996;70:966–975. doi: 10.1128/jvi.70.2.966-975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakefield J K, Wolf A G, Morrow C D. Human immunodeficiency virus type 1 can use different tRNAs as primers for reverse transcription but selectively maintains a primer binding site complementary to tRNA3Lys. J Virol. 1995;69:6021–6029. doi: 10.1128/jvi.69.10.6021-6029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilhelm M, Wilhelm F X, Keith G, Agoutin B, Heyman T. Yeast Ty1 retrotransposon: the minus-strand primer binding site and a cis-acting domain of the Ty1 RNA are both important for packaging of primer tRNA inside virus-like particles. Nucleic Acids Res. 1994;22:4560–4565. doi: 10.1093/nar/22.22.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]