Abstract
An experimental model for cytoplasmic organization is presented. We demonstrate dynamic control over protein distribution within synthetic cells comprising a lipid bilayer membrane surrounding an aqueous polymer solution. This polymer solution generally exists as two immiscible aqueous phases. Protein partitioning between these phases leads to microcompartmentation, or heterogeneous protein distribution within the “cell” interior. This model cytoplasm can be reversibly converted to a single phase by slight changes in temperature or osmolarity, such that local protein concentrations can be manipulated within the vesicle interior.
Keywords: aqueous phase separation, intracellular organization, vesicle
The interior of living cells is a crowded milieu of macromolecules, cytoskeletal filaments, and organelles. Even in cytoplasmic regions not separated by obvious barriers such as lipid membranes, differences in local composition are common. This phenomenon, referred to as microcompartmentation, is thought to have profound implications for cell function (1, 2). Understanding its role in living cells has been complicated by the lack of an experimental model system in which hypotheses could be tested. Even the mechanism(s) by which microcompartmentation is maintained remain unclear. Several possibilities have been proposed, including specific targeting and processes driven by macromolecular crowding, such as multiprotein complex formation, binding to intracellular surfaces, or phase separation (3). Aqueous phase separation occurs readily in bulk solutions of macromolecules even at much lower weight percents than are present in living cells (2). Thus, the question has been posed as to whether cytoplasm can exist without undergoing phase separation (4). Phase separation, and the accompanying partition of solutes between phases, could account for microcompartmentation of macromolecules, metabolites, and ions. Thus far, the complexity of living cells has precluded direct testing of the phase separation hypothesis.† We have encapsulated a poly(ethylene glycol) (PEG)/dextran aqueous two-phase system (ATPS) within lipid vesicles to construct synthetic cells capable of dynamic protein and nucleic acid microcompartmentation. Substantial local variations in protein concentration can be maintained in the absence of intervening membranous barriers within these ATPS-containing vesicles. Our synthetic cytoplasm is promising as an experimental model for intracellular organization in general and demonstrates that aqueous phase separation is a viable mechanism for microcompartmentation.
This work represents a bottom-up approach to understanding cell biology, in contrast to the top-down approach often adopted in biochemistry and perhaps best exemplified by efforts to generate the “minimal cell” through gene disruption in already simple organisms (6). Experimental model systems such as this one enable us to begin to test hypotheses in cell biology such as that of cytoplasmic phase separation. An analogy is lipid bilayer models of cell membranes. Differences in the diffusion of molecules in the membranes can be used to understand the role of anchored proteins and other barriers to lateral diffusion present in biological membranes but absent from the model systems (7). Model membrane studies gave researchers the ability to tease out the behaviors of these different populations and to test hypotheses in a simple, well characterized experimental system. The model cytoplasm presented here may yield similar insights into intracellular organization by separating the effects of mechanisms such as specific targeting from the non-specific biophysical consequences of macromolecular crowding and phase separation.
We have developed synthetic cells comprising two distinct aqueous phases within cell-sized lipid vesicles [or giant vesicles (GVs) (8)] as our experimental model system. In recent years, an increasing variety of materials have been encapsulated within lipid bilayer vesicles. For example, enzymatic reactions including replication and protein synthesis have been demonstrated within the aqueous interior of lipid vesicles (9-15). Cytoplasmic extracts captured within vesicles maintained enzymatic activity over several days when pore-forming proteins were incorporated in the bilayer (16). Biological polymers, such as the cytoskeletal components microtubules and actin networks, have been formed within vesicles after encapsulation of the precursor proteins (17). Several groups have also encapsulated nonbiological polymeric materials within lipid membranes, including agarose gels (18). Particularly notable is work in which pH- and ion-sensitive polymeric hydrogels coated with lipid bilayers were used to mimic secretory granules; these structures released drug molecules upon electroporation of the membrane (19). Thermo-responsive hydrogels have been microinjected into preformed GVs and GV networks, where they display characteristic sol-gel transitions (20). These hydrogel experiments were largely aimed at drug delivery but are also interesting in the present context in that their transitions are reversible, and the gel matrix mimics certain aspects of intracellular organization such as impeded diffusion. They have not been used for biomolecular microcompartmentation. Recently, temperature-induced release of fluorescent dye from smaller vesicles contained within GVs has been demonstrated by taking advantage of increased bilayer permeability during a lipid phase transition in the smaller vesicles (21). Although irreversible, this work is exciting in that it provides a route to molecular compartmentation within the larger vesicles, as well as local release without chemical caging, microinjection, or electroporation. We previously reported the encapsulation of an ATPS within GVs during synthesis by the “gentle swelling” method (22). In the present work we have used this approach to generate an experimental model of the biomacromolecular microcompartmentation that occurs in living cells even in the absence of intervening membranes.
Materials and Methods
Materials. PEG and dextran polymers, and biotin-conjugated dextran 10,000 Da were purchased from Sigma-Aldrich. Fluorescein-PEG-(N-hydroxylsuccinimide) (fluorescein-PEG-NHS) and biotin-conjugated PEG 5,000 Da were from Shearwater Polymers. Lipids 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DOPE-PEG 2000), 1,2-dioleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (sodium salt) (DOPG), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (DOPE-Rhod), and l-α-phosphatidyl choline (egg PC) were purchased as CHCl3 solutions from Avanti Polar Lipids. The Alexa Fluor 488-conjugated lectins soybean agglutinin (SBA), phytohemagglutinin (PHA-L), and Con A were purchased from Molecular Probes. Fluorescein-conjugated streptavidin was purchased from Pierce. Water used for ATPS preparation and titrations was deionized to a resistance of 18 MΩ (NANOpure Diamond, Barnstead).
Bulk Polymer Solutions and Analysis. ATPS phase diagrams were determined by cloud point titration for each polymer combination in H2O (or buffer) as described by Albertsson (23). Data sets were collected as a function of temperature and verified by preparing bulk solutions at various locations on the phase diagram and observing whether phase separation occurred. When working very close to the binodal curve, small changes in polymer composition that occur between different polymer lot numbers can result in significant alterations in the temperature-dependent phase behavior of the ATPS (i.e., a composition that is phase-separated when using one batch may not be when switching to a new batch). Therefore, we routinely generated new phase diagrams for each new batch of each polymer that we used.
Bulk partitioning of biomolecules and polymers between the PEG and dextran phases was determined by fluorescence analysis with a Jobin Yvon Horiba FL3-21 fluorimeter.
Synthetic Cell Preparation. Lipid vesicles containing the ATPS solution were prepared similarly to our previously reported modification (22) of standard lipid hydration procedures (24, 25). A key difference is that we did not routinely place synthetic cells in sucrose solutions before observation, as in our previous work (22). CHCl3 lipid solutions were added to 10 × 75-mm test tubes (Durex borosilicate glass, VWR Scientific) in the proper molar ratio as noted in the text; additional CHCl3 (100 μl) was added to ensure homogeneity. The test tube was rapidly rotated by hand under dry Ar gas to form a thin lipid film. The film was desiccated under vacuum in the dark for at least 2 h to remove residual organic solvent. Next, Ar gas was bubbled through H2O and gently blown into the test tube for 5 min to prehydrate the lipid film. After equilibrating at the proper temperature to yield a homogeneous one-phase solution for ≈5 min, 990 μl of the polymer solution was added to the test tube along with 10 μl of a stock solution of either a fluorescently labeled protein or polymer. As in the case of bulk experiments, each stock solution contained 0.1 wt/wt % of the fluorophore and 0.01 wt/wt % NaN3. For confocal microscopy experiments, the fluorophore stock solution concentration was typically 1 wt/wt % to increase the signal-to-noise ratio of the fluorophore-conjugated polymer or protein in the vesicles. The mixture was allowed to stand overnight in the dark maintained at either 37°C or 50°C for self-assembly. Afterward, the vesicle solution was allowed to stand at room temperature for 10 min and then cooled to 3°C or room temperature for at least 1 h. At this time, vesicles collected at the bulk ATPS interface.
Microscopy. Sample chambers were constructed by placing a 20 × 5-mm silicon spacer (Molecular Probes) onto a microscope slide. ATPS-containing vesicle samples were prepared by placing an aliquot of the top PEG-enriched phase from the original bulk polymer solution (lipid- and fluorophore-free) into the open sample cell, followed by an aliquot of liposome-rich sample from the bulk ATPS interface. The sample cell was then sealed with a coverslip before analysis.
Optical and fluorescence images were collected on a Nikon TE 300 inverted microscope by using a Nikon Plan Fluor ×100 oil objective (1.3 numerical aperture). Light was collected with a CoolSNAP HQ charge-coupled device camera (Photometrics, Tucson, AZ; 1,392 × 1,040 pixels) and acquired with image pro plus v.4.5 (MediaCybernetics, San Diego). Heating and cooling was managed with a Linkam PE-100 microscope stage, using a Linkam PE-94 control unit (±0.1°C). The exact temperature of the microscope stage was measured by using a microprobe (model IT-21, Harvard Apparatus; ±0.1°C) and a Physitemp BAT-12 readout unit. The heating/cooling rate was 10°C/min. The microscope stage was connected to a VWR Scientific circulating water bath (model 1160A) filled with distilled water set to 4°C. Final images were processed with photoshop v.7.0 (Adobe Systems, San Jose, CA).
Confocol microscopy was performed on an Olympus IX-70 inverted microscope, using a Plan Apo ×60 oil objective (1.4 numerical aperture). Images were collected and processed with fluoview v.4.3 (Olympus). An average fluorescence background was subtracted from PEG- and dextran-rich phase intensities before ratioing to determine K. The temperature of the microscope slide was maintained at 5°C by using the above-mentioned microscope stage and attached water bath.
Statistical Analysis. scion image v.4.0.2 (Scion, Frederick, MD) was used to measure the volume of each phase relative to the overall volume of the vesicle from DIC microscopy images. The vesicles in this case were composed of egg PC:DOPG:Rhod-DOPE (9:1:0.01) prepared in a bulk polymer phase consisting of 4.0 wt % PEG (8,000) and 3.5 wt % dextran (505,000) in H2O at 55°C. Microscopy images were collected at 21°C. At least 15 separate DIC images were collected from various parts of an undiluted sample; in conjunction, rhodamine fluorescence images were also collected to determine whether the vesicles were unilamellar. Each set of corresponding images was analyzed by eye to determine the ATPS vesicles that would be measured by selecting only vesicles that appear to be unilamellar in the fluorescence image. The Profile Plots tool in scion image was used to plot the brightness intensity changes across individual vesicles. The line width of the plot tool was set to 4 pixels, and the two distances corresponding to the 180° edge-to-edge vesicle diameter and inner phase volume were noted as shown in Fig. 5, which is published as supporting information on the PNAS web site. It was not always the case that a single measurement provided both pieces of information, depending on the orientation of the inner phase within the vesicle. The pixel values were used to determine the relative volumes by converting standardized scaling values at ×100 magnification. A histogram was prepared from six different data sets consisting of three separate ATPS preparations having two separate vesicle preparations for each.
Osmotically Controlled Reversible Phase Separation. ATPS-containing GVs were prepared by using modification of the prcedure of Akashi and coworkers (24). Vesicles were prepared by drying a mixture of 90 μl of egg PC (10 mg/ml) with 5 μl of DOPG (20 mg/ml) in CHCl3 along with 1 μl of PE-Rhod (0.2 mg/ml) under Ar. The lipids were desiccated under vacuum for 6 h before hydration overnight at 50°C with the fluorescently labeled ATPS (4.0% PEG 8,000/3.5% dextran 505,000). After hydration, the lipid solutions were cooled to room temperature, and the pink liposome “cloud” was collected via pipette from the interface between the phase-separated polymer layers and used to prepare samples for observation by microscopy.
In a typical experiment, 40 μl of ATPS vesicle solution was pipetted onto a 22 × 50-mm glass coverslip (Fisher) fitted with a 13 × 0.12-mm adhesive spacer (Molecular Probes) and immediately transferred to a sealed glass Petri dish containing wet filter paper. This chamber allowed the vesicles to settle to the bottom of the coverslip for observation without drying out. After the transfer of the sample to the microscope, the addition of 40 μl of water into the sample solution resulted in the formation of a single homogeneous phase within the vesicle interior. This process could be reversed through the addition of 5-40 μl of a 0.5 M sucrose solution.
DNA Compartmentation. Synthetic cells were prepared as described above for osmotically controlled experiments but with 0.2 μM DNA in the aqueous phase during vesicle swelling. The 88-base DNA oligonucleotide was synthesized in-house (Expedite 8909, Applied Biosystems) and had the sequence 3′-TAC GAC TTG AGA ACA CAG ACG TAC TAT CAT TGA CGC ATC AGA CAA CGT GCG TCA AAA ATT ACG TGC GGA AGG AGT TAT CCT GAA TGC G-5′-6FAM.
Results and Discussion
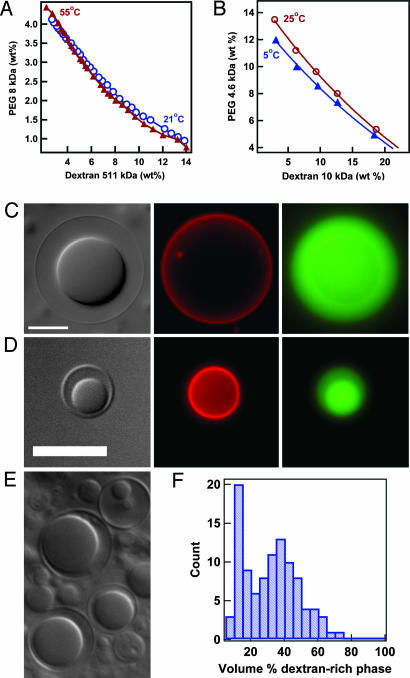
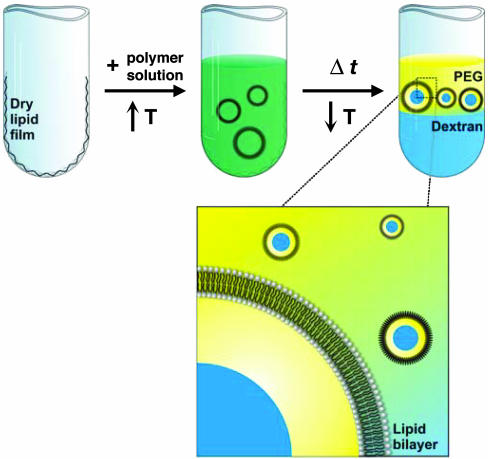
Our model cytoplasm is an aqueous polymer solution that mimics the crowded internal environment of living cells (26) and is capable of phase separation. Because macromolecular crowding is largely a physical phenomenon due to excluded volume, essentially any polymer can be used to mimic the crowding effects of cytoplasm. We have chosen the neutral polymers, PEG and dextran, which form well characterized ATPS at concentrations above a few wt % (27-30). PEG and dextran are also commonly used crowding reagents for in vitro biochemical investigations (26-28), such as those demonstrating crowding-induced changes in DNA hybridization (29), enzyme complex formation (30), and protein folding, association, and aggregation (31). To incorporate this model cytoplasm within GVs, we identified conditions under which the ATPS could be converted to a single phase (22). Phase diagrams for two different PEG/dextran systems are shown in Fig. 1 A and B. In these diagrams, phase separation is observed for compositions above the curve, whereas compositions below the curve exist as a single phase (23, 32-34). The location of this curve, referred to as the binodal, can be altered by changing the temperature of the system (22). In principle, any composition that lies between binodals for a given polymer system at two different temperatures can be encapsulated within GVs as shown in Scheme 1.
Fig. 1.
Encapsulation of APTSs. (A and B) Phase diagram for dextran 511 kDa/PEG 8 kDa and dextran 10 kDa/PEG 4.6 kDa ATPS in bulk solution. (C) Optical microscope images of synthetic cell formed at 55°C in a bulk ATPS with 3.24% dextran 511 kDa and 4.17% PEG 8 kDa, and imaged at 21°C. The three panels are transmitted light (DIC) (Left), rhodamine-tagged lipid fluorescence (Center), and Alexa Fluor 488-tagged PEG (Right). (D) Optical microscope images of synthetic cell formed at 37°C in a bulk ATPS with 9.375% PEG 4.6 kDa and 9.375% dextran 10 kDa, 5 mM phosphate buffer (pH 7.0), and imaged at 4°C. Lipid composition was 44.3:1 mol ratio DOPC/DOPE-PEG 2,000. The three panels are transmitted light (DIC) (Left), rhodamine-tagged lipid fluorescence (Center), and Alexa Fluor 488-tagged dextran fluorescence (Right). (E) Transmitted light image (DIC) showing variation in encapsulated polymer composition. (F) Histogram for estimated percent dextran-rich phase. Multiple batches of ATPS-containing vesicles are included in this data set, each prepared at 55°C in a bulk ATPS with 4.0% dextran 505 kDa and 3.5% PEG 8 kDa, and imaged at 21°C. (Scale bars, 10 μm.)
Scheme 1.
Using phase diagrams such as those shown in Fig. 1 A and B, we have developed several ATPS compositions for encapsulation, with phase-transition temperatures ranging from ≈4°C to 50°C. Vesicles are prepared at elevated temperature, where the polymer solution exists as a single phase.‡ Upon cooling, phase separation occurs both in the bulk and within the vesicles. The resulting structures are visualized by optical microscopy. Fig. 1C shows images of an ATPS-containing GV, or synthetic cell, in which both the lipid membrane and the PEG have been fluorescently tagged. Lipids were labeled with a rhodamine tag; thus, the absence of rhodamine fluorescence from the region between the two phases confirms that this is a single vesicle containing both aqueous phases. The dextran-rich phase, as determined by fluorescence from Alexa Fluor 488-labeled dextran, is the interior phase, and the PEG-rich phase is located adjacent to the lipid bilayer.
As is typical for preparations of giant unilamellar vesicles by the “gentle swelling” method used here (24, 35), we observe a variety of sizes and morphologies of vesicles in our synthetic cell preparations (Fig. 6, which is published as supporting information on the PNAS web site). Multilamellar vesicles, in which many layers of lipid are present, and vesicles in which the two aqueous phases are separated by a lipid bilayer can be readily distinguished from the desired ATPS-containing vesicles by inclusion of rhodamine-tagged lipids. Single-phase vesicles are also observed (i.e., those containing only a dextran- or PEG-rich phase). These can be identified in the transmitted light [differential interference contrast (DIC)] images. Thus, it is possible to estimate the percentage of vesicles having the desired morphology. For the sample corresponding to Fig. 1 C and E, approximately half of all GVs formed contained ATPS where no lipid bilayer separated the two aqueous phases. Of these, ≈40% were unilamellar vesicles, whereas the rest had multiple bilayers and/or additional interior lipid structures. Although these vesicles were formed while the ATPS existed as a single phase, they do not harbor identical contents, as evidenced by variation in the relative volumes of the phases (Fig. 1F). This heterogeneity may result from preferential encapsulation of one polymer over the other during vesicle formation, spatial variations in polymer concentration during swelling as a result of our proximity to the critical point of the ATPS, or changes in vesicle morphology/contents after formation (e.g., “budding”).
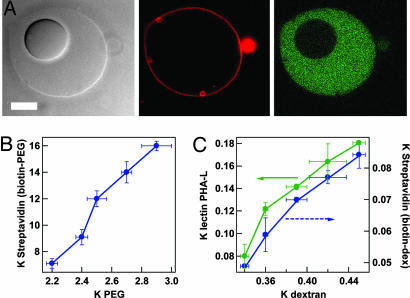
The two encapsulated phases contain different polymer concentrations and constitute separate microcompartments within the synthetic cells. We have used affinity partitioning to concentrate several different proteins within these microcompartments. Fig. 2A shows the location of fluorescently tagged streptavidin in a vesicle containing biotinylated PEG. The protein concentration in the PEG-rich outer phase is much higher than in the dextran-rich inner phase. Partitioning within these microvolumes was quantified by using confocal fluorescence microscopy images such as those in Fig. 2 A. The partition coefficient, K, in an ATPS is defined as the concentration of a solute in the top phase divided by its concentration in the bottom phase (23, 34). In bulk PEG/dextran systems, the PEG-rich phase is the upper phase. Thus, in our work large K values indicate a preferential accumulation in the PEG-rich phase, whereas fractional values indicate a preference for the dextran-rich phase. Partition coefficients for PEG and dextran polymers, as well as several proteins both in bulk ATPS and in the synthetic cells, are reported in Table 1. In all cases, we observe less dramatic partitioning for encapsulated ATPS (i.e., K closer to 1). Nonetheless, substantial differences in local protein concentration can be maintained in this cytoplasm mimic (e.g., a 4-fold difference for Con A or a nearly 7-fold difference for streptavidin in the presence of biotinylated PEG). The observed differences in partitioning between bulk solution and the vesicles can be at least partially explained based on differences in the polymer composition of the bulk and microvolumes. Fig. 2 B and C shows the dependence of protein partitioning on polymer composition in bulk solutions. When ATPS composition is adjusted to give polymer K values that match those measured in the vesicles (Table 2, which is published as supporting information on the PNAS web site), bulk protein K values also approach those observed for the vesicles.
Fig. 2.
Protein partitioning. (A) Optical microscope images of synthetic cell formed as in Fig. 1D, except that 0.004 wt % of the PEG has been biotinylated. Images were acquired on a confocal microscope at 4°C. (Left) Transmitted light. (Center) Rhodamine-tagged lipid fluorescence. (Right) Fluorescein-tagged streptavidin fluorescence. (Scale bar, 10 μm.) (B and C) Effect of ATPS composition on protein partitioning. ATPS composition is represented here by the partition coefficients for the PEG and dextran polymers, respectively.
Table 1. Macromolecule partitioning in bulk and in synthetic cells.
| Molecule | K in bulk* | K in vesicle (no. measured)*† |
|---|---|---|
| PEG 5,000 | 4.8 ± 0.11 | 1.7 ± 0.37 (22) |
| Dextran 10,000 | 0.28 ± 0.005 | 0.40 ± 0.11 (32) |
| Streptavidin | 1.7 ± 0.12 | 1.8 ± 0.40 (15) |
| Streptavidin with biotinyl PEG‡ | 39 ± 3.7 | 6.7 ± 2.9 (22) |
| Streptavidin with biotinyl dextran§ | 0.035 ± 0.0034 | 0.27 ± 0.086 (16) |
| PHA-L (lectin) | 0.048 ± 0.0033 | 0.26 ± 0.038 (31) |
| SBA (lectin) | 0.072 ± 0.0089 | 0.35 ± 0.13 (15) |
| Con A (lectin) | 0.030 ± 0.0033 | 0.33 ± 0.12 (20) |
ATPS composition: 9.375 wt/wt % PEG 4,600 Da/9.375 wt/wt % dextran 10,000 Da prepared in 5 mM phosphate buffer. Lipid composition for GVs: 44.3:1 mol ratio DOPC/DOPE-PEG 2,000.
Determined by confocal fluorescence microscopy. Because there is no top and bottom phase in the vesicles, K in vesicles is reported as concentration of the macromolecule in the PEG-rich phase divided by the concentration in the dextran-rich phase.
A total of 0.004 wt % of the PEG in this ATPS was biotinylated.
A total of 0.004 wt % of the dextran in this ATP5 was biotinylated.
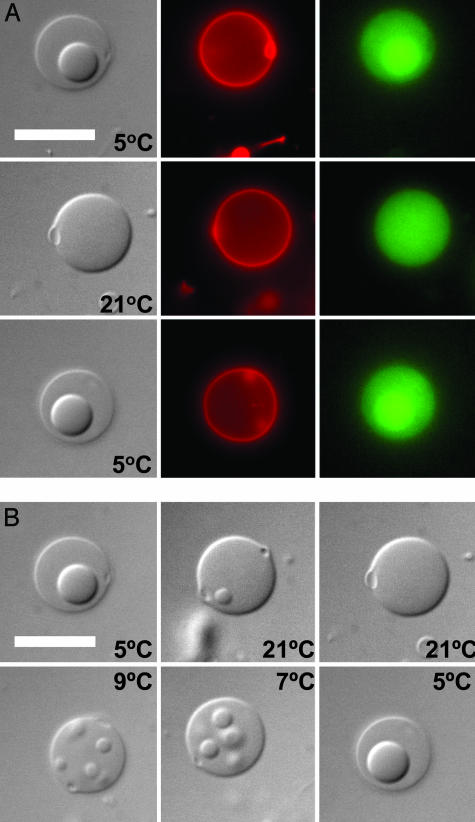
Microcompartmentation within living cells is not static; the distribution of intracellular components changes throughout the cell cycle and in response to stimuli (1, 2). Our model cytoplasm based on aqueous phase separation can provide dynamic alterations in local concentrations. Reversible microcompartmentation of the lectin, SBA, is shown in Fig. 3. The biological function of this protein is binding carbohydrates (36); it has an affinity for the dextran polymer and partitions into the dextran-rich phase of the vesicles with K = 0.35 ± 0.13. Fluorescence microscopy shows the location of the labeled protein before, during, and after a temperature-induced phase change (Fig. 3A). When these synthetic cells are heated, their aqueous interiors convert to a single phase in which both polymers are homogeneously mixed, and the SBA is uniformly distributed.§ This process is reversible; cooling results first in the formation of several small phases, which then merge to form two phases indistinguishable from their initial state (Fig. 3B; see also Fig. 7, which is published as supporting information on the PNAS web site). Thus, our data for PEG/dextran ATPS within vesicles point to a coalescence mechanism, such as has been recently proposed for the single-component α-elastin ATPS (37). We have performed similar experiments with other proteins, including two other lectins, phytohemagglutinin and Con A (Figs. 8 and 9, which are published as supporting information on the PNAS web site). For all three proteins, microcompartments having a 3- to 4-fold difference in protein concentration could be maintained or eliminated in response to an external stimulus (in this case, temperature). In contrast to bulk ATPS, the vesicle-encapsulated ATPS undergoes a more rapid phase transition and mixing because of the much smaller volumes and larger relative contact areas between the phases; conversions in microscale ATPS are complete in a few minutes, whereas hours to days can be required for unstirred bulk (5- to 10-ml total volume) ATPS of the same composition.¶
Fig. 3.
Reversibility of protein microcompartmentation. (A) Alexa Fluor 488-tagged protein (lectin SBA) partitions to the dextran-rich phase at 5°C (Top), 21°C (Middle), and 5°C (Bottom). (Left) Transmitted light (DIC). (Center) Rhodamine-tagged lipid fluorescence. (Right) Alexa Fluor 488-tagged SBA protein fluorescence. (B) Intermediate structures during heating and cooling. Synthetic cells were prepared as in Fig. 1D, with SBA added during formation. (Scale bar, 10 μm.)
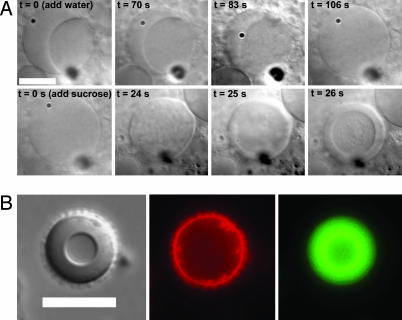
Phase separation in the model cytoplasm described here is sensitive to any condition that impacts the phase behavior of the ATPS, and the semipermeable lipid bilayer membrane provides a route to alter the interior composition of preformed vesicles. Thus, it is also possible to manipulate these structures by changing the external osmotic pressure of the solution, resulting in concentration or dilution of both polymers and a shift in the binodal to favor or disfavor phase separation, respectively. We have used hypo- and hypertonic external solutions to drive phase transitions within these synthetic cells. Fig. 4A shows the response of an ATPS-containing vesicle to increased internal volume upon addition of H2O to the external solution. The dextran-rich phase appears to grow at the expense of the PEG-rich phase, and finally the phase boundary disappears. Subsequent addition of a hypertonic sucrose solution causes phase separation. Interestingly, the relative positions of the two phases are reversed when hypertonic sucrose solutions are added to the external media. In the as-prepared structures, the PEG-rich phase borders the lipid membrane; upon exposure to sucrose solutions, this phase moves to the center and the dextran-rich phase borders the membrane. This may result from the fact that osmotically driven dehydration of the synthetic cells increases the concentration of both polymers and thus alters the interfacial tensions between the two phases as well as between each phase and the lipid membrane.
Fig. 4.
Osmotic pressure can modulate phase behavior and partitioning. (A) Osmotic pressure-induced phase transformation within synthetic cells. Addition of H2O to GV containing two aqueous phases results in conversion to a single-phase system. Subsequent addition of a hypertonic sucrose solution to the external medium causes phase separation. (B) DNA microcompartmentation in a synthetic cell. (Left) Transmitted light image (DIC). (Center) Rhodamine-tagged lipid. (Right) Alexa Fluor 488-tagged 88-mer ssDNA, which is partitioned into the dextran-rich phase. This vesicle has been immersed in a hyperosmotic sucrose solution to improve partitioning. Lipid and ATPS compositions for these vesicles are similar to those in Fig. 1C and are given in Materials and Methods. (Scale bars, 10 μm.)
Changes in the extent of microcompartmentation also occur because of changes in the partitioning of molecules between existing phases, which can be triggered by more subtle changes in temperature, cell volume, or other stimuli. We have taken advantage of osmotically driven dehydration to increase nucleic acid partitioning in synthetic cells after encapsulation. Fig. 4B shows an ATPS-containing vesicle in which single-stranded DNA oligonucleotides are partitioned into the dextran-rich phase. The DNA partitioning within these vesicles (K = 0.5 ± 0.1) was improved over that for bulk solutions (K = 0.86 ± 0.01) by dehydrating in an external sucrose solution after vesicle formation. Other factors, such as the concentration of various ions or small molecules, are also capable of shifting the position of the binodal curve and altering partitioning and/or phase behavior (29-32). In living cells one might expect such transitions in response to import or efflux of specific ions or molecules, volume changes, or cytoskeletal protein polymerization. Incorporation of ion channels or other membrane transporters in the lipid bilayer of the ATPS-containing vesicles described here could provide analogous molecule-selective response.
Conclusions
Aqueous phase separation within synthetic cells can result in the dynamic microcompartmentation of proteins and nucleic acids. If phase separation occurs in living cells, proteins themselves would act as the phase-forming polymers, and the thousands of different biomacromolecules present (38) would result in multiple phases. The number of phases could approach the number of major biomacromolecular components; Albertsson has prepared 15-phase systems from appropriate mixtures of 15 different polymers in water (23). These phases would wet the cytoskeletal network, plasma membrane, and organelles according to their volume and composition. In addition, strong and weak intermolecular binding events would modulate the composition and relative locations of the phases. This complexity has made the assessment of phase separation as a mechanism for microcompartmentation in living cells difficult. Our results for a simple polymer-based cytoplasm mimic suggest that aqueous phase separation is a viable mechanism for, and could contribute to, microcompartmentation in living cells. The synthetic cells introduced here provide an experimental model system in which the mechanisms and functional significance of microcompartmentation for processes such as sequential enzymatic reactions, protein folding, and DNA condensation can be investigated.
Supplementary Material
Acknowledgments
We thank Andrew Ewing for helpful comments on the manuscript and the National Science Foundation (Career Award 0239629), the Arnold and Mabel Beckman Institute, the Alfred P. Sloan Foundation, and Pennsylvania State University for financial support of this work. Confocal microscopy images were obtained at the Center for Quantitative Cell Analysis, a shared facility of the Huck Institutes of the Life Sciences at Pennsylvania State University.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ATPS, aqueous two-phase system; DIC, differential interference contrast; GV, giant vesicle; PEG, poly(ethylene glycol); SBA, soybean agglutinin.
See Commentary on page 5901.
Footnotes
Phase separation has been observed in eye lens cytoplasm. This is an interesting exception in that the eye lens is negatively impacted by phase separation, which causes scattering in the otherwise transparent cytoplasm of these cells. Small changes in composition such as occur during normal aging lead to phase separation in this system, resulting in opacity and vision loss (5).
For some polymer compositions in Fig. 1 A, the 55°C binodal is below the 21°C binodal. This binodal crossing is common to many of the PEG/dextran ATPSs we have studied and suggests that synthetic cells can also be prepared at lower temperatures and warmed to induce phase separation. For all of the experiments described here, we have selected polymer compositions that phase separate upon cooling.
Reversible phase transitioning within these synthetic cells requires appropriate osmotic matching of their interior and exterior solutions. This is accomplished by using the top phase of the ATPS (fluorophore- and lipid-free) to disperse the ATPS-containing vesicles gathered from the interface after their preparation. This dispersal is important for observation purposes, because one of the polymer phases is fluorescently tagged, and its presence in the external solution would result in high fluorescence backgrounds.
The time necessary for completion of bulk ATPS phase transitions depends on composition. For the dextran 10 kDa/PEG 4.6 kDa ATPS used for the experiments in Fig. 3, warm bulk solutions become cloudy after just a few minutes at 4°C and after several hours exist as two clear phases. Conversion from two to one phase requires considerably longer; samples left at 25°C for several days remain phase-separated unless stirred (agitation of the sample results in a rapid phase change).
References
- 1.Jones, D. P., ed. (1988) Microcompartmentation (CRC, Boca Raton, FL).
- 2.Walter, H. & Brooks, D. E. (1995) FEBS Lett. 361, 135-139. [DOI] [PubMed] [Google Scholar]
- 3.Pagliaro, L. (2000) Int. Rev. Cytol. 192, 303-318. [DOI] [PubMed] [Google Scholar]
- 4.Brooks, D. E. (2000) Int. Rev. Cytol. 192, 321-330. [DOI] [PubMed] [Google Scholar]
- 5.Clark, J. I. & Clark, J. M. (2000) Int. Rev. Cytol. 192, 171-187. [DOI] [PubMed] [Google Scholar]
- 6.Koonin, E. V. (2000) Annu. Rev. Genomics Hum. Genet. 1, 99-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saxton, M. J. (1999) Curr. Top. Membr. 48, 229-283. [Google Scholar]
- 8.Luisi, P. L. & Walde, P., eds. (2000) Giant Vesicles, Perspectives in Supramolecular Chemistry (Wiley, New York), Vol. 6.
- 9.Chiu, D. T., Wilson, C. F., Ryttsen, F., Stromberg, A., Farre, C., Karlsson, A., Nordholm, S., Gaggar, A., Modi, B. P., Moscho, A., et al. (1999) Science 283, 1892-1895. [DOI] [PubMed] [Google Scholar]
- 10.Walde, P. & Ichikawa, S. (2001) Biomol. Eng. 18, 143-177. [DOI] [PubMed] [Google Scholar]
- 11.Fischer, A., Franco, A. & Oberholzer, T. (2002) Chembiochem 3, 409-417. [DOI] [PubMed] [Google Scholar]
- 12.Oberholzer, T., Nierhaus, K. H. & Luisi, P. L. (1999) Biochem. Biophys. Res. Commun. 261, 238-241. [DOI] [PubMed] [Google Scholar]
- 13.Yu, W., Sato, K., Wakabayashi, M., Nakaishi, T., Ko-Mitamura, E. P., Shima, Y., Urabe, I. & Yomo, T. (2001) J. Biosci. Bioeng. 92, 590-593. [DOI] [PubMed] [Google Scholar]
- 14.Nomura, S.-i., Tsumoto, K., Hamada, T., Akiyoshi, K., Nakatani, Y. & Yoshikawa, K. (2003) Chembiochem 4, 1172-1175. [DOI] [PubMed] [Google Scholar]
- 15.Monnard, P.-A. (2003) J. Membr. Biol. 191, 87-97. [DOI] [PubMed] [Google Scholar]
- 16.Noireaux, V. & Libchaber, A. (2004) Proc. Natl. Acad. Sci. USA 101, 17669-17674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotani, H., Nomura, F. & Suzuki, Y. (1999) Curr. Opin. Coll. Interfac. Sci. 4, 358. [Google Scholar]
- 18.Viallat, A., Dalous, J. & Abkarian, M. (2004) Biophys. J. 86, 2179-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiser, P. F., Wilson, G. & Needham, D. (1998) Nature 394, 459-462. [DOI] [PubMed] [Google Scholar]
- 20.Jesorka, A., Markstrom, M. & Orwar, O. (2005) Langmuir 21, 1230-1237. [DOI] [PubMed] [Google Scholar]
- 21.Bolinger, P.-Y., Stamou, D. & Vogel, H. (2004) J. Am. Chem. Soc. 126, 8594-8595. [DOI] [PubMed] [Google Scholar]
- 22.Helfrich, M. R., Mangeney-Slavin, L. K., Long, M. S., Djoko, K. Y. & Keating, C. D. (2002) J. Am. Chem. Soc. 124, 13374-13375. [DOI] [PubMed] [Google Scholar]
- 23.Albertsson, P. A. (1971) Partition of Cell Particles and Macromolecules (Wiley, New York), 2nd Ed.
- 24.Akashi, K.-I., Miyata, H., Itoh, H. & Kinosita, K., Jr. (1996) Biophys. J. 71, 3242-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamashita, Y., Oka, M., Tanaka, T. & Yamazaki, M. (2002) Biochim. Biophys. Acta 1561, 129-134. [DOI] [PubMed] [Google Scholar]
- 26.Ellis, R. J. (2001) Trends Biochem. Sci. 26, 597-604. [DOI] [PubMed] [Google Scholar]
- 27.Zimmerman, S. B. & Minton, A. P. (1993) Annu. Rev. Biophys. Biomol. Struct. 22, 27-65. [DOI] [PubMed] [Google Scholar]
- 28.Minton, A. P. (2001) J. Biol. Chem. 276, 10577-10580. [DOI] [PubMed] [Google Scholar]
- 29.Goobes, R., Kahana, N., Cohen, O. & Minksy, A. (2003) Biochemistry 42, 2431-2440. [DOI] [PubMed] [Google Scholar]
- 30.Rohwer, J., Postma, P., Kholodenko, B. & Westerhoff, H. V. (1998) Proc. Natl. Acad. Sci. USA 95, 10547-10552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minton, A. P. (2000) Curr. Opin. Struct. Biol. 10, 34-39. [DOI] [PubMed] [Google Scholar]
- 32.Zaslavsky, B. Y. (1995) Aqueous Two-Phase Partitioning: Physical Chemistry and Bioanalytical Applications (Dekker, New York).
- 33.Hatti-Kaul, R., ed. (2000) Aqueous Two-Phase Systems: Methods and Protocols (Humana, Totowa, NJ).
- 34.Walter, H. & Johansson, G., eds. (1994) Methods Enzymol. 288.
- 35.Menger, F. M. & Angelova, M. I. (1998) Acc. Chem. Res. 31, 789-797. [Google Scholar]
- 36.Lis, H. & Sharon, N. (1998) Chem. Rev. 98, 637-674. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, Y., Mao, H. & Cremer, P. S. (2003) J. Am. Chem. Soc. 125, 15630-15635. [DOI] [PubMed] [Google Scholar]
- 38.Luby-Phelps, K. (2000) Int. Rev. Cytol. 192, 189-221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.