Abstract
As the effective use of carbon resources has become a pressing societal issue, the importance of chemical recycling of plastics has increased. The catalytic chemical decomposition for plastics is a promising approach for creating valuable products under efficient and mild conditions. Although several commodity and engineering plastics have been applied, the decompositions of stable resins composed of strong main chains such as polyamides, thermoset resins, and super engineering plastics are underdeveloped. Especially, super engineering plastics that have high heat resistance, chemical resistance, and low solubility are nearly unexplored. In addition, many super engineering plastics are composed of robust aromatic ethers, which are difficult to cleave. Herein, we report the catalytic depolymerization-like chemical decomposition of oxyphenylene-based super engineering plastics such as polyetheretherketone and polysulfone using thiols via selective carbon–oxygen main chain cleavage to form electron-deficient arenes with sulfur functional groups and bisphenols. The catalyst combination of a bulky phosphazene base P4-tBu with inorganic bases such as tripotassium phosphate enabled smooth decomposition. This method could be utilized with carbon- or glass fiber-enforced polyetheretherketone materials and a consumer resin. The sulfur functional groups in one product could be transformed to amino and sulfonium groups and fluorine by using suitable catalysts.
Subject terms: Sustainability, Synthetic chemistry methodology, Polymer chemistry
Although depolymerization methods for various commodity plastics and several engineering plastics have been developed, such methods for robust super engineering plastics that have very high heat and chemical resistance are nearly unexplored. Here, the authors report the catalytic depolymerization-like chemical decomposition of oxyphenylene-based super engineering plastics such as polyetheretherketone, polysulfone, and polyetherimide using thiols via selective carbon–oxygen main chain cleavage to form monomer-type molecules, namely electron-deficient arenes with sulfur functional groups and bisphenols.
Introduction
Organic materials and products, from commodity plastics to engineering plastics and stable super engineering plastics are indispensable for society and are utilized in a variety of fields from general-purpose products to advanced materials. However, since the organic resources that comprise them are naturally finite, future societies will be required to reuse and recycle them once consumed, rather than simply dispose of them. One of the methodologies to achieve this goal is chemical recycling, i.e., the conversion of organic products into raw materials by means of organic reactions1–14. In this scenario, gasification chemical recycling of waste plastics to produce methanol, propylene, olefins, and so on is a promising method. However, gasification requires high-temperature conditions, and the resulting products must be converted back into organic raw substrates. Thus, chemical decomposition methodologies that convert plastics directly into raw organic compounds such as monomers at lower temperatures are becoming increasingly important. Especially, plastics and polymers having relatively cleavable main chains such as an ester group are useful for this purpose and are being developed. For example, chemical recycling of polyethylene terephthalate (PET) has been extensively studied, giving usable low-weight molecules15–20.
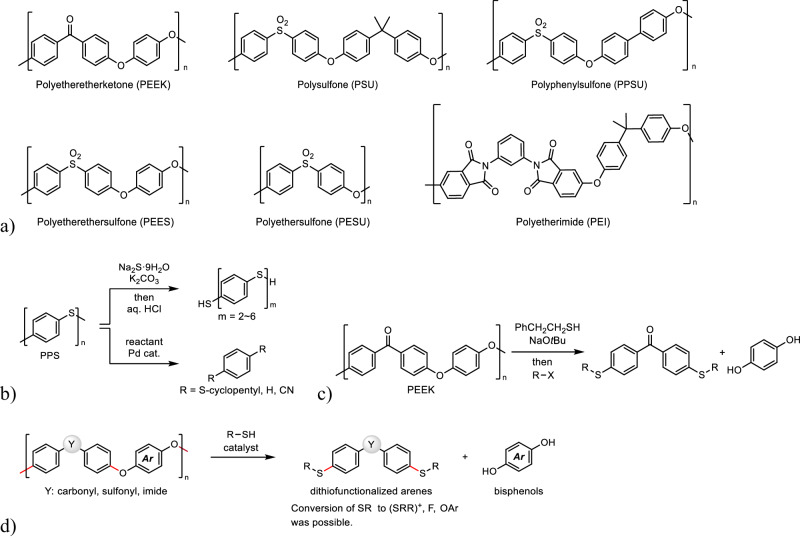
As mentioned above, many studies have developed the methodologies of the chemical decomposition of various resins, and recently, the focus is on catalytic decomposition for highly stable resins composed of strong main chains such as polyamides, polyurethanes, polyureas, thermoset resins, and super engineering plastics. For example, Nylon-6 was found to undergo decomposition in the presence of a dimethylaminopyridine21–24 or lanthanide25 catalyst to form ε-caprolactam. Catalytic hydrogenolysis was applicable to the decomposition of polyamides to produce amino alcohols26,27. Polyurethanes28,29 and polyureas30,31 were also subjected to catalytic hydrogenation to afford anilines, polyols, and amines. Decomposition of epoxy resins was developed using catalytic main-chain cleavage to provide the corresponding monomers32–34. Thus, the catalytic approach has the potential to achieve the decomposition of such stable resins to form useful low-weight molecules such as monomers. Among these stable resins, super engineering plastics are known for their excellent stability such as heat resistance and chemical resistance. Based on their high stability, these resins are indispensable to industries such as the automotive medical, aerospace, and other industries. However, catalytic decomposition of super engineering plastics remains nearly unexplored. A few catalytic decompositions of polyphenylenesulfide (PPS) composed of phenyl–sulfur bonds were reported to give low-molecular-weight molecules such as 1,4-dicyclopentylthiobenzene, benzene, and 1,4-dicyanobenzene (Fig. 1b)35–38. This scarcity of reports emphasizes the difficulty of catalytic decomposition of super engineering plastics. In addition, many super engineering plastics are composed of stable aromatic ethers, which are not easily cleaved.
Fig. 1. Chemical decomposition of super engineering plastics.
a Examples of super engineering plastics. b Decomposition of PPS. c Previous work: PEEK decomposition using sulfur nucleophiles. d This work: Catalytic depolymerization-like chemical decomposition using thiols to afford dithiofunctionalized arenes and bisphenols. R–SH, organic thiol. R–X, organic halide. Ar, aryl.
Recently, we demonstrated that thiolate reagents are highly effective for the depolymerization-like chemical decomposition of PEEK using sulfur nucleophiles, giving monomer-like products, dithiofunctionalized benzophenones and hydroquinone (Fig. 1c)39. The electron-deficient carbonyl group in the PEEK main chain enhances the reactivity of the carbon-oxygen bond at the para position such that the highly nucleophilic thiolate reagents cleave this bond selectively. We applied this system to the chemical decomposition of PSU, PESU, and PEEK using stoichiometric amounts of CsOH·H2O and CaH2 to form the corresponding bisphenols40. We expected that these stoichiometric methods have the potential to be applied to base-catalyzed chemical decomposition of various super engineering plastics. Since the previous reactions proceeded smoothly under a moderate reaction temperature (150 °C), the proposed catalytic strategy is expected to enable equally mild transformation to provide monomer-like products in high yields. Herein, we report the catalytic depolymerization-like chemical decomposition of oxyphenylene-based super engineering plastics using thiols to form monomer-like products, dithiofunctionalized arenes, and bisphenols (Fig. 1d). This method was applicable to PEEK, PSU, PPSU, and PEI. Inorganic bases and phosphazene bases were effective catalysts for this decomposition. Since the sulfur functional group acts as a leaving group for the substitution reaction under appropriate conditions41,42, the produced dithiofunctionalized arenes could be converted into sulfonium cations followed by fluorination or aryloxylation reactions.
Results and Discussion
Optimization of the reaction conditions
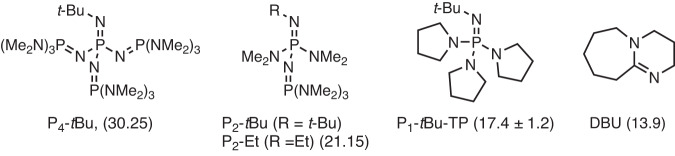
We examined the chemical decomposition of insoluble polyetheretherketone (PEEK) powder (Mw ~ 20800 and Mn ~ 10300 as catalog specifications) (1) with 2-ethyl-1-hexanethiol (2a) (2 equiv. relative to monomer unit) in 1,3-dimethyl-2-imidazolidinone (DMI) under various conditions (Table 1). The decomposition was first performed using KOH, K3PO4, KOtBu, and Cs2CO3 as catalysts (10 mol% relative to monomer unit) at 150 °C to form the corresponding decomposed products, dithiobenzophenone 4a, 1,4-hydroquinone (5) and a benzophenone-hydroquinone-type dimer intermediate 3 (Table 1, Entries 1-4). The use of Cs2CO3 was especially effective to form the final decomposition monomers, 4a and 5, in good yields (Table 1, Entry 4), indicating that large counter cation sizes as well as basicity promote the decomposition. Encouraged by these results, we expected that bulky and strongly basic organic phosphazene bases such as P4-tBu (pKBH+ 30.25 in dimethylsulfoxide (DMSO))43–48 would be promising catalysts for this decomposition (Fig. 2), which enhances the nucleophilicity of the counteranions49–63. For example, Shigeno, Korenaga, and Kondo recently reported that P4-tBu activates an alkanethiol (pKa of n-BuSH: 17.0 in DMSO)64. In this study, highly basic phosphazene bases P4-tBu and P2-tBu (pKBH+ of P2-Et: 21.15 in DMSO) exhibited good catalytic activity in comparison with weaker bases such as DBU (pKBH+ 13.9 in DMSO) and P1-tBu-TP (pKBH+ 17.4 ± 1.2 in DMSO) (Table 1, Entries 5-8). Thus, the basicity and size of the catalysts are important for this reaction to enhance the nucleophilicity of the counter anion. Increasing the amount of 2a from 2 equiv. to 2.5 equiv. enhanced the yield of 4a and 5 (Table 1, Entry 9). On the other hand, high loading of P4-tBu (20 mol%) had little effect (see Supplementary Information, Table S1, Entry 11), suggesting that increasing the amount of P4-tBu does not directly lead to an increase in yields of 4a and 5. The P4-tBu catalyst loading was successfully reduced to 5 mol%, albeit with slightly decreased yield (Table 1, Entry 10). The reaction at lower temperatures (120 and 100 °C) decreased the yield (Table 1, Entries 11 and 12). As mentioned above, PEEK is insoluble in organic solvents, but previous studies39,40 showed that solvents affect the reactivity of the decomposition. So, we checked the solvent effects for this decomposition in detail. As a result, N,N-dimethylacetamide (DMAc) was effective in the conditions whereas other solvent such as N,N-dimethylformamide (DMF), benzonitrile (PhCN), diethylene glycol diethyl ether ((C2H5OCH2CH2)2O), and xylene decreased the yield (Table 1, Entries 13-17). P4-tBu dissolves in these solvents so that the decomposition reactivity may be affected by the polarity of the solvents53. Finally, we found that the catalyst combination of P4-tBu (10 mol%) and K3PO4 (5 mol%) enhanced the reactivity of the present decomposition and gave 4a and 5 in excellent yields in DMAc solvent (Table 1, Entry 18, see Method and section 7-1 in Supplementary Methods).
Table 1.
Optimization of catalytic chemical decomposition to form monomer-like productsa
| Entry | 2a (equiv.) | Catalyst | Solvent | Temp. (ºC) | 3 (%) | 4a (%) | 5 (%) |
|---|---|---|---|---|---|---|---|
| 1 | 2 | KOH | DMI | 150 | 17 | 41 | 41 |
| 2 | 2 | K3PO4 | DMI | 150 | 12 | 54 | 56 |
| 3 | 2 | KOtBu | DMI | 150 | 12 | 62 | 62 |
| 4 | 2 | Cs2CO3 | DMI | 150 | 13 | 67 | 67 |
| 5 | 2 | DBU | DMI | 150 | 10 | 3 | 3 |
| 6 | 2 | P1-tBu-TP | DMI | 150 | 7 | 2 | 1 |
| 7 | 2 | P2-tBu | DMI | 150 | 14 | 61 | 66 |
| 8 | 2 | P4-tBu | DMI | 150 | 16 | 67 | 72 |
| 9 | 2.5 | P4-tBu | DMI | 150 | 7 | 74 | 73 |
| 10 | 2.5 | P4-tBu (5 mol%) | DMI | 150 | 12 | 58 | 58 |
| 11 | 2.5 | P4-tBu | DMI | 120 | 11 | 50 | 45 |
| 12 | 2.5 | P4-tBu | DMI | 100 | 12 | 37 | 31 |
| 13 | 2.5 | P4-tBu | DMAc | 150 | - | 95 | 84 |
| 14 | 2.5 | P4-tBu | DMF | 150 | 3 | 65 | 52 |
| 15 | 2.5 | P4-tBu | PhCN | 150 | 28 | 32 | 12 |
| 16 | 2.5 | P4-tBu | (EtOCH2CH2)2O | 150 | 19 | 10 | 3 |
| 17 | 2.5 | P4-tBu | Xylene | 150 | 19 | 11 | 2 |
| 18 | 2.5 | P4-tBu + K3PO4 (5 mol%) | DMAc | 150 | - | >99 (85) | >99 (61) |
a A mixture of 1 (powder, 0.1 mmol relative to the molecular weight of the monomer), 2a (0.2 mmol for entries 1-8, 0.25 mmol for entries 9-18), catalyst (0.01 mmol), and solvent (0.2 mL) was stirred for 16 h. Yields were determined by 1H NMR. Numbers in parentheses are isolated yields.
Fig. 2. Structure of used organic bases.
These pKa values in DMSO are shown in parentheses. Bases such as P2-tBu and P4-tBu with high basicity showed catalytic activity.
Experimental mechanistic studies
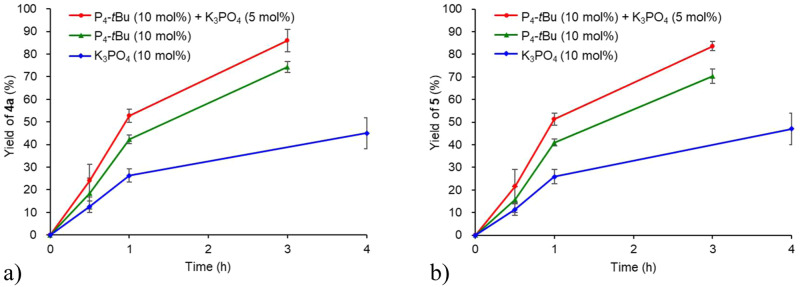
To evaluate the present catalytic decomposition reactivity, we monitored the yields of 4a and 5 during the reaction of PEEK powder 1 with 2a catalyzed by P4-tBu (10 mol%) and K3PO4 (5 mol%), P4-tBu (10 mol%), and K3PO4 (10 mol%) (Fig. 3, see section 9-1 and Table S5 in Supplementary Methods). Under the three conditions, 4a and 5 were formed after 30 minutes. Moreover, high yields of 4a and 5 were obtained after 3 h under the conditions using P4-tBu and K3PO4. These observations indicate that the decomposition proceeded rapidly. When P4-tBu catalyst was only used, decomposition proceeded faster than when K3PO4 catalyst was used. The catalyst combination of P4-tBu and K3PO4 increased the rate of formation of 4a and 5 compared to the use of P4-tBu alone. These results indicate that the use of the P4-tBu catalyst allowed for rapid decomposition. The K3PO4 assisted this catalytic activity of P4-tBu.
Fig. 3. PEEK decomposition time course.
Reaction conditions: PEEK (0.1 mmol relative to the molecular weight of monomer), 2a (2.5 equiv.) at 150 °C in the presence of P4-tBu (10 mol%) and K3PO4 (5 mol%) (red line), P4-tBu (10 mol%) (green line), and K3PO4 (10 mol%) (blue line). a Yields of 4a under various conditions are plotted as the average of the three runs with standard errors. b Yields of 5 under various conditions are plotted as the average of the three runs with standard errors.
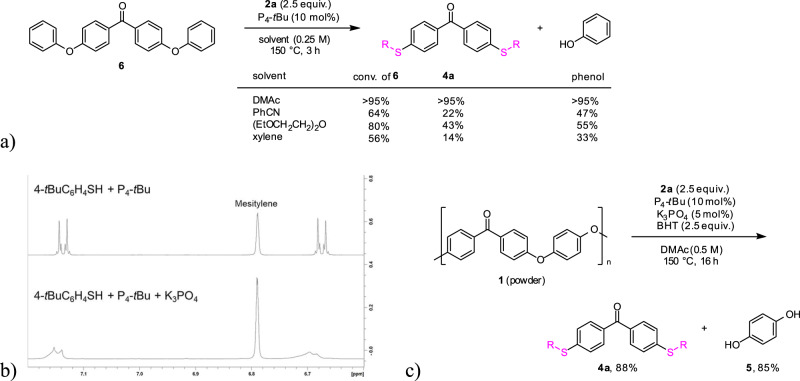
To understand the solvent effect for the decomposition of PEEK, we examined the swelling behavior of PEEK resins. PEEK granules or plates were heated in solvents such as DMAc, DMF, PhCN, (EtOCH2CH2)2O, and xylene at 150 °C for 19 h (See section 9-2, Table S6, and Fig. S1 in Supplementary Methods). As a result, these solvents increased the mass of the PEEK granules and plates (105~109 wt%) whereas the resins were apparently unchanged. These observations suggested that the swelling effect of PEEK does not affect the decomposition reactivity. Then we examined the reaction of 4,4’-diphenoxy-benzophenone (6) as a PEEK model compound with 2.5 equiv. of 2a and 10 mol% of P4-tBu at 150 °C for 3 h (Fig. 4a). The reaction using DMAc formed 4a and phenol in an excellent yield. On the other hand, use of other solvents such as PhCN, (C2H5OCH2CH2)2O, and xylene decreased the yield of 4a. In these cases, the reactions were not complete even after 22 h. Thus, DMAc as the high polar solvent enhanced the reactivity of the thiolate generated by the combination of the thiol and P4-tBu and probably promoted the cleavage of the carbon–oxygen bonds and the decomposition45.
Fig. 4. Mechanistic studies.
a Examination of the solvent effect using a model substrate 6 under P4-tBu catalyst. Yields of the products were determined by 1H NMR. b 1H NMR spectra indicating formation of reactive thiolate by reaction of 4-tert-butylphenylthiol (0.02 mmol) and P4-tBu (0.02 mmol) in the presence or absence of K3PO4 (0.02 mmol) in DMF-d7. Mesitylene was used as an internal standard. c Examination of decomposition of PEEK in the presence of 3,5-di-tert-butyl-4-hydroxytoluene (BHT). The radical inhibitor does not affect the decomposition.
Next, we carried out NMR experiments to shed light on the combination of the thiol, P4-tBu, and K3PO4. The reaction of 4-tert-butylphenylthiol (0.02 mmol) and P4-tBu (0.02 mmol) in the presence of K3PO4 (0.02 mmol) was examined in DMF-d7 (0.5 mL) at 25 °C (see section 9-3 in Supplementary Methods). As a result, a 31P{1H} NMR spectrum suggested the formation of [P4-tBu-H]+ (Fig. 4b, see Supplementary Fig. S5 compared with Fig. S2) and mass peaks were also observed at m/z 634 in ESI-TOF-(+)-MS and m/z 165 in ESI-TOF-(-)-MS mass spectra, confirming the generation of [P4-tBu-H]+·[S(C6H4-tBu)]-. The same results were observed in the absence of K3PO4 (see Supplementary Fig. S3). On the other hand, in 1H NMR spectrum, the resonances for the aryl doublets (δ 6.69 and δ 7.14) were broadened in comparison with the case in the absence of K3PO4 (Fig. 4b). In addition, these signals were different from the combination of the thiol and K3PO4 (see Supplementary Fig. S4). These results indicated that [P4-tBu-H]+·[S(C6H4-tBu)]- was initially formed and the [S(C6H4-tBu)] anion coordinated to K3PO4 in the equilibrium state. Density functional theory (DFT) calculations suggested that the NBO charge of the phenylthiolate coordinating to K3PO4 is more nucleophilic than the non-coordinating one (see section 9-4 in Supplementary Methods and Supplementary Data 2). We assumed that this catalyst combination activates the thiol for the smooth decomposition of PEEK.
Aromatic nucleophilic substitution with thiolate anions is known to proceed via the SNAr or SRA1 mechanism65–67. In the SRA1 mechanism, thiyl radicals are thought to be involved. However, this catalytic decomposition of PEEK gives hydroquinone which inhibits the generation of free radicals. We examined the decomposition with 2a under P4-tBu/K3PO4 catalyst with 3,5-di-tert-butyl-4-hydroxytoluene (BHT, 2.5 equiv.), a radical inhibitor, at 150 °C for 16 h and observed the formation of 4a and 5 in high yields (Fig. 4c). These results ruled out the possibility of a radical pathway for the decomposition. Of note, 2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPO) as a typical radical scavenger was not suitable for this experiment, which converted 2a into the corresponding disulfide in the absence of PEEK (see section 9-5 and Table S7, S8 in Supplementary Methods)68.
Proposed mechanism
A plausible pathway for the chemical decomposition catalyzed by P4-tBu and K3PO4 is shown in Fig. 5. The thiol is initially activated by P4-tBu to form a thiolate that interacts with K3PO4 in the equilibrium state. The sulfur center of the thiolate attacks the ipso-carbon bound to oxygen in the benzophenone unit in PEEK to form an anionic intermediate. The aryloxy anion is released to complete carbon–sulfur bond formation. K3PO4 may enhance the reactivity of the thiolate and assist in the release of the aryloxy anion. The generated aryloxy anion activates the thiol to form the organic thiolate and arenols. In fact, the basicity of arenols (pKa in DMSO of PhOH: 18.0; p-MeC6H4OH: 18.9)69 is higher than that of thiols (pKa in DMSO of n-BuSH: 17.0; PhSH: 10.3)64. This series of processes occurs repeatedly to generate the dithiobenzophenone 4 and hydroquinone (5).
Fig. 5. Plausible pathway for chemical decomposition of PEEK.

Cleavage of carbon-oxygen main chains by organo thiolate generated from the reaction of thiol and catalysts.
Substrate scope
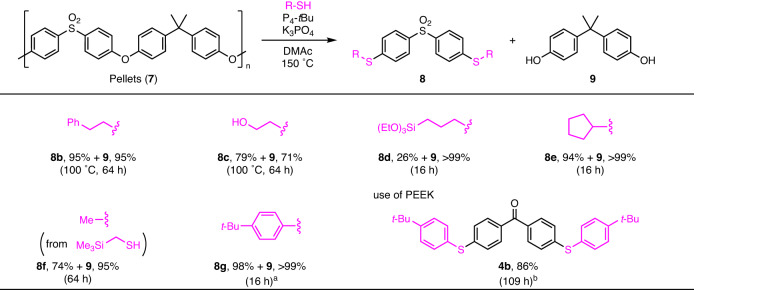
With the optimum conditions using both P4-tBu and K3PO4 in hand, we examined the chemical decomposition of other super engineering plastics such as polysulfone (PSU), polyetherethersulfone (PEES), polyphenylsulfone (PPSU), polyethersulfone (PESU), and polyetherimide (PEI) which were analyzed by high-temperature GPC analysis prior to use (see section 9-6 and Table S9 in Supplementary Methods). These resins have cleavable aryl-oxygen bonds affected by electron-withdrawing groups in a manner similar to PEEK. PSU is composed of diphenylsulfone and bisphenol A. Since thiolate anions can cleave aryl-SO2 bonds70–74, we were concerned that the present catalytic method may cleave the aryl-SO2 bond in the diphenylsulfone unit as well as the target C–O main chain. However, we found that polysulfone (PSU) pellets 7 (purchased from Sigma-Aldrich) and 7’ (purchased from Acros Organics) with different Mw (Mw 35000 and Mw 60000) in each of the catalog specifications underwent the decomposition with 2a via selective C–O bond cleavage38 to furnish the corresponding 4,4’-dialkylthiobenzosulfone (8a) and bisphenol A (9) in high yields (Table 2, Entries 1 and 2). In addition, there was no clear difference in the reaction rate between 7 and 7’ (see Supplementary Table S2). In the same way, PEES pellets (10) or PPSU powder (11) could be converted into 8a and hydroquinone (5) or 4,4’-dihydroxybiphenyl (12) in high yields (Table 2, Entries 3 and 4). In the case of PESU (13) consisting of repeating oxy-diphenylsulfone units, three products 8a, 4-alkylthio-4’-hydroxy-diphenylsulfone 14, and bisphenol S (15) were obtained (Table 2, Entry 5). PEI is composed of repeating structures of phenylene-1,3-bisphthalimide and bisphenol A. In this case, imide bonds in the phthalimide units may be cleaved by sulfur nucleophiles75. Nevertheless, the C–O main chains were successfully cleaved selectively in the decomposition of PEI pellets 16 with 2a, giving dithiofunctionalized phenylene-1,3-bisphthalimide 17 and 9 in good yields (Table 2, Entry 6).
Table 2.
Scope of super engineering plastics for the chemical decomposition with 2-ethylhexanethiol in the presence of P4-tBu and K3PO4 in DMAca
a A mixture of polymer (0.1 mmol relative to the molecular weight of the monomer), 2a (0.25 mmol), P4-tBu (0.01 mmol), K3PO4 (0.005 mmol) and DMAc (0.2 mL) was stirred for 16 h at 150 ºC. Isolated yields are shown. b 4-((4-(4-((4-((2-Ethylhexyl)thio)phenyl)sulfonyl)phenoxy)phenyl)sulfonyl)phenol was obtained in 15% yield.
We then explored the scope of thiols under the catalytic decomposition of PSU pellets 7 (Fig. 6). 2-Phenylethanethiol or 2-mercaptoethanol underwent decomposition at 100 °C to form 8b (see section 7-2 in Supplementary Methods) or 8c and bisphenol A (9) in good yields. Triethoxysilyl-substituted propanethiol and cyclopentanethiol were used in the decomposition and the corresponding decomposition products 8d and 8e were obtained. Trimethylsilylmethylthiol gave 4,4’-dimethylthiodiphenylsulfone 8f and 9 in high yields via desilylation. Not only alkanethiols but also 4-tert-butylbenzenethiol could be utilized for decomposition with only NaOtBu catalyst (20 mol%) to form the corresponding monomer 8g in 98% yield together with 9 quantitatively (see Supplementary Table S3 and section 7-3 in Supplementary Methods)). Instead of PSU, we attempted the decomposition of PEEK powder with 4-tert-butylbenzenethiol under the P4-tBu/K3PO4 or NaOtBu catalyst in DMAc but the yield of the product, 4,4’-di(arylthiol)benzophenone 4b, was low (see Supplementary Table S4 and section 7-4 in Supplementary Methods). At that time, a suspension containing precipitated 4b and its intermediates were obtained. Considering that the poor solubility of the products may have decreased the reactivity, we modified the conditions using a P4-tBu/Cs2CO3 catalytic combination in DMI to enhance the solubility. As a result, 4b was obtained in high yield, albeit with a long reaction time.
Fig. 6. Scope of thiols for the chemical decomposition of PSU.
Reaction conditions: a mixture of polymer (0.1 mmol relative to the molecular weight of monomer), thiol (0.25 mmol), P4-tBu (0.01 mmol), K3PO4 (0.005 mmol), and DMAc (0.2 mL) was stirred for the time shown at 150 °C. Isolated yields are reported. a NaOtBu (0.02 mmol) only was used as the catalyst. b Cs2CO3 (0.01 mmol) and DMI (0.2 mL) were used instead of K3PO4 and DMAc.
Utility of the decomposition method
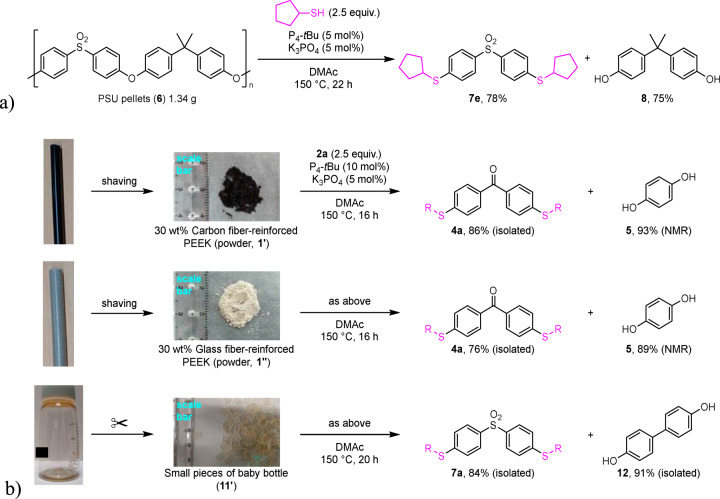
To demonstrate the scalability of the decomposition method, a gram-scale reaction of PSU pellets (7) with cyclopentanethiol catalyzed by 5 mol% of P4-tBu and K3PO4 was carried out. The desired products 8e and 9 were isolated in 78% and 75% yields, respectively (Fig. 7a, see section 7-5 in Supplementary Methods). It is worth noting that this catalytic method was applicable to composite materials. Shaved powder of 30 wt% carbon-fiber reinforced PEEK (1’) underwent the decomposition with 2a to form 4a and 5 in good yields comparable to those obtained from neat PEEK powder (Fig. 7b, see section 7-6 in Supplementary Methods). 30 wt% Glass-fiber reinforced PEEK (1’’’) was converted into 4a and 5 in the same way. In addition, small pieces of a baby bottle made up of PPSU (11’) as a representative consumer resin were transformed into products, 8a and 12, in high yields (see section 7-7 in Supplementary Methods).
Fig. 7. Utility of this decomposition method.
a Gram-scale decomposition of PSU pellets (1.34 g) with cyclopentane thiol under P4-tBu (5 mol%) and K3PO4 (5 mol%) catalysts in DMAc at 150 ºC. b Decomposition of 30% carbon fiber- or 30% glass fiber-reinforced PEEK or a baby bottle made up of PPSU with 2-ethylhexanethiol under the catalytic conditions using P4-tBu (10 mol%) and K3PO4 (5 mol%) in DMAc at 150 ºC.
Utility of products
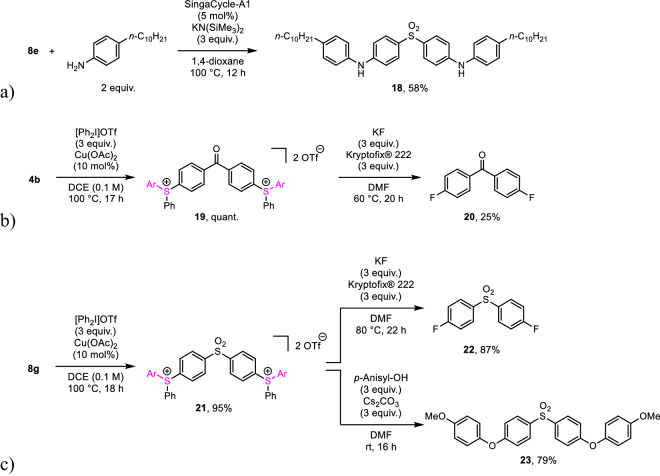
Sulfur functional groups in the products can be utilized in various transformations to yield functional molecules. For example, 8e was applicable to the double cross-coupling with 4-decylaniline under palladium-catalyzed conditions76 to give the corresponding double amination product 18 (Fig. 8a, see section 7-8 in Supplementary Methods). Double phenylation of 4b using diphenyliodonium trifluoromethanesulfonate and copper acetate catalyst in 1,2-dichloroethane at 100 °C, based on a reported method77, gave benzophenone 4,4′-bis(diarylsulfonium) salt 19 in excellent yield (Fig. 8b, see section 7-9 in Supplementary Methods). Such sulfonium groups are more reactive leaving groups than their parent sulfur functional groups. Thus, the sulfonium groups in 19 could be converted into fluorine by potassium fluoride and Kryptofix® 222 (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane) in N,N-dimethylformamide at 60 °C (see section 7-10 in Supplementary Methods)78. Of note, the product, 4,4’-difluorobenzophenone (20), is used as a monomer for PEEK79,80. PSU-depolymerized product 8g was also applicable to this transformation sequence. Double phenylation of 8g afforded diphenylsulfone 4,4′-bis(diarylsulfonium) salt 21 (Fig. 8c, see section 7-11 in Supplementary Methods). Subsequent fluorination of 21 gave bis(4-fluorophenyl)sulfone (22) in 87% yield (see section 7-12 in Supplementary Methods), which is a monomer of diphenylsulfone-based polymers such as PSU81,82, PPSU83–90, PESU91,92, and PEES93. In addition, 21 reacted with p-methoxyphenol in the presence of Cs2CO3 to give 4,4’-bis(p-anisyloxy)diphenylsulfone (23) in 79% yield (see section 7-13 in Supplementary Methods).
Fig. 8. Functionalization of products.
a Cross-coupling of 8e with 4-decylaniline to give 4,4'-sulfonylbis(N-(4-decylphenyl)aniline) (18). b Conversion of 4b into benzophenone-based disulfonium salt 19 followed by fluorination to form 4,4’-difluorobenzophenone (20). c Conversion of 8g into diphenylsulfone-based disulfonium salt 21 followed by fluorination to form di(4-fluorophenyl)sulfone (22) or etherification to form 4,4’-bis(aryloxy)diphenylsulfone 23. SingaCycle-A1: Chloro[[1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene](N,N-dimethylbenzylamine)palladium(II)]. DCE: 1,2-dichloroethane. Kryptofix® 222: 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane. DMF: N,N-dimethylformamide. p-Anisyl: 4-methoxyphenyl.
Conclusion
In this study, we demonstrated that the depolymerization-like chemical decomposition of robust super engineering plastics such as PEEK, PSU, PEES, PPSU, PESU, and PEI occurred smoothly with thiols at moderate temperature under the catalytic combination of bulky organic super bases, P4-tBu, and inorganic bases, K3PO4 or Cs2CO3. DMAc solvent also promoted the carbon-oxygen bond cleavages in a low-weight molecule and insoluble PEEK due to its polarity under the conditions. Various thiols were applied to this decomposition to afford monomer-like thiofunctionalized arenes and bisphenols in high yields. In addition, carbon fiber- or glass fiber-reinforced resins and a baby bottle made of PPSU as a representative consumer resin material were utilized in this catalytic decomposition. From a synthetic perspective, thiofunctionalities in the arene products act as leaving groups and can be transformed into various substituents such as amino groups and fluorine. Notably, fluorinated arenes are parent monomers for synthesizing super engineering plastics. This shows that the present catalytic decomposition method can be utilized not only for chemical recycling but also for upcycling. This development will expand the decomposition of other robust polymer materials with various reagents under this effective catalytic system.
Methods
General procedure for catalytic chemical decomposition of PEEK
To a mixture of PEEK powder (28.8 mg, 0.100 mmol relative to the molecular weight of the monomer), and potassium phosphate tribasic (1.1 mg, 0.0050 mmol) was added N,N-dimethylacetamide (0.20 mL), P4-tBu phosphazene base in hexane solution (1-tert-butyl-4,4,4-tris(dimethylamino)-2,2-bis[tris(dimethylamino)-phosphoranylidenamino]-2λ5,4λ5-catenadi(phosphazene), 0.8 M, 0.0125 mL, 0.010 mmol), and 2-ethylhexanethiol (36.6 mg, 0.250 mmol) in a 3 mL vial under argon atmosphere. The resultant mixture was stirred at 150 °C for 16 h. The reaction mixture was cooled to room temperature. The mixture was analyzed by 1H NMR in acetone-d6 to determine the yields of the products, 4a and hydroquinone (5), using 1,4-dioxane as an internal standard. The reaction mixture was concentrated in vacuo. The crude product was purified by column chromatography on silica gel (hexane/ethyl acetate 96:4 to 7:3) to give bis(4-(2-ethylhexylthio)phenyl)methanone (85%, 39.9 mg) and 1,4-hydroquinone (61%, 6.7 mg).
General information. See Supplementary Methods, general information (page S3).
Chemicals. See Supplementary Methods, chemicals (page S3).
NMR charts. See Supplementary Data 1, NMR spectra of obtained chemicals.
Supplementary information
Description of Additional Supplementary File
Acknowledgements
This work was supported financially by PRESTO (JPMJPR21N9 to Y.M.) from the JST, Iketani Science and Technology Foundation, Tobe Maki Scholarship Foundation, Grants-in-Aid for Scientific Research (C) (19K05481 to Y.M.) from the JSPS, and Department of Materials and Chemistry, AIST. Y.M., N.M., and Y.N. also acknowledge the DIC Corporation. Y.M. would like to thank Dr. Masanori Shigeno for discussions about the catalytic activity. Y.M would like to thank JST, ERATO (JPMJER2103), and Prof. Kyoko Nozaki and her lab members, Prof. Kohei Takahashi, Prof. Takanori Iwasaki, Prof. Shuhei Kusumoto, and Prof. Xiongjie Jin, for their discussions on this project. We would like to thank Ms. Risa Kawato for her assistance in the high-temperature GPC analysis.
Author contributions
Y.M. conceived the idea and designed the whole experiment with S.I. and N.M. Y.M., S.I., and N.M. performed the experiments. Y.M., S.I., and N.M. contributed to writing the manuscript and participated in data analyses and discussions. S.I. and N.M. contributed equally to this paper. Y.M. performed the DFT calculations. Y.N. and M.Y. supported this project. Y.M. revised the paper.
Peer review
Peer review information
Communications Chemistry thanks Eriko Sato and the other anonymous reviewer for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data obtained in this study are available within this article and its supplementary information and are also from the corresponding authors upon reasonable request. Original 1H and 13C spectra of the compounds obtained in this manuscript are available in Supplementary Data 1. The computed energy values and coordinates are available in Supplementary Data. 2.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42004-024-01120-7.
References
- 1.Ignatyev IA, Thielemans W, Beke BV. Recycling of polymers: A review. ChemSusChem. 2014;7:1579–1593. doi: 10.1002/cssc.201300898. [DOI] [PubMed] [Google Scholar]
- 2.Hong M, Chen EY-X. Chemically recyclable polymers: a circular economy approach to sustainability. Green. Chem. 2017;19:3692–3706. doi: 10.1039/C7GC01496A. [DOI] [Google Scholar]
- 3.Rahimi A, García JM. Chemical recycling of waste plastics for new materials production. Nat. Rev. Chem. 2017;1:0046. doi: 10.1038/s41570-017-0046. [DOI] [Google Scholar]
- 4.Lu Z-B, Liu Y, Zhou H. Learning nature: recyclable monomers and polymers. Chem. Eur. J. 2018;24:11255–11266. doi: 10.1002/chem.201704461. [DOI] [PubMed] [Google Scholar]
- 5.Stadler BM, Wulf C, Werner T, Tin S, de Vries JG. Catalytic approaches to monomers for polymers based on renewables. ACS Catal. 2019;9:8012–8067. doi: 10.1021/acscatal.9b01665. [DOI] [Google Scholar]
- 6.Coates GW, Getzler YDYL. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 2020;5:501–516. doi: 10.1038/s41578-020-0190-4. [DOI] [Google Scholar]
- 7.Kim JG. Chemical recycling of poly(bisphenol A carbonate) Polym. Chem. 2020;1:4830–4849. doi: 10.1039/C9PY01927H. [DOI] [Google Scholar]
- 8.Payne J, Jones MD. The chemical recycling of polyesters for a circular plastics economy: challenges and emerging opportunities. ChemSusChem. 2021;14:4041–4070. doi: 10.1002/cssc.202100400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Wan K, Zhang Y, Wang Y. Waste to wealth: chemical recycling and chemical upcycling of waste plastics for a great future. ChemSusChem. 2021;14:4123–4136. doi: 10.1002/cssc.202100652. [DOI] [PubMed] [Google Scholar]
- 10.Liguori F, Moreno-Marrodán C, Barbaro P. Valorisation of plastic waste via metal-catalysed depolymerization. Beilstein J. Org. Chem. 2021;17:589–621. doi: 10.3762/bjoc.17.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosloski-Oh SC, Wood ZA, Manjarrez Y, de Los Rios JP, Fieser ME. Catalytic methods for chemical recycling or upcycling of commercial polymers. Mater. Horiz. 2021;8:1084–1129. doi: 10.1039/D0MH01286F. [DOI] [PubMed] [Google Scholar]
- 12.Fagnani DE, et al. 100th anniversary of macromolecular science viewpoint: redefining sustainable polymers. ACS Macro Lett. 2021;10:41–53. doi: 10.1021/acsmacrolett.0c00789. [DOI] [PubMed] [Google Scholar]
- 13.Xu G, Wang Q. Chemically recyclable polymer materials: polymerization and depolymerization cycles. Green. Chem. 2022;24:2321–2346. doi: 10.1039/D1GC03901F. [DOI] [Google Scholar]
- 14.Zhang Y, Qi M-Y, Tang Z-R, Xu Y-J. Photoredox-catalyzed plastic waste conversion: nonselective degradation versus selective synthesis. ACS Catal. 2023;13:3575–3590. doi: 10.1021/acscatal.3c00301. [DOI] [Google Scholar]
- 15.Fukushima K, et al. Organocatalytic Depolymerization of Poly(ethylene terephthalate) J. Poly. Sci. A: Poly. Chem. 2011;49:1273–1281. doi: 10.1002/pola.24551. [DOI] [Google Scholar]
- 16.Tanaka S, Sato J, Nakajima Y. Capturing ethylene glycol with dimethyl carbonate towards depolymerisation of polyethylene terephthalate at ambient temperature. Green. Chem. 2021;23:9412–9416. doi: 10.1039/D1GC02298A. [DOI] [Google Scholar]
- 17.Lu H, et al. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature. 2022;604:662–667. doi: 10.1038/s41586-022-04599-z. [DOI] [PubMed] [Google Scholar]
- 18.Joseph Ng KW, et al. A facile alternative strategy of upcycling mixed plastic waste into vitrimers. Commun. Chem. 2023;6:158. doi: 10.1038/s42004-023-00949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Garcia P, et al. An archaeal lid-containing feruloyl esterase degrades polyethylene terephthalate. Commun. Chem. 2023;6:193. doi: 10.1038/s42004-023-00998-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lourenço DL, Fernandes AC. HBpin/MoO2Cl2(H2O)2 as an efficient catalytic system for the reduction of esters, lactones and polyester plastic waste. Mol. Catal. 2023;542:113128. doi: 10.1016/j.mcat.2023.113128. [DOI] [Google Scholar]
- 21.Kamimura A, Yamamoto S. A novel depolymerization of nylons in ionic liquids. Polym. Adv. Technol. 2008;19:1391–1395. doi: 10.1002/pat.1199. [DOI] [Google Scholar]
- 22.Yamamoto S, Kamimura A. Chem. Lett. 2009;38:1016–1017. doi: 10.1246/cl.2009.1016. [DOI] [Google Scholar]
- 23.Kamimura K, Shiramatsu Y, Kawamoto T. Green. Energy Environ. 2019;4:166–170. doi: 10.1016/j.gee.2019.01.002. [DOI] [Google Scholar]
- 24.Alberti C, Figueira R, Hofmann M, Koschke S, Enthaler S. Chemical recycling of end-of-Life Polyamide 6via ring closing depolymerization. ChemistrySelect. 2019;4:12638–12642. doi: 10.1002/slct.201903970. [DOI] [Google Scholar]
- 25.Wursthorn L, et al. Selective Lanthanide-organic catalyzed depolymerization of Nylon-6 to ɛ-Caprolactam. Angew. Chem. Int. Ed. 2023;62:e202212543. doi: 10.1002/anie.202212543. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A, et al. Hydrogenative depolymerization of nylons. J. Am. Chem. Soc. 2020;142:14267–14275. doi: 10.1021/jacs.0c05675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou W, et al. Depolymerization of technical-grade polyamide 66 and polyurethane materials through hydrogenation. ChemSusChem. 2021;14:4176–4180. doi: 10.1002/cssc.202002465. [DOI] [PubMed] [Google Scholar]
- 28.Liua X, Werner T. Indirect reduction of CO2 and recycling of polymers by manganese-catalyzed transfer hydrogenation of amides, carbamates, urea derivatives, and polyurethanes. Chem. Sci. 2021;12:10590–10597. doi: 10.1039/D1SC02663A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gausas L, et al. Catalytic hydrogenation of polyurethanes to base chemicals: from model systems to commercial and end-of-life polyurethane materials. JACS Au. 2021;1:517–524. doi: 10.1021/jacsau.1c00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar, A. & Luk, J. Catalytic Hydrogenation of Urea Derivatives and Polyureas. Eur. J. Org. Chem. 4546–4550 (2021).
- 31.Iwasaki T, Tsuge K, Naito N, Nozaki K. Chemoselectivity change in catalytic hydrogenolysis enabling urea-reduction to formamide/amine over more reactive carbonyl compounds. Nat. Commun. 2023;14:3279. doi: 10.1038/s41467-023-38997-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen ST, McLoughlin EA, Cox JH, Fors BP, Knowles RR. Depolymerization of hydroxylated polymers via light-driven C−C bond cleavage. J. Am. Chem. Soc. 2021;143:12268–12277. doi: 10.1021/jacs.1c05330. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen ST, et al. Chemical Recycling of thiol epoxy thermosets via light-driven C–C Bond cleavage. J. Am. Chem. Soc. 2023;145:11151–11160. doi: 10.1021/jacs.3c00958. [DOI] [PubMed] [Google Scholar]
- 34.Ahrens A, et al. Catalytic disconnection of C–O bonds in epoxy resins and composites. Nature. 2023;617:730–737. doi: 10.1038/s41586-023-05944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang SJ, Bian SG, Yan H, Xiao M, Meng YZ. Novel synthesis of macrocyclic disulfides from poly(phenylene sulfide) by depolymerization reaction. J. Appl. Poly. Sci. 2008;110:4049–4054. doi: 10.1002/app.29010. [DOI] [Google Scholar]
- 36.Lian Z, Bhawal BN, Yu P, Morandi B. Palladium-catalyzed carbon-sulfur or carbon-phosphorus bond metathesis by reversible arylation. Science. 2017;356:1059–1063. doi: 10.1126/science.aam9041. [DOI] [PubMed] [Google Scholar]
- 37.Minami Y, et al. Catalytic reductive cleavage of poly(phenylene sulfide) using a hydrosilane. Synthesis. 2021;53:3351–3354. doi: 10.1055/a-1518-9010. [DOI] [Google Scholar]
- 38.Delcaillau T, Woenckhaus-Alvarez A, Morandi B. Nickel-catalyzed cyanation of aryl thioethers. Org. Lett. 2021;23:7018–7022. doi: 10.1021/acs.orglett.1c02285. [DOI] [PubMed] [Google Scholar]
- 39.Minami Y, et al. Depolymerization of robust polyetheretherketone to regenerate monomer units using sulfur reagents. Commun. Chem. 2023;6:14. doi: 10.1038/s42004-023-00814-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minami Y, Inagaki Y, Tsuyuki T, Sato K, Nakajima Y. Hydroxylation-depolymerization of oxyphenylene-based super engineering plastics to regenerate arenols. JACS Au. 2023;3:2323–2332. doi: 10.1021/jacsau.3c00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yorimitsu H. Catalytic transformations of sulfonium salts via C-S bond activation. Chem. Rec. 2021;21:3356–3369. doi: 10.1002/tcr.202000172. [DOI] [PubMed] [Google Scholar]
- 42.Tian Z-Y, Hu Y-T, Teng H-B, Zhang C-P. Application of arylsulfonium salts as arylation reagents. Tetrahedron Lett. 2018;59:299–309. doi: 10.1016/j.tetlet.2017.12.005. [DOI] [Google Scholar]
- 43.Schwesinger R, Schlemper H. Peralkylated polyaminophosphazenes- extremely strong, neutral nitrogen bases. Angew. Chem. Int. Ed. 1987;26:1167–1169. doi: 10.1002/anie.198711671. [DOI] [Google Scholar]
- 44.Schwesinger, R., et al. Extremely strong, uncharged auxiliary bases; monomeric and polymer-supported Polyaminophosphazenes (P2–P5). Liebigs Ann. 1055–1081 (1996).
- 45.Tshepelevitsh, S., et al. On the basicity of organic bases in different media. Eur. J. Org. Chem. 6735–6748 (2019).
- 46.Seebach, D., Beck, A. K. & Studer, A. in Modern Synthetic Methods 1995 (eds Ernst, B. & Leumann, C.) 48−54 (Wiley-VCH, 1995).
- 47.Mamdani HT, Hartley RC. Tetrahedron Lett. 2000;41:747–749. doi: 10.1016/S0040-4039(99)02123-1. [DOI] [Google Scholar]
- 48.Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976;32:751–767. doi: 10.1107/S0567739476001551. [DOI] [Google Scholar]
- 49.Pietzonka T, Seebach D. Alkylations of (R,R)-2-t-Butyl-6-methyl-1,3-dioxan-4-ones which are not Possible with Lithium Amides may be Achieved with a Schwesinger P4 Base. Chem. Ber. 1991;124:1837–1843. doi: 10.1002/cber.19911240822. [DOI] [Google Scholar]
- 50.Fruchart J-S, Gras-Masse H, Melnyk O. Methyl phenylacetate enolate generated with the P4-tBu Schwesinger base: ‘naked’ or not? Tetrahedron Lett. 2001;42:9153–9155. doi: 10.1016/S0040-4039(01)01994-3. [DOI] [Google Scholar]
- 51.Solladié-Cavallo A, Liptaj T, Schmitt M, Solgadi A. iso-Propyl phenylacetate: formation of a single enolate with tBuP4 as base. Tetrahedron Lett. 2002;43:415–418. doi: 10.1016/S0040-4039(01)02157-8. [DOI] [Google Scholar]
- 52.Schwesinger R, Link R, Wenzl P, Kossek S, Keller M. Extremely base-resistant organic phosphazenium cations. Chem. -Eur. J. 2006;12:429–437. doi: 10.1002/chem.200500837. [DOI] [PubMed] [Google Scholar]
- 53.Ebisawa M, Ueno M, Oshima Y, Kondo Y. Synthesis of dictyomedins using phosphazene base catalyzed diaryl ether formation. Tetrahedron Lett. 2007;48:8918–8921. doi: 10.1016/j.tetlet.2007.10.031. [DOI] [Google Scholar]
- 54.Kolonko KJ, Reich HJ. Stabilization of Ketone and Aldehyde Enols by formation of hydrogen bonds to Phosphazene Enolates and their aldol products. J. Am. Chem. Soc. 2008;130:9668–9669. doi: 10.1021/ja804221x. [DOI] [PubMed] [Google Scholar]
- 55.Kolonko KJ, Guzei IA, Reich HJ. Structure and dynamics of α-Aryl Amide and Ketone Enolates: THF, PMDTA, TMTAN, HMPA, and Crypt-Solvated Lithium Enolates, and comparison with Phosphazenium analogues. J. Org. Chem. 2010;75:6163–6172. doi: 10.1021/jo100970r. [DOI] [PubMed] [Google Scholar]
- 56.Boileau S, Illy N. Activation in anionic polymerization: Why phosphazene bases are very exciting promoters. Prog. Polym. Sci. 2011;36:1132–1151. doi: 10.1016/j.progpolymsci.2011.05.005. [DOI] [Google Scholar]
- 57.Kawai H, Yuan Z, Tokunaga E, Shibata N. A sterically demanding organo-superbase avoids decomposition of a naked trifluoromethyl carbanion directly generated from fluoroform. Org. Biomol. Chem. 2013;11:1446–1450. doi: 10.1039/c3ob27368g. [DOI] [PubMed] [Google Scholar]
- 58.Jardel D, Davies C, Peruch F, Massip S, Bibal B. Protonated Phosphazenes: Structures and hydrogen-bonding organocatalysts for carbonyl bond activation. Adv. Synth. Catal. 2016;358:1110–1118. doi: 10.1002/adsc.201600062. [DOI] [Google Scholar]
- 59.Hong M, Chen EY-X. Towards truly sustainable polymers: a metal-free recyclable polyester from biorenewable non-strained g-Butyrolactone. Angew. Chem., Int. Ed. 2016;55:4188–4193. doi: 10.1002/anie.201601092. [DOI] [PubMed] [Google Scholar]
- 60.Luo C, Bandar JS. J. Am. Chem. Soc. 2018;140:3547–3550. doi: 10.1021/jacs.8b00766. [DOI] [PubMed] [Google Scholar]
- 61.Shigeno M, Hayashi K, Nozawa-Kumada K, Kondo Y. Organic superbase t‑Bu-P4 catalyzes amination of methoxy(hetero)arenes. Org. Lett. 2019;21:5505–5508. doi: 10.1021/acs.orglett.9b01805. [DOI] [PubMed] [Google Scholar]
- 62.Shigeno M, Hayashi K, Korenaga T, Nozawa-Kumada K, Kondo Y. Organic superbase t-Bu-P4-catalyzed demethylations of methoxyarenes. Org. Chem. Front. 2022;9:3656–3663. doi: 10.1039/D2QO00483F. [DOI] [Google Scholar]
- 63.Luo C, et al. J. Am. Chem. Soc. 2022;144:9586–9596. doi: 10.1021/jacs.1c13397. [DOI] [PubMed] [Google Scholar]
- 64.Bordwell FG, Hughes DL. Thiol Acidities and Thiolate ion reactivities toward Butyl Chloride in Dimethyl Sulfoxide Solution. The question of curvature in Brønsted plots. J. Org. Chem. 1982;47:3224–3232. doi: 10.1021/jo00138a005. [DOI] [Google Scholar]
- 65.Rossi RA, Pierini AB, Peñéñory AB. Nucleophilic substitution reactions by electron transfer. Chem. Rev. 2003;103:71–167. doi: 10.1021/cr960134o. [DOI] [PubMed] [Google Scholar]
- 66.Zhang XM, Yang DL, Liu YC. Effects of electron acceptors and radical scavengers on nonchain radical nucleophilic substitution reactions. J. Org. Chem. 1993;58:224–227. doi: 10.1021/jo00053a040. [DOI] [Google Scholar]
- 67.Zhang XM, Yang DL, Jia XQ, Liu YC. Kinetic and mechanistic studies of the nonchain radical nucleophilic substitution reactions. J. Org. Chem. 1993;58:7350–7354. doi: 10.1021/jo00078a010. [DOI] [Google Scholar]
- 68.Orsi DL, Easley BJ, Lick AM, Altman RA. Base catalysis enables access to α,α-Difluoroalkylthioethers. Org. Lett. 2017;19:1570–1573. doi: 10.1021/acs.orglett.7b00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bordwell FG, McCallum RJ, Olmstead WN. Acidities and hydrogen bonding of phenols in dimethyl sulfoxide. J. Org. Chem. 1984;49:1424–1427. doi: 10.1021/jo00182a020. [DOI] [Google Scholar]
- 70.Nove, M., Dell’Erba, C. & Sancassan, F. ipso- and tele-substitution pathways in the reactions of 1,3-Dimethyl-2,4-dinitro- and 1.3-Dimethyl-2-nitro-4-phenylsulphonylnaphthalene with Sodium Arenethiolates in Dimethyl Sulphoxide. J. Chem. Soc. Perkin. Trans. I 1145–1149 (1983).
- 71.Starosotnikov AM, Shevelev SA. Characteristic features of nucleophilic substitution in the series of 4-RSO2-6-nitro-1-phenyl-1H-indazoles and benzo[d]isoxazoles. Russ. Chem. Bull. Int. Ed. 2003;52:1797–1799. doi: 10.1023/A:1026012922607. [DOI] [Google Scholar]
- 72.Thompson A, et al. Sulfur-based protecting groups for pyrroles and the facile deprotection of 2-(2,4-Dinitrobenzene)sulfinyl and Sulfonyl Pyrroles. J. Org. Chem. 2005;70:3753–3756. doi: 10.1021/jo050077b. [DOI] [PubMed] [Google Scholar]
- 73.Zhou P, Yao J, Hu G, Fang J. Naphthalimide scaffold provides versatile platform for selective thiol sensing and protein labeling. ACS Chem. Biol. 2016;11:1098–1105. doi: 10.1021/acschembio.5b00856. [DOI] [PubMed] [Google Scholar]
- 74.Begunov RS, Valyaeva AN, Fakhrutdinov AN, Pirogova SA. Synthesis of a new monomer for sulfonated poly(arylene ether sulfones) Russ. Chem. Bull. Int. Ed. 2017;66:545–551. doi: 10.1007/s11172-017-1769-9. [DOI] [Google Scholar]
- 75.Vamisetti GB, Meledin R, Nawatha M, Suga H, Brik A. The development of a fluorescence-based competitive assay enabled the discovery of dimeric cyclic peptide modulators of ubiquitin chains. Angew. Chem. Int. Ed. 2021;60:7018–7023. doi: 10.1002/anie.202013392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sugahara T, Murakami K, Yorimitsu H, Osuka A. Palladium-catalyzed amination of aryl sulfides with anilines. Angew. Chem. Int. Ed. 2014;53:9329–9333. doi: 10.1002/anie.201404355. [DOI] [PubMed] [Google Scholar]
- 77.Takenaga, N., et al. Catalytic and non-catalytic selective aryl transfer from (mesityl)iodonium(III) salts to diarylsulfide compounds. Arkivoc 7–18 (2022).
- 78.Mu, L., et al. 18F-Radiolabeling of aromatic compounds using Triarylsulfonium salts. Eur. J. Org. Chem. 889–892 (2012).
- 79.Colquhoun HM, Hodge P, Paoloni FPV, McGrail PT, Cross P. Reversible, nondegradative conversion of crystalline aromatic Poly(ether ketone)s into Organo-Soluble Poly(ether dithioketal)s. Macromolecules. 2009;42:1955–1963. doi: 10.1021/ma8023377. [DOI] [Google Scholar]
- 80.Gunaratne HQN, Langrick CR, Puga AV, Seddon KR, Whiston K. Production of polyetheretherketone in ionic liquid media. Green. Chem. 2013;15:1166–1172. doi: 10.1039/c3gc36754a. [DOI] [Google Scholar]
- 81.Wang J, Liu Z. An efficient synthetic strategy for high performance polysulfone: ionic liquid/zwitterion as reaction medium. Green. Chem. 2012;14:3204–3210. doi: 10.1039/c2gc35704f. [DOI] [Google Scholar]
- 82.Park D-Y, Kohl PA, Beckham HW. Anion-conductive multiblock aromatic copolymer membranes: structure−property relationships. J. Phys. Chem. C. 2013;117:15468–15477. doi: 10.1021/jp311987v. [DOI] [Google Scholar]
- 83.García JM, et al. Meisenheimer complex inspired catalyst- and solvent-free synthesis of noncyclic Poly(aryl ether sulfone)s. Macromolecules. 2014;47:8131–8136. doi: 10.1021/ma501735q. [DOI] [Google Scholar]
- 84.Yang Y, Muhich CL, Green MD. Kinetics and mechanisms of polycondensation reactions between aryl halides and bisphenol A. Polym. Chem. 2020;11:5078–5087. doi: 10.1039/D0PY00740D. [DOI] [Google Scholar]
- 85.Park S-A, et al. Sustainable and recyclable super engineering thermoplastic from biorenewable monomer. Nat. Commun. 2019;10:2601. doi: 10.1038/s41467-019-10582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoshi T, Bae B, Watanabe M, Miyatake K. Synthesis and properties of sulfonated Poly(arylene ether) block copolymers as proton conductive membranes. Bull. Chem. Soc. Jpn. 2012;85:389–396. doi: 10.1246/bcsj.20110305. [DOI] [Google Scholar]
- 87.Miyame J, Watanabe M, Miyatake K. Sulfonated Poly(arylene ether phosphine oxide ketone) block copolymers as oxidatively stable proton conductive membranes. ACS Appl. Mater. Interfaces. 2013;5:5903–5907. doi: 10.1021/am401625j. [DOI] [PubMed] [Google Scholar]
- 88.Miyame J, Watanabe M, Miyatake K. Intrapolymer Heck reaction for proton conductive ladder-type aromatic block copolymers. RSC Adv. 2014;4:21049–21053. doi: 10.1039/C4RA02582B. [DOI] [Google Scholar]
- 89.Hoshi T, Miyake J, Watanabe M, Miyatake K. Synthesis and properties of sulfonated and brominated Poly(arylene ether)s as proton conductive membranes. Bull. Chem. Soc. Jpn. 2015;88:183–191. doi: 10.1246/bcsj.20140237. [DOI] [Google Scholar]
- 90.Choi J, et al. Application of spirobiindane-based microporous poly(ether sulfone)s as polymeric binder on solid alkaline exchange membrane fuel cells. J. Membr. Sci. 2018;568:67–75. doi: 10.1016/j.memsci.2018.09.048. [DOI] [Google Scholar]
- 91.Faye A, Furtos A, Brisson J. Synthesis of high molecular weight polyetherethersulfone-allyl copolymers of controlled glass transition. Macromol. Chem. Phys. 2016;217:2125–2138. doi: 10.1002/macp.201600229. [DOI] [Google Scholar]
- 92.Pirali-Hamedani M, Mehdipour-Ataei S. Effect of sulfonation degree on molecular weight, thermal stability, and proton conductivity of poly(arylene ether sulfone)s membrane. Des. Monomers Polym. 2017;20:54–65. doi: 10.1080/15685551.2016.1231035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu Z, et al. Multi-sulfonated polyhedral oligosilsesquioxane (POSS) grafted poly(arylene ether sulfone)s for proton conductive membranes. Polymer. 2017;123:21–29. doi: 10.1016/j.polymer.2017.07.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary File
Data Availability Statement
The data obtained in this study are available within this article and its supplementary information and are also from the corresponding authors upon reasonable request. Original 1H and 13C spectra of the compounds obtained in this manuscript are available in Supplementary Data 1. The computed energy values and coordinates are available in Supplementary Data. 2.