Abstract
During sexual encounters, individuals often use signals, such as display traits, to attract mates. If individuals alter their display traits with respect to the genotype of potential mates, indirect genetic effects (IGEs) may occur in which the genes of one individual influence the phenotype of another. Although IGEs between related individuals have received much attention, their occurrence between unrelated individuals during sexual encounters has not. Here, we demonstrate that in the Australian fruit fly Drosophila serrata, males assess females by using both visual and olfactory cues, resulting in a rapid plastic response (within minutes) in male cuticular hydrocarbons (CHCs), a display trait that is an important target of mate choice. Several CHCs in males exhibited significant IGEs, and IGEs were inducible on both males reared in the laboratory and on field-caught individuals. A vector describing genetic variance in multiple CHCs in females was found to be almost identical to a vector describing indirect genetic variance in male CHCs, suggesting that males might assess female CHCs during courtship. This vector displayed contributions from all female CHCs in the same direction and of similar magnitude, suggesting that female condition may be the underlying casual trait that males are assessing. Consistent with this interpretation, when measured directly in a separate experiment, genetic variance in female condition accounted for 19.8% of the indirect genetic variance in male CHCs. These indirect genetic effects have the potential to alter the response to selection of male sexual display traits.
Keywords: cuticular hydrocarbons, Drosophila serrata, indirect genetic effect, signal traits, social interaction
Quantitative genetics studies have traditionally modeled the phenotype of an individual as the product of two major components: their genes and the environment that they experience during development (1). However, when social interactions between conspecific individuals occur, one individual provides the “environment” for another, meaning that the environment can have a genetic component (2-4). This complexity introduces a new component that may affect an individual's phenotype: indirect genetic effects (IGEs).
IGEs occur whenever the genes of one individual, through their effect on that individual's phenotype, influence the phenotypic expression of a trait in an interacting individual (5). There are two requirements for IGEs to occur (6). First, the trait of interest must be phenotypically plastic with respect to some aspect of an interacting individual's phenotype. Phenotypic plasticity is the ability of a genotype to exhibit different phenotypes in different environments (6-8). Second, the trait(s) of the interacting individual acting as the environmental cue must have a genetic basis, otherwise the effect will be purely environmental (9, 10).
When these requirements are met, IGEs can occur and may influence evolutionary change (11). IGEs may create unique evolutionary dynamics for the traits involved because IGEs can alter the rate and/or the direction of a trait's response to selection (2, 4, 6, 10), and the environment, because it contains genes, may itself evolve (2, 4, 6, 10, 12-14). Under some circumstances, IGEs can allow the evolution of traits with little or no direct genetic variance (2, 10, 15-17).
To date, the primary focus of the study of IGEs has been concerned with parent-offspring interactions, such as maternal/parental effects (9, 16, 18-21), and other interactions between related individuals (5). Only recently have models been adapted to include social interactions between unrelated individuals (2, 12, 22). Although models involving related individuals have focused on morphological traits, many interactions between unrelated individuals involve behavioral traits (12). Such traits often are capable of rapid and reversible responses to various social interactions (i.e., environments) and are thus highly plastic (7). Here, we focus on one type of behavior, display traits used during sexual interactions, which often exhibit substantial phenotypic plasticity (23, 24).
IGEs in sexual display traits are of particular importance because they can affect mating success, a major component of fitness. Display traits also are often the target of sexual selection, and IGEs on these traits may alter their evolutionary trajectory (2). However, despite the potential importance of IGEs in sexual display traits, little is known about their prevalence. The existence of IGEs in Drosophila melanogaster is suggested by an experiment demonstrating that an aspect of male courtship (time to copulation) is plastic with respect to an interacting female's genotype (25). However, quantitative estimates of the IGEs on this male trait were not possible given the design of the experiment, nor were the female traits acting as the environmental cue identified.
Here, we use the Australian fruit fly Drosophila serrata to provide an experimental demonstration of the presence of IGEs in male sexual display traits. D. serrata use contact pheromones, composed of nonvolatile cuticular hydrocarbons (CHCs), as a signal system for both mutual (male and female) mate choice within populations (26-30) and species recognition (31, 32). Like many signaling systems (33, 34), mate choice in D. serrata depends on the collective presence of multiple traits (CHCs), and it is therefore necessary to consider IGEs on the signaling system as a whole. To do this, we conduct three separate experiments and apply a series of multivariate quantitative genetic analyses to uncover IGEs on male sexual displays (CHCs) and to determine how genes underlying female signal traits (CHCs) and condition may influence male sexual displays.
First, a manipulative experiment was used to determine whether male D. serrata change their CHC profile during social interactions, including mating. Because past perfuming experiments (31, 35) have shown that mating success can be altered by the physical transfer of CHCs among individuals, it was important to determine the role of IGEs in changes in male CHCs, and to determine at what stage during courtship these effects were elicited. Second, a laboratory population was used to estimate IGEs on male display traits (CHCs), resulting in an estimate of the additive genetic variance-covariance matrix of indirect genetic effects (GIGE) for male CHCs. Within the same experimental design, we also estimated the G matrix of direct genetic effects (GDGE) for the homologous set of female phenotypes. We then compared these two matrices to determine whether males were using female CHCs as an environmental cue to change their own CHCs.
Finally, increased environmental heterogeneity in nature (36, 37) may make IGEs harder to detect in field phenotypes. However, their presence would imply an important role for IGEs under natural developmental conditions. We therefore estimated IGEs on CHCs from field-caught males. In addition, because results from the second experiment suggested female condition as a possible environmental cue for IGEs on male CHCs, we also estimated the direct genetic variance in female body condition. To explore the role of female condition as an environmental cue, we estimated the direct-indirect genetic covariance between female condition and male CHCs.
Methods
Experiment 1: Manipulation of the Social Environment. Light CO2 anesthesia was used to collect virgin males and females from the Forster stock of D. serrata described in refs. 26, 27, and 32. Flies were held separately by sex in vials (five females per vial and one male per vial) with 5 ml of standard yeast medium and a small amount of live yeast sprinkled on top. When the experiment was conducted, females were 5 days posteclosion, and males were 4 days posteclosion. Except as indicated below, all handling of flies on the day of the experiment was conducted in the absence of any anesthesia.
The experiment involved seven independent “social environment” treatments designed to determine the timing and source of any changes in male CHCs. In the first treatment (control), single males were removed from their holding vials and placed directly into hexane for CHC extraction. This treatment provides a baseline for male CHCs in the absence of any social interactions with either sex. To evaluate the degree to which CHCs transfer between individuals as a by-product of physical contact, we conducted two treatments. In the first treatment, a single male and a single female were anesthetized by using CO2 and then placed together in an empty 0.3-ml glass vial and vortexed for 30 sec. The male was then removed for CHC extraction. In the second treatment, a single male and a single female were again anesthetized, and their abdomens were rubbed together by using tweezers (10 “strokes” per pair). The male was then placed in hexane for CHC extraction.
The remaining treatments were designed to determine whether males altered their CHCs in response to various social interactions. The first treatment addressed male-male interactions and involved allowing two randomly chosen males to interact in a vial for 15 min, after which one of the males was randomly chosen for CHC extraction. The final three treatments represent an escalating degree of male-female interaction characteristic of mating. In the first treatment, visual contact only was permitted by abutting two vials by their open ends with a glass slide placed between, forming a barrier to physical contact. A single male was placed in one vial, and a single female was placed in the other. Vials were filled with 35 ml of standard yeast medium, thus ensuring that the pairs made visual contact by confining them to a small area at the top of their respective vials. After 15 min, the male was removed for CHC extraction. In the next treatment, brief physical contact was permitted by placing a male and female together in a vial. During the courtship sequence, D. serrata males sometimes approach and touch the female (38); this is often immediately followed by an attempt by the male to mount. Pairs were observed, and as soon as the male touched the female, he was removed and held separately in a vial for ≈5 min, after which he had his CHCs extracted for analysis. Courtship was thus interrupted before mounting, and physical contact was limited to a single touch. The final mating treatment used the same design as the previous treatment, except that pairs were allowed to mate. After copulation terminated, the male was removed for CHC extraction.
The CHC profile of males analyzed here consists of the relative concentration of nine compounds: Z,Z-5,9-C24:2, Z,Z-5,9-C25:2, Z-9-C25:1, Z-9-C26:1, 2-Me-C26, Z,Z-5,9-C27:2, 2-Me-C28, Z,Z-5,9-C29:2, and 2-Me-C30. Assaying males on gas chromatograph (model 6890N, Agilent Technologies, Palo Alto, CA), by using the method described in ref. 31, produced peaks representing each of these compounds. The relative concentration of each compound was measured as the area under the corresponding peak divided by the total area under all peaks. To break the unit-sum constraint generated by this division, logcontrasts were generated (31, 39) by using Z,Z-5,9-C24:2 as the denominator, reducing the number of traits to eight. All analyses were conducted by using standardized logcontrasts.
A principal component analysis was performed on the covariance matrix of the eight male CHCs because a moderate degree of multicollinearity existed among these traits in this data set. To conserve degrees of freedom, the first five principal components, accounting for 97.2% of the variance in male CHCs, were used in subsequent tests for treatment effects.
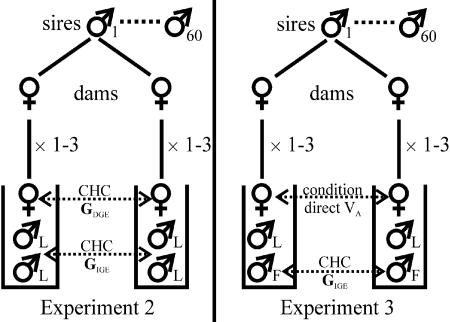
Experiment 2: IGEs on Laboratory-Reared Male CHCs. This experiment was first described in ref. 27 and was used to estimate the intersex genetic correlations among homologous male and female CHCs. Here, we use data from this experiment to estimate IGEs on male sexual display traits (CHCs). Briefly, using individuals from the Forster stock, a half-sib breeding design was established with 60 sires mated to each of two dams (Fig. 1). Female-choice mating trails were conducted in which daughters from the half-sib families were given a choice of two random males from the Forster laboratory stock. When one male successfully mated with the female, both of the males and the female then had their CHCs extracted and analyzed by using GC. Because both chosen and rejected males are included in the indirect genetic analyses below, female mate choice has not been permitted to restrict the male phenotypes that are considered.
Fig. 1.
Overview of the half-sibling mating design used in experiments 2 and 3. In both cases, males used in the mating trials are unrelated to the dams or sires and are randomly selected from either a laboratory (♂L) or field-caught (♂F) population.
The half-sib breeding design allowed the phenotypic variance to be partitioned into its various components, both genetic and environmental (40). Of interest here were the additive genetic (co)variances, estimated from the sire variance components from the standard nested linear model (the details are described in Supporting Text, which is published as supporting information on the PNAS web site). Using traits of females that were directly related to sires (as daughters) resulted in an estimate of GDGE. Because males were only indirectly related to sires through their interaction with daughters, using male traits produced an estimate of GIGE.
There are at least two methods that can be used to determine whether IGEs on male CHCs are associated with female display traits (i.e., CHCs). One approach is to estimate the direct-indirect genetic covariance matrix between female CHCs (directly affected by a sire's genes) and male CHCs (indirectly affected by a sire's genes). In our case, however, the mixed model containing the eight male and eight female traits failed to converge by using the mixed procedure in the sas statistical software package (SAS Institute, Cary, NC). In addition, this approach would have generated 64 direct-indirect genetic covariances that would have posed a considerable challenge to interpretation.
We therefore took an alternative approach that compared the eigenstructure of GDGE for female CHCs and GIGE for male CHCs. This approach estimates the response of male CHCs to the genetic (co)variance in female CHCs and resulted in a straightforward interpretation of a potentially complex set of interactions between male and female display traits. This approach allowed us to establish whether there was any similarity in the orientation of female direct genetic variance and male indirect genetic variance that would suggest that genetic variance among females in CHCs was associated with generating indirect genetic variance in male CHCs. The female GDGE and male GIGE were compared by using the geometric approach of Krzanowski (41). The utility of Krzanowski's method for the comparison of variance component-based covariance matrices were detailed in refs. 28 and 29. Briefly, a subset of principal components of each G matrix are selected to represent that part of the subspace of G that is to be compared. Selection of no more than half of the principal components is permitted. Here, we compared the orientation of the subspaces described by the maximum allowable number of principal components (four) that accounted for 93% and 89% of the female direct genetic variance and male indirect genetic variance, respectively (details are described in Supporting Text).
Experiment 3: IGEs on Field-Reared Male CHCs. From the Forster stock, 60 sires were each mated to two to three dams in a half-sib mating design (Fig. 1). From each half-sib family, 1-3 virgin daughters were collected, resulting in a total of 330 daughters. At the same time, 340 virgin males also were collected from the half-sib families, to be used as competitors for field males in the mating trials. When these lab individuals were 5 days old, 340 field males were captured from a natural population at the University of Queensland (29) and maintained on standard yeast medium for 2 days. The 7-day-old laboratory females and males and the field males were then used in female-choice mating experiments as in experiment 2. To permit their identification, male wings were clipped (field males on the right and laboratory males on the left) before conducting the mating trials. After one of the males successfully mounted the female, the field male was then removed and prepared for GC analysis (31). A total of 324 field males (227 chosen by the female and 93 rejected) went on to GC analysis.
Before conducting the indirect genetic analyses, we first estimated sexual selection on male CHCs to confirm their role as sexual display traits in field males. Sexual selection on field-male CHCs was determined by using a discriminant function analysis (42), resulting in a new univariate trait (the canonical variate) that best distinguished between chosen and rejected males. The sexual selection gradient (β) was estimated by regressing mating success onto this canonical variate, and a nonparametric randomization test (two-tailed) was used for significance testing (26). Using this approach, sexual selection on CHCs in field-reared males was significant (β = 0.059, P = 0.030), confirming the importance of CHCs as display traits during mating. Indirect genetic analysis of CHCs from field-caught males was then conducted as described in experiment 2 for the laboratory-reared individuals, resulting in an estimate of GIGE for field-caught males.
There are several methods for estimating condition, and the best approach for insects is unresolved (43). Here, we calculated female condition by using one of the most common methods: the residuals from the regression of body mass on body size (44, 45). Female (live) body mass was determined at 3 days posteclosion, and wing length, measured as the distance between the intersection of the anterior cross vein and longitudinal vein 3 to the intersection of longitudinal vein 3 and the distal wing margin (46), was used as an index of body size.
To determine whether IGEs on male CHCs are associated with female condition, we estimated the direct-indirect genetic covariance between female condition and each of the eight male CHCs. This analysis was done by including female condition in the standard nested half-sib model (40). A significant direct-indirect genetic covariance would indicate that the genes that influence female condition directly also influence male CHC expression indirectly. In addition, an estimate of the direct additive genetic variance in female condition also was possible.
A redundancy analysis (47) was used to determine the importance of female condition genes on the expression of male CHCs as a whole by estimating the percent of IGEs on male CHCs associated with genetic variance in female condition. Redundancy analysis is appropriate here, because instead of one response and multiple predictor variables, as is typically the case, our data have multiple response variables (the eight male CHCs) and only one predictor variable (female condition). Redundancy analysis allowed the effect of this predictor variable on a linear combination of the response variables to be determined. Details of how the redundancy analysis was conducted at the genetic level are given in Supporting Text.
Results
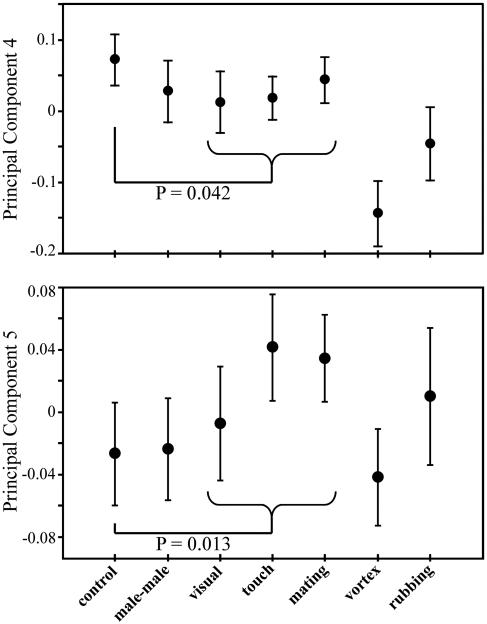
Experiment 1: Manipulation of the Social Environment. Male CHCs varied among treatments (multivariate ANOVA: Wilks' λ = 0.540, F30,834 = 4.63, P < 0.0001). Much of this variation is accounted for by the manually induced physical transfer of some CHCs between the sexes (i.e., the vortex and rubbing treatments; see Fig. 2). However, males also altered their CHCs significantly during social interactions with females in the course of mating [multivariate planned comparison of mating treatments (visual, touch, and mating) vs. control: Wilks' λ = 0.948, F5,208 = 2.28, P = 0.048]. This effect was contributed to most by principal components (PC) 4 and 5 (Fig. 2); the equivalent univariate planned comparison was significant for each (PC4: F1,212 = 4.19, P = 0.042; PC5: F1,212 = 6.28, P = 0.013). Changes in these male CHCs were not the result of physical transfer during mating (i.e., rubbing). Seeing (PC4, Fig. 2) or simply touching (PC5, Fig. 2) the female was sufficient to bring about the change, and in neither case was it enhanced by the physical act of mating (during which some physical transfer may occur).
Fig. 2.
Variation among treatments in two principal components of eight male CHCs (experiment 1). Treatments are as follows: single virgin male (control), two virgin males interacting (male-male), virgin male sees a virgin female (visual), virgin male touches a virgin female (touch), male and female mate (mating), and virgin male and female anesthetized and then vortexed together (vortex) or rubbed together by using tweezers (rubbing). Error bars are ±2 SE.
Experiment 2: IGEs on Laboratory-Reared Male CHCs. Female CHCs displayed significant additive genetic (co)variances in a number of cases (Table 1). In particular, Z,Z-5,9-C25:2, Z,Z-5,9-C27:2, and Z,Z-5,9-C29:2 displayed significant genetic variances. Two of the three genetic covariances between pairs of these CHCs also were significant, as were two other covariances involving one of these CHCs with a separate CHC (Table 1). An eigenanalysis of female GDGE revealed that 72% of the genetic variance in female CHCs was described by the first principal component (gmax) that had contributions from all traits in the same direction and of similar magnitude, with the exception of 2-Me-C26 for which the contribution was lower (Table 2).
Table 1. Additive direct genetic variance-covariance matrix (GDGE) for eight CHCs from laboratory-reared female D. serrata (experiment 2).
| Z,Z-5,9-C25:2 | Z-9-C25:1 | Z-9-C26:1 | 2-Me-C26 | Z,Z-5,9-C27:2 | 2-Me-C28 | Z,Z-5,9-C29:2 | 2-Me-C30 | |
|---|---|---|---|---|---|---|---|---|
| Z,Z-5,9-C25:2 | 0.187** | 0.153 | 0.129 | 0.004 | 0.206** | 0.094 | 0.150 | 0.125 |
| Z-9-C25:1 | 0.116 | 0.028 | 0.012 | 0.163* | 0.093 | 0.115 | 0.106 | |
| Z-9-C26:1 | 0.168 | 0.040 | 0.140 | 0.093 | 0.140 | 0.123 | ||
| 2-Me-C26 | 0.055 | 0.039 | 0.027 | 0.042 | −0.008 | |||
| Z,Z-5,9-C27:2 | 0.320*** | 0.134 | 0.270** | 0.169* | ||||
| 2-Me-C28 | 0.092 | 0.126 | 0.099 | |||||
| Z,Z-5,9-C29:2 | 0.267** | 0.152 | ||||||
| 2-Me-C30 | 0.132 |
*, P < 0.05; **, P < 0.01; ***, P < 0.001.
Table 2. Associations of female direct genetic variance with IGEs in male CHCs.
| Experiment 2
|
Experiment 3
|
|||
|---|---|---|---|---|
| a1 | b1 | gmax | CovA | |
| Z,Z-5,9-C25:2 | 0.575 | 0.575 | 0.382 | 0.053 |
| Z-9-C25:1 | 0.417 | 0.417 | 0.287 | 0.038 |
| Z-9-C26:1 | 0.317 | 0.317 | 0.301 | 0.086* |
| 2-Me-C26 | 0.124 | 0.127 | 0.063 | −0.072 |
| Z,Z-5,9-C27:2 | 0.431 | 0.431 | 0.532 | 0.052 |
| 2-Me-C28 | 0.285 | 0.281 | 0.266 | −0.060 |
| Z,Z-5,9-C29:2 | 0.204 | 0.204 | 0.464 | −0.112* |
| 2-Me-C30 | 0.266 | 0.270 | 0.329 | −0.090* |
Experiment 2: First principal vectors from a comparison of female CHC direct genetic variance (a1) and male CHC indirect genetic variance (b1), and the first principal component of GDEG (gmax). Experiment 3: Direct-indirect genetic covariance between female condition and male CHCs (CovA) for field-caught males (*, P<0.05).
A number of significant IGEs were detected on laboratory-reared male CHC phenotypes (Table 3), including indirect additive genetic variances for three traits: Z-9-C25:1, 2-Me-C26, and 2-Me-C28. The latter two methylalkanes have been shown to be highly genetically correlated (ra = 0.999) in males from this population (28), and their indirect genetic correlation of 0.965 estimated by using data from the current experiment indicates that they also have responded to the presence of females in a highly coordinated manner. In contrast, Z-9-C25:1 displays weaker negative genetic relationships with these methylalkanes.
Table 3. Additive indirect genetic variance-covariance matrix (GIGE) for male Drosophila serrata reared under laboratory conditions (boldface; experiment 2) and field conditions (experiment 3).
| Z,Z-5,9-C25:2 | Z-9-C25:1 | Z-9-C26:1 | 2-Me-C26 | Z,Z-5,9-C27:2 | 2-Me-C28 | Z,Z-5,9-C29:2 | 2-Me-C30 | ||
|---|---|---|---|---|---|---|---|---|---|
| 0.068 | 0.033 | 0.038 | 0.014 | 0.064 | 0.034 | 0.026 | 0.033 | Z,Z-5,9-C25:2 | |
| 0.217*** | 0.099* | −0.066 | 0.108* | −0.080 | 0.044 | −0.063 | Z-9-C25:1 | ||
| Z,Z-5,9-C25:2 | 0.042 | 0.000 | −0.017 | 0.078* | 0.009 | −0.013 | 0.029 | Z-9-C26:1 | |
| Z-9-C25:1 | 0.023 | 0.025 | 0.252*** | −0.006 | 0.195* | −0.050 | 0.073 | 2-Me-C26 | |
| Z-9-C26:1 | −0.047 | −0.072 | 0.095* | 0.107 | 0.005 | −0.007 | 0.007 | Z,Z-5,9-C27:2 | |
| 2-Me-C26 | −0.02 | 0.012 | −0.048 | 0.085 | 0.162* | −0.023 | 0.073 | 2-Me-C28 | |
| Z,Z-5,9-C27:2 | 0.023 | −0.004 | −0.036 | −0.027 | 0.028 | 0.000 | 0.007 | Z,Z-5,9-C29:2 | |
| 2-Me-C28 | −0.055 | 0.011 | −0.067 | 0.016 | −0.013 | 0 | 0.044 | 2-Me-C30 | |
| Z,Z-5,9-C29:2 | −0.079* | 0.034 | −0.099* | 0.076* | −0.011 | 0.050* | 0.048 | ||
| 2-Me-C30 | −0.052 | 0.008 | −0.096* | 0.093* | −0.004 | 0.070* | 0.063 | 0.027 |
*, P < 0.05, ***, P < 0.001.
Comparison of the subspaces of GDGE for female CHCs (Table 1) and GIGE for male CHCs (Table 3), as described by the first four eigenvectors of each matrix, resulted in a sum of the eigenvalues of S of 1.85. This sum can range from 0 (orthogonal subspaces) to 4 (coincident subspaces), indicating that overall similarities between the two matrices is not high when the four-dimensional subspaces are compared. However, the first eigenvalue of S was equal to 0.99996, indicating that the two subspaces shared a common dimension (a value of 1 would indicate a perfect match between the two principal vectors in each subspace). The principal eigenvectors that were compared from the two subspaces are given in Table 2. These loadings indicate that the shared direction of genetic variance is a vector with contributions from all CHCs in the same direction (all are positive) and of similar magnitude, matching results obtained for gmax of GDGE. Again, as with female gmax, 2-Me-C26 displayed the lowest contribution to this common vector.
Experiment 3: IGEs on Field-Reared Male CHCs. A number of significant IGEs were detected on field-caught male CHC phenotypes (Table 3). Only one indirect genetic variance estimate was significant (Z-9-C26:1), but two other traits, Z,Z-5,9-C29:2 and 2-Me-C30, displayed significant indirect genetic covariances with this trait. Four additional indirect genetic covariances, all involving one of the two latter CHCs, also were significant (Table 3). The indirect genetic variance estimated for the methylalkane 2-Me-C28 was zero, a result that agrees with a previous zero estimate of direct genetic variance in field males for this trait (29).
Female condition exhibited significant additive genetic variance (VA = 0.116, P = 0.040), consistent with other recent findings for a genetic basis to condition (44, 48, 49). Direct-indirect genetic covariance estimates between female condition and three male CHCs (Z-9-C26:1, Z,Z-5,9-C29:2, and 2-Me-C30) were significant (Table 2). When male CHCs were examined as a whole, the redundancy analysis indicates that 19.8% of the male CHC indirect genetic variance was explained by genetic variance in female condition.
Discussion
Individuals commonly use signals, such as display traits, to attract mates, and these traits can exhibit substantial phenotypic plasticity in response to environmental influences (23, 24). During sexual interactions, as with other social interactions, environmental influences may include interacting individuals. When the expression of a trait in one individual is influenced by interacting individuals, IGEs may result (2, 3, 12, 22). The presence of IGEs has previously been suggested for traits associated with mate choice (25, 50). Here, we have shown that IGEs occur on male display traits, quantified the level of indirect genetic variance, and shown that female signal traits and condition are genetically associated with the induction of these IGEs.
Previous studies have shown that CHCs are an important determinant of mate choice in D. serrata and are thus under sexual selection (26-28). Here, we have shown that male D. serrata respond rapidly to the presence of females by changing their CHC profiles. Although some physical transfer of CHCs can occur, as revealed by perfuming experiments (31, 35) and our current data, our results also demonstrate that changes in male CHCs can be elicited by the visual presentation of females to males where no contact is allowed. When males are allowed to touch females, additional changes in CHCs occur, but these changes are not further enhanced by allowing the male and female to continue courtship and mate. Taken together, these results suggest that both visual and olfactory cues may be involved in males changing their CHC profiles in the presence of females, and that the physical transfer of CHCs during sexual encounters (in contrast to crowded conditions used in perfuming experiments) is minimal.
Using two half-sib genetic experiments, we have established that IGEs occur during sexual encounters for a combination of CHCs in males reared under both laboratory and field conditions. In addition, we have identified two candidates for the female phenotype that acts as the environmental cue. First, in laboratory-reared males, a vector of genetic variance in female CHCs was closely associated with male IGEs. This vector displayed positive contributions from all CHC traits and was similar in orientation to the direction of greatest direct genetic variance in female CHCs (i.e., gmax). Although this result suggests that, at least in part, males responded to match females in one aspect of their CHC profiles, the overall similarity of the four-dimensional subspaces was quite low, indicating that the majority of indirect genetic variance was not associated with genetic variance in female CHCs.
Second, female condition was another potential environmental cue that was significantly associated with the generation of IGEs. In laboratory-reared males, female condition is a prime candidate for the underlying casual trait to the common dimension shared by GDGE and GIGE because all CHCs contributed positively and in similar magnitude; females in better condition may allocate more resources to CHC production. CHC production is directly affected by resource allocation to the ovaries in D. melanogaster (51) and other insects (52), and natural selection has been shown to affect the evolution of CHCs in female D. serrata (53), all of which suggest that female CHC production is likely to be affected by the availability of resources. In support of this view, by using field-caught males, we found that genetic variance in female condition accounted for 19.8% of the indirect genetic variance in male CHCs. Therefore, in two environments, female condition is implicated as an environmental cue associated with part of the indirect genetic variance in male CHCs.
Although both female CHCs and condition have been shown to be genetically related to male IGEs in this system, we are unable to determine their independent or combined effect in eliciting male IGEs by using our current data for two reasons. First, these experiments do not allow an estimate of the genetic correlation between female CHCs and female condition. It is, therefore, not known whether genetic variance in female condition explains a different part of the male IGEs than genetic variance in female CHCs. Second, even if female CHCs and condition are genetically correlated (as we suggest above), such correlative experiments would not be able to determine whether males assess female CHCs to indirectly assess condition or whether males can assess female condition directly. The importance of condition and condition-dependent expression of signal traits has proved difficult to ascertain (54). The presence of IGEs in male display traits that may be induced by female condition suggests that males consider female condition an important indicator of fitness.
The estimates of GIGE from laboratory-reared and field-caught males (Table 3) display a number of apparent differences. Our experiments, however, were not specifically designed to look for variation in the ability of males to respond to female genotypes (the two G matrices come from two different geographic populations) or the effect of the environment on the ability to detect indirect genetic effects (the matrices also derive from males raised in different environments). Given the confounded nature of the sources of variation that may have resulted in any differences between our two G matrices, we have refrained from making this comparison.
It is unlikely that we have described all of the IGEs that occur during courtship in this system with our current experiments, because female phenotypes, too, may be influenced by IGEs, introducing the potential for reciprocal effects. A detailed treatment of reciprocal effects between unrelated individuals during social interactions reveals that they may create a feedback system whereby the IGEs in one individual can feed back on the individual that caused them (2). This feedback can have important consequences for the interaction, as well as for the expression of the traits involved (2). Therefore, if IGEs also are occurring in female traits because females alter their signal in response to a male's genotype, the situation may be more complex than is considered here.
IGEs generated during sexual encounters, such as those described in the present study, may play an important role in the evolution of sexually selected traits. For example, as outlined in the theoretical treatment, the response to selection of sexually selected traits may not be accurately predicted in the absence of information on IGEs (2). Measurements of the response to selection also must take IGEs into account and be conducted after the social interactions generating them. In addition, selection that operates directly on the trait acting as the environment (here, female CHCs and/or condition) also may affect the response to selection of the interacting male trait. Therefore, the response to selection of male display traits may vary considerably from that predicted when IGEs are ignored. Full parameterization of the theoretical models (2), even for the simple situation of no reciprocal effects, will require more sophisticated genetic analyses than those presented here to result in a predicted response to selection. A comprehensive understanding of the evolution of male display traits remains a considerable challenge for evolutionary biologists.
Supplementary Material
Acknowledgments
We thank J. Hunt and two anonymous reviewers for comments on the manuscript. S.F.C., H.D.R., and M.W.B. were supported by the Australian Research Council.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CHC, cuticular hydrocarbon; DGE, direct genetic effect; IGE, indirect genetic effect.
References
- 1.Falconer, D. S. & Mackay, T. F. C. (1996) Introduction to Quantitative Genetics (Longman, New York), 4th Ed.
- 2.Moore, A. J., Brodie, E. D., III, & Wolf, J. B. (1997) Evolution (Lawrence, Kans.) 51, 1352-1362. [DOI] [PubMed] [Google Scholar]
- 3.Wolf, J. B., Moore, A. J. & Brodie, E. D., III (1997) Am. Nat. 150, 639-649. [DOI] [PubMed] [Google Scholar]
- 4.Agrawal, A., Brodie, E. D., III, & Wade, M. J. (2001) Am. Nat. 158, 308-323. [DOI] [PubMed] [Google Scholar]
- 5.Wolf, J. B. (2003) Proc. Natl. Acad. Sci. USA 100, 4655-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donohue, K. (2003) Int. J. Plant Sci. 164, S79-S92. [Google Scholar]
- 7.West-Eberhard, M. J. (1989) Annu. Rev. Ecol. Syst. 20, 249-278. [Google Scholar]
- 8.Pigliucci, M. (1992) J. Genet. 71, 135-150. [Google Scholar]
- 9.Kirkpatrick, M. & Lande, R. (1989) Evolution (Lawrence, Kans.) 43, 485-503. [DOI] [PubMed] [Google Scholar]
- 10.Wolf, J. B., Brodie, E. D., III, Cheverud, J. M., Moore, A. J. & Wade, M. J. (1998) Trends Ecol. Evol. 13, 64-69. [DOI] [PubMed] [Google Scholar]
- 11.Cheverud, J. M. (2003) Proc. Natl. Acad. Sci. USA 100, 4357-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore, A. J., Haynes, K. F., Preziosi, R. F. & Moore, P. J. (2002) Am. Nat. 160, S186-S197. [DOI] [PubMed] [Google Scholar]
- 13.Peripato, A. C. & Cheverud, J. M. (2002) Am. Nat. 160, S173-S185. [DOI] [PubMed] [Google Scholar]
- 14.Moore, J. P. & Moore, A. J. (2003) Evol. Dev. 5, 163-168. [DOI] [PubMed] [Google Scholar]
- 15.Griffing, B. (1977) in Proceedings of the International Conference on Quantitative Genetics, eds. Pollak, E., Kempthorne, O. & Bailey, T. B. (Iowa State Univ. Press, Ames, IA), pp. 413-434.
- 16.Hunt, J. & Simmons, L. W. (2002) Proc. Natl. Acad. Sci. USA 99, 6828-6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotiaho, J. S., Simmons, L. W., Hunt, J. & Tomkins, J. L. (2003) Am. Nat. 161, 852-859. [DOI] [PubMed] [Google Scholar]
- 18.Wolf, J. B. (2000) Evolution (Lawrence, Kans.) 54, 1882-1898. [DOI] [PubMed] [Google Scholar]
- 19.McAdam, A. G., Boutin, S., Reale, D. & Berteaux, D. (2002) Evolution (Lawrence, Kans.) 56, 846-851. [DOI] [PubMed] [Google Scholar]
- 20.Rauter, C. M. & Moore, A. J. (2002) J. Evol. Biol. 15, 407-417. [Google Scholar]
- 21.McAdam, A. G. & Boutin, S. (2004) Proc. R. Soc. London Ser. B 271, 75-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore, A. J., Wolf, J. B. & Brodie, E. D., III (1998) in Maternal Effects as Adaptations, eds. Mousseau, T. A. & Fox, C. W. (Oxford Univ. Press, Oxford), pp. 22-41.
- 23.Jia, F. Y., Greenfield, M. D. & Collins, R. D. (2000) Evolution (Lawrence, Kans.) 54, 953-967. [DOI] [PubMed] [Google Scholar]
- 24.Sattman, D. A. & Cocroft, R. B. (2003) Ethology 109, 981-994. [Google Scholar]
- 25.Casares, P., Carracedo, M. C., Miguel, E. S., Pineiro, R. & Garciaflorez, L. (1993) Behav. Genet. 23, 349-358. [DOI] [PubMed] [Google Scholar]
- 26.Hine, E., Lachish, S., Higgie, M. & Blows, M. W. (2002) Proc. R. Soc. London Ser. B 269, 2215-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chenoweth, S. F. & Blows, M. W. (2003) Evolution (Lawrence, Kans.) 57, 2326-2334. [DOI] [PubMed] [Google Scholar]
- 28.Blows, M. W., Chenoweth, S. F. & Hine, E. (2004) Am. Nat. 163, 329-340. [DOI] [PubMed] [Google Scholar]
- 29.Hine, E., Chenoweth, S. F. & Blows, M. W. (2004) Evolution (Lawrence, Kans.) 58, 2754-2762. [DOI] [PubMed] [Google Scholar]
- 30.Chenoweth, S. F. & Blows, M. W. (2005) Am. Nat. 165, 281-289. [DOI] [PubMed] [Google Scholar]
- 31.Blows, M. W. & Allan, R. A. (1998) Am. Nat. 152, 826-837. [DOI] [PubMed] [Google Scholar]
- 32.Higgie, M., Chenoweth, S. & Blows, M. W. (2000) Science 290, 519-521. [DOI] [PubMed] [Google Scholar]
- 33.Moore, A. J. (1997) Evolution (Lawrence, Kans.) 51, 1920-1928. [Google Scholar]
- 34.Brooks, R. & Endler, J. A. (2001) Evolution (Lawrence, Kans.) 55, 1002-1015. [DOI] [PubMed] [Google Scholar]
- 35.Coyne, J. A., Crittenden, A. P. & Mah, K. (1994) Science 265, 1461-1464. [DOI] [PubMed] [Google Scholar]
- 36.Coyne, J. A. & Beecham, E. (1987) Genetics 117, 727-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Orengo, D. J. & Prevosti, A. (1999) Heredity 82, 100-106. [DOI] [PubMed] [Google Scholar]
- 38.Hoikkala, A., Crossley, S. & Castillo-Melendez, C. (2002) J. Insect Behav. 13, 361-373. [Google Scholar]
- 39.Aitchison, J. (1986) The Statistical Analysis of Compositional Data (Chapman & Hall, London).
- 40.Lynch, M. & Walsh, B. (1998) Genetics and Analysis of Quantitative Traits (Sinauer, Sunderland, MA).
- 41.Krzanowski, W. J. (1979) J. Am. Stat. Assoc. 74, 703-707. [Google Scholar]
- 42.Endler, J. A. (1986) Natural Selection in the Wild (Princeton Univ. Press, Princeton).
- 43.Blanckenhorn, W. U. & Hosken, D. J. (2003) Behav. Ecol. 14, 612-618. [Google Scholar]
- 44.Kotiaho, J. S., Simmons, L. W. & Tomkins, J. L. (2001) Nature 410, 684-686. [DOI] [PubMed] [Google Scholar]
- 45.Ostman, O., Ekbom, B., Bengtsson, J. & Weibull, A. C. (2001) Ecol. Appl. 11, 480-488. [Google Scholar]
- 46.Partridge, L., Hoffmann, A. & Jones, J. S. (1987) Anim. Behav. 35, 468-476. [Google Scholar]
- 47.Quinn, G. P. & Keough, M. J. (2002) Experimental Design and Data Analysis for Biologists (Cambridge Univ. Press, Cambridge, U.K.).
- 48.Merila, J. (1996) Funct. Ecol. 10, 465-474. [Google Scholar]
- 49.Merila, J., Kruuk, L. E. B. & Sheldon, B. C. (2001) J. Evol. Biol. 14, 918-929. [Google Scholar]
- 50.Meffert, L. M. (1995) Genetics 139, 365-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wicker, C. & Jallon, J. M. (1995) Eur. J. Entomol. 92, 197-202. [Google Scholar]
- 52.Schal, C., Gu, X. P., Burns, E. L. & Blomquist, G. J. (1994) Arch. Insect. Biochem. Physiol. 25, 375-391. [DOI] [PubMed] [Google Scholar]
- 53.Blows, M. W. (2002) Proc. R. Soc. London Ser. B 269, 1113-1118. [Google Scholar]
- 54.Cotton, S., Fowler, K. & Pomiankowski, A. (2004) Proc. R. Soc. London Ser. B 271, 771-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.