Abstract
Subject terms: Acute myeloid leukaemia, Epidemiology
Dear Editor,
TP53 is a tumor suppressor gene located on the short arm of chromosome 17(p13), known to be involved in multiple cellular processes and regulate cell proliferation by responding to various stress [1, 2]. TP53 is a frequently mutated (m) gene found across diverse types of cancers, strongly linked to large structural and complex chromosomal abnormalities [3, 4]. It is reported in 5–10% of patients diagnosed with acute myeloid leukemia (AML), more prevalent among the elderly and those with therapy-related AML and/or complex cytogenetics (CG). AML with TP53 mutations is associated with poor prognosis and inferior responses to traditional therapies [3, 4].
Venetoclax (VEN), is a potent and selective inhibitor of B-cell leukemia/lymphoma-2 (BCL-2), approved to treat newly diagnosed patients with AML that are unable to receive intensive chemotherapy [5, 6]. Responses to venetoclax and their durability are linked to specific molecular profiles, these associations are particularly notable with mutations in DDX41, RUNX1, SRSF2, NPM1, IDH1 or IDH2 [7–11]. Resistance to venetoclax-based combinations is frequently associated with the emergence of clones that impart resistance and is often linked to specific molecular signatures or pathways (TP53, FLT3, DNMT3A or RAS) [2, 8, 9].
Prior prospective and retrospective studies comparing HMA + VEN induction therapy to other approaches in TP53m AML did not demonstrate improved OS [4, 12]. This outcome might be attributed to the persistence of clones that did not undergo apoptosis during long-term VEN + HMA therapy, as previously documented [7]. In this work, we utilized a multi-institutional real-world database to explore the outcomes of VEN in combination with HMA compared to HMA-based treatment in a larger population of adult TP53m AML patients than previously reported.
The AML database of 381 patients with TP53m AML diagnosed between 2012 and 2022 was queried and 154 (40%) (HMA [N = 50; 32%] and HMA + VEN [N = 104; 68%]) patients were eligible for this analysis. The study conducted after obtaining approval from the Institutional Review Board (IRB), adhering to the ethical standards of the Declaration of Helsinki of 1975, as revised in 2000. Details on diagnosis, response assessment and statistical analysis provided in (Supplementary methods). Bi-allelic TP53m was defined by the presence of (1) 2 or more distinct TP53m with variant allele frequency (VAF) > 10% or a single TP53m associated with (i) complex cytogenetic (CG) abnormalities (ii) involving chromosome 17p (e.g., abnormality of 17p or monosomy 17) or (iii) single TP53m with a VAF of ≥50% [13].
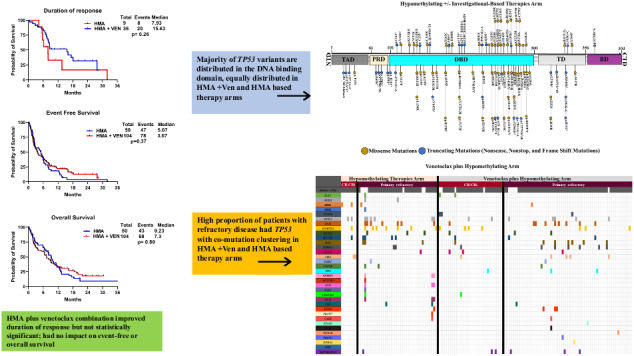
The median age at diagnosis was 74 (38–87) for the HMA group compared to 71 (29–88) for the HMA + VEN group. In the HMA group, 34 (68%) and in the HMA + VEN group, 57 (55%) patients were aged 70 years or older (p = 0.16). Characteristics and hematological features of treatment naive TP53m AML patients undergoing treatment with HMA + VEN vs. HMA, summarized in Supplementary Table 1. Secondary (s) AML (evolving from prior myelodysplasia, and/or myeloproliferative neoplasm [MPN]) was observed in 26% of patients in the HMA group, compared to 31% in the HMA + VEN group (p = 0.6); 2 (4%) and 5 (5%) patients had prior history of MPN progress to AML (MPN blast phase) in HMA and HMA+Ven group, respectively (p ≥ 0.99). Complex cytogenetics (CG) found in 86% of patients in the HMA group and in 90% of the HMA + VEN group (p = 0.6). Regarding TP53 mutation status, 68% of patients in the HMA group had bi-allelic/multi-hit TP53m (MH TP53) compared to 73% in the HMA + VEN group (p = 0.6). The proportion of patients with frequently (≥5%) occurring myeloid co-mutations (RUNX1, ASXL1, TET2, DNMT3A, RAS, and PTPN11) did not demonstrate a significant difference between the HMA and HMA + VEN groups. Additionally, the co-mutation pattern did not predict outcomes within our cohort, as indicated in (Supplementary Table 2). Supplementary Fig. 1 provides an overview of the TP53 protein structure, highlighting its domains and presenting the distribution of detected variants in our study based on different treatment options (HMA vs HMA + VEN).
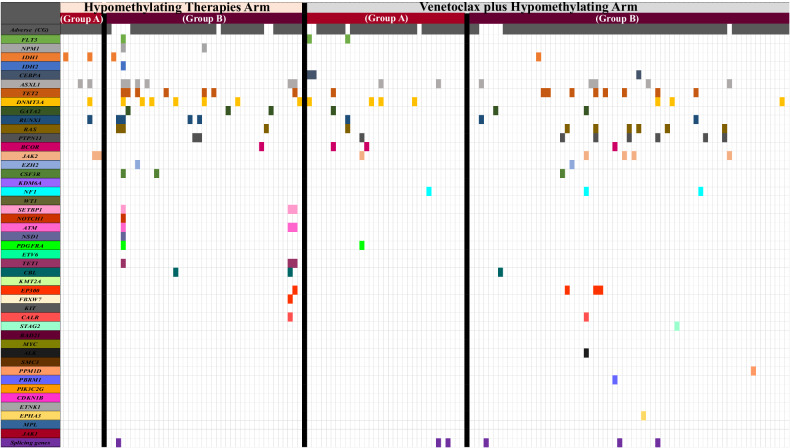
Complete remission rates were higher in the HMA + VEN group compared to the HMA-based treatment group (35% vs. 18%, p = 0.05). Similarly, the proportion of responding patients receiving allogeneic stem cell transplants (allo-HCT) after induction was higher in the HMA + VEN group compared to the HMA group (13% vs. 4%; p = 0.14). Twenty-nine (19%) patients died in first 30 days post induction; 3% in HMA vs 25% in HMA + VEN group (p = 0.03). Among these 29 patients, 18 (HMA + VEN [n = 15] and HMA [n = 3]), 5 (all in HMA + VEN group) and 4 (all in HMA + VEN group) patients died from infection, bleeding, and tumor lysis syndrome, respectively. Details on causes of death summarized in Supplementary Material. In Fig. 1 we have illustrated co-mutation patterns, and cytogenetic status among sub-group of patients who achieved CR/CRi and those who had primary refractory disease.
Fig. 1. Co-Mutation Patterns & adverse cytogenetic findings observed in the TP53 Cohort.
This figure illustrates the patterns of co-mutations identified in the TP53 cohort, categorized by treatment arm. Additionally, the data was further stratified based on two groups: the patients who achieved CR/CRi (A) and the primary refractory group (B).
The median duration of response (DOR) was higher in the HMA + VEN group (15.6 months) compared to the HMA group (7.93 months), but the difference was not statistically significant (p = 0.26) (Fig. 2A). The median follow up of the entire cohort was 6.5 months (range, 0.3–55.7). The median event free survival (EFS) and the median OS did not significantly differ between the HMA and HMA + VEN groups; (5.07 vs. 3.67 months, p = 0.37) and (9.23 vs. 7.3 months, p = 0.80) respectively (Fig. 2B, C).
Fig. 2. Kaplan–Meier survival curves by treatment regimens.
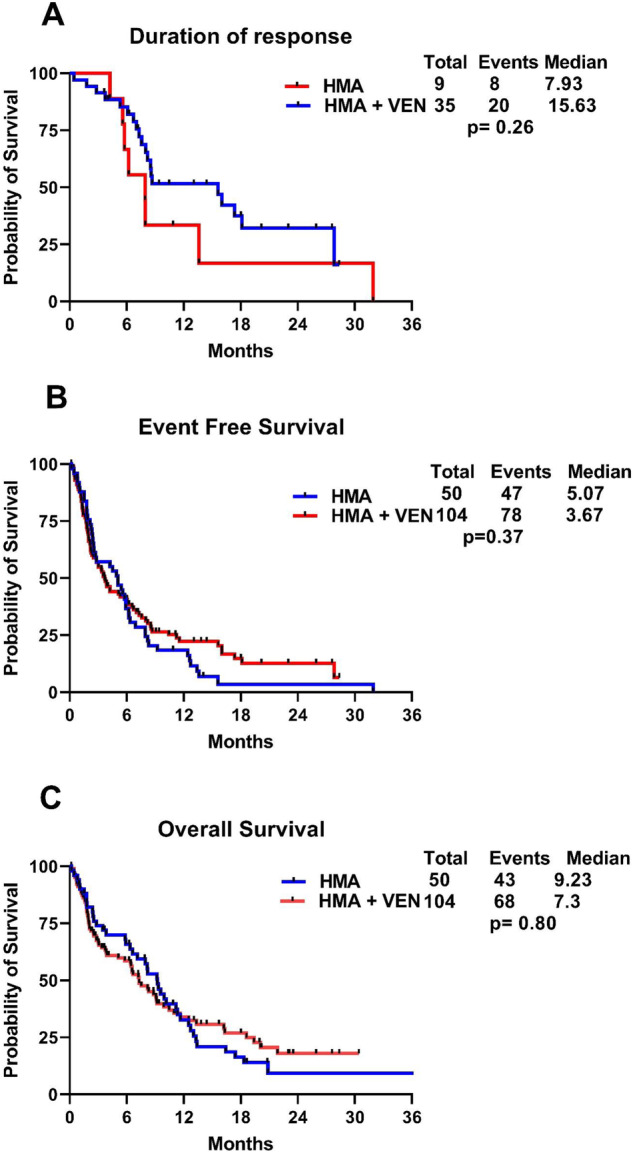
This figure illustrates Kaplan–Meier curves of treatment-naïve mutated TP53 AML patients, stratified by the treatment received—HMA + VEN vs. HMA: A Median Duration of Response. B Event-Free Survival. C Overall Survival.
We performed a multivariate analysis (MVA) for EFS and OS using variables that showed significance in univariate analysis (p < 0.05) (Supplementary Table 2). In MVA, the achievement of CR/CRi retained favorable significance for OS (HR: 0.36, 95% CI: 0.22–0.59 p < 0.001) but not for EFS (HR: 0.80, 95% CI: 0.18–3.52, p = 0.77). Similarly, allo-HCT in responding (CR/CRi) patients maintained a favorable significance for EFS (HR 0.06, 95% CI: 0.09–0.50, p = 0.009) and OS (HR 0.10, 95% CI: 0.14–0.76, p = 0.02) in MVA. Complex cytogenetics retained significance for shorter OS in MVA (HR: 2.47, 95% CI: 1.20–4.81, p = 0.01).
In this multi-institutional real-world study conducted across academic institutions in the US over a span of 10 years, while we have observed improvements in CR rates (35% vs. 18%), an extended median duration of response (15.6 vs. 7.93 months), and a higher proportion of patients progressing toward allo-HCT (13% vs. 4%) with HMA + VEN compared to HMA based treatment, this did not translate in an improvement in OS. Furthermore, no significant differences observed in baseline characteristics, co-mutation patterns, or the presence of complex cytogenetics and MH TP53m among the two groups.
In a recent pooled analysis of two large trials (NCT02993523 and NCT02203773), 54 patients diagnosed with poor-risk cytogenetics and TP53m AML treated with HMA + VEN were compared to 18 patients treated with HMAs [5, 14]. The baseline characteristics and proportion of patients with sAML were similar to those in our analysis. Among patients with TP53m AML from the pooled analysis, the median duration of response (DOR) was 6.5 months for HMA + VEN and 6.7 months for HMAs alone. Similarly, patients had a poor median OS regardless of the treatment approach; 5.2 months for HMA + VEN and 4.9 months for HMAs alone. These findings align with our observations, where we did not observe a survival advantage with the addition of venetoclax to HMA-based therapy. The majority of trials and retrospective reports in the literature on HMA + VEN outcomes in treatment naïve TP53m AML, have reported CR rates of approximately 20–40% and median OS range of (6.0–11.0 months) [5, 9]. In recently conducted real-world study on 301 AML patients, who were treated with HMA + VEN, TP53m was deemed unfavorable with inferior CR rates and overall survival [8]. The data suggest desperate need for novel and effective treatment combinations for patients with TP53m AML.
Although HMA + VEN combination is approved to managed elderly AML patients who are ineligible for intensive chemotherapy, tolerance of this combination in elderly frail patients can be challenging with prolong myelosuppression, infections and increase mortality, especially with continuous dosing of venetoclax. Based on our observation, TP53m AML patients who are frail with sub-optimal performance status can be manage with HMA based therapy alone without compromising their survival or on a reduced duration of venetoclax with HMA based on recent data that suggest no significant benefit in survival with continuous 28 days of venetoclax compared to 21 days or 14 days of venetoclax per cycle [10].
While allo-HCT is considered a potentially curative option for high-risk AML, there are conflicting reports regarding its utility in improving the survival of patients with TP53m AML. The lack of benefit was frequently attributed to the difficulty in achieving a complete response and the persistence of the TP53-mutated clone before undergoing allo-HCT [7]. In our study, among patients with CR/CRi who underwent allo-HCT, regardless of HMA or HMA + VEN induction showed improved OS, as have been reported previously [3].
In conclusion, there is a dire need for the development of more effective treatment strategies that are less susceptible to resistance to improve outcomes of patients with TP53m AML. Progress in immunotherapeutics and approaches targeting mutant TP53 protein may offer promise in improving outcomes for patients diagnosed with TP53m AML [4]. We acknowledge the limitations associated with the retrospective design of our research and the inherent selection bias. Despite these challenges, our data presents the first real-world insights from a substantial multi-center patient cohort with TP53m AML, comprehensively analyzed via NGS and closely monitored longitudinally.
Supplementary information
Acknowledgements
Research reported in this publication from Memorial Sloan Kettering Cancer Center (MSKCC) supported by the NCI of the National Institutes of Health under Award Number P30 CA016359 and P01 CA23766, and Cancer Center Support Grant/Core Grant to MSKCC (P30 CA008748). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Portion of this work was accepted at the American Society of Hematology 2023 and published (PMID: 36807649) [15].
Author contributions
BT designed the study. AN and BT collected the data, conducted the statistical analysis, and interpreted the findings. AN wrote the manuscript, with critical review and approval by all authors.
Competing interests
TB served in advisory board for Pfizer, Takeda and Morphosys. AP received research funding from Pfizer, Kronos Bio; Honoraria from AbbVie and Bristol Myers Squibb. ADG received research funding from AbbVie, Aprea, Aptose, AROG, Celularity, Pfizer, and Prelude; Consultancy and Advisory Boards with AbbVie, Astellas, Daiichi Sankyo; Honoraria from Dava Oncology. The other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-024-01000-2.
References
- 1.Bernard E, Nannya Y, Hasserjian RP, Devlin SM, Tuechler H, Medina-Martinez JS, et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat Med. 2020;26:1549–56. doi: 10.1038/s41591-020-1008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaneko H, Misawa S, Horiike S, Nakai H, Kashima K. TP53 Mutations Emerge at Early Phase of Myelodysplastic Syndrome and Are Associated With Complex Chromosomal Abnormalities. Blood. 1995;85:2189–93. doi: 10.1182/blood.V85.8.2189.bloodjournal8582189. [DOI] [PubMed] [Google Scholar]
- 3.Badar T, Atallah E, Shallis RM, Goldberg AD, Patel A, Abaza Y, et al. Outcomes of TP53-mutated AML with evolving frontline therapies: Impact of allogeneic stem cell transplantation on survival. Am J Hematol. 2022;97:E232–E5. doi: 10.1002/ajh.26546. [DOI] [PubMed] [Google Scholar]
- 4.Daver NG, Maiti A, Kadia TM, Vyas P, Majeti R, Wei AH, et al. TP53-Mutated Myelodysplastic Syndrome and Acute Myeloid Leukemia: Biology, Current Therapy, and Future Directions. Cancer Discov. 2022;12:2516–29. doi: 10.1158/2159-8290.CD-22-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17. doi: 10.1182/blood-2018-08-868752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gangat N, Tefferi A. Venetoclax-based chemotherapy in acute and chronic myeloid neoplasms: literature survey and practice points. Blood Cancer J. 2020;10:122. doi: 10.1038/s41408-020-00388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiNardo CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood. 2020;135:791–803. doi: 10.1182/blood.2019003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gangat N, Karrar O, Iftikhar M, McCullough K, Johnson IM, Abdelmagid M, et al. Venetoclax and hypomethylating agent combination therapy in newly diagnosed acute myeloid leukemia: Genotype signatures for response and survival among 301 consecutive patients. Am J Hematol. 2024;99:193–202. doi: 10.1002/ajh.27138. [DOI] [PubMed] [Google Scholar]
- 9.Gangat N, Johnson I, McCullough K, Farrukh F, Al-Kali A, Alkhateeb H, et al. Molecular predictors of response to venetoclax plus hypomethylating agent in treatment-naïve acute myeloid leukemia. Haematologica. 2022;107:2501–5. doi: 10.3324/haematol.2022.281214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karrar O, Abdelmagid M, Rana M, Iftikhar M, McCullough K, Al-Kali A, et al. Venetoclax duration (14 vs. 21 vs. 28 days) in combination with hypomethylating agent in newly diagnosed acute myeloid leukemia: Comparative analysis of response, toxicity, and survival. Am J Hematol. 2024;99:E63–E66. doi: 10.1002/ajh.27180. [DOI] [PubMed] [Google Scholar]
- 11.Begna KH, Gangat N, Al-Kali A, Litzow MR, Hogan WJ, Patnaik MM, et al. Acute myeloid leukemia after age 70 years: A retrospective comparison of survival following treatment with intensive versus HMA ± venetoclax chemotherapy. Am J Hematol. 2021;96:E108–E11. doi: 10.1002/ajh.26112. [DOI] [PubMed] [Google Scholar]
- 12.DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. 2020;383:617–29. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 13.Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka H-M, et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. 2022;140:1200–28. doi: 10.1182/blood.2022015850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollyea DA, Pratz KW, Wei AH, Pullarkat V, Jonas BA, Recher C, et al. Outcomes in Patients with Poor-Risk Cytogenetics with or without TP53 Mutations Treated with Venetoclax and Azacitidine. Clin Cancer Res. 2022;28:5272–9. doi: 10.1158/1078-0432.CCR-22-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badar T, Atallah E, Shallis R, Saliba AN, Patel A, Bewersdorf JP, et al. Survival of TP53-mutated acute myeloid leukemia patients receiving allogeneic stem cell transplantation after first induction or salvage therapy: results from the Consortium on Myeloid Malignancies and Neoplastic Diseases (COMMAND) Leukemia. 2023;37:799–806. doi: 10.1038/s41375-023-01847-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.