Abstract
Astronauts beyond the Earth's orbit are exposed to high-energy cosmic-ray nuclei with high values of linear energy transfer (LET), resulting in much more biological damage than from x-rays or γ-rays and may result in mutations and cancer induction. The relative biological effectiveness of these nuclei depends on the LET, rising to as high as ≈50 at LET values of ≈100-200 keV/μm. An endpoint of concern is germ cell mutations passed on to offspring, arising from exposure to these nuclei. A vertebrate model for germ cell mutation is Medaka fish (Oryzias latipes). We exposed wild type males to doses of 1 GeV per nucleon Fe nuclei or to 290 MeV per nucleon C nuclei. They were mated to females with recessive mutations at five-color loci. The transparent embryos from >100 days of mating (representing exposed sperm, spermatids, or spermatogonia) were observed so as to detect dominant lethal mutations and total color mutations, even though the embryos might not hatch. The relative number of mutant embryos as a function of dose were compared with those induced by γ-rays. The relative biological effectiveness values for dominant lethal mutations and total color mutations for exposed sperm and spermatids were 1.3-2.1 for exposure to C nuclei and 1.5-3.0 for exposure to Fe nuclei. (The spermatogonial data were uncertain.) These low values, and the negligible number of viable mutations, compared with those for mutations in somatic cells and for neoplastic transformation, indicate that germ cell mutations arising from exposures to cosmic ray nuclei are not a significant hazard to astronauts.
Keywords: astronaut hazards, linear energy transfer, relative biological effect
Astronauts exploring space beyond low Earth orbit are exposed to two major hazards (1), the near absence of gravity and the presence of the cosmic ray flux. The former may have serious effects on bone and muscle physiology and on the cardiovascular system. The latter includes high atomic number, high energy (HZE) nuclei, which is appreciably greater than at the Earth's surface because they are not absorbed by the atmosphere or deflected by the Earth's magnetic field. It was estimated (2) that on a three-year Mars mission, ≈30% of cells in the body would be traversed by HZE nuclei with values of Z between 10 and 28, and that virtually all cells would be traversed by nuclei with Z values between 3 and 9. Most of these HZE nuclei have high values of linear energy transfer (LET) in passing through tissues and make numerous hits on cellular molecules. The available data indicate that they have relatively high, but rather uncertain, values of relative biological effectiveness (RBE) for cell killing, mutations, and cancer induction in animals, reaching maxima of ≈50 at LETs between 100 and 200 keV/μm (3-5). The extrapolation of such data to human risk estimation is necessary if appropriate shielding is to be used on space missions. A concern to astronauts is the possible induction of germ cell mutations, mutations passed on to offspring. The radiation dose, at low LETs, to double the human germ cell mutation rate has been given a lower limit of 1 Sv with no upper bound because no significant number of mutations have been observed in the children of Japanese exposed to the atomic bombs (6). It is customary to use the doubling dose for germ cell mutations in mice as an approximation to human values. The average of a wide range of values in the mouse system is 1.4 Gy for acute exposure (7). A chronic low LET exposure would require a larger exposure, but one does not expect a dose rate effect for high LET radiation. However, there are no mouse data estimating the RBE for germ cell mutations arising from exposure to HZE nuclei. To do such experiments with mice would require an almost impossible number of animals because the observable induced mutation frequencies in the mouse system are low.
A suitable simple, precise system to measure germ cell mutation induction in a vertebrate uses Medaka fish (Oryzias latipes) (8-10), a small oviparous fresh water teleost native to Japan, South Korea, and China. The spontaneous and γ-ray induced mutation rates of the fish are very similar to those of mice (8, 9). Males (wild type ≈3 cm long, ≈6 months old) are exposed to ionizing radiation, after which individual males are mated to nonexposed tester females, recessive homozygous at several color loci. Individual eggs or embryos are collected daily (15-20 per day) and observed for dominant lethal mutations (DL) and total color mutations (TM). The big advantage of the Medaka system, other than its simplicity and relatively low cost, is the important fact that the covering of the embryos is transparent, and it is possible to observe mutations from exposed males within 2 days after fertilization. Hatching is ≈6 days, and DL and TM may be observed in the embryonic stage, even though the embryos may not hatch, something that cannot be done with mice. There are extensive Medaka data on DL and TM from exposure to γ-rays. The dose-response curves are linear (8-10).
We exposed male Medaka to 3.5 GeV 12C nuclei (LET: 13 keV/μm) and to 56 GeV 56Fe nuclei (LET: 147 keV/μm) (11) and determined DL and TM for offspring arising from exposed sperm, spermatids, and spermatogonia. The RBEs for TM, relative to γ-rays, were all <3, indicating that exposure to cosmic ray nuclei were not a significant hazard to astronauts.
Materials and Methods
Medaka Strains. Males of the HNI inbred strain, derived from the Northern population (12), were used as the wild-type parent exposed to HZE nuclei and to γ-rays. They were mated to females of T5, a tester strain with five homozygous recessive loci (b, lf, gu, i, and wl), for HZE exposed males or to T3 with three homozygous recessive loci (b, lf, and gu) for γ-ray exposed males. Males from a Sakura strain, derived from a Southern population (13), were also exposed to γ-rays and mated with T3. The average γ-ray induced frequencies at the five loci were the same at the five loci. All fish were cultured at 27 ± 2°C by using a 14-h light/10-h dark cycle. The experiments were approved by the University of Tokyo Animal Bioscience Committee and the Institutional Animal Care and Use Committee of Brookhaven National Laboratory (BNL).
Radiation Exposures. γ-ray exposures from a 137Cs-source of HNI and/or Sakura strains were done at the Research Center for Nuclear Science and Engineering of the University of Tokyo. Fish, in groups of five in T25 flasks containing aged tap water, were exposed at 0.95 Gy/min to a range of doses from 0.64 to 9.5 Gy for the analysis of mutations in exposed sperm or spermatids. Data from both strains were combined for sperm and spermatids because the spontaneous and induced mutation frequencies were the same for both. However, the induced mutation frequencies in the spermatogonia of HNI was twice that in the Sakura strain. Hence, we only used data from the HNI strain for γ-ray exposures to spermatogonia of 0, 1.90, and 4.75 Gy for exposed spermatogonia by using T3 females and the technique described below. (The small number of doses to spermatogonia resulted in large errors in the estimation of a linear dose-response curve. See Results.)
The exposures to C nuclei were carried out at the Heavy Ion Medical Accelerator in the Chiba facility at the National Institute of Radiological Sciences in Chiba, Japan. The HNI males in groups of four in T25 flasks, containing water, were exposed to doses of 2.0, 4.75, or 8.07 Gy, after which individual males were mated with nonexposed T5 females by isolating pairs in cages (14 × 18 × 12 cm3). Embryos were collected daily, washed and plated, one to a well of a 96-well microtiter plate. They were observed microscopically so as to observe TM, before and after hatching, and dead embryos (DL), before and after hatching. Embryos collected 1-3 days after mating arose from exposed sperm, from days 4 to 9 from exposed spermatids, and embryos collected after 32 days arose from exposed spermatogonia.
Exposures of 162 males to Fe nuclei were carried out at the Alternating Gradient Synchrotron at BNL. Groups of HNI males, in ≈2-liter plastic bags containing ≈1 liter of water and ≈100% O2, were shipped by Air Express from Tokyo to New York, where they were picked up and brought to BNL. After ≈1 day of acclimatization in the fish facility at BNL, the fish were exposed to Fe nuclei, and within 24 h were returned to Tokyo for mating with T5 females and subsequent daily embryo collection and mutation enumeration as described above. Because the number of males were limited, because of shipping by air, we carefully selected the best-conditioned females to obtain as many embryos as possible from the mating with the exposed males. There were two separate experiments, ≈1 year apart. The first used doses of 0.3 and 1.0 Gy, and the second used doses of 1.0 and 2.0 Gy at a dose rate of 1.0 Gy/min. About 40 fish were exposed at each dose, five per T25 flask containing water and two flasks at a time.
Enumeration of Mutations. Between 1% and 5% of the collected embryos died, independent of dose, within 24 h after fertilization. Presumably, this result is a maternal effect. These early deaths are not included in the calculations of DL, which is taken as the numbers of dead embryos, at days >1, divided by the embryos that have developed after day 1. The numbers of effective loci screened after γ-irradiation is three times the number of developed embryos (12) and for HZE nuclei exposures equals approximately five times the number of developed embryos (Table 1). The historical controls include data from crosses of nonexposed males to nonexposed T3 females in earlier experiments and T5 females in the majority of experiments. The numbers of effective loci in the radiation experiments is slightly less than five times the number of developed embryos because some severely malformed embryos were omitted from the calculation.
Table 1. Embryos from Fe and C-exposed males.
| Dose, Gy | No. (experiment) | Stage exposed* | Fraction fertilized† | Embryos developed | Effective loci, 104 | DL | TM | VM | DL frequency‡ | TM frequency,§ 10−4 |
|---|---|---|---|---|---|---|---|---|---|---|
| 56-GeV Fe-exposed males | ||||||||||
| Historical controls | 27,870 | 12.2 | 700 | 4 | 1 | 0.025 | 0.33 | |||
| 0.3 | 48 (1) | Sperm | 0.84 | 1,698 | 0.84 | 110 | 7 | 1 | 0.065 | 8.3 |
| Tids | 0.87 | 4,024 | 2.00 | 191 | 12 | 0 | 0.048 | 6.0 | ||
| Gonia | 0.86 | 11,288 | 5.89 | 520 | 11 | 1 | 0.046 | 1.9 | ||
| 1.0 | 36 (1) | Sperm | 0.86 | 1,353 | 0.67 | 143 | 19 | 1 | 0.106 | 28 |
| Tids | 0.82 | 2,925 | 1.45 | 276 | 43 | 0 | 0.094 | 30 | ||
| Gonia | 0.83 | 8,411 | 4.32 | 382 | 13 | 1 | 0.045 | 3.0 | ||
| 1.0 | 38 (2) | Sperm | 0.69 | 1,200 | 0.60 | 132 | 16 | 0 | 0.110 | 27 |
| Tids | 0.73 | 3,436 | 1.71 | 291 | 45 | 2 | 0.085 | 26 | ||
| Gonia | 0.47 | 8,359 | 4.16 | 318 | 2 | 1 | 0.038 | 0.48 | ||
| 2.0 | 40 (2) | Sperm | 0.67 | 1,466 | 0.72 | 284 | 43 | 0 | 0.194 | 59 |
| Tids | 0.69 | 3,122 | 1.54 | 548 | 78 | 5 | 0.176 | 51 | ||
| Gonia | 0.33 | 7,368 | 3.64 | 287 | 2 | 1 | 0.039 | 0.55 | ||
| 3.5-GeV C-exposed males | ||||||||||
| Historical controls | 37,968 | 18.6 | 1,027 | 6 | 1 | 0.027 | 0.32 | |||
| 2.0 | 76 | Sperm | — | 927 | .452 | 195 | 20 | 2 | 0.210 | 44 |
| Tids | — | 2,093 | 1.03 | 268 | 26 | 2 | 0.128 | 25 | ||
| Gonia | — | 5,735 | 2.99 | 327 | 6 | 1 | 0.057 | 2.0 | ||
| 4.75 | 80 | Sperm | — | 1,759 | 0.77 | 696 | 83 | 5 | 0.396 | 107 |
| Tids | — | 3,629 | 1.58 | 1,064 | 127 | 9 | 0.293 | 80 | ||
| Gonia | — | 3,189 | 1.60 | 460 | 53 | 3 | 0.144 | 33 | ||
| 8.07 | 40 | Sperm | — | 1,041 | 5.04 | 630 | 121 | 0 | 0.605 | 240 |
| Tids | — | 2,055 | 10.0 | 940 | 176 | 5 | 0.457 | 176 | ||
Matings of exposed sperm, days 1-3; spermatids (tids), days 4-9; spermatogonia (gonia), days 32-149 for experiment 1 and days 46-117 for experiment 2. Only one experiment was performed for 3.5-GeV C-exposed males.
The no. of fertilized embryos divided by the no. of embryos laid. (Fraction fertilized not included for 3.5 GeV C-exposed males because data were only partially collected.)
DL frequency per developed embryo.
TM per effective locus.
Results
We wanted to assure ourselves that the transportation by air of the fish from Tokyo to BNL and return (≈11- to 13-h flights each way) did not affect the magnitudes of mutation frequencies. In a preliminary test run, 50 HNI fish were shipped to BNL, acclimated to the new environment for ≈1 day, and then at BNL, 15 were exposed to a dose of 2.40 Gy and 15 to 4.75 Gy of 137Cs-γ-rays. These fish, plus 20 unexposed ones, were returned to Tokyo and assayed there for DL and TM. There were no significant differences from the results from fish exposed in Japan (data not shown).
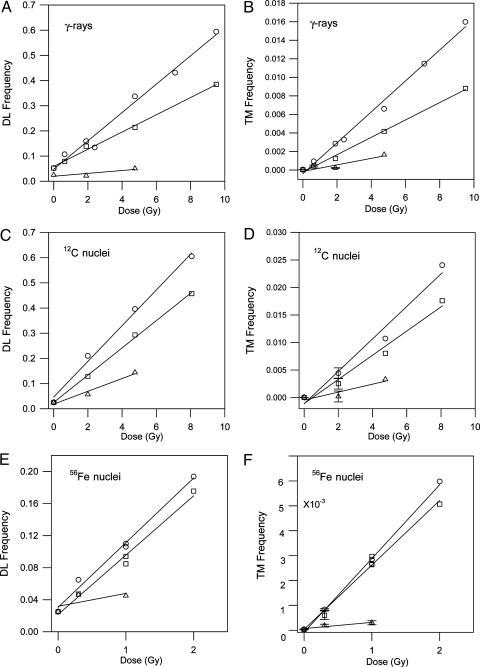
The data for γ-ray exposures have mostly been published (12). Table 1 shows the results of the exposures to Fe and C nuclei, respectively, in terms of the dose, stage exposed, fraction fertilized, embryos developed, effective genetic loci, the numbers for DL, TM, and (viable mutations) VM, and the DL and TM frequencies per locus. It is obvious that a large number of embryos were collected, ≈90,000 for Fe exposures and ≈25,000 for C exposures. The number of observed VM, for all stages and doses, is ≈2, a number with an uncertainty of ≈1.5. Although there are observable color mutations after the various exposures, the embryos did not survive after hatching. Hence, we did not further consider VM frequencies. For the two separate Fe exposures, there is reasonable agreement in the values of DL for all stages for the two exposures at 1.0 Gy. However, the values of TM for spermatogonia after 1 and 2 Gy in Experiment 2 are very low and not consistent the values in Experiment 1. The reason(s) for the inconsistently is not known but could be related to the low fertilization efficiency of these exposures in Exp. 2. Fig. 1 shows the dose-response curves for the mutation frequencies after exposures of sperm, spermatids, and spermatogonia to γ-rays, C nuclei, and Fe nuclei (only Experiment 1 for spermatogonia). Most of the dose-response curves are obviously linear. However, after exposures of spermatogonia to only two doses of γ-rays, C nuclei, or Fe nuclei, linear fits had SDs of >50% for four of six and ≈25% for the other two determinations for DL and TM. Nevertheless, we assume, for simplicity, that all of the dose-response curves are linear and have calculated their best, weighted fits to determine the slopes, in units of mutation frequencies, and their standard errors per unit dose. The results are in Table 2.
Fig. 1.
Mutations observed in the embryonic offspring of male medaka exposed to a range of ionizing radiations: γ-rays, 3.5-GeV 12C nuclei or 56-GeV 56Fe nuclei. Mutation frequencies, DL and TM, as functions of dose from γ-rays (A and B), 12C nuclei (C and D), and 56Fe nuclei (E and F) to sperm (○), spermatids (□), or spermatogonia (▵).
Table 2. Slopes of mutation frequencies vs. dose in Gy.
| γ-rays | 12C nuclei | 56Fe nuclei | |
|---|---|---|---|
| Sperm | |||
| DL | 0.057 ± 0.004 | 0.075 ± 0.005 | 0.085 ± 0.01 |
| TM | (1.50 ± 0.14) × 10−3 | (2.54 ± 0.36) × 10−3 | (2.8 ± 0.1) × 10−3 |
| Spermatids | |||
| DL | 0.035 ± 0.002 | 0.054 ± 0.002 | 0.070 ± 0.007 |
| TM | (0.85 ± 0.10) × 10−3 | (1.8 ± 0.3) × 10−3 | (2.5 ± 0.3) × 10−3 |
| Spermatogonia | |||
| DL | 0.0039 ± 0.0046 | 0.021 ± 0.005 | 0.023 ± 0.016 |
| TM | (1.8 ± 1.2) × 10−4 | (0.18 ± 0.22) × 10−3 | (0.30 ± 0.09) × 10−3 |
Values reflect best fits, linear regressions ± SD.
The ratios of the slopes of the dose-response curves of the different exposed stages for exposures to HZE nuclei to the slopes of the same stages for γ-ray exposures gives the RBEs of the HZE nuclei for the different stages exposed (Table 3). The SDs for DL and TM in exposed sperm and spermatids are less than ≈20%, but spermatogonia exposed to C nuclei, DL, and TM, the SDs are >120%, and for exposures to Fe nuclei, the RBE for DL has an uncertainty of 138% and a value for TM of 71%. These large values for RBEs arise from the large uncertainties in the spermatogonial DL and TM responses to γ-rays and from the TM response to C nuclei and of DL to Fe nuclei.
Table 3. RBE of HZE nuclei induction of mutations in sperm, spermatids, and spermatogonia.
| 3.5 GeV[12C] | 56 GeV[56Fe] | |
|---|---|---|
| Sperm | ||
| DL | 1.32 ± 0.13 | 1.49 ± 0.20 |
| TM | 1.69 ± 0.29 | 1.89 ± 0.20 |
| Spermatids | ||
| DL | 1.54 ± 0.10 | 2.00 ± 0.23 |
| TM | 2.1 ± 0.5 | 2.94 ± 0.47 |
| Spermatogonia | ||
| DL | 5.4 ± 1.0 | 6 ± 8 |
| TM | 1.0 ± 1.4 | 1.7 ± 1.2 |
Data are ± SD.
Discussion and Conclusions
The data indicate clearly that the Medaka specific locus test is a useful model for quantifying germ cell mutations induced by the exposure to HZE nuclei found beyond low-Earth orbit. It is intriguing that the RBEs for C nuclei with an LET of 13 keV/μm are only slightly less that the values for Fe nuclei (147 keV/μm), which for exposed sperm and spermatids are <3.0. Probably, only the traversal of a single nuclei of C or Fe is sufficient to inactivate a sperm or spermatid, indicating that these systems have sensitive safeguards against events that are involved in germ cell mutagenesis (12). The large statistical errors for exposed spermatogonia preclude any unique conclusions, except that those experiments probably should be repeated at a future date. The RBE values, for exposure to Fe nuclei, DL, and TM are ≈10-fold or more, less than those from the relatively few studies on cell transformation and on cancer induction in rodents (3). The results of earlier studies on Medaka (13, 14) indicated that “most of the γ-ray-induced germ cell mutations recovered as TM were accompanied by large genomic deletions, which eventually led the mutant embryos to dominant lethality.” Hence, it is not surprising that such deletions should also result from exposures to HZE, and that the observed RBE values for chromosomal instability (15, 16), chromosome aberrations (17), or cytogenetic damage (18) from HZE exposures and minisatellite mutations from exposure to fission neutrons (19) are all in the range of 2-9.
The number of VM from all exposed germ cell stages are very low and would not be expected to yield significant numbers of mutations above background levels. Moreover, the numbers of TM from exposed spermatogonia are also very low compared with the numbers of DL, indicating that stem cells damaged by exposures to HZRs in cosmic rays probably undergo apoptosis or result in abnormal sperm that would yield DL mutations.
We conclude that the hazard to male astronauts from exposures to cosmic radiation is probably temporary sterility but not significant numbers of mutagenic effects observable in progeny.
Acknowledgments
We thank Keith Thompson of BNL for his many efforts in calculating best straight lines and standard errors and James Jardine for his assistance in exposing fish to Fe nuclei. This research was supported, in part, by a grant from the National Aeronautics and Space Administration and by a Grant-in-Aid for Scientific Research (Priority Area 813) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. The C nuclei exposures were done as part of a Research Project with Heavy Ions at the National Institute of Radiological Sciences Heavy Ion Medical Accelerator in Chiba. BNL is operated by Brookhaven Science Associates, under contract with the U.S. Department of Energy.
Author contributions: A. Shimada, A. Shima, and R.B.S. designed research; A. Shimada, A. Shima, K.N., and Y.S. performed research; A. Shimada, A. Shima, and R.B.S. analyzed data; and A. Shimada and R.B.S. wrote the paper.
Abbreviations: BNL, Brookhaven National Laboratory; DL, dominant lethal mutations; HZE, high atomic number, high energy; LET, linear energy transfer; RBE, relative biological effectiveness; TM, total color mutations; VM, viable mutations.
References
- 1.Setlow, R. B. (2004) EMBO Reports 4, 1013-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curtis, S. B. (1989) Adv. Space Res. 9, 293-298. [DOI] [PubMed] [Google Scholar]
- 3.Cucinotta, C., Badhwar, G., Saganti, P., Schimmerling, W., Wilson, J. & Dicello, J. (2002) Space Radiation Cancer Risk Projections for Exploratory Missions, (Natl. Aero. Space Admin, Washington, DC) NASA Technical Publ. No. 210777.
- 4.National Research Council (1996) Radiation Hazards to Crews of Interplanetary Missions (Natl. Acad. Press, Washington, DC).
- 5.Setlow, R. B. (1999) Mutat. Res. 430, 169-175. [DOI] [PubMed] [Google Scholar]
- 6.Neel, J. V., Schull, W. J., Awa, A. A., Satoh, C., Kato, H., Otake, M. & Yoshimoto, Y. (1990) Am. J. Hum. Genet. 46, 1053-1072. [PMC free article] [PubMed] [Google Scholar]
- 7.Neel, J. V. & Lewis. S. E. (1990) Annu. Rev. Genet. 24, 327-362. [DOI] [PubMed] [Google Scholar]
- 8.Shima, A & Shimada, A. (1988) Mutat. Res. 198, 93-98. [DOI] [PubMed] [Google Scholar]
- 9.Shima, A. & Shimada, A. (1991) Proc. Natl. Acad. Sci. USA 88, 2545-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimada, A. &. Shima, A. (1998) Mutat. Res. 399, 149-165. [DOI] [PubMed] [Google Scholar]
- 11.Zeitlin, C., Heilbronn, L. & Miller, J. (1998) Radiat. Res. 149, 560-569. [PubMed] [Google Scholar]
- 12.Shima, A. & Shimada, A. (2001) Mar. Biotechnol. 3, Suppl. 1, S162-S167. [DOI] [PubMed] [Google Scholar]
- 13.Wittbrodt, J. Shima. A. & Schartl, M. (2002) Nat. Rev. Genet. 3, 53-64. [DOI] [PubMed] [Google Scholar]
- 14.Fukamachi, S., Shimada, A., Naruse, K. & Shima, A. (2001) Mutat. Res. 458, 19-29. [DOI] [PubMed] [Google Scholar]
- 15.Limoli, C. E., Ponnaiya, B., Corcoran, J. J., Giedzinski, E. & Morgan, W. F. (2000) Int. J. Radiat. Biol. 76, 1599-1606. [DOI] [PubMed] [Google Scholar]
- 16.Evans, H. H., Horng, M-F., Evans, T. E., Jordan, R. & Schwartz, J. L. (2001) Radiat. Res. 156, 186-194. [DOI] [PubMed] [Google Scholar]
- 17.Wu, H., Durante, M., George, K. & Yang, T. C. (1997) Radiat. Res. 148, S102-S107. [PubMed] [Google Scholar]
- 18.Brooks, A. L., Bao, S., Rithidech, K., Couch, L. A. & Braby, L. A. (2001) Radiat. Res. 155, 353-359. [DOI] [PubMed] [Google Scholar]
- 19.Dubrova, Y. E., Plumb, M., Brown, J., Boulton, E., Goodhead, D. & Jeffreys, A. J. (2000) Mutat. Res. 453, 17-24. [DOI] [PubMed] [Google Scholar]