Abstract
Synaptic vesicles are recycled locally within presynaptic specializations. We examined how vesicles are reused after endocytosis, using transgenic mice expressing the genetically encoded fluorescent indicator synaptopHluorin in subsets of neurons. At both excitatory and inhibitory synapses in cultured hippocampal neurons, newly endocytosed vesicles did not preferentially enter the releasable pool of vesicles. Rather, they entered the reserve pool first and subsequently the readily releasable pool over a period of several minutes. All vesicles in the recycling pool could be accessed by spaced stimuli, arguing against preferential local reuse of the readily releasable vesicles. Interestingly, nearly half the vesicles at excitatory synapses, and a third at inhibitory synapses, could not be recruited for release even by sustained stimuli. We conclude that, at presynaptic terminals in the hippocampus, most vesicles vacate release sites after exocytosis and are replaced by existing vesicles from the reserve pool, placing constraints on kiss-and-run recycling.
Keywords: endocytosis, exocytosis, hippocampus, kiss and run
Synaptic vesicles are recycled locally within the presynaptic terminal for reuse (1, 2). Several mechanisms of recycling have been proposed, including a conventional pathway involving clathrin coat formation (1, 2) and a clathrin-independent pathway involving kiss-and-run exocytosis (3-9) that might explain rapid endocytosis observed at some synapses (5, 10-12). Although recent attention has centered on modes of endocytosis, several key questions can also be raised regarding postendocytic traffic of synaptic vesicles. Where do vesicles go after endocytosis? Are recently recaptured vesicles preferentially reused? Rapid reuse of recently recaptured vesicles (13, 14) might be advantageous to synapses if it allows vesicles to become release-competent more rapidly than would be possible by recruitment from the reserve pool.
Studies in hippocampal and cortical cultures generally have focused on glutamatergic synapses or assumed that the synapses under investigation are glutamatergic synapses because they are more abundant (15). It is unclear whether inhibitory presynaptic terminals have the same mechanisms of recycling as excitatory synapses. Some differences might be anticipated based on previous studies establishing differences in the molecular composition (16, 17) and short-term synaptic dynamics (16, 18, 19). Additionally, the loss of specific proteins, for example, synapsin I (20), appears to have different consequences in glutamatergic and GABAergic synapses. Therefore, it is important to understand the properties of and mechanisms in vesicle recycling at inhibitory synapses.
Genetically encoded probes such as synaptopHluorin (spH), which is a fusion protein of the vesicle protein VAMP2 and a pH-sensitive EGFP (21), offer a method to target tracers to selected populations of neurons. We have generated lines of transgenic mice that express spH in subsets of neurons in the brain. We first show that exocytosis and endocytosis can be visualized at single synapse resolution in intact tissue. Two different lines of mice, one with expression of spH largely at glutamatergic synapses and one with spH expression confined to GABAergic synapses in cultures, allowed us to compare vesicle recycling at these two types of synapses. We find that newly endocytosed vesicles at both excitatory and inhibitory synapses do not preferentially reenter the readily releasable pool (RRP) of vesicles. Rather, they progress through a reserve pool and repopulate the RRP slowly.
Methods
Construction of Transgenic Mice. The thy-1.2 expression cassette (22) was engineered to harbor the spH gene (21) in between exons II and IV. Details can be found in Supporting Methods, which is published as supporting information on the PNAS web site. We used two lines, spH21 and spH64, in this study.
Primary Cultures and Slice Cultures. Newborn mice used for primary neuron cultures were genotyped within a day of birth. Hippocampal neurons of positive transgenic animals were obtained between postnatal days 1 and 3 as described (23). In cultured hippocampal neurons, the expression of spH took ≈10 days as determined by fluorescence intensity, which is compatible with reports that transgene expression driven by Thy1 promoter begins at postnatal days 6-10 (22). Experiments in the current study were done at 28 days in vitro, when the expression of fluorescence was strong.
Organotypic slice cultures were prepared as described (24). Briefly, transverse hippocampal slices (400 μm) from postnatal day 7 transgenic mice were prepared by using a tissue chopper and maintained in vitro on Millicell-CM filter inserts (Millipore) in a 36°C, 5% CO2, humidified incubator. The culture medium was completely exchanged every 3 days. Slices were used between 12 and 14 days in vitro.
Immunocytochemistry. Dissociated cultures were fixed in 4% paraformaldehyde and permeabilized with 0.2% Triton-X. After blocking with a mixture of horse serum and BSA, coverslips were incubated in a mixture of two primary antibodies [mouse anti-glutamic acid decarboxylase (GAD) (1:200) and rabbit anti-VGluT1 (1:1,000, Synaptic Systems, Goettingen, Germany), or mouse anti-GAD and rabbit anti-synaptotagmin I (1:1,000, Sigma)] for 1 h at room temperature. After washing, cells were incubated in medium containing secondary antibodies (Molecular Probes). Coverslips were mounted with Vectashield (Vector Laboratories) for viewing on a Zeiss LSM510 confocal microscope, with a ×40, 1.3 numerical aperture oil immersion lens.
Electrophysiology. Paired, whole-cell patch-clamp recordings were performed on hippocampal neurons by using Axopatch200B and WPC-100 amplifiers (Axon Instruments, Union City, CA). Electrodes with a tip resistance of 5-10 MΩ contained 130 mM gluconate, 10 mM NaCl, 1 mM EGTA, 2 mM MgCl2, 10 mM Hepes, 3.5 mM NaATP, and 1 mM NaGTP. The extracellular perfusion medium contained 136 mM NaCl, 2.5 mM KCl, 10 mM Hepes, 10 mM d-glucose, 2 mM CaCl2, 1.3 mM MgCl2, 0.05 mM 2-amino-5-phosphonovaleric acid, and 0.1 mM picrotoxin. Recordings were filtered at 2-5 kHz and acquired at 5-10 kHz. Action potentials (APs) were evoked in one neuron by delivering brief (1 ms) and large (150-200 mV) depolarizations in voltage-clamp mode to elicit uncontrolled action currents, and excitatory postsynaptic currents were recorded in voltage-clamp (-70mV) in a nearby neuron. Access resistance was periodically tested, and cells in which the access resistance changed by >10% were discarded. Data were acquired by using a custom-written routine in igor pro (gift from Bernardo Sabatini, Harvard University).
Fluorescence Imaging in Vitro. Organotypic slice cultures were imaged at room temperature in oxygenated saline containing 124 mM NaCl, 2 mM KCl, 1.24 mM KH2PO4, 1.3 mM MgSO4, 17.6 mM NaHCO3, 2.5 mM CaCl2, 10 mM d-glucose 10, and the glutamate receptor antagonists 2-amino-5-phosphonovaleric acid (0.05 mM) and 6-cyano-7-nitroquinoxaline-2,3-dione (0.02 mM). The schaffer collateral pathway was stimulated by using a bipolar electrode placed on the border of CA3 and CA1. Stimuli (200 μs, 50-70 μA) were controlled by using an Isoflex stimulus isolator (A.M.P.I., Jerusalem). Confocal imaging was performed with a Zeiss LSM 510, a Zeiss Achroplan 40 × 0.8 numerical aperture lens, and the 488-nm line of an argon laser to excite spH. Image acquisition and stimulation were synchronized by using transistor-transistor logic pulses.
Fluorescence imaging of dissociated cultures was done at 28 days in culture. Coverslips were mounted on a custom-built chamber equipped with a pair of parallel platinum electrodes ≈8 mm apart. Extracellular medium contained 136 mM NaCl, 2.5 mM KCl, 10 mM Hepes, 10 mM d-glucose, 2 mM CaCl2, 1.3 mM MgCl2, 0.05 mM 2-amino-5-phosphonovaleric acid, and 0.025 mM 6-cyano-7-nitroquinoxaline-2,3-dione. Bafilomycin A (Calbiochem) was stored frozen in 0.2-mM aliquots and diluted to a final concentration of 1 μM (0.2% DMSO). Electrical pulses (1-ms, 70-V square pulses) were delivered by a SD9 stimulator (Grass Instruments, Quincy, MA), whose timing was controlled by the imaging software slidebook (Intelligent Imaging Innovations, Santa Monica, CA). spH fluorescence was excited by light from a xenon lamp filtered at wavelength 470-490 nm. Emission was collected at wavelength 505-545 nm by a cooled charge-coupled device camera (PCO Sensicam, Cooke Corporation, Romulus, MI) on an Olympus inverted microscope (IX 70) with an oil lens (×60, 1.25 numerical aperture, Olympus).
Data Analysis. spH responses were analyzed by using custom routines in matlab (Mathworks, Natick, MA). Fluorescence changes are expressed in absolute units because normalizing with resting fluorescence would be inappropriate when there is a slow creep of baseline fluorescence after addition of bafilomycin. All data were normalized to the first response in the presence of bafilomycin. Data are presented as mean ± SEM, unless otherwise stated. Statistical comparisons were made by using Student's t test.
Results
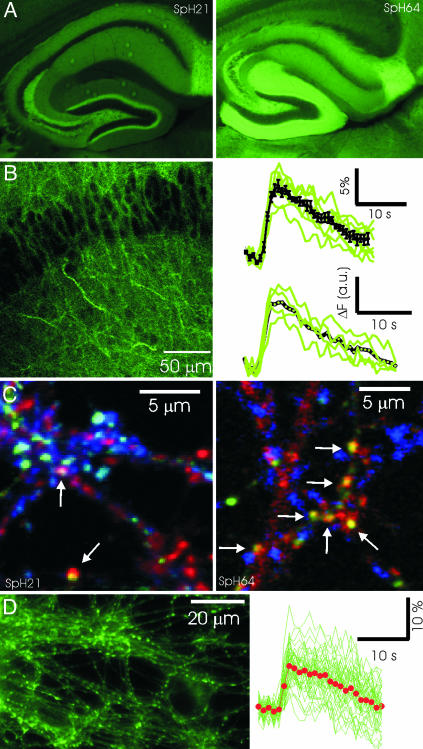
Transgenic Mice Expressing spH. We engineered mice that express spH in subsets of neurons to provide an easy source of spH-expressing neurons and to allow imaging synaptic activity in intact preparations. We chose to use the thy1 promoter because it leads to a mosaic expression resembling the classic Golgi staining method (22, 25). We recovered 12 lines that express spH in the brain and have characterized a subset (Figs. 7-9, which are published as supporting information on the PNAS web site). As shown in Fig. 1A, the pattern of fluorescence intensity in the hippocampus was different in the two different lines (spH21 and spH64) used in this study.
Fig. 1.
Characterizing transgenic mice expressing spH. (A) Fixed tissue sections from lines spH21 (Left) and spH64 (Right) showing differential pattern of fluorescence in the hippocampus. (B) Organotypic slice cultures from hippocampus exhibit robust spH fluorescence responses to brief stimuli. (Left) Image from the CA1 region showing cell bodies and dendritic regions. (Right) Responses to a stimulus of 40 APs at 20 Hz. Average responses from several boutons in individual slices are in green, and the average from six slices is shown in black. Fractional fluorescence change (Upper) and absolute change (Lower) are both shown for comparison. (C) Expression of spH in dissociated hippocampal cultures. (Left) Three-color images of spH (green), GAD (red), and VGlut1 (blue) fluorescence indicate that a majority of spH-expressing synapses in hippocampal cultures from line spH21 are glutamatergic. In this typical region, only 2 of ≈18 boutons expressing spH contain GAD and appear yellowish (arrows), the rest label with VGlut1 and appear in cyan. In contrast, virtually all spH-expressing synapses in hippocampal cultures from line spH64 are GABAergic. (Right) Arrows point to boutons with spH and GAD colocalized. (D) (Left) Sample image of spH boutons from live cultures of neurons from spH64. (Right) The response of 50 such boutons (green) to a brief stimulus of 40 APs at 20 Hz. Average response is indicated by red dots.
To determine whether synaptic activity could be measured in intact circuits, we used slice cultures of the hippocampus from line spH64, which had clearly identifiable presynaptic boutons in the different layers even at rest (Fig. 1B). This resting fluorescence in living presynaptic terminals is likely caused by a fraction of spH on the plasma membrane, as found in dissociated cultures (26, 27). Responses to brief extracellular stimuli, for example, 40 APs at 20 Hz, were easily detected at single synapse resolution (Fig. 1B). By averaging responses across multiple trials, we were able to detect responses to as few as five APs (data not shown). For the rest of the experiments in this study, we turned to dissociated cultures, where vesicle recycling has been studied extensively.
Dissociated hippocampal cultures made from line spH21 revealed a dense distribution of synapses expressing spH, a majority of which were glutamatergic (74.7 ± 9.5%, n = 12). By contrast, in hippocampal cultures made from line spH64, virtually all presynaptic boutons expressing spH colocalized with GAD (Fig. 1C), although only ≈20% of the neurons in hippocampal cultures are GABAergic (28). The fortuitous and selective labeling of inhibitory synapses with spH in line spH64 allowed us to examine their properties and compare them with those measured in glutamatergic synapses in line spH21. At synapses from both lines of mice, electrical stimulation evokes a robust increase in fluorescence intensity that returns to baseline with a time course of several seconds (Fig. 1D).
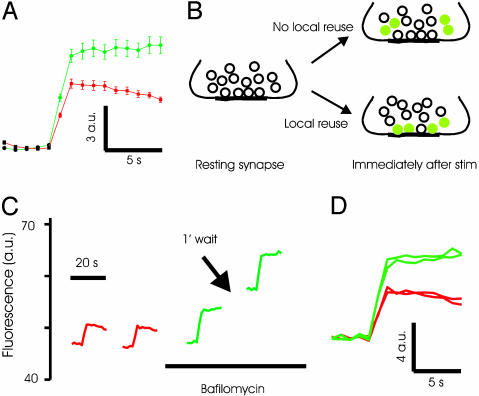
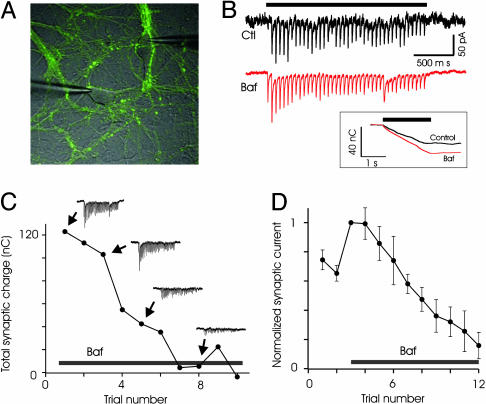
Recently Endocytosed Vesicles Are Not Preferentially Reused at Inhibitory Synapses. Experiments using styryl dyes have led to the suggestion that synaptic vesicles in the RRP are preferentially reused after exocytosis at excitatory synapses (13). To determine whether such preferential reuse occurred at inhibitory synapses, we first examined recycling at synapses in line spH64 by using alkaline trapping (27). In this method, addition of the vacuolar ATPase inhibitor bafilomycin blocks reacidification of exocytosed vesicles. Because the rate of spontaneous alkalinization is low over time scales of a few minutes, this method allows tagging vesicles that have just undergone exocytosis and recycling. As shown in Fig. 2A, the fluorescence increase at synapses triggered by a brief stimulus of 40 APs at 20 Hz does not recover in the presence of 5 μM bafilomycin. A significant increase in the peak fluorescence intensity was consistently observed after addition of bafilomycin (Fig. 2A). This increase could be caused by an increase in exocytosis after bafilomycin addition or by unmasking of rapid endocytosis that might occur during the 2-s stimulus. We favor the first explanation because electrophysiological measurements also indicated an increase in postsynaptic currents immediately after addition of bafilomycin (see below).
Fig. 2.
Alkaline trapping with bafilomycin reveals lack of immediate reuse of exocytosed vesicles at inhibitory synapses. (A) Average spH responses to a stimulus of 40 APs at 20 Hz before (red) and after (green) addition of bafilomycin. Fluorescence intensity fails to return to baseline after treatment with bafilomycin. Absolute change in fluorescence intensity is shown in arbitrary units (a.u.). n = 3 experiments, 50 boutons each. (B) The basic experimental design is schematically illustrated. (C) Typical experiment showing that the response to 40-AP stimulus in bafilomycin remains unaltered for two trials. Traces are average fluorescence intensity across a large region containing >100 spH boutons. Note that the fluorescence intensity does not return to baseline after a stimulus in the presence of bafilomycin. (D) The responses before and after addition of bafilomycin from the experiment in C are replotted. On average, the second response in bafilomycin is statistically indistinguishable from the first (103 ± 3%, n = 9 experiments, >50 boutons each, P = 0.22).
The logic of the experiments to test the preferential reuse of recently released vesicles is the following (Fig. 2B). If we trap the exocytosed vesicles in an alkaline state with bafilomycin and they are released preferentially during the next stimulus, the fluorescence change in response to the second stimulus would be substantially smaller than the first. In contrast, if the recently released and endocytosed vesicles repopulate the reserve pool, the fluorescence change evoked by the second stimulus would be comparable to the first.
We measured the response of synapses to a stimulus of 40 APs at 20 Hz before and after addition of 5 μM bafilomycin, because this stimulus is thought to release the RRP of vesicles (29). Addition of bafilomycin inhibited the return of fluorescence to baseline after stimulation. A second stimulus delivered 1 min after the first caused a fluorescence change that was indistinguishable from the first (normalized second response: 1.03 ± 0.03, n = 9, P = 0.22, Fig. 2 C and D). This result can only occur if the second stimulus causes exocytosis of fully acidified vesicles, which clearly will have to come from the reserve pool and not from the recently endocytosed vesicles that are trapped in an alkaline state. The slow spontaneous alkalinization caused by bafilomycin, measured to have a time constant of ≈60 min, is not a concern here because it will only serve to diminish the evoked response of the second stimulus, which was not observed.
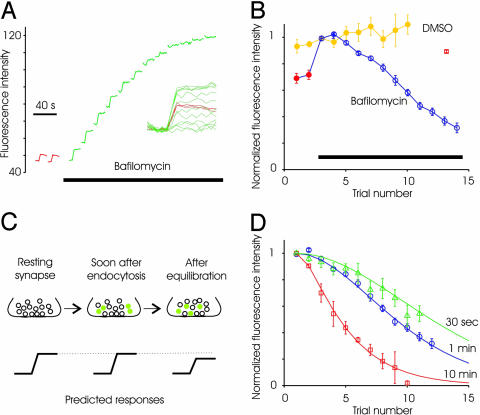
Endocytosed Vesicles Are Available for Release at Later Time Points. We demonstrated above that after releasing one round of the RRP, existing vesicles from the reserve pool refill the RRP. With multiple rounds of stimuli, however, used vesicles (trapped in the alkaline state) eventually will reach the RRP. This mixing will lead to a progressive reduction in the evoked fluorescence response. This prediction was confirmed by experiments where repeated stimuli in the continued presence of bafilomycin resulted in gradually declining response amplitudes (Fig. 3 A and B). The absolute fluorescence of the synapse continues to increase because exocytosed vesicles are cumulatively trapped in the alkaline state. The substantially reduced evoked fluorescence response in later trials is not simply an effect of continued presence of bafilomycin, because a waiting time of 10 min between the first and second trials causes only a small decrease in amplitude (Fig. 3B, red square is plotted at a point corresponding to 10 min). Control experiments done with DMSO revealed no decline in evoked responses (Fig. 3B).
Fig. 3.
Recruitment of reserve vesicles during multiple spaced stimuli. (A) Average spH responses to successive stimuli (40 APs, 20 Hz) delivered before (red) and after (green) addition of bafilomycin. The plot of absolute fluorescence intensity (after background subtraction) demonstrates several points: the lack of acidification after addition of bafilomycin, the slight spontaneous alkalinization between trials, and the progressive loss of evoked response. (Inset) All of the trials plotted with each baseline subtracted. (B) Average evoked response for successive trials before and after addition of bafilomycin (n = 9 experiments, at least 50 boutons each). Waiting time between trials was 1 min. Control responses after addition of just DMSO indicates no significant change in response size (n = 5 experiments). The response decays only slightly if the waiting time between the first and second trials in bafilomycin was 10 min (red square). (C) Schematic of the predicted outcome if mixing occurs between the RRP and reserve pool over a time scale of several minutes. (D) When the waiting time between trials was increased to 10 min, responses decline more rapidly (□, n = 5). When time interval between trials was reduced to 30 s, the response decay was similar to the 1 min waiting time (n = 4 experiments). Continuous lines are arbitrary polynomial functions showing trends in the data.
We next asked whether altering the time interval between successive stimuli alters the rate of decline of responses. For instance, if longer intervals are used between stimuli, alkaline-trapped vesicles might exchange with vesicles in the RRP (13, 30). If so, spH responses will decline more rapidly with successive trials, because alkaline-trapped vesicles will have more time to mix with the RRP (Fig. 4C cartoon). We tested this hypothesis by waiting for 10 min between trials and found a more rapid decline of responses (Fig. 3D). Longer waiting times between trials allows for a greater degree of spontaneous alkalinization, which might contribute to the acceleration of response reduction. To test for this possibility directly, we measured the response to 40 APs in the presence of bafilomycin and waited 30 min between the first and second trial. The response to the second trial was ≈90% of the first response (bafilomycin present continuously). In contrast, the response to the third trial of the 10-min wait experiment (with a total time in bafilomycin of 30 min) was <50% of the first, consistent with our model of mixing of the recycled vesicles into the releasable pool. A shorter waiting time of 30 s between successive trials did not lead to a statistically significant slowing of the decline in spH response (Fig. 3D).
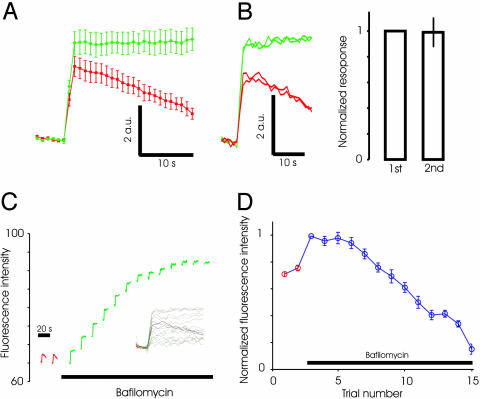
Fig. 4.
Lack of immediate reuse of exocytosed vesicles at excitatory synapses. (A) Average spH responses to a stimulus of 40 APs at 20 Hz before (red) and after (green) addition of bafilomycin in line spH21. Absolute change in fluorescence intensity is shown in arbitrary units (a.u.). n = 5 experiments, 50 boutons each. (B) Typical experiment showing that the response to 40-AP stimulus in bafilomycin (green traces) remains unaltered for two successive trials. Also shown are two trials before addition of bafilomycin (red traces). (C) Average spH responses (one experiment) to successive stimuli (40 APs, 20 Hz) delivered before (red) and after (green) addition of bafilomycin. Note the similarity to data for inhibitory synapses in Fig. 2. (Inset) All of the trials plotted with each baseline subtracted. (D) Average evoked response for successive trials before and after addition of bafilomycin (n = 5 experiments, at least 50 boutons each). Waiting time between trials was 1 min.
Preferential Reuse Is also Absent at Excitatory Synapses. We next tested whether recently exocytosed vesicles are preferentially reused at excitatory synapses by using synapses from line spH21. In this line, almost 75% of the synapses expressing spH were excitatory (Fig. 1), and the average response should be dominated by these synapses. We measured the response of synapses to a stimulus of 40 APs before and after addition of 5 μM bafilomycin. The peak response was larger after addition of bafilomycin (Fig. 4A), as observed for GABAergic synapses. A second stimulus delivered 1 min after the first caused a fluorescence change that was indistinguishable from the first (normalized second response: 0.96 ± 0.03, n = 5, P = 0.3, Fig. 4B). This finding indicates that vesicles endocytosed after the first stimulus in the presence of bafilomycin were not reused in the second round of exocytosis. We also found that repeated stimuli in the continued presence of bafilomycin resulted in gradually declining responses (Fig. 4 C and D), just as for inhibitory synapses.
Measurement of Neurotransmitter Release in Bafilomycin Support Fluorescence Data. The results from line spH21 were somewhat unexpected, given the earlier results with FM dyes. To further corroborate our results, we next tested whether preferential reuse of recently endocytosed vesicles could be detected electrophysiologically. We performed patch-clamp recordings from pairs of neurons in culture and isolated excitatory synaptic currents (Fig. 5 A and B). Responses to brief bursts of stimuli (40 APs at 20 Hz) were recorded under control conditions and after addition of bafilomycin. Addition of bafilomycin caused an increase in synaptic currents (Fig. 5B); on average, the synaptic response after bafilomycin increased to 1.59 ± 0.13 of control (n = 7, P = 0.01). This finding was unexpected because previous experiments suggested that bafilomycin does not have an acute effect on synaptic transmission, and the effect caused by the block of transmitter refilling occurs after a large number of stimuli (31). In addition, FM1-43 imaging has revealed no differences in the rate of exocytosis for sustained 10-Hz stimuli (27, 31). Although the exact reasons for the different results are unclear, the bafilomycin-induced increase in synaptic currents is probably caused by enhanced exocytosis (see also Fig. 2).
Fig. 5.
Electrophysiological recordings support lack of immediate reuse at excitatory synapses. (A) Whole-cell patch-clamp recordings were obtained from pairs of neurons. (B) Addition of bafilomycin increased synaptic responses to 20-Hz, 2-s stimulus. Shown are current traces before and after addition of bafilomycin, with the stimulus duration indicated above. (Inset) The integrated charge is also shown. (C) An example of progressive loss of synaptic responses to stimulus trains, in the presence of bafilomycin, from one pair of cells. The interval between successive trains was 1 min. Note the similarity of the first and second responses in bafilomycin. (D) Average data from seven experiments.
If recently endocytosed vesicles were to refill the RRP and be reused preferentially, bafilomycin treatment will cause a reduction in subsequent responses. To test this idea, we stimulated synapses multiple times by using the 40-AP stimulus with 1-min wait between stimuli. Synaptic currents recorded over successive trials in the presence of bafilomycin exhibit a gradual reduction in amplitude, rather than a sharp reduction expected from rapid reuse (Fig. 5 C and D). In particular, the first and the second responses in the presence of bafilomycin were statistically indistinguishable (second response: 0.99 ± 0.11, P > 0.5, n = 7). This finding again suggests limited rapid reuse of recently endocytosed vesicles. The rate of reduction of electrophysiological response is faster than that observed with spH, but the overall response profile is qualitatively similar.
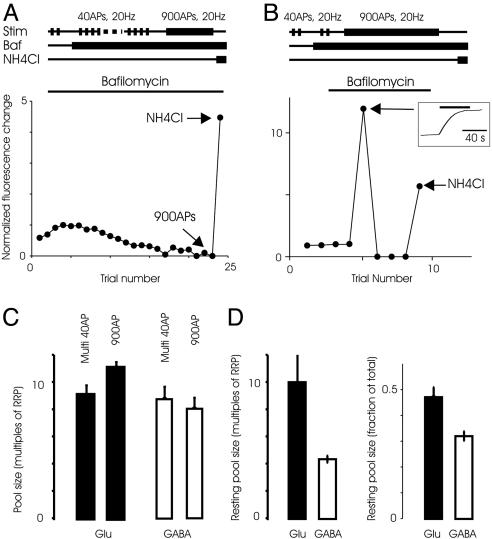
The Entire Recycling Pool Is Recruited During Spaced Release of the RRP. Experiments in the frog neuromuscular junction have indicated that the pool of vesicles that is recruited for release can vary as a function of the stimulus (32, 33). We tested whether spaced stimuli designed to release the RRP (40 APs at 20 Hz) recruits the same number of vesicles as a continuous stimulus of 900 APs at 20 Hz. The total number of vesicles released by multiple spaced stimuli was 8.8 ± 0.9 times the RRP (n = 7) for inhibitory synapses (Fig. 6). We next measured the integrated fluorescence change in response to 900 APs at 20 Hz, which was 8.1 ± 0.8 (n = 6), not significantly different from the response to multiple spaced 40-AP stimuli (P = 0.56). At excitatory synapses, the recycling pool was similar when measured with multiple spaced stimuli or a long sustained stimulus (9.1 ± 0.7 and 11.1 ± 0.4, respectively, n = 5, P = 0.07). The sizes of the recycling pool of vesicles, as a multiple of RRP, were also not different between inhibitory and excitatory synapses (P = 0.5 for multiple spaced stimuli and P = 0.08 for sustained stimuli).
Fig. 6.
Total recycling and resting pools. (A) The total pool of vesicles recruited by spaced 40-AP stimuli was measured by using the protocol illustrated (Upper). (Lower) After multiple 40-AP stimuli depleted the releasable vesicles, a sustained stimulus of 900 APs did not release additional vesicles. A final addition of ammonium chloride (NH4Cl) neutralized additional vesicles that were not recruited during the physiological stimuli. Response is normalized to the first response to 40 APs in the presence of bafilomycin. (B)(Upper) Protocol to measure the total pool of vesicles released by a 900-AP stimulus is shown. (Lower) In this typical experiment, the response to 900 APs was ≈12 times the size of the first response to a 40-AP stimulus in bafilomycin. Although additional 40-AP stimuli after 900 APs did not evoke responses, addition of NH4Cl causes a robust additional increase in fluorescence, revealing resting vesicles. (Inset) The average response to the 900-AP stimulus is shown. (C) Average size of the total recycling pool measured with a sustained stimuli (900 APs) or multiple spaced 40-AP stimuli are indistinguishable for either excitatory (Glu) synapses (n = 5 experiments each) or inhibitory (GABA) synapses (n = 6 and 7 experiments, respectively). (D) The size of the resting pool, in multiples of RRP (Left) or fraction of total pool (Right), was different at excitatory and inhibitory synapses (P < 0.004, n = 10 experiments each).
Resting Vesicle Pools at Excitatory and Inhibitory Synapses. Previous experiments with styryl dyes have indicated that only a small fraction of vesicles at excitatory hippocampal synapses participate in recycling under normal physiological conditions (30, 34). The vesicles that do not seem to recycle have been referred to as the resting pool (35). To test for the presence of the resting pool, we first depleted all of the releasable vesicles by stimulating exhaustively in the presence of bafilomycin, with either multiple spaced 40-AP stimuli or a sustained 900-AP stimulus. If there are additional vesicles at the synapse that do not participate in recycling, their lumen would remain acidic and addition of NH4Cl would cause an increase in the fluorescence intensity as their luminal pH is neutralized. Indeed, we observed a robust increase in fluorescence intensity upon addition of NH4Cl, suggesting the existence of resting vesicles (Fig. 6). The number of resting vesicles was similar when estimated by using the spaced stimuli of 40 APs or a sustained 900-AP stimulus, and the data were pooled. Our results indicate that 4.3 ± 0.3 times the RRP or 32 ± 1.8% (n = 9) of the total vesicles at an inhibitory synapse do not participate in recycling (Fig. 6). At excitatory synapses, resting vesicles constitute 10.0 ± 2.2 times the RRP, or 47.1 ± 3.9% of the total pool, significantly larger than the fraction at inhibitory synapses (P < 0.004).
Discussion
We have generated lines of transgenic mice expressing the fluorescent probe spH, which will be useful for the study of vesicle traffic in different regions of the brain in vitro, as well as for monitoring synaptic activity in the intact brain of living animals (see Fig. 10, which is published as supporting information on the PNAS web site). An important recent study described a mouse expressing spH in olfactory receptor axons, which was useful for obtaining a map of odor-evoked synaptic responses in the glomerular layer of the olfactory bulb (36). The more widespread expression of spH in our mice may permit imaging of synaptic function in different tissues. We have demonstrated the feasibility of imaging exocytic responses from single presynaptic boutons in hippocampal slice cultures.
We used cultured hippocampal neurons in our studies for ease of imaging and to take advantage of the accumulated information available on vesicle recycling in this preparation (1, 12, 37, 38). We find that once vesicles at excitatory or inhibitory terminals undergo exocytosis they are replaced by other vesicles from the reserve pool. Using the method of alkaline trapping (39), we present data arguing against significant reuse of recently released vesicles. Evoked fluorescent responses in successive trials declined gradually, indicating a slow mixing of recently endocytosed vesicles with the reserve pool. Multiple spaced stimuli can efficiently recruit all of the recycling vesicles at a synapse.
Why are our results different from recent studies in hippocampal neurons that appear to indicate kiss-and-run endocytosis and rapid reuse of vesicles? First, it is possible that different mechanisms in vesicle recycling are recruited when hypertonic stimulation is used. Many of the experiments supporting local reuse have been done with hypertonic stimuli (4, 13), although there are hints that similar phenomena might be recruited during AP stimulation (12, 37). Second, differences between techniques used in the different studies (FM1-43 and spH imaging) might account for some of the differences in the results. The membrane interaction of FM dyes might be complex, especially within confined spaces such as the synaptic cleft, complicating the interpretation of experimental results. Finally, kiss-and-run mode recycling may occur relatively infrequently (7), and we may not be able to detect it reliably.
Although the method of alkaline trapping and spH measurements makes reasonable assumptions (see Supporting Methods), we sought to corroborate our findings with electrophysiological experiments. Excitatory synaptic responses to two successive trains of stimuli in bafilomycin were very similar. Only upon further stimulation do the responses gradually decline, similar to the spH fluorescence responses. The rate of decline of the electrophysiological responses was faster than those measured with spH fluorescence. This finding might reflect some degree of uncontrolled recurrent activity in the electrophysiological experiments, which were by necessity performed in the absence of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor blockers. The slight difference between the rate of decline of spH and electrophysiological responses does not affect the basic conclusions of this study: lack of immediate reuse of endocytosed vesicles.
Preferential reuse of vesicles in the RRP was also not supported by measurements of the total recycling pool of vesicles. About 10 times the RRP of vesicles was recruited for release by repeated stimulation in the presence of bafilomycin. A similar estimate was obtained for the size of the recycling pool of vesicles released by a sustained 900-AP stimulus, suggesting that all recycling vesicles can be recruited for release during multiple spaced stimuli. This finding suggests that after one round of release vesicles from the reserve pool refill the releasable pool. Our findings are compatible with recent studies that used sustained stimulation at different frequencies to show that the recycling pool of vesicles is continuously recruited for release, indicating little local reuse (39). At the neuromuscular junction, a small pool of vesicles is selectively recycled and reused at low frequencies (14), but this pool is not strictly localized to the active zone. Therefore, selective reuse of a subset of vesicles can occur without the need for the recycled vesicles to be spatially restricted to the region of the active zone.
A puzzling feature of hippocampal synapses is the inability to recruit a large population of vesicles for release (30, 34). This population of resting vesicles can be as large as 80% of vesicles at excitatory synapses (30, 34). Here, we find that inhibitory synapses also have a population of vesicles that is resistant to release. But this population is smaller than that in excitatory synapses (30% vs. 50%). It is difficult to speculate on the mechanistic basis of this difference because little is known regarding the nature of resting vesicles. Functionally, the smaller resting vesicle pool at inhibitory synapses might allow these synapses to maintain release more efficiently during sustained activity.
In summary, we have used transgenic mice expressing spH to demonstrate the feasibility of imaging presynaptic function at individual synapses in intact tissue. In dissociated hippocampal cultures, we find little evidence for preferential local reuse of synaptic vesicles at excitatory or inhibitory synapses. These results suggest that even if vesicles are retrieved intact after exocytosis they are unlikely to remain in place for the next round of release. It will be important to determine whether there are conditions under which rapid reuse, including kiss and run, occurs.
Supplementary Material
Acknowledgments
We thank Drs. Joshua Sanes and Catherine Dulac (Harvard University) for providing the thy1.2 cassette and advice regarding its use and Dr. Hai-fei Ma and the Harvard Genome Manipulation Facility for pronuclear injections. This work was supported by grants from the National Institutes of Health (to V.N.M.), the Pew Scholars Program in Biomedical Sciences (to V.N.M.), the EJLB Foundation (to V.N.M.), the National Science Foundation (to V.N.M.), and the National Alliance for Research in Schizophrenia and Depression (to J.B.).
Author contributions: Z.L., J.B., and V.N.M. designed research; Z.L., J.B., W.J.T., K.N.H., and D.F.A. performed research; Z.L., J.B., W.J.T., K.N.H., and V.N.M. analyzed data; and Z.L., J.B., W.J.T., and V.N.M. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: spH, synaptopHluorin; GAD, glutamic acid decarboxylase; AP, action potential; RRP, readily releasable pool.
References
- 1.Murthy, V. N. & De Camilli, P. (2003) Annu. Rev. Neurosci. 26, 701-728. [DOI] [PubMed] [Google Scholar]
- 2.Sudhof, T. C. (2004) Annu. Rev. Neurosci. 27, 509-547. [DOI] [PubMed] [Google Scholar]
- 3.Fesce, R., Grohovaz, F., Valtorta, F. & Meldolesi, J. (1994) Trends Cell Biol. 4, 1-4. [DOI] [PubMed] [Google Scholar]
- 4.Stevens, C. F. & Williams, J. H. (2000) Proc. Natl. Acad. Sci. USA 97, 12828-12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klyachko, V. A. & Jackson, M. B. (2002) Nature 418, 89-92. [DOI] [PubMed] [Google Scholar]
- 6.Aravanis, A. M., Pyle, J. L. & Tsien, R. W. (2003) Nature 423, 643-647. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi, S. P. & Stevens, C. F. (2003) Nature 423, 607-613. [DOI] [PubMed] [Google Scholar]
- 8.Staal, R. G., Mosharov, E. V. & Sulzer, D. (2004) Nat. Neurosci. 7, 341-346. [DOI] [PubMed] [Google Scholar]
- 9.Verstreken, P., Kjaerulff, O., Lloyd, T. E., Atkinson, R., Zhou, Y., Meinertzhagen, I. A. & Bellen, H. J. (2002) Cell 109, 101-112. [DOI] [PubMed] [Google Scholar]
- 10.Neves, G., Gomis, A. & Lagnado, L. (2001) Proc. Natl. Acad. Sci. USA 98, 15282-15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun, J. Y., Wu, X. S. & Wu, L. G. (2002) Nature 417, 555-559. [DOI] [PubMed] [Google Scholar]
- 12.Klingauf, J., Kavalali, E. T. & Tsien, R. W. (1998) Nature 394, 581-585. [DOI] [PubMed] [Google Scholar]
- 13.Pyle, J. L., Kavalali, E. T., Piedras-Renteria, E. S. & Tsien, R. W. (2000) Neuron 28, 221-231. [DOI] [PubMed] [Google Scholar]
- 14.Rizzoli, S. O. & Betz, W. J. (2004) Science 303, 2037-2039. [DOI] [PubMed] [Google Scholar]
- 15.Benson, D. L. & Cohen, P. A. (1996) J. Neurosci. 16, 6424-6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenmund, C., Sigler, A., Augustin, I., Reim, K., Brose, N. & Rhee, J. S. (2002) Neuron 33, 411-424. [DOI] [PubMed] [Google Scholar]
- 17.Verderio, C., Pozzi, D., Pravettoni, E., Inverardi, F., Schenk, U., Coco, S., Proux-Gillardeaux, V., Galli, T., Rossetto, O., Frassoni, C. & Matteoli, M. (2004) Neuron 41, 599-610. [DOI] [PubMed] [Google Scholar]
- 18.Varela, J. A., Song, S., Turrigiano, G. G. & Nelson, S. B. (1999) J. Neurosci. 19, 4293-4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan, M. P., Wilcox, K. S. & Dichter, M. A. (2003) Synapse 50, 41-52. [DOI] [PubMed] [Google Scholar]
- 20.Terada, S., Tsujimoto, T., Takei, Y., Takahashi, T. & Hirokawa, N. (1999) J. Cell Biol. 145, 1039-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miesenbock, G., De Angelis, D. A. & Rothman, J. E. (1998) Nature 394, 192-195. [DOI] [PubMed] [Google Scholar]
- 22.Caroni, P. (1997) J. Neurosci. Methods 71, 3-9. [DOI] [PubMed] [Google Scholar]
- 23.Li, Z. & Murthy, V. N. (2001) Neuron 31, 593-605. [DOI] [PubMed] [Google Scholar]
- 24.Stoppini, L., Buchs, P. A. & Muller, D. (1991) J. Neurosci. Methods 37, 173-182. [DOI] [PubMed] [Google Scholar]
- 25.Feng, G., Mellor, R. H., Bernstein, M., Keller-Peck, C., Nguyen, Q. T., Wallace, M., Nerbonne, J. M., Lichtman, J. W. & Sanes, J. R. (2000) Neuron 28, 41-51. [DOI] [PubMed] [Google Scholar]
- 26.Sankaranarayanan, S. & Ryan, T. A. (2000) Nat. Cell Biol. 2, 197-204. [DOI] [PubMed] [Google Scholar]
- 27.Sankaranarayanan, S. & Ryan, T. A. (2001) Nat. Neurosci. 4, 129-136. [DOI] [PubMed] [Google Scholar]
- 28.Murphy, D. D., Cole, N. B., Greenberger, V. & Segal, M. (1998) J. Neurosci. 18, 2550-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schikorski, T. & Stevens, C. F. (2001) Nat. Neurosci. 4, 391-395. [DOI] [PubMed] [Google Scholar]
- 30.Murthy, V. N. & Stevens, C. F. (1999) Nat. Neurosci. 2, 503-507. [DOI] [PubMed] [Google Scholar]
- 31.Zhou, Q., Petersen, C. C. & Nicoll, R. A. (2000) J. Physiol. (London) 525, 195-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richards, D. A., Guatimosim, C. & Betz, W. J. (2000) Neuron 27, 551-559. [DOI] [PubMed] [Google Scholar]
- 33.Richards, D. A., Guatimosim, C., Rizzoli, S. O. & Betz, W. J. (2003) Neuron 39, 529-541. [DOI] [PubMed] [Google Scholar]
- 34.Harata, N., Pyle, J. L., Aravanis, A. M., Mozhayeva, M., Kavalali, E. T. & Tsien, R. W. (2001) Trends Neurosci. 24, 637-643. [DOI] [PubMed] [Google Scholar]
- 35.Sudhof, T. C. (2000) Neuron 28, 317-320. [DOI] [PubMed] [Google Scholar]
- 36.Bozza, T., McGann, J. P., Mombaerts, P. & Wachowiak, M. (2004) Neuron 42, 9-21. [DOI] [PubMed] [Google Scholar]
- 37.Sara, Y., Mozhayeva, M. G., Liu, X. & Kavalali, E. T. (2002) J. Neurosci. 22, 1608-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan, T. A. & Smith, S. J. (1995) Neuron 14, 983-989. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Alfonso, T. & Ryan, T. A. (2004) Neuron 41, 943-953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.