Abstract
Introduction
Type 2 diabetes mellitus (T2DM) is associated with huge clinical and economic burden in the Kingdom of Saudi Arabia (KSA) which can be curtailed by efficacious treatment. In order to achieve this, current treatment pathways for T2DM and associated costs need to be assessed.
Methods
A longitudinal cohort review was conducted to collect country-specific and patient-specific clinical data, over a minimum observation period of 5 years in the KSA. Patient demographics, clinical characteristics and treatment patterns were recorded. The IQVIA Core Diabetes Model (CDM) version 9.5 Plus was used to assess the burden of illness, which included long-term projections of clinical (life expectancy [LE], quality-adjusted life-years [QALYs], event rates of diabetes-related complications) and direct medical cost (per-patient annual or lifelong [50 years]) outcomes of the most commonly used first-line (1st-line) regimens for T2DM from a payer perspective in the KSA.
Results
Data were collected from a subpopulation of 638 patients from 15 participating centres. There was an equal gender representation with a majority of the patients belonging to Arabian/Saudi ethnicity (71.0%). Biguanides (81.5%), sulfonylureas (51.6%), dipeptidyl peptidase 4 (DPP4) inhibitors (26.2%) and fast-acting insulins (17.2%) were the most prescribed 1st-line agents. The most frequently used 1st-line regimens resulted in an estimated LE of 25–28 years, QALYs of 18–21 years and lifelong total cost of illness of 201,377–437,371 Saudi Arabian riyal (53,700–116,632 US dollars).
Conclusion
Our study addresses gaps in the current research by providing a complete landscape of baseline demographic, clinical characteristics and treatment patterns from a heterogeneous group of patients with T2DM in the KSA. Additionally, the burden of illness analysis using CDM showed substantially higher cost of T2DM care from a payer perspective in the KSA.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-023-02772-y.
Keywords: Burden of illness, Core diabetes model, Kingdom of Saudi Arabia, Real-world, Saudi Arabia, Type 2 diabetes mellitus
Key Summary Points
| Why carry out this study? |
| Type 2 diabetes mellitus (T2DM) is associated with a significant financial burden and low quality of life in the Kingdom of Saudi Arabia (KSA). |
| To improve the management of T2DM and reduce the prevalence of T2DM-related complications, there is a need to review the current treatment pathways and assess the economic burden in the KSA. |
| What was learned from the study? |
| Analysis of a heterogenous group of patients with T2DM showed that biguanides (monotherapy or combination) were the most commonly used 1st-line therapy. |
| The lifelong total cost of T2DM care was estimated as 201,377 to 437,371 Saudi Arabian riyal (53,700–116,632 US dollars). |
| The major cost drivers for the most frequently used 1st-line regimens were the cost of cardiovascular disease complications. |
| Since T2DM is a progressive disease, treatment intensification of patients with inadequate glycaemic control should be implemented immediately,thereby avoiding the therapeutic inertia that might delay the glycaemic control and imposes a substantial economic impact on the healthcare system in the KSA. |
Introduction
Diabetes mellitus (DM) is a chronic condition characterised by elevated glucose levels in the blood (hyperglycaemia). Type 2 diabetes mellitus (T2DM), accounting for around 90% of all cases of DM, is characterised by hyperglycaemia as a result of inability of the body to respond sufficiently to insulin (insulin resistance) followed by an inadequate production of insulin [1]. The global prevalence rate of diabetes (including all types) in adults was estimated as 536.6 million (10.5%) in 2021 [1] whilst that of T2DM was 6059 per 100,000 individuals in 2017, which continues to rise despite various public health measures [2]. The International Diabetes Federation reported the 2021 world population standardised prevalence of diabetes (including all types) as 18.7% for adults in the KSA, which is projected to reach 21.4% by 2045 [1]. Similar to global patterns, the major drivers of this rise in T2DM in the KSA could be increased life expectancy (LE) and lifestyle changes (physical inactivity and unhealthy eating) due to rapid urbanisation and industrialisation [3].
Patients with T2DM have an increased risk of complications, which can lead to decreased quality of life (QoL) especially if the disease is undiagnosed or poorly managed [1, 4]. The chronic T2DM-related microvascular complications include nephropathy, neuropathy and retinopathy [4]. Furthermore, T2DM-related chronic macrovascular complications include coronary artery disease, peripheral vascular disease and cerebrovascular disease [1]. Cardiovascular diseases (CVD) including myocardial infarction (MI) are the major cause of death and disability amongst patients with T2DM [4, 5]. These complications contribute to an increase in direct medical costs (treatment, diagnostics, hospitalisations, and inpatient and outpatient visits) and indirect costs (absenteeism, reduced productivity, lost productivity due to early mortality), and a reduced QoL [4, 6–8]. The drugs used for diabetes rank first among the most commonly used medications for chronic conditions in the KSA, thereby posing a significant financial burden [9]. A systematic review of published studies up to 2018 estimated total per-patient direct cost of 1947 US dollars ($) in the KSA for patients with T2DM [10]. The annual per-person diabetes-related healthcare expenditure in the KSA was $1743 in 2021 [1]. It was also estimated that the per capita healthcare expenditure due to diabetes for people aged 15−44 years is almost double compared to people without diabetes [11].
In order to assist healthcare providers in determining the most appropriate treatment options, Saudi National Diabetes Center (SNDC) developed Saudi Diabetes Clinical Practice Guidelines (SDCPG) in 2021 [12]. The guidelines suggest a glycosylated haemoglobin (HbA1c) level below 7% (< 53 mmol/mol) as the overall goal of management of T2DM [12]. Higher or lower targets (6.5–8%) may be appropriate in certain conditions and the targets can be individualised [12]. The SNDC guidelines prefer lifestyle modification, followed by metformin (drug class biguanide), if the disease is not controlled, as the first-line (1st line) treatment for T2DM [12]. In patients without atherosclerotic cardiovascular disease (ASCVD) or chronic kidney disease (CKD), second-line (2nd-line) combination therapy with metformin and sulfonylureas or thiazolidinediones or dipeptidyl peptidase 4 (DPP4) inhibitors or sodium-glucose co-transporter 2 inhibitors (SGLT2i) or glucagon-like peptide 1 receptor agonist (GLP-1 RA), or basal insulin is preferred if the HbA1c target is > 1.5% above the glycaemic goal. The choice of agent is selected on the basis of individual patients’ needs, cost and adverse event profile. In patients with elevated risk for ASCVD, heart failure, or CKD, a GLP-1 RA or SGLT2i is favoured. In patients who require a third-line (3rd-line) agent (with HbA1c > 9%) to achieve glycaemic goals, similar considerations are applied.
These treatment patterns for T2DM may differ in real-world (RW) practice as a result of several factors such as drug efficacy and safety, medicine cost, patient’s choices, drug side effects and physicians’ choices for drugs based on their individual experience [13, 14]. Anyhow, only about a quarter of patients with T2DM achieve the recommended HbA1c level (< 7%) in the KSA [15]. Therefore, there is the potential to curtail the economic burden of T2DM with effective diabetes management [7].
The Saudi Ministry of Health (MoH) aims to improve the management of T2DM and reduce the disease burden in the country by offering better treatment and blood glucose measurement devices, which necessitates extensive review of current treatment pathways and assessment of the associated costs. Therefore, recent baseline characteristics of patients newly diagnosed with T2DM, assigned treatments, and effects of these treatments are required. The current study aimed to collect these baseline characteristics and treatment patterns from a heterogeneous group of patients with T2DM using a retrospective patient chart review approach. Furthermore, the economic burden of T2DM in the KSA was estimated for the most commonly used 1st-line regimens (such as biguanides, sulfonylureas, insulin) and using the IQVIA Core Diabetes Model (CDM).
Methods
Study Design and Setting
This study was a longitudinal, observational study conducted at 15 participating centres (within general hospitals and specialised tertiary diabetes centres under the MoH) in the KSA over a span of 5 years. This study was conducted in accordance with the ethical principles derived from international ethics guidelines, including the Declaration of Helsinki, and the International Council for Harmonisation (ICH) guidelines for Good Clinical Practice (GCP), all applicable laws, rules, and regulations. Consistent with local regulations and prior to the enrolment of patients, the study received approval from the Saudi MoH, independent ethics committee/institutional review board (IEC/IRB). Since this research presented no more than minimal risk of harm to patients and involved no physical procedures with patients, informed consent was not required.
Study Population
The inclusion criteria for this study included a new diagnosis of T2DM over a 10-year study period (between the date of the first site initiation visit [SIV, 2021/2022] and 10 years prior [2011/2012]), with a minimum follow-up period of 5 years in any of the participating centres. Diagnosis of T2DM was based on measured HbA1c ≥ 6.5% (48.5 mmol/mol) and/or fasting plasma glucose (FPG) of ≥ 126 mg/dL.
The exclusion criteria included participation in an investigational study in which patient assessment and/or treatment was dictated by a protocol over the 10-year study period.
Data Sources and Collection
Patient data were extracted from medical records starting at the date of T2DM diagnosis (defined as baseline). Data were collected using INES (electronic Case Report Form), an online data capture and data quality management tool. Cost data from the MoH were collected to assess the costs of healthcare resource use for T2DM.
Data collected at baseline included patient demographics, risk factors and existing complications (cardiovascular, renal, ocular and foot ulcer). Follow-up data included all relevant information in the medical records after the date of diagnosis (with a minimum observation period of 5 years). This included requirement of screening tests (for eye diseases, microalbuminuria [MA], and gross renal proteinuria [GRP] and foot care programme), T2DM treatment patterns, other treatment details, newly diagnosed complications and cost of drugs and screening tests. For treatment patterns, all drugs prescribed to patients and dates of the prescriptions were recorded. A line of therapy (LoT) included all drugs prescribed to a patient within a 3-month duration. Drugs prescribed after 3 months from the previous drug were considered a new LoT.
Data Processing
The effects of treatment on the risk factors (HbA1c, lipids, and estimated glomerular filtration rate) were analysed during the 5-year follow-up period. Annual changes in risk factors were calculated using the closest follow-up data available within ± 6 months of the baseline date + follow-up year.
The incidence rate (per 100 patient-years) of each of the T2DM-related complication was calculated by dividing the number of new complications that occurred after baseline by the total number of patient-years at risk. Complications present at baseline (at date of diagnosis of T2DM) were not included in the incidence rate calculation.
Statistical Analyses
All analyses were performed using R version 3.6.1 or higher (R Foundation for Statistical Computing, Vienna, Austria). According to the nature of this study, the analyses were mainly descriptive and did not require statistical testing. Categorical variables were described by frequencies and proportions. Continuous variables were described by mean and standard deviation (SD). The calculations and proportions did not include the missing/invalid category in the denominators.
Model Description and Inputs
The IQVIA CDM version 9.5 Plus (http://www.core-diabetes.com/) was used to assess the burden of illness, which included long-term projections of clinical and cost outcomes of the most commonly used 1st-line regimens for T2DM from a payer perspective in the KSA. CDM is a proprietary, interactive computer simulation model that includes a series of interdependent Markov sub-models to perform real-time simulations of the progression of diabetes-related complications and associated mortality. CDM and its validation studies have been previously described in literature [16–18].
Clinical inputs for the model were retrieved from the retrospective phase of this study (baseline patient demographics, risk factors, ethnicity, proportion of patients using concomitant medications and screening tests, and rate of existing complications) or from published sources (cost of complications, sensitivity and specificity of screening tests [Supplementary Tables 1 and 2]). CDM default values were used for those baseline parameters which were not collected during the retrospective study. Rates of hypoglycaemia events were retrieved from published sources [19–21]. The treatment-specific efficacy data obtained during the retrospective phase of this study was used as input for the CDM.
Treatment-specific progression of risk factors (HbA1c, blood pressure, lipids and body mass index [BMI]) were applied as obtained during the 5-year retrospective phase of this study. No progression was assumed afterwards. For cardiovascular risk prediction, the general Framingham risk equation was used which is default for CDM v9.5 Plus. For mortality, combined (the United Kingdom Prospective Diabetes Study 82 [UKPDS 82]) approach was applied.
Utility values, calculated for every patient in each simulation year, were used to estimate the average quality-adjusted life-years (QALYs). The utility and disutility values were obtained from previously published sources (Supplementary Table 3) [22–24].
Only direct medical costs (associated with pharmacy, management, and T2DM complications) were included. The annual pharmacy costs for each treatment regimen were calculated using unit costs for drugs, retrieved from National Unified Procurement Company (https://www.nupco.com/en/) and drug dosage from World Health Organization Collaborating Centre for Drug Statistics Methodology (https://www.whocc.no/atc_ddd_index/). Unit costs associated with management and complications (including hospitalisation) associated with T2DM were obtained from published sources (Supplementary Table 1). Costs of complications obtained from published sources were inflated to 2021, where necessary, using consumer price index from The World Bank (https://data.worldbank.org/). The cost outcomes were also presented in US $ using an exchange rate of $1 = 3.75 SAR (Saudi Arabian riyal) [25].
The projected outcomes of the model included LE, QALYs, per-patient annual or lifelong (50 years) costs and event rates of diabetes-related complications per 1000 patient-years. The total lifelong costs were projected on the basis of the cost for treatment (drugs and glucose monitoring), management (screening tests), complications and adverse events (hypoglycaemic events). The projected annual costs were calculated by dividing the undiscounted lifelong costs by the estimated undiscounted LE.
Results
Patient Disposition
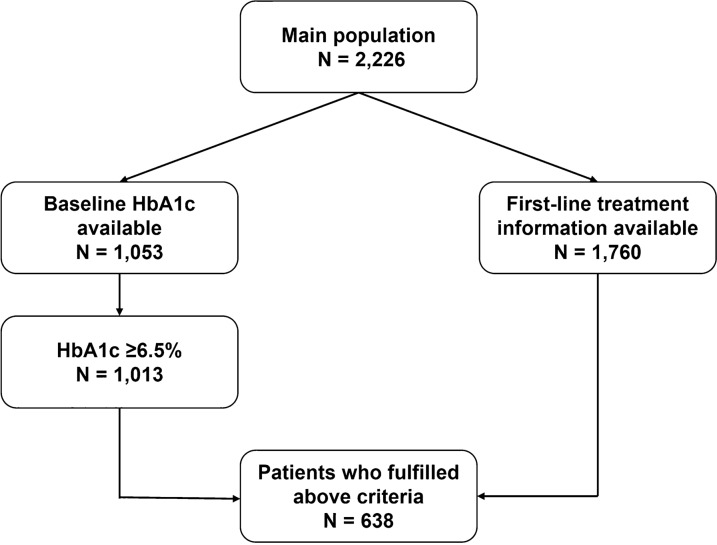
Data were collected from a total of 2226 patients (main population). The baseline demographics, risk factors and existing complications of this population are presented in Supplementary Table 4. However, as a result of many missing variables (specifically, baseline HbA1c or start date of treatment) in the collected data, the main population could not be used to provide necessary inputs for the CDM.
Therefore, for further analysis, a subpopulation was defined on the basis of the following criteria: (1) availability of baseline HbA1c, (2) baseline HbA1c ≥ 6.5%, and (3) at least one treatment with available start date (Fig. 1). This subpopulation consisted of 638 patients and all further analyses were performed for this subpopulation.
Fig. 1.
Criteria for selecting study subpopulation. N number of patients
Baseline Demographics and Clinical Characteristics
The subpopulation of 638 patients showed an equal gender representation (50% male) (Table 1). The mean (SD) age at diagnosis of diabetes was 49.1 (11.6) years, with a follow-up time of 8.0 (1.4) years. The majority of the patients belonged to Arabian/Saudi ethnicity (71.0%), followed by White (22.6%).
Table 1.
Baseline characteristics of the subpopulation
| Parameters | Description | Values (N = 638) |
|---|---|---|
| Gender | Male | 315 (49.5%) |
| Female | 322 (50.5%) | |
| Missing (n) | 1 | |
| Start age of T2DM | N | 618 |
| Mean (SD), (years) | 49.1 (11.6) | |
| Missing (n) | 20 | |
| Time of follow-upa | N | 619 |
| Mean (SD), (years) | 8.0 (1.4) | |
| Missing (n) | 19 | |
| Ethnic group | Arab/Saudi | 434 (71.0%) |
| White | 138 (22.6%) | |
| Black | 27 (4.4%) | |
| Hispanic | 0 (0.0%) | |
| Native American | 0 (0.0%) | |
| Asian/Pacific Islander | 9 (1.5%) | |
| Other | 3 (0.5%) | |
| Missing (n) | 27 | |
| Family history of T2DM | Yes | 326 (65.2%) |
| No | 174 (34.8%) | |
| Missing (n) | 138 | |
| Alcohol consumption status at initial diagnosis of T2DM | Never | 555 (100.0%) |
| Current | 0 (0.0%) | |
| Former | 0 (0.0%) | |
| Missing (n) | 83 | |
| Weekly alcohol consumptionb | N | 0 |
| Mean (SD), (oz/week) | NA | |
| Missing (n) | NA | |
| Smoking status at initial diagnosis of T2DM | Never | 481 (87.0%) |
| Current | 57 (10.3%) | |
| Former | 15 (2.7%) | |
| Missing (n) | 85 | |
| Number of cigarettes smoked per dayb | N | 37 |
| Mean (SD) | 14.1 (6.6) | |
| Missing (n) | 35 | |
| Pack yearsb | N | 36 |
| Mean (SD) | 8.9 (6.0) | |
| Missing (n) | 36 | |
| BMI | N | 598 |
| Mean (SD), (numerical, kg/m2) | 30.5 (6.1) | |
| Missing (n) | 40 | |
| BMI (categorical) | Underweight | 3 (0.5%) |
| Normal weight | 91 (15.2%) | |
| Overweight | 240 (40.1%) | |
| Obesity | 264 (44.1%) | |
| Missing (n) | 40 | |
| Waist-to-hip ratio | N | 2 |
| Mean (SD) | 0.90 (0.0071) | |
| Missing (n) | 636 | |
| Heart rate | N | 458 |
| Mean (SD), (bpm) | 83.1 (9.5) | |
| Missing (n) | 180 | |
| SBP | N | 565 |
| Mean (SD), (mmHg) | 134.1 (17.9) | |
| Missing (n) | 73 | |
| DBP | N | 565 |
| Mean (SD), (mmHg) | 79.4 (9.8) | |
| Missing (n) | 73 | |
| Haemoglobinc | N | 368 |
| Mean (SD), (g/dL) | 14.0 (1.8) | |
| Missing (n) | 270 | |
| WBCd | N | 371 |
| Mean (SD), (106/mL) | 7.2 (2.3) | |
| Missing (n) | 267 | |
| HbA1ce | N | 638 |
| Mean (SD), (%) | 9.7 (2.0) | |
| Missing (n) | 0 | |
| Serum creatininef | N | 425 |
| Mean (SD), (mg/dL) | 0.8 (0.24) | |
| Missing (n) | 213 | |
| Serum albuming | N | 251 |
| Mean (SD), (g/dL) | 3.8 (0.4) | |
| Missing (n) | 387 | |
| T-Cholh | N | 443 |
| Mean (SD), (mg/dL) | 191.8 (46.6) | |
| Missing (n) | 195 | |
| HDLh | N | 364 |
| Mean (SD), (mg/dL) | 43.7 (14.5) | |
| Missing (n) | 274 | |
| LDLh | N | 407 |
| Mean (SD), (mg/dL) | 125.0 (39.6) | |
| Missing (n) | 231 | |
| TRIGi | N | 441 |
| Mean (SD), (mg/dL) | 158.6 (92.7) | |
| Missing (n) | 197 | |
| eGFR | N | 10 |
| Mean (SD), (mL/min/1.73 m2) | 105.3 (35.2) | |
| Missing (n) | 628 | |
| UACRj | N | 10 |
| Mean (SD), (mg/mmol) | 57.9 (62.3) | |
| Missing (n) | 628 | |
| Cardiovascular | Yes | 35 (5.5%) |
| No | 598 (94.5%) | |
| Missing (n) | 5 | |
| Cardiovascular subcategoryk | MI | 9 (1.4%) |
| Angina | 4 (0.6%) | |
| PVD | 1 (0.2%) | |
| Stroke | 1 (0.2%) | |
| CHF | 5 (0.8%) | |
| Atrial fibrillation | 4 (0.6%) | |
| LVH | 1 (0.2%) | |
| Unknown | 10 (1.6%) | |
| Renal | Yes | 4 (0.6%) |
| No | 629 (99.4%) | |
| Missing (n) | 5 | |
| Renal subcategoryk | MA | 0 (0.0%) |
| GRP | 0 (0.0%) | |
| ESRD | 0 (0.0%) | |
| Unknown | 4 (0.6%) | |
| Ocular | Yes | 49 (7.7%) |
| No | 584 (92.3%) | |
| Missing (n) | 5 | |
| Ocular subcategoryk | BDR | 3 (0.5%) |
| PDR | 4 (0.6%) | |
| SVL | 14 (2.2%) | |
| ME | 0 (0.0%) | |
| Cataract | 22 (3.5%) | |
| Unknown | 6 (0.9%) | |
| Foot ulcer | Yes | 10 (1.6%) |
| No | 623 (98.4%) | |
| Missing (n) | 5 | |
| Foot ulcer subcategoryk | Uninfected foot ulcer | 4 (0.6%) |
| Infected foot ulcer | 1 (0.2%) | |
| Healed foot ulcer | 1 (0.2%) | |
| History of lower limb amputation | 0 (0.0%) | |
| Unknown | 4 (0.6%) | |
| Foot ulcer subcategory (aggregated)k | Foot ulcer (any type) | 6 (0.9%) |
| Neuropathy | Yes | 5 (0.8%) |
| No | 628 (99.2%) | |
| Missing (n) | 5 |
BDR background diabetic retinopathy, bpm beats per minute, BMI body mass index, CHF congestive heart failure, DBP diastolic blood pressure, eGFR estimated glomerular filtration rate, ESRD end-stage renal disease, GRP gross renal proteinuria, HbA1c glycosylated haemoglobin A1c, HDL high-density lipoprotein, LDL low-density lipoprotein, LVH left ventricular failure, MA microalbuminuria, ME macular oedema, MI myocardial infarction, N total number of patients with available data, NA not applicable, oz ounce, PDR proliferative diabetic retinopathy, PVD peripheral vascular disease, SBP systolic blood pressure, SD standard deviation, SVL severe visual loss, T2DM type 2 diabetes mellitus, T-Chol total cholesterol, TRIG triglycerides, UACR urinary albumin creatinine ratio, WBC white blood cell count
aDuration between diagnosis of T2DM and the site initiation visit by the patient
bFor current and former consumers
cOnly values in g/dL are used (values in mmol/L were inconsistent). Values greater than 100 g/dL were discarded
dValues greater than 30 × 106/mL were discarded
eValues in mmol/L converted to % using the following equation: (18.015 × value in mmol/L + 46.7)/28.7. Date from two patients with values of 60 and 807 mmol/L were discarded
fOnly values in mg/dL were used (values in μmol/L were inconsistent). Values greater than 3.4 mg/dL were discarded
gOnly values in g/dL were used (values in μmol/L and g/L were inconsistent). Values greater than 30 g/dL were discarded
hValues in μmol/L converted as 1 μmol/L = 38.67 mg/dL. Values lower than 10 or greater than 2000 mg/dL were discarded
iValues in μmol/L converted as 1 μmol/L = 88.57 mg/dL. Values lower than 10 or greater than 6000 mg/dL were discarded
jEight values reported in mmol/L, two in missing units. Since no conversion can be made, they were reported as if they all were in mg/mmol
kMulti-response question. Denominators for % calculation exclude patients with missing value in the main category
Furthermore, patients had a mean (SD) BMI of 30.5 (6.1) kg/m2, with 44.1% categorised as obese, and an additional 40.1% categorised as overweight (Table 1), re-emphasising that obesity is an important modifiable risk factor for onset and progression of T2DM. The rather high mean (SD) HbA1c of 9.7% (2.0%) at baseline suggests a delay in seeking medical care by these patients due to possible lack of awareness about the disease.
A small proportion of patients reported CVD (5.5%), renal (0.6%), ocular (7.7%) and foot ulcer (1.6%) complications at the diagnosis of diabetes (Table 1).
Treatment Patterns During Follow-up
Table 2 describes the treatment patterns for patients with T2DM by drug class, LoT and information about monotherapy or combinations. Whilst all 638 patients reported data for 1st LoT, a total of 352 patients initiated a 2nd LoT after a mean (SD) duration of 30.6 (22.4) months from the beginning of 1st LoT. The overall analysis of all monotherapy or combinations showed that biguanides (81.5%), sulfonylureas (51.6%), DPP4 inhibitors (26.2%) and fast-acting insulins (17.2%) were the most prescribed 1st-line agents, whilst GLP-1 analogues and SGLT2i were used in only 2.0% of patients each.
Table 2.
Description of T2DM treatments including monotherapy or combinations in 1st or 2nd LoTs
| Overall description of drugs during 1st or 2nd LoT, N (%) | |||
|---|---|---|---|
| Type of drugs and ATC codes | Drug class | Values | |
| 1st LoT (N = 638) |
2nd LoT (N = 352)a |
||
| Insulins and analogues for injectionb | |||
| A10AB | Fast-acting | 110 (17.2%) | 41 (11.6%) |
| A10AC | Intermediate-acting | 8 (1.2%) | 16 (4.5%) |
| A10AD | Intermediate- or long-acting combined with fast-acting | 37 (5.8%) | 18 (5.1%) |
| A10AE | Long-acting | 130 (20.4%) | 68 (19.3%) |
| Blood glucose-lowering drugs, excluding insulinsb | |||
| A10BA | Biguanides | 520 (81.5%) | 29 (8.2%) |
| A10BB | Sulfonylureas | 329 (51.6%) | 83 (23.6%) |
| A10BD | Combinations of oral blood glucose-lowering drugs | 0 (0.0%) | 1 (0.3%) |
| A10BG | Thiazolidinediones | 1 (0.2%) | 1 (0.3%) |
| A10BH | DPP4 inhibitors | 167 (26.2%) | 155 (44.0%) |
| A10BJ | GLP-1 analogues | 13 (2.0%) | 14 (4.0%) |
| A10BK | SGLT2 inhibitors | 13 (2.0%) | 9 (2.6%) |
| Other drugs not included above | 1 (0.2%) | ||
| Monotherapy or combinations used during 1st LoT with their durations | |||
|---|---|---|---|
| ATC codesc | Drug class | 1st LoT (N = 638) |
Duration (months) Mean (SD) |
| A10BA + A10BB | Biguanides + sulfonylureas | 189 (29.6%) | 28.8 (20.0) |
| A10BA | Biguanides | 103 (16.1%) | 31.7 (24.0) |
| A10BA + A10BB + A10BH | Biguanides + sulfonylureas + DPP4 inhibitors | 82 (12.9%) | 36.5 (22.1) |
| A10AB + A10AE | Fast-acting insulins + long-acting insulins | 48 (7.5%) | 20.2 (8.7) |
| A10BA + A10BH | Biguanides + DPP4 inhibitors | 34 (5.3%) | 24.0 (21.2) |
| A10AD + A10BA | Premixed insulins + biguanides | 19 (3.0%) | 16.9 (18.2) |
| A10AB + A10AE + A10BA | Fast-acting insulins + long-acting insulins + biguanides | 15 (2.4%) | 66.2 (38.8) |
| A10AE | Long-acting insulins | 12 (1.9%) | 16.8 (9.6) |
| A10AB | Fast-acting insulins | 11 (1.7%) | 26.5 (16.9) |
| A10AE + A10BA + A10BB + A10BH | Long-acting insulins + biguanides + sulfonylureas + DPP4 inhibitors | 10 (1.6%) | 8.5 (7.8) |
| A10AE + A10BB | Long-acting insulins + sulfonylureas | 10 (1.6%) | 19.7 (NA) |
| A10AB + A10BA + A10BH | Fast-acting insulins + biguanides + DPP4 inhibitors | 7 (1.1%) | 27.0 (28.7) |
| A10AD | Premixed insulins | 7 (1.1%) | 96.6 (NA) |
| A10BB | Sulfonylureas | 7 (1.1%) | 5.2 (0.1) |
| Other combinations | 84 (13.2%) | 35.8 (22.5) | |
ATC anatomical therapeutic chemical, DPP4 dipeptidyl peptidase 4, GLP-1 glucagon-like peptide 1, LoT line of therapy, NA not applicable, SD standard deviation, SGLT2 sodium-glucose co-transporter 2, T2DM type 2 diabetes mellitus
aOnly for patients with a 1st and 2nd line of T2DM treatment (insulins and other blood glucose-lowering drugs)
bMulti-response variable
cOnly combinations present in at least 1% of patients with a 1st LoT were reported. Some T2DM treatments, present in a low number of patients, may be included only in “Other combinations” because of this
The analysis of 1st-line regimens (Table 1) showed that the most common therapies were biguanides + sulfonylureas (regimen 1, 29.6%), biguanides (regimen 2, 16.1%), biguanides + sulfonylureas + DPP4 inhibitors (regimen 3, 12.9%), fast-acting insulins + long-acting insulins (regimen 4, 7.5%), biguanides + DPP4 inhibitors (regimen 5, 5.3%), premixed insulins + biguanides (regimen 6, 3.0%), fast-acting insulins + long-acting insulins + biguanides (regimen 7, 2.4%) and long-acting insulins (regimen 8, 1.9%). Of these, regimen 7 showed the longest mean (SD) treatment duration (66.2 [38.8] months), followed by regimen 3 (36.5 [22.1] months) during 1st LoT. The regimens used at 1st-line imply that the patients were in different stages of T2DM which is a progressive condition.
Details of 2nd-line regimens and the overall therapies used for 1st + 2nd LoT are shown in Supplementary Table 5). GLP-1 analogues and SGLT2i were used in 2.3% and 1.1% patients, respectively at 2nd LoT. Supplementary Table 6 describes the proportion of patients using other treatments (such as statins and aspirin) and screening tests.
Evolution of Risk Factors During Follow-up
The evolution of risk factors was analysed over the follow-up period and data were presented as change from baseline during each year (Table 3). After 5 years of follow-up, there was a decrease in all risk factors compared to baseline, except high-density lipoprotein (HDL), suggesting that risk of complications decreased with therapy.
Table 3.
Annual changes in risk factors between baseline and first to fifth year of follow-up per patient
| Parameters | First year | Second year | Third year | Fourth year | Fifth year |
|---|---|---|---|---|---|
| Hb1Aca | |||||
| N | 363 | 321 | 321 | 316 | 167 |
| Mean (SD), (% per year) | − 1.2 (2.4) | − 0.61 (1.1) | − 0.39 (0.7) | − 0.27 (0.59) | − 0.16 (0.41) |
| Missing (n) | 275 | 317 | 317 | 322 | 471 |
| T-Cholb | |||||
| N | 256 | 232 | 222 | 209 | 73 |
| Mean (SD), (mg/dL per year) | − 20.2 (44.0) | − 12.7 (22.4) | − 9.8 (14.9) | − 6.8 (13.0) | − 5.8 (8.7) |
| Missing (n) | 382 | 406 | 416 | 429 | 565 |
| HDLb | |||||
| N | 225 | 197 | 193 | 174 | 42 |
| Mean (SD), (mg/dL per year) | 2.2 (29.7) | -0.02 (7.0) | 0.65 (4.7) | 0.75 (3.6) | 0.14 (3.8) |
| Missing (n) | 413 | 441 | 445 | 464 | 596 |
| LDLb | |||||
| N | 231 | 199 | 199 | 178 | 45 |
| Mean (SD), (mg/dL per year) | − 9.1 (39.9) | − 6.3 (20.4) | − 4.8 (14.4) | − 2.6 (11.2) | − 1.9 (9.2) |
| Missing (n) | 407 | 439 | 439 | 460 | 593 |
| TRIGc | |||||
| N | 248 | 214 | 214 | 207 | 68 |
| Mean (SD), (mg/dL per year) | − 14.2 (76.1) | − 3.2 (38.8) | − 2.2 (26.5) | − 1.3 (18.4) | − 2.8 (17.0) |
| Missing (n) | 390 | 424 | 424 | 431 | 570 |
| eGFR | |||||
| N | 1 | 3 | 2 | 3 | 2 |
| Mean (SD), (mL/min/1.73m2 per year) | − 36.4 (NA) | − 17.7 (18.5) | − 8.1 (9.6) | − 7.5 (8.4) | − 1.5 (0.65) |
| Missing (n) | 637 | 635 | 636 | 635 | 637 |
eGFR estimated glomerular filtration rate, HbA1c glycosylated haemoglobin A1c, HDL high-density lipoprotein, LDL low-density lipoprotein, N total number of patients with available data, SD standard deviation, T-Chol total cholesterol, TRIG triglycerides
aValues in mmol/L converted to % using the following equation: (18.015 × value in mmol/L + 46.7)/28.7. Values greater than 30% were discarded
bValues in μmol/L converted as 1 μmol/L = 38.67 mg/dL. Values lower than 12 or greater than 500 mg/dL were discarded
cValues in μmol/L converted as 1 μmol/L = 88.57 mg/dL. Values lower than 10 or greater than 5000 mg/dL were discarded
The impact of the most prescribed treatments on the risk factors (HbA1c and lipids) is shown in Table 4. The maximum mean (SD) annual change from baseline HbA1c was observed with regimen 6 after first year of follow-up (− 3.2% [2.4%]) and with regimen 8 at fifth year of follow-up (− 0.68% [0.21%]).
Table 4.
Annual changes in risk factors between baseline and first to fifth year of follow-up, according to most common 1st LoT regimens
| Parameters | Regimen 1 (N = 189) |
Regimen 2 (N = 103) |
Regimen 3 (N = 82) |
Regimen 4 (N = 48) |
Regimen 5 (N = 34) |
Regimen 6 (N = 19) |
Regimen 7 (N = 15) |
Regimen 8 (N = 12) |
|---|---|---|---|---|---|---|---|---|
| Change from baseline after first year of follow-up (per year) | ||||||||
| Hb1Aca | ||||||||
| N | 152 | 55 | 59 | 4 | 14 | 9 | 7 | 2 |
| Mean (SD), (%) | − 1.1 (2.6) | − 1.1 (1.6) | − 1.1 (2.3) | − 0.52 (4.5) | − 0.57 (1.4) | − 3.2 (2.4) | − 0.86 (2.7) | − 1.6 (1.2) |
| Missing (n) | 37 | 48 | 23 | 44 | 20 | 10 | 8 | 10 |
| T-Cholb | ||||||||
| N | 112 | 30 | 47 | 2 | 10 | 5 | 5 | 1 |
| Mean (SD), (mg/dL) | − 23.3 (45.6) | − 29.7 (51.6) | − 6.7 (39.3) | − 39.5 (52.3) | − 45.8 (49.7) | − 54.7 (62.6) | − 15.5 (39.8) | 38.1 (NA) |
| Missing (n) | 77 | 73 | 35 | 46 | 24 | 14 | 10 | 11 |
| HDLb | ||||||||
| N | 102 | 20 | 44 | 2 | 9 | 4 | 4 | 1 |
| Mean (SD), (mg/dL) | 4.6 (40.3) | 1.4 (8.3) | − 0.62 (12.1) | − 5.3 (0.8) | − 3.1 (11.7) | − 11.0 (7.8) | − 3.7 (8.7) | 0.0 (NA) |
| Missing (n) | 87 | 83 | 38 | 46 | 25 | 15 | 11 | 11 |
| LDLb | ||||||||
| N | 105 | 23 | 43 | 2 | 10 | 3 | 5 | 1 |
| Mean (SD), (mg/dL) | − 7.4 (39.1) | − 25.4 (39.3) | 1.3 (33.5) | − 12.0 (26.3) | − 31.8 (37.9) | 28.7 (104.9) | 3.2 (30.3) | 44.2 (NA) |
| Missing (n) | 84 | 80 | 39 | 46 | 24 | 16 | 10 | 11 |
| TRIGc | ||||||||
| N | 110 | 24 | 46 | 2 | 10 | 6 | 6 | 1 |
| Mean (SD), (mg/dL) | − 16.7 (72.3) | 28.1 (119.4) | 3.2 (44.5) | − 62.1 (41.9) | − 16.8 (27.7) | − 4.6 (35.3) | − 33.1 (53.6) | − 107.1 (NA) |
| Missing (n) | 79 | 79 | 36 | 46 | 24 | 13 | 9 | 11 |
| Change from baseline after second year of follow-up (per year) | ||||||||
| Hb1Aca | ||||||||
| N | 112 | 59 | 50 | 7 | 14 | 6 | 6 | 2 |
| Mean (SD), (%) | − 0.63 (1.2) | − 0.51 (0.8) | − 0.48 (1.2) | − 0.38 (0.9) | − 0.59 (0.96) | − 1.4 (1.1) | − 0.63 (0.7) | − 0.09 (2.0) |
| Missing (n) | 77 | 44 | 32 | 41 | 20 | 13 | 9 | 10 |
| T-Cholb | ||||||||
| N | 102 | 32 | 34 | 3 | 10 | 4 | 4 | 0 |
| Mean (SD), (mg/dL) | − 14.7 (21.3) | − 13.3 (27.9) | − 9.3 (24.9) | 11.8 (15.0) | − 10.9 (23.7) | − 24.7 (30.1) | − 4.5 (22.1) | NA |
| Missing (n) | 87 | 71 | 48 | 45 | 24 | 15 | 11 | 12 |
| HDLb | ||||||||
| N | 89 | 22 | 31 | 3 | 12 | 3 | 3 | 0 |
| Mean (SD), (mg/dL) | 0.73 (6.2) | 0.98 (3.9) | − 2.1 (12.0) | 3.2 (4.7) | − 0.69 (4.6) | − 5.5 (11.0) | 0.43 (0.44) | NA |
| Missing (n) | 100 | 81 | 51 | 45 | 22 | 16 | 12 | 12 |
| LDLb | ||||||||
| N | 94 | 24 | 29 | 2 | 10 | 1 | 3 | 0 |
| Mean (SD), (mg/dL) | − 4.1 (17.5) | − 6.0 (24.8) | − 5.1 (21.3) | − 1.5 (8.1) | − 8.4 (16.4) | − 54.9 (NA) | − 6.0 (21.2) | NA |
| Missing (n) | 95 | 79 | 53 | 46 | 24 | 18 | 12 | 12 |
| TRIGc | ||||||||
| N | 98 | 28 | 35 | 2 | 10 | 2 | 4 | 0 |
| Mean (SD), (mg/dL) | − 5.7 (41.9) | 0.65 (32.0) | 5.7 (45.9) | − 22.7 (30.7) | 3.2 (9.4) | − 10.3 (30.5) | − 10.6 (23.1) | NA |
| Missing (n) | 91 | 75 | 47 | 46 | 24 | 17 | 11 | 12 |
| Change from baseline after third year of follow-up (per year) | ||||||||
| Hb1Aca | ||||||||
| N | 121 | 60 | 50 | 4 | 16 | 6 | 5 | 1 |
| Mean (SD), (%) | − 0.43 (0.74) | − 0.36 (0.67) | − 0.25 (0.66) | 0.29 (0.3) | − 0.41 (0.6) | − 0.46 (0.73) | − 0.13 (0.7) | − 1.5 (NA) |
| Missing (n) | 68 | 43 | 32 | 44 | 18 | 13 | 10 | 11 |
| T-Cholb | ||||||||
| N | 92 | 33 | 40 | 2 | 6 | 1 | 5 | 1 |
| Mean (SD), (mg/dL) | − 10.6 (14.3) | − 12.8 (15.0) | − 9.3 (15.4) | 3.4 (7.2) | − 8.5 (4.8) | 9.2 (NA) | − 0.94 (20.2) | 28.2 (NA) |
| Missing (n) | 97 | 70 | 42 | 46 | 28 | 18 | 10 | 11 |
| HDLb | ||||||||
| N | 84 | 21 | 40 | 1 | 6 | 2 | 5 | 1 |
| Mean (SD), (mg/dL) | 0.82 (4.3) | 0.32 (3.0) | − 0.28 (3.3) | 5.3 (NA) | 0.77 (3.1) | − 5.4 (7.7) | 0.82 (1.2) | 11.5 (NA) |
| Missing (n) | 105 | 82 | 42 | 47 | 28 | 17 | 10 | 11 |
| LDLb | ||||||||
| N | 84 | 23 | 39 | 2 | 7 | 0 | 5 | 1 |
| Mean (SD), (mg/dL) | − 3.6 (13.8) | − 9.0 (12.1) | − 3.2 (14.0) | 9.2 (4.1) | − 4.8 (15.4) | NA | 2.0 (14.3) | 23.7 (NA) |
| Missing (n) | 105 | 80 | 43 | 46 | 27 | 19 | 10 | 11 |
| TRIGc | ||||||||
| N | 91 | 29 | 40 | 2 | 6 | 4 | 5 | 1 |
| Mean (SD), (mg/dL) | − 5.8 (29.5) | − 0.069 (21.8) | 3.7 (20.0) | − 7.8 (11.0) | 12.1 (21.6) | 32.1 (55.7) | − 1.1 (33.1) | − 17.9 (NA) |
| Missing (n) | 98 | 74 | 42 | 46 | 28 | 15 | 10 | 11 |
| Change from baseline after fourth year of follow-up (per year) | ||||||||
| Hb1Aca | ||||||||
| N | 114 | 60 | 59 | 5 | 14 | 6 | 1 | 1 |
| Mean (SD), (%) | − 0.38 (0.61) | − 0.2 (0.54) | − 0.21 (0.53) | − 0.1 (1.1) | − 0.19 (0.44) | − 0.87 (0.38) | 0.62 (NA) | 0.067 (NA) |
| Missing (n) | 75 | 43 | 23 | 43 | 20 | 13 | 14 | 11 |
| T-Cholb | ||||||||
| N | 98 | 26 | 32 | 3 | 10 | 3 | 0 | 1 |
| Mean (SD), (mg/dL) | − 7.7 (11.3) | − 5.4 (20.4) | − 4.2 (13.1) | 4.0 (9.7) | − 10.5 (9.4) | − 1.6 (6.8) | NA | − 20.8 (NA) |
| Missing (n) | 91 | 77 | 50 | 45 | 24 | 16 | 15 | 11 |
| HDLb | ||||||||
| N | 82 | 15 | 34 | 1 | 11 | 2 | 0 | 1 |
| Mean (SD), (mg/dL) | 1.4 (2.9) | − 0.67 (3.8) | − 0.12 (2.8) | 3.2 (NA) | 0.22 (1.7) | − 6.9 (7.1) | NA | − 0.5 (NA) |
| Missing (n) | 107 | 88 | 48 | 47 | 23 | 17 | 15 | 11 |
| LDLb | ||||||||
| N | 80 | 25 | 32 | 2 | 10 | 1 | 0 | 0 |
| Mean (SD), (mg/dL) | − 1.9 (9.6) | − 6.0 (13.0) | − 0.17 (12.9) | 7.3 (5.8) | − 1.6 (8.8) | 19.9 (NA) | NA | NA |
| Missing (n) | 109 | 78 | 50 | 46 | 24 | 18 | 15 | 12 |
| TRIGc | ||||||||
| N | 98 | 27 | 32 | 1 | 10 | 5 | 0 | 1 |
| Mean (SD), (mg/dL) | − 1.8 (18.1) | 2.3 (19.1) | 1.7 (15.4) | − 9.1 (NA) | 3.4 (8.9) | 4.0 (24.5) | NA | NA |
| Missing (n) | 91 | 76 | 50 | 47 | 24 | 14 | 15 | 11 |
| Change from baseline after fifth year of follow-up (per year) | ||||||||
| Hb1Aca | ||||||||
| N | 37 | 35 | 25 | 3 | 9 | 4 | 8 | 2 |
| Mean (SD), (%) | − 0.21 (0.44) | − 0.13 (0.25) | − 0.18 (0.39) | − 0.034 (0.059) | − 0.045 (0.29) | − 0.24 (0.77) | − 0.0075 (0.51) | − 0.68 (0.21) |
| Missing (n) | 152 | 68 | 57 | 45 | 25 | 15 | 7 | 10 |
| T-Cholb | ||||||||
| N | 16 | 23 | 12 | 2 | 3 | 0 | 4 | 0 |
| Mean (SD), (mg/dL) | − 5.4 (9.8) | − 6.8 (9.2) | − 1.8 (10.6) | − 3.4 (1.3) | − 4.3 (4.8) | NA | − 10.0 (3.9) | NA |
| Missing (n) | 173 | 80 | 70 | 46 | 31 | 19 | 11 | 12 |
| HDLb | ||||||||
| N | 6 | 10 | 10 | 1 | 3 | 0 | 4 | 0 |
| Mean (SD), (mg/dL) | 1.5 (2.4) | 1.0 (2.9) | − 0.29 (1.5) | − 19.6 (NA) | − 0.92 (3.5) | NA | 1.3 (1.0) | NA |
| Missing (n) | 183 | 93 | 72 | 47 | 31 | 19 | 11 | 12 |
| LDLb | ||||||||
| N | 8 | 8 | 9 | 1 | 3 | 0 | 3 | 0 |
| Mean (SD), (mg/dL) | − 3.5 (12.9) | − 1.4 (7.4) | − 3.6 (8.8) | 2.4 (NA) | 5.7 (3.0) | NA | − 6.8 (1.4) | NA |
| Missing (n) | 181 | 95 | 73 | 47 | 31 | 19 | 12 | 12 |
| TRIGc | ||||||||
| N | 14 | 22 | 11 | 2 | 4 | 0 | 3 | 0 |
| Mean (SD), (mg/dL) | − 4.2 (23.8) | − 1.8 (8.9) | 5.3 (12.3) | 13.5 (10.9) | − 1.3 (14.3) | NA | − 21.9 (31.4) | NA |
| Missing (n) | 175 | 81 | 71 | 46 | 30 | 19 | 12 | 12 |
HbA1c glycosylated haemoglobin A1c, HDL high-density lipoprotein, LDL low-density lipoprotein, LoT line of therapy, N total number of patients with available data, NA not applicable, T-Chol total cholesterol, TRIG triglycerides, SD standard deviation
Regimen 1: A10BA + A10BB = biguanides + sulfonylureas
Regimen 2: A10BA = biguanides
Regimen 3: A10BA + A10BB + A10BH = biguanides + sulfonylureas + DPP4 inhibitors
Regimen 4: A10AB + A10AE = fast-acting insulins + long-acting insulins
Regimen 6: A10AD + A10BA = premixed insulins + biguanides
Regimen 7: A10AB + A10AE + A10BA = fast-acting insulins + long-acting insulins + biguanides
Regimen 8: A10AE = long-acting insulins
aValues in mmol/L converted to % using the following equation: (18.015 × value in mmol/L + 46.7)/28.7. Values greater than 30% were discarded
bValues in μmol/L converted as 1 μmol/L = 38.67 mg/dL. Values lower than 12 or greater than 500 mg/dL were discarded
cValues in μmol/L converted as 1 μmol/L = 88.57 mg/dL. Values lower than 10 or greater than 5000 mg/dL were discarded
Newly Diagnosed Complications During Follow-up
Evaluation of T2DM-related complications showed that the event rates per 100 patient-years of cardiovascular, renal, ocular, foot ulcer and neuropathy complications during the minimum 5-year follow-up period for each patient were 0.142, 0.222, 1.496, 0.283 and 0.505, respectively (Table 5).
Table 5.
Incidence rates of T2DM-related complications and proportion of other complications newly diagnosed during follow-up
| Type of complication | Unit | Values (N = 619) |
|---|---|---|
| Incidence rate of T2DM-related complicationsa | ||
| Cardiovascular | Per 100 patient-years | 0.142 |
| Renal | Per 100 patient-years | 0.222 |
| Ocular | Per 100 patient-years | 1.496 |
| Foot ulcer | Per 100 patient-years | 0.283 |
| Neuropathy | Per 100 patient-years | 0.505 |
| Other | Per 100 patient-years | 0.970 |
| Other complications | Number (N) |
|---|---|
| Hypertension | 30 |
| Hypothyroidism | 9 |
| Obesity | 2 |
| Primary hypertension | 2 |
| Adenocarcinoma cancer colon | 1 |
| Impotence | 1 |
| Non-proliferative diabetic retinopathy | 1 |
| Renal transplant | 1 |
| Right eye cataract | 1 |
T2DM type 2 diabetes mellitus
aPatients with missing follow-up time (due to unknown diabetes diagnosis date) are not included
Cost of Treatments During Follow-up
The annual per-patient costs of all treatments (T2DM treatments and other treatments) since T2DM diagnosis were evaluated (Table 6). Diabetes drugs were used by 98.8% of patients, resulting in a mean (SD) per-patient per-year cost of 1810 (4010) SAR ($483). The other concomitant medications recommended for patients with T2DM were lipid-modifying agents (73.1%), agents acting on the renin–angiotensin system (31.8%), vitamins (26.9%) and antithrombotic agents (57.0%).
Table 6.
Annual cost of all treatments (DM treatments and other treatments) since T2DM diagnosis per patient
| Parameters | Description | Overall (N = 579) |
|---|---|---|
| Use of diabetes drugs | Yes | 572 (98.8%) |
| No | 7 (1.2%) | |
| Costa | Mean (SD), (SAR/year) | 1809.6 (4010.0) |
| Use of lipid-modifying agents | Yes | 423 (73.1%) |
| No | 156 (26.9%) | |
| Cost a | Mean (SD), (SAR/year) | 55.9 (36.4) |
| Use of agents acting on the renin-angiotensin system drugs | Yes | 184 (31.8%) |
| No | 395 (68.2%) | |
| Costa | Mean (SD), (SAR/year) | 174.6 (139.4) |
| Use of vitamins | Yes | 156 (26.9%) |
| No | 423 (73.1%) | |
| Costa | Mean (SD), (SAR/year) | 29.2 (81.7) |
| Use of antithrombotic agents | Yes | 330 (57.0%) |
| No | 249 (43.0%) | |
| Costa | Mean (SD), (SAR/year) | 12.0 (23.7) |
| Use of calcium channel blockers | Yes | 73 (12.6%) |
| No | 506 (87.4%) | |
| Costa | Mean (SD), (SAR/year) | 67.3 (43.6) |
| Use of diuretics | Yes | 34 (5.9%) |
| No | 545 (94.1%) | |
| Costa | Mean (SD), (SAR/year) | 495.9 (1624.6) |
| Use of beta-blocking agents | Yes | 23 (4.0%) |
| No | 556 (96.0%) | |
| Costa | Mean (SD), (SAR/year) | 40.5 (32.6) |
| Use of anti-anaemic preparations | Yes | 6 (1.0%) |
| No | 573 (99.0%) | |
| Costa | Mean (SD), (SAR/year) | 12.6 (2.6) |
| Use of thyroid therapy | Yes | 6 (1.0%) |
| No | 573 (99.0%) | |
| Costa | Mean (SD), (SAR/year) | 61.1 (29.1) |
| Use of analgesics | Yes | 5 (0.9%) |
| No | 574 (99.1%) | |
| Costa | Mean (SD), (SAR/year) | 114.3 (110.0) |
| Use of acid-related disorders | Yes | 4 (0.7%) |
| No | 575 (99.3%) | |
| Costa | Mean (SD), (SAR/year) | 213.8 (144.7) |
| Use of other medications | Yes | 1 (0.2%) |
| No | 578 (99.8%) | |
| Costa | Mean (SD), (SAR/year) | 29.1 (NA) |
DM diabetes mellitus, N total number of patients with available data, NA not applicable, SAR Saudi Arabian riyal, SD standard deviation, T2DM type 2 diabetes mellitus
Only patients with at least one valid calculated yearly cost for one drug were included
All patients from site 10 were excluded because of systematic inconsistent recording of units and doses of treatments
aPatients not using this group of treatment were not included. Cost per-year calculated using the entire time of follow-up of the patient, including the time the treatment was not prescribed
The proportion of patients requiring eye screening, foot care, MA and GRP tests was 61.3%, 59.6%, 2.0% and 0.8%, respectively (Supplementary Table 7). The guidelines by SNDC and the American Diabetes Association suggest performing an eye examination annually (patients with retinopathy) or at least every 3 years (without any evidence of retinopathy). Also, proteinuria screening tests are recommended to be performed annually for patients with T2DM.
Burden of Illness Analysis for T2DM Using CDM
To be used as input in the CDM, the baseline physiological parameters were stratified by the eight most used 1st-line regimens (Table 7). It was observed that patients who started biguanide alone (regimen 2) had higher BMI at baseline followed by patients who initiated biguanide with insulin (regimens 6 and 7). The efficacy data describing the impact of all therapy regimens on HbA1c and lipids are presented in Table 4. The annual treatment costs of the eight most used 1st-line regimens were calculated and shown in Supplementary Table 8.
Table 7.
Baseline physiological parameters according to the most common 1st LoT regimens
| Parameters | Regimen 1 (N = 189) |
Regimen 2 (N = 103) |
Regimen 3 (N = 82) |
Regimen 4 (N = 48) |
Regimen 5 (N = 34) |
Regimen 6 (N = 19) |
Regimen 7 (N = 15) |
Regimen 8 (N = 12) |
|---|---|---|---|---|---|---|---|---|
| BMI | ||||||||
| N | 182 | 98 | 79 | 41 | 31 | 19 | 13 | 9 |
| Mean (SD), (kg/m2) | 29.0 (5.6) | 32.7 (6.6) | 30.2 (6.7) | 29.5 (4.7) | 29.9 (4.9) | 31.7 (5.0) | 32.2 (6.5) | 30.4 (6.1) |
| Missing (n) | 7 | 5 | 3 | 7 | 3 | 0 | 2 | 3 |
| SBP | ||||||||
| N | 178 | 94 | 75 | 41 | 26 | 19 | 11 | 8 |
| Mean (SD), (mmHg) | 132.7 (17.8) | 136.1 (18.5) | 130.1 (19.4) | 135.9 (14.8) | 137.0 (19.7) | 136.3 (16.4) | 129.3 (22.7) | 145.8 (24.9) |
| Missing (n) | 11 | 9 | 7 | 7 | 8 | 0 | 4 | 4 |
| DBP | ||||||||
| N | 178 | 94 | 75 | 41 | 26 | 19 | 11 | 8 |
| Mean (SD), (mmHg) | 79.6 (9.4) | 82.3 (8.6) | 79.4 (10.9) | 75.2 (10.9) | 82.0 (9.9) | 76.2 (12.1) | 74.9 (11.4) | 76.8 (12.6) |
| Missing (n) | 11 | 9 | 7 | 7 | 8 | 0 | 4 | 4 |
BMI body mass index, DBP diastolic blood pressure, LoT line of therapy, mmHg millimetres of mercury, N total number of patients with available data, SD standard deviation, SBP systolic blood pressure
Regimen 1: A10BA + A10BB = biguanides + sulfonylureas
Regimen 2: A10BA = biguanides
Regimen 3: A10BA + A10BB + A10BH = biguanides + sulfonylureas + DPP4 inhibitors
Regimen 4: A10AB + A10AE = fast-acting insulins + long-acting insulins
Regimen 5: A10BA + A10BH = biguanides + DPP4 inhibitors
Regimen 6: A10AD + A10BA = premixed insulins + biguanides
Regimen 7: A10AB + A10AE + A10BA = fast-acting insulins + long-acting insulins + biguanides
Regimen 8: A10AE = long-acting insulins
The burden of illness analysis using CDM showed that the LE and QALYs with the eight most used 1st-line regimens were 25–28 years and 18–21 years, respectively (Table 8). In addition to these health outcomes, the total lifelong disease burden varied from 201,377 to 437,371 SAR ($53,700–116,632) with the cost of CVD complications (141,973–177,466 SAR/ $37,859–47,324) being the major driver (Table 8). Besides the lifelong total cost, the model also estimated annual total costs in the range between 7368 and 16,003 SAR ($1965–4267) (Table 8).
Table 8.
Projected health and cost outcomes for eight regimens of T2DM treatments using CDM
| Parameter | Regimens | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| LE (years) | 27.331 | 28.163 | 27.913 | 26.600 | 28.008 | 25.066 | 26.343 | 26.412 |
| QALY (years) | 19.795 | 20.594 | 20.257 | 18.744 | 20.267 | 17.665 | 18.544 | 18.498 |
| Breakdown of costs (SAR, lifelong) | ||||||||
| Total costs | 230,754.13 | 201,376.61 | 327,800.81 | 383,108.06 | 338,466.28 | 437,371.19 | 404,661.22 | 335,360.13 |
| Treatment | 13,273.89 | 10,206.38 | 129,623.35 | 120,970.66 | 126,613.50 | 93,375.67 | 129,419.27 | 52,784.56 |
| Management | 5797.55 | 6028.95 | 5948.62 | 5594.13 | 5937.07 | 5220.57 | 5536.93 | 5529.25 |
| CVD | 151,935.30 | 141,972.80 | 142,296.30 | 163,195.80 | 144,120.20 | 177,466.50 | 162,430.00 | 175,395.00 |
| Renal | 14,114.57 | 5069.20 | 8281.14 | 34,899.39 | 14,797.48 | 98,813.41 | 47,176.72 | 39,262.72 |
|
Ulcer/ amputation/neuropathy |
25,877.31 | 23,026.36 | 24,076.68 | 28,162.77 | 26,933.44 | 30,999.64 | 29,201.45 | 29,008.58 |
| Eye | 19,487.22 | 15,072.94 | 16,921.26 | 23,825.04 | 19,639.38 | 28,975.44 | 24,872.34 | 26,499.22 |
| NSHE | 70.91 | – | 385.12 | 6288.60 | 313.69 | 2488.85 | 5905.13 | 6803.72 |
| SHE1 | 114.80 | – | 117.50 | 70.08 | 39.36 | 16.41 | 51.06 | 33.46 |
| SHE2 | 82.59 | – | 150.81 | 101.63 | 72.18 | 14.72 | 68.31 | 43.60 |
| Breakdown of costs (SAR, annual) | ||||||||
| Total costs | 8442.94 | 7368.07 | 11,993.74 | 14,017.35 | 12,383.97 | 16,002.75 | 14,805.94 | 12,270.32 |
| Treatment | 485.67 | 373.44 | 4742.72 | 4426.13 | 4632.60 | 3416.47 | 4735.26 | 1931.31 |
| Management | 212.12 | 220.59 | 217.65 | 204.68 | 217.23 | 191.01 | 202.59 | 202.31 |
| CVD | 5559.08 | 5194.57 | 5206.41 | 5971.09 | 5273.14 | 6493.23 | 5943.07 | 6417.44 |
| Renal | 516.43 | 185.47 | 302.99 | 1276.92 | 541.42 | 3615.43 | 1726.12 | 1436.56 |
| Ulcer/amputation/neuropathy | 946.81 | 842.50 | 880.93 | 1030.43 | 985.45 | 1134.23 | 1068.44 | 1061.38 |
| Eye | 713.01 | 551.50 | 619.12 | 871.72 | 718.58 | 1060.17 | 910.04 | 969.57 |
| NSHE | 2.59 | – | 14.09 | 230.09 | 11.48 | 91.06 | 216.06 | 248.94 |
| SHE1 | 4.20 | – | 4.30 | 2.56 | 1.44 | 0.60 | 1.87 | 1.22 |
| SHE2 | 3.02 | – | 5.52 | 3.72 | 2.64 | 0.54 | 2.50 | 1.60 |
CDM Core Diabetes Model, CVD cardiovascular disease, LE life expectancy, NSHE non-severe hypoglycaemia, QALY quality-adjusted life-years, SAR Saudi Arabian riyal, SHE1 severe hypoglycaemia (requiring non-medical assistance), SHE2 severe hypoglycaemia (requiring medical assistance), T2DM type 2 diabetes mellitus
Regimen 1: A10BA + A10BB = biguanides + sulfonylureas
Regimen 2: A10BA = biguanides
Regimen 3: A10BA + A10BB + A10BH = biguanides + sulfonylureas + DPP4 inhibitors
Regimen 4: A10AB + A10AE = fast-acting insulins + long-acting insulins
Regimen 5: A10BA + A10BH = biguanides + DPP4 inhibitors
Regimen 6: A10AD + A10BA = premixed insulins + biguanides
Regimen 7: A10AB + A10AE + A10BA = fast-acting insulins + long-acting insulins + biguanides
Regimen 8: A10AE = long-acting insulins
CDM analysis of T2DM-related complications per 1000 patient-years showed highest rates of non-severe hypoglycaemia events (Supplementary Table 9).
Discussion
This RW study was the first of its kind to perform a comprehensive analysis on the patients suffering from T2DM in the Gulf countries and to project long-term burden of illness across different 1st-line therapy options. The studied subpopulation based on the availability of HbA1c data and 1st LoT information consisted of 638 patients. Of the main population (2226 patients), baseline Hb1Ac data was available for only 1053 patients (47%), as some patients might have been diagnosed at different healthcare facilities before being referred to the study centres. Among these patients, 40 patients reported HbA1c < 6.5% which is not considered diabetes. However, these patients may have been included in the main population because their diagnosis was based on FPG level, which is a less accurate measurement than HbA1c. Therefore, we defined a subpopulation based on HbA1c levels. Availability of the 1st LoT information with non-missing start date (1760 patients among the main population) was another criterion used to define the subpopulation, which helped us assess the evolution of risk factors with different types of therapy, as it was vital for performing the analysis using CDM.
The baseline patient characteristics from our study are comparable with other RW studies conducted in the KSA [14, 15, 26, 27]. The 2013 Saudi Health Interview Survey (SHIS) reported equal numbers of male and female individuals among patients with T2DM [27]. Although a 2017 study conducted at diabetes centres in three cities in the KSA showed a higher proportion of female patients (65%) [15], our study was conducted at 15 diabetes centres and therefore covered a larger number of patients across the KSA. The participants from the KSA in a global, prospective, observational DISCOVER study reported a mean age of 52.4 years and a BMI of 31.9 kg/m2 [14]. Similar baseline characteristics were obtained in a cross-sectional analysis of patients with T2DM by Alramadan et al. and the prospective DAR-MENA T2DM study including patients from Middle East and North Africa (MENA) countries [15, 28]. The higher BMI present at baseline in patients with T2DM in the current as well as previous studies re-establishes the fact that obesity is a risk factor for T2DM and therefore there is a need to implement lifestyle changes and individualised programmes to manage obesity [29]. The rather high HbA1c at baseline in the current study corroborates with previous studies in the KSA which reported HbA1c at baseline between 8.7% and 8.8% [14, 15] and suggest the necessity to raise awareness about the disease.
The rates of complications at baseline obtained in our study are comparatively lower than a few other RW studies conducted in the KSA [13, 15, 30] and in the MENA countries (including the KSA) [28]. The DISCOVER study reported 20% (microvascular) and 11% (macrovascular) rates of complications at baseline among non-insulin users initiating a 2nd LoT [13]. AlSlail and Akil reported rates of complications varying between 3.8% (nephropathy) and 12.3% (CVD) [30]. The DAR-MENA T2DM study showed baseline rates of 32.8%, 9.2% and 8.9% for neuropathy, retinopathy and nephropathy, respectively [13, 28]. These opposing results could be attributed to the differences in study design and patient eligibility criteria. Another reason is that in our study, complications were treated at other centres because diabetes centres only provide outpatient services and, therefore, not all complications were captured.
The analysis of treatment patterns showed that biguanides (monotherapy or combination) were the most common 1st LoT (81.5%). Among the most common 1st line regimens, biguanides + sulfonylureas was used by a majority of the patients as also seen with the national data from the 2013 SHIS survey [31] and the DISCOVER study [14]. The DISCOVER study also reported that 89% of patients were using biguanides as 1st LoT [14]. The overall skewed utilisation of biguanides and sulfonylureas can be partially explained by their comparatively lower cost than newer medications such as GLP-1 RA and SGLT2i [32]. Drug cost is a major factor that influences the choice of a particular agent by patients, physicians and policy makers [32]. Furthermore, GLP-1 RA and SGLT2i were approved in the KSA only after 2014 [33] and 2018 [34], respectively. Moreover, clinical inertia, which includes inhibition to initiate or intensify treatment when indicated [35], may contribute to the preferential use of biguanides and sulfonylureas observed in the current study.
Analysis of our retrospectively collected data showed that the annual per-patient cost of drugs used for T2DM was highest amongst other drugs analysed (agents for lipid modification or acting on the renin–angiotensin system etc.). The low rates of screening tests for eye and renal disease observed in our study suggest a need for improved adherence to the guidelines to avoid diabetes-related complications. The incidence of newly diagnosed T2DM-related complications during follow-up in our study ranged between 0.222 (renal complications) and 1.496 (ocular complications) per 100 patient-years across the different groups of complications. A retrospective study conducted among adult patients with T2DM in the KSA showed frequencies of newly diagnosed complication during an average follow-up of 8 years were 32% (renal), 14% (MI), 14% (neuropathy), 5–23% (ocular) and 4% (foot infections) [4].
To the best of our knowledge, this is the first study conducted in one of the Gulf countries that collected recent baseline and follow-up clinical data for patients with T2DM and provided a comprehensive overall burden of illness using CDM in the KSA. The CDM has been previously used for cost-effectiveness analyses of therapies for T2DM across KSA [36, 37] and globally [38–41]. Moreover, a systematic review of the literature between 2008 and 2013 showed that CDM is the most widely used choice among the models used for cost-effectiveness analysis of treatments for T2DM and is routinely used for reimbursement decisions and health technology assessments [42]. A number of studies have used CDM to quantify burden of illness of T2DM [43–47]. In the USA, CDM was used to measure the clinical and economic burden associated with poor glycaemic control due to a delay in therapy for patients with T2DM [43]. CDM has also been used in China to evaluate current and future population level burden of illness for T2DM based on the current standard of care versus hypothetical alternative management strategies [44]. However, none of these studies analysed burden of illness according to different treatment regimens as considered in the current analysis.
Our burden of illness analysis showed that the most common 1st-line treatments resulted in LE of 25 to 28 years for people diagnosed at age of 49 years. Given the LE for the general population aged 49 years is 29 to 33 years [48], patients with T2DM seem to lose 4 to 5 years. These results are confirmed by another study that suggested a loss of 5.8 to 6.8 life-years if T2DM was diagnosed at the age of 40 years [49].
When we performed the burden of illness analysis including the cost of complications from published sources, the costs for complications were the major driver of the total direct cost of T2DM. Our results also showed that the higher costs were mainly driven by CVD complications. Cost of CVD complications (40−71% of total costs) was the most expensive category among other complications as also found in a previous cost of illness study performed in Iran in 2009 [50]. It has also been estimated in the USA that costs due to CVD complications accounted for 57% of total complication cost among patients with T2DM in 2012 [51]. Our burden of illness analysis showed that besides complications, the projected drug costs were highest for regimens containing DPP4 inhibitors (37−40% of total cost), fast-acting insulin (32%) or premixed insulin (21%). In Arab countries, insulin and oral drugs (mainly metformin) have been shown to contribute to 7−13% and 9−17% of the total direct cost of diabetes, respectively [52]. The per-person cost of medication for diabetes (both type 1 and type 2) have been estimated as 600−17,000 SAR whilst the costs of visits and laboratory tests varied between 1151 and 134892 SAR in the KSA in 2014 [31]. Likewise, the study conducted in Iran reported that drug costs were the major contributor of the total national direct cost in patients without complications [50].
This study has a few limitations, which are inherent to any retrospective observational analysis. Incomplete or missing data was one of the main challenges in this study, which limited the reporting of all planned analyses of the main population with the required cohort size. The study was conducted at specialist diabetes centres in the country; demographics, risk factors and disease treatment patterns of patients attending these centres might be different from those attending other general facilities. As with all studies utilising retrospective data collection, not all data relevant to the current study were recorded in the medical records, which could have resulted in information bias. The information pertaining to treatment (drug doses, start and end dates) could not be completely captured from patient charts which may have led to underestimation of costs of diabetes-related drugs. Furthermore, there is a likelihood of patients attending other general facilities for T2DM-related complications and hence the diabetes centres may not have captured the complete patient data. As a result, the cost of complications to be input in the CDM could not be collected and these had to be sourced from published articles. For analysis of burden of illness, we only included direct medical costs and did not evaluate indirect costs.
Conclusions
This RW study addresses gaps in the current research by providing a complete landscape of baseline demographic and clinical characteristics of patients with T2DM, their treatment patterns, associated costs (resource use), T2DM-related complications and clinical impact of different therapy regimens in the KSA. Additionally, the burden of illness analysis using CDM in this heterogenous group of patients showed substantially higher cost of T2DM care from a payer perspective in the KSA, with the cost of cardiovascular disease complications being the major driver.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing, Editorial, and Other Assistance.
We would like to thank Anshika Singhal, Fida Kazmi Khazi and Saurabh Trikha from IQVIA, India for the medical writing support funded by Novo Nordisk. We acknowledge the significant efforts and the contribution of their dear colleague Moataz Ghazy, Clinical Operations Manager at Novo Nordisk Saudi Araba, who contributed to managing this study. He passed away in 2022.
Author Contributions
All authors contributed to study conception and design, data collection and writing the manuscript. Mohammed AlHarbi, Abdullah Othman, Ahmed Ali Nahari, Ahmed Hamden Aljedai, Dainel Cuadras, Faisal Almalky, Fayez AlAzmi, Hajer Yousef Almudaiheem, Hamad AlShubrumi, Hameed AlSwat, Homaid AlSahafi, Kamelia Sindi, Khadija Basaikh, Majed AlQathani, Mark Lamotte, Moataz Yahia, Mohamed El-Khedr Hassan, Mohammed AlMutlaq, Mohammed AlRoaly, Somaya AlZelaye, and Zein AlGhamdi read and approved the final manuscript.
Funding
The funding for conducting this study, the journal’s Rapid Service fee, and the open access fee were provided by Novo Nordisk.
Data Availability
All data generated or analysed during this study are included in this published article or as supplementary information files.
Declarations
Conflict of Interest
IQVIA, the employer of Mark Lamotte and Daniel Cuadras, received consulting fees from NovoNordisk to conduct the current research. Mohammed AlHarbi, Abdullah Othman, Ahmed Ali Nahari, Ahmed Hamden Aljedai, Faisal Almalky, Fayez AlAzmi, Hajer Yousef Almudaiheem, Hamad AlShubrumi, Hameed AlSwat, Homaid AlSahafi, Kamelia Sindi, Khadija Basaikh, Majed AlQathani, Moataz Yahia, Mohamed El-Khedr Hassan, Mohammed AlMutlaq, Mohammed AlRoaly, Somaya AlZelaye, and Zein AlGhamdi. There is no conflict of interest to declare for the other authors.
Ethical Approval
This study was conducted in accordance with the ethical principles derived from international ethics guidelines, including the Declaration of Helsinki, and the International Council for Harmonisation (ICH) guidelines for Good Clinical Practice (GCP), all applicable laws, rules, and regulations. Consistent with local regulations and prior to the enrolment of patients, the study received approval from the Saudi MoH, independent ethics committee/institutional review board (IEC/IRB) (IRB log number 18-435, dated 28 August 2018). Only one central IRB was used for all the study sites. Since this research presented no more than minimal risk of harm to patients and involved no physical procedures with patients, informed consent was not required.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 10th ed. 2021. https://diabetesatlas.org/atlas/tenth-edition/?dlmodal=active&dlsrc=https%3A%2F%2Fdiabetesatlas.org%2Fidfawp%2Fresource-files%2F2021%2F07%2FIDF_Atlas_10th_Edition_2021.pdf. Accessed 11 Dec 2023
- 2.Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al KJ. Epidemiology of type 2 diabetes—global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10(1):107–111. doi: 10.2991/jegh.k.191028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherif S, Sumpio BE. Economic development and diabetes prevalence in MENA countries: Egypt and Saudi Arabia comparison. World J Diabetes. 2015;6(2):304–311. doi: 10.4239/wjd.v6.i2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alwakeel JS, Sulimani R, Al-Asaad H, et al. Diabetes complications in 1952 type 2 diabetes mellitus patients managed in a single institution in Saudi Arabia. Ann Saudi Med. 2008;28(4):260–266. doi: 10.5144/0256-4947.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Diabetes Federation. Diabetes and cardiovascular disease 2021. https://www.idf.org/our-activities/care-prevention/cardiovascular-disease.html. Accessed 23 May 2023.
- 6.Robert AA, Al Dawish MA, Braham R, Musallam MA, Al Hayek AA, Al Kahtany NH. Type 2 diabetes mellitus in Saudi Arabia: major challenges and possible solutions. Curr Diabetes Rev. 2017;13(1):59–64. doi: 10.2174/1573399812666160126142605. [DOI] [PubMed] [Google Scholar]
- 7.Almutairi N, Alkharfy KM. Direct medical cost and glycemic control in type 2 diabetic Saudi patients. Appl Health Econ Health Policy. 2013;11(6):671–675. doi: 10.1007/s40258-013-0065-6. [DOI] [PubMed] [Google Scholar]
- 8.Bommer C, Heesemann E, Sagalova V, et al. The global economic burden of diabetes in adults aged 20–79 years: a cost-of-illness study. Lancet Diabetes Endocrinol. 2017;5(6):423–430. doi: 10.1016/S2213-8587(17)30097-9. [DOI] [PubMed] [Google Scholar]
- 9.Moradi-Lakeh M, El Bcheraoui C, Daoud F, et al. Medication use for chronic health conditions among adults in Saudi Arabia: findings from a national household survey. Pharmacoepidemiol Drug Saf. 2016;25(1):73–81. doi: 10.1002/pds.3904. [DOI] [PubMed] [Google Scholar]
- 10.Alzaid A, Ladrón de Guevara P, Beillat M, Lehner Martin V, Atanasov P. Burden of disease and costs associated with type 2 diabetes in emerging and established markets: systematic review analyses. Expert Rev Pharmacoeconom Outcom Res. 2021;21(4):785–798. doi: 10.1080/14737167.2020.1782748. [DOI] [PubMed] [Google Scholar]
- 11.Alhowaish AK. Economic costs of diabetes in Saudi Arabia. J Family Community Med. 2013;20(1):1–7. doi: 10.4103/2230-8229.108174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saudi Health Council. Saudi Diabetes Clinical Practice Guidelines (SDCPG), Saudi National Diabetes Center (SNDC) 2021. https://shc.gov.sa/Arabic/Documents/SDCP%20Guidelines.pdf. Accessed 15 May 2023.
- 13.Al-Rubeaan K, Banah F, Alruwaily FG, et al. Longitudinal assessment of the quality of life and patterns of antidiabetic medication use in patients with type 2 diabetes, Saudi Arabia perspective, DISCOVER study. Curr Med Res Opin. 2023;39(1):27–35. doi: 10.1080/03007995.2022.2144052. [DOI] [PubMed] [Google Scholar]
- 14.Al-Rubeaan K, Bana FA, Alruwaily FG, et al. Physicians' choices in the first- and second-line management of type 2 diabetes in the Kingdom of Saudi Arabia. Saudi Pharm J. 2020;28(3):329–337. doi: 10.1016/j.jsps.2020.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alramadan MJ, Magliano DJ, Almigbal TH, et al. Glycaemic control for people with type 2 diabetes in Saudi Arabia - an urgent need for a review of management plan. BMC Endocr Disord. 2018;18(1):62. doi: 10.1186/s12902-018-0292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEwan P, Foos V, Palmer JL, Lamotte M, Lloyd A, Grant D. Validation of the IMS CORE Diabetes Model. Value Health. 2014;17(6):714–724. doi: 10.1016/j.jval.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Palmer AJ, Roze S, Valentine WJ, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin. 2004;20(S1):S27–40. doi: 10.1185/030079904X2006. [DOI] [PubMed] [Google Scholar]
- 18.Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: Projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20(S1):S5–26. doi: 10.1185/030079904X1980. [DOI] [PubMed] [Google Scholar]
- 19.Ramos M, Men P, Wang X, Ustyugova A, Lamotte M. Cost-effectiveness of empagliflozin in patients with type 2 diabetes and established cardiovascular disease in China. Cost Eff Resour Alloc. 2021;19(1):46. doi: 10.1186/s12962-021-00299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salem A, Men P, Ramos M, Zhang Y-J, Ustyugova A, Lamotte M. Cost–effectiveness analysis of empagliflozin compared with glimepiride in patients with type 2 diabetes in China. J Comparat Effect Res. 2021;10(6):469–480. doi: 10.2217/cer-2020-0284. [DOI] [PubMed] [Google Scholar]
- 21.Drugs.com. Does metformin cause hypoglycemia? 2021. https://www.drugs.com/medical-answers/metformin-hypoglycemia-3563096/. Accessed 1 May 2023.
- 22.Peasgood T, Brennan A, Mansell P, Elliott J, Basarir H, Kruger J. The impact of diabetes-related complications on preference-based measures of health-related quality of life in adults with type I diabetes. Med Decis Making. 2016;36(8):1020–1033. doi: 10.1177/0272989X16658660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P. Review of utility values for economic modeling in type 2 diabetes. Value Health. 2014;17(4):462–470. doi: 10.1016/j.jval.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Foos V, McEwan P. Conversion of hypoglycemia utility decrements from categorical units reflecting event history into event specific disutility scores applicable to diabetes decision models. Value in Health. 2018;21:S223. doi: 10.1016/j.jval.2018.04.1506. [DOI] [Google Scholar]
- 25.OANDA. Saudi riyal currency. https://www.oanda.com/currency-converter/en/currencies/majors/sar/. Accessed 11 Dec 2023.
- 26.Alzaheb RA, Altemani AH. The prevalence and determinants of poor glycemic control among adults with type 2 diabetes mellitus in Saudi Arabia. Diabetes Metab Syndr Obes. 2018;11:15–21. doi: 10.2147/DMSO.S156214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Bcheraoui C, Basulaiman M, Tuffaha M, et al. Status of the diabetes epidemic in the Kingdom of Saudi Arabia, 2013. Int J Public Health. 2014;59(6):1011–1021. doi: 10.1007/s00038-014-0612-4. [DOI] [PubMed] [Google Scholar]
- 28.Hassanein M, Al Awadi FF, El Hadidy KES, et al. The characteristics and pattern of care for the type 2 diabetes mellitus population in the MENA region during Ramadan: an international prospective study (DAR-MENA T2DM) Diabetes Res Clin Pract. 2019;151:275–284. doi: 10.1016/j.diabres.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Al Slamah T, Nicholl BI, Alslail FY, Harris L, Kinnear D, Melville CA. Correlates of type 2 diabetes and glycaemic control in adults in Saudi Arabia a secondary data analysis of the Saudi health interview survey. BMC Public Health. 2020;20(1):515. doi: 10.1186/s12889-020-08597-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.AlSlail FY, Akil YA. Treatment patterns, effectiveness, and satisfaction among patients with type 2 diabetes treated with insulin in Saudi Arabia: results of the RIMODIS Study. Diabetes Ther. 2021;12(7):1965–1978. doi: 10.1007/s13300-021-01089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mokdad AH, Tuffaha M, Hanlon M, et al. Cost of diabetes in the Kingdom of Saudi Arabia, 2014. J Diabetes Metab. 2015;6(8):575. [Google Scholar]
- 32.Al-Sofiani ME, Batais MA, Aldhafiri E, Alzaid A. Pharmacological treatment of type 2 diabetes in Saudi Arabia: a consensus statement from the Saudi Society of Endocrinology and Metabolism (SSEM) Diabetes Metab Syndr. 2021;15(3):891–899. doi: 10.1016/j.dsx.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Alkhatib NS, Almutairi AR, Alkhezi OS, Alfayez OM, Al Yami MS, Almohammed OA. Economic analysis of glucagon like peptide-1 receptor agonists from the Saudi Arabia payer perspective. Saudi Pharm J. 2022;30(4):433–439. doi: 10.1016/j.jsps.2022.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Althobaiti FM, Alsanosi SM, Falemban AH, et al. Efficacy and safety of empagliflozin in type 2 diabetes mellitus Saudi patients as add-on to antidiabetic therapy: a prospective, open-label, observational study. J Clin Med. 2022;11(16):4769. doi: 10.3390/jcm11164769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almigbal TH, Alzarah SA, Aljanoubi FA, et al. Clinical inertia in the management of type 2 diabetes mellitus: a systematic review. Medicina. 2023;59(1):182. doi: 10.3390/medicina59010182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shafie AA, Gupta V, Baabbad R, Hammerby E, Home P. An analysis of the short- and long-term cost-effectiveness of starting biphasic insulin aspart 30 in insulin-naïve people with poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2014;106(2):319–327. doi: 10.1016/j.diabres.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 37.Gupta V, Baabbad R, Hammerby E, Nikolajsen A, Shafie AA. An analysis of the cost-effectiveness of switching from biphasic human insulin 30, insulin glargine, or neutral protamine Hagedorn to biphasic insulin aspart 30 in people with type 2 diabetes. J Med Econ. 2015;18(4):263–272. doi: 10.3111/13696998.2014.991791. [DOI] [PubMed] [Google Scholar]
- 38.Ehlers LH, Lamotte M, Ramos MC, et al. The cost-effectiveness of subcutaneous semaglutide versus empagliflozin in type 2 diabetes uncontrolled on metformin alone in Denmark. Diabetes Ther. 2022;13(3):489–503. doi: 10.1007/s13300-022-01221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans M, Berry S, Malkin SJP, Hunt B, Sharma A. Evaluating the long-term cost-effectiveness of once-weekly semaglutide 1 mg versus liraglutide 1.8 mg: a health economic analysis in the UK. Diabetes Ther. 2023;14(6):1005–1021. doi: 10.1007/s13300-023-01408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franch-Nadal J, Malkin SJP, Hunt B, Martín V, Gallego Estébanez M, Vidal J. The cost-effectiveness of oral semaglutide in Spain: a long-term health economic analysis based on the PIONEER clinical trials. Adv Ther. 2022;39(7):3180–3198. doi: 10.1007/s12325-022-02156-8. [DOI] [PubMed] [Google Scholar]
- 41.Shao H, Alsaleh AJO, Dex T, Lew E, Fonseca V. Cost-effectiveness of iGlarLixi versus premix BIAsp 30 in people with type 2 diabetes suboptimally controlled by basal insulin in the US. Diabetes Ther. 2022;13(9):1659–1670. doi: 10.1007/s13300-022-01300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Charokopou M, Sabater FJ, Townsend R, Roudaut M, McEwan P, Verheggen BG. Methods applied in cost-effectiveness models for treatment strategies in type 2 diabetes mellitus and their use in Health Technology Assessments: a systematic review of the literature from 2008 to 2013. Curr Med Res Opin. 2016;32(2):207–218. doi: 10.1185/03007995.2015.1102722. [DOI] [PubMed] [Google Scholar]
- 43.Ali SN, Dang-Tan T, Valentine WJ, Hansen BB. Evaluation of the clinical and economic burden of poor glycemic control associated with therapeutic inertia in patients with type 2 diabetes in the United States. Adv Ther. 2020;37(2):869–882. doi: 10.1007/s12325-019-01199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foos V, Wang K, McEwan P, et al. Assessing the burden of type 2 diabetes in China considering the current status-quo management and implications of improved management using a modeling approach. Value Health Regional Issues. 2019;18:36–46. doi: 10.1016/j.vhri.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Bain SC, Bekker Hansen B, Hunt B, Chubb B, Valentine WJ. Evaluating the burden of poor glycemic control associated with therapeutic inertia in patients with type 2 diabetes in the UK. J Med Econ. 2020;23(1):98–105. doi: 10.1080/13696998.2019.1645018. [DOI] [PubMed] [Google Scholar]
- 46.Hellgren M, Svensson AM, Franzén S, et al. The burden of poor glycaemic control in people with newly diagnosed type 2 diabetes in Sweden: a health economic modelling analysis based on nationwide data. Diabetes Obes Metab. 2021;23(7):1604–1613. doi: 10.1111/dom.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.IMS Institute for Healthcare Informatics. Improving type 2 diabetes therapy compliance and persistence in the Kingdom of Saudi Arabia. How to address avoidable economic and societal Burden. 2016. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/diabetes-reports/improving-type-2-diabetes-therapy-compliance-and-persistence-in-the-kingdom-of-saudi-arabia.pdf. Accessed 15 May 2023.
- 48.Social Security Administration. Actuarial Life Table 2020. https://www.ssa.gov/oact/STATS/table4c6.html. Accessed 30 May 2023.
- 49.Gregg EW, Zhuo X, Cheng YJ, Albright AL, Narayan KV, Thompson TJ. Trends in lifetime risk and years of life lost due to diabetes in the USA, 1985–2011: a modelling study. Lancet Diabet Endocrinol. 2014;2(11):867–874. doi: 10.1016/S2213-8587(14)70161-5. [DOI] [PubMed] [Google Scholar]
- 50.Javanbakht M, Baradaran HR, Mashayekhi A, et al. Cost-of-illness analysis of type 2 diabetes mellitus in Iran. PLoS ONE. 2011;6(10):e26864. doi: 10.1371/journal.pone.0026864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhuo X, Zhang P, Hoerger TJ. Lifetime direct medical costs of treating type 2 diabetes and diabetic complications. Am J Prev Med. 2013;45(3):253–261. doi: 10.1016/j.amepre.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 52.Boutayeb A, Boutayeb W, Lamlili ME, Boutayeb S. Estimation of the direct cost of diabetes in the Arab region. Mediterr J Nutr Metab. 2014;7(1):21–32. doi: 10.3233/MNM-140002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article or as supplementary information files.