Abstract
Treatment of cells with tumor-promoting phorbol esters results in the activation but then depletion of phorbol ester-responsive protein kinase C (PKC) isoforms. The ubiquitin-proteasome pathway has been implicated in regulating the levels of many cellular proteins, including those involved in cell cycle control. We report here that in 3Y1 rat fibroblasts, proteasome inhibitors prevent the depletion of PKC isoforms α, δ, and ɛ in response to the tumor-promoting phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA). Proteasome inhibitors also blocked the tumor-promoting effects of TPA on 3Y1 cells overexpressing c-Src, which results from the depletion of PKC δ. Consistent with the involvement of the ubiquitin-proteasome pathway in the degradation of PKC isoforms, ubiquitinated PKC α, δ, and ɛ were detected within 30 min of TPA treatment. Diacylglycerol, the physiological activator of PKC, also stimulated ubiquitination and degradation of PKC, suggesting that ubiquitination is a physiological response to PKC activation. Compounds that inhibit activation of PKC prevented both TPA- and diacylglycerol-induced PKC depletion and ubiquitination. Moreover, a kinase-dead ATP-binding mutant of PKC α could not be depleted by TPA treatment. These data are consistent with a suicide model whereby activation of PKC triggers its own degradation via the ubiquitin-proteasome pathway.
Tumor promotion by phorbol esters involves the selective amplification of cells previously mutated in an appropriate growth-stimulatory gene (3, 17). Phorbol esters exert their effects on the protein kinase C (PKC) family of genes, which consists of genes that encode at least nine distinct isoforms that are responsive to tumor-promoting phorbol esters (9). Phorbol esters first activate phorbol ester-responsive PKC isoforms, but upon prolonged treatment, these isoforms are proteolytically degraded (16). Using a cell culture model system in which cells overexpressing c-Src were transformed by phorbol ester treatment, we recently demonstrated that the tumor-promoting effect of the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) on these cells was due to the depletion of PKC δ (7). These data suggested that PKC δ may function as a tumor suppressor. Consistent with this hypothesis, PKC δ was inactivated by tyrosine phosphorylation in cells transformed by v-Src (19) and v-Ras (2). Thus, regulation of PKC δ at the level of activity and expression may be a very important cell growth control mechanism.
PKC α has been reported to become ubiquitinated in response to bryostatin 1, an activator of PKC that prevents tumor promotion in mouse skin by TPA (6). The ubiquitin-proteasome pathway is a nonlysosomal degradation system that controls the timed destruction of cell cycle-regulatory proteins, including the tumor suppressor p53; the cyclin-dependent kinase inhibitor p27; the cyclins; the oncogene products c-Myc, c-Jun, and c-Fos; and the transcription factors NF-κB and E2F (reviewed in reference 13). This pathway involves the covalent tagging of proteins with ubiquitin, followed by proteasome-mediated degradation of tagged proteins. Conjugation of ubiquitin to substrate proteins requires three enzymes: a ubiquitin-activating enzyme (E1), a ubiquitin-conjugating enzyme (E2), and a ubiquitin ligase (E3). Both the E2 and E3 proteins belong to large families of proteins, and it is believed that different combinations of E2 proteins with different E3 ligases define a high substrate specificity. In this study, we have investigated the role of the ubiquitin-proteasome pathway in the downregulation of PKC isoforms in response to the tumor-promoting phorbol ester TPA.
MATERIALS AND METHODS
Cells and cell culture conditions.
Rat 3Y1 cells or rat 3Y1 cells expressing either v-Src or c-Src were maintained in Dulbecco’s modified Eagle medium supplemented with 10% bovine calf serum (HyClone). Cell cultures were made quiescent by growing them to confluence and then replacing the medium with fresh medium containing 0.5% newborn calf serum for 1 day. Cells expressing the kinase-dead PKC α were generated as described previously (7). The kinase-dead PKC α clone was generated by a mutation to the ATP-binding site as described previously (15).
Materials.
The PKC inhibitors staurosporine, bisindolylmaleimide II, rottlerin, and Go6976 were obtained from Calbiochem. Monoclonal antibodies for PKC α, ɛ, and ζ were obtained from Transduction Laboratories; a polyclonal antibody for PKC δ was obtained from Santa Cruz. A monoclonal antibody for ubiquitin was obtained from Zymed.
Cell lysate preparation and subcellular fractionation.
Cells grew to approximately 90% confluence in 100-mm-diameter culture dishes and were then shifted to Dulbecco’s modified Eagle medium containing 0.5% serum for 24 h. Cells were washed three times with ice-cold isotonic buffer (phosphate-buffered saline, containing 136 mM NaCl, 2.6 mM KCl, 1.4 mM KH2PO4, and 4.2 mM Na2HPO4, pH 7.2). For subcellular fractionation, cells from 100-mm-diameter dishes were washed and then scraped into 2 ml of homogenization buffer (20 mM Tris-HCl [pH 7.5], 5 mM NaCl, 1 mM EDTA, 5 mM MgCl2, 2 mM dithiothreitol, 200 μM phenylmethylsulfonyl fluoride, 10 μg of aprotinin per ml, 10 μg of leupeptin per ml). Cells were then disrupted with 20 strokes in a Dounce homogenizer (type B pestle), and the lysate was centrifuged at 100,000 × g for 1 h. The supernatant was collected as the cytosolic fraction. The membrane pellet was suspended in the same volume of homogenization buffer with 1% Triton X-100. After incubation for 30 min at 4°C, the suspension was centrifuged at 100,000 × g for 1 h. The supernatant was collected as the membrane fraction. For whole-cell lysates, cells were treated with 3 ml of homogenization buffer containing 1% Triton X-100 followed by centrifugation at 100,000 × g for 1 h. The supernatant was collected and used as the whole-cell lysate.
Immunoprecipitation and Western blot analysis.
Extraction of proteins from cultured cells was performed as previously described (7) with a modified buffer consisting of 50 mM Tris-HCl (pH 7.5), 1% Triton X-100, 150 mM NaCl, 1 mM dithiothreitol, 0.5 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride, leupeptin (12 mg/ml), aprotinin (20 μg/ml), 100 μM sodium vanadate, 100 μM sodium pyrophosphate, 1 mM sodium fluoride, 10 mM ethylmethylmaleimide, and 50 mM hemin. Cell extracts were clarified by centrifugation at 12,000 rpm, and the supernatants (1,500 μg of protein/ml) were subjected to immunoprecipitation with anti-PKC δ, α, and ɛ antibodies. After overnight incubation at 4°C, protein A-agarose beads were added and left for an additional 3 h. Immunocomplexes were then subjected to Western blot analysis as described previously (7). Western blot analysis with antiubiquitin antibody was performed with modifications described by Avantaggiati et al. (1).
RESULTS
Proteasome inhibitors block depletion of PKC isoforms.
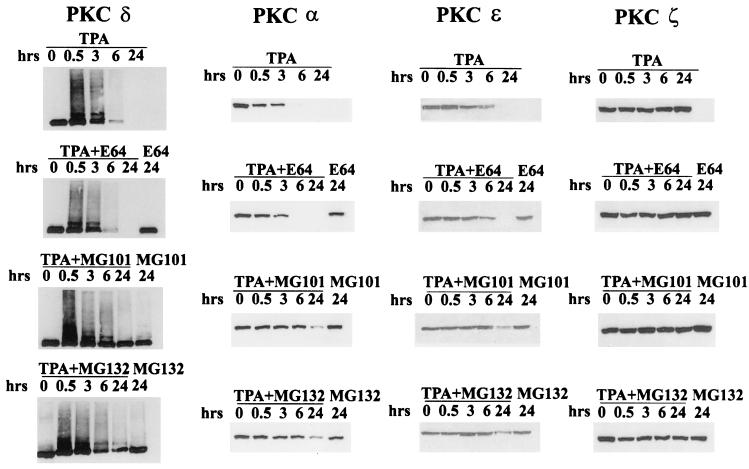
To investigate whether the ubiquitin-proteasome pathway is involved in the downregulation of PKC in response to phorbol esters, we first examined the effect of proteasome inhibitors on TPA-induced PKC depletion. MG101 and MG132, which inhibit proteasome function (11, 12), prevented the TPA-induced depletion of the α, δ, and ɛ PKC isoforms, the only TPA-responsive isoforms present in these cells (Fig. 1). E64, which shares with MG101 and MG132 the ability to inhibit calpain protease, but not the proteasome, had no effect on TPA-induced PKC depletion. We also examined the effect of these compounds on PKC ζ, a PKC isoform that is expressed in these cells but is not responsive to phorbol esters (9). As shown in Fig. 1, neither MG101 nor MG132 had any effect on PKC ζ. These data implicate the ubiquitin-proteasome pathway in the phorbol ester-induced depletion of PKC.
FIG. 1.
Proteasome inhibitors prevent TPA-induced depletion of PKC. 3Y1 cells overexpressing c-Src were treated with TPA (400 nM) for the indicated times, and PKC depletion was monitored by Western blot analysis as described previously (7). The effect of MG101, MG132, or E64 (all at 50 μM) was determined by adding these compounds 30 min prior to addition of TPA as shown. The levels of PKC δ, α, ɛ, and ζ were determined by using antibodies specific for these isoforms.
PKC isoforms become ubiquitinated upon TPA treatment.
The data in Fig. 1 demonstrate that compounds which inhibit proteasome function inhibit TPA-induced downregulation of PKC. Therefore, it is predicted that the affected PKC isoforms should become ubiquitinated in response to TPA. In Fig. 1, it was also observed that the anti-PKC δ antibody recognized several higher-molecular-weight species within 30 min after TPA treatment. The appearance of these higher-molecular-weight species of PKC δ is consistent with the rapid ubiquitination of PKC δ in response to TPA. To investigate directly whether PKC isoforms were being ubiquitinated in response to TPA, we performed Western blot analysis of PKC isoform immunoprecipitations with antiubiquitin antibody. As shown in Fig. 2, ubiquitination of PKC α, δ, and ɛ, but not PKC ζ, was detected within 30 min of TPA treatment. By 6 h, the ubiquitinated PKC isoforms were no longer detectable. However, when MG101 was used to inhibit proteasome, the ubiquitinated isoforms were still present 6 h after TPA treatment (Fig. 2). Interestingly, 24 h of treatment with MG101 alone resulted in a significant accumulation of ubiquitinated forms to a limited extent for PKC α and substantially for PKC ɛ (Fig. 2), suggesting that ubiquitination may occur in response to physiological stimuli as well as TPA. These data demonstrate that PKC isoforms α, δ, and ɛ rapidly become ubiquitinated in response to TPA treatment and that their disappearance is blocked by inhibition of proteasome.
FIG. 2.
PKC becomes ubiquitinated upon TPA treatment. The c-Src-overexpressing 3Y1 cells were treated with TPA (400 nM) for the indicated times. PKC δ, α, ɛ, and ζ were then immunoprecipitated (IP), and the level of ubiquitinated PKC was determined by Western blot analysis with an antiubiquitin antibody. The effect of MG101 (50 μM) on ubiquitination of untreated cells and cells treated with TPA is shown. Numbers on the left are molecular weights in thousands.
Degradation and ubiquitination of PKC are dependent upon PKC kinase activity.
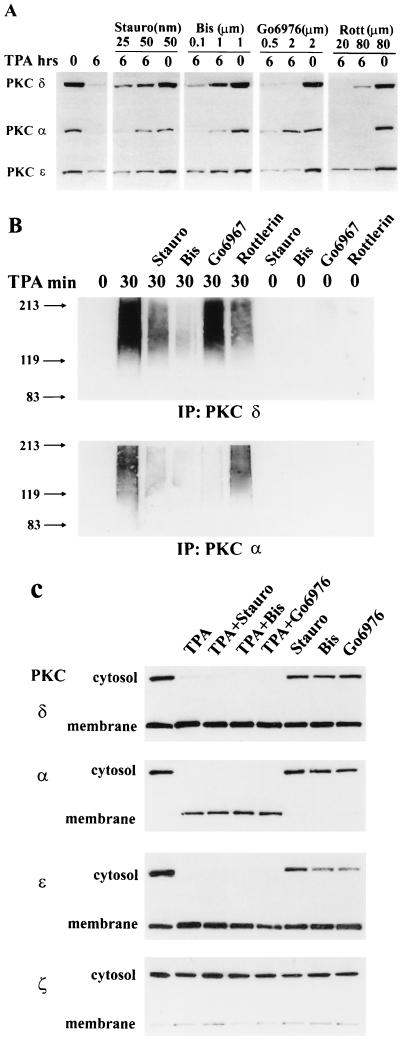
To begin to investigate the mechanism for activation of ubiquitination and proteasome degradation, we asked whether the kinase activity of PKC was important for degradation. We first investigated the effect of PKC inhibitors on the TPA-induced PKC downregulation and ubiquitination. In Fig. 3A, it is shown that the PKC inhibitors staurosporine and bisindolylmaleimide II prevented downregulation of PKC isoforms α, δ, and ɛ. Interestingly, Go6976, which specifically inhibits PKC α (8), prevented TPA-induced downregulation of the α isoform only, and rottlerin, a more specific inhibitor of PKC δ (4), prevented TPA-induced downregulation of the δ isoform only. We also investigated the effect of the PKC inhibitors on the ubiquitination of PKC isoforms α and δ, and as expected, the PKC inhibitors also prevented TPA-induced ubiquitination of these PKC isoforms with the same specificity observed for inhibition of downregulation (Fig. 3B). The PKC inhibitors did not inhibit translocation to the membrane of the PKC isoforms (Fig. 3C). Thus, the effects observed in Fig. 3A and B were not due to a lack of membrane association. These data indicate that TPA-induced downregulation and ubiquitination of the PKC isoforms require an active kinase activity. Consistent with a requirement for activation of PKC for downregulation, the inactive phorbol ester 4α-phorbol 12,13-didecanoate, which does not activate PKC (14), did not lead to the downregulation of PKC (Fig. 3D), nor did it result in the ubiquitination of PKC δ (Fig. 3E).
FIG. 3.
Degradation of PKC is dependent upon PKC kinase activity. (A) 3Y1 cells overexpressing c-Src were treated with TPA (400 nM) for 6 h to deplete the cells of PKC. This was then performed in the presence of the PKC inhibitors staurosporine (Stauro), bisindolylmaleimide II (Bis), Go6976, and rottlerin (Rott) at the indicated concentrations, and PKC levels were determined by Western blot analysis as for Fig. 1. (B) The effect of the PKC inhibitors on TPA-induced ubiquitization of PKC α and δ was determined as for Fig. 2. (C) The ability of TPA to induce translocation of the PKC isoforms from the cytosol to the membrane in the presence of PKC inhibitors was investigated by Western blot analysis of the PKC isoforms present in the cytosolic and membrane fractions before and after TPA treatment. (D and E) The effect of the inactive phorbol ester 4α-phorbol 12, 13-didecanoate (4α-PDD) (400 nM) on the induction of PKC isoform downregulation (D) and ubiquitination of PKC δ (E) was examined as for panels A and B, respectively.
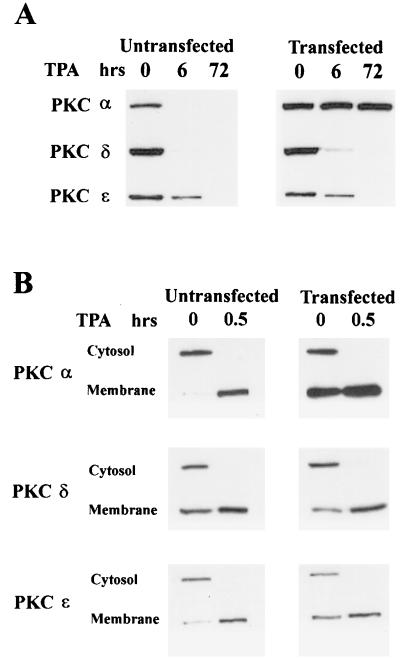
If PKC kinase activity is required for downregulation, then a kinase-dead PKC mutant should be resistant to downregulation in response to TPA. An ATP-binding site mutant of PKC α (15) that was kinase dead was introduced into the c-Src-overexpressing cell line, and the ability to downregulate PKC α with TPA was examined. As shown in Fig. 4A, this PKC α mutant was completely resistant to downregulation by TPA. Since the kinase-dead PKC α mutant could still be stimulated to associate with the membrane in response to TPA (Fig. 4B), the lack of degradation was not due to lack of membrane localization. Since PKC δ and ɛ were both activated and downregulated in these cells, activation of the ubiquitin-proteasome pathway by these PKC isoforms was apparently specific for the activated isoforms only. These data further support the conclusion that activation of the kinase activity of PKC is necessary for ubiquitination and downregulation.
FIG. 4.
A kinase-dead mutant of PKC α is not downregulated by TPA. The c-Src-overexpressing 3Y1 cells were stably transfected with a mutant PKC α gene which has a mutation in the ATP-binding site (15). (A) The mutant PKC-α-overexpressing cells were then treated with TPA (400 nM,) and the levels of PKC α, δ, and ɛ in these and the parental cells were determined at 6 and 72 h later as for Fig. 1. (B) The ability of TPA to induce translocation of the PKC isoforms from the cytosol to the membrane in the parental cells and in the cells expressing the kinase-dead PKC α was determined as for Fig. 3C.
PKC is ubiquitinated and downregulated in response to DG in a proteasome- and kinase-dependent mechanism.
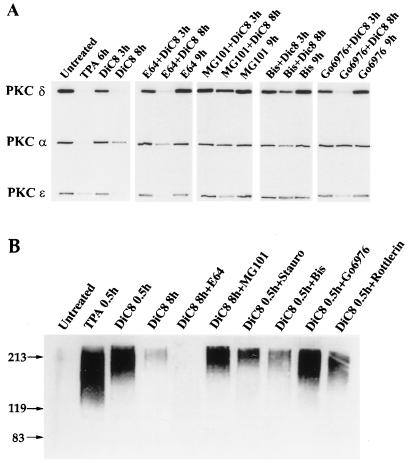
Phorbol esters bind to PKC at the site that binds the physiological activator diacylglycerol (DG) (9). As shown in Fig. 2, the proteasome inhibitor MG101 stimulated an increase in the ubiquitinated PKC isoforms α and ɛ, suggesting that ubiquitination is a physiological response and not an artifact of phorbol ester treatment. We therefore wished to investigate whether ubiquitination and downregulation of PKC occur in response to DG. As shown in Fig. 5, the α and δ isoforms and to a lesser extent the ɛ isoform were all downregulated in response to the DG dioctoylglycerol (DiC8). This downregulation was sensitive to both proteasome and PKC inhibitors (Fig. 5A). The PKC α-specific Go6976 prevented downregulation of the α isoform specifically. We also wished to determine whether DG stimulated ubiquitination of PKC isoforms. We added DiC8 to the 3Y1 cells and examined ubiquitination as in Fig. 2. In Fig. 5B, it is shown that DiC8 stimulated ubiquitination of PKC δ. The ubiquitination of PKC δ was inhibited by the PKC inhibitors staurosporine, bisindolylmaleimide II, and rottlerin but not by the proteasome inhibitor MG101 or the PKC α inhibitor Go6976 (Fig. 5B). These data suggest that PKC isoforms become ubiquitinated and downregulated by the physiological stimulus of DG as well as by the tumor-promoting stimulus of TPA and that downregulation is dependent upon an active kinase.
FIG. 5.
PKC is downregulated and ubiquitinated in response to DG in a proteasome- and kinase-dependent mechanism. (A) c-Src-overexpressing 3Y1 cells were treated with DiC8 (10 μg/ml) for the indicated times in the presence of either E64 (50 μM), MG101 (50 μM), bisindolylmaleimide II (Bis) (1 μM), or Go6976 (2 μM), and the levels of PKC α, δ, and ɛ were then determined by Western blot analysis as for Fig. 1. (B) The effect of DiC8 on the ubiquitination of PKC δ was determined in the presence of the proteasome inhibitor MG101 (50 μM) and the PKC inhibitors staurosporine (Stauro) (50 nM), bisindolylmaleimide II (1.0 μM), Go6976 (2.0 μM), and rottlerin (80 μM) as for Fig. 2.
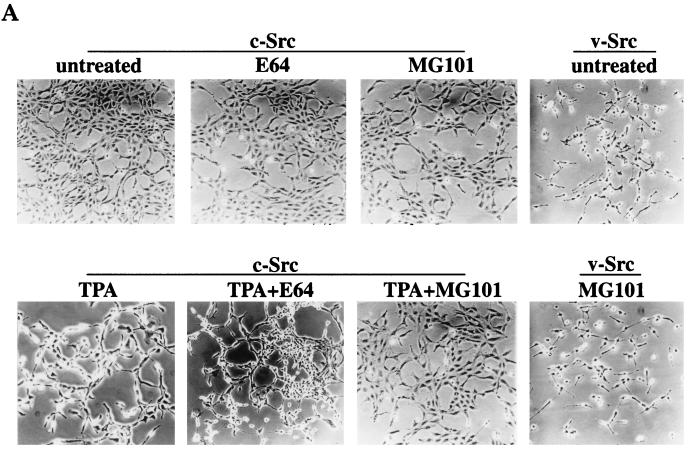
TPA-induced transformation of 3Y1 cells overexpressing c-Src is blocked by proteasome inhibitors.
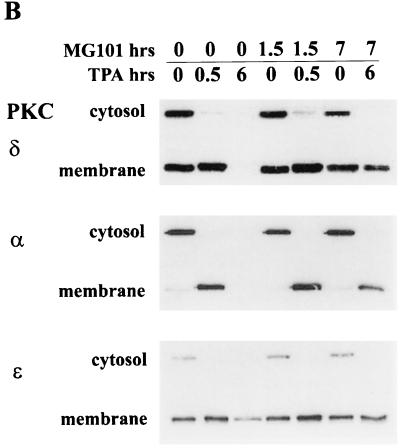
In cells overexpressing c-Src, TPA treatment causes the appearance of transformation that is due to the depletion of PKC δ (7). We therefore investigated whether inhibitors of the ubiquitin-proteasome pathway could prevent the transformed phenotype induced by TPA in the c-Src-overexpressing cells by preventing the depletion of PKC δ. As shown in Fig. 6A, the proteasome-specific inhibitor MG101 prevented the morphological transformation of the c-Src-expressing cells induced by TPA, whereas the nonspecific protease inhibitor E64 did not prevent the morphological transformation induced by TPA. The proteasome inhibitors had no effect on the transformed phenotype induced by v-Src (Fig. 6A). The ability of MG101 to prevent the TPA-induced morphological transformation was not likely due to any effects that proteasome inhibition have upon cell cycle progression (10), since aphidicolin, which blocks cells at the G1/S boundary of the cell cycle (5), had no effect on the TPA-induced morphological transformation (data not shown). In addition, MG101 had no effect on the translocation of the PKC isoforms induced by TPA (Fig. 6B). Thus, the effect observed in Fig. 6A is not due to the inability to translocate PKC isoforms to the membrane. These data suggest that PKC δ is downregulated by the ubiquitin-proteasome pathway and that this pathway is critical for the TPA-induced tumor promotion, as reported previously (7).
FIG. 6.
TPA-induced transformation of 3Y1 cells overexpressing c-Src is blocked by proteasome inhibitors. (A) 3Y1 cells overexpressing c-Src were either untreated or treated with TPA (400 nM; 10 h) in the presence of either MG101 (50 μM) or E64 (50 μM), and the morphology of the cells was examined. The effect of MG101 on v-Src-transformed 3Y1 cells is also shown. (B) The ability of TPA to induce translocation of the PKC isoforms from the cytosol to the membrane in the presence of MG101 and E64 was investigated by Western blot analysis of the PKC isoforms present in the cytosolic and membrane fractions before and after TPA treatment.
DISCUSSION
In this report, we have shown that downregulation of PKC in response to tumor-promoting phorbol esters is via the ubiqutin-proteasome pathway. In response to TPA, PKC isoforms α, δ, and ɛ all became ubiquitinated within 30 min and were degraded within 6 h in 3Y1 rat fibroblasts. Proteasome inhibitors prevented TPA-induced PKC downregulation but not ubiquitination of the PKC isoforms. Ubiquitination and downregulation of PKC isoforms were dependent on an active PKC kinase. We previously demonstrated that the downregulation of PKC δ was responsible for the tumor-promoting effects of TPA on 3Y1 cells overexpressing c-Src (7). Consistent with PKC δ downregulation being important for the tumor-promoting effects observed previously, the proteasome inhibitor MG101, which prevented PKC δ downregulation in response to TPA, also prevented the TPA-induced transformation of the c-Src-overexpressing cells. Thus, the data presented here implicate the ubiquitin-proteasome pathway in phorbol ester-induced tumor promotion.
Interestingly, treatment of 3Y1 cells with MG101 induced the appearance of PKC polyubiquitinated forms, especially for PKC ɛ, which tends to be the most constitutively activated isoform in these cells (18). This suggested that ubiquitination of PKC is a physiological response and is not unique to the response to phorbol esters. Consistent with this hypothesis, ubiquitination and downregulation were observed in response to an exogenously provided DG. DG was less potent than TPA at inducing ubiquitination and downregulation of PKC; however, this was most likely because DG can be metabolically converted to other lipids such as phosphatidic acid and monoacylglycerol.
The data presented here do not demonstrate the complete mechanism of activation of the ubiquitin-proteasome pathway; however, it is apparently regulated at the level of ubiquitination. Of special interest is the requirement for the kinase activity of the PKC isoforms. Compounds that inhibit activation of PKC prevented PKC downregulation and ubiquitination in response to TPA. Additionally, a kinase-dead PKC α was completely resistant to TPA-induced downregulation. Since phorbol esters still lead to the activation and downregulation of PKC isoforms δ and ɛ in cells expressing the kinase-dead PKC α, ubiquitination is apparently isoform specific and the activation of one PKC isoform does not stimulate ubiquitination and downregulation of other inactive PKC isoforms. Moreover, since the cells expressing the kinase-dead PKC α likely still express wild-type PKC α, which would be activated by TPA, it is not likely that PKC α activates a PKC α-specific ubiquitination system, because this would result in the degradation of the kinase-dead PKC α. Since the defect in the kinase-dead PKC α mutant that was not degraded in response to TPA was in the ATP-binding site, activation of the ubiquitin-conjugating system is likely stimulated by a conformational change in PKC that involves ATP binding or hydrolysis. This suggests a suicide model for regulation of PKC where upon activation, PKC becomes ubiquitinated and thereby targeted for degradation in a negative feedback control mechanism.
ACKNOWLEDGMENTS
We thank Robert Krauss and Neil Rosen for comments on the manuscript. We thank Shigeo Ohno for providing the PKC α kinase-dead mutant. We thank Erwin Fleissner for the continued support and encouragement our lab received during his tenure as dean.
This investigation was supported by grants from the National Institutes of Health (CA46677), the Council for Tobacco Research (3075), and the American Cancer Society (BE-243) (to D.A.F.) and by a Research Centers in Minority Institutions (RCMI) award from the Division of Research Resources, National Institutes of Health (RR-03037), to Hunter College. M.P. is supported in part by NIH grant CA66229-02.
Footnotes
This paper is dedicated to Erwin Fleissner on the occasion of his retirement as the dean of sciences and mathematics at Hunter College.
REFERENCES
- 1.Avantaggiati M L, Carbone M, Graessmann A, Nakatani Y, Howard B, Levine A S. The SV40 large T antigen and adenovirus E1a oncoproteins interact with distinct isoforms of the transcriptional co-activator, p300. EMBO J. 1996;15:2236–2248. [PMC free article] [PubMed] [Google Scholar]
- 2.Denning M F, Dlugosz A A, Howett M K, Yuspa S H. Expression of an oncogenic rasHa gene in murine keratinocytes induces tyrosine phosphorylation and reduced activity of protein kinase C δ. J Biol Chem. 1993;268:26079–26081. [PubMed] [Google Scholar]
- 3.Dingiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 4.Gschwendt M, Muller H-J, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:63–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 5.Johnston R N, Feder J, Hill A B, Sherwood S W, Schimke R T. Transient inhibition of DNA synthesis results in increased dihydrofolate reductase synthesis and subsequent increased DNA content per cell. Mol Cell Biol. 1986;6:3373–3381. doi: 10.1128/mcb.6.10.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H W, Smith L, Pettit G R, Vinitsky A, Smith J B. Ubiquitination of protein kinase C-α and degradation by the proteasome. J Biol Chem. 1996;271:20973–20976. [PubMed] [Google Scholar]
- 7.Lu Z, Hornia A, Jiang Y-W, Zang Q, Foster D A. Tumor-promotion by depleting cells of protein kinase C δ. Mol Cell Biol. 1997;17:3418–3428. doi: 10.1128/mcb.17.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martiny-Baron G, Kazanietz M G, Mischak H, Blumberg P M, Kochs G, Hug H, Marme D, Schlachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole G0 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 9.Nishizuka Y. Protein kinases 5: protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 10.Pagano M, Tam S W, Theodoras A M, Beer-Romero P, Del Sal G, Chau V, Yew P R, Draetta G F, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 11.Palombella V, Rando O, Goldberg A, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and activation of NF-κB. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 12.Rock K L, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg A L. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 13.Rolfe M, Chiu M I, Pagano M. The ubiquitin-mediated proteolitic pathway as a therapeutic area. J Mol Med. 1997;75:5–17. doi: 10.1007/s001090050081. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki A, Kozawa O, Kato K. Protein kinase C activation inhibits stress-induced synthesis of heat shock protein 27 in osteoblast-like cells: function of arachidonic acid. J Cell Biochem. 1996;62:69–75. doi: 10.1002/(SICI)1097-4644(199607)62:1%3C69::AID-JCB8%3E3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Ueda Y, Hirai S, Osada S, Suzuku A, Mizuno K, Ohno S. Protein kinase C δ activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 16.Young S, Parker P J, Ulrich A, Stabel S. Down-regulation of protein kinase C is due to an increased rate of degradation. Biochem J. 1987;244:711–717. doi: 10.1042/bj2440775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yupsa S H, Poirier M C. Chemical carcinogenesis: from animal models to molecular models in one decade. Adv Cancer Res. 1988;50:25–70. doi: 10.1016/s0065-230x(08)60434-0. [DOI] [PubMed] [Google Scholar]
- 18.Zang Q, Frankel P, Foster D A. Selective activation of protein kinase C isoforms by v-Src. Cell Growth Differ. 1995;6:1367–1373. [PubMed] [Google Scholar]
- 19.Zang Q, Lu Z, Curto M, Barile N, Shalloway D, Foster D A. Interaction between v-Src and protein kinase C δ in v-Src-transformed fibroblasts. J Biol Chem. 1997;272:13275–13280. doi: 10.1074/jbc.272.20.13275. [DOI] [PubMed] [Google Scholar]