Abstract
Corticotropin-releasing hormone (CRH) coordinates hormonal and behavioral responses to stress. The mitogen-activated protein kinase extracellular signal-related kinase 1/2 (ERK1/2) mediates several functions in different forebrain structures and recently has been implicated in CRH signaling in cultured cells. To study in vivo CRH-mediated activation of central ERK1/2, we investigated the expression pattern of the phosphorylated ERK1/2(p-ERK1/2) in the mouse brain after intracerebroventricular CRH injections. As shown by immunohistochemistry and confocal microscopy analysis, CRH administration increased p-ERK1/2 levels specifically in the CA3 and CA1 hippocampal subfields and basolateral complex of the amygdala, both structures related to external environmental information processing and behavioral aspects of stress. Other regions such as hypothalamic nuclei and the central nucleus of the amygdala, also related to central CRH system but involved in the processing of the ascending visceral information and neuroendocrine-autonomic response to stress, did not show CRH-mediated ERK1/2 activation. To dissect the involvement of CRH receptor 1 (CRHR1) and CRHR2, we used conditional knockout mice in which Crhr1 is inactivated in the anterior forebrain and limbic structures. The conditional genetic ablation of Crhr1 inhibited the p-ERK1/2 increase, underlining the involvement of CRHR1 in the CRH-mediated activation. These findings underscore the fact that CRH activates p-ERK1/2 through CRHR1 only in selected brain regions, pointing to a specific role of this pathway in mediating behavioral adaptation to stress.
Keywords: corticotropin-releasing hormone receptor 1 conditional knockout, extracellular signal-regulated kinase 1/2, mitogen-activated protein kinase
Corticotropin-releasing hormone (CRH) is a 41-aa peptide that exerts a key role in the adjustment of neuroendocrine and behavioral adaptations to stress. Hypothalamic CRH neurons drive both basal and stress-induced hypothalamicpituitary-adrenal axis (HPA) activation (1). Internal homeostasis or external environment stress changes are conveyed to the CNS by neurochemical pathways and are integrated at the hypothalamic level where they reach paraventricular CRH neurosecretory neurons controlling CRH secretion (2-5). CRH activates corticotropin (ACTH) release (6), which in turn, stimulates corticosteroid release by the adrenal glands. Besides the hypothalamus, CRH is widely distributed throughout the CNS (7, 8). CRH acts as a neurotransmitter or neuromodulator in extrahypothalamic circuits to integrate a multisystem response to stress that controls numerous behaviors such as locomotor activity, anxiety, food intake, sexual behavior, sleep, arousal, and learning (2, 9-13). Alterations in this system may influence the development of affective disorders and other stress-related clinical conditions (2, 14-18).
CRH exerts its actions by means of CRH receptors (CRHRs), of which two subtypes (CRHR1 and CRHR2) have been identified that have different localization throughout the brain (19-22). CRHR1 is expressed in high levels in neocortical areas, the basolateral and medial nucleus of the amygdala, anterior pituitary, hypothalamic nuclei, cerebellar Purkinje cells, lateral dorsal tegmentum, and pedunculopotine tegmental nucleus (19-22). CRHR2 has been detected in more discrete brain regions, including the lateral septum, ventromedial hypothalamus, and cortical nucleus of the amygdala (19-21). Both receptors have been identified in the hippocampus (19-22). CRH is a high-affinity ligand for CRHR1 and binds poorly to the CRHR2 for which other ligands such as urocortin have more affinity (16).
Both Crhr1 and Crhr2 knockout (KO) mice have been developed. Crhr1 KO mice exhibit reduced anxiety-related behavior and an impaired basal and stress-induced HPA response (23, 24). The study of Crhr2-deficient mice evidenced that CRHR2 mediates peripheral CRH/urocortin actions on the cardiovascular system and emphasizes a role for this receptor in the recovery of the HPA axis after stress mediated by CRHR1 (25, 26). Nevertheless, with regard to anxiety related behavior, there are conflicting results obtained from the three independent mouse lines developed (25-27). In addition, to dissect the specific contribution of each CRHR to the HPA regulation, double Crhr1/Crhr2 deficient mouse lines have been developed that point to the essential role of CRHR1 in triggering the stress-induced HPA activation and its recovery by CRHR2 (28, 29).
Recently, a conditional Crhr1 KO mouse line in which CRHR1 function is postnatally inactivated specifically in anterior forebrain and limbic structures, but not in the pituitary, has been described (30). In these mice, the basal activity of the HPA system remains intact. These mice present an anxiety reduced phenotype, showing a direct participation of limbic CRHR1 in anxiety related behavior. In addition, these mice, similar to Crhr2 KO mice, present a significantly slower recovery of the HPA system (corticosterone and corticotropin levels) after restraint stress, also showing a role for CRHR1 in this recovery phase. These mice help demonstrate a crucial role for limbic CRHR1 in the central control of HPA adaptation to stress. This phenotype renders these conditional Crhr1 KO mice as an interesting model to selectively dissect CRH/CRHR1 CNS pathways regulating behavior from those regulating neuroendocrine function.
The mitogen-activated protein kinase (MAPK) pathway includes several kinases, among which, the extracellular signal-regulated kinase 1/2 (ERK1/2) pathway regulates the transcription factor Elk-1. MAPKs are regulated by cAMP in several cell types. CRHRs are G protein-coupled seven-transmembrane receptors linked to a number of intracellular signaling pathways, including ligand-dependent increase of intracellular cAMP. Activation of CRHR1 by CRH increases cAMP intracellular levels and activates protein kinase A (PKA), which phosphorylates and activates its associated transcription factor CREB (31, 32). We first demonstrated that upon CRHR1 activation, corticotrophs use a MAPK signal transduction pathway that is downstream of PKA (33). This pathway depends on calcium entry through calcium-dependent voltage channels at the plasma membrane, involves calmodulin kinase II, and regulates induction and activity of target genes such as Nur77 and Nurr1. The MAPK pathway that is activated by CRH depends on PKA action and involves activation of the small G proteins Rap1 and B-Raf (33).
ERK1/2 signaling has been reported to participate in several functions of different forebrain structures (34, 35), many of which are also targets of CRH action. Thus, contextual fear conditioning requires a ERK1/2 MAPK cascade (36). Likewise, ERK1/2 inhibitors have been found to disrupt long-term potentiation in the hippocampal formation (37, 38) and in the lateral amygdala (LA) (39). To date, the in vivo link between CRH, CRHR (especially CRHR1), and ERK signaling in the CNS has not been addressed. To examine and map the CNS activation of ERK1/2 in response to CRH, we conducted functional in vivo experiments in normal mice and in mice carrying Crhr1 deletion in the anterior forebrain and limbic structures.
Methods
Animals and Housing. Adult (12-14 weeks old) male mice weighing 25-35 g were used. The animals were housed four to six per cage under standard conditions (lights were on from 6:00 a.m. to 6:00 p.m. at a temperature of 22-23°C, with a relative humidity of 40-60%) and received food and water ad libitum. Animals with intracerebroventricular (i.c.v.) cannulas were housed singly. Normal adult mice with a C57BL/6 background were used in the first set of experiments as a reference.
The CrhrloxP/loxPCamk2a-cre and its Crhr1loxP/loxPcontrol mice were obtained as originally described (for details see ref. 30). Briefly, Crhr1 targeting vector was constructed in which exons 9-13 coding for transmembrane regions 4-7 were flanked by loxP sites. Thus, the Crhr1”target” allele (Crhr1loxP) was generated by homologous recombination in embryonic stem cells. Mice homozygous for Crhr1loxP (Crhr1loxP/loxP) are sensitive to Cre recombinase-mediated inactivation of the Crhr1 locus in any cell expressing the recombinase. These Crhr1loxP/loxP mice did not evidence differences in any behavioral or neuroendocrine phenotype compared with their WT littermates (30). Afterward, Crhr1loxP/loxP mice were crossed to a mouse line expressing Cre recombinase driven by a Camk2a promoter (40), generating Crhr1loxP/loxPCamk2a-cre and Crhr1loxP/loxPcontrol mice. These animals were kept on a mixed 129/Sv X C57BL/6 background, and genotyping was performed by Southern blot analysis of XbaI-digested tail DNA by using an internal Crhr1 probe and a Cre recombinase-specific probe.
Experimental Procedures. All animal experiments were conducted in accordance with established guidelines.
Animal stereotaxis was performed as described (41, 42). Mice (n = 5 per group) were anesthetized with sodium pentobarbital (80 mg/kg, i.p.) and placed in a stereotaxic frame (Technical Scientific Equipment, Bad Homburg, Germany) (43). Coordinates for the i.c.v. implantation were checked and adjusted previously in some mice with the background identical to the experimental mice. To this end, mice were bilaterally implanted and infused with cresyl violet solution (1 μl for each side), the brains were sectioned with a cryostat, and the placement of the cannulas was histologically verified. In the experimental mice, 22-gauge stainless steel guide cannulas were implanted bilaterally at the following coordinates: anterior, 0.4 mm posterior to bregma; lateral ± 1.1 mm lateral to the midline; ventral, 1.0 mm below the exposed dura mater. The cannulas were then fixed in place by using a stainless steel screw and dental cement. A stainless steel stylet was inserted into each cannula to prevent blockage. Mice were allowed 2 weeks of recovery before CRH infusions were performed. After surgery was performed, animals were handled to prevent stress manipulation at the moment of experiment. Only animals with correct placement of the cannula were included for further analysis. Cannulated animals received i.c.v. infusions of human CRH [1.0 μg/1 μl, with an injection volume of 1 μl for each side (Bachem Biochemica, Heidelberg)] or vehicle over a period of 1 min by means of an injection cannula (31 gauge) that protruded 1.0 mm below the guide tube and was connected to a 10-μl Hamilton syringe. Because 1 μg of CRH is sufficient to evoke behavioral effects, this dose was chosen for in vivo i.c.v. administration. Ten or 30 min after i.c.v. treatment, animals were killed by decapitation after quick (<15 sec) anesthesia in saturated isoflurane vapor. For immunohistochemistry, brains were removed immediately and snap-frozen in isopentane (-40°C) and subsequently deep-frozen on dry ice.
Immunohistochemistry and Confocal Microscopy. Coronal brain sections (12 μm thick) were cut with a cryostat and mounted on slides (Superfrost, Menzel-Gläser, Merck Eurolab, Ismaning, Germany). Brain sections were stored at -80°C until they were processed. Immunohistochemistry was carried out as described (44). Fixation of mounted brain slices was performed with freshly prepared 4% paraformaldehyde-PBS (pH 7.5 at 4°C) for 5 min, followed by incubation in 0.01% Tween-20/PBS (T-PBS) for 15 min. Blocking of nonspecific sites was carried out with T-PBS/2% BSA for 40 min at room temperature. After being washed extensively with PBS, sections were incubated for 24 h at 4°C with rabbit polyclonal anti-pERK1/2 IgG that stains the activated form of ERK1/2 (Cell Signaling Technology, Beverly, MA) and rabbit polyclonal anti-ERK1/2 IgG (Cell Signaling Technology) at 1:100 dilution in T-PBS/2%BSA. Subsequently, the slices were washed three times in T-PBS for 5 min each and incubated with the corresponding biotinylated goat-anti-rabbit IgG (1:100; Vector Laboratories) for 1 h in T-PBS at room temperature. After being washed in PBS (3 × 5 min), fluorescein-conjugated streptavidin (1:50, Vector Laboratories) was added for an additional hour. Sections were then rinsed again (3 × 5 min) in PBS. Finally, the brain sections were embedded in propidium iodide (PI) containing mounting media (Vector Laboratories) to prevent rapid photobleaching of fluorescein.
Immunohistochemical analysis was carried out by using a laser-scanning confocal microscope (Fluoview FV300 BS61, Olympus, Melville, NY). Images were acquired simultaneously in two acquisition channels with the fluoview fv 300 (version 3.3) acquisition/analyzer program.
The optical density of staining was determined automatically by two different observers, with each using two different types of imaging analyzer software: a Macintosh-based nih image system and the f luoview acquisition/analyzer program. Both systems revealed similar results. f luoview-processed optical density data are shown, and all data are presented as optical density arbitrary units after correction of each image with its background signal. To minimize interslice staining variability, al least four serial tissue sections per animal and region were analyzed.
Statistics. Statistics were performed by using one-way ANOVA and a posthoc Scheffé's test. P values of < 0.05 were considered significant. Optical density measurements are expressed as the mean ± SE.
Results
CRH-Induced p-ERK1/2 Activation in Forebrain Regions. In the first group of experiments, undisturbed freely moving normal male mice received CRH (1 μg/μl) or vehicle i.c.v. injections. All sections analyzed from vehicle-treated animals exhibited very low background levels of p-ERK1/2 immunoreactivity (p-ERK1/2-ir), with the exception of the neocortex, which presented a more intense signal. Hippocampus and amygdala showed, as detailed below, a marked enhancement of the p-ERK1/2-ir both 10 and 30 min after CRH i.c.v. administration. These areas are extensively innervated by CRH fibers and are also recognized functional targets of CRH action. Therefore, we focused our study in both areas. In these areas, as in all brain regions evaluated, anti-p-ERK1/2-treated adjacent/paired slices were stained with an antibody against nonphosphorylated ERK1/2 (anti-ERK1/2). Similar expression levels of ERK1/2 were found, proving that differences in p-ERK1/2-ir between experimental groups are not caused by unequal ERK1/2 expression levels. The PI images show that cellularity of the compared brain sections is similar. Therefore, differences in immunoreactivity of p-ERK1/2 in paired images cannot be appointed to differences in cell number or constitutive unequal ERK1/2 expression. To control for the possible influence of i.c.v. injection on basal p-ERK1/2 expression levels, uncannulated animals under the same housing conditions and experimental environment as those of cannulated mice were killed and analyzed. No differences in basal levels were found between uncannulated and vehicle treated-animals (data not shown).
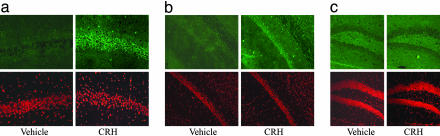
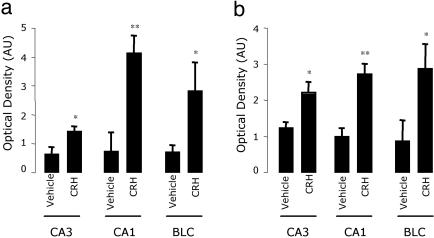
In the hippocampal formation, a great increase in p-ERK1/2-ir was detected in pyramidal cells of CA3 and CA1 subfield layers of CRH-treated mice as compared with the vehicle-treated mice (Fig. 1 a and b). Quantitative analysis of the optical density of p-ERK1/2 staining in the hippocampus shows that these differences occur at 10 and 30 min after i.c.v. CRH injection (Fig. 3). Although the dentate gyrus is a structure that expresses both CRHR1 and CRHR2, and is functionally responsive to CRH (20, 22), it did not show CRH-induced p-ERK1/2-ir (Fig. 1c).
Fig. 1.
Differential activation of p-ERK1/2-ir in hippocampal neurons after vehicle or i.c.v. CRH injections. Representative images (from five mice) of p-ERK1/2 immunohistochemistry (Upper) and control PI staining (Lower) obtained as described in Methods. Pyramidal cells of the CA3 (a) and CA1 (b) subfields strongly express p-ERK1/2 30 min after i.c.v. CRH injections. In contrast, the dentate gyrus (c) does not express basal or CRH-induced p-ERK1/2 levels. (Magnification: a, ×400; b and c, ×200.)
Fig. 3.
Quantitative analysis of p-ERK1/2-ir. Levels of p-ERK1/2 in CA3, CA1, and the BLC (BLC = LA and BLA) 10 min (a) and 30 min (b) after i.c.v. vehicle or CRH administration. Optical density data processed with the fluoview acquisition/analyzer program are shown as optical density arbitrary units (AU) after subtraction of each image with its corresponding background signal. Data are presented as mean ± SE, n = 5 animals; *, P < 0.05; **, P < 0.01 vs. the corresponding control vehicle, using ANOVA with Scheffé's test.
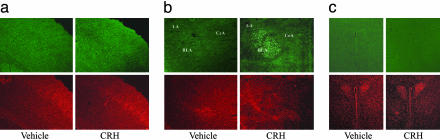
In the neocortex, a moderate level of expression was found in vehicle-treated animals, but CRH administration did not significantly enhance p-ERK1/2-ir at 30 min (Fig. 2a) or at 10 min (data not shown).
Fig. 2.
CRH induces p-ERK1/2 levels in the amygdala but not in the cortex or hypothalamus. Representative images (from five mice) of p-ERK1/2 immunohistochemistry (Upper) and control PI staining (Lower) obtained 30 min after i.c.v. CRH injections as described in Methods. (a) Cortex presents moderate levels of basal p-ERK1/2 activation in layers III and IV and CRH does not induce changes. (b) CRH strongly activates ERK1/2 in the LA and BLA. The central amygdaloid nucleus does not present basal or CRH-induced p-ERK1/2 levels. (c) The PVN of the hypothalamus remain absent of any p-ERK1/2 signal in both basal and stimulated conditions. (Magnification: ×200.)
The LA and the basolateral nucleus of the amygdala (BLA), which together constitute the basolateral complex of the amygdala (BLC), showed a significant increment of p-ERK1/2-ir at 10 and 30 min after i.c.v. CRH administration (Figs. 2b and 3). Interestingly, the central or medial nuclei (Fig. 2b) and the cortical division of the amygdala (data not shown) showed no sign of p-ERK1/2 activation.
At the hypothalamic level, no p-ERK1/2 activation was found after vehicle or CRH administration. Complete absence of p-ERK staining was observed at the level of the paraventricular nucleus (PVN) of the hypothalamus at 30 min (Fig. 2c) and 10 min (data not shown).
p-ERK1/2 Immunoreactivity in Crhr1 Conditional KO Mice. The brain structures showing CRH-induced p-ERK1/2 activation (in the hippocampus and amygdala) express in at least some of the substructures that compose them, CRHR1 and/or CRHR2. To further confirm specificity, and to analyze the CRHR subtype involved in the CRH-induced activation of p-ERK1/2, we carried out i.c.v. CRH or vehicle injections in Crhr1loxP/loxPCamk2a-cre and Crhr1loxP/loxPcontrol mice.
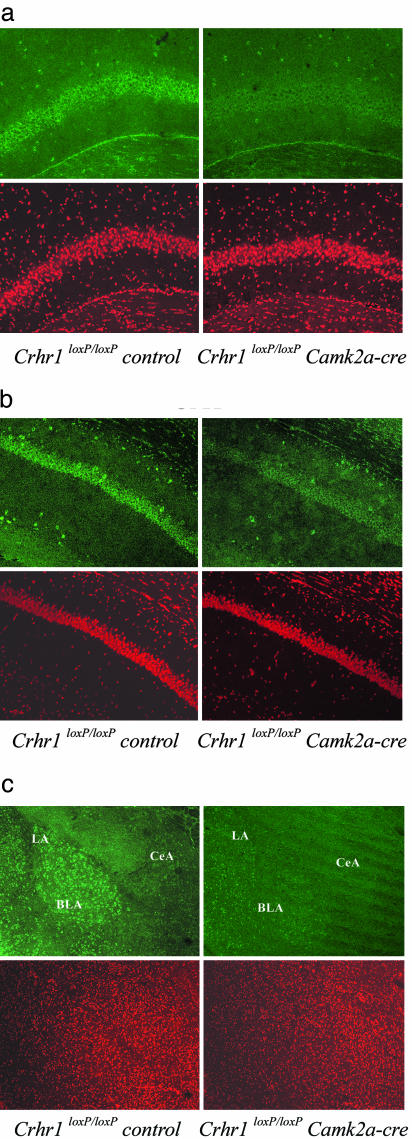
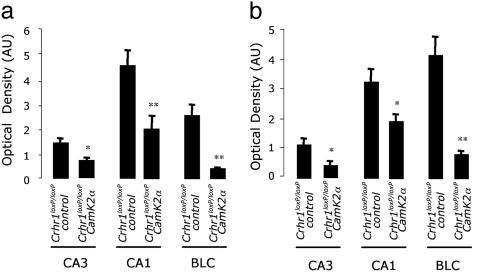
Immunohistochemical analysis of hippocampal regions showed that the CRH-induced phosphorylation of ERK1/2 in both the CA3 and CA1 subfields is strongly diminished in Crhr1loxP/loxPCamk2a-cre vs. Crhr1loxP/loxPcontrol mice (Figs. 4 and 5). Basal p-ERK1/2-ir levels in CrhrloxP/loxPCamk2a-cre mice were similar to that of Crhr1loxP/loxPcontrol mice (data not shown). These results clearly underline the relevance of CRHR1 in mediating the actions of CRH on CA3 and CA1 ERK1/2 activation.
Fig. 4.
Crhr1loxP/loxPCamk2a-cre mice show a decreased ERK1/2 activation response in CA3, CA1, and the BLC after central CRH administration. Representative images (from five mice) of p-ERK1/2 immunohistochemistry (Upper) and control PI staining (Lower) obtained 30 min after i.c.v. CRH injections as described in Methods. ERK1/2 activation is greatly reduced in CA3 (a), CA1 (b), and the BLA (c) of CRH-treated Crhr1loxP/loxPCamk2a-cre compared with CRH-treated Crhr1loxP/loxP control mice. (Magnification: ×200.)
Fig. 5.
Quantitative analysis of p-ERK1/2-ir in Crhr1loxP/loxPcontrol and Crhr1loxP/loxPCamk2a-cre mice after central CRH administration. Levels of p-ERK1/2 in CA3, CA1, and the BLC (BLC = LA and BLA) of the amygdala 10 min (a) and 30 min (b) after i.c.v. CRH administration. Optical density data processed with the fluoview acquisition/analyzer program are shown as optical density arbitrary units (AU) after subtraction of each image with its corresponding background signal. Data are presented as mean ± SE, n = 5 animals; *, P < 0.05; **, P < 0.01 vs. the corresponding Crhr1loxP/loxPcontrol mice, using ANOVA with Scheffé's test.
The significant increase in p-ERK1/2-ir in the LA and BLA after i.c.v. CRH administration was absent in the Crhr1loxP/loxPCamk2a-cre mice (Figs. 4c and 5), proving the participation of CRHR1 in CRH-induced ERK1/2 activation on these areas.
Discussion
Our results show that CRH induces ERK1/2 activation in specific areas of the CNS. The capability of i.c.v. CRH administration or different stress paradigms to activate specific areas in the brain were previously assessed by using the protein or mRNA expression patterns of the immediate-early gene c-Fos, a known general marker of activated neurons (20, 45, 46). The functional mapping of p-ERK1/2, a more selective molecular signaling pathway downstream of CRHRs, helps us to further understand, at the molecular, cellular, and cerebral levels, the direct activation of CNS pathways after in vivo central CRH administration and receptor activation. ERK1/2 MAPK, which is rapidly induced and is an early participant in signaling pathways, constitutes an interesting marker for tracking the initiation of gene expression by CRH in neurons.
At the hippocampal level, i.c.v. CRH administration caused a strong activational effect on pyramidal cells of the CA1 and CA3 fields at both 10 and 30 min after injection. Conversely, the dentate gyrus was completely devoid of p-ERK1/2-ir, underlining the specificity of the activation pattern. Interestingly, the dentate gyrus expresses low levels of both CRHR1 and CRHR2 (20-22). Thus, the low expression levels of CRHRs in this region could be responsible for the absence of p-ERK1/2-ir. However, in the dentate gyrus, an increased p-ERK1/2-ir during contextual fear conditioning (47) and an augmented Fos expression after i.c.v. CRH administration (20) has been described. This regional difference could be related to desensitization of MAPK cascades or to the use of non-MAPK alternative signaling pathways induced specifically by CRH in the dentate gyrus. In this sense, alternative coupling of CRHR1 with different G proteins as Gs, Gi, and Gq/11 has been recently described in mouse hippocampus (48). This differential recruitment of G proteins could, in fact, explain the differences in the p-ERK1/2-ir between any areas or nuclei with a similar CRHR1 expression level.
p-ERK1/2-ir was increased in some amygdaloid nuclei after CRH treatment. These nuclei participate in processes such as memory consolidation, fear and anxiety, modulation of hypothalamic circuits, and control of autonomic/neuroendocrine response (16, 49, 50). We observed also an increase in the LA and BLA p-ERK1/2-ir after CRH administration. The LA and BLA form the BLC and belong to the claustrum division of the amygdala (51). Together, both nuclei constitute the frontotemporal cortical functional component that projects to the striatum and has bidirectional communication with several cortical areas. The central nuclei, in which p-ERK1/2 induction was completely absent, is not endowed with CRHRs, and is a nucleus specialized for modulating autonomic motor outflow. Whereas hippocampus and amygdala displayed an increased CRH-induced p-ERK1/2-ir, none of the hypothalamic nuclei showed ERK1/2 activation. The PVN of the hypothalamus, a pivotal structure involved in the modulation of neuroendocrine and autonomic responses to stress, also showed an absence of p-ERK1/2-ir. The currently available data on CRHR expression on parvocellular and magnocellular divisions of this nucleus are contradictory. Whereas some reports described nonexistent or low levels of CRHR1 or CRHR2 mRNA expression in the rat brain (20, 21), a recent study evaluating CRHR1 protein distribution in the mouse (22) showed many and strongly stained neurons in the parvocellular division of the PVN. Whether by an absence of CRHRs or by a CRHR-intact/MAPK-desensitized pathway, it is interesting to note that while telencephalic structures, related to external environmental information processing and behavioral aspects of stress, have different degrees of CRH-induced MAPK activation, the hypothalamus and the central nuclei, which are involved in the processing of the ascending visceral information and neuroendocrine-autonomic response to stress, have a complete incapacity to develop a CRHR-mediated ERK1/2 activation.
Altogether, these results point to a role of ERK phosphorylation in mediating CRH-induced activation of these limbic structures, known to be involved in learning and memory, stress-related behaviors, and sensory processing of multimodal stimuli.
The involvement of ERK1/2 in several CRH actions has been described in neuronal cell cultures, such as neuroprotection against excitotoxicity in hippocampal cells (52) and the antiapoptotic activity of CRH in retinoblastoma cells (53). In cell cultures, ERK1/2 phosphorylation takes place within a range of minutes after the receptor activation (33, 54). In vivo CRH administration at 10 and 30 min is sufficient to induce a strong p-ERK1/2-ir in several brain nuclei. CRH administrated by the i.c.v. route is able to access receptor-expressing cells directly, as demonstrated by the activation of c-Fos (20). The activation of ERK1/2 10 min after CRH i.c.v. injection in our experiments, similar to the activation of c-Fos shown at 15 min after i.c.v. CRH administration (55), might reflect the extent of diffusion of i.c.v. administrated CRH into the extracellular space from the liquor and confirm its cellular accessibility.
Upon activation, ERK1/2 translocates to the nucleus of the stimulated cells, where it phosphorylates nuclear targets and modulates gene transcription rates of target cells (56-58). At each observation point, the neuronal p-ERK1/2 staining was essentially perinuclear in all evaluated nuclei and areas, as observed by overlapping the digital images of p-ERK1/2-ir (green) and PI staining (red) of brain slices. The reason for this intracellular localization remains elusive but could be explained by the times selected for killing or by a quickly nucleus-cytoplamic shuttling of the activated kinase. Nevertheless, this observation is in accordance with recent reports (47), showing that the contextual fear conditioning causes a significantly p-ERK1/2 increase of predominantly cytoplasmic localization in hippocampal cells.
At the hippocampal level, both CRHR1 and CRHR2 expression has been described. The absence of p-ERK1/2 activation in the CA3 and CA1 pyramidal cell layers of Crhr1loxP/loxPCamk2a-cre mice after central CRH treatment evidences the relevance of CRHR1 in the CRH-induced activation of the kinase. However, a weak staining remained, suggesting that coexpressed CRHR2 could be triggering lower additional ERK1/2 signals. Accordingly, a recent study (59) has demonstrated that CRHR2 in the hippocampus can mediate the ERK1/2 activation induced by stress-enhanced fear conditioning. ERK1/2 activation of the BLC was almost completely absent in the conditional Crhr1 KO mice, pointing out the critical role of CRHR1.
The hippocampal and amygdaloid CRH systems emerge as decisive players in the disregulation of limbic circuitry in depressive and anxiety-related disorders where CRHR1 activation mediates a number of depression-like symptoms (16). We observed that CRH-mediated activation of ERK1/2 in the pyramidal layer of the hippocampus and LA/BLA are impaired in Crhr1loxP/loxPCamk2a-cre mice.
The activation by CRH through CRHR1 of ERK1/2 in specific areas of the CNS involved in behavior, but not in autonomic/neuroendocrine pathways, highlights the importance of these intracellular pathways and opens the concept of the involvement of specific signaling pathways mediating responses to stress-elicited CRH.
Acknowledgments
We thank Dante Paz, Ramiro Freudenthal, Sabine Ulbricht, and Cornelia Flachskamm for their helpful contribution. This work was supported by grants from the Max Planck Institute of Psychiatry, the Argentine National Research Council, the University of Buenos Aires, Agencia Nacional de Promoción Científica y Tecnológica-Argentina, and the Antorchas Foundation. Part of the study was supported by the Freedom to Discover Award given by the Bristol-Mayer Squibb Foundation (to F.H.).
Author contributions: D.R., W.W., M.P.-P., F.H., and E.A. designed research; D.R., C.E., M.B.M., J.M.H.M.R., J.M.D., I.S., M.P.-P., and E.A. performed research; J.M.H.M.R., J.M.D., and W.W. contributed new reagents/analytic tools; D.R., M.B.M., M.P.-P., F.H., and E.A. analyzed data; and D.R., M.B.M., J.M.H.M.R., I.S., M.P.-P., F.H., and E.A. wrote the paper.
Abbreviations: CRH, corticotropin-releasing hormone; CRHR, CRH receptor; ERK1/2, extracellular-signal-regulated kinase 1/2; p-ERK1/2, phosphorylated ERK1/2; p-ERK1/2-ir, p-ERK1/2 immunoreactivity; MAPK, mitogen-activated protein kinase; i.c.v., intracerebroventricular; HPA, hypothalamic-pituitary-adrenal axis; KO, knockout; T-PBS, Tween-20/PBS; LA, lateral amygdala; BLA, basolateral nucleus of the amygdala; PVN, paraventricular nucleus; PI, propidium iodide.
References
- 1.Sawchenko, P. E., Brown, E. R., Chan, R. K., Ericsson, A., Li, H. Y., Roland, B. L. & Kovacs, K. J. (1996) Prog. Brain Res. 107, 201-222. [DOI] [PubMed] [Google Scholar]
- 2.Holsboer, F. (1999) J. Psychiatr. Res. 33, 181-214. [DOI] [PubMed] [Google Scholar]
- 3.Sawchenko, P. E., Li, H. Y. & Ericsson, A. (2000) Prog. Brain Res. 122, 61-78. [DOI] [PubMed] [Google Scholar]
- 4.Herman, J. P., Figueiredo, H., Mueller, N. K., Ulrich-Lai, Y., Ostrander, M. M., Choi, D. C. & Cullinan, W. E. (2003) Front. Neuroendocrinol. 24, 151-180. [DOI] [PubMed] [Google Scholar]
- 5.Bale, T. L. & Vale, W. W. (2004) Annu. Rev. Pharmacol. Toxicol. 44, 525-557. [DOI] [PubMed] [Google Scholar]
- 6.Ono, N., Samson, W. K., McDonald, J. K., Lumpkin, M. D., Bedran de Castro, J. C. & McCann, S. M. (1985) Proc. Natl. Acad. Sci. USA 82, 7787-7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings, S., Elde, R., Ells, J. & Lindall, A. (1983) J. Neurosci. 3, 1355-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swanson, L. W., Sawchenko, P. E., Rivier, J. & Vale, W. W. (1983) Neuroendocrinology 36, 165-186. [DOI] [PubMed] [Google Scholar]
- 9.Sutton, R. E., Koob, G. F., Le Moal, M., Rivier, J. & Vale, W. (1982) Nature 297, 331-333. [DOI] [PubMed] [Google Scholar]
- 10.Buwalda, B., de Boer, S. F., Van Kalkeren, A. A. & Koolhaas, J. M. (1997) Psychoneuroendocrinology 22, 297-309. [DOI] [PubMed] [Google Scholar]
- 11.Holahan, M. R., Kalin, N. H. & Kelley, A. E. (1997) Psychopharmacology 130, 189-196. [DOI] [PubMed] [Google Scholar]
- 12.Linthorst, A. C., Flachskamm, C., Hopkins, S. J., Hoadley, M. E., Labeur, M. S., Holsboer, F. & Reul, J. M. (1997) J. Neurosci. 17, 4448-4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radulovic, J., Ruhmann, A., Liepold, T. & Spiess, J. (1999) J. Neurosci. 19, 5016-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bissette, G., Reynolds, G. P., Kilts, C. D., Widerlov, E. & Nemeroff, C. B. (1985) J. Am. Med. Assoc. 254, 3067-3069. [PubMed] [Google Scholar]
- 15.De Souza, E. B., Whitehouse, P. J., Kuhar, M. J., Price, D. L. & Vale, W. W. (1986) Nature 319, 593-595. [DOI] [PubMed] [Google Scholar]
- 16.Reul, J. M. & Holsboer, F. (2002) Curr. Opin. Pharmacol. 2, 23-33. [DOI] [PubMed] [Google Scholar]
- 17.Wong, M. L., Kling, M. A., Munson, P. J., Listwak, S., Licinio, J., Prolo, P., Karp, B., McCutcheon, I. E., Geracioti, T. D., Jr., DeBellis, M. D., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 325-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habib, K. E., Weld, K. P., Rice, K. C., Pushkas, J., Champoux, M., Listwak, S., Webster, E. L., Atkinson, A. J., Schulkin, J., Contoreggi, C., et al. (2000) Proc. Natl. Acad. Sci. USA 97, 6079-6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chalmers, D. T., Lovenberg, T. W. & De Souza, E. B. (1995) J. Neurosci. 15, 6340-6350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bittencourt, J. C. & Sawchenko, P. E. (2000) J. Neurosci. 20, 1142-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Pett, K., Viau, V., Bittencourt, J. C., Chan, R. K., Li, H. Y., Arias, C., Prins, G. S., Perrin, M., Vale, W. & Sawchenko, P. E. (2000) J. Comp. Neurol. 428, 191-212. [DOI] [PubMed] [Google Scholar]
- 22.Chen, Y., Brunson, K. L., Muller, M. B., Cariaga, W. & Baram, T. Z. (2000) J. Comp. Neurol. 420, 305-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timpl, P., Spanagel, R., Sillaber, I., Kresse, A., Reul, J. M., Stalla, G. K., Blanquet, V., Steckler, T., Holsboer, F. & Wurst, W. (1998) Nat. Genet. 19, 162-166. [DOI] [PubMed] [Google Scholar]
- 24.Smith, G. W., Aubry, J. M., Dellu, F., Contarino, A., Bilezikjian, L. M., Gold, L. H., Chen, R., Marchuk, Y., Hauser, C., Bentley, C. A., et al. (1998) Neuron 20, 1093-1102. [DOI] [PubMed] [Google Scholar]
- 25.Bale, T. L., Contarino, A., Smith, G. W., Chan, R., Gold, L. H., Sawchenko, P. E., Koob, G. F., Vale, W. W. & Lee, K. F. (2000) Nat. Genet. 24, 410-414. [DOI] [PubMed] [Google Scholar]
- 26.Coste, S. C., Kesterson, R. A., Heldwein, K. A., Stevens, S. L., Heard, A. D., Hollis, J. H., Murray, S. E., Hill, J. K., Pantely, G. A., Hohimer, A. R., et al. (2000) Nat. Genet. 24, 403-409. [DOI] [PubMed] [Google Scholar]
- 27.Kishimoto, T., Radulovic, J., Radulovic, M., Lin, C. R., Schrick, C., Hooshmand, F., Hermanson, O., Rosenfeld, M. G. & Spiess, J. (2000) Nat. Genet. 24, 415-419. [DOI] [PubMed] [Google Scholar]
- 28.Preil, J., Muller, M. B., Gesing, A., Reul, J. M., Sillaber, I., van Gaalen, M. M., Landgrebe, J., Holsboer, F., Stenzel-Poore, M. & Wurst, W. (2001) Endocrinology 142, 4946-4955. [DOI] [PubMed] [Google Scholar]
- 29.Bale, T. L., Picetti, R., Contarino, A., Koob, G. F., Vale, W. W. & Lee, K. F. (2002) J. Neurosci. 22, 193-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller, M. B., Zimmermann, S., Sillaber, I., Hagemeyer, T. P., Deussing, J. M., Timpl, P., Kormann, M. S., Droste, S. K., Kuhn, R., Reul, J. M., et al. (2003) Nat. Neurosci. 6, 1100-1107. [DOI] [PubMed] [Google Scholar]
- 31.Dautzenberg, F. M. & Hauger, R. L. (2002) Trends Pharmacol. Sci. 23, 71-77. [DOI] [PubMed] [Google Scholar]
- 32.Luini, A., Lewis, D., Guild, S., Corda, D. & Axelrod, J. (1985) Proc. Natl. Acad. Sci. USA 82, 8034-8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovalovsky, D., Refojo, D., Liberman, A. C., Hochbaum, D., Pereda, M. P., Coso, O. A., Stalla, G. K., Holsboer, F. & Arzt, E. (2002) Mol. Endocrinol. 16, 1638-1651. [DOI] [PubMed] [Google Scholar]
- 34.Davis, M. (2002) Eur. J. Neurosci. 16, 395-398. [DOI] [PubMed] [Google Scholar]
- 35.Thomas, G. M. & Huganir, R. L. (2004) Nat. Rev. Neurosci. 5, 173-183. [DOI] [PubMed] [Google Scholar]
- 36.Schafe, G. E., Atkins, C. M., Swank, M. W., Bauer, E. P., Sweatt, J. D. & LeDoux, J. E. (2000) J. Neurosci. 20, 8177-8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.English, J. D. & Sweatt, J. D. (1997) J. Biol. Chem. 272, 19103-19106. [DOI] [PubMed] [Google Scholar]
- 38.Davis, S., Vanhoutte, P., Pages, C., Caboche, J. & Laroche, S. (2000) J. Neurosci. 20, 4563-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang, Y. Y., Martin, K. C. & Kandel, E. R. (2000) J. Neurosci. 20, 6317-6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minichiello, L., Korte, M., Wolfer, D., Kuhn, R., Unsicker, K., Cestari, V., Rossi-Arnaud, C., Lipp, H. P., Bonhoeffer, T. & Klein, R. (1999) Neuron 24, 401-414. [DOI] [PubMed] [Google Scholar]
- 41.Perez Castro, C., Penalva, R., Paez Pereda, M., Renner, U., Reul, J. M., Stalla, G. K., Holsboer, F. & Arzt, E. (1999) Endocrinology 140, 690-697. [DOI] [PubMed] [Google Scholar]
- 42.Penalva, R. G., Flachskamm, C., Zimmermann, S., Wurst, W., Holsboer, F., Reul, J. M. & Linthorst, A. C. (2002) Neuroscience 109, 253-266. [DOI] [PubMed] [Google Scholar]
- 43.Paxinos, G. & Franklin, K. (2001) The Mouse Brain in Stereotaxic Coordinates (Academic, San Diego).
- 44.Arzt, E., Buric, R., Stelzer, G., Stalla, J., Sauer, J., Renner, U. & Stalla, G. K. (1993) Endocrinology 132, 459-467. [DOI] [PubMed] [Google Scholar]
- 45.Chan, R. K., Brown, E. R., Ericsson, A., Kovacs, K. J. & Sawchenko, P. E. (1993) J. Neurosci. 13, 5126-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cullinan, W. E., Herman, J. P., Battaglia, D. F., Akil, H. & Watson, S. J. (1995) Neuroscience 64, 477-505. [DOI] [PubMed] [Google Scholar]
- 47.Sananbenesi, F., Fischer, A., Schrick, C., Spiess, J. & Radulovic, J. (2002) Mol. Cell. Neurosci. 21, 463-476. [DOI] [PubMed] [Google Scholar]
- 48.Blank, T., Nijholt, I., Grammatopoulos, D. K., Randeva, H. S., Hillhouse, E. W. & Spiess, J. (2003) J. Neurosci. 23, 700-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roozendaal, B., Brunson, K. L., Holloway, B. L., McGaugh, J. L. & Baram, T. Z. (2002) Proc. Natl. Acad. Sci. USA 99, 13908-13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merali, Z., Michaud, D., McIntosh, J., Kent, P. & Anisman, H. (2003) Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 1201-1212. [DOI] [PubMed] [Google Scholar]
- 51.Swanson, L. W. & Petrovich, G. D. (1998) Trends Neurosci. 21, 323-331. [DOI] [PubMed] [Google Scholar]
- 52.Elliott-Hunt, C. R., Kazlauskaite, J., Wilde, G. J., Grammatopoulos, D. K. & Hillhouse, E. W. (2002) J. Neurochem. 80, 416-425. [DOI] [PubMed] [Google Scholar]
- 53.Radulovic, M., Hippel, C. & Spiess, J. (2003) J. Neurochem. 84, 1074-1085. [DOI] [PubMed] [Google Scholar]
- 54.Pearson, G., Robinson, F., Beers Gibson, T., Xu, B. E., Karandikar, M., Berman, K. & Cobb, M. H. (2001) Endocr. Rev. 22, 153-183. [DOI] [PubMed] [Google Scholar]
- 55.Mansi, J. A., Rivest, S. & Drolet, G. (1996) Endocrinology 137, 4619-4629. [DOI] [PubMed] [Google Scholar]
- 56.Chen, R. H., Sarnecki, C. & Blenis, J. (1992) Mol. Cell. Biol. 12, 915-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez, F. A., Seth, A., Raden, D. L., Bowman, D. S., Fay, F. S. & Davis, R. J. (1993) J. Cell Biol. 122, 1089-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lenormand, P., Sardet, C., Pages, G., L'Allemain, G., Brunet, A. & Pouyssegur, J. (1993) J. Cell Biol. 122, 1079-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sananbenesi, F., Fischer, A., Schrick, C., Spiess, J. & Radulovic, J. (2003) J. Neurosci. 23, 11436-11443. [DOI] [PMC free article] [PubMed] [Google Scholar]