Abstract
The adrenal cortex is a major stress organ in mammals that reacts rapidly to a multitude of external and internal stressors. Adrenocorticotropin (ACTH) is the main stimulator of the adrenal cortex, activating corticosteroid synthesis and secretion. We evaluated the mechanism of action of ACTH on adrenals of male rats, preserving the architecture of the gland in vitro. We demonstrated that both sodium nitroprusside (NP), a nitric oxide (NO) donor, and ACTH stimulate corticosterone release. NO mediated the acute response to ACTH because Nω-nitro-l-arginine methyl ester, a NO synthase inhibitor, and hemoglobin, a NO scavenger, blocked the stimulation of corticosterone release induced by ACTH. NP stimulated prostaglandin E release, which in turn stimulated corticosterone release from the adrenal. Additionally, indomethacin, which inhibits cyclooxygenase, and thereby, prostaglandin release, prevented corticosterone release from the adrenal induced by both NP and ACTH, demonstrating that prostaglandins mediate acute corticosterone release. Corticosterone content in adrenals after incubation with ACTH or NP was lower than in control glands, indicating that any de novo synthesis of corticosterone during this period was not sufficient to keep up with the release of the stored hormone. The release induced by ACTH or NP depleted the corticosterone content in the adrenal by ≈40% compared with the content of glands incubated in buffer. The mechanism of rapid release is as follows: NO produced by NO synthase activation by ACTH activates cyclooxygenase, which generates PGE2, which in turn releases corticosterone stored in microvesicles and other organelles.
Keywords: cyclooxygenase, indomethacin, nitric oxide synthase, Nω-nitro-l-arginine methyl ester
The polypeptide hormone adrenocorticotropin (ACTH) is the major regulator of steroid synthesis and secretion from the adrenal cortex, inducing an acute response within a few minutes. We hypothesize that this response results from the release of stored corticosterone, followed by mobilization of cholesterol from lipid stores to the inner mitochondrial membrane, where it is converted to pregnenolone. This pathway is followed by a series of enzymatic steps that yield the final product, corticosterone. Because release of stored hormone in most, if not all, glandular tissues occurs by exocytosis through vesicles, we hypothesize that the adrenal cortex is no different. Some of our experiments specifically addressed this question. If the hypothesis is correct, there should be measurable stored hormone in the gland that may be depleted upon stimulation. We hypothesized that the mechanism of release by ACTH lies in the activation of exocytosis by nitric oxide (NO). NO releases glandular products in many cases such as that of hypothalamic peptides, anterior pituitary hormones, adrenal medullary hormones, and saliva from salivary glands (1).
Chronic effects include the increase in the transcription of steroidogenic genes in the longer term (2). However, many clinical and experimental situations have brought evidence to light that there is dissociation between plasma ACTH concentrations and cortisol secretion (3, 4). Interestingly, one study showed corticosterone secretion to peak before the elevation of ACTH induced by immobilization stress (5), and that rat pups exposed to hypoxia exhibit ACTH-independent increased corticosterone release (6). Because the sympathetic nervous system responds earlier than the hypothalamus-pituitary-adrenal axis to stressors, corticosterone release before that of ACTH action may be due to earlier stimulation of the adrenal cortex via the splanchnic nerve, possibly also through NO release. Currently, it is generally accepted that adrenocortical steroidogenesis is stimulated not only by ACTH but also by the autonomic innervation of the adrenal gland (AG) (7). The splanchnic nerve may alter corticosterone release by means of the release of catecholamines and a variety of neuropeptides, neurotransmitters, eicosanoids, and cytokines (7-11).
Regulation of adrenal function by ACTH may involve NO, which has been identified as a major biological signaling molecule exerting both inter- and intracellular effects (12, 13). NO is generated by l-arginine oxidation in a complex enzymatic reaction catalyzed by a NO synthase (NOS) family found in many tissues, including the AG (14, 15). In addition, increasing evidence suggests NO involvement in steroid biosynthesis regulation (13, 16). Because endothelial and neuronal cells have a close anatomical proximity to steroidogenic cells in the AG, and these cells release NO, one study suggested that endothelial or neural NOS could be effective in regulating steroid release (13). Moreover, NO can also be generated within steroid-producing cells (15).
Concerning the mechanisms of NO action, the most accepted is its binding to iron in heme and non-heme iron-containing enzymes. The binding of NO to the heme group of soluble guanylyl cyclase, one of the main targets for the physiological effects of NO, alters the enzyme conformation, thus increasing its activity and causing it to convert GTP to cGMP. NO also reacts with several other metalloproteins such as the cytochrome P450 side-chain cleavage enzyme, which is essential for steroidogenic reactions (17). Another target for NO action is the heme group of the enzyme cyclooxygenase (COX) (18, 19). COX metabolizes free arachidonic acid (AA) to prostaglandins (PGs) and thromboxanes. There are two known COX isoforms. COX-1 is constitutively expressed, whereas COX-2 is strongly induced during inflammation. We and others have shown that NO stimulates the production of PGs from hypothalamic explants, an action caused by activation of COX by NO interacting with a ferrous iron-heme group of the enzyme in a similar way to its action in activating guanylyl cyclase (18, 19). In addition, AA and its metabolites regulate steroid production at the rate-limiting step of cholesterol transfer from the outer to the inner mitochondrial membrane (20-22). Steroidogenic acute regulatory (StAR) protein plays a critical role in this cholesterol transfer (23). Epinephrine, one of the main products of the adrenal medulla, probably stimulates StAR expression through cAMP production (24). There are controversial reports on the effects of NO and PGs on the steroidogenic pathway (13, 16, 17). All of these studies used dispersed cells, thereby eliminating the normal architecture of the gland. Consequently, the aim of this study was to investigate the role of NO and the participation of PGs in corticosterone release stimulated from rat hemi-AGs by ACTH in vitro.
Materials and Methods
Animals and Reagents. Animals. Male Wistar rats (250-300 g) purchased from the School of Pharmacy and Biochemistry of the University of Buenos Aires were used. The animals were fed laboratory chow and water ad libitum and were maintained under controlled conditions of light (lights on from 6 a.m. to 8 p.m.) and temperature (22°C ± 2°C). The animals were kept in group cages (six rats per cage) in our animal room for 10 days before the experiment. The experimental procedures reported here were approved by the Animal Care Committee of the Center of Pharmacological and Botanical Studies of the National Council for Research of Argentina and carried out in accordance with the Declaration of Helsinki.
Chemicals. ACTH was obtained in lyophilized form from Elea Laboratories (Buenos Aires). Nω-nitro-l-arginine methyl ester (l-NAME), prostaglandin E2 standard and antiserum, corticosterone standard and antiserum, sodium nitroprusside (NP), rat hemoglobin (Hb), indomethacin (Indo), forskolin (FRSK), and N6-monobutyryladenosine 3′,5′cyclic monophosphate (mbcAMP; sodium salt) were purchased from Sigma-Aldrich (St. Louis). [1,2,6,7-3H] Corticosterone and [3H] PGE2 were purchased from NEN Life Science Products (Boston).
In Vitro Studies. The animals were decapitated, and their AGs were excised and freed of pericapsular fat. Each AG was bisected; both halves were placed in the same tube and preincubated for 15 min in a Dubnoff metabolic shaker (50 cycles per min in an atmosphere of 95% O2/5% CO2) at 37°C in Krebs-Ringer bicarbonate buffer (pH 7.4) containing 0.1% glucose. Then, the media were replaced with fresh medium or medium containing the compounds to be tested, and the tissues were incubated for another 15 min or 30 min. Then, the media and tissues were removed, processed as described below, and stored at -20°C until determinations were made.
The incubation conditions were (for 15 min): (i) Krebs-Ringer buffer (control group); (ii) NP (300-1,200 μM) as NO donor; (iii) NP plus Hb (2 μg/ml) as scavenger of NO; (iv) Indo (10-3 M), a COX inhibitor; (v) Indo plus NP; and (vi) PGE2 (10-5, 10-7, 10-9, and 10-11 M, respectively). The incubation conditions were (for 30 min): (i) Krebs-Ringer buffer (control group); (ii)ACTH (10-9 M); (iii) l-NAME (10-3 M), a specific inhibitor of NOS; (iv) ACTH plus l-NAME; (v) ACTH plus Hb (40 μg/ml due to longer incubation time); (vi) ACTH plus Indo; (vii) Indo (10-3 M); (viii) mbcAMP (10-4 M), a membrane-permeable analogue of cAMP; and (ix) FRSK (7.6 × 10-5 M), an adenylyl cyclase activator.
Determination of Corticosterone. The corticosterone contained in the tissue and released into the incubation media were measured by RIA (25). The extraction of corticosterone from the tissue was made by homogenizing the AGs in 0.5 ml of saline (NaCl, 0.9%), centrifuging at 10,000 × g, and adding three times 1.5 ml of methylene chloride. The extracts were dried in a Speedvac (Labconco, Kansas City, MO), and the pellets were stored at -20°C until measured by RIA. For the determination of corticosterone, the pellets were recovered with RIA buffer [0.05 M Tris·HCl containing 0.1 M NaCl, 0.1% BSA, and 0.1% sodium azide (pH 8)]. The tissue and media samples were incubated with rabbit anti-corticosterone for 30 min at room temperature. Then, [3H]corticosterone was added as a tracer and incubated for 1 h at 37°C. The reaction was stopped by the addition of cold dextran-coated charcoal suspension and after the incubation for 10 min at 0°C; the tubes were centrifuged at 2,000 × g for 15 min at 4°C. Scintillation mixture was added to the supernatant, and the amount of radioactivity was determined in a beta counter. The sensitivity of the assay was 30 pg per tube.
Determination of PGE. The incubation media were acidified with 1 M HCl (pH 3); the PGE was then extracted three times with one volume of ethyl acetate. The extracts were dried in a Speedvac at room temperature. These residues were stored at -20°C until measured by RIA. For the determination of PGE, the residues were resuspended with RIA buffer afterward; the Sigma antiserum was used as described (26). The sensitivity of the assay was 12.5 pg per tube. The crossreactivity of PGE2 and PGE1 was 100%, but the crossreactivity of other PGs was <0.1%. Therefore, the results were expressed as PGE. The intra- and interassay coefficients of variation for PGE were 8.2% and 12%, respectively.
Electron Microscopy. To verify the possible evidence for some mechanism of steroid storage, the ultrastructural analysis of AGs from control rats and those subjected to acute restraint stress in cylindrical tubes (duration of 30 min) was performed. We found that plasma ACTH was now increased by 100% (P < 0.001) and corticosterone was increased by 400% (P < 0.001) as compared with freely moving rats. We did not use incubated glands because the slices obtained after incubation were not suitable for ultrastructural studies. AGs (n = 3 per group) were fixed in 2% formaldehyde and 2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.3. Tissue slices were postfixed for 90 min (2% OsO4 in 0.1 M cacodylate buffer, pH 7.3), dehydrated in ethanol, and embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate, and were examined at 80 kV in a CM 10 electron microscope (Philips, Eindhoven, The Netherlands).
Statistics. Data were expressed as means ± SEM (n = 10). Comparisons between groups were performed by using one-way ANOVA followed by the Student-Newman-Keuls multiple comparison test for unequal replicates. All analyses were conducted with prism 3.0 software (GraphPad, San Diego). Differences were considered significant at P ≤ 0.05.
Results
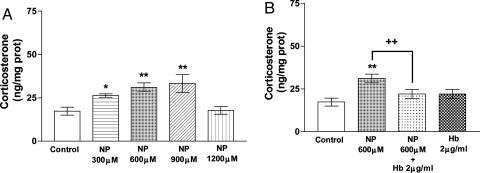
Effect of NO on Corticosterone Release from AG. NP (300-900 μM), which spontaneously releases NO in solution, significantly dose-dependently stimulated corticosterone release from AGs during an incubation of 15 min (P < 0.05 and P < 0.01, respectively), nearly doubling the release at a 900-μM dose. Corticosterone release was not increased at the highest NP dose studied (1,200 μM) (Fig. 1A). The increase in corticosterone release induced by 600 μM NP was ≈80% and was abolished in the presence of Hb (2 μg/ml), a NO scavenger, which had no effect on its own (Fig. 1B).
Fig. 1.
Effect of various concentrations of NP (A) and Hb (B) on corticosterone release by AGs incubated for 15 min in vitro. Values represent means ± SEM for 8-10 AGs per group. Data were evaluated by ANOVA, followed by a Newman-Keuls posttest. *, P < 0.05; **, P < 0.01 vs. control group; ++, P < 0.01 vs. NP group.
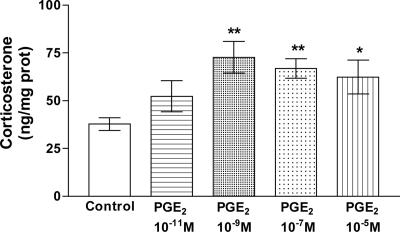
Effect of PGE2 on Corticosterone Release from AGs. To investigate the hypothesized effect of PGE2 on corticosterone release, we incubated the AGs for 15 min at various concentrations of PGE2 (10-11 M to 10-5 M). PGE2 significantly stimulated corticosterone release at the doses tested (P < 0.05 and P < 0.01, respectively) except at the lowest dose (10-11 M), which increased corticosterone, but the release was significantly less than that of the next dose tested (10-9 M) (Fig. 2). Release peaked at the dose of 10-9 M, which doubled the release.
Fig. 2.
Effect of various concentrations of PGE2 on corticosterone release by AGs incubated for 15 min in vitro. Values represent means ± SEM for 8-10 AGs per group. Data were evaluated by ANOVA, followed by a Newman-Keuls posttest. *, P < 0.05; **, P < 0.01 vs. control group.
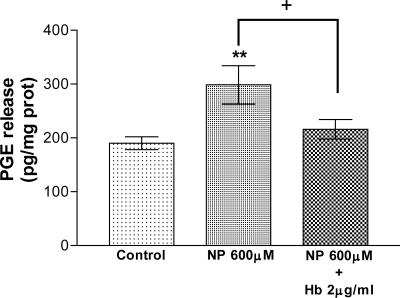
Effect of NO on PGE Release. To determine whether PGE participates in the effect of NO on corticosterone release, we first examined the effect of NO, released by NP, on PGE release. In effect, NP (600 μM) increased the release of PGE from AGs (P < 0.01) during an incubation period of 15 min. This effect was abolished by Hb (2 μg/ml), the scavenger of NO (P < 0.05) (Fig. 3).
Fig. 3.
Effect of NP and Hb on PGE release by AGs incubated for 15 min in vitro. Values represent means ± SEM for 8-10 AGs per group. Data were evaluated by ANOVA, followed by a Newman-Keuls posttest. **, P < 0.01 vs. control group; +, P < 0.05 vs. NP group.
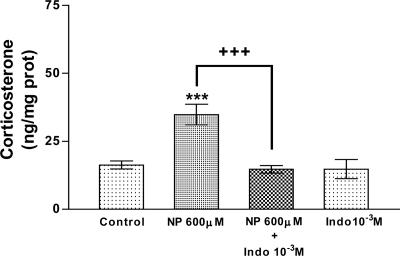
To study the participation of PGs on the effect of NO on corticosterone release in more depth, we incubated the AGs in the presence of both NP and Indo, an inhibitor of COX. Indo (10-3 M) completely blocked the stimulatory effect of NP (600 μM) on corticosterone release (P < 0.001) but had no effect on its own (Fig. 4).
Fig. 4.
Effect of Indo on corticosterone release stimulated by NP and Indo alone in AGs incubated for 15 min in vitro. Values represent means ± SEM for 8-10 AGs per group. Data were evaluated by ANOVA, followed by a Newman-Keuls posttest. ***, P < 0.001 vs. control group; +++, P < 0.001 vs. NP group.
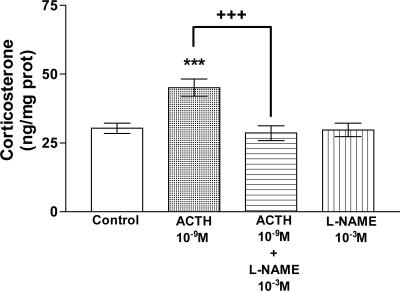
Effect of ACTH on Corticosterone Release from AGs. As we expected, ACTH (10-9 M) highly significantly (P < 0.001) stimulated (50%) the release of corticosterone over 30 min of incubation. l-NAME (10-3 M), a NOS inhibitor, abolished this stimulation (P < 0.001) but had no effect at it own (Fig. 5).
Fig. 5.
Effect of ACTH and l-NAME on corticosterone release in AGs incubated for 30 min in vitro. Values represent means ± SEM for 8-10 AGs per group. Data were evaluated by ANOVA, followed by a Newman-Keuls posttest. ***, P < 0.001 vs. control group; +++, P < 0.001 vs. ACTH group.
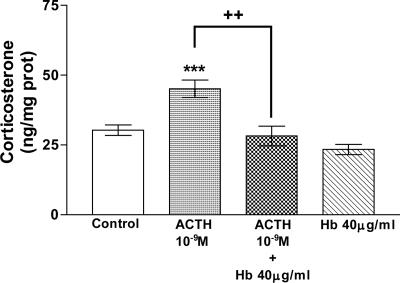
The participation of NO in corticosterone release stimulated by ACTH was confirmed by using Hb (40 μg/ml), the NO scavenger. Hb also abolished (P < 0.01) ACTH-induced stimulation of corticosterone release (Fig. 6).
Fig. 6.
Effect of ACTH and Hb on corticosterone release in AGs incubated for 30 min in vitro. Values represent means ± SEM for 8-10 AGs per group. Data were evaluated by ANOVA, followed by a Newman-Keuls posttest. ***, P < 0.001 vs. control group; ++, P < 0.01 vs. ACTH group.
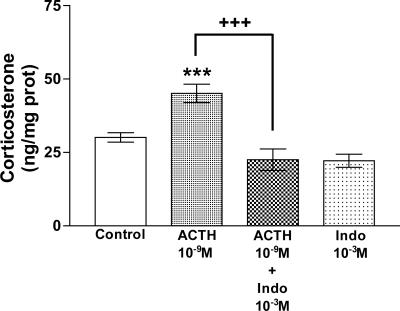
We incubated tissues with Indo to investigate the participation of PGs in the effect of ACTH on corticosterone release. Indo (10-3 M) blocked the stimulation induced by ACTH (P < 0.001) on corticosterone release (Fig. 7).
Fig. 7.
Effect of ACTH and Indo on corticosterone release in AGs incubated for 30 min in vitro. Values represent means ± SEM for 8-10 AGs per group. Data were evaluated by ANOVA, followed by a Newman-Keuls posttest. ***, P < 0.001 vs. control group; +++, P < 0.001 vs. ACTH group.
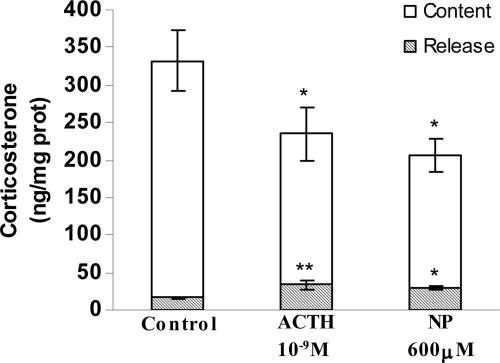
To study our hypothesis that preformed corticosterone exists in the AG and can be released, we measured the content and release of corticosterone after 30 min of stimulation with ACTH (10-9 M) and NP (600 μM). Both ACTH and NP increased the release by ≈50% and decreased corticosterone content by 40% compared with control (Krebs-Ringer buffer only) values (Fig. 8).
Fig. 8.
Effect of ACTH and NP on corticosterone content (white columns) and release (gray columns) in AGs incubated for 30 min in vitro. Values represent means ± SEM for seven to eight AGs per group. Data were evaluated by ANOVA, followed by a Newman-Keuls posttest. **, P < 0.01; *, P < 0.05 vs. respective control group.
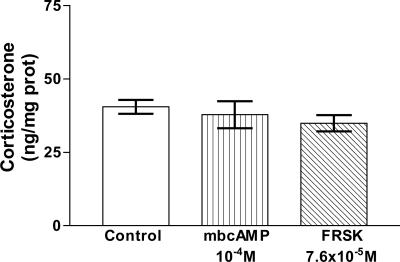
Effect of cAMP on Corticosterone Release. To evaluate whether cAMP is involved in the fast mechanism of corticosterone release as we proposed, the AGs were incubated with an analogue of cAMP, membrane permeable and resistant to hydrolysis by phosphodiesterases, mbcAMP (10-4 M), and an activator of adenylyl cyclase, FRSK (7.6 × 10-5 M). Neither of these substances modified corticosterone release from AGs at 30 min of incubation (Fig. 9).
Fig. 9.
Effect of mbcAMP and FRSK on corticosterone release in AGs incubated for 30 min in vitro. Values represent means ± SEM for 8-10 AGs per group. Data were evaluated by ANOVA, followed by a Newman-Keuls posttest.
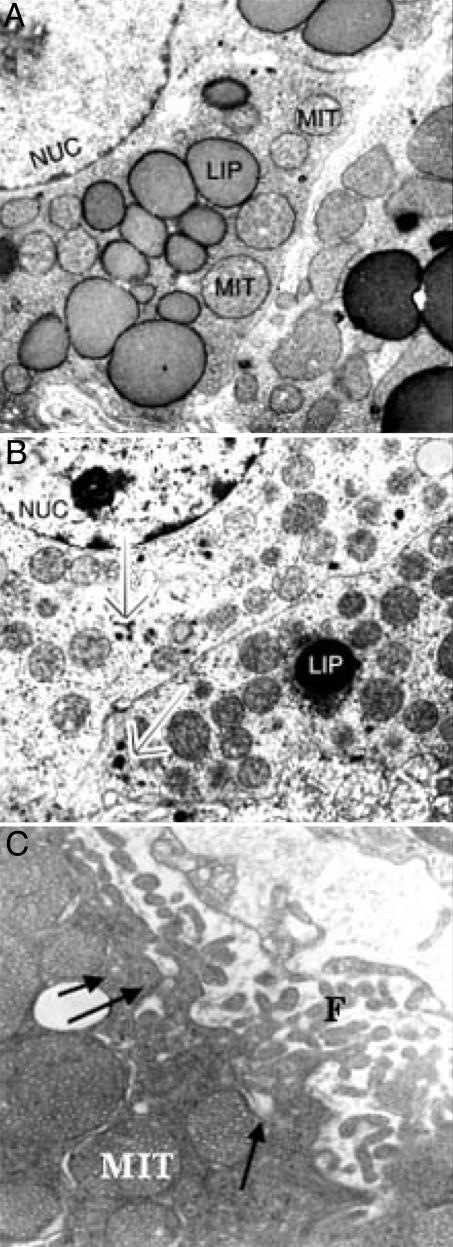
Ultrastructural Analysis. We found that the most prominent organelles in steroid-producing adrenocortical cells in control animals were round mitochondria with characteristic tubulovesicular internal membranes and numerous lipid-storing droplets constituting the substrates for steroidogenesis (Fig. 10A). There was a conspicuous increase in vascularization of the adrenal tissue after restraint stress (duration of 30 min) (Fig. 10B). After inducing stress, adrenocortical cells demonstrated a high number of mitochondria with dense internal vesicular membranes and ample smooth endoplasmic reticulum. There was a marked increase in filopodia and an increase in microcytotic vesicles along the plasma membranes (Fig. 10C). Adrenocortical cells also demonstrated sporadic single secretory granular structures reminiscent of secretory granules found in neuroendocrine cells (Fig. 10B). However, there was no significant increase in these granules in stressed animals compared with the controls, although endothelial cells were frequently found to be in direct contact with the adrenal cortical cells in stressed animals (Fig. 10C).
Fig. 10.
Electron micrographs of adrenocortical cells. (A) In the unstimulated state, adrenal cortical cells demonstrate ample liposomes (LIP), tubular vesicular mitochondria (MIT), and nucleus (NUC). (B) Activated adrenocortical cells show single clusters of secretory granules (arrows) and a decrease in liposomes. (C) The plasma membranes form extensive filopodia (F) and are in direct contact with endothelial cells. There is an increase in microcytotic vesicles (arrows) along the plasma membranes in stress-treated animals.
Discussion
NO has been postulated as an autocrine/paracrine regulator of steroidogenesis in several tissues, with effects on adrenal steroid secretion (3, 27, 28). Our present results indicate that NO stimulates corticosterone release from adrenals in vitro. These results are in concordance with those of previous authors (16), demonstrating that l-arginine, the substrate of NO, caused a dose-dependent increase in corticosterone secretion. We believe that NO leads to a rapid release of stored corticosterone from the adrenals, because release at 15 min was nearly as great as at 30 min. In addition, mbcAMP or FRSK, which increase endogenous cAMP, a known stimulator of corticosterone biosynthesis, had no effect on corticosterone release at 30 min. Also, corticosterone content measured after 30 min of incubation with ACTH or NP was lower than control values, indicating that any de novo synthesis of corticosterone was not sufficient to keep up with release over this period. The release induced by ACTH or NP depleted the corticosterone content in the adrenal by ≈40% compared with glands incubated in buffer. Our ultrastructural analysis suggests some form of storage of steroids, allowing an acute release of glucocorticoids after exposure to stress. However, morphological analysis did not allow any definitive conclusions on the mechanism allowing the release of prestored steroid molecules. Interestingly, single or small clusters of secretory granules characteristic of neuroendocrine cells with a dense core and a typical halo occur in the cytoplasm of the mesodermally derived adrenocortical cells. However, there was no significant increase in this type of granule after acute stimulation, suggesting that they were either rapidly released or contained other peptide molecules not influenced by stress. On the other hand, there was an increase of microcytotic vesicles along the markedly increased cell membranes. These filopodia frequently formed invaginations and a labyrinth in close contact with mitochondria, lipid droplets, and microcytotic vesicles, providing the structural prerequisite for a rapid release of a prestored pool of steroids.
NO acts primarily by two pathways: activation of guanylyl cyclase to yield cGMP, and activation of COX to produce PGs. Inhibition of COX by Indo blocked NP- and ACTH-induced corticosterone release. The effect of NP and ACTH appeared to be mediated by PGE2 because PGE2 released corticosterone with a minimum effective dose of 10-9 M, whereas the corticosterone releasing action of NP and ACTH was blocked by the COX inhibitor, Indo. We believe that NO causes the acute release of corticosterone due to the activation of NOS by ACTH. NO activated COX that generates PGE2, which in turn releases corticosterone from stored microvesicles that have not previously been visualized. The mechanism by which PGE2 causes exocytocis remains to be determined.
This finding leaves the role of NO in biosynthesis to be a subject of conjecture. The results from isolated cells and cells under various experimental conditions differ from ours in perfusion systems and intact tissue preparations (17, 29). Cell-cell interactions are well known in playing a potentially crucial role in the proper functioning of endocrine glands; in fact, two studies have recently discovered that interactions between chromaffin and cortical cells are important in adrenocortical steroidogenesis (7, 24).
The corticosterone biosynthesis pathway has been studied extensively, and ACTH acts on this pathway. Because mbcAMP and FRSK failed to alter corticosterone release in our system, whereas mbcAMP and FRSK would have stimulated synthesis followed by release of corticosterone, presumably with a longer time of incubation, we believe we are studying corticosterone release almost exclusively. Most glandular products are stored in secretory granules. The fusion between granules and cell membrane with extrusion of the hormone is often mediated by NO through its activation of COX and release of PGE2. We hypothesize that this is the mechanism for the ACTH's acute release action. This mechanism may also apply to other steroid-releasing tissues. Current attention has been focused on biosynthesis of corticosteroids postulated to diffuse out of the cell down a concentration gradient. The release would not be rapidly controlled in such a mechanism and would continue constitutively. Recent in vitro studies (30) have demonstrated the intracellular retention of steroids against a concentration gradient at the plasma membrane. The last few years have witnessed the description of a steroid transport mechanism involving organic anion transport. Because ACTH-stimulated corticosterone release was partially reduced by an organic anion transporter inhibitor, this mechanism could also be involved in steroid secretion from the AG other than that which is proposed here (31). The mechanism of organic acid change is of utmost importance to the transporter of corticosterone in facilitating the mobilization and fusion of the vesicles with the cell membrane and extrusion of corticosteroids in the adrenal. In any case, both NO and PGs are involved in these steps.
We have demonstrated that NO is involved in the acute response to ACTH because the NOS inhibitor l-NAME blocked the stimulation of corticosterone release induced by ACTH. Hb, a NO scavenger, also prevented this effect. NO is known to bind to the COX heme group, activating the synthesis of PGs in several tissues. We have demonstrated that NP stimulates PGE release from AGs within a short period (15 or 30 min), and that PGE2 stimulates corticosterone release from the gland. These results agree with previous work (10, 32, 33) on mammalian and frog adrenocortical tissues and cells, indicating that PGs can directly stimulate corticosteroidogenesis. In addition, we have shown that Indo, which inhibits COX, prevents corticosterone release from AGs induced by both NP and ACTH, demonstrating that PGs mediate acute corticosterone release.
Steroid hormone biosynthesis in the adrenal cortex involves two distinct responses that are temporally separated. A rapid, acute release as described here, followed by mobilization of cholesterol from lipids stored in the inner mitochondrial membrane, leading to corticosteroid neosynthesis. In the longer term, the chronic effect is a corresponding increase in steroidogenic gene transcription (2, 34).
StAR protein has recently been shown to play a critical role in trophic hormone-stimulated steroidogenesis by facilitating cholesterol transfer to the inner mitochondrial membrane; this is the rate-limiting step in steroidogenesis. Cholesterol is then converted to pregnenolone by the P450 side-chain cleavage enzyme (35-37). AA and its metabolites play an essential role acting by regulating the rate-limiting step of steroidogenesis in different steroidogenic cells through its regulation of StAR protein expression in combination with cAMP (21, 38). ACTH-induced steroidogenesis in the gland is mediated by Ca2+, cAMP, and AA metabolites. An increase in intracellular Ca2+ concentration enhances the availability of cholesterol to the cytochrome P450 side-chain cleavage enzyme. Chiyo et al. (39) have demonstrated that corticosterone specifically enhances Ca2+ signals and pregnenolone synthesis in adrenocortical cells in response to ACTH stimuli. Thus, this early corticosterone release may be necessary for the adrenal cortex's optimal response to ACTH in a nongenomic way by acting in an autocrine fashion (39).
In summary, our results provide evidence that NO and PGs mediate rapid ACTH-induced corticosterone release from stored vesicles and other organelles in the AG.
Acknowledgments
We thank Mrs. Isabel Farias (Instituto de Neurobiología, Buenos Aires) for technical microscopy assistance. This work was supported by Agencia Nacional de Promoción Cientifica, Téchnológica y de Innovación Grant PICT 03/14264, BID 1201 OC-AR.
Author contributions: C.E.M., S.M.M., and V.R. designed research; J.F.-S., A.D.L., J.P.P., C.d.l.C., R.F., and S.R.B. performed research; C.E.M., S.R.B., S.M.M., and V.R. analyzed data; and C.E.M., S.R.B., S.M.M., and V.R. wrote the paper.
Abbreviations: ACTH, adrenocorticotropin; AG, adrenal gland; NP, sodium nitroprusside;l-NAME, Nω-nitro-l-arginine methyl ester; NOS, NO synthase; Hb, hemoglobin; COX, cyclooxygenase; Indo, indomethacin; PG, prostaglandin; mbcAMP, N6-monobutyryladenosine 3′,5′cyclic monophosphate (sodium salt); FRSK, forskolin; StAR, steroidogenic acute regulatory protein; AA, arachidonic acid.
References
- 1.Machado, J. D., Segura, F., Brioso, M. A. & Borges, R. (2000) J. Biol. Chem. 257, 20274-20279. [DOI] [PubMed] [Google Scholar]
- 2.Sewer, M. B. & Waterman, M. R. (2003) Microsc. Res. Tech. 61, 300-307. [DOI] [PubMed] [Google Scholar]
- 3.Bornstein, S. R. & Chrousos, G. P. (1999) J. Clin. Endocrinol. Metab. 84, 1729-1736. [DOI] [PubMed] [Google Scholar]
- 4.Raff, H., Hong, J. J., Oaks, M. K. & Widmaier, E. P. (2003) Am. J. Physiol. 284, 78-85. [DOI] [PubMed] [Google Scholar]
- 5.Pignatelli, D., Pinto, P., Azevedo, M. E., Magalhaes, M. M. & Magalhaes, M. C. (1996) Endocr. Res. 22, 445-451. [DOI] [PubMed] [Google Scholar]
- 6.Raff, H., Lee, J. J., Widmaier, E. P., Oaks, M. K. & Engeland, W. C. (2004) Endocrinology 145, 79-86. [DOI] [PubMed] [Google Scholar]
- 7.Ehrhart-Bornstein, M., Hinson, J. P., Bornstein, S. R., Scherbaum, W. W. & Vinson, G. P. (1998) Endocrinol. Rev. 19, 101-143. [DOI] [PubMed] [Google Scholar]
- 8.O′Connell, N. A., Kumar, A., Chatzipanteli, K., Mohan, A., Agarwal, R. K., Head, C., Bornstein, S. R., Abou-Samra, A. B. & Gwosdow, A. R. (1994) Endocrinology 135, 460-467. [DOI] [PubMed] [Google Scholar]
- 9.Päth, G., Bornstein, S. R., Ehrhart-Bornstein, M. & Scherbaum, W. A. (1997) J. Clin. Endocrinol. Metab. 82, 2343-2349. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki, T., Kimoto, T., Higuchi, K., Ohta, Y., Kawato, S. & Kominami, S. (1998) Endocrinology 139, 4765-4771. [DOI] [PubMed] [Google Scholar]
- 11.Shepherd, S. P. & Holzwarth, M. A. (2001) Am. J. Physiol. 280, 61-71. [DOI] [PubMed] [Google Scholar]
- 12.Nakayama, T., Izumi, Y., Soma, M. & Kanmatsuse, K. (1996) Endocr. J. 43, 157-162. [DOI] [PubMed] [Google Scholar]
- 13.Hanke, C. J., O′Brien, T., Pritchard, K. A. & Campbell, W. B. (2000) Hypertension 35, 324-328. [DOI] [PubMed] [Google Scholar]
- 14.Palacios, M., Knowles, R. G., Palmer, R. M. & Moncada, S. (1989) Biochem. Biophys. Res. Commun. 165, 802-809. [DOI] [PubMed] [Google Scholar]
- 15.Cymeryng, C. B., Lotto, S. P., Colonia, C., Finkielstein, C., Pomeriniec, Y., Grión, N., Gadda, L., Maloberti, P. & Podestá, E. J. (2002) Endocrinology 143, 1235-1242. [DOI] [PubMed] [Google Scholar]
- 16.Cameron, L. A. & Hinson, J. P. (1993) J. Endocrinol. 139, 415-423. [DOI] [PubMed] [Google Scholar]
- 17.Cymeryng, C. B., Dada, L. A., Colonia, C., Mendez, A. F. & Podestá, E. J. (1999) Endocrinology 140, 2962-2967. [DOI] [PubMed] [Google Scholar]
- 18.Rettori, V., Gimeno, M., Lyson, K. & McCann, S. (1992) Proc. Natl. Acad. Sci. USA 89, 11543-11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvemini, D., Misko, T. P., Masferrer, J. L., Seibert, K., Curie, M. G. & Needleman, P. (1993) Proc. Natl. Acad. Sci. USA 90, 7240-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang, X. J., Walsh, L. P., Reinhart, A. J. & Stocco, D. M. (2000) J. Biol. Chem. 275, 20204-20209. [DOI] [PubMed] [Google Scholar]
- 21.Wang, X. J., Dyson, M. T., Mondillo, C., Patrignani, Z., Pignataro, O. & Stocco, D. M. (2002) Mol. Cell. Endocrinol. 188, 55-63. [DOI] [PubMed] [Google Scholar]
- 22.Wang, X. J., Dyson, M. T., Jo, Y., Eubank, D. W. & Stocco, D. M. (2003) J. Steroid Biochem. Mol. Biol. 85, 159-166. [DOI] [PubMed] [Google Scholar]
- 23.Clark, B. J., Wells, J., King, S. R. & Stocco, D. M. (1994) J. Biol. Chem. 269, 28314-28322. [PubMed] [Google Scholar]
- 24.Haidan, A., Bornstein, S. R., Liu, Z., Walsh, L. P., Stocco, D. M. & Ehrhart-Bornstein, M. (2000) Mol. Cell. Endocrinol. 165, 25-32. [DOI] [PubMed] [Google Scholar]
- 25.Etches, R. J. (1976) Steroids 28, 763-773. [DOI] [PubMed] [Google Scholar]
- 26.Mohn, C., Lomniczi, A., Faletti, A., Scorticati, C., Elverdin, J. C., McCann, S. M. & Rettori, V. (2001) Neuroimmunomodulation 9, 276-285. [DOI] [PubMed] [Google Scholar]
- 27.Adams, M. L., Nock, B., Truong, R. & Cicero, T. J. (1992) Life Sci. 50, 35-40. [DOI] [PubMed] [Google Scholar]
- 28.Riquelme, R. A., Sánchez, G., Liberona, L., Sanhueza, E. M., Giussani, D. A., Blanco, A. E., Hanson, M. A. & Lianos, A. J. (2002) J. Physiol. 544, 267-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cymeryng, C. B., Dada, L. A. & Podestá, E. J. (1998) J. Endocrinol. 158, 197-203. [DOI] [PubMed] [Google Scholar]
- 30.Beéry, E., Middel, P., Bahn, A., Willenberg, H. S., Hagos, Y., Koepsell, H., Bornstein S. R., Müller, G. A., Burckhardt, G. & Steffgen, J. (2003) Endocrinology 144, 4519-4526. [DOI] [PubMed] [Google Scholar]
- 31.Steffgen, J, Rohrbach, S., Beéry, E., Ersoy, D., Jarry, H., Mettenl, M., Bornstein S. R., Müller, G. A. & Burckhardt, G. (1999) Cell. Physiol. Biochem. 9, 72-80. [DOI] [PubMed] [Google Scholar]
- 32.Kudo, M., Kudo, T. & Matsuki, A. (1991) Masui 40, 1819-1824. [PubMed] [Google Scholar]
- 33.Kocsis, J. F., Rinkardt, N. E., Satterlee, D. G., Weber, H. & Garsia, R. V. (1999) Gen. Comp. Endocrinol. 115, 132-142. [DOI] [PubMed] [Google Scholar]
- 34.Sewer, M. B. & Waterman, M. R. (2001) Rev. Endocr. Metab. Disord. 2, 269-274. [DOI] [PubMed] [Google Scholar]
- 35.Wang, X., Liu, Z., Eimerl, S., Timberg, R., Weiss, A. M., Orly, J. & Stocco, D. M. (1998) Endocrinology 139, 3903-3912. [DOI] [PubMed] [Google Scholar]
- 36.Stocco, D. M. (2000) Biochim. Biophys. Acta 1486, 184-197. [DOI] [PubMed] [Google Scholar]
- 37.Stocco, D. M. (2001) Annu. Rev. Physiol. 63, 193-213. [DOI] [PubMed] [Google Scholar]
- 38.Csukas, S., Hanke, C. J., Rewolinski, D. & Campbell, W. B. (1998) Hypertension 31, 575-581. [DOI] [PubMed] [Google Scholar]
- 39.Chiyo, T., Yamazaki, T., Aoshika, K., Kominami, S. & Ohta, Y. (2003) Endocrinology 144, 3376-3381. [DOI] [PubMed] [Google Scholar]