Abstract
Background and Aims
Due to the COVID‐19 pandemic, a precise and reliable diagnosis of this disease is critical. The use of clinical decision support systems (CDSS) can help facilitate the diagnosis of COVID‐19. This scoping review aimed to investigate the role of CDSS in diagnosing COVID‐19.
Methods
We searched four databases (Web of Science, PubMed, Scopus, and Embase) using three groups of keywords related to CDSS, COVID‐19, and diagnosis. To collect data from studies, we utilized a data extraction form that consisted of eight fields. Three researchers selected relevant articles and extracted data using a data collection form. To resolve any disagreements, we consulted with a fourth researcher.
Results
A search of the databases retrieved 2199 articles, of which 68 were included in this review after removing duplicates and irrelevant articles. The studies used nonknowledge‐based CDSS (n = 52) and knowledge‐based CDSS (n = 16). Convolutional Neural Networks (CNN) (n = 33) and Support Vector Machine (SVM) (n = 8) were employed to design the CDSS in most of the studies. Accuracy (n = 43) and sensitivity (n = 35) were the most common metrics for evaluating CDSS.
Conclusion
CDSS for COVID‐19 diagnosis have been developed mainly through machine learning (ML) methods. The greater use of these techniques can be due to their availability of public data sets about chest imaging. Although these studies indicate high accuracy for CDSS based on ML, their novelty and data set biases raise questions about replacing these systems as clinician assistants in decision‐making. Further studies are needed to improve and compare the robustness and reliability of nonknowledge‐based and knowledge‐based CDSS in COVID‐19 diagnosis.
Keywords: clinical, computer‐assisted decision‐making, COVID‐19, decision support systems, intelligent clinical decision support system (ICDSS), medical diagnosis
1. INTRODUCTION
COVID‐19 is a respiratory disease developed by the SARS‐CoV‐2 virus. 1 Fever, fatigue, sore throat, and dry cough are the most common manifestations of this disease. 2 Other respiratory illnesses, such as influenza and respiratory syncytial virus, can also cause these symptoms and contribute to the difficulty in controlling the outbreak. 3 According to the World Health Organization (WHO), as of November 22, 2023, the coronavirus has infected 772,166,517 people worldwide and caused 6,981,263 deaths. 4 This outbreak has presented substantial challenges in delivering affordable and high‐quality healthcare services to a growing number of patients. 5 Strategies to prevent and control COVID‐19 include early diagnosis, patient isolation, contact monitoring, quarantine, and vaccination. 6
Several methods are used to diagnose COVID‐19, including clinical symptoms, epidemiological history, real‐time polymerase chain reaction (RT‐PCR) tests, chest computerized tomography (CT) scans, X‐ray imaging, enzyme‐linked immunosorbent assay (ELISA), biosensors, and point‐of‐care testing (POCT). 7 , 8 , 9 , 10 , 11 , 12 Currently, the RT‐PCR test for COVID‐19 confirmation is expensive, manual, and complex. 13 However, it has been shown to have high rates of false positives or negatives, which makes it unreliable for detection. 14 Also, the RT‐PCR test for COVID‐19 confirmation is an expensive, manual, and complex approach. 13 In some healthcare settings, commercial test kits, swabs, PCR machines, or their expertise may be less available. 13 , 15 Additionally, CT scans have been found to have high rates of false negatives. 14 This is why combining diagnostic methods could improve COVID‐19 detection accuracy.
Clinical decision support systems (CDSS) are helpful methods for healthcare providers in clinical decision‐making and the early screening of patients. 16 They integrate various information such as characteristics of individual patients, radiological images, clinical examination, and clinical guidelines and provide patient‐specific recommendations or suggestions. 16
CDSS may include artificial intelligence (AI) methodologies for assisting in quick and accurate medical diagnoses. 17 , 18 , 19 In this case, intelligent clinical decision support systems (ICDSS) are created. The CDSS based on AI are classified into two types: (1) the ICDSS based on expert system (ES) and (2) the ICDSS based on machine learning (ML). 20 , 21
The ICDSS based on ES or knowledge‐based CDSS aim to automate diagnosing COVID‐19, typically performed by medical experts. 20 , 21 These systems primarily consist of a knowledge base that contains medical expertise, an inference engine that uses the knowledge base to generate a diagnosis for the patient, and a way to communicate to the user (input and output). 20 , 21 The ICDSS based on ML or nonknowledge‐based CDSS learn to solve human problems by simulating human learning on a computer. 20 , 22 ML is the ability of machines to find patterns and learn hidden knowledge from large data sets using analytical techniques. 20 , 22 , 23 The goal is to teach automated techniques to classify and cluster data, learn behavior, generate patterns, and predict future actions using decision support systems. 20 , 22 , 23 Generally, ML can be divided into supervised and unsupervised learning. 20 , 22 , 23 The supervised methods are commonly used for disease prediction and include regression, Support Vector Machine (SVM), Random Forest (RF), Naive Bayes (NB), K‐Nearest Neighbor (KNN), Decision Tree (DT), and Artificial Neural Network (ANN). 20 , 22 , 23 A type of ML is deep learning (DL), which uses multiple‐layer artificial neural networks. 20 , 22 , 23 One of the most widely used subsets of DL is the Convolutional Neural Network (CNN), which allows the system to learn data representation. 20 , 22 , 23
Previous review studies have examined the impact of AI or ML models in screening, diagnostics, and prediction of COVID‐19, 24 , 25 , 26 , 27 , 28 ML models for diagnosing infectious diseases, 23 CDSS based on AI for early recognition of respiratory infections, 29 and CNN for the diagnosis and prognosis of COVID‐19. 30 Moreover, there is little understanding of the corresponding techniques that explain the use of different types of CDSS to assist in diagnosing COVID‐19. To our knowledge, no comprehensive review exists on the methods for developing CDSS for COVID‐19 diagnosis. Given the importance of accurate COVID‐19 diagnosis, this scoping review aimed to (1) review the types of CDSS to assist in COVID‐19 diagnosis and (2) investigate metrics for evaluating the performance accuracy of CDSS in the diagnosis process.
2. METHODS
2.1. Search strategy
The current study was a scoping review study reported based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guideline extension for scoping reviews (PRISMA‐ScR). 31 We conducted searches in the Scopus, PubMed, Web of Science, and Embase databases without time limitation up to September 2023 to identify relevant articles. The search strategy involved three sets of keywords. Synonymous words within each set were combined using the OR operator. Subsequently, the three sets of keywords were combined using the AND operator. The first set of keywords pertained to COVID‐19 (Group A), the second set was related to CDSS (Group B), and the third set was implied to diagnose (Group C) (Appendix 1). Several synonyms and spelling variations for each search term were used to capture as many articles as possible. 32 , 33
2.2. Eligibility criteria
In this study, articles were included that (1) published in English, (2) were about any CDSS, (3) reported on the detection or diagnosis of COVID‐19, (4) used various types of CDSS to assist in the diagnosis of COVID‐19. Books, book chapters, letters to the editors, and conference article abstracts were excluded, as they may be less reliable without undergoing rigorous peer review. Also, we excluded articles that used only AI or ML to diagnose COVID‐19 and did not provide a role for CDSS to assist in diagnosing COVID‐19.
2.3. Study selection and data extraction
Abstracts of articles retrieved from four scientific databases were entered into EndNote X8.0. Duplicate articles were excluded, and the remaining articles were independently screened by three researchers (A. R. A., F. S., and A. T. A.) based on the title and abstract to select relevant studies. The lists of independently selected articles were reviewed jointly by A. R. A., F. S., and A. T. A. After final approval, the full texts were independently reviewed by three researchers to extract information using a data extraction form. Three medical informatics specialists confirmed the validity of this form. The form includes fields such as authors' names, publication year, study location, sample size or data sources, types of CDSS, computational methods, performance measures, and main results. All findings in the data extraction form were re‐examined and validated by A. R. A., F. S., and A. T. A. The fourth researcher (K. B.) consulted and resolved disagreements in each stage. The extracted data were analyzed in Microsoft Excel 2016 and presented in terms of percentage, frequency, and graphs.
3. RESULTS
3.1. Study selection
This study identified a total of 2199 articles from four databases, of which 701 were repeated studies, and 1263 were unrelated upon screening based on titles and abstracts. After careful examination of 1498 remaining articles based on inclusion and exclusion criteria, 235 studies were selected for full‐text reading. For several reasons, 167 articles were excluded from the final full‐text review. These reasons included narrative reviews, brief communications, letters, protocols, and reviews. Some studies only used ML to diagnose COVID‐19, while others required a better understanding of how CDSS can assist in diagnosing COVID‐19. However, some studies utilized CDSS for other purposes, such as monitoring, resource allocation, risk and severity assessment, and reviewing prognosis and outcomes in COVID‐19 patients. These studies also recommended the use of CDSS in future research. Finally, 68 articles met all the inclusion criteria. 5 , 17 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 The flowchart of the selection process is shown in Figure 1. The authors did not assess study quality due to the review type, which was a scoping review. 31
Figure 1.
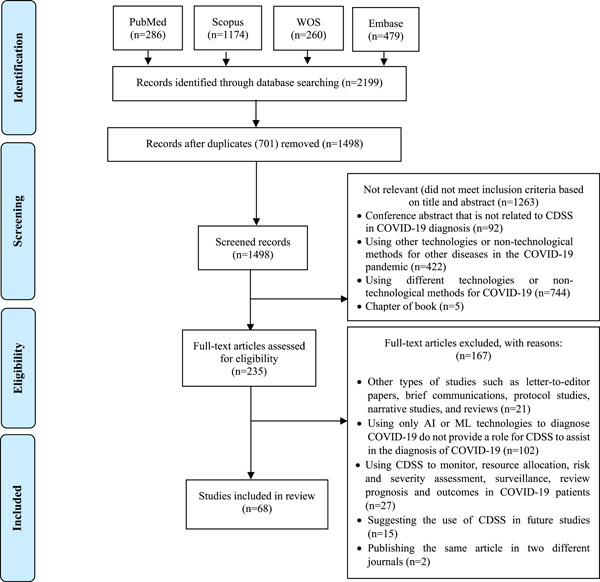
PRISMA‐ScR flowchart showing the search process. CDSS, clinical decision support systems; PRISMA‐ScR, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guideline extension for scoping reviews; WOS, Web of Sciences.
3.2. Characteristics of the included articles
The most of articles were published in 2021 (n = 23) (Figure 2). The first authors of most studies were affiliated in India (n = 12), Turkey (n = 7), the United States (n = 5), and Saudi Arabia (n = 5) (Figure 3). Chest X‐ray images and computed tomography (CT) scans of COVID‐19 patients and healthy individuals were used as data sources in most studies (n = 43) (Appendix 2). Details of the selected studies are shown in Appendix 2.
Figure 2.
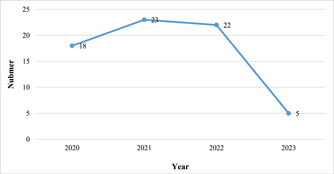
Distribution of the studies in terms of publication year.
Figure 3.
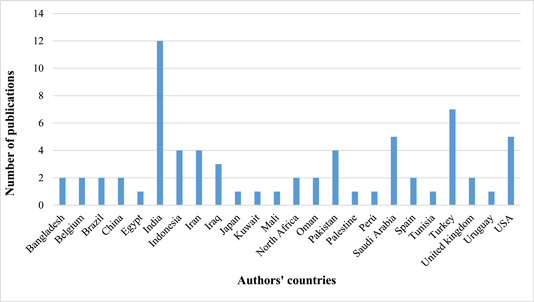
Number of publications by country based on authors' countries.
3.3. Types of CDSS to assist in diagnosing COVID‐19
Types of CDSS to assist in diagnosing COVID‐19 are shown in Figure 4. Most of the studies used ICDSS based on ML (nonknowledge‐based CDSS) (n = 52 [76.5%]). 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 In these studies, the most common methods for designing CDSS were CNN (n = 33), 38 , 40 , 41 , 42 , 45 , 46 , 47 , 49 , 50 , 51 , 52 , 54 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 71 , 72 , 78 , 82 , 83 , 84 , 85 SVM (n = 8), 35 , 36 , 39 , 43 , 44 , 54 , 56 , 57 RF (n = 7), 34 , 35 , 37 , 39 , 42 , 44 , 55 and KNN (n = 7) 36 , 37 , 39 , 42 , 43 , 55 , 56 (Table 1 and Appendix 2). Some studies used a combination of ML methods to develop CDSS (n = 14) 34 , 35 , 36 , 37 , 39 , 42 , 43 , 44 , 53 , 55 , 56 , 57 , 70 , 84 (Appendix 2). Rule sets (n = 8), 5 , 17 , 88 , 89 , 93 , 94 , 96 , 97 fuzzy mathematical models (n = 5), 86 , 90 , 91 , 92 , 95 and ontology (n = 3) 87 , 98 , 99 were used for developing ICDSS based on ES (knowledge‐based CDSS), respectively (Appendix 2).
Figure 4.
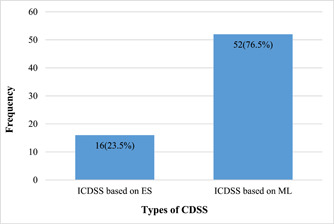
Types of CDSS in the selected studies. CDSS, clinical decision support systems; ES, expert systems; ICDSS, intelligent clinical decision support systems; ML, machine learning.
Table 1.
The computational methods of ICDSS based on ML in the selected studies.
| Computational methods in CDSS | Frequency in the studies |
|---|---|
| AdaBoost | 2 |
| ANN | 1 |
| Association rules | 1 |
| BNs | 2 |
| CART | 1 |
| Catboost | 1 |
| CNN | 33 |
| DNN | 4 |
| DT | 7 |
| GB | 1 |
| HGB | 1 |
| KNN | 7 |
| LDA | 1 |
| Lightgbm | 1 |
| LR | 5 |
| MLP | 2 |
| NB | 5 |
| PNN | 1 |
| QLDA | 1 |
| RF | 7 |
| RFC | 1 |
| SVC | 1 |
| SVM | 8 |
| XGB | 1 |
| XGBboost | 1 |
| XGBC | 1 |
| XGBoost | 2 |
Abbreviations: AdaBoost, Adaptive Boosting; ANN, Artificial Neural Network; BNs, Bayesian Networks; CART, Classification and Regression Tree; CDSS, clinical decision support systems; CNN, Convolutional Neural Networks; DNN, Deep Neural Networks; DT, Decision Tree; GB, Gradient Boosting; HGB, HistGradient Boosting; ICDSS, intelligent clinical decision support; KNN, K‐Nearest Neighbors; LDA, Linear Discriminant Analysis; LR, Logistic Regression; ML, machine learning; NB, naive Bayes; PNN, Probabilistic Neural Network; QLDA, Quadratic Linear Discriminant Analysis; RF, Random Forest; SVC, Support Vector Classification; SVM, Support Vector Machine; XGBC, XGBoost classification; XGBoost, eXtreme Gradient Boosting.
3.4. Evaluation methods of CDSS
The most common metrics for evaluating ICDSS based on ML are shown in Table 2. Most studies used a combination of performance metrics to evaluate CDSS (n = 40) (Appendix 2). In most of these studies, accuracy (n = 43), 35 , 36 , 37 , 38 , 39 , 40 , 42 , 43 , 44 , 45 , 46 , 47 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 71 , 72 , 76 , 77 , 78 , 80 , 81 , 82 , 83 , 85 sensitivity (recall) (n = 35) 35 , 36 , 37 , 39 , 41 , 42 , 43 , 44 , 46 , 47 , 49 , 50 , 51 , 52 , 53 , 54 , 56 , 57 , 61 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 71 , 72 , 76 , 78 , 81 , 82 , 83 , 84 , 85 and F1‐score (n = 26) 34 , 35 , 36 , 39 , 41 , 42 , 43 , 46 , 47 , 50 , 52 , 53 , 54 , 56 , 57 , 60 , 61 , 63 , 64 , 66 , 68 , 69 , 81 , 82 , 84 , 85 were used (Table 2). Other metrics for evaluating ICDSS based on ML were the Matthews correlation coefficient (MCC), 52 , 56 , 58 receiver operating characteristic (ROC) curve, 34 , 36 , 70 and so on (Appendix 2).
Table 2.
The most common metrics for evaluating intelligent clinical decision support based on machine learning in the selected studies.
| Evaluation criteria | Description | Frequency |
|---|---|---|
| Accuracy | The overall effectiveness of a classifier | 43 |
| Sensitivity (recall) | Effectiveness of a classifier to identify positive labels | 35 |
| F1‐score | Relations between data positive labels and those given by a classifier | 26 |
| Precision | Class agreement of the data labels with the positive labels given by the classifier | 20 |
| Specificity | How effectively does a classifier identify negative labels | 20 |
| Area under the curve (AUC) | Classifier's ability to avoid false classification | 12 |
The most common metrics for evaluating ICDSS based on ES were diagnosis rate (n = 3) 17 , 92 , 93 and accuracy (n = 3) 87 , 90 , 95 (Appendix 2). Other metrics (such as ease of use, precision, recall, and time) for evaluating these systems are shown in Appendix 2.
4. DISCUSSION
4.1. Principal findings
This scoping review examined the assistance of CDSS in COVID‐19 diagnosis. The most frequently used method for this purpose was the ICDSS based on ML (nonknowledge‐based CDSS), followed by ICDSS based on ES (knowledge‐based CDSS). Most studies have indicated that using CDSS have positively impacted the accurate diagnosis of COVID‐19. 5 , 17 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 86 , 87 , 88 , 89 , 90 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99
4.2. Nonknowledge‐based CDSS
ML was the most frequently used method to develop CDSS for COVID‐19 diagnosis. The most common ML methods in the reviewed studies were CNN. 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 In line with this result, other review studies also indicated CNN to be the most common method to develop a system for COVID‐19 diagnosis. 23 , 25 , 27 , 28 , 30 , 100 A CNN is a type of DL algorithm used for processing medical images, particularly for identifying specific features in chest radiographs of COVID‐19 patients. 30 CNN is more valuable than other methods for developing CDSS due to its excellent performance accuracy and much lower preprocessing. 25 , 30 For example, in the reviewed studies, CDSS based on CNN achieved a performance accuracy ranging from 75% to 99.62%. 38 , 40 , 41 , 42 , 45 , 46 , 47 , 49 , 50 , 51 , 52 , 54 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 71 , 72 , 78 , 82 , 83 , 84 , 85 Also, these studies indicated that CDSS based on CNN could be effective in detecting COVID‐19, assisting domain specialists, physicians, and radiologists in the decision‐making process, and enhancing radiologists' working productivity 25 , 30 , 42 , 66 , 87 It is challenging to create a CDSS using CNN due to the scarcity of big data and low quality of data sources. 25 Developing these data sets in medicine is costly and necessitates specialized labor. In addition, ethical and privacy concerns must be assessed. 30 Therefore, these findings do not mean that CDSS are a production‐ready solution because the diagnostic power of these systems relies on chest X‐ray images and CT scans of COVID‐19 patients. 50 It is important to acknowledge the bias that is introduced in these studies because someone who has a chest X‐ray or a CT scan is more likely to have COVID‐19. Many of the symptoms of COVID‐19 are nonspecific and thus difficult to differentiate from overlapping symptoms of other diseases. Future research can study some of the more unique presenting symptoms of COVID‐19 (anosmia, thromboses, etc.) in light of how CDSS could be used to assist in diagnosing these cases.
4.3. Knowledge‐based CDSS
In the present scoping review, the results of 16 studies identified a positive impact on a diagnosis of COVID‐19 with the assistance of CDSS based on ES. 5 , 17 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 In the reviewed studies, rule sets were the most common methods in developing a knowledge base of CDSS. 5 , 17 , 88 , 89 , 93 , 94 , 96 , 97 Rule‐based systems acquire contextual knowledge from extracted data stored and manipulated in other approaches. 101 The reviewed studies extracted data from various sources, such as observed symptoms, specific medical measurements, pre‐existing medical conditions or any hospitalization history, recent PCR test results, clinical guidelines, websites (e.g., WHO), and knowledge of experts to develop rule sets. 5 , 17 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 Therefore, knowledge‐based CDSS in these studies can provide a comprehensive view of the patient's health information. This feature enables health practitioners to access treatment recommendations and risk classification, recommend test lists, quickly track test results and symptoms, and access clinical guidelines in the COVID‐19 pandemic. 5 Generally, these types of CDSS require knowledge bases and input variables (e.g., fever, cough, cell blood count, respiratory rate, CT chest/RT‐PCR, family history, and age). Hak et al. conducted a literature review and found that many CDSS are inadequate due to a lack of standardization and structure in their knowledge base. 102 Therefore, understanding the different ways of representing, maintaining, and updating knowledge in rule‐based systems is important.
The reviewed studies used different metrics, including diagnostic rate, accuracy, ease of use, and time, to evaluate the performance of knowledge‐based CDSS. 5 , 17 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 The reason for this could be the widespread COVID‐19 pandemic, leading to a lack of time to evaluate these systems' performance properly. It is essential for researchers and software developers to thoroughly assess the performance of these systems before implementing them in real clinical settings for COVID‐19 diagnosis.
In summary, the present scoping review studied the types of CDDS that assist in the COVID‐19 diagnosis. Previous studies used nonknowledge‐based CDSS and knowledge‐based CDSS for this purpose. The sample size in most studies was the clinical characteristics (e.g., radiological images) of public data sets of COVID‐19 patients and healthy individuals. The performance and accuracy of CDSS may depend on extracted information from data sets to predict the infection of COVID‐19. These assumptions about performance metrics may change with the emergence and availability of new data. Therefore, these systems should be used by healthcare providers and domain experts to validate and evaluate their clinical usefulness in real workflow. Also, focusing on the early stages of COVID‐19 detection is crucial, as most methods effectively identify the disease only in advanced stages. Future studies can assess the impact of CDSS to assist in predicting and detecting the COVID‐19 disease in the initial stage. Finally, stakeholders (e.g., researchers, healthcare professionals, and health policymakers) of CDSS should first consider the type of design methods for developing a CDSS. The type of design method assists in the accuracy and precision of the diagnoses in CDSS. These issues can also be considered in future studies.
4.4. Limitations
The present study has two limitations. First, the study's exclusion of non‐English articles may cause language bias. However, we employed a comprehensive search strategy. By using this method, the likelihood of missing relevant articles may have been reduced. Our search strategy was run in January 2021 and updated in September 2023. The number of included studies may be changed after a period of time because new studies will be conducted on various CDSS to assist in diagnosing COVID‐19 at different stages of severity.
Second, we searched four scientific databases: Scopus, PubMed, Embase, and Web of Science. While searching additional databases may uncover new articles, these four databases will likely retrieve the most relevant articles.
5. CONCLUSION
This scoping review studied the assistance and impact of CDSS on the detection and diagnosis of COVID‐19. The results showed that COVID‐19 can be diagnosed by assisting nonknowledge‐based CDSS and knowledge‐based CDSS. These studies used various techniques such as ML methods (e.g., CNN, SVM, RF) and ES methods (e.g., rule sets, fuzzy mathematical models, ontologies) to design CDSS. ML methods were the most common techniques in developing CDSS for COVID‐19 diagnosis. The greater use of these techniques can be due to the availability of public data sets about chest X‐ray images and CT‐scan scans of COVID‐19 patients. Novelty and data set biases raise questions about the performance accuracy of these systems. Most models still need to be deployed enough to show their real‐world functionality. However, they can help combat the pandemic. Future studies can evaluate the usefulness and performance accuracy of CDSS for COVID‐19 diagnosis in different healthcare environments from the perspective of healthcare providers. Also, future studies can examine the impact of CDSS in other care cycle stages of COVID‐19, such as prevention, screening, surveillance, treatment, and rehabilitation, and compare the accuracy, robustness, and reliability of different methods in developing CDSS. This paper provides insights into how CDSS can be used to detect and mitigate the COVID‐19 pandemic. It will be helpful for researchers, healthcare providers, government officials, and policymakers.
ETHICS STATEMENT
Kerman University of Medical Sciences' Research Ethics Committee approved this study (Code of Ethics: IR.KMU.REC.1399.686). Informed consent was not applicable for this scoping review.
AUTHOR CONTRIBUTIONS
Arefeh Ameri: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing—original draft; writing—review and editing. Atefeh Ameri: Formal analysis; investigation; methodology; writing—original draft; writing—review and editing. Farzad Salmanizadeh: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing—original draft; writing—review and editing. Kambiz Bahaadinbeigy: Funding acquisition; validation; writing—original draft; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Farzad Salmanizadeh affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
The authors express their gratitude to the Central Library and Documentation Center of Kerman University of Medical Sciences for providing access to this study's required knowledge base references.
Ameri A, Ameri A, Salmanizadeh F, Bahaadinbeigy K. Clinical decision support systems (CDSS) in assistance to COVID‐19 diagnosis: a scoping review on types and evaluation methods. Health Sci Rep. 2024;7:e1919. 10.1002/hsr2.1919
DATA AVAILABILITY STATEMENT
All data generated and analyzed during this study are included in this published article. Upon a reasonable request, the corresponding author can provide more information on the data sets used and analyzed during the current study.
REFERENCES
- 1. Suman R, Javaid M, Haleem A, Vaishya R, Bahl S, Nandan D. Sustainability of coronavirus on different surfaces. J Clin Exp Hepatol. 2020;10(4):386‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID‐19) outbreak: what the department of radiology should know. J Am Coll Radiol. 2020;17(4):447‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao D, Yao F, Wang L, et al. A comparative study on the clinical features of coronavirus 2019 (COVID‐19) pneumonia with other pneumonias. Clin Infect Dis. 2020;71:756‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronavirus disease (COVID‐19) pandemic. World Health Organization. 2023. Accessed November 30, 2023. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/
- 5. Banjar HR, Alkhatabi H, Alganmi N, Almouhana GI. Prototype development of an expert system of computerized clinical guidelines for COVID‐19 diagnosis and management in Saudi Arabia. Int J Environ Res Public Health. 2020;17(8066):8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ohannessian R, Duong TA, Odone A. Global telemedicine implementation and integration within health systems to fight the COVID‐19 pandemic: a call to action. JMIR Public Health Surveill. 2020;6:e18810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shukla SK, Patra S, Das TR, Kumar D, Mishra A, Tiwari A. Progress in COVID research and developments during pandemic. View. 2022;3(6):20210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25(3):23‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang N, Wang L, Deng X, et al. Recent advances in the detection of respiratory virus infection in humans. J Med Virol. 2020;92(4):408‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019‐nCoV). Radiology. 2020;295(1):202‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borakati A, Perera A, Johnson J, Sood T. Diagnostic accuracy of x‐ray versus CT in COVID‐19: a propensity‐matched database study. BMJ Open. 2020;10:e042946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shatnawi M, Shatnawi A, AlShara Z, Husari G. Symptoms‐based fuzzy‐logic approach for COVID‐19 diagnosis. Int J Adv Comput Sci Appl. 2021;12(4):444‐452. [Google Scholar]
- 15. Maghdid HS, Asaad AT, Ghafoor KZ, Sadiq AS, Khan MK. Diagnosing COVID‐19 pneumonia from x‐ray and CT images using deep learning and transfer learning algorithms. arXiv. 2020;117340(12):1‐8. [Google Scholar]
- 16. Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nema B, Makki Mohialden Y, Mahmood Hussien N, Ali Hussein N. COVID‐19 knowledge‐based system for diagnosis in Iraq using IoT environment. Indones J Electr Eng Comput Sci. 2020;21(1):328‐337. [Google Scholar]
- 18. Montani S, Striani M. Artificial intelligence in clinical decision support: a focused literature survey. Yearb Med Inform. 2019;28(1):120‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Magrabi F, Ammenwerth E, McNair JB, et al. Artificial intelligence in clinical decision support: challenges for evaluating AI and practical implications. Yearb Med Inform. 2019;28(1):128‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sherimon PC, Krishnan R. OntoDiabetic: an ontology‐based clinical decision support system for diabetic patients. Arab J Sci Eng. 2016;41(3):1145‐1160. [Google Scholar]
- 21.Fawzi R, Ghazy M, Rizkallah LW. Designing Knowledge‐Based Systems for COVID‐19 Diagnosis: International Conference on Hybrid Intelligent Systems, USA, 14‐16 December 2021. Springer International Publishing; 2021:69‐75.
- 22. He C, Li Y. A Survey of Intelligent Decision Support System: 2017 7th International Conference on Applied Science, Engineering and Technology, Qingdao, China, 20‐22 May. 2017. Atlantis Press; 2017. [Google Scholar]
- 23. Alqaissi EY, Alotaibi FS, Ramzan MS. Modern machine‐learning predictive models for diagnosing infectious diseases. Comput Math Methods Med. 2022;2022:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blazek R, Hrosova L, Collier J. Internet of medical things‐based clinical decision support systems, smart healthcare wearable devices, and machine learning algorithms in COVID‐19 prevention, screening, detection, diagnosis, and treatment. Am J Med Res. 2022;9(1):65‐80. [Google Scholar]
- 25. Khan M, Mehran MT, Haq ZU, et al. Applications of artificial intelligence in COVID‐19 pandemic: a comprehensive review. Exp Syst Appl. 2021;185:115695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rasheed J, Jamil A, Hameed AA, Al‐Turjman F, Rasheed A. COVID‐19 in the age of artificial intelligence: a comprehensive review. Interdiscip Sci Comput Life Sci. 2021;13:153‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dabbagh R, Jamal A, Temsah MH, et al. Machine learning models for predicting diagnosis or prognosis of COVID‐19: a systematic review. Comput Methods Prog Biomed. 2021;205:105993. [Google Scholar]
- 28. Hassan A, Prasad D, Rani S, Alhassan M. Gauging the impact of artificial intelligence and mathematical modeling in response to the COVID‐19 pandemic: a systematic review. BioMed Res Int. 2022;2022:1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29. Ali SW, Asif M, Zia MYI, Rashid M, Syed SA, Nava E. CDSS for early recognition of respiratory diseases based on AI techniques: a systematic review. Wirel Pers Commun. 2023;131:739‐761. 10.1007/s11277-023-10432-1 [DOI] [Google Scholar]
- 30. Kugunavar S, Prabhakar CJ. Convolutional neural networks for the diagnosis and prognosis of the coronavirus disease pandemic. Vis Comput Ind Biomed Art. 2021;4(12):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med. 2018;169:467‐473. [DOI] [PubMed] [Google Scholar]
- 32. Jaspers MWM, Smeulers M, Vermeulen H, Peute LW. Effects of clinical decision‐support systems on practitioner performance and patient outcomes: a synthesis of high‐quality systematic review findings. J Am Med Inform Assoc. 2011;18(3):327‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Albahri OS, Zaidan AA, Albahri AS, et al. Systematic review of artificial intelligence techniques in the detection and classification of COVID‐19 medical images in terms of evaluation and benchmarking: taxonomy analysis, challenges, future solutions and methodological aspects. J Infect Public Health. 2020;13(10):1381‐1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Abbasi WA, Abbas SA, Andleeb S, et al. COVIDC: an expert system to diagnose COVID‐19 and predict its severity using chest CT scans: application in radiology. Inform Med Unlocked. 2021;23:100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Abdulkareem KH, Mohammed MA, Salim A, et al. Realizing an effective COVID‐19 diagnosis system based on machine learning and IOT in smart hospital environment. IEEE Internet Things J. 2021;8(21):15919‐15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Afrash MR, Erfannia L, Amrae M, et al. Machine learning‐based clinical decision support system for automatic diagnosis of COVID‐19 based on clinical data. J Biostat Epidemiol. 2022;8(1):77‐89. [Google Scholar]
- 37. Alshayeji MH, ChandraBhasi Sindhu S, Abed S. CAD systems for COVID‐19 diagnosis and disease stage classification by segmentation of infected regions from CT images. BMC Bioinform. 2022;23(1):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aulia S, Hadiyoso S, Mengko TL, Suksmono AB. COVID‐19 and Tuberculosis Classification Based on Chest X‐ray Using Convolutional Neural Network: Proceedings of the 1st International Conference on Electronics, Biomedical Engineering, and Health Informatics: ICEBEHI 2020, Surabaya, Indonesia, 8–9 October 2021. Springer Singapore; 2021.
- 39. Awal MA, Masud M, Hossain MS, Bulbul AAM, Mahmud SMH, Bairagi AK. A novel Bayesian optimization‐based machine learning framework for COVID‐19 detection from inpatient facility data. IEEE Access. 2021;9:10263‐10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Batra S, Sharma H, Boulila W, et al. An intelligent sensor based decision support system for diagnosing pulmonary ailment through standardized chest x‐ray scans. Sensors. 2022;22(19):7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carmo D, Campiotti I, Rodrigues L, et al. Rapidly deploying a COVID‐19 decision support system in one of the largest Brazilian hospitals. Health Inform J. 2021;27(3):14604582211033017. [DOI] [PubMed] [Google Scholar]
- 42. Chadaga K, Prabhu S, Bhat V, Sampathila N, Umakanth S, Chadaga R. A decision support system for diagnosis of COVID‐19 from non‐COVID‐19 influenza‐like illness using explainable artificial intelligence. Bioengineering. 2023;10(4):439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chandra TB, Singh BK, Jain D. Integrating patient symptoms, clinical readings, and radiologist feedback with computer‐aided diagnosis system for detection of infectious pulmonary disease: a feasibility study. Med Biol Eng Comput. 2022;60(9):2549‐2565. [DOI] [PubMed] [Google Scholar]
- 44. Çubukçu HC, Topcu Dİ, Bayraktar N, Gülşen M, Sarı N, Arslan AH. Detection of COVID‐19 by machine learning using routine laboratory tests. Am J Clin Path. 2022;157(5):758‐766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Devi SK, Amirthavarshini D, Anbukani RS, Harini TK. A Medical Decision Support System to Detect Covid‐19 Pneumonia Using CNN: 2021 Innovations in Power and Advanced Computing Technologies (i‐PACT), Kuala Lumpur, Malaysia, 27–29 November 2021. IEEE; 2021:1‐6.
- 46. Dutta AK, Aljarallah NA, Abirami T, et al. Optimal deep‐learning‐enabled intelligent decision support system for SARS‐CoV‐2 classification. J Healthc Eng. 2022;2022:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Echtioui A, Zouch W, Ghorbel M, Mhiri C, Hamam H. Detection methods of COVID‐19. SLAS Technol. 2020;25(6):566‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edye EO, Kurucz JF, Lois L, et al. Applying Bayesian Networks to Help Physicians Diagnose Respiratory Diseases in the Context of COVID‐19 Pandemic: IEEE URUCON, Montevideo, Uruguay, 24–26 November 2021. IEEE; 2021:368‐371.
- 49. EL Biach Fz, Imad IA, Laanaya H, Minaoui K. CovSeg‐Unet: end‐to‐end method‐based computer‐aided decision support system in lung COVID‐19 detection on CT images. Int J Adv Comput Sci Appl. 2022;13(1):1‐8. [Google Scholar]
- 50. El‐Rashidy N, El‐Sappagh S, Islam SMR, El‐Bakry HM, Abdelrazek S. End‐to‐end deep learning framework for coronavirus (COVID‐19) detection and monitoring. Electronics. 2020;9(9):1439. [Google Scholar]
- 51. Ghaderzadeh M, Asadi F, Jafari R, Bashash D, Abolghasemi H, Aria M. Deep convolutional neural network‐based computer‐aided detection system for COVID‐19 using multiple lung scans: design and implementation study. J Med Internet Res. 2021;23(4):e27468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gidde PS, Prasad SS, Singh AP, et al. Validation of expert system enhanced deep learning algorithm for automated screening for COVID‐Pneumonia on chest x‐rays. Sci Rep. 2021;11(1):23210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gomes JC, de Freitas Barbosa VA, de Santana MA, et al. Rapid protocols to support Covid‐19 clinical diagnosis based on hematological parameters. Res Biomed Eng. 2023;39:509‐539. [Google Scholar]
- 54. Heidari M, Mirniaharikandehei S, Khuzani AZ, Danala G, Qiu Y, Zheng B. Improving the performance of CNN to predict the likelihood of COVID‐19 using chest x‐ray images with preprocessing algorithms. Int J Med Inform. 2020;144:104284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jain A, Tiwari S, Choudhury T, Dewangan BK. Gradient and statistical features‐based prediction system for COVID‐19 using chest x‐ray images. Int J Comput Appl Technol. 2021;66(3‐4):362‐373. [Google Scholar]
- 56. Kadhim YA, Khan MU, Mishra A. Deep learning‐based computer‐aided diagnosis (CAD): applications for medical image datasets. Sensors. 2022;22(22):8999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kanwal S, Khan F, Alamri S, Dashtipur K, Gogate M. COVID‐opt‐aiNet: a clinical decision support system for COVID‐19 detection. Int J Imag Syst Technol. 2022;32(2):444‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Karthikeyan D, Varde AS, Wang W. Transfer Learning for Decision Support in Covid‐19 Detection From a Few Images in Big Data: 2020 IEEE International Conference on Big Data (Big Data), Atlanga, GA, USA, 10‐13 December 2020. IEEE; 2020:4873‐4881.
- 59. Lastra Leidinger M, Aragón Royón F, Etxeberria O, Balderas L. A real‐world intelligent system for the diagnosis and triage of COVID‐19 in the emergency department. Signa Vitae. 2023;19(3):91‐102. [Google Scholar]
- 60. Mansouri N, Sultan K, Ahmad A, Alseadoon I, Alkhalil A. A deep learning framework for COVID‐19 diagnosis from computed tomography. Intell Automat Soft Comput. 2022;34(2):1‐18. [Google Scholar]
- 61. Marques G, Agarwal D, de la Torre Díez I. Automated medical diagnosis of COVID‐19 through EfficientNet convolutional neural network. Appl Soft Comput. 2020;96:106691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mayya V, Karthik K, Sowmya KS, Karadka K, Jeganathan J. COVIDDX: AI‐based clinical decision support system for learning COVID‐19 disease representations from multimodal patient data. In: Pesquita C, Fred A, Gamboa H, eds. HEALTHINF 2021. SciTePress; 2021:659‐666. [Google Scholar]
- 63. Murillo‐González A, González D, Jaramillo L, et al. Medical decision support system using weakly‐labeled lung CT scans. Front Med Technol. 2022;4:980735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Niranjan K, Shankar Kumar S, Vedanth S, Chitrakala DS. An explainable AI driven decision support system for COVID‐19 diagnosis using fused classification and segmentation. Proc Comput Sci. 2023;218:1915‐1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nishio M, Noguchi S, Matsuo H, Murakami T. Automatic classification between COVID‐19 pneumonia, non‐COVID‐19 pneumonia, and the healthy on chest X‐ray image: combination of data augmentation methods. Sci Rep. 2020;10:17532. 10.1038/s41598-020-74539-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nour M, Cömert Z, Polat K. A novel medical diagnosis model for COVID‐19 infection detection based on deep features and Bayesian optimization. Appl Soft Comput. 2020;97:106580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Patni JC, Sharma HK, Sharma S, et al. COVID‐19 pandemic diagnosis and analysis using clinical decision support systems. In: Tavares JMRS, Dutta , Dutta S, Samanta D, eds. In Cyber Intelligence and Information Retrieval. Lecture Notes in Networks and Systems. Springer Singapore; 2022;291:267‐277. 10.1007/978-981-16-4284-5_23 [DOI] [Google Scholar]
- 68.Qjidaa M, Ben‐Fares A, Mechbal Y, et al. Development of a Clinical Decision Support System for the Early Detection of COVID‐19 Using Deep Learning Based on Chest Radiographic Images: 2020 International Conference on Intelligent Systems and Computer Vision (ISCV), Fez, Morocco, 9–11 June 2020. IEEE; 2020:1‐6.
- 69. Reza AW, Sorna JF, Rashel MMU, Shibly MMA. ModCOVNN: a convolutional neural network approach in COVID‐19 prognosis. Int J Adv Intell Inform. 2021;7(2):125. [Google Scholar]
- 70. Saegerman C, Gilbert A, Donneau AF, et al. Clinical decision support tool for diagnosis of COVID‐19 in hospitals. PLoS One. 2021;16(3):e0247773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saglam S, Kumrular RK, Aysel HI. COVID‐19 Detection From X‐ray Images Using a New CNN Approach: 2022 IEEE International Students' Conference on Electrical, Electronics and Computer Science (SCEECS), Bhopal, India, 19–20 February 2022. IEEE; 2022:1‐4.
- 72. Saheb SK, Narayanan B, Rao TVN. ADL‐CDF: a deep learning framework for COVID‐19 detection from CT scans towards an automated clinical decision support system. Arab J Sci Eng. 2023;48(8):9661‐9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Salah HA, Ahmed AS. Coronavirus disease diagnosis, care and prevention (COVID‐19) based on decision support system. Baghdad Sci J. 2021;18(3):0593. [Google Scholar]
- 74. Shan Z, Miao W. COVID‐19 patient diagnosis and treatment data mining algorithm based on association rules. Exp Syst. 2023;40(4):e12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shanbehzadeh M, Nopour R, Kazemi‐Arpanahi H. Determination of the most important diagnostic criteria for COVID‐19: a step forward to design an intelligent clinical decision support system. J Adv Med Biomed Res. 2021;29(134):176‐182. [Google Scholar]
- 76. Shanbehzadeh M, Nopour R, Kazemi‐Arpanahi H. Developing an intelligent system for diagnosis of COVID‐19 based on artificial neural network. Acta Med Iran. 2022:60(3)135‐143. [Google Scholar]
- 77. Shreyas SK, Rao JK. Diagnostic Decision Support for Medical Imaging and COVID‐19 Image Classification on ARM Mali GPU. In 2021 IEEE Globecom Workshops (GC Wkshps), Madrid, Spain, 7–11 December 2021. IEEE; 2021:1‐6.
- 78. Yamin Siddiqui S, Abbas S, Adnan Khan M, et al. Intelligent decision support system for COVID‐19 empowered with deep learning. Comput Mater Contin. 2021;66(2):1719‐1732. [Google Scholar]
- 79. Silahudin D, Henderi, Holidin A . Model expert system for diagnosis of COVID‐19 using naïve Bayes classifier. In IOP Conference Series: Materials Science and Engineering. 2020:1007(1). 10.1088/1757-899X/1007/1/012067 [DOI] [Google Scholar]
- 80. Subramaniam U, Subashini MM, Almakhles D, Karthick A, Manoharan S. An expert system for COVID‐19 infection tracking in lungs using image processing and deep learning techniques. BioMed Res Int. 2021;2021:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sun J, Peng L, Li T, et al. Performance of a chest radiograph AI diagnostic tool for COVID‐19: a prospective observational study. Radiol Artif Intell. 2022;4(4):e210217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Tiwari S, Jain A. Convolutional capsule network for COVID‐19 detection using radiography images. Int J Imag Syst Technol. 2021;31(2):525‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Topff L, Sánchez‐García J, López‐González R, et al. A deep learning‐based application for COVID‐19 diagnosis on CT: the imaging COVID‐19 AI initiative. PLoS One. 2023;18(5):e0285121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Verdi EB, Gok M, Mülazimoglu DD, et al. Deep learning‐based hybrid clinical decision support system algorithm for COVID‐19 diagnosis via PCR graphics and thorax CT images, preliminary data. Eur Respir J. 2022;60:1357. [Google Scholar]
- 85. Yağin Fh, Güldoğan E, Ucuzal H, Colak C. A computer‐assisted diagnosis tool for classifying COVID‐19 based on chest X‐ray images. Konuralp Tıp Dergisi. 2021;13(S1):438‐445. [Google Scholar]
- 86. Ashraf S, Abdullah S, Almagrabi AO. A new emergency response of spherical intelligent fuzzy decision process to diagnose of COVID19. Soft Comput. 2020;27;1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Çelik Ertuğrul D, Çelik Ulusoy D. A knowledge‐based self‐pre‐diagnosis system to predict Covid‐19 in smartphone users using personal data and observed symptoms. Exp Syst. 2022;39(3):e12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Goita Y, Sidibe M. Towards a Comprehensive Expert System for Coronavirus Disease: 2021 7th International Conference on Information Management (ICIM), London, United Kingdom, 27‐29 March 2021. IEEE; 2021:18‐23.
- 89. Jáuregui‐Velarde R, Molina‐Velarde P, Yactayo‐Arias C, Andrade‐Arenas L. Expert system for the timely diagnosis of infectious diseases. Int J Eng Trends Technol. 2022;71(3):215‐226. [Google Scholar]
- 90. Abbas Khan T, Abbas S, Ditta A, et al. IoMT‐based smart monitoring hierarchical fuzzy inference system for diagnosis of COVID‐19. Comput Mater Contin. 2020;65(3):2591‐2605. [Google Scholar]
- 91.Kop Naskali Y, Naskali AT, Albayrak YE. Pre‐Diagnosis Support System for Post Covid‐19 Syndrome in Turkey: International Conference on Intelligent and Fuzzy Systems, İstanbul, Turkey, 24–26 August 2021. Springer International Publishing; 2021:787‐795.
- 92. Liu W, Mostafa Khalil A, Basheer R, Lin Y. Prediction system for diagnosis and detection of coronavirus disease‐2019 (COVID‐19): a fuzzy‐soft expert system. Comput Model Eng Sci. 2023;135(3):2715‐2730. [Google Scholar]
- 93.Maulana M, Warnars HL, Setiyadi D, Qurrohman T. Model Decision Support System For Diagnosis COVID‐19 Using Forward Chaining: a Case in Indonesia: 2020 8th International Conference on Cyber and IT Service Management (CITSM), Pangkal Pinang, Indonesia, 23‐24 October 2020. IEEE; 2020:1‐4.
- 94.Mufid MR, Basofi A, Mawaddah S, Khotimah K, Fuad N. Risk Diagnosis and Mitigation System of COVID‐19 Using Expert System and Web Scraping: 2020 International Electronics Symposium (IES), Surabaya, Indonesia, 29–30 September 2020. IEEE; 2020:577‐583.
- 95. Şahin I, Akdoğan E, Aktan Me. A type‐2 fuzzy rule‐based model for diagnosis of COVID‐19. Turk J Electr Eng Comput Sci. 2023;31(1):39‐52. [Google Scholar]
- 96. Sahoo HS, Silverman GM, Ingraham NE, et al. A fast, resource efficient, and reliable rule‐based system for COVID‐19 symptom identification. JAMIA Open. 2021;4(3):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Salman FM, Abu‐Naser SS. Expert system for COVID‐19 diagnosis. Int J Acad Inf Syst Res. 2020;4(3):1‐13. [Google Scholar]
- 98. Sherimon V, Sherimon PC, Mathew R, et al. Covid‐19 ontology engineering‐knowledge modeling of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Int J Adv Comput Sci Appl. 2020;11(11):117‐123. [Google Scholar]
- 99. Sherimon V, Sherimon PC, Nair RV, et al. eCOVID19–development of ontology‐based clinical decision support system for COVID‐19. Front Health Inform. 2022;11(1):101. [Google Scholar]
- 100. Dabbagh R, Jamal A, Bhuiyan Masud JH, et al. Harnessing machine learning in early COVID‐19 detection and prognosis: a comprehensive systematic review. Cureus. 2023;15(5):1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Silva B, Hak F, Guimarães T, Manuel M, Santos MF. Rule‐based system for effective clinical decision support. Proc Comput Sci. 2023;220:880‐885. [Google Scholar]
- 102. Hak F, Guimarães T, Santos M. Towards effective clinical decision support systems: a systematic review. PLoS One. 2022;17(8):e0272846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
All data generated and analyzed during this study are included in this published article. Upon a reasonable request, the corresponding author can provide more information on the data sets used and analyzed during the current study.


