Abstract
The primary objective of this study is to record the clinical outcomes of autologous conditioned plasma (ACP) for the treatment of knee osteoarthritis (OA) based on published literature. Multiple databases (PubMed, Embase, Web of Science and Scopus) were searched using terms for “knee OA” and the intervention “ACP” for articles published in English to January 4, 2024. All clinical studies using ACP for knee OA were included. Studies not utilizing ACP alone, i.e. used as an adjunct with other modalities or not focusing on the management of knee OA, were excluded. Five studies, three randomized controlled trials (RCTs) and two real-world post-market studies conducted in a clinical practice met the inclusion/exclusion criteria and were included in this study. All studies demonstrated statistically significant improvements in various patient-reported outcome measures (PROMs), however the studies performed in the clinical practice reported non-accomplishment of minimally clinically important difference (MCID). The results demonstrated the potential of ACP for management of knee OA, however the MCID was not achieved in real-world clinical settings. Thus, more adequately powered RCTs with longer follow-up as well as real-world post-market studies are warranted to establish long-term efficacy and justify routine clinical use, respectively, of ACP in patients suffering with knee OA.
Keywords: lp prp, leukocyte-poor platelet-rich plasma, prp, platelet-rich plasma, acp max, acp, autologous conditioned plasma, orthobiologics, gonarthrosis, knee osteoarthritis
Introduction and background
Osteoarthritis (OA) is the leading joint complaint impacting millions of people globally [1]. OA is a degenerative joint condition mostly concerning large weight-bearing joints, including knees [1]. Its aetiology comprises of inflammation of synovial tissue and deterioration of articular cartilage, resulting in pain, diminished function and affected overall quality of life (QoL) [2]. Knee OA is conservatively managed via non-pharmacological modalities such as weight reduction, activity alteration, and physical therapy; pharmacological substances such as non-steroidal anti-inflammatory drugs (NSAIDs) and opioids, and intraarticular administration of corticosteroids and viscosupplementation; minimally invasive procedures such as radiofrequency ablation of genicular nerve and peripheral nerve stimulators; and surgical interventions, in advanced stages or after conventional therapies have been unproductive [1-3]. These above-mentioned treatment modalities have shortcomings and side effects, constantly intending to decrease pain instead of targeting the causal pathophysiology [1-3].
Lately, there has been a notable interest in the use of orthobiologics, including autologous peripheral blood-derived orthobiologics (APBO) for musculoskeletal regenerative medicine [4]. Various APBO include platelet-rich plasma (PRP), autologous conditioned plasma (ACP), platelet lysate (PL), autologous conditioned serum (ACS), Gold-induced cytokine (GOLDIC), plasma-rich in growth factors (PRGF), growth factor concentrate (GFC), autologous protein solution (APS), platelet-rich fibrin (PRF) and hyperacute serum (HS). PRP is the most widely used APBO, nonetheless its efficacy remains contentious, attributed to absence of standardized preparation protocol, inter- and intra-patient variables, etc. [4]. ACP (Arthrex, Naples, FL, USA), a commercially available single-spin leukocyte-poor PRP, formulated per manufacturer's instructions, is commonly used in clinical practice for regenerative medicine applications, including for knee OA treatment [5]. To date, only a limited number of studies have investigated the efficacy of ACP for the treatment of knee OA. The primary objective of this study is to record the clinical outcomes of ACP for the treatment of knee OA. The secondary objective is to document the ongoing clinical studies registered on different trial protocol repositories related to ACP for the management of knee OA.
Review
Search criteria
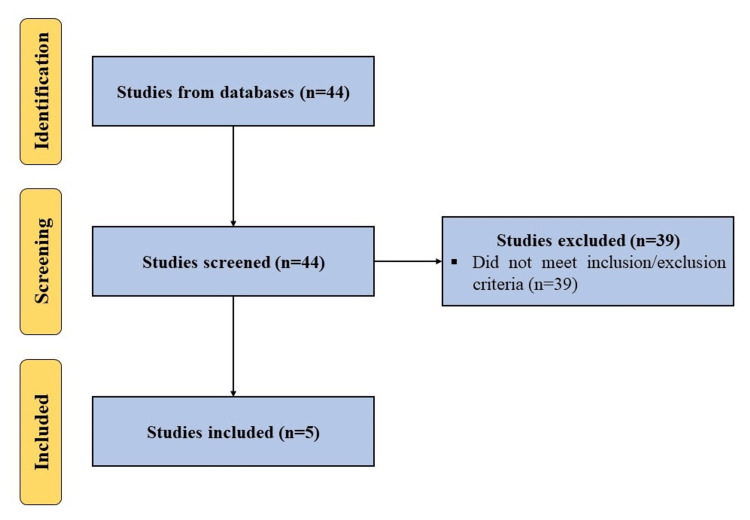
A search was performed using terms, (‘autologous conditioned plasma’ OR ‘ACP’ OR ‘leukocyte-poor platelet-rich plasma’ OR ‘LP PRP’) AND (‘knee osteoarthritis’), in databases including PubMed, Embase, Web of Science and Scopus for articles published in English to January 4, 2024, while adhering to Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. All clinical studies using ACP for knee OA were included. Studies not utilizing ACP alone or not focusing on the management of knee OA were excluded (Figure 1). In addition, we searched ClinicalTrials.gov, Clinical Trials Registry - India (CTRI), and Chinese Clinical Trial Register (ChiCTR) using the above-mentioned search terms to identify registered ongoing studies on the use of ACP for the management of knee OA.
Figure 1. A PRISMA flow diagram outlining the record identification and selection process.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Results
Clinical Studies
Korpershoek et al. [6] in a prospective case series investigated the effect of ACP in patients with knee OA. ACP was prepared per instructions from the manufacturer (Arthrex). Three weekly intra-articular injections were administered. The patient-reported outcome measures (PROMs) were collected at baseline and at three, six, and 12 months follow-up, including Knee Injury and Osteoarthritis Outcome Score (KOOS), EuroQol 5 Dimensions 5 Levels (EQ5D-5L), and numeric pain rating scale (NPRS). Two hundred sixty patients (307 knees) were included in this study. Statistically significant improvements in the KOOS and NPRS were reported at all follow-up time points compared to the baseline, however no significant improvement was noted for the EQ5D-5L. Interestingly, despite the significant improvement in the KOOS score, most of the patients did not achieve improvements above the minimally clinically important difference (MCID). A small number of patients who achieved MCID underwent repeat cycle of three weekly ACP injections. The subgroup analysis showed better improvement in older patients and bilateral treatment led to poorer outcomes. The main limitation of this study is the lack of a placebo group or active controls. In summary, MCID was not reached post-treatment with ACP in majority of the knee OA patients, and thus, was not recommended as a routine therapy in clinical settings.
Korpershoek et al. [7] in a prospective case series investigated the effectiveness of intra-articular administration of ACP in knee OA patients. ACP was prepared per instructions from the manufacturer. One hundred forty patients (158 knees) were treated with three ACP injections. The PROMs assessed included KOOS, NPRS and EQ5D at baseline, and at three, six, and 12 months follow-up. Statistically significant improvement in the KOOS was reported at all follow-up time points compared to the baseline, however no significant improvements were noted for the NPRS and EQ5D. Similar to the aforementioned study, despite statistically significant improvements in the KOOS score, the MCID was not attained in the majority of the patients. This study has several limitations including lack of control group(s), variability in dosing frequency, and loss of patients during follow-up. In summary, intraarticular administration of ACP did not lead to MCID in knee OA patients, and thus, was not recommended as a routine therapy in clinical settings.
Cole et al. [8] in a prospective, double-blind, randomized controlled trial, compared the effects of PRP to hyaluronic acid (HA) in mild to moderate knee OA patients. One hundred eleven patients were enrolled in this study and randomized 1:1 in the PRP and HA groups. Three weekly injections of PRP or HA were administered. ACP was used as a leukocyte-poor PRP formulation and formulated per manufacturer’s instructions. Patients were evaluated at baseline, treatment weeks two and three, and at six, 12, 24 and 52 weeks follow-up. The primary outcome measure was the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale. The secondary outcome measures included the International Knee Documentation Committee (IKDC), visual analog scale (VAS), and Lysholm knee score. Statistically significant improvements in the IKDC and VAS scores were observed at 24 and 52 weeks follow-up compared to the baseline. However, there was a decline in improvements in both IKDC and VAS scores for both PRP and HA groups after 24 weeks follow-up. In addition, no statistically significant differences were observed for the primary outcome measure, WOMAC pain subscale, or other secondary outcome measures at any follow-up time-points compared to the baseline. In summary, despite demonstrating no improvement in the WOMAC pain subscale, administration of PRP can lead to improvement in symptoms of knee OA patients.
Smith et al. [9] in a prospective, randomized, double-blind study investigated the safety and efficacy of ACP for the treatment of knee OA. One hundred fourteen patients were screened and 30 were included in the study. The included patients were randomized 1:1 into the ACP and saline groups and received three weekly intraarticular injections. ACP was formulated per manufacturer’s instructions. The primary outcome measure was WOMAC scale, evaluated at baseline and at one week, two weeks, and two, three, six, and 12 months follow-up. No adverse events associated with administration of ACP were reported throughout the duration of the study. Statistically significant improvements in overall WOMAC were observed beginning two weeks till 12 months follow-up for the ACP group compared to the baseline as well as the saline group. The main shortcomings of this study are small sample size and lack of active comparator. In summary, administration of ACP is safe and potentially efficacious in knee OA patients.
Cerza et al. [10] in a randomized controlled trial (RCT) evaluated the efficacy of PRP with HA in gonarthrosis patients. One hundred twenty patients were enrolled in this study. Patients were consecutively randomized to either receive four intraarticular injections of ACP or HA. The primary outcome measure was WOMAC scale, assessed at baseline and at four, 12 and 24 weeks post first injection. Statistically significant improvements in the WOMAC score were observed in the ACP group compared to both the baseline as well as the HA group at 24 weeks follow-up. The main limitations of the study include lack of blinding, small cohort size and short follow-up duration. In summary, administration of ACP led to better clinical outcomes compared to HA, and thus has potential to manage gonarthrosis patients.
Ongoing Clinical Studies
As of January 4, 2024, there are three clinical trials registered on ClinicalTrials.gov, CTRI, or ChiCTR to study the efficacy of ACP for the management of knee OA. These trials are summarized in Table 1.
Table 1. Ongoing clinical trials registered on ClinicalTrials.gov, Clinical Trials Registry – India and Chinese Clinical Trial Register till January 4, 2024, evaluating the efficacy of autologous conditioned plasma (ACP) for the management of knee osteoarthritis.
| Study Identifier | Biologic | Study Phase; Estimated Enrollment (N) | Primary Outcome Measure(s) | Recruitment Status | Country |
| NCT05765266 | ACP Max vs. Depo-Medrol | Not applicable; N=45 | Adverse Events – A comparison of the frequency and severity of all adverse events between the investigational group and the control group at 6 months; Adverse Events – Analysis of the frequency and severity of all adverse events for the investigational group at 12 months. | Not yet recruiting | USA |
| NCT03491761 | ACP vs. hylauronic acid | Phase II; N=100 | Cartilage Thickness on MRI - Evaluation of changes from baseline in central medial femorotibial compartment cartilage thickness measurements (via ordered value method) using quantitative T1 and cartilage compositional changes using T2 MRI at 6 and 12 months. | Unknown | USA |
| NCT06163573 | ACP vs. N-TEC (autologous tissue engineered cartilage graft) | Phase II; N=75 | Knee Injury and Osteoarthritis Outcome Score (KOOS) for pain - Mean change in Knee Injury and Osteoarthritis Outcome Score for pain from baseline to 24 months between groups Minimum score: 0 Maximum score :100 (higher score means no knee problems) | Not yet recruiting | Switzerland |
Discussion
The present study evaluated the therapeutic potential of ACP for the management of knee OA. Clinical studies focusing on the effect of ACP on knee OA were included. Based on our search criteria and inclusion/exclusion criteria, five clinical studies fit the scope of our manuscript. Three ongoing clinical studies are registered on clinical trial protocol registries.
The early RCTs by Smith et al. [9] and Cerza et al. [10] demonstrated that administration of intraarticular ACP led to significant improvements in WOMAC score at 12 months and 24 weeks, respectively. In contrast, RCT by Cole et al. [8] reported no significant improvement in the WOMAC score. Although significant improvements were observed in VAS and IKDC scores up to 52 weeks follow-up, a decline in scores was reported after 24 weeks [8]. Moreover, more recent studies by Korpershoek et al. [6,7] evaluated the efficacy of ACP in knee OA patients in a real-world clinical practice and reported significant improvements in the KOOS score at 12 months, however this improvement did not reach MCID. This was attributed to the low concentration of platelets (<5x compared to baseline, i.e., whole blood concentration) in the injected ACP formulation [6-8]. This is in accordance with published studies that reported that a platelet concentration between 5x-7x compared to the baseline is needed to promote cell proliferation, mesenchymal stem cell recruitment and wound healing [11,12]. To address this shortcoming, Arthrex Inc. has designed a new Arthrex MaxTM PRP system to allow for obtaining higher platelet concentrations [13], which appears to be under investigation (NCT05765266; Table 1). In addition to the low platelet concentration, a potential reason for contrasting results between studies carried out in real-world clinical practice [6,7] versus RCTs [8-10] is the controlled conditions of the RCTs, i.e., in an RCT study subjects are selected with the aim of minimizing comorbidity and the protocol is designed to ensure highest providers and participants compliance [14]. Even though RCTs are considered the most reliable and the majority of the clinical guidelines are provided based on the outcomes deduced from them, it does not represent the divergent characteristics of real-world populations [14,15]. Thus, it is essential to integrate the real-world evidence with RCTs to complement it and to enhance the entirety of evidence-based medicine assessments [14,15].
Conclusions
In conclusion, the aforementioned RCTs demonstrated the potential of ACP in alleviating symptoms associated with OA of the knee, however the MCID was not accomplished in real-world clinical settings. Thus, more adequately powered RCTs with longer follow-up as well as real-world post-market studies are warranted to establish long-term efficacy of ACP and justify its routine clinical use in patients suffering with knee OA.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Ashim Gupta
Acquisition, analysis, or interpretation of data: Ashim Gupta, Vijay Jain
Drafting of the manuscript: Ashim Gupta
Critical review of the manuscript for important intellectual content: Ashim Gupta, Vijay Jain
Supervision: Ashim Gupta
References
- 1.Allogenic umbilical cord tissue for treatment of knee osteoarthritis. Gupta A, Maffulli N. Sports Med Arthrosc Rev. 2022;30:162–165. doi: 10.1097/JSA.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Allogenic perinatal tissue for musculoskeletal regenerative medicine applications: a systematic review. Aratikatla A, Maffulli N, Rodriguez HC, Gupta M, Potty AG, Gupta A. Biomedicines. 2022;10 doi: 10.3390/biomedicines10123173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Effectiveness of the thermal genicular nerve radiofrequency ablation therapy under fluoroscopy in patients with non-operative advanced stage knee osteoarthritis: 1-year follow-up results. Kaya O, Şenel A, Batur ÖC, Gönder N, Ergen E, Peker B. Indian J Orthop. 2022;56:1033–1039. doi: 10.1007/s43465-022-00642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Efficacy and safety of platelet-rich plasma injections for the treatment of osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Xiong Y, Gong C, Peng X, et al. Front Med (Lausanne) 2023;10:1204144. doi: 10.3389/fmed.2023.1204144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autologous conditioned plasma and hyaluronic acid injection for isolated Grade 4 osteochondral lesions of the knee in young active adults. Rajeev A, Ali M, Devalia K. Cureus. 2022;14:0. doi: 10.7759/cureus.27787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Effect of autologous conditioned plasma injections in patients with knee osteoarthritis. Korpershoek JV, Vonk LA, Filardo G, Kester EC, van Egmond N, Saris DB, Custers RJ. Orthop J Sports Med. 2023;11:23259671231184848. doi: 10.1177/23259671231184848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Intra-articular injection with autologous conditioned plasma does not lead to a clinically relevant improvement of knee osteoarthritis: a prospective case series of 140 patients with 1-year follow-up. Korpershoek JV, Vonk LA, De Windt TS, et al. Acta Orthop. 2020;91:743–749. doi: 10.1080/17453674.2020.1795366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Cole BJ, Karas V, Hussey K, Pilz K, Fortier LA. Am J Sports Med. 2017;45:339–346. doi: 10.1177/0363546516665809. [DOI] [PubMed] [Google Scholar]
- 9.Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Smith PA. Am J Sports Med. 2016;44:884–891. doi: 10.1177/0363546515624678. [DOI] [PubMed] [Google Scholar]
- 10.Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Cerza F, Carnì S, Carcangiu A, et al. Am J Sports Med. 2012;40:2822–2827. doi: 10.1177/0363546512461902. [DOI] [PubMed] [Google Scholar]
- 11.A call for a standard classification system for future biologic research: the rationale for new PRP nomenclature. Mautner K, Malanga GA, Smith J, Shiple B, Ibrahim V, Sampson S, Bowen JE. PM R. 2015;7:0–9. doi: 10.1016/j.pmrj.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Giusti I, Rughetti A, D'Ascenzo S, Millimaggi D, Pavan A, Dell'Orso L, Dolo V. Transfusion. 2009;49:771–778. doi: 10.1111/j.1537-2995.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 13.Arthrex ACP MaxTM PRP system. [ Jan; 2024 ]. 2024. https://www.arthrex.com/orthobiologics/acp-max-system https://www.arthrex.com/orthobiologics/acp-max-system
- 14.Comparing clinical trial population representativeness to real-world populations: an external validity analysis encompassing 43895 trials and 5685738 individuals across 989 unique drugs and 286 conditions in England. Tan YY, Papez V, Chang WH, Mueller SH, Denaxas S, Lai AG. Lancet Healthy Longev. 2022;3:674–689. doi: 10.1016/S2666-7568(22)00186-6. [DOI] [PubMed] [Google Scholar]
- 15.Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. Kim HS, Lee S, Kim JH. J Korean Med Sci. 2018;33:0. doi: 10.3346/jkms.2018.33.e213. [DOI] [PMC free article] [PubMed] [Google Scholar]