Key Points
-
•
Among 1067 critically ill pediatric patients with HCT, survival to ICU discharge was 85%, but half of patients required ICU readmission.
-
•
For pediatric patients with HCT, overall survival 1-year after ICU admission was only 52% and survivors had excess long-term mortality risk.
Visual Abstract
Abstract
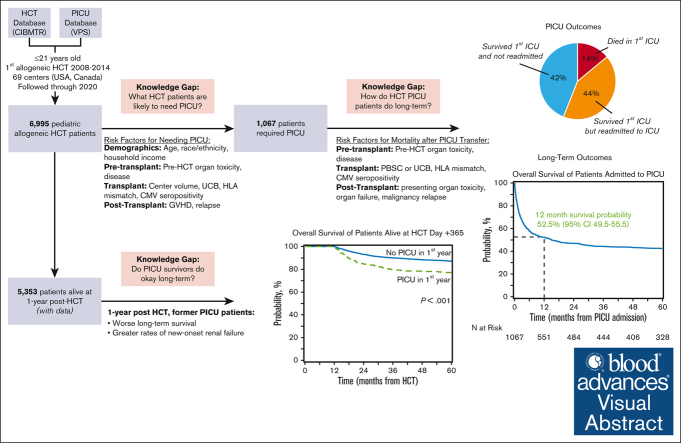
Allogeneic hematopoietic cell transplantation (HCT) can be complicated by life-threatening organ toxicity and infection necessitating intensive care. Epidemiologic data have been limited by single-center studies, poor database granularity, and a lack of long-term survivors. To identify contemporary trends in intensive care unit (ICU) use and long-term outcomes, we merged data from the Center for International Blood and Marrow Transplant Research and the Virtual Pediatric Systems databases. We identified 6995 pediatric patients with HCT aged ≤21 years who underwent first allogeneic HCT between 2008 and 2014 across 69 centers in the United States or Canada and followed patients until the year 2020. ICU admission was required for 1067 patients (8.3% by day +100, 12.8% by 1 year, and 15.3% by 5 years after HCT), and was linked to demographic background, pretransplant organ toxicity, allograft type and HLA-match, and the development of graft-versus-host disease or malignancy relapse. Survival to ICU discharge was 85.7%, but more than half of ICU survivors required ICU readmission, leading to 52.5% and 42.6% survival at 1- and 5-years post-ICU transfer, respectively. ICU survival was worse among patients with malignant disease, poor pretransplant organ function, and alloreactivity risk factors. Among 1-year HCT survivors, those who required ICU in the first year had 10% lower survival at 5 years and developed new dialysis-dependent renal failure at a greater rate (P<.001). Thus, although ICU management is common and survival to ICU discharge is high, ongoing complications necessitate recurrent ICU admission and lead to a poor 1-year outcome in select patients who are at high risk.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) offers a potential cure to over thousands of children annually across the world, including those with high-risk leukemia, immunodeficiencies, hemoglobinopathies, and other life-threatening disorders.1 However, a key barrier to HCT success is the development of acute organ failure because of chemotherapeutic toxicity, radiation exposure, infection, genetic predisposition, and impaired or dysregulated immunity.2,3 Children requiring intensive care unit (ICU) admission suffer >20% mortality, with rates exceeding 45% when intubation and mechanical ventilation are required.4, 5, 6
A prominent goal of transplant and intensive care physicians is to quickly and correctly identify patients who are at high risk to stop the advancement of critical illness and prevent irreversible organ failure.7 This is predicated based on compelling evidence indicating that organ failure can be modified by promptly recognizing and intervening in its early stages.8, 9, 10, 11 However, predicting who will require intensive care after HCT has been challenging because of relatively small number of patients spread out across multiple institutions with varying ICU admission criteria. Specifically, HCT databases do not capture ICU transfer data, and ICU databases do not rigorously phenotype HCT complexity, precluding deeper analyses of risk for critical illness.12
In addition, although recent reports have shown improved survival to ICU discharge, long-term outcomes are lacking because ICU databases do not typically follow patients after ICU discharge.4, 5, 6,13 In 1 report of pediatric patients with HCT who survived to pediatric ICU (PICU) discharge, survival at 1 year was 40% relative to 1-year survival of 65% in patients who never required PICU admission; this suggests an ongoing burden of chronic organ toxicity in these vulnerable patients.14 All major international HCT organizations recommend following all pediatric patients who undergo HCT for long-term cardiopulmonary, renal, and multiorgan toxicities as well as for neurodevelopmental outcomes and health-related quality of life.15, 16, 17 These recommendations are even more crucial in light of estimates that the number of pediatric survivors of HCT doubled between 2009 and 2020 and will likely double again between 2020 and 2030 to reach nearly 64 000 patients. Taken together, these data suggest that both short- and long-term outcomes for pediatric patients with HCT who are critically ill remain suboptimal and that the toxicity of HCT remains a major barrier to a disease-free childhood for many patients.18,19
To address ongoing knowledge gaps regarding risk-factors for critical illness and long-term outcomes, we merged records from 2 large research databases to determine the incidence of and risk-factors for post-HCT intensive care. We followed patients requiring PICU admission for a median of 5-years to determine long-term outcomes. To assess the burden of chronic toxicities, we also compared survival and organ dysfunction in 1-year transplant survivors according to a preceding need for intensive care in the first year.
Methods
Data sources
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a research collaboration between the National Marrow Donor Program/Be The Match and the Medical College of Wisconsin. It comprises over 450 transplant centers worldwide that contribute high-quality longitudinal data on consecutive allogeneic patients with HCT. The Virtual Pediatric Systems (VPS) database documents PICU admissions across 140 pediatric hospitals predominantly in the United States and Canada. Admission characteristics, severity of illness scores, critical care interventions, and critical care–related diagnosis codes are documented by trained analysts at each site, and patients are followed until hospital discharge.
The CIBMTR database was queried for patients who underwent first allogeneic HCT between 2008 and 2014 at an age of ≤21 years in the United States or Canada. Patients were excluded for allograft source other than bone marrow, peripheral blood, or umbilical cord blood (UCB); syngeneic donor; lack of consent to use data; or lack of ≥100 days follow-up because of reasons other than death. PICU admission after first allogeneic HCT was identified by matching records between CIBMTR and VPS databases as previously described4, and patients at an HCT site without any VPS matches were excluded. To avoid analyzing patients who were at low risk, PICU admissions that were both perioperative or scheduled (>12-hours notice) and lasting <2 days were excluded. All PICU admission data were benchmarked to the first HCT because data on repeat HCT could not be reliably differentiated from other cellular therapies (eg, donor lymphocyte infusion). Patients admitted to an adult ICU could not be included because they are not documented in VPS. Follow-up data were reported to CIBMTR and were abstracted for this study in the first quarter of 2020.
Outcomes
The primary outcomes were the need for intensive care and long-term mortality after intensive care. We performed a landmark analysis of all patients alive at transplant day +365 to determine if prior need for intensive care was associated with long-term mortality and morbidity, which included the occurrence and start date of the following predefined toxicities: congestive heart failure; noninfectious pulmonary dysfunction (interstitial pneumonitis and other noninfectious pulmonary abnormalities, including bronchiolitis obliterans, COP/BOOP, and diffuse alveolar hemorrhage); renal failure severe enough to warrant dialysis; noninfectious liver toxicity (including sinusoidal obstruction syndrome, cirrhosis, and other); stroke or seizure; and diabetes or hyperglycemia. Clinical changes in each toxicity, such as worsening, improvement, or resolution, were not captured in CIBMTR.
Covariates
We considered variables in the following categories: demographics, including age at HCT, sex, race, ethnicity, insurance status, ZIP code median income, weight classification at HCT; pre-HCT, including disease, disease status, organ dysfunction (estimated by the HCT comorbidity index [HCT-CI]), functional status (estimated by Lansky/Karnofsky score), history of mechanical ventilation, history of invasive fungal infection; HCT-related, including HCT center size (according to tertile of HCT volume during the study interval, <48, 48-122, or >122 allogeneic HCT performed), time from diagnosis to HCT, graft source, donor type or match, allograft manipulation, donor or recipient blood types, cytomegalovirus (CMV) infection status, sex matching, conditioning regimen intensity, conditioning regimen serotherapy, graft-versus-host disease (GVHD) prophylaxis regimen; post-HCT, including neutrophil engraftment, acute GVHD (aGVHD) grade, chronic GVHD (cGVHD) grade, hematologic malignancy relapse; ICU-related, including PICU center size (according to tertile of PICU admission volume of patient with HCT during the study interval, <5, 5-14, >14 HCT-PICU admissions), age and weight at PICU admission, time interval between HCT and PICU, organ dysfunction (estimated by Pediatric Risk of Mortality Score 3)20; use of invasive mechanical ventilation (IMV), noninvasive mechanical ventilation, or renal replacement therapy (RRT); and presence of gram-positive, gram-negative, viral, or fungal infection.
Statistical approach
Cumulative incidence of PICU admission was calculated with death as competing risk. Cox proportional hazard models were used to identify factors associated with the need for intensive care, and covariates not known at baseline (eg, aGVHD, cGVHD, and relapse) were treated as time-dependent covariates. Stepwise model selection was used to select the multivariable model. Overall survival (OS) probabilities after PICU admission were calculated using the Kaplan-Meier estimate. Cox proportional hazard regression with stepwise model selection was used to identify risk factors associated with post-ICU mortality. Next, we explored long-term survival in patients alive at 1 year after HCT. Survival after day +365 was estimated using Kaplan-Meier curves and factors associated with overall mortality were assessed using Cox proportional hazard regression with stepwise model selection. The development of post-HCT organ toxicities among patients alive at day +365 was assessed using the cumulative incidence function. Nominal significance was considered at a P value <.05.
Results
Patients
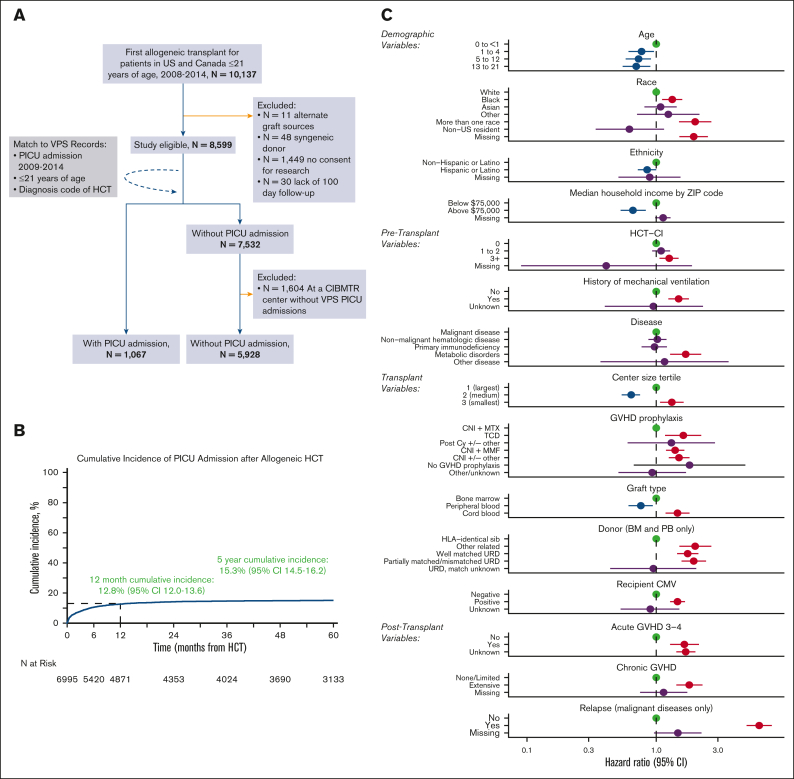
The final cohort included 6995 pediatric patients with HCT from 69 HCT centers; 1067 patients required critical care across 79 PICUs (Figure 1A). Baseline characteristics of included patients are listed in Table 1 and supplemental Table 1. The underlying reason for allogeneic HCT was malignancy in 57% of the patients. Myeloablative conditioning chemotherapy was used in 73% of patients, including total body irradiation in 35% of patients. HCT allografts were fully HLA-matched in 54% of patients and obtained most commonly from harvested bone marrow (57%). The largest third of HCT centers (23/69) accounted for 67% of all first allogeneic transplants performed.
Figure 1.
A total of 6995 pediatric patients with allogeneic HCT were included and ∼15% required intensive care by 5 years after HCT. (A) Inclusion or exclusion flow diagram. (B) Cumulative incidence of PICU admission after allogeneic HCT. (C) Factors independently associated with post–HCT-PICU admission in multivariable competing risk-regression model.
Table 1.
Baseline characteristics of patients needing PICU and non-PICU receiving allogeneic HCT between 2008 and 2014 at a center reporting to VPS
| Characteristic | PICU (n = 1 067) | Non-PICU (n = 5 928) | Total (n = 6 995) |
|---|---|---|---|
| Demographics | |||
| Age at transplant | |||
| 0 to <1 | 131 (12) | 534 (9) | 665 (10) |
| 1-4 | 250 (23) | 1 348 (23) | 1 598 (23) |
| 5-12 | 370 (35) | 2 135 (36) | 2 505 (36) |
| 13-21 | 316 (30) | 1911 (32) | 2 227 (32) |
| Sex | |||
| Male | 624 (59) | 3 480 (59) | 4 104 (59) |
| Female | 443 (41) | 2 448 (41) | 2 891 (41) |
| Race∗ | |||
| White | 692 (65) | 4 316 (73) | 5 008 (72) |
| Black or African American | 177 (17) | 810 (14) | 987 (14) |
| Asian | 53 (5) | 303 (5) | 356 (5) |
| Native Hawaiian or other Pacific Islander | 4 (0) | 13 (0) | 17 (0) |
| American Indian or Alaska native | 9 (1) | 53 (1) | 62 (1) |
| More than 1 race | 51 (5) | 128 (2) | 179 (3) |
| Ethnicity∗ | |||
| Hispanic or Latino | 234 (22) | 1 412 (24) | 1 646 (24) |
| Not Hispanic or Latino | 808 (76) | 4 309 (73) | 5 117 (73) |
| Nonresident of the United States | 11 (1) | 138 (2) | 149 (2) |
| Insurance group† | |||
| Private/military/dual insurance‡ | 246 (23) | 1 555 (26) | 1 801 (26) |
| Public insurance only | 180 (17) | 989 (17) | 1 169 (17) |
| Uninsured | 10 (1) | 44 (1) | 54 (1) |
| Median household income by ZIP code‡ | |||
| Median (IQR) | 51 985 (40 733-66 239) | 52 393 (41 536-69 484) | 52 348 (41 323-68 775) |
| BMI status at HCT§ | |||
| Underweight | 90 (8) | 493 (8) | 583 (8) |
| Normal | 497 (47) | 2 965 (50) | 3 462 (49) |
| Overweight (adult only) | 10 (1) | 64 (1) | 74 (1) |
| Obese | 114 (11) | 606 (10) | 720 (10) |
| Missing | 356 (33) | 1 800 (31) | 2 156 (32) |
| Pretransplant-related | |||
| HCT-CI∗ | |||
| 0 | 671 (63) | 3 967 (67) | 4 638 (66) |
| 1 | 152 (14) | 758 (13) | 910 (13) |
| 2 | 61 (6) | 323 (5) | 384 (5) |
| 3+ | 181 (17) | 835 (14) | 1 016 (15) |
| Performance score∗ | |||
| 100 | 554 (52) | 3 160 (53) | 3 714 (53) |
| 90 | 332 (31) | 1 832 (31) | 2 164 (31) |
| ≤80 | 164 (15) | 818 (14) | 982 (14) |
| History of mechanical ventilation∗ | |||
| No | 918 (86) | 5 358 (90) | 6 276 (90) |
| Yes | 143 (13) | 511 (9) | 654 (9) |
| History of invasive fungal infection∗ | |||
| No | 982 (92) | 5472 (92) | 6 454 (92) |
| Yes | 81 (8) | 399 (7) | 480 (7) |
| Disease group (subdisease breakdown insupplemental Table 1) | |||
| Malignant disease | 600 (56) | 3 413 (58) | 4 013 (57) |
| Nonmalignant hematologic disease | 264 (25) | 1 569 (26) | 1 833 (26) |
| Primary immunodeficiency | 134 (13) | 671 (11) | 805 (12) |
| Inherited disorders of metabolism | 66 (6) | 258 (4) | 324 (5) |
| Other disease | 3 (0) | 17 (0) | 20 (0) |
| Malignancy status before transplant‖ | |||
| Early disease | 218 (20) | 1 280 (22) | 1 498 (21) |
| Intermediate disease | 219 (21) | 1 264 (21) | 1 483 (21) |
| Advanced disease | 52 (5) | 299 (5) | 351 (5) |
| Transplant-related | |||
| Transplant center size | |||
| Tertile 1 (>122 allogeneic HCT) | 779 (73) | 3 876 (65) | 4 655 (67) |
| Tertile 2 (48-122 allogeneic HCT) | 184 (17) | 1 626 (27) | 1 810 (26) |
| Tertile 3 (<48 allogeneic HCT) | 104 (10) | 426 (7) | 530 (8) |
| Time from diagnosis to transplant for malignant diseases only (mo), median (min-max) | 7 (0-162) | 8 (0-200) | 8 (0-200) |
| Prescribed conditioning intensity | |||
| MAC | 761 (71) | 4 367 (74) | 5 128 (73) |
| RIC/NMA | 295 (28) | 1 498 (25) | 1 793 (26) |
| No conditioning | 9 (1) | 48 (1) | 57 (1) |
| Conditioning intensity/TBI use | |||
| MAC-TBI | 345 (32) | 2 091 (35) | 2 436 (35) |
| MAC-no TBI | 413 (39) | 2 262 (38) | 2 675 (38) |
| RIC/NMA | 295 (28) | 1 498 (25) | 1 793 (26) |
| No conditioning | 9 (1) | 48 (1) | 57 (1) |
| T-cell depletion, CD34+ selection, or ex vivo expansion | |||
| No | 993 (93) | 5 622 (95) | 6 615 (95) |
| Yes | 72 (7) | 302 (5) | 374 (5) |
| GVHD prophylaxis | |||
| T-cell depletion | 70 (7) | 279 (5) | 349 (5) |
| Post-CY ± other(s) | 7 (1) | 25 (0) | 32 (0) |
| CNI + MTX | 414 (39) | 2 902 (49) | 3 316 (48) |
| CNI + MMF | 374 (35) | 1 473 (29) | 2 117 (30) |
| CNI ± others | 187 (17) | 862 (15) | 1 049 (15) |
| Other/unknown | 11 (1) | 98 (2) | 109 (2) |
| No GVHD prophylaxis | 4 (0) | 19 (0) | 23 (0) |
| ATG/alemtuzumab use | |||
| ATG alone | 399 (37) | 2 307 (39) | 2 706 (39) |
| Alemtuzumab alone | 2 (0) | 10 (0) | 12 (0) |
| No ATG or alemtuzumab | 379 (36) | 2 391 (40) | 2 770 (40) |
| Missing | 287 (27) | 1 220 (21) | 1 507 (22) |
| Graft type | |||
| Bone marrow | 601 (56) | 3 421 (58) | 4 022 (57) |
| Peripheral blood | 155 (15) | 907 (15) | 1 062 (15) |
| UCB | 311 (29) | 1 600 (27) | 1 911 (27) |
| Donor type (BM and PB only)¶ | |||
| HLA-identical sibling | 186 (17) | 1 674 (28) | 1 860 (27) |
| Other related | 89 (8) | 340 (6) | 429 (6) |
| Well-matched unrelated (8/8) | 310 (29) | 1 578 (27) | 1 888 (27) |
| Partially matched unrelated (7/8)/mismatched unrelated (≤6/8) | 164 (15) | 666 (11) | 830 (12) |
| Unrelated, match unknown | 7 (1) | 66 (1) | 73 (1) |
| Donor/recipient CMV serostatus∗ | |||
| +/+ | 254 (24) | 1 217 (21) | 1 471 (21) |
| +/− | 77 (7) | 552 (9) | 629 (9) |
| −/+ | 224 (21) | 1 139 (19) | 1 363 (19) |
| −/− | 190 (18) | 1 346 (23) | 1 536 (22) |
| CB-recipient positive | 197 (18) | 841 (14) | 1 038 (15) |
| CB-recipient negative | 109 (10) | 733 (12) | 842 (12) |
| CB-recipient CMV unknown | 5 (0) | 26 (0) | 31 (0) |
| Donor/recipient ABO match† | |||
| Matched | 103 (10) | 729 (12) | 832 (12) |
| Minor mismatch | 46 (4) | 277 (5) | 323 (5) |
| Major mismatch | 37 (3) | 238 (4) | 275 (4) |
| Bidirectional | 11 (1) | 74 (1) | 85 (1) |
| CB | 252 (24) | 1 306 (23) | 1 558 (23) |
| Donor/recipient sex match∗ | |||
| M-M | 250 (23) | 1 539 (26) | 1 789 (26) |
| M-F | 165 (15) | 962 (16) | 1 127 (16) |
| F-M | 177 (17) | 1 007 (17) | 1 184 (17) |
| F-F | 163 (15) | 810 (14) | 973 (14) |
| CB-recipient M | 196 (18) | 927 (16) | 1 123 (16) |
| CB-recipient F | 115 (11) | 673 (11) | 788 (11) |
Baseline characteristics for all study participants.
ABO, ATG, antithymocyte globulin; BMI, body mass index; BM, bone marrow; CML, XX; CNI, calcineurin inhibitor; CB, cord blood; MTX, methotrexate; MMF, mycophenolate mofetil; MAC, myeloablative conditioning; NMA, nonmyeloablative conditioning; PB, peripheral blood; post-CY, posttransplant cyclophosphamide; RIC, reduced intensity conditioning; TBI, total body irradiation.
Variables with minimal missing data are noted as follows: race, n = 386 (6%); ethnicity, n = 83 (1%), HCT-CI, n = 47 (0%); performance score, n = 135 (2%); history of mechanical ventilation, n = 65 (1%); history of proven invasive fungal infection, n = 61 (1%); prescribed conditioning intensity, n = 17 (0%); conditioning intensity/TBI use, n = 34 (0%); T-cell depletion, n = 6 (0%); donor type, n = 1 (0%); donor/recipient CMV serostatus, n = 85 (1%); donor/recipient sex match, n = 11 (0%).
Variables with significant missing data are noted as follows: insurance n = 3971 (57%); ZIP code median income, n = 2630 (38%); donor/recipient ABO match, n = 3922 (56%). High missingness is due to variables only being collected for the approximately half of CIBMTR patients who participate in the data-intensive research track.
There are 8 cases who had both Medicare and Medicaid, these were grouped into dual insurance.
Children (aged from 2 to <20 years), BMI is adjusted by sex and age (months) according to Centers for Disease Control and Prevention smoothed growth charts with percentile cutoffs as follows: underweight (<5%), normal weight (5%-84.9%), overweight (85%-94.9%), and obese (≥95%). Adults aged ≥20 years, BMI is interpreted using standard weight status categories as follows: underweight (<18.5), normal weight (18.5-24.9), overweight (25.0-29.9), and obese (≥30.0). These categories are the same for men and women of all body types and ages.
Does not include NHL/HL, CML, unknown/MDS.
Donor type: multidonor n = 3 (0%).
Need for intensive care
After HCT, the cumulative incidence of PICU admission was 8.3% at day +100 (95% confidence interval [CI], 7.7-9.0), 12.8% at 1 year (95% CI, 12.0-13.6), and 15.3% at 5 years after HCT (95% CI, 14.5-16.2, Figure 1B).
Multivariable analysis with stepwise variable selection identified 15 risk factors independently associated with the need for intensive care (Figure 1C; supplemental Table 2). Among demographic factors, younger age, lower median ZIP code income, Black race (hazard ratio [HR], 1.33; 95% CI, 1.11-1.59; P = .002), multiracial background (HR, 2.0; 95% CI, 1.50-2.67; P < .001), and non-Hispanic ethnicity were each associated with PICU admission. Pretransplant organ toxicity was also associated with greater rates of PICU admission, as measured by an HCT-CI score of ≥3 (HR, 1.26; 95% CI, 1.06-1.49; P = .008) or a history of mechanical ventilation (HR, 1.49; 95% CI, 1.24-1.79; P < .001). Several transplant-related variables were associated with PICU admission, including transplantation at centers in the lowest tertile of transplant volume (HR, 1.32; 95% CI, 1.07-1.63; P = .009), HCT for underlying inborn errors of metabolism (HR, 1.69; 95% CI, 1.28-2.23; P < .001), and recipient CMV positivity (HR, 1.46; 95% CI, 1.28-1.67; P < .001). PICU admission was also associated with the use of UCB allografts (HR, 1.46; 95% CI, 1.18-1.81; P < .001) and donors other than HLA-identical siblings (eg, partially matched, unrelated donor HR, 1.95; 95% CI, 1.57-2.43; P < .001; supplemental Table 2). Finally, the development of posttransplant complications was strongly associated with subsequent PICU admission, including aGVHD grade 3 to 4 (HR, 1.65; 95% CI, 1.28-2.14; P < .001), extensive cGVHD (HR, 1.80; 95% CI, 1.43-2.28; P < .001), and post-HCT relapse of malignancy (HR, 6.26; 95% CI, 5.00-7.84; P < .001).
Outcomes after intensive care admission
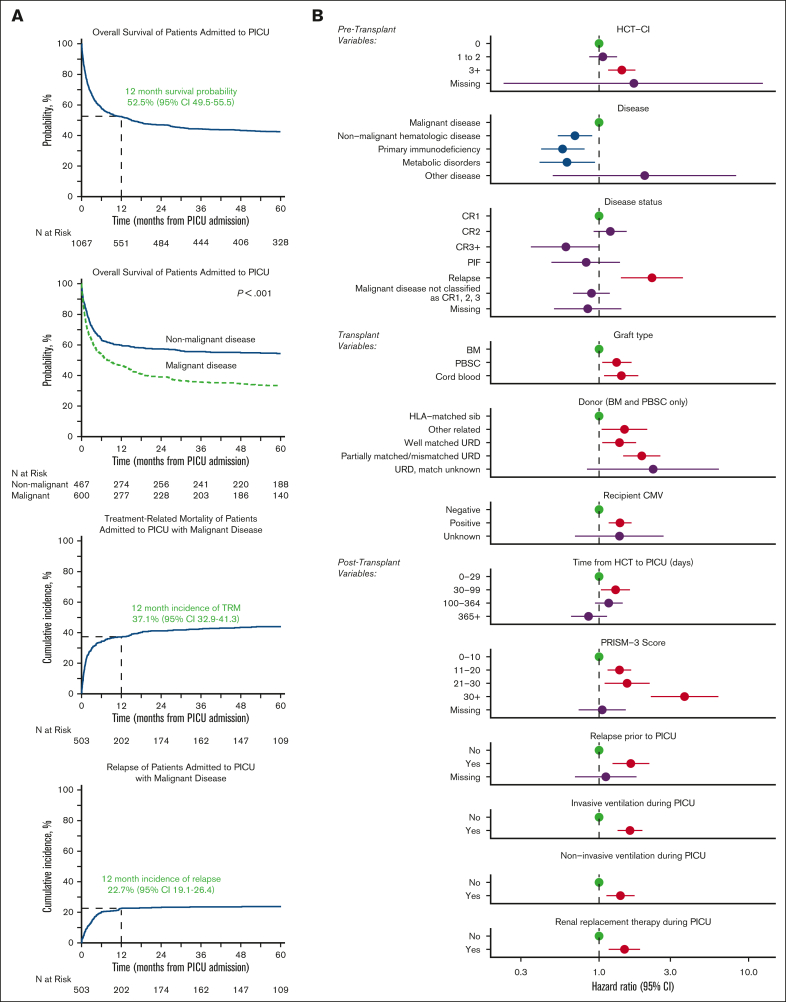
Characteristics of patients at the time of first PICU admission are listed in Table 2. The per-ICU admission survival rate was 85.7% with a median ICU length of stay of 3 days (interquartile range [IQR], 1-6) among ICU survivors and 16 days (IQR, 4-32) among ICU nonsurvivors. Among the patients requiring IMV, the median duration of IMV was 5 days (IQR, 1-10) among ICU survivors and 13 days (IQR, 3-25) among ICU nonsurvivors. Similarly, among the patients requiring RRT, the median duration of RRT was 6 days (IQR, 3-15) among ICU survivors and 9 days (IQR, 3-26) among ICU nonsurvivors. Although 14% died in the first ICU admission, 44% survived but required at least 1 additional PICU admission, and 42% of patients survived and did not require PICU readmission during the study interval. Using CIBMTR longitudinal data, patients were followed from the time of ICU admission to a median 73 months (range, 3-147). The OS from the time of first PICU admission was 52.5% at 1 year (95% CI, 49.5-55.5), 44.4% at 3 years (95% CI, 41.5-47.5), and 42.6% at 5 years (95% CI, 39.6-45.6, Figure 2A; supplemental Tables 3 and 4).
Table 2.
Additional characteristics at PICU admission (n = 1067)
| Characteristic | N (%) |
|---|---|
| ICU-related | |
| PICU size, groups created based on total number of admissions | |
| Tertile 1 (>14 HCT-PICU admissions) | 821 (77) |
| Tertile 2 (5-14 HCT-PICU admissions) | 175 (17) |
| Tertile 3 (<5 HCT-PICU admissions) | 72 (7) |
| Number of ICU admissions per patient | |
| 1 | 597 (56) |
| 2 | 248 (23) |
| 3+ | 222 (21) |
| Age at first PICU admission (y)∗ | |
| 0 to <1 | 98 (9) |
| 1-4 | 259 (24) |
| 5-12 | 365 (34) |
| 13-21 | 344 (32) |
| BMI status at PICU admission† | |
| Underweight | 90 (8) |
| Normal | 497 (47) |
| Overweight (adult only) | 10 (1) |
| Obese | 114 (11) |
| Missing | 356 (33) |
| PRISM-3 score (median, IQR) | 9 (4-14) |
| Critical care therapies in first ICU admission | |
| IMV | 395 (37) |
| Noninvasive mechanical ventilation | 175 (16) |
| RRT | 126 (12) |
| Infections in first ICU admission | |
| Gram-positive infection | 115 (11) |
| Gram-negative infection | 87 (8) |
| Fungal infection | 94 (9) |
| Viral infection | 273 (25) |
| Sepsis, Severe Sepsis, or Septic Shock | 256 (24) |
| Transplant-related | |
| Time from HCT to first ICU admission (mo), median (min-max) | 3 (0-70) |
| Neutrophil recovery relationship to first ICU admission∗ | |
| Neutrophil engrafted before first ICU admission | 761 (71) |
| Neutrophil engrafted after first ICU admission and before first ICU discharge | 89 (8) |
| Neutrophil engrafted after ICU discharge | 122 (11) |
| Never achieved neutrophil engraftment | 77 (7) |
| aGVHD relationship to first ICU admission∗,‡ | |
| No aGVHD | 299 (65) |
| aGVHD before first ICU admission | 124 (27) |
| Grade 1-2 | 53 (11) |
| Grade 3-4 | 69 (15) |
| aGVHD after first ICU admission and before first ICU discharge | 12 (3) |
| Grade 1-2 | 3 (0) |
| Grade 3-4 | 9 (2) |
| aGVHD after first ICU discharge | 27 (6) |
| Grade 1-2 | 11 (2) |
| Grade 3-4 | 16 (4) |
| cGVHD relationship to first ICU admission∗ | |
| No cGVHD | 748 (70) |
| cGVHD before first ICU admission | 130 (12) |
| Limited | 33 (3) |
| Extensive | 94 (9) |
| cGVHD after first ICU admission | 163 (15) |
| Limited | 43 (4) |
| Extensive | 119 (11) |
| Malignancy relapse before first ICU visit | 103 (10) |
Additional characteristics at time of PICU admission.
BMI, body mass index; PRISM-3; Pediatric Risk of Mortality Score.
Variables with minimal missing data are noted as follows: age at first PICU admission n = 1 (0%); neutrophil recovery relationship to first PICU admission n = 18 (2%); aGVHD grade unknown n = 2 (0%); cGVHD relationship to first PICU admission, missing n = 26 (2%), cGVHD grade unknown n = 4 (0%).
Children (aged from 2 to <20), BMI is adjusted by sex and age (mo) according to Centers for Disease Control and Prevention smoothed growth charts with percentile cutoffs as follows: underweight (<5%), normal weight (5%-84.9%), overweight (85%-94.9%), and obese (≥95%). Adults aged ≥20 years, BMI is interpreted using standard weight status categories as follows: underweight (<18.5), normal weight (18.5-24.9), overweight (25.0-29.9), and obese (≥30.0). These categories are the same for men and women of all body types and ages.
Variables with significant missing data are noted as follows: aGVHD relationship to first PICU admission, missing or not collected n = 605 (56%).
Figure 2.
Of 1067 patients requiring intensive care, OS was ∼52% by 1 year after ICU admission. (A) Kaplan Meier estimates of OS from the time of PICU admission for all patients (top left) and malignant vs nonmalignant patients (top middle). Cumulative incidence of TRM (bottom left) and relapse (bottom middle) among patients who underwent transplantation for malignancy are also shown. (B) Factors independently associated with long-term survival from the time of PICU admission to last follow-up in multivariable Cox regression.
On multivariable analysis, we identified 12 risk factors independently associated with OS among those requiring intensive care (Figure 2B; supplemental Table 5). Although several demographic factors, such as age, race, and ethnicity were associated with the need for intensive care admission, once patients were admitted to the ICU, demographics and center size were not independently associated with post-ICU survival. Although HCT for inborn errors of metabolism was associated with greater rates of ICU admission, among those admitted to the ICU, HCT for malignant disease was instead associated with worse long-term survival. This was particularly true of patients with malignancy with relapsed disease at the time of transplant (HR, 2.26; 95% CI, 1.40-3.64; P < .001) or relapsed disease after HCT (HR, 1.63; 95% CI, 1.23-2.17; P < .001). In addition to being risk-factors for ICU admission, pretransplant organ toxicity (HCT-CI ≥ 3; HR, 1.42; 95% CI, 1.15-1.75; P < .001), use of UCB allografts (HR, 1.41; 95% CI, 1.08-1.83; P = .012), and allograft donors other than HLA-identical siblings (eg, partially matched unrelated donor HR, 1.93; 95% CI, 1.45-2.57; P < .001; supplemental Table 5) were each associated with worse post-ICU survival. Interestingly, although 29% of patients did not have neutrophil engraftment at the time of PICU admission, most of these patients achieved neutrophil engraftment during or after ICU stay, and neutrophil engraftment status at the time of PICU admission was not associated with post-ICU survival (P > .05). We considered that post-HCT complications may vary according to time after HCT, with some complications appearing in the early neutropenic phase and others, months later. In this analysis, we found that patients who required PICU admission after day +30 had worse long-term survival compared with those who required PICU in the first 30 days (ie, PICU admission 30-99 days after HCT; HR, 1.29; 95% CI, 1.0-31.61; P = .029).
Analysis of 1-year survivors
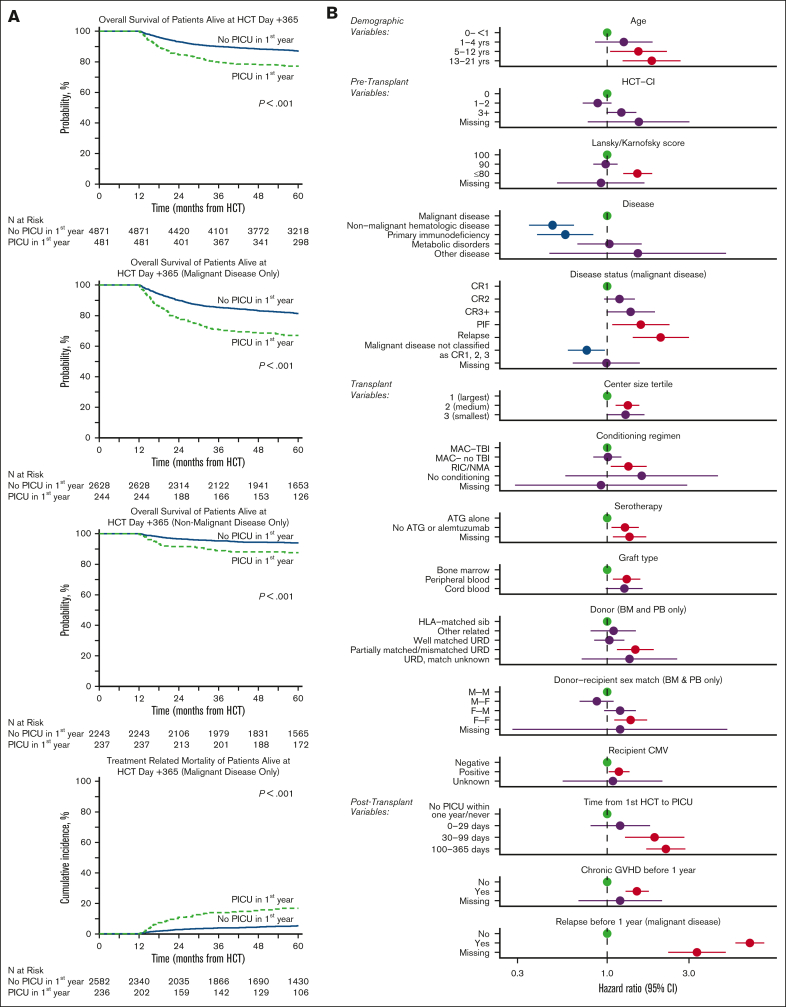
Among the subset of the cohort who were alive with follow-up at day +365 (n = 5353 patients), ∼9% required intensive care before day +365 (n = 481). When these 1-year survivors were followed to 5 years after HCT, the OS was ∼10% lower among those who had required intensive care in the first year (77.1% [95% CI, 73.2-80.8] vs 87.0% [95% CI, 86.1-88]; P < .001), corresponding to a HR of 1.82 (95% CI, 1.50-2.22; P < .001; Figure 3A; supplemental Table 6). Cause-specific mortality among the subset with malignancy showed an approximately fourfold excess burden of treatment-related mortality (TRM) by transplant year 5 in the PICU-exposed group relative to the PICU-unexposed (19.4% [95% CI, 14.1-25.2] vs 5.6% [95% CI, 4.7-6.6]; P < .001; Figure 3A). In contrast, rates of relapse after 1 year were comparable regardless of PICU exposure (supplemental Tables 7 and 8).
Figure 3.
Among 5353 patients alive at 1 year after HCT, those who survived ICU in the first year had worse 5-year survival than those who never required ICU. (A) Landmark analysis of only patients surviving to HCT day +365. Kaplan-Meier estimates of OS from transplant day +365 for all patients (top left), those with malignant disease (top middle), and those with nonmalignant disease (bottom left), stratified by need for intensive care in the first year after HCT. Cumulative incidence of TRM (bottom middle) among patients who underwent transplantation for malignancy is also shown. (B) Among those alive at HCT day +365, factors independently associated with long-term survival from transplant day +365 to last follow-up in multivariable Cox regression.
On multivariable analysis, the excess mortality after day +365 among PICU-exposed was most pronounced in those who required PICU after day +30. Specifically, PICU admission between HCT day 30 and 99 (HR, 1.89; 95% CI, 1.27-2.82) or between HCT day 100 and 365 (HR, 2.20; 95% CI, 1.69-2.86; P < .001) were associated with worse outcomes among 1-year survivors, whereas PICU admission in the first 30 days after HCT did not have excess mortality compared with patients who did not require PICU if alive at 1 year (HR, 1.19; 95% CI, 0.80-1.78; P = .395). This finding was robust to adjustment for several other factors, including pretransplant illness severity (HCT-CI, Karnofsky score), transplant variables (recipient CMV-positive, malignant disease, use of peripheral blood or UCB allograft, allograft donor other than HLA-matched sibling), and post-HCT complications (development of cGVHD or relapse before 1 year; Figure 3B; supplemental Table 9).
Organ toxicity data from CIBMTR were available for approximately half of the patients. Patients who required critical care in the first year after HCT showed a greater prevalence of renal failure, diabetes, liver toxicity, stroke or seizure, and noninfectious pulmonary dysfunction at 1-year after HCT (P < .001), demonstrating that the burden of illness persisted beyond the immediate PICU admission for some patients (supplemental Table 10). Patients who required critical care in the first year after HCT also showed significantly greater risk of developing new-onset renal failure after day +365 (increase of 4.9% in PICU-exposed vs 1.6% in PICU nonexposed, P < .001; Table 3). We did not detect a differential increase in the development of diabetes, liver toxicity, stroke or seizure, or noninfectious pulmonary dysfunction after day +365 according to whether PICU was required in the first year, although we could not account for changes or progression in the severity of existing disease.
Table 3.
Reported organ toxicities among 1-year HCT survivors
| Outcomes | Alive at day +365 without PICU (N = 2157) |
Alive at day +365 with PICU (N = 214) |
P value | ||
|---|---|---|---|---|---|
| N | Prob (95% CI), % | N | Prob (95% CI), % | ||
| Congestive heart failure | 2116 | 206 | |||
| 1-y | 2106 | 0.5 (0.3-0.9) | 206 | 0.5 (0-1.9) | |
| 2-y | 1912 | 0.8 (0.5-1.2) | 171 | 1 (0.1-2.8) | .769 |
| 3-y | 1789 | 1 (0.6-1.5) | 155 | 1 (0.1-2.8) | |
| 5-y | 1435 | 1.2 (0.7-1.7) | 126 | 1 (0.1-2.8) | |
| Renal failure requiring dialysis | 2113 | 206 | |||
| 1-y | 2081 | 1.6 (1.1-2.1) | 187 | 9.7 (6-14.1) | |
| 2-y | 1889 | 2.7 (2-3.4) | 153 | 13.6 (9.3-18.6) | <.001 |
| 3-y | 1768 | 2.9 (2.2-3.6) | 138 | 14.6 (10.1-19.8) | |
| 5-y | 1420 | 3.2 (2.5-4) | 111 | 14.6 (10.1-19.8) | |
| Diabetes/hyperglycemia | 2026 | 200 | |||
| 1-y | 1923 | 5.1 (4.2-6.1) | 174 | 13.5 (9.1-18.6) | |
| 2-y | 1738 | 5.9 (4.9-6.9) | 142 | 14 (9.5-19.2) | .371 |
| 3-y | 1622 | 6 (5-7.1) | 127 | 14 (9.5-19.2) | |
| 5-y | 1309 | 6.5 (5.4-7.6) | 105 | 14 (9.5-19.2) | |
| Noninfectious liver toxicity | 2112 | 205 | |||
| 1-y | 1718 | 18.7 (17.1-20.4) | 146 | 29.3 (23.2-35.7) | |
| 2-y | 1543 | 20.7 (19-22.4) | 118 | 30.7 (24.6-37.2) | .683 |
| 3-y | 1435 | 21.2 (19.5-23) | 107 | 31.3 (25.1-37.8) | |
| 5-y | 1102 | 22 (20.3-23.8) | 82 | 31.3 (25.1-37.8) | |
| Stroke/seizure | 2116 | 205 | |||
| 1-y | 2051 | 3.1 (2.4-3.9) | 175 | 15.1 (10.5-20.4) | |
| 2-y | 1846 | 4.6 (3.7-5.5) | 146 | 17.1 (12.2-22.5) | .081 |
| 3-y | 1723 | 5.1 (4.2-6.1) | 132 | 18.6 (13.6-24.2) | |
| 5-y | 1336 | 5.5 (4.6-6.6) | 105 | 18.6 (13.6-24.2) | |
| Noninfectious pulmonary dysfunction | 1987 | 175 | |||
| 1-y | 1845 | 7.2 (6.1-8.4) | 136 | 22.9 (16.9-29.4) | |
| 2-y | 1656 | 9.9 (8.6-11.2) | 109 | 27.5 (21.1-34.4) | .270 |
| 3-y | 1550 | 10.4 (9.1-11.7) | 102 | 27.5 (21.1-34.4) | |
| 5-y | 1252 | 10.7 (9.4-12.1) | 87 | 27.5 (21.1-34.4) | |
Organ toxicities reported in CIBMTR (evaluable for the data-intensive research track only). P values are based on significance tests comparing the cumulative incidence between the 2 subpopulations through years 2 to 5. Incidence of late effects within the first year does not have an impact on this P value. One-year estimates are reported as descriptive estimates only.
Discussion
We report an ∼15% cumulative incidence of intensive care admission within 5 years after pediatric HCT, with 85% survival to first ICU discharge and 52% survival at 1 year after first ICU admission. Importantly, ICU survivors alive at 1 year after HCT still had worse long-term outcomes, including OS, TRM, and new dialysis-dependent renal failure when followed to 5 years after HCT. Together, these data suggest an ongoing burden of toxicity in pediatric patients with HCT that continues to limit long-term survival.
First, our report of ∼8% cumulative incidence of PICU admission within the first 100 days and 15% by year 5 is less than other single-center reports citing 17% to 35%, which may be because of increased intensity of supportive care outside of the ICU, our exclusion of perioperative and planned ICU admissions, or other factors.21, 22, 23 Of 69 transplant centers, the third of centers with the highest HCT volume (n = 23 centers) accounted for approximately two-thirds of HCT volume and PICU admissions, demonstrating the regionalization of care of these patients who are at high risk. Patients who underwent transplantation at a smaller center had increased adjusted risk of ICU admission. The risk for ICU admission associated with younger age and underlying inherited disorders of metabolism may be related to more challenging fluid and airway management in these patients.24, 25, 26 The identification of Black and multiracial background and lower median ZIP code income as risk-factors for post–HCT-PICU admission merits further investigation, although these factors were not associated with survival after PICU admission.27, 28, 29, 30 Certain GVHD prophylaxis regimens, such as TCD and calcineurin inhibitor + mycophenolate mofetil were also associated with PICU admission; however, it is unclear if that is related to their association with haploidentical and/or umbilical cord transplants or some other factor that merits future exploration. Finally, the impact of measures of pre-HCT organ toxicity (higher HCT-CI and history of mechanical ventilation) emphasizes the need to optimize organ function in HCT candidates and modify the approach to HCT to increase safety for patients who are medically frail. Interestingly, time to neutrophil engraftment was not associated with need for intensive care.
Of the patients who required intensive care, we identified a significant discrepancy between survival to ICU discharge (86%) and survival to 1 year after ICU discharge (53%). Previous reports have suggested ICU survival as low as 20% to 40%, precluding long-term analyses because of fewer survivors.23,31, 32, 33, 34 Our data suggest that although survival to ICU discharge is contemporaneously feasible, nearly half of the number of patients required subsequent ICU readmission during the study interval, which could be due to pre-existing medical frailty or ongoing problems, such as alloreactivity or poor immune function. As such, survival with future ICU readmission was the most common outcome (44%), ahead of survival without future ICU admission (42%) or ICU-related death (14%). Therefore, preservation of organ function during each episode of critical illness is crucial to optimizing survival in future episodes of critical illness.35 The pediatric risk of mortality score 3, a metric of multiorgan dysfunction measured in the first 12 hours of PICU transfer, was strongly associated with long-term outcomes independent of the need for mechanical ventilation or dialysis, which indicates the importance of the timing of organ dysfunction relative to ICU care. Studies of early transfer before clinical decompensation have shown promising results in reducing adverse events.36,37 Of note, although 26% of patients had not achieved neutrophil engraftment at the time of PICU transfer, this was not associated with worse mortality, and the majority of the patients (73%) went on to achieve neutrophil engraftment, suggesting that aggressive supportive care ought not be withheld from patients purely based on neutrophil engraftment status.38
Finally, in a landmark analysis of all HCT patients alive at day +365, we found that those who required intensive care in the first year had 10% greater absolute mortality when followed for 5 years after HCT than patients who did not require intensive care in the first year, which was largely attributable to a fourfold increased risk of TRM. Studies of long-term outcomes in children who were critically ill are sparse. Duncan et al previously showed worse 1-year outcomes in survivors of intensive care; our study extends these findings to 5-years and emphasizes the chronicity of many posttransplant complications.14 This risk appeared highest in patients requiring PICU after day +30, again suggesting that transplant complications, such as alloreactivity and poor immune function may bear worse prognosis whereas early toxicities related to neutropenia, engraftment, and early fluid overload may be more manageable.
Given the high OS among patients alive at day +365, we analyzed the incidence of specific organ toxicities, including development before and after day +365. As expected, patients who used intensive care in the first year and survived to day +365 had significantly greater rates of organ toxicity in the first year. Although Broglie et al recently reported a 12% incidence of noninfectious pulmonary toxicity at 1-year in all pediatric recipients of HCT, our data suggest a much greater burden of ∼23% at 1 year among those who survived critical illness.2 Because pulmonary function test abnormalities may persist beyond 1 to 2 years after HCT in many children, this highlights the need for special attention to follow-up among patients who required intensive care.3,39,40 However, after 1 year, there was a relatively small proportion of patients who developed new noninfectious organ toxicities, ranging from 0.6% to 6%. Interestingly, those who required intensive care in the first year had a greater incidence of new-onset, dialysis-dependent renal failure after day +365, which is consistent with recent reports and suggests a subset of patients with ongoing progression of acute kidney injury or chronic kidney disease pathobiology.41 Efforts to monitor, mitigate, and address chronic toxicities of pediatric HCT should remain a priority for the field.
To our knowledge, this is the largest reported cohort of pediatric patients with HCT who are critically ill and builds on our prior work by including noncritically ill HCT controls as well as high-quality follow-up to 5 years after intensive care. Nonetheless, several limitations warrant discussion. First, transplant and ICU practices may have changed since this cohort was merged; further efforts to streamline multidatabase merging so as to deliver more contemporary data are needed. Second, repeat HCT could not be differentiated from donor lymphocyte infusion in the CIBMTR database and therefore was not addressed. Third, CIBMTR organ toxicity fields are only reported for research-level participants, include somewhat broad categories, and identify the start point of a complication but do not indicate progression or resolution. Fourth, ∼1500 patients were excluded from CIBMTR use because of lack of consent, which might introduce selection bias. Fifth, time from first allogeneic HCT to first PICU admission ranged considerably, and the factors associated with early PICU admission (ie, within 30 days) may be different than those associated with later PICU admission (ie, after day +100).
Conclusion
In summary, we report a 15% cumulative incidence of ICU admission within 5 years after pediatric HCT, with 85% survival to first ICU discharge and 52% survival at 1 year after first ICU admission. Major risk factors for critical illness included measures of pre-HCT organ toxicity and predisposition to (or development of) post-HCT alloreactivity or malignancy relapse. ICU survivors alive at 1 year after HCT had worse long-term outcomes, including OS, TRM, and new dialysis-dependent renal failure when followed to 5 years after HCT. However, accrual of other new organ toxicities was minimal among 1-year survivors. These data can be used for patient prognostication and should be targeted in future investigations focused on improving ICU outcomes after pediatric allogeneic HCT.
Conflict of interest disclosure: A.S. has received consultant fee from Spotlight Therapeutics, Medexus Inc, Vertex Pharmaceuticals, Sangamo Therapeutics and Editas Medicine. He is a medical monitor for RCI BMT CSIDE clinical trial for which he receives financial compensation. He has also received research funding from CRISPR Therapeutics and honoraria from Vindico Medical Education. A.S. is the St. Jude Children’s Research Hospital–site principal investigator of clinical trials for genome editing of sickle cell disease sponsored by Vertex Pharmaceuticals/CRISPR Therapeutics (NCT03745287), Novartis Pharmaceuticals (NCT04443907) and Beam Therapeutics (NCT05456880). The industry sponsors provide funding for the clinical trial, which includes salary support paid to A.S.’s institution. A.S. has no direct financial interest in these therapies. G.G. is the Principal Investigator of Project Sickle Cure, a Sickle Cell Transplant Advocacy and Research Alliance (STAR) study partially funded by bluebird bio. H.S. reports having received personal fees from Incyte, Janssen, Novartis, Sanofi and from the Belgian Hematological Society (BHS), as well as research grants from Novartis and the BHS, all paid to her institution and not related to this work. H.S. has also received nonfinancial support (travel grants) from Gilead, Pfizer, the European Society for Blood and Marrow Transplantation and the Center for International Bone Marrow Transplantation Research. M.S. served as a consultant and received honoraria from Jazz Pharmaceutical Canada. He received research funding from National Cancer Institute, PCORI, and Bluenote. C.C.D. reports consulting with Jazz Pharma and Alexion Inc. R.P. reports compensation from bluebird bio (advisory board) and Amgen (research funding; ended December 2021). K.M. reports significant payments: investigator initiated clinical trial sponsored by Incyte for early intervention for pulmonary dysfunction after stem cell transplant with ruxolitinib and industry sponsored clinical trial for ex vivo telomere elongation for patients with telomere biology disorders by Elixirgen Therapeutics and Proprietary Interests. K.M. has filed US provisional patent application #63/061,334, on 5 August 2021, entitled “Composition and Methods for the Treatment of Bronchiolitis Obliterans.” T.N. reports significant payments: research support to the institution for clinical trial (Novartis), research support (drug supply only) to the institution for clinical trial (Karyopharm) and relationships, advisory board membership (Medexus). The remaining authors report no competing financial interests.
Acknowledgments
Virtual Pediatric Systems (VPS) data were provided by Virtual Pediatric Systems, LLC. No endorsement or editorial restriction of the interpretation of these data or opinions of the authors has been implied or stated. This manuscript has been reviewed by the VPS Research Committee.
Funding for the study was provided by National Heart, Lung, and Blood Institute (NHLBI; NHLBI K23HL146936 (M.S.Z.). The Center for International Blood and Marrow Transplant Research is supported primarily by Public Health Service grant U24CA076518 from the National Cancer Institute, the NHLBI, and the National Institute of Allergy and Infectious Diseases; grant HHSH250201700006C from the Health Resources and Services Administration; grants N00014-21-1-2954 and N00014-23-1-2057 from the Office of Naval Research. Support was also provided by Be The Match Foundation, the Medical College of Wisconsin, the National Marrow Donor Program, and from the following commercial entities: AbbVie; Actinium Pharmaceuticals; Adaptimmune; Adaptive Biotechnologies Corporation; ADC Therapeutics; Adienne SA; Allogene; AlloVir, Inc; Amgen; Angiocrine; Anthem; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics; BeiGene; bluebird bio; Bristol Myers Squibb; CareDx; CRISPR; CSL Behring; CytoSen Therapeutics; Eurofins Viracor, DBA Eurofins Transplant Diagnostics; Gamida-Cell, Ltd; Gilead; GlaxoSmithKline; HistoGenetics; Incyte Corporation; Iovance; Janssen Research & Development, LLC; Janssen/Johnson & Johnson; Jasper Therapeutics; Jazz Pharmaceuticals; Kadmon; Karius; Kiadis Pharma; Kite, a Gilead company; Kyowa Kirin; Legend Biotech; Magenta Therapeutics; Mallinckrodt Pharmaceuticals; Medexus Pharma; Merck & Co; Mesoblast; Millennium, the Takeda Oncology Co; Miltenyi Biotec; MorphoSys; Novartis Pharmaceuticals Corporation; Omeros Corporation; OptumHealth; Orca Biosystems, Inc.; Ossium Health, Inc.; Pfizer; Pharmacyclics, LLC, an AbbVie company; Pluristem; PPD Development, LP; Sanofi; Sanofi-Aventis U.S. Inc; Sobi; StemCyte; Takeda Pharmaceuticals; Talaris Therapeutics; Terumo Blood and Cell Technologies; TG Therapeutics; Vertex Pharmaceuticals; Vor Biopharma Inc; Xenikos BV.
Authorship
Contribution: M.S.Z., R.B., J.S., S.C., S.B.-S., C.D., R.P., and C.C.D. developed study concept and designed the study; M.S.Z., R.B., J.S., S.C., S.B.-S., C.D., R.P., and C.C.D. contributed to data acquisition; all authors contributed to the analysis and interpretation of data; M.S.Z., R.B., R.P., and C.C.D. drafted the manuscript; all authors performed critical revision of the manuscript for important intellectual content; M.S.Z., R.B., J.S., S.C., S.B.S., and R.P. carried out statistical analysis; M.S.Z., R.B., J.S., S.C., S.B.S., C.D., R.P., and C.C.D. provided administrative, technical, or material support; M.S.Z., R.B., C.D., R.P., and C.C.D. supervised the study; and all authors approved the final manuscript.
Footnotes
Center for International Blood and Marrow Transplant Research (CIBMTR) supports accessibility of research in accordance with the National Institutes of Health data sharing policy and the National Cancer Institute Cancer Moonshot Public Access and Data Sharing Policy. The CIBMTR only releases deidentified data sets that comply with all relevant global regulations regarding privacy and confidentiality.
Requests for datasets will be submitted to CIBMTR and Virtual Pediatric Systems Scientific Committees for review.
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.D’Souza A, Fretham C, Lee SJ, et al. Current use and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant. 2020;26(8):e177–182. doi: 10.1016/j.bbmt.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broglie L, Fretham C, Al-Seraihy A, et al. Pulmonary complications in pediatric and adolescent patients following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(10):2024–2030. doi: 10.1016/j.bbmt.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaya Z, Weiner DJ, Yilmaz D, Rowan J, Goyal RK. Lung function, pulmonary complications, and mortality after allogeneic blood and marrow transplantation in children. Biol Blood Marrow Transplant. 2009;15(7):817–826. doi: 10.1016/j.bbmt.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Zinter MS, Logan BR, Fretham C, et al. Comprehensive prognostication in critically ill pediatric hematopoietic cell transplant patients: results from Merging the Center for International Blood and Marrow Transplant Research (CIBMTR) and Virtual Pediatric Systems (VPS) Registries. Biol Blood Marrow Transplant. 2020;26(2):333–342. doi: 10.1016/j.bbmt.2019.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zinter MS, Dvorak CC, Spicer A, Cowan MJ, Sapru A. New insights into multicenter PICU mortality among pediatric hematopoietic stem cell transplant patients. Crit Care Med. 2015;43(9):1986–1994. doi: 10.1097/CCM.0000000000001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowan CM, McArthur J, Hsing DD, et al. Acute respiratory failure in pediatric hematopoietic cell transplantation: a multicenter study. Crit Care Med. 2018;46(10):e967–e974. doi: 10.1097/CCM.0000000000003277. [DOI] [PubMed] [Google Scholar]
- 7.Elbahlawan L, Srinivasan A, Morrison RR. A critical care and transplant-based approach to acute respiratory failure after hematopoietic stem cell transplantation in children. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inwald DP, Tasker RC, Peters MJ, Nadel S, Paediatric Intensive Care Society Study Group PICS-SG Emergency management of children with severe sepsis in the United Kingdom: the results of the Paediatric Intensive Care Society sepsis audit. Arch Dis Child. 2009;94(5):348–353. doi: 10.1136/adc.2008.153064. [DOI] [PubMed] [Google Scholar]
- 9.Lee DS, Suh GY, Ryu JA, et al. Effect of early intervention on long-term outcomes of critically ill cancer patients admitted to ICUs. Crit Care Med. 2015;43(7):1439–1448. doi: 10.1097/CCM.0000000000000989. [DOI] [PubMed] [Google Scholar]
- 10.Song JU, Suh GY, Park HY, et al. Early intervention on the outcomes in critically ill cancer patients admitted to intensive care units. Intensive Care Med. 2012;38(9):1505–1513. doi: 10.1007/s00134-012-2594-0. [DOI] [PubMed] [Google Scholar]
- 11.Fausser JL, Tavenard A, Rialland F, et al. Should we pay attention to the delay before admission to a pediatric intensive care unit for children with cancer? Impact on 1-month mortality. A report from the French Children’s Oncology Study Group, GOCE. J Pediatr Hematol Oncol. 2017;39(5):e244–e248. doi: 10.1097/MPH.0000000000000816. [DOI] [PubMed] [Google Scholar]
- 12.Tamburro RF, Cooke KR, Davies SM, et al. Pulmonary complications of pediatric hematopoietic stem cell transplantation (HCT): an NIH Workshop Summary. Ann Am Thorac Soc. 2020 doi: 10.1513/AnnalsATS.202001-006OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindell RB, Gertz SJ, Rowan CM, et al. High levels of morbidity and mortality among pediatric hematopoietic cell transplant recipients with severe sepsis: insights from the Sepsis PRevalence, OUtcomes, and Therapies International Point Prevalence Study. Pediatr Crit Care Med. 2017;18(12):1114–1125. doi: 10.1097/PCC.0000000000001338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan CN, Brazauskas R, Huang J, et al. Late cardiovascular morbidity and mortality following pediatric allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2018;53(10):1278–1287. doi: 10.1038/s41409-018-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nieder ML, McDonald GB, Kida A, et al. National Cancer Institute-National Heart, Lung and Blood Institute/pediatric Blood and Marrow Transplant Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: long-term organ damage and dysfunction. Biol Blood Marrow Transplant. 2011;17(11):1573–1584. doi: 10.1016/j.bbmt.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeFilipp Z, Duarte RF, Snowden JA, et al. Metabolic syndrome and cardiovascular disease after hematopoietic cell transplantation: screening and preventive practice recommendations from the CIBMTR and EBMT. Biol Blood Marrow Transplant. 2016;22(8):1493–1503. doi: 10.1016/j.bbmt.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsons SK, Phipps S, Sung L, Baker KS, Pulsipher MA, Ness KK. NCI, NHLBI/PBMTC first international conference on late effects after pediatric hematopoietic cell transplantation: health-related quality of life, functional, and neurocognitive outcomes. Biol Blood Marrow Transplant. 2012;18(2):162–171. doi: 10.1016/j.bbmt.2011.12.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vrooman LM, Millard HR, Brazauskas R, et al. Survival and late effects after allogeneic hematopoietic cell transplantation for hematologic malignancy at less than three years of age. Biol Blood Marrow Transplant. 2017;23(8):1327–1334. doi: 10.1016/j.bbmt.2017.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung W, Ahn H, Rose SR, et al. A prospective cohort study of late sequelae of pediatric allogeneic hematopoietic stem cell transplantation. Medicine (Baltimore) 2007;86(4):215–224. doi: 10.1097/MD.0b013e31812f864d. [DOI] [PubMed] [Google Scholar]
- 20.Pollack MM, Holubkov R, Funai T, et al. The pediatric risk of mortality score: update 2015. Pediatr Crit Care Med. 2016;17(1):2–9. doi: 10.1097/PCC.0000000000000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz MA, Vicent MG, Prudencio M, et al. Predicting factors for admission to an intensive care unit and clinical outcome in pediatric patients receiving hematopoietic stem cell transplantation. Haematologica. 2002;87(3):292–298. [PubMed] [Google Scholar]
- 22.Tamburro RF, Barfield RC, Shaffer ML, et al. Changes in outcomes (1996-2004) for pediatric oncology and hematopoietic stem cell transplant patients requiring invasive mechanical ventilation. Pediatr Crit Care Med. 2008;9(3):270–277. doi: 10.1097/PCC.0b013e31816c7260. [DOI] [PubMed] [Google Scholar]
- 23.van Gestel JP, Bollen CW, van der Tweel I, Boelens JJ, van Vught AJ. Intensive care unit mortality trends in children after hematopoietic stem cell transplantation: a meta-regression analysis. Crit Care Med. 2008;36(10):2898–2904. doi: 10.1097/CCM.0b013e318186a34a. [DOI] [PubMed] [Google Scholar]
- 24.Zinter MS, Spicer AC, Liu KD, et al. Positive cumulative fluid balance is associated with mortality in pediatric acute respiratory distress syndrome in the setting of acute kidney injury. Pediatr Crit Care Med. 2019;20(4):323–331. doi: 10.1097/PCC.0000000000001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arn P, Bruce IA, Wraith JE, Travers H, Fallet S. Airway-related symptoms and surgeries in patients with mucopolysaccharidosis I. Ann Otol Rhinol Laryngol. 2015;124(3):198–205. doi: 10.1177/0003489414550154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boelens JJ, Aldenhoven M, Purtill D, et al. Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood. 2013;121(19):3981–3987. doi: 10.1182/blood-2012-09-455238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bona K, Brazauskas R, He N, et al. Neighborhood poverty and pediatric allogeneic hematopoietic cell transplantation outcomes: a CIBMTR analysis. Blood. 2021;137(4):556–568. doi: 10.1182/blood.2020006252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhandari R, Teh JB, He T, et al. Social vulnerability and risk of nonrelapse mortality after allogeneic hematopoietic cell transplantation. J Natl Cancer Inst. 2022;114(11):1484–1491. doi: 10.1093/jnci/djac150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abraham IE, Rauscher GH, Patel AA, et al. Structural racism is a mediator of disparities in acute myeloid leukemia outcomes. Blood. 2022;139(14):2212–2226. doi: 10.1182/blood.2021012830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winestone LE. Naming racism: the first step. Blood. 2022;139(14):2098–2099. doi: 10.1182/blood.2022015433. [DOI] [PubMed] [Google Scholar]
- 31.Kache S, Weiss IK, Moore TB. Changing outcomes for children requiring intensive care following hematopoietic stem cell transplantation. Pediatr Transplant. 2006;10(3):299–303. doi: 10.1111/j.1399-3046.2005.00453.x. [DOI] [PubMed] [Google Scholar]
- 32.Jacobe SJ, Hassan A, Veys P, Mok Q. Outcome of children requiring admission to an intensive care unit after bone marrow transplantation. Crit Care Med. 2003;31(5):1299–1305. doi: 10.1097/01.CCM.0000060011.88230.C8. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Garcia M, Gonzalez-Vicent M, Mastro-Martinez I, Serrano A, Diaz MA. Intensive care unit admissions among children after hematopoietic stem cell transplantation: incidence, outcome, and prognostic factors. J Pediatr Hematol Oncol. 2015;37(7):529–535. doi: 10.1097/MPH.0000000000000401. [DOI] [PubMed] [Google Scholar]
- 34.Aspesberro F, Guthrie KA, Woolfrey AE, Brogan TV, Roberts JS. Outcome of pediatric hematopoietic stem cell transplant recipients requiring mechanical ventilation. J Intensive Care Med. 2014;29(1):31–37. doi: 10.1177/0885066612457343. [DOI] [PubMed] [Google Scholar]
- 35.Hannegård Hamrin T, Eksborg S. Risks for death after admission to pediatric intensive care (PICU)-A comparison with the general population. PLoS One. 2022;17(10) doi: 10.1371/journal.pone.0265792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agulnik A, Forbes PW, Stenquist N, Rodriguez-Galindo C, Kleinman M. Validation of a pediatric early warning score in hospitalized pediatric oncology and hematopoietic stem cell transplant patients. Pediatr Crit Care Med. 2016;17(4):e146–e153. doi: 10.1097/PCC.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 37.Agulnik A, Gossett J, Carrillo AK, Kang G, Morrison RR. Abnormal vital signs predict critical deterioration in hospitalized pediatric hematology-oncology and post-hematopoietic cell transplant patients. Front Oncol. 2020;10:354. doi: 10.3389/fonc.2020.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaidman I, Mohamad H, Shalom L, et al. Survival of pediatric patients requiring admission in the intensive care unit post hematopoietic stem cell transplantation: prognostic factors associated with mortality. Pediatr Blood Cancer. 2022;69(3) doi: 10.1002/pbc.29549. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasan A, Sunkara A, Mitchell W, et al. Recovery of pulmonary function after allogeneic hematopoietic cell transplantation in children is associated with improved survival. Biol Blood Marrow Transplant. 2017;23(12):2102–2109. doi: 10.1016/j.bbmt.2017.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quigg TC, Kim YJ, Goebel WS, Haut PR. Lung function before and after pediatric allogeneic hematopoietic stem cell transplantation: a predictive role for DLCOa/VA. J Pediatr Hematol Oncol. 2012;34(4):304–309. doi: 10.1097/MPH.0b013e3182346ed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu NL, Hingorani S, Cushing-Haugen KL, Lee SJ, Chow EJ. Late kidney morbidity and mortality in hematopoietic cell transplant survivors. Transplant Cell Ther. 2021;27(5):434.e1–434.e6. doi: 10.1016/j.jtct.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.