Abstract
The protein product of the c-Cbl proto-oncogene is prominently tyrosine phosphorylated in response to insulin in 3T3-L1 adipocytes and not in 3T3-L1 fibroblasts. After insulin-dependent tyrosine phosphorylation, c-Cbl specifically associates with endogenous c-Crk and Fyn. These results suggest a role for tyrosine-phosphorylated c-Cbl in 3T3-L1 adipocyte activation by insulin. A yeast two-hybrid cDNA library prepared from fully differentiated 3T3-L1 adipocytes was screened with full-length c-Cbl as the target protein in an attempt to identify adipose-specific signaling proteins that interact with c-Cbl and potentially are involved in its tyrosine phosphorylation in 3T3-L1 adipocytes. Here we describe the isolation and the characterization of a novel protein that we termed CAP for c-Cbl-associated protein. CAP contains a unique structure with three adjacent Src homology 3 (SH3) domains in the C terminus and a region showing significant sequence similarity with the peptide hormone sorbin. Both CAP mRNA and proteins are expressed predominately in 3T3-L1 adipocytes and not in 3T3-L1 fibroblasts. CAP associates with c-Cbl in 3T3-L1 adipocytes independently of insulin stimulation in vivo and in vitro in an SH3-domain-mediated manner. Furthermore, we detected the association of CAP with the insulin receptor. Insulin stimulation resulted in the dissociation of CAP from the insulin receptor. Taken together, these data suggest that CAP represents a novel c-Cbl binding protein in 3T3-L1 adipocytes likely to participate in insulin signaling.
The mitogenic and metabolic effects of insulin are initiated by the tyrosine kinase activity of its receptor. Upon insulin binding, the insulin receptor undergoes autophosphorylation on tyrosine residues, resulting in increased kinase activity that leads to tyrosine phosphorylation of intracellular substrates (34, 45). Since the tyrosine-phosphorylated insulin receptor does not associate strongly with downstream Src homology 2 (SH2) domain-containing proteins, docking proteins are essential for insulin signaling. The tyrosine phosphorylation of these proteins, which include the insulin receptor substrates 1 and 2 (IRS-1 and IRS-2), the Shc proto-oncogene product, and the Grb2-associated protein GAB1, induces their association with proteins containing SH2 domains, resulting in activation of various signaling cascades (2, 7, 16, 24, 39, 40). Both IRS-1 and Shc have been implicated in regulating the Ras signaling pathways and the downstream mitogenic actions of insulin (27, 32, 35, 36). Binding of phosphatidylinositol 3′-kinase (PI 3′-K) to phosphorylated IRS-1 and IRS-2 leads to activation of the lipid kinase in response to insulin (3, 7, 40). Although activation of these known pathways are clearly important in insulin signal transduction, recent studies have indicated that stimulation of the mitogen-activated protein (MAP) kinase pathway and PI 3′-K may not be sufficient for the stimulation of glucose transport, lipid synthesis, or glycogen synthesis by insulin in 3T3-L1 adipocytes (19, 43, 46). These studies suggest that additional signaling pathways may exist that are initiated by the tyrosine phosphorylation of other substrates by the insulin receptor.
c-Cbl is the 120-kDa cellular homolog of the transforming v-Cbl oncogene. The primary structure of c-Cbl resembles that of DNA binding transcription factors, with a nuclear localization sequence, a zinc finger-like motif, and a leucine zipper (5, 6). However, it is localized predominately in the cytoplasm, while the truncated protein encoded by v-cbl is localized in both the cytoplasm and the nucleus, where it can bind DNA (6). c-Cbl becomes tyrosine phosphorylated in response to activation of a variety of tyrosine kinases including v-Abl and Bcr-Abl (1, 28); stimulation of the T- and B-cell antigen receptors (8, 11, 18), granulocyte-macrophage colony-stimulating factor erythropoietin, Fc gamma receptors (21, 41), and the epidermal growth factor (EGF) receptor (13, 15, 20); and during integrin-mediated cell adhesion (22). These findings suggest that c-Cbl may be an important component of signal transduction downstream of tyrosine kinases. c-Cbl contains a long proline-rich region in the COOH terminus that constitutively binds the SH3 domains of the adapters Grb2 and Nck and the Fyn and Lck tyrosine kinases independent of cell activation (8, 11, 14, 21, 31). Upon cell activation, tyrosine-phosphorylated c-Cbl binds the SH2 domains of Fyn, Lck, the p85 subunit of PI 3′-K, and c-Crk (8, 11, 13, 14, 18, 28, 37). Moreover, the association of c-Crk with tyrosine-phosphorylated c-Cbl correlates well with cellular transformation in Bcr-Abl or mutant, oncogenic c-Cbl (70Z)-expressing cells (28).
We reported recently that insulin markedly stimulated the tyrosine phosphorylation of c-Cbl in 3T3-L1 adipocytes, inducing its association with the adapter protein c-Crk and the Src family kinase Fyn. Interestingly, insulin did not stimulate c-Cbl tyrosine phosphorylation in 3T3-L1 fibroblasts or in any other cell lines expressing high levels of functional insulin receptors and c-Cbl (30). These results suggest a physiological function for c-Cbl in insulin action in the metabolically responsive 3T3-L1 adipocytes. However, we did not detect the direct association of c-Cbl with the insulin receptor, suggesting that a protein present in 3T3-L1 adipocytes but not in fibroblasts plays a critical role in the tyrosine phosphorylation of c-Cbl by the insulin receptor. We report here the isolation and the characterization of a novel signaling protein that we termed CAP for c-Cbl-associated protein. Both CAP mRNA and proteins are expressed predominately in 3T3-L1 adipocytes and not in 3T3-L1 fibroblasts. CAP interacts with both c-Cbl and the insulin receptor in 3T3-L1 adipocytes and may have an important function in the specificity of tyrosine phosphorylation events under the regulation of insulin.
MATERIALS AND METHODS
Yeast two-hybrid system.
Full-length c-Cbl fused to the C terminus of the GAL4 DNA binding domain in the yeast expression vector pGBT9 (Clontech) was constructed from full-length human c-Cbl in the pGEM4Z vector (kindly provided by L. E. Samelson). A 3T3-L1 adipocyte cDNA library was synthesized with a cDNA synthesis kit (Stratagene) and constructed in the pGAD-GH GAL4 vector (Clontech). The yeast strain Y190 was first transformed to tryptophan prototrophy with the GAL4-c-Cbl protein and then with the 3T3-L1 adipocyte cDNA library. The resulting transformants were plated on selection medium lacking tryptophan, leucine, and histidine and containing 25 mM 3-aminotriazole and were incubated at 30°C for 4 to 5 days. His+ colonies were plated onto M63GV-Trp-Leu-His medium containing 5-bromo-4-chloro-3-indolylphosphate-β-d-galactopyranoside (X-Gal) (26) and were analyzed for β-galactosidase activity. Library-derived plasmids were rescued from positive clones and were transformed into Escherichia coli HB101 for DNA sequencing. Nucleotide and amino acid sequence alignments were performed by screening GenBank and EMBL databases with the BLAST program.
Cell culture and activation.
3T3-L1 fibroblasts were maintained in Dulbecco’s modified Eagle’s medium (4,500 g of glucose/liter) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Differentiation to adipocytes was induced as previously described (33). The cells were then cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum for 2 to 8 days. Before hormonal treatment, the cells, grown in 10-cm-diameter dishes, were serum deprived for 12 to 18 h. Unless otherwise indicated, 100 nM insulin (Sigma) was added directly to the medium and the incubation was continued for the indicated times at 37°C. Chinese hamster ovary (CHO) cells were cultured in minimal Eagle’s medium containing nucleotides, 10% fetal bovine serum, and 1% penicillin-streptomycin.
Northern blot analysis.
Total cellular RNA was isolated from 3T3-L1 fibroblasts and adipocytes by the acid guanidinium thiocyanate method (9). RNA samples (20 μg) were fractionated in 1.2% agarose–2.2 M formaldehyde and transferred to a Hybond-N membrane (Amersham). Hybridizations were performed at 65°C for 20 h first with a purified EcoRI fragment from clone 2.1 (Fig. 1A) and then with a β-actin probe labeled with [α-32P]dCTP (Amersham) by the random-primer extension method. A mouse multiple-tissue Northern blot analysis (Clontech) was performed with a clone 2.1 probe by following the manufacturer’s instructions.
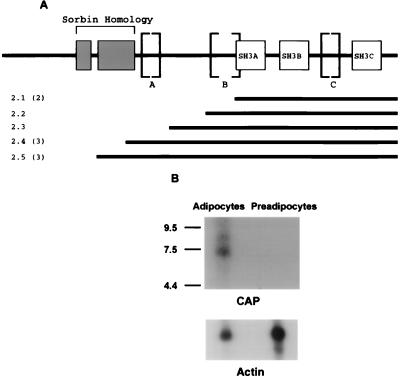
FIG. 1.
Primary structure of CAP and mRNA expression. (A) 3T3-L1 adipocyte cDNA clones isolated from the yeast two-hybrid screen with c-Cbl as bait are shown (black lines) aligned below a schematic of the domain organization of the CAP protein. Numbers in parentheses refer to the number of times each clone was isolated from the yeast two-hybrid screen. The letters A, B, and C indicate the presence of alternative spliced inserts. (B) Total RNA (20 μg) isolated from 3T3-L1 adipocytes or fibroblasts was hybridized to a probe prepared from the DNA insert of clone 2.1 labeled with [α-32P]dCTP by random-primer extension (upper). The filter was then stripped and rehybridized with a β-actin probe to estimate the relative amount of RNA in each lane (lower). The migration positions of RNA size markers (in kilobases) are on the left.
Antibodies.
For the production of anti-CAP antibodies, CAP clone 2.1 (Fig. 1A) was subcloned from the pGAD-GH plasmid into the EcoRI site of pGEX-5X-3 vector (Pharmacia Biotechnology) to facilitate expression as a glutathione S-transferase (GST) fusion protein. The GST-CAP-SH3 fusion protein was purified from bacterial cell lysates with glutathione-Sepharose beads and injected into New Zealand rabbits according to standard procedure. To affinity purify the resulting antiserum, GST-CAP-SH3 fusion protein adsorbed on the glutathione-Sepharose beads was cleaved by incubation with Factor Xa (New England BioLabs) in a buffer containing 20 mM HEPES (pH 8), 100 mM NaCl, and 2 mM CaCl2 for 12 h at room temperature. After centrifugation to remove the beads, the supernatant was incubated with benzamidine-Agarose (Sigma) for 15 min at room temperature. The CAP-SH3 protein in the supernatant was then coupled to an AminoLink column (Pierce, Rockford, Ill.) by following the instructions in the manufacturer’s manual. The affinity-purified anti-CAP antibodies were subjected to buffer exchange with Tris-buffered saline (10 mM Tris-HCl [pH 7.4], 150 mM NaCl), quantitated, and stored at −70°C. Anti-c-Cbl and anti-ERK2 antibodies were purchased from Santa Cruz Inc., anti-insulin receptor antibodies and protein G- and protein A-Agarose were purchased from Oncogene Science, and anti-c-Crk-II antibody and the antiphosphotyrosine antibody RC20H were purchased from Transduction Laboratories. Anti-Flag antibodies were purchased from Kodak, New Haven, Conn. Horseradish peroxidase-linked secondary antibodies were from Amersham.
Expression vector construct and transfection of CHO cells.
A Flag epitope-tagged full-length CAP in a eukaryotic expression vector was constructed as follows. The cDNA of the amino-terminal region of CAP was amplified by PCR with CAP cDNA in pEXlox as the template (38). The primer 5′-CGCGGATCCGCCGCCACCATGGAC TACAAGGACGACGATGACAAGAG T TC TGAATGTGAT-3′ was designed to have a BamHI restriction site followed by a coding sequence for a Flag epitope in frame with amino acid 2. The primer 5′-AATGTCTGGAGTCGG-3′ corresponds to amino acids 345 to 350. The amplified DNA fragment was digested with BamHI and SacI (present in the CAP gene) and ligated into CAP cDNA clone 2.5 (Fig. 1A) in the pGAD-GH vector digested with BamHI and SacI. The Flag-tagged full-length CAP cDNA was then liberated by digestion with SpeI and EcoRI and ligated into the PCI expression vector (Promega) at the NheI and EcoRI sites. The sequence of the Flag-tagged CAP cDNA (Flag-CAP) in this vector was confirmed by DNA sequencing.
CHO cells were transiently transfected with 10 μg of either Flag-CAP or the vector alone with the Lipofectamine reagent (Gibco-BRL) for 4 h at 37°C. The transfected cells were then washed twice with phosphate-buffered saline, and complete medium was added. Forty-eight hours after transfection, the cells were washed and lysed for further analysis.
Immunoprecipitations and immunoblotting.
Cells (0.5 × 107 to 1 × 107) were washed twice with ice-cold phosphate-buffered saline and lysed with buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% Triton X-100, 1 mM EDTA, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 100 mM NaF, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, and 1 mM phenylmethylsulfonyl fluoride on ice for 15 min. After centrifugation at 10,000 × g for 15 min at 4°C, the clarified supernatants were incubated with the indicated antibodies for 3 h at 4°C. The immune complexes were precipitated with protein G- or protein A-Agarose for 2 h and were washed extensively with lysis buffer before solubilization in Laemmli sample buffer. Bound proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Individual proteins were detected with the specified antibodies and visualized by blotting with horseradish peroxidase-linked secondary antibodies. To reprobe immunoblots, the nitrocellulose membranes were incubated for 30 min at 60°C with 62.5 mM Tris-HCl (pH 6.8)–2% SDS–0.7% 2-mercaptoethanol, and then were washed extensively with 10 mM Tris-HCl (pH 8)–150 mM NaCl.
In vitro binding assays.
The DNA fragments encoding the individual SH3 domains of CAP were generated by PCR with the following oligonucleotides (which contain BamHI and EcoRI restriction sites): 5′-CTGTGCGGATCCGGATTAGAGA-3′ and 5′-CTGTGCGAATTCCTCAGCTGGAGGAAGAAGCTC-3′ for SH3A, 5′-CTGTGCGGATCCGAATATGGAGAAGCCATTGCA-3′ and 5′-CTGTGCGAATTCAAGCACATCTACATAGGT-3′ for SH3B, and 5′-CTGTGCGGGATCCGATTTGTGTAGCTACCAAGCG-3′ and 5′-CTGTGCGAATTCTTCTTATAGATATAAAGG-3′ for SH3C. The amplification products were digested with BamHI and EcoRI and inserted in frame between the homologous sites of the GST expression vector pGEX-2T (Pharmacia). All constructs were verified by DNA sequencing through the entire portion obtained by PCR. GST fusion proteins containing the SH2 and SH3 domains of Crk were the generous gift of R. B. Birge and H. Hanafusa (4, 12). In vitro association experiments were performed with an equal amount (5 μg) of the immobilized GST-fusion proteins or GST alone as described previously (29). The bound proteins were analyzed as described above for immunoprecipitates.
Cellular fractionation.
3T3-L1 adipocytes, treated with or without insulin (100 nM) for 5 min, were washed twice with ice-cold phosphate-buffered saline and scraped into 1 ml of homogenization buffer containing 50 mM HEPES (pH 7.2), 2 mM EDTA, 2 mg of glycogen/ml, 0.2% 2-mercaptoethanol, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 10 μg of aprotinin, and 10 μg of leupeptin/ml. Cells were sonicated and centrifuged at 2,500 × g to remove nuclei and unlysed cells. The postnuclear supernatant was removed and centrifuged for 15 min at 10,000 × g to pellet the plasma membranes. The supernatant was recentrifuged for 1 h at 100,000 × g. The final supernatant was called cytosol. The plasma membrane pellets were resuspended in homogenization buffer by 10 passes through a 23-gauge needle and recentrifuged. The final pellet was resuspended as described above. Proteins (25 μg) were separated by SDS-PAGE and identified by immunoblotting with specific antibodies.
RESULTS
Isolation of a c-Cbl-binding protein with the yeast two-hybrid system.
To elucidate the signaling pathways in which c-Cbl is involved in 3T3-L1 adipocytes, we searched for adipocyte-specific c-Cbl-interacting proteins. Towards this goal, full-length c-Cbl fused to the DNA binding domain of GAL4 (GAL4-c-Cbl) was used as bait to screen a 3T3-L1 adipocyte cDNA expression library fused to the GAL4 transcriptional activation domain in the yeast two-hybrid interaction system. Of 5 × 105 total transformants, 13 colonies were subsequently positive for β-galactosidase activity when they were plated on X-Gal-containing medium. The recovered library-derived plasmids induced β-galactosidase activity only when they were coexpressed with GAL4-c-Cbl fusion protein and not with an unrelated GAL4 fusion protein containing the catalytic domain of protein phosphatase 1.
DNA sequences from the GAL4 fusion junctions of all the plasmid inserts revealed that they all encoded five independent novel protein fragments overlapping at the COOH terminus (Fig. 1A, clones 2.1, 2.2, 2.3, 2.4, and 2.5). We designated this protein CAP for c-Cbl-associated protein. The open reading frame of these cDNA inserts is followed by a stop codon and 250 bp of 3′ untranslated region. A computer-assisted sequence homology search of GenBank revealed that the COOH-terminal region of CAP contains three adjacent SH3 domains. A stretch of ∼120 amino acids of CAP (Fig. 1A, clones 2.4 and 2.5) has significant sequence similarity with the porcine peptide hormone sorbin (32% identity at the amino acid level [42]) and was termed the sorbin domain.
The cDNAs that encompass the complete coding region of CAP have been isolated from a mouse embryo cDNA expression library by using a functional screen with a defined SH3 ligand peptide (38). The open reading frames of these cDNAs contained insertions of a different sequence (types A to C) in the middle of CAP, presumably due to alternative splicing (Fig. 1A). Analysis of the coding sequences predicted proteins with molecular masses of 77 to 90 kDa. The CAP clones isolated by the two-hybrid screen had both the B and C type insertions missing (Fig. 1A). The structure and the predicted amino acid sequence of CAP suggest that it represents a family of novel signaling molecules that may function as multi-domain binding proteins.
Since CAP cDNA was isolated from a cDNA library prepared from 3T3-L1 adipocytes, we determined the expression pattern of the CAP gene in 3T3-L1 cells. Northern blot analysis of RNA isolated from both 3T3-L1 fibroblasts and fully differentiated adipocytes was performed with the cDNA insert of clone 2.1. As shown in Fig. 1B, transcripts corresponding to a major, broad band of 7 kb and a minor band of 8 kb were detected in 3T3-L1 adipocytes. Interestingly, no mRNA was detected in 3T3-L1 fibroblasts. CAP transcripts with the same sizes were also detected in RNA isolated from rat adipocytes (not shown).
Expression of CAP proteins during 3T3-L1 cell differentiation.
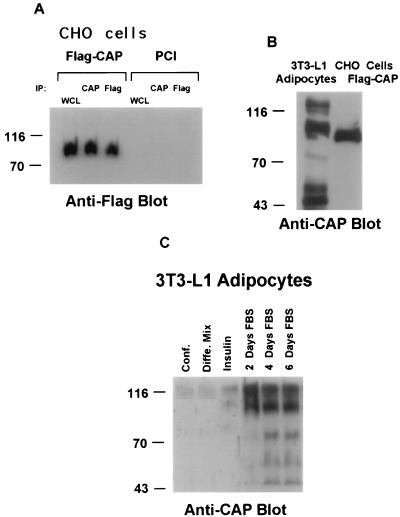
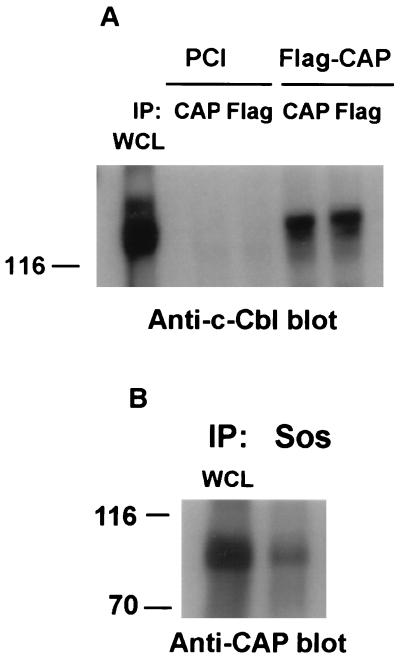
In order to characterize the proteins encoded by CAP, polyclonal antibodies were raised against the purified C-terminal domain of CAP present in clone 2.1 (Fig. 1A), expressed as a GST fusion protein. For the expression of CAP in CHO cells, CAP clone 2.5, isolated by the two-hybrid screen, was fused in frame to the N-terminal region of CAP obtained from the mouse embryo library. A plasmid containing this full-length form of CAP, with a Flag epitope tagged onto the N terminus (Flag-CAP), or the vector alone (PCI) was transiently transfected into CHO cells. Lysates prepared from the transfected cells were immunoprecipitated with antibodies to Flag (Anti-Flag) or with anti-CAP antibodies. The immunoprecipitates were separated by SDS-PAGE followed by immunoblotting with anti-Flag antibodies. As shown in Fig. 2A, The N-terminal Flag-tagged CAP was recognized by both the anti-Flag antibodies and the anti-CAP serum elicited against the C terminus of CAP. The protein encoded by this cDNA migrated as a single band with an apparent molecular mass of 85 kDa on an SDS-PAGE gel. Similar results were obtained following immunoblotting of the immunoprecipitates with the anti-CAP serum (data not shown). Both antibodies detected CAP only in cell lysates prepared from the CAP-transfected CHO cells and not in cells transfected with the vector alone. CHO cells express no detectable endogenous proteins recognized by the anti-CAP antibodies. These results indicate that our antibodies specifically recognize the protein product of the cap gene.
FIG. 2.
Identification of the CAP gene product(s). (A) CHO cells were transiently transfected with an expression vector containing full-length CAP with a Flag epitope tagged onto the N terminus (Flag-CAP) or the vector alone (PCI). Cell lysates were then immunoprecipitated (IP) with anti-Flag or anti-CAP antibodies. Whole-cell lysates (WCL) containing 20 μg of protein and the immunoprecipitates were subjected to SDS-PAGE and immunoblotted with anti-Flag antibodies. (B) Cell lysates prepared from 3T3-L1 adipocytes or CHO cells transfected with Flag-CAP were directly analyzed by immunoblotting with anti-CAP antibodies. (C) Total cellular lysates prepared from confluent 3T3-L1 fibroblasts (Conf) and at various times during differentiation into adipocytes as previously described (33) (30 μg per lane) were separated by SDS-PAGE followed by immunoblotting with anti-CAP antibodies. Molecular mass markers (in kilodaltons) are on the left. Diffe. Mix, differentiation mixture; FBS, fetal bovine serum.
We next examined the expression of CAP proteins in 3T3-L1 adipocytes (Fig. 2B). Interestingly, while in CHO cells the anti-CAP antibodies detected only the product of the Flag-tagged CAP cDNA, with similar results obtained with Flag-tagged CAP-transfected NIH 3T3 cells (data not shown), the anti-CAP antibodies detected several proteins in 3T3-L1 adipocyte cell lysates. An adipocyte protein with a molecular mass of 88 to 90 kDa migrated similarly to the CAP protein expressed in transfected CHO cells. The slower mobility of this adipocyte protein might reflect posttranslational modifications that do not occur in CHO cells. In addition to the 88-kDa protein product, the anti-CAP antibodies also detected proteins with molecular masses of 125, 75, 53, and 45 kDa with different levels of expression in 3T3-L1 adipocyte lysates. These proteins may represent different isoforms or alternatively spliced variants of CAP expressed in 3T3-L1 adipocytes, consistent with the identification of various cDNA splice forms and mRNA transcripts. It is possible that the anti-CAP antibodies were able to recognize multiple isoforms of CAP in 3T3-L1 adipocytes common in their C-terminal portion used for antibody production. However, whether each of these proteins is a distinct CAP isoform remains uncertain.
Since CAP mRNA was detected only in 3T3-L1 adipocytes (Fig. 1B), we were interested to examine the expression pattern of CAP at different stages during the conversion of 3T3-L1 fibroblasts into adipocytes. Equivalent amounts of protein, prepared from confluent 3T3-L1 preadipocytes and at various times during differentiation into adipocytes, were separated by SDS-PAGE followed by immunoblotting with anti-CAP antibodies (Fig. 2C). The 88-kDa CAP was undetectable in confluent preadipocytes, while a low level of expression of the 125-kDa protein was detected in the preadipocytes that increased during differentiation. The 88-kDa CAP was detected only 6 days after the initiation of differentiation (2 days FBS), when lipid accumulation was just starting with an increase to maximal expression after extensive differentiation. Additionally, the proteins of 75, 53, and 45 kDa were detected with anti-CAP antibodies only in fully differentiated 3T3-L1 cells. These results demonstrate that both CAP mRNA and protein expression are largely restricted to 3T3-L1 adipocytes.
Tissue distribution of CAP.
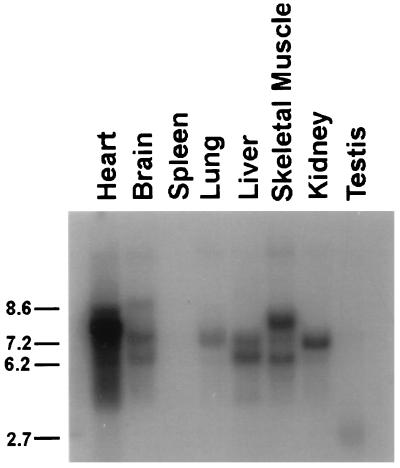
To determine the tissue distribution of CAP mRNA, a mouse multiple-tissue Northern blot was hybridized with a probe prepared from the DNA insert of clone 2.1 (Fig. 3). Several size classes of CAP mRNA transcripts were detected with tissue-specific variation in their relative abundance. CAP mRNA expression was highest in heart, liver, skeletal muscle and kidney. Lesser amounts were detected in brain and lung, while we could not detect CAP mRNA in spleen and testis, even after long exposure. The three most prominent CAP transcripts were approximately 6.5, 7.4, and 8 kb. Similar results were obtained when a rat multiple-tissue Northern blot was hybridized with the same probe (data not shown). These results are consistent with the isolation of multiple CAP cDNA variants. These may be expressed in a tissue-specific manner with distinct physiological functions. The specific expression of CAP in adipose tissue as well as liver and muscle makes CAP a candidate for a physiologically relevant effector protein in insulin signaling.
FIG. 3.
Tissue distribution of CAP mRNA. A Northern blot containing mouse poly(A+) RNA (2 μg per lane) from the indicated tissues was hybridized with the cDNA insert of clone 2.1 probe. The positions of RNA size markers in kilobases are shown on the left.
Interactions of CAP SH3 domains.
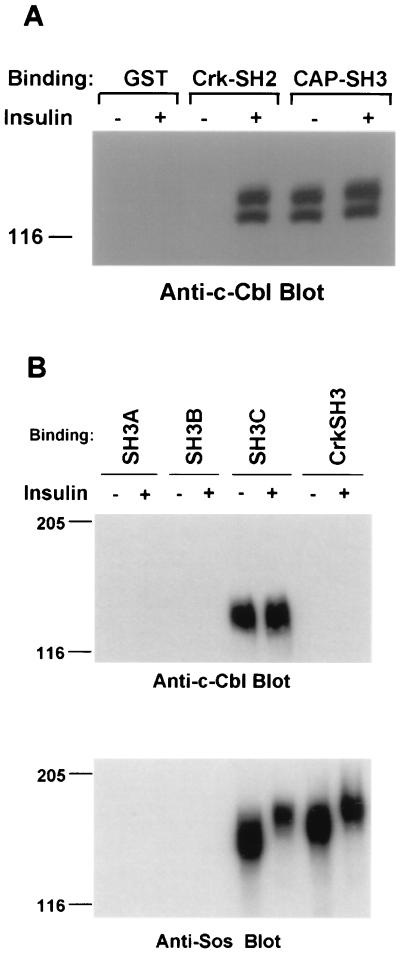
All of the activation domain-fused CAP clones isolated by the yeast two-hybrid screen contained all three SH3 domains, and one CAP clone (2.1 [Fig. 1A]) contained exclusively the three SH3 domains. This result suggested that the interaction of CAP with c-Cbl is primarily mediated by the SH3 domains binding to the proline-rich motifs in c-Cbl. To confirm that the SH3 domains of CAP interact with native c-Cbl, and to address the effect of insulin on this association, a GST fusion protein was generated with the clone 2.1 coding region (CAP-SH3). We have recently demonstrated that c-Cbl is rapidly tyrosine phosphorylated in response to insulin 3T3-L1 adipocytes. On phosphorylation, c-Cbl forms an insulin-dependent association with the SH2 domain of Crk (30). Lysates prepared from 3T3-L1 adipocytes treated with or without insulin were incubated with the GST fusion proteins containing Crk-SH2, CAP-SH3, or GST alone immobilized on glutathione-Sepharose beads. The bound proteins were separated by SDS-PAGE and were detected by immunoblotting with anti-c-Cbl antibodies (Fig. 4A). GST-CAP-SH3 fusion protein bound c-Cbl in lysates of unstimulated cells. Treatment of cells with insulin had no effect on the amount of c-Cbl associated with the GST-CAP-SH3 fusion protein, while such treatment induced the association of c-Cbl with GST-Crk-SH2. Thus, CAP and c-Cbl form a constitutive complex in 3T3-L1 adipocytes independent of insulin stimulation. The tyrosine phosphorylation of c-Cbl in response to insulin does not regulate its interaction with CAP. These data are in agreement with the two-hybrid experiments and emphasize the role of CAP-SH3 domains in the interaction with c-Cbl.
FIG. 4.
c-Cbl binds to the carboxyl-terminal SH3 domain of CAP independent of insulin stimulation. (A) 3T3-L1 adipocytes were serum starved for 18 h and then stimulated with insulin (100 nM) for 5 min or left untreated. The cell lysates were incubated with the GST fusion proteins containing Crk-SH2, the three CAP SH3 domains (CAP-SH3), or GST alone immobilized on glutathione-Sepharose beads for 90 min at 4°C. The bound proteins were eluted, resolved by SDS-PAGE, and immunoblotted with anti-c-Cbl antibodies. (B) 3T3-L1 adipocytes were stimulated with insulin (100 nM) for 5 min or left untreated. The cell lysates were then incubated with GST fusion proteins containing the first SH3 domain (SH3A, closest to the N terminus), the middle SH3 domain (SH3B), the carboxyl-terminal SH3 domain of CAP (SH3C), and the Crk-SH3 domain. The resulting precipitated proteins were separated by SDS-PAGE and immunoblotted with anti-c-Cbl or anti-Sos antibodies. Molecular mass markers (in kilodaltons) are on the left.
To define the interaction between CAP and c-Cbl in more detail, each of the SH3 domains of CAP was expressed as a GST fusion protein. Lysates prepared from 3T3-L1 adipocytes stimulated with insulin or left untreated were incubated with GST fusion protein containing the individual SH3 domains of CAP and the Crk-SH3 domain as a control. The bound proteins were immunoblotted with anti-c-Cbl or anti-Sos antibodies. As shown in Fig. 4B (anti-c-Cbl blot), the carboxyl-terminal SH3 domain of CAP (SH3C) was able to bind c-Cbl in unstimulated 3T3-L1 adipocytes. As described above, insulin had no effect on the association state of the CAP-SH3C–c-Cbl complex. Immunoblotting the above-described bound proteins with anti-Sos antibodies (Fig. 4B, anti-Sos blot) indicated that the carboxyl-terminal SH3 domain of CAP (SH3C) may bind Sos in lysates of unstimulated cells. Interestingly, similar to the ability of insulin to induce the phosphorylation-dependent dissociation of Grb2-Sos and to a lesser extent Crk-Sos complexes (23, 44), there was an insulin-dependent dissociation of the CAP-SH3C–Sos complex. The first SH3 domain (SH3A, closest to the N terminus) and the middle SH3 domain (SH3B) did not independently associate with either c-Cbl or Sos. However, using a phage-displayed library, we identified peptide ligands to each of the isolated SH3 domains of CAP, demonstrating that these are functional SH3 domains (data not shown).
Association of CAP with c-Cbl and Sos in intact cells.
The association of CAP and c-Cbl were examined in CHO cells transfected with Flag-CAP or the vector alone (PCI). Lysates prepared from transfected CHO cells were immunoprecipitated with anti-CAP or anti-Flag antibodies followed by immunoblotting with anti-c-Cbl antibodies (CHO cells express endogenous c-Cbl). c-Cbl was coimmunoprecipitated with anti-CAP or anti-Flag antibodies from Flag-CAP-transfected CHO cell lysates but not from cells transfected with the vector alone (Fig. 5A).
FIG. 5.
In vivo association of CAP with c-Cbl and Sos. (A) CHO cells transfected with Flag-CAP or the vector alone (PCI) were lysed and immunoprecipitated with anti-CAP or anti-Flag antibodies. Following SDS-PAGE, the immunoprecipitates (IP) were immunoblotted with anti-c-Cbl antibodies. (B) CHO cells transfected with Flag-CAP were lysed and immunoprecipitated with anti-Sos antibodies. The immunoprecipitates were separated by SDS-PAGE and immunoblotted with anti-CAP antibodies. WCL, whole-cell lysates. Molecular mass markers (in kilodaltons) are on the left.
As the SH3 domain of CAP could also bind Sos in vitro, we tested whether this association also occurs in cells. CHO cells transfected with Flag-CAP were lysed followed by immunoprecipitation with anti-Sos antibodies. As shown in Fig. 5B, CAP was immunoprecipitated with anti-Sos antibodies from the transfected cells. These experiments indicated that CAP interacts with c-Cbl and Sos to form stable complexes in intact cells through binding of the CAP carboxy-terminal SH3 domain.
Endogenous CAP associates with c-Cbl in 3T3-L1 adipocytes.
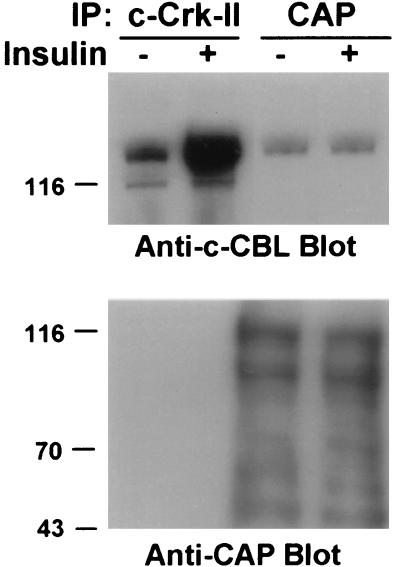
To gain further insight regarding the functional importance of the c-Cbl-CAP association, we analyzed the association of endogenous CAP and c-Cbl in intact 3T3-L1 adipocytes and the effect of insulin stimulation on the in vivo interaction. Lysates of unstimulated or insulin-stimulated 3T3-L1 adipocytes were immunoprecipitated with anti-CAP or anti-c-Crk-II antibodies as a control. The resulting immunocomplexes were blotted with anti-c-Cbl antibodies. As shown in Fig. 6 (anti-c-Cbl blot), c-Cbl coimmunoprecipitated with the endogenous CAP in quiescent, unstimulated 3T3-L1 adipocytes. Insulin treatment did not affect this association. In the control experiment, and as we have recently shown (30), after insulin-dependent tyrosine phosphorylation, c-Cbl forms complexes with endogenous c-Crk. The anti-CAP immunoblotting of the above-described immunoprecipitates revealed that CAP proteins detected in 3T3-L1 adipocyte cell lysates (Fig. 2B) were also immunoprecipitated by the anti-CAP antibodies (Fig. 6, anti-CAP blot). Equal amounts of these proteins were immunoprecipitated from unstimulated or insulin-stimulated 3T3-L1 adipocytes. These results show that endogenous c-Cbl and CAP interact in vivo in a constitutive manner in 3T3-L1 adipocytes. We could not detect any tyrosine phosphorylation of CAP proteins in unstimulated or insulin-stimulated 3T3-L1 adipocytes (data not shown).
FIG. 6.
CAP associates with c-Cbl in intact 3T3-L1 adipocytes. 3T3-L1 adipocytes were serum starved for 18 h and then stimulated with insulin (100 nM) for 5 min or left untreated. The cell lysates were immunoprecipitated with anti- c-Crk or anti-CAP antibodies. The resulting immunoprecipitates (IP) were separated by SDS-PAGE and subjected to immunoblotting with anti-c-Cbl antibodies. The blot was then stripped of the anti-c-Cbl antibodies and reprobed with anti-CAP antibodies. WCL, whole-cell lysates. Molecular mass markers (in kilodaltons) are on the left.
Association of CAP with the insulin receptor in 3T3-L1 adipocytes.
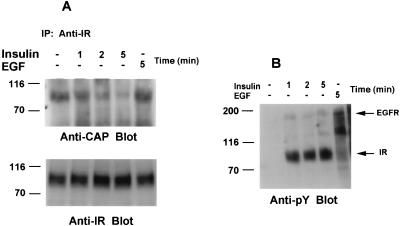
Given the predominant expression of CAP in differentiated versus undifferentiated 3T3-L1 cells, we examined whether endogenous CAP could form a complex with the insulin receptor. 3T3-L1 adipocytes were stimulated with and without insulin for the indicated times, and the cell lysates were incubated with anti-insulin receptor antibodies. The resulting immunoprecipitates were separated by SDS-PAGE and were analyzed by immunoblotting with anti-CAP antibodies. As shown in Fig. 7A (anti-CAP blot), one major CAP isoform coimmunoprecipitated with the insulin receptor in unstimulated 3T3-L1 adipocytes. Interestingly, addition of insulin caused a time-dependent dissociation of the CAP-insulin receptor complex. After 5 min of insulin stimulation, only a fraction of CAP was detected in the anti-insulin receptor immunoprecipitate. To ensure that the dissociation of CAP from the insulin receptor is due to insulin stimulation, 3T3-L1 adipocytes were treated with EGF. EGF stimulation had no effect on the association state of CAP with the insulin receptor, and the same levels of CAP were detected in the anti-insulin receptor immunoprecipitates from unstimulated and EGF-stimulated 3T3-L1 adipocytes. The blot was completely stripped of the anti-CAP antibodies and reprobed with anti-insulin receptor antibodies (Fig. 7A, anti-IR blot). The insulin receptors were precipitated equally from all samples. Antiphosphotyrosine immunoblotting of the whole-cell lysate samples used in these experiments confirmed that insulin and EGF stimulated the tyrosine autophosphorylation of the insulin receptor β-subunit and the EGF receptor, respectively (Fig. 7B, anti-pY blot). The dissociation of CAP from the insulin receptor was not due to dephosphorylation of the receptor, as it remains tyrosine phosphorylated during this time. The finding that in 3T3-L1 adipocytes predominantly one form of CAP associates with the insulin receptor, and that this interaction decreased following insulin receptor activation, raises the possibility that CAP may link c-Cbl to the insulin receptor. The functional domains within CAP and the insulin receptor that mediate these interactions remain to be determined.
FIG. 7.
Association of CAP with the insulin receptor in 3T3-L1 adipocytes. (A) Cell lysates prepared from serum-starved 3T3-L1 adipocytes treated with insulin (100 nM) for the indicated times or with EGF (100 ng/ml) for 5 min were immunoprecipitated with anti-insulin receptor (Anti-IR) antibodies. The immunoprecipitates (IP) were subjected to SDS-PAGE and immunoblotted with anti-CAP antibodies. The blot was then stripped of the anti-CAP antibodies and reprobed with anti-IR antibodies. (B) Equal protein amounts (30 μg per lane) of the cell lysates used in panel A were directly analyzed by immunoblotting with antiphosphotyrosine antibodies (anti-pY). The positions of the EGF receptor (EGFR) and the insulin receptor (IR) are indicated by arrows. WCL, whole-cell lysates. Molecular mass markers (in kilodaltons) are on the left.
Localization of CAP in 3T3-L1 adipocytes.
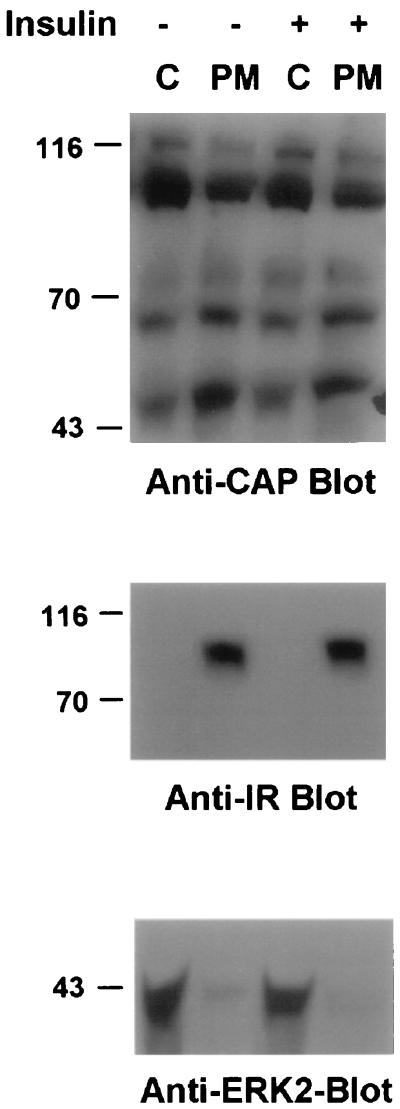
We next analyzed the subcellular localization of CAP proteins. Differentiated 3T3-L1 cells were treated with or without insulin and then fractionated into cytosolic and membrane fractions. Immunoblotting of the fractions with the anti-CAP antibodies showed both that CAP proteins are present in the cytosolic and membrane fractions of unstimulated 3T3-L1 adipocytes and that insulin stimulation did not significantly alter the subcellular distribution of CAP (Fig. 8, anti-CAP blot). The purity of the fractions was analyzed with the anti-insulin receptor antibodies that detected the insulin receptor exclusively in the membrane fraction (Fig. 8, anti-IR blot), while anti-ERK2 antibodies were used to detect ERK2 in the cytosolic fraction (Fig. 8, anti-ERK2 blot). The multiple SH3 domains of CAP may direct the localization of some CAP to the membrane fraction of the cells, as this motif is often found in cytoskeleton-associated proteins (25).
FIG. 8.
Subcellular localization of CAP proteins in 3T3-L1 adipocytes. Serum-starved differentiated 3T3-L1 cells were stimulated with and without insulin (100 nM) for 5 min. Subcellular fractionation was obtained as described in Materials and Methods, and fractions are identified as C (cytosolic fraction) and M (membrane fraction). Equal amounts of protein (25 μg per lane) were analyzed by immunoblotting with anti-CAP, anti-insulin receptor, and anti-ERK2 antibodies. Molecular mass markers (in kilodaltons) are on the left.
DISCUSSION
Protein tyrosine phosphorylation is an early event in insulin-initiated intracellular signaling cascades (34, 45). Recently, we have demonstrated that insulin stimulates the tyrosine phosphorylation of the c-Cbl proto-oncogene product, and this phosphorylation shows specificity for the differentiated adipocyte phenotype. Following insulin stimulation, the tyrosine-phosphorylated c-Cbl specifically associates with the adapter c-Crk and the Fyn tyrosine kinase in vivo and in vitro (30). Because we could not detect c-Cbl tyrosine phosphorylation in response to insulin in a variety of other cell types expressing functional insulin receptor (30), we suspected that 3T3-L1 adipocytes might express a specific adapter protein that may be involved in the tyrosine phosphorylation of c-Cbl.
To search for such novel proteins we used the full-length c-Cbl as a target protein to screen a 3T3-L1 adipocyte cDNA library in the yeast two-hybrid system. Multiple, independent c-DNA inserts, which encoded different fragments of the same protein were cloned from this library and designated CAP for c-Cbl-associated protein. These interactions were then verified in several experiments by using GST fusion proteins and coimmunoprecipitation. Analysis of the predicted amino acid sequences of these clones revealed three adjacent SH3 domains at the carboxyl terminus and a novel putative sorbin homology domain at the N terminus. The structure of CAP suggests that it may participate in multiple signaling cascades. cDNA sequencing and Northern blot analysis indicated that there are multiple splice variants of CAP that may result in a family of different isoforms. Interestingly, both CAP mRNA and proteins are expressed predominately in 3T3-L1 adipocytes and not in 3T3-L1 fibroblasts, implying a restricted signaling role for CAP in these cells.
The three SH3 domains of CAP were cloned as a fragment from the yeast two-hybrid library, suggesting that the association of CAP with c-Cbl was SH3 mediated. This was further suggested by an examination of the proline-rich regions of c-Cbl, revealing multiple potential SH3 binding motifs (6). In vitro binding assays with GST fusion proteins containing the individual SH3 domains of CAP confirmed that the functional association of CAP with c-Cbl is mediated primarily by the carboxyl-terminal SH3 domain of CAP. Interestingly, in 3T3-L1 adipocytes, which normally express both CAP and c-Cbl, CAP and c-Cbl associate in a constitutive manner independent of insulin stimulation. The specific tyrosine phosphorylation of c-Cbl and CAP expression in the differentiated adipocytes suggest that the CAP-c-Cbl complex may have a specialized signaling function in insulin action in these cells. The observation that one major CAP isoform associated with the insulin receptor suggests that at least one role of CAP might be to facilitate the interaction of c-Cbl with the insulin receptor, allowing for the phosphorylation of c-Cbl and its association with c-Crk and Fyn. Indeed, c-Cbl tyrosine phosphorylation is observed only in 3T3-L1 adipocytes, which uniquely express CAP. Moreover, receptor activation leads to rapid dissociation of the insulin receptor-CAP complex. The kinetics of the insulin receptor-CAP dissociation parallels the time course of insulin-stimulated tyrosine phosphorylation of c-Cbl in these cells (30). The structural requirements for this interaction remain to be determined.
The c-cbl gene was cloned as the cellular homolog of the v-cbl oncogene, which is transforming in early B-lineage and myeloid cells (5, 6). However, overexpression of c-Cbl does not induce cellular transformation, and the function of the proto-oncogene product remains unclear. The potential signaling function of c-Cbl downstream of tyrosine kinases depends on the association of its proline-rich region with SH3 domain-containing signaling proteins such as Grb2 and Nck (8, 11, 14, 21, 31) and the tyrosine phosphorylation sites with SH2 domain-containing proteins (8, 11, 13, 14, 18, 28, 37). Since Grb2 and Nck are ubiquitous in their expression, CAP with its specific expression in 3T3-L1 adipocytes may represent a specialized component in the signal transduction role of c-Cbl in these cells. Thus, different pools of c-Cbl may associate with different proteins forming various signaling complexes, each with distinct cellular function.
The ability of insulin to stimulate glucose uptake and storage of glucose as glycogen and lipids is significantly increased after 3T3-L1 differentiation. The expression of many fat cell-specific genes critical to insulin action is increased during adipocyte differentiation, such as GLUT4 (10, 17). Thus, changes in the level of CAP expression and c-Cbl tyrosine phosphorylation might contribute to increased insulin responsiveness observed in differentiated 3T3-L1 adipocytes. CAP was also found to associate with the nucleotide exchange protein Sos. Insulin treatment of 3T3-L1 adipocytes induces a dissociation of the CAP-SH3 domain from Sos similar to the dissociation of the Grb2/Sos complex (44). CAP, CAP/c-Cbl, and CAP/Sos may represent specific endogenous regulators in insulin-activated signal transduction pathways in 3T3-L1 adipocytes.
ACKNOWLEDGMENT
We thank Roman Herrera for helpful discussions.
REFERENCES
- 1.Andoniou C E, Thien C B F, Langdon W Y. Tumour induction by activated abl involves tyrosine phosphorylation of the product of the cbl oncogene. EMBO J. 1994;13:4515–4523. doi: 10.1002/j.1460-2075.1994.tb06773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki E, Lipes M A, Patti M E, Bruning J C, Haag B, Johnson R S, Kahn R C. Alternative pathway of insulin signaling in mice with targeted disruption of the IRS-1 gene. Nature (London) 1994;372:186–190. doi: 10.1038/372186a0. [DOI] [PubMed] [Google Scholar]
- 3.Backer J M, Mayers M G, Jr, Shoelson S E, Chin D J, Sun X-J, Miralpeix M, Patrick H, Margolis B, Skolnik E Y, Schlessinger J, White M F. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J. 1992;11:3469–3479. doi: 10.1002/j.1460-2075.1992.tb05426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birge R B, Fajardo E J, Reichman C, Shoelson S E, Songyang Z, Cantley L C, Hanafusa H. Identification and characterization of a high-affinity interaction between v-Crk and tyrosine-phosphorylated paxillin in CT10-transformed fibroblasts. Mol Cell Biol. 1993;13:4648–4656. doi: 10.1128/mcb.13.8.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake T, Heath K, Langdon W. The truncation that generated the v-cbl oncogene reveals an ability for nuclear transport, DNA binding and acute transformation. EMBO J. 1993;12:2017–2026. doi: 10.1002/j.1460-2075.1993.tb05851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blake T, Shapiro M, Morse H, Langdon W. The sequences of the human and mouse c-cbl proto-oncogenes show v-cbl was generated by a large truncation encompassing a proline-rich domain and a leucine zipper-like motif. Oncogene. 1991;6:653–657. [PubMed] [Google Scholar]
- 7.Bruning J C, Winnay J, Cheatham B, Kahn C R. Differential signaling by insulin receptor substrate 1 (IRS-1) and IRS-2 in IRS-1-deficient cells. Mol Cell Biol. 1997;17:1513–1521. doi: 10.1128/mcb.17.3.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buday L, Khwaja A, Sipeki S, Farago A, Downward J. Interactions of Cbl with two adapter proteins, Grb2 and Crk, upon T cell activation. J Biol Chem. 1996;271:6159–6163. doi: 10.1074/jbc.271.11.6159. [DOI] [PubMed] [Google Scholar]
- 9.Chomcynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 10.de Herreros A G, Birnbaum M J. The acquisition of increased insulin-responsive hexose transporter in 3T3-L1 adipocytes correlates with expression of a novel transporter gene. J Biol Chem. 1989;264:19994–19999. [PubMed] [Google Scholar]
- 11.Donovan J A, Wagner R L, Langdon W Y, Samelson L E. The protein product of the c-cbl protooncogene is the 120-kDa tyrosine-phosphorylated protein in Jurkat cells activated via the T cell antigen receptor. J Biol Chem. 1994;269:22921–22924. [PubMed] [Google Scholar]
- 12.Feller M S, Knudsen B, Hanafusa H. Cellular proteins binding to the first src homology 3 (SH3) domain of the proto-oncogene product c-Crk indicates Crk-specific signaling pathways. Oncogene. 1995;10:1465–1473. [PubMed] [Google Scholar]
- 13.Fukazawa T, Miyake S, Band V, Band H. Tyrosine phosphorylation of Cbl upon epidermal growth factor (EGF) stimulation and its association with EGF receptor and downstream signaling proteins. J Biol Chem. 1996;271:14554–14559. doi: 10.1074/jbc.271.24.14554. [DOI] [PubMed] [Google Scholar]
- 14.Fukazawa T, Reedquist K A, Traub T, Soltoff S, Panchamoorthy G, Druker B, Cantley L, Shoelson S E, Band H. The SH3 domain binding T cell tyrosyl phosphoprotein p120. Demonstration of its identity with the c-cbl protooncogene product and in vivo complexes with Fyn, Grb2, and phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:19141–19150. doi: 10.1074/jbc.270.32.19141. [DOI] [PubMed] [Google Scholar]
- 15.Galisteo M L, Dikic I, Batzer A G, Langdon W Y, Schlessinger J. Tyrosine phosphorylation of the c-cbl proto-oncogene protein product and association with epidermal growth factor (EGF) receptor upon EGF stimulation. J Biol Chem. 1995;270:20242–20245. doi: 10.1074/jbc.270.35.20242. [DOI] [PubMed] [Google Scholar]
- 16.Holgado-Madruga M, Emlet D R, Moscatello D K, Godwin A K, Wong A J. A Grb2-associated docking protein in EGF- and insulin-receptor signaling. Nature (London) 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- 17.Kandror K V, Stephens J M, Pilch P F. Expression and compartmentalization of caveolin in adipocytes cells: coordinate regulation with and structural segregation from GLUT4. J Cell Biol. 1995;129:999–1006. doi: 10.1083/jcb.129.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim T J, Kim Y T, Pillai S. Association of activated phosphatidylinositol 3′-kinase with p120cbl in antigen receptor-ligated B cells. J Biol Chem. 1995;270:27504–27509. doi: 10.1074/jbc.270.46.27504. [DOI] [PubMed] [Google Scholar]
- 19.Lazar F D, Wiese R J, Brady M J, Mastik C M, Waters S B, Yamauchi K, Pessin J E, Cuatrecasas P, Saltiel A R. Mitogen-activated protein kinase kinase inhibition does not block the stimulation of glucose utilization by insulin. J Biol Chem. 1995;270:20801–20807. doi: 10.1074/jbc.270.35.20801. [DOI] [PubMed] [Google Scholar]
- 20.Levkowitz G, Klapper L N, Tzahar E, Freywald A, Sela M, Yarden Y. Coupling of the c-Cbl protooncogene product to ErbB-1/EGF-receptor but not to other ErbB proteins. Oncogene. 1996;12:1117–1125. [PubMed] [Google Scholar]
- 21.Odai H, Sasaki K, Iwamatsu A, Hanazono Y, Tanaka T, Mitani K, Yazaki Y, Hirai H. The proto-oncogene product c-Cbl becomes tyrosine phosphorylated by stimulation with GM-CSF or Epo and constitutively binds to the SH3 domain of Grb2/Ash in human hematopoietic cells. J Biol Chem. 1995;270:10800–10805. doi: 10.1074/jbc.270.18.10800. [DOI] [PubMed] [Google Scholar]
- 22.Ojaniemi M, Martin S S, Dolfi F, Olefsky J M, Vuori K. The proto-oncogene product p120cbl links c-Src and phosphatidylinositol 3′-kinase to the integrin signaling pathway. J Biol Chem. 1997;272:3780–3787. doi: 10.1074/jbc.272.6.3780. [DOI] [PubMed] [Google Scholar]
- 23.Okada S, Pessin J E. Interactions between Src homology (SH)2/SH3 adapter proteins and the guanylnucleotide exchange factor SOS are differentially regulated by insulin and epidermal growth factor. J Biol Chem. 1996;271:25533–25538. doi: 10.1074/jbc.271.41.25533. [DOI] [PubMed] [Google Scholar]
- 24.Okada S, Yamauchi K, Pessin J E. Shc isoform-specific tyrosine phosphorylation by the insulin and epidermal growth factor receptors. J Biol Chem. 1995;270:20737–20741. doi: 10.1074/jbc.270.35.20737. [DOI] [PubMed] [Google Scholar]
- 25.Pawson T. Protein modules and signaling networks. Nature (London) 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 26.Printen J A, Sprague J F. Protein-protein interactions in the yeast pheromone response pathway: Ste5p interacts with all members of the MAP kinase cascade. Genetics. 1994;138:609–619. doi: 10.1093/genetics/138.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pronk G J, Vries-Smith A M, Buday L, Downward J, Maasen J A, Medema R H, Bos J L. Involvement of Shc in insulin- and epidermal growth factor-induced activation of p21ras. Mol Cell Biol. 1994;14:1575–1581. doi: 10.1128/mcb.14.3.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribon V, Hubbell S, Herrera R, Saltiel A R. The product of the cbl oncogene forms stable complexes in vivo with endogenous Crk in a tyrosine phosphorylation-dependent manner. Mol Cell Biol. 1996;16:45–52. doi: 10.1128/mcb.16.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ribon V, Saltiel A R. Nerve growth factor stimulates the tyrosine phosphorylation of endogenous Crk-II and augments its association with p130Cas in PC-12 cells. J Biol Chem. 1996;270:10800–10805. doi: 10.1074/jbc.271.13.7375. [DOI] [PubMed] [Google Scholar]
- 30.Ribon V, Saltiel A R. Insulin stimulates tyrosine phosphorylation of the proto-oncogene product of c-Cbl in 3T3-L1 adipocytes. Biochem J. 1997;324:839–846. doi: 10.1042/bj3240839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivero-Lezcano O, Sameshima J, Marcilla A, Robbins K. Physical association between src homology 3 elements and the protein product of the c-cbl proto-oncogene. J Biol Chem. 1994;269:17363–17366. [PubMed] [Google Scholar]
- 32.Rose, D. W., A. R. Saltiel, M. Majumdar, S. J. Decker, and J. M. Olefsky. Insulin receptor substrate 1 is required for insulin-mediated mitogenic signal transduction. Proc. Natl. Acad. Sci. USA 91:797–801. [DOI] [PMC free article] [PubMed]
- 33.Rubin C S, Lai E, Rosen O M. Acquisition of increased hormone sensitivity during in vitro adipocyte development. J Biol Chem. 1977;252:3554–3557. [PubMed] [Google Scholar]
- 34.Saltiel A R. Diverse signaling pathways in the cellular actions of insulin. Am J Physiol. 1996;270:E375–E385. doi: 10.1152/ajpendo.1996.270.3.E375. [DOI] [PubMed] [Google Scholar]
- 35.Sasoka T, Draznin B, Leitner J W, Langlois W J, Olefsky J M. Shc is the predominant signaling molecule coupling insulin receptor to activation of guanine nucleotide releasing factor and p21ras-GTP formation. J Biol Chem. 1994;269:10734–10738. [PubMed] [Google Scholar]
- 36.Skolnik E Y, Lee C H, Batzer A, Vicentini L M, Zhou M, Daly R, Myers M J, Baker J M, Ullrich A, White M F, Schlessinger J. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and SHC: implications for insulin control of ras signaling. EMBO J. 1993;12:1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soltoff S P, Cantley L C. p120cbl is a cytosolic adapter protein that associates with phosphoinositide 3-kinase in response to epidermal growth factor in PC12 and other cells. J Biol Chem. 1996;271:563–567. doi: 10.1074/jbc.271.1.563. [DOI] [PubMed] [Google Scholar]
- 38.Sparks A B, Hoffman N G, McConnell S J, Fowlkes D M, Kay B K. Cloning of ligand targets: systematic isolation of SH3 domain containing proteins. Nat Biotechnol. 1996;14:741–745. doi: 10.1038/nbt0696-741. [DOI] [PubMed] [Google Scholar]
- 39.Sun X-J, Rothenberg P, Kahn C R, Backer J M, Araki E, Wilden P A, Cahill D A, Goldstein B J, White M F. Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature (London) 1991;352:73–77. doi: 10.1038/352073a0. [DOI] [PubMed] [Google Scholar]
- 40.Sun X-J, Wang L M, Zhang Y, Yenush L, Myers M G, Glasheen E, Lane W S, Pierce J H, White M F. Role of IRS-2 in insulin and cytokine signaling. Nature (London) 1995;377:173–177. doi: 10.1038/377173a0. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka S, Neff L, Baron R, Levy J B. Tyrosine phosphorylation and translocation of the c-Cbl protein after activation of tyrosine kinase signaling pathways. J Biol Chem. 1995;270:14347–14351. doi: 10.1074/jbc.270.24.14347. [DOI] [PubMed] [Google Scholar]
- 42.Vagne-descroix M, Panasu D, Jornvall H, Carlquist M, Guignard H, Jourdan G, Desvigne G, Collinet M, Caillet C, Mutt V. Isolation and characterization of porcine sorbin. Eur J Biochem. 1991;201:53–59. doi: 10.1111/j.1432-1033.1991.tb16254.x. [DOI] [PubMed] [Google Scholar]
- 43.van den Berghe N, Ouwens D M, Maassen J A, van Mackelenbergh M G H, Sips H C M, Krans H M J. Activation of the Ras/mitogen-activated protein kinase signaling pathway alone is not sufficient to induce glucose uptake in 3T3-L1 adipocytes. Mol Cell Biol. 1994;14:2372–2377. doi: 10.1128/mcb.14.4.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waters S B, Yamauchi K, Pessin J E. Insulin-stimulated dissociation of the SOS-Grb2 complex. Mol Cell Biol. 1995;15:2791–2799. doi: 10.1128/mcb.15.5.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White M F, Kahn C R. The insulin signaling system. J Biol Chem. 1994;269:1–4. [PubMed] [Google Scholar]
- 46.Wiese R J, Mastik C M, Lazar F D, Saltiel A R. Activation of mitogen-activated protein kinase and phosphatidylinositol 3′-kinase is not sufficient for the hormonal stimulation of glucose uptake, lipogenesis, or glycogen synthesis in 3T3-L1 adipocytes. J Biol Chem. 1995;270:3442–3446. doi: 10.1074/jbc.270.7.3442. [DOI] [PubMed] [Google Scholar]