Summary
Background
Pharmacogenomics (PGx) holds promise to revolutionize modern healthcare. Although there are several prospective clinical studies in oncology and cardiology, demonstrating a beneficial effect of PGx-guided treatment in reducing adverse drug reactions, there are very few such studies in psychiatry, none of which spans across all main psychiatric indications, namely schizophrenia, major depressive disorder and bipolar disorder. In this study we aim to investigate the clinical effectiveness of PGx-guided treatment (occurrence of adverse drug reactions, hospitalisations and re-admissions, polypharmacy) and perform a cost analysis of the intervention.
Methods
We report our findings from a multicenter, large-scale, prospective study of pre-emptive genome-guided treatment named as PREemptive Pharmacogenomic testing for preventing Adverse drug REactions (PREPARE) in a large cohort of psychiatric patients (n = 1076) suffering from schizophrenia, major depressive disorder and bipolar disorder.
Findings
We show that patients with an actionable phenotype belonging to the PGx-guided arm (n = 25) present with 34.1% less adverse drug reactions compared to patients belonging to the control arm (n = 36), 41.2% less hospitalisations (n = 110 in the PGx-guided arm versus n = 187 in the control arm) and 40.5% less re-admissions (n = 19 in the PGx-guided arm versus n = 32 in the control arm), less duration of initial hospitalisations (n = 3305 total days of hospitalisation in the PGx-guided arm from 110 patients, versus n = 6517 in the control arm from 187 patients) and duration of hospitalisation upon readmission (n = 579 total days of hospitalisation upon readmission in the PGx-guided arm, derived from 19 patients, versus n = 928 in the control arm, from 32 patients respectively). It was also shown that in the vast majority of the cases, there was less drug dose administrated per drug in the PGx-guided arm compared to the control arm and less polypharmacy (n = 124 patients prescribed with at least 4 psychiatric drugs in the PGx-guided arm versus n = 143 in the control arm) and smaller average number of co-administered psychiatric drugs (2.19 in the PGx-guided arm versus 2.48 in the control arm. Furthermore, less deaths were reported in the PGx-guided arm (n = 1) compared with the control arm (n = 9). Most importantly, we observed a 48.5% reduction of treatment costs in the PGx-guided arm with a reciprocal slight increase of the quality of life of patients suffering from major depressive disorder (0.935 versus 0.925 QALYs in the PGx-guided and control arm, respectively).
Interpretation
While only a small proportion (∼25%) of the entire study sample had an actionable genotype, PGx-guided treatment can have a beneficial effect in psychiatric patients with a reciprocal reduction of treatment costs. Although some of these findings did not remain significant when all patients were considered, our data indicate that genome-guided psychiatric treatment may be successfully integrated in mainstream healthcare.
Funding
European Union Horizon 2020.
Keywords: Preemptive pharmacogenomics, Clinical implementation, Schizophrenia, Major depressive disorder, Bipolar disorder, Cost-effectiveness
Research in context.
Evidence before this study
Genome-guided therapeutics aims to modernize medical practice. Although there are several prospective clinical studies in oncology and cardiology, demonstrating a beneficial effect of genome-guided treatment in reducing adverse drug reactions and maximizing treatment efficacy, there are very few such studies in psychiatry, and interestingly none of which include patients from all main psychiatric indications, namely schizophrenia, major depressive disorder and bipolar disorder.
Added value of this study
This study is the largest prospective clinical study on PGx-guided treatment in psychiatry, including 1076 patients from all three different major psychiatric indications, namely schizophrenia, major depressive disorder and bipolar disorder, into a homogenous cohort. Furthermore, it included, along with the provided clinical evidence, results from a thorough economic analysis, particularly using raw real-life clinical data, demonstrating that genome-guided treatment for major depressive disorders patients is cost-effective, accompanied by a slight increase of the quality of life of these patients.
Implications of all the available evidence
Our large-scale prospective study of pre-emptive PGx-guided therapeutics clearly demonstrates that genome-guided treatment can have a beneficial effect in treating the disease in psychiatric patients by reducing the incidence of adverse drug reactions, accompanied with a reciprocal reduction of treatment costs, providing the necessary evidence for integrating genome-guided psychiatric treatment in mainstream clinical practice.
Introduction
Genome-guided drug treatment, also known as pharmacogenomics (PGx), is nowadays considered to be the cornerstone of modern medical practice. By aiming to rationalize drug use, PGx-guided treatment can maximize therapeutic efficacy and/or minimize toxicity due to adverse drug reactions (ADRs).1 As such, PGx testing can positively impact on today's healthcare, not only by improving the quality of life of the patients but also by reciprocally minimizing healthcare expenditures. Since the conception of the term “pharmacogenetics” in the late 1960s, there is continuous growing evidence for the association of variants in genes encoding for enzymes mostly involved in drug metabolism and transport. However, there is a limited number of prospective PGx clinical studies that demonstrate the clinical utility of PGx testing.2 The latter, together with the lack of evidence for the cost-effectiveness of genome-guided therapeutic interventions, are the two key factors that hinder the integration of PGx into routine clinical care, including their broad reimbursement by payers.3
The PREPARE study (PREemptive Pharmacogenomic testing for preventing Adverse drug REactions; ClinicalTrials.gov NCT03093818) was launched in March 2017, as part of the European Commission-funded Ubiquitous Pharmacogenomics (U-PGx; www.upgx.eu) project. The PREPARE study was an investigator-initiated, open-label, multi-center, cluster-randomised crossover implementation study conducted in 18 hospitals, 9 community health centers, and 28 community pharmacies in 7 European countries, namely Austria, Greece, Italy, the Netherlands, Slovenia, Spain, and United Kingdom that investigated the clinical utility of a pre-emptive genotyping strategy using a 12-gene/46 PGx variants panel.4 The PREPARE study is the first, large scale, prospective clinical PGx study in real-world clinical settings that investigates the effect of genome-guided drug prescription in ADRs occurrence as its name implies. According to Swen and coworkers (2023), a 30% decrease of the risk of developing clinically relevant ADRs was demonstrated across diverse European health-care settings, indicating that large-scale pre-emptive PGx implementation could help to make drug treatment safer.5 Indeed, it was shown that in patients recruited within the scope of the PREPARE study with an actionable PGx test result for any index drug (i.e. one of the drugs that were included in the study design and were prescribed to patients for the first study), a clinically relevant ADR occurred in 152 of 725 patients (21.0%) in the study arm and in 231 (27.7%) of 833 patients in the control arm (p = 0.0075), while when all patients were taken into account, the incidence was 628 of 2923 patients (21.5%) in the study arm and 934 of 3270 patients (28.6%) in the control arm (p < 0.0001). This significant reduction of ADRs occurrence is vital and illustrates the clinical effectiveness of PGx-guided treatment.
Here, we provide both clinical and economic evidence of PGx testing in psychiatric disorders with data derived from a large number of psychiatric patients enrolled within the scope of the PREPARE study in Greece, suffering from schizophrenia (n = 330 in both arms), major depressive disorders (MDD; n = 494 in both arms) and bipolar disorder (n = 252 in both arms). Our aim was to demonstrate that PGx-guided treatment in psychiatry can not only be clinically beneficial by reducing the number and severity of clinically relevant ADRs, hospitalisation days, deaths, the need for polypharmacy and the overall treatment costs but also may improve psychiatric patients’ quality of life.
Methods
Study design
Both clinical and economic data derived from the PREPARE study records. Recruitment has taken place in two clinical sites in Greece: (a) the Psychiatric clinic of Patras University General Hospital and, (b) the 2nd Psychiatric Clinic of Athens University General Hospital “ATTIKON” from May 29th, 2017 until June 30th, 2020. Data on the PREPARE study protocol was previously reported.4 During this period, 1321 psychiatric patients suffering from schizophrenia, MDD, bipolar disorder and other psychiatric indications were recruited in both sites. However, for the purpose of this study, only patients belonging to the three distinct disease subgroups, namely schizophrenia, MDD and bipolar disorder (n = 1076) were included, since the remaining 245 patients suffering from other psychiatric indications was a rather heterogeneous group and hence were excluded from further analysis (Fig. 1a). An informed consent form was provided, discussed and signed by each participant before any study's related assessment while detailed medical records were documented in source documents and in the study's electronic case report system (eCRF) for all subjects.
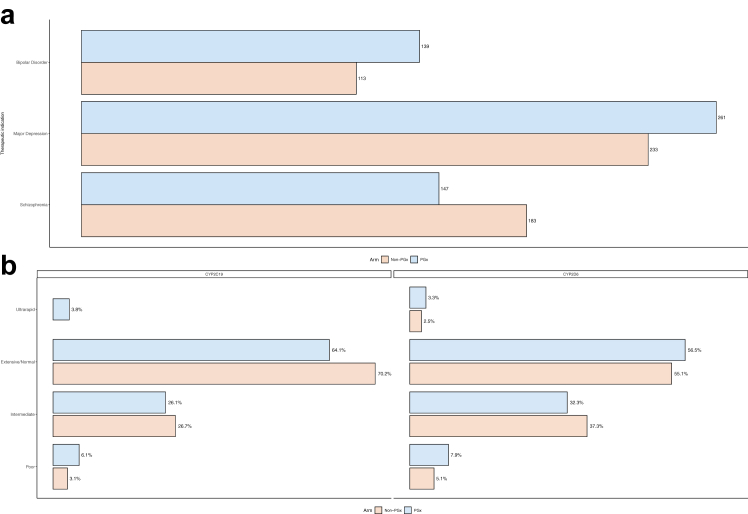
Fig. 1.
Barplot of (a) indications and (b) the metaboliser status in each study arm in the Greek PREPARE patient cohort.
Study participants
All inclusion and exclusion criteria of the study are briefly described below. Adult (≥18 years of age) patients of any ethnicity, with a clinical diagnosis of a psychiatric disorder, namely psychosis, MDD and bipolar disorder that were treatment naïve to 13 psychiatric medications with proven clinical actionability based on DPWG guidelines,4,5 namely Amitriptyline, Aripiprazole, Carbamazepine, Citalopram, Clomipramine, Doxepine, Escitalopram, Haloperidol, Paroxetine, Pimozide, Sertraline, Venlafaxine, Zuclopenthixol, hadn't undertaken any genetic testing in the past for CYP2C19 and/or CYP2D6 genes, consented to be followed up for at least 12 weeks and could give blood or saliva sample were eligible to participate in the study. Patients were excluded in case that (a) they did not provide signed informed consent, (b) were pregnant or breastfeeding, (c) were suffering from advanced liver failure (stage Child-Pugh C) or had an existing impaired hepatic or renal function (estimated glomerular filtration rate (MDRD) of less than 15 ml/min per 1.73 m2 in a subject with a functioning graft), (d) their estimated life expectancy was less than three months and (e) had no fixed address or an assigned general practitioner. Physicians participating in the study established the diagnosis of psychiatric disorder, by the DMS-V criteria, the life expectancy of patient and medical history of each patient relying on all available clinical data. More detailed description of inclusion and exclusion criteria is presented by van der Wouden and coworkers (2017).4
Procedures
Within PREPARE, participating countries were randomized to start with either PGx-guided treatment or standard of care. Greece was allocated to start with PGx-guided treatment. At the end of each study arm, a new set of subjects were recruited. In Greece, the PGx-guided arm run from May 29th, 2017, until October 31st, 2018 and the control arm from November 1st, 2018 until June 30th, 2020. All study participants were followed-up for a minimum of 12 weeks and no more than 19 months. Patients belonging to the control arm followed a non-tailored treatment strategy based on the common clinical routine, whereas patients belonging to the PGx-guided arm received a genome-guided treatment based on each patient's CYP2C19 or CYP2D6 genotyping results and recommendations from the DPWG.6 Patients' samples were genotyped for 12 genes of the pre-emptive panel but only genotyping results for CYP2C19 or CYP2D6 pharmacogenes were taken into consideration, since these were related to the metabolism of psychiatric medications. During the study, subjects were asked to complete two online questionnaires at week 2 and at week 8 and to perform four interviews, called nurse assessments, on baseline, week 4, week 12 and upon completion of the arm period (19 months). Those nurse assessments were conducted either via phone calls remotely or via on-site interviews by trained research personnel and included questions about the occurrence of any ADRs, disease progression, subject's quality of life, use of any concomitant medication or procedure and any hospitalisation event.
On baseline visit, trained physicians discussed with participants all study requirements and obtained biological material for DNA isolation, either blood or saliva samples, follow-up visits, interviews and provided them with the informed consent form. Genetic results for patients belonging to the PGx-guided arm were available within 7 days upon sample collection. Physicians then reviewed each patient's results to adjust the patient's treatment either by adjusting the drug dose or by changing medication in accordance with the DPWG relevant guidelines. Therefore, for patients belonging to the PGx-guided arm, drug treatment was decided up to a week upon patient's enrollment.
Basic participants’ demographics information including gender, age, body mass index (BMI), smoking and alcohol consumption status along with clinical data such as comorbidities, medication use was recorded at the baseline visit (Table 1). Data related to ADRs, utilities, visits to emergency units, hospital admissions were collected via the nurse assessments as mentioned above. Genotyping was performed using the LGC Genomics platform as described previously.4,5 Psychiatric index drugs included in our analysis were Carbamazepine, Pimozide, Doxepine, Sertraline, Venlafaxine, Escitalopram, Citalopram, Paroxetine, Amitriptyline, Clomipramine, Haloperidol, Aripiprazole, Zuclopenthixol, Carbamazepine, Pimozide and Doxepine (Table 2). Other common psychiatric drugs were not included in our procedures, as they do not have an actionable PGx test, according to the DPWG guidelines.4,5
Table 1.
Demographics of the Greek PREPARE patient cohort. Continuous variables are summarised as median (Q1, Q3), and continuous as n (%).
| N = 1076 |
p-value | ||
|---|---|---|---|
| PGx-guided 0N = 547a | Control N = 529a | ||
| Age | 49 (39, 58) | 49 (40, 58) | >0.9b |
| Gender | 0.4 | ||
| Female | 285 (52.10%) | 262 (49.53%) | |
| Male | 262 (47.90%) | 267 (50.47%) | |
| BMI | 0.11c | ||
| Underweight | 7 (1.28%) | 9 (1.70%) | |
| Healthy weight | 177 (32.36%) | 207 (39.13%) | |
| Overweight | 224 (40.95%) | 192 (36.29%) | |
| Obese | 139 (25.41%) | 121 (22.87%) | |
| Smoking status | 0.057c | ||
| Current | 316 (57.77%) | 323 (61.06%) | |
| Ex-smoker | 50 (9.14%) | 63 (11.91%) | |
| Non-smoker | 181 (33.09%) | 143 (27.03%) | |
| Alcohol consumption (units/week) | 0.2d | ||
| <1 | 380 (69.47%) | 369 (69.75%) | |
| 1–5 | 103 (18.83%) | 115 (21.74%) | |
| 6–14 | 42 (7.68%) | 23 (4.35%) | |
| 15–21 | 10 (1.83%) | 5 (0.94%) | |
| 22–49 | 7 (1.28%) | 9 (1.70%) | |
| >50 | 5 (0.91%) | 4 (0.76%) | |
| N/A | 0 (0%) | 4 (0.76%) | |
Median (IQR); n (%).
Wilcoxon rank sum test.
Pearson's Chi-squared test.
Fisher's exact test.
Table 2.
Average dosage (in milligrams|) administered per index drug, presented as mean and standard deviation.
| First index drug | Average dose (SD) | ||
|---|---|---|---|
| Antidepressants | Sertraline | PGx-guided = 86 | Control = 92 |
| 78.55 (43.42) | 96.74 (35.4) | ||
| Venlafaxine | PGx-guided = 75 | Control = 98 | |
| 180.2 (76.3) | 182.14 (61.87) | ||
| Escitalopram | PGx-guided = 85 | Control = 68 | |
| 12.24 (5.09) | 14.45 (5.05) | ||
| Citalopram | PGx-guided = 71 | Control = 41 | |
| 19.51 (8.5) | 19.02 (8.53) | ||
| Paroxetine | PGx-guided = 16 | Control = 7 | |
| 21.25 (8.06) | 24.29 (5.35) | ||
| Amitriptyline | PGx-guided = 16 | Control = 4 | |
| 20.56 (25.48) | 65 (48.13) | ||
| Clomipramine | PGx-guided = 6 | Control = 5 | |
| 167.92 (66.9) | 150 (53.03) | ||
| Antipsychotics | Haloperidol | PGx-guided = 64 | Control = 133 |
| 20.84 (12.41) | 22.19 (14.8) | ||
| Aripiprazole | PGx-guided = 110 | Control = 72 | |
| 21.68 (37.53) | 20.66 (8.67) | ||
| Zuclopenthixol | PGx-guided = 13 | Control = 8 | |
| 42.54 (13.37) | 61.88 (26.72) |
Note: Carbamazepine, Pimozide and Doxepine are not included in the table (Carbamazepine was removed from PREPARE's list of index drugs and was only prescribed to 3 patients, all in the PGx-guided arm, while Pimozide and Doxepine were only prescribed in one patient each).
Perspective of the economic analysis
The perspective of this study's economic analysis was that of the Greek healthcare system. In this analysis, types of direct costs such as ADRs costs, hospitalisation costs in the psychiatric clinic, cost of psychiatric medications, follow-up costs, genetic testing cost and therapist sessions along with the relevant induced costs were included. All those costs were reimbursed by the payers in Greece. Indirect costs such as loss of productivity due to absenteeism were not taken into consideration for the present analysis.
Missing data in the economic analysis
Dealing with missing data is a very common issue in economic analysis and their proper handling might improve the analysis’ conclusions. Each value has a different impact in the observed outcome. For instance, missing baseline values can have a great impact on the analysis, on the ground that it might be necessary to use those missing values to predict subsequent outcomes. For this reason, single imputation method was applied for baseline utility in each treatment arm, by filling the missing values with the average of the observed cases. For intermittent missing data in quality-of-life related answers, when possible, linear interpolation method was used between measurement points while multiple imputation method with five imputed datasets was done for the rest of them. No data censoring was performed, and it was assumed that all patients remained on study and were observed for 18 months in each arm.
Utility values and costing methodology
The Visual Analogue Scale (VAS) score was the valuation method used to elicit PGx testing utility. Participants had to complete a relevant question at baseline visit, week 4, week 12 and 19 months. Quality-Adjusted Life Years (QALYs) were measured by calculating the integral of the product of individual's life expectancy multiplied by weighted VAS score and adjusting the baseline measures of utility within a covariate regression framework. Total cost included a) the cost of ADRs, b) the cost of daily hospitalisation in psychiatric clinic, c) follow-up costs, d) the cost of index drugs used, e) the cost of genetic testing applicable only for PGx-guided group.
More precisely, ADRs costs were based on the description of Diagnosis Related Groups (DRGs) that are designed and implemented for the Greek healthcare system, while only events characterized with severity grade 3 and above were included in the present analysis. Furthermore, it was assumed that ADRs of lower severity grade, (grade 1 and 2), didn't produce any worth-measurable costs and were excluded from the analysis. Based on the available raw data, it was observed that management of mild severity ADRs didn't incorporate any hospital admission or any other costly assessment and thus there weren't any costs to be estimated.
Reimbursement tariffs for costs of DRGs, hospitalisation costs, follow up costs, session with therapists’ costs were retrieved from the official sourced of Greek Ministry of Health and of Greek National Healthcare Services Organisation and were applicable to all public hospitals and public payers of Greece [www.eopyy.gov.gr7 (content in Greek); Table S1]. Prices of index drugs prescribed and used during the study were taken from the national reimbursement list published in December 2022. Finally, price of PGx testing for the two genes of interest (CYP2C19 and CYP2D6) was derived from an economic study in Italy,8 owing to the lack of an official tariff in Greece. Similarly with a previous pharmacoeconomic analysis, patient-level resource utilisation data were combined with unit cost data and then aggregated to compute total treatment cost per patient.9 Due to limited time horizon of this observational study, discount rate was not applied.
Finally, each of three psychiatric diseases were analysed separately. The Incremental Cost-Effectiveness Ratio (ICER) was determined as the ratio of the difference in costs between PGx-guided group versus control group divided by the difference in QALYs. All patients were included in the analysis and not only the ones with an actionable genotype.
Statistics
Baseline patient characteristics, including age, gender, alcohol consumption and body mass index (BMI) are presented in Tables S1 and S2. BMI specifically, was categorized based on the classification system provided by the center of Disease Control and Prevention (CDC, https://www.cdc.gov/obesity/basics/adult-defining.html). Continuous variables are reported with their respective mean and standard deviation if normally distributed, or their median and interquartile range (IQR) otherwise, while for categorical variables, absolute and relative frequencies are provided. Normality was assessed by implementing the Shapiro–Wilk normality test, while for hypothesis testing, the appropriate test among Wilcoxon Mann Whitney U test (for continuous variables), Pearson's chi-square or Fisher's exact test (for categorical variables, depending on the number of available instances) was conducted.
Outcomes
The primary outcome of the study was the occurrence of any clinically relevant ADR with severity grade 2, 3, 4, or 5 according to NCI-CTCAE criteria, that were related to the (first or second) index drug.10 Secondary outcomes include the occurrence of any ADRs, irrespective of the severity grade, as well as the occurrence of non-ADR-related hospitalisations or re-admissions and quality of life. Moreover, genome-guided drug or dose changes, frequency of ADRs, frequency and duration of non-ADR-related hospitalisations and re-admissions were explored too. Analyses were performed initially for patients having a metabolizer status for which DPWG guidelines were available for their prescribed first index drug (hereinafter mentioned as “actionable patients”) and subsequently for the entire study population.
In addition, the occurrence of clinically relevant and any ADRs, as well as the occurrence of non-ADR-related hospitalisations were compared between the control and PGx-guided arms, after stratifying for the therapeutic class of the first index drug. The comparisons were originally performed in the entire study population, and for those patients receiving antidepressants, the same hypotheses were tested in the actionable patients too. Due to the small number of actionable patients among those treated with antipsychotics, no further test was performed in this subgroup.
Logistic regression was utilized to model the primary outcome, as well as the outcomes of presenting with any ADR or non-ADR related hospitalisations, against the intervention (PGx-guided pharmacotherapy or standard-of-care). Patient's age, baseline quality of life, total number of concomitant medications (both psychiatric and non-psychiatric drugs), as well as patient's diagnosis were included as covariates.5 Patient perceived health and quality of life was determined using the visual analogue scale, and ranges from zero (worst possible state) to 100 (best possible state). Due to the limited number of actionable patients, the respective logistic regression models included only age and number of concomitant medications as covariates, along with the attributed arm. The same approach was also applied for the non-ADR hospitalisations outcome within the actionable subpopulation. Odds ratios and confidence intervals are provided, as well as p-values for each covariate, derived from the Wald test. For any other evaluations, appropriate hypothesis tests were applied. When the frequency of individual ADRs (both grade-specific and overall) was evaluated, the Benjamini-Hochberg correction for multiple testing was applied to reduce the risk of false positive results.11 All analyses were performed in R statistical language (R Development Core Team, 2023) version 4.2.1, while significance level (α) was set to 0.05.12
Role of the funders
The funders had no influence on the design or conduct of the trial and were not involved in data collection or analysis, in the writing of the manuscript, or in the decision to submit it for publication.
Ethics
For the realisation of the study in Greece, approvals from the Institutional Review Board of the Patras University General hospital (825/28.12.2016) and the Athens University General Hospital “ATTIKON” (17/28.11.2017) were received. Signed and written informed consent was provided by all patients that have participated in the study and it was previously approved by local hospitals’ ethical committees. Data from the PREPARE study are not publicly available but are planned to be made available after preplanned analyses have been completed.
Results
In total, 1076 psychiatric patients were included in this analysis, from which 547 patients belonged in the PGx-guided arm and 529 patients in the control arm. From these patients, 330 patients were suffering from schizophrenia (n = 147 in the PGx-guided arm and n = 183 in the control arm), 494 patients were suffering from MDD (n = 261 in the PGx-guided arm and n = 233 in the control arm) and 252 patients were suffering from bipolar disorder (n = 139 in the PGx-guided arm and n = 113 in the control arm; Fig. 1a). The distribution of the patients according to their metabolizer status is shown in Fig. 1b. Both study arms were found to be comparable in terms of their baseline characteristics and composition. Median age was 48 (36–57) years in the PGx-guided arm and 47 (38–57) years in the control arm. Moreover, more than half of the patients were overweight or obese, and over 60% of the patients in both arms were either current or previous smokers. Finally, the most common index psychiatric drug was haloperidol (n = 197), followed by aripiprazole (n = 182), and sertraline (n = 178) (Table 2). In total nine patients were lost to follow-up, eight of them from the control arm and one from the PGx-guided arm.
Actionable diplotypes
With respect to the 12 pharmacogenes assessed in the PREPARE study (CYP2B6, CYP3A5, SLCO1B1, VKORC1, CYP2D6, DPYD, CYP2C9, UGT1A1, CYP2C19, F5, TPMT and HLA-B), only one of the 1076 Greek psychiatric patients was not a carrier of a potentially actionable PGx diplotype (0.09%). 10 patients (0.93%) had actionable diplotype in one gene, 72 (6.69%) in two genes, 193 (17.94%) in three genes, 316 (29.37%) in four genes, 273 (25.37%) in five genes, 143 (13.29%) in six genes, 56 (5.2%) in seven genes, 9 (0.84%) in eight genes, and 3 (0.28%) in nine genes. Overall, a subject in our study was found to carry on average a diplotype with potential impact for drug response in at least four genes (4.38).
DPWG recommendations and adherence
Based on the genotyping analysis results, DPWG recommendations for index drug change or dose adjustment were available for 136 out of 547 patients (24.86%) in the PGx-guided arm. Treating physicians adhered to recommendations for 110 out of 136 cases (80.88%). Indeed, dose was amended in 97 patients, index drug was changed in 10 patients, while increasing surveillance was decided for one patient and medication withdrawal was applied for two patients. Among the 97 patients in which the dose was tailored, the average modified dose was 52.5% (SD 20.8) of the standard dose. More specifically, the drug was altogether stopped in one patient, in 3 patients the dose was left to the standard dose (100%), and in one patient it was increased to 150% of the standard dose. The remaining patients received drug doses that varied between 10% and 80% of the standard.
Similarly, in the control arm, 126 patients for which DPWG recommendations were available for their respective drug-metabolizer status combination were identified. For the first index drug, the highest numbers of patients having an actionable variant were observed for venlafaxine (85/173 [49.13%]—40 in the PGx-guided and 45 in the control arm), sertraline (56/178 [31.66%]—26 the PGx-guided and 30 in the control arm), escitalopram (38/153 [24.84%]—20 in the PGx-guided and 18 in the control arm) and citalopram (29/112 [25.89%]—17 in the PGx-guided and 12 in the control arm).
Primary outcome
Occurrence of clinically relevant ADRs
Within the actionable patients (n = 262), clinically relevant ADRs were observed at 10.73% of the patients of the PGx-guided arm, compared to 19.05% of the control arm patients. This protective effect of PGx-guided arm remains statistically significant upon adjusting for patient's age and number of concomitant medications (OR: 0.48 (0.23, 0.98), p-value = 0.049; Table 3). When the comparison was extended to include the entire study population, this effect was diluted, with the occurrence of ADRs in the PGx arm being 12%, compared to 15% in the control arm (p = 0.2).
Table 3.
Adjusted comparison of clinically significant ADRs across treatment arms.
| Entire study |
Actionable patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control N = 529a | PGx-guided N = 547a | OR (96% CI)b | p-valueb | Control N = 126a | PGx-guided N = 136a | OR (96% CI)b | p-valueb | |
| Clinically relevant ADRs | 81 (15%) | 68 (12%) | 0.79 (0.59, 1.13) | 0.2 | 24 (19.05%) | 14 (10.37%) | 0.48 (0.23, 0.98) | 0.049 |
| Age | 49 (40.58) | 49 (39.58) | 0.98 (0.97, 0.99) | 0.002 | 51 (44.58) | 50 (39.58) | 0.98 (0.95, 1.01) | 0.2 |
| Co-medications | 3.00 (2.00, 6.00) | 3.00 (2.00, 5.00) | 1.08 (1.02, 1.15) | 0.013 | 3.00 (1.00, 5.00) | 3.00 (2.00, 5.00) | 1.18 (1.05, 1.33) | 0.006 |
| Baseline QoL | 0.60 (0.40, 0.70) | 0.59 (0.40, 0.70) | 0.23 (0.10, 0.55) | 0.001 | 0.50 (0.40, 0.70) | 0.52 (0.40, 0.70) | ||
Median (IQR); n (%).
Logistic regression of any clinically relevant ADRs on treatment arm, age, concomitant medications, diagnosis and baseline QoL (entire study) or treatment arm, age and concomitant medications (actionable patients).
With regards to the specific ADRs presented in the entire population, among the psychiatric patients investigated in the Greek component of the PREPARE study, the overall occurrence of ADR is lower than that reported in the entire study,5 for either of the two arms. Statistically significant differences in the distribution of NCI-CTCAE grade 2–5 ADRs were observed for gastrointestinal disorders (2.8% in the control arm compared to 0.7% in the PGx-guided arm, p-value = 0.009, Pearson's Chi-squared test), musculoskeletal and connective tissue disorders (2.0% in the control compared to 0.2% in the PGx-guided arm, p-value = 0.004, Pearson's Chi-squared test), general disorders and administration site conditions (2.1% in the control arm compared to 0.6% in the PGx-guided arm, p-value = 0.027, Pearson's Chi-squared test), nervous system disorders (6.4% in the control arm compared to 2.8% in the PGx-guided arm, p-value = 0.004, Pearson's Chi-squared test) and psychiatric disorders (2.7% in the control arm compared to 0.6% in the PGx-guided arm, p-value = 0.006, Pearson's Chi-squared test). Nevertheless, these differences did not remain statistically significant after applying a correction for multiple testing (Table S3). From the aforementioned ADRs, those related to nervous system disorders (grade 2–5) remained statistically significant in the DPWG actionable patients (7.94% in the control arm compared to 1.48% in the PGx-guided arm, p-value = 0.013, Pearson's Chi-squared test), as well as grade 1 psychiatric disorders, that are not present at all in the PGx-guided arm (6.35% in the control arm, p-value = 0.003, Fisher's exact test). Of them, the difference in grade 1 psychiatric disorders remained statistically significant after correction (Table S3).
Among the patients that presented with a clinically relevant ADR in the entire cohort, the most common prescribed index drugs in the PGx arm were haloperidol (28/68, 41%), aripiprazole (9/68, 13%) and sertraline (9/68, 13%). Similarly, in the control arm, the most commonly prescribed index drugs among patients with clinically relevant ADRs were venlafaxine (33/81, 41%), haloperidol (18/81, 22%), and sertraline (11/81, 14%).
Secondary outcomes
Occurrence of any ADRs
When investigating the effect of drug prescription in the occurrence of any ADR (including those with severity grade 1), a reduction in the occurrence of ADRs, both in the actionable patients (n = 262, 18.52% in the PGx-guided arm compared to 28.57% in the control arm, p-value = 0.055) and in the entire population of the study (n = 1076, 20% in the PGx-guided arm compared to 24% in the control arm, p-value = 0.13; Table 4) was observed, without however being statistically significant.
Table 4.
Adjusted comparison of any ADRs across treatment arms.
| Entire patient cohort |
Actionable patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control N = 529a | PGx-guided N = 547a | OR (96% CI)b | p-valueb | Control N = 126a | PGx-guided N = 136a | OR (96% CI)b | p-valueb | |
| Any ADR | 128 (24%) | 110 (20%) | 0.79 (0.59, 1.07) | 0.13 | 36 (28.57%) | 25 (18.52%) | 0.56 (0.31, 1.01) | 0.055 |
| Age | 49 (40, 58) | 49 (39, 58) | 0.97 (0.96, 0.99) | <0.001 | 51 (44, 58) | 50 (39, 58) | 0.99 (0.96, 1.01) | 0.3 |
| Co-medications | 3.00 (2.00, 6.00) | 3.00 (2.00, 5.00) | 1.07 (1.02, 1.13) | 0.006 | 3.00 (1.00, 5.00) | 3.00 (2.00, 5.00) | 1.14 (1.03, 1.27) | 0.011 |
| Baseline QoL | 0.60 (0.40, 0.70) | 0.59 (0.40, 0.70) | 0.30 (0.15, 0.63) | 0.001 | 0.50 (0.40, 0.70) | 0.52 (0.40, 0.70) | ||
Median (IQR); n (%).
Logistic regression of any ADRs on treatment arm, age, concomitant medications, diagnosis and baseline QoL (entire study) or treatment arm, age and concomitant medications (actionable patients).
Hospitalisations
As far as hospitalisations are concerned in the entire study population, our results showed significant differences in terms of number of patients hospitalized, that remain statistically significant when adjusting for age and number of concomitant medications. In particular, 110 out of 547 patients (20.11%) in the PGx-guided arm were hospitalized, compared to 187 out of 529 patients (35.25%) belonging to the control arm (OR 0.46 (0.34, 0.61), p < 0.001). As far as actionable patients are concerned, 22 out of 136 patients (16.18%) in the PGx-guided arm were hospitalized, compared to 30 out of 126 patients (23.81%) in the control arm (p = 0.11; Table 5), although this difference was not found to be statistically significant.
Table 5.
Adjusted comparisons of hospitalisations and re-admissions across treatment arms.
| Entire study |
Actionable patients |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control N = 529a | PGx-guided N = 547a | OR (96% CI)b | p-valueb | Control N = 126a | PGx-guided N = 136a | OR (96% CI)b | p-valueb | |
| Hospitalisation | 187 (35.35%) | 110 (20.11%) | 0.46 (0.34, 0.61) | <0.001 | 30 (23.81%) | 22 (16.18%) | 0.60 (0.32, 1.11) | 0.11 |
| Age | 49 (40, 58) | 49 (39, 58) | 0.98 (0.97, 1.00) | 0.004 | 51 (44, 58) | 50 (39, 58) | 0.98 (0.96, 1.01) | 0.2 |
| Co-medications | 3.00 (2.00, 6.00) | 3.00 (2.00, 5.00) | 1.02 (0.97, 1.08) | 0.4 | 3.00 (1.00, 5.00) | 3.00 (2.00, 5.00) | 1.06 (0.95, 1.18) | 0.3 |
| Baseline QoL | 0.60 (0.40, 0.70) | 0.59 (0.40, 0.70) | 0.23 (0.10, 0.55) | 0.6 | 0.50 (0.40, 0.70) | 0.52 (0.40, 0.70) | ||
Median (IQR); n (%).
Logistic regression of hospitalisation on treatment arm, age, concomitant medications, diagnosis and baseline QoL (entire study) or treatment arm, age and concomitant medications (actionable patients).
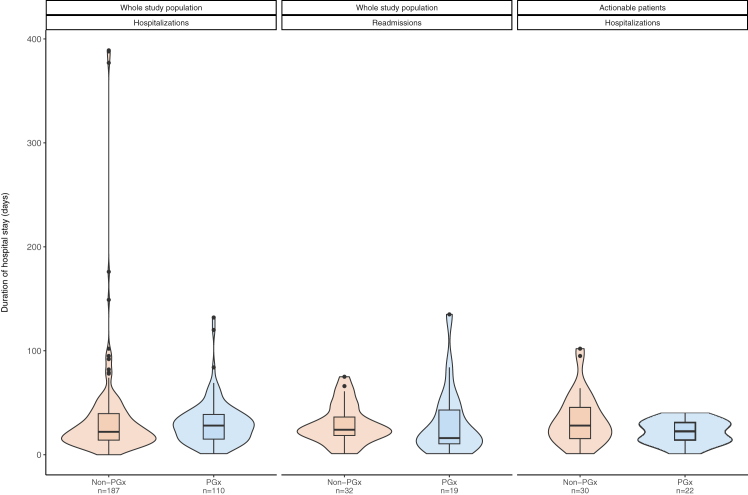
Among hospitalized patients, approximately 17% required a subsequent hospitalisation when the entire cohort was evaluated, while in the case of actionable patient cohort, 33.33% of patients in the control arm and 23.64% of the patients in the PGx-guided arm were readmitted. The total hospitalisations days for the entire patient cohort appear to be markedly different when comparing the two arms, with 3305 days in the PGx-guided patient group (110 patients) versus 6517 days in the control group (187 patients), while similar observations are derived from the actionable patient cohort (477 days in the PGx-guided patients versus 1001 days in the control patient cohort; Table 6). It must be noted, however, that a small number of patients were outliers, namely hospitalised for prolonged periods of time in both study arms, reflected both in the median number of primary hospitalisation days between the two arms, or the median re-admission days (Table 6; Fig. 2). More specifically, during the primary hospitalization, actionable patients of the PGx-guided arm spent 14–30 days in the hospital, compared to 16–46 days for the patients of the control arm (p = 0.12, Wilcoxon rank sum test). When the entire study population was considered the median hospitalization time was 14–40 days for the patients in the control arm and 15–39 days for the patients in the PGx-guided arm (p = 0.3, Wilcoxon rank sum test). With regards to subsequent hospitalizations and after normalizing for the number of times a patient was re-admitted to the hospital, we observe that both in the entire cohort [PGx-guided arm: 9–30 days, control arm: 16–30 days, p = 0.3, Wilcoxon rank sum test], as well as inside the subpopulation of actionable patients [PGx-guided arm: 14–36 days, control arm: 9–44 days, p > 0.9, Wilcoxon rank sum test], patients in the PGx-guided arm are hospitalised for less days than patients in the control arm, although this differences are not statistically significant (Table 6).
Table 6.
Detailed description of non-ADR related hospitalisations, including the frequency and duration of hospitalisations and subsequent re-admissions, within the hospitalised patients.
| All hospitalized patients | Entire cohort |
Actionable patients |
||||
|---|---|---|---|---|---|---|
| Control N = 187 | PGx-guided N = 110 | p-value | Control N = 30 | PGx-guided N = 22 | p-value | |
| Total days hospitalized | 6517 [4322]e | 3305 [2969]e | 1001 [804]e | 477 | ||
| Duration of hospitalisations (days) | 22 (14–40)a | 28 (15–39)a | 0.3d | 28 (16–46)a | 22 (14–30)a | 0.12d |
| Re-admitted | 32 (17.11%)b | 19 (17.27%)b | >0.9c | 10 (33.33%)b | 3 (13.64%)b | 0.11c |
| All re-admitted patients | Control N = 32 | PGx-guided N = 19 | p-value | Control N = 10 | PGx-guided N = 3 | p-value |
| Frequency of re-admissions | 1 (1–1)a | 1 (1–1.5)a | 0.5d | 1 (1–2)a | 1 (1–1.5)a | 0.8d |
| Duration of re-admissions | 24 (18–36)a | 16 (10–43)a | 0.3d | 28 (20–48)a | 28 (21–43)a | >0.9d |
| Average days/re-admission/patient | 23 (16–30)a | 14 (9–30)a | 0.15d | 23 (9–44)a | 14 (14–36)a | >0.9d |
| Total days of hospitalisation upon readmission | 928 [787]e | 579 | 341 | 100 | ||
With regards to total days hospitalised or re-admitted per patient, the calculations have been also performed after excluding outliers (15 patients from the control group and 3 from the PGx group for the entire cohort, and 2 patients from the control group within actionable patients) and these values are presented within square brackets.
Median (IQR).
n (%).
Pearson's Chi-squared test.
Wilcoxon rank sum test.
Excluding outliers, defined as patients with total hospitalisation/re-admission days over Q3 + 1.5∗IQR or below Q1−1.5∗IQR.
Fig. 2.
Distribution of days hospitalised/re-admitted in the entire study population and the actionable patients separately. Median values and respective IQRs for each case are presented in Table 6.
Overall, this analysis indicates a benefit for patients receiving PGx-guided therapy with respect to the odds of being hospitalised. Although the duration of hospitalisation, when it occurs, is not affected, there is a substantial decrease in the chances of requiring to be hospitalised in the first place for these patients.
Polypharmacy
It was observed that a quarter of psychiatric patients are under polypharmacy in terms of disease management and not in monotherapy. In the present analysis, we defined as polypharmacy the use of at least four psychiatric medications. Indeed, 22.67% of patients in the PGx-guided arm were under 4 psychiatric drugs compared to 27.03% of the control arm (Table 7). In the entire study population, there is a statistically significant difference in the average number of psychiatric drugs administered on top of index drug 1. Furthermore, when we focused only on antipsychotic treatment and polypharmacy (in this case we considered only patients receiving at least two antipsychotic drugs), it was concluded that 18.53% patients of the control group compared to 12.98% in the PGx-guided arm were under treatment with at least two antipsychotic drugs (Table S4).
Table 7.
Number of patients under psychiatric polypharmacy.
| Entire patient cohort | Control N = 529a | PGx-guided N = 547a | p-valueb |
|---|---|---|---|
| At least 4 psychiatric drugs | 143 (27.03%) | 124 (22.67%) | 0.2b |
| Less than 4 psychiatric drugs | 291 (55.01%) | 310 (56.67%) | |
| Only index drug (No co-administered drugs) | 95 (17.96%) | 113 (20.66%) | |
| Average number of co-administered psychiatric drugs on top of index drug | 2 (1–4) | 2 (1–3) | 0.021c |
|
Actionable cohort |
Control N = 126a |
PGx-guided N = 136a |
p-valueb |
| At least 4 psychiatric drugs | 29 (23.02%) | 28 (20.59%) | 0.8b |
| Less than 4 psychiatric drugs | 69 (54.76%) | 74 (54.41%) | |
| Only index drug (No co-administered drugs) | 28 (22.22%) | 34 (25.00%) | |
| Average number of co-administered psychiatric drugs on top of the index drug | 2 (1–3) | 2 (0.75–3) | 0.8c |
n (%); Median (IQR).
Pearson's Chi-squared test.
Wilcoxon rank sum test.
Cost-effectiveness analysis
We then explored whether the clinically relevant differences seen previously between the two study arms in terms of ADRs and hospitalisations, could also have an impact on an economic level. Our data show that the costs associated with treating patients suffering from MDD and belonging to the PGx-guided arm were substantially less (€1302) compared to those belonging to the control arm (€2526). A much smaller difference, in favor of the PGx-guided arm, was found between the two study arms in terms of QALYs and life-years (LYs). In particular, the mean estimate for QALYs in MDD patients in the PGx-guided group was 0.93 (95% CI, 0.85–0.99) versus 0.92 (95% CI, 0.86–0.99) for the control group (Table 8). As far as bipolar and schizophrenia patients are concerned, we have only seen a very minor difference in both costs and overall quality of life. In particular, the mean estimate for QALYs in schizophrenia patients in the PGx-guided group was 0.967 compared to 0.97 of the control group, respectively, while QALYs in bipolar patients was 0.96 for PGx-guided group and 0.97 for the control group, respectively. Similar differences were noticed in costs as well. In the cohort of patient suffering from schizophrenia, the PGx-guided group had a slightly higher average cost (€1243) in contrast to control group (€1115), a trend that is followed in bipolar disorder cohort as well, namely €940 for the PGx-guided group versus €1027 for the control group, respectively.
Table 8.
Differences in the total costs and quality of life in both the PGx-guided and the control study arms.
| PGx-guided arm |
Control arm |
|||
|---|---|---|---|---|
| QALYa | Total cost (€)a | QALYa | Total cost (€)a | |
| Schizophrenia | 0.9697 | 1243 | 0.9768 | 1115 |
| Major depressive disorder | 0.935 | 1302 | 0.925 | 2526 |
| Bipolar Disorder | 0.96 | 940 | 0.97 | 1027 |
The data have been calculated using bootstrapping analysis.
Refers to average values.
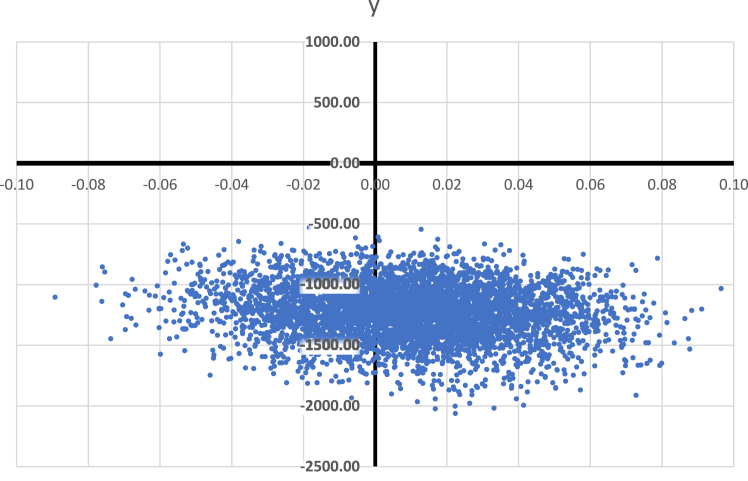
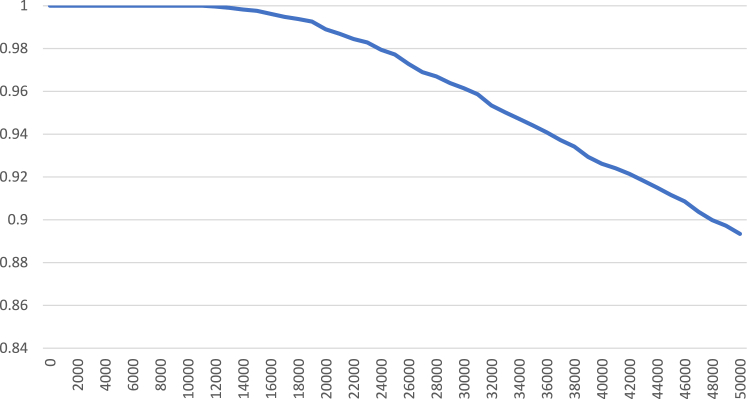
Τhe results of the probabilistic sensitivity analysis on 5000 bootstraps simulations have been illustrated in the cost-effectiveness plane showing how the difference of experiments for cost and Quality-Adjusted Life Years (QALYs) is distributed (Fig. 3). PGx-guided treatment represents a cost-effective option in 97% of experiments at €30,000 per QALY willingness to pay (WTP) threshold. Furthermore, in accordance with the acceptability curve, the probability of PGx-guided treatment being cost-effective increases at a lower willingness to pay (WTP) threshold (Fig. 4). Notably, at €50,000 per QALY, the probability PGx-guided treatment of being cost-effective is around 89%, whereas in lower WTP such as under €18,000, the probability is well over 99%.
Fig. 3.
Bootstrapping simulation results on cost-effectiveness plane of the PGx-guided arm versus the Control arm for MDD patients.
Fig. 4.
Acceptability curve of the PGx-guided arm treatment strategy for MDD patients.
Discussion
Mental illness, especially serious mental diseases such as MDD, schizophrenia and bipolar disorder affect millions of individuals of people worldwide. Importantly, almost 250 million people are diagnosed with MDD while over 81 million patients live with schizophrenia or bipolar disorder and are under chronic treatment.13,14 Besides the global burden caused by psychiatric disorders, the lack of new psychotropic medications in combination with the difficulty to determine an efficient drug regimen due to several parameters including interindividual genetic variability to drug response, leads to frequent ADRs, increased health expenditures and unsatisfactory patient management.13,15
To our knowledge, this study: (a) is the largest prospective clinical study on PGx-guided treatment in psychiatry, (b) managed to include in substantial numbers of patients from three different major psychiatric indications into a homogenous cohort, (c) included a follow-up time of up to 18 months, while most studies in the literature had much shorter duration (24 weeks at most), (d) included well-balanced cohorts in terms of demographics, contrary to previous studies possibly leading to a potential bias, (e) was characterized by more generic clinical endpoints compared to other studies in the field, allowing to observe three distinct mental diseases in an effective way and to collect both clinical and economic data, and (f) included, along with the provided clinical evidence, data from a thorough economic analysis, particularly using raw real-life clinical data contrary to the majority (more than 69%) of the economic evaluation studies that are employing simulated data.16,17
Our findings clearly demonstrate that PGx-guided treatment is effective in both clinical and economic endpoints. Indeed, individuals included in the PGx-guided treatment arm suffered almost 50% less ADRs compared to the control arm, had 40% less hospitalisations in the psychiatric clinic and almost 50% less days spent under hospitalisation compared to psychiatric patients included in the control arm. Furthermore, our study's treating physicians followed and adhered to the official DPWG guidelines in 80.8% of the cases, a percentage that is much higher compared to other studies18,19 and, also compared to the Pan-European PREPARE study.5
These findings are congruent with the literature. According to Greden and coworkers (2019) in the GUIDED study, it was demonstrated that within 8 weeks of PGx-guided treatment, patients suffering from MDD had experienced significant less ADRs compared to patients on non-PGx guided medication regime [6.5% (5/77) versus 16.5% (22/136), p = 0.045] and showed better health outcomes in terms of disease symptoms.20 Perlis and colleagues (2020) reported similar findings.21 In particular, patients suffering from MDD that were under PGx-guided medication regime had stable or slightly improved disease progression and greater likelihood of remission.21 Furthermore, in the Oslon and coworkers (2017) study, PGx guided treatment in patients diagnosed with neuropsychiatric disorder had reduced the occurrence of ADRs.22 Indeed, 23% more patients in the control group reported at least one ADR compared to the PGx-guided group while 20% of patients in control group had suffered at least two ADRs in contrast with 5% of their counterparts.22
Moreover, in the Papastergiou and coworkers (2020) study, it was indicated that patients suffering from MDD in the PGx-guided arm had an impressive improvement in depression severity and symptoms (36% compared to 18%), accompanied by better results in quality-related assessments.17 Interestingly, Bradley and coworkers (2018) showed that 94% of ADRs reported were not severe (severity grade 1–3) and the remaining 6% of severe ADRs were distributed equally between the control and the PGx-guided arms,18 a fact that is in line with our results.
Polypharmacy perspective is an important barrier and concern in mental diseases’ drug management. According to the literature, a great percentage of bipolar disorder and schizophrenia patients are under extensive polypharmacy by being prescribed more than five psychotropic medications especially in elder patients23 and they frequently experienced side-effects owing to drug–drug interactions. In our study, it was observed that less participants of the PGx-guided arm (22.67%) were under psychiatric polypharmacy compared to the control arm (27.03%), and that patients in the PGx-guided arm consumed fewer antipsychotic medications. These observations imply that PGx-guided treatment may have a positive impact in this crucial aspect of drug management.
Most importantly, one of the innovative components of this study was not only the encouraging clinical findings but also the outcomes of the economic analysis, as well. In particular, when assessing the cost-effectiveness of PGx-guided treatment in patients suffering from MDD, the largest patient cohort from our study sample which would allow safer conclusions, it was shown that PGx-guided treatment could be a cost-effective option compared to the standard-of-care regimen. This observation is also well aligned with the literature. According to Carta and coworkers (2022), in a cohort of Italian patients suffering from MDD, PGx-guided treatment was cost-effective for a €75,000 willingness to pay (WTP) threshold in 58% and 63% of the Monte Carlo replications.24 In addition, in the Najafzadeh and coworkers study (2017), it was demonstrated that PGx-guided treatment could represent a dominant strategy to guide treatment rationalisation in patients suffering from depression and anxiety in a 3-year time horizon.25 Moreover, based on our recent systematic review15 focusing on the cost-effectiveness of PGx-guided treatment in psychiatry, of the 18 studies included in our analysis, 16 studies (88.89%) drew conclusions in favor of the PGx-guided treatment, of which 9 (50%) indicated that PGx-guided interventions were cost-effective and 7 (38.9%) were less costly compared to the standard-of-care treatment based on the cost analysis.
The cohort of participants suffering from schizophrenia or bipolar disorder wasn't taken into account in extraction of conclusions because of the minor differences between the two groups. This could be explained, as indicated by our clinicians, from the fact that schizophrenia and manic bipolar patients, when in an active episode upon enrolment, may overestimate their quality of life, because of lack of insight and elated mood. At the end of the study, when mostly in remission, the self-report of quality of life is more accurate, and usually lower.
In general, there are many studies attempting to assess the cost-effectiveness of PGx-guided treatment in psychiatry, indicating that most of them are cost-effective and in some of them even dominant. Morris and coworkers (2022) concluded in their systematic review that out of 108 articles, 44% demonstrated that PGx-guided treatment was cost-effective and in 27% cost-saving.17 Among those studies, 11 referred to antidepressant drugs. Verbelen and coworkers (2017) reported similar results in their systematic review, indicating that most economic evaluations posed a positive attitude towards PGx-guided treatment and highlighted that in 27% of the published economic analysis, PGx-guided treatment was dominant compared to other conventional treatment schemes while 30% of the studies concluded that the PGx-guided option was cost-effective.26
It is noteworthy that PREPARE's PGx testing was performed using a panel of 12 pharmacogenes that are related to the metabolism of more than 42 different medications prescribed for different indications, such as oncology, cardiology, etc. Patients' actionability for all genes tested was found to be high. Indeed, Swen and coworkers (2023) stated that almost 94% of all participants shared at least one actionable variant for any of the study's index drugs and a quarter of them had an actionable variant for their index drug.5 This observation is in accordance with our own findings described herein, owing to the fact that only one of the 1076 Greek psychiatric patients was not a carrier of a potentially actionable PGx diplotype (0.09%).
Other genomic studies have also reported similar findings. According to Lteif and coworkers (2024), 96% of study's participants was demonstrated to share an actionable genotype and around 30% had an actionable genotype for their main prescribed drug. These authors also stated that underserved patients or uninsured patients without proper accessibility to healthcare benefited from pre-emptive PGx testing, while their medication adherence and satisfaction were significantly increased.27 Moreover, based on the McInnes and coworkers (2021) study, who focused only on 14 clinically relevant pharmacogenes, it was concluded that at least one potentially actionable PGx variant was identified in 99.5% of the participants, while on average 3.7 actionable variants were found in each individual. From those patients, 25% were receiving a drug for which they had an actionable genotype.28 These data, in conjunction with our own findings, highlight the clinical and social impact of pre-emptive PGx testing since it refers to almost a quarter of the general population.27,28
Our study has some limitations. Firstly, we only examined the impact of PGx-guided treatment in reducing the occurrence of ADRs only; it would also be interesting if such study was extended to assess the impact of PGx-guided treatment on drug efficacy. Nevertheless, it should be indicated that it is rather difficult to define a precise endpoint for efficacy for a wide range of psychiatric drugs and multiple psychiatric indications as those covered in the present study. This could very well be the focus of future studies which may likely reveal an equally positive impact on psychiatric drug efficacy in those patient cohorts. Also, the fact that this study was block-randomised could be also considered as another limitation. Although block randomisation of different centers across time was preferable for the PREPARE study as a whole, the selection of using only 2 blocks per center might end up exposing the individual centers to time-dependent differences, for example changes in the standard of care, costs, as well as in the level of experience and knowledge of the clinicians with respect to PGx. That said, allowing multiple cross-over points per center or even randomization at patient level would decrease this risk. However, such an arrangement would have been followed by significant logistic and practical complexity, which could in turn allow for more errors to occur, undermining the findings of the study. Furthermore, in several cases, the findings did not reach statistical significance due to the relatively small number of patients, while in other cases statistical significance was lost when the entire patient population was considered and not just the actionable patients (Table 3) that only constituted approximately 25% of the entire study sample. Nevertheless, a clear statistical trend was observed in these cases that would be indicative of the importance of these findings.
Overall, our large-scale prospective study of pre-emptive PGx-guided treatment showed in a large number of psychiatric patients suffering from schizophrenia, MDD and bipolar disorder that PGx-guided treatment can lead to less ADRs, less hospitalisations, less hospital re-admissions, shorter durations of initial hospitalisations and re-admissions, less drug dose administrated per drug, less polypharmacy, smaller average number of co-administered psychiatric drugs and smaller number of deaths compared to the standard-of-care drug treatment modalities although in some cases statistical significance could not be established. This study also showed a drastic reduction of drug treatment costs with a reciprocal slight increase of the quality of life of patients suffering from MDD in the PGx-guided compared to the control arm.
Importantly, this study also shows a trend towards changing clinician behaviour. In other words, provision of relevant PGx data to the clinicians results in drug dose adjustments. That is indeed very encouraging, since much of the medical education literature often improvises strategies to change clinicians’ behavior.29 Hence, in this case of PGx-guided therapeutics, clinicians appropriately interpret the genotyping results, based on which they change prescribing, leading to ADRs reduction.
Although some of the findings of our study did not reach statistical significance, for the reasons described above, our data demonstrate that PGx-guided treatment may have a beneficial effect in treating the disease in psychiatric patients with a reciprocal reduction of treatment costs, providing evidence for integrating genome-guided psychiatric treatment in mainstream healthcare for reducing ADRs.
Contributors
GPP was the principal investigator of the U-PGx study in Greece. MS, EET and PF were responsible for patient recruitment. EET performed nurse assessments. MB, SS, KK, and TK performed genotyping analysis of the patient samples. MIK, MTP and CM performed the cost-effectiveness analysis. GPP, MIK and MTP prepared the first draft of the manuscript. MIK and MTP wrote the statistical analysis plan and performed the statistical analysis of the study. All authors critically reviewed the manuscript and approved the final version before submission. All authors had full access to all the data in the study. The corresponding author had final responsibility for the decision to submit for publication. GPP and MS accessed and verified the data.
Data sharing statement
A complete de-identified dataset will be made accessible, together with a data dictionary, for a minimum of 5 years. Requests for access to the data can be made by sending an email together with a research plan to the corresponding author and will be evaluated by and require authorisation from the Ubiquitous Pharmacogenomics Consortium Executive Board. The criteria for data access (e.g., who will be granted access, for what types of analyses, and by what mechanism) have yet to be determined; the procedure is under development by the consortium's Executive Board and will be published separately.
Declaration of interests
We declare no competing interests.
Acknowledgements
We thank all patients that have participated in the present study. This study was funded by the EU Horizon 2020 Programme (grant agreement number 668353 [U-PGx]). We are indebted to the Special Account for Research Funding of the University of Patras for generously supporting the Article Processing Charges of this open-access publication.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2024.105009.
Contributor Information
George P. Patrinos, Email: gpatrinos@upatras.gr.
Consortium of the PREPARE study in Greece:
Konstantinos Assimakopoulos, Eleni Georgila, Philippos Gourzis, Aikaterini Karaivazoglou, Olympia Prodromaki, George Rigas, Georgia Voukelatou, Vassiliki Zacharopoulou, Evangelia Barba, Konstantina Chalikiopoulou, Dimitra Dedousi, Georgia Emmanouil, Panagiotis Giannopoulos, Ouliana Ivantsik, Marina Kalogeropoulou, Manoussos E. Kambouris, Filippos Kanellakis, Alexandra Kolliopoulou, Panagiotis Kollios, Zoi Kordou, Ioannis Liopetas, Efrossyni Mendrinou, Konstantinos Mitropoulos, Georgia-Chryssa Samiou, Theano Stamopoulou, Andreas Stathoulias, Apostolos Stratopoulos, Athina Tsikrika, Athanassios Douzenis, Charilaos Gerassimou, Maria-Angeliki Voziki, and Athanassios Vozikis
Appendix A. Supplementary data
References
- 1.Squassina A., Manchia M., Manolopoulos V.G., et al. Realities and expectations of pharmacogenomics and personalized medicine: impact of translating genetic knowledge into clinical practice. Pharmacogenomics. 2010;11(8):1149–1167. doi: 10.2217/pgs.10.97. [DOI] [PubMed] [Google Scholar]
- 2.van Westrhenen R., Aitchison K.J., Ingelman-Sundberg M., Jukić M.M. Pharmacogenomics of antidepressant and antipsychotic treatment: how far have we got and where are we going? Front Psychiatry. 2020;11:94. doi: 10.3389/fpsyt.2020.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koufaki M.I., Karamperis K., Vitsa P., Vasileiou K., Patrinos G.P., Mitropoulou C. Adoption of pharmacogenomic testing: a marketing perspective. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.724311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Wouden C.H., Cambon-Thomsen A., Cecchin E., et al. Implementing pharmacogenomics in Europe: design and implementation strategy of the ubiquitous pharmacogenomics consortium. Clin Pharmacol Ther. 2017;101(3):341–358. doi: 10.1002/cpt.602. [DOI] [PubMed] [Google Scholar]
- 5.Swen J.J., van der Wouden C.H., Manson L.E., et al. A 12-gene pharmacogenetic panel to prevent adverse drug reactions: an open-label, multicentre, controlled, cluster-randomised crossover implementation study. Lancet. 2023;401(10374):347–356. doi: 10.1016/S0140-6736(22)01841-4. [DOI] [PubMed] [Google Scholar]
- 6.Brouwer J.M.J.L., Nijenhuis M., Soree B., et al. Dutch Pharmacogenetics Working Group (DPWG) guideline for the gene-drug interaction between CYP2C19 and CYP2D6 and SSRIs. Eur J Hum Genet. 2022;30(10):1114–1120. doi: 10.1038/s41431-021-01004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.https://www.eopyy.gov.gr/ Accessed on 30 Apr 2023.
- 8.Fragoulakis V., Roncato R., Fratte C.D., et al. Estimating the effectiveness of DPYD genotyping in Italian individuals suffering from cancer based on the cost of chemotherapy-induced toxicity. Am J Hum Genet. 2019;104(6):1158–1168. doi: 10.1016/j.ajhg.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koufaki M.I., Fragoulakis V., Díaz-Villamarín X., et al. Economic evaluation of pharmacogenomic-guided antiplatelet treatment in Spanish patients suffering from acute coronary syndrome participating in the U-PGx PREPARE study. Hum Genomics. 2023;17(1):51. doi: 10.1186/s40246-023-00495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf Accessed on 30 Apr 2023.
- 11.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57(1):289–300. [Google Scholar]
- 12.R Development Core Team R . (Version 4.2.1 (2022-06-23) -- "Funny-Looking Kid") R Foundation for statistical computing. 2023. A language and environment for statistical computing.https://www.r-project.org [Google Scholar]
- 13.Routhieaux M., Keels J., Tillery E. The use of pharmacogenetic testing in patients with schizophrenia or bipolar disorder: a systematic review. Ment Health Clin. 2018;8(6):294–302. doi: 10.9740/mhc.2018.11.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jukic M., Milosavljević F., Molden E., Ingelman-Sundberg M. Pharmacogenomics in treatment of depression and psychosis: an update. Trends Pharmacol Sci. 2022;43(12):1055–1069. doi: 10.1016/j.tips.2022.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y., Lauschke V.M. Population pharmacogenomics: an update on ethnogeographic differences and opportunities for precision public health. Hum Genet. 2022;141(6):1113–1136. doi: 10.1007/s00439-021-02385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karamperis K., Koromina M., Papantoniou P., et al. Economic evaluation in psychiatric pharmacogenomics: a systematic review. Pharmacogenomics J. 2021;21(4):533–541. doi: 10.1038/s41397-021-00249-1. [DOI] [PubMed] [Google Scholar]
- 17.Morris S.A., Alsaidi A.T., Verbyla A., et al. Cost effectiveness of pharmacogenetic testing for drugs with clinical pharmacogenetics implementation consortium (CPIC) guidelines: a systematic review. Clin Pharmacol Ther. 2022;112(6):1318–1328. doi: 10.1002/cpt.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papastergiou J., Quilty L.C., Li W., et al. Pharmacogenomics guided versus standard antidepressant treatment in a community pharmacy setting: a randomized controlled trial. Clin Transl Sci. 2021;14(4):1359–1368. doi: 10.1111/cts.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradley P., Shiekh M., Mehra V., et al. Improved efficacy with targeted pharmacogenetic-guided treatment of patients with depression and anxiety: a randomized clinical trial demonstrating clinical utility. J Psychiatr Res. 2018;96:100–107. doi: 10.1016/j.jpsychires.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Greden J.F., Parikh S.V., Rothschild A.J., et al. Impact of pharmacogenomics on clinical outcomes in major depressive disorder in the GUIDED trial: a large, patient- and rater-blinded, randomized, controlled study. J Psychiatr Res. 2019;111:59–67. doi: 10.1016/j.jpsychires.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Perlis R.H., Dowd D., Fava M., Lencz T., Krause D.S. Randomized, controlled, participant- and rater-blind trial of pharmacogenomic test-guided treatment versus treatment as usual for major depressive disorder. Depress Anxiety. 2020;37(9):834–841. doi: 10.1002/da.23029. [DOI] [PubMed] [Google Scholar]
- 22.Olson M.C., Maciel A., Gariepy J.F., et al. Clinical impact of pharmacogenetic-guided treatment for patients exhibiting neuropsychiatric disorders: a randomized controlled trial. Prim Care Companion CNS Disord. 2017;19(2) doi: 10.4088/PCC.16m02036. [DOI] [PubMed] [Google Scholar]
- 23.Aguglia A., Natale A., Fusar-Poli L., et al. Complex polypharmacy in bipolar disorder: results from a real-world inpatient psychiatric unit. Psychiatry Res. 2022;318 doi: 10.1016/j.psychres.2022.114927. [DOI] [PubMed] [Google Scholar]
- 24.Carta A., Del Zompo M., Meloni A., et al. Cost-utility analysis of pharmacogenetic testing based on CYP2C19 or CYP2D6 in major depressive disorder: assessing the drivers of different cost-effectiveness levels from an Italian societal perspective. Clin Drug Investig. 2022;42(9):733–746. doi: 10.1007/s40261-022-01182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Najafzadeh M., Garces J.A., Maciel A. Economic evaluation of implementing a novel pharmacogenomic test (IDgenetix®) to guide treatment of patients with depression and/or anxiety. Pharmacoeconomics. 2017;35(12):1297–1310. doi: 10.1007/s40273-017-0587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verbelen M., Weale M.E., Lewis C.M. Cost-effectiveness of pharmacogenetic-guided treatment: are we there yet? Pharmacogenomics J. 2017;17(5):395–402. doi: 10.1038/tpj.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lteif C., Eddy E., Terrell J., Cavallari L.H., Malaty J., Duarte J.D. Feasibility of preemptive pharmacogenetic testing and improvement of medication treatment satisfaction among medically underserved patients. Clin Transl Sci. 2024;17(1) doi: 10.1111/cts.13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McInnes G., Lavertu A., Sangkuhl K., Klein T.E., Whirl-Carrillo M., Altman R.B. Pharmacogenetics at scale: an analysis of the UK biobank. Clin Pharmacol Ther. 2021;109(6):1528–1537. doi: 10.1002/cpt.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Just K.S., Steffens M., Swen J.J., Patrinos G.P., Guchelaar H.J., Stingl J.C. Medical education in pharmacogenomics-results from a survey on pharmacogenetic knowledge in healthcare professionals within the European pharmacogenomics clinical implementation project Ubiquitous Pharmacogenomics (U-PGx) Eur J Clin Pharmacol. 2017;73(10):1247–1252. doi: 10.1007/s00228-017-2292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.