Summary
About 500,000 patients with rare adult solid cancers (RASC) are diagnosed yearly in Europe. Delays and unequal quality of management impact negatively their survival. Since 2017, European reference networks (ERN) aim to improve the quality of care of patients with rare disease. The steering committee of EURACAN, including physicians, researchers and patients review here the previous actions, present objectives of the ERN EURACAN dedicated to RASC. EURACAN promoted management in reference centres, and equal implementation of excellence and innovation in Europe and developed 22 clinical practice guidelines (CPGs). Additionally, fourteen information brochures translated in 24 EU languages were developed in collaboration with patient advocacy groups (ePAGs) and seventeen training session were organized. Nevertheless, connections to national networks in the 26 participating countries (106 centres), simplification of cross-border healthcare, international multidisciplinary tumour boards, registries and monitoring of the quality of care are still required. In this Health Policy, evaluation criteria of the performances of the network and of health care providers are proposed.
Keywords: Rare cancers, Rare diseases, Clinical practice guidelines, Surgery, Relapse, Survival, Reference centres, European reference networks
Introduction
Rare cancers represent 22% of all human cancers but cause 30% of all cancer deaths.1,2 Each year in Europe, nearly 500,000 people will be diagnosed with a rare cancer. Rare cancers represent an extremely fragmented group of diseases. There are probably over 300 different rare cancer entities, as defined by an incidence under the threshold level of 6/100,000/year. Within these rare cancer groups there is a further fragmentation, with many different histological or molecular subtypes. For example, for sarcomas or brain tumours, which represent 1–2% of all cancers, over 150 different subtypes are identified.
Patients with rare cancers do not have access to the same levels of scientific evidence to build clinical practice guidelines as compared to patients with frequent cancers, because of the limited number of clinical trials and frequent lack of centralised care in the past. Specialized care with experienced teams is often difficult to find for these patients. Patients with rare cancers therefore frequently face delays in access to diagnosis and care, difficulties in accessing expert reference centres, have fewer options for clinical trials and frequently receive inadequate treatment.1, 2, 3, 4 All these factors contribute to a worse prognosis of rare cancer patients which is consistently reported in the literature.
EURACAN in 2024
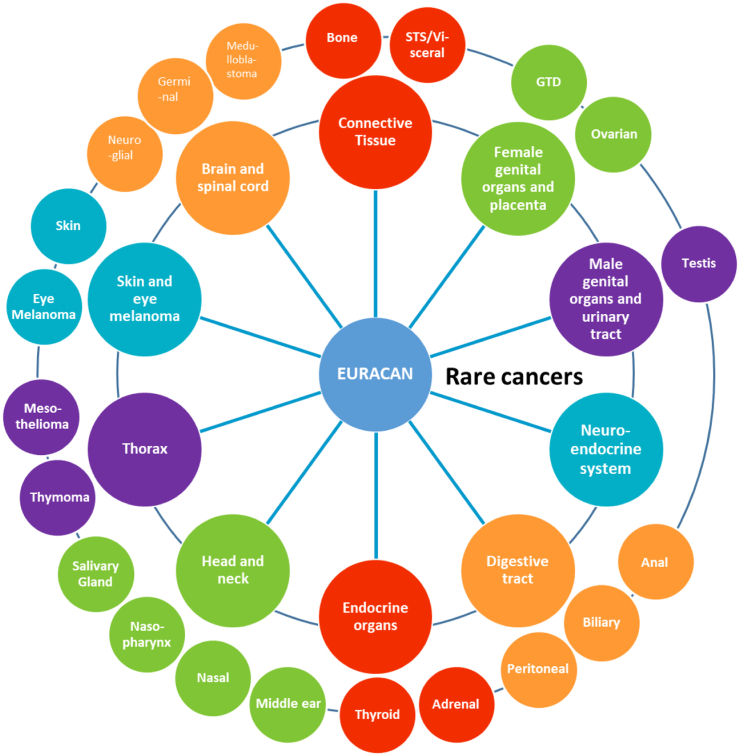
In 2017, the European Commission launched 24 European Reference Networks (ERNs) in order to improve the quality of care of patients with rare diseases.5 Four ERNs are dedicated to cancer: GENTURIS (for patients with rare genetic tumour risk syndromes), PAEDCAN (for paediatric cancers), EUROBLOODNET (for haematological malignancies), and EURACAN (for rare adult solid tumours).6 EURACAN includes ten clinical domains representing the ten different groups of rare adult solid cancers1: sarcomas, rare gynaecological malignancies, rare urological malignancies, neuroendocrine neoplasms (NEN), rare gastrointestinal tract tumours, endocrine cancers, head and neck rare cancers, rare thoracic cancers, rare skin and eye tumours, and central nervous system –CNS- tumours, including subdomains for several of the domains (Fig. 1). There were initially 67 full member centres across 16 countries, working closely with 14 patient advocacy groups (ePAGs) and 22 scientific societies and rare cancer networks to meet the objectives set within the first grant period.
Fig. 1.
Cancer groups and subgroups covered by EURACAN.
With European funding of 250 K€ per year from March 2017 to February 2022, this ERN has produced several actions and achievements (Table 1), matching its initial objectives and the general objectives of all ERNs. Guidelines, training sessions, websites and information documents in multiple languages were produced. Connections between reference centres within and across countries have started, international multidisciplinary tumour boards were initiated in some domains, dedicated clinical and translational research programmes conducted with national cooperative groups and the European Organisation for Research and Treatment of Cancer (EORTC) were launched and interactions with other ERNs led to state-of-the-art guidelines, research publications and cooperation, significantly improving the attention given to this underserved group of cancer patients.5, 6, 7, 8, 9, 10, 11, 12 Yet, substantial parts of EURACAN’s mission still require to be further developed to improve service delivery and outcomes for rare adult solid cancer patients. Much has been learned also from this five-year experience on how to provide optimal treatment to all rare cancer patients using available resources and funding. ERN EURACAN has also recently expanded substantially with now 106 centres across 25 countries, 15 ePAGs and 24 associated partners and stakeholders (scientific societies, national rare cancer networks).
Table 1.
EURACAN achievements 2017–2022.
| Clinical practice guidelines: Development, update or endorsement in each rare cancer group |
|
Website development www.Euracan.eu including information on all rare adult solid cancers, guidelines, specific sections for healthcare professionals and patients, news … |
|
Training courses Free ESO courses on rare adult solid cancers on sarcomas, gynae tumours, head and neck cancers Moodle e-learning platform with courses on rare thoracic tumours |
|
Information material: 11 patient brochures for 2 domains (gynae and endocrine tumours) translated into 25 EU languages EURACAN general leaflet translated into 25 EU languages |
|
Patient pathways Organized around national networks including EURACAN centres. Limited for cross border health care. |
|
Monitoring quality of cancer Variable pathology review and registries per countries |
|
European MDTBs Occasional use of CPMS for complex cases |
Innovation
|
The objectives of this Health Policy are to describe the current vision of the aims of EURACAN and to propose methodology and criteria for the evaluation of the activities of this ERN which could be relevant for the next five years, following the recommendations of the EU Joint Action on Rare Cancers (JARC).13
At the time when a Bridging Grant increased almost three-fold to support the ERN activities, this reinforced support of the ERNs by the European Commission invites all ERNs to rethink their strategies. Many of the proposed actions, and mode of evaluation in this Health Policy could be shared with other ERNs. However, the diseases treated within each ERN have their own specificities which may lead to individual propositions. In the case of EURACAN, the very large cumulative number of patients with rare adult solid cancers (over 400,000 per year in the EU), the rapid development of precision medicine in oncology, and the increasing number of rare molecular entities within «common» cancers are characteristics which are not universally observed in other rare non neoplastic diseases. The objectives and activities of EURACAN are presented in Table 2 and Fig. 2. The goal for this article is also to propose simple criteria of evaluation of the activities of this ERN. These criteria are based on the deliverables and the ERN performance indicators (Supplementary Tables S1 and S2) shared with the EC within the framework of the next grant period (2023–2027). They build upon the Network’s previous achievements to foster its development in the upcoming years. A more detailed explanation regarding the different activities is given in the following paragraphs.
Table 2.
Objectives for EURACAN in relation to previous achievements.
| Previous achievements 2017–2023 | Objectives in 5 years |
|---|---|
|
Clinical practice guidelines See list of CPGs (Table 1) |
Clinical practice guidelines Complete for those missing Continuous updates Measure of their implementation |
|
Website Euracan.eu including specific sections for healthcare professionals and patients |
Website Updated information materials on rare cancers, and productions of EURACAN, information for patient access to reference centres |
|
Training courses ESO courses on rare adult solid cancers Moodle e-learning platform with courses on rare thoracic tumours |
Training courses Number of training courses for HCPs and patients ESMO sarcoma and rare cancer meeting Specialized training per domains |
|
Information material Information material (patient brochures) for 2 domains Translated in 25 languages and Ukrainian |
Information material Information material for 10 domains Translated in 25 EU languages |
|
Patient pathways Organized around national networks including EURACAN centres. Limited for cross border health care |
Patient pathways To define healthcare pathways for all patients affected by rare adult options, links, between local, national centres with |
|
Monitoring quality of cancer care Variable pathology review and Registries per countries |
Monitoring quality of cancer care Organised networks of centralized expert diagnosis in each country |
|
International MDTB Occasional use of CPMS for complex cases |
International MDTB Each country for all patients Measuring number of MDTs for each domain per year, per centre, domain and EURACAN; Number of patients presented to MDTs per year via international MDTs |
|
Innovation Two clinical projects launched 1032 patients included |
Innovation Number of trials launched with EURACAN support, number of new trials for rare cancer in EU, number of patients included, including cross border |
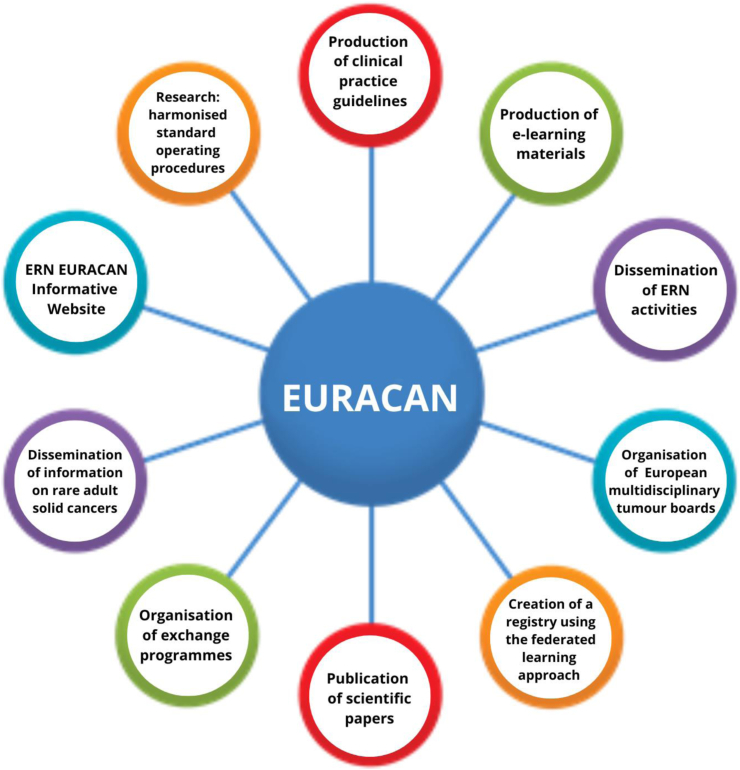
Fig. 2.
Graphical abstract of EURACAN activities.
The deliverables and milestones of EURACAN were reviewed by its steering committee, including physicians, researchers and patients. The activities of each individual ten tumour domains and transversal tasks forces were reviewed internally then shared with the whole steering committee. New objectives and criteria of evaluation were proposed.
Connection and integration within national healthcare systems
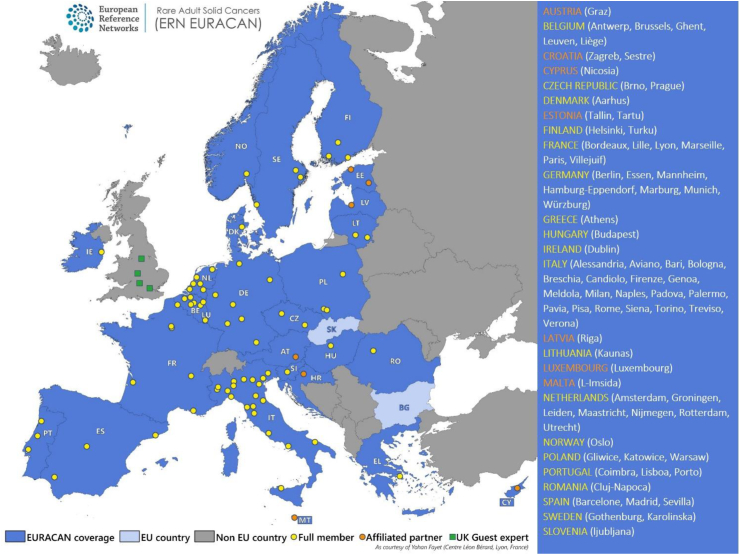
Fig. 3 describes the different centres (Full members, Affiliated centres, Invited experts) in the different EU countries hosting a HCP, as well as countries without HCPs yet. The different countries participating in EURACAN have established different policies for the management of rare cancers (all types). In very few countries, all rare cancer patients must be referred to a rare cancer reference centre for diagnosis and treatment. This is sometimes required in order to obtain proper reimbursement of care for these patients. In some countries, it is only optional to refer patients to reference centres. For some, networks of reference centres for rare cancers have not been implemented yet. Clinical research groups dedicated to rare cancers also have variable organisation and structures within countries and across Europe. For some domains (e.g., sarcomas, CNS) and countries, national groups have been operating for more than a decade. For other rare cancer types, clinical research is organised at a more global level, connected to international scientific societies (e.g., ENETS, EANO, EOTTD), working with association or federation of national research groups (e.g., ESGO), or with international research organisations such as the European Organisation for Research and Treatment of Cancer (EORTC).
Fig. 3.
EURACAN coverage as of February 2023.
In this heterogeneous national context, EURACAN operates as a network of national networks, specifically helping the countries which lack a rare cancer network, while countries with pre-existing organisations may be invited to connect these within the EURACAN network. Indeed, most organized national networks of rare cancers in all countries are connected to EURACAN through one or multiple centres. The connection of ERN EURACAN to the national health care system may use this principle of subsidiarity, serving mostly countries without organized reference Networks, and sometimes connecting these with reference networks in neighbouring countries for expert opinion through multi-disciplinary teams (MDTs). ERNs have the mission to complement what is not available nationally to best serve patients with rare cancers across the whole EU. This requires dedicated budget and funding, as human resources in the field of rare cancers are limited in all countries.
In this heterogenous national context, EURACAN may operate as an overarching European network connecting across countries established national networks for rare adult solid cancers as well as national centres for rare adult solid cancers in those countries not having yet national networks. The overall objective is to define clear healthcare pathways for patients affected by rare adult solid cancers in Europe, establishing solid links between local, national centres with highly specialised expert centres.
EURACAN’s mission is to support patients affected by a rare adult solid cancer with the best expertise, either directly or via the treating physician, to access a timely, accurate diagnosis and treatment. This requires dedicated budget and funding as human resources in the field of rare cancers are limited in all countries.
A comprehensive and well-implemented Cross-Border Healthcare Directive at the EU level is key to ensure the proper functioning of the ERNs. By assessing the number of cancer patients that seek diagnostic and therapy abroad through the ERNs, one can assess the level of application and implementation of the Directive, as well as how it facilitates patients’ access to cross-border healthcare.
An important criterion of evaluation of EURACAN will therefore be the description and evolution of the individual situation in EU countries for each of the ten disease domains, and how EURACAN centres can support the management of rare adult solid cancer patients in countries without national networks, for instance by e.g., building a referral MDT system, or contributing to the establishment of the national network.
Providing information on rare adult solid cancers
The access to information on rare adult solid cancers is identified as a major challenge by patients, patient advocacy groups, general practitioners and specialists in all countries. The group of patient organisations associated with EURACAN has made an outstanding contribution to EURACAN by preparing and disseminating documents and tools which provide information on the natural history of specific rare adult solid cancers, and on strategies for their diagnosis and treatment. Information and guidance documents have been prepared for the lay person, non-specialist physicians, nurses and social workers. Patients, families and also caregivers have thus access to reliable and updated information and resources for the optimal management of their rare tumour.12 These documents and tools are available on the open euracan.eu website.
On the EURACAN website, dedicated flyers and communication tools are now available and translated into multiple languages, and represented a major set of deliverables in the first period of EURACAN. For the next period, these information tools will need to be updated and developed for cancer types where they are not yet available.
Another criterion of evaluation of this action will be the capacity of the EURACAN network to disseminate information on rare cancers widely and in a timely fashion. Monitoring of the number of connections to EURACAN website may help to evaluate this, and it will be interesting to study the correlation with improvement of compliance to clinical practice guidelines within the organisation in place in the different countries.14, 15, 16 Once the EURACAN registry is implemented, the number of annually registered cases will be an important measure of the impact of the network at the pan-EU level. In countries which recently developed their national reference networks, this proved to be an important tool to assess progresses made in the management of patients over years.14,17
Guidelines for the management of rare adult solid cancer patients
The making, implementation, and measurement of the impact of clinical practice guidelines for rare adult solid cancers are essential steps to improve survival of patients with these rare cancers (as well as in frequent cancers).7, 8, 9,11,16 The goals of guidelines are to improve diagnostic accuracy and facilitate quick access to the best standard of care deployed in a reference centre to which the patient is referred. The development of guidelines is particularly challenging for all rare adult solid cancers where the level of evidence of published data is most often inferior to that available for frequent cancers. In this case, clinical practice guidelines (CPGs) are more often based on the consensus opinion of experts18; they also may propose several therapeutic options with similar levels of evidence when comparison of strategies is not available.7, 8, 9 Across the different disease domains of EURACAN, the quality of existing CPGs might vary and sometimes only address partial aspects of the disease, e.g., diagnostic aspects or treating metastatic disease. Guidelines are however not universal tools to be used as guidance for treatment in any centre. They may guide the initial steps of diagnosis outside of a reference centre; but it is recommended that they are applied in reference centres to maximize the chances of extended survival and cure rates.15,17
In the field of rare adult solid cancers, where so many different subtypes are now identified, creating of individual guidelines for each type of rare cancer represents a substantial workload. One of the important achievements of EURACAN in its first five years was the development of clinical practice guidelines for all patients with rare adult solid cancers within the ten “domains” of EURACAN7, 8, 9,11,19 (Supplementary Table S3). The guidelines were created by bringing together the existing guideline teams from scientific societies (ESMO and other domain-specific societies), the EURACAN group of experts and representatives of patient organisations focussing on rare adult solid tumours. Where tumours overlapped different domains or different ERNs (e.g., osteosarcoma overlapping with paediatric cancers), a multidisciplinary approach was taken.9 For some domains, e.g., neuroendocrine tumours (NETs), the guidelines were largely adapted from a pre-existing set of guidelines developed by the relevant scientific society looking after these tumours. EURACAN guidelines also served as starting points for European projects on rare cancers in other continents, e.g., South America20,21 for the SELNET EU program. Moreover, for some rare cancers, EURACAN in joint collaboration with scientific societies was able to develop guidelines in fields where no guidelines were available before (i.e., adult medulloblastoma).22
Guidelines are therefore essential tools for the actions of all ERNs, and the complexity of their development sometimes requires a coordinated action across multiple scientific societies. A proposed criterion of evaluation of the EURACAN will include the continuous development and updating of guidelines aiming to cover all rare adult solid cancers, newly identified, and not yet covered by guidelines, as well as, ultimately, a measure of their implementation at the level of the network and beyond in nationwide cohorts of patients.20,23 This has been so far conducted only at national levels to our knowledge. Generalizing such analysis at the EU level is challenging and will require the involvement of each nation and HCP for data collection. Registries collecting prospectively the key steps of patient management have been used successfully for this purpose. This requires significant resources and funding, but it is the ultimate measure of what needs to be done.
Training and education for health care workers and patients
Training about rare adult solid cancers in medical schools across the different domains, and across different countries varies significantly in quantity and quality. This heterogeneity is observed both during medical school and during specialization in transversal oncology or organ specialties. There is also an important global need to develop continuous medical education and training courses in rare cancers, where the state of the art is rapidly evolving and requires regular update for healthcare professionals and patients. But such training courses are not uniformly available in different countries. A “global” curriculum for rare adult solid cancer training is a challenging goal given the transversal nature of these cancers observed across different organ specialties. This is however becoming more relevant in the era of precision medicine, particularly regarding the field of agnostic therapies which treat similar molecular targets in different cancer types with similar efficacy, in marked contrast with some of the first generation of targeted cancer treatments.24, 25, 26 In the early days of EURACAN, yearly training courses were developed in collaboration with the European School of Oncology (ESO), the European Society for Medical Oncology (ESMO), and other domain specific scientific societies associated with EURACAN. This training was impacted by the COVID-19 pandemic, but the development of these internationally recognised/accredited courses focussing on rare adult solid cancers for oncologists, organ specialists, nurses and patient advocates remain an important need and deliverable.
Staff exchanges are as well important tools to disseminate the expertise. The patient representatives in EURACAN—ePAGs—have also the possibility to participate in the programme for visiting a EURACAN centre within their disease area. These exchanges of short or mid-term duration are encouraged and supported within all ERNs including EURACAN. This must be among the foremost tasks of EURACAN: the implementation of: multi-national, multi-university training programmes. Part of the evaluation criteria for ERN EURACAN at five years must be the number of training courses (and participants) for the different domains and overall, within EURACAN, which must be periodically delivered (e.g., yearly meetings). In the longer term, the goal should be the development of yearly training courses incorporating a European Continuing Medical Education diploma.
Additionally, online trainings/webinars for patients co-organised by ePAGs (patient representatives in EURACAN) and clinicians in the different clinical domains of EURACAN are encouraged. For instance, the ‘first webinar for patients with EURACAN ERN physician experts on thymoma and thymic carcinoma’ took place in May 2022, co-organised by the patient organisation T.U.T.O.R (nominated ePAG) and Prof Nicolas Girard, chair of the clinical domain for rare thoracic tumours.
Improving diagnostic accuracy of rare adult solid cancers
It is well demonstrated that delays in diagnosis and diagnostic accuracy are two of the challenges most affecting the optimal care of rare adult solid cancer patients. In many rare adult solid cancer patients, their correct diagnosis is not identified in a timely fashion. Inaccurate histological diagnostic is also common in some rare adult solid cancer types, and understandably results in inappropriate treatment, thus affecting the chances of cure of a patient or, if not cure, then potential extended survival. Examples can be provided for sarcomas, for which misdiagnosis for a benign tumour or for a lymphoma was reported in up to 30% of cases before centralization of the pathology review.27, 28, 29, 30, 31, 32 Also, expert revision of pathology samples of patients with gastro-entero-pancreatic neuroendocrine tumours had a clinical impact in 36.0% of patients, leading to a new therapeutic indication in 26.3%. Indeed, central pathology review is one of the key tools to improve this situation. Though the volume of activity is challenging, it has been shown to significantly reduce the number of inaccurate diagnoses from 30% to under 10% in countries where central pathology review has been implemented.14,17,33 The cost of central pathology review is counterbalanced by the reduction of the use of inadequate or incorrect treatments, and lowered potentially the reduced need for second line therapeutics.27 While this is not necessary for all rare adult solid cancer histotypes and domains, central pathology review is crucial for many domains (for example sarcomas, gynaecological cancers, neuroendocrine tumours, etc), and should be implemented at national level.
The organisation of such a central review also enables accurate recording of all cases prospectively and exhaustively in registries, enabling to document inaccurate diagnosis, and its evolution over time.
Central pathology review by an expert team is essential here: registries not including this service are at high risk of containing a large proportion of inaccurate diagnoses. When well established, these registries enable important differences in the epidemiology of rare adult solid cancers to be explored.34, 33, 35, 36, 27, 28, 29, 30, 31, 32 Although this exhaustive centralised pathology review requires significant mobilises resources, it is cost effective by preventing inadequate treatments and costs associated with potential relapses.37 For this purpose, the improvement of universal staging and grading, ENETS—an associated partner of EURACAN–has also proposed and introduced several synoptic, or standardized reporting themes in the field of neuroendocrine neoplasms.
In this context, the proposed criteria for evaluation of EURACAN may include the presence of organised networks in each country to ensure proper diagnosis for all patients. When funding is available, registries (national or international) should prospectively collect this information, as well as the number of patients with rare adult solid cancers diagnosed per year per country, and ultimately the dates between first symptoms and confirmed, accurate diagnosis. This is extremely challenging for a small entity to implement in all member states in the longer run. But it is however a cheap, efficient method to improve cure rate/extended survival in many rare adult solid cancers. “It is cheaper to implement registries and collect this vital information”—this sentence should be widely communicated.
Expert MDTBs for all rare adult solid cancer patients
Multidisciplinary tumour boards (MDTBs) are essential to ensure optimal guidance for the treatment of patients with rare adult solid cancers. They are ideally local or regional structures, organised within national networks. One of the missions of the ERN is to facilitate their development when non-existing. The organisation of the referral of patients to the closest expert reference centre is a key step to improve the quality of care and chances of cure/extended survival for patients. As an alternative, cases may be presented remotely to a distant MDTB to obtain guidance while limiting travel. It is also clear in some cancer types that treatment in the reference centre is associated with improved outcome, and sometimes survival.16 In reference centres, the number of patients analysed weekly in MDTBs is important, through the referral process. A streamlined MDTB process to cope with the number of patients to be discussed must be organised. This is one of the challenges of the European Commission’s tool known as the Clinical Patient Management System (CPMS) which is essentially a virtual MDTB for all ERNs to use. While securing data exchange, this tool was initially not customized to adapt to the specificities of individual ERNs. It remains, even after customisation, too time-consuming and difficult to use for the weekly discussion of all patient cases, which are often conducted in few minutes in the large MDTBs within the healthcare professional groups of EURACAN. Discussion and presentation of a case may require up to 30 min of preparation and attendance using the CPMS. Conversely, the CPMS may be adapted to the presentation of ultrarare cancer cases, in particular those with an incidence of 1/10 million per year.17,38
One of the objectives of EURACAN is to test the CPMS for these rarest adult solid cancer types, also known as ultra-rare cancers,39 recognising that it is impracticable to present the case of 500,000 patients diagnosed with a rare adult solid tumour each year in Europe. EURACAN MDTs may work with countries lacking such networks with a subsidiarity principle, and with the aim of helping the implementation of the network of MDTBs in the country. In addition, several domains of EURACAN have started to implement international, monthly MDTBs for complex cases, reviewing five to ten cases in 30–90 min, at a pace more compatible with the clinical workload of each healthcare professional. As a general principle, it is crucial that the work of a physician in an MDTB should occur through the simplest possible process, as human resources are limited in many countries.
In this context, the proposed evaluation criteria of the centres and of the network should be a simple numerical criterion: 1) the presence of organised networks of MDTBs in each country to ensure therapeutic guidance for all patients, 2) the number of MDTBs for each domain per year, per centre, domain and whole network; and 3) number of patients with rare adult solid cancers presented to MDTBs per year via international MDTBs. In the longer term, and when available, there should be the chronicling of these activities in national and international disease bases registries, including the number of new MDTBs established within the different countries represented in EURACAN.
Access to innovation for rare adult solid cancer patients
Access to innovation, for diagnostic and therapeutic tools, varies considerably across countries. While priority must be given to the optimal first line curative treatment in rare adult solid cancer as for all cancers, the rapid pace of development of precision medicine generates novel therapeutic options with curative potential for selected molecular subtypes of cancers.24, 25, 26 Rare adult solid cancers are particularly represented in these subgroups, but historically have had limited access to clinical trials. Providing access to innovation to a larger proportion of rare adult solid cancer patients is therefore important.
EURACAN is ideally positioned to communicate the availability of clinical trials in the different centres. Patient referral is generally easy within a single country, but much more challenging across national borders. To enable access for all rare adult solid cancer patients to innovative programmes, the organisation of cross border healthcare must be streamlined further to mitigate the generally reduced access of rare adult solid cancer patients to therapeutic opportunities in other countries. EURACAN has generated clinical trials exploring molecular diagnostic tools in the SPECTA EORTC programme (subprogram Arcagen, SPECTA NCT 02834884). In this programme, over 1000 patients with a rare adult solid cancer have so far benefited from molecular screening for somatic mutation using a commercial panel enabling the identification of actionable alterations in close to 50% of the enrolled patients and therapeutic options for 15% of patients.10
Facilitating access for all EU patients to trials not only benefits the patient but also reinforces the capacity of Europe to conduct clinical trials in rare entities, a final general goal of the whole ERN -border healthcare programme. Barriers to cross border healthcare must thus be identified in order to optimally use the opportunity offered by EURACAN to provide access to innovation to all EU patients. MDTBs dedicated to providing patient access to new drugs in clinical trials were recently initiated within EURACAN.
The barriers to cross border healthcare and cross-border research must thus be identified in order to use optimally the opportunity offered by EURACAN to prove access to innovation to all EU patients with rare adult solid cancers. MDTBs dedicated to providing relevant patients with access to new drugs in clinical trials have been recently initiated within EURACAN.
However, access to cross-border clinical trials is neither covered by the EU Clinical Trial Regulation nor is in the scope of the EU Directive 2011/24/EU on the application of patients’ rights in cross-border healthcare There is a current lack of EU legislation or guidelines to facilitate patients’ participation in trials in other locations than in their home country. However, European legal clarifications and/or guidelines are very much needed to facilitate access to cross-border clinical trials, including in the field of paediatric research.38
This important topic of access to innovation, the proposed criteria for the evaluation of EURACAN’s actions adult solid may include the number of clinical trials started for these rare adult solid cancers through EURACAN’s activities, the number of rare adult solid cancer patients included in the EURACAN HCP network (and in national networks when available), and the number of patients with rare adult solid cancer included in trials in another country than their home country.
Research
Facilitating the development of research and the access of rare cancer patients to research is one of the goals of ERNs in general and of EURACAN. The resources for this goal must be obtained from additional grants. Without support of EJPRD to the EURACAN project, the EURACAN members turned to collaborations with academic research structures (EORTC) with support from pharma or diagnosis industry. The Arcagen project, a subset of the SPECTA project of the EORTC enabled the collection of molecular data for 1032 patients with rare cancers.10,40 Another project launched under the umbrella of EURACAN is the Tracking project, collecting the outcome of patients with rare molecular alterations (https://euracan.eu/registries/tracking/). Since 2022, EURACAN has been working on the elaboration of unified Standard Operating Procedures to be shared and adopted with the aim to perform prospective and retrospective observational studies, including those relying on EURACAN registry (ies). Focussing on General Management and Personal Data Protection, their implementation across EURACAN institutions is an important goal to be reached within the next five years.
Nations and Europe: who does what?
Bringing the ambitions of EURACAN to success requires considerable interactions between national health care systems, ERN Patient Advocacy Groups (ePAG), scientific societies, professional societies, associated partners, and the European Reference Network itself. EURACAN must stand as a Network of Networks of reference centres on rare cancers, most with a national coverage. Guidelines are naturally coordinated at a multinational level with the ERN members and the scientific societies. The monitoring of their implementation should be conducted at a national level, with the help of registries. For this, simple quality indicators are required. Compliance to key steps of clinical practice guidelines, measure of patient survival, as well as development of specific QoL measures need to be implemented in routine practice. This was demonstrated to be feasible in several countries within Euracan centres.41, 42, 43 Development of specific HRQoL for rare cancer is ongoing in the EORTC, an associate partner of EURACAN.44
Training sessions have also a natural multinational coverage with the ERN’s, PAGs’, scientific societies’ support, as for the production of information materials. National registries should aim to converge towards a federated registry, as proposed by the STARTER project (https://euracan.eu/registries/starter/).
Multidisciplinary tumour boards are naturally conducted at the national level, and complex cases or when experts are not available in a country could be organized at the ERN level (international then) with the customized CPMS currently under evolution. Finally, research programs on rare cancers require active support from the current European Union Mission Cancer and Beating Cancer plan, as support for research in other rare disease is less available for rare cancers.
Conclusions
In this Heath Policy, we have attempted to summarize some of the key steps necessary to improve the outcome of rare adult solid cancer patients in Europe, the actions developed by EURACAN, and the forthcoming objectives which need to be implemented in this network in the next five years. Evaluation criteria of the network’s performance and HCPs are proposed. The increased budget allocated to ERNs and the work of The Special Committee on Beating Cancer (BECA) and the Cancer Mission, offer the potential of more ambitious actions within EURACAN and all ERNs. This could significantly improve the quality of care and outcome of patients with rare adult solid cancers across Europe.
Contributors
Conceptualisation: was proposed by J-YB, PC, IR-C,MJS, JG, WWdH, MC, H-JK, OG, LW, RP, LL, NG, SP-N, EK, AI, EF, AT, A-MF, PH, NH, JM-B, JS, MR, SL, KO, FdL,AW. Literature search was conducted by J-YB, PC, IR-C,MJS, JG, WWdH, MC, H-JK, OG, LW, RP, LL, NG, SP-N, EK, AI, EF, AT, A-MF, PH, NH, JM-B, JS, MR, SL, KO, FdL, AW Funding acquisition was conducted by J-YB, PC, IR-C,MJS, JG, WWdH, MC, H-JK, OG, LW, RP, LL, NG, SP-N, EK, AI, EF, AT, A-MF, PH, NH, JM-B, JS, MR, SL, KO, FdL, AW Project administration involved J-YB, PC, IR-C, MJS, JG, WWdH, MC, H-JK, OG, LW, RP, LL, NG, SP-N, EK, AI, JS, MR, SL, KO, FdL,AW. Supervision of the work was conducted by J-YB, MR, JS. The first draft was written by J-YB, IR-C. Writing-review and editing, and final approval were obtained from all authors.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
WwdH Consulting fees Novartis, Ipsen, Camurus, ITM Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events Novartis, Ipsen, Camurus, ITM (personal).
AMF: Grants or contracts from any entity Advenchen Laboratories, Amgen DompÈ, AROG Pharmaceuticals, Bayer, Blueprint Medicines, Daiichi Sankyo, Deciphera, Eisai, Eli Lilly, Epizyme Inc, GlaxoSmithKline, Karyopharm Pharmaceuticals, Novartis, Pfizer, PharmaMar, SpringWorks. Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid EURACAN Coordination team G1 ISG Coordination of the WG dedicated to education (Institution).
AI: Grants or contracts from any entity: Carthera Transgene NutrithÈragËne Sanofi Consulting fees: Novocure Leo Pharma Novartis Boehringer Ingelheim Polytone Laser INC. Support for attending meetings and/or travel Carthera, Enterome (To my institution, unrelated to the submitted work)/DSMB: NCT02331498 (unpaid).
HJK: Participation on a Data Safety Monitoring Board or Advisory Board Janssen, Astra Zeneca, MSD (Advisory board, payments to my institution).
JM-B Grants or contracts from any entity (PharmaMar Novartis Eisai IMMIX Biopharma Boehringer Ing. PTC Bio (Preclinical grant, to the institution); Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events PharmaMar Invited speaker Eli-Lilly Invited speaker Bayer Invited speaker Eisai Invited speaker Roche Invited speaker Daichii Invited speaker; IDMC: Tracon –(personal).
LL: Grants or contracts: Adlai Nortye, Astrazeneca, BMS, DEbiopharm Eisai Eli Lilly, Exeliixis, Hoffman la Roche Isa therapeutics, Kura oncology, Merck Serono, MSD, MSD, Nektar, Novartis, Regeneron, Roche, Sanofi, Syneos, Sun Pharma, Incyte Bioscienc ee international, Gilead Science, Genmab, Merck KGa (to the institution); Consulting fees: MSD IT, Merck Serono, Spa Healthcare professional, Merck Healthcare Kga, GSK, Hoffman la Roche, ALX oncology, EMD Serono Research and Development Inst. , Boehringer Ingelheim Int. Payment or honoraria for lectures: Merck Serono Spa, Merck Healthcare Kga, Neutron therapeutics Inc, Merck& Co IOnc., Astrazeneca, MSD IT, EMDSerono Research & Development Institute Inc, Mirati THerapeutics inc, F Hoffmann la Roche Ltd, Novartis Pharma Spa, Janssen Research and Development LLC, SEagen International GmbH, Eisai Europe Limited, Genmab US Inc, AstraZeneca uK Ltd, Abbvie Srl (Personal).
MC: Consulting fees AAA-NOVARTIS To me IPSEN Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events AAA-NOVARTIS IPSEN INCA (personal).
MJS: Consulting fees:Gamida (personal).
PGC: Grants or contracts: Advenchen, Amgen, Dompé, AROG Pharma, Bayer, Blueprint Medicines, Boehringer ingelheim, Diichi Sankyo, Deciphera, Eisai, Eli Lilly, Epizyme inc, Foghorn Ther. Inc, Glaxo, Hutchinson Mredipharma Ltd, Inhibrx Inc, Karyopharm pharmaceuticals, PTC ther, Novartis, Pfize, Pharmamar, Rain Oncology, Springworks (to institution).
JYB Grants or contracts: EURACAN grant French NCI (INCA) NETSARC + grant, INTERSARC + grant, LYRICAN + grant; DEpGYN RHU; Fondation ARC:ACSE grant (to the institution); Ligue Nationale contre le Cancer ACSE Grant; Comité de l’Ain de la Ligue contre le Cancer Grant (all for the institution).
The other authors report no conflict of interest related to this work.
Acknowledgements
Funding: Jean-Yves Blay: NetSARC (INCA & DGOS) and RREPS (INCA & DGOS), RESOS (INCA & DGOS), INTERSARC, and LYRICAN (INCA-DGOS-INSERM 12563), Institut Convergence PLASCAN (17-CONV-0002), Eurosarc (FP7-278742), la Fondation ARC, Infosarcome, InterSARC (INCA), LabEx DEvweCAN (ANR-10-LABX-0061), Ligue de L’Ain contre le Cancer, La Ligue contre le Cancer.
This work is generated within the European Reference Network EURACAN (EC 739521).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2024.100861.
Appendix ASupplementary data
References
- 1.Gatta G., van der Zwan J.M., Casali P.G., et al. RARECARE working group Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer. 2011;47:2493–2511. doi: 10.1016/j.ejca.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Gatta G., Capocaccia R., Botta L., et al. RARECAREnet working group. Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol. 2017;18:1022–1039. doi: 10.1016/S1470-2045(17)30445-X. [DOI] [PubMed] [Google Scholar]
- 3.Blay J.Y., Casali P., Bouvier C., et al. EURACAN Network European Reference Network for rare adult solid cancers, statement and integration to health care systems of member states: a position paper of the ERN EURACAN. ESMO Open. 2021;6(4) doi: 10.1016/j.esmoop.2021.100174. Epub 2021 Jun 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blay J.Y., Coindre J.M., Ducimetiere F., Ray-Coquard I. The value of research collaborations and consortia in rare cancers. Lancet Oncol. 2016;17:e62–e69. doi: 10.1016/S1470-2045(15)00388-5. [DOI] [PubMed] [Google Scholar]
- 5.Tumiene B., Graessner H., Mathijssen I.M., et al. European Reference Networks: challenges and opportunities. J Community Genet. 2021;12(2):217–229. doi: 10.1007/s12687-021-00521-8. Epub 2021 Mar 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blay J.Y., Fenaux P., Ladenstein R., Hoogerbrugge N. Continue rare cancers collaboration with European reference networks after brexit. Lancet. 2021;397(10276):793. doi: 10.1016/S0140-6736(21)00264-6. PMID: 33640061. [DOI] [PubMed] [Google Scholar]
- 7.Casali P.G., Blay J.Y., Abecassis N., et al. ESMO Guidelines Committee. EURACAN and GENTURIS Gastrointestinal stromal tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:20–33. doi: 10.1016/j.annonc.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Strauss S.J., Frezza A.M., Abecassis N., et al. ESMO Guidelines Ommittee. EURACAN. GENTURIS. ERN PaedCan Bone sarcomas: ESMO-EURACAN- GENTURIS-ERN PaedCan clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2021;32:1520–1536. doi: 10.1016/j.annonc.2021.08.1995. [DOI] [PubMed] [Google Scholar]
- 9.Gronchi A., Miah A.B., Dei Tos A.P., et al. ESMO Guidelines Committee. EURACAN. GENTURIS Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann Oncol. 2021;32:1348–1365. doi: 10.1016/j.annonc.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Morfouace M., Stevovic A., Vinches M., et al. First results of the EORTC-SPECTA/Arcagen study exploring the genomics of rare cancers in collaboration with the European reference network EURACAN. ESMO Open. 2020;5(6) doi: 10.1136/esmoopen-2020-001075. PMID: 33262201; PMCID: PMC7709508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desmoid Tumor Working Group The management of desmoid tumours: a joint global consensus-based guideline approach for adult and paediatric patients. Eur J Cancer. 2020;127:96–107. doi: 10.1016/j.ejca.2019.11.013. [DOI] [PubMed] [Google Scholar]
- 12.https://euracan.eu/patients/how-euracan-helps-you/
- 13.https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5c8385d19&appId=PPGMS
- 14.Martin-Broto J., Hindi N., Cruz J., et al. Relevance of reference centers in sarcoma care and quality item evaluation: results from the prospective registry of the Spanish group for research in sarcoma (GEIS) Oncologist. 2019;24:e338–e346. doi: 10.1634/theoncologist.2018-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blay J.Y., Soibinet P., Penel N., et al. Improved survival using specialized multidisciplinary board in sarcoma patients. Ann Oncol. 2017;28:2852–2859. doi: 10.1093/annonc/mdx484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blay J.Y., Honoré C., Stoeckle E., et al. NETSARC/REPPS/RESOS and French Sarcoma Group–Groupe d’Etude des Tumeurs Osseuses (GSF-GETO) Networks Surgery in reference centers improves survival of sarcoma patients: a nationwide study. Ann Oncol. 2019;30:1143–1153. doi: 10.1093/annonc/mdz124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Pinieux G., Karanian M., Le Loarer F., et al. NetSarc/RePPS/ResSos and French Sarcoma Group- Groupe d’Etude des Tumeurs Osseuses (GSF-GETO) Networks Nationwide incidence of sarcomas and connective tissue tumors of intermediate malignancy over four years using an expert pathology review network. PLoS One. 2021;16(2) doi: 10.1371/journal.pone.0246958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lok C., van Trommel N., Massuger L., Golfier F., Seckl M. Clinical Working Party of the EOTTD. Practical clinical guidelines of the EOTTD for treatment and referral of gestational trophoblastic disease. Eur J Cancer. 2020;130:228–240. doi: 10.1016/j.ejca.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Oldenburg J., Berney D.M., Bokemeyer C., et al. ESMO Guidelines Committee Electronic address: clinicalguidelines@esmo.org; EURACAN. Testicular seminoma and non-seminoma: ESMO-EURACAN Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:362–375. doi: 10.1016/j.annonc.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Blay J.Y., Palmerini E., Bollard J., et al. SELNET clinical practice guidelines for bone sarcoma. Crit Rev Oncol Hematol. 2022;174 doi: 10.1016/j.critrevonc.2022.103685. Epub 2022 Apr 21. [DOI] [PubMed] [Google Scholar]
- 21.Blay J.Y., Hindi N., Bollard J., et al. SELNET clinical practice guidelines for soft tissue sarcoma and GIST. Cancer Treat Rev. 2022;102 doi: 10.1016/j.ctrv.2021.102312. Epub 2021 Nov 14. [DOI] [PubMed] [Google Scholar]
- 22.Franceschi E., Hofer S., Brandes A.A., et al. EANO-EURACAN clinical practice guideline for diagnosis, treatment, and follow-up of post-pubertal and adult patients with medulloblastoma. Lancet Oncol. 2019;20:e715–e728. doi: 10.1016/S1470-2045(19)30669-2. [DOI] [PubMed] [Google Scholar]
- 23.Blay J.Y., Penel N., Gouin F., Le Cesne A., Toulmonde M. Improving at a nationwide level the management of patients with sarcomas with an expert network. Ann Oncol. 2022;33(6):659–661. doi: 10.1016/j.annonc.2022.02.221. [DOI] [PubMed] [Google Scholar]
- 24.Hyman D.M., Puzanov I., Subbiah V., et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373(8):726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le D.T., Uram J.N., Wang H., et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drilon A., Laetsch T.W., Kummar S., et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen T.W., Chiang R.C., Le Cesne A., et al. Soft tissue sarcoma incidences and clinical characteristics are significantly different in France and Taiwan. Cancer. 2022;128:3360–3369. doi: 10.1002/cncr.34372. [DOI] [PubMed] [Google Scholar]
- 28.de Herder W.W., Fazio N., O’Toole D.J. ENETS standardized (synoptic) reporting in neuroendocrine tumours. J Neuroendocrinol. 2022;34 doi: 10.1111/jne.13054. [DOI] [PubMed] [Google Scholar]
- 29.Hicks R.J., Dromain C., de Herder W.W., et al. ENETS standardized (synoptic) reporting for molecular imaging studies in neuroendocrine tumours. J Neuroendocrinol. 2022;34 doi: 10.1111/jne.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borbath I., Pape U.F., Deprez P.H., et al. Members of the advisory board of the European neuroendocrine tumor society (ENETS) ENETS standardized (synoptic) reporting for endoscopy in neuroendocrine tumors. J Neuroendocrinol. 2022;34 doi: 10.1111/jne.13105. [DOI] [PubMed] [Google Scholar]
- 31.Dromain C., Vullierme M.P., Hicks R.J., et al. ENETS standardized (synoptic) reporting for radiological imaging in neuroendocrine tumours. J Neuroendocrinol. 2022;34 doi: 10.1111/jne.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Velthuysen M.F., Couvelard A., Rindi G., et al. ENETS standardized (synoptic) reporting for neuroendocrine tumour pathology. J Neuroendocrinol. 2022;34 doi: 10.1111/jne.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mastrangelo G., Fadda E., Cegolon L., et al. A European project on incidence, treatment, and outcome of sarcoma. BMC Publ Health. 2010;10:188. doi: 10.1186/1471-2458-10-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lurkin A., Ducimetière F., Vince D.R., et al. Epidemiological evaluation of concordance between initial diagnosis and central pathology review in a comprehensive and prospective series of sarcoma patients in the Rhone-Alpes region. BMC Cancer. 2010;10:150. doi: 10.1186/1471-2407-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ducimetière F., Lurkin A., Ranchère-Vince D., et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011;6 doi: 10.1371/journal.pone.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Merola E., Zandee W., de Mestier L., et al. Histopathological Revision for Gastroentero pancreatic Neuroendocrine Neoplasms in Expert Centers: Does It Make the Difference? Neuroendocrinology. 2021;111:170–177. doi: 10.1159/000507082. [DOI] [PubMed] [Google Scholar]
- 37.Perrier L., Rascle P., Morelle M., et al. The cost-saving effect of centralized histological reviews with soft tissue and visceral sarcomas, GIST, and desmoid tumors: the experiences of the pathologists of the French Sarcoma Group. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0193330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lalova T., Padeanu C., Negrouk A., et al. Cross-border access to clinical trials in the EU: exploratory study on needs and reality. Front Med. 2020;7 doi: 10.3389/fmed.2020.585722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stacchiotti S., Frezza A.M., Blay J.Y., et al. Ultra-rare sarcomas: a consensus paper from the Connective Tissue Oncology Society community of experts on the incidence threshold and the list of entities. Cancer. 2021;127:2934–2942. doi: 10.1002/cncr.33618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamarca A., Morfouace M., Tejpar S., et al. Molecular profiling and precision medicine in rare gastrointestinal cancers within EURACAN in the SPECTA Arcagen study (EORTC-1843): too few patients with matched treatment in Europe. Ann Oncol. 2022;33:1200–1202. doi: 10.1016/j.annonc.2022.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Tong T.M.L., Bastiaannet E., Speetjens F.M., et al. Time trends in the treatment and survival of 5036 uveal melanoma patients in The Netherlands over a 30-year period. Cancers. 2023;15:5419. doi: 10.3390/cancers15225419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olthof P.B., Franssen S., van Keulen A.M., van der Geest L.G., Hoogwater F.J.H., Coenraad M., Dutch Hepatocellular & Cholangiocarcinoma Group (DHCG) Nationwide treatment and outcomes of intrahepatic cholangiocarcinoma. HPB (Oxford) 2023;25:1329–1336. doi: 10.1016/j.hpb.2023.06.019. [DOI] [PubMed] [Google Scholar]
- 43.Blay J.Y., Penel N., Valentin T., et al. Improved nationwide survival of sarcoma patients with a network of reference centers. Ann Oncol. 2024;9 doi: 10.1016/j.annonc.2024.01.001. S0923-7534(24)00008-5. [DOI] [PubMed] [Google Scholar]
- 44.Padilla C.S., Tesselaar M.T.E., van der Graaf W.T.A., Husson O. Measuring health-related quality of life in solid rare cancer patients: a study protocol. Rare. 2024;2 doi: 10.1016/j.rare.2023.100012. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.