Abstract
Phototropins (phot1 and phot2) are plant-specific blue light receptors for phototropism, chloroplast movement, leaf expansion, and stomatal opening. All these responses are thought to optimize photosynthesis by helping to capture light energy efficiently, reduce photodamage, and acquire CO2. However, experimental evidence for the promotion of plant growth through phototropins is lacking. Here, we report dramatic phototropin-dependent effects on plant growth. When plants of Arabidopsis thaliana wild type, the phot1 and phot2 mutants, and the phot1 phot2 double mutant were grown under red light, no significant growth differences were observed. However, if a very low intensity of blue light (0.1 μmol m−2 s−1) was superimposed on red light, large increases in fresh weight up to threefold were found in those plants that carried functional PHOT1 genes. When the intensity of blue light was increased to 1 μmol m−2 s−1, the growth enhancement was also found in the phot1 single mutant, but not in the double mutant, indicating that phot2 mediated similar responses as phot1 with a lower sensitivity. The effects occurred under low photosynthetically active radiation in particular. The well-known physiological phototropin-mediated responses, including chloroplast movement, stomatal opening, and leaf expansion, in the different lines tested indicated an involvement of these responses in the blue light–induced growth enhancement. We conclude that phototropins promote plant growth by controlling and integrating a variety of responses that optimize photosynthetic performance under low photosynthetically active radiation in the natural environment.
INTRODUCTION
Efficient use of solar energy for photosynthesis is important for plant growth and survival, especially in low light environments. Plants use light not only as an energy source for photosynthesis but also as an environmental signal and respond to its intensity, wavelength, and direction. Light is perceived by plant photoreceptors such as phytochromes, cryptochromes, and phototropins, and plants generate a wide range of specific physiological responses through these receptors.
In Arabidopsis thaliana, at least 10 photoreceptors, including five phytochromes (phyA through phyE), three cryptochromes (cry1, cry2, and cry3), and two phototropins (phot1 and phot2), have been identified (Arabidopsis Genome Initiative, 2000). Phytochromes that absorb red/far-red light and cryptochromes that sense UV-A/blue light coordinately regulate photomorphogenetic processes, including deetiolation, vegetative growth, flowering induction, and circadian rhythms (Smith, 2000; Lin, 2002; Morelli and Ruberti, 2002; Wang and Deng, 2002). Phytochrome also regulates seed germination and shade avoidance. By contrast, phototropins that absorb UV-A/blue light have been suggested to play an important role in photo-induced movement responses (Briggs and Christie, 2002).
A phototropin was first cloned as a blue light receptor responsible for phototropic bending, using an Arabidopsis mutant impaired in phototropism (Huala et al., 1997). The mutants lacked light-dependent phosphorylation of a 120-kD protein that appeared related to phototropism. The action spectrum of in vivo phosphorylation of this protein and the fluence dependency of the phosphorylation were similar to those of physiological phototropic responses. The cloned gene, initially named NPH1 (for NONPHOTOTROPIC HYPOCOTYL 1), contained two repeated motifs assigned as LOV (Light, Oxygen, Voltage) domains in the N terminus and a Ser/Thr protein kinase domain in the C terminus (Huala et al., 1997; Briggs and Christie, 2002). The gene later was renamed PHOT1. Thereafter, a PHOT1 homolog (NPH1-like 1) has been cloned from Arabidopsis and was renamed PHOT2 (Jarillo et al., 1998; Briggs and Christie, 2002). phot1 underwent autophosphorylation in response to blue light in insect cells and was demonstrated to be an autophosphorylating receptor kinase (Christie et al., 1998, 1999).
Using an Arabidposis mutant impaired in chloroplast movement, phot2 has been demonstrated to be responsible for the strong-light avoidance response (Jarillo et al., 2001; Kagawa et al., 2001; Kasahara et al., 2002). Moreover, phot2 also is responsible for phototropic curvature in response to relatively high intensities of blue light (Sakai et al., 2001). Using the Arabidopsis phot1 phot2 double mutant, it has been demonstrated that phot1 and phot2 redundantly mediate stomatal opening; the blue light–dependent H+-pumping activity that drives stomatal opening is lost in the guard cells of mutant plants (Kinoshita et al., 2001; Doi et al., 2004). More recently, both phot1 and phot2 have been suggested to mediate leaf expansion (Sakamoto and Briggs, 2002), and phot1 has been implied to be involved in the rapid inhibition of hypocotyl growth (Folta and Spalding, 2001). In contrast with this growth inhibition that is specifically mediated by phot1, genetic studies have revealed that phot1 and phot2 have partially overlapping functions in mediating phototropism, chloroplast movements, stomatal opening, and leaf expansion (Kagawa et al., 2001; Kinoshita et al., 2001; Sakai et al., 2001; Briggs and Christie, 2002). phot1 seems to be more sensitive to blue light than phot2 in triggering these responses, although sufficient documentation is yet to be provided for leaf expansion.
The blue light–induced responses mediated by phototropins optimize photosynthesis by improving the efficiency of light capture, reducing photodamage, and regulating the gas exchange between leaves and atmosphere. If so, we might expect to find an enhancement of plant growth in response to blue light through the stimulation of photosynthesis. However, no experimental evidence for the promotion of plant growth through phototropins is available to date.
In this study, we provide evidence that phototropin-mediated responses elicit a significant increase in plant growth, particularly under low photosynthetically active radiation (PAR). We also demonstrate that both phot1 and phot2 mediate this response and that the phot1-dependent pathway is more sensitive than the phot2-dependent one.
RESULTS
Phototropins Mediate Plant Growth under Light-Limited Conditions in Response to Blue Light
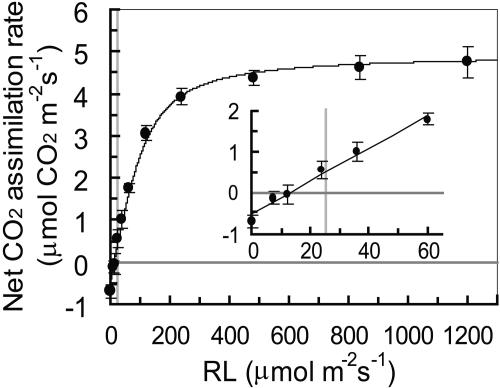
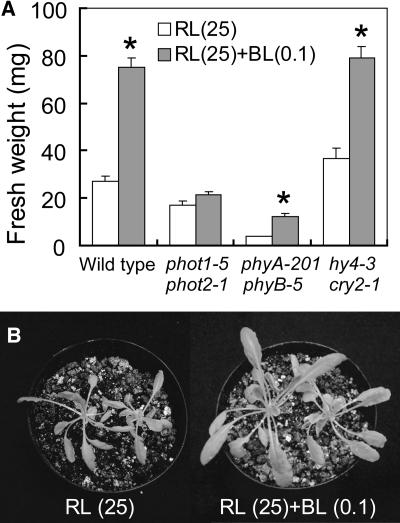
It is possible that blue light enhances plant growth if phototropins optimize photosynthesis. To test this hypothesis, we initially determined the light-response curve of photosynthetic CO2 fixation in Arabidopdsis leaves under red light. We found the light compensation point at 12.8 μmol m−2 s−1 and a half-saturation intensity of 75.0 μmol m−2 s−1 (Figure 1). When Arabidopsis seedlings of the wild type were grown under light-limited conditions for CO2 fixation (i.e., at 25 ± 4 μmol m−2 s−1 red light) for 4 weeks (Figure 1, gray line), the fresh weight of the green tissue was 27.1 ± 2.4 mg per plant (Figure 2A). If blue light at very low intensity (defined here as 0.1 ± 0.01 μmol m−2 s−1) was superimposed on red light during the growth period, the green tissue fresh weight was increased threefold to 75.1 ± 4.0 mg per plant (Figure 2A). Figure 2B shows a typical case of 6-week-old plants grown under red light and red light supplemented with very low intensity of blue light, respectively. Because the blue light intensity did not amount to >0.4% of PAR in these experiments, blue light must have acted as a signal triggering the response.
Figure 1.
Light-Response Curve of Net CO2 Assimilation Rate in Leaves of the Wild Type.
Red light (RL) intensity (equivalent to PAR) varied from 7.2 to 1200 μmol m−2 s−1. Each point represents the mean of three measurements ± se. Vertical gray line indicates the light intensity used in growth tests (25 μmol m−2 s−1).
Figure 2.
Blue Light–Induced Enhancement of Plant Growth under Low PAR of Red Light.
(A) Fresh weight of green tissues of wild-type and mutant plants. Wild-type, phot1-5 phot2-1, phyA-201 phyB-5, and hy4-3 cry2-1 plants were grown for 4 weeks under red light (RL) (25 μmol m−2 s−1) with or without blue light (BL) (0.1 μmol m−2 s−1). Bars represent means ± se (n = 24). Asterisks indicate significant differences in fresh weights between red light and red light with blue light (P < 0.01).
(B) Wild-type plants were grown for 5 to 6 weeks under red light (25 μmol m−2 s−1) with or without blue light (0.1 μmol m−2 s−1).
We attempted to identify blue light receptors responsible for this response using photoreceptor mutants of Arabidopsis. As in the wild type, the blue light–induced growth enhancement was found in phyA phyB (phyA-201 phyB-5) as well as cry1 cry2 (hy4-3 cry2-1) double mutants, but not in the phot1 phot2 (phot1-5 phot2-1) double mutant (Figure 2A). However, growth of the phyA phyB double mutant was very slow, probably because of the disturbance of seed germination and deetiolation (Wang and Deng, 2002). These results suggested that the growth enhancement by blue light is mediated by phot1 and/or phot2, but not by other photoreceptors.
Both phot1 and phot2 Mediate Blue Light–Induced Growth Enhancement
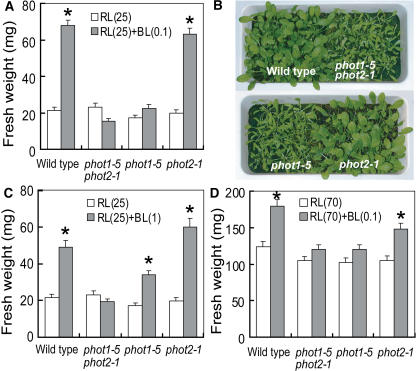
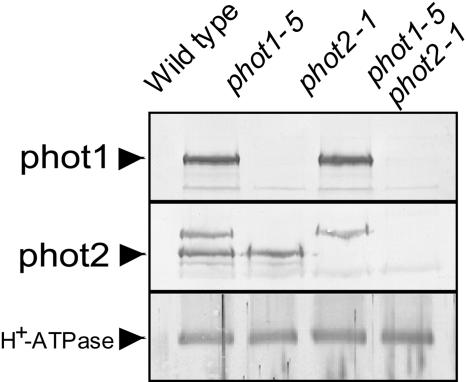
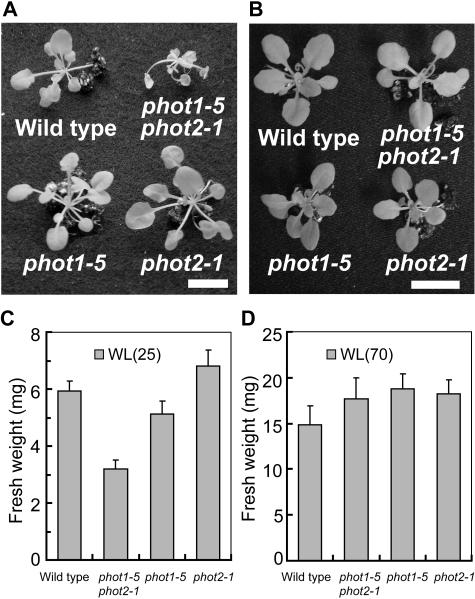
We further characterized the significance of phototropins for the blue light–induced growth enhancement using phototropin single (phot1-5 and phot2-1) and double (phot1-5 phot2-1) mutants. When wild-type and mutant plants were grown for 4 weeks under light-limited red light conditions, no significant differences were observed in their fresh weight (white bars in Figure 3A). When very low blue light as defined above was supplemented, growth was enhanced in the wild type and phot2-1, but not in the phot1-5 and the phot1-5 phot2-1 double mutant (gray bars in Figures 3A and 3B). Thus, phot1 was responsible for the growth enhancement, and phot2 did not seem to be involved in the response to very low blue light. Because it appeared possible that expression of the phot2 protein was specifically suppressed under our growth conditions, we confirmed that phot2 was expressed in both the wild type and phot1-5 to similar extents (Figure 4). The phot1 protein was expressed in both the wild type and phot2-1.
Figure 3.
Phototropin-Mediated Growth Enhancement.
(A), (C), and (D) Fresh weight of green tissues of the wild type and mutants. Wild-type, phot1-5, phot2-1, and phot1-5 phot2-1 plants were grown for 4 weeks under red light (RL) (25 μmol m−2 s−1) with or without blue light (BL) (0.1 μmol m−2 s−1) (A), red light (25 μmol m−2 s−1) with or without blue light (1 μmol m−2 s−1) (C), and red light (70 μmol m−2 s−1) with or without blue light (0.1 μmol m−2 s−1) (D). Bars represent means ± se (n = 24). Asterisks indicate significant differences in fresh weights between red light and red light with blue light (P < 0.01).
(B) Five- to six-week-old wild-type and mutant plants grown under the same conditions as in Figure 3A.
Figure 4.
Expression of phot1 and phot2 Proteins Determined by Protein Blot Analysis.
Microsomal membranes were obtained from leaves of the wild type and mutants grown under the same conditions as in Figure 3A. Amounts of protein used were 50 μg for phot1, 80 μg for phot2, and 20 μg for H+-ATPase, respectively. The top band in the panel marked phot2 is phot1.
Because phot2 is known to mediate phototropic responses, chloroplast movement, and stomatal opening at higher blue light intensities, we examined the effect of 1.0 instead of 0.1 μmol m−2 s−1 blue light on plant growth. Under these conditions, phot1-5 responded to blue light, whereas the phot1-5 phot2-1 double mutant did not (Figure 3C). This result indicated that phot2 also mediates blue light–induced growth responses, but that the phot1-dependent pathway is more sensitive than the phot2-dependent one. We confirmed that the phot1 protein was neither expressed in the single mutant phot1-5 nor in the double mutant phot1-5 phot2-1 (Figure 4). Furthermore, we found that the expression of phot proteins was not affected in the absence of blue light (data not shown).
PHOT1 Restores Blue Light–Induced Growth Enhancement in the phot1 phot2 Double Mutant
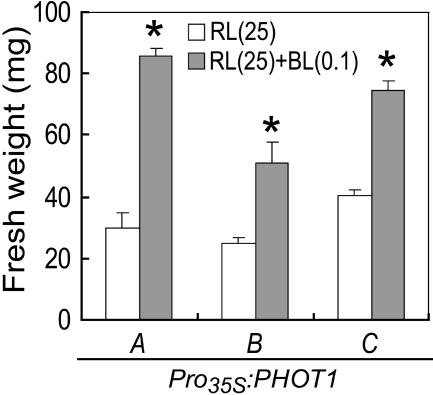
We transformed the phot1 phot2 double mutant with the 35S-controlled PHOT1 gene and obtained three lines (A, B, and C), with the highest transcript levels of PHOT1 in A (data not shown). The blue light–dependent growth response was restored in response to a very low intensity of blue light in all of the transgenic lines and was most prominent in line A, demonstrating unambiguously that phot1 was responsible for the growth enhancement (Figure 5).
Figure 5.
Complementation of Blue Light–Induced Growth Enhancement by Transformation of the phot1 phot2 Double Mutant with PHOT1.
Fresh weight of green tissues of transgenic plants. Three independent lines (A, B, and C) of phot1-5 phot2-1 transformed with the 35S-controlled PHOT1 were grown for 4 weeks under the same conditions as in Figure 3A. BL, blue light; RL, red light.
Chloroplast Accumulation, Stomatal Opening, and Leaf Expansion Mediated by Phototropins May Contribute to Growth Enhancement
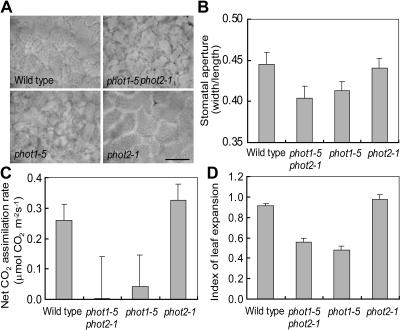
To determine whether known phototropin-mediated physiological reactions correlate with the blue light–induced growth enhancement shown here, we measured chloroplast distribution and stomatal aperture in the wild type and mutants under our standard growth conditions. If very low blue light (0.1 μmol m−2 s−1) was superimposed on red light, chloroplasts accumulated at the periclinal cell surfaces in both the wild type and phot2, but not in phot1 and phot1 phot2 (Figure 6A). Similarly, wider stomatal apertures were observed in the wild type and phot2 than in phot1 and phot1 phot2 (Figure 6B). Chloroplast accumulation increases light absorption, and increased stomatal conductance enhances CO2 uptake into the leaves; both factors optimize the efficiency of photosynthetic CO2 fixation.
Figure 6.
Phototropin-Mediated Physiological Responses under Red Light (25 μmol m−2 s−1) with Blue Light (0.1 μmol m−2 s−1).
(A) Distribution of chloroplasts in mesophyll cells of wild-type and mutants leaves. Micrographs were taken from the adaxial side of leaves. Bar = 50 μm.
(B) Stomatal aperture in leaves of the wild type and mutants. Apertures are expressed as the ratio of width to length of the guard cell pair, as described in Methods. Bars represent means ± se (n = 30).
(C) Net CO2 assimilation rate in leaves of the wild type and mutants. Bars represent means ± se (n = 10).
(D) Leaf expansion of the wild type and mutants. The degree of expansion is expressed as the ratio of the projection of the leaf before and after artificial uncurling. Bars represent means ± se (n = 5).
We measured the rate of photosynthetic CO2 fixation under standard growth conditions to determine whether it actually was increased by the phot1-mediated responses. As shown in Figure 6C, photosynthetic CO2 fixation rates in both wild-type and phot2 mutant plants were higher than in phot1 and phot1 phot2 mutants. Essentially the same results were obtained when CO2 fixation was expressed on the basis of chlorophyll content (data not shown). The enhanced rates of photosynthesis in the wild type and phot2 corresponded to ∼5% of the maximum rate under light-saturated conditions (Figure 1). The increased photosynthetic CO2 fixation appeared correlated to the plant's capacity to show blue light–dependent growth responses (Figure 2A).
We also found that under standard growth conditions, leaves of phot1 and phot1 phot2 curled downward (Sakai et al., 2001; Sakamoto and Briggs, 2002), but those of the wild type and phot2 did not (Figure 3B). We compared the surface area of artificially flattened leaves with the area of their projection in the undisturbed, more or less curled, state and found that the leaf area directly exposed to the light was reduced by ∼50% because of curling in phot1 and phot1 phot2 (Figure 6D). Presumably, leaf curling decreases the photosynthetic efficiency by reducing the effective leaf area of light capturing, and this appears to contribute largely to the growth reduction of the mutants.
Phototropins Mediate Plant Growth under More Natural Light Conditions
As shown in previous reports, no obvious difference in green tissue growth was observed between wild-type plants and the phot mutant plants under the relatively high light intensities (Kasahara et al., 2002; Sakamoto and Briggs, 2002). Therefore, phototropins do not appear to improve light capture characteristics significantly under high light conditions. To see whether very low blue light affects growth under moderate PAR, we increased red light from 25 to 70 μmol m−2 s−1, a value close to the half-saturating intensity for Arabidopsis photosynthesis (Figure 1). In this environment, plants grew considerably faster than under low PAR conditions, but blue light still significantly enhanced green tissue fresh weight by 40% in both wild-type and phot2 plants (Figure 3D).
Natural sunlight differs from the irradiation applied under our standard growth conditions in that its blue light portion is relatively larger than its red light fraction. To determine plant growth under more natural conditions, plants were cultivated under white light whose emission properties resembled those of sunlight. Under weak white light (25 μmol m−2 s−1), in which PAR was the same as that of our standard growth conditions, the wild type, phot1-5, and phot2-1 all grew considerably faster than phot1-5 phot2-1 (Figures 7A and 7C). The growth difference between the phot1-5 and pho2-1 mutants was eliminated under this condition, suggesting that phot2 functions to enhance plant growth under white light. If the intensity of white light was increased to 70 μmol m−2 s−1, the growth differences observed under low PAR were greatly reduced (Figures 7B and 7D). These results demonstrate that phototropin-mediated blue light–induced growth enhancement occurs in natural environments at low PAR and that phot2 as well as phot1 take part in the response.
Figure 7.
Plants Grown under White Light (12 h Photoperiod).
(A) and (B) Plants of the wild type, phot1-5, phot2-1, and phot1-5 phot2-1 grown for 4 weeks at 25 μmol m−2 s−1 white light (A) and at 70 μmol m−2 s−1 white light (B). Bars = 1 cm.
(C) and (D) Fresh weight of green tissues of the wild type and mutants grown for 4 weeks at 25 μmol m−2 s−1 white light (WL) (C) and at 70 μmol m−2 s−1 white light (D). Bars represent means ± se (n = 24).
DISCUSSION
Phototropins Mediate Growth Enhancement in Response to Blue Light
In this study, we demonstrated that a low intensity of blue light superimposed on red light induced increased growth in Arabidopsis green tissue and that the growth enhancement was particularly prominent when the plants were cultivated under low PAR of red light (Figures 2 and 3). The green tissue fresh weight reached threefold values when plants were grown under low PAR if only 0.4% of the PAR was supplemented as blue light. Such a dramatic growth enhancement was probably due to the fact that the PAR chosen was close to the light compensation point for CO2 fixation. In accordance with this notion, the degree of growth enhancement by blue light was decreased (Figure 3D) when PAR was increased to the moderate level of 70 μmol m−2 s−1. These findings may become important in the commercial cultivation of vegetable crops.
We have demonstrated that the blue light–dependent growth enhancement was mediated by phototropins. The involvement of other blue light absorbing receptors could be ruled out because the double mutants phyA phyB and cry1 cry2 exhibited clear growth enhancement in response to blue light (Figure 2A). These findings suggested that phototropins functioned to optimize photosynthetic performance. Our attempts to estimate photosynthetic efficiency by monitoring green tissue growth substantiated this interpretation.
phot1 Is More Sensitive to Blue Light in Promoting Plant Growth than phot2
We found that both phot1 and phot2 took part in the growth enhancement, but the phot1-mediated pathway was more sensitive than the phot2-mediated one (Figure 3). This may be accounted for by the depression of PHOT2 expression under our growth conditions because the expression depends on blue light irradiation (Jarillo et al., 2001; Kagawa et al., 2001). However, phot2 was expressed under red light, and its levels were not increased by additional blue light (0.1 μmol m−2 s−1; Figure 4) or by white light (70 μmol m−2 s−1; data not shown) in which the blue light intensity was much higher than under standard conditions (Figure 7B). The difference in the sensitivity of plant growth to blue light is in accordance with biochemical properties of phototropins (Salomon et al., 2000; Christie et al., 2002; Kasahara et al., 2002; Chen et al., 2004). A flavin-cysteinyl adduct, which forms between Cys residues in LOV domains and flavin mononucleotide, is produced in the phototropin molecule under blue light. This photoproduct, which in turn leads to activate a kinase, has a longer life time in phot1 than in phot2 in the dark (Christie et al., 2002; Harper et al., 2003; Chen et al., 2004). Moreover, it is in line with the recent finding that phot1 mediates a rise in cytosolic Ca2+ in response to 0.1 μmol m−2 s−1 blue light, whereas the analogous phot2-dependent effect requires >1 μmol m−2 s−1 blue light (Harada et al., 2003).
Physiological Responses Mediated by Phototropins
The growth response to very low intensity of blue light was surprisingly strong. Under our standard growth conditions, we found chloroplast accumulation at periclinal cell surfaces and increased average stomatal apertures in both the wild type and phot2, but not in phot1 and phot1 phot2 (Figures 6A and 6B). These blue light responses are likely to increase the efficiency of photosynthetic CO2 fixation, particularly under low PAR. Therefore, we measured photosynthetic CO2 fixation and found increased rates in the wild type and phot2 under standard growth conditions (Figure 6C). Although the enhancement of the photosynthetic CO2 fixation rate corresponded to only 5% of the maximum rate, it may promote overproportionate growth increments at light intensities close to the compensation point for CO2 fixation, as in our experiments (Figure 1).
We further found that the surface area of leaves exposed to light was reduced by 50% because of curling in phot1 and phot1 phot2 (Figure 6D). Leaf curling may decrease the photosynthetic efficiency by reducing the effective light-capturing leaf area. Interestingly, leaves curled in the wild type as well as in the double mutant if irradiated with low and moderate red light (see supplemental data online).
It would appear possible that the growth enhancement observed is caused by a phototropin-mediated transcriptional regulation of growth-relevant genes. However, recent microarray analyses suggest that phototropins only play a minor role in blue light–controlled transcriptional regulation (Ohgishi et al., 2004).
Role of Phototropins in the Natural Environment
Under normal illumination (∼100 μmol m−2 s−1 white light), no significant differences in growth became evident between the Arabidopsis wild type and phototropin mutants (Kasahara et al., 2002; Sakamoto and Briggs, 2002; Figure 7). However, under low PAR, phot1 mediates a remarkable increase of green tissue fresh weight in response to blue light (Figures 2 and 3). The simultaneous optimization of chloroplast localization and stomatal opening and the maximization of effective leaf area under low PAR (Figure 6) probably contribute to increased photosynthetic rates and, consequently, accelerated growth. Furthermore, a substantial phot1-dependent growth enhancement was observed also under moderate PAR (Figure 3D) corresponding to frequently encountered light intensities in the natural environment (Vogelmann, 2002). Moreover, phot2 mediated growth responses under light-limited conditions in addition to the phot1-dependent effect, when the supplemental blue light intensity was increased (Figure 3C).
In natural environments, phototropism and sun tracking also play an important role in plant growth (Briggs and Christie, 2002), in contrast with our defined experimental conditions where the light source was fixed. The phototropin-mediated responses are integrated to optimize photosynthesis and provide distinct advantages for the survival of plants growing in low-light environments, including dense canopies, dawn, and cloudy daytime, and may have played an important role in higher plant evolution. It is interesting to note the role of fern photoreceptor phytochrome3 (phy3), a chimeric protein with a red/far-red-light–absorbing phytochrome and a phototropin (Nozue et al., 1998). phy3 greatly enhances the sensitivity of phototropism and chloroplast movement to white light in fern Adiantum (Kawai et al., 2003) and is likely to play a central role in the divergence and proliferation of polypod fern in a low light environment, which had been made after the proliferation of angiosperms in the Cretaceous period (Schneider et al., 2004). Finally, we note that recent study, using wild-type, phot1, phot2, and nph3 mutants, suggested the adaptive function of phototropins in the seedling emergence in the field conditions (Galen et al., 2004).
METHODS
Plant Materials
Arabidopsis thaliana plants of the wild type (ecotype Columbia), phot1-5 (Huala et al., 1997), phot2-1 (Kagawa et al., 2001), phot1-5 phot2-1 (Kagawa et al., 2001), hy4-3 cry2-1 (ecotype Wassilewskija; Ahmad et al., 1998), and phyA-201 phyB-5 (ecotype Landsberg; obtained from the ABRC) were grown from seeds at 22 ± 3°C for 4 to 6 weeks under continuous red light (25 ± 4 μmol m−2 s−1) and blue light (0.1 ± 0.01 μmol m−2 s−1) unless otherwise stated (standard growth conditions). Three transformant lines of homozygous T2 generation plants (A, B, and C; see below) were grown under the same conditions. To determine red light–dependent light-response curves of the CO2 assimilation rate, rosette leaves of 5- to 6-week-old plants grown under fluorescent light (55 μmol m−2 s−1) were used.
Red light and blue light were provided by light-emitting photodiodes (LED-R, maximum intensity at 660 nm, and Stick-B-32, maximum intensity at 470 nm; Eyela, Tokyo, Japan). For the determination of the light-response curves, red light was obtained by passing light from a halogen lamp (MHF-G150-LR; Moritex, Tokyo, Japan) through a red glass filter (Corning 2-61; Corning, NY). White light was obtained from fluorescent lamps (FL 40S N-SDL; National, Tokyo, Japan). Photon flux density was measured using a light meter (LI-250; Li-Cor, Lincoln, NE) equipped with a light sensor (LI-190 SA; Li-Cor).
Generation of Transgenic Arabidopsis
The cDNA encoding full-length phot1 of Arabidopsis was cloned using specific primers (5′-CCGGATCCAAGATGGAACCAACAGAAAAAC-3′ and 5′-CCGGATCCCTCAAAAAAACATTTGTTTGCAG-3′). The phot1-5 phot2-1 double mutant was transformed with 35S Cauliflower mosaic virus promoter-driven PHOT1 cDNA, which was inserted into the pBI121 vector through Agrobacterium tumefaciens (Doi et al., 2004). Homozygous T2 generations, named A, B, and C, were obtained.
Immunological Analysis of Phototropin Proteins
Microsomal fractions were prepared from rosette leaves of 4-week-old wild-type and phototropin mutants. Proteins of microsomal fractions were separated by SDS-PAGE and transferred onto nitrocellulose membranes (Laemmli, 1970). Immunodetection of phot1 and phot2 was performed with polyclonal antibodies raised against Arabidopsis phot1 and phot2, respectively (Doi et al., 2004). phot1 antibodies recognized the protein specifically, whereas phot2 antibodies recognized both phot1 and phot2 (Figure 4). Plasma membrane H+-ATPase was quantified immunologically with polyclonal antibodies as described previously (Kinoshita and Shimazaki, 1999). Total protein was determined as described before (Bradford, 1976).
Determination of Growth and Physiological Parameters
For green tissue fresh weight determination, aerial parts of 4-week-old plants were excised and weighed immediately. Chloroplast localization in living mesophyll cells was monitored using micrographs taken from the adaxial sides of detached rosette leaves (Kasahara et al., 2002).
For the measurement of stomatal apertures, rosette leaves were detached and infiltrated immediately with water under vacuum. Stomata on the abaxial sides of the leaves were examined with a microscope (Optiphot; Nikon, Tokyo, Japan). The apertures were expressed as the ratio of stoma width (distance between outer edges of guard cell dorsal walls) and the length of the guard cells.
Leaf surface area was determined in rosette leaves that were detached and photographed. The leaves were uncurled, and photographs were taken again. Leaf areas before and after artificial flattening were compared.
Gas exchange was measured with a portable gas exchange system (LI-6400; Li-Cor) equipped with an Arabidopsis leaf chamber (6400-15; Li-Cor). Measurements were done at 24°C, with a reference CO2 concentration of 350 μmol mol−1 and relative humidity of 45 to 60%. To measure photosynthetic CO2 fixation under our standard growth conditions, gas exchange characteristics were determined in plants in the growth room. Curled leaves of mutants were flattened artificially before measurement. Chlorophyll was determined as detailed elsewhere (Porra et al., 1989).
Supplementary Material
Acknowledgments
We thank M. Wada (Metropolitan University, Tokyo, Japan) for providing the phot1 phot2 double mutant and M. Wada and I. Terashima (Osaka University, Japan) for critical reading of the manuscript. This work was supported in part by a Grant-in-Aid for Scientific Research in Priority Areas from the Ministry of Education, Science, Sports, and Culture of Japan to K.S. (Grant 13139202).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Ken-ichiro Shimazaki (kenrcb@mbox.nc.kyushu-u.ac.jp).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.030049.
References
- Ahmad, M., Jarillo, J.A., Smirnova, O., and Cashmore, A.R. (1998). Cryptochrome blue-light photoreceptors of Arabidopsis implicated in phototropism. Nature 392, 720–723. [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815. [DOI] [PubMed] [Google Scholar]
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Briggs, W.R., and Christie, J.M. (2002). Phototropins 1 and 2: Versatile plant blue-light receptors. Trends Plant Sci. 7, 204–210. [DOI] [PubMed] [Google Scholar]
- Christie, J.M., Reymond, P., Powell, G.K., Bernasconi, P., Raibekas, A.A., Liscum, E., and Briggs, W.R. (1998). Arabidopsis NPH1: A flavoprotein with the properties of a photoreceptor for phototropism. Science 282, 1698–1701. [DOI] [PubMed] [Google Scholar]
- Christie, J.M., Salomon, M., Nozue, K., Wada, M., and Briggs, W.R. (1999). LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): Binding sites for the chromophore flavin mononucleotide. Proc. Natl. Acad. Sci. USA 96, 8779–8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie, J.M., Swartz, T.E., Bogomolni, R.A., and Briggs, W.R. (2002). Phototropin LOV domains exhibit distinct roles in regulating photoreceptor function. Plant J. 32, 205–219. [DOI] [PubMed] [Google Scholar]
- Chen, M., Chory, J., and Fankhauser, C. (2004). Light signal transduction in higher plants. Annu. Rev. Genet. 38, 87–117. [DOI] [PubMed] [Google Scholar]
- Doi, M., Shigenaga, A., Emi, T., Kinoshita, T., and Shimazaki, K. (2004). A transgene encoding a blue-light receptor, phot1, restores blue-light responses in the Arabidopsis phot1 phot2 double mutant. J. Exp. Bot. 55, 517–523. [DOI] [PubMed] [Google Scholar]
- Folta, K.M., and Spalding, E.P. (2001). Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J. 26, 471–478. [DOI] [PubMed] [Google Scholar]
- Galen, C., Huddle, J., and Liscum, E. (2004). An experimental test of the adaptive evolution of phototropins: Blue-light photoreceptors controlling phototropism in Arabidopsis thaliana. Evolution 58, 515–523. [PubMed] [Google Scholar]
- Harada, A., Sakai, T., and Okada, K. (2003). phot1 and phot2 mediate blue light-induced transient increases in cytosolic Ca2+ differently in Arabidopsis leaves. Proc. Natl. Acad. Sci. USA 100, 8583–8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper, S.M., Neil, L.C., and Gardner, K.H. (2003). Structural basis of a phototropin light switch. Science 301, 1541–1544. [DOI] [PubMed] [Google Scholar]
- Huala, E., Oeller, P.W., Liscum, E., Han, I.S., Larsen, E., and Briggs, W.R. (1997). Arabidopsis NPH1: A protein kinase with a putative redox-sensing domain. Science 278, 2120–2123. [DOI] [PubMed] [Google Scholar]
- Jarillo, J.A., Ahmad, M., and Cashmore, A.R. (1998). PHR2: A Novel Arabidopsis gene related to the blue-light photoreceptor/photolyase family. Plant Physiol. 117, 719. [Google Scholar]
- Jarillo, J.A., Gabrys, H., Capel, J., Alonso, J.M., Ecker, J.R., and Cashmore, A.R. (2001). Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 410, 952–954. [DOI] [PubMed] [Google Scholar]
- Kagawa, T., Sakai, T., Suetsugu, N., Oikawa, K., Ishiguro, S., Kato, T., Tabata, S., Okada, K., and Wada, M. (2001). Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science 291, 2138–2141. [DOI] [PubMed] [Google Scholar]
- Kasahara, M., Kagawa, T., Oikawa, K., Suetsugu, N., Miyao, M., and Wada, M. (2002). Chloroplast avoidance movement reduces photodamage in plants. Nature 420, 829–832. [DOI] [PubMed] [Google Scholar]
- Kasahara, M., et al. (2002). Photochemical properties of the flavin mononucleotide-binding domains of the phototropins from Arabidopsis, rice, and Chlamydomonas reinhardtii. Plant Physiol. 129, 762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, H., Kanegae, T., Christensen, S., Kiyosue, T., Sato, Y., Imaizumi, T., Kadota, A., and Wada, M. (2003). Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature 421, 287–290. [DOI] [PubMed] [Google Scholar]
- Kinoshita, T., Doi, M., Suetsugu, N., Kagawa, T., Wada, M., and Shimazaki, K. (2001). phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414, 656–660. [DOI] [PubMed] [Google Scholar]
- Kinoshita, T., and Shimazaki, K. (1999). Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J. 18, 5548–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural protein during assembly of the head of bacteriphageT4. Nature 27, 680–685. [DOI] [PubMed] [Google Scholar]
- Lin, C. (2002). Blue light receptors and signal transduction. Plant Cell 14 (suppl.), S207–S225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli, G., and Ruberti, I. (2002). Light and shade in the photocontrol of Arabidopsis growth. Trends Plant Sci. 7, 399–403. [DOI] [PubMed] [Google Scholar]
- Nozue, K., Kanegae, T., Imaizumi, T., Fukuda, S., Okamoto, H., Yeh, K.-C., Lagarias, J.C., and Wada, M. (1998). A phytochrome from the fern Adiantum with features of the putative photoreceptor NPH1. Proc. Natl. Acad. Sci. USA 95, 15826–15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgishi, M., Saji, K., Okada, K., and Sakai, T. (2004). Functional analysis of each blue light receptor, cry1, cry2, phot1, and phot2, by using combinatorial multiple mutants in Arabidopsis. Proc. Natl. Acad. Sci. USA 101, 2223–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra, R.J., Thompson, W.A., and Kriedemann, P.E. (1989). Determination of accurate extinction coefficients and simultaneous-equation for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents—Verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochim. Biophys. Acta 975, 348–394. [Google Scholar]
- Sakai, T., Kagawa, T., Kasahara, M., Swartz, T.E., Christie, J.M., Briggs, W.R., Wada, M., and Okada, K. (2001). Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. USA 98, 6969–6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto, K., and Briggs, W.R. (2002). Cellular and subcellular localization of phototropin 1. Plant Cell 14, 1723–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon, M., Christie, J.M., Knieb, E., Lempert, U., and Briggs, W.R. (2000). Photochemical and mutational analysis of the FMN-binding domains of the plant blue light receptor, phototropin. Biochemistry 39, 9401–9410. [DOI] [PubMed] [Google Scholar]
- Schneider, H., Schuettpelz, E., Pryer, K.M., Cranfill, R., Magallon, S., and Lupia, R. (2004). Ferns diversified in the shadow of angiosperms. Nature 428, 553–557. [DOI] [PubMed] [Google Scholar]
- Smith, H. (2000). Phytochromes and light signal perception by plants—An emerging synthesis. Nature 407, 585–591. [DOI] [PubMed] [Google Scholar]
- Vogelmann, T.C. (2002). Photosynthesis: Physiological and ecological considerations. In Plant Physiology, 3rd ed., L. Taiz and E. Zeiger, eds (Sunderland, MA: Sinauer Associates), pp. 171–192.
- Wang, H., and Deng, X.W. (2002). Phytochrome signaling mechanism. In The Arabidopsis Book, C.R. Somerville and E.M. Meyerowitz, eds (Rockville, MD: American Society of Plant Biologists), doi/10.1199/tab.0074, http://www.aspb.org/publications/arabidopsis/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.