Abstract
Background
Branch retinal vein occlusion (BRVO) is the second most common cause of retinal vascular abnormality after diabetic retinopathy. Persistent macular oedema develops in 60% of eyes with a BRVO. Untreated, only 14% of eyes with chronic macular oedema will have a visual acuity (VA) of 20/40 or better. Macular grid laser photocoagulation is used for chronic non‐ischaemic macular oedema following BRVO and has been the mainstay of treatment for over 20 years. New treatments are available and a systematic review is necessary to ensure that the most up‐to‐date evidence is considered objectively.
Objectives
To examine the effects of macular grid laser photocoagulation in the treatment of macular oedema following BRVO.
Search methods
We searched CENTRAL, Ovid MEDLINE, EMBASE, Web of Science Conference Proceedings Citation Index, the metaRegister of Controlled Trials (mRCT), ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform. We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 21 August 2014.
Selection criteria
We included randomised controlled trials (RCTs) comparing macular grid laser photocoagulation treatment to another treatment, sham treatment or no treatment.
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included five studies conducted in Europe and North America. Four separate trials compared grid laser to no treatment, sham treatment, intravitreal bevacizumab and intravitreal triamcinolone. One further trial compared subthreshold to threshold laser. Two of these trials were judged to be at high risk of bias in one or more domains.
In one trial of grid laser versus observation, people receiving grid laser were more likely to gain visual acuity (VA) (10 or more ETDRS letters) at 36 months (RR 1.75, 95% confidence interval (CI) 1.08 to 2.84, 78 participants, moderate‐quality evidence). The effect of grid laser on loss of VA (10 or more letters) was uncertain as the results were imprecise (RR 0.68, 95% CI 0.23 to 2.04, 78 participants, moderate‐quality evidence). On average, people receiving grid laser had better improvement in VA (mean difference (MD) 0.11 logMAR, 95% CI 0.05 to 0.17, high‐quality evidence). In a trial of early and delayed grid laser treatment versus sham laser (n = 108, data available for 99 participants), no participant gained or lost VA (15 or more ETDRS letters). At 12 months, there was no evidence for a difference in change in VA (from baseline) between early grid laser and sham laser (MD ‐0.03 logMAR, 95% confidence interval (CI) ‐0.07 to 0.01, 68 participants, low‐quality evidence) or between delayed grid laser and sham laser (MD 0.00, 95% CI ‐0.04 to 0.04, 66 participants, low‐quality evidence).
The relative effects of subthreshold and threshold laser were uncertain. In one trial, the RR for gain of VA (15 or more letters) at 12 months was 1.68 (95% CI 0.57 to 4.95, 36 participants, moderate‐quality evidence); the RR for loss of VA (15 or more letters) was 0.56 (95% CI 0.06 to 5.63, moderate‐quality evidence); and at 24 months the change in VA from baseline was MD 0.07 (95% CI ‐0.10 to 0.24, moderate‐quality evidence).
The relative effects of macular grid laser and intravitreal bevacizumab were uncertain. In one trial, the RR for gain of 15 or more letters at 12 months was 0.67 (95% CI 0.39 to 1.14, 30 participants, low‐quality evidence). Loss of 15 or more letters was not reported. Change in VA at 12 months was MD 0.11 logMAR (95% CI ‐0.36 to 0.14, low‐quality evidence).
The relative effects of grid laser and 1mg triamcinolone were uncertain at 12 months. RR for gain of VA (15 or more letters) was 1.13 (95% CI 0.75 to 1.71, 1 RCT, 242 participants, moderate‐quality evidence); RR for loss of VA (15 or more letters) was 1.20 (95% CI 0.63 to 2.27, moderate‐quality evidence); MD for change in VA was ‐0.03 letters (95% CI ‐0.12 to 0.06, moderate‐quality evidence). Similar results were seen for the comparison with 4mg triamcinolone. Beyond 12 months, the visual outcomes were in favour of grid laser at 24 months and 36 months with people in the macular grid group gaining more VA.
Four studies reported on adverse effects. Laser photocoagulation appeared to be well tolerated in the studies. One participant (out of 71) suffered a perforation of Bruch's membrane, but this did not affect visual acuity.
Authors' conclusions
Moderate‐quality evidence from one RCT supports the use of grid laser photocoagulation to treat macular oedema following BRVO. There was insufficient evidence to support the use of early grid laser or subthreshold laser. There was insufficient evidence to show a benefit of intravitreal triamcinolone or anti‐vascular endothelial growth factor (VEGF) over macular grid laser photocoagulation in BRVO. With recent interest in the use of intravitreal anti‐VEGF or steroid therapy, assessment of treatment efficacy (change in visual acuity and foveal or central macular thickness using optical coherence tomography (OCT)) and the number of treatments needed for maintenance and long‐term safety will be important for future studies.
Keywords: Humans; Anti‐Inflammatory Agents; Anti‐Inflammatory Agents/therapeutic use; Antibodies, Monoclonal, Humanized; Antibodies, Monoclonal, Humanized/administration & dosage; Bevacizumab; Laser Coagulation; Laser Coagulation/adverse effects; Laser Coagulation/methods; Macular Edema; Macular Edema/etiology; Macular Edema/surgery; Randomized Controlled Trials as Topic; Retinal Vein Occlusion; Retinal Vein Occlusion/complications; Triamcinolone; Triamcinolone/administration & dosage; Visual Acuity; Visual Acuity/drug effects
Plain language summary
Grid laser photocoagulation for macular oedema after branch retinal vein occlusion (BRVO)
Review question: We assessed the role of macular grid laser (laser performed in a grid pattern) compared to other new treatments.
Background When a vein is blocked ('occluded') in the retina at the back of the eye, swelling of the macula (the central retina) can occur, which reduces vision. The options are to wait to see whether the swelling clears up on its own, or to treat the swelling by applying laser to the macula, injecting the eye with steroids or injecting the eye with anti‐vascular endothelial growth factor (anti‐VEGF). Laser has been an established mode of treatment ever since a landmark study, (the Branch Vein Occlusion Study in 1984) showed the advantage of laser compared to no treatment. However, over the last 10 years laser technology has evolved, and new injection treatments have become available.
Search date The electronic databases were searched on 21 August 2014.
Study characteristics We include five studies with a total of 715 participants. Three studies were from Italy and two were from the USA.
Key results We looked primarily at the proportion of participants gaining or losing significant vision. The trial comparing grid laser to no laser showed a clear benefit for grid laser. The result of the trial comparing early grid laser to delayed grid laser for macular BRVO (a subgroup of BRVO in which the occlusion is limited to a small vessel draining a sector of the macular region) was uncertain, and the quality of the evidence was low. We could not be certain that bevacizumab injections were better than grid laser treatment, because the effect was imprecise and the quality of the evidence was low. We could not be certain if subthreshold diode laser treatment was better than threshold laser treatment because the results were imprecise. The trial comparing grid laser treatment to triamcinolone (steroid) injection was imprecise, but there was a suggestion of a benefit for grid laser over 1 mg triamcinolone at 36 months and a benefit for grid laser over 4 mg triamcinolone at 24 months. Two of the five studies were at risk of bias, meaning that there were problems with the design and execution of two of the five studies which raised questions about the validity of these two studies.
Four of the five studies reported on adverse outcomes. Grid laser was well tolerated within these studies. One participant had an apparent perforation of Bruch's membrane (a membrane under the macula) following laser but this did not affect their vision. Bevacizumab injection was also well tolerated with only minor local side effects (transient red eye and superficial bleeding). Participants receiving triamcinolone injection were at risk of developing a raised eye pressure that required medication or surgery, at risk of developing a cataract, and at risk of developing a serious eye infection (endophthalmitis).
Quality of the evidence Good‐quality evidence was available from one trial to support macular grid laser treatment for macular swelling following a blocked vein. There is insufficient evidence to recommend early grid laser, subthreshold laser, bevacizumab injections or triamcinolone injections over grid laser. Anti‐VEGF and steroid treatments are becoming increasingly popular for treating eye conditions. However, more studies are needed to assess the longer‐term outcome of these treatments against grid laser treatment in the management of macular oedema after branch retinal vein occlusions.
Background
Description of the condition
Branch retinal vein occlusion (BRVO) is the second most common cause of retinal vascular abnormality after diabetic retinopathy (BVOS 1984) and a frequent cause of visual loss. Population‐based studies have reported a prevalence of 0.6% (Klein 2000) to 1.6% (Mitchell 1996) and an incidence rate of 2.14 per 1000 people in those aged 40 and older (David 1988).
Branch retinal vein occlusions (BRVOs) most commonly occur at an arteriovenous crossing. The common adventitia (connective tissue covering of the vessels) binds the artery and vein together at the arteriovenous crossing, and the thickening of the arterial wall compresses the vein, leading to turbulent blood flow in the vein, endothelial vascular damage, intravascular thrombus formation and vein occlusion (Zhao 1993). This is believed to be the main cause of BRVO.
Up to two‐thirds of BRVOs occur in the superotemporal quadrant, while 22% to 43% of BRVOs occur with occlusion of the inferotemporal branch vein. Nasal BRVOs are often asymptomatic, so that people with this type of BRVO do not seek medical help, causing diagnosis of BRVO in the nasal quadrants to be rare and accidental (Rehak 2008).
The level of visual impairment is determined by the degree of macular involvement. In the acute phase, superficial retinal haemorrhages, retinal oedema and cotton‐wool spots are seen in the distribution of the affected retinal vein. Vision may be reduced from macular oedema, retinal haemorrhage or perifoveal retinal capillary occlusion. The visual outcome is good in general, with 50% to 60% of eyes recovering vision to 20/40 or better without any treatment (Gutman 1974; Hayreh 1983; Michels 1974).
The extent of perifoveal capillary damage and retinal ischaemia are important visual prognostic factors, because the retinal haemorrhages resolve with time, while capillary compensation and the formation of collateral vessels allow the resolution of oedema and an improvement in visual function. However, more permanent visual loss occurs from macular ischaemia, cystoid macular oedema, subretinal fibrosis and epiretinal membrane formation. Persistent macular oedema develops in 60% of eyes with a BRVO (Finkelstein 1986). Untreated, only 14% of eyes with chronic macular oedema will have a visual acuity (VA) of 20/40 or better, while 86% will have a VA of 20/50 or worse (Gutman 1977).
In eyes with large areas of non‐perfusion and retinal ischaemia, neovascularisation may develop, resulting in vitreous haemorrhage and rhegmatogenous or tractional retinal detachments. Retinal neovascularisation develops in 25% of eyes with BRVO (Hayreh 1983). Neovascularisation of the iris and neovascular glaucoma are rare in BRVOs.
Description of the intervention
Photocoagulation therapy is currently considered for the two major complications of a BRVO.
1. Macular grid laser photocoagulation is used for chronic non‐ischaemic macular oedema and is the intervention of interest in this review. 2. Scatter photocoagulation is used for retinal neovascularisation.
The Branch Vein Occlusion Study (BVOS) demonstrated that macular grid laser photocoagulation was effective in the treatment of macular oedema (BVOS 1984). The recommendation is to wait for at least three months to see if the patient's vision spontaneously improves with the resolution of the retinal haemorrhages and retinal oedema. If the vision is reduced to 20/40 or worse, a fluorescein angiogram should be performed once there has been sufficient clearing of retinal haemorrhages. Macular grid laser photocoagulation is then performed in eyes where the fluorescein angiogram shows the perifoveal capillaries to be intact (i.e. adequate perfusion) and that macular oedema is responsible for the decrease in vision. The BVOS Group found that treated eyes were more likely to gain two lines of VA (65%) compared with the untreated control group (37%) (BVOS 1984). Treated eyes were also more likely to have 20/40 or better vision at three years of follow‐up (60% compared with 34% untreated) (BVOS 1986). If, however, the fluorescein angiogram reveals macular non‐perfusion, laser therapy is not warranted and observation is recommended.
How the intervention might work
Retinal grid laser treatment improves oxygenation of the inner retina both in animals (Molnar 1985; Stefansson 1981) and in humans with diabetic retinopathy (DR) (Stefánsson 1992). The photocoagulation of the photoreceptors reduces the oxygen consumption of the outer retina and allows oxygen to diffuse from the choroid to the inner retina, where it raises the oxygen tension and relieves hypoxia (Alder 1987; Molnar 1985; Stefansson 1981; Stefánsson 1992). Increasing oxygen tension in the inner retina results in autoregulatory vasoconstriction and more resistance in the arterioles, leading to reduced hydrostatic pressure in the capillaries and venules (Molnar 1985; Stefansson 1981). According to Starling’s law, this will decrease the fluid flux from the intravascular compartment into the tissue and reduce tissue oedema, assuming that the oncotic pressures are constant. The decrease in hydrostatic pressure will at the same time cause the venules to constrict and shorten according to the law of Laplace and the study by Kylstra 1986. This hypothesis has previously been tested in diabetic macular oedema by showing its disappearance after grid laser treatment of the macula (Gottfredsdóttir 1993; Kristinsson 1997). In experimentally‐produced BRVO, the veins upstream from the occlusive site dilate (between 10% and 20%) and become more tortuous (Attariwala 1997; Hamilton 1979; Kohner 1970). In monkeys it has been demonstrated that the retinal capillary area decreases around the site of laser photocoagulation (Wilson 1988), indicating vasoconstriction. Laser treatment of macular oedema in BRVO in humans leads to shortening and constriction of the occluded venule and the adjacent arteriole (Arnarsson 2000).
Why it is important to do this review
Branch retinal vein occlusions have a reasonable prognosis because a significant proportion of people with the condition regain good VAs. Laser treatment has been the mainstay of treatment for the complications of BRVOs on the basis of the findings of the BVOS Group over 20 years ago. New treatments are now available for macular oedema secondary to BRVOs. However, laser technology has also progressed. A systematic review is necessary to ensure that all the most up‐to‐date evidence for laser photocoagulation is considered objectively, and also to allow a comparison of laser photocoagulation with recently‐available treatments. People with BRVOs and their clinicians would benefit from having recommendations that take into account the latest information.
Objectives
To examine the effects of macular grid laser photocoagulation in the treatment of macular oedema following BRVO.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐randomised RCTs.
Types of participants
People with BRVO, in one or both eyes, irrespective of age or sex. We included participants only if there was at least one year of follow‐up after treatment. Where there were trials that met the inclusion criteria but reported on less than one year of follow‐up data, we contacted the trialists for any available additional follow‐up data. We documented trials with follow‐up data of less than one year but did not include their data in the primary analysis.
Types of interventions
We included trials where grid laser photocoagulation treatment has been a reported intervention for treating macular oedema following BRVO. We considered RCTs that reported on laser photocoagulation for BRVO through the use of different laser sources and photocoagulation techniques. We considered trials where comparisons have been made between laser treatment and: 1. no intervention or sham treatment; 2. pharmacologic treatments (intravitreal steroids and intravitreal anti‐vascular endothelial growth factor (VEGF) compounds); 3. surgery (pars plana vitrectomy, vitrectomy and arteriovenous decompression); 4. a combination of pharmacologic treatments and surgery.
Types of outcome measures
Primary outcomes
Our primary outcomes were:
Proportion of participants with at least 15 Early Treatment Diabetic Retinopathy Study (ETDRS) letters (i.e. three ETDRS lines or 0.3 logMAR) improvement in visual acuity at one year or more.
Proportion of participants with at least 15 ETDRS letters worsening in visual acuity at one year or more.
Secondary outcomes
We include the following secondary outcome measures:
Change in best‐corrected visual acuity (BCVA) compared with baseline visual acuity as a continuous score at one year or more.
Anatomic measures for macular oedema: presence of macular oedema with stereoscopic fundus photography or biomicroscopy, presence of leakage on intravenous fluorescein angiography (IVFA) and assessment of central macular thickness on optical coherence tomography (OCT) at one year or more.
In addition:
We describe (where available) other functional measures: contrast sensitivity; quality of life assessment through validated questionnaires.
We document and summarise the frequency and severity of ocular and systemic adverse events.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register), (2014, Issue 7), Ovid MEDLINE, Ovid MEDLINE In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to August 2014), EMBASE (January 1980 to August 2014), Web of Science Conference Proceedings Citation Index ‐ Science (CPCI‐S) (January 1990 to August 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 21 August 2014.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), CPCI‐S (Appendix 4), mRCT (Appendix 5), ClinicalTrials.gov (Appendix 6) and the ICTRP (Appendix 7).
Searching other resources
We searched through the reference lists of other reviews, guidelines and included (and excluded) studies for additional RCTs. We tracked ongoing trials for possible inclusion in this review on their completion.
Data collection and analysis
Selection of studies
Three authors independently assessed the titles and abstracts resulting from the electronic searches for inclusion. We obtained full‐text copies of all relevant or potentially relevant trials and assessed these according to the 'Criteria for considering studies for this review'. The authors were not masked to the names of trial authors, institutions, journal of publication or results when making their assessments. We resolved disagreements about whether a trial should be included by discussion and consensus. In cases where we needed additional information before we could decide whether to include a trial, we tried to obtain this information by contacting the trial authors.
Data extraction and management
Two authors independently extracted the data for the primary and secondary outcomes on to standard data extraction forms developed by the Cochrane Eyes and Vision Group. One author entered data into Review Manager 5 (RevMan 2014) and two other authors checked the data entered into RevMan to make sure there were no mistakes. The authors resolved any differences by discussion. Where there was doubt about the data of a trial, we contacted the authors of the trial. Where studies were reported in more than one publication, we extracted data from each report separately and collated the information from the multiple data collection forms.
Assessment of risk of bias in included studies
Three authors assessed trial quality according to the methods set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used The Cochrane Collaboration's 'Risk of bias' tool and considered six domains to assess the risk of bias:
sequence generation;
allocation sequence concealment;
blinding (masking) of participants and personnel;
masking of outcome assessment;
incomplete outcome data;
selective outcome reporting.
Three authors assessed the risk of bias for each parameter and judged each as being at low risk of bias, high risk of bias or 'unclear' risk of bias (where there is insufficient information). We resolved disagreements by discussion and consensus. Where a domain had been designated as 'unclear', we contacted the trial authors for further information. If we did not receive a response within four weeks, we made a decision on the basis of the available information.
Measures of treatment effect
Dichotomous data
We calculated the risk ratios (RRs) and 95% confidence intervals (CIs) for our two primary visual outcomes:
1. proportion of participants with at least 15 letters improvement; 2. proportion of participants with at least 15 letters worsening in visual acuity.
Where macular oedema was documented only as a dichotomous variable (i.e. present or absent on clinical examination, IVFA or OCT or both), we also generated RRs and their 95% CIs.
Continuous data
We identified the mean and standard deviation from the trial papers for:
1. the change in BCVA from baseline (our secondary outcome); 2. the change in central macular thickness.
Unit of analysis issues
We carried out data extraction and analysis treating each eye of an individual participant as the unit of analysis. If both eyes from one person were included in a trial, we would have extracted the data and performed analyses to properly account for the non‐independence between eyes, following Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).However, only one eye per participant was included in all the included trials.
Dealing with missing data
Where data were missing, we attempted to contact the original investigators to request missing data. When we could not obtain these data, we performed an available‐case analysis for the main analysis. We considered the potential impact of the missing data on the results and have highlighted the potential impact in the 'Discussion' section of the review.
Data synthesis
We were unable to conduct any meta‐analyses because of the methodological and clinical heterogeneity we observed between studies. The different studies compared different interventions, had different inclusion criteria and used separate time points for outcome measurements. We have therefore provided a narrative report, with illustrative non‐pooled forest plots.
If additional trials become available in future, we will analyse them as follows: we will continue to calculate risk ratios for dichotomous outcomes. We will combine data in a meta‐analysis where appropriate, using the random‐effects model .We will assess heterogeneity on the basis of the Chi² test, I² value, the overlap of confidence intervals in the forest plots, and by comparing the overall characteristics of the studies.
Summary of findings table
In a modification to our published protocol, we planned to prepare a summary of findings table presenting relative and absolute risks for the outcomes listed below. However, as only one trial was available for each comparison we do not include any summary of findings tables for the current review.
The overall quality of the evidence for each outcome was graded using the GRADE classification (www.gradeworkinggroup.org/).
In future updates, we will include the following outcomes in the summary of findings table.
1. Proportion of participants with at least 15 ETDRS letters improvement in visual acuity 2. Proportion of participants with at least 15 ETDRS letters worsening in visual acuity 3. Change in best‐corrected visual acuity compared with baseline visual acuity 4. Presence of macular oedema 5. Central macular thickness as measured by optical coherence tomography 6. Quality of life 7. Adverse events
Follow‐up: one year
Results
Description of studies
Results of the search
The electronic searches yielded a total of 1248 records (Figure 1). After deduplication the Trials Search Co‐ordinator scanned 860 records and discarded 691 records as they were not relevant to the scope of the review. We screened the remaining 169 records and rejected a further 140 records after reading the abstracts. We obtained full‐text reports of 29 records for further assessment. We included six reports of five studies in the review (see Characteristics of included studies tables) and have identified two ongoing studies which we will assess when data become available (see Characteristics of ongoing studies tables). We excluded the remaining 21 records (see Characteristics of excluded studies tables).
1.
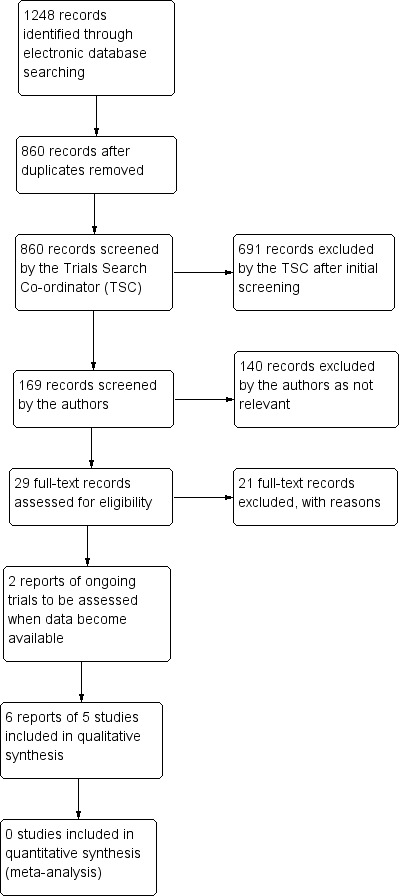
Results from searching for studies for inclusion in the review.
Included studies
We included five studies in the review (Battaglia Parodi 1999; BVOS 1984; Parodi 2006; Russo 2009; SCORE 2009). See Characteristics of included studies tables for further information.
Types of participants
Battaglia Parodi 1999 included 108 participants (data on 99 participants only) with significant macular oedema and a visual acuity (VA) of less than 0.6 from 'macular BRVO' occurring within 15 days. Macular branch retinal vein occlusion (BRVO), in this study, was defined as "a subgroup of branch retinal vein occlusion in which the occlusion is limited to a small vessel draining a sector of the macular region".
BVOS 1984 included 139 participants (78 with three‐year follow‐up) with macular oedema secondary to BRVO reducing VA to 20/40 or less occurring three to 18 months earlier.
Parodi 2006 included 36 eyes of 36 participants with macular oedema secondary to BRVO reducing best‐corrected visual acuity (BCVA) to 20/40 or less occurring three to 18 months earlier. They excluded people with "macular BRVO".
Russo 2009 included 30 eyes of 30 participants with "perfused BRVO" for three months or more, macular leakage on fundus fluorescein angiography (FFA), a BCVA of 0.4 logMAR or less, and a central macular thickness of 300 μm or more.
SCORE 2009 included 411 participants with macular oedema secondary to BRVO (including hemi‐retinal vein occlusion), BCVA of 73 Early Treatment Diabetic Retinopathy Study (ETDRS) letters or less (Snellen ≤ 20/40) and 19 ETDRS letters or more (Snellen ≥ 20/400), and mean central subretinal thickness of 250 μm or more.
Types of interventions
Battaglia Parodi 1999 randomised eyes to three arms: 1) Early grid laser with the krypton laser ‐ laser performed soon after the three months, or 2) Delayed grid laser ‐ laser performed after a period of six to 18 months, or 3) Sham laser (control group).
BVOS 1984 compared argon grid laser according to a standard protocol with additional photocoagulation if untreated leaking areas and foveal oedema persisted with continued VA loss, and no treatment (control group).
Parodi 2006 compared subthreshold grid laser with infrared diode laser (125 μm laser spot diameter, 0.2 second exposure, 10% duty cycle; laser power determined by single test burn to bring about a medium white burn), and threshold grid laser with krypton laser (100 μm spot diameter, 0.1 seconds; medium white burn).
Russo 2009 compared grid laser with the argon green laser (100 μm spots, 100 ms, one half to one burn‐width apart, covering leaking area outside capillary‐free zone as shown by FFA) with retreatment if no VA improvement and oedema still present after three months as detected by optical coherence tomography (OCT), and bevacizumab intravitreal injection (1.25 mg (0.05 ml) of bevacizumab via the pars plana) with retreatment until macular oedema resolved as detected by OCT.
SCORE 2009 randomised eyes into three arms: 1) Standard care with grid laser photocoagulation with green to yellow wavelength (50 μm to 100 μm laser spot, 0.05 to 0.1 seconds exposure, mild intensity, covers areas of diffuse retinal thickening and treat any focal leaks, one to two burn‐widths apart (500 μm to 3000 μm from centre of fovea)), or 2) 1 mg triamcinolone (preservative‐free, single‐use, Trivaris, 1 mg), or 3) 4 mg triamcinolone (preservative‐free, single‐use, Trivaris, 4 mg). Participants were retreated if required.
The treatment comparisons of the included trials are summarised in Table 1.
1. Treatment comparisons in included trials.
| Trial | Treatment group 1 | Treatment group 2 | Treatment group 3 | Control |
| Battaglia Parodi 1999 | Early grid laser | Delayed grid laser | ‐ | Sham laser |
| BVOS 1984 | Grid laser | ‐ | ‐ | Observation |
| Parodi 2006 | Subthreshold grid laser | Threshold grid laser | ‐ | ‐ |
| Russo 2009 | Grid laser | Intravitreal bevacizumab | ‐ | ‐ |
| SCORE 2009 | Grid laser | 1 mg intravitreal triamcinolone | 4 mg intravitreal triamcinolone | ‐ |
Types of outcomes
Battaglia Parodi 1999 did not define primary and secondary outcomes. Outcomes measured in the study were mean logMAR VA (at baseline, 3, 12, and 24 months), improvement of macular oedema based on stereophotography and FFA at end of follow‐up, and laser complications.
BVOS 1984 did not define primary and secondary outcomes.
Outcomes reported in the study were third‐year outcomes compared with baseline of the percentage of eyes which had gained two or more lines of VA on the Diabetic Retinopathy Study charts for at least two consecutive visits; the percentage of eyes which had lost two or more lines for at least two consecutive visits; the percentage of eyes with visual acuity 20/40 or better at third‐year visit; the percentage of eyes with visual acuity 20/400 or worse at third‐year visit; average visual acuity at third‐year visit; average number of lines gained at third‐year visit; and laser complications.
Parodi 2006 designated the decrease in OCT mean foveal thickness as the primary outcome. Secondary outcomes were changes in total macular volume over follow‐up, the proportion of eyes that gained 10 or more letters on standard ETDRS charts (approximately two or more lines of VA gain) at 12 months and 24 months, and the timing of macular oedema resolution. Additional outcomes reported were the number of participants with retreatment in each group.
Russo 2009 did not define primary and secondary outcomes. Outcome measures used were mean logMAR BCVA, mean logMAR BCVA change versus baseline, number of participants with increased BCVA by more than three ETDRS lines, mean central macular thickness, mean central macular thickness change versus baseline at one, three, six and 12 months. The study also reported on the number of treatments received by study participants, and on complications.
SCORE 2009 defined the percentage of participants with a gain in visual acuity letter score of 15 or more letters at month 12 as the primary outcome. Secondary outcomes were change in VA scores at month 12; mean change from baseline in mean VA score; percentage of participants gaining 15 or more letters; percentage of participants losing 15 or more letters; and OCT‐measured centre point thickness for every four months up to 36 months. Safety outcomes (elevated intra‐ocular pressure (IOP), cataract, infectious endophthalmitis, minor ocular adverse events, systemic adverse events) were also reported.
Excluded studies
We examined and excluded 21 full‐text articles. Further details on the reasons for the exclusion of these studies are summarised in the Characteristics of excluded studies tables.
Risk of bias in included studies
The risk of bias assessment is summarised in Figure 2 and Figure 3.
2.
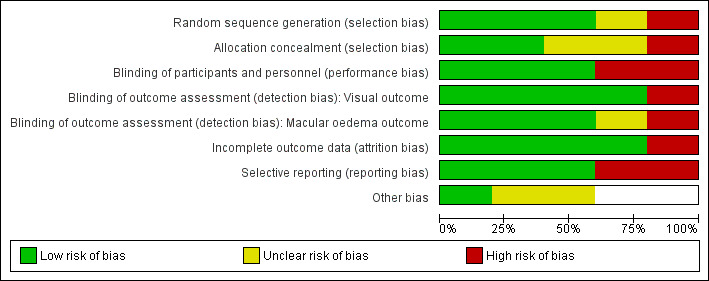
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
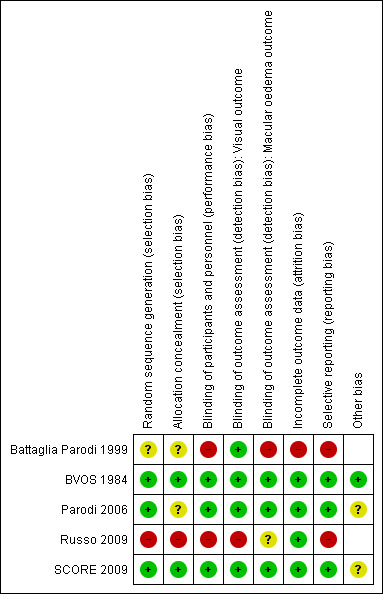
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation and allocation concealment (selection bias)
Battaglia Parodi 1999 was a randomised controlled study; however the method of random sequence generation and allocation concealment was not revealed in the paper. We contacted the corresponding author who advised us that sealed envelopes were used for the process. There were three types of envelope (sham treatment, early treatment, and delayed treatment) and a single envelope was assigned to each participant. As we were still unclear on how random sequence generation was performed and how allocation concealment was maintained, we deemed this study to be at unclear risk of selection bias.
BVOS 1984 was assessed to be at a low risk for selection bias because a computer‐generated random allocation schedule was used to randomise and the allocation for participating centres was done at a co‐ordinating centre.
Parodi 2006 demonstrated adequate random sequence generation, as the participants were randomised following a computer‐generated list using a block randomisation scheme. However there was no information provided on how allocation concealment was maintained. We contacted the corresponding author for details regarding allocation concealment but did not receive any further details for this study.
Russo 2009 was assessed as being at a high risk of bias in the allocation of participants, as they were assigned to a treatment group on the basis of clinic chart numbers, whereby the allocation was non‐random and could be predicted.
SCORE 2009 was assessed to be at a low risk of bias for random sequence generation and for allocation concealment, as participants were randomly assigned centrally through a web‐based system and the random sequence of treatment assignments was generated using a separate, permuted block design with random block sizes.
Blinding
Masking of participants and personnel (performance bias) and of outcome assessment (detection bias)
Battaglia Parodi 1999 was assessed as being at a high risk of performance bias from the lack of masking of personnel, but at a low risk of detection bias due to the adequate masking of outcome assessments. There was no mention in the paper of what measures had been taken to mask the clinic personnel to the intervention group of participants. In addition, the study authors assessed the fluorescein angiograms and macular stereophotographs of the study participants, and the study authors may have also performed the laser treatments. The participants in the study would not be aware of the intervention group to which they had been randomised, as the study author advised us that they performed sham laser. We rated the risk of detection bias to be low for the assessment of visual acuity, as this was measured by a co‐worker unaware of the treatment allocation, but high for the assessment of the change in macular oedema, although this risk was reduced by having the two different assessors review the stereophotographs and FFAs independently.
BVOS 1984 was assessed as being at a low risk of performance and detection bias. There was a clear protocol for the treatment and follow‐up of all study participants, the assessors of visual acuity were masked to treatment allocations, and the assessments of macular oedema were undertaken at a co‐ordinating centre not directly involved in the care of the study participants.
Parodi 2006 was assessed to have adequate masking of participants and personnel and adequate masking of outcome assessment. At each examination, an independent examiner refracted the study eye and performed the OCT scans; retreatment criteria were based on objective OCT data, which was performed by an independent examiner.
Russo 2009 was assessed as being at a high risk of performance and detection bias from the lack of masking. Clinic and study personnel would be able to tell from the clinic chart numbers which intervention group the participants were in, because they had been assigned on the basis of odd and even clinic chart numbers.
SCORE 2009 was assessed as being at a low risk of performance and detection bias due to the high quality of masking. Participants and physicians were masked to the intravitreal dose of triamcinolone. Although participants and physicians were not masked to treatment assignment of standard care versus intravitreal triamcinolone, there was a clear visit schedule where the examinations and investigations that were to be performed at each visit were predetermined. The retreatment protocol was based on objective outcome data obtained by masked outcome assessors who were certified, and were following a standardised protocol. We assessed the risk of detection bias to be low, as outcome assessors for VA and change in macular oedema were masked to intervention group and were using predefined protocols to grade outcomes.
Incomplete outcome data
Battaglia Parodi 1999 was assessed as being at a high risk of bias from incomplete outcome data, as nine participants were lost to follow‐up with eight of them having been randomised to the laser group but refusing treatment. However, when we contacted the author to clarify the number of participants that were lost from each group, the author informed us that three participants were lost to follow‐up in each group.
BVOS 1984 was assessed as being at a low risk of attrition bias. Of the 139 participants, 78 were analysed at three‐year follow‐up. However, analyses were performed on the basis of the intention to treat, and statistical methods were used to adjust for varying lengths of follow‐up, for loss to follow‐up and death (17 participants).
Parodi 2006 and Russo 2009 were assessed as being at a low risk of bias from incomplete outcome data, as all randomised participants completed the planned follow‐up of the studies.
SCORE 2009 was assessed as being at a low risk of attrition bias. Although the month‐12 primary outcome visit was completed by 88%, 89% and 91% of participants respectively in the three arms of the trial, the rates of death and premature withdrawal or missed visits were similar among groups. Sensitivity analyses taking into account this missing data to explore extreme possible estimates of treatment effects and a per protocol analysis had been performed in the study. They found in these analyses that the conclusions of the study for the primary outcomes were unchanged.
Selective reporting
BVOS 1984 mentioned a study protocol. The study report contains all the outcomes expected to address the prespecified research question. The two eyes that received treatment too close to the area by study protocol were excluded from analysis. This is probably not significant.
SCORE 2009 published their study protocol and the baseline characteristics of the study participants.
Battaglia Parodi 1999; Parodi 2006; and Russo 2009 did not mention a study protocol.
Other potential sources of bias
BVOS 1984 did not show significant baseline differences. Twenty‐two (16%) participants had a duration of BRVO of greater than 18 months. This is because people who entered the study and had been placed in the 'eyes at risk of the development of neovascularisation' group or the 'eyes at risk of development of vitreous haemorrhage' group to investigate the effect of scatter photocoagulation were still eligible for randomisation to grid laser treatment. However, prior randomisation to scatter photocoagulation did not have a significant effect on the change in VA in this study.
Parodi 2006 did not show significant baseline differences in age, sex, or systemic disorders.
SCORE 2009 baseline characteristics were described in detail in SCORE Study Report 3. There was a baseline imbalance in which a lower proportion of the participants in the laser group had cortical opacities. The significance of this imbalance is unknown. Cataract surgery was more frequent in the 4 mg triamcinolone group compared to the 1 mg triamcinolone group or the grid laser group. However, the authors of the study had also performed an analysis limited to eyes that were pseudophakic at baseline, which demonstrated no statistically significant difference among the treatment groups with respect to change in VA.
Effects of interventions
Macular grid laser versus control
Two studies compared macular grid laser to control (observation or sham treatment) (BVOS 1984; Battaglia Parodi 1999).
BVOS 1984 included participants with a recent BRVO (onset within three to 18 months) with cystoid macular oedema reducing VA to 20/40 or worse. Participants were randomised to grid laser or observation. Grid photocoagulation was applied to the involved fundus segment identified by fluorescein angiography using argon laser.
Primary outcomes
Proportion of participants with at least 15 ETDRS letters improvement in visual acuity at one year or more.
The number of people gaining at least 15 ETDRS letters was not reported. A greater proportion of participants in the grid laser group (28/43) gained 10 or more letters of vision at 36 months compared to the observation group (13/35) (risk ratio (RR) 1.75, 95% CI 1.08 to 2.84, P = 0.02). (Analysis 1.1)
1.1. Analysis.
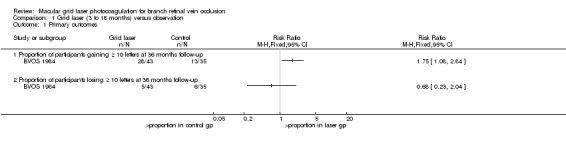
Comparison 1 Grid laser (3 to 18 months) versus observation, Outcome 1 Primary outcomes.
We judged this to be moderate‐quality evidence; we downgraded for imprecision.
Proportion of participants with at least 15 ETDRS letters worsening in visual acuity at one year or more.
Again the 15 letter cutpoint was not reported. At 36 months, 5/43 people treated with grid laser lost 10 or more letters compared to 6/35 in the control group (RR 0.68, 95% CI 0.23 to 2.04, P 0.49).(Analysis 1.1)
We judged this to be moderate‐quality evidence; we downgraded for imprecision.
Secondary outcomes
Change in BCVA from baseline
The mean difference in the change in BCVA from baseline to 36 months between the two groups was 0.11 logMAR (95% CI 0.05 to 0.17, P < 0.0001; 78 participants). (Analysis 1.2)
1.2. Analysis.

Comparison 1 Grid laser (3 to 18 months) versus observation, Outcome 2 Change in BCVA at 36 months.
We judged this to be high‐quality evidence.
Change in macular oedema
Although eyes were assessed with stereophotography at each visit and fluorescein angiography was performed at the first visit, at the first return visit and then at annual intervals, the study did not report changes in anatomical measures of macular oedema.
Number of treatments
Before exclusion and drop out, 53/69 eyes were treated once, 10 eyes were treated twice, two eyes were treated three times, and four eyes were treated five times.
Frequency and severity of ocular and systemic adverse events
One participant experienced an apparent perforation of Bruch's membrane, but this did not affect visual acuity. No other complications were observed.
Battaglia Parodi 1999 compared early grid laser within three months with delayed grid laser between six and 18 months with observation. However, the study was examining the treatment of macular oedema following 'macular branch retinal vein occlusion' (macular BRVO). The corresponding author of the study advised us that in this study macular BRVO was defined as "a BRVO form involving a small venous vessel within the temporal arcades." Some ophthalmologists may define this as a macular tributary branch vein occlusion, which is clinically different from the four other studies analysed in this review. Battaglia Parodi 1999 included participants with an occurrence of macular BRVO within 15 days with a visual acuity less than 0.6 logMAR. Krypton laser was directed to areas of capillary leakage identified by fluorescein angiography.
Primary outcomes
Proportion of participants with at least 15 ETDRS letters improvement in visual acuity at one year or more.
No participants (early treatment, delayed treatment and control) gained 15 or more letters after 12 months follow‐up.
Proportion of participants with at least 15 ETDRS letters worsening in visual acuity at one year or more.
No participants lost 15 or more letters after 12 months follow up.
Secondary outcomes
Change in BCVA from baseline
At 12 months, no difference could be demonstrated in the change in mean BCVA (from baseline) between early grid laser and sham laser (mean difference (MD) ‐0.03, 95% confidence interval (CI) ‐0.07 to 0.01, P = 0.12; 68 participants) or between delayed grid laser and sham laser (MD 0.00, 95% CI ‐0.04 to 0.04, P = 1; 66 participants). At 24 months, there was also no statistically significant difference in the change in mean BCVA (from baseline) between early grid laser and sham laser (MD ‐0.03, 95% CI ‐0.07 to 0.01, P = 0.12; 68 participants) or between delayed grid laser and sham laser (MD 0.02, 95 % CI ‐0.02 to 0.06, P = 0.34; 66 participants). (Analysis 2.1; Analysis 2.2)
2.1. Analysis.
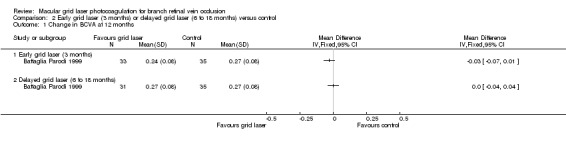
Comparison 2 Early grid laser (3 months) or delayed grid laser (6 to 18 months) versus control, Outcome 1 Change in BCVA at 12 months.
2.2. Analysis.
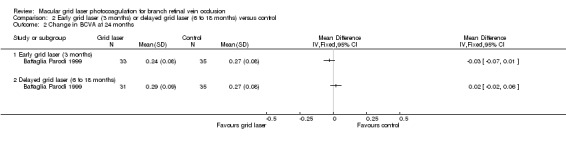
Comparison 2 Early grid laser (3 months) or delayed grid laser (6 to 18 months) versus control, Outcome 2 Change in BCVA at 24 months.
We judged this to be low‐quality evidence; we downgraded for risk of bias and imprecision.
Change in macular oedema
No difference could be demonstrated between groups when assessing improvement in macular oedema with stereophotography or fluorescein angiography at 24 months. In people treated with laser within 3 months 29/33 people experienced an improvement compared to 28/35 in the control group (RR 1.10, 95% 0.89, 1.35). In people treated with laser between 6‐18 months 25/31 people experienced an improvement compared to 28/35 in the control group (RR 1.01, 95% 0.79, 1.28). (Analysis 2.3; Analysis 2.4)
2.3. Analysis.
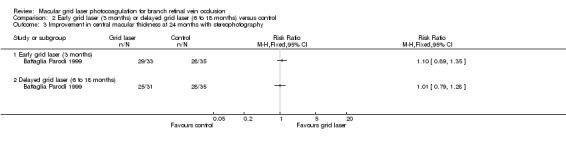
Comparison 2 Early grid laser (3 months) or delayed grid laser (6 to 18 months) versus control, Outcome 3 Improvement in central macular thickness at 24 months with stereophotography.
2.4. Analysis.
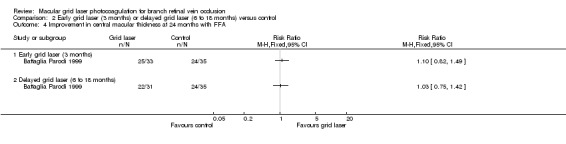
Comparison 2 Early grid laser (3 months) or delayed grid laser (6 to 18 months) versus control, Outcome 4 Improvement in central macular thickness at 24 months with FFA.
We judged this to be low‐quality evidence; we downgraded for risk of bias and imprecision.
Number of treatments
No participant was retreated.
Frequency and severity of ocular and systemic adverse events
No complications or adverse events were observed in any of the groups.
Subthreshold grid laser versus threshold grid laser
Only one study reported this comparison. Parodi 2006 randomised 36 participants to either subthreshold laser or threshold grid laser.
Primary outcomes
Proportion of participants with at least 15 ETDRS letters improvement in visual acuity at one year or more.
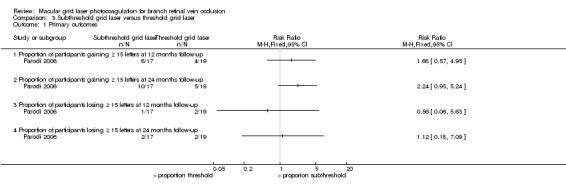
A greater proportion of the participants in the subthreshold grid laser group gained 15 or more letters at 12 months (RR 1.68, 95% CI 0.57 to 4.95, P = 0.35), and the effect appeared to be greater at 24 months (RR 2.24, 95% CI 0.95 to 5.24, P = 0.06). However, the wide confidence intervals rendered these effect estimates imprecise. (Analysis 3.1)
3.1. Analysis.
Comparison 3 Subthreshold grid laser versus threshold grid laser, Outcome 1 Primary outcomes.
We judged this to be moderate‐quality evidence; we downgraded for imprecision.
Proportion of participants with at least 15 ETDRS letters worsening in visual acuity at one year or more.
No difference could be demonstrated in the proportion of participants losing 15 or more letters when comparing the subthreshold laser and threshold laser group at 12 months (RR 0.56, 95% CI 0.06 to 5.63, P 0.61) or at 24 months (RR 1.12, 95% CI 0.18 to 7.09, P = 0.91). (Analysis 3.1)
We judged this to be moderate‐quality evidence; we downgraded for imprecision.
Secondary outcomes
Change in BCVA from baseline
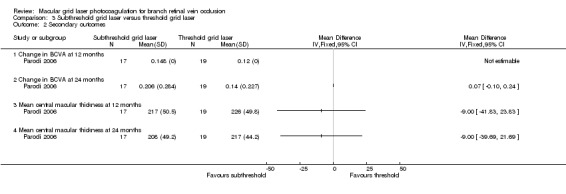
At 12 months, the mean BCVA in the subthreshold grid laser group increased by 0.024 logMAR more than the threshold grid laser group. However, there was some discrepancy in the 12‐month BCVA data presented in Figure 4 of the paper (from where we extracted the data) and Figure 3 of the paper. If data from figure 3 were used instead, then the change in BCVA at 12 months from baseline would have been an increase of 0.15 logMAR in the subthreshold group and an increase of 0.11 logMAR for the threshold group, so that the difference in the change in BCVA between the two groups would have been 0.04 logMAR. Therefore, despite the discrepancy, the difference between the two groups was still small. Nevertheless, we were unable to estimate a standard deviation or to generate a 95% confidence interval for this comparison. At 24 months, there was no statistical difference in the gain in mean BCVA from baseline between the subthreshold grid laser group and the threshold grid laser group (MD 0.07, 95% CI ‐0.10 to 0.24, P = 0.42).(Analysis 3.2)
3.2. Analysis.
Comparison 3 Subthreshold grid laser versus threshold grid laser, Outcome 2 Secondary outcomes.
We judged this to be moderate‐quality evidence; we downgraded for imprecision.
Change in macular oedema
No difference could be demonstrated in the mean foveal thickness between the subthreshold group and the threshold grid laser group at 12 months (MD ‐9.00, 95% CI ‐41.83 to 23.83; participants = 36) or at 24 months (MD ‐9.00, 95% CI ‐39.69 to 21.69; participants = 36).(Analysis 3.2)
We judged this to be moderate‐quality evidence; we downgraded for imprecision.
Number of treatments
Three participants in the subthreshold group and one participant in the threshold group who did not show macular oedema resolution at the 12‐month examination were retreated.
Frequency and severity of ocular and systemic adverse events
This was not reported.
Grid laser versus intravitreal bevacizumab
Only one study offered this comparison. Russo 2009 randomised 30 participants to either grid laser or intravitreal bevacizumab.
Primary outcomes
Proportion of participants with at least 15 ETDRS letters improvement in visual acuity at one year or more.
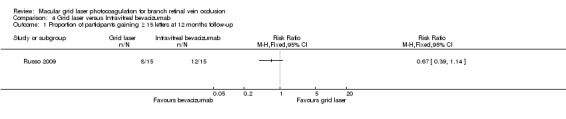
A smaller proportion of the grid laser group gained 15 or more letters compared to the intravitreal bevacizumab group but this did not appear to be statistically significant due to the wide confidence intervals (RR 0.67, 95% CI 0.39 to 1.14, P = 0.14).(Analysis 4.1)
4.1. Analysis.
Comparison 4 Grid laser versus Intravitreal bevacizumab, Outcome 1 Proportion of participants gaining ≥ 15 letters at 12 months follow‐up.
We judged this to be low‐quality evidence; we downgraded for risk of bias and imprecision.
Proportion of participants with at least 15 ETDRS letters worsening in visual acuity at one year or more.
This was not estimable as there were no data provided on the number of participants who lost 15 or more letters.
Secondary outcomes
Change in BCVA from baseline
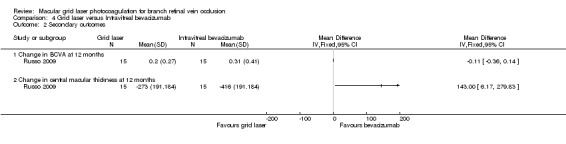
The study reported that the group receiving bevacizumab had better BCVA at one, three, six and 12 months (P < 0.05). The intravitreal bevacizumab group had a greater improvement in the change in BCVA at 12 months from baseline than the grid laser group by 0.11 (SD 0.14706). However, this effect estimate was imprecise with wide confidence intervals and we found that the difference between the groups was not statistically significant (95% CI ‐0.36 to 0.14, P = 0.39).(Analysis 4.2)
4.2. Analysis.
Comparison 4 Grid laser versus Intravitreal bevacizumab, Outcome 2 Secondary outcomes.
We judged this to be low‐quality evidence; we downgraded for risk of bias and imprecision.
Change in macular oedema
At 12 months, the reduction in mean central macular thickness was significantly greater in the intravitreal bevacizumab group (MD 143, 95% CI 6.17 to 279.83). The study reported that the group receiving bevacizumab had lower central macular thickness values at one, three, six and 12 months (P < 0.05). (Analysis 4.2)
We judged this to be low‐quality evidence; we downgraded for risk of bias and imprecision.
Number of treatments
In the grid laser photocoagulation group 5/15 participants (33.3%) received two laser treatments and 3/15 participants (20%) received three laser treatments. In the intravitreal bevacizumab group, 7/15 (46.6%) received two injections and 3/15 (20%) received three injections.
Frequency and severity of ocular and systemic adverse events
There were no laser complications. The injections were well tolerated and there was no uveitis, endophthalmitis, ocular toxicity, systemic adverse events, significant intra‐ocular pressure or lens status changes. However, minor local adverse events (conjunctival hyperaemia and subconjunctival haemorrhage) occurred in nine participants during the first week.
Grid laser versus 1 mg intravitreal triamcinolone versus 4 mg triamcinolone
Only one study presented this comparison. SCORE 2009 randomised 411 participants to either grid laser treatment or intravitreal triamcinolone. At the time of study closeout, 89% of study participants had 12‐month outcomes assessed, 58% of study participants had 24‐month outcomes assessed and 31% had 36‐month outcomes assessed.
Primary outcomes
Proportion of participants with at least 15 ETDRS letters improvement in visual acuity at one year or more.
The proportion of participants gaining 15 or more letters was similar in the grid laser, 1 mg triamcinolone and 4 mg triamcinolone groups at 12 and 24 months. At 36 months a greater proportion of the grid laser group gained 15 or more letters compared to the 1 mg triamcinolone group (RR 1.91, 95% CI 1.06 to 3.47, P = 0.03; 90 participants); however, there was no difference between grid laser and the 4 mg triamcinolone group (RR 0.96, 95% CI 0.62 to 1.48, P = 0.84; 84 participants).(Analysis 5.1)
5.1. Analysis.
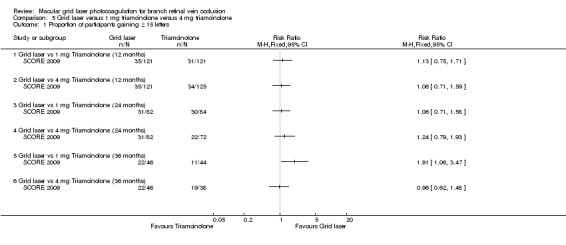
Comparison 5 Grid laser versus 1 mg triamcinolone versus 4 mg triamcinolone, Outcome 1 Proportion of participants gaining ≥ 15 letters.
We judged this to be moderate‐quality evidence; we downgraded for imprecision.
Proportion of participants with at least 15 ETDRS letters worsening in visual acuity at one year or more.
At 12 months, no difference could be demonstrated between the grid laser and the 1 mg triamcinolone group (RR 1.20, 95% CI 0.63 to 2.27, P 0.45; 242 participants) or the 4 mg triamcinolone group (RR 1.24, 95% CI 0.65 to 2.35, P = 0.51; 246 participants).
At 24 and 36 months, the grid laser group was less likely to lose 15 or more letters compared to the 1 mg triamcinolone group and the 4 mg triamcinolone group. However, the point estimates for the 24‐ and 36‐month follow‐ups were imprecise with wide confidence intervals. At these time points, the only comparison that seemed to be statistically significant was the comparison between grid laser and 4 mg triamcinolone at 24 months where the proportion of participants losing 15 or more letters was significantly lower in the grid laser group (RR 0.29, 95% CI 0.10 to 0.87, P = 0.03; 154 participants). (Analysis 5.2)
5.2. Analysis.
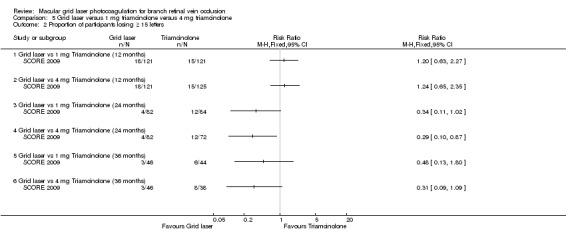
Comparison 5 Grid laser versus 1 mg triamcinolone versus 4 mg triamcinolone, Outcome 2 Proportion of participants losing ≥ 15 letters.
We judged this to be moderate‐quality evidence; we downgraded for imprecision.
Secondary outcomes
Change in BCVA from baseline
At 12 months, no difference could be demonstrated in the change of mean logMAR from baseline between grid laser and 1 mg triamcinolone (MD ‐0.03 letters, 95% CI ‐0.12 to 0.06, P = 0.49; 242 participants) or grid laser and 4 mg triamcinolone (MD 0.00, 95% CI ‐0.09 to 0.09, P = 1.00; 246 participants). At 24 months and 36 months, the mean improvement from baseline was greater in the grid laser group compared with 1 mg or 4 mg triamcinolone. These improvements were statistically significant only when comparing grid laser to 4 mg triamcinolone at 24 months (MD 0.15 logMAR, 95% CI 0.04 to 0.26, P = 0.01; 154 participants); and grid laser treatment to 1 mg triamcinolone at 36 months (MD 0.17 logMAR, 95% CI 0.02 to 0.32, P = 0.03: 90 participants; Analysis 5.3; see Figure 4).
5.3. Analysis.
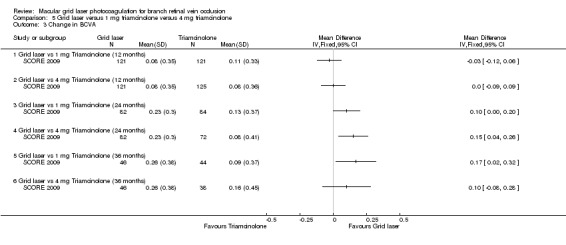
Comparison 5 Grid laser versus 1 mg triamcinolone versus 4 mg triamcinolone, Outcome 3 Change in BCVA.
4.
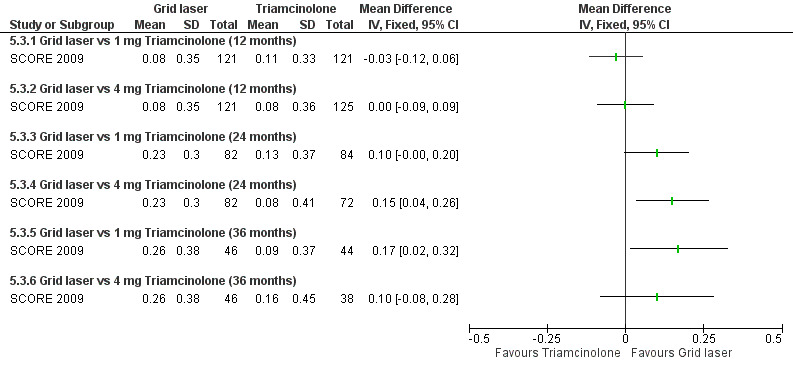
Forest plot of comparison: 5 Grid laser versus 1 mg triamcinolone versus 4 mg triamcinolone, outcome: 5.3 Change in BCVA.
We judged this to be moderate‐quality evidence; we downgraded for imprecision.
Change in macular oedema
All groups showed decreases in centre point thickness, but the grid laser group had a greater decrease at 12, 24 and 36 months compared to 1 mg and 4 mg triamcinolone. This was statistically significant at all time periods (Analysis 5.4). When assessed with fluorescein angiogram, the area of fluorescein leakage and capillary non‐perfusion were similar in all groups at 12 months.
5.4. Analysis.
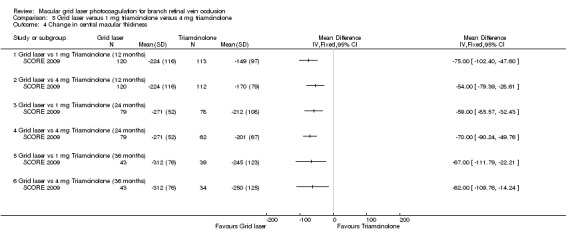
Comparison 5 Grid laser versus 1 mg triamcinolone versus 4 mg triamcinolone, Outcome 4 Change in central macular thickness.
We judged this to be high‐quality evidence.
Number of treatments
Mean number of laser treatments by 12 months was 1.8 (95% CI 1.6 to 2.0) in eyes without dense macular haemorrhage at baseline, and 0.7 (95% CI 0.5 to 1.0) in eyes with dense macular haemorrhage at baseline; in the latter group the initial laser treatment was deferred until haemorrhage cleared sufficiently. In comparison, the mean number of injections was 2.2 (95% CI 2.1 to 2.4) in the 1 mg triamcinolone group and 2.1 (95% CI 2.0 to 2.3) in the 4 mg triamcinolone group.
Frequency and severity of ocular and systemic adverse events
Intra‐ocular pressure‐lowering medication was initiated in 41% of eyes in the 4 mg triamcinolone group compared to 7% in the 1 mg triamcinolone and 2% in the laser group throughout the first 12 months (P = 0.03 for laser versus 1 mg triamcinolone; P < 0.001 for laser versus 4 mg triamcinolone). None underwent surgery during the 12 months but by 24 months, one eye in the 4 mg triamcinolone had trabeculectomy and one eye in the 4 mg triamcinolone had tube shunt.
Among eyes that were phakic at baseline, lens opacity developed or progressed in 13% of the laser group during the first 12 months, compared with 25% and 35% of the 1 mg and 4 mg triamcinolone groups respectively (P = 0.03 for laser versus 1 mg triamcinolone; P < 0.001 for laser versus 4 mg triamcinolone). Throughout 12 months, cataract surgery was performed in three eyes in laser, none in 1 mg triamcinolone and four in 4 mg triamcinolone groups. However, by 24 months these increased to six (out of 137) participants in the laser, eight (out of 136) participants in the 1 mg triamcinolone and 35 (out of 138) participants in the 4 mg triamcinolone group (P = 0.59 for laser versus 1 mg triamcinolone; P < 0.001 for laser versus 4 mg triamcinolone).
There was no infectious endophthalmitis in the laser or 1 mg triamcinolone groups, but one case with 4 mg triamcinolone that grew coagulase‐negative staphylococcus on vitreous culture. Other minor ocular adverse events that were reported only in the triamcinolone groups were vitreous floaters, conjunctival haemorrhage, and silicone oil droplets in vitreous.
Discussion
Summary of main results
Early grid laser (three months) versus delayed grid laser (six to 18 months) versus control
Battaglia Parodi 1999 (108 participants) did not show a benefit of early or delayed laser treatment over control for macular branch retinal vein occlusion (MBRVO). For our primary outcome, no participants gained or lost 15 or more letters and we were unable to calculate risk ratios. For our secondary outcomes, the effects on change in best‐corrected visual acuity (BCVA), or improvement in central macular thickness at 24 months follow‐up were uncertain (very low‐quality evidence).
Grid laser (3 to 18 months) versus observation
BVOS 1984 (139 participants) showed a benefit of grid laser treatment over observation. For our primary outcomes, the risk ratios for gaining 10 or more letters showed a benefit for grid laser treatment and no significant difference between the two groups for losing 10 or more letters. For our secondary outcomes, the mean improvement in logMAR BCVA was greater with grid laser treatment. Results for change in mean BCVA were only reported at 36 months. The study authors did not include data for visual acuity at baseline or at 36 months. No anatomical measures of macular oedema were available.
Subthreshold grid laser versus threshold grid laser
In Parodi 2006 (36 participants), the effect of subthreshold grid laser versus the threshold grid laser on the proportion of participants gaining or losing 15 or more letters, change in mean BCVA or change in mean central macular thickness was imprecise, and compatible with either benefit or harm.
Grid laser versus intravitreal bevacizumab
In Russo 2009 (30 participants), the effect of grid laser versus the intravitreal bevacizumab on the proportion of participants gaining or losing 15 or more letters or in the change in mean BCVA was uncertain. However, the reduction in the central macular thickness for the intravitreal bevacizumab group was significantly greater than the grid laser group at 12 months.
Grid laser versus 1 mg intravitreal triamcinolone versus 4 mg triamcinolone
In SCORE 2009 (411 participants), the visual acuity outcomes (proportion of participants gaining or losing 15 or more letters; change in mean BCVA) between grid laser treatment and 1 mg or 4 mg intravitreal triamcinolone were imprecise. However, there was a possible benefit in grid laser over 1 mg intravitreal triamcinolone at 36 months, and a possible benefit in grid laser over 4 mg triamcinolone at 24 months. Anatomically, the grid laser group had a greater reduction in macular thickness compared to 1 mg triamcinolone groups and 4 mg triamcinolone at 12, 24, and 36 months. Grid laser had a superior safety profile with respect to elevation of intra‐ocular pressure, cataract surgery and risk of endophthalmitis.
Overall completeness and applicability of evidence
The five studies in this review addressed our objectives and answered a variety of questions pertaining to the use of grid laser photocoagulation in people with chronic macular oedema following branch retinal vein occlusions i.e.
whether grid laser treatment was beneficial compared to observation alone;
whether early or delayed grid laser treatment was beneficial over sham laser treatment;
whether subthreshold grid laser treatment was beneficial over standard grid laser treatment;
whether intravitreal anti‐vascular endothelial growth factor injections offer a benefit over grid laser photocoagulation;
whether intravitreal steroid injections offer a benefit over grid laser photocoagulation.
However, there was only one study for each of these research questions.
Two studies (BVOS 1984 and SCORE 2009) dealing with research questions 1 and 5 were deemed to be of good methodological quality. These studies presented all the relevant outcomes and data using the appropriate methods. The evidence from these studies therefore addressed these questions well. However, both these studies were performed in the United States with a predominantly white population. Caution could therefore be required before applying this evidence internationally in different healthcare systems with different modes of practice. Cost implications and the availability of resources could also render one treatment more feasible over the others in different countries.
The remaining three of the five studies (Battaglia Parodi 1999; Parodi 2006; Russo 2009) addressing research questions 2 to 4 above were relatively small in size (although Parodi 2006 was of good methodological quality). In addition, the study which addressed the question of whether early grid laser treatment was beneficial over standard grid laser treatment (Battaglia Parodi 1999) recruited only participants with a subtype of BRVO, called 'macular BRVO'. The authors defined this in their study as "a subgroup of branch retinal vein occlusion in which the occlusion is limited to a small vessel draining a sector of the macular region". The results of this study therefore cannot be applied more generally to all people with macular oedema with branch retinal vein occlusions. In view of these issues, we consider that these three studies do not provide sufficient evidence to answer research questions 2, 3 and 4, and that further study is required to address these issues.
Quality of the evidence
Three of the five included trials in this systematic review were of good methodological quality. These three trials compared grid laser treatment versus observation (BVOS 1984), grid laser treatment versus subthreshold grid laser (Parodi 2006) and grid laser treatment versus intravitreal triamcinolone (SCORE 2009). We found Russo 2009, which compared grid laser treatment versus intravitreal bevacizumab, to be at high risk of bias. Battaglia Parodi 1999, comparing early versus late grid laser for a subgroup of participants with branch vein occlusions (i.e. tributary vein occlusions), was also potentially at high risk of bias.
Early grid laser (three months) versus delayed grid laser (six to 18 months) versus observation in 'macular BRVO'
Battaglia Parodi 1999 was the only randomised controlled trial (RCT) to address this comparison. There were methodological issues. The study did not find a difference between the treatment and the control groups. The study only looked at a subgroup of BRVO participants and it would be difficult to apply this more generally to other people with BRVO and macular oedema. Due to the limitations in the design and implementation, indirectness of evidence and imprecision of the results, the quality of the evidence was downgraded to very low.
Grid laser (three to 18 months) versus observation
BVOS 1984 was the only RCT to address this comparison. The evidence was in favour of grid laser treatment. The methodological quality of the study was good and the magnitude of effect of treatment was large. The number of participants lost to follow‐up was not deemed to be significant because the authors used Kaplan‐Meier statistics, the logrank test and the Cox proportional hazards model to adjust for varying length of follow‐up for the effect of co‐variables and for the loss to follow‐up and death; and every eye randomised was included in their analyses. However, we downgraded the quality of evidence to moderate because of the relatively small sample size and wide confidence intervals which rendered the results imprecise.
Subthreshold grid laser versus threshold grid laser
Parodi 2006 was the only RCT to address this comparison. This study was deemed to be at low risk of bias. However, the study sample was small and the confidence intervals were wide, rendering the results imprecise.The quality of evidence for subthreshold laser was therefore downgraded for imprecision to moderate.
Grid laser versus intravitreal bevacizumab
Russo 2009 was the only RCT to address this comparison, but there were serious issues with the design and implementation of the study, suggesting a high likelihood of bias. In addition, the number of participants in the study was small and the confidence intervals were wide, rendering the results imprecise. The evidence for the comparison between grid laser and intravitreal bevacizumab was therefore downgraded by three levels to a very low quality of evidence.
Grid laser versus 1 mg intravitreal triamcinolone versus 4 mg triamcinolone
SCORE 2009 was the only RCT to address this comparison. The evidence showed no difference in visual outcomes between the groups at 12 months, but suggested a possible benefit for grid laser treatment at 24 and 36 months. However, the confidence intervals were wide. We noted no dose‐response gradient in the intravitreal triamcinolone groups. The grid laser group had a greater reduction in macular thickness at all time points. The study was of good methodological quality, but we downgraded the evidence one level to moderate because the wide confidence intervals rendered the results imprecise.
Potential biases in the review process
We included only RCTs in this systematic review, and would therefore have excluded all observational studies even if they were otherwise of good methodological quality with large treatment effects.
Agreements and disagreements with other studies or reviews
We identified two other systematic reviews (McIntosh 2007, Glanville 2014) which also included macular grid laser photocoagulation for the treatment of macular oedema following BRVO.
McIntosh 2007 included five trials on laser photocoagulation, of which three compared grid macular laser photocoagulation with observation (Battaglia Parodi 1999; Battaglia 1999; BVOS 1984) while one compared grid macular laser photocoagulation and intravitreal triamcinolone (Avitabile 2005). We did not include Avitabile 2005 as this study only had nine months of follow‐up presented and there were only six participants with BRVO, allocating two participants to each of the three arms (i.e. intravitreal injections of triamcinolone acetonide, macular grid laser photocoagulation, and a combination both triamcinolone injection and macular grid laser).
We also did not include Battaglia 1999, as this appeared to be a duplicate publication of Battaglia Parodi 1999 relating to the same RCT. The corresponding author subsequently advised us that Battaglia 1999 was a conference report, while Battaglia Parodi 1999 was the full paper. In their systematic review, McIntosh 2007 also included one other RCT evaluating intravitreal steroid, three RCTs evaluating haemodilution, one RCT evaluating ticlopidine and one RCT including troxerutin. We did not include these RCTs as they did not include macular grid laser photocoagulation as one of their intervention arms. McIntosh 2007 concluded that there was limited level‐one evidence for any interventions for BRVO. They reported that BVOS 1984 showed that macular grid laser photocoagulation was an effective treatment for macular oedema, improving vision in eyes with VA of 20/40 to 20/200, but that the effectiveness of the other treatments that they evaluated was unsupported by the evidence at that time. McIntosh 2007 did not include Parodi 2006, Russo 2009 or SCORE 2009. Parodi 2006 was published in the same year that their review was submitted for publication.
Glanville 2014 aimed to assess the efficacy of available treatments for macular oedema secondary to vein occlusions, i.e. ranibizumab, bevacizumab, intravitreal triamcinolone and macular laser photocoagulation and to assess the feasibility of conducting an indirect comparison between these therapies. The work was funded by Novartis, Basel, Switzerland. Glanville 2014 included three trials on grid macular laser photocoagulation. Two comparing grid macular laser photocoagulation with observation (Battaglia 1999; BVOS 1984) and one comparing grid macular laser photocoagulation with intravitreal bevacizumab (Russo 2009). Glanville 2014 included data from Battaglia 1999 but not Battaglia Parodi 1999.
Glanville 2014 also included one other RCT evaluating intravitreal ranibizumab (0.3 and 0.5mg) vs. sham treatment (Campochiaro 2010). We did not include this study because grid macular laser photogoaculation was given to participants who did not achieve sufficient improvement in BCVA after month 3, irrespective of whether they received ranibizumab or sham treament.
Glanville 2014 concluded that data from RCTs for ranibizumab and dexamethasone IVT demonstrate that both new agents constitute significant improvements over the previously widely accepted standard of care (laser therapy) for the treatment of BRVO. This contrasts with our findings where we found moderate quality evidence to support the use of grid laser photocoagulation to treat macular oedema following BRVO, and insufficient evidence to show a benefit of intravitreal triamcinolone or intravitreal anti‐VEGF over macular grid laser photocoagulation.
Ongoing trials
We found two unpublished RCTs. NCT00562406 is a pilot study with the primary objective of comparing the functional and anatomic outcomes of chronic macular oedema secondary to BRVO treated with argon laser photocoagulation versus intravitreal ranibizumab (Lucentis®) injection versus a combination of both. Although this study was completed in September 2010, no publications from this RCT are currently available. NCT00642226 is an open‐label RCT comparing the effectiveness of pars plana vitrectomy in combination with triamcinolone acetate versus macular grid laser photocoagulation in the treatment of macular oedema due to BRVO. This study was completed in November 2013. Additional details are listed in the Characteristics of ongoing studies tables.
Authors' conclusions
Implications for practice.
There is moderate‐quality evidence from one adequately‐powered RCT supporting the use of grid laser photocoagulation to treat macular oedema following BRVO. However, there was little evidence to support the early or late use of macular grid laser photocoagulation or the use of subthreshold laser, although the study evaluating early grid laser was only looking at a particular subset of BRVO. There was insufficient evidence to show a benefit of intravitreal bevacizumab or intravitreal triamcinolone over grid macular laser photocoagulation for the treatment of macular oedema following BRVO.
Implications for research.
There is recent interest in the use of intravitreal injections in the treatment of macular oedema for BRVO. Future studies of agents used in intravitreal injections should include macular grid laser photocoagulation as one of the intervention arms. Assessments of treatment efficacy including the proportion of participants gaining or losing 15 or more letters of visual acuity, as well as the change in mean visual acuity, will be important. With greater use of optical coherence tomography imaging in patient management, reports on objective measures of changes in foveal or central macular thickness will also be important. Due to the natural history of spontaneous improvement in many cases of BRVO, follow‐up should be for at least 12 months and the number of treatments needed for maintenance and long‐term safety will be important for future studies.
Acknowledgements
The Cochrane Eyes and Vision Group created and executed the search strategies. We thank:
Ahmad Al Moujahed for his contributions to the protocol;
Catey Bunce and Mariacristina Parravano for their comments on the protocol and review;
Jennifer Evans for her help in writing the review;
Gianni Virgili for his comments and assistance during the writing of this review; and
Anupa Shah and Iris Gordon for their assistance through the whole review process.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Retinal Vein Occlusion #2 MeSH descriptor Retinal Vein #3 retina* near/3 (vein* or occlu* or obstruct* or clos* or stricture* or steno* or block* or embolism*) #4 branch venous occlu* #5 BRVO or RVO #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Lasers #8 laser* #9 MeSH descriptor Laser Coagulation #10 photocoagulat* #11 photo near/1 coagulat* #12 coagulat* or argon or diode #13 (#7 OR #8 OR #9 OR #10 OR #11 OR #12) #14 (#6 AND #13)
Appendix 2. MEDLINE (OvidSP) search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp retinal vein occlusion/ 14. exp retinal vein/ 15. ((vein$ or occlu$ or obstruct$ or clos$ or stricture$ or steno$ or block$ or embolism$) adj3 retina$).tw. 16. branch venous occlu$.tw. 17. (BRVO or RVO).tw. 18. or/13‐17 19. exp lasers/ 20. laser$.tw. 21. exp laser coagulation/ 22. photocoagulat$.tw. 23. (photo adj1 coagulat$).tw. 24. (coagulat$ or argon or diode).tw. 25. or/19‐24 26. 12 and 18 and 25
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp retinal vein occlusion/ 34. exp retina vein/ 35. ((vein$ or occlu$ or obstruct$ or clos$ or stricture$ or steno$ or block$ or embolism$) adj3 retina$).tw. 36. branch venous occlu$.tw. 37. (BRVO or RVO).tw. 38. or/33‐37 39. exp lasers/ 40. laser$.tw. 41. exp laser coagulation/ 42. photocoagulat$.tw. 43. (photo adj1 coagulat$).tw. 44. (coagulat$ or argon or diode).tw. 45. or/39‐44 46. 32 and 38 and 45
Appendix 4. Web of Science CPCI‐S search strategy
# 9 #4 AND #8 # 8 #5 OR #6 OR #7 # 7 TS=(coagulat* or argon or diode) # 6 TS=photocoagulat* # 5 TS=laser photocoagulation # 4 #1 OR #2 OR #3 # 3 TS= (BRVO OR RVO) # 2 TS=branch retinal vein # 1 TS=retinal vein occlusion
Appendix 5. metaRegister of Controlled Trials search strategy
laser AND branch retinal
Appendix 6. ClinicalTrials.gov search strategy
laser AND branch retinal
Appendix 7. ICTRP search strategy
laser AND branch retinal
Data and analyses
Comparison 1. Grid laser (3 to 18 months) versus observation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Proportion of participants gaining ≥ 10 letters at 36 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Proportion of participants losing ≥ 10 letters at 36 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in BCVA at 36 months | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
Comparison 2. Early grid laser (3 months) or delayed grid laser (6 to 18 months) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in BCVA at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Early grid laser (3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Delayed grid laser (6 to 18 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Change in BCVA at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Early grid laser (3 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Delayed grid laser (6 to 18 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Improvement in central macular thickness at 24 months with stereophotography | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Early grid laser (3 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Delayed grid laser (6 to 18 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Improvement in central macular thickness at 24 months with FFA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Early grid laser (3 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Delayed grid laser (6 to 18 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Subthreshold grid laser versus threshold grid laser.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Proportion of participants gaining ≥ 15 letters at 12 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Proportion of participants gaining ≥ 15 letters at 24 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Proportion of participants losing ≥ 15 letters at 12 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Proportion of participants losing ≥ 15 letters at 24 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Secondary outcomes | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Change in BCVA at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Change in BCVA at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Mean central macular thickness at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Mean central macular thickness at 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Grid laser versus Intravitreal bevacizumab.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants gaining ≥ 15 letters at 12 months follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Secondary outcomes | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Change in BCVA at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Change in central macular thickness at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Grid laser versus 1 mg triamcinolone versus 4 mg triamcinolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants gaining ≥ 15 letters | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Grid laser vs 1 mg Triamcinolone (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Grid laser vs 4 mg Triamcinolone (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Grid laser vs 1 mg Triamcinolone (24 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Grid laser vs 4 mg Triamcinolone (24 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Grid laser vs 1 mg Triamcinolone (36 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Grid laser vs 4 mg Triamcinolone (36 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Proportion of participants losing ≥ 15 letters | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Grid laser vs 1 mg Triamcinolone (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Grid laser vs 4 mg Triamcinolone (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Grid laser vs 1 mg Triamcinolone (24 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Grid laser vs 4 mg Triamcinolone (24 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Grid laser vs 1 mg Triamcinolone (36 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Grid laser vs 4 mg Triamcinolone (36 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Change in BCVA | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Grid laser vs 1 mg Triamcinolone (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Grid laser vs 4 mg Triamcinolone (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Grid laser vs 1 mg Triamcinolone (24 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Grid laser vs 4 mg Triamcinolone (24 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Grid laser vs 1 mg Triamcinolone (36 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Grid laser vs 4 mg Triamcinolone (36 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in central macular thickness | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Grid laser vs 1 mg Triamcinolone (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Grid laser vs 4 mg Triamcinolone (12 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Grid laser vs 1 mg Triamcinolone (24 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Grid laser vs 4 mg Triamcinolone (24 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Grid laser vs 1 mg Triamcinolone (36 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Grid laser vs 4 mg Triamcinolone (36 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Battaglia Parodi 1999.
| Methods | Randomised controlled trial 108/137 participants fulfilled inclusion criteria and were randomised. Study reported on 99 participants and did not provide information on 9 participants excluded after randomisation. Quote: "Nine patients were lost to follow‐up (one patient died 1 month after the inclusion in the study, and eight patients refused the laser treatment)". Study duration/length of follow‐up: 24 months (planned and actual) |
|
| Participants | Primary diagnosis/diagnoses: Macular BRVO ‐ "a subgroup of branch retinal vein occlusion in which the occlusion is limited to a small vessel draining a sector of the macular region." Inclusion criteria: recent occurrence (within 15 days), significant macular oedema (retinal thickening one optic disc area or greater in size, involving the fovea, assessed by stereophotography), VA < 0.6 Exclusion criteria: media opacities, previous laser Rx, previous surgical Rx, any other retinal pathology Demographic characteristics: 1. 59 men, 40 women 2. Mean age 69.9 3. Italy 4. Includes 77 participants of Battaglia 1999 |
|
| Interventions | Interventions: Krypton laser (100 microns, 100 ‐ 200 mW, 0.5s, directed at area of capillary leakage identified on FFA, avoiding foveal avascular zone). intervention groups:
|
|
| Outcomes | No primary or secondary outcomes were defined. Outcomes measured:
Intervals at which outcomes assessed: Baseline, 3, 12, 24 months (BCVA, stereophotography, FFA) Number of participants included in analysis by group: 33 group E, 31 group D, 35 group C. Subgroup analyses (prespecified and post hoc): None |
|
| Notes | Study data source: 1) Published data only (unpublished not sought) Funding source(s): none declared Note correspondence with investigators (e.g., unpublished data sought, clarification of methods): We contacted the corresponding author for further details regarding their methodology and for details regarding the participants lost to follow‐up to assist in our 'Risk of bias' assessments. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to no laser treatment or to laser treatment." Materials and methods section. Comment: Method for randomisation not mentioned in paper. We contacted the study author and the response was "sealed envelopes". Quote: "The grid laser treatment was performed soon after the 3‐month control in one subgroup...... and after 6‐18 months in another subgroup....." Materials and methods section. Comment: After randomisation into treatment and control group, participants in treatment group were subdivided into the early treatment group (group E) or the delayed treatment group (group D). We contacted the corresponding author of the study to enquire how the participants in the treatment group were subdivided into the early treatment or delayed treatment group. The response that we received was: Quote: "We had 3 types of envelopes: sham treatment, early treatment, delayed treatment. a single envelope was assigned to each patient" |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not mentioned in paper. We contacted the corresponding author of the study who advised that the participants were randomised with "sealed envelopes". |
| Blinding of participants and personnel (performance bias) Visual outcome | High risk | Quote: "The patients were randomly assigned to no laser treatment or to laser treatment. The grid laser treatment was performed soon after the 3‐month control in one subgroup of eyes (early treatment group, defined as group E), and after a period of 6–18 months in another subgroup of eyes (delayed treatment group, defined as group D), in order to evaluate the effect of different timing of laser treatment.
Comments: The corresponding author informed us that they "performed sham laser to all patients". Quote: "Worsening or the improvement of the macular edema was assessed by comparing the stereophotographs and the fluorescein angiographs (late phases of10 min). Two authors (M.B.P., S.S.) had interobserver concordance of 96% and 94% of cases, respectively." Comment: The study authors who were reviewing the fluoresceins do not appear to have been blinded to treatment group. It is unclear from the paper if the clinic personnel and the personnel involved in the laser interventions were also blinded to the intervention groups, It is likely that blinding was not performed. We contacted the corresponding author of the study to enquire how the blinding of participants and personnel was performed. The response we received was "we performed sham laser to all patients". |
| Blinding of outcome assessment (detection bias) Visual outcome | Low risk | Quote: “The best‐corrected visual acuity was registered at each time, and according to the protocol, the co‐worker who measured visual acuity was unaware of the treatment allocation.” (Materials and Methods section) Comment: There was adequate masking of the assessor of visual acuity. |
| Blinding of outcome assessment (detection bias) Macular oedema outcome | High risk | Quote: “Two authors (M.B.P., S.S.) had interobserver concordance of 96% and 94% of (stereophotographs and fluorescein angiographs) cases, respectively). A third author (G.R.) was consulted in uncertain cases." (Materials and Methods section) Comments: The assessors would be able to tell from the stereophotographs and FFA whether a participant had laser treatment or not. The assessors do not appear to have been blinded to treatment group when assessing stereophotographs and IVFA. Therefore there was inadequate blinding of the assessors of the change in macular oedema. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Nine patients were lost to follow‐up (one patient died 1 month after the inclusion
in the study, and eight patients refused the laser treatment), and thus only 99 patients (59 males and 40 females) were considered for the study." (Results section). Comment: There is significant risk of bias due to incomplete outcome data. 108 participants fulfilled the established criteria for randomisation. 9 were lost to follow‐up but the number of participants lost to follow‐up were not balanced between the intervention groups, with 8 appearing to have been randomised to the laser intervention group. We contacted the corresponding author of the study for further information on which intervention group the participants who were lost to follow‐up belonged to. The response we received was "3 pts in control group and 3 in treatment groups (1 early and 2 delayed treatment)" |
| Selective reporting (reporting bias) | High risk | Quote: "We considered changes of at least 2 lines of Snellen chart." (Methods section) The authors reported in their methods section that changes of at least 2 lines of vision would be considered but they did not present any data on the proportion/number of participants gaining or losing at least 2 lines on the Snellen chart. |
BVOS 1984.
| Methods | Multi‐centre randomised controlled trial. 5 centres in USA 139 randomised (68 controls, 71 treated), 78 (with 3‐year follow‐up) analysed. Study duration/length of follow‐up (planned and actual): 1st July 1977 to 28th Feb 1984 (3‐year outcome reported) |
|
| Participants | Primary diagnosis/diagnoses: BRVO with macular oedema reducing VA to ≤ 20/40 Other inclusion/exclusion criteria: ‐ "Group III (eyes at risk for vision loss from macular oedema)" ‐ If the time of onset of BRVO was uncertain, the presence of segmental intraretinal haemorrhage was accepted as evidence of a recent occlusion. Inclusion: BRVO with macular oedema reducing VA to ≤ 20/40; onset 3 ‐ 18 month (however 22/139 participants had onset > 18 months because those who entered the study in Groups I, II or X were still considered eligible to be included into Group III), refracted VA ≤ 20/40, FFA evidence of macular oedema involving fovea, sufficient clearing of intraretinal haemorrhage to permit evaluation of FFA and safe laser photocoagulation, absence of haemorrhage directly in the fovea, absence of other ocular disease threatening VA. Participants who were using an anticoagulant for the BRVO discontinued its use before entry into the study. Exclusion: people using an anticoagulant (e.g. aspirin) for systemic conditions who could not discontinue medication were excluded. Demographic characteristics:
|
|
| Interventions | Intervention group:
Intervals at which outcomes assessed:
Number of participants included in analysis by group (note if number of participants analysed differs by outcome):
Subgroup analyses (prespecified and post hoc):
Quote: "This study was not designed to study early vs later treatment" (Results section). Comment: Post hoc analysis. |
|
| Outcomes | No primary or secondary outcomes were defined. Outcomes reported in the study were outcomes at the 3‐year follow‐up compared with baseline of:
Intervals at which outcomes assessed: 4 monthly (with FFA). Number of participants included in analysis by group (note if number of participants analysed differs by outcome): 78 (with 3‐year follow‐up at least; 43 treated, 35 control). Subgroup analyses (prespecified and post hoc) Subgroup analyses (pre‐specified and post hoc):
|
|
| Notes | Study data source: 1) Published data only (unpublished not sought) Funding source(s): National Eye Institute, Bethesda, MD Note correspondence with investigators (e.g., unpublished data sought, clarification of methods) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "assignment as either the treatment or control group from a computer‐generated random allocation schedule.” (Subjects and Methods section) |
| Allocation concealment (selection bias) | Low risk | Quote: ".... the participating center contacted the Coordinating Center... If the patient was eligible, the study coordinator issued the patient management assignment as either the treatment or control group from a computer‐generated random allocation schedule." |
| Blinding of participants and personnel (performance bias) Visual outcome | Low risk | Quote: "The protocol specified that the individual who measured visual acuity should be unaware of ("masked" in regard to) the treatment allocation." (Subjects and Methods section). Quote: "Photocoagulation was performed in all centers according to a standard protocol." (Subjects and Methods section: Treatment). Quote: "Stereoscopic color photographs and fluorescein angiograms on all patients were forwarded to the Coordinating Center..... Eligibility and treatment compliance were assessed...." (Subjects and Methods section: Evaluation of fundus photographs and fluorescein angiography). Quote: "Return visits were scheduled for all patients at four‐month intervals." (Subjects and Methods section: Patient follow‐up). Comment: A clear standard protocol for the study was available with clear instructions on treatments, the role of various personnel, evaluations carried out at each visit and follow‐up schedule. The adherence to this protocol was overseen by the Coordinating Center. |
| Blinding of outcome assessment (detection bias) Visual outcome | Low risk | Quote: "... individual who measured VA should be unaware of (“masked” in regard to) the treatment allocation. If the examiner inadvertently became aware of the treatment allocation before VA measurement, this was recorded on the protocol form submitted to the Coordinating Center.” (Subjects and Methods section). Comment: Outcome assessment for VA is masked. Inadvertent awareness of treatment allocation did still occur and it was reported that at the third‐year visit 78% of examinations were obtained by a masked examiner. |
| Blinding of outcome assessment (detection bias) Macular oedema outcome | Low risk | Quote: "Stereoscopic color photographs and fluorescein angiograms on all patients were forwarded to the Coordinating Center... ", "the Coordinating Center evaluated and analysed all data from clinics, including photographic documentation. Coordinating Center statisticians analysed the study data". (Subjects and Methods section). Comments: Photos and FFAs were assessed in a separate centre by individuals not directly involved in the care of the study participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Every eye randomized into the study was included in the following analyses in the group to which the eye was originally assigned." Quote: "Of the 71 eyes assigned to treatment, two were not treated, three were found to be ineligible after randomization, and two received treatment too close to the area by study protocol. Two of the control eyes were treated outside the protocol and three were found not to have met the eligibility criteria". Comment: The analysis was undertaken on an intention‐to‐treat basis. Both controls and treated group had similar number excluded after treatment assignment. 43 treated and 35 controls were included in analysis. Quote: "Seventeen patients were lost to follow‐up...." (Results section) From Table 2: 11 participants (23.9%) in the control group and 6 participants (12.2%) in the treatment group had dropped out or died. Quote: "Kaplan‐Meier statistics, the logrank test and the Cox proportional hazards model were also employed to adjust for varying length of follow‐up, for the effect of covariables, and for loss to follow‐up and death." (Methods section). Comment: As an intention‐to‐treat analysis was performed and statistical methods were used to adjust for the varying length of follow‐up and loss to follow‐up, we consider that the risk of bias was low despite the difference in the proportion of participants lost to follow‐up between the 2 intervention arms. |
| Selective reporting (reporting bias) | Low risk | The BVOS study was designed in part to answer the question "Can photocoagulation improve visual acuity in eyes with macular edema reducing vision to 20/40 or worse?". The report contains all expected outcomes required to answer this question. |
| Other bias | Low risk | Quote: "A number of baseline variables were examined for differences between the treated and control groups. These included duration of branch vein occlusion, medications for hypertension, age, initial visual acuity, diabetes, use of anticoagulants between onset of the branch vein occlusion and entry into the study, sex and study eye." (Results section). Comment: there was no statistically significant difference in the baseline characteristics of the treatment and control groups. Quote: "Patients who entered the study in Groups I, II or X were considered eligible to be entered into Group III at a later date even if the duration of the occlusion to entry into Group III was more than 18 months. Consequently, 22 (16%) had a duration beyond 18 months" (Intro section). Quote: "Treament in Groups I or II, were found to have no significant effect on the change in visual acuity" (Results section) Quote: "The treatment effect was not found to differ in patients with times of onset before and after one year (p=0.92); therefore it should not be inferred that late treatment is not effective. This study was not designed to study early vs later treatment". (Results section) Quote: "This study was not designed to determine how long after branch vein occlusion a patient should be treated. There is no evidence that the benefit of laser photocoagulation varies with the duration of occlusion" (Discussion section) Comment: 22/139 had macular oedema beyond 18 months. However, this probably did not affect the overall result. |
Parodi 2006.
| Methods | Randomised controlled trial pilot 44 participants considered. 36 eyes of 36 participants who met inclusion and exclusion criteria randomised – 17 subthreshold grid laser treatment (SGLT), 19 threshold grid laser treatment (TGLT). Length of follow‐up (planned and actual): 24 months |
|
| Participants | Primary diagnosis/diagnoses: Macular oedema secondary to BRVO Diagnosis: biomicroscopy (superficial and/or deep retinal haemorrhages in a retinal quadrant, congestion and tortuousness of corresponding venous vessels, with presence of exudates), FFA (delayed filling of involved vein) Inclusion criteria: BRVO occurring 3 ‐ 18 months earlier, macular oedema involving fovea, sufficient clearing of retinal haemorrhages, BCVA ≤ 20/40 Exclusion criteria: retinal capillary non‐perfusion ≥ 5 disc diameters on FFA, sensory detachment on OCT, coexistence of any other chorioretinal diseases, glaucoma, media opacities, cataract extraction or lens implantation in last 12 months, previous laser treatment, macular BRVO (better prognosis than BRVO) Demographic characteristics:
|
|
| Interventions | Subthreshold grid laser (17 eyes): infrared diode laser (Iris Medical Oculight); 125 μm laser spot diameter, 0.2 second exposure, 10% duty cycle; laser power determined by single‐test burn delivered in a macular area involved by oedema, which brought about a medium white burn in a continuous wave. Threshold grid laser (19 eyes): krypton laser (Novus Omni); 100 μm spot diameter, 0.1 seconds; medium white burn Supplementary treatment in both groups if unchanged or increased foveal thickness after 12 months. |
|
| Outcomes | Primary outcome: decrease in OCT mean foveal thickness Secondary outcomes:
Additional outcome: number of participants with retreatment in each group Intervals at which outcomes assessed: complete ophthalmological examination, including determination of BCVA on standard Early Treatment Diabetic Retinopathy Study charts, OCT, and fluorescein angiography at 6‐month intervals with a planned follow‐up of 24 months Number of participants included in analysis by group: 17 SGLT, 19 TGLT Subgroup analyses (prespecified and post hoc): None |
|
| Notes | Study data source: 1) Published data only (unpublished not sought) Funding source(s): None Note correspondence with investigators (e.g., unpublished data sought, clarification of methods): None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were assigned randomly to either SGLT or TGLT following a computer‐generated list using a block randomization scheme.” (Materials and Methods section) |
| Allocation concealment (selection bias) | Unclear risk | No information provided in paper. |
| Blinding of participants and personnel (performance bias) Visual outcome | Low risk | Quote: "At each examination, an independent examiner refracted the study eye and performed the OCT scans.” (Materials and Methods section) Quote: "Supplementary treatment with the same protocol as the primary treatment was planned in those eyes showing unchanged or increased FT on OCT after 12 months." Comments: Retreatment criteria based on objective OCT data which was performed by an independent examiner. |
| Blinding of outcome assessment (detection bias) Visual outcome | Low risk | Quote: "At each examination, an independent examiner refracted the study eye and performed the OCT scans.” (Materials and Methods section) |
| Blinding of outcome assessment (detection bias) Macular oedema outcome | Low risk | Quote: "At each examination, an independent examiner refracted the study eye and performed the OCT scans.” (Materials and Methods section) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 36 randomised participants completed 24 months follow‐up |
| Selective reporting (reporting bias) | Low risk | Quote: “The primary efficacy outcome was the decrease in mean FT on OCT. Secondary efficacy outcomes included changes of the TMV over the follow‐up, the proportion of eyes that gained at least 10 letters (approximately 2 lines of VA gain) at the 12‐ and 24‐month examinations, and the timing of macular edema resolution.” (Methods section) Comment: The study protocol was not available. However the study reported on all the outcomes specified in their methods section and they report on the outcomes that would be expected of such a study. |
| Other bias | Unclear risk | Quote: "The 2 groups turned out to be similar with regard to mean age, gender distribution, and systemic disorders." From Table 1: hypertension, cardiovascular disease, diabetes, glaucoma. (Results section) Comment: The baseline characteristics appeared to be equal in both groups. |
Russo 2009.
| Methods | Randomised controlled trial. 30 eyes of 30 participants randomised ‐ randomisation based on retina clinic chart number (participants with even numbers were assigned to Bevacizumab and participants with odd numbers were assigned to laser (chart numbers were not assigned until the day of treatment). Length of follow‐up: 12 months (planned and actual) |
|
| Participants | Primary diagnosis/diagnoses: macular oedema secondary to perfused BRVO Inclusion criteria: perfused BRVO ≥ 3 months, macular leakage on FFA, logMAR ETDRS BCVA ≤ 0.4, central macular thickness ≥ 300 μm on Stratus III OCT Exclusion criteria: diabetic retinopathy, AMD, previous cataract surgery Demographic characteristics:
|
|
| Interventions | Grid laser (15 participants): Argon green laser, 100 μm spots, 100 ms, ½ ‐ 1 burn width apart, covering leaking area outside capillary‐free zone as shown by FFA. Retreatment criteria: repeat laser if no VA improvement and oedema still present after 3 months as detected by OCT‐3. Bevacizumab intravitreal injection (15 participants): 1.25 mg (0.05 ml) of bevacizumab via the pars plana. Retreatment criteria: reinject at follow‐up until macular oedema resolved as detected by OCT‐3. |
|
| Outcomes | No primary or secondary outcomes were defined. Outcome measures:
Intervals at which outcomes assessed: baseline (just before laser or Avastin), then 1, 3, 6 and 12 months after treatment Number of participants included in analysis by group – 15 in each group Subgroup analyses (prespecified and post hoc) – None |
|
| Notes | Study data source: 1) Published data only (unpublished not sought) Funding source(s) ‐ None Note correspondence with investigators (e.g., unpublished data sought, clarification of methods) ‐ none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: “Eyes were assigned to either grid laser photocoagulation (GLP) or to intravitreal bevacizumab (IB) based on retina clinic chart number (patients with even numbers were assigned to IB treatment and patients with odd numbers were assigned to GLP treatment), chart numbers were not assigned until day of treatment.” (Methods section) |
| Allocation concealment (selection bias) | High risk | Comment: As the patients were assigned intervention on the basis of odd and even chart numbers, the sequence was predictable and upcoming allocations from those involved in enrolment into the trial could be biased. |
| Blinding of participants and personnel (performance bias) Visual outcome | High risk | Quote: “Eyes were assigned to either grid laser photocoagulation (GLP) or to intravitreal bevacizumab (IB) based on retina clinic chart number..." (Methods section) Comment: It would be clear to participants which group they had been randomised to. Personnel would also be able to tell from chart numbers which group the participants had been randomised to. |
| Blinding of outcome assessment (detection bias) Visual outcome | High risk | Quote: "...patients with even numbers were assigned to IB treatment and patients with odd numbers were assigned to GLP treatment..." (Methods section) Comment: Assessors can easily tell from the chart numbers which group the patient had been randomised to. |
| Blinding of outcome assessment (detection bias) Macular oedema outcome | Unclear risk | Quote: "...patients with even numbers were assigned to IB treatment and patients with odd numbers were assigned to GLP treatment..." (Methods section) Comment: Assessors could easily tell from the chart numbers which group the patient had been randomised to. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “All 30 patients completed all follow‐up visits.” (Results section) Comments: All patients completed follow‐up |
| Selective reporting (reporting bias) | High risk | There was no study protocol available and there were no primary or secondary outcomes specified in their Methods section. While the number of participants gaining 3 or more ETDRS lines of visual acuity were reported, they did not report on the number of participants who lost 3 or more ETDRS lines of visual acuity. |
SCORE 2009.
| Methods | Multicentre randomised controlled trial 411 participants were randomised using random sizes block permutation Study duration/length of follow‐up: 12 ‐ 36 months (planned and actual); actual length of follow‐up depended on the randomisation date relative to the common close‐out date of February 28, 2009. The prespecified primary efficacy evaluation was performed at month 12. |
|
| Participants | 411 participants with macular oedema secondary to BRVO, includes hemi‐retinal vein occlusion. 1 eye per participant was enrolled in the trial. Inclusion criteria: BCVA ≤ 73 ETDRS letters (Snellen ≤ 20/40) and ≥ 19 ETDRS letters (Snellen ≥ 20/400); macular oedema secondary to BRVO; mean central subretinal thickness of 2 OCT fast macular scans ≥ 250 μm; media clarity, pupillary dilation, subject co‐operation sufficient for fundus photos. Exclusion criteria: Macular oedema from other cause; ocular condition such that VA would not improve from resolution of oedema (e.g. foveal atrophy); substantial cataract estimated reduced VA by ≥ 3 lines; prior intravitreal corticosteroids at any time or peribulbar steroid injection within 6 months prior to randomisation; history of focal/grid macular laser within 15 weeks (3.5 months) or PRP within 4 months prior to randomisation or anticipated need for PRP within 4 months following randomisation; prior pars plana vitrectomy; major ocular surgery (including cataract extraction) within prior 6 months or anticipated within the next 6 months following randomisation; YAG capsulotomy within 2 months prior to randomisation; IOP ≥ 25 mmHg, OAG (POAG or other causes of OAG), or steroid‐induced IOP elevation requiring IOP‐lowering treatment or PXF; aphakia. Includes participants with prior grid/sector/PRP, dense macular haemorrhage Demographic characteristics:
|
|
| Interventions | 411 participants were randomised into 3 groups: 1) Standard care (n = 137)
2) 1 mg Triamcinolone (preservative‐free, single‐use, Trivaris, 1‐mg) (n = 136) 3) 4 mg Triamcinolone (preservative‐free, single‐use, Trivaris, 4‐mg) (n = 138) Retreatment criteria: At 4 months (min 105 days) according to original treatment assigned at randomisation. Defer if:
Alternative treatment criteria: Loss of BCVA ≥ 15 letter score at 2 consecutive 4‐month interval visits, due to persistent or recurrent macular oedema documented on OCT. |
|
| Outcomes |
Primary outcome: Percentage of participants with a gain in visual acuity letter score of ≥ 15 letters at month 12 (measured 2 months before target date to 3 months after target date) Secondary outcomes: Visual outcomes: change in visual acuity score at month 12; mean change from baseline in mean VA score, percentage of participants losing ≥ 15 letters, percentage of participants gaining ≥ 15 letters Imaging outcome: OCT‐measured centre point thickness decreased from baseline throughout follow‐up Safety outcome: elevated IOP, cataract, infectious endophthalmitis, minor ocular adverse events, systemic adverse events Intervals at which outcomes assessed: Baseline, every 4 months through 36 months Subgroup analyses (prespecified and post hoc): 12‐month visual acuity outcomes were analysed for prespecified baseline subgroups:
|
|
| Notes | Study data source: 1) Published data only (unpublished not sought) Funding source(s): The study was supported by the National Eye Institute (National Institutes of Health, Department of Health and Human Services) grants 5U10EY014351, 5U10EY014352, and 5U10EY014404; and in part by Allergan Inc. Note correspondence with investigators (e.g., unpublished data sought, clarification of methods): None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Within baseline VA strata of good/moderate/poor, participants were randomly assigned centrally through a Web‐based data entry system maintained at the Data Coordinating Center (The EMMES Corporation, Rockville, Maryland), with equal probability to receive standard care/1mg triamcinolone/4mg triamcinolone using a permuted blocks design with random block sizes." (Methods: Randomization section) Quote: “The random sequence of treatment assignments was constructed before trial onset, using a separate, permuted block design with random block sizes of (3, 6) within each stratum” (SCORE report 3) |
| Allocation concealment (selection bias) | Low risk | Quote: “random assigned centrally through a Web‐based data entry system maintained at the Data Coordinating Center... using a permuted blocks design with random block sizes” (Methods: Randomization section) |
| Blinding of participants and personnel (performance bias) Visual outcome | Low risk | Quote: "Study visits were planned for every 4 months through 36 months." (Methods section: visit schedule) Quote: “Participants and physicians were masked to the intravitreal triamcinolone dose (1 mg vs 4 mg) but not to the treatment assignment of standard care vs intravitreal triamcinolone.” (Methods section) Quote: “All participants who received intravitreal triamcinolone had safety visits at 4 days (±3 days) and at 4 weeks (±1 week) following each injection;” Quote: “Participating study personnel such as physician‐investigators and study coordinators, were certified by the data coordinating center". Quote: "Photographers and technicians who took the fundus photographs and OCT images for this study were certified by the reading center.” (Methods section: Visit schedule) Comments: Although, there was no masking to intervention group, there was a clear visit schedule where the examinations and investigations that were to be performed at each visit were predetermined. The retreatment protocol was based on objective outcome data obtained by masked outcome assessors who were certified and who were following a standardised protocol. |
| Blinding of outcome assessment (detection bias) Visual outcome | Low risk | Quote: “At baseline and at each follow‐up visit, BCVA letter score was measured at 3m by a masked, certified tester using the E‐ETDRS method.” (Methods section: Visit schedule) Quote: “All [FFA] images were sent to the reading center (University of Wisconsin Fundus Photograph Reading Center, Madison, Wisconsin) for analysis, where they were graded in a masked fashion.” Quote: “The reading center graders, without knowledge of treatment assignment or participant clinical data, followed a standardised protocol to grade the area of macular oedema and retinal haemorrhage using stereoscopic fundus photographs.” (Methods section: Assessment of macular oedema section) Comment: The masking of the outcome assessors to the intervention group was of very high quality. |
| Blinding of outcome assessment (detection bias) Macular oedema outcome | Low risk | Quote: “All [FFA] images were sent to the reading center (University of Wisconsin Fundus Photograph Reading Center, Madison, Wisconsin) for analysis, where they were graded in a masked fashion.” Quote: “The reading center graders, without knowledge of treatment assignment or participant clinical data, followed a standardised protocol to grade the area of macular oedema and retinal haemorrhage using stereoscopic fundus photographs.” (Methods section: Assessment of macular oedema section) Comment: The masking of the outcome assessors to the intervention group was of very high quality. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The month 12 primary outcome visit was completed by 88%, 89%, and 91% of participants in the standard care, 1‐mg, and 4‐mg groups, respectively." (Results section: Follow‐up) Quote: “Additional analyses were performed to assess the consistency of the primary efficacy results and included a sensitivity analysis in which outcomes were assigned to participants without month 12 data so as to explore extreme possible estimates of treatment effects". (Methods section: Statistical analysis) Quote: "A per protocol analysis was also conducted, including only study eyes that have 12‐month VA data and excluded participants who, before 12 months, received an alternative treatment (i.e. treatment cross‐overs) or nonprotocol treatments; who did not meet the eligibility criteria; or who did not receive the treatment assigned at randomization.” (Methods section: Statistical analysis) Quote: "The sensitivity of the primary efficacy conclusions to missing data was investigated, and no pattern of outcomes among missing participants overturns the conclusion that 1 mg or 4 mg of intravitreal triamcinolone is not superior to standard care at 12 months. Furthermore, the per‐protocol analysis gave results that were qualitatively similar to the primary analysis." (Results: Visual acuity outcomes) Comment: Sensitivity analyses performed for primary outcomes accounting for participants without 12 month data were performed and this did not impact on the conclusions drawn on the primary outcomes of the trial. |
| Selective reporting (reporting bias) | Low risk | Comment: A published study protocol is available as the SCORE Study Report 3. Quote: “Few treatment protocol deviations prior to 12 months... 1) an anti‐VEGF drug in 1 participant in the 4mg triamcinolone group and 1 participant in the standard care group; 2) non study formulation triamcinolone in 1 participant in the 4mg triamcinolone group and 3 participants in the standard care group. These participants were included in the primary analyses but not in the per‐protocol analyses.” |
| Other bias | Unclear risk | Quote: "Lower proportion in laser group (13%) compared with the triamcinolone groups (28% and 32%) had cortical opacities (P = 0.0025; P* = 0.22), a fact which we attribute to type I error." Comment: There appears to be a baseline imbalance. Significance of this imbalance is unknown. Quote: “eyes could receive the alternate treatment when there was a loss from baseline in best‐corrected visual acuity letter score of 15 or more that was present at 2 consecutive 4‐month interval visits........ Study eyes that received an alternate treatment were analysed in the arm to which they were randomised.” (Methods section: Alternative treatment criteria section) Quote: "Cataract surgery was more frequent between months 12 and 24 in the 4‐mg group, with 35 study eyes receiving such surgery, compared with 8 in the 1‐mg study group and 6 in the standard care group (log‐rank test between 1 and 2 years, standard care vs 1 mg of triamcinolone, P=.59; standard care vs 4 mg of triamcinolone, P.001; and 1 mg vs 4 mg of triamcinolone, P.001)." (Results section (Safety outcomes)) Comment: The higher rate of cataract surgery in the triamcinolone group would bias the results in favour of triamcinolone However, an analysis performed by the authors of the SCORE study limited to eyes that were pseudophakic at baseline also demonstrated no statistically significant difference among the treatment groups with respect to change in visual acuity over time. |
AMD: age‐related macular degeneration BCVA: best‐corrected visual acuity BRVO: branch retinal vein occlusion CMT: central macular thickness ETDRS: Early Treatment Diabetic Retinopathy Study FFA: fundus fluorescein angiography FT: mean foveal thickness IOP: intra‐ocular pressure IVFA: intravenous fluorescein angiography OAG: open‐angle glaucoma OCT: optical coherence tomography OPD: outpatient department POAG: primary open‐angle glaucoma PRP: pan‐retinal photocoagulation PXF: pseudoexfoliation7 Rx: treatment TMV: total macular volume VA: visual acuity VEGF: vascular endothelial growth factor YAG: Yttrium Aluminum Garnet Laser
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avitabile 2005 | Prospective, randomised, interventional, parallel, three‐arm clinical trial to evaluate the outcome of cystoid macular oedema treated with intravitreal injections of triamcinolone acetonide, macular grid laser photocoagulation or both triamcinolone injections combined with macular grid laser photocoagulation. This study was excluded because only 6 participants had BRVO (2 in each of the 3 arms) and only 9 months of follow‐up data were available. |
| Balashevich 1986 | Not a RCT. [In Russian]. |
| Bouzikas 1989 | Not a RCT |
| BVOS 1986 | Scatter laser for prevention of neovascularisation and vitreous haemorrhage |
| Campbell 1973 | Not a RCT |
| Chew 2009 | Commentary. Not a RCT. |
| Donati 2012 | RCT of macular grid combined with intravitreal bevacizumab versus intravitreal bevacizumab alone. This did not meet our protocol criteria for inclusion. |
| Erdol 2000 | A study examining the effect of arterial crimping with laser. Both arms of study received grid laser treatment. |
| Finkelstein 1986 | Review paper. |
| Goyal 1998 | Study looking at sector photocoagulation within 3 months of the onset of branch retinal vein occlusion. (AAO Abstract). |
| Hayreh 1993 | Scatter photocoagulation. Not macular grid laser. |
| Klemen 1980 | Not a RCT |
| Lang 1993 | Not a RCT |
| Manaviat 2008 | Not a RCT |
| Mason 2004 | Not a RCT of macular grid laser but an RCT of intravitreal triamcinolone versus focal laser versus observation. (AAO Abstract). |
| Park 2002 | Not a RCT. Compared outcomes of laser in people with and without posterior vitreous detachment. (ARVO Abstract). |
| Parodi 2008 | RCT examining intravitreal triamcinolone versus sham injection. Both arms received subthreshold grid laser treatment. |
| Qiao 2009 | RCT of laser versus Xue Shuan Tong tablets. This study was excluded because the laser treatment was not standard grid laser photocoagulation treatment. |
| Saxena 1997 | Review |
| Vidic 1981 | Not a RCT |
| Zhang 2009 | Not a RCT |
AAO: American Academy of Ophthalmology ARVO: Association for Research in Vision and Ophthalmology RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT00562406.
| Trial name or title | Ranibizumab for branch retinal vein occlusion associated macular edema study (RABAMES) |
| Methods | Study type: Interventional Study design:
|
| Participants | Enrolment: 30 participants
|
| Interventions | 3 intervention arms: 1. Laser photocoagulation to the retina at the area of oedema 2. Intravitreal injection of 0.5 mg ranibizumab after randomisation at week 4 and week 8 3. Laser photocoagulation to the retina at the area of oedema and intravitreal injection of ranibizumab at week 4 and week 8 |
| Outcomes |
Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | November 2007 |
| Contact information | Principal Investigator: Lars‐Olof Hattenbach, MD, Privatdozent. Department of Ophthalmology, Ludwigshafen Hospital. |
| Notes | Primary completion date: September 2010 (Final data collection date for primary outcome measure) Sponsors and Collaborators: Klinikum Ludwigshafen, Norvartis Pharma, Nuremberg, Germany Co‐ordination centre for clinical studies, Mainz, Germany |
NCT00642226.
| Trial name or title | A randomized study comparing combined vitrectomy and triamcinolone to laser grid in patients with macular edema secondary to branch retinal vein occlusion (BRVO) |
| Methods | Study type: Interventional Study design:
|
| Participants | Estimated enfoldment: 34 participants Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | 2 intervention arms: 1. ETDRS grid laser photocoagulation of macular oedema 2. Pars plana vitrectomy with induction of posterior vitreous detachment followed by an injection of 20 mg purified triamcinolone acetate |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | November 2006 |
| Contact information | Study director: Johan Seland, PhD, Helse Stavanger HF |
| Notes | Estimated primary completion date: November 2013 (Final data collection date for primary outcome measure) Location: Stavanger University Hospital, Department of Ophthalmology, Stavanger, Norway, 4018 Sponsors and Collaborators: Helse Stavanger HF |
Differences between protocol and review
In Battaglia Parodi 1999, the proportion of participants gaining or losing three or more lines of vision was not reported. For BVOS 1984, the outcome measure used in the study was the percentage of participants gaining or losing two or more lines on the Diabetic Retinopathy Study charts for at least two consecutive visits, while our protocol had specified the proportion of participants gaining or losing at least 15 letters on the Early Treatment Diabetic Retinopathy Study chart.
We included quasi‐RCTs as there were not many RCTs.
Contributions of authors
Conceiving the review: FCL Designing the review: FCL Co‐ordinating the review: FCL Data collection for the review ‐ Designing search strategies: Cochrane Eyes and Vision Group Editorial Base ‐ Undertaking manual searches: Cochrane Eyes and Vision Group ‐ Screening search results: FCL, SNC, RML ‐ Organising retrieval of papers: FCL ‐ Screening retrieved papers against inclusion criteria: FCL, SNC, RML ‐ Appraising quality of papers: FCL, SNC, RML ‐ Extracting data from papers: FCL, SNC, RML ‐ Writing to authors of papers for additional information: FCL ‐ Providing additional data about papers: ‐ Obtaining and screening data on unpublished studies: Data management for the review ‐ Entering data into RevMan: FCL, RML, SNC Analysis of data: FCL, SNC, RML Interpretation of data ‐ Providing a methodological perspective: FCL, RML, SNC ‐ Providing a clinical perspective: FCL, SNC, RML ‐ Providing a policy perspective: ‐ Providing a consumer perspective: Writing the review: FCL, SNC, RML Providing general advice on the review:
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
Declarations of interest
FCL: none known RML: none known SNC: none known
New
References
References to studies included in this review
Battaglia Parodi 1999 {published data only}
- Battaglia Parodi M, Saviano S, Ravalico G. Grid laser treatment in macular branch retinal vein occlusion. Graefe's Archive for Clinical and Experimental Ophthalmology 1999;237(12):1024‐7. [DOI] [PubMed] [Google Scholar]
BVOS 1984 {published data only}
- Anonymous. Argon laser photocoagulation for macular edema in branch vein occlusion. The Branch Vein Occlusion Study Group. American Journal of Ophthalmology 1984;98(3):271‐82. [DOI] [PubMed] [Google Scholar]
Parodi 2006 {published data only}
- Parodi MB, Spasse S, Iacono P, Stefano G, Canziani T, Ravalico G. Subthreshold grid laser treatment of macular edema secondary to branch retinal vein occlusion with micropulse infrared (810 nanometer) diode laser. Ophthalmology 2006;113(12):2237‐42. [DOI] [PubMed] [Google Scholar]
Russo 2009 {published data only}
- Russo V, Barone A, Conte E, Prascina F, Stella A, Nocci ND. Bevacizumab compared with macular laser grid photocoagulation for cystoid macular edema in branch retinal vein occlusion. Retina 2009;29(4):511‐5. [DOI] [PubMed] [Google Scholar]
SCORE 2009 {published data only}
- Ip MS, Oden NL, Scott IU, VanVeldhuisen PC, Blodi BA, Figueroa M, et al. SCORE Study report 3: study design and baseline characteristics. Ophthalmology 2009;116(9):1770‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott IU, Ip MS, Veldhuisen PC, Oden NL, Blodi BA, Fisher M, et al. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular Edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) study report 6. Archives of Ophthalmology 2009;127(9):1115‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Avitabile 2005 {published data only}
- Avitabile T, Longo A, Reibaldi A. Intravitreal triamcinolone compared with macular laser grid photocoagulation for the treatment of cystoid macular edema. American Journal of Ophthalmology 2005;140(4):695‐702. [DOI] [PubMed] [Google Scholar]
Balashevich 1986 {published data only}
- Balashevich LI, Boĭka EV. Comparative evaluation of the late outcomes in retinal vein occlusions after conservative and laser treatment. Vestnik Oftalmologii 1986;102(3):59‐63. [PubMed] [Google Scholar]
Bouzikas 1989 {published data only}
- Bouzikas SP, Tsirou M, Economidis I, Pollalis SP. Argon‐green laser photocoagulation in the treatment of cystoid macular edema secondary to branch vein occlusion. Journal Francais d'Ophtalmologie 1989;12(12):909‐14. [PubMed] [Google Scholar]
BVOS 1986 {published data only}
- Anonymous. Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. A randomized clinical trial. Branch Vein Occlusion Study Group. Archives of Ophthalmology 1986;104(1):34‐41. [DOI] [PubMed] [Google Scholar]
Campbell 1973 {published data only}
- Campbell CJ, Wise GN. Photocoagulation therapy of branch vein obstructions. American Journal of Ophthalmology 1973;75(1):28‐31. [DOI] [PubMed] [Google Scholar]
Chew 2009 {published data only}
- Chew EY. Laser photocoagulation and intravitreal injection of triamcinolone for retinal vein occlusion. JAMA 2009;302(15):1693‐5. [DOI] [PubMed] [Google Scholar]
Donati 2012 {published data only}
- Donati S, Barosi P, Bianchi M, Al Oum M, Azzolini C. Combined intravitreal bevacizumab and grid laser photocoagulation for macular edema secondary to branch retinal vein occlusion. European Journal of Ophthalmology 2012;22(12):607‐14. [DOI] [PubMed] [Google Scholar]
Erdol 2000 {published data only}
- Erdol H, Akyol N. Arterial crimping in branch retinal vein occlusion with macular edema. Acta Ophthalmologica Scandinavica 2000;78(4):456‐9. [DOI] [PubMed] [Google Scholar]
Finkelstein 1986 {published data only}
- Finkelstein D. Argon laser photocoagulation for macular edema in branch vein occlusion. Ophthalmology 1986;93(7):975‐77. [DOI] [PubMed] [Google Scholar]
Goyal 1998 {published data only}
- Goyal M, Tweari HK, Kumar A, Honavar SG. Photocoagulation in branch retinal vein occlusion (BRVO): comparative evaluation of laser wavelengths. American Academy of Ophthalmology. 1998:139.
Hayreh 1993 {published data only}
- Hayreh SS, Rubenstein L, Podhajsky P. Argon laser scatter photocoagulation in treatment of branch retinal vein occlusion. A prospective clinical trial. Ophthalmologica 1993;206(1):1‐14. [DOI] [PubMed] [Google Scholar]
Klemen 1980 {published data only}
- Klemen UM, Freyler H, Gnad HD, Preskavec FH. Indications for laser coagulation in cases of branch vein occlusion [author's translation]. Klinische Monatsblätter für Augenheilkunde 1980;177(6):715‐8. [PubMed] [Google Scholar]
Lang 1993 {published data only}
- Lang GE, Händel A. Results of laser coagulation of retinal branch vein occlusions. Klinische Monatsblätter für Augenheilkunde 1993;203(3):180‐8. [DOI] [PubMed] [Google Scholar]
Manaviat 2008 {published data only}
- Manaviat MR, Rashidi M. Intravitreal triamcinolone acetonide for macular edema in retinal vein occlusion. International Journal of Ophthalmology 2008;8(2):230‐3. [Google Scholar]
Mason 2004 {published data only}
- Mason JO, Roberts DR, Emond TL, White MF, Feist RM, Thomley ML, et al. Intravitreal triamcinolone acetonide for macular edema associated with branch retinal vein occlusion. American Academy of Ophthalmology. 2004:226.
Park 2002 {published data only}
- Park YH, Cha SC, Song SJ, Kim YT, Lee DS. The results of laser photocoagulation in case of branch retinal vein oclusion with or without posterior vitreous detachment. Investigative Ophthalmology and Visual Science 2002;43(Suppl 1):500. [Google Scholar]
Parodi 2008 {published data only}
- Parodi MB, Iacono P, Ravalico G. Intravitreal triamcinolone acetonide combined with subthreshold grid laser treatment for macular oedema in branch retinal vein occlusion: a pilot study. British Journal of Ophthalmology 2008;92(8):1046‐50. [DOI] [PubMed] [Google Scholar]
Qiao 2009 {published data only}
- Qiao LF, Lei CT, Fan YC. Clinical research of 810nm laser for treating branch retinal vein occlusion. International Journal of Ophthalmology 2009;9(12):2341‐3. [Google Scholar]
Saxena 1997 {published data only}
- Saxena S. Laser photocoagulation in retinal vein occlusion: branch vein occlusion study and central vein occlusion study recommendations. Indian Journal of Ophthalmology 1997;45(2):125‐8. [PubMed] [Google Scholar]
Vidic 1981 {published data only}
- VIdic B, Hiti H, Schuhmann G, Hubel K. Development of retinal vein thrombosis after argon laser photocoagulation. Klinische Monatsblätter für Augenheilkunde 1981;179(6):415‐7. [DOI] [PubMed] [Google Scholar]
Zhang 2009 {published data only}
- Zhang DW, Kong FH, Zhang R, Hua W. Clinical observation on treatment of fundus laser combined with compound anisodine for retinal vein occlusion. International Journal of Ophthalmology 2009;9(8):1592‐3. [Google Scholar]
References to ongoing studies
NCT00562406 {unpublished data only}
- NCT00562406. Ranibizumab for branch retinal vein occlusion associated macular edema study (RABAMES). clinicaltrials.gov/show/NCT00562406 (accessed 13 April 2015).
NCT00642226 {unpublished data only}
- NCT00642226. Combined vitrectomy and triamcinolone in macular edema secondary to branch retinal vein occlusion (BRVO). clinicaltrials.gov/show/NCT00642226 (accessed 13 April 2015).
Additional references
Alder 1987
- Alder VA, Cringle SJ, Brown M. The effect of regional photocoagulation on vitreal oxygen tension. Investigative Ophthalmology and Visual Science 1987;28(7):1078‐85. [PubMed] [Google Scholar]
Arnarsson 2000
- Arnarsson A, Stefansson E. Laser treatment and the mechanism of edema reduction in branch retinal vein occlusion. Investigative Ophthalmology and Visual Science 2000;41(3):877‐9. [PubMed] [Google Scholar]
Attariwala 1997
- Attariwala R, Jensen PS, Glucksberg MR. The effect of acute experimental retinal vein occlusion on cat retinal vein pressures. Investigative Ophthalmology and Visual Science 1997;38(13):2742‐9. [PubMed] [Google Scholar]
Battaglia 1999
- Battaglia Parodi M, Saviano S, Bergamini L, Gavalico G. Grid laser treatment of macular edema in macular branch retinal vein occlusion. Documenta Ophthalmologica 1999;97(3‐4):427‐31. [DOI] [PubMed] [Google Scholar]
Campochiaro 2010
- Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, Murahashi WY, Rubio RG. Ranibizumab for macular edema following branch retinal vein occlusion: six‐month primary end point results of a phase III study. Ophthalmology 2010;117(6):1102‐1112. [DOI] [PubMed] [Google Scholar]
David 1988
- David R, Zangwill L, Badarna M, Yassur Y. Epidemiology of retinal vein occlusion and its association with glaucoma and increased intraocular pressure. Ophthalmologica 1988;197(2):69‐74. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Glanville 2014
- Glanville J, Patterson J, McCool R, Ferreira A, Gairy K, Pearce I. Efficacy and safety of widely used treatments for macular oedema secondary to retinal vein occlusion: a systematic review. BMC Ophthalmology 2014;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gottfredsdóttir 1993
- Gottfredsdóttir MS, Stefánsson E, Jónasson F, Gíslason I. Retinal vasoconstriction after laser treatment for diabetic macular edema. American Journal of Ophthalmology 1993;115(1):64‐7. [DOI] [PubMed] [Google Scholar]
Gutman 1974
- Gutman FA, Zegarra H. The natural course of temporal retinal branch vein occlusion. Transactions of the American Academy of Ophthalmology and Otolaryngology 1974;78(2):OP178‐92. [PubMed] [Google Scholar]
Gutman 1977
- Gutman FA. Macular edema in branch retinal vein occlusion: prognosis and management. Transactions of the American Academy of Ophthalmology and Otolaryngology 1977;83(3 Pt 1):488‐95. [PubMed] [Google Scholar]
Hamilton 1979
- Hamilton AM, Kohner EM, Rosen D, Bird AC, Dollery CT. Experimental retinal branch vein occlusion in rhesus monkey, I: clinical appearances. British Journal of Ophthalmology 1979;63(6):377‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayreh 1983
- Hayreh SS, Rojas P, Podhajsky P, Montague P, Woolson RF. Ocular neovascularization with retinal vascular occlusion‐III. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology 1983;90(5):488‐506. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Klein 2000
- Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Transactions of the American Ophthalmological Society 2000;98:133‐41. [PMC free article] [PubMed] [Google Scholar]
Kohner 1970
- Kohner EM, Dollery CT, Shakib M, Henkind P, Paterson JW, Oliveira LN, et al. Experimental retinal branch vein occlusion. American Journal of Ophthalmology 1970;69(5):778‐825. [DOI] [PubMed] [Google Scholar]
Kristinsson 1997
- Kristinsson JK, Gottfredsdóttir MS, Stefánsson E. Retinal vessel dilatation and elongation precedes diabetic macular oedema. British Journal of Ophthalmology 1997;81(4):274‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kylstra 1986
- Kylstra JA, Wierzbicki T, Wolbarsht ML, Landers MB 3rd, Stefánsson E. The relationship between retinal vessel tortuosity, diameter, and transmural pressure. Graefe's Archive for Clinical and Experimental Ophthalmology 1986;224(5):477‐80. [DOI] [PubMed] [Google Scholar]
McIntosh 2007
- McIntosh RL, Mohamed Q, Saw SM, Wong TY. Interventions for branch retinal vein occlusion: an evidence‐based systematic review. Ophthalmology 2007;114(5):835‐46. [DOI] [PubMed] [Google Scholar]
Michels 1974
- Michels RG, Gass JD. The natural course of retinal branch vein obstruction. Transactions of the American Academy of Ophthalmology and Otolaryngology 1974;78(2):OP166‐77. [PubMed] [Google Scholar]
Mitchell 1996
- Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study. Archives of Ophthalmology 1996;114(10):1243‐7. [DOI] [PubMed] [Google Scholar]
Molnar 1985
- Molnar I, Poitry S, Tsacopoulos M, Gilody N, Leuenberger PM. Effect of laser photocoagulation on oxygenation of the retina in miniature pigs. Investigative Ophthalmology and Visual Science 1985;26(10):1410‐4. [PubMed] [Google Scholar]
Rehak 2008
- Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Current Eye Research 2008;33(2):111‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Stefansson 1981
- Stefansson E, Landers MB 3rd, Wolbarsht ML. Increased retinal oxygen supply following pan‐retinal photocoagulation, vitrectomy and lensectomy. Transactions of the American Ophthalmological Society 1981;79:307‐34. [PMC free article] [PubMed] [Google Scholar]
Stefánsson 1992
- Stefánsson E, Machemer R, Juan E, McCuen BW 2nd, Peterson J. Retinal oxygenation and laser treatment in patients with diabetic retinopathy. American Journal of Ophthalmology 1992;113(1):36‐8. [DOI] [PubMed] [Google Scholar]
Wilson 1988
- Wilson J, Finkelstein D, Quigley HA, Green R. Macular grid photocoagulation. An experimental study on the primate retina. Archives of Ophthalmology 1988;106(1):100‐5. [DOI] [PubMed] [Google Scholar]
Zhao 1993
- Zhao J, Sastry SM, Sperduto RD, Chew EY, Remaley NA. Arteriovenous crossing patterns in branch retinal vein occlusion. The Eye Disease Case‐Control Study Group. Ophthalmology 1993;100(3):423‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lam 2010
- Lam FC, Al Moujahed AM. Macular grid laser photocoagulation for branch retinal vein occlusion. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008732] [DOI] [PMC free article] [PubMed] [Google Scholar]


