Abstract
In the fission yeast Schizosaccharomyces pombe, the onset of sexual development is controlled mainly by two external signals, nutrient starvation and mating pheromone availability. We have isolated a novel gene named rcd1+ as a key factor required for nitrogen starvation-induced sexual development. rcd1+ encodes a 283-amino-acid protein with no particular motifs. However, genes highly homologous to rcd1+ (encoding amino acids with >70% identity) are present at least in budding yeasts, plants, nematodes, and humans. Cells with rcd1+ deleted are sterile if sexual development is induced by nitrogen starvation but fertile if it is induced by glucose starvation. This results largely from a defect in nitrogen starvation-invoked induction of ste11+, a key transcriptional factor gene required for the onset of sexual development. The striking conservation of the gene throughout eukaryotes may suggest the presence of an evolutionarily conserved differentiation controlling system.
When starved for nutrient, the fission yeast Schizosaccharomyces pombe arrests in G1, but if mating partners are available, it resumes sexual development. The cells that have committed to sexual development subsequently perform conjugation, meiosis, and sporulation. The commitment to this alternative pathway requires the action of the Ste11 transcriptional factor. This factor is essential for the activation of many genes needed for the initiation and progression of conjugation and meiosis. Among them are mating-type genes, ste genes (including ste11+), mei2+, and rep1+ (11, 19, 24, 33, 34, 38, 40). Therefore, it is conceivable that the ste11+ gene and its product serve as key targets for the regulation of the onset of sexual development.
Nitrogen starvation and carbon starvation are two major nutrient exhaustion signals that trigger sexual development. The cyclic AMP (cAMP)-Pka1 pathway mediates mostly a carbon source signal and partly a nitrogen source signal and negatively regulates ste11+ expression (2, 10, 15, 16, 33). The pac2+ gene, whose physiological role is unknown, also represses ste11+ expression (20). In addition, a stress signal transduced by the Wis1-Phh1/Sty1/Spc1 mitogen-activated protein kinase kinase–mitogen-activated protein kinase cascade is essential for ste11+ induction in response to nutrient starvation (18), which is mediated by the Atf1/Gad7 transcriptional factor (17, 35). Despite extensive studies, little is understood about the specific factors and mechanisms responsible for nitrogen starvation-invoked ste11+ induction.
In the regulation of sexual development, Pat1/Ran1 kinase plays a unique role (1, 13, 14). Its primary role is to block the onset of meiosis until conjugation takes place, by inactivating Mei2, a key factor triggering the onset and progression of meiosis (39). The function of Ste11 protein itself is modulated by direct phosphorylation by Pat1 kinase, although its function in starting conjugation seems to be unchanged (22). Consequently, inactivation of Pat1 kinase in haploid cells unconditionally induces lethal meiosis, which can be suppressed by inactivation of the mei2+ gene. Since ste11+ is required for the expression of mei2+ (33), any factors that inhibit ste11+ expression or its function would rescue the pat1 lethality. Based on this assumption, we recently screened a gene library for multicopy suppressors of the pat1 lethality and isolated a new gene, named rcd1+, that is required for ste11+ expression specifically induced by nitrogen starvation. Strikingly, genes highly homologous to rcd1+ are present in many eukaryotes including humans. In this communication, we report the structure and function of this new gene and discuss the possibility of the presence of a highly conserved differentiation control mechanism throughout the eukaryotes.
MATERIALS AND METHODS
Yeast manipulations.
The strains of S. pombe used in this study are listed in Table 1. Media were prepared as described previously (4, 8, 25, 28). EMM medium (25) contained 0.5% NH4Cl and 2% glucose unless specified and was sterilized by filtration.
TABLE 1.
Fission yeast strains used in this study
| Strain | Genotype |
|---|---|
| L972 | h− |
| L968 | h90 |
| SO3 | h−leu1-32 |
| TI-120 | h90leu1-32 |
| SO5 | h−pat1-114 ura4-294 |
| SO6 | h−pat1-114 leu1-32 |
| DP2 | h−/h+ade6-M210/ade6-M216 ura4-D18/ura4D18 leu1-32/leu1-32 |
| NN1 | h−/h+ade6-M210/ade6-M216 ura4-D18/ura4D18 leu1-32/leu1-32 |
| NS1 | h−/h+ade6-M210/ade6-M216 ura4-D18/ura4D18 leu1-32/leu1-32 rcd1::ura4+/rcd1::ura4+ |
| NS2 | h−rcd1::ura4+ ura4-D18 |
| NS3 | h+rcd1::ura4+ ura4-D18 |
| NS4 | h90rcd1::ura4+ ura4-D18 |
| NS5 | h90rcd1::ura4+ ura4-D18 leu1-32 |
| NS6 | h−pat1-114 |
| NS7 | h−pat1-114 rcd1::ura4+ ura4-D18 |
| NS8 | h−pat1-114 rcd1::ura4+ ura4-D18 leu1-32 |
| NS9 | h−ste11-1 |
| NS10 | h−mei2-2 |
| NS11 | h90pka1::ura4+ ura4-D18 |
| NS12 | h90pka1::ura4+ rcd1::ura4+ ura4-D18 |
| NS13 | h90wis1::his1+ his1-102 |
| NS14 | h90wis1::his1+ rcd1::ura4+ his1-102 ura4-D18 |
| NS15 | h90pac2::ura4+ ura4-D18 |
| NS16 | h90pac2::ura4+ rcd1::ura4+ ura4-D18 |
| NS17 | h90cig2::ura4+ ura4-D18 |
| NS18 | h90cig2::ura4 rcd1::ura4+ ura4-D18 |
Conjugation capability was measured as follows. Cells were cultured to the mid-log phase (4 × 106 to 6 × 106 cells/ml) at 30°C in EMM medium and resuspended at 107 cells/ml in NH4Cl-free EMM medium or in EMM medium with the indicated concentration of NH4Cl and glucose. After incubation overnight or for the indicated times, aliquots of the cells were gently sonicated and the conjugated cells were counted by microscopy.
The ability to perform meiosis and sporulation was assayed as follows. Heterothallic h− rcd1 and h+ rcd1 cells were grown on YES plates (25) overnight, diluted to 107 cells/ml with H2O, and spotted on ME plates (25). Both conjugated and sporulated cells were counted, and the frequencies of conjugation and sporulation of conjugated cells were calculated.
Flow cytometry was performed as described previously (36) with the FACScan system and the CellFIT cell cycle analysis program (Becton Dickinson).
DNA manipulations.
The S. pombe cDNA expression library used in this study has been described previously (29) and a Sau3AI genomic library was constructed by inserting partial Sau3AI-digested L972 genomic DNAs into the BamHI-digested pBluescriptII KS+ vector (34). The pALSK+ and the simian virus 40 early promoter-driven pcL vectors have been described previously (12, 26). The rcd1+ cDNA was isolated by suppression of SO5 (h− pat1-114 ura4-294) as described previously (36). The genomic DNA fragment containing rcd1+ was isolated from an S. pombe Sau3A1 genomic library by colony hybridization. The human RCD1 cDNA was isolated from the human foreskin fibroblast cDNA library pcD2-Basinger (3) by colony hybridization with a partial human RCD1 sequence amplified by PCR against the cDNA library with primers that were synthesized based on the sequence information from the Wash U-Merck EST project (R38452 yh89b11.r1 cDNA clone 136893 5′). The DNA sequence was determined by the dideoxynucleotide method (30).
Gene disruption.
Two types of rcd1 deletion mutants were constructed. In one type, the 0.75-kb NruI-AatII fragment containing 88% of the rcd1+ coding sequence was replaced by the ura4+ gene. In the other, the SpeI fragment corresponding to amino acids 77 to 129 was replaced by ura4+ (7). Inactivation of rcd1+ in each construct was confirmed by its inability to rescue the pat1-114 mutant. The diploid strain DP2 was transformed with each disrupted rcd1 fragment, stable ura+ transformants were selected, and successful disruption was confirmed by Southern blot hybridization or by PCR detection. The ura+ diploid cells were sporulated and germinated to obtain haploid rcd1 disruptant cells.
Northern blot analysis.
S. pombe cells were grown in EMM medium to 5 × 106 cells/ml. An aliquot of the cells was harvested, and the remainder were inoculated into EMM medium without NH4Cl or EMM medium with 0.5% glucose at 107 cells/ml and incubated, with sampling of cell aliquots at the indicated times. Total RNA was prepared and Northern blot analysis was performed as described previously (27). The Northern blot membrane filter for human tissues used in this study was purchased from Clontech Laboratories, Inc. (human multiple-tissue Northern blots II). The hybridization probes used were the 0.85-kb fragment of ste11+, the 0.87-kb fragment of rcd1+, the 1.1-kb fragment of fbp1+, the 0.95-kb fragment of matPc+ and matPi+, the 1.7-kb BanII fragment of human RCD1, and the 3.3-kb PvuII-HindIII fragment of mei2+ (38). These probes were obtained by PCR amplification of cDNA libraries with appropriate primers.
Nucleotide sequence accession numbers.
The DDBJ, EMBL, and GenBank accession number of the rcd1+ gene is D87956, and that of human RCD1 is D87957.
RESULTS
Isolation of the rcd1+ gene.
To identify new elements controlling the onset of cell differentiation, we screened an S. pombe expression cDNA library for genes that suppress the lethality of the temperature-sensitive pat1-114 mutation, as described previously (28), and isolated several distinct clones. One new cDNA clone, named rcd1+ (required for cell differentiation [see below]), was characterized further. The rcd1+ cDNA placed under the control of the simian virus 40, cytomegalovirus or nmt1+ promoter effectively suppressed the temperature-sensitive lethality of the pat1-114 mutant at 35°C or higher temperatures (data not shown).
rcd1+ encodes a highly conserved protein.
The rcd1+ cDNA was 1.8 kb long. Because it had an in-frame termination codon upstream of the assigned initiation codon and was similar in size to the transcript determined by Northern blot hybridization, it was judged to contain the entire coding region, which is capable of encoding a 283-amino-acid protein (Fig. 1). The predicted protein product is leucine rich but has no apparent motifs or significant homology to any proteins with known function. However, it has strikingly high level of homology to the products of putative genes in four eukaryotes, Saccharomyces cerevisiae, Arabidopsis, Caenorhabditis elegans, and Homo sapiens, all of which were identified by the genome project (Fig. 1). Amino acid identity in the homologous region exceeds 70% among these species. However, there are some distinctions. The putative S. cerevisiae homolog of Rcd1 has an N-terminal extension, whereas the C. elegans homolog has a C-terminal extension. The Arabidopsis and H. sapiens homologs deposited in the DNA database appeared to be truncated at the N or C terminal, perhaps because of isolation of incomplete genes. We cloned a full-size cDNA for the human RCD1 homolog. The sequence shown in Fig. 1 is the one determined from this cDNA.
FIG. 1.
Amino acid identity among Rcd1 and its homologs from S. cerevisiae (Z71564), C. elegans (U13875 C26E6.3) Arabidopsis (Z29188), and H. sapiens. Amino acids that match in any three proteins among these are shown against a black background, and those that match in any two proteins are against a shaded background. The DDBJ, EMBL, and GenBank accession numbers for the rcd1+ and human RCD1 are D87956 and D87957, respectively.
Comparison of the rcd1+ cDNA with the genomic sequence isolated by colony hybridization indicated that there is no intron in the protein coding sequence. The rcd1+ gene is transcribed into a 1.8-kb mRNA with no obvious oscillation in the mRNA level during nutrient starvation (data not shown).
Cells disrupted for rcd1+ are sterile.
To investigate the physiological role of rcd1+, we constructed two types of rcd1 null mutants. Two nonfunctional rcd1 fragments in which either 30% of the middle of the coding region (SpeI-SpeI) or 80% of the coding region (NruI-AatII) was replaced with the ura4+ cassette were constructed and transfected into a diploid strain. The resulting rcd1+/rcd1 disruptants were identified by Southern blotting, sporulated, and germinated to obtain haploid disruptants. The two types of haploid disruptants were phenotypically indistinguishable. Therefore, the SpeI disruptant was chosen as representative and further characterized.
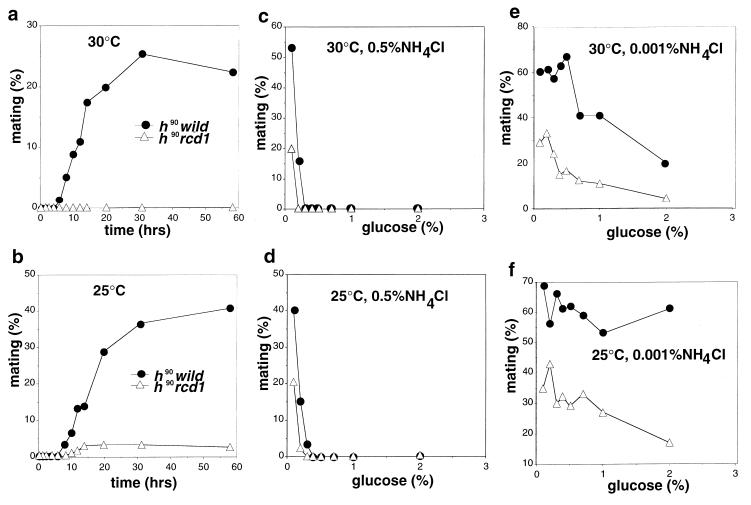
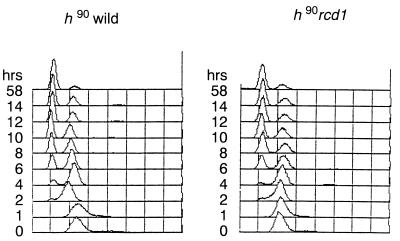
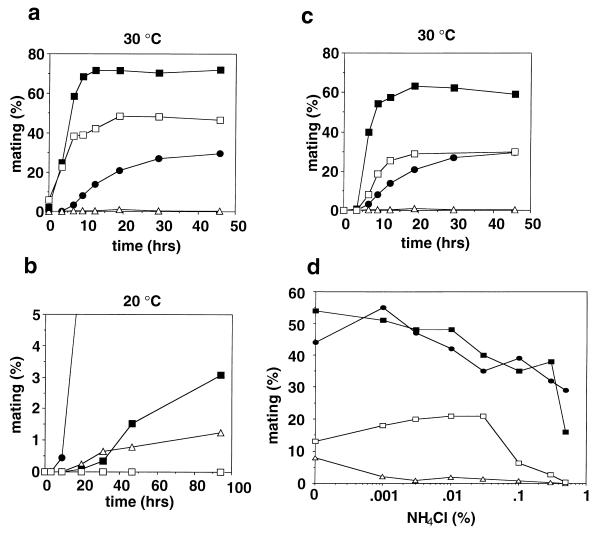
The disruptant germinated and proliferated with the same growth ability as did wild-type cells. The only noticeable difference was slight elongation of the cells. Because multicopy rcd1+ blocked sexual development induced by pat1+ inactivation, we anticipated that the rcd1 disruptant would be highly proficient for sexual development. However, instead, the disruptant was highly sterile when sexual development was induced by nitrogen starvation (Fig. 2a). This sterility was the result of rcd1+ disruption because it was suppressed by ectopic expression of rcd1+ and always cosegregated with rcd1+ disruption during repeated backcrossing with the standard ura4 strain. Interestingly, the sterility was slightly alleviated at 25°C, a suboptimal temperature for growth (Fig. 2b). In contrast, when starved for glucose, the disruptant was grossly normal and actively conjugated, albeit slightly (approximately twofold) less efficiently than wild-type cells at both temperatures (Fig. 2c and d). Starvation for both nitrogen and glucose also effectively induced the onset of sexual development of the disruptant, indicating that the disruptant was responsive to glucose starvation irrespective of the presence or absence of nitrogen starvation (Fig. 2e and f). The efficiency and viability of the spores formed by glucose starvation were the same as those of wild-type cells. The severe sterility is not a consequence of loss of the ability of the cell to sense nitrogen availability and starvation or of arrest in G1 in response to nitrogen starvation, because the disruptant grew at the same rate and arrested in G1 upon nitrogen starvation with the same time course and extent of G1 arrest as those of wild-type cells (Fig. 3).
FIG. 2.
Mating frequencies of the rcd1 disruptant under various conditions. (a and b) Mating efficiencies of rcd1 cells induced by nitrogen starvation. The h90 wild-type cells (L968) and h90 rcd1 cells (NS4) were grown in EMM medium to 5 × 106 cells/ml, washed, resuspended at 107 cells/ml in NH4Cl-free EMM medium, and incubated at 30°C (a) or 25°C (b). (c and d) Mating efficiencies of rcd1 cells induced by glucose starvation. The h90 wild-type cells (L968) and h90 rcd1 cells (NS4) were grown in EMM medium to 5 × 106 cells/ml, washed, resuspended at 107 cells/ml in EMM medium containing 0.5% NH4Cl and with the indicated concentration of glucose and incubated at 30°C (c) or 25°C (d). (e and f) Mating efficiencies of rcd1 cells induced by dual starvation for glucose and nitrogen. The h90 wild-type cells (L968) and h90 rcd1 cells (NS4) were grown in EMM medium to 5 × 106 cells/ml, washed, resuspended at 107 cells/ml in EMM medium containing 0.001% NH4Cl and with the indicated concentrations of glucose and incubated at 30°C (e) or 25°C (f). Conjugated cells were counted at the indicated times, and their populations were calculated as percent mating.
FIG. 3.
rcd1 disruptant responds to nitrogen starvation and arrest in G1 in the same time course and to the same extent as wild-type cells. The same samples as for Fig. 2a were analyzed by flow cytometry. Fission yeast cells in logarithmic growth show only a 2C DNA content because of prolonged G2 phase and late cell separation.
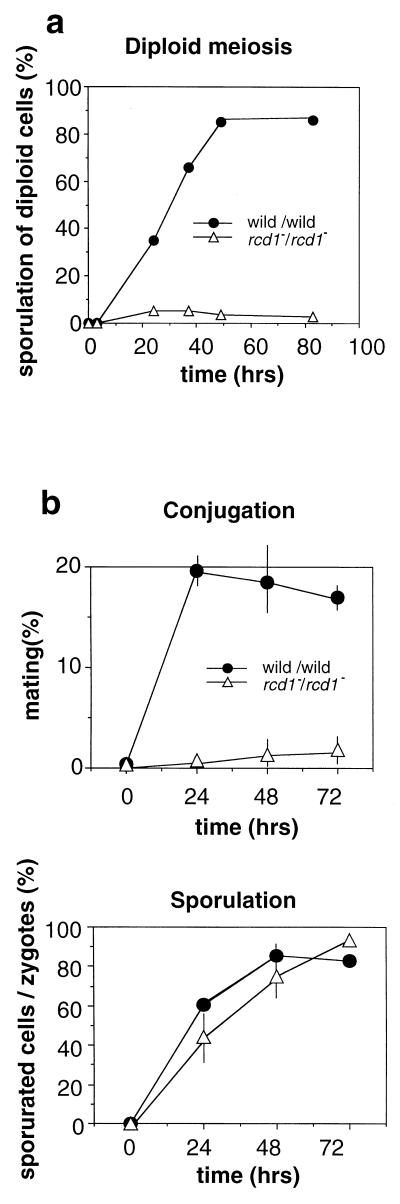
To gain a deeper insight into the function of rcd1+, we examined the effect of rcd1 disruption on the pat1 phenotype. Contrary to expectation but consistent with the sterility, inactivation of rcd1+ markedly suppressed the pat1 phenotype, and the heterothallic pat1-114 rcd1 double mutant grew at a nonpermissive temperature of 34°C without undergoing haploid meiosis (data not shown). The reason for the paradoxical pat1 suppression by both overexpression and disruption of rcd1+ is discussed below. When the rcd1 disruptant was converted to diploid cells and the established rcd1/rcd1 diploid cells were tested for their ability to perform meiosis and sporulation, they showed poor sporulation (Fig. 4a). However, this was caused not by a defect in meiosis or sporulation but by a defect in the initiation of sexual development. When the disruptant was induced to conjugate and the resulting poorly conjugated diploid cells were examined for their sporulation, they were indistinguishable from wild-type cells in sporulation efficiency (Fig. 4b) and also in both morphology and spore viability (data not shown); this is consistent with the lack of apparent defect in their conjugation, meiosis, and sporulation when induced by glucose starvation. The poor ability of the disruptant to perform diploid meiosis is likely to be a consequence of the absolute requirement of some factors controlling the onset of sexual development for meiosis and sporulation, such as ste11+ for the expression of mei2+ (33, 38).
FIG. 4.
rcd1+ is not required for meiosis and sporulation after onset of sexual differentiation. (a) The homozygous rcd1 diploid (NS1) and wild-type diploid cells (NN1) were grown in EMM medium to 2 × 106 cells/ml, washed, resuspended in NH4Cl-free EMM medium at 5 × 106 cells/ml, and incubated at 30°C. (b) The heterothallic h− cells (L972), h− rcd1 cells (NS2), h+ cells (L975), and h+ rcd1 cells (NS3) were separately grown on YES plates overnight and mixed with opposite-mating-type but otherwise genotypically identical cells at a concentration of 107 cells/ml in H2O and spotted on ME plates. Conjugated cells and sporulated cells were counted by microscopy. Conjugation was carried out on agar plates but not in liquid medium to increase the mating frequencies of the rcd1 disruptant. The percentage of sporulated cells was calculated by counting the number of spore asci per conjugated cell. The data shown are means ± standard deviations in two independent experiments.
Nitrogen starvation-induced ste11+ expression is defective in Δrcd1 cells.
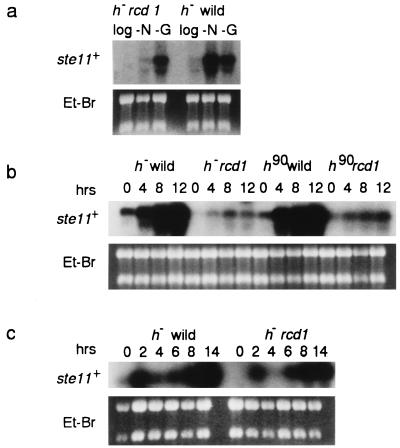
Because ste11+ plays a key role in the initiation of sexual development, we examined the effect of rcd1+ inactivation on the induction of ste11+. Glucose starvation and nitrogen starvation are two major nutrient exhaustion signals for ste11+ induction. In heterothallic wild-type cells, ste11+ mRNA was drastically induced during a 6-h starvation for nitrogen or glucose (Fig. 5a). However, in the rcd1 disruptant, nitrogen starvation failed to induce ste11+ but, consistent with the fertility, glucose starvation involved ste11+ induction. To confirm these results, the time course of ste11+ induction was examined not only for heterothallic cells but also for self-conjugative homothallic disruptant and wild-type cells. Again, irrespective of mating types, nitrogen starvation failed to strongly induce ste11+ transcription in the disruptant whereas the response to glucose starvation was the same as that of wild-type cells (Fig. 5b and c). Nevertheless, it should be pointed out that nitrogen starvation-invoked ste11+ induction was not totally lost in the disruptant, since a significant level of ste11+ mRNA was detected in the nitrogen-starved disruptant. Moreover, in the homothallic disruptant, ste11+ mRNA seemed to be induced slightly more strongly.
FIG. 5.
The rcd1 disruptant is defective in ste11+ induction in response to nitrogen starvation but not to glucose starvation. (a) The h− wild-type (L972) and h− rcd1 (NS2) cells were grown in EMM medium to 5 × 106 cells/ml, washed, resuspended in NH4Cl-free EMM medium or in 0.5% glucose containing EMM medium at 107 cells/ml, and incubated at 30°C for 6 h. Total RNA was then extracted from the cells. (b) The h− wild-type (L972), h− rcd1 (NS2), h90 wild-type (L968), and h90 rcd1 cells (NS4) were grown and incubated in NH4Cl-free EMM medium under the same conditions as in panel a. The cells were harvested at the indicated times, and total RNA was extracted. (c) The h− wild-type (L972) and h− rcd1 (NS2) cells were grown and incubated in EMM medium containing 0.5% glucose under the same conditions as in panel a. The cells were harvested at the indicated times, and total RNA was extracted. The level of the ste11+ transcript was determined by Northern blot hybridization.
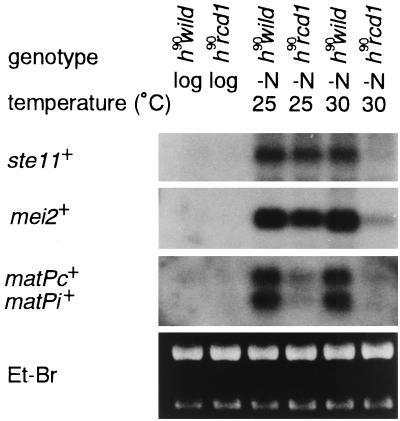
These results were obtained at 30°C. However, as mentioned above, the sterility of the disruptant was slightly alleviated at 25°C (Fig. 2). To examine any direct correlation between the degree of sterility and the extent of ste11+ induction, the levels of nitrogen starvation-invoked ste11+ induction in the homothallic disruptant and wild-type cells were compared at 25 and 30°C. As expected, nitrogen starvation induced ste11+ to a higher level at 25°C than at 30°C, although the level was still slightly lower than in wild-type cells (Fig. 6). The induction of mei2+ closely paralleled the ste11+ induction. By contrast, in wild-type cells, the level of nitrogen starvation-invoked ste11+ induction was the same at both temperatures. Perhaps reflecting the significant induction of ste11+ at 25°C, matPc+ and matPi+ were partially induced in the disruptant. However, at this temperature, the level of matP+ induction and the mating frequency (Fig. 2b) relative to ste11+ induction were still noticeably low. These results indicate that rcd1+ is required for nitrogen starvation-invoked ste11+ mRNA induction at 30°C but not at 25°C. Furthermore, these results suggest that rcd1+ is likely to play additional roles, such as induction of matPc+ and matPi+, independent of transcriptional regulation of ste11+ expression.
FIG. 6.
The defect of the rcd1 disruptant in nitrogen starvation-responsive ste11+ induction is partially suppressed at 25°C. The h90 wild-type (L968) and h90 rcd1 cells (NS4) were grown in EMM medium to 5 × 106 cells/ml, washed, resuspended in NH4Cl-free EMM medium at 107 cells/ml, and incubated for 6 h at 30 or 25°C. Total RNA was then extracted from the cells, and the levels of the ste11+, mei2+, matPc+, and matPi+ mRNAs were determined by Northern blot hybridization.
Pat1+s inactivation-triggered ste11+ induction is also defective in Δrcd1 cells.
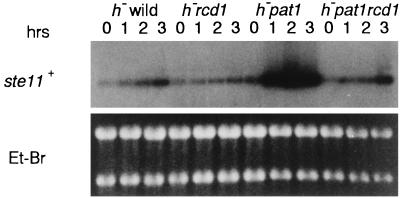
As mentioned above, deletion of rcd1+ suppressed the lethality of pat1. To investigate the possibility of mechanistic similarity, we examined the level of ste11+ induction in both pat1-114 and pat1-114 rcd1 cells after the shift to 34°C. ste11+ was induced in the pat1-114 mutant upon the shift to the nonpermissive temperature (Fig. 7). In the double mutant, however, ste11+ induction was markedly diminished. Diminished ste11+ induction was indeed the cause of pat1 suppression because ectopic expression of ste11+ or mei2+ in the double mutant effectively led to reversion of the suppressed pat1 lethality (Table 2). Thus, the rcd1 disruptant responded to glucose starvation but not to nitrogen starvation or inactivation, indicating that rcd1+ is required for ste11+ induction invoked at 30°C by nitrogen starvation and by Pat1+s inactivation.
FIG. 7.
The rcd1 disruptant is defective in ste11+ mRNA induction invoked by Pat1+s inactivation. The h− wild-type (L972), h− pat1-114 (NS6), and h− pat1-114 rcd1 (NS7) cells were grown in EMM medium at 23°C. When the cell density reached 107 cells/ml, each culture was shifted to 34°C and incubated for the indicated times. Total RNA was extracted from the cells, and the level of the ste11+ mRNA was determined by Northern blot hybridization.
TABLE 2.
Ectopic expression of ste11+ reduces the growth ability of the pat1 and pat1 rcd1 mutants at the restrictive temperaturesa
| Strains | Plasmid | % Suppressionb |
|---|---|---|
| h− pat1-114 | pcLX | 0.1 |
| pcLrcd1+ | 53.1 | |
| pcLste11+ | <0.1 | |
| pcLmei2+ | <0.1 | |
| h− pat1-114 rcd1 | pcLX | 37.1 |
| pcLrcd1+ | 42.2 | |
| pcLste11+ | 2.1 | |
| pcLmei2+ | 2.7 |
The h− pat1-114 leu1 and h− pat1-114 rcd1 leu1 cells were transfected with the indicated plasmids. The transfected cells were spread on MMA medium plates and incubated at 23°C overnight and then at the restrictive temperature of 34 or 23°C. The numbers of leu+ colonies were counted after incubation for 3 days.
The percent suppression of pat1-114 mutation was calculated by dividing the number of colonies that grew at 34°C by the number that grew at 23°C.
Ectopic expression of ste11+ restores fertility to rcd1 cells.
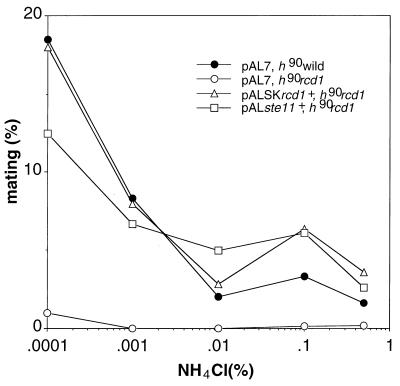
To resolve whether the sterility of the disruptant was attributable largely to poor ste11+ induction, we tested if ectopic expression of ste11+ could restore fertility to the disruptant. As shown in Fig. 8, expression of ste11+ indeed restored fertility to the disruptant to the same extent as in wild-type cells or cells obtained by expression of rcd1+. This led us to conclude that the sterility of the rcd1 disruptant was caused largely by poor induction of ste11+ in response to nitrogen starvation.
FIG. 8.
Ectopic expression of ste11+ restores fertility to the rcd1 disruptant. The h90 rcd1 leu− cells harboring empty pAL7, pAL7 carrying ste11+, or pALSK carrying rcd1+ were grown in EMM medium to 5 × 106 cells/ml. The cells were washed, resuspended in EMM medium with the indicated concentration of NH4Cl at 107 cells/ml, and incubated at 25°C for 22 h. The numbers of zygotes and nonzygotes were counted. The pAL7 vector was described previously (28).
rcd1+ is required for ste11+ induction independently of the cAMP-Pka1 and Wis1-Phh1 pathways and other known factors.
The cAMP-Pka1 and Wis1-Phh1 pathways mediate mainly glucose and stress signals, respectively, and critically control the onset of sexual development (18, 42). We therefore investigated the possible link of rcd1+ to these pathways. For this purpose, a homothallic rcd1 pka1 double mutant was constructed and compared to each single mutant for the ability to perform nitrogen starvation-induced conjugation. The pka1 cells are highly proficient for conjugation even if not starved for nitrogen (23), whereas the rcd1 cells were sterile, as described above. The rcd1 pka1 cells were, however, fertile and showed an intermediate level of mating frequency, indicating that rcd1+ is independent of the cAMP-Pka1 pathway (Fig. 9a).
FIG. 9.
rcd1+ controls differentiation independently of the cAMP-Pka1 (a) and Wis1-Phh1 (b) pathways, Pac2 (c), and Cig2 (d). (a to c) The h90 wild-type (solid circles), h90 rcd1 (open triangles), h90 pka1 (solid squares), and h90 pka1 rcd1 (open squares) cells (a), the h90 wild-type (solid circles), h90 rcd1 (open triangles), h90 wis1 (solid squares), and h90 rcd1 wis1 (open squares) cells (b), and the h90 wild-type (solid circles), h90 rcd1 (open triangles), h90 pac2 (solid squares), and h90 rcd1 pac2 (open squares) cells (c) were grown in EMM medium to 5 × 106 cells/ml, washed, resuspended in NH4Cl-free EMM medium at 107 cells/ml, and incubated at 30°C for the indicated times. Conjugated cells were counted, and their percent populations were calculated by dividing twice the number of conjugated cells by the total cell number. (d) The h90 wild-type (solid circles), h90 rcd1 (open triangles), h90 cig2 (solid squares), and h90 rcd1 cig2 (open squares) cells were grown in EMM medium to 5 × 106 cells/ml, washed, resuspended in EMM medium with the indicated concentrations of NH4Cl at 107 cells/ml, and incubated at 30°C for 24 h. Conjugated cells were counted at the indicated times, and their percent populations were calculated as above.
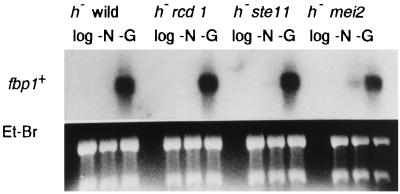
Similarly, a homothallic rcd1 wis1 double mutant was constructed and examined for its ability to perform conjugation. Both rcd1 and wis1 single mutants are highly but not completely sterile, each conjugating at low frequencies of 1 to 5% under the conditions used (18, 32) (Fig. 2). The rcd1 wis1 double mutant was, however, completely sterile, and we failed to detect any conjugated cells throughout the entire experiment, indicating that the action of rcd1+ is also independent of the Wis1-Phh1 stress signal pathway (Fig. 9b). The independence of rcd1+ from both pathways was further confirmed by the lack of effect of rcd1+ deletion on the glucose starvation-invoked fbp1+ induction, which is regulated by both the cAMP-Pka1 and Wis1-Phh1 pathways via Atf1 (10, 32) (Fig. 10).
FIG. 10.
fbp1+ mRNA is fully inducible in rcd1 disruptants in response to glucose starvation. The h− wild-type (L972), h− rcd1 (NS2), ste11 (NS9), and h− mei2 (NS10) cells were grown in EMM medium to 5 × 106 cells/ml, washed, resuspended in NH4Cl-free EMM medium (−N) or EMM medium containing 0.5% glucose (−G) at 107 cells/ml, and incubated at 30°C for 6 h. Total RNA was extracted from the cells, and the level of the fbp1+ transcript was determined by Northern blot hybridization. Fbp1, fructose-1,6-bisphosphatase.
Overexpression of the pac2+ gene blocks the onset of sexual development by repressing ste11+ expression, and cells defective in pac2+ could express ste11+ and enter sexual development under incomplete starvation conditions, although the biological significance of pac2+ is not understood (20). The possible relationship between pac2+ and rcd1+ was therefore examined by the same analysis. Again, rcd1+ was suggested to be independent of pac2+ (Fig. 9c).
The Cig2/Cyc17 cyclin inhibits sexual development and promotes the start of the cell cycle (5, 27). This cyclin inhibits sexual development by a mechanism independent of transcriptional regulation of ste11+ (41). A similar analysis was carried out for the relation between cig2+ and rcd1+. As shown in Fig. 9d, the double mutant became fertile but not to the level of wild-type cells, indicating that rcd1+ is independent of cig2+, as expected.
The human RCD1 homolog is expressed in various tissues.
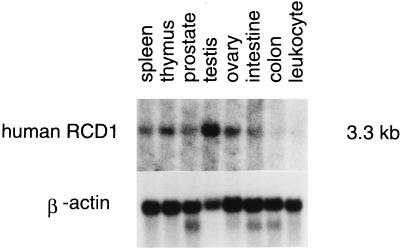
Given the biological role of rcd1+ in fission yeast, we examined tissue-specific expression of the human RCD1+ homolog by Northern blot analysis. As shown in Fig. 11, it was expressed in a variety of human tissues, but its expression was particularly high in the testes, ovaries, and thymus, the tissues in which cell growth and differentiation are actively taking place.
FIG. 11.
Tissue-specific expression of human RCD1. Each lane contains approximately 2 μg of poly(A)+ RNA prepared from human spleen, thymus, prostate, testes, ovaries, small intestine, colon (mucosal lining), or peripheral blood leukocytes probed with human RCD1 (top) or with the β-actin gene (bottom).
DISCUSSION
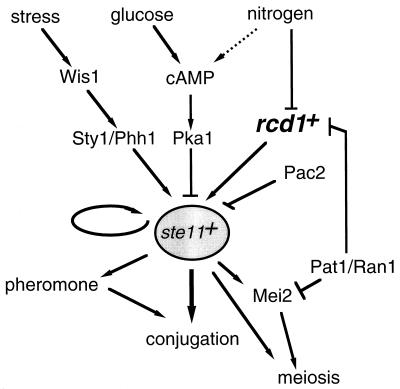
As is apparent from the data presented above, rcd1+ is a newly identified differentiation-controlling factor that is crucial for nitrogen starvation-invoked onset of sexual development in fission yeast. Although the molecular mechanism by which rcd1+ controls sexual development is not fully understood, rcd1+ is at least essential for ste11+ induction in response to nitrogen starvation at the normal growth temperature. To date, three different factors or pathways have been identified that regulate the expression of ste11+: (i) the cAMP-Pka1 cascade, which mainly mediates a signal for carbon source; (ii) the Wis1-Phh1 pathway, which mediates a stress signal; and (iii) Pac2, whose physiological role is unknown. As is known, starvation for nitrogen source is the most effective signal for the induction of the onset of sexual development. However, none of these ste11+ regulatory systems seems to specifically mediate the nitrogen starvation signal. Rcd1 is the fourth factor that controls ste11+ expression. All the data indicate that the system involving Rcd1 is independent of these three ste11+-regulatory systems and is uniquely responsible for controlling ste11+ expression by nitrogen starvation and Pat1+s inactivation signals (Fig. 12). Rcd1 is essential for nitrogen starvation-invoked ste11+ induction but is not a component of the general nitrogen signal cascade, because cells lacking rcd1+ still respond to nitrogen starvation and can arrest in G1 in a time course indistinguishable from that of wild-type cells (Fig. 3). Interestingly, sterility and lack of ste11+ induction in the rcd1 disruptant are partially suppressed at 25°C or lower temperatures. The reason for this phenomenon is unknown. Rcd1 might be involved in stabilizing the possibly heat-labile nitrogen starvation-responsive ste11+ regulatory system, or there might be two redundant factors, one of which is Rcd1 and functions exclusively at relatively high temperatures. rcd1+ seems to play another role besides the transcriptional control of ste11+. At 25°C, ste11+ was significantly induced in the rcd1 disruptant by nitrogen starvation, yet both induction of matPi+ and matPc+ and conjugation were poor (Fig. 6). These results suggest that rcd1+ is likely to control differentiation by a mechanism independent of transcriptional regulation of ste11+ expression. This mechanism may involve posttranscriptional control of ste11+, because the sterility of the rcd1 disruptant was effectively rescued by ectopic overexpression of ste11+ (Fig. 8).
FIG. 12.
Proposed model for the regulation of ste11+ expression by Rcd1 and other known factors. Rcd1 is required for ste11+ induction in response to nitrogen starvation and Pat1+s inactivation. In addition to ste11+ induction, Rcd1 controls differentiation via a mechanism independent of ste11+ regulation, which is not shown here because of ignorance of its molecular target. The Wis1-Sty1/Phh1 stress signal pathway (18) positively regulates ste11+ expression, and the cAMP-Pka1 pathway (15) and Pac2 (20) negatively regulate ste11+ expression. In addition, the Ste11 molecule positively autoregulates its own expression (33).
Deletion of rcd1+ suppresses the pat1-114 mutant, because rcd1+ is required for ste11+ induction by Pat1+s inactivation (Fig. 7). Paradoxically, rcd1+ was initially isolated as a multicopy suppressor of the pat1-114 lethality. An entirely different mechanism seems to be involved in this suppression. The rcd1+ gene was also independently isolated and found to inhibit the activity of a dominant active mutant of the Mei2 protein and to strongly bind to Mei2 in the budding yeast two-hybrid system (39a). The inhibition of Mei2 seems to be evident only in an overproduced situation, since deletion of rcd1+ apparently did not influence the efficiency of meiosis and sporulation following conjugation (Fig. 4b).
One striking finding in the present work is the evolutionary conservation of the rcd1+ gene throughout eukaryotes. Budding yeast, plants, worms, and humans all contain its structural homologs. Amino acid homology exceeds 70% among the products of these homologs. Despite such a high level of similarity, rcd1+ could not functionally be substituted, at least by the human homolog (29a). Although the physiological role of those homologs is not known because they were identified by the genome-sequencing project, the level of tissue-specific expression of the human RCD1 homolog, which is particularly high in the testes, ovaries, and thymus, is certainly consistent with its possible involvement in differentiation control. Conservation of regulatory factors is not restricted to Rcd1. Ste11 contains a typical high-mobility group (HMG) motif (33). The recently identified TCF1 is a HMG protein and is essential for the terminal differentiation of lymphocytes (37), whereas p38, a homolog of Phh1, mediates stress signals and controls the growth and differentiation of lymphocytes (6, 9, 21). Moreover, cAMP is a well-identified regulator of the growth and differentiation of mammalian cells (31). Such similarity in several distinct factors may suggest that the system controlling the onset of differentiation found in fission yeast might well be conserved throughout the eukaryotes.
ACKNOWLEDGMENTS
We thank Chikashi Shimoda for the fbp1+ plasmid and the ste11-1 and mei2-2 strains. We also thank Tomoko Ishihara-Obara, Tomohisa Kato, Jr., Koichi Tanaka, and Kappei Tsukahara for the plasmids and yeast strains used in this study.
This work was supported in part by grants from Ministry of Education and Science and from HESP to H.O.
REFERENCES
- 1.Beach D, Rodgers L, Gould J. ran1+ controls the transition from mitotic division to meiosis in fission yeast. Curr Genet. 1985;10:297–311. doi: 10.1007/BF00365626. [DOI] [PubMed] [Google Scholar]
- 2.Byrne S M, Hoffman C S. Six git genes encode a glucose-induced adenylate cyclase activation pathway in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1993;105:1095–1100. doi: 10.1242/jcs.105.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egel R, Egel-Mitani M. Premeiotic DNA synthesis in fission yeast. Exp Cell Res. 1974;88:127–134. doi: 10.1016/0014-4827(74)90626-0. [DOI] [PubMed] [Google Scholar]
- 5.Fisher D L, Nurse P. A single fission yeast mitotic cyclin B p34cdc2 kinase promotes both S-phase and mitosis in the absence of G1 cyclins. EMBO J. 1996;15:850–860. [PMC free article] [PubMed] [Google Scholar]
- 6.Freshney N W, Rawlinson L, Guesdon F, Jones E, Cowley S, Hsuan J, Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994;78:1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- 7.Grimm C, Kohli J, Mundrell K. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4+ gene as a selectable marker. Mol Gen Genet. 1988;215:81–86. doi: 10.1007/BF00331307. [DOI] [PubMed] [Google Scholar]
- 8.Gutz H, Heslot V, Leupold V, Loprieno N. Schizosaccharomyces pombe. In: King R C, editor. Handbook of genetics. Vol. 1. New York, N.Y: Plenum Press; 1974. pp. 395–446. [Google Scholar]
- 9.Han J, Lee J D, Bibbs L, Ulevitch R J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman C S, Winston F. Glucose repression of transcription of the Schizosaccharomyces pombe fbp1 gene occurs by a cAMP signaling pathway. Genes Dev. 1991;5:561–571. doi: 10.1101/gad.5.4.561. [DOI] [PubMed] [Google Scholar]
- 11.Hughes D A, Fukui Y, Yamamoto M. Homologous activators of ras in fission and budding yeast. Nature. 1990;344:355–357. doi: 10.1038/344355a0. [DOI] [PubMed] [Google Scholar]
- 12.Igarashi M, Nagata A, Jinno S, Suto K, Okayama H. Wee1+-like gene in human cells. Nature. 1991;353:80–83. doi: 10.1038/353080a0. [DOI] [PubMed] [Google Scholar]
- 13.Iino Y, Yamamoto M. Mutants of Schizosaccharomyces pombe which sporulate in the haploid state. Mol Gen Genet. 1985;198:416–421. doi: 10.1007/BF00332932. [DOI] [PubMed] [Google Scholar]
- 14.Iino Y, Yamamoto M. Negative control for the initiation of meiosis in Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1985;82:2447–2451. doi: 10.1073/pnas.82.8.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isshiki T, Mochizuki N, Maeda T, Yamamoto M. Characterization of a fission yeast gene, gpa2, that encodes a G alpha subunit involved in the monitoring of nutrition. Genes Dev. 1992;6:2455–2462. doi: 10.1101/gad.6.12b.2455. [DOI] [PubMed] [Google Scholar]
- 16.Jin M, Fujita M, Culley B M, Apolinario E, Yamamoto M, Maundrell K, Hoffman C S. sck1, a high copy number suppressor of defects in the cAMP-dependent protein kinase pathway in fission yeast, encodes a protein homologous to the Saccharomyces cerevisiae SCH9 kinase. Genetics. 1995;140:457–467. doi: 10.1093/genetics/140.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanoh J, Watanabe Y, Ohsugi M, Iino Y, Yamamoto M. Schizosaccharomyces pombe gad7+ encodes a phosphoprotein with a bZIP domain, which is required for proper G1 arrest and gene expression under nitrogen starvation. Genes Cells. 1996;1:391–408. doi: 10.1046/j.1365-2443.1996.d01-247.x. [DOI] [PubMed] [Google Scholar]
- 18.Kato T, Jr, Okazaki K, Murakami H, Stettler S, Fantes P A, Okayama H. Stress signal, mediated by a Hog1-like MAP kinase, controls sexual development in fission yeast. FEBS Lett. 1996;378:207–212. doi: 10.1016/0014-5793(95)01442-x. [DOI] [PubMed] [Google Scholar]
- 19.Kelly M, Burke J, Smith M, Klar A, Beach D. Four mating-type genes control sexual differentiation in the fission yeast. EMBO J. 1988;7:1537–1547. doi: 10.1002/j.1460-2075.1988.tb02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunitomo H, Sugimoto A, Wilkinson C R, Yamamoto M. Schizosaccharomyces pombe pac2+ controls the onset of sexual development via a pathway independent of the cAMP cascade. Curr Genet. 1995;28:32–38. doi: 10.1007/BF00311879. [DOI] [PubMed] [Google Scholar]
- 21.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 22.Li P, McLeod M. Molecular mimicry in development: identification of ste11+ as a substrate and mei3+ as a pseudosubstrate inhibitor of ran1+ kinase. Cell. 1996;87:869–880. doi: 10.1016/s0092-8674(00)81994-7. [DOI] [PubMed] [Google Scholar]
- 23.Maeda T, Watanabe Y, Kunitomo H, Yamamoto M. Cloning of the pka1 gene encoding the catalytic subunit of the cAMP-dependent protein kinase in Schizosaccharomyces pombe. J Biol Chem. 1994;269:9632–9637. [PubMed] [Google Scholar]
- 24.Miyamoto M, Tanaka K, Okayama H. res2+, a new member of the cdc10+/SWI4 family, controls the ‘start’ of mitotic and meiotic cycles in fission yeast. EMBO J. 1994;13:1873–1880. doi: 10.1002/j.1460-2075.1994.tb06456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 26.Nagata A, Igarashi M, Jinno S, Suto K, Okayama H. An additional homolog of the fission yeast cdc25+ gene occurs in humans. New Biol. 1991;3:959–968. [PubMed] [Google Scholar]
- 27.Obara-Ishihara T, Okayama H. A B-type cyclin negatively regulates conjugation via interacting with cell cycle ‘start’ genes in fission yeast. EMBO J. 1994;13:1863–1872. doi: 10.1002/j.1460-2075.1994.tb06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okazaki K, Okazaki N, Kume K, Jinno S, Tanaka K, Okayama H. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 1990;18:6485–6489. doi: 10.1093/nar/18.22.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okazaki N, Okazaki K, Tanaka K, Okayama H. The ste4+ gene, essential for sexual differentiation of Schizosaccharomyces pombe, encodes a protein with a leucine zipper motif. Nucleic Acids Res. 1991;19:7043–7047. doi: 10.1093/nar/19.25.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Okazaki, N., et al. Unpublished observations.
- 30.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sassone-Corsi P. Transcription factors responsive to cAMP. Annu Rev Cell Dev Biol. 1995;11:355–377. doi: 10.1146/annurev.cb.11.110195.002035. [DOI] [PubMed] [Google Scholar]
- 32.Stettler S, Warbrick E, Prochnik S, Mackie S, Fantes P. The wis1 signal transduction pathway is required for expression of cAMP-repressed genes in fission yeast. J Cell Sci. 1996;109:1927–1935. doi: 10.1242/jcs.109.7.1927. [DOI] [PubMed] [Google Scholar]
- 33.Sugimoto A, Iino Y, Maeda T, Watanabe Y, Yamamoto M. Schizosaccharomyces pombe ste11+ encodes a transcription factor with an HMG motif that is a critical regulator of sexual development. Genes Dev. 1991;5:1990–1999. doi: 10.1101/gad.5.11.1990. [DOI] [PubMed] [Google Scholar]
- 34.Sugiyama A, Tanaka K, Okazaki K, Nojima H, Okayama H. A zinc finger protein controls the onset of premeiotic DNA synthesis of fission yeast in a Mei2-independent cascade. EMBO J. 1994;13:1881–1887. doi: 10.1002/j.1460-2075.1994.tb06457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeda T, Toda T, Kominami K, Kohnosu A, Yanagida M, Jones N. Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase. EMBO J. 1995;14:6193–6208. doi: 10.1002/j.1460-2075.1995.tb00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka K, Okazaki K, Okazaki N, Ueda T, Sugiyama A, Nojima H, Okayama H. A new cdc gene required for S phase entry of Schizosaccharomyces pombe encodes a protein similar to the cdc 10+ and SWI4 gene products. EMBO J. 1992;11:4923–4932. doi: 10.1002/j.1460-2075.1992.tb05599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald H R, Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe Y, Iino Y, Furuhata K, Shimoda C, Yamamoto M. The S. pombe mei2 gene encoding a crucial molecule for commitment to meiosis is under the regulation of cAMP. EMBO J. 1988;7:761–767. doi: 10.1002/j.1460-2075.1988.tb02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe Y, Shinozaki-Yabana S, Chikashige Y, Hiraoka Y, Yamamoto M. Phosphorylation of RNA-binding protein controls cell cycle switch from mitotic to meiotic in fission yeast. Nature. 1997;386:187–190. doi: 10.1038/386187a0. [DOI] [PubMed] [Google Scholar]
- 39a.Watanabe, Y., and M. Yamamoto. Unpublished data.
- 40.Willer M, Hoffmann L, Styrkarsdottir U, Egel R, Davey J, Nielsen O. Two-step activation of meiosis by the mat1 locus in Schizosaccharomyces pombe. Mol Cell Biol. 1995;15:4964–4970. doi: 10.1128/mcb.15.9.4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaguchi, S., H. Murakami, and H. Okayama. A WD repeat protein controls the cell cycle and differentiation by negatively regulating Cdc2/B-type cyclin complexes. Mol. Biol. Cell, in press. [DOI] [PMC free article] [PubMed]
- 42.Yamamoto M. The molecular control mechanisms of meiosis in fission yeast. Trends Biochem Sci. 1996;21:18–22. [PubMed] [Google Scholar]