Abstract
The Arabidopsis thaliana suppressor of npr1-1, constitutive 1 (snc1) mutant contains a gain-of-function mutation in a Toll Interleukin1 receptor-nucleotide binding-Leu-rich repeat–type resistance gene (R-gene), which leads to constitutive activation of disease resistance response against pathogens. In a screen for suppressors of snc1, a recessive mutation, designated mos3 (for modifier of snc1,3), was found to suppress the constitutive pathogenesis-related gene expression and resistance to virulent Pseudomonas syringae maculicola ES4326 and Peronospora parasitica Noco2 in snc1. In addition, mos3 is also compromised in resistance mediated by Resistance to Peronospora parasitica 4 (RPP4), Resistance to Pseudomonas syringae pv maculicola (RPM1), and Resistance to Pseudomonas syringae 4 (RPS4). Single mutant mos3 plants exhibited enhanced disease susceptibility to P. s. pv maculicola ES4326, suggesting that MOS3 is required for basal resistance to pathogens as well. mos3-1 was identified by map-based cloning, and it encodes a protein with high sequence similarity to human nucleoporin 96. Localization of the MOS3-green fluorescent protein fusion to the nuclear envelope further indicates that MOS3 may encode a nucleoporin, suggesting that nuclear and cytoplasmic trafficking plays an important role in both R-gene–mediated and basal disease resistance.
INTRODUCTION
In eukaryotic cells, transcription takes place in the nucleus while proteins are synthesized in the cytoplasm. Trafficking of proteins and RNAs between the nucleus and cytoplasm uses nuclear pore complexes (NPCs) located in the nuclear envelope. In yeast, the molecular mass of the NPC is ∼66 MD, containing ∼35 to 50 unique proteins (Yang et al., 1998; Rout et al., 2000). The mammalian NPC is a much larger complex (∼125 MD) composed of ∼80 to 100 unique proteins (Gorlich and Kutay, 1999), collectively known as nucleoporins. The structure of the NPCs and the function of individual nucleoporins have been extensively studied in yeast and mammalian systems; however, there are very few studies involving NPCs in plants.
In the Arabidopsis thaliana snc1 mutant, a gain-of-function mutation in a disease resistance gene results in constitutive pathogenesis-related (PR) gene expression and pathogen resistance (Li et al., 2001; Zhang et al., 2003). SNC1 encodes a protein containing an N-terminal Toll Interleukin1 receptor (TIR) domain, a central nucleotide binding site (NBS), and a C-terminal Leu-rich repeat (LRR) domain, which are shared among a large number of resistance proteins (R-proteins) (Ellis et al., 2000; Dangl and Jones, 2001; Meyers et al., 2003). The snc1 mutation is located in the linker region between the NBS and LRR, and it may affect the interaction between SNC1 and its negative regulator. Interestingly, mutations in the corresponding linker region of NOD2, a mammalian NBS-LRR–containing protein involved in host defense against pathogens, also results in constitutive activation of NOD2 (Tanabe et al., 2004). One of the potential negative regulators of SNC1 is BON1. Loss-of-function mutations in BON1 constitutively activate SNC1-dependent PR gene expression and pathogen resistance (Yang and Hua, 2004). The genetic interaction between BON1 and SNC1 resembles the interactions between RIN4 and RPS2 or RPM1, as rin4 constitutively activate defense response in an RPM1- and RPS2-dependent fashion (Belkhadir et al., 2004a). Whereas RIN4 associates with RPM1 and RPS2 in a multiprotein complex (reviewed in Belkhadir et al., 2004b), it remains to be determined whether BON1 and SNC1 interact with each other in vivo.
Two genes have been shown to be required for the activation of downstream signaling in snc1 (Li et al., 2001; Zhang et al., 2003). One is EDS1, which was originally identified as a gene required for RPP5-mediated resistance to Peronospora parasitica Noco2 (P.p. Noco2) (Parker et al., 1996). The other is PAD4, a gene that was initially identified to be required for synthesis of camalexin in response to infection by the virulent bacterial pathogen Pseudomonas syringae pv maculicola ES4326 (P.s.m. ES4326) (Glazebrook et al., 1996). EDS1 and PAD4 both encode lipase-like proteins (Falk et al., 1999; Jirage et al., 1999), and they interact with each other in vivo (Feys et al., 2001). On the other hand, the eds5-3 mutation that blocks salicylic acid (SA) synthesis (Nawrath and Métraux, 1999; Nawrath et al., 2002) only partly affects the snc1-mediated defense signaling, suggesting that SA synthesis is only moderately required for the manifestation of the snc1 mutant phenotype (Zhang et al., 2003).
Previously NDR1, RAR1, SGT1b, and HSP90 have also been shown to be important regulators of R-gene function (reviewed in Muskett and Parker, 2003). Whereas EDS1 and PAD4 are essential for the resistance specified by the TIR-NBS-LRR proteins, NDR1 is only important for the resistance conferred by several coiled-coil–NB-LRR proteins (Century et al., 1995; Aarts et al., 1998). Unlike EDS1, PAD4 and NDR1, RAR1 and SGT1 are required for resistance mediated by R-proteins in both groups (Shirasu et al., 1999; Austin et al., 2002; Azevedo et al., 2002; Liu et al., 2002; Muskett et al., 2002; Peart et al., 2002; Tor et al., 2002; Tornero et al., 2002). HSP90 associated with both RAR1 and SGT1 and has been shown to be essential for resistance mediated by multiple R-proteins (Hubert et al., 2003; Lu et al., 2003; Takahashi et al., 2003; Liu et al., 2004). Although ndr1-1 does not suppress snc1-mediated resistance (Li et al., 2001), it is not yet known whether RAR1, SGT1b, or HSP90 are required for snc1-mediated resistance signaling.
To identify additional components required for snc1 signaling, we performed a genetic screen to search for mutations that suppress the phenotypes of snc1. mos3-1 is one of the mutants that was characterized in detail. The mos3-1 mutation abolishes both the constitutive PR gene expression and resistance to P.s.m. ES4326 and P.p. Noco2 in snc1. In addition, the mos3 single mutant is more susceptible to the virulent bacterial pathogen P.s.m. ES4326. We cloned mos3 using a map-based approach and found that it encodes a protein with high similarity to human nucleoporin 96.
RESULTS
Identification of the mos3-1 Mutant
Mutants that lost the snc1 morphological phenotypes of small stature and curly dark-green leaves were identified in the M2 population of fast neutron-mutagenized snc1 or snc1 npr1-1. The progeny of these plants were further analyzed for the expression of the pBGL2–β-glucuronidase (GUS) reporter gene in the M3 generation. Approximately 50 recessive mutants with no constitutive GUS staining were obtained and subsequently named mos (for modifier of snc1). Among them, three are mutant alleles of PAD4 (Glazebrook et al., 1996), a gene that was previously shown to be required for snc1 signaling (Zhang et al., 2003). The rest fall into 14 complementation groups mapped to loci that contain no known essential components of R-gene signaling. Here, we report the characterization and cloning of mos3-1.
The isolated mos3-1 snc1 plants are of intermediate size. The leaves of these plants are lighter green and no longer curly (Figure 1A), and the mos3-1 mutant flowers slightly earlier than wild-type plants. Real-time RT-PCR was used to compare the SNC1 expression level in snc1 and mos3-1 snc1, and no significant difference was observed (data not shown). The constitutive pBGL2-GUS reporter gene expression in snc1 was completely suppressed by the mos3-1 mutation (Figure 1B). RT-PCR analysis showed that endogenous PR-2 (BGL2) was no longer constitutively expressed (Figure 2). The constitutive expression of PR-1 in snc1 was suppressed in mos3-1 snc1 as well (Figure 2).
Figure 1.
Phenotypic Analysis of the mos3-1 Mutant.
(A) Morphology of snc1, the wild type (Col), mos3-1 snc1, and mos3-1. All plants were grown on soil and photographed when they were 4 weeks old.
(B) Suppression of snc1-induced pBGL2-GUS reporter gene expression in mos3-1 snc1. Twenty-day-old seedlings grown on MS plates were stained for GUS activity as previously described (Bowling et al., 1994).
Figure 2.
PR Gene Expression in mos3-1 snc1.
RNAs were prepared from 20-d-old plants grown on MS media and reverse transcribed to obtain total cDNA. The cDNA samples were normalized by real-time PCR using an Actin1 probe. PR-1, PR-2, and Actin1 were amplified by 35 cycles of PCR using equal amounts of total cDNA, and the products were analyzed by agarose gel electrophoresis and ethidium bromide staining. The experiment was repeated once with the same results.
When mos3-1 snc1 (pBGL2-GUS) was backcrossed with snc1 (pBGL2-GUS), the F1 progeny exhibited snc1 morphology, indicating that mos3-1 is a recessive mutation. Among the 44 F2 progeny, 35 had GUS staining, suggesting that the suppression of pBGL2-GUS expression by mos3-1 is caused by a single recessive mutation (expected ratio: 3:1, χ 2 = 0.48; P > 0.1).
mos3-1 Suppresses the Elevated SA Level in snc1
Previously, snc1 was shown to accumulate high levels of SA (Li et al., 2001). To determine whether mos3-1 affects the SA level in snc1, total SA and free SA in the mos3-1 snc1 plants were determined. As shown in Figure 3, the free SA in mos3-1 snc1 is approximately fourfold lower than that in snc1, whereas the total SA in mos3-1 snc1 is ∼10-fold lower, indicating that the signal leading to increased SA synthesis in snc1 is absent or dramatically reduced by the mos3-1 mutation. The SA level in the single mutant was similar to that in the wild-type plants (Figure 3).
Figure 3.
SA Levels in snc1, Wild-Type (Col), mos3-1 snc1, and mos3-1 Plants.
Leaves of 4-week-old soil-grown plants were collected, and SA was extracted and analyzed with high-pressure liquid chromatography using a previously described procedure (Li et al., 1999). (A), Free SA; (B), total SA levels in the leaves. The values presented are averages of four replicates ± standard deviations. The experiment was repeated once with similar results. FW, fresh weight.
mos3-1 Suppresses the Pathogen Resistance in snc1
To test whether the constitutive pathogen resistance in snc1 was affected by the mos3-1 mutation, mos3-1 snc1 plants were inoculated with the virulent bacterial pathogen P.s.m. ES4326 or oomycete pathogen P.p. Noco2. Whereas snc1 plants were resistant to these two pathogens, the mos3-1 snc1 double mutant lost the resistance completely. The bacterial growth in mos3-1 snc1 was significantly higher than that in wild-type plants but considerably lower than the bacterial growth in the snc1 pad4-1 plants (Figure 4).
Figure 4.
Growth of P.s.m. ES426 and P.p. Noco2 in snc1, Wild-Type (Col), snc1 pad4-1, and mos3-1 snc1 Plants.
(A) Growth of P.s.m. ES4326. The leaves of 4-week-old soil-grown plants were infiltrated with a suspension of the bacteria at OD600 = 0.0001. Leaf discs within the inoculated areas were taken 3 d after infection. Four replicates were taken for each treatment. The experiment was repeated once with similar results. cfu, colony-forming units.
(B) Growth of P.p. Noco2. Two-week-old seedlings were sprayed with Noco2 spores at a conidiospore suspension concentration of 3 × 104 spores per milliliter of water. The experiment was repeated twice with similar results. The infection was rated as follows on 20 plants 7 d after infection by counting the number of conidiophores per infected leaf: 0, no conidiophores on the plants; 1, no more than five conidiophores per infected leaf; 2, 6 to 20 conidiophores on a few of the infected leaves; 3, 6 to 20 conidiophores on most of the infected leaves; 4, five or more conidiophores on all infected leaves; 5, 20 or more conidiophores on all infected leaves.
Both Basal and R-Gene–Mediated Resistance Are Compromised in mos3-1 Plants
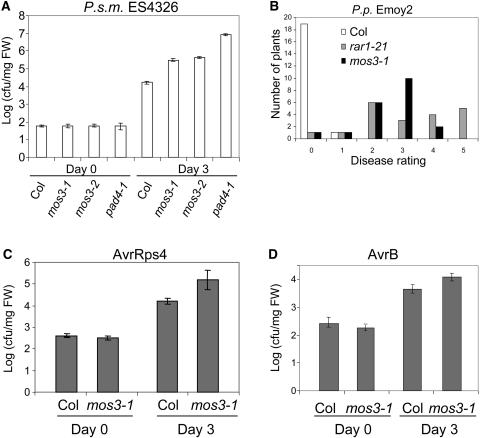
To determine whether the mos3 single mutant is more susceptible to the virulent bacterial pathogen P.s.m. ES4326, bacterial growth in mos3-1, pad4-1, and Columbia wild-type plants was compared. As shown in Figure 5A, bacterial growth in mos3-1 is ∼20-fold higher than that in the wild-type plants. In pad4-1 plants, the bacterial growth is one order of magnitude higher compared with the mos3-1 plants.
Figure 5.
Growth of P.s.m. ES4326, P.p. Emoy2, P. syringae pv tomato DC3000 with AvrRps4, and P.s.m. ES4326 with AvrB on mos3-1.
(A) Growth of P.s.m. ES4326 in mos3-1 and mos3-2 plants. The leaves of 4-week-old soil-grown plants were infiltrated with a suspension of the bacteria (OD600 = 0.0001). Leaf discs within the inoculated areas were taken 3 d after infection. Four replicates were taken for each treatment. cfu, colony-forming units; FW, fresh weight.
(B) Growth of P.p. Emoy2 on mos3-1 plants. Two-week-old seedlings were sprayed with Emoy2 spores at a conidiospore suspension concentration of 5 × 104 spores per milliliter of water. The infection was rated as described in Figure 4 on 20 plants 7 d after infection by counting the number of conidiophores per infected leaf.
(C) and (D) Growth of P. syringae pv tomato DC3000 avrRps4 (C) or P.s.m. ES4326 avrB (D) in mos3-1 plants. The leaves of 4-week-old soil-grown plants were infiltrated with a suspension of the bacteria (OD600 = 0.001). Leaf discs within the inoculated areas were taken 3 d after infection. Four replicates were taken for each treatment. All experiments were repeated at least twice with similar results.
To test whether mos3-1 affects functions of genetically defined R-genes, mos3-1 plants were inoculated with P. parasitica Emoy2 (P.p. Emoy2). In the Columbia ecotype, wild-type plants are resistant to P.p. Emoy2, and the resistance is mediated by RPP4 (van der Biezen et al., 2002), a TIR-NB-LRR–type R-gene closely related to SNC1. As shown in Figure 5B, mos3-1 was more susceptible than the wild type, but less susceptible than the rar1-21 control plants, suggesting that RPP4-mediated resistance to P.p. Emoy2 is partially compromised by mos3-1 mutation. P.p. Emoy2 infected mos3-1 leaves also displayed trailing necrosis (see Supplemental Figure 1 online), which is a phenotype often associated with partial loss of R-gene–mediated resistance. In addition, mos3-1 supports significantly higher growth of bacterial strains carrying either avrRps4 or avrB (Figures 5C and 5D), suggesting that mos3-1 partially affects the resistance mediated by RPS4 and RPM1 as well.
Map-Based Cloning of MOS3
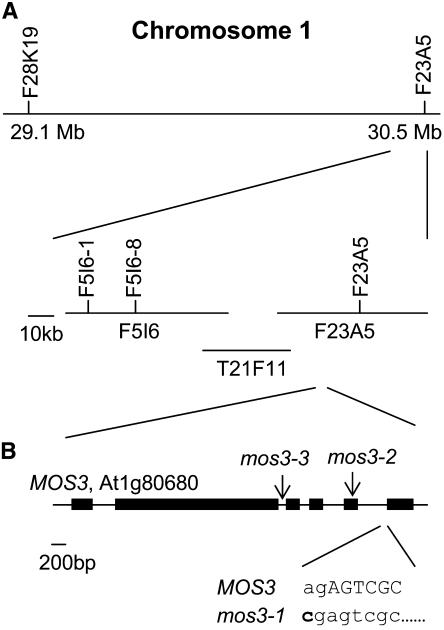
To map the mos3-1 mutation, mos3-1 snc1 (in the Columbia ecotype background with pBGL2-GUS) was crossed with Landsberg erecta (Ler)-snc1 (with no pBGL2-GUS) to generate a segregating population. For crude mapping, 24 plants homozygous at the mos3-1 locus were identified in the F2 progeny on the basis of lack of snc1 morphology. Linkage was found on the bottom of chromosome 1. When the marker F28K19 on chromosome 1 was used, two recombinants were found, whereas when the marker F23A5 was used, no recombinant was found, indicating mos3-1 is south of F28K19 and closely linked to F23A5 (Figure 6A). Because F23A5 is the last BAC clone on the south end of chromosome 1, mos3-1 is most likely to be flanked by these two markers.
Figure 6.
Map-Based Cloning of mos3-1
(A) Map of the mos3-1 locus on chromosome 1. Positions of the markers used for the mapping are indicated. Six recombinants pointed the mos3-1 mutation to the south of marker F5I6-1, and four recombinants pointed the mutation to the south of marker F5I6-8.
(B) Exon/intron structure of MOS3. The coding regions are indicated with boxes. The position of the mos3-1 mutation is indicated, and its effects on the splicing of MOS3 are detailed below the wild-type sequence. Lowercase letters mark intron sequences, and uppercase letters indicate exon sequences. The arrows indicate the positions of T-DNA insertions in mos3-2 and mos3-3.
For fine mapping, 720 randomly chosen F3 plants derived from F2 plants heterozygous for mos3-1 and carrying the homozygous pBGL2-GUS reporter gene were genotyped with the markers F28K19 and F23A5. A total of 51 recombinants between these two markers were found, and their seeds were collected. The phenotypes of the recombinants were subsequently determined by assessing the segregation of GUS staining and the snc1 morphological phenotype in their progeny. The phenotypes of all 51 recombinants were perfectly correlated with the genotypes at marker F23A5, suggesting that mos3-1 is tightly linked to this marker. Further analysis of the 51 recombinants with additional markers indicated that mos3-1 was positioned to the south of F5I6-8, located at position 89 kb of the BAC clone F5I6 (Figure 6A).
The distance between marker F5I6-8 and the south end of chromosome 1 is ∼165 kb. This region in mos3-1 snc1 was amplified by PCR and sequenced. A single A-to-C point mutation was found in At1g80680. The cDNA of the gene was then obtained by sequencing three overlapping RT-PCR fragments covering the gene and found to be consistent with the annotation of At1g80680 (GenBank accession number NM_106716). The GenBank accession number for MOS3 is AY942798. At1g80680 is identical to PRE (GenBank accession number AF411839), suggesting that mos3-1 may be allelic to pre, which was reported at the Arabidopsis Conference 2001 to have an early flowering phenotype (C. Alonso-Blanco, I. Ausin, L. Ruiz-Garcia, and J.M. Martinez-Zapater, Molecular analysis of FVE and PRE: Two genes involved in the autonomous flowering promotion pathway. 12th International Conference on Arabidopsis Research, 23–27 June, 2001, Madison, WI.). Microarray analysis of snc1 and wild-type plants indicated that there is no significant difference in the expression level of At1g80680 in the wild type and snc1 (data not shown). Comparison of the cDNA sequence and the genomic sequence revealed that At1g80680 consists of six exons. The A-to-C mutation in mos3-1 occurs at the 3′ intron acceptor site at the junction between the fifth intron and the sixth exon (Figure 6B). This mutation results in a deletion of 52 nucleotides in the cDNA. BLAST analysis of At1g80680 shows that it encodes a protein with high similarity to the human nucleoporin Nup96 and the C-terminal part of the yeast nucleoporin Nup145 (Figure 7).
Figure 7.
Amino Acid Sequence Alignments of MOS3 (AtNup96), Human Nup96 (hNup96), and Nup145 of S. cerevisiae (ScNup145).
Identical amino acids are shaded in black, and similar amino acids are shaded in gray. Alignment was performed using ClustalW (http://www.ebi.ac.uk/clustalw/). Boxshade was produced by BOXSHADE 3.21 (http://www.ch.embnet.org/software/BOX_form.html). AtNup96, accession number NP_178183; hNup96, accession number NP_057404, amino acids 864 to 1800; ScNup145, accession number CAA54057, amino acids 606 to 1317.
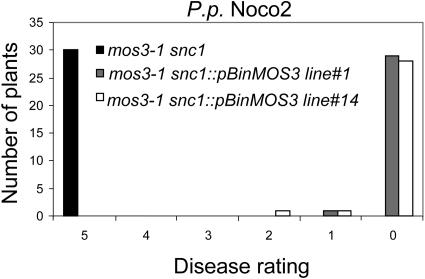
To confirm that MOS3 is At1g80680, a plasmid expressing the cDNA of At1g80680 driven by the 35S promoter of Cauliflower mosaic virus was transformed into mos3-1 snc1. Among 12 transgenic plants obtained, 10 displayed snc1 morphology. Expression of the pBGL2-GUS reporter gene was also restored in the transgenic plants with snc1 morphology, suggesting that MOS3 is At1g80680. Furthermore, progeny of two independent transgenic lines carrying the MOS3 transgene were tested for resistance against P.p. Noco2. As shown in Figure 8, constitutive resistance to P.p. Noco2 was restored in the transgenic plants.
Figure 8.
Complementation of mos3-1 snc1 by the MOS3 cDNA Clone.
Two-week-old T3 plants homozygous for the MOS3 cDNA transgene in mos3-1 snc1 background were sprayed with P.p. Noco2 spores (5 × 104 spores/mL). The infection was rated as described in Figure 4 on 20 plants 7 d after infection by counting the number of conidiophores per infected leaf. The experiment was repeated once with similar results.
At the same time, we obtained two additional mutant alleles of At1g80680 from the ABRC. mos3-2 (Salk_109959) contains a T-DNA insertion in the fifth exon, and mos3-3 (Salk_117966) contains a T-DNA insertion in the second intron of At1g80680 (Figure 6B). Because the mos3-1 single mutant is more susceptible to the virulent bacterial pathogen P.s.m. ES4326, we tested mos3-2 for enhanced disease susceptibility to this pathogen. As shown in Figure 5A, mos3-2 supports ∼20-fold more bacterial growth than wild-type plants
To test whether mos3-2 and mos3-3 are allelic to mos3-1, the homozygous lines were crossed with mos3-1 snc1. Because morphology of snc1 is recessive (Li et al., 2001), plus snc1 and mos3-1 are located on different chromosomes, if mos3-2 is not allelic to mos3-1, then 3/16 of the F2 progeny is expected to have snc1 morphology. Among 240 F2 plants from the cross between mos3-2 and mos3-1 snc1, none has the snc1 morphology, suggesting that mos3-2 is allelic to mos3-1. Similar results were obtained from the cross between mos3-1 snc1 and mos3-3. Furthermore, five independent mos3-2 snc1 lines were obtained from the F2, and the morphology of these lines is indistinguishable from that of mos3-1 snc1, further suggesting that MOS3 is At1g80680.
Subcellular Localization of MOS3
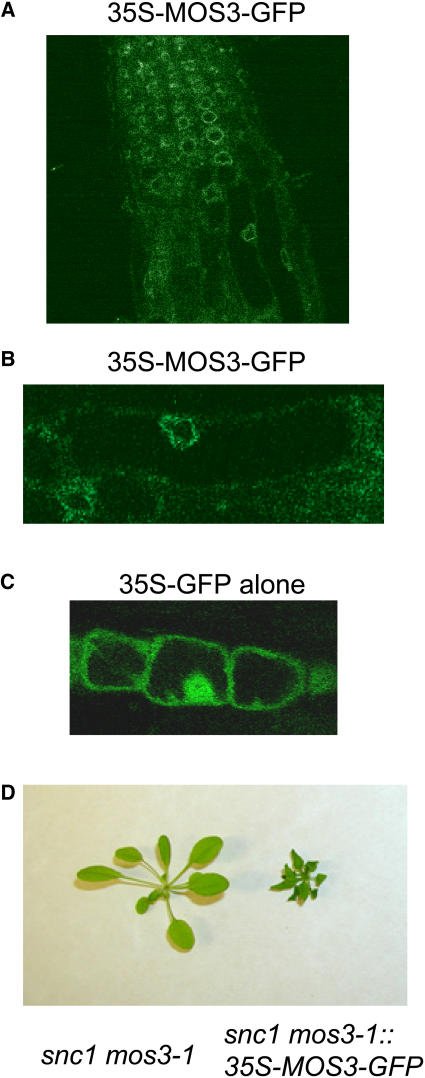
To determine the subcellular localization of the MOS3 protein, green fluorescent protein (GFP) was fused to the C terminus of MOS3. In wild-type transgenic plants expressing the MOS3-GFP fusion protein under the 35S promoter of Cauliflower mosaic virus, GFP fluorescence was observed predominantly on the nuclear rim (Figures 9A and 9B), suggesting that MOS3 is localized to the nuclear envelope. By comparison, GFP in the 35S-GFP control plants was present in both cytoplasm and nucleus (Figure 9C). To determine whether the MOS3-GFP protein is functional, mos3-1 snc1 plants were transformed with the pBI-MOS3-GFP construct. As shown in Figure 9D, transgenic plants expressing the MOS3-GFP fusion protein displayed snc1 morphology, suggesting that the MOS3-GFP can complement the mos3-1 mutation, and the fusion protein is correctly localized in Arabidopsis plants.
Figure 9.
Subcellular Localization of MOS3.
(A) Roots of 2-week-old Arabidopsis transgenic plants expressing the MOS3-GFP fusion protein were examined by confocal microscopy, and the picture was taken from the root tip region of a representative plant.
(B) Magnified view of (A). Arabidopsis root cells expressing the MOS3-GFP fusion protein.
(C) Two-week-old Arabidopsis root cells expressing GFP alone.
(D) Complementation of mos3-1 with MOS3-GFP. Morphology of a representative T1 transgenic plant expressing MOS3-GFP protein in mos3-1 snc1 background.
DISCUSSION
To elucidate the components of R-gene–mediated resistance signaling, a screen for suppressor mutations of snc1 was performed. Although multiple defense response pathways are constitutively activated in snc1, no cell death was observed (Li et al., 2001), providing a clean background for identifying genes that are required for pathogen resistance in the absence of hypersensitive response. One of the mutants, designated as mos3-1, was identified based on its lack of snc1 mutant morphology and constitutive PR gene expression. The mos3-1 mutation also suppresses the elevated SA level and constitutive pathogen resistance in snc1. In addition, mos3-1 partially affects the resistance mediated by RPP4, RPS4, and RPM1. However, the effects of mos3-1 mutation on resistance mediated by these R-genes seem to be much smaller than its effect on snc1-mediated resistance. Similarly, pad4-1 completely suppresses the snc1-mediated resistance response (Zhang et al., 2003) but is only partially required for resistance mediated by RPP4, RPP5, and RPS4 (Feys et al., 2001; van der Biezen et al., 2002). One possibility is that the loss of resistance caused by mos3-1 and pad4 is partially compensated by the strong resistance resulting from hypersensitive response.
MOS3 is not only required for the activation of downstream defense pathways by the snc1 mutation, it also plays an essential role in basal resistance against the bacterial pathogen P.s.m. ES4326. Growth of P.s.m. ES4326 in mos3-1 and mos3-2 is ∼20-fold higher than that in the wild-type plants. Previously, PAD4 and EDS1 have been shown to be essential for snc1 signaling. Interestingly, mutations in PAD4 and EDS1 also result in enhanced disease susceptibility to virulent pathogens (Glazebrook et al., 1996; Parker et al., 1996). Microarray analysis of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen P. syringae indicated that the difference between these two kinds of interactions is largely quantitative (Tao et al., 2003). The identification of MOS3 as another component shared between R-gene signaling and basal resistance further suggests that there is significant overlap between the signal transduction pathways of basal resistance and R-gene–mediated resistance responses.
Map-based cloning of MOS3 revealed that it encodes a protein with high similarity to mammalian nucleoporin 96 (Fontoura et al., 1999) and yeast C-Nup145p (Fabre et al., 1994; Wente and Blobel, 1994). In mammals, the gene encoding Nup96 also encodes another nucleoporin, Nup98. Proteolytic cleavage of a 186-kD precursor protein yields both Nup96 and Nup98 (Fontoura et al., 1999). The yeast Nup96 homolog, C-Nup145p, is also produced through proteolytic cleavage of a precursor protein that yields both C-Nup145p and N-Nup145p, the Nup98 equivalent in yeast (Dockendorff et al., 1997; Emtage et al., 1997; Teixeira et al., 1997). Database searches revealed that MOS3 is the only Nup96 homolog and the message for MOS3 only encodes AtNup96. There are two Arabidopsis proteins that show high similarity to Nup98. These two putative Nup98 homologs, At1g10390 and At1g59660, are located on the top and middle of chromosome 1, respectively. AtNup96 and AtNup98 are encoded at different chromosomal locations. Therefore, unlike in mammals and yeast, they cannot be part of a polyprotein.
Embedded in the double membrane nuclear envelop (NE), NPCs form a ring-like structure surrounding a central pore that is believed to facilitate bidirectional transport of RNAs, proteins, ribonucleoprotein particals, and larger cargoes through the NE, and at the same time allow diffusion of small molecules and ions across the NE (reviewed in Fahrenkrog et al., 2004). The overall three-dimensional architectures of the NPCs seem to be conserved from yeast to mammals. Most nucleoporins are believed to have a symmetrical distribution on both the cytoplasmic and nuclear sides of the NPC.
In yeast, the gene encoding Nup145p has been shown to be important for RNA export (Dockendorff et al., 1997; Emtage et al., 1997). Cells lacking C-Nup145 grew at 23 and 30°C, but were unable to grow at 37°C. A shift to 37°C led to a dramatic and rapid nuclear accumulation of poly(A) RNA in the mutant cell. However, deleting up to 200 amino acids from the C terminus did not affect the cell viability at 37°C (Dockendorff et al., 1997). In human cell lines, Nup96, along with Nup98, a nucleoporin encoded by the same gene encoding NUP96, is induced by interferon (Enninga et al., 2002). The upregulation of Nup96 and Nup98 appears to play an important role in antiviral responses because treatment with interferon-gamma or transfection of a cDNA encoding Nup96 and Nup98 reverses the inhibition of mRNA nuclear export by a viral protein (Enninga et al., 2002). It is unclear whether both NUP96 and NUP98 are required in the reverse of the inhibition of mRNA export. The identification of mos3-1 as a suppressor of snc1 suggests that the Arabidopsis Nup96 also plays an important role in defense against pathogens. It is unclear how MOS3 regulates the snc1-mediated defense responses and basal resistance. One simple explanation is that MOS3 is involved in the nuclear export of an RNA encoding a positive regulator important for activation of disease resistance signaling. A block in the nuclear export of this RNA in mos3 mutants might result in suppression of snc1 mutant phenotypes and reduced basal resistance.
On the other hand, physiological roles of the NPCs are not limited to nucleocytoplasmic transport. Studies from yeast and mammalian systems indicate that nucleoporins play essential roles in many other processes, including regulation of gene expression, chromatin organization, chromosome positioning, apoptosis, and the secretory pathway (reviewed in Fahrenkrog et al., 2004). In yeast, Nup145 has been shown to be involved in the organization of a nuclear subdomain, and deletion of C-Nup145p results in derepression of a subtelomeric URA3 reporter gene (Galy et al., 2000). If MOS3 has a similar function, mutations in MOS3 may result in increased expression of genes that are normally silenced. The suppression of snc1 and reduced basal resistance could be a result of overexpression of a negative regulator of disease resistance. Moreover, human Nup96 stably interacts with Sec13, a protein that is located in both endoplasmic reticulum (ER) and the NPC (Enninga et al., 2003). At the ER, Sec13 is involved in the biogenesis of COPII-coated vesicles. Because Sec13 shuttles between the cytoplasm and the nucleus, Nup96 may help to couple and regulate functions between these two compartments through its association with Sec13 (Enninga et al., 2003). In plants, MOS3 may play a similar role in the crosstalk between the NPC and the ER, which in turn regulates disease resistance signaling. Further characterization and identification of the other mos mutants will help us address the question on how MOS3 regulates snc1-mediated resistance mechanistically. At the same time, the mos3 mutant will be an important tool for general understanding of nuclear-cytoplasmic trafficking that seems to be understudied in plants.
METHODS
Mutant Screen and Characterization
All plants were grown at 22°C under 16-h-light/8-h-dark cycles. The snc1 or snc1 npr1-1 mutant seeds were treated with fast neutron at a dose of 60 Gy by Andrea Kodym (Agriculture and Biotechnology Laboratory, International Atomic Energy Agency, Vienna, Austria). In the primary screen, ∼150,000 M2 plants representing ∼10,000 M1 families were grown on soil and screened for loss of the snc1 morphological phenotype. Seeds from the putative mutants were subsequently plated on MS medium and tested for loss of the constitutive pBGL2-GUS reporter gene expression by GUS staining. Mutants with no constitutive GUS staining were backcrossed with snc1, and the morphology of the F1 plants was analyzed to determine whether the mutation is dominant or recessive.
RNA used for gene expression analysis was extracted from 20-d-old plants grown on MS medium using the Totally RNA kit from Ambion (Austin, TX). Reverse transcription was performed using the RT-for-PCR kit from Clontech (Palo Alto, CA). Real-time PCR was performed using the QuantiTect SYBR Green PCR kit from Qiagen (Valencia, CA). The primers used for amplification of Actin1, PR-1, PR-2, and SNC1 were described previously (Zhang et al., 2003).
Infection of wild-type and mutant Arabidopsis thaliana with Pseudomonas syringae maculicola ES4326 was performed on 4-week-old plants and infection of Peronospora parasitica Noco2 was performed on 2-week-old seedlings as described (Li et al., 1999). SA was extracted and measured using a previously described procedure (Li et al., 1999).
Map-Based Cloning of mos3
To introgress the Columbia snc1 mutation into the Landsberg ecotype background, snc1 was backcrossed with wild-type Landsberg plants six times. A single line homozygous for snc1 was selected from the F2 population of the sixth backcross and designated Ler-snc1. This line was confirmed to contain homozygous Landsberg sequences at each arm of the five chromosomes. To map the mos3-1 mutation, mos3-1 was crossed with Ler-snc1. Crude mapping was performed on the F2 plants homozygous for mos3-1, and fine mapping was performed on F3 plants derived from F2 plants heterozygous for mos3-1 while carrying the homozygous pBGL2-GUS reporter gene. Both morphology and GUS staining of the progeny were used to confirm the phenotypes of the mapping lines.
The markers used for mapping were designed according to the Monsanto Arabidopsis polymorphism and Landsberg sequence collections (Jander et al., 2002). Marker F28K19 was amplified with primers 5′-CTTAATAAAGTTGGTTCAACCG-3′ and 5′-GTTGCCATTAGCAAGCTGTC-3′, and the Columbia fragment was 29 bp shorter than the Landsberg fragment. Marker F23A5 was amplified with primers 5′-AAGTTTTCGAGATGCGCTGC-3′ and 5′-CACCTTTTGCTTTGGCCGTC-3′, and the Columbia fragment was 35 bp shorter than the Landsberg fragment. Marker F5I6-1 was amplified with primers 5′-AACTATACAGGCCGCATTAAC-3′ and 5′-GCTCCGCCTTTGCCACGCCA-3′, and the Columbia fragment was 99 bp shorter than the Landsberg fragment. Marker F5I6-8 was amplified with primers 5′-CGTGACAGCGCTGGCTGAG-3′ and 5′-CGTTCTGGTTTCGTCTGGAG-3′, and the single nucleotide polymorphism was detected by sequencing the PCR fragments.
To confirm that the mutation identified in At1g80680 is mos3-1, full-length cDNA was amplified by RT-PCR, cloned into pBI1.4T (Mindrinos et al., 1994) and transformed into mos3-1 snc1 plants by the floral dipping method (Clough and Bent, 1998). Transgenic plants were selected on MS plates containing 50 μg/mL of kanamycin.
The T-DNA insertion mutants mos3-2 and mos3-3 were obtained from the ABRC (Alonso et al., 2003). Plants homozygous for the T-DNA insertions were identified by PCR using primers 5′-GGGATTTGTTGCACAGCTTC-3′ and 5′-ACTCATTCACGCTTCTAAGG-3′, which flank the insertions.
To identify the mos3-1 single mutant, mos3-1 snc1 was crossed with wild-type plants containing pBGL2-GUS. F2 plants were genotyped for the mos3-1 and snc1 mutations by PCR. Lines homozygous for mos3-1 with no snc1 mutation were kept as mos3-1 single mutants and used for further analysis.
Subcellular Localization of MOS3
To fuse MOS3 to the GFP gene, full-length MOS3 cDNA was amplified by PCR and cloned into the pBS-GFP5 vector (Haseloff et al., 1997). The resulting plasmid was sequenced to confirm that the fusion was in frame without PCR mistakes. The fragment containing the MOS3-GFP fusion was subsequently excised and cloned into pBI1.4T to obtain pBI-MOS3-GFP. pBI-MOS3-GFP was used to transform mos3-1 snc1 and the wild-type plants; transgenic plants were selected on MS medium containing 50 μg/mL of kanamycin. Roots of the transgenic seedlings were examined for GFP by confocal microscopy as described previously (Haseloff et al., 1997).
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession number AY942798.
Supplementary Material
Acknowledgments
We thank Mark Tessaro, Yu-ti Cheng, Arthur Lau, and Gary Wong for their excellent technical assistance; Jane Glazebrook for pad4-1 seeds; Jeff Dangl for rar1-21 seeds; Jane Parker for P. parasitica strains; Brian Staskawitz and Roger Innes for strains of P. syringae carrying avirulence genes AvrRps4 and AvrB; Greg Lampard and Brian Ellis for Arabidopsis transgenic lines with 35S-GFP; ABRC and SALK for mos3-2 and mos3-3; Hugo Zheng and the University of British Columbia Electron Microscopy Lab for help with confocal microscopy; Jim Kronstad, Kristoffer Palma, and Sandra Goritschnig for critical reading of the manuscript; and three anonymous reviewers for helpful comments and suggestions. We are grateful for the financial support to X.L. from the University of British Columbia, the Natural Sciences and Engineering Research Council of Canada, and the Canadian Foundation for Innovation.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Xin Li (xinli@interchange.ubc.ca).
Online version contains Web-only data.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.104.029926.
References
- Aarts, N., Metz, M., Holub, E., Staskawicz, B.J., Daniels, M.J., and Parker, J.E. (1998). Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. USA 95, 10306–10311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso, J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657. [DOI] [PubMed] [Google Scholar]
- Austin, M.J., Muskett, P., Kahn, K., Feys, B.J., Jones, J.D., and Parker, J.E. (2002). Regulatory role of SGT1 in early R gene-mediated plant defenses. Science 295, 2077–2080. [DOI] [PubMed] [Google Scholar]
- Azevedo, C., Sadanandom, A., Kitagawa, K., Freialdenhoven, A., Shirasu, K., and Schulze-Lefert, P. (2002). The RAR1 interactor SGT1, an essential component of R gene-triggered disease resistance. Science 295, 2073–2076. [DOI] [PubMed] [Google Scholar]
- Belkhadir, Y., Nimchuk, Z., Hubert, D.A., Mackey, D., and Dangl, J.L. (2004. a). Arabidopsis RIN4 negatively regulates disease resistance mediated by RPS2 and RPM1 downstream or independent of the NDR1 signal modulator and is not required for the virulence functions of bacterial type III effectors AvrRpt2 or AvrRpm1. Plant Cell 16, 2822–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkhadir, Y., Subramaniam, R., and Dangl, J.L. (2004. b). Plant disease resistance protein signaling: NBS-LRR proteins and their partners. Curr. Opin. Plant Biol. 7, 391–399. [DOI] [PubMed] [Google Scholar]
- Bowling, S.A., Guo, A., Cao, H., Gordon, A.S., Klessig, D.F., and Dong, X. (1994). A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 6, 1845–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Century, K.S., Holub, E.B., and Staskawicz, B.J. (1995). NDR1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and a fungal pathogen. Proc. Natl. Acad. Sci. USA 92, 6597–6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Dangl, J.L., and Jones, J.D.G. (2001). Plant pathogens and integrated defence responses to infection. Nature 411, 826–833. [DOI] [PubMed] [Google Scholar]
- Dockendorff, T.C., Heath, C.V., Goldstein, A.L., Snay, C.A., and Cole, C.N. (1997). C-terminal truncations of the yeast nucleoporin Nup145p produce a rapid temperature-conditional mRNA export defect and alterations to nuclear structure. Mol. Cell. Biol. 17, 906–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, J., Dodds, P., and Pryor, T. (2000). Structure, function and evolution of plant disease resistance genes. Curr. Opin. Plant Biol. 3, 278–284. [DOI] [PubMed] [Google Scholar]
- Emtage, J.L., Bucci, M., Watkins, J.L., and Wente, S.R. (1997). Defining the essential functional regions of the nucleoporin Nup145p. J. Cell Sci. 110, 911–925. [DOI] [PubMed] [Google Scholar]
- Enninga, J., Levay, A., and Fontoura, B.M. (2003). Sec13 shuttles between the nucleus and the cytoplasm and stably interacts with Nup96 at the nuclear pore complex. Mol. Cell. Biol. 23, 7271–7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enninga, J., Levy, D.E., Blobel, G., and Fontoura, B.M. (2002). Role of nucleoporin induction in releasing an mRNA nuclear export block. Science 295, 1523–1525. [DOI] [PubMed] [Google Scholar]
- Fabre, E., Boelens, W.C., Wimmer, C., Mattaj, I.W., and Hurt, E.C. (1994). Nup145p is required for nuclear export of mRNA and binds homopolymeric RNA in vitro via a novel conserved motif. Cell 78, 275–289. [DOI] [PubMed] [Google Scholar]
- Fahrenkrog, B., Koser, J., and Aebi, U. (2004). The nuclear pore complex: A jack of all trades? Trends Biochem. Sci. 29, 175–182. [DOI] [PubMed] [Google Scholar]
- Falk, A., Feys, B.J., Frost, L.N., Jones, J.D., Daniels, M.J., and Parker, J.E. (1999). EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc. Natl. Acad. Sci. USA 96, 3292–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feys, B.J., Moisan, L.J., Newman, M.-A., and Parker, J.E. (2001). Direct interaction between the Arabidopsis disease resistance signalling proteins, EDS1 and PAD4. EMBO J. 20, 5400–5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontoura, B.M., Blobel, G., and Matunis, M.J. (1999). A conserved biogenesis pathway for nucleoporins: Proteolytic processing of a 186-kilodalton precursor generates Nup98 and the novel nucleoporin, Nup96. J. Cell Biol. 144, 1097–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galy, V., Olivo-Marin, J.C., Scherthan, H., Doye, V., Rascalou, N., and Nehrbass, U. (2000). Nuclear pore complexes in the organization of silent telomeric chromatin. Nature 403, 108–112. [DOI] [PubMed] [Google Scholar]
- Glazebrook, J., Rogers, E.E., and Ausubel, F.M. (1996). Isolation of Arabidopsis mutants with enhanced disease susceptibility by direct screening. Genetics 143, 973–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlich, D., and Kutay, U. (1999). Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell Dev. Biol. 15, 607–660. [DOI] [PubMed] [Google Scholar]
- Haseloff, J., Siemering, K.R., Prasher, D.C., and Hodge, S. (1997). Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc. Natl. Acad. Sci. USA 94, 2122–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert, D.A., Tornero, P., Belkhadir, Y., Krishna, P., Takahashi, A., Shirasu, K., and Dangl, J.L. (2003). Cytosolic HSP90 associates with and modulates the Arabidopsis RPM1 disease resistance protein. EMBO J. 22, 5679–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jander, G., Norris, S.R., Rounsley, S.D., Bush, D.F., Levin, I.M., and Last, R.L. (2002). Arabidopsis map-based cloning in the post-genome era. Plant Physiol. 129, 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirage, D., Tootle, T.L., Reuber, T.L., Frost, L.N., Feys, B.J., Parker, J.E., Ausubel, F.M., and Glazebrook, J. (1999). Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc. Natl. Acad. Sci. USA 96, 13583–13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Clarke, J.D., Zhang, Y., and Dong, X. (2001). Activation of an EDS1-mediated R-gene pathway in the snc1 mutant leads to constitutive, NPR1-independent pathogen resistance. Mol. Plant Microbe Interact. 14, 1131–1139. [DOI] [PubMed] [Google Scholar]
- Li, X., Zhang, Y., Clarke, J.D., Li, Y., and Dong, X. (1999). Identification and cloning of a negative regulator of systemic acquired resistance, SNI1, through a screen for suppressors of npr1-1. Cell 98, 329–339. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Burch-Smith, T., Schiff, M., Feng, S., and Dinesh-Kumar, S.P. (2004). Molecular chaperone Hsp90 associates with resistance protein N and its signaling proteins SGT1 and Rar1 to modulate an innate immune response in plants. J. Biol. Chem. 279, 2101–2108. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Schiff, M., Serino, G., Deng, X.W., and Dinesh-Kumar, S.P. (2002). Role of SCF ubiquitin-ligase and the COP9 signalosome in the N gene-mediated resistance response to Tobacco mosaic virus. Plant Cell 14, 1483–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, R., Malcuit, I., Moffett, P., Ruiz, M.T., Peart, J., Wu, A.J., Rathjen, J.P., Bendahmane, A., Day, L., and Baulcombe, D.C. (2003). High throughput virus-induced gene silencing implicates heat shock protein 90 in plant disease resistance. EMBO J. 22, 5690–5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers, B.C., Kozik, A., Griego, A., Kuang, H., and Michelmore, R.W. (2003). Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15, 809–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindrinos, M., Katagiri, F., Yu, G.-L., and Ausubel, F.M. (1994). The A. thaliana disease resistance gene RPS2 encodes a protein containing a nucleotide-binding site and leucine-rich repeats. Cell 78, 1089–1099. [DOI] [PubMed] [Google Scholar]
- Muskett, P., and Parker, J. (2003). Role of SGT1 in the regulation of plant R gene signalling. Microbes Infect. 5, 969–976. [DOI] [PubMed] [Google Scholar]
- Muskett, P.R., Kahn, K., Austin, M.J., Moisan, L.J., Sadanandom, A., Shirasu, K., Jones, J.D., and Parker, J.E. (2002). Arabidopsis RAR1 exerts rate-limiting control of R gene-mediated defenses against multiple pathogens. Plant Cell 14, 979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath, C., Heck, S., Parinthawong, N., and Métraux, J.-P. (2002). EDS5, an essential component of salicylic acid–dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14, 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath, C., and Métraux, J.-P. (1999). Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11, 1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, J.E., Holub, E.B., Frost, L.N., Falk, A., Gunn, N.D., and Daniels, M.J. (1996). Characterization of eds1, a mutation in Arabidopsis suppressing resistance to Peronospora parasitica specified by several different RPP genes. Plant Cell 8, 2033–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peart, J.R., et al. (2002). Ubiquitin ligase-associated protein SGT1 is required for host and nonhost disease resistance in plants. Proc. Natl. Acad. Sci. USA 99, 10865–10869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout, M.P., Aitchison, J.D., Suprapto, A., Hjertaas, K., Zhao, Y., and Chait, B.T. (2000). The yeast nuclear pore complex: Composition, architecture, and transport mechanism. J. Cell Biol. 148, 635–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasu, K., Lahaye, T., Tan, M.W., Zhou, F., Azevedo, C., and Schulze-Lefert, P. (1999). A novel class of eukaryotic zinc-binding proteins is required for disease resistance signaling in barley and development in C. elegans. Cell 99, 355–366. [DOI] [PubMed] [Google Scholar]
- Takahashi, A., Casais, C., Ichimura, K., and Shirasu, K. (2003). HSP90 interacts with RAR1 and SGT1 and is essential for RPS2-mediated disease resistance in Arabidopsis. Proc. Natl. Acad. Sci. USA 100, 11777–11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe, T., et al. (2004). Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 23, 1587–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao, Y., Xie, Z., Chen, W., Glazebrook, J., Chang, H.S., Han, B., Zhu, T., Zou, G., and Katagiri, F. (2003). Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. Plant Cell 15, 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira, M.T., Siniossoglou, S., Podtelejnikov, S., Benichou, J.C., Mann, M., Dujon, B., Hurt, E., and Fabre, E. (1997). Two functionally distinct domains generated by in vivo cleavage of Nup145p: A novel biogenesis pathway for nucleoporins. EMBO J. 16, 5086–5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tor, M., Gordon, P., Cuzick, A., Eulgem, T., Sinapidou, E., Mert-Turk, F., Can, C., Dangl, J.L., and Holub, E.B. (2002). Arabidopsis SGT1b is required for defense signaling conferred by several downy mildew resistance genes. Plant Cell 14, 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero, P., Merritt, P., Sadanandom, A., Shirasu, K., Innes, R.W., and Dangl, J.L. (2002). RAR1 and NDR1 contribute quantitatively to disease resistance in Arabidopsis, and their relative contributions are dependent on the R gene assayed. Plant Cell 14, 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Biezen, E.A., Freddie, C.T., Kahn, K., Parker, J.E., and Jones, J.D. (2002). Arabidopsis RPP4 is a member of the RPP5 multigene family of TIR-NB-LRR genes and confers downy mildew resistance through multiple signalling components. Plant J. 29, 439–451. [DOI] [PubMed] [Google Scholar]
- Wente, S.R., and Blobel, G. (1994). NUP145 encodes a novel yeast glycine-leucine-phenylalanine-glycine (GLFG) nucleoporin required for nuclear envelope structure. J. Cell Biol. 125, 955–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Q., Rout, M.P., and Akey, C.W. (1998). Three-dimensional architecture of the isolated yeast nuclear pore complex: Functional and evolutionary implications. Mol. Cell 1, 223–234. [DOI] [PubMed] [Google Scholar]
- Yang, S., and Hua, J. (2004). A haplotype-specific resistance gene regulated by BONZAI1 mediates temperature-dependent growth control in Arabidopsis. Plant Cell 16, 1060–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y., Goritschnig, S., Dong, X., and Li, X. (2003). A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. Plant Cell 15, 2636–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.