Abstract
Nitric oxide (NO) serves as an evolutionarily conserved signaling molecule that plays an important role in a wide variety of cellular processes. Extensive studies in Drosophila melanogaster have revealed that NO signaling is required for development, physiology, and stress responses in many different types of cells. In neuronal cells, multiple NO signaling pathways appear to operate in different combinations to regulate learning and memory formation, synaptic transmission, selective synaptic connections, axon degeneration, and axon regrowth. During organ development, elevated NO signaling suppresses cell cycle progression, whereas downregulated NO leads to an increase in larval body size via modulation of hormone signaling. The most striking feature of the Drosophila NO synthase is that various stressors, such as neuropeptides, aberrant proteins, hypoxia, bacterial infection, and mechanical injury, can activate Drosophila NO synthase, initially regulating cellular physiology to enable cells to survive. However, under severe stress or pathophysiological conditions, high levels of NO promote regulated cell death and the development of neurodegenerative diseases. In this review, I highlight and discuss the current understanding of molecular mechanisms by which NO signaling regulates distinct cellular functions and behaviors.
Keywords: Drosophila, Neurodegenerative disease, Nitric oxide, Nitric oxide synthase, Stress response
Graphical abstract

Highlights
-
•
Nitric oxide (NO) serves as a multifunctional signaling molecule within and between cells in Drosophila.
-
•
The Drosophila NO synthase produces NO in response to various types of stressors including aberrant proteins and hypoxia.
-
•
The combinatorial control by multiple NO signaling pathways seems to regulate diverse cellular processes in Drosophila.
-
•
NO signaling in Drosophila plays a protective or destructive role in various stress conditions.
INTRODUCTION
Nitric oxide (NO), which serves as a gaseous signaling molecule in vertebrates and invertebrates, was shown to play essential roles in several physiological systems including the cardiovascular, nervous, and immune systems (Lee et al., 2022a, Lee et al., 2022b, Lundberg and Weitzberg, 2022, Seo et al., 2023). Owing to its ability to rapidly pass through the hydrophobic membrane, NO generated in the brain cortex homogenously diffuses in all directions (Meulemans, 1994). Therefore, NO can function both as a paracrine and autocrine signal. NO is endogenously produced by a family of NO synthase (NOS) enzymes (Garthwaite, 2016). Three different NOS isoforms are found in mammals, that is, endothelial NOS, neuronal NOS, and cytokine-inducible NOS. However, the Drosophila genome encodes only 1 NOS enzyme (dNOS) (Andreakis et al., 2011). These NOS enzymes share an N-terminal oxygenase domain, a central calmodulin (CaM)-binding motif, and a C-terminal reductase domain (Andreakis et al., 2011). The oxygenase domain contains evolutionarily conserved binding sites for heme and tetrahydrobiopterin (BH4) while the reductase domain has binding sites for flavin mononucleotide, flavin adenine dinucleotide, and nicotinamide adenine dinucleotide phosphate (NADPH) (Gonzalez-Domenech and Munoz-Chapuli, 2010, Jeandroz et al., 2016). Upon Ca2+-CaM binding, these 2 domains act in consort to convert L-arginine to NO and L-citrulline through NADPH- and O2-dependent oxidation reactions (Griffith and Stuehr, 1995, Marletta et al., 1998). Two flavin cofactors flavin adenine dinucleotide and flavin mononucleotide transfer electrons, which are generated from NADPH, to the heme iron during the oxidation reactions (Griffith and Stuehr, 1995). Recently, BH4 was identified as a potential electron donor to the heme-dioxy species (Hurshman and Marletta, 2002, Stuehr and Haque, 2019). Biochemical studies suggest that the catalytic and regulatory properties of dNOS are most similar to those of mammalian neuronal NOS (Ray et al., 2007a, Ray et al., 2007b, Sengupta et al., 2003). Evolutionary conservation in domain structure and enzymatic and regulatory properties between vertebrate and Drosophila NOSs strongly suggests that these enzymes have many common elements in the cellular functions and regulatory mechanisms mediated by NO signaling.
Indeed, the canonical NO signaling in vertebrates and invertebrates is commonly mediated by soluble guanylate cyclase (sGC) that catalyzes the production of cyclic guanosine monophosphate (cGMP) from guanosine triphosphate (GTP) when activated upon NO binding to the heme of sGC (Fig. 1; Martínez-Ruiz et al., 2011). Cyclic nucleotide-gated (CNG) ion channels and protein kinase G (PKG) are the 2 classes of downstream target proteins that are directly regulated by cGMP in both vertebrates and invertebrates (Steinert et al., 2010). Moreover, the reactive free radical NO in these animal groups appears to regulate various cellular processes through S-nitrosation and 3-nitrotyrosination (Fig. 1; Robinson et al., 2018; Steinert et al., 2010; Tegeder et al., 2011). Interestingly, NO signaling in vertebrates was shown to play a crucial role in cellular stress response (Kim et al., 2023, Suzuki et al., 2023, Thomas et al., 2008). Several stressors and stress-associated signaling molecules that increase the activity of the dNOS enzyme are found in Drosophila model animals (Fig. 1). Those include neuropeptides, aberrant proteins, hypoxia, bacterial infection, and mechanical injury (Fig. 1). These findings support the idea that the major signaling pathways mediated by NO appear to be conserved between Drosophila and vertebrates. However, there are some potential limitations to research in model animals, such as Drosophila, due to the anatomical, developmental, and genetic differences between the fruit fly and vertebrate systems (Jeibmann and Paulus, 2009, Marsh and Thompson, 2006). Therefore, some intracellular mediators of NO signaling may be vertebrate-specific or fly-specific (Jeibmann and Paulus, 2009, Marsh and Thompson, 2006).
Fig. 1.
Nitric oxide signaling pathways in Drosophila. The dNOS that is activated by several stress-associated stimuli including neuropeptides, aberrant stress proteins, hypoxia, bacterial infection, and mechanical injury, produces gaseous NO. NO signaling can be mediated by the activation of sGC and Ser/Thr phosphatase CaN or by the inhibition of nuclear receptor E75. In addition, NO-induced nitrosation and nitrotyrosination also regulate a wide range of cellular physiologies. GFAP, glial fibrillary acidic protein; PTTH, prothoracicotropic hormone; dNOS, Drosophila nitric oxide synthase, NO, nitric oxide; CaN, calcineurin; sGC, soluble guanylate cyclase; cGMP, cyclic GMP; CNGs, cyclic nucleotide-gated ion channels; PKG, protein kinase G; E75, nuclear receptor E75.
In vertebrates, cGMP-dependent PKG was shown to reduce vascular smooth muscle tone and also to modulate synaptic excitability through the phosphorylation of specific ion channels and GluR1, respectively (Farah et al., 2018, Steinert et al., 2010). In Drosophila, the V-type H+ adenosine 5'-triphosphatase (V-type H+ ATPase), which generates the proton gradient across the tubule principal cell membranes and potentiates fluid secretion, appears to be a target of PKG and/or cGMP (Fig. 1; Davies et al., 2013). In the Drosophila innate immune response, the Ser/Thr phosphatase calcineurin A1 present in hemocytes is activated by NO signaling, although the underlying mechanism is not clear (Dijkers and O'Farrell, 2007). In contrast with sGC, NO binding to the heme of nuclear receptor E75 was shown to negatively regulate the transcriptional repressor activity of E75 (Fig. 1; Cáceres et al., 2011). Whether the function and regulation of these mediators have diverged between vertebrates and invertebrates requires further studies.
It is clear that Drosophila research in many fields has led to corresponding studies in other invertebrates as well as vertebrates, contributing to new discoveries and expanding our understanding (Bellen et al., 2010, Rubin and Lewis, 2000). Therefore, in this review, I summarize and discuss the functional roles and regulatory mechanisms of Drosophila NO signaling not only in normal physiological conditions but also in pathophysiological and stress conditions.
LEARNING AND MEMORY FORMATION
Recently, NO was found to act as a cotransmitter in a few types of dopaminergic neurons (DANs) that are involved in either aversive or attractive olfactory learning (Aso et al., 2019). In wild-type flies, the optogenetic activation of the posterior protocerebrum lateral 1 cluster of DANs induces an aversive response, whereas in tyrosine hydroxylase (TH) mutants, characterized by lack of production of dopamine, the activation of the same cluster results in an attractive response (Aso et al., 2019). Interestingly, this valence-inversion phenotype is also observed in the reward-related protocerebral anterior medial (PAM) cluster of DANs. The optogenetic activation of the PAM cluster induces an attractive response in wild-type, but an aversive response in TH mutant flies (Aso et al., 2019). Knockdown of dNOS using RNA interference (RNAi) or the competitive NOS inhibitor Nω-nitro-L-arginine (L-NNA) robustly suppresses the positive-valence memory observed in TH mutant flies (Aso et al., 2019). Furthermore, the NO-dependent memory formation depends on sGC Gycβ100B expressed in Kenyon cells (KCs) (Aso et al., 2019). These findings suggest that both dNOS in DANs and sGC in KCs play a pivotal role in the formation of olfactory memory. However, the mechanism by which NO acts as an antagonist to dopamine is not clear. Further studies demonstrated that NO is also involved in limiting memory retention and promoting fast update of memory (Aso et al., 2019).
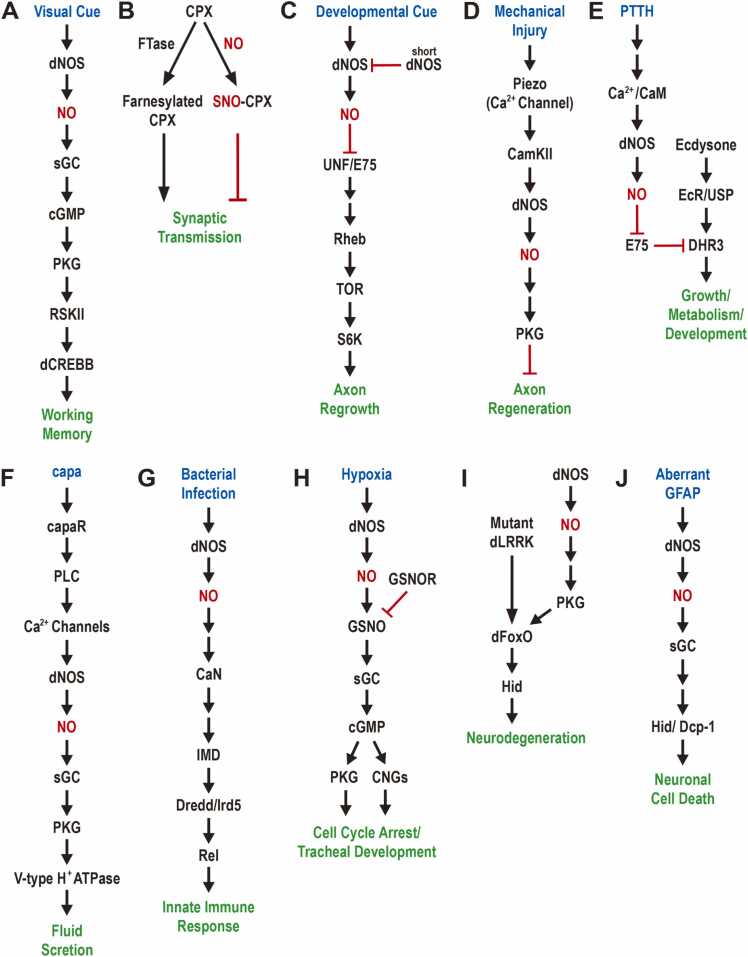
NO/cGMP signaling pathway is involved in the formation of visual short-term working memory in addition to its long-term memory function. Visual working memory can be defined as a short-term memory of the visual information, including shape, color, and location, that is used for performing ongoing cognitive tasks (Luck and Vogel, 2013). Loss-of-function (LOF) mutant alleles in NO signaling components, such as dNOS, sGC, foraging (for, PKG), Ribosomal-S6-kinase II, and dCREBB, caused a significant impairment in visual working memory (Fig. 2A; Kuntz et al., 2017). Moreover, RNAi-mediated knockdown of 2 CNG nonselective cation channels (CNGA and CNGL) substantially decreased the visual working memory, indicating the key role of elevated cGMP and Ca2+ levels in visual working memory (Baumann et al., 1994, Chowdhury et al., 2023, Kuntz et al., 2017, Miyazu et al., 2000). Consistent with this, genetical and pharmacological inhibition of cGMP-dependent phosphodiesterase 6 robustly rescued the visual memory deficit observed in either dNOS, ellipsoid-body open, or cystathionine β-synthase LOF mutants (Kuntz et al., 2017). These results combined with dNOS expression detected at presynapses of the ellipsoid body ring 3 neurons led to a hypothesis that NO/cGMP signaling contributes to the maintenance of short-term visual information through the activation of PKG and the gating of CNG cation channels (Fig. 2A; Baumann et al., 1994; Kuntz et al., 2017; Miyazu et al., 2000).
Fig. 2.
Schematic illustration of the regulation of diverse cell functions by nitric oxide signaling in Drosophila. NO is involved in the regulation of various cellular functions through seemingly different signaling pathways: (A) visual working memory; (B) synaptic transmission; (C) axon regrowth; (D) axon regeneration; (E) cell growth, metabolism, and development; (F) fluid secretion; (G) innate immune response; (H) cell cycle arrest and tracheal development; (I) neurodegeneration; (J) neuronal cell death. dNOS, Drosophila nitric oxide synthase; NO, nitric oxide; sGC, soluble guanylate cyclase; cGMP, cyclic GMP; PKG, protein kinase G; RSKII, ribosomal-S6-kinase II; dCREBB, Drosophila cAMP response element binding protein B; CPX, complexin; FTase, farnesyltransferase; SNO, S-nitrosothiols; UNF, nuclear receptor UNF; E75, nuclear receptor E75; Rheb, Ras homolog enriched in brain; TOR, target of rapamycin; S6K, ribosomal protein S6 kinase; Piezo, nonselective cation channel Piezo; CamKII, Ca2+/calmodulin-dependent kinase II; PTTH, prothoracicotropic hormone; CaM, calmodulin; EcR, ecdysone receptor; USP, ultraspiracle; DHR3, Drosophila hormone receptor 3; capa, capability; capaR, capability receptor; PLC, phospholipase C; CaN, calcineurin; IMD, immune deficiency; Dredd, death related ced-3/Nedd2-like caspase; Rel, relish; GSNOR, S-nitrosoglutathione reductase; GSNO, S-nitrosoglutathione; CNGs, cyclic nucleotide-gated ion channels; dLRRK, Drosophila leucine-rich repeat kinase; dFoxO, Drosophila forkhead box O; Hid, head involution defective; Dcp-1, death caspase-1; GFAP, glial fibrillary acidic protein.
SYNAPTIC TRANSMISSION
NO and cGMP signaling was shown to modulate synaptic transmission of glutaminergic motoneurons and cholinergic olfactory sensory neurons (OSNs). Fluorescent dye-based imaging of the recycling of synaptic vesicles demonstrated that NO and cGMP signaling promotes vesicle release at the Drosophila neuromuscular junction (NMJ) in a Ca2+-independent manner (Wildemann and Bicker, 1999a). In addition, pharmacological studies in motor axon terminals showed that elevated presynaptic cGMP production can be induced by electric activity-mediated activation of dNOS/sGC (Shakiryanova and Levitan, 2008). Since NADPH-diaphorase staining failed to detect dNOS activity in both presynaptic boutons and postsynaptic muscle fibers, it is not clear whether NO functions as an anterograde or a retrograde signal at the larval NMJ (Wildemann and Bicker, 1999b). Interestingly, whole-cell recording studies provided functional evidence that NO/cGMP signaling inhibits cholinergic synaptic transmission of OSNs to olfactory projection neurons in the Drosophila antennal lobes (Duan et al., 2012). Given the heavy but inhomogeneous dNOS activity detected in OSNs (Müller and Buchner, 1993), these findings indicate the presynaptic function of NO in the processing of olfactory information. Furthermore, NO/cGMP-mediated contrasting modulations of synaptic transmission have also been demonstrated in vertebrates (Feil and Kleppisch, 2008), suggesting the evolutionarily conserved synaptic mechanism of NO/cGMP signaling.
Another mechanism by which NO modulates protein function is via nonenzymatic addition of NO to the thiol group of a protein's cysteine (Cys) to form S-nitrosothiols, a process referred to as S-nitrosation (Stamler et al., 2001). In Drosophila, the fusion-clamp protein complexin (CPX), which is required for the release of synaptic transmitter at the NMJ, can be functionally downregulated by either its increased S-nitrosation or reduced farnesylation (Fig. 2B; Robinson et al., 2018). Consistently, NO-induced S-nitrosation of CPX suppressed evoked and spontaneous synaptic release by inhibiting farnesylation (Robinson et al., 2018). In contrast, upregulation of denitrosation through genetic and pharmacological manipulation that led to elevated glutathione (GSH) levels reversed NO-mediated suppression of synaptic function (Robinson et al., 2018). These findings demonstrate that S-nitrosation and denitrosation are dynamic regulatory mechanisms of protein function involving reversible posttranslational modification. On the other hand, NO signaling activity is required for the induction of a permanent change to coordinated neural network function during a critical period (mid-stage 17) of embryonic neural development (Giachello et al., 2021). Optogenetic stimulation of neural network activity during the critical period resulted in an increased duration of spontaneous rhythmic current in anterior corner cell (aCC) motoneurons, indicating increased synaptic excitation (Giachello et al., 2021). This increased synaptic activity was almost abolished by pretreatment with the NOS inhibitor, Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME), and further potentiated by the NOS donor, sodium nitroprusside (Giachello et al., 2021). These results suggest a key role of NO signaling in establishing the coordinated patterned activity of neural networks during the embryonic critical period.
SYNAPTIC CONNECTIONS
During pupal development, NO/cGMP retrograde signaling is required for normal synaptic connections of the photoreceptor axons with optic lobe interneurons (Gibbs and Truman, 1998). Immunohistochemical studies demonstrated high levels of cGMP in the photoreceptor axons after treatment with NO donor whereas dNOS was observed in the lamina and medulla of the optic lobe (Gibbs and Truman, 1998). Suppression of NO and cGMP production using pharmacological inhibitors during the early metamorphosis often led to the projection of photoreceptor axons beyond their normal target layers in the optic lobe (Gibbs and Truman, 1998). A similar but slight overshooting phenotype of photoreceptor axons was also observed in Gcα1 LOF mutants that showed highly reduced activity of sGC in the visual system (Gibbs et al., 2001). These results suggest that NO may serve as an arrest signal for extending the growth cone and/or as a target recognition signal (Bicker, 2005, Gibbs and Truman, 1998).
AXON DEGENERATION AND REGENERATION
The Drosophila mushroom body (MB), which is composed of 3 distinct subtypes of KCs: α/β, α’/β’, and γ neurons, found in both larval and adult brains is required for olfactory learning and memory (Busto et al., 2010). In the early stage of pupal development, the γ neurons of larval MB undergo pruning of their dorsal and medial axon lobes through a local degeneration mechanism (Watts et al., 2004). Subsequently, these γ neurons regrow their axons to form adult-specific medial lobes (Luo and O'Leary, 2005). NO signaling has been shown to regulate the pruning and regrowth of the MB γ neurons (Rabinovich et al., 2016). CaM-dependent activation of dNOS promotes axon pruning of the MB γ neurons whereas downregulation of dNOS by inhibitory short dNOS isoform is required for their axon regrowth (Fig. 2C; Rabinovich et al., 2016; Stasiv et al., 2004). Given that 2 heme-binding nuclear receptors, UNF and E75, through the Rheb/TOR/S6K pathway promote the regrowth of the MB γ axons, high NO levels appear to disrupt the formation of UNF-E75 heterodimer, inhibiting the axon regrowth (Fig. 2C; Rabinovich et al., 2016; Reinking et al., 2005; Yaniv et al., 2012). Interestingly, NO-induced axon pruning does not occur through the canonical sGC/cGMP signaling (Rabinovich et al., 2016). To summarize, opposing control of NO signaling provides a switching mechanism between axon pruning and regrowth during metamorphosis.
After severing the axon of mechanosensitive class III dendritic arborization (da) neurons in second instar Drosophila larvae, limited axonal regeneration is observed for 3 days (Song et al., 2012). The Drosophila Piezo, which was initially identified to play an important role in mechanosensory nociception, is responsible for this limited axon regeneration observed in wild-type da neuron injury model (Fig. 2D; Kim et al., 2012; Song et al., 2019). Mechanical injury of sensory axons triggers local calcium transients through activation of the nonselective cation channel Drosophila Piezo, which then activates dNOS ostensibly in a Ca2+/CaM-dependent manner (Song et al., 2019). In addition, genetic interaction analyses revealed that Drosophila PKG Foraging inhibits mechanical injury-induced axon regeneration downstream of dNOS activation (Fig. 2D; Song et al., 2019). In contrast, the phosphatase and tensin homolog (PTEN)/Akt growth signaling pathway was shown to promote axon regeneration in the central nervous system where the da neuron axons barely regenerate (Song et al., 2012). A similar antagonistic relationship between NO and PTEN/Akt signaling pathways was found in the control of the pruning and regrowth of the MB γ neurons (Rabinovich et al., 2016). Moreover, the small lateral ventral neurons of dNOS-deficient mutants showed enlarged and abnormal axon branching patterns, leading to an increase in the area of the axonal arbor (Kozlov et al., 2020). This abnormal axon morphology of the key pacemaker neurons small lateral ventral neurons was suggested to contribute to the arrhythmic circadian behavior observed in dNOS deficiency flies (Kozlov et al., 2020). Collectively, these findings support the notion that NO signaling activity negatively regulates axonal growth and probably synaptic connections under normal physiological conditions.
CELL CYCLE REGULATION DURING DEVELOPMENT
The size of organs and bodily structures in adults is determined by the combined action of cell proliferation, differentiation, and apoptosis during development (Hafen and Stocker, 2003). Histochemical staining for the NADPH-diaphorase activity of dNOS showed intense staining patterns in the wing, eye, haltere, and genital disks of third-instar larvae (Kuzin et al., 1996). A reduction in dNOS activity in the developing larvae causes enlarged appendages and organs, whereas increased NOS activity leads to reduced size of fly limbs (Fig. 2E; Kuzin et al., 1996). These results suggest that NO functions as an antiproliferative signaling molecule in controlling cell division during Drosophila organ development. Furthermore, genetic and pharmacological manipulation of dNOS activity in the developing eye revealed that NO-mediated suppression of cell cycle progression can be achieved in cooperation with the retinoblastoma pathway that is key to the G1-S transition (restriction point) of the cell cycle (Dyson, 1998, Kuzin et al., 2000).
Genetic manipulation of NO-mediated inhibition of the nuclear receptor E75 was shown to affect feeding behavior, lipid metabolism, and development (Fig. 2E; Cáceres et al., 2011). When dNOS was downregulated in the prothoracic gland by an RNAi transgene, most of the larvae did not form pupae on time and instead continued feeding until their body size increased to approximately 1.5 times that of the wild-type (Cáceres et al., 2011). In contrast, overexpression of constitutively active mouse macrophage NOS in the prothoracic gland produced small-sized larvae (Cáceres et al., 2011). RNAi-induced knockdown of E75 or overexpression of Drosophila hormone receptor 3 (DHR3), which functions as an E75 heterodimer partner, robustly suppressed the overgrowth phenotype observed in dNOS knockdown larvae, suggesting that E75 and DHR3 in the prothoracic gland are key mediators of NO signaling (Fig. 2E; Cáceres et al., 2011). An intriguing question relates to the mechanism by which NO negatively regulates the activity of the heme-binding nuclear receptor E75. In the absence of NO, dimerization of DHR3 with E75 compromises DHR3 transcriptional activity, whereas binding of NO to the reduced form of E75 heme appears to interfere with protein interaction between E75 and DHR3, leading to the activation of DHR3 (Reinking et al., 2005). In another study, the binding of NO to E75 resulted in reduced interaction between E75 and SMRT-related and ecdysone receptor interacting factor (SMRTER), showing that the function of E75 as a transcriptional repressor is mediated by the recruitment of the corepressor SMRTER (Johnston et al., 2011). Further studies are required to elucidate the specific regulatory mechanism underlying NO-induced inhibition of E75.
OSMOREGULATION AND FLUID HOMEOSTASIS
NO signaling plays an important role in osmoregulation and fluid homeostasis by the Malpighian tubules (Davies, 2000, Dow et al., 1994). Malpighian tubule principal cells increase the production of NO and cGMP, leading to increased fluid secretion in response to nitridergic neuropeptide capability (capa) (Pollock et al., 2004). Upon binding to capa, its cognate G-protein-linked receptor capaR triggers Ca2+ release through Ca2+ channels present in the endoplasmic reticulum and plasma membrane, activating the Ca2+/CaM-sensitive dNOS and subsequently sGC (Fig. 2F; Davies et al., 2013). The V-type H+-ATPase, which is responsible for fluid secretion, appears to be upregulated by cGMP-dependent protein kinase (cGK) (Fig. 2F; Davies et al., 2013). Given the close relationship between fluid secretion and desiccation tolerance, NO/cGMP signaling in principal cells contributes to appropriate cellular response to external stressors.
INNATE IMMUNE RESPONSE
In the Drosophila immune system, NO was identified as a key signaling molecule that mediates innate immune responses to parasitoid wasp and Gram-negative bacteria (Nappi et al., 2000, Foley and O'Farrell, 2003). When third instar larvae were fed Gram-negative bacteria, recognition of pathogen-associated molecular patterns, such as peptidoglycan found in the bacterial cell wall, via pattern recognition receptors in the gut appeared to induce increased production of reactive oxygen species, which subsequently upregulated the dNOS messenger RNA level and NO production (Fig. 2G; Foley and O'Farrell, 2003; Wu et al., 2012). This intestinal NO is required for the activation of the immune deficiency (IMD) pathway in the fat body, which activates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) family transcription factor Relish (Rel) through phosphorylation and proteolytic cleavage, triggering the expression of antimicrobial peptides including Diptericin (Fig. 2G; Foley and O'Farrell, 2003; Myllymäki et al., 2014). Interestingly, hemocytes seem to respond to the intestinal NO signal and relay it to the fat body to induce an IMD-based innate immune response (Fig. 2G; Foley and O'Farrell, 2003; Wu et al., 2012). Furthermore, NO-induced activation of the IMD pathway in the fat body depends on the function of calcineurin A1, which is 1 of the 3 catalytic subunits showing the Ca2+/CaM-dependent phosphatase activity in hemocytes (Fig. 2G; Dijkers and O'Farrell, 2007). Currently, the mechanism by which hemocytes receiving NO signal stimulate antimicrobial peptide production in the fat body is not clear. However, these results underline the important role of NO signaling in innate immune communication among the gut, hemocytes, and fat body. A recent study found that the inflammatory function of NO/cGMP/PKG signaling significantly contributes to Notch-PI3K/Akt oncogenic cooperation in the Drosophila eye (Villegas et al., 2018). Unexpectedly, the NO produced by eye tumor cells also seems to mediate the suppression of antitumor immune cells (Villegas et al., 2018).
HYPOXIA RESPONSE
Since oxygen is an essential molecule for the generation of energy in mitochondria, aerobic organisms need to sense and adapt to low oxygen (hypoxia) (Bartz and Piantadosi, 2010, Lee et al., 2020). Fruit flies also exhibit cellular, developmental, and behavioral adaptations in response to hypoxia (Wingrove and O'Farrell, 1999). Hypoxic exposure during embryonic development induces a rapid but reversible arrest of the cell cycle, and hypoxic larvae show more exploratory behavior (Wingrove and O'Farrell, 1999). These cellular and behavioral phenotypes were reproduced after increased production of NO and were suppressed when either NO production or PKG activity decreased (DiGregorio et al., 2001, Wingrove and O'Farrell, 1999). In addition, tracheal ramification in larvae, which is believed to correlate with oxygen need, is promoted by increased NO production and is compromised by pharmacological inhibition of dNOS or reduced PKG activity (Fig. 2H; Wingrove and O'Farrell, 1999). A study using hypoxia-induced translocation of green fluorescent protein (GFP)-tagged Relish in a Drosophila cell line led to a better understanding of the hypoxia-induced NO signaling pathway. Mitochondrial NO and its oxidized form nitrite (NO2−) contribute to hypoxia response and S-nitrosoglutathione, which is formed via nitrosation of glutathione, can act as a hypoxia-mimetic to activate sGC (Fig. 2H; Dijkers and O'Farrell, 2009). Moreover, mobilization of calcium and potassium through CNG ion channel and hyperpolarization-activated CNG potassium channel, respectively, downstream of cGMP signaling is required for hypoxia-induced translocation of GFP-tagged Relish in cultured cells (Fig. 2H; Dijkers and O'Farrell, 2009).
NEURODEGENERATIVE DISEASES
Parkinson’s disease (PD) is a common neurodegenerative disease characterized by movement difficulty and neuropsychiatric problems (Armstrong and Okun, 2020, Lee et al., 2022a, Lee et al., 2022b). The onset of PD is closely associated with the loss of DANs in the substantia nigra that is responsible for motor control and reward functions (Poewe et al., 2017). Several missense mutations in the gene encoding Leucine-rich repeat kinase 2 (LRRK2) were shown to cause autosomal dominant late-onset PD (Fig. 2I; Paisán-Ruíz et al., 2004; Zimprich et al., 2004). In aged flies, overexpression of the Drosophila orthologue of human LRRK2 (dLRRK), which contains one of the PD-associated mutations (Y1383C and I1915T), in DANs resulted in a significant loss of DANs (Imai et al., 2008). This neurotoxic activity may be mediated by LRRK2/dLRRK-induced phosphorylation of the forkhead box transcription factor FoxO and the resulting upregulation of a proapoptotic protein Hid (Fig. 2I; Kanao et al., 2010). Recently, the Drosophila cGK Foraging (For) was shown to stimulate FoxO-transcriptional activity through phosphorylation of the same Ser residue as phosphorylated by LRRK2/dLRRK (Kanao et al., 2012). Further genetic and pharmacological studies demonstrated the contribution of NO/cGMP/cGK signaling to the FoxO-mediated neurodegeneration of DANs (Fig. 2I; Kanao et al., 2012).
Similarly, the neurotoxic activity of NO was also demonstrated in the Alexander disease (AxD) model. AxD is a rare genetic leukodystrophy that is largely caused by astrocyte dysfunction and anomalies in the cerebral white matter (Sawaishi, 2009). In most cases, dominant gain-of-function mutations in the GFAP gene, which encodes an intermediate filament protein, are responsible for both early- and later-onset of AxD (Messing et al., 2012). Overexpression of AxD-associated mutant forms of GFAP in the Drosophila glial cells led to increased production of reactive oxygen species, NO, and cGMP, subsequently promoting cell death in both glial and neuronal cells and increasing seizure frequency (Fig. 2J; Wang et al., 2015). Glial fibrillary acidic protein-mediated glial and neuronal cell death appears to require the activation of Caspase, Hid, and Drosophila inhibitor of apoptosis (DIAP), but glial cell death involves DNA damage and upregulated p53 (Fig. 2J; Wang et al., 2015).
CONCLUSION AND PERSPECTIVES
NO is undoubtedly a multifunctional signaling molecule within and between cells. Indeed, NO was shown to act as a cotransmitter in a few types of DANs whereas NO signaling at presynapses of the ellipsoid body ring 3 neurons is required for the development of visual short-term working memory. In addition, the glutaminergic synaptic transmission of motoneurons can be either enhanced or suppressed by NO signaling. During pupal development, precise synaptic connections between the photoreceptor axons and optic lobe interneurons in part depend on NO retrograde signaling activity. Sequential activation and inhibition of dNOS induce the axon pruning and regrowth of the MB γ neurons, respectively. NO can also be used as an antiproliferative signal in cell cycle regulation. These findings suggest that NO signaling is essential for the normal development and cellular physiology of Drosophila. However, the characterization of 2 different amorphic alleles, such as dNOSC and dNOSΔ15, showed that these homozygous mutant flies were viable and exhibited no obvious developmental defects (Yakubovich et al., 2010). Interestingly, a growing body of evidence from fruit flies has implicated NO signaling as one of the pathways inducing cellular stress responses. The dNOS is activated in response to various types of stressors including hypoxia, bacterial infection, aberrant stress proteins, and mechanical injury. The generated NO triggers multiple but distinct signal transduction cascades that appear to depend on the type, intensity, and duration of stress as well as the physiological features of cells in which NO signaling occurs. NO-mediated signaling pathways initially seem to play a protective role in ensuring cell survival and maintaining cellular homeostasis under stress situations. In contrast, highly elevated NO signaling induced by prolonged and/or noxious stress can result in regulated cell death and the development of NO-associated diseases. The molecular mechanisms by which NO signaling plays a protective or destructive role in response to stress are poorly understood. One interesting question is, through what mechanism do differences in the concentration of NO and the resulting differences in NO signaling activity lead to opposite roles in the same cells? This question is also related to several other issues. First, exactly how do the aforementioned stressor molecules activate the dNOS? Second, how does NO signaling integrate with other types of stress signaling to affect cellular physiology? Third, identifying downstream target proteins that are directly affected by NO or mediators of NO signaling is considered an essential prerequisite for better elucidating the NO signaling pathway. Furthermore, it is very challenging to determine which of the downstream target proteins activated or inhibited by NO signaling are directly responsible for the development of NO-associated diseases. Considering that NO signaling is evolutionarily well conserved between Drosophila and vertebrates, a better understanding of NO-mediated opposing functions and their underlying regulatory mechanisms in the fruit fly can help characterize the pathophysiological processes and facilitate targeted drug discovery for NO-associated diseases.
Author Contributions
S.J. contributed the literature search, generation of figures, and the manuscript preparation.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MIST) (2021R1I1A3059555 and 2018R1A2B6008037).
References
- Andreakis N., D'Aniello S., Albalat R., Patti F.P., Garcia-Fernàndez J., Procaccini G., Sordino P., Palumbo A. Evolution of the nitric oxide synthase family in metazoans. Mol. Biol. Evol. 2011;28:163–179. doi: 10.1093/molbev/msq179. [DOI] [PubMed] [Google Scholar]
- Armstrong M.J., Okun M.S. Diagnosis and treatment of Parkinson disease: a review. JAMA. 2020;323:548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- Aso Y., Ray R.P., Long X., Bushey D., Cichewicz K., Ngo T.T., Sharp B., Christoforou C., Hu A., Lemire A.L., et al. Nitric oxide acts as a cotransmitter in a subset of dopaminergic neurons to diversify memory dynamics. Elife. 2019;8 doi: 10.7554/eLife.49257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz R.R., Piantadosi C.A. Clinical review: oxygen as a signaling molecule. Crit. Care. 2010;14:234. doi: 10.1186/cc9185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann A., Frings S., Godde M., Seifert R., Kaupp U.B. Primary structure and functional expression of a Drosophila cyclic nucleotide-gated channel present in eyes and antennae. EMBO J. 1994;13:5040–5050. doi: 10.1002/j.1460-2075.1994.tb06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen H.J., Tong C., Tsuda H. 100 years of Drosophila research and its impact on vertebrate neuroscience: a history lesson for the future. Nat. Rev. Neurosci. 2010;11:514–522. doi: 10.1038/nrn2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicker G. STOP and GO with NO: nitric oxide as a regulator of cell motility in simple brains. Bioessays. 2005;27:495–505. doi: 10.1002/bies.20221. [DOI] [PubMed] [Google Scholar]
- Busto G.U., Cervantes-Sandoval I., Davis R.L. Olfactory learning in Drosophila. Physiology. 2010;25:338–346. doi: 10.1152/physiol.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres L., Necakov A.S., Schwartz C., Kimber S., Roberts I.J., Krause H.M. Nitric oxide coordinates metabolism, growth, and development via the nuclear receptor E75. Genes Dev. 2011;25:1476–1485. doi: 10.1101/gad.2064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury M.A.R., An J., Jeong S. The pleiotropic face of CREB family transcription factors. Mol. Cells. 2023;46:399–413. doi: 10.14348/molcells.2023.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S. Nitric oxide signalling in insects. Insect Biochem. Mol. Biol. 2000;30:1123–1138. doi: 10.1016/s0965-1748(00)00118-1. [DOI] [PubMed] [Google Scholar]
- Davies S.A., Cabrero P., Povsic M., Johnston N.R., Terhzaz S., Dow J.A. Signaling by Drosophila capa neuropeptides. Gen. Comp. Endocrinol. 2013;188:60–66. doi: 10.1016/j.ygcen.2013.03.012. [DOI] [PubMed] [Google Scholar]
- DiGregorio P.J., Ubersax J.A., O'Farrell P.H. Hypoxia and nitric oxide induce a rapid, reversible cell cycle arrest of the Drosophila syncytial divisions. J. Biol. Chem. 2001;276:1930–1937. doi: 10.1074/jbc.M003911200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkers P.F., O'Farrell P.H. Drosophila calcineurin promotes induction of innate immune responses. Curr. Biol. 2007;17:2087–2093. doi: 10.1016/j.cub.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkers P.F., O'Farrell P.H. Dissection of a hypoxia-induced, nitric oxide-mediated signaling cascade. Mol. Biol. Cell. 2009;20:4083–4090. doi: 10.1091/mbc.E09-05-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow J.A., Maddrell S.H., Davies S.A., Skaer N.J., Kaiser K. A novel role for the nitric oxide-cGMP signaling pathway: the control of epithelial function in Drosophila. Am. J. Physiol. 1994;266:R1716–R1719. doi: 10.1152/ajpregu.1994.266.5.R1716. [DOI] [PubMed] [Google Scholar]
- Duan J., Li W., Yuan D., Sah B., Yan Y., Gu H. Nitric oxide signaling modulates cholinergic synaptic input to projection neurons in Drosophila antennal lobes. Neuroscience. 2012;219:1–9. doi: 10.1016/j.neuroscience.2012.05.068. [DOI] [PubMed] [Google Scholar]
- Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- Farah C., Michel L.Y.M., Balligand J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018;15:292–316. doi: 10.1038/nrcardio.2017.224. [DOI] [PubMed] [Google Scholar]
- Feil R., Kleppisch T. NO/cGMP-dependent modulation of synaptic transmission. Handb. Exp. Pharmacol. 2008;184:529–560. doi: 10.1007/978-3-540-74805-2_16. [DOI] [PubMed] [Google Scholar]
- Foley E., O'Farrell P.H. Nitric oxide contributes to induction of innate immune responses to gram-negative bacteria in Drosophila. Genes Dev. 2003;17:115–125. doi: 10.1101/gad.1018503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J. From synaptically localized to volume transmission by nitric oxide. J. Physiol. 2016;594:9–18. doi: 10.1113/JP270297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachello C.N.G., Fan Y.N., Landgraf M., Baines R.A. Nitric oxide mediates activity-dependent change to synaptic excitation during a critical period in Drosophila. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-99868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs S.M., Becker A., Hardy R.W., Truman J.W. Soluble guanylate cyclase is required during development for visual system function in Drosophila. J. Neurosci. 2001;21:7705–7714. doi: 10.1523/JNEUROSCI.21-19-07705.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs S.M., Truman J.W. Nitric oxide and cyclic GMP regulate retinal patterning in the optic lobe of Drosophila. Neuron. 1998;20:83–93. doi: 10.1016/s0896-6273(00)80436-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Domenech C.M., Munoz-Chapuli R. Molecular evolution of nitric oxide synthases in metazoans. Comp. Biochem. Physiol. Part D Genom. Proteom. 2010;5:295–301. doi: 10.1016/j.cbd.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Griffith O.W., Stuehr D.J. Nitric oxide synthases: properties and catalytic mechanism. Annu. Rev. Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- Hafen E., Stocker H. How are the sizes of cells, organs, and bodies controlled? PLoS Biol. 2003;1 doi: 10.1371/journal.pbio.0000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurshman A.R., Marletta M.A. Reactions catalyzed by the heme domain of inducible nitric oxide synthase: evidence for the involvement of tetrahydrobiopterin in electron transfer. Biochemistry. 2002;41:3439–3456. doi: 10.1021/bi012002h. [DOI] [PubMed] [Google Scholar]
- Imai Y., Gehrke S., Wang H.Q., Takahashi R., Hasegawa K., Oota E., Lu B. Phosphorylation of 4E-BP by LRRK2 affects the maintenance of dopaminergic neurons in Drosophila. EMBO J. 2008;27:2432–2443. doi: 10.1038/emboj.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeandroz S., Wipf D., Stuehr D.J., Lamattina L., Melkonian M., Tian Z., Zhu Y., Carpenter E.J., Wong G.K., Wendehenne D. Occurrence, structure, and evolution of nitric oxide synthase-like proteins in the plant kingdom. Sci. Signal. 2016;9:re2. doi: 10.1126/scisignal.aad4403. [DOI] [PubMed] [Google Scholar]
- Jeibmann A., Paulus W. Drosophila melanogaster as a model organism of brain diseases. Int. J. Mol. Sci. 2009;10:407–440. doi: 10.3390/ijms10020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D.M., Sedkov Y., Petruk S., Riley K.M., Fujioka M., Jaynes J.B., Mazo A. Ecdysone- and NO-mediated gene regulation by competing EcR/Usp and E75A nuclear receptors during Drosophila development. Mol. Cell. 2011;44:51–61. doi: 10.1016/j.molcel.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanao T., Venderova K., Park D.S., Unterman T., Lu B., Imai Y. Activation of FoxO by LRRK2 induces expression of proapoptotic proteins and alters survival of postmitotic dopaminergic neuron in Drosophila. Hum. Mol. Genet. 2010;19:3747–3758. doi: 10.1093/hmg/ddq289. [DOI] [PubMed] [Google Scholar]
- Kanao T., Sawada T., Davies S.A., Ichinose H., Hasegawa K., Takahashi R., Hattori N., Imai Y. The nitric oxide-cyclic GMP pathway regulates FoxO and alters dopaminergic neuron survival in Drosophila. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.E., Coste B., Chadha A., Cook B., Patapoutian A. The role of Drosophila Piezo in mechanical nociception. Nature. 2012;483:209–212. doi: 10.1038/nature10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.K., Choi W., Deshar B., Kang S., Kim J. Golgi stress response: New insights into the pathogenesis and therapeutic targets of human diseases. Mol. Cells. 2023;46:191–199. doi: 10.14348/molcells.2023.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov A., Koch R., Nagoshi E. Nitric oxide mediates neuro-glial interaction that shapes Drosophila circadian behavior. PLoS Genet. 2020;16 doi: 10.1371/journal.pgen.1008312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz S., Poeck B., Strauss R. Visual working memory requires permissive and instructive NO/cGMP signaling at presynapses in the Drosophila central brain. Curr. Biol. 2017;27:613–623. doi: 10.1016/j.cub.2016.12.056. [DOI] [PubMed] [Google Scholar]
- Kuzin B., Regulski M., Stasiv Y., Scheinker V., Tully T., Enikolopov G. Nitric oxide interacts with the retinoblastoma pathway to control eye development in Drosophila. Curr. Biol. 2000;10:459–462. doi: 10.1016/s0960-9822(00)00443-7. [DOI] [PubMed] [Google Scholar]
- Kuzin B., Roberts I., Peunova N., Enikolopov G. Nitric oxide regulates cell proliferation during Drosophila development. Cell. 1996;87:639–649. doi: 10.1016/s0092-8674(00)81384-7. [DOI] [PubMed] [Google Scholar]
- Lee J.A., Kwon Y.W., Kim H.R., Shin N., Son H.J., Cheong C.S., Kim D.J., Hwang O. A novel pyrazolo[3,4-d]pyrimidine Induces heme oxygenase-1 and exerts anti-inflammatory and neuroprotective effects. Mol. Cells. 2022;45:134–147. doi: 10.14348/molcells.2021.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P., Chandel N.S., Simon M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020;21:268–283. doi: 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Kim J., Kim H., Han J.E., Kim S., Kang K.H., Kim D., Kim J.M., Koh H. Pyruvate dehydrogenase kinase protects dopaminergic neurons from oxidative stress in Drosophila DJ-1 null mutants. Mol. Cells. 2022;45:454–464. doi: 10.14348/molcells.2022.5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S.J., Vogel E.K. Visual working memory capacity: from psychophysics and neurobiology to individual differences. Trends Cogn. Sci. 2013;17:391–400. doi: 10.1016/j.tics.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J.O., Weitzberg E. Nitric oxide signaling in health and disease. Cell. 2022;185:2853–2878. doi: 10.1016/j.cell.2022.06.010. [DOI] [PubMed] [Google Scholar]
- Luo L., O'Leary D.D. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- Marletta M.A., Hurshman A.R., Rusche K.M. Catalysis by nitric oxide synthase. Curr. Opin. Chem. Biol. 1998;2:656–663. doi: 10.1016/s1367-5931(98)80098-7. [DOI] [PubMed] [Google Scholar]
- Marsh J.L., Thompson L.M. Drosophila in the study of neurodegenerative disease. Neuron. 2006;52:169–178. doi: 10.1016/j.neuron.2006.09.025. [DOI] [PubMed] [Google Scholar]
- Martínez-Ruiz A., Cadenas S., Lamas S. Nitric oxide signaling: classical, less classical, and nonclassical mechanisms. Free Radic. Biol. Med. 2011;51:17–29. doi: 10.1016/j.freeradbiomed.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Messing A., Brenner M., Feany M.B., Nedergaard M., Goldman J.E. Alexander disease. J. Neurosci. 2012;32:5017–5023. doi: 10.1523/JNEUROSCI.5384-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulemans A. Diffusion coefficients and half-lives of nitric oxide and N-nitroso-L-arginine in rat cortex. Neurosci. Lett. 1994;171:89–93. doi: 10.1016/0304-3940(94)90612-2. [DOI] [PubMed] [Google Scholar]
- Miyazu M., Tanimura T., Sokabe M. Molecular cloning and characterization of a putative cyclic nucleotide-gated channel from Drosophila melanogaster. Insect Mol. Biol. 2000;9:283–292. doi: 10.1046/j.1365-2583.2000.00186.x. [DOI] [PubMed] [Google Scholar]
- Müller U., Buchner E. Histochemical localization of NADPH-diaphorase in the adult Drosophila brain. Is nitric oxide a neuronal messenger also in insects? Naturwissenschaften. 1993;80:524–526. doi: 10.1007/BF01140811. [DOI] [PubMed] [Google Scholar]
- Myllymäki H., Valanne S., Rämet M. The Drosophila imd signaling pathway. J. Immunol. 2014;192:3455–3462. doi: 10.4049/jimmunol.1303309. [DOI] [PubMed] [Google Scholar]
- Nappi A.J., Vass E., Frey F., Carton Y. Nitric oxide involvement in Drosophila immunity. Nitric Oxide. 2000;4:423–430. doi: 10.1006/niox.2000.0294. [DOI] [PubMed] [Google Scholar]
- Paisán-Ruíz C., Jain S., Evans E.W., Gilks W.P., Simón J., van der Brug M., López de Munain A., Aparicio S., Gil A.M., Khan N., et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Poewe W., Seppi K., Tanner C.M., Halliday G.M., Brundin P., Volkmann J., Schrag A.E., Lang A.E. Parkinson disease. Nat. Rev. Dis. Primers. 2017;3 doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- Pollock V.P., McGettigan J., Cabrero P., Maudlin I.M., Dow J.A., Davies S.A. Conservation of capa peptide-induced nitric oxide signalling in Diptera. J. Exp. Biol. 2004;207:4135–4145. doi: 10.1242/jeb.01255. [DOI] [PubMed] [Google Scholar]
- Rabinovich D., Yaniv S.P., Alyagor I., Schuldiner O. Nitric oxide as a switching mechanism between axon degeneration and regrowth during developmental remodeling. Cell. 2016;164:170–182. doi: 10.1016/j.cell.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S.S., Sengupta R., Tiso M., Haque M.M., Sahoo R., Konas D.W., Aulak K., Regulski M., Tully T., Stuehr D.J., et al. Reductase domain of Drosophila melanogaster nitric-oxide synthase: redox transformations, regulation, and similarity to mammalian homologues. Biochemistry. 2007;46:11865–11873. doi: 10.1021/bi700805x. [DOI] [PubMed] [Google Scholar]
- Ray S.S., Tejero J., Wang Z.Q., Dutta T., Bhattacharjee A., Regulski M., Tully T., Ghosh S., Stuehr D.J. Oxygenase domain of Drosophila melanogaster nitric oxide synthase: unique kinetic parameters enable a more efficient NO release. Biochemistry. 2007;46:11857–11864. doi: 10.1021/bi700803p. [DOI] [PubMed] [Google Scholar]
- Reinking, Lam J., Pardee M.M., Sampson K., Liu H.M., Yang S., Williams P., White S., Lajoie W., Edwards G., et al. The Drosophila nuclear receptor e75 contains heme and is gas responsive. Cell. 2005;122:195–207. doi: 10.1016/j.cell.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Robinson S.W., Bourgognon J.M., Spiers J.G., Breda C., Campesan S., Butcher A., Mallucci G.R., Dinsdale D., Morone N., Mistry R., et al. Nitric oxide-mediated posttranslational modifications control neurotransmitter release by modulating complexin farnesylation and enhancing its clamping ability. PLoS Biol. 2018;16 doi: 10.1371/journal.pbio.2003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G.M., Lewis E.B. A brief history of Drosophila's contributions to genome research. Science. 2000;287:2216–2218. doi: 10.1126/science.287.5461.2216. [DOI] [PubMed] [Google Scholar]
- Sawaishi Y. Review of Alexander disease: beyond the classical concept of leukodystrophy. Brain Dev. 2009;31:493–498. doi: 10.1016/j.braindev.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Sengupta R., Sahoo R., Mukherjee S., Regulski M., Tully T., Stuehr D.J., Ghosh S. Characterization of Drosophila nitric oxide synthase: a biochemical study. Biochem. Biophys. Res. Commun. 2003;306:590–597. doi: 10.1016/s0006-291x(03)01003-9. [DOI] [PubMed] [Google Scholar]
- Seo J.Y., Kim T.H., Kang K.R., Lim H., Choi M.C., Kim D.K., Chun H.S., Kim H.J., Yu S.K., Kim J.S. 7α,25-Dihydroxycholesterol-induced oxiapoptophagic chondrocyte death via the modulation of p53-Akt-mTOR axis in osteoarthritis pathogenesis. Mol. Cells. 2023;46:245–255. doi: 10.14348/molcells.2023.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiryanova D., Levitan E.S. Prolonged presynaptic posttetanic cyclic GMP signaling in Drosophila motoneurons. Proc. Natl. Acad. Sci. U.S.A. 2008;105:13610–13613. doi: 10.1073/pnas.0802131105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Li D., Farrelly O., Miles L., Li F., Kim S.E., Lo T.Y., Wang F., Li T., Thompson-Peer K.L., et al. The mechanosensitive ion channel Piezo inhibits axon regeneration. Neuron. 2019;102:373–389. doi: 10.1016/j.neuron.2019.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Ori-McKenney K.M., Zheng Y., Han C., Jan L.Y., Jan Y.N. Regeneration of Drosophila sensory neuron axons and dendrites is regulated by the Akt pathway involving Pten and microRNA bantam. Genes Dev. 2012;26:1612–1625. doi: 10.1101/gad.193243.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J.S., Lamas S., Fang F.C. Nitrosylation: the prototypic redox-based signaling mechanism. Cell. 2001;106:675–683. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- Stasiv Y., Kuzin B., Regulski M., Tully T., Enikolopov G. Regulation of multimers via truncated isoforms: a novel mechanism to control nitric-oxide signaling. Genes Dev. 2004;18:1812–1823. doi: 10.1101/gad.298004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert J.R., Chernova T., Forsythe I.D. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist. 2010;16:435–452. doi: 10.1177/1073858410366481. [DOI] [PubMed] [Google Scholar]
- Stuehr D.J., Haque M.M. Nitric oxide synthase enzymology in the 20 years after the Nobel Prize. Br. J. Pharmacol. 2019;176:177–188. doi: 10.1111/bph.14533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Takahashi J., Yamamoto M. Molecular basis of the KEAP1-NRF2 signaling pathway. Mol. Cells. 2023;46:133–141. doi: 10.14348/molcells.2023.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeder I., Scheving R., Wittig I., Geisslinger G. SNO-ing at the nociceptive synapse? Pharmacol. Rev. 2011;63:366–389. doi: 10.1124/pr.110.004200. [DOI] [PubMed] [Google Scholar]
- Thomas D.D., Ridnour L.A., Isenberg J.S., Flores-Santana W., Switzer C.H., Donzelli S., Hussain P., Vecoli C., Paolocci N., Ambs S., et al. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic. Biol. Med. 2008;45:18–31. doi: 10.1016/j.freeradbiomed.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas S.N., Gombos R., García-López L., Gutiérrez-Pérez I., García-Castillo J., Vallejo D.M., Da Ros V.G., Ballesta-Illán E., Mihály J., Dominguez M. PI3K/Akt cooperates with oncogenic Notch by inducing nitric oxide-dependent inflammation. Cell Rep. 2018;22:2541–2549. doi: 10.1016/j.celrep.2018.02.049. [DOI] [PubMed] [Google Scholar]
- Wang L., Hagemann T.L., Kalwa H., Michel T., Messing A., Feany M.B. Nitric oxide mediates glial-induced neurodegeneration in Alexander disease. Nat. Commun. 2015;6 doi: 10.1038/ncomms9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts R.J., Schuldiner O., Perrino J., Larsen C., Luo L. Glia engulf degenerating axons during developmental axon pruning. Curr. Biol. 2004;14:678–684. doi: 10.1016/j.cub.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Wildemann B., Bicker G. Nitric oxide and cyclic GMP induce vesicle release at Drosophila neuromuscular junction. J. Neurobiol. 1999;39:337–346. doi: 10.1002/(sici)1097-4695(19990605)39:3<337::aid-neu1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Wildemann B., Bicker G. Developmental expression of nitric oxide/cyclic GMP synthesizing cells in the nervous system of Drosophila melanogaster. J. Neurobiol. 1999;38:1–15. doi: 10.1002/(sici)1097-4695(199901)38:1<1::aid-neu1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Wingrove J.A., O'Farrell P.H. Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell. 1999;98:105–114. doi: 10.1016/S0092-8674(00)80610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.C., Liao C.W., Pan R.L., Juang J.L. Infection-induced intestinal oxidative stress triggers organ-to-organ immunological communication in Drosophila. Cell Host Microbe. 2012;11:410–417. doi: 10.1016/j.chom.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Yakubovich N., Silva E.A., O'Farrell P.H. Nitric oxide synthase is not essential for Drosophila development. Curr. Biol. 2010;20:R141–R142. doi: 10.1016/j.cub.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv S.P., Issman-Zecharya N., Oren-Suissa M., Podbilewicz B., Schuldiner O. Axon regrowth during development and regeneration following injury share molecular mechanisms. Curr. Biol. 2012;22:1774–1782. doi: 10.1016/j.cub.2012.07.044. [DOI] [PubMed] [Google Scholar]
- Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R.J., Calne D.B., et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]