Abstract
The intracellular domain of the prolactin (PRL) receptor (PRLr) is required for PRL-induced signaling and proliferation. To identify and test the functional stoichiometry of those PRLr motifs required for transduction and growth, chimeras consisting of the extracellular domain of either the α or β subunit of human granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor (GM-CSFr) and the intracellular domain of the rat PRLr were synthesized. Because the high-affinity binding of GM-CSF results from the specific pairing of one α- and one β-GM-CSFr, use of GM-CSFr/PRLr chimera enabled targeted dimerization of the PRLr intracellular domain. To that end, the extracellular domains of the α- and β-GM-CSFr were conjugated to one of the following mutations: (i) PRLr C-terminal truncations, termed α278, α294, α300, α322, or β322; (ii) PRLr tyrosine replacements, termed Y309F, Y382F, or Y309+382F; or, (iii) PRLr wild-type short, intermediate, or long isoforms. These chimeras were cotransfected into the cytokine-responsive Ba/F3 line, and their expression was confirmed by ligand binding and Northern and Western blot analyses. Data from these studies revealed that heterodimeric complexes of the wild type with C-terminal truncation mutants of the PRLr intracellular domain were incapable of ligand-induced signaling or proliferation. Replacement of any single tyrosine residue (Y309F or Y382F) in the dimerized PRLr complex resulted in a moderate reduction of receptor-associated Jak2 activation and proliferation. In contrast, trans replacement of these residues (i.e., αY309F and βY382F) markedly reduced ligand-driven Jak2 activation and proliferation, while cis replacement of both tyrosine residues in a single intracellular domain (i.e., αY309+382F) produced an inactive signaling complex. Analysis of these GM-CSFr–PRLr complexes revealed equivalent levels of Jak2 in association with the mutant receptor chains, suggesting that the tyrosine residues at 309 and 382 do not contribute to Jak association, but instead to its activation. Heterodimeric pairings of the intracellular domains from the known PRLr receptor isoforms (short-intermediate, short-long, and intermediate-long) also yielded inactive receptor complexes. These data demonstrate that the tyrosine residues at 309 and 382, as well as additional residues within the C terminus of the dimerized PRLr complex, contribute to PRL-driven signaling and proliferation. Furthermore, these findings indicate a functional requirement for the pairing of Y309 and Y382 in trans within the dimerized receptor complex.
The protein hormone prolactin (PRL) regulates the development and maturation of mammary tissues (20, 51). PRL also modulates immunoresponsiveness by promoting antigen-driven lymphocyte proliferation and inhibiting glucocorticoid-induced lymphocyte apoptosis (4, 13–15, 26, 34, 42). PRL is expressed by breast epithelium (12) and T cells (55) in an autocrine or paracrine-manner, and its receptor (PRLr) appears to be expressed ubiquitously in these tissues. In rats, three PRLr isoforms have been cloned: the short (PRLr-S) isoform, the long (PRLr-L) isoform, and the intermediate mutation (PRLr-I) (2, 5, 6). The three isoforms are identical in their ligand-binding extracellular domains and differ in the lengths of their intracellular domains, with 57, 160, or 358 amino acids (aa) within PRLr-S, PRLr-I, or PRLr-L, respectively. Synthesis of PRLr-S mRNA occurs through alternative splicing, resulting in a C-terminal truncation. The PRLr-I isoform lacks 198 aa (i.e., aa 323 to 520) from the central portion of the intracellular domain found within PRLr-L (2). Like other members of the cytokine receptor superfamily (3), the PRLr lacks an intrinsic tyrosine kinase catalytic domain. PRLr transduction is mediated by associated signaling proteins, such as Jak2 (7, 41, 62), Fyn (10), Grb2/Sos1 (25), Raf (16), and Vav (11) that are activated as a consequence of ligand-induced receptor dimerization. Structure-function studies have indicated that three structural motifs within the intracellular domain of the superfamily of growth factor receptors, namely the box 1, variable box (V box), and box 2, may contribute to interactions with these signaling proteins (37). The box 1 motif consists of a hydrophobic proline-rich region and presents some similarity to the Src homology 3 (SH3) binding sites. The box 2 motif, present in PRLr-I and PRLr-L but absent in PRLr-S, is rich in hydrophobic and acidic amino acid residues. The intervening region between box 1 and box 2 is the V-box; only a partial sequence of this motif is found within PRLr-S. On the carboxyl side of the box 2 motif resides the extended box 2 domain (X box), a region poorly conserved between the cytokine receptors, required for the function of some members of the cytokine receptor family (46). Functional analyses of the different PRLr isoforms in a transient promoter-reporter assay system have found that both PRLr-L and PRLr-I, but not PRLr-S, can initiate transcription from a PRL-responsive promoter (1, 43, 53). When stably transfected into the cytokine-responsive line Ba/F3, the PRLr-L and PRLr-I isoforms were comparable at stimulating PRL-driven cell proliferation and gene expression (53), while the PRLr-S isoform lacked such activity.
Coexpression of the PRLr isoforms occurs at various levels in PRL-responsive tissues and is regulated in part by ovarian hormones (49). Because no PRL-responsive tissue has been shown to express only a single PRLr isoform, the formation of heterodimeric complexes in vivo may be more of a rule than an exception. To test the functional significance of PRLr isoform heterodimerization, our laboratory recently utilized chimeric receptor constructs (8). These chimeric receptors contained the extracellular domains of the human granulocyte-macrophage colony-stimulating factor receptor (hGM-CSFr) α or β subunit, termed α or β, respectively, and the transmembrane and intracellular domains of the rat PRLr-I or -S isoform, termed I or S, respectively. When these chimeras (termed αS, αI, βS, or βI) were coexpressed in the interleukin 3 (IL-3)-dependent B-lymphocyte Ba/F3 line, ligand-induced dimerization of the extracellular domains induced a specific one-to-one pairing of the PRLr intracellular domains. While transfectants expressing the αI/βI homodimer demonstrated ligand-induced function equivalent to that of the wild-type PRLr, Ba/F3 transfectants of either homo- or heterodimers of the PRLr-S isoform (αS/βS, αS/βI, or αI/βS) were incapable of ligand-driven proliferation and receptor-associated signaling. As such, the PRLr-S isoform acted as a “ligand trap” and demonstrated that paired copies of structures other than the box 1 motif, such as the V box, box 2, X box, and carboxyl tail, are required for mitogenesis and activation of Jak2 and Fyn in the dimerized PRLr complex.
In this study, we now examine how the structural stoichiometry of the PRLr complex modulates ligand-induced cellular proliferation and signaling. Specifically, the functional contributions of the membrane-proximal region of the PRLr intracellular domain (i.e., box 1, V-box, box 2, and X-box motifs) and tyrosines within the X box and C terminus were tested. In addition, the function of heterodimeric pairings of the long isoform of the PRLr with the short or intermediate isoforms was also tested. These aims were accomplished through the use of the GM-CSFr/PRLr chimera into which deletions or replacements of these conserved structural motifs were introduced. These data demonstrate that structural motifs present in the distal intracellular domain of the PRLr are necessary for Jak2 activation and proliferation. As such, these data also support a transactivation mechanism of the PRLr-signal transduction complex following ligand-induced receptor dimerization.
MATERIALS AND METHODS
Chimeric constructs and mutagenesis experiments.
Cassettes of the extracellular domains of the hGM-CSFr α or β subunits (termed α or β, respectively) and the transmembrane and intracellular domains of the rat PRLr-L, PRLr-I or PRLr-S isoforms (termed L, I, or S, respectively) were generated by PCR from a full-length cDNA (8). The syntheses of the truncation mutations α278, α294, α300, and α- or β322, as well as the single- and double-point mutations αY309F, αY382F, βY382F, and αY309+382F, were performed by PCR-based site-directed mutagenesis (Stratagene) on a full length of PRLr-I intracellular domain cDNA subcloned into the TA vector (Invitrogen) as a template. Both the wild-type and mutated cassettes were ligated to the α or β subunit and subcloned into the pREP9 or pREP4 expression vector containing a neomycin (G418) or hygromycin B resistance gene (Invitrogen), respectively. Sequences of the chimeric constructs were confirmed by the dideoxynucleotide chain-termination method.
Cell culture, transfection, and proliferation assays.
A mouse IL-3-dependent pro-B-cell line, Ba/F3 (54), was maintained in RPMI 1640 medium (GIBCO BRL) supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin in the presence of 1 ng of IL-3 per ml (PeproTech). Ba/F3 cells (107) were sequentially cotransfected (8) with 50 μg of XmnI-linearized GM-CSFr α or β construct cDNA by exposure to a single voltage pulse (0.6 kV [25 μF] for 0.1 s) in a Gene Pulser electroporator (Bio-Rad). Selection utilized 750 μg of G418 per ml (GIBCO BRL) and 375 μg of hygromycin B per ml (Boehringer Mannheim) in the complete medium. Clones were obtained by limiting dilution after 2 weeks of selection. To assess ligand-induced cellular proliferation, 106 washed transfectants were aliquoted in medium consisting of RPMI 1640 medium supplemented with sodium selenide, linoleic acid, insulin, and transferrin (ITS+; Calbiochem) in the presence or absence of hGM-CSF (PeproTech). After overnight culture, cells were pulsed with 0.5 μCi (1 Ci = 37 GBq) of [3H]thymidine at 37°C for 8 h. Incorporation of radiolabel was determined by scintillography.
Northern blot analysis.
Total RNA from cells was isolated by extraction with TRIzol reagent (Life Technologies) (8). Ten micrograms of total RNA was denatured and subjected to electrophoresis on 1% agarose formaldehyde gels and transferred onto a nylon membrane. Probe inserts were labeled with [α-32P]dCTP by the random primer method. Membranes were hybridized under stringent conditions as described with 2.5 × 107 cpm of randomly labeled α or β cDNA probes.
Flow cytometry.
Ligand binding by transfected cells was assessed with the Fluorokine kit (R & D Systems) (8). Washed cells (105) were incubated with 110 ng of phycoerythrin (PE)-conjugated recombinant hGM-CSF or 110 ng of control PE-conjugated streptavidin per ml at 4°C for 1 h. After being washed, 104 cells were analyzed at 488 nm with a FACSTAR flow cytometer (Becton Dickinson).
In vitro kinase and immunoprecipitation assays.
Autokinase activities were measured by a modification of a method previously described (8). Washed lysates, obtained as described above, were immunoprecipitated by sequential incubation with anti-mouse Jak2 (5 μl; Upstate Biotechnology, Inc.) or anti-mouse Fyn (10 μl; Santa Cruz Biotechnology) antibody followed by protein A- and G-conjugated Sepharose beads. Washed immunoprecipitates were resuspended in kinase buffer (50 mM HEPES [pH 7.1], 0.1 mM EDTA, 0.1 mg of bovine serum albumin per ml, 0.1% 2-mercaptoethanol, 0.15 M NaCl, 0.15 mM ATP, 20 mM MgCl2) containing 10 μCi of [γ-32P]ATP for 20 min. Immunocomplexes were boiled in 2× sodium dodecyl sulfate (SDS) sample buffer for 5 min, resolved by SDS–10% polyacrylamide gel electrophoresis (PAGE), and visualized by autoradiography.
For the sequential immunoprecipitation and immunoblot assays, cell lysates were immunoprecipitated with an anti-GM-CSFr α subunit antibody (Pharmingen). Immunocomplexes were isolated with protein A and G beads, resolved by SDS–10% PAGE, and transferred to nitrocellulose. Antigen was detected as previously described (11) with anti-Jak2 or anti-GM-CSFr antibody (Santa Cruz Biotech) and enhanced chemiluminescence (Amersham).
RESULTS
Rationale and synthesis of the GM-CSFr/PRLr chimera.
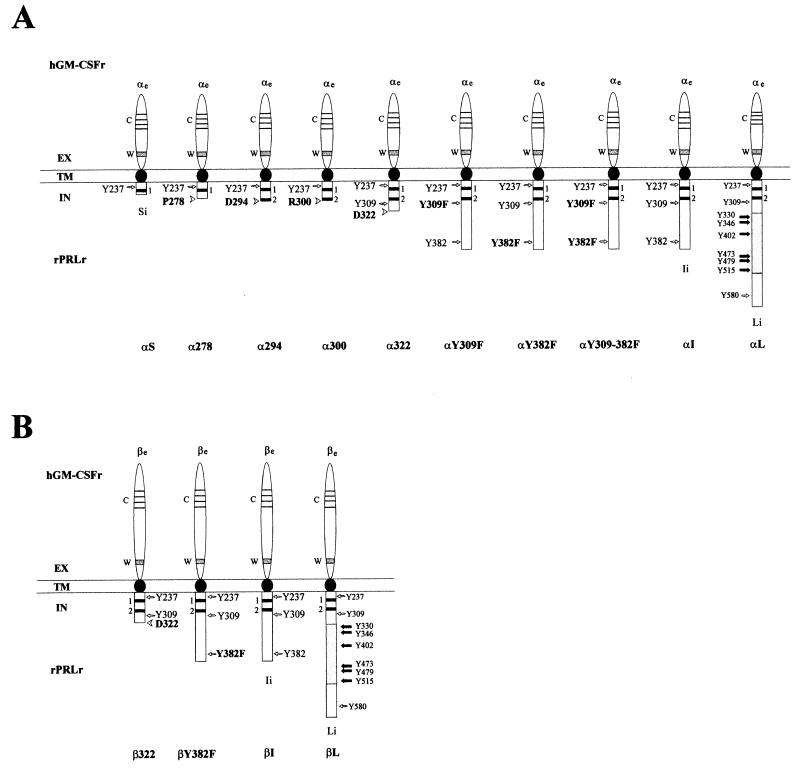
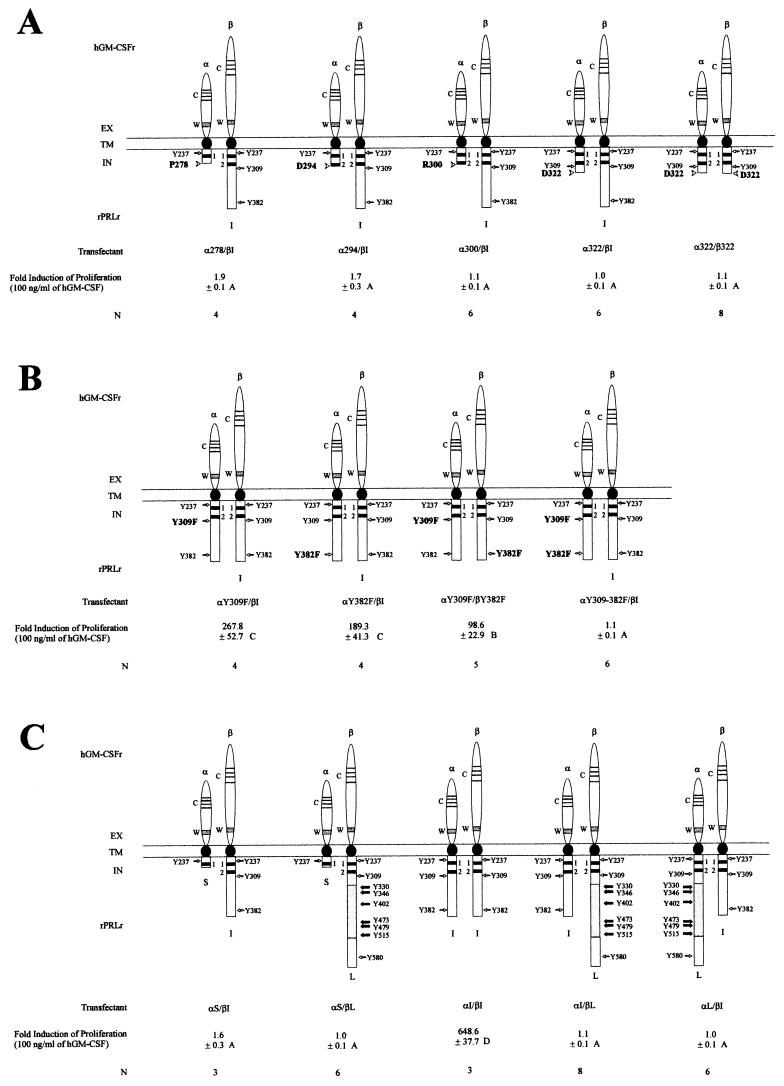
To examine the functional stoichiometry of PRLr structure, seven mutations were introduced into an αI chimeric construct. These mutations, termed α278, α294, α300, α322, αY309F, αY382F, and αY309+382F were synthesized to test specifically the contribution of the V box, box 2, and C-terminal tyrosines to PRLr-associated signaling and proliferation (Fig. 1A). In contrast to αS, which has a box 1 and partial V-box motif, the four truncation mutations contained a complete box 1 in addition to the following domains: α278, full-length V box; α294, full-length V box, partial box 2; α300, full-length V box, box 2; and α322, full-length V box, box 2, X box. The function of the two terminal tyrosine residues within the X box and the C-terminus of the αI were tested by the synthesis of point mutations αY309F, αY382F, and αY309+382F, which replaced one or both of the tyrosine residues with phenylalanine. In addition to these mutant chimeric constructs of αI, three chimera consisting of α and the wild-type intracellular domains of the S, I, and L isoforms of the PRLr were constructed (Fig. 1A). In parallel with these α chimera, four β chimera, termed β322, βY382F, βI, and βL, were also generated (Fig. 1B). These chimeric mutations were cotransfected as expression constructs into the IL-3-dependent, murine pro-B-cell line Ba/F3. Previous work from our laboratory had demonstrated that ligand stimulation of any single α or β chimeric transfectant was nonfunctional with respect to PRLr-associated signaling and proliferation (8). When cotransfected into Ba/F3, however, α/β cotransfectants did demonstrate specific binding of the GM-CSF ligand. Indeed, ligand stimulation of αI/βI double transfectants induced the incorporation of [3H]thymidine to levels observed in the IL-3-stimulated parental line (8). Data from these studies also indicated that the chimeric constructs were functionally symmetric; e.g., cotransfection of either the αS/βI or αI/βS chimera failed to induce PRLr-associated signaling and proliferation in response to ligand.
FIG. 1.
Schematic representation of the chimeric GM-CSFr/PRLr. The extracellular, transmembrane, and intracellular domains are abbreviated as EX, TM, and IN, respectively. The chimeric receptors consisted of the extracellular domain of the hGM-CSFr α or β subunit, termed αe or βe and the transmembrane and intracellular domains of the rat PRLr-S (rPRLr-S) PRLr-I, or PRLr-L isoforms, termed Si, Ii, or Li, respectively. Syntheses of chimera between αe or βe and Si, Ii, or Li were termed αS, αI, αL, βI, or βL, respectively. Mutations of αI termed α278, α294, α300, or α322 represented truncations immediately C terminal to the V box, a partial box 2, a full-length box 2, and the X box, respectively. A single truncation to βI, termed β322, was also synthesized. Replacements of the tyrosines with phenylalanine at residue 309 or 382 were respectively termed αY309F, αY382F, αY309+382F, or βY382F. The conserved extracellular domain regions including the four cysteine residues and a WSXWS motif are respectively indicated by C and W. Two thick lines labeled 1 and 2 indicate the conserved box 1 and box 2 motifs.
Expression and ligand binding of the GM-CSFr/PRLr chimera.
Northern blot analysis of the cotransfected Ba/F3 revealed expression of chimeric transcripts of the anticipated size (Fig. 2). To confirm synthesis at the protein level, anti-hGM-CSFr α- or β-chain immunoprecipitates from these cotransfectants were probed by immunoblot analysis with the same antibodies. This analysis demonstrated that chimeric receptors of the appropriate size were expressed at approximately equivalent levels in the cotransfectant subclones (see Fig. 6). The ability of the α/β cotransfectants to engage ligand at the cell surface was also examined with a flow cytometric assay that utilized a PE-conjugated hGM-CSF. As demonstrated in Fig. 3, all α/β cotransfectants demonstrated ligand binding, whereas the Ba/F3 parent line lacked the ability to bind PE-conjugated hGM-CSF. Analysis of these cotransfectants revealed a comparable level of ligand binding (mean cellular fluorescence, 65.8 ± 11.7 U) between each cotransfectant clone, with 60 to 80% of the cells of each clone demonstrating specific ligand binding. Thus, these data further confirm that the cotransfectant clones obtained expressed similar levels of receptor chimera that were capable of binding ligand with high affinity.
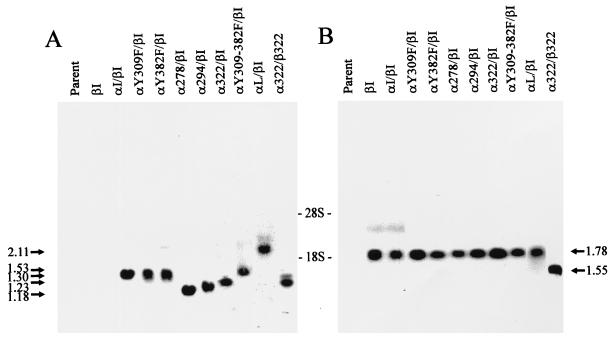
FIG. 2.
Expression of the GM-CSFr/PRLr chimeric receptors at the RNA level. As shown in panels A and B, Northern blot analysis of total cellular RNA from Ba/F3 cells transfected with GM-CSFr/PRLr chimeras. Ten micrograms of total RNA per lane was hybridized with the extracellular domains of the α or β subunit of hGM-CSFr as probes (panels A and B, respectively). Transfectants are indicated on top of each lane. The sizes of different chimeric transcripts in kilobases are indicated on the sides; the relative locations of the 18S and 28S bands are indicated between panels A and B. This blot was representative of several blots; not pictured here is the cotransfectant α300/βI, which demonstrated similar levels of transcript.
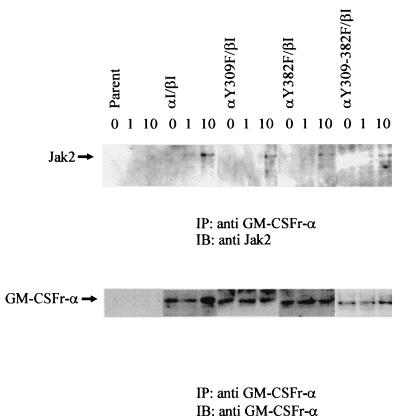
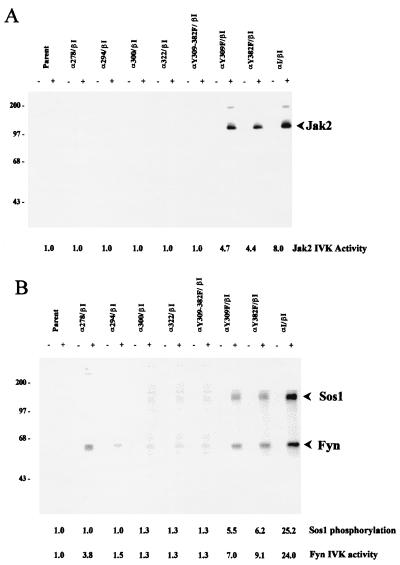
FIG. 6.
Mutations within the GM-CSFr/PRLr chimera do not alter Jak2 association. Lysates from the indicated Ba/F3 cotransfectants were sequentially immunoprecipitated (IP) with an anti-α GM-CSFr antibody and immunoblotted (IB) with an anti-Jak2 antibody. Comparable levels of ligand-induced Jak2 association were observed with each transfectant. Stripping and reprobing of this blot with the anti-α GM-CSFr antibody revealed equivalent levels of receptor loading and expression.
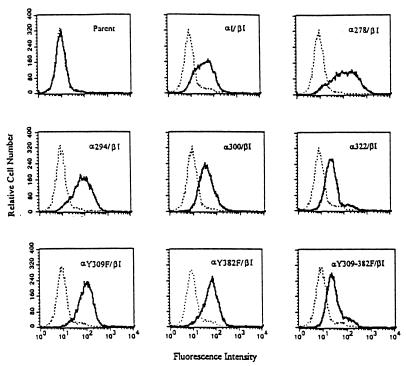
FIG. 3.
GM-CSFr/PRLr chimeras are expressed at the cell surface and specifically bind hGM-CSF. Binding of ligand was assessed by flow cytometric analysis of transfected Ba/F3 cells incubated with excess PE-conjugated hGM-CSF. Binding of this ligand conjugate (bold curves), in excess of that seen in either the parental Ba/F3 line or controls incubated with an irrelevant PE-conjugated streptavidin control (dashed curves), was observed in 60 to 80% of the cells within each cloned cotransfectant.
Both PRLr C termini are necessary for ligand-induced signaling and proliferation in the dimerized receptor complex.
Cotransfectants of the α/β chimeric receptors were tested for their ability to proliferate in response to exogenous hGM-CSF (Fig. 4). As previously demonstrated (8), those Ba/F3 cotransfectants that received both the αI and βI chimeric receptors showed robust proliferation in response to 100 ng of hGM-CSF/ml of culture medium. In contrast, cotransfectants expressing either αS or any of the αI truncation mutations (α278, α294, α300, or α322) with βI were incapable of stimulating proliferation in response to ligand as measured by [3H]thymidine incorporation. In parallel to these findings, a lack of significant activation of Jak2 and Fyn was observed in all of the cotransfectants expressing the various α truncation mutations (Fig. 5). Another measure of the signaling competency of these chimeric receptors was assessed through the evaluation of the phosphorylation of the guanine nucleotide exchange factor Sos that coimmunoprecipitates with Fyn (8, 44). Comparable to the activation of Jak2 and Fyn, Sos phosphorylation occurred only in ligand-stimulated cotransfectants expressing the wild-type αI/βI chimera (Fig. 5). Thus, like the wild-type PRLr-S isoform (53), C-terminal mutations of the PRLr through the V box, box 2, and X box were incapable of mediating ligand-induced signaling or proliferation. Since the truncated α chimeras were appreciably shorter than their ligand-juxtaposed βI partner, the possibility existed that the “unpaired” C terminus of the βI might sterically inhibit a proximal and critical signaling function. To evaluate this possibility, a cotransfectant clone expressing both α322 and β322 chimeras was generated and tested for its ability to proliferate in response to ligand. Since this cotransfectant failed to respond to ligand (Fig. 4), it is unlikely that steric hindrance is responsible for the lack of ligand-induced signaling and proliferation observed in the other α truncation cotransfectants. Instead, the most probable interpretation of these data suggests that an intact C terminus in each signaling domain is necessary for ligand-induced transduction.
FIG. 4.
Stoichiometric requirements for PRLr intracellular motifs necessary for ligand-induced mitogenesis as determined by GM-CSFr/PRLr chimeras. (A) Truncation mutations. (B) Tyrosine replacements. (C) Receptor isoforms. The ratio of GM-CSF (100 ng/ml)-stimulated to unstimulated [3H]thymidine incorporation (counts per minute) was used to calculate the fold induction of proliferation by ligand. The mean incorporation of [3H]thymidine by the parental Ba/F3 line in the presence or absence of hGM-CSF was 294 ± 60 or 342 ± 77 cpm per 106 cells, respectively. EX, TM, and IN, extracellular, transmembrane, and intracellular domains respectively. rPRLr, rat PRLr. Each value represents the mean ± standard error of three to eight separate experiments (shown as the experimental number [N]). Analysis of these results demonstrated a statistically significant difference by one-way analysis of variance between transfectants designated A and B (P < 0.01), B and C (P < 0.01), or C and D (P < 0.01).
FIG. 5.
Stoichiometric requirements for PRLr intracellular structural motifs necessary for ligand-induced signaling. Recombinant hGM-CSF-induced phosphorylation of Jak2, Fyn, and Sos through chimeric receptors in Ba/F3 transfectants was assessed by in vitro autokinase (IVK) assay. Resting cotransfectants (2 × 106) (indicated on top) were stimulated with 0 or 100 ng of hGM-CSF per ml (indicated as − or +, respectively). Cell lysates were immunoprecipitated with 5 μl of rabbit polyclonal antibody against murine Jak2 (A) or 10 μl of rabbit polyclonal antibody against Fyn (B) and immobilized on protein A- and G-agarose beads, followed by incubation in vitro with 10 μCi of [γ-32P]ATP in the presence of 20 mM Mg2+ for 20 min. Proteins were resolved by SDS–10% PAGE followed by autoradiography. The relative masses of the molecular standards are indicated in kilodaltons.
The functional requirement for trans pairing of tyrosine residues in the PRLr complex.
To test the stoichiometric requirements for the tyrosine residues at positions 309 and 382, Ba/F3 cotransfectants, which expressed tyrosine replacements both in cis and trans within the dimerized receptor complex (αY309F/βI, αY382F/βI, αY309+382F/βI, and αY309F/βY382), were tested for their ability to proliferate and signal in response to ligand. Analysis of αY309F/βI or αY382F/βI cotransfectants demonstrated a similar phenotype. When stimulated with ligand, both cotransfectants incorporated 30 to 40% of the [3H]thymidine observed in the αI/βI cotransfectant (Fig. 4). In contrast to the chimeras expressing single tyrosine replacements, the Ba/F3 cotransfectant expressing αY309+ 382F/βI was incapable of ligand-induced proliferation. Further examination of αY309F/βI, αY382F/βI, and αY309+382F/βI revealed that the ligand-induced activation of associated signaling factors paralleled the magnitude of induced [3H]thymidine uptake (Fig. 4 and 5). These data indicate that both tyrosine residues at positions 309 and 382 contribute to PRLr-associated signaling and proliferation.
Replacement of the tyrosine residues with phenylalanine at position 382 in both PRLr signaling chains has been found to yield a functionally inactive receptor complex (39). One interpretation of these data suggests that the pairing in trans of tyrosine residues in the PRLr intracellular domain is required for signal transduction. To test this hypothesis further, a cotransfectant was generated that expressed chimeric receptors in which one opposed tyrosine residue was replaced in each signaling chain (αY309F/βY382F). When stimulated with ligand, this cotransfectant demonstrated a significant decrease in [3H]thymidine uptake, to approximately 15% of the levels observed in the αI/βI cotransfectant. Taken together, these data strongly suggest that the ligand-induced juxtaposition of adjacent tyrosine residues at both positions 309 and 382 (i.e., pairing in trans), is necessary for efficient PRLr transduction.
Heterodimeric complexes of the known PRLr isoforms fail to mediate ligand-induced proliferation.
All tissues that demonstrate the binding of PRL have been found to variably coexpress each of the PRLr isoforms (23). Given that each of the PRLr isoforms shares an identical extracellular ligand binding domain, recent data demonstrating ligand-induced heterodimerization of these isoforms was not unexpected (56). Prior research has demonstrated that the pairing of the short with a short, or intermediate, PRLr isoform (i.e., αS/βS, αS/βI, or αI/βS) produced an inactive receptor complex (8), while homodimeric complexes of the intermediate isoform were functionally active (8, 53). To extend these preliminary studies, the functions of heterodimeric pairings of the long with the short and intermediate PRLr isoforms were tested by the ligand stimulation of αS/βL, αI/βL, and αL/βI cotransfectants (Fig. 4). Consistent with our previous findings, each of the novel heterodimeric cotransfectants was incapable of mediating ligand-induced proliferation.
Activation, but not association, of Jak2 by GM-CSFr/PRLr chimera requires Y309 and/or Y382.
Previous studies with homodimeric PRLr truncations have indicated that the C terminus of the PRLr is not required for the association or activation of the Jak2 (22, 31, 40). The data presented in Fig. 4 and 5 with the chimeric PRLr truncation and tyrosine replacement mutants clearly indicate, however, that some contribution from the C terminus and Y309 and/or Y382 is necessary for ligand-induced Jak2 activation. To determine whether this was secondary to the inability of Jak2 to associate with the chimeric mutants, immunoprecipitates of lysates obtained from the αI/βI, αY309F/βI, αY382F/βI, and αY309+382F/βI cotransfectants with an anti-GM-CSFr α-chain antibody, were subjected to immunoblot analysis with an anti-Jak2 antibody (Fig. 6). These data demonstrate that ligand induced comparable levels of Jak2 association with each of the α/PRLr chimeras, with an 8.3 ± 1.3-fold increase in associated Jak2 after 10 min of stimulation. Thus, while Y309 and Y382 appear not to directly contribute to the association of Jak2 with the PRLr, as previously documented (21), these data suggest that Y309 and Y382 and/or proteins associated with these residues are necessary for Jak2 activation in this receptor complex.
DISCUSSION
The GM-CSFr/PRLr chimeric receptor system was developed to examine the stoichiometry of structure-function relationships within the PRLr. To enable the targeted dimerization of the associated intracellular PRLr domains, the extracellular domains of the α- and β-chains of GM-CSFr were utilized, given their 1:1 engagement of ligand. As with other receptor chimeric constructs (50, 52, 67), ligand stimulation of the GM-CSFr/PRLr chimera resulted in the transduction of intracellular domain-associated signals. With this system, previous data from our laboratory determined that pairing of the intracellular domain of the PRLr-S with itself or the intracellular domain of the PRLr-I isoform was incapable of mediating ligand-driven proliferation and receptor-associated signaling (8). While the PRLr-S isoform contains a box 1 motif, it lacks the C-terminal portion of its V box and the box 2 and X-box motifs as the result of alternative splicing. Thus, structure-function comparisons between PRLr-S and the PRLr-I indicated that the paired distal signaling domains present in the αI/βI complexes were necessary for PRLr-associated signaling and proliferation. Candidate motifs located in the C-terminal domain of PRLr-I that could mediate such function were the full-length V-box, box 2, and X-box domains and two tyrosine residues (Y309 and Y382).
To characterize further the functional stoichiometry of these PRLr C-terminal motifs, several novel GM-CSFr/PRLr chimeric receptor mutations were constructed and tested for their mitogenic and signaling properties in response to ligand in the IL-3-dependent Ba/F3 cell line. Specifically, the PRLr intracellular domain mutations consisted of staggered deletions encompassing all or portions of the V box (aa 251 to 278), box 2 (aa 279 to 300), and X box (aa 300 to 322), or one or both of the C-terminal tyrosine residues (Y309 and Y382). Ligand-induced dimerization of these αGM-CSFr/PRLr chimeric mutations with the βGM-CSFr/PRLr-I wild-type chimera revealed that in the absence of the ligand-paired PRLr C termini, these proximal PRLr motifs were insufficient for the induction of either signaling or proliferation. The data presented here both confirm and contrast with the results obtained from three independent laboratories examining the function of mutated homodimeric PRLr complexes, summarized as follows. (i) Transfectants of the IL-3-dependent, promyeloid 32D line expressing a rat PRLr mutation truncated C terminal to the V box did not demonstrate PRL-induced Jak2 or Stat activation, gene transcription, or cellular proliferation in response to ligand. In contrast, 32D transfectants expressing PRLr truncations C terminal to the X box (i.e., containing box 1, V box, box 2, and X box and Y309) could fully activate the Jak2-Stat pathway and the transcription of ornithine decarboxylase mRNA, but could only partially induce cellular proliferation (21, 22), in response to ligand. (ii) Transfection of similar constructs into the human 293 fibroblast subline LA (a constitutive overexpressor of Jak2) produced dissimilar results when stimulated with ligand. Ligand stimulation of transfectants expressing PRLr truncations C terminal to both the V and X boxes induced the activation of Jak2. However, stimulation of both of these transfectants failed to induce the expression of a PRL-responsive promoter (β-casein) reporter construct (24, 40). (iii) Similar to the first study, CHO transfectants expressing rabbit PRLr truncations C terminal to the V box poorly activated Jak2, Stat5, and a cotransfected, PRL-responsive (β-lactoglobulin promoter) reporter construct. In addition, a transfectant expressing a PRLr truncation C terminal to the X box was found to fully activate Jak2 and Stat5, but induced marginal PRL-dependent gene expression (31). Taken together, the current and previous studies indicate that in the absence of Jak2 overexpression, elements within the V box, box 2, X box, and C terminus are necessary for ligand-induced gene expression and/or proliferation. The results presented here, however, stand in contrast to the previous studies, in that the homodimeric pairing of the α322/β322 or any of the heterodimeric truncations were incapable of mediating ligand-induced Jak2 activation. The observation that the αY382F/βI cotransfectant was capable of partially mediating proliferation and Jak2 activation, while the α322/βI or α322/β322 cotransfectants were not, argues that residues within the C terminus other than Y382 contribute to PRLr-mediated proliferation. Alternatively, these findings may be trivially explained by inherent differences between the cell lines utilized and their relative abundance of signaling constituents. This possibility should be shortly resolved, because the introduction of the GM-CSFr/PRLr chimera into other PRL-responsive cell lines is currently under way in our laboratory.
The results presented also revealed that replacement of either tyrosine residue within the X box (Y309F) or the C terminus (Y382F) in one receptor chain within the ligand-dimerized PRLr complex resulted in a partial reduction of PRL-induced signal transduction and proliferation. While replacement of both of these residues in one chain resulted in a functionally inactive complex, the replacement of one of the Y309 and Y382 residues (i.e., αY309F/βY382F) in opposing receptor intracellular chains resulted in a nearly eightfold reduction in overall ligand-induced proliferation. Prior studies examining the role of the terminal tyrosine residues in mutated homodimeric PRLr complexes (i.e., complexes in which tyrosine residues in both PRLr chains were mutated) transfected into the Jak2-overexpressing 293/LA line indicated that the removal of Y309 (via deletion of the X box) did not ablate PRL-driven expression of a β-casein promoter reporter construct (39). In contrast, replacement of Y382 completely ablated such expression in the 293/LA transfectants. When stimulated with ligand, however, both the Y309del and Y382F transfectants, as well as a Y309del + Y382F transfectant, demonstrated Jak2 phosphorylation in response to ligand. Collectively, these previous results have been interpreted to indicate that proximal motifs, such as the well-recognized contribution of the box 1 motif (40), within the PRLr were sufficient for both the association and activation of Jak2, while the Y382 residue was necessary for the induction of PRL-responsive gene expression. The data presented here, however, argue that the amino acid residues Y309 and Y382, while not directly contributing to the association of Jak2, do contribute to the activation of Jak2, Fyn, and Sos. The basis for the differences between our current study and previous findings may relate to fundamental differences in the cell systems and receptor mutants utilized. In particular, the previous data (40) relied on a cell subline which overexpressed Jak2 and examined the functional significance of Y309, not by replacement, but by deletion of the entire X box.
Previous data have indicated that both Y309 and Y382 are phosphorylated during ligand-induced PRLr dimerization (41). The significant reduction in ligand-induced proliferation (and signaling) observed in the αY309F/βY382F and αY309+Y382F/βI cotransfectants would argue that at least one pair of tyrosines either at Y309 or Y382 in trans are necessary for effective signaling from the dimerized PRLr complex. Taken together, these data suggest that transphosphorylation of the tyrosine residues significantly contributes to proximal PRLr transduction. The seminal importance of receptor transphosphorylation of growth factor receptors containing tyrosine kinase domains, such as epidermal growth factor receptor, platelet-derived growth factor receptor, and the insulin receptor is well recognized (38, 57, 58, 65). To the best of our knowledge, however, our findings represent the first elaboration of this phenomenon among the superfamily of cytokine receptors to which the PRLr belongs. Paralleling the phosphorylation of these tyrosine residues within the PRLr, both Jak2 and Fyn are activated with similar kinetics (7, 10). One interpretation of these data would support a model in which the dimerization of the PRLr complex initiates signaling via transphosphorylation of the receptor by an associated kinase. In light of our findings, it is conceivable that the activation of Jak2 associated with the PRLr may require the initial activation of an additional kinase or phosphatase associated with the PRLr at either Y309 or Y382.
The inability of ligand-induced heterodimers of the PRLr isoforms may be of considerable functional significance. While PRLr-I is only known to exist within the Nb2 cell in rodents (2), the PRLr-I isoform in humans occurs naturally as a splice variant and is thought to represent the predominate isoform within normal and malignant human breast tissues (9). Whether the heterodimeric PRLr complexes are inactive with respect to other cellular processes or associated signaling cascades remains an active area of investigation for this laboratory. The basis for the inability of PRLr-I and PRLr-L to function as heterodimers, when perfectly capable of functioning as homodimers, is currently open to additional consideration. One interpretation of the findings presented here, however, suggests that the ligand-induced pairing of functional motifs within the PRLr intracellular domain (such as Y382 and/or other C-terminal residues) must occur in spatial proximity and/or the appropriate register. Such a theory could explain why the αI/βL and αL/βI cotransfectants were functionally inactive and is readily testable through the use of additional chimeric constructs.
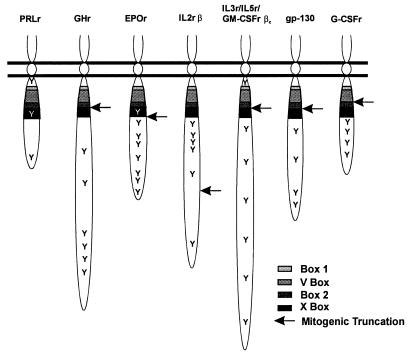
Use of the GM-CSFr/PRLr chimera has revealed that the structural basis for PRLr-associated proliferation is dissimilar from that observed for many other members of the cytokine receptor family, including growth hormone (17, 32, 33, 66); erythropoietin (18, 19, 28, 36, 59, 61); GM-CSF, IL-3, and IL-5 (βc-chain) (48, 60, 64); gp 130 (47); and granulocyte-CSF (27). As summarized in Fig. 7, C-terminal truncations of these cytokine receptors in the proximity of either the box 2 or X-box motifs do not ablate ligand-induced cell proliferation, although the loss of activation of specific signal transduction and gene expression pathways associated with cellular differentiation has been reported in some receptor mutations (63). One specific example of this structure-function relationship was found in the receptor for erythropoietin. C-terminal truncations distal to the X box produced a receptor mutation fully capable of mediating erythropoietin-induced cell proliferation, but unable to phosphorylate and activate Stat5 (61). In terms of the proliferative requirement for its C-terminal tail, the PRLr bears the greatest structural similarity to the β-chain of the IL-2r (35). Truncations proximal to or replacement of either of the two terminal tyrosine residues in this receptor preclude ligand-induced proliferation (29, 30, 45). These findings suggest that PRLr and IL-2rβ may require intracellular domain motifs in addition to those found in box 1, the V box, box 2, and the X box. Analysis of the amino acid sequences surrounding these C-terminal tyrosines in the PRLr and IL-2rβ, however, reveals no significant homology, suggesting that the associated signaling machinery utilized by these receptor complexes at these sites may differ. Given the relatively promiscuous utilization of Jak and Stat pathways by the cytokine receptor superfamily, such alternative signaling mechanisms could significantly contribute to the generation of specificity in cytokine receptor signaling. Thus, further study of those motifs and proteins associated with the C terminus and/or residues Y309 and Y382 of the PRLr may provide further insights into the structural basis for the specific growth and differentiation signals arising from the PRLr complex.
FIG. 7.
Structure-function relationships with the cytokine receptor superfamily. Schema demonstrating the location of the conserved box 1, V-box, box 2, and X-box motifs (as sequentially indicated by increased shading) and tyrosine residues (indicated by Y) in various cytokine receptors. The arrow indicates the shortest C-terminal truncation that produces a mutation capable of initiating ligand-induced proliferation. Like the PRLr, an appreciable portion of the C terminus of the IL-2r β chain (IL2rβ) is necessary for the induction of cell proliferation. GHr, growth hormone receptor; EPOr, erythropoietin receptor; G-CSFr, granulocyte–colony-stimulating factor.
ACKNOWLEDGMENTS
We thank Armen Shanafelt for providing the GM-CSFr α cDNA, Atsushi Miyajima for GM-CSFr β cDNA, and Paul Kelly for the PRLr-I and PRLr-S cDNAs. We are grateful to Seong-Joo Jeong and the Lucille Markey Flow Cytometry Unit at the University of Pennsylvania for excellent support.
This work was supported in part by funding from National Institutes of Health grants R29 AI33510 and R01 CA69294. C.V.C. is a recipient of an American Cancer Society Junior Faculty Research Award.
REFERENCES
- 1.Ali S, Edery M, Pellegrini I, Lesueur L, Paly J, Djiane J, Kelly P A. The Nb2 form of prolactin receptor is able to activate a milk protein gene promoter. Mol Endocrinol. 1992;6:1242–1248. doi: 10.1210/mend.6.8.1406702. [DOI] [PubMed] [Google Scholar]
- 2.Ali S, Pellegrini I, Kelly P A. A prolactin-dependent immune cell line (Nb2) expresses a mutant form of prolactin receptor. J Biol Chem. 1991;266:20110–20117. [PubMed] [Google Scholar]
- 3.Bazan J F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci USA. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernton E W, Meltzer M S, Holaday J W. Suppression of macrophage activation and T-lymphocyte function in hypoprolactinemic mice. Science. 1988;239:401–404. doi: 10.1126/science.3122324. [DOI] [PubMed] [Google Scholar]
- 5.Boutin J M, Edery M, Shirota M, Jolicoeur C, Lesueur L, Ali S, Gould D, Djiane J, Kelly P. Identification of a cDNA encoding a long form of prolactin receptor in human hepatoma and breast cancer cells. Mol Endocrinol. 1989;3:1455–1461. doi: 10.1210/mend-3-9-1455. [DOI] [PubMed] [Google Scholar]
- 6.Boutin J M, Jolicoeur C, Okamura H, Gagnon J, Edery M, Shirota M, Banville D, Dusanter-Fourt I, Djiane J, Kelly P A. Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell. 1988;53:69–77. doi: 10.1016/0092-8674(88)90488-6. [DOI] [PubMed] [Google Scholar]
- 7.Campbell G S, Argetsinger L S, Ihle J N, Kelly P A, Rillema J A, Carter-Su C. Activation of Jak2 tyrosine kinase by prolactin receptors in Nb2 cells and mouse mammary gland explants. Proc Natl Acad Sci USA. 1994;91:5232–5236. doi: 10.1073/pnas.91.12.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang W-P, Clevenger C V. Modulation of growth factor receptor function by isoform heterodimerization. Proc Natl Acad Sci USA. 1996;93:5947–5952. doi: 10.1073/pnas.93.12.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clevenger C V, Chang W-P, Ngo W, Pasha T L M, Montone K T, Tomaszewski J E. Expression of prolactin and prolactin receptor in human breast carcinoma: evidence for an autocrine/paracrine loop. Am J Pathol. 1995;146:1–11. [PMC free article] [PubMed] [Google Scholar]
- 10.Clevenger C V, Medaglia M V. The protein tyrosine kinase p59fyn is associated with prolactin (PRL) receptor and is activated by PRL stimulation of T-lymphocytes. Mol Endocrinol. 1994;8:674–681. doi: 10.1210/mend.8.6.7935483. [DOI] [PubMed] [Google Scholar]
- 11.Clevenger C V, Ngo W, Luger S M, Gewirtz A M. Vav is necessary for prolactin-stimulated proliferation and is translocated into the nucleus of a T-cell line. J Biol Chem. 1995;270:13246–13253. doi: 10.1074/jbc.270.22.13246. [DOI] [PubMed] [Google Scholar]
- 12.Clevenger C V, Plank T L. Prolactin as an autocrine/paracrine growth factor in breast tissue. J Mammary Gland Biol Neoplasia. 1997;2:59–68. doi: 10.1023/a:1026325630359. [DOI] [PubMed] [Google Scholar]
- 13.Clevenger C V, Russell D H, Appasamy P M, Prystowsky M B. Regulation of IL2-driven T-lymphocyte proliferation by prolactin. Proc Natl Acad Sci USA. 1990;87:6460–6464. doi: 10.1073/pnas.87.16.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clevenger C V, Sillman A L, Hanley-Hyde J, Prystowsky M B. Requirement for prolactin during cell cycle regulated gene expression in cloned T-lymphocytes. Endocrinology. 1992;130:3216–3222. doi: 10.1210/endo.130.6.1534539. [DOI] [PubMed] [Google Scholar]
- 15.Clevenger C V, Thickman K, Ngo W, Chang W-P, Takayama S, Reed J C. Role of Bag-1 in the survival and proliferation of the cytokine-dependent lymphocyte lines, Ba/F3 and Nb2. Mol Endocrinol. 1997;11:608–618. doi: 10.1210/mend.11.5.9925. [DOI] [PubMed] [Google Scholar]
- 16.Clevenger C V, Torigoe T, Reed J C. Prolactin induces rapid phosphorylation and activation of prolactin receptor associated Raf-1 kinase in a T-cell line. J Biol Chem. 1994;269:5559–5565. [PubMed] [Google Scholar]
- 17.Colosi P, Wong K, Leong S R, Wood W I. Mutational analysis of the intracellular domain of the human growth hormone receptor. J Biol Chem. 1993;268:12617–12623. [PubMed] [Google Scholar]
- 18.Damen J E, Wakao H, Miyajima A, Krosl J, Humphries R K, Cutler R L, Krystal G. Tyrosine 343 in the erythropoietin receptor positively regulates erythropoietin-induced cell proliferation and Stat5 activation. EMBO J. 1995;14:5557–5568. doi: 10.1002/j.1460-2075.1995.tb00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.D’Andrea A D, Yoshimura A, Youssoufian H, Zon L I, Koo J-W, Lodish H F. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth-regulatory domains. Mol Cell Biol. 1991;11:1980–1987. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das R, Vonderhaar B K. Prolactin as a mitogen in mammary cells. J Mammary Gland Biol Neoplasia. 1997;2:29–39. doi: 10.1023/a:1026369412612. [DOI] [PubMed] [Google Scholar]
- 21.DaSilva L, Howard O M Z, Rui H, Kirken R A, Farrar W L. Growth signaling and JAK2 association mediated by membrane-proximal cytoplasmic regions of prolactin receptors. J Biol Chem. 1994;269:18267–18270. [PubMed] [Google Scholar]
- 22.DaSilva L, Rui H, Erwin R A, Howard O M Z, Kirken R A, Malabarba M G, Hackett R H, Larner A C, Farrar W L. Prolactin recruits STAT1, STAT3 and STAT5 independent of conserved receptor tyrosines TYR402, TYR479, TYR515, and TYR580. Mol Cell Endocrinol. 1996;117:131–140. doi: 10.1016/0303-7207(95)03738-1. [DOI] [PubMed] [Google Scholar]
- 23.Dusanter-Fourt I, Gaye P, Belair L, Petridou B, Kelly P A, Djiane J. Prolactin receptor gene expression in the rabbit: identification, characterization and tissue distribution of several prolactin receptor messenger RNAs encoding a unique precursor. Mol Cell Endocrinol. 1991;77:181–192. doi: 10.1016/0303-7207(91)90073-2. [DOI] [PubMed] [Google Scholar]
- 24.Edery M, Levi-Meyrueis C, Paly J, Kelly P A, Djiane J. A limited cytoplasmic region of the prolactin receptor critical for signal transduction. Mol Cell Endocrinol. 1994;102:39–44. doi: 10.1016/0303-7207(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 25.Erwin R A, Kirken R A, Malabarba M G, Farrar W L, Rui H. Prolactin activates ras via signaling proteins Shc, growth factor receptor bound 2, and son of sevenless. Endocrinology. 1995;136:3512–3517. doi: 10.1210/endo.136.8.7628388. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher-Chiappini S E, Comptom M M, Lavoie H A, Day E B, Witorsch R J. Glucocorticoid-prolactin interactions in Nb2 lymphoma cells: antiproliferative versus anticytolytic effects. Proc Soc Exp Biol Med. 1993;202:345–352. doi: 10.3181/00379727-202-43545. [DOI] [PubMed] [Google Scholar]
- 27.Fukunaga R, Ishizaka-Ikeda E, Pan C-X, Seto Y, Nagata S. Functional domains of the granulocyte colony-stimulating factor receptor. EMBO J. 1991;10:2855–2865. doi: 10.1002/j.1460-2075.1991.tb07835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gobert S, Chretien S, Gouilleux F, Muller O, Pallard C, Dusanter-Fourt I, Groner B, LaCombe C, Gisselbrecht S, Mayeux P. Identification of tyrosine residues within the intracellular domain of the erythropoietin receptor crucial for STAT5 activation. EMBO J. 1996;15:2434–2441. [PMC free article] [PubMed] [Google Scholar]
- 29.Goldsmith M A, Lai S Y, Xu W, Amaral C, Kurczek E S, Parent L J, Mills G B, Tarr K L, Longmore G D, Greene W C. Growth signal transduction by the human interleukin-2 receptor requires cytoplasmic tyrosines of the β chain and non-tyrosine residues of the γc chain. J Biol Chem. 1995;270:21729–21737. doi: 10.1074/jbc.270.37.21729. [DOI] [PubMed] [Google Scholar]
- 30.Goldsmith M A, Xu W, Amaral C, Kuczek E S, Greene W C. The cytoplasmic domain of the interleukin-2 receptor β chain contains both unique and functionally redundant signal transduction elements. J Biol Chem. 1994;269:14698–14704. [PubMed] [Google Scholar]
- 31.Goupille O, Daniel N, Bignon C, Jolivet J, Djiane J. Prolactin signal transduction to milk protein genes: carboxy-terminal part of the prolactin receptor and its tyrosine phosphorylation are not obligatory for JAK2 and STAT5 activation. Mol Endocrinol. 1997;127:155–169. doi: 10.1016/s0303-7207(97)04005-7. [DOI] [PubMed] [Google Scholar]
- 32.Hackett R H, Wang Y-D, Larner A C. Mapping of the cytoplasmic domain of the human growth hormone receptor required for the activation of Jak2 and Stat proteins. J Biol Chem. 1995;270:21326–21330. doi: 10.1074/jbc.270.36.21326. [DOI] [PubMed] [Google Scholar]
- 33.Hansen L H, Wang X, Kopchick J J, Bouchelouche P, Nielsen J H, Galsgaard E D, Billestrup N. Identification of tyrosine residues in the intracellular domain of the growth hormone receptor required for transcriptional signaling and Stat 5 activation. J Biol Chem. 1996;271:12669–12673. doi: 10.1074/jbc.271.21.12669. [DOI] [PubMed] [Google Scholar]
- 34.Hartmann D P, Holoday J W, Bernton E W. Inhibition of lymphocyte proliferation by antibodies to prolactin. FASEB J. 1989;3:2194–2202. doi: 10.1096/fasebj.3.10.2787766. [DOI] [PubMed] [Google Scholar]
- 35.Hatakeyama M, Tsudo M, Minamoto S, Kono T, Doi T, Miyata T, Miyasaka M, Taniguchi T. Interleukin-2 receptor β chain gene: generation of three receptor forms by cloned human α and β chain cDNA’s. Science. 1989;244:551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- 36.He T-C, Jiang N, Zhuang H, Quelle D E, Wojchowski D M. The extended box subdomain of the erythropoietin receptor is nonessential for Jak2 activation yet critical for efficient mitogenesis of FDC-ER cells. J Biol Chem. 1994;269:18291–18294. [PubMed] [Google Scholar]
- 37.Ihle J N, Kerr I M. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 38.Lammers R, Van Obberghen E, Ballotti R, Schlessinger J, Ullrich A. Transphosphorylation as a possible mechanism for insulin and epidermal growth factor receptor activation. J Biol Chem. 1990;265:16886–16890. [PubMed] [Google Scholar]
- 39.Lebrun J-J, Ali S, Goffin V, Ullrich A, Kelly P A. A single phosphotyrosine residue of the prolactin receptor is responsible for activation of gene transcription. Proc Natl Acad Sci USA. 1995;92:4031–4035. doi: 10.1073/pnas.92.9.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lebrun J-J, Ali S, Ullrich A, Kelly P A. Proline-rich sequence-mediated Jak2 association to the prolactin receptor is required but not sufficient for signal transduction. J Biol Chem. 1995;270:10664–10670. doi: 10.1074/jbc.270.18.10664. [DOI] [PubMed] [Google Scholar]
- 41.Lebrun J J, Ali S, Sofer L, Ullrich A, Kelly P A. Prolactin-induced proliferation of Nb2 cells involves tyrosine phosphorylation of the prolactin receptor and its associated tyrosine kinase JAK2. J Biol Chem. 1994;269:14021–14026. [PubMed] [Google Scholar]
- 42.Leff M A, Buckley D J, Krumenacker J S, Reed J C, Miyashita T, Buckley A R. Rapid modulation of the apoptosis regulatory genes, bcl-2 and bax, by prolactin in rat Nb2 lymphoma cells. Endocrinology. 1996;137:5456–5462. doi: 10.1210/endo.137.12.8940371. [DOI] [PubMed] [Google Scholar]
- 43.Lesueur L, Edery M, Ali S, Paly J, Kelly P A, Djiane J. Comparison of long and short forms of the prolactin receptor on prolactin-induced milk protein gene transcription. Proc Natl Acad Sci USA. 1991;88:824–828. doi: 10.1073/pnas.88.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li B-Q, Subleski M, Fusaki N, Yamamoto T, Copeland T, Princler G L, Kung H-F, Kamata T. Catalytic activity of the mouse guanine nucleotide exchanger mSOS is activated by Fyn tyrosine protein kinase and the T-cell antigen receptor in T-cells. Proc Natl Acad Sci USA. 1996;93:1001–1005. doi: 10.1073/pnas.93.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu K, Lai S Y, Goldsmith M A, Greene W C. Identification of a variable region within the cytoplasmic tail of the IL-2 receptor β chain that is required for growth signal transduction. J Biol Chem. 1995;270:22176–22181. doi: 10.1074/jbc.270.38.22176. [DOI] [PubMed] [Google Scholar]
- 46.Miura O, Cleveland J L, Ihle J N. Inactivation of erythropoietin receptor function by point mutations in a region having homology with other cytokine receptors. Mol Cell Biol. 1993;13:1788–1795. doi: 10.1128/mcb.13.3.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murakami M, Narazaki M, Hibi M, Yawata H, Yasukawa K, Hamaguchi M, Taga T, Kishimoto T. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine family. Proc Natl Acad Sci USA. 1991;88:11349–11353. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Muto A, Watanabe S, Miyajima A, Yokota T, Arai K. High affinity chimeric granulocyte-macrophage colony-stimulating factor receptor carrying the cytoplasmic domains of the β subunit but not the α subunit transduces growth promoting signals in Ba/F3 cells. Biochem Biophys Res Commun. 1995;208:368–375. doi: 10.1006/bbrc.1995.1347. [DOI] [PubMed] [Google Scholar]
- 49.Nagano M, Kelly P A. Tissue distribution and regulation of rat prolactin receptor gene expression. J Biol Chem. 1994;269:13337–13345. [PubMed] [Google Scholar]
- 50.Nelson B H, Lord J D, Greenberg P D. Cytoplasmic domains of the interleukin-2 receptor beta and gamma chains mediate the signal for T-cell proliferation. Nature. 1994;369:333–336. doi: 10.1038/369333a0. [DOI] [PubMed] [Google Scholar]
- 51.Nicoll C S. Physiological actions of prolactin. In: Knobil E, Sawyer W H, editors. Handbook of physiology. Vol. 4. 1974. pp. 253–291. , part 2. American Physiological Society, Washington, D.C. [Google Scholar]
- 52.Ohashi H, Maruyama K, Liu Y-C, Yoshimura A. Ligand-induced activation of chimeric receptors between the erythropoietin receptor and receptor tyrosine kinases. Proc Natl Acad Sci USA. 1994;91:158–162. doi: 10.1073/pnas.91.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Neal K D, Yu-Lee L-Y. Differential signal transduction of the short, Nb2, and long prolactin receptors. Activation of interferon regulatory factor-1 and cell proliferation. J Biol Chem. 1994;269:26076–26082. [PubMed] [Google Scholar]
- 54.Palacios R, Steinmetz M. IL3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985;41:727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- 55.Pellegrini I, Lebrun J-J, Ali S, Kelly P A. Expression of prolactin and its receptor in human lymphoid cells. Mol Endocrinol. 1992;6:1023–1031. doi: 10.1210/mend.6.7.1508218. [DOI] [PubMed] [Google Scholar]
- 56.Perrot-Applanet M, Gualillo O, Pezet A, Vincent V, Edery M, Kelly P A. Dominant negative and cooperative effects of mutant forms of prolactin receptor. Mol Endocrinol. 1997;11:1020–1032. doi: 10.1210/mend.11.8.9954. [DOI] [PubMed] [Google Scholar]
- 57.Qian X, Dougall W C, Hellman M E, Greene M I. Kinase-deficient neu proteins suppress epidermal growth factor receptor function and abolish cell transformation. Oncogene. 1994;9:1507–1514. [PubMed] [Google Scholar]
- 58.Qian X, LeVea C M, Freeman J K, Dougall W C, Greene M I. Heterodimerization of epidermal growth factor receptor and wild-type or kinase-deficient Neu: a mechanism of interreceptor kinase activation and transphosphorylation. Proc Natl Acad Sci USA. 1994;91:1500–1504. doi: 10.1073/pnas.91.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quelle D E, Wojchowski D M. Localized cytosolic domains of the erythropoietin receptor regulate growth signaling and down-modulate responsiveness to granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci USA. 1991;88:4801–4805. doi: 10.1073/pnas.88.11.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quelle F W, Sato N, Witthuhn B A, Inhorn R C, Eder M, Miyajima A, Griffen J D, Ihle J N. JAK2 associates with the βc chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol Cell Biol. 1994;14:4335–4341. doi: 10.1128/mcb.14.7.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quelle F W, Wang D, Nosaka T, Thierfelder W E, Stravopodis D, Weinstein Y, Ihle J N. Erythropoietin induces activation of Stat5 through association with specific tyrosines on the receptor that are not required for a mitogenic response. Mol Cell Biol. 1996;16:1622–1631. doi: 10.1128/mcb.16.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rui H, Lebrun J-J, Kirken R A, Kelly P A, Farrar W L. JAK2 activation and cell proliferation induced by antibody-mediated prolactin receptor dimerization. Endocrinology. 1994;135:1299–1306. doi: 10.1210/endo.135.4.7925093. [DOI] [PubMed] [Google Scholar]
- 63.Sato N, Sakamaki K, Terada N, Arai K-I, Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common beta subunit responsible for different signaling. EMBO J. 1993;12:4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato N, Sakamaki K, Terada N, Arai K, Miyajima A. Signal transduction by the high-affinity GM-CSF receptor: two distinct regions of the common beta subunit responsible for different signaling. EMBO J. 1993;12:4181–4189. doi: 10.1002/j.1460-2075.1993.tb06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tartare S, Ballotti R, Lammers R, Alengrin F, Dull T, Schlessinger J, Ullrich A, Van Obberghen E. Insulin-EGF receptor chimerae mediate tyrosine transphosphorylation and serine/threonine phosphorylation of kinase-deficient EGF receptors. J Biol Chem. 1991;266:9900–9906. [PubMed] [Google Scholar]
- 66.Wang X, Darus C J, Xu B C, Kopchick J J. Identification of growth hormone receptor (GHR) tyrosine residues required for GHR phosphorylation and JAK2 and STAT5 activation. Mol Endocrinol. 1996;10:1249–1260. doi: 10.1210/mend.10.10.9121492. [DOI] [PubMed] [Google Scholar]
- 67.Zon L I, Moreau J-F, Koo J-W, Mathey-Prevot B, D’Andrea A D. The erythropoietin receptor transmembrane region is necessary for activation by the Friend spleen focus-forming virus gp55 glycoprotein. Mol Cell Biol. 1992;12:2949–2957. doi: 10.1128/mcb.12.7.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]