Abstract
The heritability of eating disorder (ED) symptoms increases dramatically across gonadarche in girls. Past studies suggest these developmental differences could be due to pubertal activation of estrogen, but findings have been limited to only one ED symptom (i.e., binge eating). The current study examined whether estrogen contributes to gonadarcheal differences in genetic influences on overall levels of ED symptoms as well as key cognitive symptoms (i.e., weight/shape concerns) that are present across all EDs and are early risk factors for eating pathology. Given that binge eating frequently co-occurs with all of these symptoms, analyses also examined whether estrogen effects exist for overall levels of ED symptoms and body weight/shape concerns after accounting for the known effects of estrogen on genetic risk for binge eating. Participants included 964 female twins (ages 8–16) from the Michigan State University Twin Registry. Overall levels of ED symptoms were assessed with the Minnesota Eating Behavior Survey (MEBS) total score. Weight/shape concerns were assessed with a latent factor modeled using subscales from the MEBS and the Eating Disorder Examination Questionnaire. Estradiol levels were assessed with saliva samples. Twin moderation models were used to examine whether genetic influences on overall levels of ED symptoms and weight/shape concerns differed significantly across estradiol levels. Although initial models suggested modest differences in genetic influences on overall levels of ED symptoms across estradiol levels, these effects were eliminated when binge eating was accounted for in the models. In addition, weight/shape concerns did not show significant moderation of genetic influences by estradiol in models with or without binge eating. Taken together, results are significant in suggesting that individual differences in estradiol levels during gonadarche have a unique and specific impact on genetic risk for binge eating, while other etiologic factors must contribute to increased heritability of cognitive ED symptoms during this key developmental period in girls.
1. Introduction
Eating disorders (EDs), including anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED), are associated with substantial psychiatric (e.g., mood disorders, anxiety) and medical (e.g., cardiac abnormalities) comorbidity and the highest mortality rates of all psychiatric disorders (e.g., Zerwas et al., 2015). Importantly, ED symptoms (i.e., weight/shape concerns, binge eating) are more prevalent than EDs and have been shown to be early risk factors for the development of full threshold EDs (see Jacobi, 2005). Given the prevalence and substantial morbidity associated with these symptoms (e.g., Mond et al., 2013), more studies are needed to better understand their development.
Key epidemiological patterns in the prevalence and developmental emergence of ED symptoms provide important clues to etiology. ED symptoms tend to be more common in females than males (e.g., Culbert et al., 2013) and substantially increase in prevalence across adolescence, particularly during/after gonadarche in girls (Culbert et al. 2013; Klump, 2013). Interestingly, patterns of genetic influences on ED symptoms mimic these epidemiological features; the heritability of ED symptoms increases from ~0% in pre-early gonadarche (i.e., the stage of puberty in which gonadal hormones increase, leading to outward physical changes) to ≥50% in mid-post-gonadarche in girls (Culbert et al., 2009; Klump et al., 2007, 2012, 2017a). These gonadarcheal differences are present for a range of ED symptoms (i.e., total symptom scores, binge eating, weight/shape concerns) even after controlling for age and other potential confounds (e.g., body mass index (BMI), anxiety) (Klump et al., 2017a; O’Conner et al., 2020). Importantly, increases in genetic effects across gonadarche are found in girls but not boys (Culbert et al., 2017; Klump et al., 2012), highlighting the presence of female-specific processes.
One female-specific process that may contribute is the activation of estrogen during gonadarche in girls. Estrogen becomes activated in girls (but not boys) during gonadarche and increases linearly across this period (Wilson et al., 1998). Changes in estradiol levels are similar to the linear increases in genetic effects that are present for ED symptoms (Culbert et al., 2009; Klump et al., 2007; Klump et al., 2017b; O’Connor et al., 2020). Moreover, estrogen is a steroid hormone that directly regulates gene transcription in neural systems (e.g., serotonin, dopamine) that are disrupted in EDs (Ostlund et al., 2003) and could contribute to increased genetic influences on disordered eating during gonadarche.
To date, only one study has investigated estrogen effects on genetic influences for any ED symptom during gonadarche. Klump et al. (2018) found that the heritability of binge eating was significantly higher in girls with lower (69%) versus higher (2%) estradiol levels during gonadarche, even after controlling for age, BMI, and the physical changes of puberty (e.g., breast development, skin changes). Progesterone did not contribute to differences in genetic effects, most likely because progesterone activation occurs much later (i.e., after first ovulation; Wilson et al., 1998) than the increases in genetic effects observed during mid-gonadarche. At first glance, stronger genetic influences at lower estradiol levels seem to contradict increases in genetic effects across gonadarche. However, data show that estrogen is protective against binge eating in adulthood, such that lower estradiol levels are associated with increased phenotypic and genetic risk for binge eating in humans (Klump et al., 2013, 2014). Findings from Klump et al. (2018) suggest that these protective effects may extend to gonadarche as well, such that increases in genetic effects on binge eating during gonadarche are driven by girls with lower estradiol levels. Lower estradiol levels may disrupt normative genomic and developmental processes, leading to increased genetic risk for binge eating across the pubertal period.
Nonetheless, there is a complete lack of data on estrogen effects for other ED symptoms that exhibit increases in genetic effects during gonadarche and substantially contribute to the development of clinical EDs. For example, body weight and shape concerns are common symptoms across all ED diagnoses and are some of the strongest early predictors of the later development of clinical eating pathology (see Jacobi, 2005). Likewise, omnibus measures of ED symptoms (e.g., the total score on the Minnesota Eating Behavior Survey [MEBS]) have strong links to a range of clinical EDs (von Ranson et al., 2005) and are routinely used as screening instruments in the field (Maguen et al., 2018). Both body weight/shape concerns and these omnibus measures show increases in heritability across gonadarche in girls (Culbert et al., 2009; Klump et al., 2012; O’Connor et al., 2020) but have never been examined for estrogen effects during this key developmental period.
An important consideration in the examination of estrogen effects on body weight/shape concerns and overall measures of ED symptoms will be accounting for overlap with binge eating. Binge eating and shape/weight concerns typically co-occur (Jacobi, 2005), and omnibus ED symptom measures often include binge eating as a symptom domain (e.g., the MEBS total score). Given the strong effects of estrogen on binge eating across development (Klump et al., 2017b), analyses should control for binge eating to ensure estrogen effects are unique to weight/shape concerns and other ED symptoms. Past studies have shown unique effects of gonadarche on genetic influences on weight/shape concerns and total ED symptoms even when controlling for binge eating (O’Connor et al., 2020). Thus, it is likely that estrogen effects will remain after controlling for binge eating, but direct tests of this hypothesis are needed.
Given the above, this study aimed to examine the effects of estrogen on genetic influences on weight/shape concerns and total ED symptoms during gonadarche in a large, population-based sample of female twins (ages 8–16). We examined these effects with and without controls for binge eating to identify the unique effects of estrogen on other ED symptoms. We focused our primary analyses on estrogen rather than progesterone given that progesterone activation occurs late in gonadarcheal development (e.g., after first ovulation and observed increases in genetic effects on weight/shape concerns and total ED symptom scores), but we also include supplementary models of progesterone. We hypothesized that there would be stronger genetic effects on weight/shape concerns and total ED symptoms at lower versus higher estradiol levels that would remain even after controlling for levels of binge eating.
2. Methods
2.1. Participants
The sample consisted of 964 (MZ = 464 (48%); DZ = 500 (52%)) female twins ages 8 to 16 (M = 11.75, SD = 2.03) who participated in the Twin Study of Mood, Behavior, and Hormones during Puberty (TSMBH; Klump et al., 2018) from within the population-based Michigan State University Twin Registry (MSUTR; for details, see Burt & Klump (2019)). This is the same sample used in the study of the moderating effect of estradiol on genetic influences on binge eating (Klump et al., 2018). The MSUTR recruits twins using birth records in collaboration with the Michigan Department of Health and Human Services. TSMBH response rates (~65%) were on par or better than response rates for other large-scale twin registries (Baker et al., 2002; Hay et al., 2002). MSUTR and TSMBH (see Table 1) twins are representative of the recruitment region in terms of race, ethnicity, and socioeconomic status (Burt & Klump, 2019).
Table 1.
Sociodemographic Data and Descriptive Statistics
| Sociodemographic Variables | N | % |
|---|---|---|
|
| ||
| Racial Identity | ||
| White | 812 | 81.4 |
| Black | 78 | 7.9 |
| Asian/Pacific Islander | 6 | 0.6 |
| Native American | 2 | 0.2 |
| Multiracial | 78 | 7.7 |
| Missing | 22 | 2.1 |
| Hispanic/Latinx Ethnicity | 38 | 4.0 |
|
| ||
| Parental Income | ||
| Under $20,000 | 56 | 5.6 |
| $20,000 – $40,000 | 128 | 12.8 |
| $40,000 – $60,000 | 150 | 15.0 |
| $60,000 – $100,000 | 266 | 26.7 |
| Over $100,000 | 346 | 34.7 |
|
| ||
| Study Variables | Observed Range | Mean (SD) |
|
| ||
| Age | 8–16 | 11.75 (2.03) |
| MEBS Total Score | 0–27 (0–30 possible range) | 4.32 (4.56) |
| MEBS Body Dissatisfaction | 0–6 (0–6 possible range) | .92 (1.46) |
| MEBS Weight Preoccupation | 0–8 (0–8 possible range) | 1.70 (1.98) |
| EDE-Q Shape Concerns | 0–5.88 (0–6 possible range) | .73 (1.08) |
| EDE-Q Weight Concerns | 0–5.40 (0–6 possible range) | .70 (1.03) |
| Weight/Shape Factor Score | −.52 to .86 | −.08 (0.38) |
| MEBS Binge Eating | 0–7 (0–74 possible range) | .88 (1.32) |
| Estradiol (pg/mL) | 0.11–6.78 | 1.47 (0.90) |
| PDS Score | 1–4 (1–4 possible range) | 2.25 (0.90) |
| Body mass index (BMI) | 10.70–46.55 | 19.53 (4.47) |
Note. MEBS = Minnesota Eating Behavior Survey; EDE-Q = Eating Disorder Examination Questionnaire; PDS = Pubertal Development Scale. The Weight/Shape Factor Score included the body dissatisfaction and weight preoccupation subscales of the MEBS, as well as the weight concerns and shape concerns subscales of the EDE-Q. Confirmatory factor analysis was used to model a single factor that provided an excellent fit to the data (CFI = .995, TLI = .984, SRMR = .014). Within the standardized weight/shape factor score, a “zero” value indicates a close to average score for the sample, negative scores indicate below sample average, and positive scores indicate above sample average.
Because TSMBH focused on hormone regulation during gonadarche, twins were required to meet the following inclusion/exclusion criteria: 1) no hormonal contraceptive use within the past 3 months; 2) no psychotropic or steroid medications within the past 4 weeks; 3) no pregnancy or lactation within the past 6 months; and 4) no history of genetic/medical conditions known to influence hormone functioning or appetite/weight. Despite these criteria, the TSMBH twins are representative of the recruitment region in terms of race/ethnicity, and they do not differ on measures of disordered eating (e.g., body dissatisfaction, weight concerns) from other MSUTR female twins in the same age range (Cohen’s d = 0.02–0.14, all p’s >.05). The study was approved by the Michigan State University Institutional Review Board (protocol #01–052M) and informed consent was obtained from participants’ parents along with assent from participants.
2.2. Measures
2.2.1. Zygosity Determination.
Zygosity was determined using a well-validated physical similarity questionnaire that is over 95% accurate when compared to genotyping (Lykken et al., 1990; Peeters et al., 1998). The twins’ mother and trained research assistants independently completed the questionnaire for all twins. Any rater discrepancies were resolved using questionnaire responses, review of photographs of the twins by the principal investigator (KLK), and twin concordance across several single nucleotide polymorphisms.
2.2.2. Overall Levels of ED Symptoms.
The total score from the MEBS1 (von Ranson et al., 2005) was used to assess overall levels of disordered eating in the areas of body dissatisfaction (6 items; dissatisfaction with body size and/or shape), weight preoccupation (8 items; preoccupation with body weight and dieting), compensatory behaviors (6 items; use of, or thoughts of using, self-induced vomiting or other inappropriate compensatory behaviors to control weight) and binge eating (7 items; thinking about or engaging in binge eating, secretive eating, and/or preoccupation with food). The MEBS is a 30-item, true/false questionnaire that is appropriate for use in children as young as 8 years old (e.g., Luo et al., 2016; von Ranson et al., 2005). The MEBS total score shows strong internal consistency in preadolescents and adolescents in past work (alpha = .86–.89; von Ranson et al., 2005) as well as the current study (alpha = .87). Females diagnosed with clinical EDs also score significantly higher on the MEBS total score as compared to controls (von Ranson et al., 2005). Finally, this score shows good stability (r = 0.61) across a 3-year test-retest period (von Ranson et al., 2005).
2.2.3. Weight/Shape Concerns.
We assessed weight/shape concerns using the MEBS body dissatisfaction and weight preoccupation subscales described above, as well as the weight concerns (5 items assessing fears of weight gain and impact of weight on self-evaluation) and shape concerns (8 items assessing body dissatisfaction and impact of body shape on self-evaluation) subscales of the Eating Disorder Examination Questionnaire (EDE-Q; Fairburn and Beglin, 1994). EDE-Q items are rated from 0 (no days/not at all) to 6 (every day/markedly) for the past 28 days. These subscales from the MEBS and EDE-Q show good internal consistency in past work with preadolescent and adolescent samples (; Goossens & Braet, 2010; Van Durme et al., 2015; von Ranson et al., 2005) as well as in the current sample (). The EDE-Q weight/shape concerns subscales show good convergent validity with interview measures of weight/shape concerns (e.g., the Eating Disorders Examination interview; Van Durme et al., 2015) and the MEBS weight preoccupation and body dissatisfaction subscales successfully discriminate between girls with and without diagnosed EDs (ds = .67–1.46; von Ranson et al., 2005).
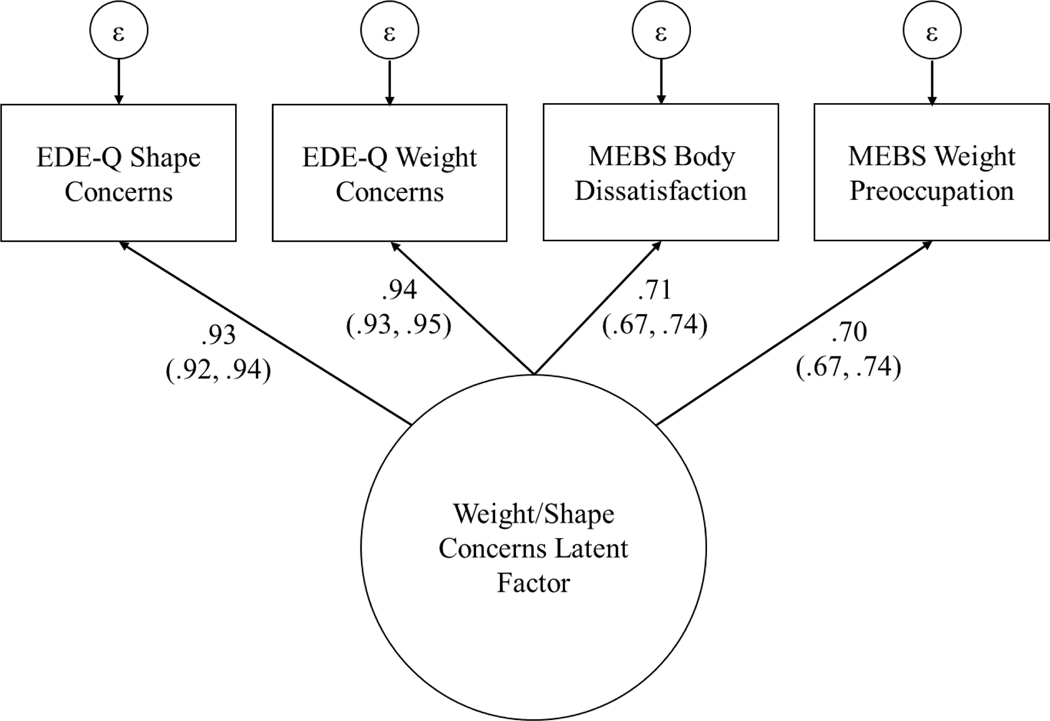
The four MEBS and EDE-Q weight/shape concerns scales are highly conceptually related and were strongly correlated in our sample (rs = .57–.90), suggesting they measure a single underlying construct. Therefore, we modeled weight/shape concerns as a latent factor with these four scales as indicators. An advantage of this approach is that a latent factor is theoretically a more accurate measure of the underlying weight/shape concerns construct than any single observed indicator because it is error-free. All indicators were log transformed prior to factor analysis due to positive skew. We first confirmed that the four scales formed a single factor using exploratory factor analysis (EFA). EFA with orthogonal varimax rotation suggested that all four indicators loaded onto a single latent factor (see Figure 2). Specifically, the first factor extracted through EFA had an eigenvalue of 2.79, while the second factor had an eigenvalue of less than .001. Confirmatory factor analysis also indicated excellent fit for a single latent factor model (CFI = .995, TLI = .984, SRMR = .014). Standardized factor scores estimated using full information maximum likelihood (which makes use of all available data) were extracted from the CFA for the data analyses described below.
Figure 2. Weight/Shape Concerns Latent Factor Model.
EDE-Q = Eating Disorder Examination Questionnaire; MEBS = Minnesota Eating Behavior Survey. Standardized factor loadings are presented with 95% confidence intervals in parentheses.
Binge eating.
Epidemiological research has found very low rates of threshold EDs characterized by binge eating (i.e., BN, BED) in population-based samples in childhood/early adolescence (Nagl et al., 2016; Tanofsky-Kraff et al., 2020) and this was also true in the current sample (i.e., only 2 participants with BN or BED; 0.2%). Binge eating symptoms were therefore measured continuously rather than categorically (i.e., the degree to which participants had any symptoms related to binge eating pathology rather than whether they had threshold BED) using the 7-item MEBS binge eating scale. Notably, this is the same measure of binge eating used in our earlier study examining effects of estrogen on genetic influences on binge eating during gonadarche in this sample (Klump et al., 2018), facilitating direct comparison with those results.
The MEBS binge eating scale shows adequate internal consistency in past studies (alpha = .65–.69; von Ranson et al., 2005) and our current sample (alpha = .69). The binge eating scale exhibits good criterion-related validity, as women with bulimia nervosa (BN) score significantly higher on the scale than controls (von Ranson et al., 2005). In the current sample, twins who self-reported one or more episodes of objective binge eating on the EDE-Q scored significantly higher on the MEBS binge eating scale than controls (t(992) = 15.67, p < .001; d = 1.02).
2.2.4. Hormone Levels.
Salivary samples were used to obtain estradiol levels. Saliva samples represent a less invasive collection method than other measures (e.g., venipuncture, blood spots), particularly for younger participants who, in our population-based lab studies, are much less likely to agree to blood draws. Previous research has also shown that saliva samples are associated with higher compliance rates and, in some cases, more robust hormone-ED associations than blood-spot sampling (Edler et al., 2007). Estradiol values from matched serum and saliva samples show a linear relationship for females (r = .80; Shirtcliff et al., 2000). Notably, this relationship is the same for the Salimetrics assays used in the current study (serum-saliva r = .80 – see Salimetrics, 2022).
Following previously reported procedures (Klump et al., 2018), twins were asked to refrain from eating or drinking for four hours prior to providing a saliva sample and to refrain from smoking, brushing their teeth, or chewing gum for 30 minutes prior to saliva collection. The twins were then instructed to passively drool through a straw into a cryovial until ≥4 ml was produced. All saliva collections occurred between 2:00 pm and 5:00 pm. We chose to collect during these times because afternoon-early evening diurnal variations in ovarian hormones during gonadarche tend to be minimal (Angold et al., 1999; Grumbach & Styne, 1998), and most families were available for assessments during this time.
All saliva samples were processed in duplicate by Salimetrics, LLC (State College, PA) using enzyme immunoassay kits designed specifically for analyzing ovarian hormones in saliva. These are the same assays used in our prior study examining moderation of genetic influences on binge eating by estrogen during gonadarche (Klump et al., 2018). These assays show excellent intra- and inter-assay coefficients of variation (estradiol = 7.1% and 7.5%), as well as assay sensitivity (measured by interpolating the mean optical density minus 2 SDs of 10–20 replicates at the 0-pg/ml level; estradiol = 0.10 pg/ml) and method accuracy (determined by spike recovery and linearity; estradiol = 104.2% and 99.4%). Importantly, all twins in the current study had estradiol levels that fell above the minimum detection limits for the assays.
2.2.5. Covariates.
We controlled for age, BMI, and the physical changes of puberty in analyses. This approach is consistent with past work (e.g., Klump et al., 2019; O’Connor et al., 2020) and ensures that differences in genetic effects are due to estradiol rather than other factors that change during gonadarche.
BMI (weight in kg/height in m2) was calculated using height and weight measured in-person with a wall-mounted ruler and digital scale, respectively. Raw and sex and age-adjusted BMI scores were nearly identical in the current sample (r > .99), with minimal mean differences (mean difference = .038, SD = .03). We therefore used raw BMI values in current analyses to remain consistent with our prior paper examining differences in genetic influences on binge eating across estradiol levels during puberty (Klump et al, 2018). This allowed us to more directly compare effects in the current study with prior results regarding binge eating. Our approach is also consistent with prior developmental twin studies (Culbert et al., 2009; Culbert, Burt, Sisk, & Klump, 2013; Klump et al., 2012, 2003; Klump, Burt, et al., 2007; Klump, Burt, McGue, Iacono, & Wade, 2010; Klump, Holly, Iacono, McGue, & Willson, 2000; Klump, Keel, et al., 2010).
The physical changes of puberty were measured with twin self-report on the Pubertal Development Scale (PDS; Petersen et al., 1988). The PDS assesses growth spurts, skin changes, body hair growth, and breast development on a 4-point scale from (1) development has not yet begun to (4) development seems complete. Menarche status on the PDS is assessed with a yes (coded 4)/no (coded 1) question about whether an individual has had their first period as an indicator of pubertal stage, and no additional questions are asked about missed/skipped periods after they have started menstruating. Although we did not have a direct measure of missed periods, twins were unlikely to have secondary amenorrhea due to a medical condition because we excluded twins with medical or genetic conditions likely to influence hormone functioning (e.g., Turner syndrome, polycystic ovary syndrome). Maternal reports on the PDS were used for a subset of twins (n = 16; 1% of sample) who were missing self-reported PDS scores. Consistent with past studies (Culbert et al., 2009; Klump et al., 2018; O’Connor et al., 2020), PDS items were averaged to create a total score for analyses. Previous studies of the PDS have supported its reliability (median alpha = .77; Petersen et al., 1988) and validity, including high correlations with clinician ratings of pubertal development (85–100% agreement within one stage; Schmitz et al., 2004). Self-report measures of pubertal status such as the PDS are also more acceptable to adolescents and their families than physician examinations in research with non-clinical samples, facilitating higher participation rates (Walker et al., 2020).
2.3. Statistical Analyses
2.3.1. Data preparation.
Missing scores on the MEBS total score, MEBS subscales, and EDE-Q subscales were prorated when ≤10% of the items were missing and coded missing if >10% of items were missing. Both the MEBS total score and the weight/shape concerns factor score were standardized prior to analyses. We conducted all analyses with age, BMI, and PDS scores regressed out of each twin’s MEBS total score and the weight/shape concerns factor score. Notably, these were the same variables partialed out in prior analyses of estrogen effects on genetic influences on binge eating (Klump et al., 2018).
To determine whether binge eating accounted for any observed estrogen effects, we also conducted models controlling for binge eating scores. Specifically, we re-calculated the MEBS total score without the 7 binge eating items and included this modified total score in analyses. For the weight/shape concerns factor score, we regressed MEBS binge eating scores out of the factor score and included the standardized residuals in twin analyses. These approaches for controlling for binge eating are identical to those used in our previous work examining differences in genetic effects across gonadarche (see O’Connor et al., 2020).
2.3.2. Twin moderation models.
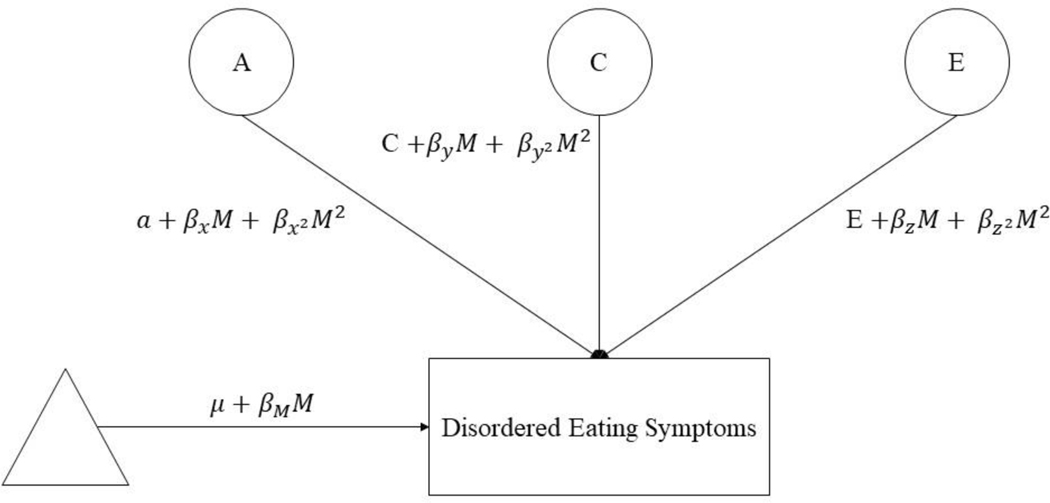
We used extended, univariate twin moderation models (van der Sluis et al., 2012) to examine differences in additive genetic (A; total effects summed across genes), shared environmental (C; common environmental factors that are shared by siblings raised in the same family and contribute to their behavioral similarity), and nonshared environmental (E; factors that are unique to siblings raised in the same family and contribute to behavioral differences, including measurement error) influences on disordered eating across estradiol levels (see Figure 1). These models estimate: 1) path coefficients (i.e., a, c, e) assessing genetic/environmental influences at the lowest level of estradiol; 2) linear moderators(, , ) assessing linear increases/decreases in etiologic influences across estradiol levels; and 3) quadratic moderators (, , ) assessing non-linear increases/decreases in genetic/environmental influences across estradiol levels. We first fit the “full” model that included all parameters. We then compared the fit of this model to nested submodels that differentially constrained linear and quadratic moderators to 0. We initially focused on submodels that are commonly tested in twin moderation analyses (i.e., models that constrained all moderators to 0, models that constrained the quadratic moderators only to 0) as well as those that directly tested our theory of genetic moderation (i.e., models that constrained the genetic moderators to 0). Because of the large number of submodels that could potentially be fit, we used parameter estimates from the full model to identify additional submodels for analysis. This approach allowed us to test relevant submodels without unduly increasing the number of tests.
Figure 1. Path Diagram for the Full Twin Moderation Model for One Twin Only.
A = additive genetic effects; c = shared environmental effects; e = nonshared environmental effects; M = moderator (i.e., estradiol levels); triangle = mean for MEBS total score or weight/shape concerns factor score; phenotypic regression coefficient; a, c, and e = paths or intercepts; , , = linear moderators; , , = quadratic moderators.
We used continuous estradiol values in all analyses. Prior to model-fitting, minimum estradiol levels were “floored” to 0. Models were then fit to the raw data. Best-fitting models were those that minimized Akaike’s information criterion (AIC), Bayesian information criterion (BIC), and sample-size adjusted BIC (SBIC) and had a non-significant difference in minus twice the log-likelihood (−2lnL) from the full model. Unstandardized parameter estimates from the full and best-fitting models are reported in figures, as they more accurately depict absolute differences in etiologic effects than standardized estimates that represent differences as proportions of the total variance.
In addition to our primary models, we conducted some initial, supplementary models to ensure that our approach to model-fitting was appropriate. First, an important assumption in twin moderation models is that the moderator (i.e., estradiol) is genetically independent of the dependent variable (i.e., overall levels of ED symptoms, weight/shape concerns). If there is not independence, then genetic mediation (i.e., gene-environment correlations or rGE) may be present, such that the same genes that influence hormone levels also influence ED symptoms. These types of rGE are troublesome in the context of twin moderation models, as rGE could conceivably “masquerade” as hormone moderation effects. To test this possibility, we first fit “gene × environment (GxE) in the presence of rGE” models (Purcell, 2002) to examine whether the genetic covariance between estradiol and our ED measures could be constrained to zero. The genetic covariance with estradiol could be constrained to zero without worsening model fit for both total ED symptoms (, p = .678; , p = .705; with and without BE items, respectively) and weight/shape concerns (, p = .823; , p = .825; with and without BE items, respectively), ruling out the possibility of rGE. We therefore focused our analyses on the twin moderation models without rGE described above.
Second, we confirmed that progesterone did not significantly moderate genetic effects on disordered eating using the same model-fitting approach as Klump et al. (2018). We dichotomized estradiol and progesterone levels and used two moderator models to examine whether estradiol alone, progesterone alone, or estradiol × progesterone interactions moderated genetic effects. Importantly, the best-fitting model for the MEBS total score constrained all progesterone moderators (including the estradiol × progesterone interaction) to 0 (i.e., comparison with full model: , p = .450), but retained the estradiol moderators (see a description of these models in Figure S1 in Supplemental Material). The best-fitting model for the weight/shape factor score also constrained the progesterone moderator and the estradiol × progesterone interaction to 0 (i.e., comparison with full model: , p = .620). These results support our focus on estradiol in primary analyses.
Finally, to ensure that our inclusion of all twins in analyses regardless of menarche status did not unduly influence results, we conducted model-fitting in only those twins who were pre-menarcheal (65.5%). Hormone values are more variable in post-menarcheal twins and dependent upon menstrual cycle phase. Model-fitting results in this subsample were nearly identical to results in the full sample for both the MEBS total score and the weight/shape factor score (see Figure S2 in Supplemental Material). Thus, we focused our findings on the full twin models that included both pre- and post-menarcheal twins.
3. Results
3.1. Descriptive Statistics and Phenotypic Correlations
Descriptive statistics are presented in Table 1. The MEBS total score and the four weight/shape concerns subscales showed ample variability, with means and standard deviations that are in line with past studies of adolescent girls (Culbert et al., 2009; Goossens & Braet, 2010). Additionally, 3.8% of our participants scored above the MEBS total score clinical cutoff (i.e., total score ≥ 15.55; von Ranson et al., 2005). In line with past work in binge eating (Klump et al., 2018), correlations were small between estradiol levels and the MEBS total score and our weight/shape concerns factor score (r = .10; p = .002 for both measures). Importantly, a small phenotypic correlation between an outcome and putative moderator does not preclude etiologic moderation; in fact, it is often an indicator of significant moderation (Hayes, 2018). Indeed, estradiol levels were previously found to be only modestly correlated with binge eating (r = .01; p =.662) despite significantly moderating genetic influences on binge eating during gonadarche (Klump et al., 2018).
3.2. Twin Moderation Models.
3.2.1. Overall ED Symptoms.
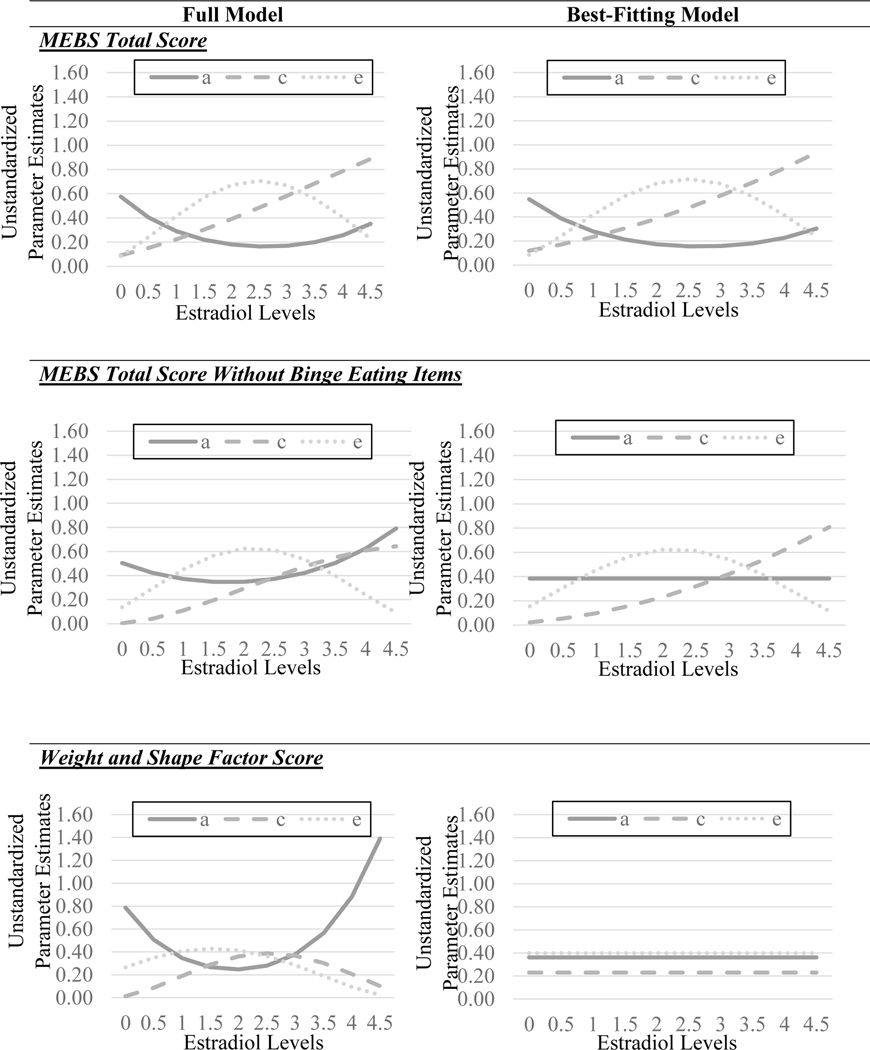
The full model for the MEBS total score with binge eating items included suggested some differences in genetic influences across estradiol levels (see Figure 3), with greater genetic influences at lower estradiol levels. By contrast, shared environmental effects appeared stronger at higher versus lower estradiol levels. Nonshared environmental influences showed a more complex and non-linear pattern of differences, with lower nonshared environmental influences at both lower and higher estradiol. Model fit comparisons (see Table 2) showed that the model constraining all genetic, shared environmental, and nonshared environmental moderators to 0 provided a poor fit to the data (i.e., significant chi-square change and the highest AIC and SBIC values), suggesting significant moderation. Of the remaining submodels, the model that constrained the C quadratic moderator to zero was best fitting, as this model had a non-significant change in chi-square and the lowest AIC and SBIC values. Unstandardized parameter estimates (see Figure 3) from this model were strikingly similar to those from the full model and showed greater genetic effects at lower versus higher estradiol levels.
Figure 3. Unstandardized Parameter Estimates for Additive Genetic (a), Shared Environmental (c), and Nonshared Environmental (e) Effects from the Full and Best-Fitting Twin Moderation Models.
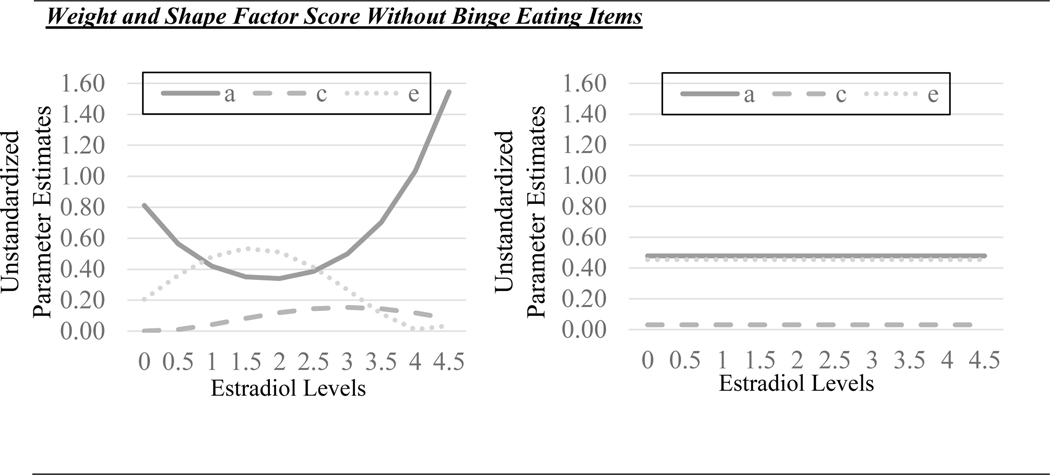
The best fitting models were the models that: 1) constrained the shared environmental quadratic moderator for the original MEBS total score; 2) constrained the shared environmental quadratic moderator and all genetic moderators for MEBS total score without binge eating; and 3) constrained all moderators (genetic, shared environmental, and non-shared environmental) for the weight/shape factor score with and without binge eating. It is important to note that the estradiol values in the figures were floored to 0 for the twin models (see Methods), and that the raw estradiol values range from 0.15–4.61 pg/ml.
Table 2.
Fit Statistics for the Full Twin Moderation Models and Nested Submodels.
| Models | −2lnL | df | AIC | BIC | SBIC | p | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| MEBS Total Score | ||||||||
| Full Model | 2224.718 | 15 | 2254.718 | 2315.178 | 2267.579 | -- | -- | -- |
| Nested Submodels: | ||||||||
| No moderation | 2250.654 | 9 | 2268.655 | 2304.931 | 2276.372 | 25.936 | 6 | <.001 |
| No a quadratic moderator | 2226.122 | 14 | 2254.122 | 2310.551 | 2266.126 | 1.404 | 1 | .24 |
| No c quadratic moderator | 2224.756 | 14 | 2252.755 | 2309.185 | 2264.759 | 0.038 | 1 | .85 |
| No e quadratic moderator | 2237.096 | 14 | 2265.097 | 2321.526 | 2277.101 | 12.378 | 1 | <.001 |
|
| ||||||||
| MEBS Total Score without Binge Eating Items | ||||||||
| Full Model | 2209.644 | 15 | 2239.644 | 2300.104 | 2252.505 | -- | -- | -- |
| Nested Submodels: | ||||||||
| No moderation | 2231.202 | 9 | 2249.201 | 2285.477 | 2256.918 | 21.558 | 6 | .001 |
| No a quadratic moderator | 2210.742 | 14 | 2238.742 | 2295.172 | 2250.746 | 1.098 | 1 | .29 |
| No c quadratic moderator | 2210.048 | 14 | 2238.048 | 2294.477 | 2250.052 | 0.404 | 1 | .53 |
| No e quadratic moderator | 2218.990 | 14 | 2246.989 | 2303.419 | 2258.993 | 9.346 | 1 | .002 |
| No a or c quadratic moderators | 2210.780 | 13 | 2236.780 | 2289.179 | 2247.927 | 1.136 | 2 | .57 |
| No a moderation | 2210.348 | 13 | 2236.349 | 2288.748 | 2247.496 | 0.704 | 2 | .70 |
| No a moderation or c quadratic moderator | 2210.408 | 12 | 2234.408 | 2282.776 | 2244.697 | 0.764 | 3 | .86 |
|
| ||||||||
| Weight and Shape Factor Score | ||||||||
| Full Model | 2165.636 | 15 | 2195.636 | 2256.097 | 2208.498 | -- | -- | -- |
| Nested Submodels: | ||||||||
| No moderation | 2170.786 | 9 | 2188.786 | 2225.062 | 2196.503 | 5.150 | 6 | .53 |
| No a quadratic moderator | 2168.066 | 14 | 2196.066 | 2252.495 | 2208.070 | 2.430 | 1 | .12 |
| No c quadratic moderator | 2168.318 | 14 | 2196.318 | 2252.748 | 2208.322 | 2.682 | 1 | .10 |
| No e quadratic moderator | 2167.724 | 14 | 2195.725 | 2252.154 | 2207.728 | 2.088 | 1 | .15 |
| No a moderation | 2169.022 | 13 | 2195.022 | 2247.421 | 2206.169 | 3.386 | 2 | .18 |
| No c moderation | 2168.184 | 13 | 2194.183 | 2246.582 | 2205.330 | 0.050 | 2 | .98 |
| No e moderation | 2168.108 | 13 | 2194.108 | 2246.507 | 2205.254 | 2.472 | 2 | .29 |
|
| ||||||||
| Weight and Shape Factor Score without Binge Eating Items | ||||||||
| Full Model | 2184.698 | 15 | 2214.698 | 2275.158 | 2227.559 | -- | -- | -- |
| Nested Submodels: | ||||||||
| No moderation | 2190.546 | 9 | 2208.545 | 2244.821 | 2216.262 | 5.848 | 6 | .44 |
| No a quadratic moderator | 2186.390 | 14 | 2214.390 | 2270.82 | 2226.394 | 1.692 | 1 | .19 |
| No c quadratic moderator | 2184.798 | 14 | 2212.797 | 2269.227 | 2224.801 | 0.100 | 1 | .75 |
| No e quadratic moderator | 2188.262 | 14 | 2216.263 | 2272.692 | 2228.267 | 3.564 | 1 | .06 |
| No a moderation | 2188.404 | 13 | 2214.404 | 2266.803 | 2225.551 | 3.706 | 2 | .16 |
| No c moderation | 2185.074 | 13 | 2211.075 | 2263.474 | 2222.221 | 0.376 | 2 | .83 |
| No e moderation | 2188.336 | 13 | 2214.337 | 2266.736 | 2225.484 | 3.638 | 2 | .16 |
Note. Full model = model with all paths and all moderators; a = additive genetic effect; c = shared environmental influences; e = non-shared environmental influences; MEBS = Minnesota Eating Behavior Survey; binge eating = MEBS binge eating subscale score; −2lnL = minus twice the log-likelihood; df = degrees of freedom; AIC = Akaike information criterion; BIC = Bayesian information criterion; = change in chi-square compared to the full model; = change in the degrees of freedom compared to the full model. The best fitting models are noted by borders and bolded text.
However, results from the models with the revised MEBS total score without binge eating items clearly indicated that differences in genetic effects were due to binge eating. Findings from the full and best-fitting models (see Table 2) showed much more attenuated differences in genetic influences across estradiol levels after removing binge eating items. In the full model, there appeared to be somewhat stronger genetic influences at higher versus lower estradiol levels, an effect that is opposite to that described above and for binge eating (see Klump et al., 2018) where genetic influences were stronger at lower estradiol levels. However, the best-fitting model indicated that these modest differences were not statistically significant. Indeed, the best-fitting model constrained the additive genetic moderation effects (and quadratic shared environmental moderation effects) to zero, suggesting that once binge eating items were removed from the MEBS total score, there were no significant differences in genetic influences on overall ED symptoms by estradiol levels.
3.2.2. Weight/Shape Concerns.
The full model for the original weight/shape concerns factor score (without controlling for binge eating) suggested differences in genetic influences across estradiol levels that were once again in the opposite direction from those for binge eating and the original MEBS total score (see Figure 3). The full model showed stronger genetic influences at higher versus lower estradiol levels, although the nature of the differences was non-linear (see Figure 3). By contrast, both shared and nonshared environmental effects appeared to show minimal differences across estradiol levels. Despite indications of differences in genetic effects, model fit comparisons (see Table 2) showed that the model constraining all genetic, shared environmental, and nonshared environmental moderators to 0 provided the best fit to the data (i.e., non-significant chi-square change and the lowest AIC, BIC, and SBIC values), suggesting no significant moderation by estradiol levels. Results controlling for binge eating were very similar, with the no moderation model again providing the best fit to the data despite some indication that there could be greater genetic influences at higher, rather than lower, estradiol levels in the full model. In aggregate, these findings suggest that estrogen has minimal effects on genetic and environmental influences on weight/shape concerns, and that factors other than estrogen likely contribute to developmental differences in weight/shape concerns during gonadarche in girls.
4. Discussion
This was the first large-scale study of estrogen effects on genetic risk for overall ED symptoms and weight/shape concerns that are significant risk factors for later development of clinical EDs. Although estradiol levels significantly moderated genetic influences on overall ED symptoms, these effects were no longer significant after controlling for binge eating. Further, there were no significant moderation effects of estradiol levels on a latent score of weight/shape concerns, with or without controls for binge eating. These results substantially extend our understanding of developmental differences in genetic influences during gonadarche for ED symptoms by highlighting a specific role for estrogen in developmental differences in genetic risk for binge eating that is not present for other key symptoms. In other words, estrogen may not be the mechanism underlying gonadarcheal shifts in genetic influences on aspects of ED risk other than binge eating, including core cognitive symptoms such as weight/shape concerns.
Lack of genetic moderation on ED symptoms beyond binge eating was unexpected, particularly given prior data suggesting increases in genetic influences on overall ED symptoms (e.g., Klump et al., 2007) and weight/shape concerns (O’Connor et al., 2020) across gonadarche. However, our findings align with previous research in adults suggesting that ovarian hormone changes across the menstrual cycle have a greater impact on binge eating and emotional eating (i.e., eating in response to negative emotions) (Klump et al., 2013, 2014) than cognitive symptoms such as weight preoccupation (Hildebrandt et al., 2015). Animal research has shown that ovarian hormones have a strong impact on food intake (Butera, 2010) and binge eating (Klump et al., 2020), as well as mechanisms underlying binge eating (i.e., reward processing; Ma et al., 2020). Indeed, in earlier studies (Klump et al., 2018), we hypothesized that the moderating effects of estrogen on binge eating could be due to its impact on the development of brain reward pathways (e.g., dopamine; Barth et al., 2015; Ma et al., 2020) that are involved in palatable food intake. Our findings are consistent with these hypotheses and suggest that estrogen may specifically impact the development and organization of brain reward pathways or basic physiological processes implicated in binge eating (e.g., satiety and food intake) but not other ED symptoms. These findings provide insight into the types of mechanisms that are important to investigate in future studies of binge eating risk during puberty.
The lack of estrogen moderation for the other ED symptoms (e.g., weight/shape concerns) highlights the need to identify non-hormonal factors that contribute to increasing genetic influences across gonadarche in girls. Puberty is a critical period for increased attention to social consequences and peer relations (e.g., Nelson et al., 2005), and these social/cognitive changes are undergirded by neurobiological development in brain regions such as the prefrontal cortex that are critical for social awareness and metacognition (thinking about the self, including self-esteem and self-image) (e.g., Delevich et al., 2021; Parrish et al., 2018; Somerville et al., 2013). While hormones play a role in the maturation of the prefrontal cortex and social evaluation during adolescence, other biological processes (e.g., neuronal myelination, increased innervation from other brain regions) are also key (Caballero et al., 2016; Nelson et al., 2005) and are genetically-based (van Soelen et al., 2012). Developmental effects of genes not under direct hormonal regulation may contribute to increased genetic influences on cognitive ED symptoms (e.g., weight/shape concerns) during gonadarche. It may also be that the increasingly complex social demands of adolescence “uncover” latent genetically-based differences in neural regions relevant for self-concept and social evaluation that were less impactful at earlier stages in development through genotype x environment interactions. In other words, genetic individual differences that impact social evaluation and metacognition may not be fully expressed phenotypically until an individual encounters increasingly complex and nuanced social demands during puberty/adolescence. Additional research investigating other genetic and neurobiological processes during puberty is needed to identify potentially separable mechanisms underlying risk for binge eating and weight/shape concerns during adolescence.
Though additional work is required before these findings can directly inform prevention or intervention, our results suggest risk factors for binge eating and other disordered eating symptoms may diverge somewhat during puberty, potentially indicating the need for symptom-specific prevention/intervention efforts to target partially distinct underlying mechanisms. In particular, activities or behaviors that lead to low estradiol levels in females with underlying genetic risk during puberty (e.g., intense physical activity and/or insufficient caloric intake; Dipla et al., 2021) could potentially be more impactful for binge eating than other disordered eating symptoms.
Before closing, we should note some study limitations. Pubertal development was measured using youth self-report. Although discrepancies between self-reported PDS scores and pubertal stage based on Tanner staging are small (Schmitz et al., 2004) and self-report measures are more acceptable to adolescent participants (Walker et al., 2020), pubertal stage may have been misclassified in some cases. We also used self-report measures of ED symptoms and weight/shape concerns rather than interview-based measures of diagnoses and/or symptoms. Although questionnaire measures can overestimate rates of binge eating (Field et al., 2004), self-report questionnaires are comparable to interviews for other cognitive and behavioral ED symptoms (e.g., shape/weight concerns; Berg et al., 2011). Use of continuous measures was also consistent with participants’ developmental stage, as rates of threshold EDs characterized by binge eating are very low in childhood/early adolescence (Nagl et al., 2016; Tanofsky-Kraff et al., 2020). Prior research suggests activation of genetic influences on disordered eating may precede onset of threshold EDs because these genetic effects organize the developing nervous system to impact later risk (Schulz & Sisk, 2016; Klump et al., 2018). The organizational effects of hormones would therefore be most evident prior to the developmental period when threshold EDs typically emerge. Indeed, prior research in animal models has shown that ovarian hormones have the greatest impact on organizing risk for binge eating prior to emergence of marked individual differences in behavior (Klump et al., 2020). In other words, although few youth had threshold EDs at the time of analysis in the current study, the impact of hormones on genetic influences on dimensional symptoms may have important implications for development of more severe ED symptoms as youth move into later and post-puberty. The MEBS total score and subscales also show strong reliability/validity in youth/adolescents (Luo et al., 2016), which may not be true for categorical measures typically developed for adults (Tanofsky-Kraff et al., 2020). Nevertheless, it is possible associations between estradiol and ED symptoms could be stronger in children/adolescents who already have clinically significant symptoms, and this is an important area to investigate in future research with clinical samples.
We focused exclusively on core ED symptoms and did not include measures of non-ED psychopathology that may be associated with weight/shape concerns and binge eating (e.g., internalizing symptoms such as anxiety and depression; Ulfvebrand et al., 2015). However, research suggests associations between internalizing symptoms and disordered eating are weaker prior to mid-puberty (Vo et al., 2021) and that pubertal increases in genetic influences on disordered eating are observed even when controlling for internalizing symptoms (Klump et al., 2007).
As noted in Klump et al. (2018), there is less published literature on the reliability/validity of salivary hormone values, and normative data for girls in our age range and during puberty are lacking. More data on reliability/validity of salivary measures could confirm that our findings are not unduly influenced by measurement error, particularly at the lower levels of estradiol. It is important to note, however, that measurement error loads on the nonshared environment in twin models, and thus would result in increased nonshared environmental influences at lower estradiol levels (where, presumably, error of measurement would be greater). Because we did not see any increase in nonshared environmental influences at lower estradiol levels, it seems unlikely that measurement error unduly influenced our results, but additional replications using salivary and serum measures of estradiol are needed to confirm our findings.
Although our sample was population based and demographically representative of our recruitment region, the sample was predominately White and did not include a large proportion of girls from socioeconomically disadvantaged backgrounds. Results may not fully generalize to girls from other racial/ethnic backgrounds or girls experiencing financial stressors. Research has found that socioeconomic disadvantage is associated with earlier potentiation of genetic influences on overall ED symptoms in girls (Mikhail et al., 2021). Additional research is needed to determine whether estrogen differentially impacts genetic influences on ED symptoms in girls experiencing environmental stressors such as disadvantage or discrimination. Further, our data were cross-sectional rather than longitudinal in nature. We were therefore unable to follow the same sample of twins to examine the impact of changing estradiol levels over time. Additional longitudinal research is needed to confirm that findings replicate using within-person measures of changes in estradiol across gonadarche.
Supplementary Material
Table 3.
Unstandardized Path and Moderator Estimates for the Full and Best-Fitting Models.
| Path Estimates | Linear Moderator Estimates | Nonlinear Moderator Estimates | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Model | a | c | e | ||||||
|
| |||||||||
| MEBS Total Score | |||||||||
| Full Model | −.81 (−1.22, .55) | .48 (−.06, .89) | .37 (.03, .65) | .66 (−.12, .95) | .18 (−.33, .62) | .53 (.13, .86) | −.07 (−.15, .03) | −.02 (−.11, .08) | −.18 (−.25, −.04) |
| Best-Fitting Model | .69 (−.09, 1.03) | .47 (.21, .73) | .56 (.27, .78) | −.35 (−.77, .30) | .12 (−.04, .20) | .17 (−.06, .46) | .05 (−.04, .14) | — | −.04 (−.11, −.00) |
| MEBS Total Score without Binge Eating Items | |||||||||
| Full Model | .71 (−.01, .98) | .06 (−.64, .73) | −.37 (−.62, −.07) | −.14 (−.59, .44) | .30 (−.21, .68) | −.39 (−.79, −.12) | .04 (−.06, .11) | −.03 (−.11, .05) | .09 (.04, .19) |
| Best-Fitting Model | 0.62 (.17, .77) | 0.15 (−.35, .56) | 0.39 (.13, .62) | — | 0.17 (.003, .30) | 0.37 (.13, .70) | — | — | −0.08 (−.18, .03) |
| Weight and Shape Factor Score | |||||||||
| Full Model | .89 (.53, 1.24) | .11 (−.53, .76) | .52 (.30, .73) | −.40 (−.81, .00) | .40 (−.19, .99) | .18 (−.05, .41) | .10 (.02, .20) | −.08 (−.21, .05) | −.06 (−.11, −.01) |
| Best-Fitting Model | .60 (.38, .82) | .48 (.24, .72) | .63 (.57, .69) | — | — | — | — | — | — |
| Weight and Shape Factor Score without Binge Eating Items | |||||||||
| Full Model | .90 (.62, 1.18) | −.03 (−.98, .93) | .45 (.22, .68) | −.35 (−.74, .04) | .28 (−.55, 1.11) | .35 (.03, .66) | .09 (.00, .19) | −.05 (−.26, .16) | −.11 (−.21, −.01) |
| Best-Fitting Model | .69 (.48, .91) | .18 (−.54, .89) | .67 (.61, .74) | — | — | — | — | — | — |
Note. MEBS = Minnesota Eating Behavior Survey; binge eating = MEBS binge eating subscale score; a = genetic path estimate; c = shared environmental path estimate; e = non-shared environmental path estimate; = linear moderator of genetic path estimate; = linear moderator of shared environmental path estimate; = linear moderator of non-shared environmental path estimate; = nonlinear moderator of genetic path estimate; = nonlinear moderator of shared environmental path estimate; = nonlinear moderator of non-shared environmental path estimate. Estimates are followed by 95% confidence intervals in parentheses. Confidence intervals that do not overlap with zero indicate statistical significance (bolded) at p < .05. In the “Full” model, genetic, shared environmental, and non-shared environmental estimates are allowed to vary both linearly and nonlinearly across levels of the moderator (i.e., estradiol). The best fitting model is listed below the full model.
Acknowledgments
This research was supported by grants from the National Institute of Mental Health (R01 MH111715 awarded to KLK and SAB) and National Science Foundation (NSF-GRFP awarded to MEM). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or NSF.
Footnotes
Declarations of Interest: none
The Minnesota Eating Behavior Survey (MEBS; previously known as the Minnesota Eating Disorder Inventory) was adapted and reproduced by special permission of Psychological Assessment Resources, Inc., 16204 North Florida Avenue, Lutz, FL 33549, from the Eating Disorder Inventory (collectively, EDI and EDI-2) by Garner, Olmstead, and Polivy (1983) by the Psychological Assessment Resources, Inc. Further reproduction of the MEBS is prohibited without prior permission from Psychological Assessment Resources, Inc.
References
- Angold A, Costello EJ, Erkanli A, & Worthman CM (1999). Pubertal changes in hormone levels and depression in girls. Psychological Medicine, 29(5), 1043–1053. 10.1017/s0033291799008946 [DOI] [PubMed] [Google Scholar]
- Baker LA, Barton M, & Raine A. (2002). The southern California twin register at the University of Southern California. Twin Research, 5(5), 456–459. 10.1375/136905202320906273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C, Villringer A, & Sacher J. (2015). Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Frontiers in Neuroscience, 9. 10.3389/fnins.2015.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CB, Middlemass K, Taylor B, Johnson C, & Gomez F. (2017). Food insecurity and eating disorder pathology. International Journal of Eating Disorders, 50(9), 1031–1040. Portico. 10.1002/eat.22735 [DOI] [PubMed] [Google Scholar]
- Berg KC, Peterson CB, Frazier P, & Crow SJ (2011). Convergence of scores on the interview and questionnaire versions of the Eating Disorder Examination: A meta-analytic review. Psychological Assessment, 23(3), 714–724. 10.1037/a0023246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, & Klump KL (2019). The Michigan state university twin registry (MSUTR): 15 years of twin and family research. Twin Research and Human Genetics, 22(6), 741–745. 10.1017/thg.2019.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera PC (2010). Estradiol and the control of food intake. Physiology & Behavior, 99(2), 175–180. 10.1016/j.physbeh.2009.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero A, Granberg R, & Tseng KY (2016). Mechanisms contributing to prefrontal cortex maturation during adolescence. Neuroscience & Biobehavioral Reviews, 70, 4–12. 10.1016/j.neubiorev.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Breedlove SM, Sisk CL, Burt SA, & Klump KL (2013). The emergence of sex differences in risk for disordered eating attitudes during puberty: A role for prenatal testosterone exposure. Journal of Abnormal Psychology, 122(2), 420–432. 10.1037/a0031791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Burt SA, & Klump KL (2017). Expanding the developmental boundaries of etiologic effects: The role of adrenarche in genetic influences on disordered eating in males. Journal of Abnormal Psychology, 126(5), 593–606. 10.1037/abn0000226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Burt SA, McGue M, Iacono WG, & Klump KL (2009). Puberty and the genetic diathesis of disordered eating attitudes and behaviors. Journal of Abnormal Psychology, 118(4), 788–796. 10.1037/a0017207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevich K, Klinger M, Okada NJ, & Wilbrecht L. (2021, October). Coming of age in the frontal cortex: The role of puberty in cortical maturation. In Seminars in Cell & Developmental Biology (Vol. 118, pp. 64–72). Academic Press. [DOI] [PubMed] [Google Scholar]
- Dipla K, Kraemer RR, Constantini NW, & Hackney AC (2021). Relative energy deficiency in sports (RED-S): Elucidation of endocrine changes affecting the health of males and females. Hormones, 20, 35–47. [DOI] [PubMed] [Google Scholar]
- Edler C, Lipson SF, & Keel PK (2006). Ovarian hormones and binge eating in bulimia nervosa. Psychological Medicine, 37(1), 131–141. 10.1017/s0033291706008956 [DOI] [PubMed] [Google Scholar]
- Field AE, Taylor CB, Celio A, & Colditz GA (2004). Comparison of self-report to interview assessment of bulimic behaviors among preadolescent and adolescent girls and boys. International Journal of Eating Disorders, 35(1), 86–92. [DOI] [PubMed] [Google Scholar]
- Grumbach MM, Styne DM Puberty: Ontogeny, neuroendocrinology, physiology, and disorders. In: Wilson JD, Foster DW, Kronenberg HM, Larsen PR, editors Williams Textbook of Endocrinology 9th. Philadelphia, PA: W.B. Saunders Company; 199815091625 [Google Scholar]
- Hayes Andrew F. (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. Journal of Educational Measurement, 51(3), 335–337. Portico. 10.1111/jedm.12050 [DOI] [Google Scholar]
- Hay DA, McStephen M, Levy F, & Pearsall-Jones J. (2002). Recruitment and attrition in twin register studies of childhood behavior: The example of the australian twin ADHD project. Twin Research, 5(5), 324–328. 10.1375/136905202320906039 [DOI] [PubMed] [Google Scholar]
- Hildebrandt BA, Racine SE, Keel PK, Burt SA, Neale M, Boker S,…Klump KL (2015). The effects of ovarian hormones and emotional eating on changes in weight preoccupation across the menstrual cycle. International Journal of Eating Disorders, 48(5), 477–486. 10.1002/eat.22326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi C, 2005. Psychosocial risk factors for eating disorders. In: Wonderlich SA,Mitchell JE, deZwaan M, Steiger H. (Eds.), Eating Disorders Review. Radcliffe Publishing Ltd, Oxford, UK, pp. 59–85. [Google Scholar]
- Klump KL (2013). Puberty as a critical risk period for eating disorders: A review of human and animal studies. Hormones and Behavior, 64(2), 399–410. 10.1016/j.yhbeh.2013.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Burt SA, McGue M, & Iacono WG (2007). Changes in genetic and environmental influences on disordered eating across adolescence: a longitudinal twin study. Archives of general psychiatry, 64(12), 1409–1415. [DOI] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, & Sisk CL (2017b). Sex differences in binge eating: Gonadal hormone effects across development. Annual Review of Clinical Psychology, 13, 183–207. 10.1146/annurev-clinpsy-032816-045309 [DOI] [PubMed] [Google Scholar]
- Klump KL, Racine SE, Hildebrandt B, Burt SA, Neale M, Sisk CL,…Keel PK (2014). Influences of ovarian hormones on dysregulated eating: A comparison of associations in women with versus women without binge episodes. Clinical Psychological Science, 2(5), 545–559. 10.1177/2167702614521794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, O’Connor S, Fowler N, & Burt SA (2017a). The significant effects of puberty on the genetic diathesis of binge eating in girls. International Journal of Eating Disorders, 50(8), 984–989. 10.1002/eat.22727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, Slane JD, Burt SA, Sisk CL, & Nigg JT (2012). The effects of puberty on genetic risk for disordered eating: Evidence for a sex difference. Psychological Medicine, 42(3), 627–637. 10.1017/s0033291711001541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Fowler N, Mayhall L, Sisk CL, Culbert KM, & Burt SA (2018). Estradiol moderates genetic influences on binge eating during puberty: Disruption of normative processes?. Journal of Abnormal Psychology, 127(5), 458. 10.1037/abn0000352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Perkins PS, Burt AS, McGue M, & Iacono WG (2007). Puberty moderates genetic influences on disordered eating. Psychological Medicine, 37(05), 627. 10.1017/s0033291707000189 [DOI] [PubMed] [Google Scholar]
- Klump KL, Sinclair EB, Hildebrandt BA, Kashy DA, O’Connor S, Mikhail ME, … & Sisk CL (2020). The disruptive effects of estradiol removal before puberty on risk for binge eating in female rats. Clinical Psychological Science, 8(5), 839–856. 10.1177/2167702620921343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Donnellan MB, Burt SA, & Klump KL (2016). The dimensional nature of eating pathology: Evidence from a direct comparison of categorical, dimensional, and hybrid models. Journal of Abnormal Psychology, 125(5), 715. 10.1037/abn0000174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykken DT, Bouchard TJ, McGue M, & Tellegen A. (1990). The Minnesota twin family registry: Some initial findings. Acta Geneticae Medicae et Gemellologiae: Twin Research, 39(1), 35–70. 10.1017/s0001566000005572 [DOI] [PubMed] [Google Scholar]
- Ma R, Mikhail ME, Culbert KM, Johnson AW, Sisk CL, & Klump KL (2020). Ovarian hormones and reward processes in palatable food intake and binge eating. Physiology, 35(1), 69–78. 10.1152/physiol.00013.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguen S, Hebenstreit C, Li Y, Dinh JV, Donalson R, Dalton S, … & Masheb R. (2018). Screen for disordered eating: Improving the accuracy of eating disorder screening in primary care. General Hospital Psychiatry, 50, 20–25. 10.1016/j.genhosppsych.2017.09.004 [DOI] [PubMed] [Google Scholar]
- Mikhail ME, Carroll SL, Clark DA, O’Connor S, Burt SA, & Klump KL (2021). Context matters: Neighborhood disadvantage is associated with increased disordered eating and earlier activation of genetic influences in girls. Journal of Abnormal Psychology, 130(8), 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhail ME, & Klump KL (2020). A virtual issue highlighting eating disorders in people of black/African and Indigenous heritage. International Journal of Eating Disorders, 54(3), 459–467. 10.1002/eat.23402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J, Mitchison D, Latner J, Hay P, Owen C, & Rodgers B. (2013). Quality of life impairment associated with body dissatisfaction in a general population sample of women. BMC Public Health, 13(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimetrics. (2022, March 9). Salivary estradiol. Salimetrics. https://salimetrics.com/analyte/salivary-estradiol/#technicalInfo [Google Scholar]
- Nagl M, Jacobi C, Paul M, Beesdo-Baum K, Höfler M, Lieb R, & Wittchen HU (2016). Prevalence, incidence, and natural course of anorexia and bulimia nervosa among adolescents and young adults. European child & adolescent psychiatry, 25, 903–918. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, & Pine DS (2005). The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine, 35(2), 163–174. [DOI] [PubMed] [Google Scholar]
- O’Connor SM, Culbert KM, Mayhall LA, Burt SA, & Klump KL (2020). Differences in genetic and environmental influences on body weight and shape concerns across pubertal development in females. Journal of Psychiatric Research, 121, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund H, Keller E, Hurd YL (2003). Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences 1007, 54–63. 10.1196/annals.1286.006 [DOI] [PubMed] [Google Scholar]
- Parrish MH, Inagaki TK, Muscatell KA, Haltom KE, Leary MR, & Eisenberger NI (2018). Self-compassion and responses to negative social feedback: The role of fronto-amygdala circuit connectivity. Self and Identity, 17(6), 723–738. [Google Scholar]
- Peeters H, Van Gestel S, Vlietinck R, Derom C, & Derom R. (1998). Validation of a telephone zygosity questionnaire in twins of known zygosity. Behavior Genetics, 28(3), 159–163. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A. (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. [DOI] [PubMed] [Google Scholar]
- Purcell S. (2002). Variance components models for gene–environment interaction in twin analysis. Twin Research and Human Genetics, 5(6), 554–571. [DOI] [PubMed] [Google Scholar]
- Schulz KM, & Sisk CL (2016). The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neuroscience & Biobehavioral Reviews, 70, 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Schwartz EB, Curran MJ, Booth A, & Overman WH (2000). Assessing estradiol in biobehavioral studies using saliva and blood spots: Simple radioimmunoassay protocols, reliability, and comparative validity. Hormones and Behavior, 38(2), 137–147. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, & Casey BJ (2013). The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychological Science, 24(8), 1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfvebrand S, Birgegård A, Norring C, Högdahl L, & von HausswolffJuhlin Y. (2015). Psychiatric comorbidity in women and men with eating disorders results from a large clinical database. Psychiatry Research, 230(2), 294–299 [DOI] [PubMed] [Google Scholar]
- Van der Sluis S, Posthuma D, & Dolan CV (2011). A note on false positives and power in G × E modelling of twin data. Behavior Genetics, 42(1), 170–186. doi: 10.1007/s10519-011-9480-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Durme K, Craeynest E, Braet C, & Goossens L. (2015). The detection of eating disorder symptoms in adolescence: A comparison between the children’s eating disorder examination and the children’s eating disorder examination questionnaire. Behaviour Change, 32(3), 190–201. [Google Scholar]
- Vo PT, Fowler N, Rolan EP, Culbert KM, Racine SE, Burt SA, & Klump KL (2021). The effects of puberty on associations between mood/personality factors and disordered eating symptoms in girls. International Journal of Eating Disorders, 54(9), 1619–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ranson KM, Klump KL, Iacono WG, & McGue M. (2005). The Minnesota Eating Behavior Survey: A brief measure of disordered eating attitudes and behaviors. Eating Behaviors, 6(4), 373–392. [DOI] [PubMed] [Google Scholar]
- van Soelen IL, Brouwer RM, Peper JS, van Leeuwen M, Koenis MM, van Beijsterveldt TC, … & Boomsma DI (2012). Brain SCALE: Brain structure and cognition: An adolescent longitudinal twin study into the genetic etiology of individual differences. Twin Research and Human Genetics, 15(3), 453–467. [DOI] [PubMed] [Google Scholar]
- Wade TD, Hansell NK, Crosby RD, Bryant-Waugh R, Treasure J, Nixon R, Byrne S, & Martin NG (2013). A study of changes in genetic and environmental influences on weight and shape concern across adolescence. Journal of Abnormal Psychology, 122(1), 119–130. 10.1037/a0030290 [DOI] [PubMed] [Google Scholar]
- Walker IV, Smith CR, Davies JH, Inskip HM, & Baird J. (2020). Methods for determining pubertal status in research studies: Literature review and opinions of experts and adolescents. Journal of Developmental Origins of Health and Disease, 11(2), 168–187. [DOI] [PubMed] [Google Scholar]
- Wilksch SM, & Wade TD (2010). Risk factors for clinically significant importance of shape and weight in adolescent girls. Journal of Abnormal Psychology, 119(1), 206. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Foster DW, Kronenberg HM, Larsen PR Williams Textbook of Endocrinology 9th. Philadelphia, PA: W.B. Saunders Company; 1998. [Google Scholar]
- Zerwas S, Larsen JT, Peterson L, Thornton LM, Mortensen PB, & Bulik CM (2015). The incidence of eating disorders in a Danish register study: Associations with suicide risk and mortality. Journal of Psychiatric Research, 65,16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.