Abstract
Background
Migraine is a multiphasic neurovascular disorder, where headache can be succeeded by postdromal symptoms. However, there are limited research on postdromal symptoms. This study aimed to investigate the proportion of individuals with migraine from a tertiary care unit reporting postdromal symptoms in adherence with the ICHD-3 definition. We also aimed to examine how the means of enquiry might influence the estimated proportions. Additionally, we explored whether any clinical features might affect the likelihood of reporting postdromal symptoms. Finally, we assessed to what extend the postdromal symptoms might impact the disease burden.
Methods
In a cross-sectional study, we enrolled adult participants diagnosed with migraine who were asked to report their postdromal symptoms (i.e., unprompted reporting). Subsequently, a 16-item list was used to further ascertain the occurrence of postdromal symptoms (i.e., prompted reporting). Clinical characteristics were obtained through a semi-structured interview. Moreover, electronic questionnaires were used to assess the disease burden, i.e., the Six-Item Headache Impact Test (HIT-6), Migraine Disability Assessment (MIDAS), and the World Health Organization Disability Assessment 2.0 (WHODAS 2.0).
Results
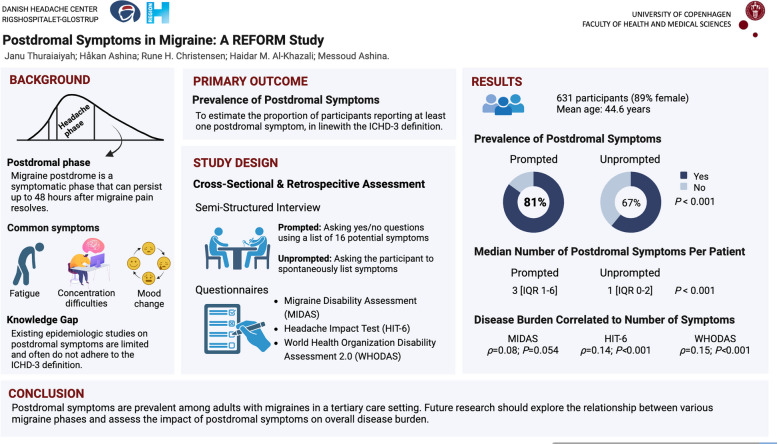
Among 631 participants with migraine, a higher proportion experienced at least one postdromal symptom when prompted (n = 509 [80.7%]) compared with unprompted reporting (n = 421 [66.7%], P < 0.001). Furthermore, the total number of postdromal symptoms experienced was greater with prompted than unprompted reporting (medians 3 [IQR 1 – 6] versus 1 [IQR 0 – 2]; P < 0.001). Furthermore, the likelihood of reporting postdromal symptoms increased with the presence of premonitory symptoms and decreased with higher number of monthly migraine days. Weak correlations were identified between the number of postdromal symptoms reported and both HIT-6 (ρ = 0.14; P < 0.001) and WHODAS scores (ρ = 0.15; P < 0.001), whilst no correlation was observed with MIDAS score (ρ = 0.08; P = 0.054).
Conclusions
Postdromal symptoms are prevalent in individuals with migraine from a tertiary care unit. However, reported estimates warrant cautious interpretation as they depend on the means of enquiry, presence of premonitory symptoms, and frequency of monthly migraine days. Moreover, a weak correlation was identified between the number of postdromal symptoms and both HIT-6 and WHODAS scores, indicating only a marginal influence on the disease burden.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-024-01716-3.
Keywords: Migraine hangover, Tiredness, Premonitory, Disease burden
Introduction
Migraine is a disabling neurologic disorder, afflicting more than one billion people worldwide [21]. Central to our current understanding is the description of migraine in distinct phases: interictal, prodrome, aura, headache, and postdrome [6]. Most research has been directed towards dissecting the prodromal (i.e., premonitory), aura, and headache phases, whereas the postdromal phase has historically received less attention.
The postdromal phase, often referred to as the “migraine hangover”, represents the aftermath of a migraine attack [1]. According to the 3rd edition of the International Classification of Headache Disorders (ICHD-3), these symptoms must occur within 48 h after the resolution of pain in migraine attacks [24]. Common postdromal symptoms include tiredness, concentrations difficulties, and mood changes [4]. However, available epidemiologic literature regarding these symptoms is sparse and has not applied the ICHD-3 definition [4]. Other important considerations also remain unanswered. For instance, it needs to be determined whether the means of enquiry influence the reported count of postdromal symptoms. In addition, potential interrelationships between various migraine phases continue to be enigmatic [10]. Lastly, a clear grasp of postdromal symptoms and their impact on the disease burden might shed light on a possibly overlooked but essential aspect of migraine.
To address gaps in our current knowledge, we performed a cross-sectional study involving a large sample of adult participants with migraine. Our results stand as the first to examine the estimated proportion of postdromal symptoms using the ICHD-3 definition. Furthermore, we investigated whether the likelihood of reporting postdromal symptoms is associated to specific clinical features, including other phases of migraine. Finally, we aimed to explore the impact of postdromal symptoms on the disease burden attributable to migraine.
Methods
The present investigation is part of the larger parental Registry for Migraine (REFORM) study and was carried out at a single tertiary care unit [11]. The study protocol received approval from the relevant ethics committee and data protection agency. Furthermore, the study was conducted in adherence with the principles outlined in Helsinki Declaration, 7th revision [25]. All participants provided written informed consent before any study-related tasks or procedures were performed. A detailed description of the methods used in the parental study can be found elsewhere [11].
Design
The results presented herein are based on cross-sectional, retrospective observational data. The primary recruitment source was adult individuals diagnosed with migraine from the outpatient clinic of a single tertiary care unit. The enrolment period spanned from September 2020 to June 2022. Potential participants underwent an initial screening via phone. Following this, eligible participants were invited for an on-site visit. Each participant underwent a neurologic examination and a semi-structured interview. Furthermore, they were asked to complete various electronic patient-reported outcome measures (PROMs).
Participants
Eligible participants were individuals aged 18 years and above, diagnosed with either migraine with or without aura in accordance with the ICHD-3 criteria [24]. Furthermore, participants were required to have experienced, on average, a minimum of 4 monthly migraine days in the three months before enrolment. The key exclusion criteria were onset of migraine after 50 years of age, history of cluster headache or hemiplegic migraine, inability to distinguish migraine from other types of headaches, and being pregnant or breastfeeding. For the present investigation, we also excluded individuals who experienced continuous and unremitting daily headache. For a complete list of inclusion and exclusion criteria, please refer to Supplemental Table 1.
Procedures
The semi-structured interview was conducted to obtain demographics, headache characteristics, and full medical history. Within the course of the interview, participants were educated on the distinct phases of a migraine attack. This was followed by a series of questions about both premonitory and postdromal symptoms. To ascertain the latter, participants were asked to list all symptoms manifesting within 48 h after the resolution of pain in a migraine attack (i.e., unprompted reporting). If clarity was required, the question was rephrased or explained using a drawing of the migraine phases, omitting mention of specific symptoms. Following this, participants were enquired regarding the occurrence of 16 pre-defined symptoms using close-ended questions (prompted reporting). Participants were also offered the option to introduce additional symptoms. Data regarding premonitory symptoms and triggers factors were also obtained in a similar manner. In addition, participants were asked about comorbid conditions – these were verified using electronic medical records when available.
PROMs were administered and stored using the Research Electronic Data Capture (REDCap) software. The administered questionnaires comprised the Migraine Disability Assessment (MIDAS), Six-Item Headache Impact Test (HIT-6), World Health Organization Disability Assessment 2.0 (WHODAS 2.0), alongside other PROMs reported elsewhere [11].
Definitions
Postdromal symptoms were defined in accordance with the ICHD-3 definition [24]. The latter characterizes postdromal symptoms, lasting up to 48-h, and follows the resolution of pain in a migraine attack with or without aura. Moreover, premonitory symptoms were defined as symptoms occurring 2 to 48 h prior to the onset of pain in a migraine attack. This definition aligns with the description in both ICHD-2 and ICHD-3β [26, 27].
Migraine disability assessment
The MIDAS questionnaire is a self-report instrument, evaluating headache-related disability in individuals with migraine [20]. MIDAS accounts for the number of days on which work/school, household chores, or non-work activities were prevented or impaired due to headache. The appraisal period is the past 90 days. The calculated score can be stratified into little or no disability (0–5), mild disability (6–10), moderate disability (11–20) and severe disability (≥ 21). MIDAS’ upper limit is a score of 450.
Headache impact test
HIT-6 is a self-report instrument with six items, assessing headache-related burden [23]. The appraisal period is the past four weeks for the final three questions, while there is no explicit appraisal period for the remaining questions. Each item is scored from 6 to 13, with a total score ranging from 36 to 78 points. The total score can then be categorized into little or no impact (≤ 49), some impact (50–59), substantial impact (56–59) and severe impact (60–78).
World Health Organization disability assessment schedule 2.0
The WHODAS 2.0 questionnaire is a 12-item instrument to assess disability due to health conditions [5]. The appraisal period is the past 30 days, and each question is rated on a 5-point scale, ranging from “not at all” to “extremely difficult”. The total score ranges from 12 to 60 points.
Outcomes
The primary objective was to estimate the proportion of participants listing postdromal symptoms (i.e., ≥ 1 symptom) using prompted reporting and in adherence with the ICHD-3 definition [24]. The secondary objectives were exploring differences between prompted and unprompted reporting in terms of the proportion of participants with ≥ 1 postdromal symptom. A similar comparison was made for the total number of postdromal symptoms per participant using the two means of enquiry. Another objective was to examine whether the likelihood of reporting postdromal symptoms was affected by certain clinical factors. This included age, gender, migraine with aura, chronic migraine, medication-overuse headache (MOH), monthly migraine days, migraine duration, migraine severity, current use of triptans, current use of preventive migraine medication, premonitory symptoms, and trigger factors. Moreover, we explored whether MIDAS, HIT-6 and WHODAS 2.0 scores/grades differed between participants with and without postdromal symptoms. Finally, we assessed if correlations existed between the number of postdromal symptoms with the total score of MIDAS, HIT-6, and WHODAS 2.0 individually.
Statistical analysis
Participant demographics and clinical characteristics were summarized using descriptive statistics. Categorical data are presented as frequency counts (n) and percentages (%). The distribution of continuous data was assessed for normality using the Kolmogorov–Smirnov test. Means and standard deviations (SD) were then used for data following a normal distribution, while medians and interquartile ranges (IQR) were used for skewed data.
McNemar’s test was used for analyses of paired nominal data, while the Mann–Whitney U test was performed non-parametric continuous data. For categorical data involving three or more groups, Fisher’s exact test was used. Correlation analyses were performed using Spearman’s rank correlation and presented with the correlation coefficient, ρ, and P values. To assess factors that influence the likelihood of reporting postdromal symptoms, we applied a 2-step approach. First, each pre-defined factor was assessed using logistic regression. Those that were significant, exhibiting P values ≤ 0.05, were then tested using binominal logistic regression and reported as odds ratios (ORs) with 95% confidence intervals (95% CI) and corresponding P values. The Bonferroni correction was applied to the binominal logistic regression. The threshold for statistical significance was set at P-values ≤ 0.05. All analyses were performed in R (version 4.2.0).
Results
Participants
A total of 633 adult individuals with migraine participated in the study. Of these, two did not provide information on postdromal symptoms, which rendered data from 631 participants suitable for analyses. The mean participant age was 44.6 (SD, 12.0) years, with most being female (n = 562 [89%]).
The most common diagnosis was migraine without aura (n = 620 [98%]), but 198 of these participants also reported migraine with aura, while 11 participants reported experiencing only migraine with aura. Furthermore, 380 (60%) participants had chronic migraine and 222 (35%) participants met the ICHD-3 criteria for MOH. Current use of triptans was reported by 570 (90%) participants, and 350 (55%) were on preventive migraine medication. Regardless of the assessment method, whether prompted or unprompted, 447 (71%) participants reported experiencing premonitory symptoms, whereas 511 (81%) documented postdromal symptoms. Table 1 provides a detailed overview of participant demographics and clinical characteristics.
Table 1.
Demographics and baseline characteristics of the study population
| Demographics and Clinical Characteristics | |||
|---|---|---|---|
| Reporting ≥ 1 postdromal symptoms | |||
| Yes n = 511 | No n = 120 | Overall n = 631 | |
| Gender, n (%) | |||
| Female | 464 (90.8%) | 98 (81.7%) | 562 (89.1%) |
| Male | 47 (9.2%) | 22 (18.3%) | 69 (10.9%) |
| Age, mean (SD) | 44.9 (11.8) | 43.4 (12.9) | 44.6 (12.0) |
| BMI, mean (SD) | 25.0 (4.9) | 25.0 (5.2) | 25.0 (5.0) |
| Race, n (%) | |||
| White | 469 (91.8%) | 115 (95.8%) | 584 (92.6%) |
| Middle Eastern or North African | 31 (6.1%) | 5 (4.2%) | 36 (5.7%) |
| Black | 6 (1.2%) | 0 (0%) | 6 (1.0%) |
| Asian or Pacific Islander | 4 (0.8%) | 0 (0%) | 4 (0.6%) |
| Hispanic or Latin American | 1 (0.2%) | 0 (0%) | 1 (0.2%) |
| Native American | 0 (0%) | 0 (0%) | 0 (0%) |
| Headache Diagnoses, n (%)a | |||
| Migraine without Aura | 502 (98.2%) | 118 (98.3%) | 620 (98.3%) |
| Migraine with Aura | 166 (32.5%) | 32 (26.7) | 198 (31.4%) |
| Chronic Migraine | 300 (58.7%) | 80 (66.7%) | 380 (60.2%) |
| Medication Overuse Headache | 179 (35.0%) | 43 (35.8%) | 222 (35.2%) |
| Headache Frequency, mean (SD) | |||
| Monthly Migraine Days | 13.7 (6.1) | 18.6 (9.4) | 14.6 (7.1) |
| Total Headache Days Per Month | 17.2 (6.5) | 20.8 (8.4) | 17.9 (7.1) |
| Migraine Intensity, n (%) | |||
| Mild | 1 (0.2%) | 2 (1.7%) | 3 (0.5%) |
| Moderate | 148 (29.0%) | 37 (30.8%) | 185 (29.3%) |
| Severe | 362 (70.8%) | 81 (67.5%) | 443 (70.2%) |
| Clinical Features, n (%) | |||
| Pulsating | 392 (76.7%) | 87 (72.5%) | 479 (75.9%) |
| Pressing | 226 (44.2%) | 65 (54.2%) | 291 (46.1%) |
| Aggravated by physical activity | 465 (91.0%) | 106 (88.3%) | 571 (90.5%) |
| Presence of Nausea | 481 (94.1%) | 107 (89.2%) | 588 (93.2%) |
| Presence of Photophobia | 492 (96.3%) | 112 (93.3%) | 604 (95.7%) |
| Presence of Phonophobia | 481 (94.1%) | 105 (87.5%) | 586 (92.9%) |
| Presence of Autonomic Symptoms | 291 (56.9%) | 51 (42.5%) | 342 (54.2%) |
| Current Use of Treatment, n (%) | |||
| Current Use of Acute Medication | 501 (98.0%) | 115 (95.8%) | 616 (97.6%) |
| Current Use of Triptans | 469 (91.8%) | 101 (84.2%) | 570 (90.3%) |
| Current Use of Preventive Medication | 291 (56.9%) | 59 (49.2%) | 350 (55.5%) |
| Reports Premonitory Symptoms, n (%) | 413 (80.8%) | 34 (28.3%) | 447 (70.8%) |
| Reports Trigger Factors, n (%) | 493 (96.5%) | 113 (94.2%) | 606 (96.0%) |
BMI Body Mass Index, SD Standard Deviations
aHeadache diagnoses were not mutually exclusive. Participants experiencing migraine with aura (MA) and without aura (MO) were categorized under both MA and MO. Those meeting the criteria for chronic migraine (CM), based on headache frequency, and medication-overuse headache (MOH), due to acute medication use, were concurrently assigned the diagnoses of MA and/or MO, CM, and MOH. This approach was adopted to comprehensively represent the diverse migraine presentations within our study population
Postdromal symptoms
Of 631 participants, 509 (80.7%) noted experiencing ≥ 1 postdromal symptom when presented with a pre-defined list of symptoms (prompted reporting). In contrast, 421 (66.7%) experienced ≥ 1 postdromal symptom when the enquiry was unprompted (P < 0.001; Table 2). The mean number of postdromal symptoms also differed between prompted and unprompted reporting (medians 3 [IQR 1 – 6] versus 1 [IQR 0 – 2]; P < 0.001; Table 2). Furthermore, all individual postdromal symptoms were more often reported with prompting than without (P < 0.001 for all tests).
Table 2.
Relative frequency of postdromal symptoms reported unprompted and prompted
| Postdromal symptom (PS) | Unprompted (n = 631) | Prompted (n = 631) | P-value |
|---|---|---|---|
| Reported PS (≥ One Symptom) | 421 (66.7%) | 509 (80.7%) | < 0.001 |
| Number of Symptoms Reported, Median [IQR] | 1 [0–2] | 3 [1–6] | < 0.001 |
| Reported one PS, n (%) | 157 (24.9%) | 86 (13.6%) | |
| Reported two PS, n (%) | 128 (20.3%) | 78 (12.4%) | |
| Reported three PS, n (%) | 75 (11.9%) | 64 (10.1%) | |
| Reported four PS, n (%) | 35 (5.5%) | 69 (10.9%) | |
| Reported five or more PS, n (%) | 26 (4.1%) | 212 (33.6%) | |
| Specific symptoms, n (%) | |||
| Difficulty Concentrating | 58 (9.2%) | 213 (33.8%) | < 0.001 |
| Difficulty Writing and Reading | 2 (0.3%) | 61 (9.7%) | < 0.001 |
| Dizziness | 17 (2.7%) | 57 (9.0%) | < 0.001 |
| Facial Paleness | 1 (0.2%) | 81 (12.8%) | < 0.001 |
| Hunger | 58 (9.2%) | 155 (24.6%) | < 0.001 |
| Irritability | 21 (3.3%) | 123 (19.5%) | < 0.001 |
| Mood Changes | 67 (10.6%) | 159 (25.2%) | < 0.001 |
| Nausea or Vomiting | 35 (5.5%) | 84 (13.3%) | < 0.001 |
| Neck Pain | 29 (4.6%) | 142 (22.5%) | < 0.001 |
| Phonophobia | 22 (3.5%) | 123 (19.5%) | < 0.001 |
| Photophobia | 28 (4.4%) | 137 (21.7%) | < 0.001 |
| Frequency of Urination | 6 (1.0%) | 57 (9.0%) | < 0.001 |
| Tendency Sweating | 2 (0.3%) | 28 (4.4%) | < 0.001 |
| Thirst | 35 (5.5%) | 192 (30.4%) | < 0.001 |
| Tiredness | 317 (50.2%) | 426 (67.5%) | < 0.001 |
| Urge to Yawn | 3 (0.5%) | 76 (12.0%) | < 0.001 |
| Other | 146 (23.1%) | 38 (6.0%) | < 0.001 |
IQR Interquartile ranges, PS Postdromal symptom(s)
When using prompted reporting, the three most common postdromal symptoms were tiredness (n = 426 [67.5%]), concentrations difficulties (n = 213 [33.8%]), and thirst (n = 192 [30.4%]). Unprompted reporting, in turn, revealed tiredness (n = 317 [50.2%]) to be the most prevalent postdromal symptom followed by mood changes (n = 67 [10.6%]). Beyond the pre-defined list of 16 individual symptoms, an additional 38 distinct symptoms were reported unprompted.
Associated factors
Logistic regression analyses revealed several clinical factors associated with the likelihood of experiencing postdromal symptoms. Foremost among these was reporting premonitory symptoms, with an odds ratio of 10.66 (95% CI: 6.83–16.97; P < 0.001). Also being female carried an increased likelihood of having postdromal symptoms (OR: 2.22, 95% CI: 1.26–3.81; P = 0.005), as did reporting current use of triptans (OR: 2.10, 95% CI: 1.15–3.72; P = 0.013). Conversely, a higher number of monthly migraine days inversely affected the likelihoods of reporting postdromal symptoms (OR: 0.91, 95% CI: 0.89–0.94; P < 0.001). The other factors evaluated did not reach a level of statistical significance, as detailed in Table 3.
Table 3.
Presence of postdromal symptoms and risk factors
| Simple Logistic Regression (Univariate) | Binomial Logistic Regression (Multivariate) | |||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value | |
| Age | 1.01 (0.99–1.02) | 0.21 | ||
| Female | 2.22(1.26–3.81) | 0.005 | 2.09 (1.08–3.96) | 0.03 |
| Migraine With Aura | 1.32 (0.86–2.09) | 0.22 | ||
| Chronic Migraine | 0.80 (0.52–1.22) | 0.31 | ||
| Medication-Overuse Headache | 0.97 (0.64–1.47) | 0.87 | ||
| Monthly Migraine Days | 0.91 (0.89–0.94) | < 0.001 | 0.94 (0.91–0.97) | < 0.001* |
| Migraine Duration | 1.00 (0.998–1.01) | 0.15 | ||
| Migraine Intensity (NRS score) | 1.06 (0.93–1.22) | 0.37 | ||
| Current Use of Triptans as Acute Treatment | 2.10 (1.15–3.72) | 0.013 | 1.14 (0.55–2.28) | 0.72 |
| Current Use of Preventive Medication | 1.37 (0.92–2.04) | 0.12 | ||
| Presence of Premonitory Symptoms | 10.66 (6.83–16.97) | < 0.001 | 8.77 (5.53–14.14) | < 0.001* |
| Presence of Trigger Factors | 1.70 (0.65–4.00) | 0.25 | ||
Each clinical factor was assessed using simple logistic regression, and those that exhibiting P values ≤ 0.05, were then tested using binominal logistic regression. The Bonferroni correction was applied to the binominal logistic regression. NRS Numerical Rating Scale
*Significant following Bonferroni correction
The identified significant factors were then tested using binomial logistic regression. Among them, three factors remained significantly associated with reporting postdromal symptoms. These were experiencing premonitory symptoms (OR: 8.77, 95% CI: 5.53–14.14; P < 0.001), being female (OR: 2.09, 95% CI: 1.08–3.96; P = 0.03), and the number of monthly migraine days (OR: 0.94, 95% CI: 0.91–0.97; P < 0.001). However, current use of triptans did not remain significant (OR: 1.14, 95% CI: 0.55–2.28; P = 0.72). After Bonferroni correction, significant associations were only present for reporting premonitory symptoms and the number of monthly migraine days.
Burden measures
For the MIDAS questionnaire, 466 participants (91.2%) with postdromal symptoms and 112 participants (93.3%) without such symptoms responded (Table 4). The median MIDAS scores did not differ between these groups: 53.0 [IQR 29.0–96.0] points for participants with symptoms and 69.5 [IQR 26.8–112.0] points for those without (P = 0.09). The lack of statistical significance remained even after adjusting for the number of monthly migraine days (P < 0.001) and the presence of comorbid chronic pain conditions (P = 0.01). The MIDAS grades also did not differ based on the presence of postdromal symptoms (P = 0.62). Moreover, the MIDAS scores did not correlate the number of postdromal symptoms reported, both when assessed prompted (ρ = 0.08; P = 0.054) or unprompted (ρ = 0.005; P = 0.90).
Table 4.
Postdromal symptoms and migraine burden
| Reporting postdromal symptoms | P-value | ||
|---|---|---|---|
| Yes | No | ||
| MIDAS | |||
| Number of Subjects with Replies | 466 | 112 | |
| Median score [IQR] | 53.0 [29.0–96.0] | 69.5 [26.8–112.0] | 0.09 |
| Grading, n (%) | 0.62 | ||
| No or Little Disability | 9 (1.9%) | 4 (3.6%) | |
| Mild Disability | 15 (3.2%) | 4 (3.6%) | |
| Moderate Disability | 54 (11.6%) | 8 (7.1%) | |
| Severe Disability | 388 (83.3%) | 96 (85.7%) | |
| HIT-6 | |||
| Number of Subjects with Replies | 470 | 112 | |
| Median score [IQR] | 64 [62–66] | 64 [61.8–67] | 0.68 |
| Grading, n (%) | 0.71 | ||
| No or Little Disability | 1 (0.2%) | 1 (0.9%) | |
| Mild Disability | 17 (3.6%) | 5 (4.5%) | |
| Moderate Disability | 37 (7.9%) | 10 (8.9%) | |
| Severe Disability | 415 (88.3%) | 96 (85.7%) | |
| WHODAS | |||
| Number of Subjects with Replies | 416 | 112 | |
| Median Score [IQR] | 21 [16–27] | 22 [16–29] | 0.47 |
HIT-6 Six-Item Headache Impact Test, IQR Interquartile ranges, MIDAS Migraine Disability Assessment, WHODAS World Health Organization Disability Assessment
For the HIT-6 questionnaire, 470 (92.0%) participants with postdromal symptoms and 112 (93.3%) without such symptoms provided responses (Table 4). The median HIT-6 scores were comparable between these groups; 64 (IQR 62.0–66.0) points for those with symptoms and 64 (IQR 61.8–67) points for those without (P = 0.68). This non-significance persisted, even after adjusting for the number of monthly headache days (P < 0.001) and comorbid chronic pain conditions (P = 0.51). The HIT-6 grades also did not differ between the two groups (P = 0.71). Moreover, a weak correlation was identified between the HIT-6 scores and the number of postdromal symptoms reported when prompted (ρ = 0.14; P < 0.001), but not unprompted (ρ = 0.06; P = 0.17).
For the WHODAS 2.0 questionnaire, 416 (81.4%) participants with postdromal symptoms and 112 (93.3%) without such symptoms responded (Table 4). The median WHODAS scores did not differ between these two groups (22 [16–29] vs. 21 [16–27]; P = 0.47). This remained insignificant after adjusting for the number of monthly headache days (P < 0.001), comorbid chronic pain conditions (P < 0.001), current major depressive disorder (P < 0.001) and current anxiety disorder (P = 0.17). Moreover, the WHODAS score correlated weakly with the number of postdromal symptoms reported prompted (ρ = 0.15; P < 0.001). No such correlation was observed with the corresponding unprompted responses (ρ = 0.02; P = 0.56).
Discussion
The present study offers novel insights into the migraine postdrome and stands as the first investigation to use the ICHD-3 definition of postdromal symptoms. Our findings reveal a high prevalence of these symptoms among adults with migraine from a tertiary care population. Approximately 80% of our participants reported experiencing postdromal symptoms, with the most common ones being tiredness and concentration difficulties. These observations accord well with estimates from a recent meta-analysis [4] and support our clinical impression that migraine is not limited to the headache phase. It seems evident that various symptoms present themselves both before (i.e., premonitory) and after (i.e., postdromal) the actual migraine pain.
Assessing postdromal symptoms
Another important finding is the discrepant reporting of postdromal symptoms when assessed prompted versus unprompted. Our results reveal that the relative frequency pertaining to both the total number of symptoms and each individual symptom is greater when assessed prompted than unprompted. One explanation might be that most do experience postdromal symptoms but fail to associate these symptoms with their migraine. However, prompted reporting carries the risk of acquiescence bias [2]. It is also worth noting that the means of enquiry similarly impacts the reporting of premonitory symptoms in individuals with migraine from the same cohort [28]. Taken together, a standardized assessment method is warranted to aid recognition of postdromal symptoms while minimizing the risk of acquiescence bias and false attribution.
In contrast to previous studies that used a 24-h limit [16, 18], our investigation evaluated postdromal symptoms presenting up to 48 h after the resolution of headache. It is therefore interesting that despite our extended duration, the proportion of individuals reporting postdromal symptoms accords well with previous studies [4]. This indicates that the onset of postdromal symptoms most often occur within 24 h after the resolution of headache. However, the duration of these symptoms might still extend beyond 24 h. One previous investigation reported that about one-third of participants with migraine experienced postdromal symptoms lasting more than 24 h [15]. Another retrospective, cross-sectional study found that postdromal symptoms persisted on average for 25.2 h [13]. However, some important questions remain unanswered and warrant further investigation. The onset and duration of individual postdromal symptoms needs to be firmly established. In this context, future enquires should also ascertain whether certain postdromal symptoms manifest in antecedent phases of migraine and then persist in subsequent phases. Such continuity, if confirmed, invites a more nuanced perspective on the description of migraine phases.
Associated factors
Our findings revealed an inverse relationship between the number of monthly migraine days and the reporting of postdromal symptoms. In addition, the presence of premonitory symptoms increased the likelihood of reporting postdromal symptoms. These observations seem conceivable, as high-frequency migraine renders it difficult to distinguish between different phases of migraine. The ICHD-3 indeed also states that for high-frequency migraine, it can be impossible in some cases to distinguish one attack from another. Thus, future studies might consider exclusion of individuals with high-frequency migraine to accurately assess the occurrence of premonitory and postdromal symptoms. However, a growing body of evidence indicate that non-headache symptoms can persist throughout the premonitory, headache, and postdromal phases of migraine [3, 9, 12, 16]. This continuity not only reaffirms the clinical similarities but also alludes to a shared pathophysiologic substrate across different phases of migraine.
Burden measures
Multiple studies have found that the migraine postdrome has negative effects on patients’ daily functioning and overall quality of life [3, 9, 15, 22]. Our study revealed a weak positive correlation between the number of postdromal symptoms reported prompted and both HIT-6 and WHODAS scores. It is also worth noting that no such correlation was observed with the MIDAS scores, which is congruent with a previous investigation [18]. The discrepant findings between MIDAS and HIT-6 might be attributed to their different objectives. MIDAS quantifies days missed or with reduced productivity due to migraine, while HIT-6 considers the broader impact of headache on quality of life, encompassing factors such as pain, social and cognitive functioning, and physiological distress [19].
From a patient perspective, the adverse effects of migraine also extend into the postdromal phase, negatively impacting work, social interactions, and family life [3, 15, 17]. Indeed, a large online survey revealed that about half of individuals with migraine felt very or extremely limited in completing daily tasks during the postdromal phase [8]. Another study found that those with postdromal symptoms tend to report increased work or school absenteeism [13]. Collectively, it is evident that postdromal symptoms are burdensome and can contribute to the overall impact of migraine. This lends support to our findings regarding HIT-6 and WHODAS. The number of postdromal symptoms rather than just their presence might thus be relevant to consider in both clinical and research settings.
Strengths and limitations
The present study stands as the first to use the ICHD-3 definition for postdromal symptoms in a well-characterized population of adults with migraine [24]. The issue of acquiescence bias was also somewhat accounted for via the use of both prompted and unprompted means of enquiry. However, several limitations should be considered when interpreting our results. First, retrospective assessment carries the risk of recall bias, which can lead to underreporting of postdromal symptoms [9]. Second, the onset, duration, and severity of each postdromal symptoms was not evaluated nor were the side effects experienced when using acute medications. Third, the potential for selection bias must be acknowledged. Our study population was primarily recruited from a tertiary care setting and predominantly diagnosed with chronic migraine. This approach skews our sample towards people with more severe disease manifestations, thereby limiting the extrapolation of our results to the broader migraine population. Lastly, our participants’ high migraine frequency might complicate the clear differentiation of migraine phases, increasing the likelihood of symptom misattribution. However, it is pertinent to note that existing literature suggests that the actual duration of premonitory and postdromal symptoms typically does not reach the 48-h maximum outlined in the ICHD-3 criteria [7, 9, 13–15]. This suggests that the theoretical maximum duration might not accurately reflect the lived reality of most people with migraine, as the occurrence of symptoms persisting for the full 48-h window is relatively rare. Thus, restricting the investigation of premonitory and postdromal symptoms to people with only episodic migraine introduces different biases and limit the generalizability of the findings.
Perspectives
To more accurately delineate the migraine postdrome and its associated symptoms, it is essential to use standardized case definitions and robust methods of data collection. Prospective, population-based studies, particularly those using electronic diaries with regular entries and time stamps, would be highly beneficial. These studies should adhere to the ICHD-3 definition of postdromal symptoms. In addition, comprehensive recording of each non-headache symptom, including its onset, duration, and severity across all phases of migraine, is important to achieve a more complete understanding of the migraine postdrome. Furthermore, the present findings indicated a positive correlation between the number of postdromal symptoms and the disease burden. This is an area ripe for exploration and might reveal the impact of postdromal symptoms on the disease burden attributed to migraine. To this end, the development of questionnaires specifically tailored to evaluate the burden associated with postdromal symptoms is essential.
Conclusions
Our study identified a widespread prevalence of postdromal symptoms among adults with migraine from a tertiary care population. These symptoms might be a common disease feature, although confirmation requires population-based data. It is also important to consider that symptom reporting is influenced by the means of enquiry, in addition to the presence of premonitory symptoms and migraine frequency. Future, prospective, observational studies should assess the intricate relationship between differences phases of migraine, as well as the contribution of postdromal symptoms to the overall disease burden.
Supplementary Information
Acknowledgements
For their invaluable contributions to this study, we would like to thank the following investigators and staff members of the Danish Headache Center:
Sub-investigators: Andreas Vinther Thomsen, Betel Tesfay, Christopher Kjær Cullum, Lili Kokoti, Nadja Bredo Rasmussen, Navid Noory, Rogelio Domínguez-Moreno, Thien Phu Do, William Kristian Karlsson and Zixuan Alice Zhuang.
Clinical research nurses: Anne Mette Autzen, Susanne Leed, and Marianne Hestad.
Medical secretaries: Ane Lundgaard Dahl, Dianna Bartolin Christiansen, and Pia Frydendall.
Research administrator: Kateryna Kolkova.
Medical students: Amanda Poulsen, Amenah Ayyoub, Amir Al-Saoudi, Astrid Wiggers, Emil Gazolov, Johanne Gry Larsen, Kathrine Rose, Mikkel Johannes Henningsen, Mohammed Bakir Ahmad Lafta, Sarra Al-Khazali, Sarah Hugger, Shan Elahi Goandal.
Our sincere appreciation goes out to them for their dedicated efforts and expertise, which contributed greatly to making this study a success.
Abbreviations
- BMI
Body Mass Index
- CI
Confidence Interval
- HIT-6
Six-Item Headache Impact Test
- ICHD
International Classification of Headache Disorders
- IQR
Interquartile Range
- MIDAS
Migraine Disability Assessment
- MOH
Medication-Overuse Headache
- OR
Odds Ratio
- PROMs
Patient-Peported Outcome Measures
- REDCap
Research Electronic Data Capture
- REFORM
Registry for Migraine
- SD
Standard Deviations
- WHODAS 2.0
World Health Organization Disability Assessment 2.0
Authors’ contributions
Concept and design: MA, HA. Acquisition, analysis, or interpretation of data: JT, HA, RHC, HMA, MA. Drafting of the manuscript: JT, HA, RHC, HMA, MA. Critical revision of the manuscript for important intellectual content: JT, HA, RHC, HMA, MA. Statistical analysis: JT. Administrative, technical, or material support: MA, HA. Supervision: MA, HA.
Funding
Open access funding provided by Copenhagen University The Lundbeck Foundation professor grant (R310-2018–3711) provided funding for this study.
Availability of data and materials
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol for the parental studied received approval from the ethics committee of the Capital Region of Denmark and the Danish Data Protection Agency (H‐20033264 and H‐20047793). Furthermore, the study was conducted in adherence with the principles outlined in Helsinki Declaration, 7th revision. All participants provided written informed consent before any study-related tasks or procedures were performed.
Consent for publication
Not applicable.
Competing interests
JT and RHC declare that they have no competing interests. HA reports personal fees from Lundbeck and Teva, outside of the submitted work. HMA reports personal fees from Pfizer, outside of the submitted work. MA reports receiving personal fees from AbbVie, Amgen, Eli Lilly, Lundbeck, Novartis, Pfizer and Teva Pharmaceuticals outside of the submitted work. MA received institutional grants from Lundbeck Foundation, Novo Nordisk Foundation, and Novartis. MA reports serving as associate editor of Cephalalgia, associate editor of The Journal of Headache and Pain, and associate editor of Brain.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blau JN. Resolution of migraine attacks: sleep and the recovery phase. J Neurol Neurosurg Psychiatry. 1982;45:223–226. doi: 10.1136/jnnp.45.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowling A. Mode of questionnaire administration can have serious effects on data quality. J Public Health (Bangkok) 2005;27:281–291. doi: 10.1093/pubmed/fdi031. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho IV, Fernandes CS, Damas DP, et al. The migraine postdrome: Clinical characterization, influence of abortive treatment and impact in the quality of life. Clin Neurol Neurosurg. 2022;221:107408. doi: 10.1016/j.clineuro.2022.107408. [DOI] [PubMed] [Google Scholar]
- 4.Christensen RH, Eigenbrodt AK, Ashina H, et al. What proportion of people with migraine report postdromal symptoms? a systematic review and meta-analysis of observational studies. Cephalalgia. 2023;43:03331024231206376. doi: 10.1177/03331024231206376. [DOI] [PubMed] [Google Scholar]
- 5.Federici S, Bracalenti M, Meloni F, Luciano JV. World Health Organization disability assessment schedule 2.0: an international systematic review. Disabil Rehabil. 2017;39:2347–2380. doi: 10.1080/09638288.2016.1223177. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari MD, Goadsby PJ, Burstein R, et al. Migraine. Nat Rev Dis Prim. 2022;8:2. doi: 10.1038/s41572-021-00328-4. [DOI] [PubMed] [Google Scholar]
- 7.Gago-Veiga AB, Pagán J, Henares K, et al. To what extent are patients with migraine able to predict attacks? J Pain Res. 2018;11:2083–2094. doi: 10.2147/JPR.S175602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbs SN, Shah S, Deshpande CG, et al. United States patients’ perspective of living with migraine: country-specific results from the global “My Migraine Voice” Survey. Headache. 2020;60:1351–1364. doi: 10.1111/head.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giffin NJ, Lipton RB, Silberstein SD, et al. The migraine postdrome. Neurology. 2016;87:309–313. doi: 10.1212/WNL.0000000000002789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goadsby PJ, Holland PR, Martins-Oliveira M, et al. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. 2017;97:553–622. doi: 10.1152/physrev.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karlsson WK, Ashina H, Cullum CK, et al. The Registry for Migraine (REFORM) study: methodology, demographics, and baseline clinical characteristics. J Headache Pain. 2023;24:70. doi: 10.1186/s10194-023-01604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karsan N, Peréz-Rodríguez A, Nagaraj K, et al. The migraine postdrome: spontaneous and triggered phenotypes. Cephalalgia. 2021;41:721–730. doi: 10.1177/0333102420975401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelman L. The postdrome of the acute migraine attack. Cephalalgia. 2006;26:214–220. doi: 10.1111/j.1468-2982.2005.01026.x. [DOI] [PubMed] [Google Scholar]
- 14.Kelman L. The premonitory symptoms (Prodrome): a tertiary care study of 893 migraineurs. Headache. 2004;44:865–872. doi: 10.1111/j.1526-4610.2004.04168.x. [DOI] [PubMed] [Google Scholar]
- 15.Martelletti P, Schwedt TJ, Lanteri-Minet M, et al. My Migraine Voice survey: a global study of disease burden among individuals with migraine for whom preventive treatments have failed. J Headache Pain. 2018;19:115. doi: 10.1186/s10194-018-0946-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messina R, Cetta I, Colombo B, Filippi M. Tracking the evolution of non-headache symptoms through the migraine attack. J Headache Pain. 2022;23:1–9. doi: 10.1186/s10194-022-01525-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng-Mak DS, Fitzgerald KA, Norquist JM, et al. Key concepts of migraine postdrome: a qualitative study to develop a post-migraine questionnaire. Headache. 2011;51:105–117. doi: 10.1111/j.1526-4610.2010.01817.x. [DOI] [PubMed] [Google Scholar]
- 18.Quintela E, Castillo J, Muñoz P, Pascual J. Premonitory and resolution symptoms in migraine: a prospective study in 100 unselected patients. Cephalalgia. 2006;26:1051–1060. doi: 10.1111/j.1468-2982.2006.01157.x. [DOI] [PubMed] [Google Scholar]
- 19.Sauro KM, Rose MS, Becker WJ, et al. HIT-6 and MIDAS as measures of headache disability in a headache referral population. Headache. 2010;50:383–395. doi: 10.1111/j.1526-4610.2009.01544.x. [DOI] [PubMed] [Google Scholar]
- 20.Stewart WF, Lipton RB, Whyte J, et al. An international study to assess reliability of the Migraine Disability Assessment (MIDAS) score. Neurology. 1999;53:988–994. doi: 10.1212/wnl.53.5.988. [DOI] [PubMed] [Google Scholar]
- 21.Stovner LJ, Nichols E, Steiner TJ, et al. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954–976. doi: 10.1016/S1474-4422(18)30322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent M, Viktrup L, Nicholson RA, et al. The not so hidden impact of interictal burden in migraine: a narrative review. Front Neurol. 2022;13:1032103. doi: 10.3389/fneur.2022.1032103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang M, Rendas-Baum R, Varon SF, Kosinski M. Validation of the Headache Impact Test (HIT-6™) across episodic and chronic migraine. Cephalalgia. 2011;31:357–367. doi: 10.1177/0333102410379890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.(2018) Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38:1–211 [DOI] [PubMed]
- 25.(2013a) World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. Jama 310:2191–2194 [DOI] [PubMed]
- 26.(2004) Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 24 Suppl 1:9–160. 10.1055/s-2004-813350 [DOI] [PubMed]
- 27.(2013b) Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 33:629–808 [DOI] [PubMed]
- 28.Thuraiaiyah J, Ashina H, Christensen RH et al (2024) Premonitory symptoms in migraine: A REFORM Study. Cephalalgia [In Press]. 10.1177/03331024231223979 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used and/or analyzed during the current study are available from the corresponding author on reasonable request.