Abstract
Paediatric acute respiratory distress syndrome (PARDS) is a heterogeneous clinical syndrome that is associated with high rates of mortality and long-term morbidity. Factors that distinguish PARDS from adult acute respiratory distress syndrome (ARDS) include changes in developmental stage and lung maturation with age, precipitating factors, and comorbidities. No specific treatment is available for PARDS and management is largely supportive, but methods to identify patients who would benefit from specific ventilation strategies or ancillary treatments, such as prone positioning, are needed. Understanding of the clinical and biological heterogeneity of PARDS, and of differences in clinical features and clinical course, pathobiology, response to treatment, and outcomes between PARDS and adult ARDS, will be key to the development of novel preventive and therapeutic strategies and a precision medicine approach to care. Studies in which clinical, biomarker, and transcriptomic data, as well as informatics, are used to unpack the biological and phenotypic heterogeneity of PARDS, and implementation of methods to better identify patients with PARDS, including methods to rapidly identify subphenotypes and endotypes at the point of care, will drive progress on the path to precision medicine.
Introduction
Acute respiratory distress syndrome (ARDS) is a heterogeneous clinical syndrome that contributes to high rates of mortality and long-term morbidity.1,2 Although the initial case series of 12 patients with ARDS, described by Ashbaugh and colleagues in 1967,3 included an 11-year-old boy and four adolescents, important differences exist between paediatric ARDS (PARDS) and adult ARDS related to developmental stages and lung maturation with age, epidemiology, comorbidities, and outcomes.4,5 Risk factors for PARDS also differ between children and adults, with the specific effects of viral or bacterial causative agents possibly contributing to PARDS heterogeneity (similar to differences reported for adult phenotypes between COVID-19-related ARDS and non-COVID-19-related ARDS6). Furthermore, there is substantial heterogeneity among patients with PARDS in the response to supportive therapies, so determining whether a treatment is likely to afford clinically meaningful benefit for a particular child is a crucial goal in the provision of safe and effective clinical care.7 A precision medicine approach is therefore needed to identify characteristics of an individual patient that might be useful to guide therapeutic decisions. Recent efforts in PARDS research have aimed to better understand the heterogeneity of ARDS and to advance a precision medicine approach, although optimal strategies to personalise care are still unclear or unknown.
Adoption of a precision medicine approach requires further progress in our understanding of the clinical and biological heterogeneity of PARDS. Use of clinical, biomarker, and transcriptomic data, as well as informatics, to elucidate biological and phenotypic heterogeneity might help to improve the identification of patients with PARDS who would benefit from specific mechanical ventilation strategies or ancillary treatments, such as prone positioning, and enable the development of novel therapeutic approaches. In this Series paper, we consider the definition of PARDS and the diversity of the patient population, and review current knowledge about the clinical and biological heterogeneity of PARDS. We discuss how improved understanding might advance treatment options for PARDS and the implementation of precision medicine, and thereby drive improvements in outomes for patients. Finally, we highlight ongoing clinical trials and observational studies of PARDS, and outline priorities for future research.
Definition of PARDS
Identification of patients with ARDS is crucial to determine appropriate therapy, but ARDS is often under-recognised by clinicians. This issue has been exacerbated in PARDS because, for decades, paediatric intensivists have used the prevailing adult-oriented definitions of ARDS—ie, the American–European Consensus Conference definition8 and the Berlin definition9—for diagnosis, management, and risk stratification of children with ARDS. However, limitations in the applicability of these criteria to children, such as differences in risk factors, the presence of comorbidities, and lower use of indwelling arterial lines in children, stimulated the Pediatric Acute Lung Injury Consensus Conference (PALICC) Group to publish a specific definition for PARDS (table 1),10 with guidelines for management and future research (table 2).11–30
Table 1:
PALICC definition of PARDS
| PALICC criteria | |
|---|---|
|
| |
| Age | ≤18 years; patients with perinatal-related lung disease excluded |
| Timing of hypoxaemia and radiographic changes | Within 7 days of known clinical insult |
| Origin of oedema | Not fully explained by cardiac failure or fluid overload |
| Chest imaging | Findings of new infiltrate(s) consistent with acute pulmonary parenchymal disease |
| Oxygenation | |
| Non-invasive mechanical ventilation | PARDS (no severity stratification): full face mask bi-level ventilation or CPAP ≥5 cm H2O; PaO2/FiO2 ratio ≤300 mm Hg; SpO2/FiO2 ratio ≤264* |
| Invasive mechanical ventilation | Mild PARDS: OI 4 to <8; OSI 5 to <7·5* Moderate PARDS: OI 8 to <16; OSI 7·5 to <12·3* Severe PARDS: OI ≥16; OSI ≥12·3* |
| Special populations | |
| Cyanotic heart disease | Standard criteria above for age, timing, origin of oedema, and chest imaging, with acute deterioration in oxygenation that is not explained by underlying cardiac disease† |
| Chronic lung disease | Standard criteria above for age, timing, and origin of oedema, with chest imaging consistent with new infiltrate and acute deterioration in oxygenation from baseline that meets oxygenation criteria for PARDS† |
| Left ventricular dysfunction | Standard criteria above for age, timing, and origin of oedema, with chest imaging consistent with new infiltrate and acute deterioration in oxygenation from baseline that meets oxygenation criteria for PARDS and is not explained by left ventricular dysfunction |
Modified from The Pediatric Acute Lung Injury Consensus Conference Group,10 by permission of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. CPAP=continuous positive airway pressure. FiO2=fraction of inspired oxygen. OI=oxygenation index: (FiO2 × mean airway pressure × 100)/PaC2. OSI=oxygen saturation index: (FiO2 × mean airway pressure × 100)/Sp02. PaO2=partial pressure of oxygen in arterial blood. PALICC=Pediatric Acute Lung Injury Consensus Conference. PARDS=paediatric acute respiratory distress syndrome. SpO2=pulse-oximetric oxygen saturation.
Use OI if available; if PaO2 not available, wean FO2 to maintain SpO2 at ≤97% to calculate OSI or SpO2/FiO2 ratio.
PARDS severity groups stratified by OI or OSI should not be applied to children with chronic lung disease who normally receive invasive mechanical ventilation or to children with cyanotic congenital heart disease.
Table 2:
PALICC recommendations for the management of PARDS
| Summary of evidence from paediatric studies | PALICC recommendations | |
|---|---|---|
|
| ||
| Lung-protective ventilation | • Threshold for injurious tidal volume not established (systematic review11) • Higher mortality with lower PEEP relative to FiO2 than that recommended by the ARDS Network12 (observational study13) • Improved oxygenation but no improvement in clinical outcomes with HFOV (small RCTs,14–17 systematic review,18 observational study19); PROSpect 2 × 2 factorial, response-adaptive RCT (prone positioning and HFOV; NCT03896763) ongoing |
• Use tidal volume of 5–8 mL/kg predicted bodyweight if respiratory system compliance preserved (3–6 mL/kg predicted bodyweight if respiratory system compliance reduced)20 • Maintain plateau pressure of ≤28 cm H2O (29–32 cm H2O if chest wall elastance increased)20 • Consider HFOV as an alternative approach if lung-protective ventilation targets cannot be maintained20 • Maintain SpO2 at 92–97% (88–92% for severe PARDS and PEEP ≥10 cm H2O)20 • Allow permissive hypercapnia: target pH 7–15-7–3020 |
| Recruitment manoeuvres | No paediatric studies | Use incremental and decremental PEEP titration, with monitoring of markers of oxygen delivery, respiratory system compliance, and haemodynamics20 |
| Fluid management | Fluid overload associated with worse outcomes (oxygenation and fewer ventilator-free days; systematic review21) | Use a goal-directed fluid-management protocol to maintain intravascular volume while minimising fluid overload22 |
| Prone positioning | No reduction in ventilator-free days (RCT23); PROSpect RCT ongoing | Not recommended for routine use; consider in patients with severe PARDS24 |
| Nitric oxide | Improved oxygenation but no improvement in survival (RCTs, observational studies, literature review24) | Not recommended for routine use; consider in patients with documented pulmonary hypertension or severe right ventricular failure, or as rescue from or bridge to ECMO24 |
| Surfactant | Improved oxygenation but no improvement in survival (RCTs, observational studies, literature review24) | Not recommended for routine use24 |
| Steroids | No effect on survival or duration of ventilation (RCT25); longer duration of mechanical ventilation with prolonged steroid administration (observational study26) | Not recommended for routine use24 |
| Neuromuscular blockade | Improved oxygenation27 but longer duration of mechanical ventilation and paediatric ICU stay28 (observational studies) | Consider if sedation alone is deemed to be inadequate to achieve effective mechanical ventilation22 |
| Sedation | No effect on duration of mechanical ventilation with reduced sedation exposure (RCT29) | • Use minimal yet effective targeted sedation to facilitate tolerance to mechanical ventilation and to optimise oxygen delivery, oxygen consumption, and work of breathing22 • Use validated pain and sedation scales22 |
| ECMO | No paediatric RCTs | Consider in patients with severe PARDS when lung-protective ventilation strategies result in inadequate gas exchange; disease process must be deemed reversible30 |
PALICC recommendations from the Pediatric Acute Lung Injury Consensus Conference Group.10 ARDS=acute respiratory distress syndrome. ECMO=extracorporeal membrane oxygenation. FiO2=fraction of inspired oxygen. HFOV=high-frequency oscillatory ventilation. PALICC=Paediatric Acute Lung Injury Consensus Conference. PARDS=paediatric acute respiratory distress syndrome. PEEP=positive end-expiratory pressure. PROSpect=Prone and Oscillation Pediatric Clinical Trial. RCT=randomised controlled trial. SpO2=pulse-oximetric oxygen saturation.
The PALICC definition of PARDS10 builds on the Berlin definition of ARDS,9 but has important modifications to improve the diagnosis of PARDS. In the PALICC definition, oxygenation severity is quantified by the oxygenation index, which incorporates ventilator mean airway pressure in addition to partial pressure of arterial oxygen (PaO2) and fraction of inspired oxygen (FiO2). Use of the oxygenation index is important because paediatric ventilator practice is highly variable with respect to the use of positive end-expiratory pressure (PEEP) and strategies for lung recruitment, factors that can substantially alter the PaO2/FiO2 ratio. Furthermore, there is high practice variability in the use of arterial blood gas analysis for diagnosis and assessment of the severity of hypoxaemia in children, which prompted the PALICC Group to allow an oxygenation severity metric using pulse-oximetric oxygen saturation (SpO2), such as the oxygenation saturation index, for children on invasive mechanical ventilation, or the SpO2/FiO2 ratio for those on non-invasive ventilation. A multinational study confirmed that nearly 40% of patients who met PARDS criteria did not have an arterial blood gas measurement within the first 3 days of an ARDS diagnosis.31 Radiographic criteria were simplified by eliminating the need for bilateral infiltrates to account for high interobserver variability in the identification of bilateral infiltrates and their limited prognostic relevance in PARDS. The PARDS criteria also include modifications to enable more objective diagnosis in children with chronic cardiopulmonary disease, and have established specific criteria to identify children treated with non-invasive respiratory support who are at risk for PARDS.10 The PALICC definition excludes perinatal lung diseases, which are addressed in the neonatal definition of ARDS (the Montreux definition), which has many similarities to the PALICC definition, but retains the criteria for bilateral infiltrates on chest imaging to diagnose neonatal ARDS.32
Since the publication of the PALICC definition of PARDS,10 there has been a large increase in the number of studies using the PALICC criteria. The epidemiological and prognostic analyses in these studies33 have provided validation and have helped to consolidate the PALICC definition among the paediatric intensivist community. The PALICC guidelines are currently being updated (PALICC-2) to accommodate changes in clinical practice. For example, high-flow nasal oxygen (HFNO) and other modes of non-invasive respiratory support are increasingly being used throughout paediatric critical care, including in children who probably have ARDS pathophysiology; however, the current PALICC criteria preclude the diagnosis of PARDS in children receiving HFNO. This consideration could be even more important in resource-limited settings, where invasive mechanical ventilation is not readily available.
There is clear evidence that higher pulmonary dead space is independently associated with mortality and duration of mechanical ventilation in patients with PARDS after controlling for oxygenation severity.34–36 Dead space reflects areas of the respiratory system that receive ventilation without perfusion, and might indicate endothelial and vascular pathology in ARDS.37 Several studies have validated the use of time-based or volumetric capnography, paired with arterial blood gas analysis, to calculate measures or surrogates of different components of dead space (physiological, alveolar, airway).38 Surrogates of capnography derivatives of dead space, including the ventilatory ratio, have prognostic value for adults with ARDS.39 However, the ventilatory ratio requires arterial blood gas analysis and is based on the estimation of predicted minute ventilation. Estimating predicted minute ventilation is challenging in children, even within similar age groups, because size, weight, and anatomical dead space can differ markedly between children. Moreover, metabolic states in critically ill children also vary. Not surprisingly, the ventilatory ratio has not performed well against capnography-based measures of dead space in patients with PARDS.40 Dead space is likely to be a crucial variable for risk stratification and phenotyping in PARDS, but advances in non-invasive and continuous methods to estimate dead space are needed to ensure availability of this measure in all patients with PARDS.
Epidemiology, risk factors, and comorbidites
PARDS has a lower incidence and mortality than does ARDS in adults. A meta-analysis of paediatric studies that used the American–European Consensus Conference definition8 or the Berlin definition9 of ARDS estimated the incidence of ARDS in children to be 3·5 cases per 100 000 person-years (95% CI 2·2–5·7) and the prevalence in paediatric intensive care units (ICUs) to be 2·3% (1·9–2·9%).1 Pooled mortality for PARDS was 33·7%. By contrast, the Large Observational Study to Understand the Global Impact of Severe Acute Respiratory Failure (LUNG-SAFE) study in adults reported a prevalence of 10·4% of ICU admissions and 23·4% of patients requiring mechanical ventilation using the Berlin definition of ARDS.41 Overall mortality for patients admitted to an ICU with ARDS was 35·3%, and 46·1% in those with severe ARDS. Using the PALICC definition of PARDS, the Paediatric ARDS International Epidemiology (PARDIE) study investigated the epidemiology, risk factors, and causes of PARDS in a cohort of 23 280 patients admitted to 145 paediatric ICUs in 27 middle-income and high-income countries.38 3·2% of all paediatric ICU patients and 6·1% of those who were mechanically ventilated fulfilled criteria for PARDS. Overall mortality in this cohort was 17%, but in patients with severe PARDS, mortality was 33%.38 These results also showed that epidemiological trends have barely changed over the past two decades1 and that mortality differs according to geo-economic variation and resource availability, with higher mortality in middle-income than in high-income countries.1,38 Taking into account age, gender, and race or ethnicity disparities, PARDIE showed significantly higher mortality only in Hispanic children compared with other cohorts.
The PARDIE study showed that pneumonia or lower respiratory tract infection is the most common risk factor for PARDS, followed by non-pulmonary sepsis, whereas aspiration and trauma are less likely to be risk factors.38 Pre-existing comorbidities are also common, with up to 63% of patients with PARDS having comorbidities including chronic lung disease, prematurity, chronic respiratory support, congenital heart disease, or immune suppression. Comorbidities and PARDS triggers both contribute to the heterogeneity of this syndrome,38 and there might be differences between children and adults in the precipitating conditions for ARDS; however, direct comparisons between PARDS and adult ARDS are difficult in the absence of uniformity in the reporting of trigger factors. A better understanding of the unique features of PARDS will be key to the development of a personalised approach to care (panel 1).
Phenotyping of PARDS
The underlying pathobiology of PARDS includes disruption of the endothelial and epithelial permeability barrier, accumulation of protein-filled fluid in the alveolar airspace, dysregulated inflammation and coagulation, and fibrosis.42,43 No specific pharmacological treatment has proved to be effective in decreasing ARDS-related mortality or morbidity in adults or children.44,45 In large part, this is because ARDS is a complex, heterogeneous syndrome rather than a distinct pathological entity.45 In addition to the pathophysiological complexity, there is further heterogeneity in PARDS owing to variations in age and stage of development (of the lungs, chest wall, and immune system), comorbidities (which differ from those in adults), and risk factors (eg, pneumonia, sepsis; panel 1).31,42
Strategies to facilitate prognostic and predictive enrichment are a focus of current ARDS research aimed at transcending the heterogeneity inherent in this syndrome to meet the needs of individual patients. Prognostic enrichment refers to the identification of subgroups of patients who are at high risk of an outcome such as mortality or new morbidity, whereas predictive enrichment refers to the identification of subgroups of patients who are likely to respond to a treatment on the basis of their underlying pathobiology. Therefore, prognostic and predictive enrichment together hold promise for the characterisation of subgroups most likely to benefit from therapies that target common underlying biological mechanisms.45 Subgroups that share a risk factor, clinical trait, diagnostic feature, expression marker, mortality risk, or outcome in response to treatment are known as subphenotypes.46 Subgroups that are distinguished by differences in the underlying pathobiology—as is the case for predictive enrichment—are referred to as endotypes.46 A subphenotype might have shared underlying pathobiological mechanism and hence meet the definition of an endotype. Alternatively, a subphenotype might be composed of two or more endotypes.
In the past 5 years, several studies have aimed to identify subgroups of patients with acute hypoxaemic respiratory failure and PARDS (table 3).47,49–51,53 In a single-centre study, the biomarker-based risk-stratification tool developed by Wong and colleagues for children with sepsis54,55 was adapted for use in PARDS (n=122 patients with PARDS).53 These investigators used a classification and regression tree model and found that three biomarkers—interleukin-8 (IL-8), C-C motif chemokine 3, and heat shock protein 70 kDa 1B—and age accurately predicted mortality, with a good area under the receiver operating characteristic curve (AUROC) of 0·85 (95% CI 0·73–0·92).53
Table 3:
Studies of PARDS-related phenotypes and endotypes
| Studytype | Participants | Sample type | Variables | Analysis | Key findings | |
|---|---|---|---|---|---|---|
|
| ||||||
| Dahmer and colleagues (2022)47 | Secondary analysis of data from the RESTORE trial29 and BALI ancillary study48 | 304 patients (≥2 weeks and <18 years) with PARDS | Plasma | Demographic and clinical variables and protein biomarkers | Latent class analysis to identify PARDS phenotypes and association with clinically relevant outcomes | Two phenotypes, hyperinflammatory and hypoinflammatory, identified with characteristics similar to those in adults; hyperinflammatory phenotype associated with worse outcomes |
| Grunwell and colleagues (2021)49 | Prospective observational cohort study | 74 patients (2 days to 18 years) recruited within 72 h of intubation for acute hypoxaemic respiratory failure (41 [55%] with PARDS) | Tracheal aspirate | Targeted metabolites | Clustering and partial least squares-discriminant analysis to explore clusters of metabolites and association with acute hypoxaemia severity and ventilator-free days | Three clusters of amino acid metabolites important to acute hypoxaemia severity correlated with ventilator-free days |
| Yehya and colleagues (2020)50 | Prospective observational cohort study | 96 patients (>1 month and <18 years) with PARDS* | mRNA from whole blood | Genome-wide transcripts | K-means clustering of expression profiles to identify subphenotypes and association with PICU mortality and ventilator-free days | Three subphenotypes identified with different clinical characteristics and prognoses |
| Yehya and colleagues (2019)51 | Secondary analysis of a microarray-based study of paediatric sepsis52 | 67 patients (≤10 years) with sepsis-associated acute hypoxaemic respiratory failure (PaO2/FiO2 ratio ≤200 mm Hg) | mRNA from whole blood | Expression of 100 genes | Analysis of visual gene-expression patterns to test whether endotypes identified in paediatric sepsis are applicable to paediatric acute hypoxaemic respiratory failure† | Endotypes of paediatric acute hypoxaemic respiratory failure secondary to sepsis identified with differential risk for poor outcomes |
| Yehya and Wong (2018)53 | Prospective observational cohort study | 122 mechanically ventilated patients (>1 month and <18 years) with PARDS* | Plasma | Protein biomarkers | Classification and regression tree analysis to derive a risk prediction model for PARDS | Biomarker-based risk-stratification tool designed and validated for paediatric sepsis adapted for use in PARDS |
BALI=Genetic Variation and Biomarkers in Children with Acute Lung Injury. FiO2=fraction of inspired oxygen. PaO2=partial pressure of arterial oxygen. PARDS=paediatric acuterespiratory distress syndrome. RESTORE=Randomised Evaluation of Sedation Titration for Respiratory Failure.
Defined using the Berlin criteria.9
Using the Gene Expression Dynamics Inspector software.
A transcriptomics approach has also been used to distinguish PARDS subgroups. Using a cohort of children categorised into one of two septic shock endotypes, data from a subgroup of 67 children with acute hypoxaemic respiratory failure secondary to sepsis who met oxygenation criteria for PARDS were reanalysed.52,56 Results indicated that these sepsis-defined endotypes might also be present in PARDS.51 Whole-blood transcriptome gene-expression profiling of 96 patients with PARDS from a single centre identified three transcriptome-based subphenotypes with divergent clinical characteristics and outcomes.50 Despite these advances in PARDS subphenotyping, the relationship between the three subphenotypes and the septic shock-related endotypes previously identified in children with acute hypoxaemic respiratory failure52,56 is not clear.
A recent latent class analysis approach using demographic, clinical, and plasma biomarker data (about 30 variables) identified hyperinflammatory and hypoinflammatory subphenotypes in patients with PARDS (n=304) who were enrolled in the multicentre Genetic Variation and Biomarkers in Children with Acute Lung Injury (BALI) study, an ancillary study of the Randomized Evaluation of Sedation Titration for Respiratory Failure (RESTORE) clinical trial.47 The two subphenotypes differed, in part, by the concentrations of biomarkers, with the hyperinflammatory endotype having higher concentrations of inflammatory biomarkers (IL-6, IL-8, and soluble tumour necrosis factor receptor 1), lung vascular and epithelial cell markers (angiopoietin 2 and receptor for advanced glycation end products), and the coagulation marker plasminogen activator inhibitor 1 than did the hypoinflammatory subphenotype, in which inflammatory biomarkers were also elevated but to a lesser degree. Children with the hyperinflammatory subphenotype had worse outcomes than did those with the hypoinflammatory subphenotype, with a higher rate of mortality (13·8% vs 2·2%, p=0·0001) and longer duration of mechanical ventilation in survivors (8·7 days [IQR 5·5–15·4] vs 6·4 days [4·1–10·5], p=0·003).47 These two PARDS subphenotypes were similar to those identified by Calfee and collaborators in adults with ARDS, which could be distinguished by many of the same inflammatory biomarkers.57–59 In addition, a parsimonious three-variable model developed in adults60 had good discriminatory power in children (AUROC 95%),47 but the optimal cutoff point for discrimination between ARDS subphenotypes differed between adults and children. This difference in discriminatory ability of the model suggests that there are some differences between children and adults in the hypoinflammatory and hyperinflammatory subphenotypes. Prospective validation and development of point-of-care testing for the elements in the parsimonious model are needed before it can be used for enrichment and stratification in clinical trials.
Additional approaches to identify PARDS subgroups could be explored, including metabolomics, proteomics, and physiological parameters that are part of electronic health record data, but validation is needed before these approaches can be used to deliver personalised care. A recent metabolomics study of airspace fluid in 74 children with acute hypoxaemic respiratory failure (41 of whom met PARDS criteria) identified three subgroups that differed by severity of hypoxaemia and outcome.49 Although blood leukocytes and plasma are the primary sampling sites in children for phenotyping and endotyping studies, biomarkers found in the blood compartment might not reflect the underlying pathobiology in the lung. However, lung sampling in PARDS is not straightforward because bronchoscopy is rarely performed for diagnostic and therapeutic purposes in critically ill children. Furthermore, routine endotracheal tube suctioning and return of saline-diluted airspace fluid and cells might not capture the exact pathobiology of the distal airways and alveolar spaces that are damaged in PARDS. Although an attempt to derive subphenotypes from airway cells obtained from tracheal aspirates of intubated patients with PARDS has not yet been done, transcriptomic profiles of neutrophils obtained from tracheal aspirates have been reported that differentiate those at risk of PARDS from those with PARDS.61 In addition, electronic health record data have been used to identify subphenotypes of paediatric multiple organ dysfunction syndrome.62
Heterogeneity clearly exists among patients with PARDS. Subgroups can be identified that might differ in prognostic (outcomes) and predictive (underlying pathobiology) features. This highlights the potential feasibility of identifying patients with PARDS who are at highest risk of poor outcomes and endotypes that share underlying biological pathways; however, further research is needed (panel 2). First, larger studies are needed to replicate and validate the biomarkers identified in smaller, single-centre studies of PARDS. Second, research into how subphenotypes identified using different approaches (eg, transcriptomics vs latent class analysis) relate to each another is needed. Third, work is needed to establish whether knowledge of the biological pathways specific to an endotype can be used successfully to develop and test targeted treatments. Fourth, bedside point-of-care methods to rapidly identify subphenotypes or endotypes in near real-time must be developed and deployed before those at highest risk of poor outcomes can be identified and before endotype-targeted treatments can become a practical reality. Subphenotyping and endotyping hold promise for the delivery of personalised care, with the ultimate goal of reducing morbidity and mortality associated with PARDS.
Individualised ventilator management
Titrating ventilator settings in PARDS to the individual needs of the patient and the specific timepoint in the disease trajectory requires a good understanding of the physics and mechanics of a mechanical ventilator, the function of and required settings for a mode of respiratory support (including non-invasive ventilation), the physiology of the patient (ie, respiratory mechanics and respiratory system conditions), and the pathophysiology of the disease (figure 1).
Figure 1: Potential strategies for personalised ventilator management of PARDS.
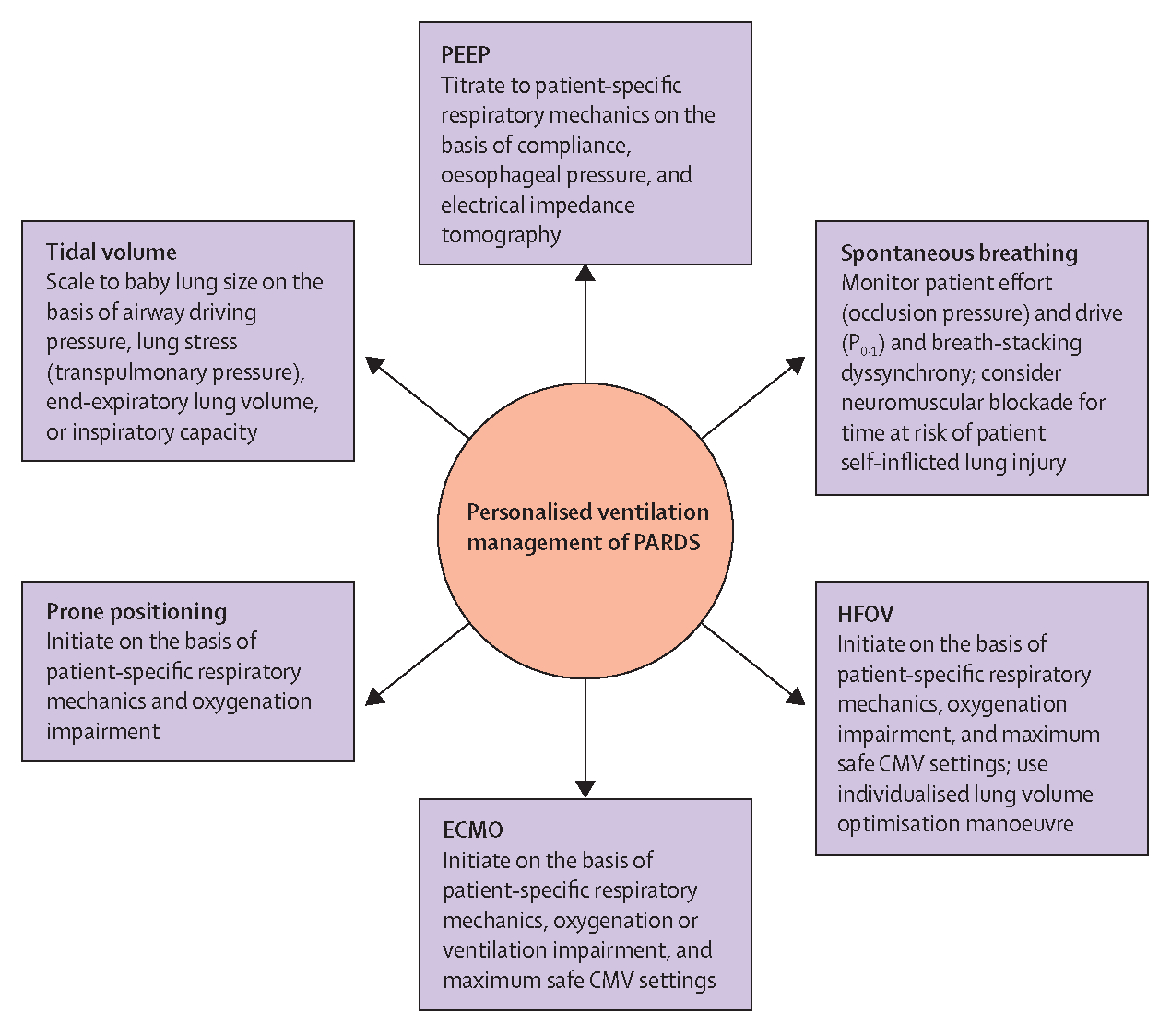
CMV=conventional mechanical ventilation. ECMO=extracorporeal membrane oxygenation. HFOV=high-frequency oscillatory ventilation. P0·1=pressure generated during the first 100 ms of a breath. PARDS=paediatric acute respiratory distress syndrome. PEEP=positive end-expiratory pressure.
To date, most ventilatory practices in PARDS have been based on personal experience and data extrapolated from adults. Both overdistension (ie, volutrauma from the delivery of inappropriate tidal volume, excessive distending, or plateau pressure) and atelectrauma (ie, the repetitive opening and closure of alveoli) have been identified as key pathophysiological mechanisms underlying ventilation-induced lung injury, although there might be marked age-related differences in the thresholds for injurious ventilation.63,64
Lung-protective and diaphragm-protective ventilation entails limiting inspiratory volume, plateau pressure, and driving pressure, individualising PEEP, and finding a balance between insufficient and excessive assistance to prevent respiratory muscle atrophy in ARDS.65,66 From a physiological perspective, such an approach is also warranted in PARDS. The National Heart, Lung, and Blood Institute ARDS Network trial in adults with ARDS showed that ventilation with a lower tidal volume of 6 mL/kg ideal bodyweight and a plateau pressure of less than 30 cm H2O had more favourable outcomes than did a tidal volume of 12 mL/kg ideal bodyweight and a plateau pressure of less than 50 cm H2O.12 However, there is no paediatric equivalent of this trial and paediatric observational data are inconclusive on the optimal tidal volume in PARDS.11 PALICC guidelines recommend the use of patient-specific tidal volumes according to disease severity: tidal volumes of 3–6 mL/kg predicted bodyweight for patients with poor respiratory system compliance, and closer to the physiological range of 5–8 mL/kg ideal bodyweight for patients with relatively well preserved respiratory system compliance.20 These recommendations were based on the conceptual model known as the ARDS baby lung, which describes lower functional lung volume in ARDS due to the collapse of dependent regions (ie, the lowest regions of the lung in relation to gravity); however, the amount of aerated lung tissue correlates well with respiratory system compliance, suggesting that tidal volume should not be set by bodyweight but by diseased functional lung size.67 This would involve a move away from ventilator modes that require the operator to set a prespecified tidal volume in patients with lung injury. The baby lung model has evolved clinically into the concept of driving pressure, which describes the end-inspiratory ratio of tidal volume over respiratory system compliance under zero-flow conditions.68,69 Reducing driving pressure by limiting plateau pressure and increasing PEEP was associated with increased survival in adults with ARDS;70 paediatric data that report similar associations are emerging,71 although whether a ventilation strategy that uses driving pressure improves patient outcomes remains to be determined.
PALICC guidelines recommend that moderately elevated levels of PEEP (10–15 cm H2O) titrated to the observed oxygenation and haemodynamic response be used in severe PARDS, but that higher levels might be required.20 However, paediatric critical care practitioners tend to use low levels of PEEP and inherently accept higher FiO2.72,73 These practices might negatively affect patient outcomes, as demonstrated in a study13 that showed higher mortality in patients managed with lower PEEP relative to FiO2 than that recommended by the ARDS Network,12 especially in those with severe PARDS. In adults with ARDS, unselective use of higher PEEP does not improve outcomes, and might be associated with a risk of harm in subsets of patients.74 This finding underscores the need for individualised PEEP setting, balancing the potential benefit of keeping the lung fully open against the risk of lung overinflation.74,75 Numerous combinations of physiological markers can be used to gauge PEEP response, including respiratory system mechanics such as respiratory system compliance, stress index, and the lower inflection point of the pressure–volume curve, oesophageal pressure, gas exchange such as dead space or oxygenation, and regional ventilation using electrical impedance tomography, but so far there are no clear paediatric data to suggest the best approach for individualising PEEP. Oesophageal pressure-guided PEEP titration might enhance individualised PEEP setting, but has not been systematically investigated in PARDS, although adult data suggest that much higher levels of PEEP than are used in routine paediatric practice are likely to be needed to achieve a transpulmonary pressure at end-exhalation that is close to or (slightly) above 0 cm H2O, the therapeutic target when using oesophageal pressure-guided PEEP.76,77 While awaiting these data, PALICC guidelines recommended that markers of oxygen delivery, respiratory system compliance, and haemodynamics be closely monitored as PEEP is increased20—an approach supported by the concept that cardiopulmonary function is optimised on the basis of respiratory system compliance analysis.78
There has been increased use of alternatives to conventional invasive mechanical ventilation in PARDS (figure 2), such as non-invasive ventilation with continuous positive airway pressure (CPAP) or bi-level intermittent positive airway pressure (BiPAP), high-frequency oscillatory ventilation (HFOV), and extracorporeal membrane oxygenation, without clear data on who is likely to benefit or be harmed by these therapies.19,79,80 Currently, the global two-by-two factorial, response-adaptive Prone and Oscillation Pediatric Clinical Trial (PROSpect) is enrolling children with moderate-to-severe PARDS (ie, oxygenation index >12 or oxygenation saturation index >9·0), who are randomised to receive conventional invasive mechanical ventilation versus HFOV and to prone versus supine positioning (NCT03896763). The open-lung HFOV protocol is designed to target ventilator mean airway pressure through an individualised titration algorithm. PARDS heterogeneity is being addressed by stratifying for age categories and for direct versus indirect lung injury. Results of this trial should provide important evidence on whether prone positioning and HFOV should be considered for use in severe cases in which conventional invasive mechanical ventilation is insufficient to maintain safe and acceptable gas exchange.
Figure 2: The pathway to precision medicine for PARDS.
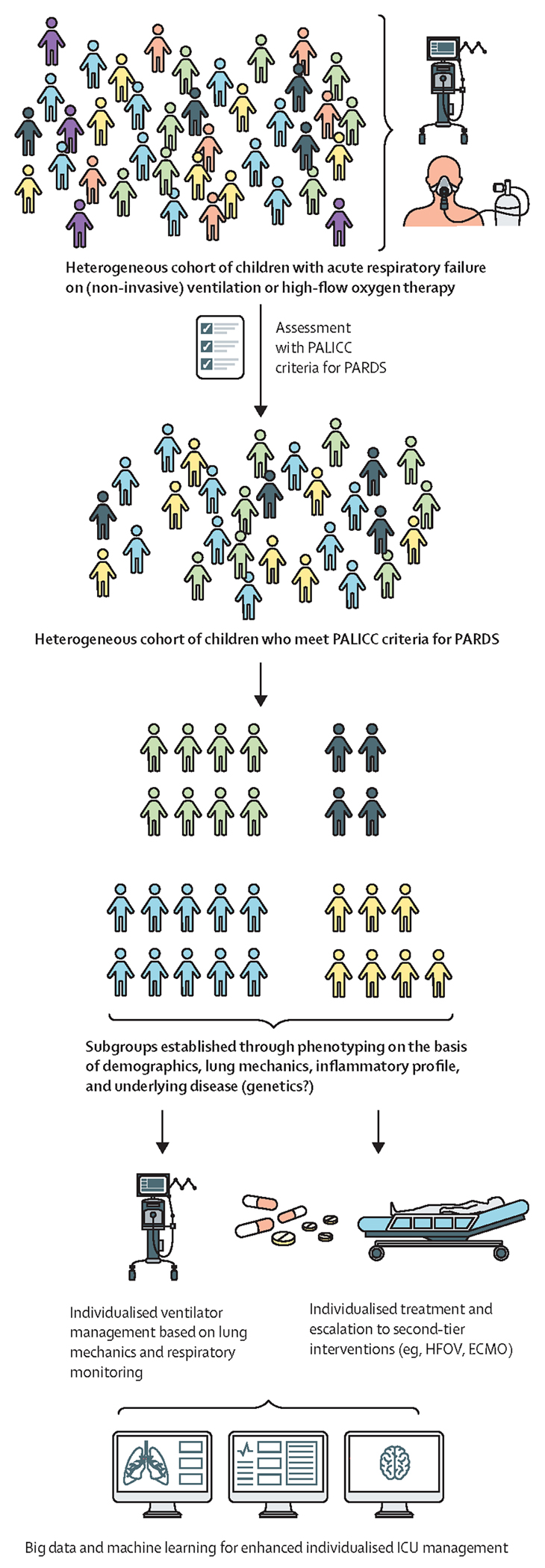
A personalised or precision medicine approach to PARDS is needed to meet the needs of individuals in this diverse patient population, which encompasses differences in age, sex, race, ethnicity, socioeconomic status, and geographical region. The pathway to precision medicine starts with a heterogeneous cohort of children admitted to the paediatric ICU with acute respiratory failure. A range of factors including age (stage of development and lung maturation), sex, causative infectious agent, underlying disease, genetics, and comorbidities, among others, might contibute to clinical and biological heterogeneity. Most of these patients are supported with high-flow oxygen therapy or non-invasive mechanical ventilation. Some of these patients meet the PALICC criteria for PARDS—ie, acute onset of disease with a triggering event <7 days before confirmation with PARDS criteria, one or more infiltrates on chest x-ray, and impaired oxygenation (ie, OI ≥4).10 Severity of PARDS is stratified into mild (OI 4 to <8), moderate (OI 8 to <16), or severe (OI ≥16).10 This heterogeneous cohort of children could be subphenotyped on the basis of charactersitics such as demographics, lung mechanics, inflammatory profile, and underlying disease (and, potentially, genetics in the future). Such phenotyping would enable individualised ventilator management based on lung mechanics and respiratory monitoring, and individualised treatment and escalation to second-tier interventions such as HFOV or ECMO. In future, individualised care, including the use of novel, targeted treatments, could be advanced using big data analysis and machine learning. ECMO=extracorporeal membrane oxygenation. HFOV=high-frequency oscillatory ventilation. ICU=intensive care unit. OI=oxygenation index. PALICC=Pediatric Acute Lung Injury Consensus Conference. PARDS=paediatric acute respiratory distress syndrome.
Monitoring and modulation of respiratory effort
Monitoring of respiratory effort allows individual titration of ventilator settings in relation to the patient’s disease trajectory. Paediatric preclinical studies have shown that supraphysiological pressures and tidal volume during mechanical ventilation can induce or amplify lung injury.64 Maintenance of spontaneous breathing has long been strongly advocated for patients with ARDS because it was found to lead to a reduced shunt fraction and decreased dead space.81–83 More recently, it has been recognised that unregulated respiratory effort in ARDS might also contribute to lung injury, a phenomenon known as patient self-inflicted lung injury.84–87 Experimental work has shown that in injured lungs, unassisted spontaneous breathing promotes regional strain and progression of strain heterogeneity, which are biomechanical phenomena that are both closely linked to lung damage.88,89 By contrast, controlled low-tidal-volume mechanical ventilation prevented regional strain and heterogeneity progression and thus lung damage.88,89
A priori, it seems logical that ventilation-induced lung injury and patient self-inflicted lung injury might share the same patterns of damage; however, the direction of the lung strain and stress vector (positive or negative pressure, respectively) is not negligible, and can generate nuances related to the side of the blood–gas barrier that is primarily affected. Experimental data suggest that patient self-inflicted lung injury induces vascular injury that is disproportionately greater than epithelial injury (which is predominant in ventilation-induced lung injury), potentially related to negative-pressure swings and increased pulmonary blood flow.84,90,91 Vascular stress due to high pulmonary blood flow and oscillations in the right ventricular stroke volume have been proposed to explain lung injury development during unregulated respiratory effort.87,89 Blood flow oscillation might be intensified during unassisted spontaneous breathing as cyclic inspiratory negative pressures increase venous return, in the absence of PEEP-related resistance to vascular flow.
There is indirect evidence for the existence of patient self-inflicted lung injury in children. A recent secondary analysis found that pre-intubation use of non-invasive ventilation and duration of use are associated with worse outcomes in children with acute respiratory failure.92 Another study identified worse outcomes in immune-compromised children who received non-invasive respiratory support before intubation.93 However, whether these adverse outcomes can be attributed to disease severity (associated with non-invasive ventilation failure) or to patient self-inflicted lung injury is not clear. Furthermore, the potential benefit of respiratory effort suppression with neuromuscular blockade in patients with moderate-to-severe ARDS is subject to debate. Large trials in adults have produced conflicting results.94–97 In PARDS, the use of neuromuscular blockade was associated with longer duration of invasive mechanical ventilation in a multicentre observational study.28 A pilot trial also suggested that maintenance of a controlled level of spontaneous breathing in PARDS could decrease the duration of mechanical ventilation.98 Experimental studies by Yoshida and colleagues84,90 showed that although spontaneous breathing during mechanical ventilation might be beneficial in mild ARDS, spontaneous breathing might amplify damage by increasing transpulmonary pressure, atelectasis, cyclic collapse, and histological signs of damage in severe ARDS. However, the paradox of spontaneous breathing and lung damage cannot be fully explained by the severity of ARDS. Respiratory drive has a key role. In an experimental model of severe ARDS supported with extracorporeal carbon dioxide removal, low-intensity respiratory effort did not result in progression of ARDS when compared with ultra-protective invasive mechanical ventilation plus neuromuscular blockade.99 Balancing the benefit of reducing spontaneous ventilation versus the risks associated with intubation, sedation, and paralysis remains a major clinical challenge.
Thus, the monitoring and modulation of respiratory effort are very relevant in PARDS and key to the personalisation of clinical care. By relying on clinical judgement and vital signs, respiratory effort might be underestimated or overestimated.100,101 Hence, additional tools are needed to determine the level of respiratory effort. The gold standard is measurement of change in oesophageal pressure, reflecting the change in pleural pressure, but this is rarely performed in clinical practice owing to the expertise required for appropriate oesophageal manometry catheter placement and data interpretation. A recent study in PARDS explored a simpler and more readily available method for the monitoring of respiratory effort.102 The magnitude of change in airway pressure (plateau pressure minus peak pressure) during an inspiratory hold while on pressure control or pressure support ventilation was correlated with change in oesophageal pressure and enabled identification of patients with excessively high respiratory effort.102
Other widely available clinical measurements exist to estimate different aspects of a patient’s inspiratory effort. Airway occlusion pressure during the entire breath or during the first 100 ms of the breath (P0·1) might provide a reliable marker of respiratory drive or effort in children.103 Monitoring of diaphragm electrical activity using specific nasogastric tubes also provides an estimate of patient ventilatory drive. Inspiratory diaphragm electrical activity is correlated with change in oesophageal pressure,104 and the combination of both measurements can be used to assess respiratory muscle efficiency.105 In the absence of diaphragm dysfunction, respiratory muscle ultrasonographic measurements, such as diaphragmatic and parasternal intercostal thickening, can be used as indicators of inspiratory effort.106 Negative inspiratory force, also known as maximum inspiratory pressure, quantifies respiratory muscle strength but might not reflect respiratory drive. Further research is required to identify the appropriate methods and thresholds to determine excessive breathing effort in children, and the clinical scenarios in which respiratory effort should be modulated to facilitate the individualised titration of ventilation.
Ancillary treatments
Evidence on the use of ancillary treatments in paediatric patients is limited, with most studies focusing on prone positioning. A planned secondary analysis of the multinational PARDIE study conducted in 145 paediatric ICUs showed significant practice variability in the use of pulmonary-specific (eg, inhaled nitric oxide and corticosteroids) and non-pulmonary (eg, sedation and fluid management) ancillary treatments.107 PARDS severity and the presence of comorbidities such as prematurity and congenital heart disease appeared to drive decisions about treatments, many of which are not proven to be beneficial in adults. The analysis showed that prone positioning was often used in a subset of patients, probably driven by evidence from adult studies because the only randomised controlled trial (RCT) in children to date did not support its use.107
The physiological rationale for using prone positioning is probably similar for children and adults. ARDS is a heterogeneous lung disease with consolidation in gravitationally dependent dorsal areas and aeration in ventral areas. In the supine position, during passive mechanical ventilation, dorsal consolidation, the cephalad movement of diaphragm from abdominal contents,108,109 compression from the heart,110 and tougher posterior chest wall all increase dorsal pleural pressure, while the more compliant anterior chest wall reduces ventral pleural pressure. This generates a higher dorsal–ventral pleural pressure gradient,111 resulting in ventilation inhomogeneity.112–114 Children, especially infants, have higher chest wall compliance, lower functional residual capacity approximating closing capacity, and small airway diameters with high resistance, further increasing the risk of ventilation inhomogeneity (compared with adults) in ARDS.112 With prone positioning, consolidation shifts ventrally whereas ventilation shifts dorsally,115 resulting in a lowered dorsal–ventral pleural pressure gradient113,114,116 and improved homogeneity.113 Furthermore, given the higher density of pulmonary vessels in dorsal areas, which are unaffected by gravity, perfusion remains dorsal, resulting in better ventilation–perfusion matching.117–119 Together, these changes result in improved oxygenation in ARDS,120–125 which, in addition to improved mechanics, might reduce the right ventricular afterload, especially in preload-replete patients.113,126–130
Recent meta-analyses131–133 and a large RCT134 in adults with moderate-to-severe ARDS showed that deploying prone positioning for more than 16 h/day imparts a significant survival benefit, indicating that a reduction in ventilation-induced lung injury underlies improved survival.135 In preclinical models, pronation reduced and redistributed lung injury136 and delayed progression of ventilation-induced lung injury.137 The lowered pleural pressure gradient, elongation of the lung, improved ventilation homogeneity (lower hyperinflation and tidal recruitment),113,138 and improved shape matching between the lung and the chest wall139 with prone positioning are likely to affect stress and strain distribution,140 which is a plausible mechanism for reduced burden of ventilation-induced lung injury.
So far, there are no paediatric-specific data showing that prone positioning results in improved outcomes. A multicentre RCT of prone positioning for more than 20 h/day for a maximum of 7 days in patients with PARDS (PaO2/FiO2 ratio <300 mm Hg) showed no difference between groups in the number of ventilator-free days, mortality, time to recovery of lung injury, or overall functional outcome, despite improvements in oxygenation.23 However, it could be argued that this study did not specifically target the patients who were most likely to benefit—ie, those with severe lung injury.141
Prone positioning is likely to have a beneficial effect on outcome only when combined with lung-protective mechanical ventilation.134 Moderate-to-high PEEP113,142 probably improves lung homogeneity during pronation, especially because prone positioning reduces hyperinflation and tidal recruitment as well as chest wall compliance.125 As highlighted above, however, the optimal PEEP titration approach in children is unclear.
PALICC guidelines recommend considering the use of prone positioning in severe PARDS,10 but further study is warranted to determine which patients will truly benefit. An oxygenation response is common with prone positioning124 but might not truly capture reductions in the risk of ventilation-induced lung injury. Additional monitoring tools that can be used to measure regional ventilation and help to gauge the balance between recruitment and overdistension when moving to the prone position are likely to be important to achieve personalised therapeutic targets in children.
Clinical informatics and data science
Clinical informatics and data science are increasingly making an impact in critical care research and patient care.143 Among all forms of critical illness in children, PARDS is likely to benefit most from novel data-driven technologies. The large amount of data generated by patients with PARDS combined with the complexity of PARDS and the need for better evidence-based care makes the use of data science and informatics particularly relevant in this population. Some of the most important future uses of these data-driven technologies include prediction of children at risk of PARDS, early identification and stratification of patients with PARDS, improved adherence to best practices and facilitation of personalised care with clinical decision support (CDS) systems, and enabling of multicentre PARDS research.
Areas in which informatics might contribute to individualised management of PARDS include recognition and timely diagnosis of PARDS, and better adherence to best practices through computerised CDS systems.144 Data in adults showing better recognition of ARDS and improved tidal volume compliance support the implementation of these systems.145–153 In one paediatric study, implementation of a ventilator-management CDS tool for patients with PARDS was associated not only with improved compliance with best practices, but also with a reduction in the duration of invasive mechanical ventilation when compared with historical controls.98
Current and future CDS systems for PARDS, including those enabled by machine learning algorithms, have the potential to improve care and reduce costs. Prediction of PARDS risk, early identification and stratification, automated chest imaging analysis and interpretation, open and closed-loop ventilator management, personalised PARDS care, and ventilator liberation are some of the many uses for the next generation of data-driven CDS systems for PARDS.154–161 Although the potential benefits of CDS systems are evident, successful implementation of these systems requires economic, human, and technical investment, as well as clinician training and endorsement. Rigorous scientific development and validation as well as institutional support and funding are all necessary for the success of these systems.162
Conclusions
The substantial clinical and biological heterogeneity of PARDS poses a challenge in the clinical management of patients with PARDS in terms of titrating therapies to the individual needs of the child and the specific timepoint in the disease trajectory. Age and developmental stage of lung maturation, epidemiology, risk factors, and comorbidities might all be associated with the PARDS phenotype. There is also considerable heterogeneity among patients with PARDS in the response to supportive therapies. The PARDS research agenda should, therefore, not focus exclusively on whether existing or novel preventive or therapeutic interventions improve clinically relevant outcomes but, more importantly, aim to understand which patient subgroups might benefit from such interventions and the underlying biological mechanisms of action. This approach requires improved PARDS definitions and the acquisition of highly granular biological, physiological, and clinical data as the new standard across studies, as well as multidisciplinary, interprofessional collaborative efforts across the critical care community (panel 2). The currently enrolling PROSpect trial is one of the first studies to test commonly used interventions such as prone positioning and HFOV in patients with PARDS and, as such, provides a good example of a much-needed RCT in this patient population. Ongoing clinical trials and observational studies in patients with PARDS are presented in table 4 and the appendix (pp 1–3). Further understanding of the clinical and biological heterogeneity of PARDS will ultimately enable the research and clinical communities to develop novel preventive and therapeutic strategies, and to implement a precision medicine approach to the care of patients with this syndrome (figure 2).
Table 4:
Ongoing intervention and observational studies in PARDS
| Studytype | Participants | Intervention or assessment | |
|---|---|---|---|
|
| |||
| Paediatric Ards Neuromuscular Blockade Study (PAN; NCT02902055) | Multicentre, double-blind, phase 4 RCT | 178 patients (<5 years) with early moderate-to-severe PARDS (OI ≥12 or OSI ≥9·09) | Continuous neuromuscular blockade (rocuronium 1 mg/kg per h) vs placebo for 48 h |
| Real-Time Effort Driven Ventilator Management (REDvent; NCT03266016) | Single-centre, single-blind, phase 2 RCT | 276 patients (>1 month to ≤18 years) with PARDS (pulmonary parenchymal disease and OI ≥4 or OSI ≥5) | Ventilator management using a CDS tool for lung and diaphragm protection vs usual care |
| Identifying PARDS Endotypes (NCT03539783) | Single-centre case-control study | 60 patients (1 month to 18 years) admitted to the PICU: 30 intubated patients with PARDS (acute changes on chest x-ray; OI ≥4 or OSI ≥5) and 30 patients with non-lung-injury-related conditions | Bronchial epithelial cell brushing for gene-expression profiling |
| Long Term Follow up of Children Enrolled in the REDvent Study (NCT03709199) | Single-centre, prospective observational follow-up study | 240 patients enrolled in REDvent | Ventilator management using a CDS tool for lung and diaphragm protection vs usual care |
| Prone and Oscillation Pediatric Clinical Trial (PROSpect; NCTO3896763) | Multicentre, open-label, 2 × 2 factorial, response-adaptive RCT | 800 patients (2 weeks to 20 years) with moderate-to-severe PARDS* | CMV vs HFOV; prone vs supine positioning |
| Clinical Decision Support Tool in PARDS Pilot Study (NCT04068012) | Multicentre observational study | 180 patients (>1 month to ≤18 years) with PARDS (pulmonary parenchymal disease and OI ≥4 or OSI ≥5) | Ventilator management using a CDS tool for lung and diaphragm protection and to guide liberation from the ventilator |
| Pediatric Acute Respiratory Distress Syndrome Asia Study (PARDSPROASIA; NCT04068038) | Multicentre, prospective cohort study | 800 patients (≤21 years) receiving ventilatory support for PARDS* | Screening of all PICU admissions and collection of epidemiological and clinical data |
| Linking Endotypes and Outcomes in Pediatric Acute Respiratory Distress Syndrome (LEOPARDS; NCT04113434) | Multicentre, prospective cohort study | 500 patients (44 weeks to <17·5 years) with respiratory failure receiving invasive mechanical ventilation (bilateral infiltrates on chest x-ray; OI ≥4 or OSI ≥5) | Assessment of plasma protein biomarkers and peripheral blood gene expression |
| Infants With Severe Acute Respiratory Distress Syndrome: The Prone Trial (NCT05002478) | Single-centre, open-label RCT | 14 patients (>36 weeks to <24 months) with severe PARDS (OSI ≥12·3) | Prone vs supine positioning after surfactant administration |
| Continuous Infusion Versus Intermittent Boluses of Cisatracurium in the Early Management of Pediatric ARDS (NCT05153525) | Single-centre, open-label, phase 4 RCT | 60 patients (1 month to 18 years) with PARDS* | Intermittent boluses of cisatracurium vs intravenous infusion of cisatracurium for 24 h |
| ARDS in Children and ECMO Initiation Strategies Impact on Neurodevelopment (ASCEND; NCT05388708) | Multicentre, prospective cohort study (PROSpect ancillary study) | 550 patients with moderate-to-severe PARDS† from the ELSO registry; 800 patients from PROSpect | ECMO vs protocolised therapies (CMV vs HFOV; prone vs supine positioning) |
| Endotypes in Children with Severe Acute Respiratory Distress Syndrome: Impact on Response to Treatment (ENSNARE) | Multicentre observational study (PROSpect ancillary study) | 300 patients enrolled in PROSpect | Assessment of plasma protein biomarkers and wholeblood genome-wide gene expression; latent class analysis to relate expression profiles to treatment responses |
| Microbiome and Nutrition in Severe PARDS Trial (MANTIS) | Multicentre observational study (PROSpect ancillary study) | 800 patients enrolled in PROSpect | Stool and endotracheal aspirate sampling for assessment of gut and lung microbiomes |
For details of eligibility criteria, key outcomes, and study status, see appendix (pp 1–3). ARDS=acute respiratory distress syndrome. CDS=computerised decision support. CMV=conventional mechanical ventilation. ECMO=extracorporeal membrane oxygenation. ELSO=Extracorporeal Life Support Organization. HFOV high-frequency oscillatory ventilation. OI=oxygenation index. OSI=oxygen saturation index. PARDS=paediatric acute respiratory distress syndrome. PICU=paediatric intensive care unit. RCT=randomised controlled trial.
Defined by PALICC criteria.10
Defined by OI or OSI criteria in patients with bilateral lung disease on chest x-ray (one OI ≥16 or two OIs ≥12 and ≤16 at least 4 h apart, or two OSIs ≥10 at least 4 h apart, or one OI ≥12 and ≤16 and one OSI ≥10 at least 4 h apart).
Supplementary Material
Key messages.
PARDS is a heterogeneous clinical syndrome that contributes to high rates of mortality and long-term morbidity; precipitating factors, comorbidities, and changes from infancy to adulthood in lung development and maturation distinguish PARDS from adult ARDS
The paediatric-specific PALICC definition of ARDS enables improved identification and prognostication of patients with PARDS; as for adult ARDS, no specific treatment is available for PARDS and management is supportive, focused on mechanical ventilation and ancillary treatments
The substantial clinical and biological heterogeneity of PARDS poses a challenge for clinical management in terms of titrating therapies to the individual needs of the patient and the specific timepoint in the disease trajectory
Phenotyping of PARDS using clinical or biological data might enable individualised ventilator management (including selection of tidal volume, setting of positive end-expiratory pressure, and use of second-tier interventions such as high-frequency oscillatory ventilation or extracorporeal membrane oxygenation) and the development of novel preventive and therapeutic options
A latent class analysis approach using demographic, clinical, and plasma biomarker data (about 30 variables) has enabled identification of hyperinflammatory and hypoinflammatory subphenotypes of PARDS
To realise the potential of precision medicine in PARDS, further work is needed to identify subphenotypes and endotypes of PARDS, to develop point-of-care methods for their rapid identification, and to understand their association with response to treatment, clinical course, and clinical outcomes
ARDS=acute respiratory distress syndrome. PALICC=Pediatric Acute Lung Injury Consensus Conference. PARDS=paediatric acute respiratory distress syndrome.
Panel 1: Features that distinguish PARDS from adult ARDS.
Growth and development
Changing patterns from infancy to adulthood in lung maturation and alveolarisation, innate and adaptive immunity, and respiratory system mechanics (lung compliance, chest wall compliance, and airway resistance) distinguish PARDS from adult ARDS and contribute to heterogeneity in PARDS. Subgrouping by stage of development and lung maturation in clinical studies could help in the development of a precision medicine approach.
Comorbidities
Unique comorbidities of infancy and childhood that affect respiratory mechanics (eg, chronic lung disease of infancy, [congenital] neuromuscular disorders, and congenital heart disease) and the significant proportion of children receiving home invasive mechanical ventilation before the onset of PARDS set this population apart from the adult population with ARDS. Comorbidities should be considered in the individualised titration of mechanical ventilation and potentially in the selection of pulmonary-specific and non-specific ancillary treatments. Any injurious event during (early) childhood could affect pulmonary function in later life, so persisting or emerging morbidity after PARDS is likely to differ from that in adults after ARDS.
ARDS triggers
PARDS has a lower incidence and mortality than does ARDS in adults, and a lower prevalence among admissions to the intensive care unit. PARDS is associated with a very high rate of lower respiratory tract infection triggers compared with adult ARDS, particularly viral infections in younger children. ARDS triggers (eg, a specific viral or bacterial causative agent) might be associated with phenotypic or endotypic features of PARDS.
Phenotypes and endotypes
The extent to which subphenotypes and endotypes of PARDS and adult ARDS overlap is unclear at present. Preliminary evidence suggests that there might be differences in the hypoinflammatory and hyperinflammatory subphenotypes identified in children and in adults with ARDS. A better understanding of the unique clinical and biological features of PARDS will be key to the development of safe and effective therapeutic options for this patient population.
Diagnosis
Compared with adult ARDS, PARDS diagnosis depends on less frequent use of arterial blood gases (pulse-oximetry-based criteria) and variable use of positive end-expiratory pressure (incorporation of mean airway pressure with oxygenation index), and is associated with high variability in the interpretation of bilateral infitrates with unclear PARDS mortality risk (simplified radiographic criteria). In future, the use of rapid diagnostic tests that incorporate biomarkers associated with different subphenotypes (eg, hypoinflammatory and hyperinflammatory) might support a precision medicine approach to treatment.
ARDS=acute respiratory distress syndrome. PARDS=paediatric acute respiratory distress syndrome.
Panel 2: Priorities for future studies of PARDS phenotypes and endotypes.
Conduct of larger observational studies or biomarker studies in the context of large, multicentre RCTs to replicate, validate, and extend previous findings on subphenotypes and endotypes
Assessment of the association of subphenotypes and endotypes with response to treatment
Investigation of associations between longitudinal trajectories of PARDS and post-PARDS outcomes and subphenotypes and endotypes, including studies of the stability of phenotypic and endotypic features
Assessment of the effects of developmental age on subphenotypes and endotypes
Investigation of the relationships between subphenotypes and endotypes identified using different approaches (eg, proteomics, transcriptomics, and metabolomics)
Development of high-throughput, human-based lung epithelial–endothelial models that can be used to understand pathophysiological mechanisms of PARDS and facilitate drug discovery related to subphenotypes and endotypes
Development of new targeted treatments aimed at biological mechanisms that underpin specific endotypes
Development of point-of-care methods to rapidly identify subphenotypes and endotypes
Development of machine learning models using clinical and biological data to identify subphenotypes and endotypes in real-time to optimise clinical trial enrolment and timely interventions
PARDS=paediatric acute respiratory distress syndrome. RCT=randomised controlled trial.
Search strategy and selection criteria.
We searched PubMed for articles published in English from the past 20 years from Jan 1, 2002 up to Sept 16, 2022, using combinations of the following search terms: “personalized medicine”, “acute respiratory distress syndrome”, “children”, “ventilation”, “tidal volume”, “PEEP”, “prone positioning”, “monitoring”, “biomarkers”, “informatics”, “big data”, and “artificial intelligence”. Articles resulting from this literature search and appropriate references cited in those articles were reviewed and included on the basis of relevance to the topics covered in this Series paper. We searched ClinicalTrials.gov for ongoing clinical trials and observational studies using the search term “ARDS”.
Acknowledgments
No funding was received for the writing of this Series paper. MCJK receives research funding from Nederlandse Organisatie voor Gezondheidsonderzoek en Zorginnovatie (ZonMw; 80–84800-98–41034), University Medical Center Groningen (Groningen, Netherlands), Stichting Vrienden Beatrix Kinderziekenhuis, and the US National Institutes of Health (NIH; UH3 HL141736, U24 HL141723). RGK (1RO11HL134666–01, 1RO11HL134666–04S1, 1R13 HD102137–01, 1RL1HD107785–01), AB (K23HL153756), MKD (R01 HL149910–03, R21 HD097387–03), JG (K23 HL151897), and LNS-P (R01HD105939, R21GM146159) are also funded by the NIH. PC receives grant funding from the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT, Chile). GE is funded by the Fonds de Recherche du Québec – Santé (FRQS, Canada). YML-F is funded by the Instituto de Salud Carlos III (Madrid, Spain; PI19/00141).
Footnotes
Declaration of interests
MCJK reports personal fees from Vyaire and Metran, and research funding from Vyaire and Applied Biosignals, outside of the submitted work. RGK reports personal fees from Orange Med Nihon Kohden; he has received reimbursement for participation on data safety monitoring boards for the PREVENT study (funded by the NIH) and the FIRST-ABC study (funded by the UK National Institute for Health and Care Research), outside of the submitted work. GE has received financial support from Maquet for an investigator-led study, outside of the submitted work. PCR has received personal fees from Getinge, outside of the submitted work. The other authors declare no competing interests.
This is the third in a Series of three papers about acute respiratory distress syndrome (papers 1 and 2 appear in The Lancet). For the Acute Respiratory Distress Syndrome 2022 Series see www.thelancet.com/series/ARDS-2022
Contributor Information
Martin C J Kneyber, Department of Paediatrics, Division of Paediatric Critical Care Medicine, Beatrix Children’s Hospital, University Medical Center Groningen, University of Groningen, Groningen, Netherlands; Critical Care, Anaesthesiology, Perioperative and Emergency Medicine, University of Groningen, Groningen, Netherlands.
Robinder G Khemani, Department of Anesthesiology and Critical Care Medicine, Children’s Hospital Los Angeles, Los Angeles, CA, USA; Department of Paediatrics, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Anoopindar Bhalla, Department of Anesthesiology and Critical Care Medicine, Children’s Hospital Los Angeles, Los Angeles, CA, USA; Department of Paediatrics, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Robert G T Blokpoel, Department of Paediatrics, Division of Paediatric Critical Care Medicine, Beatrix Children’s Hospital, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Pablo Cruces, Facultad de Ciencias de la Vida, Universidad Andres Bello, Santiago, Chile.
Mary K Dahmer, Department of Pediatrics, Division of Critical Care, University of Michigan, Ann Arbor, MI, USA.
Guillaume Emeriaud, Department of Pediatrics, CHU Sainte Justine, Université de Montréal, Montreal, QC, Canada.
Jocelyn Grunwell, Department of Pediatrics, Division of Critical Care, Emory University, Atlanta, GA, USA.
Stavroula Ilia, Pediatric Intensive Care Unit, University Hospital, School of Medicine, University of Crete, Heraklion, Crete, Greece.
Bhushan H Katira, Department of Pediatrics, Division of Critical Care Medicine, Washington University in St Louis, St Louis, MO, USA.
Yolanda M Lopez-Fernandez, Pediatric Intensive Care Unit, Department of Pediatrics, Cruces University Hospital, Biocruces-Bizkaia Health Research Institute, Bizkaia, Spain.
Prakadeshwari Rajapreyar, Department of Pediatrics (Critical Care), Medical College of Wisconsin and Children’s Wisconsin, Milwaukee, WI, USA.
L Nelson Sanchez-Pinto, Department of Pediatrics (Critical Care), Northwestern University Feinberg School of Medicine and Ann & Robert H Lurie Children’s Hospital of Chicago, Chicago, IL, USA.
Peter C Rimensberger, Division of Neonatology and Paediatric Intensive Care, Department of Paediatrics, University Hospital of Geneva, University of Geneva, Geneva, Switzerland.
References
- 1.Schouten LR, Veltkamp F, Bos AP, et al. Incidence and mortality of acute respiratory distress syndrome in children: a systematic review and meta-analysis. Crit Care Med 2016; 44: 819–29. [DOI] [PubMed] [Google Scholar]
- 2.Quasney MW, Lopez-Fernandez YM, Santschi M, Watson RS, Pediatric Acute Lung Injury Consensus Conference Group. The outcomes of children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015; 16: S118–31. [DOI] [PubMed] [Google Scholar]
- 3.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet 1967; 2: 319–23.4143721 [Google Scholar]
- 4.Thomas NJ, Jouvet P, Willson D. Acute lung injury in children--kids really aren’t just “little adults”. Pediatr Crit Care Med 2013; 14: 429–32. [DOI] [PubMed] [Google Scholar]
- 5.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med 2005; 171: 995–1001. [DOI] [PubMed] [Google Scholar]
- 6.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 2020; 201: 1299–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beitler JR, Goligher EC, Schmidt M, et al. Personalized medicine for ARDS: the 2035 research agenda. Intensive Care Med 2016; 42: 756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernard GR, Artigas A, Brigham KL, et al. The American–European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149: 818–24. [DOI] [PubMed] [Google Scholar]
- 9.ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307: 2526–33. [DOI] [PubMed] [Google Scholar]
- 10.Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015; 16: 428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jager P, Burgerhof JG, van Heerde M, Albers MJ, Markhorst DG, Kneyber MC. Tidal volume and mortality in mechanically ventilated children: a systematic review and meta-analysis of observational studies*. Crit Care Med 2014; 42: 2461–72. [DOI] [PubMed] [Google Scholar]
- 12.Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–08. [DOI] [PubMed] [Google Scholar]
- 13.Khemani RG, Parvathaneni K, Yehya N, Bhalla AK, Thomas NJ, Newth CJL. PEEP lower than the ARDS Network protocol is associated with higher pediatric ARDS mortality. Am J Respir Crit Care Med 2018; 198: 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold JH, Hanson JH, Toro-Figuero LO, Gutiérrez J, Berens RJ, Anglin DL. Prospective, randomized comparison of high-frequency oscillatory ventilation and conventional mechanical ventilation in pediatric respiratory failure. Crit Care Med 1994; 22: 1530–39. [PubMed] [Google Scholar]
- 15.El-Nawawy A, Moustafa A, Heshmat H, Abouahmed A. High frequency oscillatory ventilation versus conventional mechanical ventilation in pediatric acute respiratory distress syndrome: a randomized controlled study. Turk J Pediatr 2017; 59: 130–43. [DOI] [PubMed] [Google Scholar]
- 16.Samransamruajkit R, Prapphal N, Deelodegenavong J, Poovorawan Y. Plasma soluble intercellular adhesion molecule-1 (sICAM-1) in pediatric ARDS during high frequency oscillatory ventilation: a predictor of mortality. Asian Pac J Allergy Immunol 2005; 23: 181–88. [PubMed] [Google Scholar]
- 17.Samransamruajkit R, Rassameehirun C, Pongsanon K, et al. A comparison of clinical efficacy between high frequency oscillatory ventilation and conventional ventilation with lung volume recruitment in pediatric acute respiratory distress syndrome: a randomized controlled trial. Indian J Crit Care Med 2016; 20: 72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Junqueira FMD, Nadal JAH, Brandão MB, Nogueira RJN, de Souza TH. High-frequency oscillatory ventilation in children: a systematic review and meta-analysis. Pediatr Pulmonol 2021; 56: 1872–88. [DOI] [PubMed] [Google Scholar]
- 19.Bateman ST, Borasino S, Asaro LA, et al. Early high-frequency oscillatory ventilation in pediatric acute respiratory failure. A propensity score analysis. Am J Respir Crit Care Med 2016; 193: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rimensberger PC, Cheifetz IM, Pediatric Acute Lung Injury Consensus Conference Group. Ventilatory support in children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015; 16: S51–60. [DOI] [PubMed] [Google Scholar]
- 21.Ingelse SA, Wösten-van Asperen RM, Lemson J, Daams JG, Bem RA, van Woensel JB. Pediatric acute respiratory distress syndrome: fluid management in the PICU. Front Pediatr 2016; 4: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valentine SL, Nadkarni VM, Curley MA. Nonpulmonary treatments for pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015; 16 (suppl 1): S73–85. [DOI] [PubMed] [Google Scholar]
- 23.Curley MA, Hibberd PL, Fineman LD, et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA 2005; 294: 229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamburro RF, Kneyber MC. Pulmonary specific ancillary treatment for pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015; 16 (suppl 1): S61–72. [DOI] [PubMed] [Google Scholar]
- 25.Drago BB, Kimura D, Rovnaghi CR, et al. Double-blind, placebo-controlled pilot randomized trial of methylprednisolone infusion in pediatric acute respiratory distress syndrome. Pediatr Crit Care Med 2015; 16: e74–81. [DOI] [PubMed] [Google Scholar]
- 26.Yehya N, Servaes S, Thomas NJ, Nadkarni VM, Srinivasan V. Corticosteroid exposure in pediatric acute respiratory distress syndrome. Intensive Care Med 2015; 41: 1658–66. [DOI] [PubMed] [Google Scholar]
- 27.Wilsterman MEF, de Jager P, Blokpoel R, et al. Short-term effects of neuromuscular blockade on global and regional lung mechanics, oxygenation and ventilation in pediatric acute hypoxemic respiratory failure. Ann Intensive Care 2016; 6: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudolph MW, Kneyber MCJ, Asaro LA, Cheifetz IM, Wypij D, Curley MAQ. Early neuromuscular blockade in moderate-to-severe pediatric acute respiratory distress syndrome. Crit Care Med 2022; 50: e445–57. [DOI] [PubMed] [Google Scholar]
- 29.Curley MA, Wypij D, Watson RS, et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. JAMA 2015; 313: 379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalton HJ, Macrae DJ. Extracorporeal support in children with pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015; 16 (suppl 1): S111–17. [DOI] [PubMed] [Google Scholar]
- 31.Khemani RG, Smith LS, Zimmerman JJ, Erickson S, Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: definition, incidence, and epidemiology: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med 2015; 16: S23–40. [DOI] [PubMed] [Google Scholar]
- 32.De Luca D, van Kaam AH, Tingay DG, et al. The Montreux definition of neonatal ARDS: biological and clinical background behind the description of a new entity. Lancet Respir Med 2017; 5: 657–66. [DOI] [PubMed] [Google Scholar]
- 33.Rudolph M, van Dijk J, de Jager P, et al. Performance of acute respiratory distress syndrome definitions in a high acuity paediatric intensive care unit. Respir Res 2021; 22: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghuman AK, Newth CJ, Khemani RG. The association between the end tidal alveolar dead space fraction and mortality in pediatric acute hypoxemic respiratory failure. Pediatr Crit Care Med 2012; 13: 11–15. [DOI] [PubMed] [Google Scholar]
- 35.Bhalla AK, Belani S, Leung D, Newth CJ, Khemani RG. Higher dead space is associated with increased mortality in critically ill children. Crit Care Med 2015; 43: 2439–45. [DOI] [PubMed] [Google Scholar]
- 36.Yehya N, Bhalla AK, Thomas NJ, Khemani RG. Alveolar dead space fraction discriminates mortality in pediatric acute respiratory distress syndrome. Pediatr Crit Care Med 2016; 17: 101–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caudron M, Holt T, Cuvelier G, Dmytrowich J, Hansen G. Pulmonary thromboses in pediatric acute respiratory distress syndrome. Respir Care 2019; 64: 209–16. [DOI] [PubMed] [Google Scholar]
- 38.Khemani RG, Smith L, Lopez-Fernandez YM, et al. Paediatric acute respiratory distress syndrome incidence and epidemiology (PARDIE): an international, observational study. Lancet Respir Med 2019; 7: 115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinha P, Calfee CS, Beitler JR, et al. Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med 2019; 199: 333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhalla AK, Dong J, Klein MJ, Khemani RG, Newth CJ. The association between ventilatory ratio and mortality in children and young adults. Respir Care 2021; 66: 205–12. [DOI] [PubMed] [Google Scholar]
- 41.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315: 788–800. [DOI] [PubMed] [Google Scholar]
- 42.Sapru A, Flori H, Quasney MW, Dahmer MK, Pediatric Acute Lung Injury Consensus Conference Group. Pathobiology of acute respiratory distress syndrome. Pediatr Crit Care Med 2015; 16: S41–50. [DOI] [PubMed] [Google Scholar]
- 43.Huppert LA, Matthay MA, Ware LB. Pathogenesis of acute respiratory distress syndrome. Semin Respir Crit Care Med 2019; 40: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frank AJ, Thompson BT. Pharmacological treatments for acute respiratory distress syndrome. Curr Opin Crit Care 2010; 16: 62–68. [DOI] [PubMed] [Google Scholar]
- 45.Prescott HC, Calfee CS, Thompson BT, Angus DC, Liu VX. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med 2016; 194: 147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy K, Sinha P, O’Kane CM, Gordon AC, Calfee CS, McAuley DF. Subphenotypes in critical care: translation into clinical practice. Lancet Respir Med 2020; 8: 631–43. [DOI] [PubMed] [Google Scholar]
- 47.Dahmer MK, Yang G, Zhang M, et al. Identification of phenotypes in paediatric patients with acute respiratory distress syndrome: a latent class analysis. Lancet Respir Med 2022; 10: 289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dahmer MK, Quasney MW, Sapru A, et al. Interleukin-1 receptor antagonist is associated with pediatric acute respiratory distress syndrome and worse outcomes in children with acute respiratory failure. Pediatr Crit Care Med 2018; 19: 930–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grunwell JR, Rad MG, Stephenson ST, et al. Cluster analysis and profiling of airway fluid metabolites in pediatric acute hypoxemic respiratory failure. Sci Rep 2021; 11: 23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yehya N, Varisco BM, Thomas NJ, Wong HR, Christie JD, Feng R. Peripheral blood transcriptomic sub-phenotypes of pediatric acute respiratory distress syndrome. Crit Care 2020; 24: 681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yehya N, Thomas NJ, Wong HR. Evidence of endotypes in pediatric acute hypoxemic respiratory failure caused by sepsis. Pediatr Crit Care Med 2019; 20: 110–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong HR, Cvijanovich N, Lin R, et al. Identification of pediatric septic shock subclasses based on genome-wide expression profiling. BMC Med 2009; 7: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yehya N, Wong HR. Adaptation of a biomarker-based sepsis mortality risk stratification tool for pediatric acute respiratory distress syndrome. Crit Care Med 2018; 46: e9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong HR, Salisbury S, Xiao Q, et al. The pediatric sepsis biomarker risk model. Crit Care 2012; 16: R174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong HR, Weiss SL, Giuliano JS Jr, et al. Testing the prognostic accuracy of the updated pediatric sepsis biomarker risk model. PLoS One 2014; 9: e86242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong HR, Cvijanovich NZ, Anas N, et al. Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am J Respir Crit Care Med 2015; 191: 309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Famous KR, Delucchi K, Ware LB, et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med 2017; 195: 331–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014; 2: 611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calfee CS, Delucchi KL, Sinha P, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med 2018; 6: 691–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sinha P, Calfee CS, Delucchi KL. Practitioner’s guide to latent class analysis: methodological considerations and common pitfalls. Crit Care Med 2021; 49: e63–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grunwell JR, Rad MG, Stephenson ST, et al. Machine learning-based discovery of a gene expression signature in pediatric acute respiratory distress syndrome. Crit Care Explor 2021; 3: e0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ye J, Sanchez-Pinto LN. Three data-driven phenotypes of multiple organ dysfunction syndrome preserved from early childhood to middle adulthood. AMIA Annu Symp Proc 2020; 2020: 1345–53. [PMC free article] [PubMed] [Google Scholar]
- 63.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013; 369: 2126–36. [DOI] [PubMed] [Google Scholar]
- 64.Kneyber MC, Zhang H, Slutsky AS. Ventilator-induced lung injury. Similarity and differences between children and adults. Am J Respir Crit Care Med 2014; 190: 258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goligher EC, Jonkman AH, Dianti J, et al. Clinical strategies for implementing lung and diaphragm-protective ventilation: avoiding insufficient and excessive effort. Intensive Care Med 2020; 46: 2314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Juffermans NP, Rocco PRM, Laffey JG. Protective ventilation. Intensive Care Med 2022; 48: 1629–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med 2005; 31: 776–84. [DOI] [PubMed] [Google Scholar]
- 68.Grieco DL, Chen L, Dres M, Brochard L. Should we use driving pressure to set tidal volume? Curr Opin Crit Care 2017; 23: 38–44. [DOI] [PubMed] [Google Scholar]
- 69.Chiumello D, Carlesso E, Brioni M, Cressoni M. Airway driving pressure and lung stress in ARDS patients. Crit Care 2016; 20: 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015; 372: 747–55. [DOI] [PubMed] [Google Scholar]
- 71.van Schelven P, Koopman AA, Burgerhof JGM, Markhorst DG, Blokpoel RGT, Kneyber MCJ. Driving pressure is associated with outcome in pediatric acute respiratory failure. Pediatr Crit Care Med 2022; 23: e136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khemani RG, Markovitz BP, Curley MA. Characteristics of children intubated and mechanically ventilated in 16 PICUs. Chest 2009; 136: 765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Santschi M, Jouvet P, Leclerc F, et al. Acute lung injury in children: therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med 2010; 11: 681–89. [DOI] [PubMed] [Google Scholar]
- 74.Walkey AJ, Del Sorbo L, Hodgson CL, et al. Higher PEEP versus lower PEEP strategies for patients with acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc 2017; 14 (suppl 4): S297–303. [DOI] [PubMed] [Google Scholar]
- 75.Rouby JJ, Lu Q, Goldstein I. Selecting the right level of positive end-expiratory pressure in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2002; 165: 1182–86. [DOI] [PubMed] [Google Scholar]
- 76.Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008; 359: 2095–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beitler JR, Sarge T, Banner-Goodspeed VM, et al. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 2019; 321: 846–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suter PM, Fairley B, Isenberg MD. Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N Engl J Med 1975; 292: 284–89. [DOI] [PubMed] [Google Scholar]
- 79.Kneyber MCJ, de Luca D, Calderini E, et al. Recommendations for mechanical ventilation of critically ill children from the Paediatric Mechanical Ventilation Consensus Conference (PEMVECC). Intensive Care Med 2017; 43: 1764–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rimensberger PC, Cheifetz IM, Kneyber MCJ. The top ten unknowns in paediatric mechanical ventilation. Intensive Care Med 2018; 44: 366–70. [DOI] [PubMed] [Google Scholar]
- 81.Putensen C, Rasanen J, Lopez FA. Ventilation-perfusion distributions during mechanical ventilation with superimposed spontaneous breathing in canine lung injury. Am J Respir Crit Care Med 1994; 150: 101–08. [DOI] [PubMed] [Google Scholar]
- 82.Putensen C, Rasanen J, Lopez FA, Downs JB. Effect of interfacing between spontaneous breathing and mechanical cycles on the ventilation-perfusion distribution in canine lung injury. Anesthesiology 1994; 81: 921–30. [DOI] [PubMed] [Google Scholar]
- 83.Putensen C, Mutz NJ, Putensen-Himmer G, Zinserling J. Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 1999; 159: 1241–48. [DOI] [PubMed] [Google Scholar]
- 84.Yoshida T, Torsani V, Gomes S, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med 2013; 188: 1420–27. [DOI] [PubMed] [Google Scholar]
- 85.Yoshida T, Amato MBP, Kavanagh BP, Fujino Y. Impact of spontaneous breathing during mechanical ventilation in acute respiratory distress syndrome. Curr Opin Crit Care 2019; 25: 192–98. [DOI] [PubMed] [Google Scholar]
- 86.Yoshida T, Uchiyama A, Fujino Y. The role of spontaneous effort during mechanical ventilation: normal lung versus injured lung. J Intensive Care 2015; 3: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med 2017; 195: 438–42. [DOI] [PubMed] [Google Scholar]
- 88.Hurtado DE, Erranz B, Lillo F, et al. Progression of regional lung strain and heterogeneity in lung injury: assessing the evolution under spontaneous breathing and mechanical ventilation. Ann Intensive Care 2020; 10: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guldner A, Pelosi P, Gama de Abreu M. Spontaneous breathing in mild and moderate versus severe acute respiratory distress syndrome. Curr Opin Crit Care 2014; 20: 69–76. [DOI] [PubMed] [Google Scholar]
- 90.Yoshida T, Uchiyama A, Matsuura N, Mashimo T, Fujino Y. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med 2012; 40: 1578–85. [DOI] [PubMed] [Google Scholar]
- 91.Mascheroni D, Kolobow T, Fumagalli R, Moretti MP, Chen V, Buckhold D. Acute respiratory failure following pharmacologically induced hyperventilation: an experimental animal study. Intensive Care Med 1988; 15: 8–14. [DOI] [PubMed] [Google Scholar]
- 92.Kopp W, Gedeit RG, Asaro LA, McLaughlin GE, Wypij D, Curley MAQ. The impact of preintubation noninvasive ventilation on outcomes in pediatric acute respiratory distress syndrome. Crit Care Med 2021; 49: 816–27. [DOI] [PubMed] [Google Scholar]
- 93.Lindell RB, Fitzgerald JC, Rowan CM, et al. The use and duration of preintubation respiratory support is associated with increased mortality in immunocompromised children with acute respiratory failure. Crit Care Med 2022; 50: 1127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363: 1107–16. [DOI] [PubMed] [Google Scholar]
- 95.Hua Y, Ou X, Li Q, Zhu T. Neuromuscular blockers in the acute respiratory distress syndrome: a meta-analysis. PLoS One 2020; 15: e0227664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.National Heart, Lung, and Blood Institute PETAL Clinical Trials Network, Moss M, Huang DT, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med 2019; 380: 1997–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tarazan N, Alshehri M, Sharif S, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: updated systematic review and meta-analysis of randomized trials. Intensive Care Med Exp 2020; 8: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hotz JC, Bornstein D, Kohler K, et al. Real-time effort driven ventilator management: a pilot study. Pediatr Crit Care Med 2020; 21: 933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dubo S, Oviedo V, Garcia A, et al. Low spontaneous breathing effort during extracorporeal membrane oxygenation in a porcine model of severe acute respiratory distress syndrome. Anesthesiology 2020; 133: 1106–17. [DOI] [PubMed] [Google Scholar]
- 100.Banner MJ, Kirby RR, Kirton OC, DeHaven CB, Blanch PB. Breathing frequency and pattern are poor predictors of work of breathing in patients receiving pressure support ventilation. Chest 1995; 108: 1338–44. [DOI] [PubMed] [Google Scholar]
- 101.Tulaimat A, Patel A, Wisniewski M, Gueret R. The validity and reliability of the clinical assessment of increased work of breathing in acutely ill patients. J Crit Care 2016; 34: 111–15. [DOI] [PubMed] [Google Scholar]
- 102.Kyogoku M, Shimatani T, Hotz JC, et al. Direction and magnitude of change in plateau from peak pressure during inspiratory holds can identify the degree of spontaneous effort and elastic workload in ventilated patients. Crit Care Med 2021; 49: 517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Telias I, Junhasavasdikul D, Rittayamai N, et al. Airway occlusion pressure as an estimate of respiratory drive and inspiratory effort during assisted ventilation. Am J Respir Crit Care Med 2020; 201: 1086–98. [DOI] [PubMed] [Google Scholar]
- 104.Essouri S, Baudin F, Mortamet G, Beck J, Jouvet P, Emeriaud G. Relationship between diaphragmatic electrical activity and esophageal pressure monitoring in children. Pediatr Crit Care Med 2019; 20: e319–25. [DOI] [PubMed] [Google Scholar]
- 105.Crulli B, Kawaguchi A, Praud JP, Petrof BJ, Harrington K, Emeriaud G. Evolution of inspiratory muscle function in children during mechanical ventilation. Crit Care 2021; 25: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Umbrello M, Formenti P, Lusardi AC, et al. Oesophageal pressure and respiratory muscle ultrasonographic measurements indicate inspiratory effort during pressure support ventilation. Br J Anaesth 2020; 125: e148–57. [DOI] [PubMed] [Google Scholar]
- 107.Rowan CM, Klein MJ, Hsing DD, et al. Early use of adjunctive therapies for pediatric acute respiratory distress syndrome: a PARDIE study. Am J Respir Crit Care Med 2020; 201: 1389–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Froese AB, Raja SN. Gravity, the belly, and the diaphragm: you can’t ignore physics. Anesthesiology 2006; 104: 193–96. [DOI] [PubMed] [Google Scholar]
- 109.Froese AB, Bryan AC. Effects of anesthesia and paralysis on diaphragmatic mechanics in man. Anesthesiology 1974; 41: 242–55. [DOI] [PubMed] [Google Scholar]
- 110.Albert RK, Hubmayr RD. The prone position eliminates compression of the lungs by the heart. Am J Respir Crit Care Med 2000; 161: 1660–65. [DOI] [PubMed] [Google Scholar]
- 111.Yoshida T, Amato MBP, Grieco DL, et al. Esophageal manometry and regional transpulmonary pressure in lung injury. Am J Respir Crit Care Med 2018; 197: 1018–26. [DOI] [PubMed] [Google Scholar]
- 112.Bryan AC. Conference on the scientific basis of respiratory therapy. Pulmonary physiotherapy in the pediatric age group. Comments of a devil’s advocate. Am Rev Respir Dis 1974; 110: 143–44. [DOI] [PubMed] [Google Scholar]
- 113.Katira BH, Osada K, Engelberts D, et al. Positive end-expiratory pressure, pleural pressure, and regional compliance during pronation: an experimental study. Am J Respir Crit Care Med 2021; 203: 1266–74. [DOI] [PubMed] [Google Scholar]
- 114.Mutoh T, Guest RJ, Lamm WJ, Albert RK. Prone position alters the effect of volume overload on regional pleural pressures and improves hypoxemia in pigs in vivo. Am Rev Respir Dis 1992; 146: 300–06. [DOI] [PubMed] [Google Scholar]
- 115.Gattinoni L, Pelosi P, Vitale G, Pesenti A, D’Andrea L, Mascheroni D. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure. Anesthesiology 1991; 74: 15–23. [DOI] [PubMed] [Google Scholar]
- 116.Terzi N, Bayat S, Noury N, et al. Comparison of pleural and esophageal pressure in supine and prone positions in a porcine model of acute respiratory distress syndrome. J Appl Physiol 1985; 2020: 1617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Richter T, Bellani G, Scott Harris R, et al. Effect of prone position on regional shunt, aeration, and perfusion in experimental acute lung injury. Am J Respir Crit Care Med 2005; 172: 480–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Glenny RW, Lamm WJ, Albert RK, Robertson HT. Gravity is a minor determinant of pulmonary blood flow distribution. J Appl Physiol 1985; 1991: 620–29. [DOI] [PubMed] [Google Scholar]
- 119.Glenny RW, Polissar L, Robertson HT. Relative contribution of gravity to pulmonary perfusion heterogeneity. J Appl Physiol 1985; 1991: 2449–52. [DOI] [PubMed] [Google Scholar]
- 120.Curley MA, Thompson JE, Arnold JH. The effects of early and repeated prone positioning in pediatric patients with acute lung injury. Chest 2000; 118: 156–63. [DOI] [PubMed] [Google Scholar]
- 121.Casado-Flores J, Martinez de Azagra A, Ruiz-Lopez MJ, Ruiz M, Serrano A. Pediatric ARDS: effect of supine-prone postural changes on oxygenation. Intensive Care Med 2002; 28: 1792–96. [DOI] [PubMed] [Google Scholar]
- 122.Relvas MS, Silver PC, Sagy M. Prone positioning of pediatric patients with ARDS results in improvement in oxygenation if maintained > 12 h daily. Chest 2003; 124: 269–74. [DOI] [PubMed] [Google Scholar]
- 123.Douglas WW, Rehder K, Beynen FM, Sessler AD, Marsh HM. Improved oxygenation in patients with acute respiratory failure: the prone position. Am Rev Respir Dis 1977; 115: 559–66. [DOI] [PubMed] [Google Scholar]
- 124.Mure M, Martling CR, Lindahl SG. Dramatic effect on oxygenation in patients with severe acute lung insufficiency treated in the prone position. Crit Care Med 1997; 25: 1539–44. [DOI] [PubMed] [Google Scholar]
- 125.Pelosi P, Tubiolo D, Mascheroni D, et al. Effects of the prone position on respiratory mechanics and gas exchange during acute lung injury. Am J Respir Crit Care Med 1998; 157: 387–93. [DOI] [PubMed] [Google Scholar]
- 126.Lai C, Adda I, Teboul JL, et al. Effects of prone positioning on venous return in patients with acute respiratory distress syndrome. Crit Care Med 2021; 49: 781–89. [DOI] [PubMed] [Google Scholar]
- 127.Jozwiak M, Teboul JL, Anguel N, et al. Beneficial hemodynamic effects of prone positioning in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2013; 188: 1428–33. [DOI] [PubMed] [Google Scholar]
- 128.Gattinoni L, Tognoni G, Pesenti A, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med 2001; 345: 568–73. [DOI] [PubMed] [Google Scholar]
- 129.Guerin C, Gaillard S, Lemasson S, et al. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA 2004; 292: 2379–87. [DOI] [PubMed] [Google Scholar]
- 130.Mancebo J, Fernández R, Blanch L, et al. A multicenter trial of prolonged prone ventilation in severe acute respiratory distress syndrome. Am J Respir Crit Care Med 2006; 173: 1233–39. [DOI] [PubMed] [Google Scholar]
- 131.Sud S, Friedrich JO, Taccone P, et al. Prone ventilation reduces mortality in patients with acute respiratory failure and severe hypoxemia: systematic review and meta-analysis. Intensive Care Med 2010; 36: 585–99. [DOI] [PubMed] [Google Scholar]
- 132.Gattinoni L, Carlesso E, Taccone P, Polli F, Guerin C, Mancebo J. Prone positioning improves survival in severe ARDS: a pathophysiologic review and individual patient meta-analysis. Minerva Anestesiol 2010; 76: 448–54. [PubMed] [Google Scholar]
- 133.Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone position for acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc 2017; 14 (suppl 4): S280–88. [DOI] [PubMed] [Google Scholar]
- 134.Guerin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368: 2159–68. [DOI] [PubMed] [Google Scholar]
- 135.Abroug F, Ouanes-Besbes L, Dachraoui F, Ouanes I, Brochard L. An updated study-level meta-analysis of randomised controlled trials on proning in ARDS and acute lung injury. Crit Care 2011; 15: R6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Broccard A, Shapiro RS, Schmitz LL, Adams AB, Nahum A, Marini JJ. Prone positioning attenuates and redistributes ventilator-induced lung injury in dogs. Crit Care Med 2000; 28: 295–303. [DOI] [PubMed] [Google Scholar]
- 137.Valenza F, Guglielmi M, Maffioletti M, et al. Prone position delays the progression of ventilator-induced lung injury in rats: does lung strain distribution play a role? Crit Care Med 2005; 33: 361–67. [DOI] [PubMed] [Google Scholar]
- 138.Lupton-Smith A, Argent A, Rimensberger P, Frerichs I, Morrow B. Prone positioning improves ventilation homogeneity in children with acute respiratory distress syndrome. Pediatr Crit Care Med 2017; 18: e229–34. [DOI] [PubMed] [Google Scholar]
- 139.Gattinoni L, Taccone P, Carlesso E, Marini JJ. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit Care Med 2013; 188: 1286–93. [DOI] [PubMed] [Google Scholar]
- 140.Perchiazzi G, Rylander C, Vena A, et al. Lung regional stress and strain as a function of posture and ventilatory mode. J Appl Physiol 1985; 2011: 1374–83. [DOI] [PubMed] [Google Scholar]
- 141.Kavanagh BP. Prone positioning in children with ARDS: positive reflections on a negative clinical trial. JAMA 2005; 294: 248–50. [DOI] [PubMed] [Google Scholar]
- 142.Cornejo RA, Diaz JC, Tobar EA, et al. Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2013; 188: 440–48. [DOI] [PubMed] [Google Scholar]
- 143.Sanchez-Pinto LN, Luo Y, Churpek MM. Big data and data science in critical care. Chest 2018; 154: 1239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med 2020; 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Herasevich V, Yilmaz M, Khan H, Hubmayr RD, Gajic O. Validation of an electronic surveillance system for acute lung injury. Intensive Care Med 2009; 35: 1018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Koenig HC, Finkel BB, Khalsa SS, et al. Performance of an automated electronic acute lung injury screening system in intensive care unit patients. Crit Care Med 2011; 39: 98–104. [DOI] [PubMed] [Google Scholar]
- 147.Azzam HC, Khalsa SS, Urbani R, et al. Validation study of an automated electronic acute lung injury screening tool. J Am Med Inform Assoc 2009; 16: 503–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bagga S, Paluzzi DE, Chen CY, et al. Better ventilator settings using a computerized clinical tool. Respir Care 2014; 59: 1172–77. [DOI] [PubMed] [Google Scholar]
- 149.Blum JM, Stentz MJ, Maile MD, et al. Automated alerting and recommendations for the management of patients with preexisting hypoxia and potential acute lung injury: a pilot study. Anesthesiology 2013; 119: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bourdeaux CP, Thomas MJ, Gould TH, et al. Increasing compliance with low tidal volume ventilation in the ICU with two nudge-based interventions: evaluation through intervention time-series analyses. BMJ Open 2016; 6: e010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Castellanos I, Martin M, Kraus S, et al. Effects of staff training and electronic event monitoring on long-term adherence to lung-protective ventilation recommendations. J Crit Care 2018; 43: 13–20. [DOI] [PubMed] [Google Scholar]
- 152.Eslami S, Abu-Hanna A, Schultz MJ, de Jonge E, de Keizer NF. Evaluation of consulting and critiquing decision support systems: effect on adherence to a lower tidal volume mechanical ventilation strategy. J Crit Care 2012; 27: 425.e1–8. [DOI] [PubMed] [Google Scholar]
- 153.Eslami S, de Keizer NF, Abu-Hanna A, de Jonge E, Schultz MJ. Effect of a clinical decision support system on adherence to a lower tidal volume mechanical ventilation strategy. J Crit Care 2009; 24: 523–29. [DOI] [PubMed] [Google Scholar]
- 154.Walsh BK, Smallwood C, Rettig J, Kacmarek RM, Thompson J, Arnold JH. Daily goals formulation and enhanced visualization of mechanical ventilation variance improves mechanical ventilation score. Respir Care 2017; 62: 268–78. [DOI] [PubMed] [Google Scholar]
- 155.Mayampurath A, Churpek MM, Su X, et al. External validation of an acute respiratory distress syndrome prediction model using radiology reports. Crit Care Med 2020; 48: e791–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zeiberg D, Prahlad T, Nallamothu BK, Iwashyna TJ, Wiens J, Sjoding MW. Machine learning for patient risk stratification for acute respiratory distress syndrome. PLoS One 2019; 14: e0214465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ding XF, Li JB, Liang HY, et al. Predictive model for acute respiratory distress syndrome events in ICU patients in China using machine learning algorithms: a secondary analysis of a cohort study. J Transl Med 2019; 17: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Newth CJL, Khemani RG, Jouvet PA, Sward KA. Mechanical ventilation and decision support in pediatric intensive care. Pediatr Clin North Am 2017; 64: 1057–70. [DOI] [PubMed] [Google Scholar]
- 159.Newth CJ, Hotz JC, Khemani RG. Ventilator liberation in the pediatric ICU. Respir Care 2020; 65: 1601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Khemani RG, Hotz JC, Sward KA, Newth CJL. The role of computer-based clinical decision support systems to deliver protective mechanical ventilation. Curr Opin Crit Care 2020; 26: 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Taoum A, Mourad-Chehade F, Amoud H. Evidence-based model for real-time surveillance of ARDS. Biomed Signal Process Control 2019; 50: 83–91. [Google Scholar]
- 162.Morris AH, Stagg B, Lanspa M, et al. Enabling a learning healthcare system with automated computer protocols that produce replicable and personalized clinician actions. J Am Med Inform Assoc 2021; 28: 1330–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


