Abstract
Context:
TwinKleen™ and Triton™ are newer all-in-one irrigants that have simultaneous action on both organic and inorganic contents. Studies comparing their tissue dissolving ability (TDA) either at room temperature (RT) or on prewarming (PW) and continuous warming (CW) are not yet reported.
Aims:
To evaluate and compare the effect of 3% sodium hypochlorite (NaOCl), Twin Kleen™, and Triton™, on the bovine TDA.
Materials and Methods:
One hundred and twenty tissue specimens (size 4 mm × 4 mm × 2 mm and weight 37 ± 3 mg) were divided into Group 1, normal saline (negative control); Group 2, 3% NaOCl (positive control); Group 3, Twin Kleen™; and Group 4, Triton™ (n = 30) Each group is further divided into three subgroups with ten samples each as sub group a – at room temperature (RT), sub group b – on pre warming (PW) and sub group c– on continuous warming (CW), Tissue specimens were immersed in test tubes with 5 ml of respective irrigants replenished thrice every 15 min. The percentage difference in tissue weights was calculated after 5, 10, and 15 min.
Statistical Analysis Used:
Multiple intergroup comparisons were done using Tukey’s multiple-comparison test, using SPSS software version 23.0.
Results:
Both Triton™ and 3% NaOCl showed significantly higher dissolution than normal saline and Twin Kleen™ on CW followed by PW than at RT. Twin Kleen™ showed significantly less dissolution at all the tested temperatures.
Conclusions:
Heating enhances the TDA of Triton™ and 3% NaOCl but not Twin Kleen™. CW showed significantly higher dissolution than PW.
Keywords: Continuous warming, prewarming, sodium hypochlorite, temperature, tissue dissolution
INTRODUCTION
Sodium hypochlorite (NaOCl) is the most favored root canal irrigant because of its unique tissue-dissolving ability (TDA), which depends on its concentration, volume, temperature, periodic replenishment, and its agitation within the canal.[1] Irrigation with warm NaOCl has the additional advantage of better apical cleaning with enhanced irrigation dynamics.[2] Altering the temperature either by prewarming (PW) or continuous warming (CW) of NaOCl is preferred by many clinicians. Woodmansey reported an increase in dissolution rate by two hundred and ten times when heated to its boiling temperature.[3] However, studies have shown that the efficiency of NaOCl irrigant reaches its plateau over 60°C.[4] de Hemptinne et al. stated that preheated NaOCl reaches equilibrium with a normal body temperature of approximately 35°C within 4 min of irrigation, thus reducing their therapeutic window.[5] Iandolo et al. stated that by continuous intracanal warming, irrigants can be maintained at higher temperatures for longer time intervals and could achieve cleaner canals than preheated solutions.[6]
Recently, newer all-in-one irrigants namely Twin Kleen™ (Maarc Dental, India) and Triton™ (Brasseler, USA) were introduced with dual function of tissue dissolving and chelating properties within a single solution. Twin Kleen™ is a combination of 18% etidronate (HEDP) and 5% NaOCl. This mixture acts as a soft chelate with the least effect on root dentin microhardness[7] and comparatively lower organic solvent action than 5% NaOCl. Heating of the NaOCl/HEDP mixtures had a detrimental effect on free available chlorine (FAC).[8]
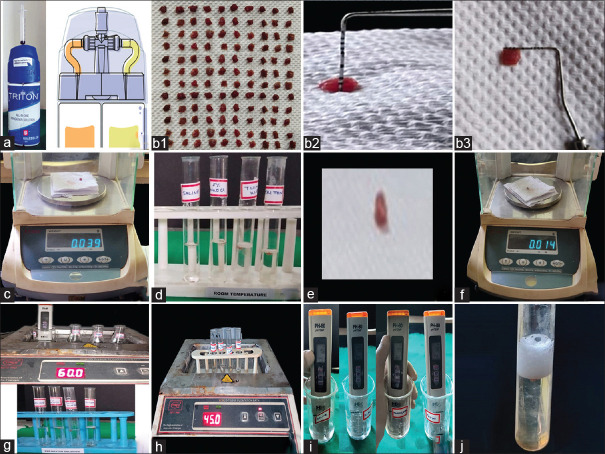
Triton™ (Brasseler, USA, Savannah, GA) is a recently introduced all-in-one solution with 8% NaOCl and fourteen different chelators along with sodium lauryl sulfate surfactant available in separate bottles with a unique delivery system as stated by the manufacturer [Figure 1a].[9] The TDA of Triton™ is not yet reported either at room temperature (RT) or at elevated temperatures.
Figure 1.
(a) Triton all-in-one irrigating solution, (b) b1-b3 - Bovine muscle tissue specimens, (c) Preweighing, (d) Irrigants at RT, (e) Blot drying, (f) Re-weighing, (g) prewarming (PW) of irrigants, (h) continuous warming (CW) of irrigants, (i) pH estimation of irrigants, (j) effervescence in Twin Kleen™ on PW/CW
Hence, the objective of this study was to evaluate and compare the TDA of Triton™ with Twin Kleen™ and 3% NaOCl after 5, 10, and 15 min at RT, PW, and CW.
MATERIALS AND METHODS
This tissue dissolution study was conducted on bovine muscle tissue using four different irrigants – normal saline, 3% NaOCl, Twin Kleen™, and Triton™ after obtaining the institutional ethical committee clearance. The composition and manufacturer details of each irrigant used in the study are shown in Table 1. One hundred and twenty samples of fresh bovine muscle tissue each with a standardized dimension of 4 mm × 4 mm × 2 mm and an initial weight of 3.7 ± 0.3 mg as measured using a precision balance (Essae – Teraoka Pvt Ltd. Germany Model No. F300) were obtained [Figure1b1-b3 and c] and divided into four groups (n = 30). This experiment was done with each irrigant at RT 22°C, on PW to 60°C, and CW at 45°C, and tissue dissolution was assessed and compared after 5, 10, and 15 min with periodic replenishment of fresh irrigant after each tested time interval. Accordingly, each group was divided into three subgroups (n = 10) with a mean total weight of 37 ± 3 mg as follows:
Table 1.
Materials used in the study
| Material | Manufacturer (lot number) | Composition |
|---|---|---|
| Triton™ | Brasseler, Savannah, GA, USA (lot number 20220919) | Part A |
| 2-Phosphono-1,2,4-butanetricarboxylic acid | ||
| Citric acid | ||
| Sodium dodecylbenzenesulfonate | ||
| Alcohols, C9-11, ethoxylated, liquids | ||
| Polyethylene glycol 4-(tert-octylphenyl) ether, liquid | ||
| Sodium lauryl sulfate | ||
| 2-Ethylhexyl sodium sulfate | ||
| Sodium cumenesulphonate | ||
| Sodium hydroxide | ||
| Part B | ||
| ≤10% NaOCl | ||
| ≤12% Sodium hydroxide | ||
| Twin Kleen™ | Maarc dental, Maharashtra, India (lot number 3014/01/112020) | 18% HEBP (HEBP/HEDP) |
| Procedure: 2 capsules of Twin Kleen™ are freshly mixed in 10 mL of 5% NaOCl | ||
| NaOCl | Vishal dentocare Pvt. Ltd., Ahmedabad, India (lot number VM-122) | 3% NaOCl |
NaOCl: Sodium hypochlorite, HEBP: Etidronic acid
Group 1 – Normal saline
Group 2 – 3% NaOCl
Group 3 – Twin Kleen™
-
Group 4 – Triton™
- Subgroups 1a, 2a, 3a, and 4a – RT
- Subgroups 1b, 2b, 3b, and 4b–PW
- Subgroups 1c, 2c, 3c, and 4c – CW.
Tissue dissolution
Procedure at room temperature: (1a, 2a, 3a, and 4a)
Ten test tubes of each group were filled with 5 mL of respective irrigating solution. The preweighed tissue specimens were submerged in the solution for 5 min [Figure 1d]. The specimens were then taken out and immediately immersed in distilled water to remove the irrigant. These samples were then blot-dried [Figure 1e] and reweighed for comparison with initial values [Figure 1f]. This procedure was repeated thrice at 5-min intervals using freshly replenished solutions with the same test samples to compare initial weights and change in weights after 5, 10, and 15 min.
Procedure for prewarming: (1b, 2b, 3b, and 4b)
A temperature-controlled water bath (Yorca YSI-133D, Yorco Sales Pvt. Ltd., India) was maintained at 60°C. Four beakers each containing 50 mL of respective irrigants were placed in a water bath until the solutions in the beaker attained 45°C, which was confirmed with a digital thermometer. The solutions were then transferred into respective test tubes of 5 mL each [Figure 1g] and preweighed tissue specimens were immersed. The same experimental procedure as done at RT was followed using freshly preheated test solutions at all tested time intervals.
Procedure for controlled warming: (1c, 2c, 3c, and 4c)
Each group containing ten test tubes of 5 ml solution was immersed in a temperature-controlled water bath to attain 45°C [Figure 1h]. The preweighed samples were submerged in their respective irrigating solutions, and CW of the irrigant was carried out by maintaining the test tubes in the water bath throughout the experimental procedure. The difference in weights of the specimens was noted after 5, 10, and 15 min similarly as done at RT.
The pH of all the test solutions was recorded at RT/PW/CW [Figure 1i]. The weight of the tissue specimen after each tested time interval was recorded. The percentage weight loss after 5, 10, and 15 min was calculated and subjected to statistical analysis.
Statistical analysis
Weight loss was expressed as mean values and standard deviation, after 5, 10, and 15 min of submersion in respective irrigants at RT, PW, and CW [Table 2]. One-way analysis of variance and Tukey’s multiple post hoc tests were used to detect differences between the groups at the same immersion periods using SPSS software version 23.0 for Windows (SPSS Inc., Chicago, IL, USA), [Table 3]. All the hypotheses were tested at 99% confidence level.
Table 2.
Mean tissue dissolution by various groups after 5, 10, and 15 min in milligrams
| Name of the groups | Sub-groups | pH | Mean±SD | ||
|---|---|---|---|---|---|
|
| |||||
| T5 | T10 | T15 | |||
| Group 1 | 1a-RT | 7.7 | 39±0.94 | 37.92±3.42 | 39.01±0.94 |
| 1b-PW | 7.7 | 39±0.47 | 39.16±0.35 | 39±0.47 | |
| 1c-CW | 7.7 | 38.81±0.43 | 39.3±0.67 | 39±0.00 | |
| Group 2 | 2a-RT | 10 | 32.73±0.89 | 30.03±0.50 | 23.1±0.99 |
| 2b-PW | 8 | 29.40±0.70 | 27±0.47 | 24±0.47 | |
| 2c-CW | 8 | 14±0.47 | 3.86±0.35 | 0.11±0.28 | |
| Group 3 | 3a-RT | 7 | 35.2±0.92 | 37.06±0.38 | 37.06±0.38 |
| 3b-PW | 5.5 | 32.6±0.00 | 33.69±0.95 | 33.7±1.27 | |
| 3c-CW | 4.9 | 32.6±0.00 | 34±0.00 | 34.8±0.00 | |
| Group 4 | 4a-RT | 8.3 | 31.93±0.35 | 27.2±0.45 | 19.71±0.47 |
| 4b-PW | 9.9 | 18.69±0.69 | 1.9±0.57 | 0±0.00 | |
| 4c-CW | 10 | 2.99±0.03 | 0.07±0.22 | 0.00±0.09 | |
RT: Room temperature, PW: Prewarming, CW: Continuous warming, SD: Standard deviation
Table 3.
Comparison of differences in time taken for dissolution of pulp after 5, 10, and 15 min using Tukey’s multiple post hoc test
| Pair of groups | Sub group a-RT | Sub group b-PW | Sub group c-CW | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| T5 | T10 | T15 | T5 | T10 | T15 | T5 | T10 | T15 | |
| Group (1 vs. 2) | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* |
| Group (1 vs. 3) | 0.0001* | 0.8392 | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* |
| Group (1 vs. 4) | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* |
| Group (2 vs. 3) | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* |
| Group (2 vs. 4) | 0.1206 | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 1.0000 |
| Group (3 vs. 4) | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* | 0.0001* |
*Statistically significant at p<0.05, RT: Room temperature, PW: Prewarming, CW: Continuous warming
RESULTS
The results of this study revealed that Triton™ had the significantly highest TDA followed by 3% NaOCl. Twin Kleen™ had the least TDA either at RT/PW/CW. All the groups showed a greater percentage of tissue dissolution with an increase in the time of exposure to irrigants (P < 0.05). On comparing the TDA using PW and CW solutions, it was observed that there was a significantly higher amount of dissolution in Triton™ and 3% NaOCl, whereas Twin Kleen™ showed no difference in its solvent action. The amount of tissue dissolution achieved with Triton™ on CW was 92%, 99.81%, and 100% after 5, 10, and 15-min intervals respectively, while that of NaOCl was 62.4%, 89.64%, and 99.70% respectively.
DISCUSSION
The manuscript of this laboratory study has been written according to Preferred Reporting Items for Laboratory Studies in Endodontology 2021 guidelines. It was reported that hypochlorite showed increased TDA on either PW or continuous intracanal heating. However, on warming, NaOCl/HEDP mixtures showed mixed results due to chemical interactions between them.[10] Triton™ being a newer single irrigant, assessing its TDA is most warranted before its clinical use. Hence, the present tissue dissolving experiment at different temperatures was undertaken.
In the present study, bovine muscle tissue was selected because of its availability and easier standardization of samples to a size of 4 mm × 4 mm × 2 mm to achieve a uniform surface area for the action of irrigants.
The TDA of NaOCl depends on FAC, which can be either in the form of hypochlorous acid (HOCl) or hypochlorite ions (OCl-). OCl-ions are predominantly responsible for TDA, whereas HOCl shows bactericidal effect.[11] Studies have shown that OCl-ions predominate in alkaline pH (>7.6) and HOCl predominates in acidic pH (<7).[12] Hence, in the present study, the pH of all four irrigants was measured at RT/PW/CW to determine the change in pH with a temperature rise. It was reported that NaOCl loses most of its TDA within 2 min of contact with organic tissue and the irrigants rapidly equilibrate from 66°C to 41°C in 15 s and to 36°C in 4 min.[13] Hence, in this study, each solution was replenished every 5 min.
Most of the authors preferred heating NaOCl to 60°C as it reaches a plateau around 60°C, and no additional benefit was noted above these temperatures due to the exhaustion of available chlorine ions. An increased rate of chemical reaction between chelators and NaOCl was reported at or above 60°C with a rapid loss of FAC.[14,15] Hence, in this study, PW was done to the maximum acceptable temperature of 60°C, while CW was done at 45°C.
Tissue samples immersed in saline for 5 min showed weight gain. This could be due to its hypotonic nature resulting in the movement of fluid into the tissue cells. Among the tested irrigants, Triton™ was found to have the highest TDA followed by 3% NaOCl at all tested time intervals. This suggests higher TDA when exposed to longer durations.
Both Triton™ and NaOCl resulted in significantly faster tissue dissolution in PW and CW than at RT. Heating of these irrigants might have resulted in the liberation of OCl-ions at a faster rate as indicated by their alkaline pH values. Thus, the availability of these ions at higher concentrations could have enhanced their TDA. In the present study, CW showed significantly higher and faster tissue dissolution than PW. This could be due to the rapid attainment of equilibrium to RT by prewarmed solutions within a few seconds.[5] In CW, the solutions were maintained at higher temperatures throughout the procedure. This enhances irrigant efficacy by continuous release of OCl-ions with increased molecular movement and rate of collision with tissue specimens and consequently its dissolution at a faster rate.
Twin Kleen™ showed the least tissue dissolution than 3% NaOCl and Triton™. The specimens showed a minimal weight gain due to the hydration of tissues after initial dissolution. Twin Kleen™ attained an acidic pH of 4.9 at RT, suggesting the presence of FAC in the form of HOCl, which has a lesser TDA. On heating Twin Kleen™, effervescence was observed [Figure 1j] as a result of an exothermic reaction indicating evaporation of chlorine gas. Similarly, Jaiswal et al. in their study reported that pulp dissolution by continuous chelation was lower than hypochlorite even after warming.[16] Zollinger et al. stated that heating of NaOCl and HEDP is not beneficial as HEDP decomposes to acetic acid and phosphonic acid, which is neutralized by NaOCl.[17] This chemical interaction with NaOCl might have resulted in a reduction of FAC. However, these findings are contradictory to previous studies that stated the TDA of NaOCl is retained in combination with HEDP at RT.[10] This could be due to differences in methodology. The authors stated that NaOCl when mixed with HEDP retained its TDA at RT but lost its effect on heating to higher temperatures.[10,16,17]
In the present study, Triton™ showed significantly greater TDA than Twin Kleen™ and 3% NaOCl at all the tested conditions. The greater tissue dissolution ability of Triton™ could be attributed to its composition and alkaline pH. Triton™ has a unique combination of 8% NaOCl with a blend of 14 different chelating agents and surfactant (sodium lauryl sulfate - SLS) resulting in a freshly mixed solution equivalent to 4% NaOCl as stated by the manufacturer. SLS provides better wettability to the irrigant allowing closer contact with the tissue surface and, thus, better TDA. It was found in this study that complete tissue dissolution was only possible in Triton™ – PW (within 12 min) and CW (in 2.5 min) groups. None of the other groups showed complete dissolution. This suggests increased efficiency of Triton™ at elevated temperatures, which can be explained by its alkaline pH with predominant OCl-ions responsible for tissue dissolution. A pH of 8.3 was recorded for freshly mixed Triton™ solution at RT and 9.8 on PW and CW. It can also be hypothesized that there is no chemical interaction between hypochlorite and the chelators on heating, and, Triton™ was chemically stable even on heating to 60°C, thus maintaining its TDA.
CONCLUSIONS
Within the limitations of our study, we can conclude that Triton™ has the fastest rate of dissolution than 3% NaOCl and can be used as an alternative for conventional sequential irrigation protocols. The TDA of Triton™ can be enhanced by both PW and CW without any adverse chemical interactions. Twin Kleen™ showed the least tissue dissolution among all the tested irrigants.
Clinical significance
CW of NaOCl is preferred during root canal irrigation. Triton™ can be considered an ideal tissue solvent during biomechanical preparation. The fastest way of achieving tissue dissolution is by CW of Triton™. Twin Kleen™ showed the least TDA among the tested irrigants.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. S B Javali, Senior Associate Professor in Statistics, Department of Community Medicine, USM KLE IMP, Belagavi, Karnataka, India.
REFERENCES
- 1.Kandaswamy D, Venkateshbabu N. Root canal irrigants. J Conserv Dent. 2010;13:256–64. doi: 10.4103/0972-0707.73378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gopikrishna V, Ashok P, Kumar AP, Narayanan LL. Influence of temperature and concentration on the dynamic viscosity of sodium hypochlorite in comparison with 17% EDTA and 2% chlorhexidine gluconate: An in vitro study. J Conserv Dent. 2014;17:57–60. doi: 10.4103/0972-0707.124142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodmansey KF. Intracanal heating of sodium hypochlorite solution: An improved endodontic irrigation technique. Dent Today. 2005;24:114, 116. [PubMed] [Google Scholar]
- 4.Rossi-Fedele G, De Figueiredo JA. Use of a bottle warmer to increase 4% sodium hypochlorite tissue dissolution ability on bovine pulp. Aust Endod J. 2008;34:39–42. doi: 10.1111/j.1747-4477.2007.00110.x. [DOI] [PubMed] [Google Scholar]
- 5.de Hemptinne F, Slaus G, Vandendael M, Jacquet W, De Moor RJ, Bottenberg P. In vivo intracanal temperature evolution during endodontic treatment after the injection of room temperature or preheated sodium hypochlorite. J Endod. 2015;41:1112–5. doi: 10.1016/j.joen.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Iandolo A, Amato M, Dagna A, Poggio C, Abdellatif D, Franco V, et al. Intracanal heating of sodium hypochlorite: Scanning electron microscope evaluation of root canal walls. J Conserv Dent. 2018;21:569–73. doi: 10.4103/JCD.JCD_245_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dineshkumar MK, Vinothkumar TS, Arathi G, Shanthisree P, Kandaswamy D. Effect of ethylene diamine tetra-acetic acid, MTAD™, and HEBP as a final rinse on the microhardness of root dentin. J Conserv Dent. 2012;15:170–3. doi: 10.4103/0972-0707.94587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biel P, Mohn D, Attin T, Zehnder M. Interactions between the tetrasodium salts of EDTA and 1-Hydroxyethane 1,1-Diphosphonic acid with sodium hypochlorite irrigants. J Endod. 2017;43:657–61. doi: 10.1016/j.joen.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Triton (Part A) SDS No 210301;Brasseler, USA;2021. [[Last accessed on 2023 Nov 15]]. 2021. [[Last accessed on 2023 Nov 15]]. Available from: https://brasselerusadental.com/wpcontent/uploads/sites/9/2022/01/Brasseler-Triton-Part-A-US-SDS-EN-210301.pdf . Triton (Part B), SDS No. 210301; Brasseler, USA. Available from: https://brasselerusadental.com/wpcontent/uploads/sites/9/2022/01/Brasseler-Triton-Part-B-US-SDS-EN-210301.pdf .
- 10.Wright PP, Kahler B, Walsh LJ. The effect of heating to intracanal temperature on the stability of sodium hypochlorite admixed with etidronate or EDTA for continuous chelation. J Endod. 2019;45:57–61. doi: 10.1016/j.joen.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Bloomfield SF, Miles GA. The antibacterial properties of sodium dichloroisocyanurate and sodium hypochlorite formulations. J Appl Bacteriol. 1979;46:65–73. doi: 10.1111/j.1365-2672.1979.tb02582.x. [DOI] [PubMed] [Google Scholar]
- 12.Fukuzaki S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006;11:147–57. doi: 10.4265/bio.11.147. [DOI] [PubMed] [Google Scholar]
- 13.Clarkson RM, Moule AJ, Podlich H, Kellaway R, Macfarlane R, Lewis D, et al. Dissolution of porcine incisor pulps in sodium hypochlorite solutions of varying compositions and concentrations. Aust Dent J. 2006;51:245–51. doi: 10.1111/j.1834-7819.2006.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 14.Tartari T, Guimarães BM, Amoras LS, Duarte MA, Silva e Souza PA, Bramante CM. Etidronate causes minimal changes in the ability of sodium hypochlorite to dissolve organic matter. Int Endod J. 2015;48:399–404. doi: 10.1111/iej.12329. [DOI] [PubMed] [Google Scholar]
- 15.Vyavahare N, Srinidhi SR, Desai N, Hindlekar A, Balsaraf O, Surwade P. Effect of different chelating agents on bovine tissue dissolving capacity of sodium hypochlorite. Endodontology. 2020;32:216–9. [Google Scholar]
- 16.Jaiswal S, Gupta S, Nikhil V, Bhadoria A, Raj S. Effect of intracanal and extracanal heating on pulp dissolution property of continuous chelation irrigant. J Conserv Dent. 2021;24:544–8. doi: 10.4103/jcd.jcd_230_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zollinger A, Mohn D, Zeltner M, Zehnder M. Short-term storage stability of NaOCl solutions when combined with Dual Rinse HEDP. Int Endod J. 2018;51:691–6. doi: 10.1111/iej.12875. [DOI] [PubMed] [Google Scholar]