Abstract
Borrelia miyamotoi is a relapsing fever spirochete carried by Ixodes spp. ticks throughout the northern hemisphere. The pathogen is acquired either transovarially (vertically) or horizontally through blood-feeding and passed transtadially across life stages. Despite these complementary modes of transmission, infection prevalence of ticks with B. miyamotoi is typically low (<5%) in natural settings and the relative contributions of the two transmission modes have not been studied extensively. Horizontal transmission of B. miyamotoi (strain CT13–2396 or wild type strain) was initiated using infected Ixodes scapularis larvae or nymphs to expose rodents, which included both the immunocompetent CD-1 laboratory mouse (Mus musculus) and a natural reservoir host, the white-footed mouse (Peromyscus. leucopus), to simulate natural enzootic transmission. Transovarial transmission was evaluated using I. scapularis exposed to B. miyamotoi as either larvae or nymphs feeding on immunocompromised SCID mice (M. musculus) and subsequently fed as females on New Zealand white rabbits. Larvae from infected females were qPCR-tested individually to assess transovarial transmission rates. Tissue tropism of B. miyamotoi in infected ticks was demonstrated using in situ hybridization. Between 1 and 12% of ticks were positive (post-molt) for B. miyamotoi after feeding on groups of CD-1 mice or P. leucopus with evidence of infection, indicating that horizontal transmission was inefficient, regardless of whether infected larvae or nymphs were used to challenge the mice. Transovarial transmission occurred in 7 of 10 egg clutches from infected females. Filial infection prevalence in larvae ranged from 3 to 100% (median 71%). Both larval infection prevalence and spirochete load were highly correlated with maternal spirochete load. Spirochetes were disseminated throughout the tissues of all three stages of unfed ticks, including the salivary glands and female ovarian tissue. The results indicate that while multiple transmission routes contribute to enzootic maintenance of B. miyamotoi, transovarial transmission is likely to be the primary source of infected ticks and therefore risk assessment and tick control strategies should target adult female ticks.
Keywords: Borrelia miyamotoi, Ixodes scapularis, Transovarial transmission, In situ hybridization, Peromyscus leucopus
1. Introduction
Borrelia miyamotoi is a relapsing fever group spirochete carried by Ixodes ticks in Holarctic regions (Fukunaga et al., 1995; Scoles et al., 2001; Richter et al., 2003; Krause et al., 2015). Human cases of B. miyamotoi disease (BMD) have been reported from Asia, Europe, and North America (Platonov et al., 2011; Hovius et al., 2013; Krause et al., 2013), though the extent of morbidity is unclear (Krause et al., 2014; Molloy et al., 2015; Sinski et al., 2016; Jobe et al., 2016; Krause et al., 2018). In the United States, Ixodes scapularis (the blacklegged tick) and Ixodes pacificus (the western blacklegged tick) are naturally infected vectors of B. miyamotoi (Scoles et al., 2001; Tsao et al., 2004; Mun et al., 2006; Kingry et al., 2017a; Johnson et al., 2018).
Strategies aimed at preventing human exposure to zoonotic disease agents such as B. miyamotoi benefit from an understanding of fundamental biological relationships at the pathogen-vector-host interface, which can identify crucial points for disruption of enzootic transmission cycles. Similar to the Lyme disease spirochete Borrelia burgdorferi sensu stricto (s.s.), the most common tick-borne disease agent in North America, B. miyamotoi can be transmitted horizontally, from infected ticks to vertebrate hosts and then back from infectious hosts to new ticks (Scoles et al., 2001; van Duijvendjik et al., 2016). Unlike B. burgdorferi s. s., B. miyamotoi is also transmitted transovarially (vertically) from infected female Ixodes ticks, including I. scapularis and the closely related Ixodes ricinus (the castor bean tick) in Europe (Scoles et al., 2001; Richter et al., 2012; Rollend et al., 2013) to their offspring. This mode of transmission may present a more effective means of perpetuating the spirochetes in nature compared with horizontal transmission (Lynn et al., 2018). Despite having the apparent advantage of multiple transmission routes, in natural settings the prevalence of B. miyamotoi is often 10-fold (or more) lower in nymphal and adult I. scapularis ticks compared with B. burgdorferi s.s. (Tsao et al., 2004; Barbour et al., 2009; Krause et al., 2015; Nelder et al., 2016; Johnson et al., 2018). Borrelia miyamotoi has been detected in wild rodents, which are among the many vertebrate hosts for immature stages of I. scapularis (Bunikis and Barbour, 2005; Barbour et al., 2009; Salkeld et al., 2018). However, the transmission dynamics of this organism have not been investigated in depth since it was recently recognized as a human pathogen, and reservoir competence has only been confirmed for the white-footed mouse (Peromyscus. leucopus) (Scoles et al., 2001). Accordingly, the question remains as to how effectively B. miyamotoi is maintained via horizontal transmission relative to transovarial transmission.
Only a few previous studies have investigated the efficiency of transmission of B. miyamotoi from infected ticks to naïve hosts and/or from infectious hosts to feeding ticks (Scoles et al., 2001; van Duijvendjik et al., 2016; Breuner et al., 2017; Breuner et al., 2018). Similarly, there exist only a few reports where PCR was used to determine B. miyamotoi infection rates in transovarially-infected larval clutches, comprising a total of 15 I. scapularis clutches (Scoles et al., 2001; Breuner et al., 2018; Han et al., 2019) and a single I. ricinus clutch (Richter et al., 2012). To compare transovarial and horizontal transmission routes, we used a combination of I. scapularis ticks infected experimentally by feeding on infected mice in the laboratory and I. scapularis with transovarially acquired infections, descended from naturally infected field-collected females. Horizontal transmission was initiated using infected larvae as well as infected nymphs to expose rodents, which included both immunocompetent laboratory mice (Mus musculus), and P. leucopus to simulate a natural route of enzootic transmission. P. leucopus were included as hosts in this study because this species (i) is ubiquitous in the eastern and north central United States (Kays and Wilson, 2009), where (ii) it is an important host for I. scapularis immatures (Spielman et al., 1984; Piesman and Schwan 2010), (iii) has been found naturally infected with B. miyamotoi (Bunikis and Barbour, 2005; Barbour et al., 2009), and (iv) is considered a natural reservoir for other horizontally maintained I. scapularis-borne human pathogens, including B. burgdorferi s.s., Anaplasma phagocytophilum, Ehrlichia muris eauclairensis, and Babesia microti (Donahue et al., 1987; Spielman et al., 1994; Scoles et al., 2001; Tsao et al., 2004; Barbour et al., 2009; Castillo et al., 2015; Lynn et al., 2017; Eisen et al., 2017).
Our primary objectives were to provide an expansive experimental assessment of the efficiencies of horizontal and transovarial transmission of B. miyamotoi, and to comprehensively illustrate the distribution of spirochetes among different tissue types in larval, nymphal and female I. scapularis ticks.
2. Materials and methods
2.1. Regulatory compliance
All animal procedures were approved by the Centers for Disease Control and Prevention, Division of Vector-Borne Diseases Animal Care and Use Committee, in compliance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (National Research Council Committee for the Update of the Guide for the and Use of Laboratory 2011).
2.2. Horizontal B. miyamotoi transmission with laboratory mouse hosts challenged by horizontally infected ticks
The infection chain was started with I. scapularis nymphs from the Oklahoma State University Tick Rearing Facility (Stillwater, OK, USA) that acquired B. miyamotoi (CT13–2396) infection as larvae by feeding on infectious immunocompromised SCID mice (M. musculus) (Charles River Laboratories, Wilmington, MA, USA), as previously described by Lynn et al. (2019). The infection prevalence in the challenge nymphs (ticks fed with the intention of exposing naïve hosts to infection) was expected to be 20–25%, based on previous testing of subsets of more than 200 nymphs of the same cohort exposed to infectious mice (Lynn et al., 2019).
Nymphal ticks that were exposed as larvae to infectious mice were placed freely on three groups of five CD-1 mice (Charles River Laboratories), where Groups 1 and 2 (G1 and G2) received 15 nymphs per mouse and Group 3 (G3) received 25 nymphs per mouse (Fig. 1A). The first two groups of mice were limited to 15 nymphs based on the allowed volume of blood that could be extracted from hosts while also allowing for blood loss during subsequent but overlapping larval feeding. Engorged nymphs were collected after detaching and the number recovered was recorded for each mouse. Each of the mice from the three groups was then exposed to 50 freely placed uninfected larval (xenodiagnostic) ticks sourced from the OSU Tick Rearing Facility. The larvae were placed on the mice at different time points after the potentially infectious nymphs were introduced: 2 d later for G1, 4 d later for G2 and 10 d later for G3. While it is unknown how long after exposure to B. miyamotoi mice are most infectious for ticks, these time points were chosen on the criteria that transmission of B. miyamotoi from ticks to mice can occur as soon as 24 h after tick attachment, and spirochetemia is detectable in needle-inoculated immunocompetent mice for up to two weeks (Breuner et al., 2017; Lynn et al., 2019). In this study, challenge nymphs and xenodiagnostic larvae temporally overlapped in their feeding periods for up to 3 d for G1 mice and for up to 1 d for G2 mice, whereas the nymphal and larval feeding periods were temporally separate for G3 mice. Larval ticks were allowed to feed to repletion, and then were collected after detaching from mice and housed within glass desiccators at 95% relative humidity, in a growth chamber maintained at 21 to 23 °C with a 16:8 h light:dark cycle. PCR-based detection and quantification of B. miyamotoi spirochetes in molted nymphs resulting from the xenodiagnostic larvae followed methodology described below. Since exposure to at least 15 ticks infected at 20–25% infection prevalence was expected to result in the exposure of all mice to spirochetes, engorged nymphs were collected and collectively housed for molting and subsequent use as breeding adults, with the result that infection status for the nymphs was not determined.
Fig. 1.
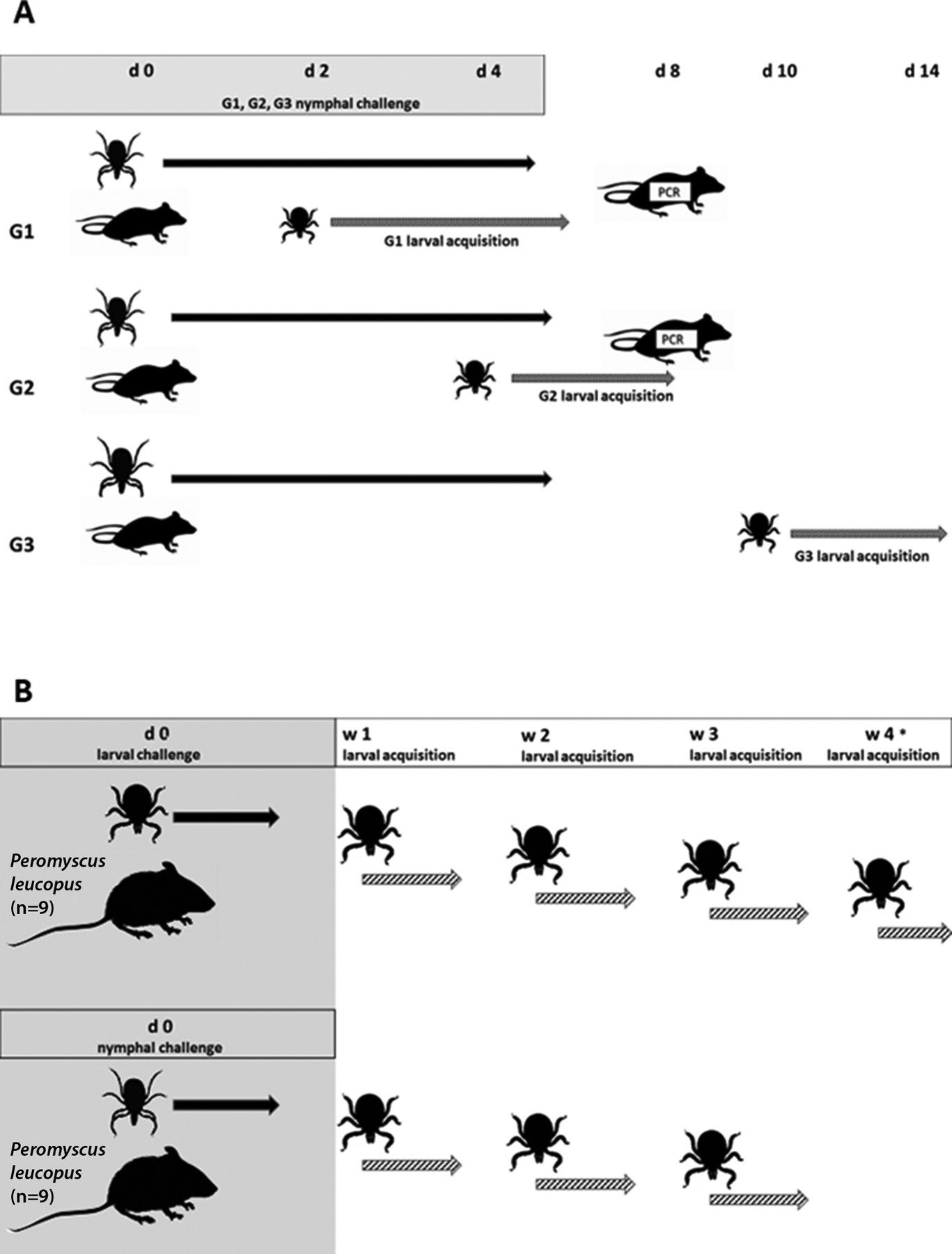
Transmission experiment diagrams. A. Horizontal B. miyamotoi transmission with laboratory mouse (CD-1) hosts challenged by horizontally infected ticks. Three groups of 5 CD-1 mice (G1, G2, G3) were challenged with Ixodes scapularis nymphs from a cohort infected with B. miyamotoi (infection prevalence 25%). G1 and G2 were infested with 15 nymphs and G3 was infested with 25 nymphs. Mice were infested with uninfected larvae at 2 days (G1), 4 days (G2) and 10 days (G3) post-challenge (day 0). Larval feeding overlapped with that of nymphs on G1 and G2 mice. Replete larvae were collected, allowed to molt and qPCR tested for B. miyamotoi as nymphs. Blood and tissue from G1 and G2 were collected 8 days post challenge for qPCR. B. Horizontal B. miyamotoi transmission with natural mouse hosts challenged by transovarially-infected ticks. Peromyscus leucopus were challenged with I. scapularis larvae or nymphs from cohorts with transovarially acquired B. miyamotoi. Mice were infested with uninfected larvae at one week (7 day) intervals following challenge. Replete ticks were collected and allowed to molt, and infection status was determined via qPCR. * No ticks used for challenge were recovered from two mice. These mice were subjected to a second challenge so that larval challenges were delayed by one week.
G1 and G2 mice were euthanized 8 d after initiation of nymphal challenge and blood and tissue samples (heart and spleen) were collected. The methods for PCR-based detection and quantification of B. miyamotoi spirochetes from mouse blood and tissue samples are described in Section 2.5. Because xenodiagnostic infections were confirmed for all mice in G3, tissues were not PCR-tested for this group.
2.3. Horizontal B. miyamotoi transmission with natural mouse hosts challenged by transovarially-infected ticks
We exposed P. leucopus mice (8–10 wk old females from the Peromyscus Genetic Stock Center at the University of South Carolina; Columbia, SC, USA) to B. miyamotoi through tick feeding using transovarially-infected I. scapularis (Fig. 1B). Ticks used for challenge were either second generation nymphs (88% infection prevalence) originating from a female collected in Minnesota (Breuner et al., 2018), or first generation transovarially-infected I. scapularis larvae (100% infection prevalence) derived from a female infected with CT13–2396 as a larva on a SCID mouse (Lynn et al., 2019). Eight mice were challenged with 2 ticks each (4 mice with 2 larvae each and 4 mice with 2 nymphs each); to ensure recovery of fed ticks, these few ticks were placed within feeding capsules attached to the shaved backs of the mice (Mbow et al., 1994; Breuner et al., 2017). An additional 10 mice received larger numbers of freely placed ticks: 5 mice were challenged with 6 larvae each and 5 mice with 6–7 nymphs each. A single CD-1 mouse was challenged with 25 nymphs as a positive process control from which 15 engorged nymphs were recovered (data not shown). Challenge ticks were collected after detaching from mice and were housed during molting as described in Section 2.2. Each mouse was then exposed three times (weekly) over a 3-wk period to 100 freely placed uninfected xenodiagnostic larvae (Medical Entomology Laboratory at the Centers for Disease Control and Prevention; Atlanta, GA, USA). Specifically, larvae were introduced at 7, 14 and 21 days after challenge ticks were placed on the mice. Larvae completed feeding in 5 or fewer days so that no temporal overlap occurred between cohorts of xenodiagnostic larvae. Engorged larvae were collected and housed after detaching, and then tested for B. miyamotoi infection within 6 wk after molting into nymphs.
2.4. Transovarial transmission
Mated female I. scapularis exposed to B. miyamotoi as either larvae or nymphs feeding on SCID mice (Lynn et al., 2019) were fed on New Zealand white rabbits (Charles River Laboratories). Replete females were housed in vented polystyrene vials incubated at 21–23 °C (Section 2.2) until oviposition was completed. qPCR-based detection and quantification of B. miyamotoi spirochetes in spent females is described in Section 2.5. Larvae from infected females were tested individually, and 15 to 25 larvae from 34 negative females were tested either individually or in pools of up to 25.
2.5. DNA extraction/qPCR detection of B. miyamotoi
DNA was extracted from mouse blood and unfed ticks as previously described in Lynn et al. (2019). For mouse tissues, between 10 and 20 mg of tissue was removed using a sterile scalpel and placed in a 2 mL microcentrifuge tube with 950 μL lysis buffer (810 μL ATL buffer, 90 μL Proteinase K, 50 μL DX reagent, Qiagen, Valencia, CA, USA). DNA was extracted from 50 μl blood per mouse. Spent female ticks were placed in 2 mL tubes with beads (2.0 mm Very High Density Yttria stabilized zirconium oxide beads, GlenMills, Clifton, NJ, USA) and 1000 μL lysis buffer. A Mini-Beadbeater-96 (BioSpec Products, Bartlesville, OK, USA) was used for evisceration, followed by incubation for 10 min at 56 °C to lyse cells. Tissue triturate from each mouse or spent females was split into three equal parts. For several spent females, failure of tick actin to amplify indicated that qPCR was inhibited by excessive extra-nucleic contaminants such as hemoglobin carried through the extraction process, requiring a second DNA purification using Qiagen DNA Easy extraction kit and columns. Cleaned DNA was re-eluted in the same volume as it had existed prior to the second purification and successfully amplified, with resulting quantitation cycle (Cq) values within the normal range observed for specimens that amplified on the first attempt. qPCR on tick samples was performed using a duplex assay targeting the B. miyamotoi adenylosuccinate lyase (purB) gene (forward primer - TCC TCA ATG AAA GCT TTA, reverse primer - GGA TCA ACT GTC TCT TTA ATA AAG, probe – CalRd610-TCG ACT TGC AAT GAT GCA AAA CCT-BHQ2) (Graham et al., 2016) and the I. scapularis actin gene (forward primer - GCC CTG GAC TCC GAG CAG, reverse primer - CCG TCG GGA AGC TCG TAG G, probe – Quas705-CCA CCG CCT CTT CC-BHQ3) (Hojgaard et al., 2014). To estimate whole tick spirochete load, a standard curve using a recombinant plasmid containing the purB sequence was used to convert quantitation cycle (Cq values) into gene copy numbers, as described in Lynn et al. (2019). This result was divided by the fraction of eluted DNA included in the qPCR reaction. For mouse blood and tissues, the actin primers and probe were exchanged with primer/probes targeting rodent GAPDH (Applied Biosystems® Taq-Man® Rodent GAPDH ControlReagents kit; ThermoFisher Scientific, Houston, TX, USA). Reactions were performed as described in Lynn et al. (2018) and real-time cycling conditions followed a previously described protocol (Graham et al., 2016). qPCR samples were analyzed using CFX Manager 3.1 software (Bio-Rad Laboratories, Hercules, CA, USA) with Cq determination set to regression.
2.6. In situ hybridization/imaging
To demonstrate the efficacy of our probe, CT13–2396 was cultured as previously described (Lynn et al., 2019; Replogle et al., 2021), centrifuged at low speed for 5 min and re-suspended in a small volume of PBS. The spirochete suspension was mixed into liquid 2% agarose gel, allowed to solidify, and fixed for 24 h in 10% neutral buffered formalin solution (ThermoFisher Scientific). Ticks used for in situ hybridization were unfed and included larvae, nymphs, females and males that had acquired infection transovarially. Ticks were surface rinsed in Milli-Q water (Millipore Co. Billerica, MA, USA), dried on Whatman filter paper and fixed in formalin for 96 h. Following fixation, ticks were positioned in 2% agarose gel. Blocks of gel were paraffin embedded and sectioned at 5 μm thickness onto Leica bond plus slides (Leica Biosystems Inc., Buffalo Grove, IL, USA) at the Colorado State University Veterinary Diagnostic Laboratory (Fort Collins, CO, USA).
Slides were baked at 60 °C for 60 min prior to paraffin removal using HistoClear (National Diagnostics, Atlanta, GA, USA). In situ hybridization (ISH) was performed using the ViewRNA ISH Tissue 1-Plex Assay for RNA (Invitrogen, ThermoFisher) according to manufacturer’s instructions. Gill’s Hemotoxylin (Electron Microscopy Sciences, Hatfield, PA, USA) was used to stain cell nuclei, and Diamond Prolong with DAPI (Thermofisher Scientific) was used to mount coverslips. A custom ViewRNA DNA probe set (Cat # VF1–6,000,540) specific for CT13–2396 (GenBank accession #CP017126, Kingry et al., 2017b) was hybridized to spirochete RNA. The probe contained a set of 20 oligonucleotide pairs targeting the 16S rRNA. Sequence was specifically selected to prevent hybridization with the I. scapularis endosymbiont Rickettsia buchneri (accession # JFKF01000080; locus tag REISMN_04040) (Kurtti et al., 2015). Probes were hybridized with a label probe that used Fast Red (2-amino-5-chlorotoluenehydrochloride tablets dissolved in napthol) to provide red labeling. The ISH assay was previously used successfully on whole sectioned ticks with an Ehrlichia-specific probe and validated with transmission electron microscopy (Lynn et al., 2015). An Olympus BX53X microscope with a DP73 camera was used for imaging histological specimens and Adobe Photoshop Elements software was used to edit images. References used for anatomical identification of tick tissues included Sonenshine (1991), Balashov (1972), and Roshdy (1969).
3. Results
3.1. Horizontal B. miyamotoi transmission with laboratory mouse (CD-1) hosts challenged by horizontally infected ticks
For the five G1 mice, where xenodiagnostic larvae were placed on mice two d after the start of challenge with potentially infected nymphs, there was no evidence of B. miyamotoi acquisition by any of the 64 molted xenodiagnostic nymphs (Table 1). Infection was detected in heart and spleen tissue from one of the mice, but none of 11 unfed nymphs from larval xenodiagnosis of this mouse were PCR-positive. The exposure status for the other four mice, from which a total of 18 engorged nymphs were recovered, could not be determined definitively because of both the transient nature of B. miyamotoi in immunocompetent hosts (Lynn et al., 2019), and because the replete ticks used for challenge were reserved for use as adults in reproduction rather than PCR tested.
Table 1.
Molecular and xenodiagnostic evaluation of horizontal transmission of Borrelia miyamotoi by Ixodes scapularis nymphs to three groups of five naïve CD-1 mice. Mouse blood and tissues were tested by PCR for evidence of infection, and larvae used for xenodiagnosis were PCR tested for infection after molting to nymphs. The time periods from the start of the nymphal challenge to placement of xenodiagnostic larvae on mice were 2 d for G1, 4 d for G2, and 10 d for G3. Infection status is shown for G1 and G2 mice at postmortem, 8 d following the start of infestation with 15 nymphs per mouse from a cohort with 20–25% infection prevalence.
| Mouse | No. engorged challenge nymphs recovered* | Infection of mouse blood and tissues | infected xenodiagnostic nymphs (no. infected/tested) | % infected xenodiagnostic ticks | ||
|---|---|---|---|---|---|---|
| Blood | Heart | Spleen | ||||
| 11 | 3 | − | + | + | 0/11 | 0% |
| 12 | 2 | − | − | − | 0/16 | 0% |
| 13 | 7 | − | − | − | 0/11 | 0% |
| 14 | 6 | − | − | − | 0/10 | 0% |
| 15 | 3 | − | − | − | 0/16 | 0% |
| G1 total | 21 | 0/64 | 0% | |||
| 21 | 4 | + | − | − | 0/15 | 0% |
| 22 | 4 | − | + | + | 0/31 | 0% |
| 23 | 9 | − | + | + | 2/33 | 6.1% |
| 24 | 5 | − | − | − | 1/19 | 5.3% |
| 25 | 7 | − | − | − | 0/18 | 0% |
| G2 total | 29 | 3/116 | 2.6% | |||
| 31 | 11 | 2/14 | 14.3% | |||
| 32 | 19 | 3/25 | 12.0% | |||
| 33 | 14 | 3/18 | 16.7% | |||
| 34 | 15 | 1/12 | 8.3% | |||
| 35 | 17 | 1/16 | 6.3% | |||
| G3 total | 76 | 10/85 | 11.8% | |||
due to host grooming, not all ticks could be recovered and some mice may not have been exposed to infected ticks.
Of the five G2 mice, where xenodiagnostic larvae were placed on mice four d after the potentially infected nymphs were introduced, four had evidence of infection with B. miyamotoi either via mouse blood or tissue samples or xenodiagnostic ticks. Of three mice with evidence of infection in mouse blood or tissue samples, two failed to produce infected molted xenodiagnostic nymphs, whereas the infection prevalence in xenodiagnostic nymphs from the third mouse was 6.1% (Table 1). A fourth mouse without evidence of infection in mouse blood or tissue samples produced one infected molted xenodiagnostic nymph (5.3% infection prevalence). In total, three of 98 (3.1%) G2 molted nymphs from the four mice with confirmed exposure acquired
B. miyamotoi.
Xenodiagnostic larvae were placed on G3 mice ten d after the potentially infected nymphs were introduced, and all five mice yielded at least one infected molted xenodiagnostic nymph. The infection prevalence in xenodiagnostic nymphs (fed as larvae) ranged from 6.3 to 16.7% across individual mice, with an overall infection prevalence of 11.8%, which was significantly higher than for ticks from G2 mice with confirmed infections (χ2 = 5.4; d.f. = 1; P = 0.022).
3.2. Horizontal B. miyamotoi transmission with natural mouse hosts (P. leucopus) challenged by transovarially-infected ticks
Because none of the larvae used to challenge two of the mice (2 and 9, see Table 2) were recovered at the conclusion of the first challenge, these mice were re-exposed to 10 additional larvae originating from the same cohort used for challenge, which delayed the first xenodiagnostic infestations by one week. Two PCR-positive ticks were recovered from the second challenge of mouse 2, however no xenodiagnostic nymphs from this mouse were positive. Conversely, larvae used for challenge were not recovered from mouse 9 after the second challenge, yet a single nymph from the 3rd week xenodiagnostic feeding tested positive. No differences in success of transmission of B. miyamotoi were apparent for ticks fed within capsules versus freely on the mice, and therefore xenodiagnostic results for mice were grouped according to the tick life stage used for challenge (Table 2). Replete challenge ticks that tested positive for B. miyamotoi were collected from 15 of 18 P leucopus mice. This included 8 mice exposed to infected larvae (range: 1–6 larvae per mouse) and seven mice exposed to infected nymphs (range: 1–4 nymphs per mouse). A xenodiagnostic nymph was also produced from a mouse lacking confirmed exposure due to a failure to recover larvae used from this individual. Infection prevalence for all xenodiagnostic nymphs resulting from mice challenged with PCR-confirmed infected ticks (larvae or nymphs) was 1.0% (8/762). A total of eight nymphs out of 213 (3.8%) acquired infection via larval feed from the 5 mice with xenodiagnostically confirmed infections. These included 5/175 (2.9%) nymphs from four of the nine mice challenged with larvae. Only one of seven mice fed on by infected nymphs supported horizontal transmission to naïve ticks, yet this mouse yielded at least one xenodiagnostic tick in each of the three weeks after challenge, totaling 3/38 (7.9%). Eight of 114 (7.0%) ticks acquired infection when at least one positive xenodiagnostic tick was recovered from the same mouse during that specific week. Because none of the infected larvae used to challenge mouse 38 were recovered after the initial challenge, a second challenge was performed with ten larvae, none of which were recovered. Nevertheless, successful challenge was confirmed via positive xenodiagnosis, although because host exposure could have occurred during either of the two infestations, larval acquisition of spirochetes may have occurred at either three weeks or four weeks after challenge.
Table 2.
Evaluation of horizontal transmission of Borrelia miyamotoi by Ixodes scapularis larvae or nymphs to naïve Peromyscus leucopus mice, by means of xenodiagnostic larvae PCR-tested for infection after molting to nymphs.
| Mouse | Tick challenge | PCR positive challenge ticks recovered | Infected xenodiagnostic ticks by week of feeding after challenge (no. infected/tested) | ||||
|---|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | Weeks 1–4 combined | |||
| 1 | larvae | 1 | 0/22 | 1/21 | 0/14 | 1/57 | |
| 2 | larvae | 2 | 0/16 | 0/13 | 0/30 | 0/59 | |
| 3 | larvae | 6 | 0/17 | 0/7 | 0/21 | 0/45 | |
| 4 | larvae | 2 | 1/14 | 0/4 | 0/11 | 1/29 | |
| 5 | larvae | 1 | 0/9 | 0/4 | 0/13 | 0/26 | |
| 6 | larvae | 1 | 2/25 | 0/5 | 0/17 | 2/47 | |
| 7 | larvae | 2 | 0/27 | 0/14 | 0/9 | 0/50 | |
| 8 | larvae | 2 | 0/25 | 0/8 | 0/12 | 0/45 | |
| 9 | larvae | 0/20 | 0/6 | 1/16 | 1/42 | ||
| Total from positive (xenodiagnostic) mice (n = 4) | 5/175 (2.9%) | ||||||
| Total from mice exposed to infected larvae (n = 9) | 3/139 (2.2%) | 1/99 (1.0%) | 0/116 (0%) | 1/46 (2.2%) | 5/400 (1.3%) | ||
| 11 | nymphs | 1 | 0/15 | 0/6 | 0/9 | 0/30 | |
| 13 | nymphs | 2 | 0/28 | 0/18 | 0/4 | 0/50 | |
| 14 | nymphs | 3 | 0/16 | 0/8 | 0/21 | 0/45 | |
| 15 | nymphs | 2 | 0/22 | 0/16 | 0/25 | 0/63 | |
| 16 | nymphs | 4 | 0/9 | 0/24 | 0/9 | 0/42 | |
| 17 | nymphs | 1 | 0/26 | 0/18 | 0/50 | 0/94 | |
| 18 | nymphs | 4 | 1/9 | 1/6 | 1/23 | 3/38 | |
| Total from mice exposed to infected nymphs (n = 7) | 1/125 (0.8%) | 1/96 (1%) | 1/141 (0.7%) | 3/362 (0.8%) | |||
| 12 | nymphs | 0 | 0/9 | 0/5 | 0/5 | 0/19 | |
| 19 | nymphs | 0 | 0/33 | 0/9 | 0/5 | 0/47 | |
| Total from mice exposed to negative nymphs (n = 2) | |||||||
3.3. Transovarial transmission
All pools and individual larval offspring from 34 females PCR-negative for B. miyamotoi also tested negative (data not shown). Of the 10 females that tested positive for B. miyamotoi and successfully ovi-posited, seven females produced infected larvae (Table 3). These females included both those infected as larvae (IsF13, IsF6, and IsFB), and as nymphs (IsF2, IsF3, IsF9, IsF27, and IsF29). Filial infection rate in larval batches ranged from 3.3 to 100%, and the median rate was 71%. Low filial infection rates (3–4%) and larval spirochete loads were associated with low maternal purB copies (<7 × 102) in the females (r2 = 0.86, 0.74), indicating a strong relationship between maternal load and transovarial transmission (Fig. 2A and B). Maternal spirochete load was estimated to be less than 3 × 103 copies in 5 positive females, each of which produced a clutch with an infection prevalence estimated between 0 and 4%. Three of these females did not fully engorge, and produced relatively small clutches (20–100 larvae), which suggests that inadequate feeding may have limited spirochete growth and potentially, transovarial transmission.
Table 3.
Transovarial transmission success and spirochete load for Ixodes scapularis females experimentally infected with Borrelia miyamotoi and their offspring. Spirochete load is represented by purB copies.
| Female | Female purB copies | No. larvae tested | % larval infection prevalence | 95% C.I. for % larval infection prevalence | Mean larval purB copy number | |
|---|---|---|---|---|---|---|
| IsF2 3 | 2.09 × 108 | 50 | 96.0 | 86.5 – 98.9 | 11,650 | |
| IsF2 9 | 1.88 × 109 | 100 | 71.0 | 61.5 – 79.0 | 15,891 | |
| IsF2 27 | 1.34 × 108 | 100 | 81.0 | 72.2 – 87.5 | 30,459 | |
| IsF2 29 | 2.09 × 108 | 100 | 65.0 | 55.3 – 73.6 | 21,132 | |
| IsF1 13 | 1.91 × 107 | 15 | 100.0 | 79.6 – 100 | 24,816 | |
| IsF1 14 | 1.79 × 102 | 24 | 0 | 0 – 13.8 | 0 | |
| IsF1 15 | 2.52 × 103 | 25 | 0 | 0 – 13.3 | 0 | |
| IsF1 6 | 6.26 × 102 | 24 | 4.2 | 1.0 – 20.2 | 35 | a |
| IsF1 A | 1.21 × 103 | 22 | 0 | 0 – 14.9 | 0 | b |
| IsF1 B | 6.61 × 102 | 30 | 3.3 | 1.0 – 16.7 | 121 | b |
reduced engorgement, many unhatched eggs.
minimal engorgement, less than 50 larvae.
Fig. 2.
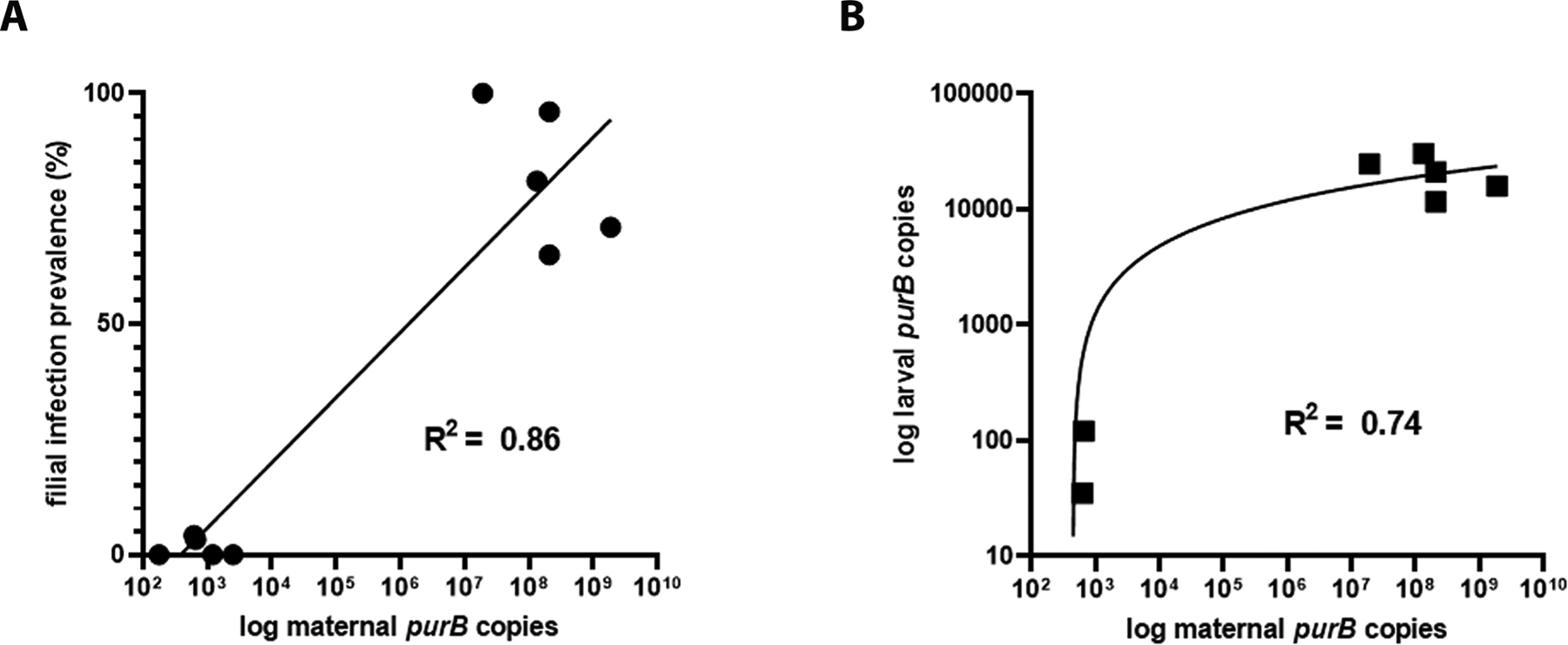
Vertical transmission of Borrelia miyamotoi in Ixodes scapularis. (A) maternal purB copy representing spirochete load correlated with likelihood of transmission to larvae (filial infection prevalence). (B) maternal spirochete load correlated with spirochete load in offspring.
3.4. Tick histology
Non-specific binding was not observed in the whole sectioned uninfected ticks (Fig. 3A) that were assayed, while cultured CT13–2396 B miyamotoi spirochetes suspended in agarose and labeled with the 16S rDNA probe were readily identified by microscopy (Fig. 3B). Spirochete RNA was apparent and well-disseminated in sectioned whole larval, nymphal, and adult female and male ticks (Figs. 4–8). Infection was apparent in a variety of tissues including the acini and ducts of salivary glands (Fig. 4A and B), basal lamina of midgut diverticulae (Fig. 5A), epithelium of Malpighian tubules (Fig. 5B), female ovarian tissue (Figs. 5C, D and 6B, 7, 8), synganglion (central nervous system), especially the neuropile (Figs. 3A and B, 6A), various epithelial tissues (Figs. 7 and 8) and male testes (not shown). Furthermore, spirochetes could also be distinguished near internal mouthparts including the chelicerae (Fig. 4A).
Fig. 3.
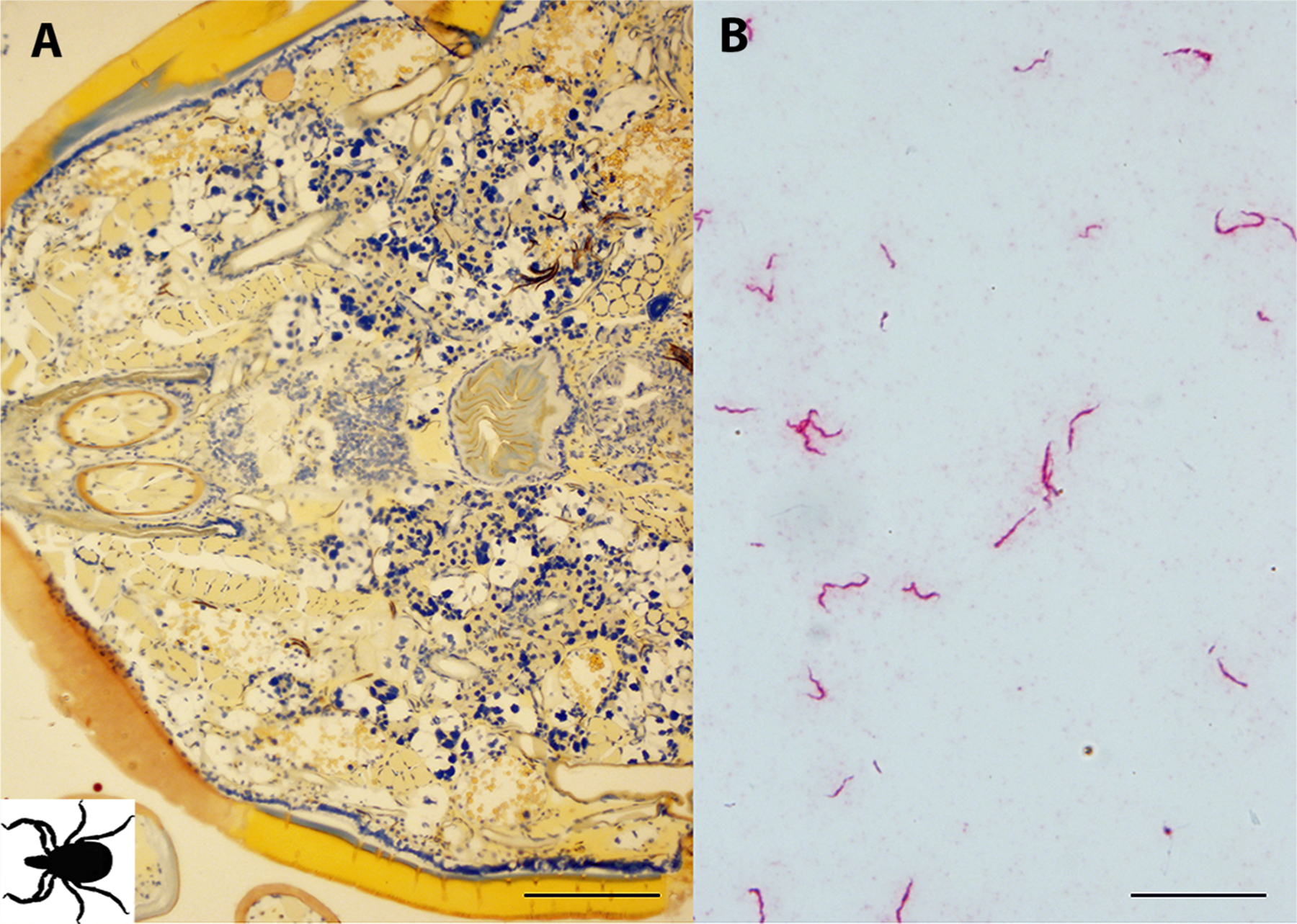
Controls for in situ hybridization (ISH) assay. (A) Uninfected adult Ixodes scapularis female sectioned ventrally and subjected to ISH with Borrelia miyamotoi-specific DNA probe demonstrating a lack of non-specific hybridization. The black figure in the lower left corner indicates directional orientation of sectioned tick. (B) cultured spirochetes suspended in agarose demonstrating successful labeling using a DNA probe targeting 16S rRNA sequence. 16S rDNA probe hybridization to spirochetes is indicated by red staining, blue indicates nuclear staining. Scale bars A = 200 μm, B = 20 μm.
Fig. 4.
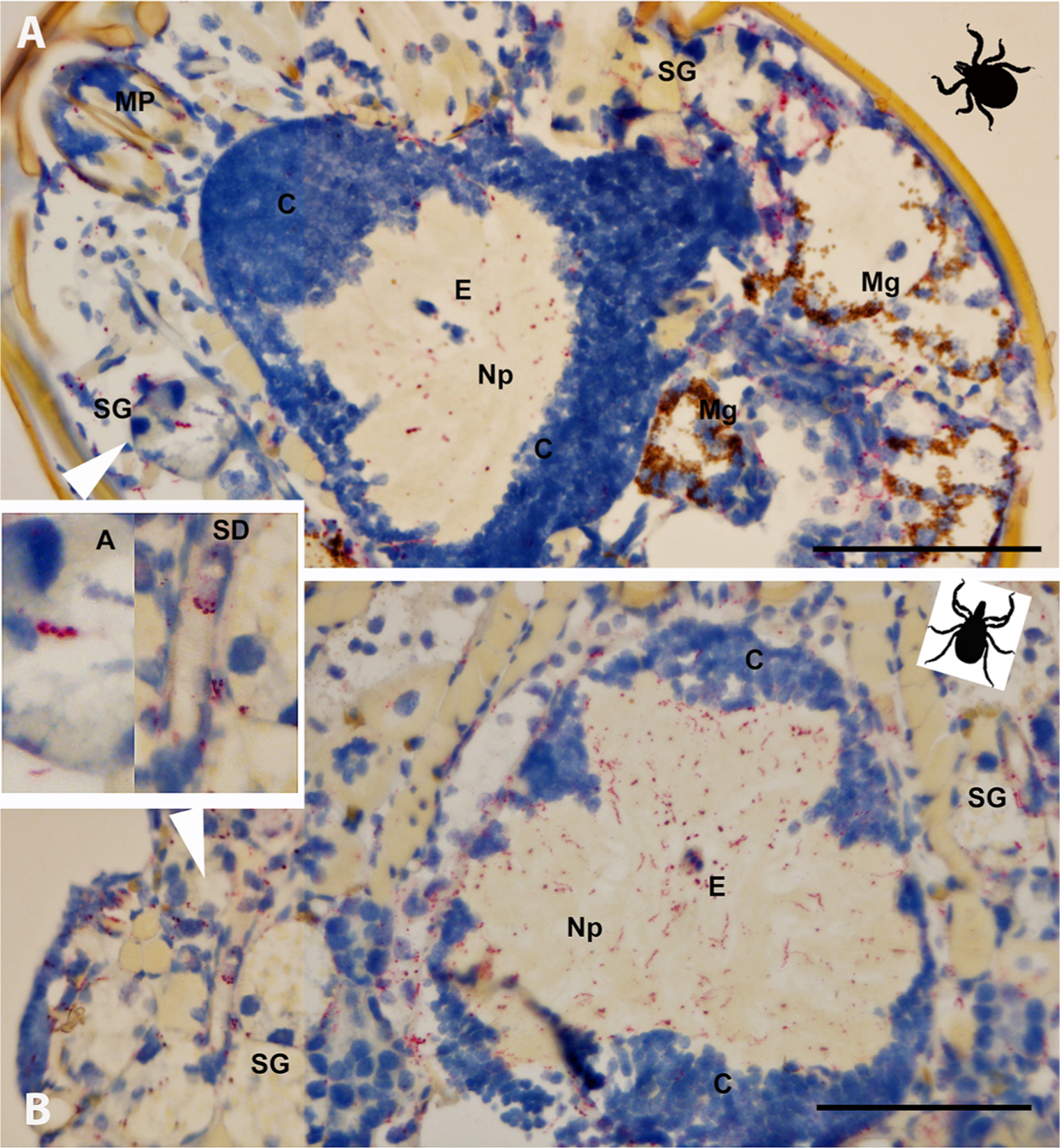
Borrelia miyamotoi-infected Ixodes scapularis ticks sectioned ventrally (coronal plane) and subjected to in situ hybridization assay. (A) Unfed larva with systemic infection, including infection of salivary glands (acini [A] shown in left inset). (B) Unfed nymph with systemic infection including salivary ducts (SD) shown in the right inset. 16S rDNA probe hybridization to spirochetes is indicated by red staining, blue indicates nuclear staining. See key for anatomical feature abbreviations. The black figures in the upper right corners indicate directional orientation of sectioned ticks. Scale bars = 100 μm.
Fig. 8.
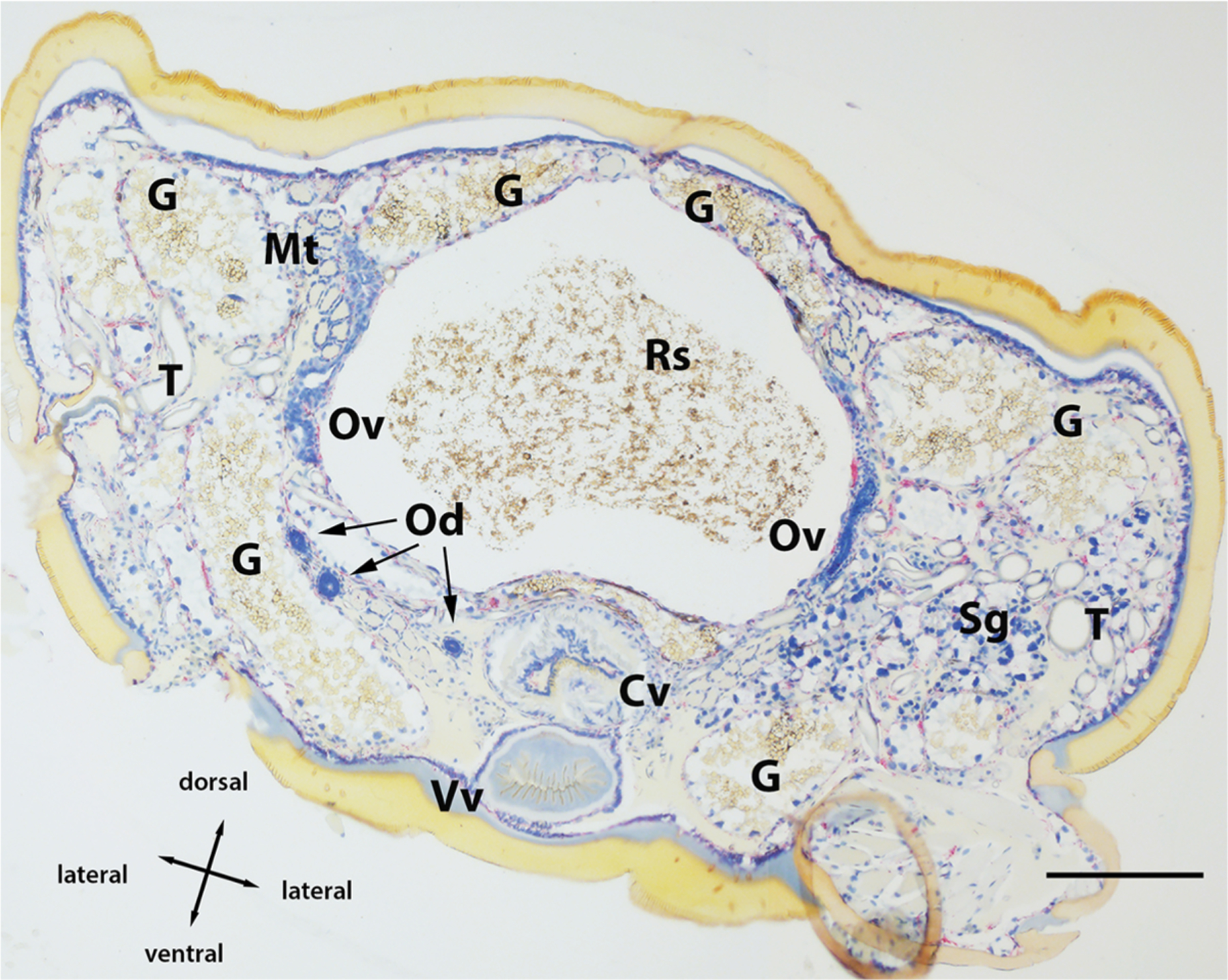
Montage of Borrelia miyamotoi-infected female Ixodes scapularis transversally sectioned and subjected to in situ hybridization assay. 16S rDNA probe hybridization to spirochetes is indicated by red staining, blue indicates nuclear staining. See key for anatomical feature abbreviations. Scale bar = 200 μm.
Fig. 5.
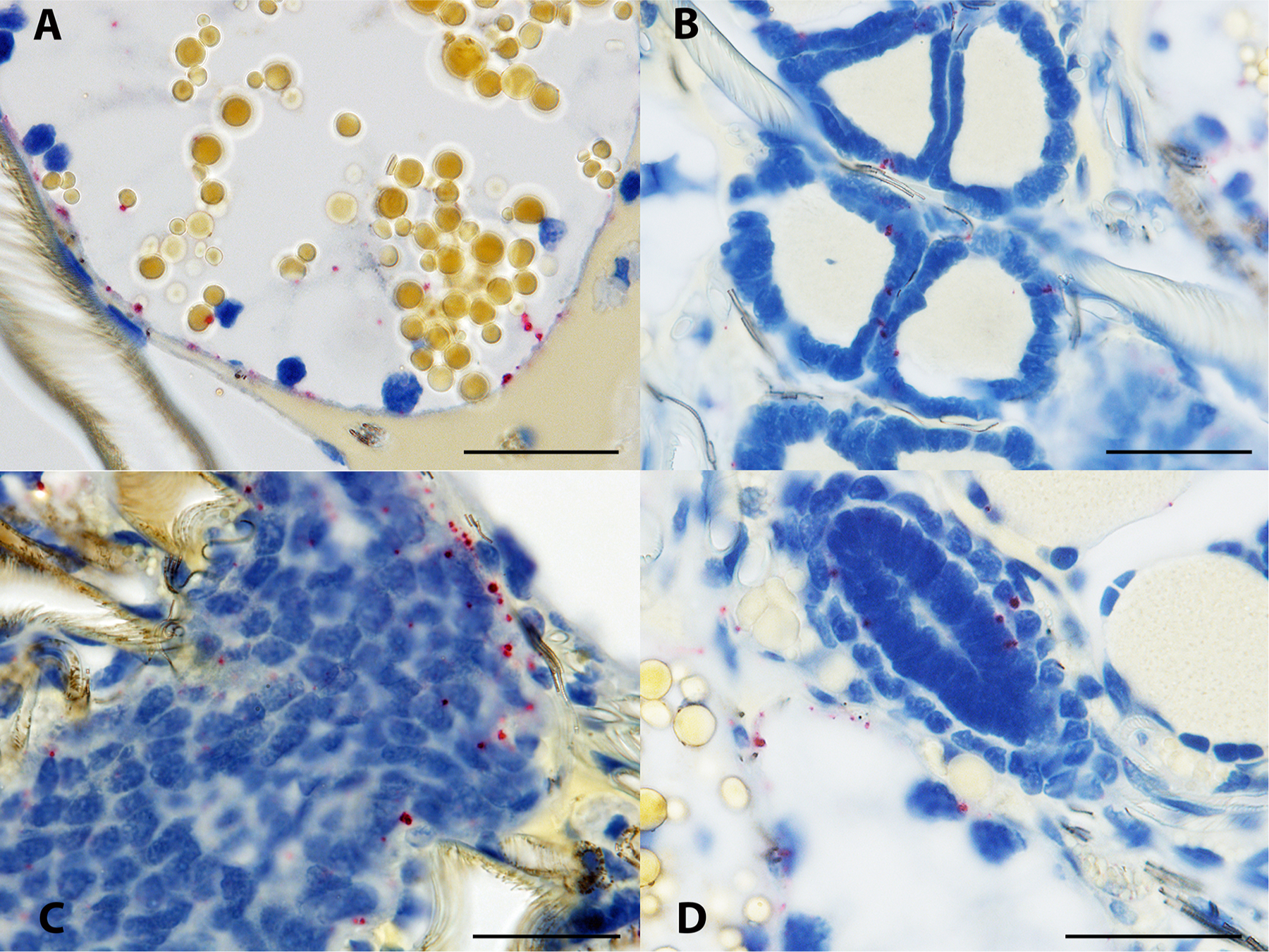
Borrelia miyamotoi-infected female Ixodes scapularis subjected to in situ hybridization assay. (A) spirochetes are apparent near the basal lamina of a distal diverticulum of the midgut, recognizable by the many straw-colored inclusion bodies evident within the digestive cells. (B) spirochetes are present within the densely packed epithelial cells of a Malpighian tubule. (C) spirochetes are present in the ovarian tissue of an unfed female. (D) spirochetes are apparent within and in the vicinity of an oviduct, shown as the ovaloid cluster of nuclei center image. 16S rDNA probe hybridization to spirochetes is indicated by red staining, blue indicates nuclear staining. Scale bars = 30 μm.
Fig. 6.
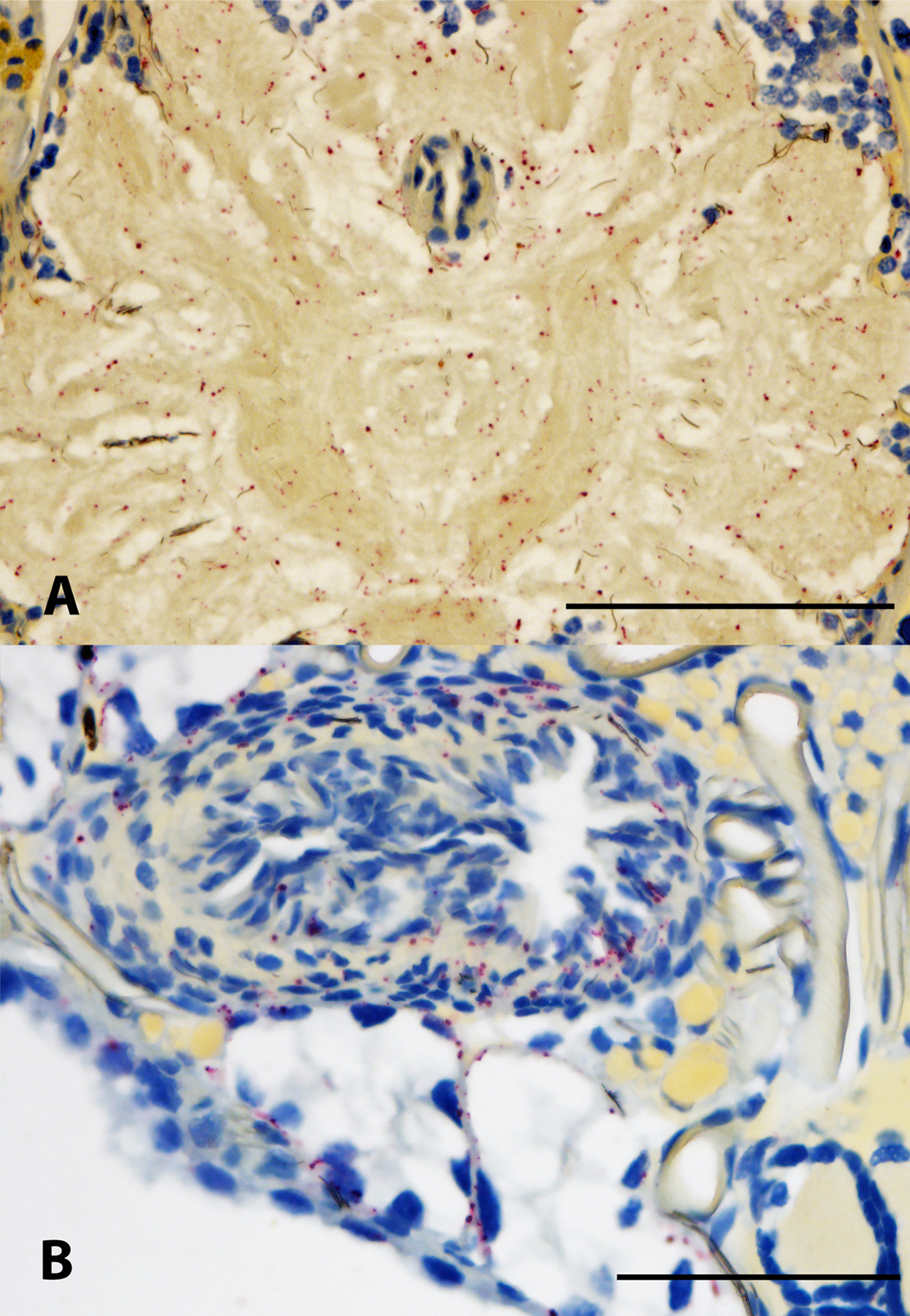
Borrelia miyamotoi-infected female Ixodes scapularis subjected to in situ hybridization assay. (A) Extensive infection of the neuropile region of the synganglion (note blue nuclei of the esophagus just above center image). B) spirochetes present at the lower (near terminus) regions of an ovary. 16S rDNA probe hybridization to spirochetes is indicated by red staining, blue indicates nuclear staining. Scale bars A = 50 μm, B = 100 μm.
Fig. 7.
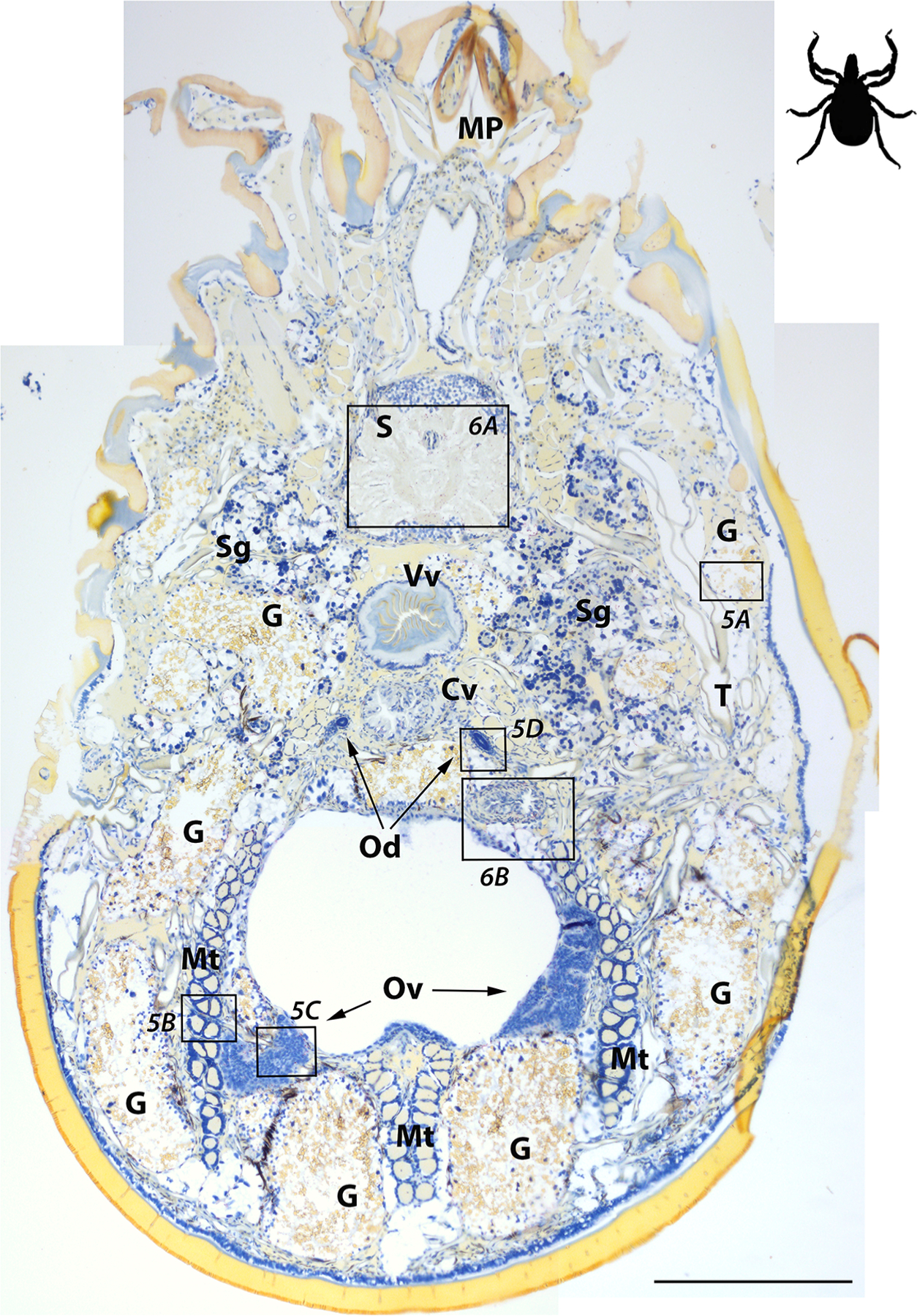
Montage of Borrelia miyamotoi-infected adult female Ixodes scapularis sectioned ventrally and subjected to in situ hybridization assay. Locations of images shown in Figs. 5 and 6 are indicated by boxes. 16S rDNA probe hybridization to spirochetes is indicated by red staining, blue indicates nuclear staining. See key for anatomical feature abbreviations. Scale bar = 400 μm. The black figure in the upper right corner indicates directional orientation of sectioned tick.
4. Discussion
Since it was formally recognized more than two decades ago, only a few studies have investigated the efficiency of transmission routes of B. miyamotoi, and most have included limited sample sizes owing in part to low prevalence in naturally infected ticks, and the challenges of producing infected ticks experimentally. Recent improvements in clinical recognition of human cases have sparked renewed interest in the ecology of B. miyamotoi. In this study, we evaluated both horizontal and transovarial (vertical) transmission routes under controlled experimental conditions, enabling a relative comparison. Using multiple stages of ticks to infect both a laboratory model mouse strain (CD-1) and a natural murine host (P. leucopus), we found that horizontal acquisition of B. miyamotoi by uninfected ticks was consistently inefficient, regardless of tick life stage used for the preceding infection challenge or the in-host incubation period between infected tick challenge and acquisition feed. In contrast, transovarial transmission was a substantially more effective means of producing newly infected ticks.
When a laboratory strain of mouse was used to evaluate horizontal transmission, we observed that acquisition of B. miyamotoi infection was absent or minimal in xenodiagnostic ticks fed shortly (two or four days) after CD-1 mice were challenged by nymphs infected via SCID mice as larvae. Importantly, these groups included between one and three days overlap in the feeding periods of ticks used for transmission and acquisition, allowing for the potential of co-feeding transmission. While exposure of some mice to challenge with infected ticks could only be inferred, acquisition success was low even for the subset of ticks fed on G1 and G2 mice with confirmed infection (5) via tissue or xenodiagnoses (3/109, 2.8%). Acquisition of infection increased modestly to 11.8% of xenodiagnostic ticks fed on G3 mice (larval infestation at 10 days post-challenge), which is likely the result of these mice having had exposure to a greater number of infected nymphs during challenge compared to mice in G1 and G2.
Horizontal transmission was similarly low among ticks fed on P. leucopus. Of xenodiagnostic ticks fed on mice challenged with I. scapularis with PCR-confirmed infection, 1% acquired a transstadially persistent infection; from mice with at least one positive xenodiagnostic tick, only 3.8% of molted nymphs were positive. Interestingly, xenodiagnostic acquisition occurred in each of the three weeks following challenge with infected ticks yet did not appear to be significantly more likely in any specific week following challenge, an observation that held for groups challenged by either larvae or nymphs.
We observed transovarial transmission to the larval progeny in seven of 10 clutches produced by B. miyamotoi-infected females and filial infection prevalence ranged from 3.3 to 100%, consistent with reported ranges for transovarial transmission in captive, field-collected I. scapularis (Scoles et al., 2001; Han et al., 2019). Infection was detected in two thirds of larvae from positive clutches and over 60% of total larvae tested from positive females. Filial infection prevalence and larval spirochete burden were both strongly correlated with maternal spirochete load. Interestingly, three of the positive females that engorged poorly with reduced ovulation or larval viability also had among the lowest maternal and larval spirochete loads and filial infection prevalence.
The similar success in horizontal transmission initiated by either infected larvae or nymphs suggests a potentially greater importance for larval contribution to enzootic maintenance of B. miyamotoi in natural settings given the tendency of small vertebrate hosts to host larger burdens of larvae than nymphs, especially in regions where active periods of these life stages are asynchronous. In line with this logic, Barbour et al. (2009) reported prevalence of B. miyamotoi in captured mice was highest during the period of greatest larval activity. This observation was contrasted with prevalence of B. burgdorferi s.s., which was highest in hosts during peak nymphal activity, in advance of larval peak (Barbour et al., 2009). Transovarial transmission is not considered important for natural enzootic maintenance of B. burgdorferi s.s., and acquisition of infection occurs during the larval or nymphal feed, with the initial vectorial opportunity occurring during the subsequent nymphal or adult stage (Kurokawa et al., 2020). However, interstage differences in horizontal transmission of B. miyamotoi are likely insignificant in comparison to the disproportionately greater role of transovarial transmission in natural maintenance.
Finally, assaying histological sections of whole ticks allowed an expansive anatomical evaluation of B. miyamotoi tissue tropism in all three life stages of I. scapularis. Our results corroborate previous reports where qPCR and immunofluorescence microscopy were used to describe presence of B. miyamotoi spirochetes in nymphal salivary glands and midguts (Breuner et al., 2017; Lynn et al., 2019). As Barbour et al. (2009) predicted, we observed systemic infections in unfed larvae, including the presence of spirochetes in salivary glands and internal mouthparts that facilitate a short window between tick attachment and transmission of spirochetes to hosts (Breuner et al., 2017, 2018). As in larvae, highly disseminated infections were readily apparent in nymphs, males, and female ticks with transovarially-acquired infections. In addition to salivary glands and the basal regions of midgut epithelial cells, other sites of infection included synganglion, Malpighian tubules, ovary, and various epithelial and secretory tissues. This broad pattern of dissemination closely follows a previous description of what were most likely B. miyamotoi spirochetes in Ixodes pacificus (Lane and Burgdorfer, 1987). Multiple foci of B. miyamotoi RNA observed in the ovarian tissue of unfed females indicates that spirochetes are likely to have a ubiquitous presence in the germinal tissue of infected females, though the exact time point at which spirochetes enter oocytes requires further study. Our qPCR data for unfed and spent females included in this study and its precursor (Lynn et al., 2019), combined with histological images strongly suggest that spirochete replication occurs during the female bloodmeal followed by migration into the oocytes during the period between engorgement and the development of the oocyte chorion (outer shell).
Interestingly, we observed that the synganglion was a focal point of infection in all life stages. Likewise, similar heavy colonization of this organ by Ehrlichia muris eauclairensis was also reported in I. scapularis, where intracellular ehrlichiae were concentrated within the ganglia of the outer cortex (Lynn et al., 2015). In ticks infected with B. miyamotoi, we observed a clear tropism of spirochetes for the internally located neuropile, that may be more accessible for highly mobile spirochetes. A strong association between borreliae and nerve tissues has previously been noted in various species of soft ticks and lice, and in I. scapularis, B. miyamotoi exhibits a broader tissue tropism that is more analogous to that of soft tick relapsing fever group spirochetes in ticks than B. burgdorferi s.s. (Barbour and Hayes, 1986).
Our study is not without limitations. Our transovarially-infected larvae were the offspring of a female experimentally infected with a previously characterized isolate of B. miyamotoi (CT13–2396) cultured from a tick collected in Connecticut, whereas infected nymphs also used to challenge P. leucopus were descended from a wild-collected naturally infected female tick (also from Connecticut). Secondly, some female ticks from the first cohort used to evaluate transovarial transmission failed to feed, or fed minimally on the rabbit, and were excluded from results. It is possible that successful engorgement and oviposition by females could have altered our results for transovarial infection rate, though we have no expectation of this. Due to sample size limitations, we did not assess acquisition rates in fed larvae in comparison to molted nymphs, which limits our ability to determine if acquisition rates were low, or if infections were lost during the transstadial molt from larva to nymph. And finally, active infection was not detected in several CD-1 mice, leaving some uncertainty of whether they were exposed to spirochetes. Relapsing fever spirochete numbers are known to fluctuate rapidly in blood as serotype switching occurs and it is likely that the volume of blood tested reflected low spirochetemia in some individuals (Barbour and Hayes, 1986; Lynn et al., 2019). Unfortunately, we were not successful in our attempt to use Western blots to diagnose exposure. However, for the two G1 mice (13 and 14) fed on to repletion by six and seven nymphs belonging to a nymphal cohort with an infection prevalence of ~25%, it is unlikely that both mice avoided exposure to B. miyamotoi. Our previous work showed that seven of eight mice exposed to a complete blood meal by a single feeding B. miyamotoi-infected larvae had serologic evidence of exposure, and nearly two thirds of mice exposed to infected ticks lacking PCR detectable levels of B. miyamotoi spirochetemia nevertheless seroconverted (Breuner et al., 2018). Combined with our results, this suggests that infected ticks are efficient at transmission of B. miyamotoi to mice, yet spirochetemia may not reach the limits of PCR detection when testing is performed on low volumes of blood, and perhaps these levels also limit xenodiagnostic acquisition. We expect low host spirochetemia, and poor maintenance of infection during the primary transstadial transmission event in ticks (Lynn et al., 2019) to be the primary factors limiting horizontal transmission.
Acknowledgments
We would like to thank Dr. Luke Kingry and Adam Replogle for materials, Christine Graham for technical advice, as well as Dr. Stephen Wikel for helpful discussions on tick anatomy. We thank Todd Bass for histological sectioning.
Abbreviations:
- A
acinus
- C
cortex of synganglion
- Cv
cervical vagina
- E
esophagus (as depicted in the synganglion)
- G
gut; Mg, midgut
- MP
mouth parts (cheliceral sheaths)
- Mt
Malpighian tubule
- Od
oviduct
- Ov
ovary
- Np
neuropile of synganglion
- Rs
rectal sac
- Sd
salivary duct
- Sg
salivary gland
- T
trachea
- Vv
vestibular vagina
Footnotes
CRediT authorship contribution statement
Geoffrey E. Lynn: Conceptualization, Methodology, Investigation, Formal analysis, Visualization, Writing – original draft. Nicole E. Breuner: Investigation, Writing – review & editing. Andrias Hojgaard: Methodology, Resources, Investigation. Jonathan Oliver: Visualization, Writing – review & editing. Lars Eisen: Conceptualization, Methodology, Formal analysis, Writing – review & editing. Rebecca J. Eisen: Conceptualization, Methodology, Formal analysis, Writing – review & editing.
Declaration of Competing Interest
None.
References
- Balashov YS, 1972. Bloodsucking ticks (Ixodoidea) - vectors of diseases of man and animals. Misc. Publ. Entomol. Soc. Am 8, 161–376. [Google Scholar]
- Barbour AG, Hayes SF, 1986. Biology of Borrelia species. Microbiol. Rev 50, 381–400. 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Bunikis J, Travinsky B, Gatewood Hoen A, Diuk-Wasser MA, Fish D, Tsao JI, 2009. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am. J. Trop. Med. Hyg 81, 1120–1131. 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner NE, Dolan MC, Replogle AJ, Sexton C, Hojgaard A, Boegler KA, Clark RJ, Eisen L, 2017. Transmission of Borrelia miyamotoi sensu lato relapsing fever group spirochetes in relation to duration of attachment by Ixodes scapularis nymphs. Ticks Tick Borne Dis. 8, 677–681. 10.1016/j.ttbdis.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuner NE, Hojgaard A, Replogle AJ, Boegler KA, Eisen L, 2018. Transmission of the relapsing fever spirochete, Borrelia miyamotoi, by single transovarially-infected larval Ixodes scapularis ticks. Ticks Tick Borne Dis. 9, 1464–1467. 10.1016/j.ttbdis.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikis J, Barbour AG, 2005. Third Borrelia species in white-footed mice. Emerg. Infect. Dis 11, 1150–1151. 10.3201/eid1107.041355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo CG, Eremeeva ME, Paskewitz SM, Sloan LM, Lee X, Irwin WE, Tonsberg S, Pritt BS, 2015. Detection of human pathogenic Ehrlichia muris-like agent in Peromyscus leucopus. Ticks Tick Borne Dis. 6, 155–157. [DOI] [PubMed] [Google Scholar]
- Donahue JG, Piesman J, Spielman A, 1987. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am. J. Trop. Med. Hyg 36, 92–96. 10.4269/ajtmh.1987.36.92. [DOI] [PubMed] [Google Scholar]
- Eisen RJ, Kugeler KJ, Eisen L, Beard CB, Paddock CD, 2017. Tick borne zoonoses in the United States: persistent and emerging threats to human health. ILAR J. 58, 319–335. 10.1093/ilar/ilx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, Nakao M, 1995. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int. J. Syst. Bacteriol 45, 804–810. 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- Graham CB, Pilgard MA, Maes SE, Hojgaard A, Eisen RJ, 2016. Paired real-time PCR assays for detection of Borrelia miyamotoi in North American Ixodes scapularis and Ixodes pacificus (Acari: ixodidae). Ticks Tick Borne Dis. 7, 1230–1235. 10.1016/j.ttbdis.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Lubelczyk C, Hickling GJ, Belperron AA, Bockenstedt LK, Tsao JI, 2019. Vertical transmission rates of Borrelia miyamotoi in Ixodes scapularis collected from white-tailed deer. Ticks Tick Borne Dis. 10, 682–689. 10.1016/j.ttbdis.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojgaard A, Lukacik G, Piesman J, 2014. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti, with two different multiplex PCR assays. Ticks Tick Borne Dis. 5, 349–351. 10.1016/j.ttbdis.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Hovius JW, de Wever B, Sohne M, Brouwer MC, Coumou J, Wagemakers A, Oei A, Knol H, Narasimhan S, Hodiamont CJ, Jahfari S, Pals ST, Horlings HM, Sprong H, van Oers MHJ, 2013. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 382, 658. 10.1016/S0140-6736(13)61644-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe DA, Lovrich SD, Oldenburg DG, Kowalski TJ, Callister SM, 2016. Borrelia miyamotoi infection in patients from Upper Midwestern United States, 2014–2015. Emerg. Infect. Dis 22, 1471–1473. 10.3201/eid2208.151878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TL, Graham CB, Maes SE, Hojgaard A, Fleshman A, Boegler KA, Delory MJ, Slater KS, Karpathy SE, Bjork JK, Neitzel DF, Schiffman EK, Eisen RJ, 2018. Prevalence and distribution of seven human pathogens in host-seeking Ixodes scapularis (Acari: ixodidae) nymphs in Minnesota, USA. Ticks Tick Borne Dis. 9, 1499–1507. 10.1016/j.ttbdis.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kays RW, Wilson DE, 2009. Mammals of North America: second edition, pp. 174. Princeton University Press. Princeton, NJ, USA. ProQuest Ebook Central. https://ebookcentral.proquest.com/lib/tamucs/detail.action?docID=539789. [Google Scholar]
- Kingry LC, Replogle A, Dolan M, Sexton C, Padgett KA, Schriefer ME, 2017a. Chromosome and large linear plasmid sequences of a Borrelia miyamotoi strain isolated from Ixodes pacificus ticks from California. Genome Announc 5. 10.1128/genomeA.00960-17 e00960–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingry LC, Replogle A, Batra D, Rowe LA, Sexton C, Dolan M, Connally N, Petersen JM, Schriefer ME, 2017b. Toward a complete North American Borrelia miyamotoi genome. Genome Announc 5. 10.1128/genomeA.01557-16 e01557–16Published 2017 Feb 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Carroll M, Fedorova N, Brancato J, Dumouchel C, Akosa F, Narasimhan S, Fikrig E, Lane RS, 2018. Human Borrelia miyamotoi infection in California: serodiagnosis is complicated by multiple endemic Borrelia species. PLoS ONE 13, e0191725. 10.1371/journal.pone.0191725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, Lepore T, Barbour A, Fish D, 2013. Human Borrelia miyamotoi infection in the United States. N. Engl. J. Med 368, 291–293. 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Fish D, Narasimhan S, Barbour AG, 2015. Borrelia miyamotoi infection in nature and in humans. Clin. Microbiol. Infect 21, 631–639. 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause PJ, Narasimhan S, Wormser GP, Barbour AG, Platonov AE, Brancato J, Lepore T, Dardick K, Mamula M, Rollend L, Steeves TK, Diuk-Wasser M, Usmani-Brown S, Williamson P, Sarksyan DS, Fikrig E, Fish D, 2014. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg. Infect. Dis 20, 1183–1190. 10.3201/eid2007.131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa C, Lynn GE, Pedra J, Pal U, Narasimhan S, Fikrig E, 2020. Interactions between Borrelia burgdorferi and ticks. Nat. Rev. Microbiol 18, 587–600. 10.1038/s41579-020-0400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RS, Burgdorfer W, 1987. Transovarial and transstadial passage of Borrelia burgdorferi in the western black-legged tick, Ixodes pacificus (Acari: ixodidae). Am. J. Trop. Med. Hyg 37, 188–192. 10.4269/ajtmh.1987.37.188. [DOI] [PubMed] [Google Scholar]
- Lynn GE, Oliver JD, Nelson CM, Felsheim RF, Kurtti TJ, Munderloh UG, 2015. Tissue distribution of the Ehrlichia muris-like agent in a tick vector. PLoS ONE 10, e0122007. 10.1371/journal.pone.0122007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn GE, Oliver JD, Cornax I, O’Sullivan MG, Munderloh UG, 2017. Experimental evaluation of Peromyscus leucopus as a reservoir host of the Ehrlichia muris-like agent. Parasit. Vectors 10, 48. 10.1186/s13071-017-1980-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn GE, Graham CB, Horiuchi K, Eisen L, Johnson TL, Lane RS, Eisen RJ, 2018. Prevalence and geographic distribution of Borrelia miyamotoi in host-seeking Ixodes pacificus (Acari: ixodidae) nymphs in Mendocino County California. J. Med. Entomol. 55, 711–716. 10.1093/jme/tjx258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn GE, Breuner NE, Eisen L, Hojgaard A, Replogle AJ, Eisen RJ, 2019. An immunocompromised mouse model to infect Ixodes scapularis ticks with the relapsing fever spirochete, Borrelia miyamotoi. Ticks Tick Borne Dis. 10, 352–359. 10.1016/j.ttbdis.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbow ML, Christe M, Rutti B, Brossard M, 1994. Absence of acquired resistance to nymphal Ixodes ricinus ticks in Balb/C mice, developing cutaneous reactions. J. Parasitol 80, 81–87. [PubMed] [Google Scholar]
- Molloy PJ, Telford SR, Chowdri HR, Lepore TJ, Gugliotta JL, Weeks KE, Hewins ME, Goethert HK, Berardi VP, 2015. Borrelia miyamotoi disease in the Northeastern United States: a case series. Ann. Intern. Med 163, 91–98. 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- Mun J, Eisen RJ, Eisen L, Lane RS, 2006. Detection of a Borrelia miyamotoi sensu lato relapsing-fever group spirochete from Ixodes pacificus in California. J. Med. Entomol 43, 120–123. 10.1093/jmedent/43.1.120. [DOI] [PubMed] [Google Scholar]
- Nelder MP, Russell CB, Sheehan NJ, Sander B, Moore S, Li Y, Johnson S, Patel SN, Sider D, 2016. Human pathogens associated with the blacklegged tick Ixodes scapularis: a systematic review. Parasit. Vectors 9, 265. 10.1186/s13071-016-1529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J, Schwan TG, 2010. Ecology of borreliae and their arthropod vectors. In: Samuels DS, Radolf JD, Borrelia (Eds.), Molecular Biology, Host Interaction and Pathogenesis. Caister Academic Press, Norfolk, UK, pp. 251–278. [Google Scholar]
- Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, Maleev VV, Fish D, Krause PJ, 2011. Humans infected with relapsing fever spirochete Borrelia miyamotoi. Rus. Emerg. Infect. Dis 17, 1816–1823. 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Replogle AJ, Sexton C, Young J, Kingry LC, Schriefer ME, Dolan M, Johnson TL, Connally NP, Padgett KA, Petersen JM, 2021. Isolation of Borrelia miyamotoi and other borreliae using a modified BSK medium. Sci. Rep 11, 1926. 10.1038/s41598-021-81252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D, Schlee DB, Matuschka FR, 2003. Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerg. Infect. Dis 9, 697–701. 10.3201/eid0906.020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D, Debski A, Hubalek Z, Matuschka FR, 2012. Absence of Lyme disease spirochetes in larval Ixodes ricinus ticks. Vector Borne Zoonotic Dis. 12, 21–27. doi: 10.1089/vbz.2011.0668. [DOI] [PubMed] [Google Scholar]
- Rollend L, Fish D, Childs JE, 2013. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: a summary of the literature and recent observations. Ticks Tick Borne Dis. 4, 46–51. 10.1016/j.ttbdis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Roshdy MA, 1969. Structure of the female reproductive system of Ixodes ricinus (L.), and its bearing on the affinity of Ixodes to other ixodid genera. J. Parasitol 55, 1078–1083. [Google Scholar]
- Salkeld DJ, Nieto NC, Bonilla DL, Yoshimizu MH, Padgett KA, 2018. Borrelia miyamotoi infections in small mammals. California, USA. Emerg. Infect. Dis 24, 2356–2359. 10.3201/eid2412.171632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoles GA, Papero M, Beati L, Fish D, 2001. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic. Dis 1, 21–34. 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- Siński E, Welc-Falęciak R, Zajkowska J, 2016. Borrelia miyamotoi: a human tick-borne relapsing fever spirochete in Europe and its potential impact on public health. Adv. Med. Sci 61, 255–260. 10.1016/j.advms.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE, 1991. Biology of Ticks. Volume 1. Oxford University Press, New York, NY, USA. [Google Scholar]
- Spielman A, Levine JF, Wilson ML, 1984. Vectorial capacity of North American Ixodes ticks. Yale J. Biol. Med 57, 507–513. [PMC free article] [PubMed] [Google Scholar]
- Tsao JI, Wootton JT, Bunikis J, Luna MG, Fish D, Barbour AG, 2004. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc. Natl. Acad. Sci. U. S. A 101, 18159–18164. 10.1073/pnas.0405763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijvendijk G, Coipan C, Wagemakers A, Fonville M, Ersoz J, Oei A, Foldvari G, Hovius J, Takken W, Sprong H, 2016. Larvae of Ixodes ricinus transmit Borrelia afzelii and B. miyamotoi to vertebrate hosts. Parasit. Vectors 9, 97. 10.1186/s13071-016-1389-5. Published 2016 Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]


