ABSTRACT
This scoping review examines the role of digital solutions in active, participant-centered surveillance of adverse events following initial release of COVID-19 vaccines. The goals of this paper were to examine the existing literature surrounding digital solutions and technology used for active, participant centered, AEFI surveillance of novel COVID-19 vaccines approved by WHO. This paper also aimed to identify gaps in literature surrounding digital, active, participant centered AEFI surveillance systems and to identify and describe the core components of active, participant centered, digital surveillance systems being used for post-market AEFI surveillance of WHO approved COVID-19 vaccines, with a focus on the digital solutions and technology being used, the type of AEFI detected, and the populations under surveillance. The findings highlight the need for customized surveillance systems based on local contexts and the lessons learned to improve future vaccine monitoring and pandemic preparedness.
KEYWORDS: Adverse events following immunization (AEFI), vaccine safety surveillance, active surveillance, post-marketing surveillance, participant reporting, pharmacovigilance, COVID-19 vaccine
Introduction
Post-market surveillance for adverse events following immunization (AEFI) from COVID-19 vaccines is a key priority amongst public health stakeholders and policy makers, with emphasis placed on the necessity for adequate, comprehensive, and adaptable population level safety monitoring.1–3 Broadly speaking, AEFI surveillance can either be passive (unprompted, spontaneous reporting of events)4,5 or active (deliberate prompting of participants and/or active case seeking to solicit event reporting),6 with data typically sourced from either healthcare providers, vaccinees, or both to monitor a population for safety signals.4 An increasingly recognized and emerging form of AEFI monitoring is active, participant-centered surveillance, which collects solicited health and/or reactogenicity information from vaccinees.5 In addition to classic analog approaches to active, participant-centered AEFI surveillance, such as health diary cards and interviews, a number of systems are employing digital solutions and technology, such as e-mail and short-message-system (SMS).5
Digital solutions have been utilized in many facets of public health measures one of which is pandemic planning and responses.7–10 The technology has been implemented for some AEFI surveillance for monitoring the safety of vaccines, for example the CDC’s V-Safe app.11 As the COVID-19 pandemic subsides there is an opportunity to learn from the implementation of the various digital solutions implemented in multiple jurisdictions. This information can be valuable for future pandemic as well as non-pandemic settings. Digital systems have various advantages. The major advantage is that they facilitate more real time AEFI surveillance which allows for more rapid detection of AEFI signals. Another advantage is that digital systems can be more easily standardized, which is key in implementing a “gold-standard” that is translatable across the international community, and they can capture large volumes of data and information.12 A disadvantage surrounding digital systems is data privacy concerns. Patients may have concerns regarding how their health information is digitally handled which could hinder patient trust in the system.13 Digital systems also exclude individuals who may not be fluent with technology, such as older adults in long-term care. Finally, they are more expensive to implement and require more maintenance.13–15
To assist in this regard, we conducted a scoping review to better understand the role of different technological and digital approaches to active, participant-centered, AEFI surveillance of COVID-19 vaccines during the early stages of the pandemic.
Methods
Our objectives were
To identify the published research (describe the extent, range, and nature of research activity)12–14 of digital solutions and technology used for active, participant centered, AEFI surveillance of novel COVID-19 vaccines approved by the World Health Organization (WHO).15
To identify gaps in literature surrounding digital, active, participant centered AEFI surveillance systems
To identify and describe the core components of active, participant centered, digital surveillance systems being used for post-market AEFI surveillance of WHO approved COVID-19 vaccines, with a focus on the digital solutions and technology being used, the type of AEFI detected, and the populations under surveillance.
For the purpose of this review, “digital” was defined as any tool that used electronic technology for capturing and processing data through digital signals. “Active, participant-centered, AEFI surveillance” was defined as an approach which proactively searched for AEFIs and included purposeful solicitation of health events and/or symptom information specifically from vaccinees following immunization, where clear prompting for and elicitation of data occurred, with cases actively sought out.
Methodological approach
This scoping review followed a detailed and structured approach, informed by PRISMA Extension for Scoping Review (PRISMA-ScR) guidelines to identify, plot, and describe the peer-reviewed literature landscape within the area of active, participant centered, digital AEFI surveillance for WHO approved COVID-19 vaccines.14
Information sources
Three bibliographic databases (Embase Classic + Embase, OVID-Medline, and EBM Review – Cochrane Central Register of Controlled Trials) were searched for published, peer-reviewed literature ranging from January 1st, 1946 to December 15th, 2022. The search strategy was created in collaboration with, and executed by, an experienced medical librarian. A detailed description of the search strategy, including the specific search terms selected and conventions applied, is found in Appendix 1. The final search result records were uploaded to Covidence, where additional deduplication automatically occurred. Screening was conducted by four independent investigators (DS, DZ, MS, and NK). Grey literature was searched for and accessed in order to provide additional contextual information for the identified digital solutions extracted from included records.
Selection of sources of evidence
Pre-determined inclusion and exclusion criteria (Table 1) were first applied to all titles and abstracts by two independent investigators (DZ, DS, NK, and MS) followed by full text screening completed independently in duplicate, with a third-party (BB and KW) resolving decision conflicts. Studies that were included in the scoping review underwent data extraction by one investigator (NK), with a second performing verification (MS).
Table 1.
Applied inclusion and exclusion criteria.
| Inclusion Criteria (Included Studies Must Satisfy All the Following to Be Included) |
|---|
| The vaccine under surveillance must be approved by the World Health Organisation (Novavax (NVX-CoV2373), COVOVAX (Novavax formulation), Moderna (mRNA-1273), Pfizer/BioNTech (BNT162b2), Janssen (Ad26.COV2.S), Oxford/AstraZeneca (AZD1222), Covishield, Covaxin, Sinopharm (BBIBP-CorV -Vero Cells), Sinovac (CoronaVac)15 |
| The surveillance method described must include a digital solution/technology, such as an app, e-mail, SMS, website, and/or e-questionnaire (a mix of digital and non-digital was allowed as long as results were separated out, with digital outcomes specifically reported on, such as response rate) |
| The study describes post-market AEFI surveillance (Phase IV studies, post-market reports, etc.) |
| The manuscript is primary research |
| The AEFI surveillance type is “active,” defined as being directly instructed/prompted to respond (solicited AEFI reports) and is patients/participants/vaccinees centered |
| English language full text, manuscripts are available (matching language proficiencies of investigators) |
Study must report the following:
|
| Exclusion Criteria (Record Excluded if Any of the Following) |
| The vaccine under surveillance is not approved by the World Health Organization (mix was allowed if results were separated out with specific outcomes reported for approved vaccines) |
| The publication is not primary research (narrative review, editorial, letter, comment, opinion piece) |
| The study does not describe post-market AEFI surveillance |
| Abstract and/or poster only available which do not provide sufficient detail for interpretation and data extraction |
| Surveillance reports came from manufacturers, healthcare providers, and/or non-vaccinee/patient/participant sources |
| Passive surveillance or administrative data studies (mix was allowed if results were separated out with specific outcomes for active surveillance components) |
| Non-digital solution/technology used for AEFI surveillance, including phone interviews, assisted calling, diary cards, paper forms, etc. (Mix was allowed if results were separated out with specific outcomes for digital AEFI surveillance components) |
| Full text, in English to meet investigator language proficiency, is not available and/or accessible |
| The method of surveillance and the components of digital surveillance were unclear, and interpretation of the approach taken was not possible due to an absence of description and details regarding the methods applied. |
Data charting process & items
Data was collected from included records and inputted into tables with prespecified categories. Extraction endpoints included reference details (authorship and publication year), study design, surveillance approach details (period of data collection, population(s) under surveillance, technology and digital solutions used for AEFI data collection, and reporting schedule, any formal system name (e.g., V-Safe etc.), the type of COVID-19 vaccine(s) under surveillance, types of adverse events reported (local, systemic, serious, or severe), and management approach(es) for serious events.
Synthesis and presentation of results
Characteristics of included studies (authorship, publication year, country, study design, data collection period, and sample size), characteristics and components of the digital surveillance (population(s) monitored, vaccine(s) covered, response rate(s), participant communication methods, data collection methods, and AEFI surveillance timing) and human resources required to carry out surveillance (human follow-up approaches, operating costs, and associated public health agencies) were summarized in table format.
Results
Selection of sources of evidence and included studies
The applied search strategy, after initial deduplication by the medical librarian using referencing software, identified 3796 records. After additional automatic deduplication by Covidence (n = 1),16 title and abstract screening excluded 3443 records, from which an additional 296 were subsequently excluded after full-text review. A detailed description of the screening process is presented in a PRISMA flow-chart (Figure 1). 56 studies were included in the present scoping review (Table 2). Two of these publications, one by Zhang et al. (2021) and the other by Zhu et al. (2021), performed their analyses using the same dataset; accordingly, we included 56 papers from 55 unique studies.
Figure 1.
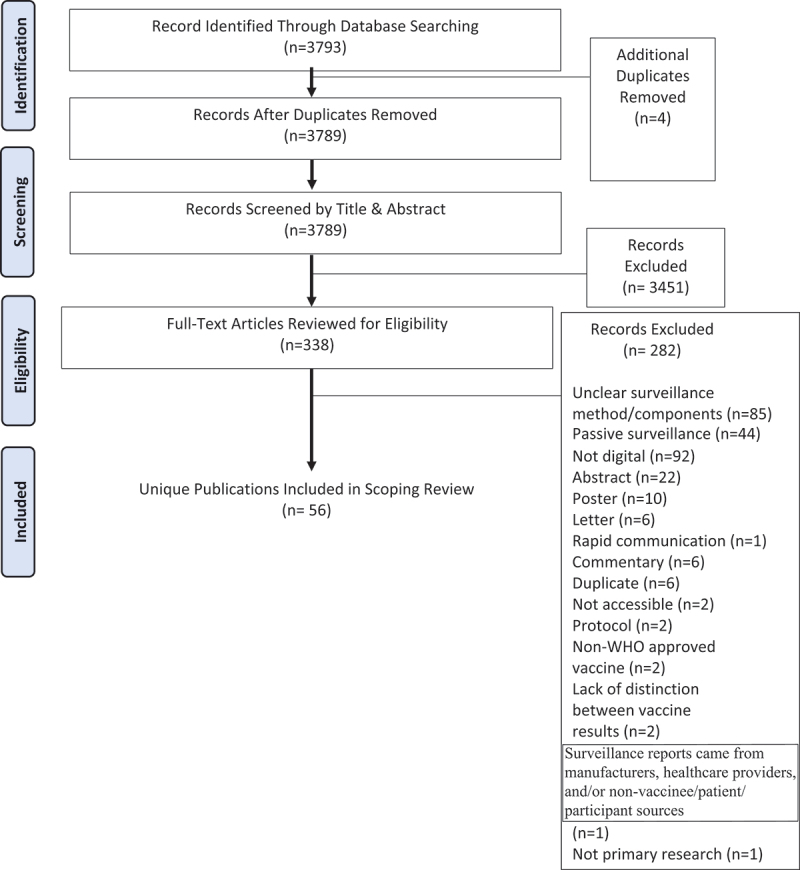
PRISMA chart.
PRISMA Flow-Chart (COVID-19 Vaccine Active, Participant Centered, Digital AEFI Surveillance)
Table 2.
Study characteristics.
| Reference Number First Author and Country study conducted | Population Monitored & Data Collection Date | Vaccines Included | Primary Communication Method | Study Design & Data Collection Method | Name of Surveillance System(s) or Software Used | Timing of AEFI Reporting After Vaccination |
|---|---|---|---|---|---|---|
| Alhowaymel et al17– Saudi Arabia | Adults received AstraZeneca in Riyadh -March – May 2021 | Oxford-AstraZeneca | Not specified | Cross-sectional-Online survey link | Google Forms | Not specified |
| Amodio et al18– Italy | Vaccinated persons Palermo University Hospital-January – April 2021 | Pfizer-BioNTech | Cohort-Online survey link | Google Forms | 7 days after 1st and 2nd dose | |
| Angkasekwinai et al.19 – Thailand | Healthy Thai HCW’s Siriraj Hospital-February – July 2021 | Sinovac and Oxford-AstraZeneca | Not specified | Cohort-Online survey link | Google Forms | 7 days after each vaccination |
| Azzolini et al20 – Italy | 4156 HCW’s tertiary care hospital Northern Italy-December 2020 – April 2021 | Pfizer-BioNTech | Not specified | Cohort-Online questionnaire | Online questionnaire | Following each dose |
| Beatty et al21 – United States | 18 years + with internet access and smartphone-March 2020 – May 2021 | Pfizer-BioNTech | Phone number | Cohort-Mobile app/web-based software | Eureka | Daily, weekly, monthly surveys |
| Bettinger et al22 – Canada | Vaccinated Canadians from 7 provinces and territories-December 2020 – February 2022 | Pfizer, Moderna, Oxford-AstraZeneca | Cohort-Online questionnaire | CANVAS | 7 days after each vaccination | |
| Briggs et al23 – USA | Multiple sclerosis patients registered in the iConquer MS research network-March 22, 2021 – June 9, 2021 | Pfizer-BioNTech, Moderna, Johnson & Johnson, Oxford-AstraZeneca | iConquer MS web portal | Cross-sectional -Digital invitation to the web-based survey was sent to subjects via the iConquer MS web portal | iConquerMS | Not specified |
| Bsoul et al24 – USA | University of Texas Health San Antonio dentistry community-January – March 2021 | Pfizer-BioNTech | Cross-sectional- Online link | Qualtrics | 47 days after | |
| Chalermphanchai et al.25 – Thailand | Participants 18+ years completed 2 doses of 3 week Sinovac in Lampang-August – September 2021 | Sinovac | Not specified | Cohort-Online survey | Online side effect monitoring survey | Day 1, 7, 30 after injection |
| Cuschieri et al.26– Italy | Healthcare workers in the state hospital in Malta, Italy-March 29, 2021 – April 9, 2021 | Pfizer-BioNTech | Cross-sectional-Online survey link was sent to subjects via e-mail | Google Forms | Not specified | |
| D’Arminio Monforte et al.27 – Italy | HCW’s two large hospitals Milan, Italy-January – February 2021 | Pfizer-BioNTech | Cohort-Online questionnaire | Online questionnaire | Just before second dose, two weeks after second dose | |
| Deng et al28 – Australia | 16 years + vaccination sites Australia-February – August 2021 | Oxford-AstraZeneca, Pfizer-BioNTech | Email, SMS | Cohort-Link to online survey | AusVaxSafety | 0–3 days, 4–7 days after vaccination |
| Ebinger et al.29 – USA | Healthcare workers at Cedar-Sinai Medical Centre in the USA-Dec 17, 2020 – Feb 10, 2021 | Pfizer-BioNTech | Not specified | Cohort-Not specified | REDCap | Participants were prospectively instructed to complete the survey between 8–21 days after each vaccine dose |
| Figueroa30 – Mexico | HCW’s High Specialty Regional Hospital in Yucatan-January – February 2021 | Pfizer-BioNTech | Cohort-Online link | SurveyMonkey | Not specified | |
| Lai et al31 – Hong Kong | Recruited 16+ years receiving 1st dose Sinovac or Pfizer at community vaccination centers Hong Kong-February – July 2021 | Sinovac, Pfizer-BioNTech | SMS | Cohort-Online survey | Qualtrics | Up to 14 days after vaccine |
| Gepner et al32 – Israel | Not found to be COVID positive receive 2nd dose Pfizer January – March 2021 | Pfizer-BioNTech | Not specified | Cohort-Mobile application | PerMed | 15 days after |
| Goldlin et al33 – India | Vaccinees tertiary teaching hospital Tamil Nadu-January – February 2021 | Oxford-AstraZeneca | Mobile number | Cohort-Mobile phone | Mobile phone | 2 weeks after |
| Guan et al34 – Israel | Israel 2nd or 3rd BNT dose-January – September 2021 | Pfizer-BioNTech | Mobile phone | Cohort-Mobile phone questionnaire | Smartwatch/smartphone questionnaire | Up to 14 days after vaccination |
| Hammad et35 – Egypt | Healthcare workers Zagazig Faculty of Medicine Egypt -June 2021 – March 2022 | Oxford-AstraZeneca | Not specified | Cohort-Online survey link | Google Forms | Monitored after each vaccine |
| Hyun et al36 – Korea | Korean HCW’s Gangnam Severance Hospital-March – August 2021 | Oxford-AstraZeneca | Not specified | Cohort-Online survey link | Google Forms | 14 days after vaccination |
| Inoue et al37 – Japan | Medical staff Yamagata University Hospital-March – August 2021 | Pfizer-BioNTech | Not specified | Cross-sectional-Online survey link | Google Forms | Not specified |
| Javed et al38 – Saudi Arabia | HCW’s Maternity and Children Hospital Buraidah-March – April 2021 | Oxford-AstraZeneca | WhatsApp, Email | Cohort-Online survey link | Google Forms | 15–20 days post vaccination |
| Jeon et al.39 – South Korea | Healthcare workers aged <65 years at a teaching hospital in South Korea-Not specified | Oxford-AstraZeneca | SMS | Cohort-Daily web-based survey links were sent to subjects via SMS | MVAERS | Twice daily from days 0–7 after vaccination |
| Kim et al40 – South Korea | Healthcare workers in 3 referral teaching hospitals in South Korea-April, 2021 | Pfizer-BioNTech | SMS/e-mail | Cross-sectional-Online survey link was sent to subjects via SMS/e-mail | Google Forms | Range: 5–41 days after vaccination |
| Lee et al41 – South Korea | HCW’s at Hanyang University Hospital who received 2 doses-March 2021 – May 2021 | Oxford-AstraZeneca | Not specified | Cohort-Online survey | Online survey | 7 days after each dose |
| Levy et al42 – Israel | HCW’s vaccinated with Pfizer Sheba Medical Centre-December 2020 – April 2021 | Pfizer-BioNTech | SMS | Cohort-Online questionnaire | Text message questionnaire | 7 days after each dose |
| Lim et al43 – Singapore | Healthcare workers at the National University Hospital in Singapore-February 8, 2021 – April 12, 2021 | Pfizer-BioNTech | Not specified | Cross-sectional-FormSG web-based survey platform | FormSG | 7 days after vaccination |
| Lotan et al44 – Israel | Multiple sclerosis patients at Rabin Medical Centre, Israel-March 15, 2021 – April 17, 2021 | Pfizer-BioNTech | Cross-sectional-Online survey link was sent to subjects via e-mail | REDCap | Not specified | |
| Low et al45 – Singapore | Lactating HCW’s Singapore-February – March 2021 | Pfizer-BioNTech | BNT | Cohort-FormSG web-based survey platform | FormsSG | 28 days after |
| Maruyama et al.46 – Japan | HCW’s vaccinated with BNT in Japan-March – July 2021 | Pfizer-BioNTech | Not specified | Cohort-Website | Website | 8 days after injection |
| Mofaz et al47 – Israel | Participants who received more than 1 BNT Israel-November 2020 – September 2021` | Pfizer-BioNTech | Not specified | Cohort-Mobile application | PerMed mobile application | Monitored 37 days, 7 days before vaccination |
| Nachtigall et al48 – Germany | Employees of hospitals of Helios group-May – June 2021 | Pfizer-BioNTech, Moderna, Oxford-AstraZeneca | Cross-sectional-Online survey | Online survey | More than 5 days after injection | |
| Nittner-Marszalska et al.49 – Poland | Medical students and professionals at Wroclaw University in Poland -44,229 | Pfizer-BioNTech | Cross-sectional-Online survey link was sent to subjects via e-mail | Google Forms | Variable – vaccination dates varied, and survey was distributed on one day only | |
| Okumura et al.50 – Japan | Individuals at Keio University School of Pharmacy-June 2021 – June 2022 | Moderna | Cohort-Online survey link | Google Forms | Day 1, 3, 7 after each dose questionnaire administered online | |
| Park et al.51 – South Korea | Hospital staff at a university hospital in Daegu, South Korea-June 2, 2021 – June 18, 2021 | Pfizer-BioNTech | SMS | Cross-sectional-Online survey link was sent to subjects via SMS | NAVER Form | 3 SMS prompts from June 2–18, 2021 |
| Pellegrino et al.52 – Italy | IBD patients at University of Campania ‘Luigi Vanvitelli’-April 2021 – January 2022 | Pfizer-BioNTech | Not specified | Cohort-Online questionnaire | Online questionnaire | 9 ± 2 days after vaccination |
| Presby et al.53 – USA | Individuals wearing WHOOP device Boston MA-44317 | Oxford-AstraZeneca, Johnson, Pfizer-BioNTech, Moderna | Biometric device | Cross-sectional-Survey | Wearable biometric device | 1 week before and after |
| Rahmani et al.54 – Italy | Resident physicians at University of Genoa-January 11, 2021 – March 16, 2021 | Pfizer-BioNTech | Cross-sectional-Online survey link was sent to subjects via e-mail | LimeSurvey | 3 reminder e-mails within 7 days after vaccination | |
| Rolfes et al.55 – Netherlands | People vaccinated in Dutch immunization program-March 2021 – May 2021 | Pfizer-BioNTech, Oxford-AstraZeneca, Johnson & Johnson, Moderna | Not specified | Cohort-Online questionnaire | Lareb Intensive Monitoring System | First survey sent 7 days after vaccination, 6 questionnaires sent over period of 6 months |
| Sadarangani et al.56 – Canada | Pregnant women Canada-December 2020 - November 2021 | Pfizer-BioNTech, Moderna, AstraZeneca | Email, telephone number | Cohort-Online survey | REDCap | 7 days after vaccination |
| Sen et al.57 – Various countries | COVAD study multiple countries-April – September 2021 | Multiple vaccines | Not specified | Cross-sectional-Online survey | Online survey | 7 days after vaccination |
| Shimamura et al.58– Japan | Healthcare workers in hospital in Japan-March 2021 – January 2022 | Pfizer-BioNTech | SMS | Cross-sectional- Online survey link was sent to subjects via SMS | Microsoft Forms | After each vaccine, for two days |
| Song et al.59 – South Korea | Healthcare workers aged 20–64 years at Inje University Ilsan Paik Hospital in South Korea-March 17, 2021 – March 21, 2021 | Oxford-AstraZeneca | SMS | Cross-sectional-Online survey link was sent to subjects via text | Google Forms | Range: 5–9 days after vaccination |
| Supangat et al.60– Indonesia | Medical students in clerkship programs at Soebandi General Hospital in Indonesia-February, 2021 | Sinovac | Cross-sectional-Online survey link was sent to subjects via WhatsApp after each vaccine dose | Google Forms | 7 days after each dose of the vaccine | |
| Tani et al.61 – Japan | HCW’s who received 3 Pfizer doses at Fukuoka City Hospital-March 2021 – January 2022 | Pfizer-BioNTech | Not specified | Cohort-Web-based questionnaire | Web-based questionnaire | 7 days after |
| Tawinprai et al.62 – Thailand | Individuals at Chulabhorn Hospital in Bangkok, Thailand 18+ years negative for anti-SARS-CoV2 antibody were eligible-March 31 2021 – May 5 2021 | Oxford-AstraZeneca | SMS | Cohort-Online questionnaire through SMS | SMS questionnaire | Day 1 and 7 post-vaccination |
| Toussia-Cohen et al.63 – Israel | Pregnant who received 2 doses BNT to pregnant women receiving 3 doses-January – November 2021 | Pfizer-BioNTech | Not specified | Cohort-Digital questionnaire | Digital questionnaire | 2–4 wks after vaccination |
| Vigezzi et al.64 – Italy | Hospital staff to San Raffaele Hospital-January 4, 2021 – April 27, 2021 | Pfizer-BioNTech | Cross-sectional-Online survey link was sent to subjects via e-mail | SurveyMonkey | Not specified | |
| Walmsley et al.65 – Canada | Persons receiving vaccine at Ontario vaccine distribution centers-May – July 2021 | Pfizer-BioNTech, Moderna, Oxford-AstraZeneca | Cohort-Electronic questionnaire | Electronic questionnaire | 7 days after each dose | |
| Warkentin et al.66 – USA | Individuals at primary care practices or vaccination centers in Bavaria, Germany-April 2021 – August 2021 | Pfizer-BioNTech, Moderna, Oxford-AstraZeneca |
Cohort-Web-based survey | REDCap | 14–19 and 40–59 days after vaccination | |
| Wei et al.67 – China | Staff Guizhou Provincial Staff Hospital-January 2021 – January 2022 | Sinopharm, Oxford-AstraZeneca | Mobile phone | Cross-sectional-Mobile phone questionnaire | Mobile phone | 3 days after vaccination |
| Yamazaki et al.68 – Japan | HCW’s Chiba University Hospital Comirnaty vaccinees-March – April 2021 | Pfizer-BioNTech | Email/Mobile phone | Cross-sectional-Respon:sum | Respon:sum | 14 days after |
| Yechezkel et al.69 – Israel | Retrospective from Maccabi Health Services and Prospective from PerMed study-December 2021 – July 2022 | Pfizer-BioNTech | Mobile phone | Cohort-Mobile phone/smartwatch | Smartwatch/mobile application | 42 days after vaccination |
| Zhang et al.70 – China | Hospital staff in a tertiary hospital in Taizhou, China-February 24, 2021 – March 7, 2021 | Sinovac | WeChat/e-mail | Cross-sectional-Online survey link was sent to subjects via WeChat/e-mail | Wen-Juang-Xing platform | Not specified |
| Zhu et al.71 – China | Hospital staff in a tertiary hospital in Taizhou, China-February 24, 2021 – March 7, 2021 | Sinovac | WeChat/e-mail | Cross-sectional-Online survey link was sent to subjects via WeChat/e-mail | Wen-Juang-Xing platform | Not specified |
Characteristics of studies
The studies included came from 19 unique countries. 7 of the studies were conducted in Italy, 7 in Israel, 6 in the United States, 6 in South Korea, 6 in Japan, and 3 from Canada.
There were various study designs implemented. As expected, all studies were observational in nature and can be further classified as cross sectional or cohort in nature. It was found that 22 studies were classified as cross-sectional, and 33 studies were classified as cohort.
Populations examined included healthcare workers which accounted for 55.3% of all studies (N = 31/56), the general population at 33.9% (N = 19/56), patients with various illnesses at 5.4% (N = 3/56), and pregnant people at 3.6% (N = 2/56).
Multiple different COVID-19 vaccines were examined in these studies and some studies had multiple vaccines. The most common was the Pfizer-BioNTech (BNT162b2) vaccine, which was found in 39 studies, followed by the Oxford-AstraZeneca (AZD1222) vaccine, which was found in 20 studies, the third most common was the Moderna (mRNA-1273) vaccine which was found in 9 different studies. Other vaccines that were included in these studies were the Sinovac (CoronaVac) vaccine, the Johnson & Johnson (Ad26.COV2.S) vaccine, and the Sinopharm (BBIBP-CorV) vaccine.
Digital AEFI surveillance solutions
There were two broad categories of digital solutions identified. A small percentage of publications (N = 5.6%; N = 3/56) employed specifically designed AEFI digital surveillance systems (either purpose built or adapted from publicly available software) such as CANVAS, CANIM, Voxiva, TeleWatch, or SmartVax. However, most of the papers reported using publicly available software for data capture. The most frequently used software platforms were Google Forms (21.4%; N = 12/56) and REDCap (N = 7.1%; N = 4/56). These two categories overlap as the first set of solutions may leverage publicly available software. A comprehensive list of digital technologies used for surveillance, and their attributes, can be seen in Table 3. Crucially, many studies that were excluded from our review did not provide necessary details concerning their digital surveillance tool (N = 54) to determine what was used.
Table 3.
Response rates.
| Any reaction N(%) |
Severe Reaction N(%) |
Medically attended | Follow-up | Gender/Sex N(%) |
Age | Race/Religion/Cultural Group N(%) |
Vaccine type N(%) |
|
|---|---|---|---|---|---|---|---|---|
| Alhowaymel et al.17 - Saudi Arabia | 174/222(78.4) | N/A | N/A | N/A |
M–165(74.3) F–57(25.7) |
N(%) 18–29: 66(29.8) 30–40: 68(30.6) 41–51: 60(27.0) >52: 28(12.6) |
Saudi-138(62.2) Non-Saudi-84(37.8) |
AZ- 222(100) |
| Amodio et al.18 - Italy | 242/293(82.6) | N/A | N/A | Seven questionnaires sent for a week following first and second dose | F − 134/293(45.7) M − 159/293(54.3) |
Median(IQR) 36(29–52) |
N/A | Pfizer 293(100) |
| Angkasekwinai et al.19 - Thailand | CoronaVac v ChAdOx1 1st dose 152/180(84.4) vs. 119/180(66.1) CoronaVac v ChAdOx1 2nd dose 136/180(75.6) vs. 109/180(60.6) |
0(0) | N/A | 7 days after vaccination | F − 303(84.2) M − 57(15.8) |
Median(IQR) 35(29–44) |
N/A | CoronaVac 180(50) ChAdOx1 180(50) |
| Azzolini et al.20 - Italy | 1621/4156 | N/A | 8/2211 events(0.36) | 10 days after 2nd dose | M − 1589(38) F − 2567(62) |
Median 37 IQR 27–48 | N/A | Pfizer 4156(100) |
| Beatty et al.21 - United States | 1 dose of BNT162b2 or mRNA-1273 5629/8680(64.9) 2 doses of BNT162b2 or mRNA-1273 or 1 dose of JNJ-78436735 8947/11140(80.3) |
1 dose of BNT162b2 or mRNA-1273 26/8680(0.3) 2 doses of BNT162b2 or mRNA-1273 or 1 dose of JNJ-78436735 27/11140(0.2) |
N/A | Monthly surveys from January 14 – May 19, 2021 | M − 6024/19586(30.9) F – 13,281/19586(68.1) Transgender- 46/19586(0.23) Genderqueer-110/19586(0.6) Other-60/19586(0.3) |
Median (IQR) 54 (38–66) |
American Indian or Alaska Native 286(1.5) Asian 1506(7.8) Black 443(2.3) Native Hawaiian/Pacific Islander 87(0.4) White 17294(89.4) Other/Unknown 617(3.2) Hispanic 1476(7.6) |
Did not separate out for each |
| Bettinger et al.22 - Canada | 234174/683847(34.2) | 2055/683847(0.3) | 10092/683847(1.5) | 8 days after each of 3 doses sent questionnaire | M − 291144/683847(42.6) F − 391528/683847(57.3) Intersex/Decline − 1175/683847(0.17) |
20–29: 72750(10.6) 30–39: 111493(16.3) 40–49: 66067(9.7) 50–64: 138524(20.3) 65–79: 160767(23.5) 80+:23246(3.4) |
Black 2947/369351(0.8) Asian 9204/369351(2.5) Indigenous 1909/369351(0.5) Latino 3512/369351(0.9) Arabic 3871/369351(1.0) Indian/Pakistani 6188/369351(1.7) Southeast Asian 3503/369351(0.9) White 215626/369351(58.4) Mixed 5491/369351(1.5) Other/Unknown 112262/369351(30.4) Declined 4838/369351(1.3) |
Pfizer 369406(54) Moderna 201314(29.4) AZ 113127(16.5) |
| Briggs et al.23 - USA | 1st dose 459/719(63.8) 2nd dose 327/442(74.0) |
1st dose 122(16.9) 2nd dose 99(22.4) |
N/A | N/A | 1st dose F − 608(84.6) M − 111(15.4) 2nd dose F- 371(83.9) M-71(16.1) |
Mean(SD) 1st dose 53.0 (SD 11.8) 2nd dose 53.5 (SD 12.2) |
1st dose White − 677(94.2) Non-white − 32(4.4) Unknown − 10(1.4) 2nd dose White − 419(94.6) Non-white − 21(4.8) Unknown − 3(0.7) |
1st dose Pfizer-409(56.9) Moderna-258(35.9) Johnson-31(4.3) AZ-20(2.8) Other-1(0.1) 2nd dose Pfizer-269(60.9) Moderna-166(37.6) Johnson-0(0) AZ-6(1.4) Other-1(0.2) |
| Bsoul et al.24 - USA | 296(78) | 30(8) | N/A | N/A | F − 241(64) M- 134(35) Prefer not to answer − 4(1) |
18–24:56(15)25–34:101(27) 35–44:51(13)45–54:58(15)55+:113(30) |
Asian:62(16) Black:10(3)Hispanic:124(33) Other:12(3)White:171(45) |
Pfizer 379(100) |
| Chalermphanchai et al.25 - Thailand | 20/42(47.6) | 0(0) | N/A | Followed for 30 days | M − 12(28.3) F − 30(71.4) |
Mean 48 Range 23–62 |
N/A | Pfizer 42(100) |
| Cuschieri et al.26 - Italy | 34–1316/1480(2.3–88.9) across several symptoms | 2–186/1480(0.1–12.6) across several symptoms | N/A | Not specified | M − 493(33.3) F − 987(66.7) |
18–24 144(9.7) 25–34 474(32) 35–44 291(19.7)45–54 328(22.2)55–64 235(15.9)65+ 80(5.4) |
N/A | Pfizer 4885(100) |
| D’Arminio Monforte et al.27 - Italy | First dose 1836/3078(59.6) Second dose 2238/3049(73.4) |
0(0) | 0(0) | Two weeks after 2nd dose | F − 1980/3078(64.3) M − 1098/3078(35.7) |
Median(IQR) 47(34–56) |
Italian 2856(92.8) Other 222(7.2) |
Pfizer 3078(100) |
| Deng et al.28 - Australia | Pfizer Dose 1–483 003/1 346 308(35.9) Pfizer Dose 2–521 748/953 704(54.7) AZ Dose 1 - 228 685/433 427(52.8) AZ Dose 2 - 66 726/302 544(22.0) |
N/A | Pfizer Dose 1–8699/1 346 308(0.65) Pfizer Dose 2–13 073/953 704(1.4) AZ Dose 1–5260/433 427(1.2) AZ Dose 2–1266/302 544(0.42) |
N/A | Pfizer Dose 1 M: 160 764/565 158(28.4) F: 320 712/777 187(41.3) Other: 782/1700 (46) Pfizer Dose 2 M: 181 950/399 392(45.6) F: 338 101/551 535(61.3) Other: 690/1021(68) AZ Dose 1 M: 93 652/199 643(46.9) F: 133 113/230 019(57.9) Other: 199/285(70) AZ Dose 2 M: 23 052/136 689(16.9) F: 43 174/163 831(26.4) Other: 40/92 (44) |
Median (IQR) Pfizer Dose 1 42 (33–49) Pfizer Dose 2 44 (37–49) AZ Dose 1 61 (52–68) AZ Dose 2 62 (54–70) |
Pfizer Dose 1 Indigenous7443/20 245(36.8) Non-indigenous 467 856/1 303 080(35.9) Pfizer Dose 2 Indigenous 6447/12 228(52.7) Non-indigenous 498 268/910 202(54.7) AZ Dose 1 Indigenous 2230/4 551(49.0) Non-indigenous 219 938/416 314(52.8) AZ Dose 2 Indigenous 625/3019(20.7) Non-indigenous 62 624/284 443(22.0) |
Pfizer Dose 1 1 346 308/3 035 983(44.3) Pfizer Dose 2 953 704/3 035 983(31.4) AZ Dose 1 433 427/3 035 983(14.3) AZ Dose 2 66 726/3 035 983(2.2) |
| Ebinger et al.29 - USA | After dose 1 614/1032(60.0) After dose 2 752/1032(73.6) |
N/A | N/A | N/A | F − 691(67.4) M − 341(32.6) |
Average Age (SD) 43.3(12.6) |
Non-Hispanic Asian 280(27.1) Non-Hispanic Black 33(3.2) Non-Hispanic White 493(47.8) Hispanic/Latinx 126(12.2) Other 100(9.7) |
Pfizer 1032(100) |
| Figueroa et al.30 - Mexico | First dose 68/79(86) Second dose 64/79(81) |
First dose 0(0) Second dose 0(0) |
N/A | N/A | F − 51(64.6) M- 28(35.4) |
Median 42 years (IQR 35–46) | Mexican 79(100) |
Pfizer 79(100) |
| Lai et al.31 - Hong Kong | 80/160(50) | N/A | N/A | N/A | F − 90/160(56.25) M − 70/160(43.75) |
21–78 Median 40 |
N/A | Pfizer 160(100) |
| Gepner et al.32 - Israel | 422/1323(31.9) | 1/625(0.16) | 2/625(0.32) Hospitalization |
Followed up at the end of 1st and 2nd week after vaccination | M − 676(51.1) F − 647(48.9) |
<30: 239/422(56.6) 30–59: 169/422(39.8) 60+: 14/422(3.6) |
N/A | Covishield 1323(100) |
| Goldlin et al.33 - India | Second vaccine 102/355(30.4) Third vaccine 404/1179(34.2) |
Second vaccine 52(15.6) Third vaccine 120(10.2) |
N/A | N/A | Second vaccine M − 149 (42.1) F − 206(57.9) Third vaccine M − 512(43.4) F − 667(56.6) |
Second vaccine Average 51.8 Third vaccine 50.0 |
N/A | Second vaccine 355(100) Third vaccine 1179(100) |
| Guan et al.34 - Israel | 212/255(83.1) | 0(0) | 0(0) | Followed for 6 months after completing vaccine schedule | F − 144(56.5) M − 111(43.5) |
Mean(SD) 40.7(11.4) |
N/A | AZ 255(100) |
| Hammad et al.35 - Egypt | First dose 199/232(85.78) Second dose 136/232(58.62) |
N/A | N/A | N/A | M − 26(11.21) F − 206(88.79) |
Average 39(SD 9.97) |
N/A | AZ 232(100) |
| Hyun et al.36 - Korea | First dose 1450/1586(91.4) Second dose 1194/1306(91.4) |
First dose 0(0) Second dose 0(0) |
N/A | N/A | First dose M − 522/1586(32.9) F − 1064/1586(67.1) Second dose M − 388/1306(29.7) F − 918/1306(70.3) |
First dose 20–29546 (34.4%) 30–39402 (25.3%) 40–49336 (21.2%) 50–59220 (13.9%) 60–82 (5.2%) Second dose 20–29 427 (32.7%) 30–39 321 (24.6%) 40–49 288 (22.1%) 50–59 197 (15.1%) 60- 73 (5.6%) |
N/A | Pfizer First dose 1586(100) Second dose 1306(100) |
| Inoue et al.37 - Japan | 1st dose 975/994(98.1) 2nd dose 661/727(90.9) |
1/994(0.1) | 1st dose 13/994(1.3) 2nd dose 5/727(0.7) |
7 days following vaccination | 1st dose F − 762(76.7) M − 232(23.4) 2nd dose F − 559(76.9) M − 168(23.1) |
1st dose Mean 35.7 Range 19–63 2nd dose Mean 36.7 Range 20–63 |
N/A | AZ 1384(100) |
| Javed et al.38 - Saudi Arabia | 324/564(57.4) | 0(0) | N/A | 15–20 days Google Forms after vaccination | M − 210(37.2) F − 354(62.8) |
25 and below:108(18.4) 26–35:288(51.1) 36–45:110(19.5) 45+:62(11) |
N/A | AZ 564(100) |
| Jeon et al.39 - South Korea | Pfizer Dose 1 Local reactions: 996/1406(70.8) Systemic reactions: 850/1406(60.5) Pfizer Dose 2 Local reactions: 849/1168(72.7) Systemic reactions: 1010/1168(86.5) AZ Local reactions: 1195/1679(71.2) Systemic reactions: 1501/1679(89.4) |
N/A | Pfizer Dose 1 26/1406(1.8) Pfizer Dose 2 38/1168(3.3) AZ 143/1679(8.5) |
Not specified | F − 3216/4253(75.6) M − 1037/4253(24.4) |
20–29 1448(34) 30–39 1184(27.8) 40–49 903(21.2)50–59 561(13.2)60+ 157(3.7) |
N/A | Pfizer 2500/6385(39.2) AZ 3885/6385(60.8) |
| Kim et al.40 - South Korea | Pfizer 801/969(82.7) CoronaVac 543/1129(48.1) |
Adjusted odds ratios (severe allergic reaction) for those who received CoronaVac vs Pfizer Odds ratio (95% confidence interval) 0.62 (0.36–1.06) Adjusted odds ratios (severe allergic reaction) from the second dose compared with the first dose Odds ratio (95% confidence interval) CoronaVac 1.15 (0.62–2.15) Pfizer 2.01 (1.21–3.33), p < .05 |
N/A | Followed up 2 weeks after 2nd dose | Pfizer M − 498/969(51.4) F − 471/969(48.6) CoronaVac M − 527/1129(46.7) F − 602/1129(53.3) |
Pfizer Mean(SD) 43.13(16.54) CoronaVac 46.49(24.42) |
N/A | Pfizer 869/1998(43.5) CoronaVac 1129/1998(56.5) |
| Lee et al.41 - South Korea | 434/447(97.1) | 206(46.1) | N/A | 7 days after 2 injections | F − 388(86.8) M − 59(13.2) |
Mean(SD) 40.6(10.9) |
N/A | AZ 447(100) |
| Levy et al.42 - Israel | 1st dose 711/831(85.6) 2nd dose 673/738(91.2) |
N/A | N/A | Seven days after each dose, received text message | F − 627/831(75.5) M − 204/831(24.5) |
Mean 46.5(SD 11.8) |
N/A | Pfizer 831(100) |
| Lim et al.43 - Singapore | Dose 1 3–975/1704(0.2–57.2) Dose 2 13–1195/1704(0.8–70.1) |
0(0) | 196/1704(11.5) | 1 week after both doses | F − 1340(78.6) M − 364(21.4) |
Median (Range) 35(18–76) |
N/A | Pfizer 6101(100) |
| Lotan et al.44 - Israel | 136/239(56.9) | N/A | 8/36(22.2) | Week following vaccination | F- 199/262(75.9) M − 63/262(24.0) |
Median(range) 42(22–79) |
N/A | Pfizer 425(100) |
| Low et al.45 - Singapore | 57/88(68.4) | 0(0) | 0(0) | N/A | F − 88(100) | Mean 33.2(SD 3.3) | Chinese77(87.5) Malay 6 (6.8) Indian 2 (2.3) Others 3 (3.4) |
Pfizer 88(100) |
| Maruyama et al.46 - Japan | 348/374(93.0) | N/A | N/A | 8 days after vaccination for both doses | F − 225(60.2) M − 149(39.8) |
Mean(SD) 42.44(12.71) |
N/A | Pfizer 374(100) |
| Mofaz et al.47 - Israel | 1st dose 217/1609(13.5) 2nd dose 586/1609(36.4) 3rd dose 637/1609(39.6) |
N/A | N/A | N/A | F − 854(53.08) M − 755(46.92) |
Median 52 Range 18–88 |
N/A | Pfizer 1609(100) |
| Nachtigall et al.48 - Germany | 12084/16207(74.6) | 9/16207(0.05) | N/A | Not specified | F − 6131/8269(74.1) M − 2138/8269(25.9) |
18–30: 1096/8269(13.3) 31–40: 1817/8269(30.0) 41–50: 2044/8269(24.7) 51–60: 2515/8269(30.4) >61:627/8269(7.6 Invalid: 170/8269(2.1) |
N/A | Pfizer-Pfizer 4179/8246(50.7) Moderna-Moderna 207/8246(2.5) AZ-AZ 748/8246(9.1) AZ-Pfizer 1465/8246(17.8) AZ-Moderna 284/8246(3.4) Missing information 1363/8246(16.5) |
| Nittner-Marszalska et al.49 - Poland | 1st dose 1571/1707(92.03) 2nd dose 1587/1707(92.97) |
1st dose 0(0) 2nd dose 0(0) |
1st dose Pharmacological intervention 128(7.5) Medical consultation 6(0.35) 2nd dose Pharmacological intervention 567(33.2) Medical consultation 64(3.7) |
Not specified | M − 356(20.85) F- 1351(79.15) |
20–29: 585(34.27) 30–39: 554(32.45) 40–49: 264(15.47) 50–59: 160(9.37)60+: 144(8.44) |
N/A | Pfizer 1707(100) |
| Okumura et al.50 - Japan | 252/301(83.7) | 0(0) | First dose Day 0: 1/301(0.3) Day 1: 0 (0) Day 3: 1/196(0.5) Day 7: 0(0) Second dose Day 0: 1/179(0.6) Day 1: 0(0) Day 3: 1/138(0.7) Day 7: 0(0) Third dose Day 0: 0(0) Day 1: 1/38(2.6) Day 3: 0(0) Day 7: 0(0) |
1 week follow-up after each dose | M − 128/301(42.5) F- 172/301(57.1) |
18–29 238/301(79.1) 30–69 63/301(20.9) |
N/A | Moderna 301(100) |
| Park et al.51 - South Korea | AZ 1st dose 4–205/299(1.3–68.6) across several symptoms 2nd dose 1–174/304(0.3–57.2)across several symptoms Pfizer 1st dose 0–13/19(0–68.4) across several symptoms 2nd dose 0–15/22(0–68.2) across several symptoms |
Severe interference with work 161/603(26.7) Severe interference with daily life 195/644(30.3) |
AZ 1st dose 28/299(9,4) 2nd dose 3/299(0.1) Pfizer 1st dose 1/19(5.3) 2nd dose 1/22(4.5) |
N/A | AZ 1st dose M-80(26.8) F-219(73.2) 2nd dose M-80(26.3) F-224(73.7) Pfizer 1st dose M-2(10.5) F-17(89.5) 2nd dose M-2(9.1) F-20(90.9) |
AZ 1st dose 20–29:96(32.1) 30–39:56(18.7) 40–49:54(18.1) 50–59:81(27.1) 60+:12(4.0) AZ 2nd dose 20–29: 87(28.6) 30–39: 56(18.4) 40–49: 66(21.7) 50–59: 83(27.3) 60+:12(3.9) Pfizer 1st dose 20–29:11(57.9) 30–39:4(21.1) 40–49:1(5.3) 50–59: 3(15.8) 60+:0(0) Pfizer 2nd dose 20–29:10(45.5) 30–39:7(31.8) 40–49: 3(13.6) 50–59:2(9.1) 60+: 0(0) |
N/A | AZ 368/395(93.2) Pfizer 27/395(6.8) |
| Pellegrino et al.52 - Italy | after 1st, 2nd, 3rd dose Local 26.25% (21/80), 58.75% (47/80), and 28.37% (21/74) Systemic 52.2% (42/80), 48.75% (39/80), and 43.24% (32/74) |
0(0) | N/A | N/A | M − 42/80(52.5) F − 36/80(37.5) |
Median 47.5 |
N/A | Pfizer 80(100) |
| Presby et al.53 - USA | AZ First Dose 2915/3547(84.3) AZ Second Dose 191/325(58.7) Johnson 3751/4584(81.8) Moderna First Dose 10763/17632(61.0) Moderna Second Dose 14963/16987(88.1) Pfizer First Dose 14825/29366(50.5) Pfizer Second Dose 19854/27084(73.3) |
N/A | N/A | N/A | M − 65334/99435(65.7) F − 34101/99435(34.3) |
18–29 28107(28.3) 30–39 38928(39.1) 40–54 26164(26.3) 55+ 6236(6.27) |
N/A | AZ 3782(3.8) Johnson 4584(4.6) Moderna 34619(34.8) Pfizer 56450(56.8) |
| Rahmani et al.54 - Italy | Dose 1 2–285/296(0.7–96.3) across several local and systemic reactions Dose 2 6–257/275(2.2–93.5) across several local and systemic reactions |
Dose 1 0–6/296(0–2.0) across several local and systemic reactions Dose 2 0–17/275(0–6.2) across several local and systemic reactions |
Dose 1 0(0) Dose 2 0(0) |
7 days after both doses | F − 272(53.2) M − 240(0.47) |
Mean(SD) 28.9(2.7) |
N/A | Pfizer 512(100) |
| Rolfes et al.55 - Netherlands | 13959/22184(62.9) | N/A | N/A | 6 months after vaccination | M − 3199/13959(22.9) F − 10760/13959(77.1) |
0–50 6419(46) 51–60 3090(22.1) 61–79 3539(25.3) 80+ 911(6.5) |
N/A | Pfizer 10724/22590(47.5) AZ 8778/22590(38.9) Johnson 1508/22590(6.7) Spikevax 1508/22590(6.7) Unknown 72/2590(2.8) |
| Sadarangani et al.56 - Canada | Dose 1 Not pregnant 10950/174765(6.3) Pregnant 226/5597(4.0) Dose 2 Not pregnant 10254/91131(11.3) Pregnant 227/3108(7.3) |
Dose 1 Not pregnant 733/174765(0.4) Pregnant 31/5597(0.6) Dose 2 Not pregnant 343/91131(0.4) Pregnant 19/3108(0.6) |
Y for severe reactions | N/A | Dose 1 Not pregnant Woman:170674(97.7) Man:819(0.5) Non-binary:2380(1.4) Two-spirit:148(0.1) Other: 139(0.1) Unknown:605(0.3) Pregnant Woman: 5579(99.7) Man: 3(0.1) Non-binary:12(0.2) Two-spirit:0(0) Other:1(<0.1) Unknown:2(<0.1) Dose 2 Not pregnant Woman:89176(97.9) Man:341(0.4) Non-binary:1254(1.4) Two-spirit:49(0.1) Other:69(0.1) Unknown:242(0.3) Pregnant Woman:3091(99.5) Man:1(<0.1) Non-binary:13(0.4) Two-spirit:0(0) Other:0(0) Unknown: 3(0.1) |
Dose 1 Not pregnant 15–29: 59263(33.9) 30–49: 115502(66.1) Pregnant 15–29:1417(25.3) 30–49:4180(74.7) Dose 2 Not pregnant 15–29: 26324(28.9) 30–49:64807(71.1) Pregnant 15–29:716(23.0) 30–49:2392(77.0) |
Dose 1 Not pregnant White: 47539(27.2) Black:1155(0.7) East Asian:3367(1.9) South Asian:1977(1.1) Southeast Asian:1552(0.9) Indigenous:699(0.4) Middle Eastern:1352(0.8) Latino:1392(0.8) Mixed:2714(1.6) Unknown or Other:113018(64.7) Pregnant White: 1818(32.5) Black:18(0.3) East Asian:117(2.1) South Asian:95(1.7) Southeast Asian:47(0.8) Indigenous:22(0.4) Middle Eastern:44(0.8) Latino:48(0.9) Mixed :92(1.6) Unknown or Other:3296(58.9) Dose 2 Not pregnant White:50779(55.7) Black:1169(1.3) East Asian:3471(3.8) South Asian:2041(2.2) Southeast Asian:1587(1.7) Indigenous:703(0.8) Middle Eastern:1361(1.5) Latino: 1460(1.6) Mixed:2800(3.1) Unknown or Other:25758(28.3) Pregnant White:1778(57.2) Black:22(0.7) East Asian:106(3.4) South Asian:104(3.3) Southeast Asian:45(1.4) Indigenous:21(0.7) Middle Eastern:44(1.4) Latino:50(1.6) Mixed:88(2.8) Unknown or Other:850(27.3) |
Dose 1 Not pregnant Pfizer 107121/180362(59.4) Moderna 67644/180362(37.5) Pregnant Pfizer 3414/180362(1.9) Moderna 2183/180362(1.2) Dose 2 Not pregnant Pfizer 53077/94239(56.3) Moderna 38054/94239(40.4) Pregnant Pfizer 1892/94239(2.0) Moderna 1216/94239(1.3) |
| Sen et al.57 - Various countries | 8573/10900(79) | 351/10900(3) | 38/10900(0.3) | N/A | M − 2834/10900(26) F − 8066/10900(74) |
Median(IQR) 42(30–55) |
Caucasian 4972(45) African American 83(0.7) Asian 2018(18) Hispanic 1193(11) Native American/Indigenous/Pacific Islander 342(3) Do not wish to disclose 449(4) Other 865(8) Unanswered 1672(15) |
Pfizer 4339(39) AZ 1456(13) Johnson 95(1) Moderna 910(8) Novavax 14(0.1) Covishield 1194(11) Covaxin 248(2) Sputnik 204(2) Sinopharm 1821(17) Not sure 62(0.5) Others 563(5) |
| Shimamura et al.58 - Japan | 40–1910/1990(2.0–96.0) across several local and systemic reactions | 4/1990(0.2) | N/A | 2 days following each of 3 doses | M − 418(21) F − 1572(79) |
Median 32 |
N/A | Pfizer 1990(100) |
| Song et al.59 – South Korea | 809/998(81.1) | 0(0) | N/A | N/A | F − 779(78.1) M − 219(21.9) |
20–29: 380(38.1 30–39: 216(21.6) 40–49: 185(18.5) 50–59: 180(0.18) 60–64: 37(3.7) |
N/A | AZ 998(100) |
| Supangat et al.60 - Indonesia | 68/144(47.2) | N/A | N/A | Followed for 1 week after both doses | M − 38/144(26.4) F − 106/144(73.6) |
Average age range 21–25 | N/A | CoronaVac 144(100) |
| Tani et al.61 - Japan | 278/281(98.9) | N/A | N/A | Data collected until 7 days after booster dose | F − 204/281(72.6) M − 77/281(27.4) |
Median(IQR) 41(33–50) |
N/A | Pfizer 281(100) |
| Tawinprai et al.62 - Thailand | 322/538(59.9) | 0–41(7.62) across various local and systemic reactions |
N/A | Data collected until 7 days after vaccination | F − 517/794(65.1) | Median(IQR) 40(30–57) |
N/A | AZ 794(100) |
| Toussia-Cohen et al.63 - Israel | Second vaccination 73/78(93.6) Third vaccination 61/84(72.6) |
Second vaccination 0(0) Third vaccination 0(0) |
Second vaccination 0(0) Third vaccination 0(0) |
N/A | Second vaccinationF-78(100) Third vaccinationF-84(100) |
Second vaccination Mean 32.85(SD3.49) Third vaccination 33.23(3.95) |
N/A | Second vaccination Pfizer-78(100) Third vaccination Pfizer-84(100) |
| Vigezzi et al.64 - Italy | Male 376/720 (52.2) Female 1,286/1,939(66.3) |
285/2,659 (10.7) | N/A | N/A | F − 2600/5668(45.9) M − 3068/5668(54.1) |
Median 42 Range 19–76 |
N/A | Pfizer 5668(100) |
| Walmsley et al.65 - Canada | First dose 26/37(0.70) Second dose 906/955(94.9) |
N/A | N/A | 38(4%) reported persistent adverse events thought related to the vaccine at month 1, decreasing to 10(1%) at month five. | F or non-binary 760/1193(63.7) M 433/1193(36.3) |
30–50 41[36, 45] 70+ 73[71, 76] |
Arab/West Indian 10/1193(0.8) Black 20/1193(1.7) Indigenous 5/1193(0.4) Latin American 7/1193(0.6) South Asian 15/1193(1.3) Southeast Asian 32/1193(2.7) White 1045/1193(87.6) Other 52/1193(4.4) |
2 doses Pfizer 733(61.4) 2 doses Moderna 131(11.0) 1 dose Pfizer/1 dose Moderna 201(16.8) 1 dose AZ/1 dose Pfizer or Moderna 69(5.8) Other or unknown 36(3.0) |
| Warkentin et al.66 - USA | ChAdOx1/ChAdOx1 475/552(86) ChAdOx1/mRNA 1382/2383(58) mRNA/mRNA 4721/6212(0.76) |
N/A | ChAdOx1/ChAdOx1 69/462(14.9) ChAdOx1/mRNA 287/1638(17.5) mRNA/mRNA 796/5004(15.9) |
Followed up to 56 days after vaccination | F − 4661/8145(57.2) M − 3483/8145(42.8) Diverse - 1/8145(0.01) |
ChAdOx1/ChAdOx1 Mean(SD) 55.87(15.3) ChAdOx1/mRNA 47.6(13.89) mRNA/mRNA 45.87(15.14) |
N/A | ChAdOx1/ChAdOx1 487/8145(6.0) ChAdOx1/mRNA 1943/8145(23.9) mRNA/mRNA 5715/8145(70.2) |
| Wei et al.67 - China | Homologous Vero Cell Booster Group 62/635(9.8) Homologous CHO Cell Booster Group 13/75(17.3) Heterologous Mixed Vaccines Booster Group 17/82(20.7) |
N/A | N/A | N/A | F − 597/792 (75.4) M − 195/792(24.6) |
Ages 18–60 | N/A | Homologous Vero Cell Booster Group 635/792(80.1) Homologous CHO Cell Booster Group 75/792(9.5) Heterologous Mixed Vaccines Booster Group 82/792(10.4) |
| Yamazaki et al.68 - Japan | 1st dose 2134/2406(88.7) 2nd dose 2168/2347(92.4) |
1st dose 27(1.1) 2nd dose 154(6.6) |
1st dose 9(0.4) 2nd dose 13(0.6) |
N/A | 1st dose M − 921(38.3) F − 1485(61.7) 2nd dose M-898(38.3) F-1449(61.7) |
1st dose 20–29:657(27.3) 30–39:772(32.1) 40–49:551(22.9) 50–59:335(13.9) 60+:91(3.8) 2nd dose 20–29: 627(26.7)30–39: 750(32.0)40–49: 541(23.1)50–59: 339(14.4)60+: 90(3.8) |
N/A | Pfizer 1st dose 2406(100) 2nd dose 2347(100) |
| Yechezkel et al.69 - Israel | Retrospective Cohort 2–203(<1–1.1) |
Retrospective Cohort 0–170 (0–1) |
N/A | N/A | Prospective First booster M: 866/1785(48.5) F: 919/1785(51.5) Unspecified: 0/1785(0) Second booster M: 348/699(48.8) F: 350/699(50.2) Unspecified: 1/699(<1) First and Second Booster M: 215/446(48.2) F: 231/446(51.8) Unspecified: 0/446(0) Retrospective First booster M: 45208/94169(48.0) F: 48961/94169(52.0) Unspecified: 0/94169(0) First and Second Booster M: 8879/17814(49.8) F: 8935/1781(50.2) Unspecified: 0/1781(0) |
Prospective Median(IQR) First booster 52(34–61) Second booster 62(53–68) First and second booster 64(57–70) Retrospecitve First booster 47(31–61) First and second booster 69(62–76) |
Prospective First booster Jewish 1678(94) Arab 18(1.0) Unspecified89(5.0) Second Booster Jewish 672(96.1) Arab 1(<1) Unspecified26(3.7) First and second booster Jewish 434(97.3) Arab 0(0) Unspecified12(2.7) Retrospective First booster Jewish 90106(95.7) Arab 4046(4.3) Unspecified17(<1) First and second booster Jewish 17513(98.3) Arab 300(1.7) Unspecified1(<1) |
Pfizer 254698(100) |
| Zhang et al.70 - China | Female 0–124/1107(0–11.2) across several systemic and local reactions Male 0–25/290(0–8.6) across several systemic and local reactions |
N/A | N/A | 1 week following 2 doses | F − 1107/1397(79.2) M − 290/1397(20.8) |
F – Mean (SD) 34.7(8.6) M – Mean (SD) 38.7(9.9) |
N/A | CoronaVac 3013(100) |
| Zhu et al.71 - China | Female 0–124/1107(0–11.2) across several systemic and local reactions Male 0–25/290(0–8.6) across several systemic and local reactions |
N/A | N/A | 1 week following 2 doses | F − 1107/1397(79.2) M − 290/1397(20.8) |
F – Mean (SD) 34.7(8.6) M – Mean (SD) 38.7(9.9) |
N/A | CoronaVac 3013(100) |
A variety of communication mediums were used when reaching out to individuals within the various studies. Email was the most frequent (26.8%; N = 15/56), followed by SMS (23.2%; N = 13/56), cell-phone app notification (7.1%; N = 4/56), web-portal notification (1.7%; N = 1/56, or a combination of methods (8.9%; N = 3/56). 17 studies did not clearly specify how participants were communicated with (27.1%; N = 17/56), although it appears that prospective instructions were given to participants in person in some instances.
Response rates
There was a wide range of the response rates in the studies reflecting the heterogeneity of the technologies and study designs (See Table 3. for more information). In some instances, there is a higher response rate reported for specific genders or age groups. For example, in Vigezzi et al, females had a higher response rate (66.3%, N = 1286/1939) (p < .01) compared to males (52.2%, N = 376/720) (p < .01).
Discussion
This scoping review provides an overview of published research on the digital technologies used for active, participant-centered AEFI surveillance of COVID-19 vaccines approved by the World Health Organization (WHO) during the early stages of the pandemic. Our review provides a sample of the breadth of programs that were utilized during the pandemic. We observed a diversity of programs, with some appearing to be more specifically built for AEFI surveillance and others identifying existing software that could facilitate this function. There was a diversity in data collected among programs, methods of communicating with participants and participant response rates. We limited the search to this time period as we wanted to examine AEFI reporting in the context of the COVID-19 pandemic which was a highly unusual event and atypical situation for standard AEFI reporting. Future studies could expand this review to examine AEFI reporting of COVID-19 vaccines beyond the initial pandemic release of vaccines.
We also noted variability on the level of detail reported on these systems, particularly with respect to evaluation criteria and a substantial difference in response rates. Future research would benefit from further exploration of the best strategies to ensure optimal reporting of AEFI’s. Ultimately, however, surveillance systems need to be custom built for the local environment in which they will be implemented. Federal jurisdictions face challenges with respect to the collection of public health data from regional governments which unitary states do not.72 There is also a diversity of challenges for high income countries versus low- and middle-income countries.73 In many low- and middle-income countries (LIMCs), there is a lack of a formal vaccine safety monitoring system. Vaccines are often used without extensive post-licensure experience.74 For example, vaccines that target novel threats such as Lassa and Nipah viruses are employed in such environments. In response, sentinel sites, which are designed healthcare facilities, are provided the tools and resources to collect data from individuals who experience an adverse event post-vaccination.74 This approach has been successful in Mali and Niger when evaluating a new meningococcal vaccine.
The difference between females and males regarding AEFI response rate is still to be understood fully. This could be due to selection bias or behavior in terms of who response to online surveys or biological differences that may influence AEFI occurrence.75 The COVID-19 pandemic and subsequent vaccine roll-out demonstrated the need for AEFI surveillance systems and the value of digital technologies in supporting these systems. The rapid roll-out of a multitude of new vaccines, some using novel platforms, required post-market surveillance systems to ensure both the safety and effectiveness of these vaccines. COVID vaccines approve for use on an emergency basis further emphasized the need for robust post-market surveillance. AEFI surveillance systems were critical as they identified the risk of vaccine-induced immune thrombotic thrombocytopenia (VITT) with the ChAdOx1 CoV-19 vaccine and the risk of myocarditis from mRNA vaccines, quantified these risks and guided vaccine recommendations.76,77
This review can guide public health AEFI surveillance. Robust AEFI surveillance systems need to be in place in anticipation of future pandemic vaccines as well as to enhance monitoring of existing vaccine programs and the roll-out of novel vaccines.74 Standardization of AEFI surveillance and reporting of these systems is a priority of the WHO.78 For example, we observed that many studies found within this review would have benefitted from having a comparison group to serve as a control. Having a comparison group that is representative of the vaccinated population would allow the studies examined to have a more accurate assessment of AEFI risks and benefits.
The international community should prioritize the adoption of standardized definitions for events, using established frameworks such as those provided by the Brighton Collaboration.79 To ensure global consistency and facilitate seamless integration across digital systems, it is imperative to implement a WHO standard. This involves the development of an Adverse Events Following Immunization (AEFI) reporting framework that incorporates standardized forms or templates for comprehensive data collection, covering essential information such as patient demographics, vaccination details, and a detailed description of AEFI.79,80
In collaboration with the World Health Organization (WHO), the international community could further enhance this framework by developing a recognized system for coding AEFI events, akin to established medical coding systems like the International Classification of Diseases (ICD) or the Diagnostic and Statistical Manual of Mental Disorders (DSM).78 This holistic approach, combining standardized definitions, digital system integration, and a universally accepted coding system, would significantly contribute to the global effort in ensuring the safety of vaccines and streamlining the reporting and analysis of vaccine safety data.
AEFI data extracted through digital surveillance technologies
The studies included in this scoping review clearly defined the type of AEFI being detected in their respective participant populations. All studies reported local and systemic events (N = 56), although there was less consistency with respect to defining and reporting severe events, serious adverse events (SAEs) and medically attended adverse events (MAEs). Twenty-two studies report medically attended events and seven of the twenty-two studies report that participants experienced ‘severe’ events, although the term is not defined. Further information is provided in Table 3. including follow-up protocols for SAE surveillance where applicable.
Limitations
The protocol that was generated internally and used to conduct this scoping review was not registered. This scoping review did not conduct an environmental scan, thus it only included peer reviewed published articles and did not search the gray literature which encompasses non-published materials, such as newspaper articles, policy documents, conference abstracts, reports and any other forms of unpublished research. Due to investigator language proficiency, only records that were available in English could be included, which presents an issue due to the global scope of our study. Further, this scoping review only included articles up to December 31st, 2022, therefore, limiting our study inclusion and analyses to approximately the first two waves of the COVID-19 pandemic which encompassed the vaccine rollout of the primary vaccine and a 2nd booster in Canada. Our intent, however, was to examine AEFI systems for the release of the emerging vaccines during the pandemic period which would largely have occurred by this time period. AEFI reporting during the post-pandemic phase of COVID-19 would be similar to other AEFI reporting which we have previously reported on.81 Due to the rapidly evolving nature of the pandemic, newly emerging, COVID-19 vaccines, and changing landscape of active, participant-centered AEFI surveillance systems in response to these innovations, future studies should incorporate longer-term follow-up and continued evaluation of these surveillance systems as the pandemic progresses.
Conclusion and future directions
The scoping review has explored the different approaches and digital solutions for AEFI surveillance during the early stages of the COVID-19 pandemic. The rapid creation or repurposing of AEFI surveillance systems was a major challenge for public health systems during the pandemic. Learnings from each other experience can allow these systems to be better prepared for future pandemics as well as further augment their existing AEFI surveillance systems.
Appendix 1. Search Strategies
Embase Classic+Embase <1947 to 2022 December 15>
Ovid MEDLINE(R) ALL < 1946 to December 15, 2022>
EBM Reviews - Cochrane Central Register of Controlled Trials <November 2022>
COVID-19 Vaccines/36188
((coronavirus or 2019 ncov or 2019-ncov or covid or COVID-19 or COVID-19 virus or COVID-19 or COVID-19 virus or COVID-19 or COVID-19 virus or coronavirus disease 19 or coronavirus disease 2019 or coronavirus disease 2019 virus or coronavirus disease-19 or sars cov 2 or sars coronavirus 2 or sars-cov-2 or sars2) adj3 (vaccin* or immuni*)).tw,kf.63512
((mRNA or messenger RNA) adj3 vaccin*).tw,kf.14674
(BNT162b2 or BNT 162b2).tw,kf.8949
pfizer vaccin×.tw,kf.660
moderna vaccin×.tw,kf.773
astra zeneca vaccin×.tw,kf.46
(AZD1222 or azd 1222).tw,kf.1310
(mRNA-1273 or mRNA1273).tw,kf.3897
johnson vaccin×.tw,kf.154
Vaxzevria.tw,kf.638
astrazenica.tw,kf.70
Covishield.tw,kf.680
Spikevax.tw,kf.510
BNT162b1.tw,kf.63
ChAdOx1-S.tw,kf.367
or/1-16 76,261
(adverse event* or side effect*).tw,kf.1493402
Adverse Drug Reaction×.tw,kf.56363
exp “Drug-Related Side Effects and Adverse Reactions”/752180
((local or systemic) adj2 reaction*).tw,kf.36448
reactogenicity.tw,kf. or ae.fs. or aefi.tw,kf.3505587
risk/or risk factors/or patient safety/or “drug-related side effects and adverse reactions”/2873320
or/18-23 7,147,377
17 and 2419156
Vaccines, Synthetic/ae and COVID-19/95
25 or 26 19,156
product surveillance, postmarketing/or pharmacovigilance/27593
Adverse Drug Reaction Reporting Systems/13099
(pharmacovigilance or monitor* or drug evaluation*).tw,kf.2412654
Adverse Drug Reaction Reporting Systems/13099
Drug Evaluation/247384
surveillance.mp.650989
Self Report/190401
((self or patient) adj2 report*).tw,kf.728881
survey×.mp.3025822
questionnaire×.mp.2282175
or/28–37 7,685,377
or/28–38 7,685,377
27 and 395422
40 use medall1920
limit 41 to dt = 20211209–202212161196
exp SARS-CoV-2 vaccine/46143
((coronavirus or 2019 ncov or 2019-ncov or covid or COVID-19 or COVID-19 virus or COVID-19 or COVID-19 virus or COVID-19 or COVID-19 virus or coronavirus disease 19 or coronavirus disease 2019 or coronavirus disease 2019 virus or coronavirus disease-19 or sars cov 2 or sars coronavirus 2 or sars-cov-2 or sars2) adj3 (vaccin* or immuni*)).tw.62869
((mRNA or messenger RNA) adj3 vaccin*).tw.14094
(BNT162b2 or BNT 162b2).tw.8752
pfizer vaccin×.tw.627
moderna vaccin×.tw.737
astra zeneca vaccin×.tw.44
(AZD1222 or azd 1222).tw.1271
(mRNA-1273 or mRNA1273).tw.3784
johnson vaccin×.tw.147
Vaxzevria.tw.615
astrazenica.tw.70
Covishield.tw.640
Spikevax.tw.471
BNT162b1.tw.61
ChAdOx1-S.tw.359
or/43–58 77,013
vaccination reaction/or exp adverse drug reaction/752180
(adverse event* or side effect*).tw.1475630
AEFI.tw.1385
((local or systemic) adj2 reaction*).tw.36238
reactogenicity.tw.7637
or/60–64 2,100,903
59 and 65 11,633
exp SARS-CoV-2 vaccine/ae7833
exp SARS-CoV-2 vaccine/and (risk/or risk factor/or patient safety/)2331
66 or 67 or 68 17,452
postmarketing surveillance/or drug surveillance program/or active surveillance/46398
pharmacovigilance/7477
(surveillance or pharmacovigilance or monitor* or drug evaluation*).tw.2844349
drug screening/254360
self report/190401
((self or patient) adj2 report*).tw.720219
(survey* or questionnaire*).mp.4344459
or/70–76 7,600,425
69 and 774992
78 use emczd3113
limit 79 to dc = 20211209–202212162439
COVID-19 Vaccines/36188
((coronavirus or 2019 ncov or 2019-ncov or covid or COVID-19 or COVID-19 virus or COVID-19 or COVID-19 virus or COVID-19 or COVID-19 virus or coronavirus disease 19 or coronavirus disease 2019 or coronavirus disease 2019 virus or coronavirus disease-19 or sars cov 2 or sars coronavirus 2 or sars-cov-2 or sars2) adj3 (vaccin* or immuni*)).tw,kw.68854
((mRNA or messenger RNA) adj3 vaccin*).tw,kw.14268
(BNT162b2 or BNT 162b2).tw,kw.8874
pfizer vaccin×.tw,kw.655
moderna vaccin×.tw,kw.771
astra zeneca vaccin×.tw,kw.46
(AZD1222 or azd 1222).tw,kw.1306
(mRNA-1273 or mRNA1273).tw,kw.3866
johnson vaccin×.tw,kw.147
Vaxzevria.tw,kw.634
astrazenica.tw,kw.70
Covishield.tw,kw.673
Spikevax.tw,kw.508
BNT162b1.tw,kw.63
ChAdOx1-S.tw,kw.367
or/81–96 80,358
(adverse event* or side effect*).tw,kw.1515106
Adverse Drug Reaction×.tw,kw.82184
100exp “Drug-Related Side Effects and Adverse Reactions”/752180
101((local or systemic) adj2 reaction*).tw,kw.36275
102reactogenicity.tw,kw. or ae.fs. or aefi.tw,kw.3505562
103risk/or risk factors/or patient safety/or “drug-related side effects and adverse reactions”/2873320
104or/98–103 7,175,226
10597 and 104 19,496
106Vaccines, Synthetic/ae and COVID-19/95
107105 or 106 19,496
108product surveillance, postmarketing/or pharmacovigilance/27593
109Adverse Drug Reaction Reporting Systems/13099
110(pharmacovigilance or monitor* or drug evaluation*).tw,kw.2396986
111Adverse Drug Reaction Reporting Systems/13099
112Drug Evaluation/247384
113surveillance.mp.650989
114Self Report/190401
115((self or patient) adj2 report*).tw,kw.722922
116survey×.mp.3025822
117questionnaire×.mp.2282175
118or/108–117 7,670,074
119107 and 1185458
120119 use cctr141
121limit 120 to yr=“2022”66
12242 or 80 or 1213701
123remove duplicates from 1222717
Ovid MEDLINE(R) ALL <1946 to December 15, 2022>
COVID-19 Vaccines/16941
((coronavirus or 2019 ncov or 2019-ncov or covid or COVID-19 or COVID-19 virus or COVID-19 or COVID-19 virus or COVID-19 or COVID-19 virus or coronavirus disease 19 or coronavirus disease 2019 or coronavirus disease 2019 virus or coronavirus disease-19 or sars cov 2 or sars coronavirus 2 or sars-cov-2 or sars2) adj3 (vaccin* or immuni*)).tw,kf.29013
((mRNA or messenger RNA) adj3 vaccin*).tw,kf.6420
(BNT162b2 or BNT 162b2).tw,kf.3648
pfizer vaccin×.tw,kf.234
moderna vaccin×.tw,kf.314
astra zeneca vaccin×.tw,kf.14
(AZD1222 or azd 1222).tw,kf.369
(mRNA-1273 or mRNA1273).tw,kf.1358
johnson vaccin×.tw,kf.59
Vaxzevria.tw,kf.177
astrazenica.tw,kf.3
Covishield.tw,kf.214
Spikevax.tw,kf.130
BNT162b1.tw,kf.18
ChAdOx1-S.tw,kf.139
or/1-16 33,413
(adverse event* or side effect*).tw,kf.493062
Adverse Drug Reaction×.tw,kf.19521
exp “Drug-Related Side Effects and Adverse Reactions”/129577
((local or systemic) adj2 reaction*).tw,kf.12741
reactogenicity.tw,kf. or ae.fs. or aefi.tw,kf.1954820
risk/or risk factors/or patient safety/or “drug-related side effects and adverse reactions”/1117659
or/18-23 3,175,642
17 and 247348
Vaccines, Synthetic/ae and COVID-19/94
25 or 267348
product surveillance, postmarketing/or pharmacovigilance/10588
Adverse Drug Reaction Reporting Systems/8665
(pharmacovigilance or monitor* or drug evaluation*).tw,kf.966031
Adverse Drug Reaction Reporting Systems/8665
Drug Evaluation/42048
surveillance.mp.277616
Self Report/41786
((self or patient) adj2 report*).tw,kf.279327
survey×.mp.1217430
questionnaire×.mp.913528
or/28–37 2,803,321
or/28–38 2,803,321
27 and 391920
Embase Classic+Embase <1947 to 2022 December 15>
exp SARS-CoV-2 vaccine/27938
((coronavirus or 2019 ncov or 2019-ncov or covid or COVID-19 or COVID-19 virus or COVID-19 or COVID-19 virus or COVID-19 or COVID-19 virus or coronavirus disease 19 or coronavirus disease 2019 or coronavirus disease 2019 virus or coronavirus disease-19 or sars cov 2 or sars coronavirus 2 or sars-cov-2 or sars2) adj3 (vaccin* or immuni*)).tw.32738
((mRNA or messenger RNA) adj3 vaccin*).tw.7628
(BNT162b2 or BNT 162b2).tw.5040
pfizer vaccin×.tw.399
moderna vaccin×.tw.434
astra zeneca vaccin×.tw.30
(AZD1222 or azd 1222).tw.867
(mRNA-1273 or mRNA1273).tw.2376
johnson vaccin×.tw.91
Vaxzevria.tw.431
astrazenica.tw.53
Covishield.tw.424
Spikevax.tw.354
BNT162b1.tw.38
ChAdOx1-S.tw.204
or/1-16 42,179
vaccination reaction/or exp adverse drug reaction/618761
(adverse event* or side effect*).tw.792306
AEFI.tw.776
((local or systemic) adj2 reaction*).tw.20492
reactogenicity.tw.2959
or/18-22 1,286,882
17 and 237196
exp SARS-CoV-2 vaccine/ae4332
exp SARS-CoV-2 vaccine/and (risk/or risk factor/or patient safety/)1940
24 or 25 or 26 10,427
postmarketing surveillance/or drug surveillance program/or active surveillance/41029
pharmacovigilance/4339
(surveillance or pharmacovigilance or monitor* or drug evaluation*).tw.1590371
drug screening/199586
self report/145953
((self or patient) adj2 report*).tw.390578
(survey* or questionnaire*).mp.2650343
or/28–34 4,521,044
27 and 353113
EBM Reviews – Cochrane Central Register of Controlled Trials <November 2022>
COVID-19 Vaccines/210
((coronavirus or 2019 ncov or 2019-ncov or covid or COVID-19 or COVID-19 virus or COVID-19 or COVID-19 virus or COVID-19 or COVID-19 virus or coronavirus disease 19 or coronavirus disease 2019 or coronavirus disease 2019 virus or coronavirus disease-19 or sars cov 2 or sars coronavirus 2 or sars-cov-2 or sars2) adj3 (vaccin* or immuni*)).tw,kw.1462
((mRNA or messenger RNA) adj3 vaccin*).tw,kw.342
(BNT162b2 or BNT 162b2).tw,kw.165
pfizer vaccin×.tw,kw.13
moderna vaccin×.tw,kw.13
astra zeneca vaccin×.tw,kw.1
(AZD1222 or azd 1222).tw,kw.58
(mRNA-1273 or mRNA1273).tw,kw.109
johnson vaccin×.tw,kw.3
Vaxzevria.tw,kw.20
astrazenica.tw,kw.14
Covishield.tw,kw.25
Spikevax.tw,kw.11
BNT162b1.tw,kw.6
ChAdOx1-S.tw,kw.20
or/1–161627
(adverse event* or side effect*).tw,kw.229879
Adverse Drug Reaction×.tw,kw.29100
exp “Drug-Related Side Effects and Adverse Reactions”/3842
((local or systemic) adj2 reaction*).tw,kw.3114
reactogenicity.tw,kw. or ae.fs. or aefi.tw,kw.140676
risk/or risk factors/or patient safety/or “drug-related side effects and adverse reactions”/31571
or/18–23 356,402
17 and 24664
Vaccines, Synthetic/ae and COVID-19/0
25 or 26664
product surveillance, postmarketing/or pharmacovigilance/123
Adverse Drug Reaction Reporting Systems/95
(pharmacovigilance or monitor* or drug evaluation*).tw,kw.108677
Adverse Drug Reaction Reporting Systems/95
Drug Evaluation/5750
surveillance.mp.9469
Self Report/2662
((self or patient) adj2 report*).tw,kw.56917
survey×.mp.70305
questionnaire×.mp.163449
or/28–37 329,913
27 and 38141
Disclosure statement
KW is Chief Scientists of CANImmunize Inc and has served as a member of the independent data safety advisory board for Medicago and Moderna. KAT receives research support from the Coalition of Epidemic Preparedness Innovations for vaccine safety studies. During the conduct of this work, D. B. F. worked for the University of Ottawa and had academic appointments at the Children’s Hospital of Eastern Ontario Research Institute and ICES; she is currently employed by Pfizer.
Financial support
This work was supported by the Public Health Agency of Canada and Canadian Institute of Health Research through the Canadian Immunization Research Network (FRN#151944).
References
- 1.Kochhar S, Salmon DA.. Planning for COVID-19 vaccines safety surveillance. Vaccine. 2020;38(40):6194–28. doi: 10.1016/j.vaccine.2020.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petousis-Harris H. Assessing the safety of COVID-19 vaccines: a primer. Drug Saf. 2020;43(12):1205–10. doi: 10.1007/s40264-020-01002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee GM, Romero JR, Bell BP. Postapproval vaccine safety surveillance for COVID-19 vaccines in the US. JAMA - J Am Med Assoc. 2020;324(19):1937. doi: 10.1001/jama.2020.19692. [DOI] [PubMed] [Google Scholar]
- 4.Crawford NW, Clothier H, Hodgson K, Selvaraj G, Easton ML, Buttery JP. Active surveillance for adverse events following immunization. Expert Rev Vaccines. 2014;13(2):265–76. doi: 10.1586/14760584.2014.866895. [DOI] [PubMed] [Google Scholar]
- 5.Cashman P, Macartney K, Khandaker G, King C, Gold M, Durrheima DN. Participant-centred active surveillance of adverse events following immunisation: a narrative review. Int Health. 2017;9(3):164–76. doi: 10.1093/inthealth/ihx019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heininger U, Holm K, Caplanusi I, et al. Guide to active vaccine safety surveillance: report of CIOMS working group on vaccine safety – executive summary. Vaccine. 2017;35(32):3917–21. doi: 10.1016/j.vaccine.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Whitelaw S, Mamas MA, Topol E, van Spall HGC. Applications of digital technology in COVID-19 pandemic planning and response. Lancet Digit Health. 2020;2(8):e435–e440. doi: 10.1016/S2589-7500(20)30142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du J, Xiang Y, Sankaranarayanapillai M, Zhang M, Wang J, Si Y, HA Pham, Xu H, Chen Y, Tao C. Extracting postmarketing adverse events from safety reports in the vaccine adverse event reporting system (VAERS) using deep learning. J Am Med Inform Assn. 2021;28(7):1393–400. doi: 10.1093/jamia/ocab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mbunge E, Akinnuwesi B, Fashoto SG, Metfula AS, Mashwama P. A critical review of emerging technologies for tackling COVID-19 pandemic. Hum Behav Emerg Technol. 2021;3(1):25–39. doi: 10.1002/hbe2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odone A, Gianfredi V, Sorbello S, Capraro M, Frascella B, Vigezzi GP, Signorelli C. The use of digital technologies to support vaccination programmes in Europe: state of the art and best practices from experts’ interviews. Vaccines (Basel). 2021;9(10):1126. doi: 10.3390/vaccines9101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) . V-safe after vaccination Health checker. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html.
- 12.Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5(1). doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol Theory Pract. 2005;8(1):19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 14.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization . World Health Organization (WHO): 10 vaccines approved for use by WHO. https://covid19.trackvaccines.org/agency/who/.
- 16.Covidence . Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org. [Google Scholar]
- 17.Alhowaymel F, Abdelmalik MA, Mohammed AM, Mohamaed MO, Alenezi A. Reported side effects of COVID-19 vaccination among adults in Saudi Arabia: a cross-sectional study. SAGE Open Nurs. 2022;8:23779608221103210. doi: 10.1177/23779608221103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amodio E, Minutolo G, Casuccio A, Costantino C, Graziano G, Mazzucco W, Pieri A, Vitale F, Zarcone M, Restivo V. Adverse reactions to anti-SARS-CoV-2 vaccine: a Prospective Cohort study based on an active surveillance system. Vaccines (Basel). 2022;10(3):345. doi: 10.3390/vaccines10030345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angkasekwinai N, Sewatanon J, Niyomnaitham S, et al. Comparison of safety and immunogenicity of CoronaVac and ChAdOx1 against the SARS-CoV-2 circulating variants of concern (alpha, Delta, beta) in Thai healthcare workers. Vaccine. 2022;10:100153. doi: 10.1016/j.jvacx.2022.100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azzolini E, Canziani LM, Voza A, et al. Short-term adverse events and antibody response to the BNT162b2 SARS-CoV-2 vaccine in 4156 Health care professionals. Vaccines (Basel). 2022;10(3):439. doi: 10.3390/vaccines10030439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beatty AL, Peyser ND, Butcher XE, Cocohoba JM, Lin F, Olgin JE, Pletcher MJ, Marcus GM. Analysis of COVID-19 vaccine type and adverse effects following vaccination. JAMA Netw Open. 2021;4(12):e2140364. doi: 10.1001/jamanetworkopen.2021.40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettinger JA, Sadarangani M, De Serres G, et al. Protocol: the Canadian National vaccine safety network: surveillance of adverse events following immunisation among individuals immunised with the COVID-19 vaccine, a cohort study in Canada. BMJ Open. 2022;12(1):51254. doi: 10.1136/BMJOPEN-2021-051254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Briggs FBS, Mateen FJ, Schmidt H, et al. COVID-19 vaccination reactogenicity in persons with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;9(1). doi: 10.1212/NXI.0000000000001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bsoul EA, Loomer PM, Patel SKS. COVID-19 vaccination experience among United States dental professionals and students: safety, confidence, concerns, and side effects. PLoS One. 2022;17(2):e0264323. doi: 10.1371/journal.pone.0264323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalermphanchai N, Arunothong W, Jettavan N, et al. Safety, tolerability, and antibody response after intradermal vaccination of PFE-BNT in adults who have completed two-doses of verocell (inactivated vaccine). Vaccine. 2022;10:100148. doi: 10.1016/j.jvacx.2022.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuschieri S, Gauci C, Agius S, Grech V. Vaccine hesitancy among Maltese healthcare workers vis-a-vis influenza and COVID-19 vaccination. Malta Medical Journal. 2022;34(3):39–49. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emexb&NEWS=N&AN=2017807278. [Google Scholar]
- 27.d’Arminio Monforte A, Tavelli A, Perrone PM, et al. Association between previous infection with SARS CoV-2 and the risk of self-reported symptoms after mRNA BNT162b2 vaccination: data from 3,078 health care workers. EClinicalMedicine. 2021;36:100914. doi: 10.1016/j.eclinm.2021.100914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng L, Glover C, Dymock M, et al. The short term safety of COVID-19 vaccines in Australia: AusVaxSafety active surveillance, February – August 2021. Med J Aust. 2022;217(4):195–202. doi: 10.5694/mja2.51619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebinger JE, Lan R, Sun N, et al. Symptomology following mRNA vaccination against SARS-CoV-2. Prev Med. 2021;153. doi: 10.1016/J.YPMED.2021.106860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Figueroa-Hurtado E, Ortiz-Farias DL, Sada-Ovalle I, Maldonado-Ortiz SY, Valdivieso-Jimenez JA, Cortes-Telles A. Humoral immune response in healthcare workers after two doses of a BNT162b2 mRNA vaccine in Yucatan, Mexico. Respirol Case Rep. 2022;10(4):e0920. doi: 10.1002/rcr2.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai FTT, Chua GT, Chan EWW, et al. Adverse events of special interest following the use of BNT162b2 in adolescents: a population-based retrospective cohort study. Emerg Microbes Infect. 2022;11(1):885–893. doi: 10.1080/22221751.2022.2050952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gepner Y, Mofaz M, Oved S, Yechezkel M, Constantini K, Goldstein N, Eisenkraft A, Shmueli E, Yamin D. Utilizing wearable sensors for continuous and highly-sensitive monitoring of reactions to the BNT162b2 mRNA COVID-19 vaccine. Commun Med. 2022;2(1):27. doi: 10.1038/s43856-022-00090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldlin T, Kalyanaraman S, Ravichandran M, Ramya J. A pharmacovigilance study of covishield in a tertiary care teaching hospital in Tamil Nadu. J Pharmacol Pharmacother. 2021;12(3):131–136. doi: 10.4103/jpp.jpp_63_21. [DOI] [Google Scholar]
- 34.Guan G, Mofaz M, Qian G, Patalon T, Shmueli E, Yamin D, Brandeau ML. Higher sensitivity monitoring of reactions to COVID-19 vaccination using smartwatches. npj Digit Med. 2022;5(1):140. doi: 10.1038/s41746-022-00683-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammad NM, Kadry HM, Malek MM, Bahgat SM, Abdelsalam NM, Afifi AHM, Abo-Alella DA. Maintenance of antibody response in Egyptian healthcare workers vaccinated with ChAdOx1 nCoV-19 vaccine during Delta and omicron variants pandemic: a Prospective study. Vaccines (Basel). 2022;10(10):1706. doi: 10.3390/vaccines10101706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyun J, Park Y, Song YG, et al. Reactogenicity and immunogenicity of the ChAdOx1 nCOV-19 coronavirus disease 2019 vaccine in South Korean healthcare workers. Yonsei Med J. 2022;63(12):1078–87. doi: 10.3349/ymj.2022.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoue S, Igarashi A, Morikane K, Hachiya O, Watanabe M, Kakehata S, Sato S, Ueno Y. Adverse reactions to BNT162b2 mRNA COVID-19 vaccine in medical staff with a history of allergy. Respir Investig. 2022;60(2):248–255. doi: 10.1016/j.resinv.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Javed D, Hadi Alharbi Y, Javed A, Iqbal J. Symptoms and side effects of the ChAdOx1 nCoV-19 vaccine (AZD1222) among healthcare workers. PJMHS. 2021;15(12):3202–3204. doi: 10.53350/pjmhs2115123202. [DOI] [Google Scholar]
- 39.Jeon M, Kim J, Oh CE, Lee JY. Adverse events following immunization associated with the first and second doses of the chadox1 ncov-19 vaccine among healthcare workers in korea. Vaccines (Basel). 2021;9(10):1096. doi: 10.3390/vaccines9101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim T, Park SY, Yu S, JW Park, Lee E, MH Jeon, TH Kim, EJ Choo.Impacts of side effects to bnt162b2 and the first dose of chadox1 anti‐sars‐cov‐2 vaccination on work productivity, the need for medical attention, and vaccine acceptance: a multicenter survey on healthcare workers in referral teaching hospitals in the republic of korea. Vaccines (Basel). 2021;9(6). doi: 10.3390/VACCINES9060648/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee GM, Romero JR, Bell BP. Postapproval vaccine safety surveillance for COVID-19 vaccines in the US. JAMA. 2020;324(19):1937–8. doi: 10.1001/JAMA.2020.19692. [DOI] [PubMed] [Google Scholar]
- 42.Levy I, Levin EG, Olmer L, et al. Correlation between adverse events and antibody titers among healthcare workers vaccinated with BNT162b2 mRNA COVID-19 vaccine. Vaccines (Basel). 2022;10(8):1220. doi: 10.3390/vaccines10081220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim SM, Chan HC, Santosa A, Quek SC, Liu EHC, Somani J. Safety and side effect profile of pfizer-BioNTech COVID-19 vaccination among healthcare workers: a tertiary hospital experience in Singapore. Ann Acad Med Singapore. 2021;50(9):703–11. doi: 10.47102/ANNALS-ACADMEDSG.2021160. [DOI] [PubMed] [Google Scholar]
- 44.Lotan I, Wilf-Yarkoni A, Friedman Y, Stiebel-Kalish H, Steiner I, Hellmann MA. Safety of the BNT162b2 COVID-19 vaccine in multiple sclerosis (MS): early experience from a tertiary MS center in Israel. Eur J Neurol. 2021;28(11):3742–8. doi: 10.1111/ENE.15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Low JM, Lee LY, Ng YPM, Zhong Y, Amin Z. Breastfeeding mother and child clinical outcomes after COVID-19 vaccination. J Hum Lact. 2022;38(1):37–42. doi: 10.1177/08903344211056522. [DOI] [PubMed] [Google Scholar]
- 46.Maruyama A, Sawa T, Teramukai S, Katoh N. Adverse reactions to the first and second doses of Pfizer-BioNTech COVID-19 vaccine among healthcare workers. J Infect Chemother. 2022;28(7):934–942. doi: 10.1016/j.jiac.2022.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mofaz M, Yechezkel M, Guan G, Brandeau ML, Patalon T, Gazit S, Yamin D, Shmueli E. Self-reported and physiologic reactions to Third BNT162b2 mRNA COVID-19 (booster) vaccine dose. Emerg Infect Dis. 2022;28(7):1375–1383. doi: 10.3201/eid2807.212330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nachtigall I, Bonsignore M, Hohenstein S, et al. Effect of gender, age and vaccine on reactogenicity and incapacity to work after COVID-19 vaccination: a survey among health care workers. BMC Infect Dis. 2022;22(1):291. doi: 10.1186/s12879-022-07284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nittner-Marszalska M, Rosiek-Biegus M, Kopeć A, Pawłowicz R, Kosińska M, Łata A, Szenborn L. Pfizer-BioNTech COVID-19 vaccine tolerance in allergic versus non-allergic individuals. Vaccines (Basel). 2021;9(6):553. doi: 10.3390/VACCINES9060553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okumura K, Hara A, Inada I, Sugiyama D, Hoshino T, Yakoh T, Yokoyama H, Urushihara H. Real-time survey of vaccine safety of the mRNA-1273 SARS-CoV-2 vaccine in workplace vaccination at Keio University. Vaccines (Basel). 2022;10(9):1461. doi: 10.3390/vaccines10091461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park C, Sakong J, Jo S, Kim M, Baek K. Adverse effects on work and daily life interference among healthcare workers after the first and second chadox1 and bnt162b2 COVID-19 vaccine doses. Vaccines (Basel). 2021;9(8):926. doi: 10.3390/vaccines9080926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pellegrino R, Pellino G, Selvaggi L, Selvaggi F, Federico A, Romano M, AG Gravina. 2022. BNT162B2 MRNA COVID-19 vaccine safety in inflammatory bowel disease patients on biologic therapy: a monocentric real-world study. United European Gastroenterol J. 10(Supplement 8):1088. doi: 10.1002/ueg2.12290. [DOI] [PubMed] [Google Scholar]
- 53.Presby DM, Capodilupo ER. Biometrics from a wearable device reveal temporary effects of COVID-19 vaccines on cardiovascular, respiratory, and sleep physiology. J Appl Physiol. 2022;132(2):448–458. doi: 10.1152/japplphysiol.00420.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rahmani A, Dini G, Orsi A, et al. Reactogenicity of BNT162b2 mRNA COVID-19 vaccine in a Young Working age population: a survey among Medical School residents, within a Mass vaccination campaign, in a regional reference teaching Hospital in Italy. Vaccines (Basel). 2021;9(11):1269. doi: 10.3390/VACCINES9111269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rolfes L, Harmark L, Kant A, Van Balveren L, Van Hunsel F. Cohort event monitoring of COVID-19 vaccine reactogenicity in the Netherlands using patient reported outcomes. Drug Saf. 2021;44(12):1418–1419. doi: 10.1007/s40264-021-01129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sadarangani M, Soe P, Shulha P, et al. Safety of COVID-19 vaccines in pregnancy: a Canadian National vaccine safety (CANVAS) network cohort study. Lancet Infect Dis. 2022;22(11):1553–1564. doi: 10.1016/S1473-3099(22)00426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sen P, R N, Nune A, et al. POS1260 COVID-19 vaccination-related adverse events among autoimmune disease patients: results from the COVID-19 vaccination in autoimmune diseases (COVAD) study. Ann Rheum Dis. 2022;81(Suppl 1):966–967. doi: 10.1136/annrheumdis-2022-eular.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shimamura Y, Anbo Y, Furuta Y. Post-vaccination adverse reactions after receiving the Pfizer-BioNTech coronavirus disease 2019 vaccines among healthcare workers in Sapporo, Japan. Cureus. 2022;14(3):e23549. doi: 10.7759/cureus.23549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song JE, Oh GB, Park HK, Lee SS, Kwak YG. Survey of adverse events after the First dose of the ChAdOx1 nCoV-19 vaccine: a single-center experience in Korea. Infect Chemother. 2021;53(3):557. doi: 10.3947/IC.2021.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Supangat, Sakinah EN, Nugraha MY, Qodar TS, Mulyono BW, Tohari AI. COVID-19 vaccines programs: adverse events following immunization (AEFI) among medical clerkship Student in Jember, Indonesia. BMC Pharmacol Toxicol. 2021;22(1). doi: 10.1186/S40360-021-00528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tani N, Ikematsu H, Goto T, et al. Correlation of post-vaccination fever with specific antibody response to SARS-CoV-2 BNT162b2 booster and no significant influence of antipyretic medication. medRxiv. Published online 2022. doi: 10.1101/2022.07.25.22277569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tawinprai K, Siripongboonsitti T, Porntharukchareon T, Dechates B, Monprach H, Sornsamdang G, Wittayasak K, Soonklang K, Mahanonda N. Persistence of immunogenicity, contributing factors of an immune response, and reactogenicities after a single dose of the ChAdOx1 (AZD1222) COVID-19 vaccine in the Thai population. Hum Vaccin Immunother. 2022;18(1):2035573. doi: 10.1080/21645515.2022.2035573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toussia-Cohen S, Yinon Y, Peretz-Machluf R, et al. Early adverse events and immune response Following Second and Third COVID-19 vaccination in pregnancy. J Clin Med. 2022;11(16):4720. doi: 10.3390/jcm11164720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vigezzi GP, Lume A, Minerva M, Nizzero P, Biancardi A, Gianfredi V, Odone A, Signorelli C, Moro M. Safety surveillance after BNT162b2 mRNA COVID-19 vaccination: results from a cross-sectional survey among staff of a large Italian teaching hospital. Acta Biomed. 2021;92(S6). doi: 10.23750/ABM.V92IS6.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walmsley S, Szadkowski L, Wouters B, et al. Safety and efficacy of preventative COVID vaccines: the StopCoV study.medRxiv. Published online 2022. doi: 10.1101/2022.02.09.22270734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Warkentin L, Zeschick N, Kuhlein T, Steininger P, Überla K, Kaiser I, Gall C, Sebastião M, Hueber S. Reactogenicity after heterologous and homologous COVID-19 prime-boost vaccination regimens: descriptive interim results of a comparative observational cohort study. BMC Infect Dis. 2022;22(1):504. 0531. doi: 10.1186/s12879-022-07443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei Y, Wang Y, Liu J, et al. Investigation of adverse events experienced by healthcare workers following immunization with Homologous orHeterologous COVID-19 booster vaccinations. Vaccines (Basel). 2022;10(11):1869. doi: 10.3390/vaccines10111869. 1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamazaki S, Watanabe K, Okuda Y, et al. Adverse effect investigation using application software after vaccination against SARS-CoV-2 for healthcare workers. Journal of Infection and Chemotherapy. 2022;28(6):791–796. doi: 10.1016/j.jiac.2022.02.020. 0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yechezkel M, Mofaz M, Painsky A, Patalon T, Gazit S, Shmueli E, Yamin D. Safety of the fourth COVID-19 BNT162b2 mRNA (second booster) vaccine: a prospective and retrospective cohort study. Lancet Respir Med. 2023;11(2):139–150. Published online 20221118. doi: 10.1016/S2213-2600(22)00407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang MX, Zhang TT, Shi GF, Cheng F-M, Zheng Y-M, Tung T-H, Chen H-X. Safety of an inactivated SARS-CoV-2 vaccine among healthcare workers in China. Expert Rev Vaccines. 2021;20(7):891–898. doi: 10.1080/14760584.2021.1925112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu JS, Zhang MX, Chien CW, Yang W-Y, Shi G-F, Qiu S, Tung T-H, Chen H-X. Sex differences in adverse reactions to an inactivated SARS-CoV-2 vaccine among Medical staff in China. Front Med. 2021;8:731593. doi: 10.3389/FMED.2021.731593/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson K, McDougall C, Upshur R. The new international Health Regulations and the federalism dilemma. PLoS Med. 2006;3(1):30–34. doi: 10.1371/JOURNAL.PMED.0030001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Straus W, Rubin H. COVID-19 vaccine safety monitoring in low and middle income countries – time for a bold new approach. Vaccine. 2022;40(32):4301. doi: 10.1016/J.VACCINE.2022.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buttery JP, Clothier H. Information systems for vaccine safety surveillance. Hum Vaccin Immunother. 2022;18(6). doi: 10.1080/21645515.2022.2100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harris T, Nair J, Fediurek J, Deeks SL. Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012–15. Vaccine. 2017;35(19):2600–4. doi: 10.1016/J.VACCINE.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 76.Klok FA, Pai M, Huisman MV, Makris M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022;9(1):e73–e80. doi: 10.1016/S2352-3026(21)00306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wong HL, Hu M, Zhou CK, et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet. 2022;399(10342):2191–9. doi: 10.1016/S0140-6736(22)00791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.WHO . Performance indicators. Accessed June 26, 2023. https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/aefi/performance-indicators
- 79.Kohl KS, Bonhoeffer J, Braun MM, RT Chen, Duclos P, Heijbel H, Heininger U, Loupi E, SM Marcy. The brighton collaboration: creating a global standard for case definitions (and guidelines) for adverse events following immunization. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Published online 2005. Accessed November 14, 2023. https://www.ncbi.nlm.nih.gov/books/NBK20507/ [PubMed]
- 80.Gold MS, Gidudu J, Erlewyn-Lajeunesse M, Law B. Can the Brighton collaboration case definitions be used to improve the quality of adverse event following immunization (AEFI) reporting?: anaphylaxis as a case study. Vaccine. 2010;28(28):4487–98. doi: 10.1016/J.VACCINE.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 81.Psihogios A, Brianne Bota A, Mithani SS, Greyson D, Zhu DT, Fung SG, Wilson SE, Fell DB, Top KA, Bettinger JA, et al. A scoping review of active, participant-centred, digital adverse events following immunization (AEFI) surveillance: a Canadian immunization research network study. Vaccine. 2022;40(31):4065–80. doi: 10.1016/J.VACCINE.2022.04.103. [DOI] [PubMed] [Google Scholar]


